Abstract
Acute respiratory tract infections (ARTIs) are among the leading causes of morbidity and mortality in children. The viral etiology of ARTIs was investigated over 3 years (October 2012–September 2015) in 2575 children in Parma, Italy, using indirect immunofluorescent staining of respiratory samples for viral antigens, cell culture, and molecular assays. Respiratory viruses were detected in 1299 cases (50.44%); 1037 (79.83%) were single infections and 262 (20.17%) mixed infections. The highest infection incidence was in children aged >6 months to ≤3 years (57.36%). Human respiratory syncytial virus (27.12%) and human adenovirus (23.58%) were the most common viruses identified. The virus detection rate decreased significantly between the first and third epidemic season (53.9% vs. 43.05%, P < 0.0001). The simultaneous use of different diagnostic tools allowed us to identify a putative viral etiology in half the children examined and to provide an estimate of the epidemiology and seasonality of respiratory viruses associated with ARTIs.
Abbreviations: ARTI, acute respiratory tract infection; BAL, bronchial alveolar lavage; BAS, bronchial aspirate; BSA, bovine serum albumin; CPE, cytopathic effect; CV, coxsackievirus; ECHO, echovirus; HADV, human adenovirus; HBOV, human bocavirus; HCOV, human coronavirus; HEP-2, human larynx epidermoid carcinoma; HEV, human enterovirus; HMPV, human metapneumovirus; HPIV, human parainfluenza virus; HRSV, human respiratory syncytial virus; IV, influenza virus; IAV, influenza virus A; IBV, influenza virus B; IIF, indirect immunofluorescence assay; Intestine 407, embryonic human intestine; IV, influenza virus; LLC-MK2, rhesus monkey-kidney; mAb, monoclonal antibody; MDCK-SIAT1, Madin-Darby Canine Kidney with enhanced expression of 6-linked sialic acids; MRC-5, human lung fibroblasts; NPA, nasopharyngeal aspirate; NS, nasal swab; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; RT-PCR, real-time PCR; rt-RT-PCR, reverse transcription RT-PCR; SP, sputum; TS, throat swab; Vero, African green monkey kidney
Keywords: Acute respiratory tract infections, Respiratory viruses, Molecular assays, Virus isolation in cell culture, Epidemiology, Laboratory diagnosis
Highlights
-
•
Respiratory viruses were assessed in children from October 2012 to September 2015.
-
•
Viruses were detected using antigen and molecular assays, and cell culture.
-
•
Respiratory syncytial virus and adenovirus were the most common viruses detected.
-
•
Influenza virus and respiratory syncytial virus detection showed seasonal variation.
-
•
Respiratory virus detection was highest in children aged >6 months to ≤3 years.
1. Introduction
Acute respiratory tract infections (ARTIs) are a persistent public health problem (Lu et al., 2013).
ARTIs affect the upper respiratory tract, leading to colds, rhinosinusitis, pharyngitis, laryngitis, tracheitis, and otitis media, as well as the lower respiratory tract, causing tracheitis, bronchitis, bronchiolitis, and pneumonia (Bicer et al., 2013). Although the majority of ARTIs remain confined to the upper tract (Tregoning and Schwarze, 2010), they can cause severe manifestations when affecting the lower tract (Zappa et al., 2008).
Viruses are frequently the cause of ARTIs characterized by high risks of morbidity and mortality (Taylor et al., 2017). The viruses most frequently detected are: influenza viruses A and B (IAV, IBV), human parainfluenza virus (HPIV), human adenovirus (HADV), human metapneumovirus (HMPV), and human respiratory syncytial virus (HRSV). This list is constantly evolving along with improvement in the performance of diagnostic tests (Ljubin-Sternak et al., 2016, Tregoning and Schwarze, 2010) and the discovery of more recent viruses, for example, human bocavirus (HBOV) (Allander et al., 2005), human coronaviruses (HCOVs) NL63 and HKU1, and new human enterovirus (HEV), parechovirus, and rhinovirus strains (Berry et al., 2015).
The main diagnostic methods for respiratory virus infections are: virus isolation in cell culture, viral antigen/nucleic acid detection, and virus-specific serology. Virus isolation is labor-intensive (Landry, 1997), making it impractical to determine a diagnosis during the acute infection phase. Shell vial cultures and viral antigen detection reduce the time for diagnosis (Engler and Preuss, 1997, Gardner and McQuillin, 1974). Nearly all these methods are being replaced by highly sensitive multiplex PCR (Kim et al., 2009, Tregoning and Schwarze, 2010).
ARTI prevalence in children varies by geographic area, season, and year (Goktas and Sirin, 2016, Moura et al., 2009, Panda et al., 2017). Recent Italian data are limited, apart from those relating to influenza (Pariani et al., 2015, Trucchi et al., 2017).
This three-year (October 2012–September 2015) hospital-based survey in Parma (Northern Italy) aimed to determine the prevalence of respiratory virus infections, their seasonality, and any patterns of mixed infections in children with ARTIs by using indirect immunofluorescent staining of respiratory samples for viral antigens, cell culture, and molecular assays.
2. Materials and methods
2.1. Study setting
The study was conducted at the Virology Unit of the University Hospital of Parma (Northern Italy), a 1300-bed tertiary care center with more than 50,000 admissions per year from the city and surroundings with approximately 450,000 inhabitants. Laboratory diagnosis was performed upon medical request. Patients' identities and medical information were protected.
The inclusion criteria were: patients ≤14 years old, acute fever, respiratory symptoms, and illness onset within 2 days.
From October 1st, 2012 to September 30th, 2015, 2892 samples from 2575 children (1148 females, 44.58%, and 1427 males, 55.42%; 2311 inpatients, 89.75%, and 264 outpatients, 10.25%) were collected by bronchoalveolar lavages (BALs), bronchial aspirates (BASs), nasopharyngeal aspirates (NPAs), nasal swabs (NSs), sputum (SP), and throat swabs (TSs). NPAs, NSs and TSs were stored in viral transport medium (De Conto et al., 2018) and all samples were kept at 4 °C until submitted to laboratory (within 2 h of sampling).
Age was available for 2570 children (99.8%) and ranged from ≥2 days to 14 years (mean age: 3 years 6 months; median age: 2 years 1 month). Children were divided into four age groups: 0 to ≤6 months (21.98%), >6 months to ≤3 years (37.78%), >3 to ≤6 years (18.95%), and > 6 to 14 years (21.28%). Of note, 236 children (9.16%) were examined at least twice during the survey, with a time interval between care provider visits of 3 days to 8 months, presumably for either worsening symptoms of a current infection or infection recurrence.
