Abstract
Internalization of bacteria into mammalian host cells has been studied extensively in the past two decades. These studies have highlighted the amazingly diverse strategies used by bacterial pathogens to induce their entry in non-phagocytic cells. The roles of actin and of the whole cytoskeletal machinery have been investigated in great detail for several invasive organisms, such as Salmonella, Shigella, Yersinia and Listeria. Recent results using Listeria highlight a role for the endocytosis machinery in bacterial entry, suggesting that clathrin-dependent endocytic mechanisms are also involved in internalization of large particles. This contrasts with the generally accepted dogma but agrees with previous studies of bacterial and viral infections and also of phagocytosis.
Introduction
The plasma membrane is a dynamic structure that separates the intracellular compartment from the extracellular milieu and enables communication between the cell and its environment. Ions and small molecules, such as amino acids and sugars, can traverse the plasma membrane through membrane channels. Macromolecules and larger structures enter cells by endocytosis, which is defined as the uptake by invagination and pinching-off of portions of the plasma membrane containing lipids and membrane proteins, extracellular ligands and/or soluble molecules 1, 2, 3. Several types of endocytosis have been described, differing in the size of the endocytic vesicle, the nature of the cargo and the mechanism of vesicle formation. They include phagocytosis, macropinocytosis, clathrin-mediated endocytosis, caveolin-mediated endocytosis and clathrin- and caveolin-independent endocytosis [1].
Phagocytosis is restricted to ‘professional phagocytes’, such as neutrophils, macrophages or dendritic cells, whereas the other internalization events occur in almost all cell types. Both phagocytosis and macropinocytosis produce extensions of the plasma membrane, driven by actin polymerization, whereby cells engulf particles and/or extracellular fluid. The other entry processes have long been considered to be actin-independent events.
Clathrin-mediated endocytosis is the major process by which transmembrane proteins are internalized from the plasma membrane. It starts at the so-called coated pits, which are formed by the assembly of clathrin on the cytoplasmic side of the plasma membrane. Transmembrane proteins are selectively recruited to these coated pits by binding to clathrin adaptors or other coat-associated proteins. The coated pit then pinches off from the membrane and forms a coated vesicle inside the cell [4]. Clathrin in coated vesicles is structured as a triskelion, formed by three heavy (192 kDa) and three light (25–29 kDa) chains that bind to each other in a polyhedral lattice [5]. Data from in vitro reconstitution studies and models of clathrin triskelion suggest that clathrin can support the entry of vesicles with a maximal diameter of 120 nm 6, 7, 8. This led to the dogma that particles with a diameter larger that 120 nm cannot enter cells by clathrin-mediated endocytosis [1]. In addition to enabling the entry of macromolecules (e.g. low density lipoprotein, α2 macroglobulin or growth factor receptors), clathrin-mediated endocytosis is a well documented portal of entry for a large number of viruses (e.g. influenza viruses, adenoviruses, vesicular stomatitis virus (VSV), reoviruses, Semliki forest virus, Ebola virus, SARS coronavirus or hepatitis B and C viruses) to invade host cells 9, 10, 11, 12, 13, 14.
Invasive bacteria, like viruses, induce their own uptake by non-phagocytic host cells. In this intracellular niche, bacteria are protected from hostile events occurring in the extracellular environment, such as complement deposition or antibody binding. They can then multiply and invade neighbouring cells after direct cell-to-cell spread or after cell lysis. Invasive bacteria can be separated into two well-differentiated groups on the basis of their entry mechanisms, the ‘zipper’ and the ‘trigger’ mechanisms (Box 1 ). The entry of Listeria monocytogenes, Yersinia pseudotuberculosis and other pathogenic bacteria that invade host cells by the zipper mechanism is triggered by the interactions between bacterial surface-exposed proteins and cellular receptors. For example, the Listeria proteins InlA and InlB interact with cellular E-cadherin (an adhesion protein) and Met (also known as hepatocyte growth factor (HGF) receptor), respectively, and Yersinia invasin binds to β1-integrins. These interactions induce signalling cascades that result in the activation of the actin cytoskeleton and relatively small membrane extensions that zip around the bacterium and engulf it [15]. Salmonella and Shigella are the paradigms of bacteria that use the second mechanism of entry, the trigger mechanism. Bacteria in this group actively inject effectors into the host cytoplasm using a specialized secretory apparatus, the type III secretion system. Some of these bacterial effectors modulate the actin cytoskeleton, producing massive polymerization of actin and membrane ruffling. This results in the formation of pseudopods loosely attached to bacteria, that enable bacterial internalization in a process similar to macropinocytosis 15, 16. The two entry pathways share a requirement for actin polymerization.
