Abstract
Background
Severe acute respiratory syndrome (SARS) is a life-threatening form of pneumonia caused by SARS coronavirus (SARS-CoV). From late 2002 to mid 2003, it infected more than 8000 people worldwide, of which a majority of cases were found in China. Owing to the absence of definitive therapeutic Western medicines, Houttuynia cordata Thunb. (Saururaceae) (HC) was shortlisted by Chinese scientists to tackle SARS problem as it is conventionally used to treat pneumonia.
Aim of the study
The present study aimed to explore the SARS-preventing mechanisms of HC in the immunological and anti-viral aspects.
Results
Results showed that HC water extract could stimulate the proliferation of mouse splenic lymphocytes significantly and dose-dependently. By flow cytometry, it was revealed that HC increased the proportion of CD4+ and CD8+ T cells. Moreover, it caused a significant increase in the secretion of IL-2 and IL-10 by mouse splenic lymphocytes. In the anti-viral aspect, HC exhibited significant inhibitory effects on SARS-CoV 3C-like protease (3CLpro) and RNA-dependent RNA polymerase (RdRp). On the other hand, oral acute toxicity test demonstrated that HC was non-toxic to laboratory animals following oral administration at 16 g/kg.
Conclusion
The results of this study provided scientific data to support the efficient and safe use of HC to combat SARS.
Keywords: Houttuynia cordata, Immunomodulation, SARS, Anti-viral, 3C-like protease, RNA-dependent RNA polymerase
1. Introduction
Houttuynia cordata Thunb. (Saururaceae) (HC) is a traditional Chinese medicine (TCM) used for hundreds of year to relieve lung-related symptoms such as lung abscess, phlegm, cough and dyspnea (State Pharmacopoeia Commission of People's Republic of China, 2005) and is effective in treating pneumonia, infectious disease, refractory hemoptysis as well as malignant pleural effusion (Anon, 1997). Recently, several studies also provided scientific data to support and unveil its anti-inflammatory (Park et al., 2005, Lu et al., 2006), anti-allergic (Li et al., 2005, Kim et al., 2007), virucidal (Hayashi et al., 1995, Chiang et al., 2003), anti-oxidative (Chen et al., 2003, Cho et al., 2003, Ng et al., 2007) and anti-cancer (Chang et al., 2001, Kim et al., 2001) activities.
During severe acute respiratory syndrome (SARS) outbreak in late 2002 to mid 2003, there were more than 8000 probable cases reported to WHO, of which over 7000 cases were found in China (http://www.who.int/en/). Owing to the high infectious rate and the absence of definitive therapeutic Western medicines, State Administration of Traditional Chinese Medicine of the People's Republic of China proposed six TCM formulae to general public as preventive measures on 24 April 2003 (http://www.satcm.gov.cn/zhuanti/jbfz/20060901/100052.shtml). Houttuynia cordata was one of the component herbs in a heat-removing and detoxifying formula. In order to reveal its underlying mechanisms in preventing SARS, HC was investigated for its immunomodulatory effect in mouse splenic lymphocytes and inhibitory activity on SARS coronaviral 3C-like protease (3CLpro) and RNA-dependent RNA polymerase (RdRp) in this work.
2. Materials and methods
2.1. Preparation of herbal extract
Dried HC, harvested in SiChuan Province of China, was purchased from an herbal supplier in Hong Kong. It was authenticated by morphological characterizations and thin layer chromatography (TLC, Merck silica gel G, hexane:ethyl acetate = 9:1 as the solvent system and visualized with staining solution of dinitrophenylhydrazine TS) against methyl nonyl ketone (National Institute for the Control Pharmaceuticals and Biological Products, Beijing, PRC) as the chemical marker in accordance with the Chinese Pharmacopoeia (State Pharmacopoeia Commission of People's Republic of China, 2005). A voucher specimen of HC was deposited in the museum of Institute of Chinese Medicine, The Chinese University of Hong Kong (Voucher specimen number: 2005-2606).
Water extract was prepared by boiling 300 g of HC in 3 l distilled water under reflux for 1 h twice. The extract was then centrifuged and filtered to remove tiny debris. The filtrate was eventually lyophilized into dry powder for experiments. The extraction yield was about 14%. HPLC profile of the extract for standardization was developed with Beckman-high performance liquid chromatography system gold equipped with Beckman ultra-sphere ODS column (4.6 mm × 250 mm i.d., particle size 5 μm) and photodiode array detector (Beckman 168 DAD detector) (Liu, 2007). The extract was dissolved in 30% ethanol to make a solution at concentration of 10 mg/ml and filtered with a 0.45-μm membrane prior to injection. Ten microliters of the solution was injected into the HPLC system with the detector set at 254 and 320 nm and eluted at a flow rate of 1 ml/min with a gradient profile of 0.1% formic acid in H2O as solvent A and acetonitrile as solvent B (starting with 5% of B, from 5% to 15% of B in 15 min; 15% to 25% of B in next 15 min; 25% to 55% of B in next 20 min and 55% to 90% of B in next 5 min).
2.2. Animals
Balb/c mice were supplied by Laboratory Animal Services Center, The Chinese University of Hong Kong. They were housed under the conditions of 22–25 °C and a 12-h light–dark cycle. They were supplied with standard animal chow (PicoLab Rodent Diet 20, PMI Nutrition International, Inc., Brentwood, MO, USA) ad libitum with free access to tap water.
