Abstract
Newcastle disease (ND) and infectious bronchitis (IB) are two major viral diseases affecting the respiratory tracts of birds and whose impact on African poultry is still poorly known. In the present study we aimed at assessing NDV and IBV prevalences in Ivory-Coast by molecular screening of > 22,000 avian swabs by nested PCR and by serology testing of close to 2000 avian sera from 2010 through 2012. The NDV and IBV seroprevalences over the study period reached 22% and 72%, respectively. We found 14.7% pooled swabs positive by PCR for NDV and 14.6% for IBV. Both pathogens are therefore endemic in Ivory-Coast. Economic losses associated with NDV and IBV infections still need to be evaluated.
Keywords: Newcastle disease, Infectious bronchitis, Avian influenza virus, PCR, ELISA, Poultry, Ivory-Coast
Highlights
-
•
Surveillance of avian influenza virus is found negative in backyard poultry farm.
-
•
Study of Newcastle disease and infectious bronchitis as differential diagnosis diseases
-
•
Newcastle disease virus seroprevalence is much lower than infectious bronchitis virus.
-
•
First study of infectious bronchitis revealed high prevalence in backyard poultry.
-
•
Both pathogens are endemic, cause economic losses and need to be fully evaluated.
1. Introduction
Newcastle disease (ND) and infectious bronchitis (IB) are two viral diseases affecting the respiratory tracts of many species of birds and placing a severe economic burden on the poultry industry (Alexander, 1997, Cavanagh and Gelb, 2008, Jackwood et al., 2012).
ND has a worldwide distribution. In Africa, it is the major constraint of chicken development, mainly in rural areas (Maminiaina et al., 2010, Couacy-Hymann et al., 2012a). The infectious agent of ND, Newcastle disease virus (NDV), is a single stranded, non-segmented, negative-sense RNA virus belonging to the order Mononegavirales, family Paramyxoviridae, sub-family Paramyxovirinae, and genus Avulavirus (Lamb and Parks, 2007, Cattoli et al., 2011). However, only virulent strains of NDV cause ND when they infect birds. This genus contains at least 9 serogroups of avian paramyxoviruses (APMV-1 to -9) previously described and recently 3 more serogroups have been added: APVM10 (Miller et al., 2010), APMV11 (Briand et al., 2012) and APVM12 (Terregino et al., 2013). According to their virulence in poultry, APMV-1 isolates can be grouped into three pathotypes: lentogenic, mesogenic or velogenic (Alexander, 1997, Cattoli et al., 2009). The velogenic strains may cause 100% mortality in infected chicken flocks (Kho et al., 2000); they are further classified as neurotropic or viscerotropic based on their pathological manifestations (Alexander, 1998, Wise et al., 2004). Mesogenic strains cause primarily respiratory disease while lentogenic isolates are of low virulence and may cause mild respiratory or enteric infections. The virulent NDV isolates (mesogens and velogens) are notifiable agents that require reporting to the OIE (OIE, 2000).
IB, in contrast, remains less known in Africa, and is found mainly in the backyard poultry production system. It is a highly contagious upper-respiratory tract disease of chickens. The causative agent, infectious bronchitis virus (IBV), is a coronavirus, an enveloped, positive-strand RNA virus with a genome of about 27 kb. It belongs to the family Coronaviridae and subfamily Coronavirinae within the genera of Gammacoronaviridae (Jackwood et al., 2012). Clinical signs of IB disease in chickens are watery eyes, mucus in the nares and trachea, gasping, coughing, and tracheal rales. The disease can also cause a decrease in egg production and egg quality and some strains of the virus can cause an interstitial nephritis (Jackwood et al., 2012). Morbidity is close to 100%, while mortality can be variable, ranging from 14% to 82%, depending on the age of the birds, strain of the virus and secondary infections (Cavanagh and Gelb, 2008).
Up to now little is known about the distribution and impact of IBV in sub-Saharan African countries including Ivory-Coast. A recent study undertaken on chickens from commercial farms, live bird markets and backyard farms in Nigeria and Niger revealed the presence of IBV genome. Phylogenetic analysis of the S1 coding sequence revealed a new genotype of IBV. This strain did not cross-react with antisera against known strains such as IT02, M41, D274 or Connecticut in virus neutralisation tests (Ducatez et al., 2009). In Ivory-Coast, poultry technicians report on a regular basis the presence of IB in commercial farms and recommend the use of vaccine, mainly based on the M41 strain, although there is no prior study of the presence of IBV in the country or on the type of strains circulating. These reports, based on clinical signs, were never confirmed by the laboratory.
Both ND and IB affect the respiratory tract, so the differential diagnosis between them and with respect to other respiratory diseases such as Mycoplasma gallisepticum (chronic respiratory disease), infectious laryngotracheitis, Haemophilus paragallinarum (infectious coryza) and avian influenza virus (AIV) infections, remains a challenge (Ducatez et al., 2009).
The present study took advantages of the surveillance for avian influenza viruses carried out within Ivory-Coast to determine the prevalence of NDV and IBV in poultry farms (both backyard and commercial farms) and at live poultry markets.