2.2. HRSV antigen detection by indirect immunofluorescence assay (IIF)
NPAs, NSs, and TSs were centrifuged (1000 rpm, 10 min, 4 °C) and the pellets resuspended in phosphate-buffered saline (PBS, pH 7.4; 7 mM Na2HPO4, 1.5 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl), before cytocentrifugation (1000 rpm, 5 min). Cell sediments on slides were dried in a laminar-flow hood and fixed in acetone (10 min, −20 °C). These were then hydrated with PBS, blocked with 1% bovine serum albumin (BSA) in PBS, and the slides were incubated (1 h, 37 °C) with anti-HRSV monoclonal antibodies (mAbs) (1:40; Argene/BioMérieux, France) in 0.2% BSA in PBS. After washes with PBS, they were incubated with fluorescein isothiocyanate-conjugated anti-mAbs (1:200; Argene/BioMérieux) in 0.0001% Evans Blue in PBS (45 min, 37 °C). After further washes with PBS, the sediments were mounted in glycerol and analyzed by epifluorescence microscopy (DMLB Leica, Germany).
2.3. Cells
Madin-Darby Canine Kidney cells with 6-linked sialic acids enhanced expression (MDCK-SIAT1) came from Sigma-Aldrich (Italy), with human lung fibroblasts (MRC-5) from LGC Standards (Italy). Human larynx epidermoid carcinoma (HEP-2), embryonic human intestine (Intestine 407), rhesus monkey kidney (LLC-MK2), and African green monkey kidney (Vero) cells came from the “Istituto Zooprofilattico Sperimentale della Lombardia e dell'Emilia-Romagna” (Italy). Cells were grown in Earle's Modified Minimum Essential Medium (E-MEM) supplemented with 2 mM L-glutamine, 10% fetal bovine serum, and antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin), and maintained at 37 °C in a 5% CO2 atmosphere. Reagents were from Life Technologies (Italy).
2.4. HADV, HPIV, HRSV, and IV detection by rapid culture method
After aspiration of the growth medium, MDCK-SIAT1, HEP-2, and Intestine 407 cells grown in 8-well chamber slides (Fisher Scientific, Italy) were incubated with samples (1 h, 37 °C). After restoring the E-MEM, incubation was carried out in E-MEM for 24 h before IIF with mAbs against HADV, HPIV, HRSV, and influenza virus (IV) (Argene/BioMérieux), as described above.
2.5. Respiratory virus detection by conventional culture method
HADV, HEV, HPIV, HRSV, and IV isolation was performed in LLC-MK2, MDCK-SIAT1, HEP-2, Intestine 407, MRC-5, and Vero cells grown in 24-well plates (Sigma-Aldrich). After aspirating the E-MEM, cells were incubated with samples (1 h, 37 °C). After restoring the E-MEM, incubation in E-MEM was continued until the appearance of cytopathic effect (CPE). If the cells showed no CPE after 7 days, they were scraped, and cell suspension was collected for a second inoculation. When CPE was observed, cells were scraped in PBS and cytocentrifuged, before IIF. When CPE suggested HEV infection, neutralization assays were performed.
2.6. Neutralization assays
HEVs serotype identification was performed with type-specific antisera (Statens Serum Institut, Denmark), according to Lim and Benyesh-Melnick (1960).
2.7. Respiratory virus detection by molecular assays
Samples were screened for HADV, HBOV, HCOV, HMPV, HPIV, HRSV, and IV nucleic acids with real-time (RT)-PCR or reverse transcription (rt)-RT-PCR assays. Nucleic acid extraction was performed with the NucliSENS® easyMAG® kit on the EasyMAG extractor (BioMérieux, Italy) and rt with the RT-kit plus (ELITechGroup Molecular Diagnostics, France) on the GeneAmp PCR System 9700 thermocycler (Applied Biosystems, Thermo Fischer Scientific-Waltham, USA). Extracted nucleic acids were tested with Influenza A/B Q-PCR Alert AmpliMIX kit (ELITechGroup) on the 7300 RT-PCR system (Applied Biosystems). The Respiratory Multi Well System (MWS) r-gene™ assay (Argene/BioMérieux) was used to detect ADV/HBOV, HCOV/HPIV, and RSV/HMPV on the Rotor-Gene Q system (Qiagen, Germany). Assays were performed according to the manufacturer's instructions.
2.8. Statistical analysis
A chi-square test was performed using GraphPad Prism software. P < 0.05 was considered statistically significant.
3. Results
3.1. Prevalence of respiratory viruses
A total of 2892 respiratory secretions from 2575 children were analyzed as described in the Methods section; 1408 samples (48.69%) from 1299 (50.44%) children (714 males, 54.97%, and 585 females, 45.03%) were virus-positive (Table 1 and Supplementary Table 1).
Table 1.
Rate of respiratory viral infections in children with ARTIs in Northern Italy (October 2012–September 2015).