2.3. Splenic lymphocytes preparation
Female Balb/c mice (6–8 weeks) were sacrificed by cervical dislocation and spleens were removed. Single cell suspension was obtained by pressing the spleens through a sterile wire sieve into complete RPMI medium. The single cell suspension was carefully layered onto the Ficoll-Paque Plus solution (Amersham Biosciences, Piscataway, NJ, USA) with a density gradient of 1.077 g/ml. The splenic lymphocytes were collected at the interface after centrifugation at 660 × g for 20 min at room temperature. The splenic lymphocytes were washed with RPMI medium and then suspended in complete RPMI medium to a concentration of 5 × 106 cells/ml.
2.4. 3H-thymidine incorporation assay
Water extract of HC was dissolved in complete RPMI medium and incubated with 10 μg/ml of polymyxin B sulfate (Sigma-Aldrich Co., St. Louis, MO, USA) at 37 °C for 1 h to mask the effect of probable presence of endotoxin in the extract. Splenic lymphocytes (5 × 105 cells/well) were seeded in a 96-well plate and incubated with HC extract at 0, 50, 100, 200 or 400 μg/ml for 48 or 72 h. Subsequently, 3H-thymidine (0.5 μCi per well) was added and the plate was incubated at 37 °C for 6 h. The cells were harvested with Unifilter-96 Harvester (PerkinElmer Inc., Waltham, MA, USA) and 3H-thymidine incorporation was then measured by TopCount NXT liquid-scintillation counter (PerkinElmer Inc.).
2.5. T cell population determination
Mouse splenic lymphocytes (5 × 106 cells) were treated for 24, 48 or 72 h in 12-well plate at 37 °C with HC water extract at 0, 100, 200 or 400 μg/ml that was pre-incubated with polymyxin B sulfate as described above. Splenic lymphocytes were then washed with cold staining buffer (2% heat inactivated FBS and 0.05% sodium azide in PBS) and blocked with mouse and rat IgG. Splenic lymphocytes were incubated with fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD4 antibody and phycoerythrin (PE)-conjugated rat anti-mouse CD8 antibody (BD Pharmingen, San Diego, CA, USA) on ice for 30 min. Cells were then washed with staining buffer and fixed with 0.8 ml of 1% paraformaldehyde (Sigma) and analyzed by FACSort flow cytometer (Becton, Dickinson & Co., Franklin Lakes, NJ, USA).
2.6. ELISA
Cell culture supernatant from mouse splenic lymphocytes treated with HC extract for 24, 48 or 72 h was tested for the presence of cytokines including IL-2, IL-4, IL-10 and IFN-γ by ELISA. It was carried out according to the manufacturer's protocol (BD Pharmingen).
2.7. SARS-CoV 3C-like protease (3CLpro) assay
2.7.1. Construction of His-3CLpro and protease substrate expression plasmids
DNA fragment encoding SARS-CoV main protease was amplified by PCR using primers [5′-TAGCTAGAATTCGGATCCAGTGGTTTTAGGAAAATG-3′] and [5′-TAACTAAAGCTTTCATTGGAAGGTAACACCAGA-3′], followed by digestion by BamHI and HindIII. The DNA was ligated with digested pET3a plasmid, which contains the gene of maltose binding protein (MBP). The resulting plasmid encodes the protease with an N-terminal His-MBP tag. For construction of the substrate, the primers [5′-GCCGCCGAGCTCACCAGCGCGGTGCTGCAGAGCGGCTTTCGCAAGATG-3′] and [5′-TATGCAGGTACCTTACTTGTACAGCTCGTCCAT-3′] were used to produce DNA fragment of substrate sequence with yellow fluorescent protein (YFP). After digestion by SacI and KpnI, the DNA was ligated with linearized pET3a plasmid cloned with cyan fluorescent protein (CFP) previously, so that the resulting plasmid encodes a protein-containing substrate sequence cloned in between CFP and YFP.
2.7.2. Expression and purification of His-tagged SARS-CoV 3CLpro and 3CLpro substrate proteins
Escherichia coli stain BL21 (DE3) (Novagen, EMD Chemicals Inc., Darmstadt, Germany) was used to express the recombinant pET-3a-3CLpro protein. BL21 (DE3) cells were transformed with pET-3a expression vector containing the pET-3a-3CLpro insert, as described above. The transformants were cultured in 500 ml of LB medium containing 0.4% glucose, 1 mM MgSO4, 100 μg/ml ampicillin and 50 μg/ml chloramphenicol at 37 °C until Abs600 nm reached 0.8. After that, they were induced with 0.4 mM isopropyl β-d-thiogalactoside (IPTG) at 37 °C for 4 h. The cells were then harvested by centrifugation at 4 °C.