2. Materials and methods
2.1. Sampling sites
Outbreaks of avian influenza due to H5N1 strains were detected in Ivory-Coast in 2006. From that date on a continuous surveillance of poultry farms, both backyard and commercial production systems, has been implemented. Every month, the team of the Virology Laboratory was sent to the field to collect tracheal and cloacal swabs and serum samples. These samples were collected in the southern regions (Agneby, District of Abidjan, South Comoe), which are the biggest large-scale poultry production areas in the country. In addition, the south-eastern region (South Comoe) includes lakes and rivers with large populations of various water bird species (Fig. 1 ). The sampling was carried out following a validated protocol previously described with data from 2007 through 2009 previously reported (Couacy-Hymann et al., 2012a). In each region, a minimum of 5 villages were randomly selected from a known list of villages. In addition, following the same protocol, 5 commercial farms were selected per region. However, any commercial farm, having reported any diseases to the veterinary field technician, was systematically included in the survey (in addition to the 5 commercial farms randomly selected). Within a selected village, any backyard poultry's owner having a poultry flock (flock size varying between 5 and 20 birds per household) was systematically included in the survey. At live bird markets (mainly one big live market per region), 5 vendors were randomly selected (average number of vendors per market = 10). In addition, farmers were interviewed regarding the case mortality that occurred on their farms.
Fig. 1.
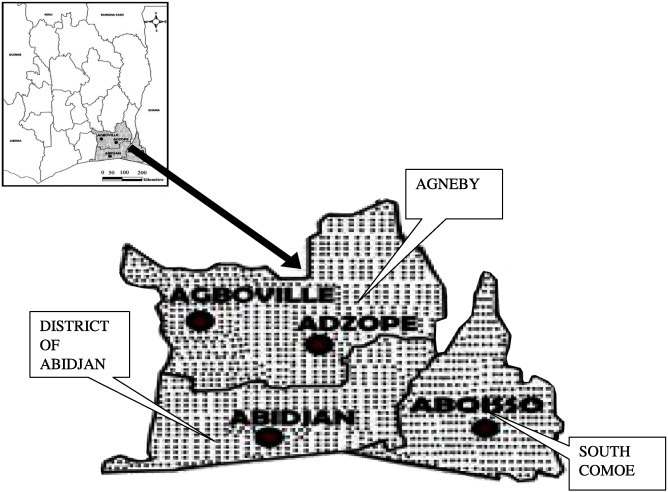
Sampling sites.
2.2. Sample collection
At the sampling sites (backyard and commercial poultry farms, live-poultry markets), clinical examination of each bird (chicken, guinea fowl or duck) was undertaken for any signs of disease prior to sampling. In each selected village, a minimum of 30 birds were sampled. From a commercial farm, 30 to 50 chickens were selected and at live bird market, 5 birds were selected from each selected vendor in a given market. Any dead or sick animals were systematically included in the survey at any sampling sites and sampled. Blood samples were obtained from examined animals and processed to yield serum. Individual sterile swabs were used in this survey. Tracheal and cloacal swabs were also collected from the same birds and placed in viral transport medium (VTM) (50% sterile glycerin; 45% sterile PBS 1 M, pH 7.2–7.4; 2% antibiotic solution with Penicillin and Streptomycin; 0.5% Gentamycin; 1% Nystatin; 1.5% Polymyxin B) with the final antibiotic concentration of Penicillin 1000 units/mL, Streptomycin 200 μg/mL, Nystatin 50 units/mL, Gentamycin 250 μg/mL, and Polymyxin B 100 units/mL. Each tracheal and cloacal swab was stored in a sterile individual tube containing the VTM. In the field, collected swab samples were kept in liquid nitrogen to prevent any degradation of biological materials. At the laboratory, serum samples were stored at − 20 °C and swabs were transferred to a − 80 °C freezer until used for analysis (Table 1, Table 2 ).
Table 1.
Collected serum samples from live bird markets and serological results on the period 2010–2012.