| TS | NPA | BAS | BAL | NS | SP | Total | |
|---|---|---|---|---|---|---|---|
| No. of samples examined | 2172 | 703 | 10 | 3 | 3 | 1 | 2892 |
| Single infections (%) | 764 (67.73) | 359 (31.82) | 2 (0.18) | 2 (0.18) | 0 (0) | 1 (0.09) | 1128 (39) |
| HRSV | 130 (11.52) | 209 (18.53) | 0 (0) | 1 (0.09) | 0 (0) | 0 (0) | 340 (30.14) |
| HADV | 240 (21.28) | 22 (1.95) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 262 (23.23) |
| HCOV | 104 (9.22) | 33 (2.92) | 0 (0) | 0 (0) | 0 (0) | 1 (0.09) | 138 (12.23) |
| HBOV | 62 (5.50) | 27 (2.40) | 1 (0.09) | 0 (0) | 0 (0) | 0 (0) | 90 (7.98) |
| IAV | 67 (5.94) | 18 (1.60) | 0 (0) | 1 (0.09) | 0 (0) | 0 (0) | 86 (7.62) |
| HPIV | 56 (4.96) | 26 (2.30) | 1 (0.09) | 0 (0) | 0 (0) | 0 (0) | 83 (7.36) |
| HMPV | 35 (3.10) | 16 (1.42) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 51 (4.52) |
| IBV | 47 (4.17) | 3 (0.26) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 50 (4.43) |
| CV | 11 a (0.97) | 3 b (0.26) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 14 (1.24) |
| ECHO | 5 c (0.44) | 2 d (0.18) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (0.62) |
| Not typeable HEV | 7 (0.62) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (0.62) |
| Mixed infections (%) | 200 (71.43) | 79 (28.21) | 0 (0) | 0 (0) | 1 (0.36) | 0 (0) | 280 (9.68) |
| HADV + HRSV | 22 (7.85) | 15 (5.35) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 37 (13.21) |
| HADV + HCOV | 34 (12.14) | 2 (0.71) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 36 (12.85) |
| HRSV + HCOV | 13 (4.64) | 22 (7.85) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 35 (12.5) |
| HADV + HBOV | 20 (7.14) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 20 (7.14) |
| HRSV + HBOV | 11 (3.93) | 5 (1.78) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 16 (5.71) |
| HRSV + IAV | 6 (2.14) | 3 (1.07) | 0 (0) | 0 (0) | 1 (0.36) | 0 (0) | 10 (3.57) |
| HCOV + IAV | 7 (2.5) | 3 (1.07) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 10 (3.57) |
| HADV + HPIV | 8 (2.85) | 1 (0.36) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 9 (3.21) |
| HADV + HMPV | 6 (2.14) | 2 (0.71) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 8 (2.85) |
| HCOV + HMPV | 5 (1.78) | 3 (1.07) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 8 (2.85) |
| HBOV + HCOV | 6 (2.14) | 1 (0.36) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (2.5) |
| HBOV + IAV | 6 (2.14) | 1 (0.36) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (2.5) |
| HRSV + IBV | 7 (2.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (2.5) |
| HADV + IAV | 4 (1.43) | 1 (0.36) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (1.78) |
| HBOV + HPIV | 3 (1.07) | 2 (0.71) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (1.78) |
| HRSV + HPIV | 4 (1.43) | 1 (0.36) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (1.78) |
| HCOV + IBV | 2 (0.71) | 2 (0.71) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (1.43) |
| HADV + HCOV + HBOV | 4 (1.43) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (1.43) |
| HADV + HCOV + HRSV | 1 (0.36) | 3 (1.07) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (1.43) |
| HADV + IBV | 1 (0.36) | 2 (0.71) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (1.07) |
| HBOV + HMPV | 1 (0.36) | 2 (0.71) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (1.07) |
| HCOV + HPIV | 2 (0.71) | 1 (0.36) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (1.07) |
| Less frequent mixed infections § | 27 (9.64) | 7 (2.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 34 (12.14) |
ARTI = acute respiratory tract infection; BAL = bronchial alveolar lavage; BAS = bronchial aspirate; CV = coxsackievirus; ECHO = echovirus; HADV = human adenovirus; HBOV = human bocavirus; HCOV = human coronavirus; HEV = human enterovirus; HMPV = human metapneumovirus; HPIV = human parainfluenza virus; HRSV = human respiratory syncytial virus; IAV = influenza virus A; IBV = influenza virus B; NPA = nasopharyngeal aspirate; NS = nasal swab; SP = sputum; TS = throat swab.
The results of HEV typing are listed below: a 1 CV A16, 1 CV B1, 4 CV B4, 5 CV B5; b 3 CV B5; c 2 ECHO 6, 1 ECHO 11, 1 ECHO 21, 1 ECHO 24; d 1 ECHO 23, 1 ECHO 27.
The less frequent mixed infections are detailed in Supplementary Table 1.
Single infections were detected in 1128 (80.11%) samples from 1037 (79.83%) children. Mixed infections were detected in 280 (19.89%) samples from 262 (20.17%) children. There was a significant difference in the frequency of single vs. mixed infections among positive samples (80.11% vs. 19.89%; P < 0.0001) as well as among total examined samples (39% vs. 9.68%; P < 0.0001). Among mixed infections, two viruses were observed in 252 (90.00%) samples, three viruses in 26 (9.29%) samples, and four viruses in 2 (0.71%) samples. The percentage difference in the number of mixed infections with two viruses (90.00%) and four viruses (0.71%) was significant (P < 0.0001).
Overall, 1718 viruses were detected. HRSV (466/1718: 27.12%) and HADV (405/1718: 23.58%) were the most common viruses identified, followed by HCOV (262/1718: 15.25%), IV (198/1718: 11.53%), HBOV (161/1718: 9.37%), HPIV (114/1718: 6.64%), HMPV (76/1718: 4.42%), and HEV (36/1718: 2.09%).
HRSV and HADV were found in single (30.14% and 23.23%, respectively) and mixed infections (126/280: 45% and 143/280: 51.07%, respectively) (Table 1). HRSV co-infected with HADV (37/126: 29.36%), HCOV (35/126: 27.78%), IV (17/126: 13.5%), HBOV (16/126: 12.7%), HPIV (5/126: 3.97%), HEV (2/126: 1.6%), and HMPV (1/126: 0.8%), while HADV with HRSV (37/143: 25.87%), HCOV (36/143: 25.17%), HBOV (20/143: 13.99%), HPIV (9/143: 6.29%), HMPV (8/143: 5.6%), IV (8/143: 5.6%), and HEV (4/143: 2.8%).
3.2. Seasonality of respiratory viruses
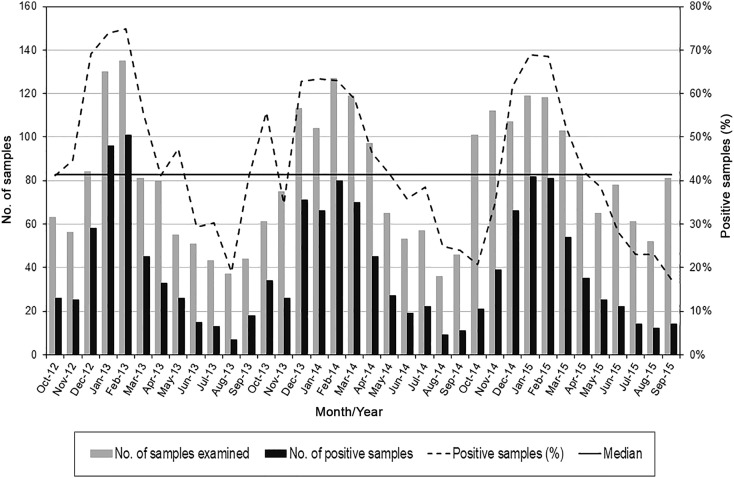
The virus detection rate decreased from 53.9% (463/859) in the first season (October 2012–September 2013) to 50.37% (480/953) in the second (October 2013–September 2014) to 43.05% (465/1080) in the third (October 2014–September 2015) (53.9% vs. 43.05%, P < 0.0001) (Fig. 1 ). The infection rate exceeded the median from November 2012 to March 2013, in May and October 2013, from December 2013 to May 2014, and from December 2014 to April 2015. Major peaks occurred in February 2013 (74.81%), February 2014 (62.99%), and January 2015 (68.91%).
Fig. 1.
Seasonality of human respiratory viruses detected in 1408 samples belonging to children with ARTIs in Northern Italy (October 2012–September 2015).
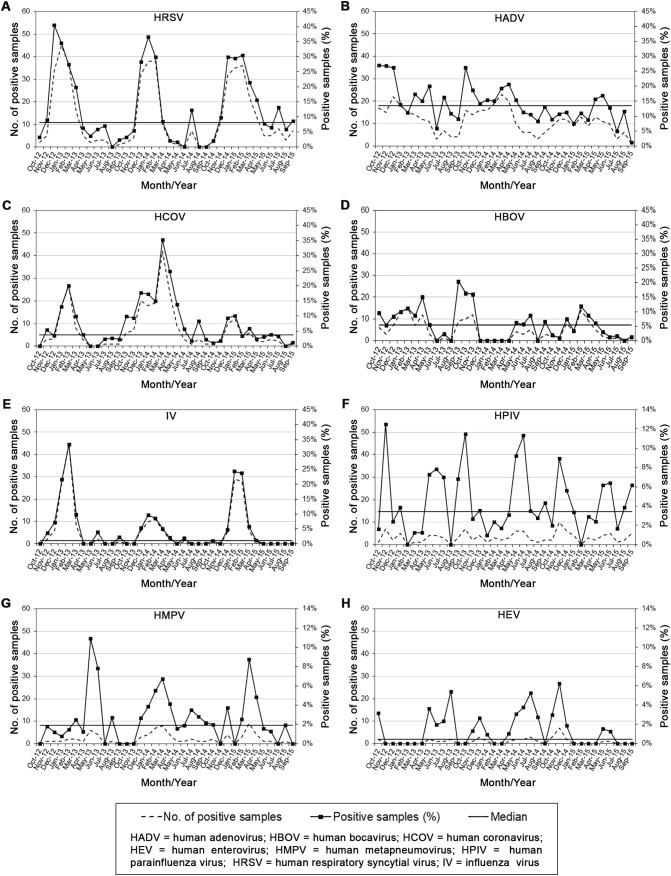
HRSV (Fig. 2A) was systematically detected, except in August 2013, and in June, August, and September 2014, prevailing in fall and early spring. The infection rate exceeded the median by more than twice from December 2012 to March 2013, from December 2013 to February 2014, and from December 2014 to March 2015. Major peaks came in December 2012 (40.48%), January 2014 (36.54%), and February 2015 (30.51%).
Fig. 2.
Monthly infection rates of human respiratory viruses detected in 1408 samples belonging to children with ARTIs in Northern Italy (October 2012–September 2015): (A) HRSV; (B) HADV; (C) HCOV; (D) HBOV; (E) IV; (F) HPIV; (G) HMPV; (H) HEV.
HADV (Fig. 2B) circulated throughout the survey, showing the highest prevalence from fall to spring in the first two seasons, and in April and May 2015. The detection rate exceeded the median by more than twice in October 2012. Major peaks occurred in October 2012 (26.98%) and 2013 (26.23%).
HCOV (Fig. 2C) usually circulated in winter with a higher prevalence in the second season. The infection rate passed the median by more than twice from January to March 2013, from October 2013 to May 2014, in August 2014, and from December 2014 to January 2015. Major peaks occurred in February 2013 (20%) and March 2014 (35.29%).
The HBOV prevalence (Fig. 2D) exceeded the median by more than twice in February and April 2013, from September to November 2013, and in February 2015. Major peaks occurred in April (15%) and September (20.45%) 2013.
IV (Fig. 2E) was mainly detected from late fall to early spring. The infection prevalence passed the median by more than twice from November 2012 to March 2013, in June and September 2013, from December 2013 to March 2014, and from December 2014 to March 2015. Major peaks occurred in February 2013 (33.33%) and January 2015 (24.37%).
HPIV (Fig. 2F) circulated almost every month, exceeding the median by more than twice in November 2012, from May to July 2013, in October 2013, in May, June, and November 2014. Major peaks came in November 2012 (12.5%), October 2013 (11.48%), and June 2014 (11.32%).
The HMPV detection rate (Fig. 2G) exceeded the median by more than twice in May and June 2013, from January to April 2014, and in March and April 2015. Major peaks occurred in May 2013 (10.91%) and March 2015 (8.74%).
HEV (Fig. 2H) showed the highest detection rate in spring and summer in the first seasons, but in fall and early winter in the third. The infection prevalence exceeded the median by more than twice in October 2012, from May to August 2013, from November to December 2013, from April to August 2014, from October to December 2014, and in May and June 2015. Major peaks came in August 2013 (5.41%), July 2014 (5.26%), and November 2014 (6.25%).
3.3. Age distribution of the respiratory viruses
For the 1295 children with viral ARTIs and age available, the mean and median age were 2 years 9 months and 1 year 7 months, respectively.