For His-tagged SARS-CoV 3CLpro protein purification, the cell pellet was first resuspended in buffer A [20 mM Tris–HCl (pH 7.8), 20 mM NaCl, 10 mM imidazole] and disrupted on ice by sonication. The lysate was subsequently centrifuged to collect the supernatant for loading onto a nickel-chelated (Ni-NTA) column. The bound protein was eluted in a gradient of 0–100% buffer B [20 mM Tris–HCl (pH 7.8), 20 mM NaCl, 300 mM imidazole]. Purity of the isolated protein was examined by SDS-PAGE, followed by Coomassie blue staining. Thereafter, the semi-purified 3CLpro protein was pooled and dialyzed against buffer C [20 mM Tris–HCl (pH 8.0), 50 mM NaCl, 2 mM CaCl2, 10 mM 2-mercaptoethanol] overnight. The resulting protein samples were subjected to factor Xa cleavage at room temperature for 10 h. The His-tags were later removed by the nickel hi-trap column. At the end, the proteins so obtained were further purified using the S75 gel filtration column [buffer D: 20 mM Tris–HCl (pH 7.8), 20 mM NaCl, 10 mM 2-mercaptoethanol]. The protein concentration was determined by Bradford method (Loffler and Kunze, 1989) for protease activity assay.
Similarly, the plasmid pET-3a-3CLpro substrate was transformed into the E. coli stain BL21 (DE5) (Novagen), and the protein was expressed at 22 °C for 16 h with 0.4 mM IPTG induction. His-tagged SARS-CoV pET-3a-3CLpro substrate protein was isolated by means of the nickel-chelating column chromatography. Briefly, the pellet was resuspended in buffer A [20 mM Tris–HCl (pH 7.8), 20 mM NaCl, 10 mM imidazole] and disrupted on ice by sonication. The lysate was centrifuged. The supernatant resulted was allowed to bind onto a Ni-NTA column. Meanwhile, any unbound proteins were washed away thoroughly with buffer A. Then, the His-3CLpro substrate was eluted in a gradient of 0–100% buffer B [20 mM Tris–HCl (pH 7.8), 20 mM NaCl, 300 mM imidazole]. The pure protein fractions were pooled and dialyzed against buffer D [20 mM Tris–HCl (pH 7.8), 20 mM NaCl, 10 mM 2-mercaptoethanol] overnight. Again, the protein concentration was determined by the Bradford method and it was ready for the protease activity assay.
2.7.3. Fluorogenic SARS-CoV 3CLpro activity assay
The effect of HC water extract on SARS-CoV 3CLpro was studied as previously reported with minor modification (Kuo et al., 2004). HC extract, at different concentrations in reaction buffer (150 mM Tris, 150 mM NaCl, 10 mM 2-mercaptoethanol, pH 7.8), was incubated with 80 μl of 3CLpro (final concentration: 2 μM) for 15 min at room temperature. After that, 80 μl of 3CLpro substrate (20 μM) was added to the reaction mixture and fluorescence signals (Ex430 nm, Em486 nm, Em530 nm) were measured immediately at 15-s interval for 25 min by multilabel fluorescence reader (PerkinElmer 2101 EnVision, PerkinElmer Inc.).
2.8. SARS-CoV RNA-dependent RNA polymerase (RdRp) assay
2.8.1. Construction of GST-RdRp full-length expression plasmid
A 2.5 kb DNA fragment corresponding to the full length of the SARS-CoV RdRp CUHK W-1 (NCBI accession code: AAP13566) was amplified using the forward [5′-GTGGTGGGATCCTCTGCGCGGATGCATCAACGTTTTT-3′] and reverse [5′-TATGCGGCCGCTCACTGCAAGACTGTATGTGGTGTGTA-3′] primer set. The two primers contain BamHI and NotI sites, respectively. The amplified DNA fragment was digested at BamHI and NotI sites and subcloned into pGEX-6p2 vector (Amersham Biosciences) to form the pGEX-6p2–RdRp construct. At the 5′-end of the RdRp gene, a sequence encoding the GST protein was attached. The open reading frame of the final construct and the encoding of SARS-CoV RdRp (residues 1–932) were confirmed by DNA sequencing.
2.8.2. Expression and purification of GST-fusion SARS-CoV RdRp full-length protein
The pGEX-6p2–RdRp plasmid was transformed into Origami (DE3) E. coli strain (Novagen). A single colony was grown at 37 °C for 16 h in 10 ml of 2× YTG medium supplemented with ampicillin (0.1 mg/ml). The cell culture was further grown at 37 °C in 1L of 2× LB medium containing ampicillin (0.1 mg/ml) until OD600 reached 0.6–0.8. Protein expression was induced with 0.4 mM IPTG at 28 °C for 6 h before harvesting.
Protein purification was carried out using affinity chromatography with glutathione Sepharose™ Fast Flow (Amersham Biosciences). The cell pellet was resuspended in buffer A (50 mM Tris–HCl, pH 8.0, 500 mM NaCl, 2 mM DTT) containing 1 mM PMSF and 1 mM benzamidine and lysed on ice by sonication. The lysate was centrifuged at 40,000 × g and 4 °C for 1 h. The supernatant was loaded onto a glutathione Sepharose 4B column equilibrated with the washing buffer (buffer A). The column was washed with buffer A and the bound protein was then eluted with five column volumes of buffer B (10 mM reduced glutathione, 50 mM Tris–HCl, pH 8.0). The eluted fraction were pooled and concentrated. The concentrated proteins were dialyzed against buffer C (50 mM Tris–HCl, pH 8.0, 10% glycerol, 1 mM DTT) for 24 h. SDS-PAGE analysis were performed to check the purity and quality of the protein samples at every stage of purification. The purified proteins were characterized by western blot, mass spectrometry and polymerase activity assay. The protein concentration was determined by the Bradford method.
2.8.3. Radiometric RdRp assay—filter-binding enzyme assay
The filter-binding polymerase assay was modified from that used in polymerase activity assay of hepatitis C virus (HCV) RdRp (Yamashita et al., 1998). A total volume of 50 μl polymerase-containing reaction mixture included 50 mM Tris–HCl, pH 8.0, 7.5 mM KCl, 8 mM MgCl2, 10 mM DTT, 1%BSA, 3.5 μl of 1 mM UTP, 3.33 μCi of [α-32P] UTP (Amersham Pharmacia Biotech, Sweden), 3.12 μg/ml of poly A (Roche Applied Science, Switzerland), 5 μg/ml of RNA, 1 μg/ml of oligoU16 (Tech Dragon Ltd., Hong Kong, China), 20 units of RNAase inhibitor (Promega Bioscience, Madison, USA) and with or without HC water extract. The mixture was incubated at 37 °C for 1 h and reaction was stopped by adding a cold buffer solution containing 20 mM sodium pyrophosphate and 5% trichloroacetic acid. The solution was dotted on the Whatman GF/C glass microfiber filter and washed with the cold buffer five times and then 75% ethanol once. The incorporated triphosphate was assayed by measuring 32P using liquid scintillation counter (Beckman LS 6000SC).
2.9. Acute oral toxicity test
The acute oral toxicity test was carried out according to the Procedures and Methods for Toxicological Assessment on Food Safety, Peoples Republic of China. Balb/c mice (male and female) each weighed between 17 and 20 g were used. Twelve hours prior to dosing, all food was removed to fast the animals before initiating the test. On the day of the test, animals were identified and body weights recorded. The dosage to be administered was calculated based on the animal's body weight. The dosage of HC water extract was 16 g/kg body weight. The maximum volume is 0.4 ml/10 g body weight. The control group was treated with same amount of distilled water. Animals were closely observed for gross toxicological effects at 1, 3 and 6 h immediately after a single dose administration of the sample and then daily for a 7-day observation period. Test animals’ body weights, a sensitive indicator of toxic insult, were recorded during the observation period.
2.10. Statistical analysis
Data were analyzed using Mann–Whitney U test with SPSS 14.0 for Window. Statistical significance was accepted at p < 0.05.
3. Results
3.1. Proliferation of splenic lymphocytes
Splenic lymphocytes of Balb/c mice were incubated with HC extract at a concentration of 0–400 μg/ml in the presence of polymyxin B sulfate for 48 and 72 h. The proliferation response was shown in Fig. 1 . HC extract was found to significantly stimulate the proliferation of mouse splenic lymphocytes in a dose-dependent manner. However, the stimulation at 48 h is higher than that of 72 h.
Fig. 1.
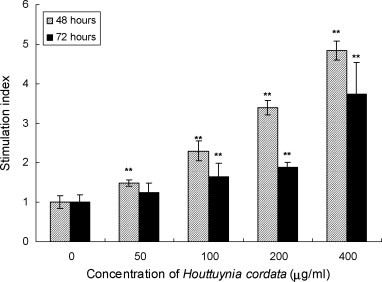
Lymphoproliferation activity of Houttuynia cordata extract on mouse splenic lymphocytes after 48 and 72 h treatment. The results are expressed as mean ± S.D. of six values from one representative experiment. **p < 0.01 vs. control (0 μg/ml).
3.2. Effect on CD4+ and CD8+ T cells in vitro
The above data raised our interest to investigate whether HC extract stimulates T cell population. Splenic lymphocytes were cultured with various concentrations of HC extract in the presence of polymyxin B sulfate for 24, 48 and 72 h. The numbers of CD4+ and CD8+ T cells were measured by flow cytometry. It was found that HC extract increased the proportion of CD4+ T cells in a dose-dependent manner (Table 1 ). HC extract also increased the proportion of CD8+ T cells after treatment (Table 2 ). These results indicated that HC stimulates T cells proliferation in vitro.
Table 1.
Percentage of CD4+ T cells in mouse splenic lymphocytes after HC treatment
| Concentration of HC extract (μg/ml) |
||||
|---|---|---|---|---|
| 0 | 100 | 200 | 400 | |
| 24 h | 31.8 ± 0.9 | 37.3 ± 1.4* | 36.8 ± 0.5* | 37.4 ± 1.0* |
| 48 h | 31.8 ± 0.7 | 35.5 ± 0.9* | 36.3 ± 0.4* | 38.0 ± 0.5* |
| 72 h | 27.1 ± 2.1 | 31.8 ± 0.5* | 32.8 ± 1.5* | 35.1 ± 0.7* |
Data are means ± S.D. of four values.
p < 0.05.
Table 2.
Percentage of CD8+ T cells in mouse splenic lymphocytes after HC treatment
| Concentration of HC extract (μg/ml) |
||||
|---|---|---|---|---|
| 0 | 100 | 200 | 400 | |
| 24 h | 10.0 ± 0.3 | 11.2 ± 0.6* | 10.9 ± 0.2* | 10.2 ± 0.3 |
| 48 h | 7.3 ± 0.4 | 9.1 ± 0.4* | 9.2 ± 0.2* | 9.2 ± 0.3* |
| 72 h | 4.8 ± 0.2 | 7.9 ± 0.2* | 8.6 ± 0.6* | 8.0 ± 0.2* |
Data are means ± S.D. of four values.
p < 0.05.
3.3. Effect on cytokine secretion
In addition to examining the effect of HC on T cell activation, we investigated its effect on helper T cell differentiation by measuring IL-2, IL-4, IL-10 and IFN-γ secretion. After 48 and 72 h treatment, HC extract increased the level of IL-2 in a dose-dependent manner (Fig. 2A). HC extract also caused a significant increase in secretion of IL-10 by mouse splenic lymphocytes (Fig. 2B). However, the levels of IL-4 did not change (Fig. 2C) and the level of IFN-γ decreased in a dose-dependent manner after treatment (Fig. 2D).
Fig. 2.
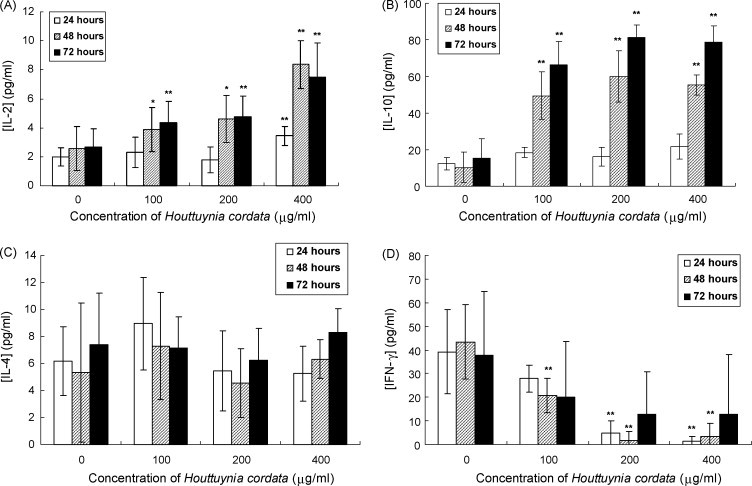
Effect of Houttuynia cordata on IL-2 (A), IL-10 (B), IL-4 (C) and IFN-γ (D) production in mouse splenic lymphocytes. Mouse splenic lymphocytes were treated with HC extract for 24, 48 and 72 h. Cytokine production was measured by ELISA. Data are expressed as mean ± S.D. of values from at least three independent experiments. *p < 0.05, **p < 0.01 vs. control (0 μg/ml).
3.4. Effect on SARS-CoV 3CLpro activity
The effect of HC on SARS-CoV 3CLpro was evaluated by a protein-based fluorogenic assay. HC, at concentration of 200 μg/ml or above, could significantly inhibit the activity of 3CLpro (p < 0.05). At the highest testing dose (1000 μg/ml), the 3CLpro activity was decreased to 50% of control. This result indicated that HC exhibited a dose-dependent inhibition on 3CLpro activity significantly (Fig. 3 ).
Fig. 3.
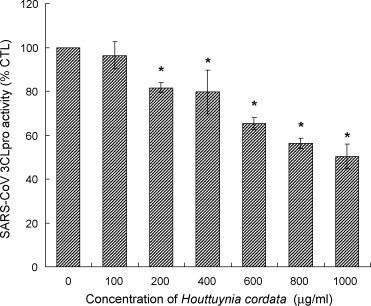
Effect of Houttuynia cordata on the SARS coronaviral 3CLpro activity. Data are expressed as mean ± S.D. of values from four independent experiments. *p < 0.05 vs. control (0 μg/ml).
3.5. Effect on RdRp activity
The RdRp activities of the protein samples were further confirmed by the conventional [α-32P] UTP incorporation using filter-binding polymerase assay modified from that used for HCV RdRp (Yamashita et al., 1998). During RNA synthesis, the [α-32P] UTP was incorporated into the newly synthesized RNA. Afterwards, the RNA binding on the filter was separated from the free unincorporated nucleotides and the radioactivity was measured by liquid scintillation counting.
The purified GST-RdRp exhibited a dose-dependent RdRp activity when polyA was used as templates (Data not shown). To study the effect of HC on the polymerase activity, different concentrations of HC (50, 100, 200, 400 and 800 μg/ml) were tested. All concentrations of HC showed a significant decrease in RdRp activity. At the highest dose (800 μg/ml), the RdRp activity was decreased to 26% of control. This result indicated that HC exhibited a dose-dependent inhibition on RdRp activity significantly (Fig. 4 ).
Fig. 4.
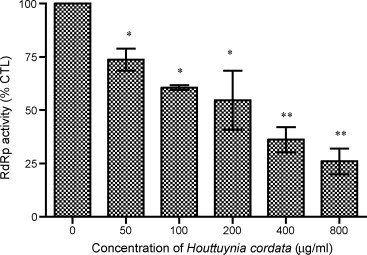
Effect of Houttuynia cordata on the SARS-CoV RdRp activity using radiometric RdRp assay by [α-32P] UTP incorporation. Data are expressed as mean ± S.D. from three individual experiments. *p < 0.05, **p < 0.01 vs. control.
3.6. Acute oral toxicity test
Twenty Balb/c mice (10 male, 10 female) were administrated a single oral dose of HC water extract at 16 g/kg. All animals appeared normal throughout the 7-day observation period. There was no difference of body weights between HC group and control group (p > 0.05) (Table 3 ). According to Procedures and Methods for Toxicological Assessment on Food Safety, Peoples Republic of China, HC water extract was tested as specified and considered to be essentially non-toxic to laboratory animals following oral administration at 16 g/kg.
Table 3.
Change of body weights of animals in the oral acute toxicity study of Houttuynia cordata water extract
| Group | Number of animal | Dose (g/kg) | Mortality (%) | Average body weight (g) |
Body weight gain (g) | p-value vs. control | |
|---|---|---|---|---|---|---|---|
| Initial | Final | ||||||
| HC extract | M 10 | 16 | 0 | 18.9 ± 0.5 | 20.5 ± 0.7 | 1.6 ± 0.5 | >0.05 |
| F 10 | 16 | 10 | 17.2 ± 0.6 | 17.9 ± 0.8 | 0.9 ± 0.6 | >0.05 | |
| Control | M 10 | – | 0 | 18.7 ± 0.6 | 20.8 ± 0.5 | 2.1 ± 0.4 | |
| F 10 | – | 0 | 19.0 ± 0.7 | 20.4 ± 1.0 | 1.4 ± 0.7 | ||
Data are means ± S.D.
4. Discussion and conclusion
T cells are essential for adaptive immunity against viral infections. Anti-viral CD4+ helper T cells (Th) help the production of virus-specific antibodies by B cells, while CD8+ cytotoxic T cells (Tc) can kill virus-infected host cells. Lymphopenia was usually observed in SARS patients at the initial phase of infection (Li et al., 2003, Yu et al., 2003, He et al., 2005). Virus-induced apoptosis of lymphocytes has been suggested as a cause for lymphopenia seen in SARS patients (O’Donnell et al., 2003). Since monocytes and T cells are involved in both the innate and adaptive immune response, destruction of these cells may result in a compromised immune response and the development of disease. Our results demonstrated that HC extract could exhibit immunostimulatory effect (Fig. 1; Table 1, Table 2). It was found to stimulate the proliferation of CD4+ helper T cells and CD8+ cytotoxic T cells, which may help to prevent SARS-CoV infection.
CD4+ helper T cells can be divided into different subtypes, based on the specific profiles of cytokines that they release. Th1 cells have been found to produce IL-2, IFN-γ and lymphotoxin-α, whereas Th2 cells produce IL-4, IL-5, IL-9 and IL-13 (Romagnani, 2006). Th1 cells assist in activating macrophages and Tc cells, which are particularly useful for eliminating intracellular infections; Th2 cells mediate anti-helminth and allergic responses. Type 1 regulatory T (Tr1) cells are distinct from the classical Th1 or Th2 cells. They produce high levels of IL-10, with or without TGF-β, IFN-γ and IL-5, and low or no IL-2 and IL-4 (Veldman et al., 2006). IL-10 has a key effect on the suppression of Th1 cell responses and has a role in host protection against harmful effects of an exacerbated cellular immune response during acute infection. In order to investigate how HC extract affect the T cell differentiation, we measured the levels of IL-2, IL-4, IL-10 and IFN-γ secreted by mouse splenic lymphocytes. From our results, IFN-γ and IL-4 that are secreted by Th1 and Th2, respectively, did not increase. On the contrary, IL-10 that is secreted by Tr1 increased significantly after treatment. We speculated that HC extract caused the induction of Tr1. In the 3H-thymidine incorporation assay, the stimulatory response in 72 h is lower than that of 48 h (Fig. 1). This may due to the negative feedback by Tr1 cells that suppress Th cells response. Induction of Tr1 cells can assist in turning off immune response after pathogen elimination and prevent harmful effect. However, further in-depth studies are needed to confirm the induction of Tr1 by HC extract.
The SARS-CoV main protease, 3CLpro , is responsible for releasing the key replicative enzymes such as RdRp and helicase from the polyprotein precursors (Thiel et al., 2003). This functional importance of 3CLpro in the life cycle of virus makes it a key target for the development of drugs directed against SARS (Anand et al., 2003, Gan et al., 2006, Wu et al., 2006) and for screening the anti-SARS effect of traditional Chinese medicines (Lin et al., 2005). In order to study the effect of HC extract on SARS-CoV 3CLpro, a convenient protein-based fluorescence resonance energy transfer (FRET) assay system was set up for measuring the enzyme activity. The substrate for assay is a recombinant protein that has a cleavable linker sequence (TSAVLQ↓SGFRK) between two fluorescent proteins, viz CFP and YFP. The former one is the donor protein of which the emission spectrum overlaps with the excitation spectrum of the latter acceptor protein. If these two fluorophores are very close to each other, FRET will occur. When excited at 430 nm, CFP will emit fluorescent at 486 nm that will in turn excite YFP and cause it to emit fluorescent at 530 nm. If the SARS-CoV 3CLpro is active, the linker sequence will be cleaved, two fluorophores separated and the fluorescence ratio at 530/486 decreased. HC extract, at 200 μg/ml or above, could effectively inhibit the decrease in fluorescence ratio of the substrate, implying that it could inactivate the SARS-CoV 3CLpro.
The SARS-CoV RNA-dependent RNA polymerase is a key enzyme responsible for both positive and negative strand RNA synthesis. It is the essential enzyme in a replicase complex that is expected to contain additional viral and cellular proteins (Thiel et al., 2003). Given the crucial role of RdRp in the virus life cycle and the success obtained with polymerase inhibitors in the treatment of viral infections including human immunodeficiency virus type 1 (HIV-1), human hepatitis B virus (HBV) (Korba et al., 2006), HCV and herpes virus, SARS-CoV RdRp is an attractive target for the development of anti-SARS drugs. At present, there are no structural and very limited biochemical data on coronavirus polymerase. Thus, study on the polymerization mechanisms is likely to aid the development of anti-SARS agent. It is reported that during purification, the full-length enzyme is found to be hydrolytically cleaved into three main fragments: an N-terminal p12 fragment, a middle p30 fragment, and a C-terminal p64 fragment which comprises the polymerase catalytic domain (Cheng et al., 2005). Currently, the recombinant SARS-RdRp expressed in E. coli is characterized by radioactive assay (Al et al., 1998, Yamashita et al., 1998, Cheng et al., 2005) and we demonstrated that radiolabeled nucleotides incorporation increased with increasing SARS-CoV RdRp protein (Data not shown). HC extract, at 50 μg/ml or above, could effectively inhibit [α-32P] UTP incorporation, implying that it can inactivate the SARS-CoV RdRp activity.
Taking all the results into account, the actions of HC extract on SARS may be biphasic. Before the invasion of SARS-CoV, HC extract may activate the cell-mediated immunity to prevent viral infection. In case of infected, HC extract may slow down the viral replication process by inhibiting the pivotal enzymes and trigger negative feedback control in immune system.
Acknowledgments
This work was supported by the Research Fund for the Control of Infectious Diseases (02040332), Health, Welfare and Food Bureau, The Government of the Hong Kong Special Administrative Region (HKSAR). We would also like to thank Mrs. Yeung Yeo G.Y. and Miss Chong L.T. for their technical support.
References
- Al R.H., Xie Y., Hagedorn C.H. Expression of recombinant hepatitis C virus non-structural protein 5B in Escherichia coli. Virus Research. 1998;53:141–149. doi: 10.1016/s0168-1702(97)00147-0. [DOI] [PubMed] [Google Scholar]
- Anand K., Ziebuhr J., Wadhwani P., Mesters J.R., Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300:1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- Anon . Herba Houttuynia. In: Zheng H.Z., Dong Z.H., She J., editors. vol. 3. Xue Yuan Press; Beijing: 1997. pp. 2983–3003. (Modern Study of Traditional Chinese Medicine). [Google Scholar]
- Chang J.S., Chiang L.C., Chen C.C., Liu L.T., Wang K.C., Lin C.C. Antileukemic activity of Bidens pilosa L. var. minor (Blume) Sherff and Houttuynia cordata Thunb. American Journal of Chinese Medicine. 2001;29:303–312. doi: 10.1142/S0192415X01000320. [DOI] [PubMed] [Google Scholar]
- Chen Y.Y., Liu J.F., Chen C.M., Chao P.Y., Chang T.J. A study of the antioxidative and antimutagenic effects of Houttuynia cordata Thunb. using an oxidized frying oil-fed model. Journal of Nutritional Science & Vitaminology. 2003;49:327–333. doi: 10.3177/jnsv.49.327. [DOI] [PubMed] [Google Scholar]
- Cheng A., Zhang W., Xie Y., Jiang W., Arnold E., Sarafianos S.G., Ding J. Expression, purification, and characterization of SARS coronavirus RNA polymerase. Virology. 2005;335:165–176. doi: 10.1016/j.virol.2005.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang L.C., Chang J.S., Chen C.C., Ng L.T., Lin C.C. Anti-Herpes simplex virus activity of Bidens pilosa and Houttuynia cordata. American Journal of Chinese Medicine. 2003;31:355–362. doi: 10.1142/S0192415X03001090. [DOI] [PubMed] [Google Scholar]
- Cho E.J., Yokozawa T., Rhyu D.Y., Kim H.Y., Shibahara N., Park J.C. The inhibitory effects of 12 medicinal plants and their component compounds on lipid peroxidation. American Journal of Chinese Medicine. 2003;31:907–917. doi: 10.1142/S0192415X03001648. [DOI] [PubMed] [Google Scholar]
- Gan Y.R., Huang H., Huang Y.D., Rao C.M., Zhao Y., Liu J.S., Wu L., Wei D.Q. Synthesis and activity of an octapeptide inhibitor designed for SARS coronavirus main proteinase. Peptides. 2006;27:622–625. doi: 10.1016/j.peptides.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Kamiya M., Hayashi T. Virucidal effects of the steam distillate from Houttuynia cordata and its components on HSV-1, influenza virus, and HIV. Planta Medica. 1995;61:237–241. doi: 10.1055/s-2006-958063. [DOI] [PubMed] [Google Scholar]
- He Z., Zhao C., Dong Q., Zhuang H., Song S., Peng G., Dwyer D.E. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. International Journal of Infectious Diseases. 2005;9:323–330. doi: 10.1016/j.ijid.2004.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I.S., Kim J.H., Kim J.S., Yun C.Y., Kim D.H., Lee J.S. The inhibitory effect of Houttuynia cordata extract on stem cell factor-induced HMC-1 cell migration. Journal of Ethnopharmacology. 2007;112:90–95. doi: 10.1016/j.jep.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Kim S.K., Ryu S.Y., No J., Choi S.U., Kim Y.S. Cytotoxic alkaloids from Houttuynia cordata. Archives of Pharmacal Research. 2001;24:518–521. doi: 10.1007/BF02975156. [DOI] [PubMed] [Google Scholar]
- Korba B.E., Furman P.A., Otto M.J. Clevudine: a potent inhibitor of hepatitis B virus in vitro and in vivo. Expert Review of Anti-infective Therapy. 2006;4:549–561. doi: 10.1586/14787210.4.4.549. [DOI] [PubMed] [Google Scholar]
- Kuo C.J., Chi Y.H., Hsu J.T., Liang P.H. Characterization of SARS main protease and inhibitor assay using a fluorogenic substrate. Biochemical & Biophysical Research Communications. 2004;318:862–867. doi: 10.1016/j.bbrc.2004.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G.Z., Chai O.H., Lee M.S., Han E.H., Kim H.T., Song C.H. Inhibitory effects of Houttuynia cordata water extracts on anaphylactic reaction and mast cell activation. Biological & Pharmaceutical Bulletin. 2005;28:1864–1868. doi: 10.1248/bpb.28.1864. [DOI] [PubMed] [Google Scholar]
- Li T., Qiu Z., Han Y., Wang Z., Fan H., Lu W., Xie J., Ma X., Wang A. Rapid loss of both CD4+ and CD8+ T lymphocyte subsets during the acute phase of severe acute respiratory syndrome. Chinese Medical Journal. 2003;116:985–987. [PubMed] [Google Scholar]
- Lin C.W., Tsai F.J., Tsai C.H., Lai C.C., Wan L., Ho T.Y., Hsieh C.C., Chao P.D. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antiviral Research. 2005;68:36–42. doi: 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.C. Study on HPLC fingerprint of flavoniods in ultramicro-powders of Houttuynia Cordata Thunb. Science & Technology Review. 2007;25:45–49. [Google Scholar]
- Loffler B.M., Kunze H. Refinement of the Coomassie brilliant blue G assay for quantitative protein determination. Analytical Biochemistry. 1989;177:100–102. doi: 10.1016/0003-2697(89)90021-3. [DOI] [PubMed] [Google Scholar]
- Lu H.M., Liang Y.Z., Yi L.Z., Wu X.J. Anti-inflammatory effect of Houttuynia cordata injection. Journal of Ethnopharmacology. 2006;104:245–249. doi: 10.1016/j.jep.2005.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L.T., Yen F.L., Liao C.W., Lin C.C. Protective Effect of Houttuynia cordata extract on Bleomycin-induced pulmonary fibrosis in rats. American Journal of Chinese Medicine. 2007;35:465–475. doi: 10.1142/S0192415X07004989. [DOI] [PubMed] [Google Scholar]
- O’Donnell R., Tasker R.C., Roe M.F. SARS: understanding the coronavirus: apoptosis may explain lymphopenia of SARS. British Medical Journal. 2003;327:620–627. doi: 10.1136/bmj.327.7415.620-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E., Kum S., Wang C., Park S.Y., Kim B.S., Schuller-Levis G. Anti-inflammatory activity of herbal medicines: inhibition of nitric oxide production and tumor necrosis factor-alpha secretion in an activated macrophage-like cell line. American Journal of Chinese Medicine. 2005;33:415–424. doi: 10.1142/S0192415X05003028. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Regulation of the T cell response. Clinical and Experimental Allergy. 2006;36:1357–1366. doi: 10.1111/j.1365-2222.2006.02606.x. [DOI] [PubMed] [Google Scholar]
- State Pharmacopoeia Commission of People's Republic of China . vol. I. Chemical Industry Press; Beijing: 2005. p. 155. (Pharmacopoeia of the People's Republic of China 2005 ed). [Google Scholar]
- Thiel V., Ivanov K.A., Putics A., Hertzig T., Schelle B., Bayer S., Weissbrich B., Snijder E.J., Rabenau H., Doerr H.W., Gorbalenya A.E., Ziebuhr J. Mechanisms and enzymes involved in SARS coronavirus genome expression. Journal of General Virology. 2003;84:2305–2315. doi: 10.1099/vir.0.19424-0. [DOI] [PubMed] [Google Scholar]
- Veldman C., Nagel A., Hertl M. Type I regulatory T cells in autoimmunity and inflammatory diseases. International Archives of Allergy & Immunology. 2006;140:174–183. doi: 10.1159/000092576. [DOI] [PubMed] [Google Scholar]
- Wu Y.S., Lin W.H., Hsu J.T., Hsieh H.P. Antiviral drug discovery against SARS-CoV. Current Medicinal Chemistry. 2006;13:2003–2020. doi: 10.2174/092986706777584988. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Kaneko S., Shirota Y., Qin W., Nomura T., Kobayashi K., Murakami S. RNA-dependent RNA polymerase activity of the soluble recombinant hepatitis C virus NS5B protein truncated at the C-terminal region. Journal of Biological Chemistry. 1998;273:15479–15486. doi: 10.1074/jbc.273.25.15479. [DOI] [PubMed] [Google Scholar]
- Yu X.Y., Zhang Y.S., Han C.W., Wang P., Xue X.J., Cong Y.L. Change of T lymphocyte and its activated subsets in SARS patient. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2003;25:542–546. [PubMed] [Google Scholar]