Box 1. The zipper and trigger models.
The zipper mechanism
Listeria (Figure Ia, top), Neisseria and Yersinia are examples of bacteria that enter using the zipper mechanism. These bacteria express proteins on their surfaces that interact with cellular receptors, initiating signalling cascades that result in close apposition of the cellular membrane around the entering bacteria (Figure 1a, bottom). Actin polymerization and modest membrane extensions finally lead to bacterial internalization. For example, the listerial protein InlB interacts with Met, promoting Met auto-phosphorylation, the recruitment of the protein adaptors Gab1, Cbl and Shc and activation phosphatidylinositol 3-kinase (PI 3-kinase) and the small GTPase Rac, which in turn promotes actin polymerization through the actin-binding Arp2/3 and Wave complexes. This interaction of InlB with Met also promotes ubiquitination of the receptor and the recruitment of the clathrin-dependent endocytic machinery, including clathrin and dynamin, to the bacterial entry site. Finally, bacteria are endocytosed in a clathrin-coated vesicle. It is possible that actin polymerization is also associated with the endocytic machinery in an Arp2/3-independent manner, as it has been shown that the actin-binding protein cortactin and CD2AP are important for Listeria internalization.
Figure I.
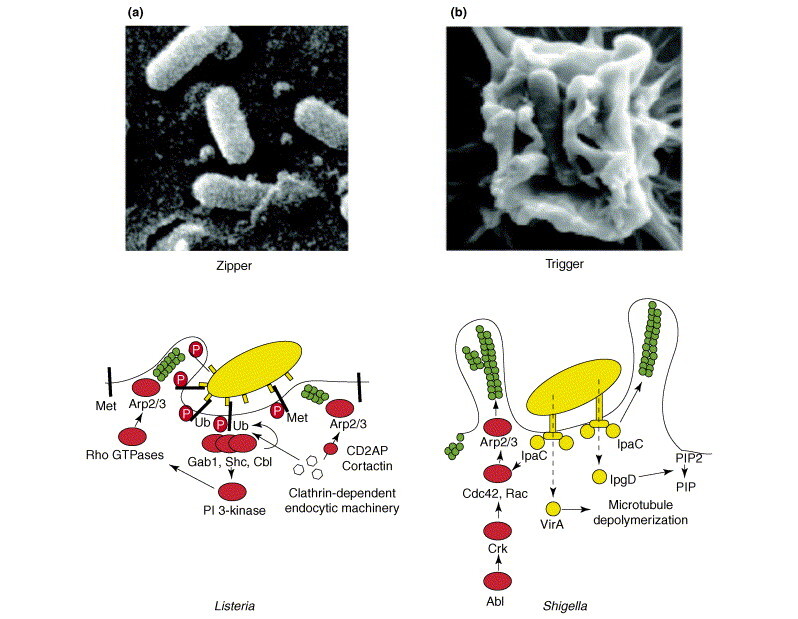
(a) A scanning electron microscopy image (top) of a L. monocytogenes bacterium entering an epithelial cell. Reproduced with permission from [73]. The diagram below shows a model of the signalling produced by the interaction of listerial protein InlB with Met. (b) A scanning electron micrograph (top) of a Shigella bacterium entering into an epithelial cell. The diagram below is a representation of the signalling cascades produced during Shigella invasion. Green circles represent actin.
The trigger mechanism
Shigella (Figure Ib, top) and Salmonella are the best-studied examples of bacteria entering cells using the trigger mechanism. These bacteria bypass the necessity to bind to a cellular receptor and use type III secretion systems to inject protein effectors that interact with the actin cytoskeleton. Type III secretion systems are complex protein machineries that enable the secretion of bacterial effectors directly from the bacterial cytoplasm into the cellular cytosol. The injected bacterial effectors promote massive actin polymerization and formation of macropinocytic membrane extensions loosely attached to the bacteria, and which eventually lead to bacterial internalization. For example, the Shigella protein IpaC triggers actin polymerization directly or in a manner dependent on the small GTPases Cdc42 and Rac. The Shigella effector VirA, which binds to tubulin hetero-oligomers and inhibits microtubule formation, can indirectly promote actin polymerization. Shigella infection also promotes the activation of the kinases Abl and Arg and phosphorylation of the adaptor protein Crk, which can activate Cdc42 and Rac in a process similar to that observed in filopodia formation. Another Shigella effector, IpgD, hydrolyzes phosphatidylinositol (4,5)bisphosphate (PIP2) to phosphatidylinositol (5) phosphate (PIP) and disconnects cortical actin from the membrane [74].
For many years, drugs that interfere with the actin cytoskeleton (e. g. cytochalasin D) have been used as tools to distinguish between clathrin-dependent endocytosis and phagocytosis. It was generally thought that clathrin-mediated endocytosis was actin-independent (see, for example, Ref. [17]). As bacteria require the participation of the actin cytoskeleton to enter into host cells 15, 16, and as the size of the vesicle harbouring a bacterium far exceeds the maximal size expected for a clathrin-coated vesicle, the hypothesis was that bacteria enter into non-phagocytic cells by phagocytic-like mechanisms. However, receptors used by zippering bacteria to enter into cells (e.g. Met, E-cadherin and integrins) can be endocytosed by a clathrin-dependent mechanism, and an increasing number of articles start to point to the necessary role of actin cytoskeleton in clathrin-mediated endocytosis 3, 18, 19. The requirement of the actin cytoskeleton was therefore not incompatible with the participation of clathrin-dependent endocytosis in bacterial entry. Challenging the dogma, it has been shown that Listeria invade non-epithelial cells by hijacking the clathrin-dependent endocytic machinery. There are also data implicating clathrin-mediated uptake for several other bacteria. Here, we discuss the possible role of clathrin-mediated endocytosis in bacterial entry.
A shift in paradigm, the Listeria model
Listeria monocytogenes is a Gram-positive bacterium responsible for listeriosis, a severe human infection with an overall 30% mortality rate. The clinical features include severe gastroenteritis, mother-to-child infections and central nervous system infections. L. monocytogenes can cross the intestinal, blood–brain and placental barriers. L monocytogenes is a facultative intracellular pathogen and can invade and replicate in epithelial cells and macrophages 15, 20. As mentioned earlier, two bacterial proteins involved in entry have been described: InlA, which interacts with E-cadherin on epithelial cells, and InlB, a surface protein that enables L. monocytogenes to enter into most non-phagocytic cells by interaction with its cellular receptor Met. Met belongs to the receptor tyrosine kinase (RTK) family and is involved in diverse cellular functions, such as scattering, invasion, proliferation, morphogenesis and angiogenesis. It is also implicated in a large number of human tumours, correlating closely with metastasis and poor prognosis 21, 22. These tumorigenic activities occur when Met is overexpressed or when Met signalling after ligand (HGF) binding is not down-regulated [23]. The normal way used by cells to stop signalling downstream of Met and other RTKs is the ligand-dependent endocytosis and subsequent degradation of the activated receptor 21, 23.
Interaction of Met with soluble InlB results in the clathrin-dependent endocytosis of Met 24, 25. As observed by immunofluorescence, clathrin and dynamin, the major proteins involved in clathrin-dependent endocytosis [1], localize at bacterial entry sites (Figure 1 and see the supplementary material online) [25]. Cbl, the ubiquitin ligase responsible for ubiquitination and subsequent endocytosis of Met and other growth factors 26, 27, 28, 29, also localizes at bacterial entry sites [25]. The possible role in bacterial entry of the major proteins involved in the clathrin-dependent endocytosis of growth factor receptors, including clathrin heavy chain, dynamin and Cbl, was tested by small interfering RNA (siRNA), which resulted in great reduction in bacterial entry [25]. Moreover, the relevance of other components of the endocytic machinery, such as growth factor receptor-binding protein (Grb2), epidermal growth factor pathway substrate 15 (eps15), Cbl-interacting protein of 85 kDa (CIN85) and CD2 associated protein (CD2AP), all of which are proteins necessary for ligand-induced endocytosis of receptor tyrosine kinases 2, 19, 30, 31 during bacterial entry was shown by siRNA knock-down. Decreased expression of these proteins strongly inhibited L. monocytogenes entry [25], demonstrating a major role of the clathrin-dependent endocytic machinery during L. monocytogenes infections.
Figure 1.
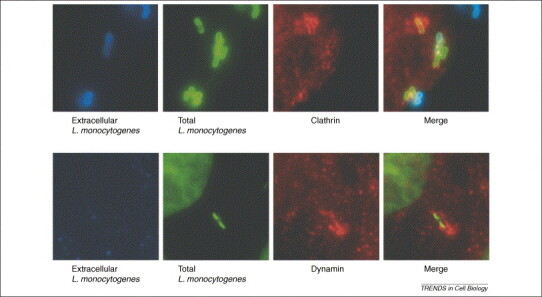
Localization of clathrin and dynamin at L. monocytogenes entry sites into HeLa cells. (a) Extracellular bacteria are in blue, total bacteria in green and clathrin in red. (b) Extracellular bacteria are in blue, total bacteria and the cellular nucleus in green and dynamin in red. The merged images clearly show clathrin and dynamin surrounding bacteria that are entering the cell. Reproduced with permission from Ref. [15].
Bacteria might therefore use the clathrin-dependent endocytosis machinery to enter non-phagocytic cells, challenging established ideas about the maximum size permitted for a clathrin-coated vesicle. Note that Listeria is a bacillus reaching 2–6 μm in length.
Clathrin and other bacteria
Several other pathogenic bacteria entering by the zipper mechanism recognize membrane receptors that are susceptible to being endocytosed. A possible role of clathrin in the entry of such bacteria had been suggested, reinforcing the data obtained with Listeria and leading to the conclusion that a role for clathrin-dependent endocytosis in bacterial entry is probably the rule rather than the exception.
Yersinia pseudotuberculosis
Yersinia pseudotuberculosis, a Gram-negative enteroinvasive bacterium, enters nonphagocytic cells by the interaction between a bacterial surface protein called invasin and host β1-integrins [32]. Expressing the inv gene (encoding invasin) confers to non-pathogenic Escherichia coli (E. coli inv+) the ability to enter into non-phagocytic cells, mimicking the entry of Y. pseudotuberculosis [33]. Electron microscopy (EM) studies show that invasion of epithelial cells by E. coli inv+ occurs through electron dense structures resembling clathrin-coated vesicles [34]. In addition, potassium depletion, which has been shown to inhibit clathrin-mediated endocytosis [35], also inhibits bacterial entry [34]. Moreover, microinjection into host cells of antibodies against clathrin or AP2 (the major clathrin adaptor in the plasma membrane [36]) also inhibits bacterial entry [34]. Together, these results point to a role for the endocytosis machinery in Yersinia invasion.
Rickettsiae
Rickettsiae are obligate intracellular Gram-negative bacteria with a life cycle that includes an arthropod vector and mammalian hosts. The ubiquitous protein Ku70 has been recently identified as a cellular receptor used by Rickettsia conorii to infect mammalian host epithelial cells [37]. In the same study, the ubiquitin ligase Cbl was shown to localize with R. conorii during entry. Knocking down Cbl by siRNA inhibits bacterial internalization, suggesting a role for clathrin-dependent endocytosis in R. conorii invasion similar to that observed in L. monocytogenes.
Other bacteria
Data coming from studies using drugs inhibiting endocytosis and microscopy studies also suggest a possible role of clathrin-dependent endocytosis in the entry of E. coli 38, 39, 40, 41, Staphylococcus aureus 42, 43, Streptococcus pneumoniae 44, 45, Streptococcus dysgalactiae [46], Ehrlichia risticii 17, 47, Brucella abortus [48], Klebsiella pneumoniae [49], Legionella pneumophila [50], Campylobacter jejuni [51] and Citrobacter freundii [51]. More controversial is the role of clathrin-dependent endocytosis in Neisseria and Chlamydia invasion.
Neisseria gonorrhoeae, the etiologic agent of the sexually transmitted disease gonorrhoea, can infect epithelial cells by binding to the asialoglycoprotein receptor (ASGPR) [52]. Endocytosis of ASGPR is clathrin-dependent and bacteria localize with clathrin and ASGPR during entry. In addition, monodansylcadaverine, which inhibits clathrin-dependent endocytosis, inhibits bacterial entry in such cells [53]. However, other studies show that treatment of epithelial cells with monodansylcadaverine had very weak or no effect on N. gonorrhoeae entry, and no clathrin was observed in the N. gonorrhoeae entry sites [54].
Chlamydiae are Gram-negative obligate intracellular bacteria involved in a wide spectrum of human and other vertebrate diseases. EM studies show that Chlamydia psittaci and Chlamydia trachomatis enter epithelial cells using clathrin-coated pits 55, 56, 57. In these studies, the infective forms of Chlamydia, called elementary bodies, were observed at coated pits and coated vesicles, and anti-clathrin immunostaining indicated the presence of clathrin at the bacterial entry sites [56]. However, EM studies of Chlamydia invasion failed to find clathrin-coated pits associated with entry of the bacterium, and monodansylcadaverine had no effect on invasion [58]. Moreover, in cells expressing mutants of dynamin (dynamin I K44A) and of Eps15 (Eps15 Δ95–295) [59], which inhibit clathrin-dependent endocytosis 60, 61, the entry of C. trachomatis remained unaffected [59], suggesting a clathrin-independent entry. These conflicting reports on the role of clathrin-dependent endocytosis suggest that Neisseria and Chlamydia could use different and alternative entry pathways; some of these could be clathrin-dependent and some not, probably depending on the receptor engaged.
Conclusion
Taken together, these data indicate that bacteria can use clathrin-dependent endocytosis as a mechanism to enter host cells. Thus, endocytosis clearly supports entry of particles larger than 1 μm, an observation that has broad implications for cell biology, immunology and infectious disease processes. The fact that clathrin can enable the entry of large vesicles was supported by EM studies of viral infections of epithelial cells, showing viral particles (VSV, influenza, parvovirus) in clathrin-coated pits or vesicles larger that 120 nm 62, 63, 64.
The role of clathrin in the internalization of large particles was also pointed out many years ago in studies of latex bead internalization in macrophages using the deep-etch replica technique 65, 66. These studies clearly showed clathrin lattices surrounding phagocytosed beads (Figure 2 ). However, the role of clathrin-mediated endocytosis in phagocytosis has remained controversial, and no role for clathrin was found in the phagocytosis of immunoglobulinG-coated particles larger than 3 μm [67].
Figure 2.
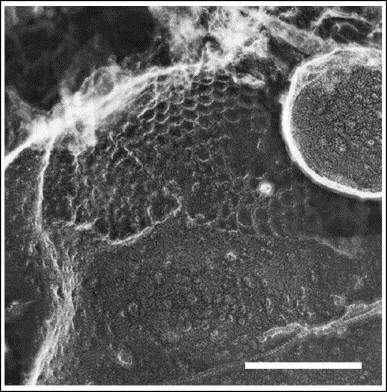
Image of a bead being phagocytosed revealing a large clathrin basketwork immediately below the advancing lip of a nascent phagosome. The scale bar represents 0.2 μm. Reproduced with permission from Ref. [65].
Our view on endocytosis is continuously changing, and further studies of pathogen–host interactions will probably contribute to a better understanding of how cells communicate with their environment. Bacteria, or beads coated with ligands of cellular receptors, could be used as tools to understand the basic mechanisms of endocytosis. As bacteria or coated beads are larger than soluble macromolecules, they can be detected easily and the proteins involved can be spatially and temporally followed.
Given that the interactions between bacterial proteins with their cellular receptors are highly specific, the presence of the receptors determines which cell types and species are susceptible to a given pathogen. The efficiency of invasion processes, as well as the exact mechanism of uptake used by each bacterium, depends mainly on the specific receptor engaged, which is unknown for the majority of pathogens. The shape of the pathogen could also have a role in bacterial endocytosis, as has been shown during phagocytosis [68].
In agreement with results showing that the time required to complete the assembly of the endocytic complex is proportional to the size of cargo molecules [69], Listeria using the InlB-Met pathway or InlB-coated beads take 4 min to enter epithelial cells (our unpublished results), which is longer than the endocytosis of macromolecules. It is thus possible that other bacteria that are far larger than macromolecules also take more time to enter into host cells. This prolonged internalization time, versus a transient signal, could affect the signalling and trafficking of the internalized particle.
The key issue that remains unsolved is the architecture of clathrin around entering bacteria. It is known that in endosomes there are zones of flat or irregular clathrin 70, 71, 72. Thus, it could be possible that clathrin assembles as flat layers at the entry sites of bacteria and then cover the invaginating membrane, as previously shown for entering beads (Figure 2) [65]. This point deserves further investigation.
Footnotes
Supplementary videos associated with this article can be found online at doi:10.1016/j.tcb.2006.08.005.
Appendix A. Supplementary material
References
- 1.Conner S.D., Schmid S.L. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 2.Dupre S., et al. Ubiquitin and endocytic internalization in yeast and animal cells. Biochim. Biophys. Acta. 2004;1695:89–111. doi: 10.1016/j.bbamcr.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Engqvist-Goldstein A.E., Drubin D.G. Actin assembly and endocytosis: from yeast to mammals. Annu. Rev. Cell Dev. Biol. 2003;19:287–332. doi: 10.1146/annurev.cellbio.19.111401.093127. [DOI] [PubMed] [Google Scholar]
- 4.Sorkin A. Cargo recognition during clathrin-mediated endocytosis: a team effort. Curr. Opin. Cell Biol. 2004;16:392–399. doi: 10.1016/j.ceb.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Fotin A., et al. Structure of an auxilin-bound clathrin coat and its implications for the mechanism of uncoating. Nature. 2004;432:649–653. doi: 10.1038/nature03078. [DOI] [PubMed] [Google Scholar]
- 6.Zaremba S., Keen J.H. Assembly polypeptides from coated vesicles mediate reassembly of unique clathrin coats. J. Cell Biol. 1983;97:1339–1347. doi: 10.1083/jcb.97.5.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keen J.H. Clathrin assembly proteins: affinity purification and a model for coat assembly. J. Cell Biol. 1987;105:1989–1998. doi: 10.1083/jcb.105.5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMahon H.T. Endocytosis: an assembly protein for clathrin cages. Curr. Biol. 1999;9:R332–R335. doi: 10.1016/s0960-9822(99)80206-1. [DOI] [PubMed] [Google Scholar]
- 9.Blanchard E., et al. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J. Virol. 2006;80:6964–6972. doi: 10.1128/JVI.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper A., Shaul Y. Clathrin-mediated endocytosis and lysosomal cleavage of hepatitis B virus capsid-like core particles. J. Biol. Chem. 2006;281:16563–16569. doi: 10.1074/jbc.M601418200. [DOI] [PubMed] [Google Scholar]
- 11.Lakadamyali M., et al. Endocytosis of influenza viruses. Microbes Infect. 2004;6:929–936. doi: 10.1016/j.micinf.2004.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsh M., Helenius A. Virus entry: open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meier O., Greber U.F. Adenovirus endocytosis. J. Gene Med. 2004;6(Suppl 1):S152–S163. doi: 10.1002/jgm.553. [DOI] [PubMed] [Google Scholar]
- 14.Sieczkarski S.B., Whittaker G.R. Dissecting virus entry via endocytosis. J. Gen. Virol. 2002;83:1535–1545. doi: 10.1099/0022-1317-83-7-1535. [DOI] [PubMed] [Google Scholar]
- 15.Cossart P., Sansonetti P.J. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science. 2004;304:242–248. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- 16.Pizarro-Cerda J., Cossart P. Bacterial adhesion and entry into host cells. Cell. 2006;124:715–727. doi: 10.1016/j.cell.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Messick J.B., Rikihisa Y. Characterization of Ehrlichia risticii binding, internalization, and proliferation in host cells by flow cytometry. Infect. Immun. 1993;61:3803–3810. doi: 10.1128/iai.61.9.3803-3810.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yarar D., et al. A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol. Biol. Cell. 2005;16:964–975. doi: 10.1091/mbc.E04-09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch D.K., et al. A Cortactin-CD2-associated protein (CD2AP) complex provides a novel link between epidermal growth factor receptor endocytosis and the actin cytoskeleton. J. Biol. Chem. 2003;278:21805–21813. doi: 10.1074/jbc.M211407200. [DOI] [PubMed] [Google Scholar]
- 20.Dussurget O., et al. Molecular determinants of Listeria monocytogenes virulence. Annu. Rev. Microbiol. 2004;58:587–610. doi: 10.1146/annurev.micro.57.030502.090934. [DOI] [PubMed] [Google Scholar]
- 21.Hicke L. Protein regulation by monoubiquitin. Nat. Rev. Mol. Cell Biol. 2001;2:195–201. doi: 10.1038/35056583. [DOI] [PubMed] [Google Scholar]
- 22.Trusolino L., Comoglio P.M. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat. Rev. Cancer. 2002;2:289–300. doi: 10.1038/nrc779. [DOI] [PubMed] [Google Scholar]
- 23.Bache K.G., et al. Defective downregulation of receptor tyrosine kinases in cancer. EMBO J. 2004;23:2707–2712. doi: 10.1038/sj.emboj.7600292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li N., et al. The Listeria protein internalin B mimics hepatocyte growth factor-induced receptor trafficking. Traffic. 2005;6:459–473. doi: 10.1111/j.1600-0854.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 25.Veiga E., Cossart P. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat. Cell Biol. 2005;7:894–900. doi: 10.1038/ncb1292. [DOI] [PubMed] [Google Scholar]
- 26.Dikic I., et al. Cbl signaling networks in the regulation of cell function. Cell. Mol. Life Sci. 2003;60:1805–1827. doi: 10.1007/s00018-003-3029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dikic I., Giordano S. Negative receptor signalling. Curr. Opin. Cell Biol. 2003;15:128–135. doi: 10.1016/s0955-0674(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 28.Haglund K., et al. Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem. Sci. 2003;28:598–603. doi: 10.1016/j.tibs.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Marmor M.D., Yarden Y. Role of protein ubiquitylation in regulating endocytosis of receptor tyrosine kinases. Oncogene. 2004;23:2057–2070. doi: 10.1038/sj.onc.1207390. [DOI] [PubMed] [Google Scholar]
- 30.Stang E., et al. Cbl-dependent ubiquitination is required for progression of EGF receptors into clathrin-coated pits. Mol. Biol. Cell. 2004;15:3591–3604. doi: 10.1091/mbc.E04-01-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrelli A., et al. The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met. Nature. 2002;416:187–190. doi: 10.1038/416187a. [DOI] [PubMed] [Google Scholar]
- 32.Isberg R.R., Leong J.M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 33.Isberg R.R., Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985;317:262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- 34.Van Nhieu G.T., et al. Mutations in the cytoplasmic domain of the integrin beta1 chain indicate a role for endocytosis factors in bacterial internalization. J. Biol. Chem. 1996;271:7665–7672. doi: 10.1074/jbc.271.13.7665. [DOI] [PubMed] [Google Scholar]
- 35.Larkin J.M., et al. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell. 1983;33:273–285. doi: 10.1016/0092-8674(83)90356-2. [DOI] [PubMed] [Google Scholar]
- 36.Owen D.J., et al. Adaptors for clathrin coats: structure and function. Annu. Rev. Cell Dev. Biol. 2004;20:153–191. doi: 10.1146/annurev.cellbio.20.010403.104543. [DOI] [PubMed] [Google Scholar]
- 37.Martinez J.J., et al. Ku70, a component of DNA-dependent protein kinase, is a mammalian receptor for Rickettsia conorii. Cell. 2005;123:1013–1023. doi: 10.1016/j.cell.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 38.Oelschlaeger T.A., et al. Some structures and processes of human epithelial cells involved in uptake of enterohemorrhagic Escherichia coli O157:H7 strains. Infect. Immun. 1994;62:5142–5150. doi: 10.1128/iai.62.11.5142-5150.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green B.T., Brown D.R. Differential effects of clathrin and actin inhibitors on internalization of Escherichia coli and Salmonella choleraesuis in porcine jejunal Peyer's patches. Vet. Microbiol. 2006;113:117–122. doi: 10.1016/j.vetmic.2005.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nazli A., et al. Enterocyte cytoskeleton changes are crucial for enhanced translocation of nonpathogenic Escherichia coli across metabolically stressed gut epithelia. Infect. Immun. 2006;74:192–201. doi: 10.1128/IAI.74.1.192-201.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jouve M., et al. Adhesion to and invasion of HeLa cells by pathogenic Escherichia coli carrying the afa-3 gene cluster are mediated by the AfaE and AfaD proteins, respectively. Infect. Immun. 1997;65:4082–4089. doi: 10.1128/iai.65.10.4082-4089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almeida R.A., et al. Staphylococcus aureus invasion of bovine mammary epithelial cells. J. Dairy Sci. 1996;79:1021–1026. doi: 10.3168/jds.S0022-0302(96)76454-8. [DOI] [PubMed] [Google Scholar]
- 43.Ellington J.K., et al. Mechanisms of Staphylococcus aureus invasion of cultured osteoblasts. Microb. Pathog. 1999;26:317–323. doi: 10.1006/mpat.1999.0272. [DOI] [PubMed] [Google Scholar]
- 44.Radin J.N., et al. beta-Arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect. Immun. 2005;73:7827–7835. doi: 10.1128/IAI.73.12.7827-7835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cundell D.R., et al. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 46.Calvinho L.F., Oliver S.P. Characterization of mechanisms involved in uptake of Streptococcus dysgalactiae by bovine mammary epithelial cells. Vet. Microbiol. 1998;63:261–274. doi: 10.1016/s0378-1135(98)00239-9. [DOI] [PubMed] [Google Scholar]
- 47.Rikihisa Y., et al. