Abstract
Purpose
Observational research focused upon emerging infectious diseases such as Ebola virus, Middle East respiratory syndrome, and Zika virus has been challenging to quickly initiate. We aimed to determine the duration of start-up procedures and barriers encountered for an observational study focused upon such infectious outbreaks.
Materials and methods
At 1 pediatric and 5 adult intensive care units, we measured durations from protocol receipt to a variety of outbreak research milestones, including research ethics board (REB) approval, data sharing agreement (DSA) execution, and patient study screening initiation.
Results
The median (interquartile range) time from site receipt of the protocol to REB submission was 73 (30-126) days; to REB approval, 158 (42-188) days; to DSA completion, 276 (186-312) days; and to study screening initiation, 293 (269-391) days. The median time from REB submission to REB approval was 43 (13-85) days. The median time for all start-up procedures was 335 (188-335) days.
Conclusions
There is a lengthy start-up period required for outbreak-focused research. Completing DSAs was the most time-consuming step. A reactive approach to newly emerging threats such as Ebola virus, Middle East respiratory syndrome, and Zika virus will likely not allow sufficient time to initiate research before most outbreaks are advanced.
Keywords: Outbreak, Pandemic, Research, Canada, Critical, Intensive care
Highlights
-
•
Start-up period required for observational studies focused on outbreak surveillance is time consuming.
-
•
The median time for all start-up procedures was 335 (188-335) days.
-
•
Completing data sharing agreements was the most time-consuming step, taking 9 months on average.
-
•
There is a need to have a nationally and internationally coordinated approach, with context-appropriate, tiered case report forms and preparatory work—protocol and case report form generation, data sharing agreements, and REB submissions—completed during the pre- and interoutbreak periods.
-
•
A reactive approach to newly emerging threats will likely not allow sufficient time to initiate research before most outbreaks are advanced or completed.
1. Introduction
New emerging and reemerging infections such as Ebola virus, Middle East respiratory syndrome (MERS-CoV), and Zika virus are a concern for the public, clinicians, health systems, and public health agencies. Outbreaks and pandemics are perceived to occur at increasing frequency; however, they remain unpredictable in their time and location of onset [1]. Outbreaks increase patient morbidity and mortality, and cause additional burden on health care workers, facilities, and health agencies [2], [3], [4]. Surveillance can identify cases at an early stage and lead to prevention of broader spread. Severe acute respiratory syndrome [5]; pandemic influenza A (H1N1) 2009-2010 [6]; and, more recently, Ebola virus [7], MERS-CoV [8], and Zika virus have been characterized by challenges initiating observational research and a near inability to rapidly undertake interventional trials necessary to inform best practice and improve care of patients [9], [10], [11]. This has prompted calls from patients, clinicians, funders, and policy makers to improve preparedness, including the capacity to undertake real-time research during such events. However, conducting studies and trials involves time-consuming start-up steps such as development of study protocol, establishing a budget and obtaining funding, research ethics board (REB) approval, organizing multisite collaboration, and data sharing agreements. The objective of this study was to determine the delay from protocol completion to study initiation and determine time spent in each of the necessary steps to identify and collect data in real time for new and emerging infection-related critical illness.
2. Methods
2.1. Design and setting
This is a time-in-motion study accompanying a prospective surveillance project to assess the feasibility of screening and real-time data collection for severe acute respiratory infection (SARI)- and outbreak-related critical illness. The parent prospective study aimed to screen all hospitalized critically ill patients on a daily basis for up to 72 hours after admission to detect all cases of SARI, the details of which are published elsewhere [12]. The study included 1 pediatric and 5 adult intensive care units (ICUs) across 6 Canadian provinces. Paper and electronic case report forms and daily and weekly screening log sheets were made available to all the sites to be used for data collection (Appendix). The study was approved by each participating site's REB and was funded by the Public Health Agency of Canada, Canadian Critical Care Trials Group, and Heart and Stroke Foundation (Ontario office).
2.2. Data collection
For the purpose of this study, the following data were collected: time required from protocol receipt by the site to REB submission, time required from REB submission to REB approval, time required from REB approval to data sharing agreement execution, time required from data sharing agreement execution to screening initiation, time required from protocol receipt to data sharing agreement execution, time required from protocol receipt to screening initiation, and overall time required for start-up procedures.
2.3. Statistical analysis
Categorical variables are presented as numbers and proportions. Durations are presented as median, interquartile range (IQR), and ranges. All statistical tests were 2-tailed, and the significance level was set at P < .05.
3. Results
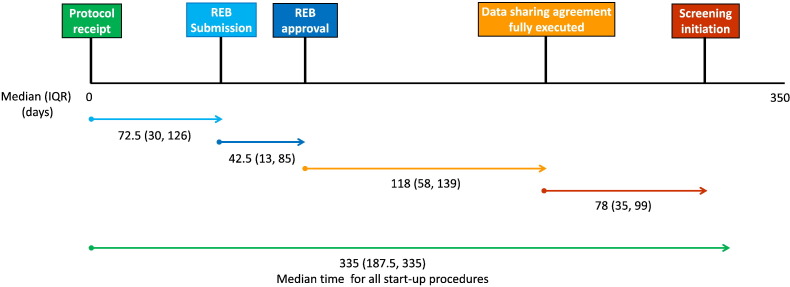
Table 1 shows the median time required in each step along the pathway to initiate an observational study of outbreak surveillance in ICUs. Overall start-up procedures required a median (IQR) of 335 (188-335) days (range, 128-335). Median (IQR) duration from protocol receipt to REB submission was 73 (30-126) days (range, 3-127) and protocol receipt to REB approval was 158 (42-188) days (range, 31-218 days). Time from protocol receipt to data sharing agreement receipt was 92 (92-104) days (range, 92-104), protocol receipt to signed data sharing agreement was 276 (186-312) days (range, 177-335), and protocol receipt to screening initiation was 293 (269-391) days (range, 258-412). Time from REB submission to REB approval was 43 (13-85) days (range, 9-178), REB approval to data sharing agreement completion was 118 (58-139) days (range, 8-142), and REB approval to screening initiation was 123 (92-237) days (range, 71-238). Time from data sharing agreement receipt to data sharing agreement completion was 185 (89-215) days (range, 74-244), and data sharing agreement completion to screening initiation was 78 (35-99) days (range, 6-103) (Fig. 1 ).
Table 1.
Median time (in days) spent from receipt of protocol, REB submission, and finalization of data sharing agreements to task completion at study sites
| Duration | Median (d) | IQR (d) | Range (d) |
|---|---|---|---|
| Protocol receipt to REB submission | 72.5 | 30.0-126.0 | 3-127 |
| Protocol receipt to REB approval | 158.0 | 42.0-188.0 | 31-218 |
| Protocol receipt to DSA receipt | 92.0 | 92.0-104.0 | 92-104 |
| Protocol receipt to DSA signed | 276.0 | 186.0-311.5 | 177-335 |
| Protocol receipt to screening initiation | 293.0 | 268.5-391.0 | 258-412 |
| REB submission to REB approval | 42.5 | 13.0-85.0 | 9-178 |
| REB approval to DSA completion | 118.0 | 58.0-139.0 | 8-142 |
| REB approval to screening initiation | 123.0 | 92.0-237.0 | 71-238 |
| DSA receipt to DSA completion | 185.0 | 89.0-214.5 | 74-244 |
| DSA completion to screening initiation | 78.0 | 35.0-99.0 | 6-103 |
| All Start-up procedures | 335.0 | 187.5-335.0 | 128-335 |
DSA indicates data sharing agreement.
Fig. 1.
Diagrammatic representation of median time (in days) spent from receipt of protocol, REB submission, and finalization of data sharing agreements to task completion at study sites.
4. Discussion
In this multicenter study of severe acute respiratory infections, we observed that it took nearly 1 year to complete all necessary start-up procedures before enrolment in the study could begin at all sites. Obtaining an interinstitutional legal data sharing agreement required approximately 9 months from protocol receipt to completion—the most time-consuming process. It took sites approximately 2½ months after protocol receipt to be ready to submit to their REB yet only approximately 1½ months for REB approval. Our findings indicate that despite an existing in-ICU infrastructure and capability for real-time data collection and reporting, observational research during an outbreak or pandemic is at risk of failing because of the time required for start-up procedures. Seasonal influenza outbreaks provide a compelling annual example. If we do not initiate the study start-up process immediately after influenza season, we will not be ready for screening at the next.
The time necessary for appropriate and necessary REB vetting and approvals has been reported previously for various clinical trials [13], [14], [15], [16], [17], [18]. However, none of the studies have identified the actual time required in initiating outbreak-related research at multiple sites. Efficient research initiation during an outbreak or pandemic is critical considering the potential for outbreak expansion and greater morbidity and mortality without better understanding of risk factors for illness and transmission, clinical course, outcomes, and responses to treatment. Although we studied timelines to initiate observational research, it is possible and in fact likely that start-up time for a clinical experimental trial would be even longer. This has been the experience during severe acute respiratory syndrome, pandemic influenza, MERS-CoV, Ebola virus, and now Zika virus [9], [10], [11].
There are various reasons for delays in initiating outbreak-focused observational research both at the investigator level and at the administrative level. Some of these reasons include (1) developing the study protocol and case report forms in a short span of time [13], (2) preparing REB applications, (3) fixed meeting dates of institutional ethics boards followed by important and necessary back-and-forth communications [16], (4) drafting and finalizing the data sharing agreements, (5) lack of parallel reviewing of REB applications and data sharing agreements across institutions, and (6) finalizing budget and arranging funding. There may be several possible ways to overcome these delays and be prepared ahead of time to conduct an outbreak-related study or trial. First, there is a need to have research-ready protocols-in-waiting for periods when seasonal or outbreak-related infections increase. This can be achieved through research-ready outbreak-related observational studies and trials using national and international networks [19], undertaking preemptive REB review of generic outbreak-related observational study case report forms and protocols, establishing data sharing agreements where necessary ahead of time, and helping other centers similarly prepare.
Although ethical approval is mandatory for research involving human subjects, there are provisions in many jurisdictions for exempt reviews for studies involving public health emergencies, typically consisting of observational studies collecting already available and anonymized data [20], [21]. Similarly, collecting data as “quality assurance” or “quality improvement” does not require REB approval in some provinces. If a multicenter observational study intends to collect nonidentifiable data from available information collected as a part of routine clinical care, which can be rapidly and efficiently used to generate new evidence, mechanisms are often in place to grant rapid assessments, and there exist guidelines to exempt certain studies from certain aspects of the review process [22].
Another approach will be to identify certain steps that take the longest duration among start-up procedures. In this study, we identified that data sharing agreements took 6 to 9 months to be fully executed. We found that some sites had limited research administration and regulatory staff, that some sites were busy with other ongoing research-related activities and that starting an unplanned research project introduced substantial demand on a system with already stretched capacity. Hospitals often have unique research administrative structures. Some university-affiliated hospitals were required to obtain REB approval from a university authority first and then from the local hospital to proceed, whereas others required data sharing agreements to be finalized before issuing the final REB approval. Improving efficiency and parallel administrative activities for certain types of low-risk observational studies are a potential mechanism to mitigate delays in start-up procedures. Centralized ethics approvals for pandemic research at provincial, state, and national levels may also help to improve efficiency and lessen workload for individual sites [23]. Having durable (5-10 years) protocols and generic approvals, to include anticipated ranges of pathogens and/or outbreaks meeting prespecified criteria, may also be more appropriate for outbreak and pandemic-related research as opposed to annual reapproval. Having a tiered case report form that seeks to collect either a minimal amount of core clinical information or more detailed data, depending upon the clinical research resources of individual sites, might assist in both start-up and actual study-related workload and translate to greater enthusiasm, capacity, and shorter start-up times. The World Health Organization–International Severe Acute Respiratory and Emerging Infection Consortium Clinical Characterization Protocol provides one such example [19].
Planning and preparedness before the next outbreak or pandemic strikes, during interpandemic periods, are essential for an effective research and subsequent clinical and health system response. Because of previous experiences in delays, there is a need to have a strategic plan for the surveillance of these emerging infections, if not at all times then during times of increased local or national risk, and to develop a mechanism to augment existing public health reporting with richer clinical data.
Recent examples of research responses to new infectious diseases events include funding and initiation of interpandemic clinical trials by groups within the Platform for European Preparedness Against (Re)emerging Epidemics [24] and coordination of funding efforts through the formation of the Global Research Collaboration for Infectious Disease Preparedness [25].
Models of informed consent are one other important consideration for outbreak- and pandemic-related research [26]. Obtaining truly informed consent for research involving time-sensitive interventions, during critical illness, in the midst of an outbreak or pandemic is challenging. It can sometimes be difficult to locate and fully inform substitute decision-makers of critically ill patients in a timely manner for interventions targeted at prehospital care or during the period primary resuscitation. Deferred consent may be appropriate for select emergency and time-sensitive interventions [27]. Waived consent may, occasionally, be appropriate when evaluating select interventions that fall firmly within the standard of care.
The strengths of this study include prospective data collection; use of internationally employed case definitions and eligibility criteria for, in this case, SARI-related outbreak activity; a fully operational Web-accessible case reporting system [28]; and an experienced research team with expertise in outbreak and pandemic specific research. This is the first study to report the actual duration of time spent in each step to initiate multisite outbreak-related research. Limitations to this study include lack of qualitative data from participating site research staff to better understand their perspectives regarding delays. Future studies may focus upon this complementary aspect. Also, the study was limited to major hospitals already carrying out critical care research, and therefore, we may be underestimating required timelines among centers without staff already familiar with the processes necessary for study start-up. Finally, although this study was focused upon surveillance of SARI during a period of global concern for many outbreak-causing pathogens—influenza A (H7N9, H1N1, H5N1) and MERS-CoV—it was initiated during an interoutbreak period in Canada; start-up time may be shorter or longer during an actual outbreak and has generalizable lessons for nonrespiratory outbreaks such as Ebola and Zika virus.
5. Conclusions
In this study, we found that there is substantial start-up time required to initiate outbreak-related observational research that may impede on our ability to conduct research, generate knowledge to help care for patients, and prepare for future threats. Our study stresses the need to have a nationally and internationally coordinated approach, with context-appropriate, tiered case report forms and preparatory work—protocol and case report form generation, data sharing agreements, and REB submissions—completed during the pre- and interoutbreak periods. To have the research mechanisms functional for real-time data collection and reporting when they are required, durable administrative and ethical approvals and data sharing agreements must be planned and executed before outbreaks and pandemics occur.
Footnotes
Funding: This work was supported by the Public Health Agency of Canada, Canadian Critical Care Trials Group, and Heart and Stroke Foundation (Ontario office).
Conflicts of interest: none.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jcrc.2017.02.009.
Appendix A. Supplementary data
Supplementary Material 1
Supplementary Material 2
References
- 1.Kilbourne E.D. Influenza pandemics of the 20th century. Emerg Infect Dis. 2006;12(1):9–14. doi: 10.3201/eid1201.051254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowler R.A., Abdelmalik P., Wood G., Foster D., Gibney N., Bandrauk N. Critical care capacity in Canada: results of a national cross-sectional study. Crit Care. 2015;19:133. doi: 10.1186/s13054-015-0852-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christian M.D., Hawryluck L., Wax R.S., Cook T., Lazar N.M., Herridge M.S. Development of a triage protocol for critical care during an influenza pandemic. CMAJ. 2006;175(11):1377–1381. doi: 10.1503/cmaj.060911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eastman N., Philips B., Rhodes A. Triaging for adult critical care in the event of overwhelming need. Intensive Care Med. 2010;36(6):1076–1082. doi: 10.1007/s00134-010-1862-0. [DOI] [PubMed] [Google Scholar]
- 5.Fowler R.A., Lapinsky S.E., Hallett D., Detsky A.S., Sibbald W.J., Slutsky A.S. Critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290(3):367–373. doi: 10.1001/jama.290.3.367. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A., Zarychanski R., Pinto R., Cook D.J., Marshall J., Lacroix J., Canadian Critical Care Trials Group H1N1 Collaborative Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302(17):1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 7.Schieffelin J.S., Shaffer J.G., Goba A., Gbakie M., Gire S.K., Colubri A., KGH Lassa Fever Program, Viral Hemorrhagic Fever Consortium, WHO Clinical Response Team Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014;371(22):2092–2100. doi: 10.1056/NEJMoa1411680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A., KSA MERS-CoV Investigation Team Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fowler R.A., Webb S.A., Rowan K.M., Sprung C.L., Thompson B.T., Randolph A.G. Early observational research and registries during the 2009-2010 influenza A pandemic. Crit Care Med. 2010;38(Suppl. 4):e120–e132. doi: 10.1097/CCM.0b013e3181d20c77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy M.M., Baylor M.S., Bernard G.R., Fowler R., Franks T.J., Hayden F.G. Clinical issues and research in respiratory failure from severe acute respiratory syndrome. Am J Respir Crit Care Med. 2005;171(5):518–526. doi: 10.1164/rccm.200405-621WS. [DOI] [PubMed] [Google Scholar]
- 11.Aoyama K., Fowler R.A. The challenges of treating Ebola virus disease with experimental therapies. Lancet Respir Med. 2015;3(7):503–504. doi: 10.1016/S2213-2600(15)00237-4. [DOI] [PubMed] [Google Scholar]
- 12.Dominguez-Cherit G., De la Torre A., Rishu A., Pinto R., Ñamendys-Silva S.A., Camacho-Ortiz A. Influenza A (H1N1pdm09)–related critical illness and mortality in Mexico and Canada. Crit Care Med. 2014 doi: 10.1097/CCM.0000000000001830. [in press] [DOI] [PubMed] [Google Scholar]
- 13.Hall D.E., Hanusa B.H., Stone R.A., Ling B.S., Arnold R.M. Time required for institutional review board review at one veterans affairs medical center. JAMA Surg. 2015;150(2):103–109. doi: 10.1001/jamasurg.2014.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Feo G., Frontini L., Rota S., Pepe A., Signoriello S., Labianca R. Time required to start multicentre clinical trials within the Italian medicine agency programme of support for independent research. J Med Ethics. 2015;41(10):799–803. doi: 10.1136/medethics-2012-100803. [DOI] [PubMed] [Google Scholar]
- 15.Wang-Gillam A., Williams K., Novello S., Gao F., Scagliotti G.V., Govindan R. Time to activate lung cancer clinical trials and patient enrollment: a representative comparison study between two academic centers across the Atlantic. J Clin Oncol. 2010;28(24):3803–3807. doi: 10.1200/JCO.2010.28.1824. [DOI] [PubMed] [Google Scholar]
- 16.Green L.A., Lowery J.C., Kowalski C.P., Wyszewianski L. Impact of institutional review board practice variation on observational health services research. Health Serv Res. 2006;41(1):214–230. doi: 10.1111/j.1475-6773.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dziak K., Anderson R., Sevick M.A., Weisman C.S., Levine D.W., Scholle S.H. Variations among institutional review board reviews in a multisite health services research study. Health Serv Res. 2005;40(1):279–290. doi: 10.1111/j.1475-6773.2005.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenyon G.M., Mendelow A.D., Gregson B.A., Rowan E. Obtaining regulatory approval for multicentre randomised controlled trials: experiences in the STICH II trial. Br J Neurosurg. 2011;25(3):352–356. doi: 10.3109/02688697.2010.551675. [DOI] [PubMed] [Google Scholar]
- 19.International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) https://isaric.tghn.org/protocols/ [accessed 11.07.16]
- 20.Orchard J. For debate: should observational clinical studies require ethics committee approval? J Sci Med Sport. 2008;11(3):239–242. doi: 10.1016/j.jsams.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Moser B., Röggla G. Should observational clinical studies require ethics committee approval? J Sci Med Sport. 2008;11(5):518. doi: 10.1016/j.jsams.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Neff M.J. Institutional review board consideration of chart reviews, case reports, and observational studies. Respir Care. 2008;53(10):1350–1353. [PubMed] [Google Scholar]
- 23.Kaufmann P., O'Rourke P.P. Central institutional review board review for an academic trial network. Acad Med. 2015;90(3):321–323. doi: 10.1097/ACM.0000000000000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Platform for European Preparedness Against (Re-)emerging Epidemics (PREPARE) http://www.prepare-europe.eu [accessed 12.12.16]
- 25.Global Research Collaboration for Infectious Disease Preparedness (GloPID-R) http://www.glopid-r.org [accessed 12.12.16]
- 26.Cook D., Burns K., Finfer S., Kissoon N., Bhagwanjee S., Annane D. Clinical research ethics for critically ill patients: a pandemic proposal. Crit Care Med. 2010 Apr;38(Suppl. 4):e138–e142. doi: 10.1097/CCM.0b013e3181cbaff4. [DOI] [PubMed] [Google Scholar]
- 27.Gobat N.H., Gal M., Francis N.A., Hood K., Watkins A., Turner J. Key stakeholder perceptions about consent to participate in acute illness research: a rapid, systematic review to inform epi/pandemic research preparedness. Trials. 2015;16:591. doi: 10.1186/s13063-015-1110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.iDataFax user guide Clinical DataFax systems Inc. http://clarityrand.mcmaster.ca/datafax/ [accessed 12.12.16]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1
Supplementary Material 2