| Year | Region | Localities | Species/prod syst | Collected serum | NDV positive | IBV positive | NDV prevalence (× 100) | IBV Prevalence (× 100) |
|---|---|---|---|---|---|---|---|---|
| 2010 | Agneby | Agboville/Adzope | BYC | 382 | 91 | 244 | 23.8 | 63.9 |
| CMF | – | – | – | – | – | |||
| Ducks | 7 | 0 | – | – | – | |||
| Guinea fowl | 5a | 3 | – | 60 | – | |||
| District of Abidjan | Bingerville/Abidjan Market | BYC | 17 | 7 | 11 | 41.2 | 64.7 | |
| CMF | – | – | – | – | – | |||
| Ducks | 25 | – | 4 | – | 16 | |||
| Guinea fowl | – | – | – | – | – | |||
| South Comoe | Aboisso | BYC | 292 | 110 | 217 | 37.6 | 74.3 | |
| CMF | – | – | – | – | – | |||
| Ducks | 23 | 9 | 1 | 39.1 | 4.3 | |||
| Guinea fowl | – | – | – | – | – | |||
| Subtotal | 751 | 220 | 477 | 29.3 | 63.9 | |||
| 2011 | Agneby | Agboville/Adzope | BYC | 91 | 8 | 77 | 8.8 | 84.6 |
| CMF | – | – | – | – | – | |||
| Ducks | 31 | 0 | – | – | – | |||
| Guinea fowl | – | – | – | – | – | |||
| District of Abidjan | Bingerville/Abidjan Market | BYC | – | – | – | – | – | |
| CMF | – | – | – | – | – | |||
| Ducks | – | – | – | – | – | |||
| Guinea fowl | – | – | – | – | – | |||
| South Comoe | Aboisso | BYC | 351 | 62 | 293 | 17.7 | 83.5 | |
| CMF | – | – | – | – | – | |||
| Ducks | 9 | 1 | – | 11.1 | – | |||
| Subtotal | 482 | 71 | 370 | 14.7 | 76.8 | |||
| 2012 | Agneby | Agboville/Adzope | BYC | 310 | 33 | 284 | 10.6 | 91.6 |
| CMF | – | – | – | – | – | |||
| Ducks | – | – | – | – | – | |||
| Guinea fowl | – | – | – | – | – | |||
| District of Abidjan | Bingerville/Abidjan Market | BYC | 8 | 3 | 8 | 37.5 | 100 | |
| CMF | – | – | – | – | – | |||
| Ducks | 6 | – | 2 | – | 33.3 | |||
| Guinea fowl | – | – | – | – | – | |||
| South Comoe | Aboisso | BYC | 386 | 93 | 260 | 24.1 | 67.4 | |
| CMF | – | – | – | – | – | |||
| Ducks | – | – | – | – | – | |||
| Subtotal | 710 | 129 | 554 | 18.2 | 78 | |||
| Total | 1943 | 420 | 1401 | 21.6 | 72.3 | |||
BYC: backyard chicken. CMF: commercial poultry farm. Prod syst: production system.
Minus 5 guinea fowl for IBV total serum.
Table 2.
Collected swab samples from poultry's farms and at live bird markets and corresponding RT-PCR results on the period 2010–2012.
| Year | Region | Localities | Species/prod syst | Collected samples TS + ClS |
Total pooled samples TS + ClS |
Results PCR NDV-pools |
Results PCR IBV-pools |
||
|---|---|---|---|---|---|---|---|---|---|
| TS | ClS | TS | ClS | ||||||
| 2010 | Agneby | Agboville/Adzope | BYC | 2934 | 586 | 26 | 16 | 8 | 27 |
| CMF | 952 | 190 | 20 | 10 | 10 | 32 | |||
| Ducks | 20 | 4 | 0 | 0 | 0 | 0 | |||
| Guinea fowl | 80 | 16 | 0 | 0 | 1 | 3 | |||
| District of Abidjan | Bingerville/Abidjan Market | BYC | 564 | 112 | 13 | 11 | 3 | 5 | |
| CMF | 838 | 168 | 18 | 10 | 10 | 28 | |||
| Ducks | 190 | 38 | 0 | 0 | 0 | 0 | |||
| Guinea fowl | 30 | 6 | 0 | 0 | 2 | 2 | |||
| South Comoe | Aboisso | BYC | 2550 | 510 | 50 | 30 | 22 | 40 | |
| CMF | 272 | 54 | 8 | 5 | 0 | 0 | |||
| Ducks | 160 | 32 | 4 | 2 | 1 | 5 | |||
| Guinea fowl | 6a | 2a | 0 | 0 | 0 | 0 | |||
| 2010-total | 8596 | 1718 | 139 (16.2%) | 84 (9.8%) | 57 (6.6%) | 142 (16.5%) | |||
| 2011 | Agneby | Agboville/Adzope | BYC | 920 | 184 | 20 | 11 | 15 | 19 |
| CMF | 1620 | 324 | 29 | 18 | 21 | 44 | |||
| Ducks | 180 | 36 | 6 | 2 | 3 | 5 | |||
| Guinea fowl | 110 | 22 | 2 | 1 | 3 | 1 | |||
| District of Abidjan | Bingerville/Abidjan Market | BYC | 240 | 48 | 7 | 2 | 8 | 6 | |
| CMF | 870 | 174 | 12 | 9 | 19 | 20 | |||
| Ducks | – | – | – | – | – | – | |||
| Guinea fowl | 70 | 14 | 2 | 0 | 0 | 2 | |||
| South Comoe | Aboisso | BYC | 2710 | 542 | 48 | 19 | 27 | 48 | |
| CMF | 680 | 136 | 16 | 8 | 14 | 18 | |||
| Ducks | 60 | 12 | 0 | 0 | 2 | 0 | |||
| 2011-total | 7460 | 1492 | 142 (19%) | 70 (9.4%) | 112 (15%) | 163 (21.8%) | |||
| 2012 | Agneby | Agboville/Adzope | BYC | 1990 | 398 | 42 | 22 | 24 | 40 |
| CMF | 1190 | 238 | 27 | 13 | 16 | 20 | |||
| Ducks | 20 | 4 | – | – | – | – | |||
| Guinea fowl | 30 | 6 | – | – | – | 1 | |||
| District of Abidjan | Bingerville/Abidjan Market | BYC | 140 | 28 | 5 | 2 | 1 | 1 | |
| CMF | 780 | 156 | 12 | 8 | 7 | 10 | |||
| Ducks | 14a | 4a | – | – | – | – | |||
| Guinea fowl | – | – | – | – | – | – | |||
| South Comoe | Aboisso | BYC | 2460 | 492 | 67 | 30 | 25 | 44 | |
| CMF | 120 | 24 | 4 | 3 | 1 | 2 | |||
| Ducks | 6a | 2a | – | – | – | – | |||
| 2012-total | 6750 | 1352 | 157 (23.2%) | 78 (11.5%) | 74 (10.9%) | 118 (17.4%) | |||
| 2010–2012-total prevalence | 22,806 | 4562b | 438 (19.2%) | 232 (10.2%) | 243 (10.7%) | 423 (18.5%) | |||
14 individual samples (7 tracheal swabs and 7 cloacal swabs giving 2 pools of each with 3 and 4 individual samples, respectively) and 6 individual samples (3 tracheal swabs and 3 cloacal swabs giving 1 pool of 3 individual samples each).