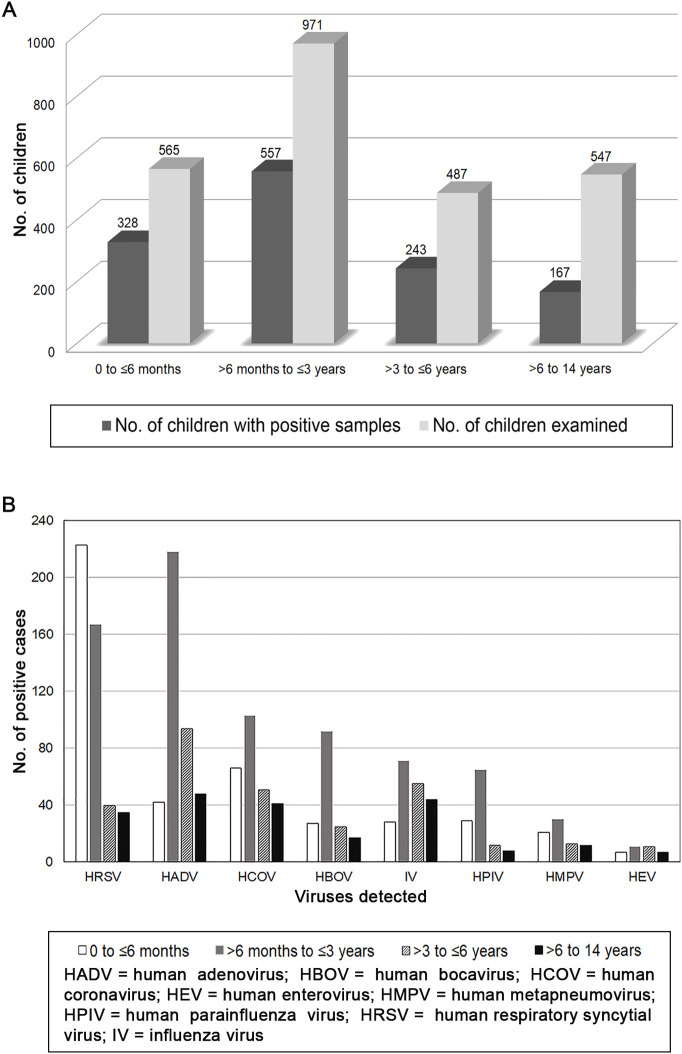
The infection rate was highest in children between >6 months to ≤3 years (57.36%; 43.01% among positive cases), followed by the 0 to ≤6 months (58.05%; 25.33% among positive cases) and > 3 to ≤6 years (49.9%; 18.76% among positive cases) groups (Fig. 3A).
Fig. 3.
(A) Age and (B) virus distribution in different age groups of 1295 children with ARTIs in Northern Italy (October 2012–September 2015).
Significant differences in the infection rate were evidenced among children aged 0 to ≤3 years and > 3 to ≤6 years (P < 0.05), among children aged 0 to ≤3 years and > 6 to 14 years (P < 0.0001), and among children aged >3 to ≤6 years and > 6 to 14 years (P < 0.0001), but not among children 0 to ≤6 months and > 6 months to ≤3 years (P > 0.05).
HRSV had the highest prevalence (223/328: 67.98%) in children aged 0 to ≤6 months, followed by HCOV (66/328: 20.12%) and HADV (42/328: 12.8%), while HADV prevailed in children >6 months to ≤3 years (218/557: 39.13%), followed by HRSV (167/557: 29.98%) and HCOV (103/557: 18.49%) (Fig. 3B). In children >3 to ≤6 and > 6 to 14 years, HADV prevailed (94/243: 38.68% and 48/167: 28.74%, respectively), followed by IV (55/243: 22.63% and 44/167: 26.34%, respectively) and HCOV (51/243: 20.98% and 41/167: 24.55%, respectively). The highest detection rates of HADV (218/402: 54.22%), HCOV (103/261: 39.46%), HBOV (92/161: 57.14%), IV (71/198: 35.85%), HPIV (65/114: 57.02%), and HMPV (30/76: 39.47%) occurred in children aged >6 months to ≤3 years.
3.4. Effectiveness of the diagnostic assays
Of the 1408 virus-positive samples, 1373 were detected by molecular assays and 426 by culture (97.51% vs. 30.25%, P < 0.0001) (Table 2 ). Most of the culture-positive samples (421, 29.9%) were also positive by molecular methods, although different virus combinations were detected in 9 mixed infections. In discordant culture-negative samples molecular assays detected 262 HCOV (18.6%), 161 HBOV (11.43%), and 76 HMPV (5.4%). Finally, of the 466 samples positive for HRSV by PCR, 233 (50%) were also detected by IIF (100% vs. 50%, P < 0.0001).
Table 2.
Detection rate of the diagnostic methods employed for respiratory samples from children with ARTIs in Northern Italy (October 2012–September 2015).
| Total | Culture method | Molecular method | P value | |
|---|---|---|---|---|
| No. of samples positive for respiratory viruses (%) | 1408 | 426 (30.25) | 1373 (97.51) | <0.0001 |
| Total | IIF | Molecular method | P value | |
| No. of samples positive for HRSV (%) | 466 | 233 (50) | 466 (100) | <0.0001 |
ARTI = acute respiratory tract infection; HRSV = human respiratory syncytial virus; IIF = indirect immunofluorescence assay.
4. Discussion
This survey carried out in Parma (Northern Italy) from October 2012 to September 2015 describes the detection of viruses associated with respiratory samples from 2575 patients presenting with symptoms consistent with acute respiratory tract infections. Overall, 50.44% of cases were positive by the simultaneous employment of different diagnostic assays. Other studies have reported similar rates, although they refer to a single epidemic season and fewer number of cases examined exclusively by PCR (Annan et al., 2016, Pratheepamornkull et al., 2015, Zuccotti et al., 2011). However, different results have also been described (Do et al., 2011, Venter et al., 2011). It must be considered that many factors could lead to prevalence variations, such as the virus panel examined, diagnostic methods, study setting, and geographic area.
HRSV (27.12%) and HADV (23.58%) prevailed; HCOV (15.25%), IV (11.53%), and HBOV (9.37%) showed a higher frequency than HPIV (6.64%), HMPV (4.42%), and HEV (2.09%).
Single infections predominated over mixed infections (80.11% vs. 19.89%, P < 0.0001), which was consistent with previous observations (Martin et al., 2012, Paranhos-Baccalà et al., 2008). Of multiple infections, dual infection (90%) was predominant, in accordance with other authors (Martin et al., 2012, Peng et al., 2009). HRSV, HADV, and HCOV were mainly involved in mixed infections, as would be expected from their overlapping seasonal distribution. Although HRSV and HADV are among the viruses most commonly involved in mixed infections (Cantais et al., 2014, Harada et al., 2013), the relevance of rhinoviruses, HPIV, and IV has also been assessed (Lu et al., 2013). Very complex combinations of mixed viral infections have been observed (Lu et al., 2013, Martin et al., 2012, Paranhos-Baccalà et al., 2008) and certain pathogens appear to have higher multiple infection frequency (Brunstein et al., 2008). In this scenario, superinfection exclusion mechanisms preventing a secondary infection with the same or a closely related virus should be envisaged (Folimonova, 2012).
Although mixed infections have been widely assessed in ARTIs (Moesker et al., 2016, Seo et al., 2014, Turner et al., 2013), there is still no clear relationship with prolonged virus shedding, asymptomatic virus persistence, and disease severity. However, there is evidence that genome quantification could clarify the role of each virus in mixed infections (Franz et al., 2010, Martin et al., 2012). Accordingly, high viral loads have been related to severe diseases (Franz et al., 2010, Hasegawa et al., 2014, Jansen et al., 2011), but conflicting results have been reported (Adams et al., 2015, Wu et al., 2012). In addition, some viruses have been detected in healthy children (Advani et al., 2012, Moe et al., 2016). In this study, molecular assays showed greater sensitivity when compared to culture assays (97.51% vs. 30.25%, P < 0.0001) and HRSV antigen detection (100% vs. 50%, P < 0.0001). Conversely, culture assays allowed the detection of infectious viruses in 426 (30.25%) of the 1408 positive samples, eventually providing a clue to discriminate the main causative agent in co-infections. Nevertheless, cautious conclusions should be drawn, since the growth difficulties of some viruses may have decreased the number of those found by culture assays, while the long-term viral excretion after clinical recovery in children with frequent ARTIs may have increased the mixed infection rate.
The epidemic season of respiratory viruses ranged from 5 to 6 months, with peaks in February (2013 and 2014) or January (2015). The virus detection rate decreased significantly between the first and third seasons (53.9% vs. 43.05%, P < 0.0001), but environmental factors may have influenced the frequency and severity of ARTIs (Nenna et al., 2017).
HRSV showed a sharp winter/early-spring seasonality. When compared to previous reports, in the Parma area the HRSV epidemic season was shorter (Medici et al., 2006).
HADV circulated persistently throughout the study with higher rates in fall and spring of the first two years of surveillance, eventually indicating a reduction of circulation due to the accumulation of immunity in the population.
The IV infection seasonality was consistent with previous reports (Del Manso et al., 2015a, Del Manso et al., 2015b).
No definite seasonality was shown by HBOV, HCOV, HMPV, HPIV, and HEV.
A correct viral diagnosis allows highlighting virus seasonality, which could favor both preventive measures and reduction of overuse of antibiotics. Accordingly, in children with HRSV or IV, bacterial infections are less prevalent than in those without a viral infection (Purcell and Fergie, 2004, Smitherman et al., 2005).
The virus detection rate was significantly higher in children aged 0 to ≤3 years (68.34%), when compared to the >3 to ≤6 years (18.76%, P < 0.05) and > 6 to 14 years (12.9%, P < 0.0001) groups, suggesting that immaturity of the immune system and absence of previous immunity could influence the severity of ARTIs (Hammond et al., 2007, Monto, 2004).
HRSV prevailed (67.98%) in younger infants (0 to ≤6 months), while HADV was prevalent in all other age groups studied. Accordingly, HRSV causes severe diseases in young children (Moe et al., 2017, Nguyen et al., 2017, Shi et al., 2017).
For all the remaining respiratory viruses the highest detection rate was among children aged >6 months to ≤3 years. The attribution of an etiological role in ARTIs to HBOV has been extensively debated (Debiaggi et al., 2012, Schildgen et al., 2008). In this study, HBOV was most frequently found in single infections (55.9%) than in co-infections, strengthening the idea that it can be considered a pathogen.
In conclusion, although this study has certain limitations, such as the lack of rhinovirus detection, it does provide relevant information on respiratory virus circulation in children with ARTIs in an area of Northern Italy with a temperate climate. The simultaneous use of different diagnostic approaches made it possible to carry out in-depth analysis of a large number of cases spanning over 3 years.
These findings contribute to estimate the disease burden associated with respiratory viruses, reinforcing the need for timely virologic diagnosis and continuous surveillance to optimize the prediction and control of ARTIs in children.
The following is the supplementary data related to this article.
Rate of less frequent mixed respiratory viral infections in children with ARTIs in Northern Italy (October 2012-September 2015).
Compliance with Ethical Standards
Funding
This study was funded by grants from the University of Parma (Fondi di Ateneo FIL 2014 - FIL2014_DECONTO_MCS).
Conflict of interest
Flora De Conto, Francesca Conversano, Maria Cristina Medici, Francesca Ferraglia, Federica Pinardi, Maria Cristina Arcangeletti, and Carlo Chezzi hereby declare that they have no conflict of interest. Adriana Calderaro declares that she is an Editorial Member of the journal.
Ethical approval
This article does not describe any studies with human participants or animals performed by any of the authors.
Informed consent
Respiratory virus detection was performed according to the medical order; hence there was no need to obtain informed consent for the epidemiological analysis of the related data.
Authors' Contributions
FDC, MCM: conceived the study; FDC, MCM, FC: wrote the manuscript; FC: collected the data; FDC, MCM, FC, FF, FP, MCA: analyzed the data; CC, AC: critically revised the manuscript; FDC, MCM, FC, FF, FP, MCA, CC, AC: approved the definitive version of the manuscript.
Footnotes
Declaration of interest statement: Adriana Calderaro is an Editorial Member of Diagnostic Microbiology and Infectious Disease.
References
- Adams O., Weis J., Jasinska K., Vogel M., Tenenbaum T. Comparison of human metapneumovirus, respiratory syncytial virus and rhinovirus respiratory tract infections in young children admitted to hospital. J Med Virol. 2015;87(2):275–280. doi: 10.1002/jmv.24025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advani S., Sengupta A., Forman M., Valsamakis A., Milstone A.M. Detecting respiratory viruses in asymptomatic children. Pediatr Infect Dis J. 2012;31(12):1221–1226. doi: 10.1097/INF.0b013e318265a804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allander T., Tammi M.T., Eriksson M., Bjerkner A., Tiveljung-Lindell A., Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A. 2005;102(36):12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annan A., Ebach F., Corman V.M., Krumkamp R., Adu-Sarkodie Y., Eis-Hübinger A.M. Similar virus spectra and seasonality in paediatric patients with acute respiratory disease, Ghana and Germany. Clin Microbiol Infect. 2016;22(4):340–346. doi: 10.1016/j.cmi.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M., Gamieldien J., Fielding B.C. Identification of new respiratory viruses in the new millennium. Viruses. 2015;7(3):996–1019. doi: 10.3390/v7030996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicer S., Giray T., Çöl D., Erdağ G.Ç., Vitrinel A., Gürol Y. Virological and clinical characterizations of respiratory infections in hospitalized children. Ital J Pediatr. 2013;39:22. doi: 10.1186/1824-7288-39-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstein J.D., Cline C.L., McKinney S., Thomas E. Evidence from multiplex molecular assays for complex multipathogen interactions in acute respiratory infections. J Clin Microbiol. 2008;46(1):97–102. doi: 10.1128/JCM.01117-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantais A., Mory O., Pillet S., Verhoeven P.O., Bonneau J., Patural H. Epidemiology and microbiological investigations of community-acquired pneumonia in children admitted at the emergency department of a university hospital. J Clin Virol. 2014;60(4):402–407. doi: 10.1016/j.jcv.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Conto F., Fazzi A., Razin S.V., Arcangeletti M.C., Medici M.C., Belletti S. Mammalian diaphanous-related formin-1 restricts early phases of influenza A/NWS/33 virus (H1N1) infection in LLC-MK2 cells by affecting cytoskeleton dynamics. Mol Cell Biochem. 2018;437(1–2):185–201. doi: 10.1007/s11010-017-3107-9. [DOI] [PubMed] [Google Scholar]
- Debiaggi M., Canducci F., Ceresola E.R., Clementi M. The role of infections and coinfections with newly identified and emerging respiratory viruses in children. Virol J. 2012;9:247. doi: 10.1186/1743-422X-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Manso M., Rota M.C., Declich S., Giannitelli S., Nacca G., Rizzo C. InfluNet: sistema di sorveglianza sentinella delle sindromi influenzali in Italia. Rapporto sulla stagione influenzale 2013–2014. Istituto Superiore di Sanità, Roma (Rapporti ISTISAN 15/48) 2015. http://www.iss.it/flue/index.php?lang=1&id=142&tipo=21
- Del Manso M., Rota M.C., Declich S., Giannitelli S., Nacca G., Rizzo C. InfluNet: sistema di sorveglianza sentinella delle sindromi influenzali in Italia. Rapporto sulla stagione influenzale 2012–2013. Istituto Superiore di Sanità, Roma (Rapporti ISTISAN 15/47) 2015. http://www.iss.it/flue/index.php?lang=1&id=142&tipo=21
- Do A.H., van Doorn H.R., Nghiem M.N., Bryant J.E., Hoang T.H., Do Q.H. Viral etiologies of acute respiratory infections among hospitalized vietnamese children in Ho Chi Minh City, 2004-2008. PLoS One. 2011;6(3) doi: 10.1371/journal.pone.0018176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H.D., Preuss J. Laboratory diagnosis of respiratory virus infections in 24 hours by utilizing shell vial cultures. J Clin Microbiol. 1997;35(8):2165–2167. doi: 10.1128/jcm.35.8.2165-2167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folimonova S.Y. Superinfection exclusion is an active virus-controlled function that requires a specific viral protein. J Virol. 2012;86(10):5554–5561. doi: 10.1128/JVI.00310-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz A., Adams O., Willems R., Bonzel L., Neuhausen N., Schweizer-Krantz S. Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. J Clin Virol. 2010;48(4):239–245. doi: 10.1016/j.jcv.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner P.S., McQuillin J. 1st ed. Butterworth; London: 1974. Rapid virus diagnosis: Application of immunofluorescence. [Google Scholar]
- Goktas S., Sirin M.C. Prevalence and seasonal distribution of respiratory viruses during the 2014-2015 season in Istanbul. Jundishapur J Microbiol. 2016;9(9) doi: 10.5812/jjm.39132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond S., Chenever E., Durbin J.E. Respiratory virus infection in infants and children. Pediatr Dev Pathol. 2007;10(3):172–180. doi: 10.2350/07-02-0238.1. [DOI] [PubMed] [Google Scholar]
- Harada Y., Kinoshita F., Yoshida L.M., Minh le N., Suzuki M., Morimoto K. Does respiratory virus coinfection increases the clinical severity of acute respiratory infection among children infected with respiratory syncytial virus? Pediatr Infect Dis J. 2013;32(5):441–445. doi: 10.1097/INF.0b013e31828ba08c. [DOI] [PubMed] [Google Scholar]
- Hasegawa K., Jartti T., Mansbach J.M., Laham F.R., Jewell A.M., Espinola J.A. Respiratory syncytial virus genomic load and disease severity among children hospitalized with bronchiolitis: multicenter cohort studies in the United States and Finland. J Infect Dis. 2014;211(10):1550–1559. doi: 10.1093/infdis/jiu658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R.R., Wieringa J., Koekkoek S.M., Visser C.E., Pajkrt D., Molenkamp R. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J Clin Microbiol. 2011;49(7):2631–2636. doi: 10.1128/JCM.02094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.R., Ki C.S., Lee N.Y. Rapid detection and identification of 12 respiratory viruses using a dual priming oligonucleotide system-based multiplex PCR assay. J Virol Methods. 2009;156(1–2):111–116. doi: 10.1016/j.jviromet.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry M.L. Rapid viral diagnosis. In: Rose N.R., Conway de Macario E., Folds J.D., Lane H.C., Nakamura R.M., editors. Manual of clinical laboratory immunology. 5th ed. ASM Press; Washington DC: 1997. pp. 608–617. [Google Scholar]
- Lim K.A., Benyesh-Melnick M. Typing of viruses by combinations of antiserum pools. Application to typing of enteroviruses (Coxsackie and ECHO) J Immunol. 1960;84:309–317. [PubMed] [Google Scholar]
- Ljubin-Sternak S., Marijan T., Ivković-Jureković I., Čepin-Bogović J., Gagro A., Vraneš J. Etiology and clinical characteristics of single and multiple respiratory virus infections diagnosed in croatian children in two respiratory seasons. J Pathog. 2016;2016:2168780. doi: 10.1155/2016/2168780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Wang S., Zhang L., Xu C., Bian C., Wang Z. Epidemiology of human respiratory viruses in children with acute respiratory tract infections in Jinan, China. Clin Dev Immunol. 2013;2013:210490. doi: 10.1155/2013/210490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E.T., Kuypers J., Wald A., Englund J.A. Multiple versus single virus respiratory infections: viral load and clinical disease severity in hospitalized children. Influenza Other Respi Viruses. 2012;6(1):71–77. doi: 10.1111/j.1750-2659.2011.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici M.C., Arcangeletti M.C., Rossi G.A., Lanari M., Merolla R., Di Luzio Paparatti U. Four year incidence of respiratory syncytial virus infection in infants and young children referred to emergency departments for lower respiratory tract diseases in Italy: the “Osservatorio VRS” study (2000-2004) New Microbiol. 2006;29:35–43. [PubMed] [Google Scholar]
- Moe N., Pedersen B., Nordbø S.A., Skanke L.H., Krokstad S., Smyrnaios A. Respiratory virus detection and clinical diagnosis in children attending day care. PLoS One. 2016;11(7) doi: 10.1371/journal.pone.0159196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe N., Stenseng I.H., Krokstad S., Christensen A., Skanke L.H., Risnes K.R. The burden of human metapneumovirus and respiratory syncytial virus infections in hospitalized norwegian children. J Infect Dis. 2017;216(1):110–116. doi: 10.1093/infdis/jix262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moesker F.M., van Kampen J.J., van Rossum A.M., de Hoog M., Koopmans M.P., Osterhaus A.D. Viruses as sole causative agents of severe acute respiratory tract infections in children. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto A.S. Occurrence of respiratory virus: time, place and person. Pediatr Infect Dis J. 2004;23(1 Suppl):S58–S64. doi: 10.1097/01.inf.0000108193.91607.34. [DOI] [PubMed] [Google Scholar]
- Moura F.E., Perdigão A.C., Siqueira M.M. Seasonality of influenza in the tropics: a distinct pattern in northeastern Brazil. Am J Trop Med Hyg. 2009;81(1):180–183. [PubMed] [Google Scholar]
- Nenna R., Evangelisti M., Frassanito A., Scagnolari C., Pierangeli A., Antonelli G. Respiratory syncytial virus bronchiolitis, weather conditions and air pollution in an Italian urban area: an observational study. Environ Res. 2017;158:188–193. doi: 10.1016/j.envres.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V.H., Dubot-Pérès A., Russell F.M., Dance D.A.B., Vilivong K., Phommachan S. Acute respiratory infections in hospitalized children in Vientiane, Lao PDR - the importance of respiratory syncytial virus. Sci Rep. 2017;7(1):9318. doi: 10.1038/s41598-017-09006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S., Mohakud N.K., Suar M., Kumar S. Etiology, seasonality, and clinical characteristics of respiratory viruses in children with respiratory tract infections in Eastern India (Bhubaneswar, Odisha) J Med Virol. 2017;89(3):553–558. doi: 10.1002/jmv.24661. [DOI] [PubMed] [Google Scholar]
- Paranhos-Baccalà G., Komurian-Pradel F., Richard N., Vernet G., Lina B., Floret D. Mixed respiratory virus infections. J Clin Virol. 2008;43(4):407–410. doi: 10.1016/j.jcv.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariani E., Amendola A., Piatti A., Anselmi G., Ranghiero A., Bubba L. Ten years (2004-2014) of influenza surveillance in Northern Italy. Hum Vaccin Immunother. 2015;11(1):198–205. doi: 10.4161/hv.35863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D., Zhao D., Liu J., Wang X., Yang K., Xicheng H. Multipathogen infections in hospitalized children with acute respiratory infections. Virol J. 2009;6:155. doi: 10.1186/1743-422X-6-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratheepamornkull T., Ratanakorn W., Samransamruajkit R., Poovorawan Y. Causative agents of severe community acquired viral pneumonia among children in Eastern Thailand. Southeast Asian J Trop Med Public Health. 2015;46(4):650–656. [PubMed] [Google Scholar]
- Purcell K., Fergie J. Concurrent serious bacterial infections in 912 infants and children hospitalized for treatment of respiratory syncytial virus lower respiratory tract infection. Pediatr Infect Dis J. 2004;23(3):267–269. doi: 10.1097/01.inf.0000116759.21252.29. [DOI] [PubMed] [Google Scholar]
- Schildgen O., Müller A., Allander T., Mackay I.M., Völz S., Kupfer B. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin Microbiol Rev. 2008;21(2):291–304. doi: 10.1128/CMR.00030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Y.B., Song J.Y., Choi M.J., Kim I.S., Yang T.U., Hong K.W. Etiology and clinical outcomes of acute respiratory virus infection in hospitalized adults. Infect Chemother. 2014;46(2):67–76. doi: 10.3947/ic.2014.46.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T., McAllister D.A., O'Brien K.L., Simoes E.A.F., Madhi S.A., Gessner B.D. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390(10098):946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smitherman H.F., Caviness A.C., Macias C.G. Retrospective review of serious bacterial infections in infants who are 0 to 36 months of age and have influenza A infection. Pediatrics. 2005;115(3):710–718. doi: 10.1542/peds.2004-1112. [DOI] [PubMed] [Google Scholar]
- Taylor S., Lopez P., Weckx L., Borja-Tabora C., Ulloa-Gutierrez R., Lazcano-Ponce E. Respiratory viruses and influenza-like illness: epidemiology and outcomes in children aged 6 months to 10 years in a multi-country population sample. J Infect. 2017;74(1):29–41. doi: 10.1016/j.jinf.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregoning J.S., Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010;23(1):74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucchi C., Alicino C., Orsi A., Paganino C., Barberis I., Grammatico F. Fifteen years of epidemiologic, virologic and syndromic influenza surveillance: a focus on type B virus and the effects of vaccine mismatch in Liguria region, Italy. Hum Vaccin Immunother. 2017;13(2):456–463. doi: 10.1080/21645515.2017.1264779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P., Turner C., Watthanaworawit W., Carrara V., Cicelia N., Deglise C. Respiratory virus surveillance in hospitalised pneumonia patients on the Thailand-Myanmar border. BMC Infect Dis. 2013;13:434. doi: 10.1186/1471-2334-13-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter M., Lassaunière R., Kresfelder T.L., Westerberg Y., Visser A. Contribution of common and recently described respiratory viruses to annual hospitalizations in children in South Africa. J Med Virol. 2011;83(8):1458–1468. doi: 10.1002/jmv.22120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu U.I., Wang J.T., Chen Y.C., Chang S.C. Severity of pandemic H1N1 2009 influenza virus infection may not be directly correlated with initial viral load in upper respiratory tract. Influenza Other Respi Viruses. 2012;6(5):367–373. doi: 10.1111/j.1750-2659.2011.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappa A., Perin S., Amendola A., Bianchi S., Pariani E., Ruzza M. Epidemiological and molecular surveillance of influenza and respiratory syncytial viruses in children with acute respiratory infections (2004/2005 season) Microbiol Med. 2008;23(1) doi: 10.4081/mm.2008.2592. [DOI] [Google Scholar]
- Zuccotti G., Dilillo D., Zappa A., Galli E., Amendola A., Martinelli M. Epidemiological and clinical features of respiratory viral infections in hospitalized children during the circulation of influenza virus A (H1N1) 2009. Influenza Other Respi Viruses. 2011;5(6):e528–e534. doi: 10.1111/j.1750-2659.2011.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Rate of less frequent mixed respiratory viral infections in children with ARTIs in Northern Italy (October 2012-September 2015).