2281 TS + 2281 ClS.
2.3. Serological tests
2.3.1. Detection of anti-NDV antibodies
Serum samples (n = 1943) were screened for anti-NDV antibodies using the haemagglutination/haemagglutination inhibition test (HA/HAI), the gold standard test, following the reference method (OIE, 2012) with reference NDV antigens (batch no. 1/08 — Ulster 2C) and corresponding reference positive serum as positive control. The reference reagents were provided free of charge by the World Organization for Animal Health (OIE)/Food and Agriculture Organization of the United Nations (FAO) Reference Laboratory in Padova (Italy) and by St Jude Children's Research Hospital, Memphis (TN, USA).
2.3.2. Detection of anti-IBV antibodies
Serum samples were also screened for specific anti-IBV antibodies by ELISA using the IDEXX IBV kit (IDEXX, The Netherlands with specificity = 100% and sensitivity > 90%) according to the protocol recommended by the manufacturer. Only 1938 serum samples were used for this analysis, as five (5) serum samples from guinea fowl were not available anymore to perform this test.
2.4. Molecular detection of avian viral genomes
Tracheal and cloacal swabs were processed as described (Kho et al., 2000, Snoeck et al., 2009). In the laboratory, each individual swab in an individual tube with VTM was processed and the suspension was kept individually. Then 5 individual swab-suspensions were pooled from the same species, farm or vendor in the live market. Finally, the samples were screened in pools of 5 swabs (Couacy-Hymann et al., 2012a). However some pools could contain less than 5 individual samples depending upon the number of available samples. The procedure for RNA isolation was as recommended by the manufacturer, using the RNeasy Mini Kit (Qiagen, Germany). The RNA was eluted in 50 μL of nuclease-free water. The RT step was performed by using random hexamer primers (Introgen, Carlsbad, CA., USA) with 10 μL of extracted RNA and the First-strand cDNA Synthesis Kit (GE Healthcare Europe GmBH, Orsay, France) as recommended by the manufacturer's protocol. Then, 5 μL of the cDNA obtained was used as the template for the PCR step with each outer set of primers specific for NDV F (Kho et al., 2000) or IBV S1 (Akin et al., 2001). Conventional PCR was carried out with the GeneAmp PCR System 2400 (Perkin-Elmer, Applied-Biosystems, Paris, France) using a 50 μL reaction mixture as previously described (Couacy-Hymann et al., 2012b, Kho et al., 2000, Akin et al., 2001). Nested PCR with inner primer sets specific for NDV (Kho et al., 2000) or IBV (Akin et al., 2001) was carried out in the same tubes, using the whole of the first stage PCR, to prevent any contamination (Kho et al., 2000, Akin et al., 2001).
The matrix gene was targeted for AIV detection in a single RT-PCR reaction (Couacy-Hymann et al., 2009, Starick et al., 2000).
2.5. Statistical analysis
A measure of precision of the prevalence estimate was obtained using 95% confidence intervals. The chi-squared test was used to compare the prevalence of NDV and IBV between species, production system and locations while the McNemar's test was used to compare the prevalence of NDV and IBV between cloacal and tracheal swab samples.
All experimental and animal management procedures were undertaken in accordance with the requirements of the Animal Care and Ethics Committees of LANADA.
3. Results
3.1. Clinical examination and sample collection
Individual sampled birds were clinically examined before sampling. Over the sampling period, a total of 234 dead chickens were found, 95 in 2010, 92 in 2011 and 47 in 2012. Regarding clinical signs (respiratory and nervous signs, inappetence, diarrhoea), a total of 1254 chickens (11%) of the total 11,403 birds sampled presented apparent signs of disease (340 in 2010, 503 in 2011 and 411 in 2012). The total number of dead and sick chickens was 1488 from which we collected both cloacal and tracheal swabs giving a total of 2976 swab samples.
A total of 22,806 samples, consisting of 11,403 cloacal and 11,403 tracheal swabs, were collected during the period 2010–2012 during the monthly surveys, including the samples from dead and sick chickens. These collected materials were pooled using maximum of 5 individual samples per pool, giving 4562 pools of samples (2281 pools of each type of swab) including 595 pools from sick and dead birds. During the same period, 1943 serum samples were collected (serum sampling only every 3 months) with 186 sera from apparently sick chickens (9.6%). These samples were obtained from backyard poultry farms, commercial farms and at live-bird markets within the three selected regions and involved samples from chickens, ducks and guinea fowl, with chickens representing 95.6% (23,667/24,749), duck, 3.04% (753/24,749) and guinea fowl, 1.3% (329/24,749) of the total collected samples, including serum samples (Table 1, Table 2). An average of 687.5 samples (24,749/36) was collected each month during the survey period. Samples collected from live bird markets represented 19% (4704/24,749) of the total.
3.2. NDV- and IBV-specific antibodies
Of a total of 1943 serum samples screened using the HA/HI test, 420 sera were positive, with an overall NDV prevalence of 21.6% (95% CI 19.8, 23.5%). Yearly prevalence ranged from 18.2% (95% CI 15.4, 21.0) in 2012 to 29.3 (95% CI 26.0, 32.5) in 2010. Species prevalence was 22.1% (95% CI 20.2, 24.0) (407 positive/1837) in chickens, 9.9 (95% CI 4.1, 5.7) (10/101) in ducks and 60% (95% CI 17.1, 100.9) (3/5) in guinea fowl. Among these positive serum samples, 156 were from the 186 serum samples collected from sick chickens (84%). The same serum samples (minus 5 guinea fowl samples) were screened for IBV antibodies by ELISA and 1401 samples were found positive out of 1938 serum samples giving 72.3% (95% CI 70.3, 74.3) IBV seroprevalence in the population as a whole. This ranged from 63.9% (95% CI 60.4, 67.3) in 2010 to 78% (95% CI 75.0, 78.0) in 2012. Species prevalence was 74.9% (95% CI 72.9, 76.9) (1387/1851) in chickens (including 13.8% (95% CI 7.1, 20.5) from sick chickens) and 13.9% (95% CI 7.2, 20.6) (14/101) in ducks. Regarding the locations, the NDV prevalence ranged from 13.2% (95% CI 11.7, 14.7) (265/2008) in Agneby region, 14.8% (95% CI 12.3, 17.3) (111/748) in District of Abidjan to 16.3% (95% CI 14.6, 18.0) (294/1806) in South Comoe region. The IBV prevalence ranged from 13.8% (95% CI 12.2, 15.4) (249/1806) in South Comoe region, 14.6% (95% CI 13.1, 16.1) (293/2008) in Agneby region to 16.6% (95% CI 14.0, 19.2) (124/748) in District of Abidjan (Table 1).
3.3. Detection of viral genomes
The 4562 pooled samples were analysed using nested-PCR on cDNA generated with random hexamers. This analysis found that 670 (14.7% (95% CI 13.7, 15.7)) and 666 (14.6% (95 CI 13.6, 15.6)) pools were positive for NDV or IBV respectively, all years, types of swabs or hosts taken together. However, regarding especially backyard poultry (chicken, duck and guinea fowl) the prevalence of IBV was 12.7% (95% CI 11.5, 13.9) (394/3098) and 12.5% (95% CI 11.3, 13.7) (363/2900) in backyard chicken only. IBV prevalence in commercial farms' chicken was 18.6% (95% CI 16.6, 20.6) (272/1464).
On a yearly basis, the prevalence of NDV in swab samples was 13% (95% CI 11.4, 14.6) (223/1718) in 2010, 13.2% (95% CI 11.5, 14.9) (212/1492) in 2011 and 17.4% (95% CI 15.2, 19.6) (235/1352) in 2012, while the prevalence of IBV in the samples was 11.6% (95% CI 10.1, 13.1) (199/1718), 18.4% (95% CI 16.4, 20.4) (275/1492) and 14.2% (95% CI 12.3, 16.1) (192/1352) in the same years. From the 595 pools of samples collected from dead and sick chickens, 572 pools were NDV positive (96.1% (95% CI 94.5, 97.7)) and 17 pools, IBV positive (2.8% (95% CI 1.4, 4.1)) with 3 pools positive for both viruses (Table 2).
The statistical analysis using the chi-squared test showed that the difference in NDV prevalence between backyard poultry and commercial chickens was not significant (p > 0.3) while this difference was significant regarding IBV prevalence (p < 0.0001). Between locations (Agneby, District of Abidjan and South Comoe), the difference in NDV prevalence was significant (p < 0.03) while the IBV prevalence was not significantly different between these regions (p > 0.05). About species (chicken, duck, guinea fowl), the difference in both NDV and IBV prevalences was not significant (p > 0.05 and p > 0.1, respectively).
When the results were broken down in terms of the type of swab, this survey found 19.2% (95% CI 17.6, 20.8) (438/2281) tracheal swab pools versus 10.2% (95% CI 8.9, 11.4) (232/2281) cloacal swab pools positive for NDV genome: NDV was more common in the tracheae of birds (p < 0.0001, McNemar's test). IBV prevalence, in contrast, was higher in the cloacal than tracheal swabs: 18.5% (95% CI 17.0, 20.1) (423/2281) and 10.7% (95% CI 9.4, 12.0) (243/2281) prevalence in cloacal and tracheal swabs, respectively (p < 0.0001, McNemar's test). We found a total of 49 pools that were positive for both NDV and IBV, of which 21 were tracheal and 28 cloacal. The detailed breakdown of the results is shown in Table 2.
4. Discussion
The avian influenza crisis, starting in Asia, reached Africa and in particular Ivory-Coast in 2006, causing huge economic losses. This situation greatly affected local poultry industries along with the loss of an important source of proteins for middle income and poor populations. Interestingly, the avian crisis highlighted the importance of ND (of which the main concern is the velogenic form) alongside other respiratory diseases such as IB. We took advantage of the ongoing surveillance for avian influenza virus within Ivory-Coast which followed the detection of 12 outbreaks of H5N1 (Couacy-Hymann et al., 2009). Birds that were sampled were clinically examined for any signs of disease. Animals showing clinical signs were included in the survey along with dead animals found on the site of sampling. The collected samples were screened for avian influenza virus type A RNA and for specific subtype H5, H7 and H9 antibodies and the overall result remained negative (Couacy-Hymann et al., 2012b).
These same samples have been screened in the present study for the presence of NDV and IBV, using assays for both genetic material and antibodies, for the period 2010–2012. The study has demonstrated the importance of ND in these mainly rural areas with poor populations, whose backyard poultry farms contribute significantly to household income and so contribute to poverty alleviation. Particularly, essentially all chickens found dead or sick were positive for NDV genome, with 96.3% prevalence. Partial sequencing of the F gene from samples collected on dead chickens showed the presence of polybasic sequence at the F protein cleavage site, corresponding with that expected for a velogenic strain of NDV. NDV-specific antibody prevalence ranged from 18.2% to 29.3% over the period of the study, with an overall average value of 21.6%, while the NDV F gene detection gave an overall prevalence of 14.7%, showing widespread distribution of the virus even among apparently healthy animals. These results confirm a previous study undertaken in Ivory-Coast on the burden of NDV in backyard poultry units, when compared to commercial farms where vaccinations are implemented in a correctly and thoroughly applied programme (Couacy-Hymann et al., 2012a). In rural regions, no vaccination against NDV is implemented on free range poultry. Among the three avian species studied, chickens, with 22.1% seroprevalence, are of main concern. The widespread nature of NDV in these populations contributes to the maintenance of the endemic pattern of the disease, causing mass seasonal death and impacting negatively on food security and poverty alleviation in those rural populations.
If ND is well known and studied in Africa, this is not the case with IB, which remains less investigated, with few data available presently (Ducatez et al., 2009). Cases of IB are reported mainly from commercial layer farms based on clinical signs such as respiratory distress, decline of the egg production, and damage of the shape of the eggs. Vaccination against the disease is strongly recommended in commercial poultry farms. Although field veterinary personnel and rural farmers report from time to time cases of low egg size or change of the shape of the eggs, any respiratory signs in the field are associated with, and reported as, ND. Little is also known on IB in backyard poultry units, since ND is still reported as the most important disease in that type of poultry farm. Our study shows that IBV is widespread in such units, albeit causing largely inapparent or subclinical infections; the seroprevalence in the period 2010–2012 was 72.3%, much higher than the seroprevalence for NDV, while the prevalence of the viral genomes was similar for the two viruses (12.7% IBV positive, 14.2% NDV positive). Chickens, with a seroprevalence of 74.9%, appear to be the main host of IBV in backyard poultry species. Our results demonstrate the high levels of circulation of IBV in poultry farms in free range (backyard) poultry farms as in commercial farms. Our results are in agreement with previous studies in Nigeria and Senegal, which also found high levels of circulation of IBV in backyard poultry farms, with seroprevalence rates above 70% (Owoade et al., 2006, Emikpe et al., 2010, Ntirandekura, 2011). The IBV virus itself was so far only reported in Africa in Morocco in 1982–1983 (el Houadfi and Jones, 1985), in Egypt in 2003 (Abdel-Moneim et al., 2006), and in Nigeria in 2006–2007 (Ducatez et al., 2009), likely more because very few research teams looked for the virus on the continent rather than because it is not present.
While we observed a much higher seroprevalence for IBV than for NDV (72% versus 22%), the virus prevalences were similar for both viruses. The sequenced NDV F cleavage sites highlighted the circulation of velogenic strains of the virus in the country. Taken together these results suggest that while healthy birds have been detected positive for NDV velogenic strains, the pathogen likely causes severe mortality in the field that may explain a lower seroprevalence for NDV than for IBV. A recent study in domestic poultry reported 8.7% NDV prevalence by virus isolation in Uganda with circulation of mainly velogenic viruses as well. In the Ugandan study, 28.6% (6/21) and 9.0% (108/1229) of the chickens from which NDV could be isolated were sick and healthy, respectively, confirming both the morbidity caused by velogenic NDV in the field and the detection of these strains in asymptomatic birds (Byarugaba et al., 2014).
Forty nine (49) of the pooled samples were positive for both NDV and IBV. Since each pool contained material from 5 birds, this result could be that the two viruses came from different birds or was a dual infection of the same individual bird. To clarify this situation, further work clearly needs to be done on individual samples from each positive pool.
This study on IBV in free range poultry farms is the first investigation on this disease undertaken in the country. Commercial poultry farms in the country used to vaccinate their flocks with vaccine having the Massachusett 41 (M41) strain of IBV while several serotypes circulate worldwide and there is not always cross-protection from one serotype to another (reviewed in Cavanagh, 2003). There needs to be fuller investigation to determine the genotype(s) and serotype(s) of the strains which are present in a concerned area prior to any vaccination. Re-use of samples collected for AIV surveillance may provide the opportunity to characterise the IBV strains currently circulating in Ivory-Coast.
Acknowledgements
We are grateful to Pr. C.P. Muller and Dr C. Snoeck, Laboratoire National de Santé, Centre de Recherche Public Santé, Luxembourg, who provided the ELISA kit for the IBV antibody detection.
We would like to thank the field veterinary services and personnel for their collaboration as well as all the poultry owners, vendors and other stakeholders for their cooperation during this study.
We specially thank Dr M. Baron, The Pirbright Institute, Ash Road, Pirbright GU24 ONF, UK., for his comments and the editing of this manuscript.
This study was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services under contract no. HHSN266200700005C, by the American Lebanese Syrian Associated Charities (ALSAC).
References
- Abdel-Moneim A.S., El-Kady M.F., Ladman B.S., Gelb J., Jr. S1 gene sequence analysis of a nephropathogenic strain of avian infectious bronchitis virus in Egypt. Virol. J. 2006;3:78. doi: 10.1186/1743-422X-3-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin A., Lin T.L., Wu C.C., Bryan T.A., Hooper T., Schrader D. Nucleocapsid protein gene sequence analysis reveals close genomic relationship between turkey coronavirus and avian infectious bronchitis virus. Acta Virol. 2001;45:31–38. [PubMed] [Google Scholar]
- Alexander D.J. Newcastle disease and other Paramyxoviridae infections. In: Calnek B.W., Barnes H.J., Beard C.W., McDougalg L., Saif J.Y.M., editors. Diseases of Poultry. 10th ed. Iowa State University; Ames, Iowa: 1997. pp. 541–569. [Google Scholar]
- Alexander D.J. Newcastle disease virus and other avian paramyxoviruses. In: Swayne D.E., Glisson J.R., Jackwood M.W., Pearson J.E., Reed W.M., editors. A Laboratory Manual for the Isolation and Identification of Avian Pathogens. 4th ed. American Association of Avian Pathologists; Kennett Square, PA: 1998. pp. 156–168. [Google Scholar]
- Briand F.X., Henry A., Massin P., Jestin V. Complete genome sequence of a novel avian paramyxovirus. J. Virol. 2012;86(14):7710. doi: 10.1128/JVI.00946-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byarugaba D.K., Mugimba K.K., Omony J.B., Okitwi M., Wanyana A., Otim M.O., Kirunda H., Nakavuma J.L., Teillaud A., Paul M.C., Ducatez M.F. High pathogenicity and low genetic evolution of avian paramyxovirus type I (Newcastle disease virus) isolated from live bird markets in Uganda. Virol. J. 2014;11:173. doi: 10.1186/1743-422X-11-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattoli G., Monne I., Fusaro A., Tony M.J., Lombin L.H., Aly M.M., Arafa A.S., Sturm-Ramirez K.M., Couacy-Hymann E., Awuni J.A., Batawui K.B., Awoume K.A., Aplogan G.L., Sow A., Ngangnou A.C., El Nasri I.M., Gamatie H.D., Dauphin D., Domenech J.M., Capua I. Highly pathogenic avian influenza virus subtype H5N1 in Africa: a comprehensive phylogenetic analysis and molecular characterization of isolates. PLoS One. 2009;4(3):e4842. doi: 10.1371/journal.pone.0004842. (1–9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattoli G., Susta L., Terregino C., Corrie B. Newcastle disease: a review of field recognition and current methods of laboratory detection. J. Vet. Diagn. Investig. 2011;23:637–657. doi: 10.1177/1040638711407887. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32:567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D., Gelb J., Jr. Infectious bronchitis. In: Saif Y.M., editor. Diseases of Poultry. 12th ed. Blackwell Publishing; Ames, Iowa: 2008. pp. 117–135. [Google Scholar]
- Couacy-Hymann E., Danho T., Keita D., Bodjo S.C., Kouakou C., Koffi Y.M., Beudje F., Tripodi A., De Benedictis P., Cattoli G. The first specific detection of a highly pathogenic avian influenza virus (H5N1) in Ivory Coast. J. Vet. Med. Zoonoses Public Health. 2009;58:10–15. doi: 10.1111/j.1863-2378.2008.01167.x. [DOI] [PubMed] [Google Scholar]
- Couacy-Hymann E., Kouakou A.V., Kouamé K.C., Kouassi L.A., Koffi Y.M., Godji P., Lana P., Tarnagda Z., Akoua-Koffi C. Surveillance for avian influenza and Newcastle disease in backyard poultry flocks in Côte-d'Ivoire, 2007–2009. Rev. Sci. Tech. Off. Int. Epizoot. 2012;31(3):821–828. doi: 10.20506/rst.31.3.2158. [DOI] [PubMed] [Google Scholar]
- Couacy-Hymann E., Kouakou A.V., Aplogan G.L., Awoumé F., Kouakou K.C., Kakpo L., Sharp B.R., McClenaghan L., McKenzie P., Webster R.G., Webby R.J., Ducatez M.F. Surveillance for influenza viruses in poultry and swine, West Africa, 2006–2008. Emerg. Infect. Dis. 2012;18(9):1446–1452. doi: 10.3201/eid1809.111296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducatez M.F., Martin A.M., Owoade A.A., Olatoye I.O., Alkali B.R., Maikano I., Snoeck C.J., Sausy A., Cordioli P., Muller C.P. Characterisation of a new genotype and serotype of infectious bronchitis virus in Western Africa. J. Gen. Virol. 2009;90:2679–2685. doi: 10.1099/vir.0.012476-0. [DOI] [PubMed] [Google Scholar]
- el Houadfi M., Jones R.C. Isolation of avian infectious bronchitis viruses in Morocco including an enterotropic variant. Vet. Rec. 1985;116:445. doi: 10.1136/vr.116.16.445-a. [DOI] [PubMed] [Google Scholar]
- Emikpe B.O., Ohore O.G., Olujonwo M., Akpavie S.O. Prevalence of antibodies to infection bronchitis virus (IBV) in chickens in southwestern Nigeria. Afr. J. Microbiol. Res. 2010;4(1):92–95. [Google Scholar]
- Jackwood M.W., Hall D., Handel A. Molecular evolution and emergence of avian gammacoronaviruses. Infect. Genet. Evol. 2012;12:1305–1311. doi: 10.1016/j.meegid.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho C.L., Mohd-Azmi M.L., Arshad, Yusoff K. Performance of an RT-nested PCR ELISA for detection of Newcastle disease virus. J. Virol. Methods. 2000;86:71–83. doi: 10.1016/s0166-0934(99)00185-8. [DOI] [PubMed] [Google Scholar]
- Lamb R.A., Parks G.D. Paramyxoviridae: the viruses and their replication. In: Howley D.M., Wolters P.M., editors. Fields Virology, 5th Knipe. Kluwer–Lippincott Williams & Wilkins; Philadelphia, PA: 2007. pp. 1449–1496. [Google Scholar]
- Maminiaina O.F., Gil P., Briand F.-X., Albina E. Newcastle disease virus in Madagascar: identification of genotype possibly deriving from a died out ancestor of genotype IV. PLoS One. 2010;5(11):1–12. doi: 10.1371/journal.pone.0013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P.J., Afonso C.L., Spackman E., Scott M.A., Pedersen J.C., Senne D.A., Brown J.D., Fuller C.M., Uhart M.M., Karesh W.B., Brown I.H., Alexander D.J., Swayne D.E. Evidence for a new avian paramyxovirus serotype 10 detected in rockhopper penguins from the Falkland Islands. J. Virol. 2010;84(21):11496–11504. doi: 10.1128/JVI.00822-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntirandekura J.B. en santé publique vétérinaire; 2011. Séroprévalence de la bronchite infectieuse en aviculture traditionnelle au Sénégal. (Mémoire de diplôme de Master) (41 pages) [Google Scholar]
- OIE . International Health Code. 9th ed. Office International des Epizooties; Paris, France: 2000. Newcastle disease. [Google Scholar]
- OIE . 7th edn. Office International des Epizooties; Paris: 2012. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. [Google Scholar]
- Owoade A.A., Ducatez M.F., Muller C.P. Seroprevalence of avian influenza virus, infectious bronchitis virus, reovirus, avian pneumovirus, infectious laryngotracheitis virus, and avian leucosis virus in Nigerian poultry. Avian Dis. 2006;50:222–227. doi: 10.1637/7412-071505R.1. [DOI] [PubMed] [Google Scholar]
- Snoeck C.J., Ducatez M.F., Owoade A.A., Faleke O.O., Alkali B.R., Tahita M.C., Tarnagda Z., Ouedraogo J.-B., Maikano I., Mbah P.O., Kremer J.R., Muller C.P. Newcastle disease virus in West Africa: new virulent strains identified in non-commercial farms. Arch. Virol. 2009;154:47–54. doi: 10.1007/s00705-008-0269-5. [DOI] [PubMed] [Google Scholar]
- Starick E., Romer-Oberdorfer A., Werner O. Type and subtype-specific RT-PCR assays for avian influenza A viruses (AIV) J. Vet. Med. B. 2000;47(4):295–301. doi: 10.1046/j.1439-0450.2000.00386.x. [DOI] [PubMed] [Google Scholar]
- Terregino C., Aldous E.W., Heidari A., Fuller C.M., De Nardi R., Manvell R.J., Beato M.S., Shell W.M., Monne I., Brown I.H., Alexander D.J., Capua I. Antigenic and genetic analyses of isolate APMV/wigeon/Italy/3920-1/2005 indicate that it represents a new avian paramyxovirus (APMV-12) Arch. Virol. 2013;158:2233–2243. doi: 10.1007/s00705-013-1735-2. [DOI] [PubMed] [Google Scholar]
- Wise M.G., Suarez D.L., Seal B.S., Pedersen J.C., Senne D.A., King D.J., Kapczynski D.R., Spackman E. Development of a real-time reverse-transcription PCR for detection of Newcastle disease virus RNA in clinical samples. J. Clin. Microbiol. 2004;42:329–338. doi: 10.1128/JCM.42.1.329-338.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


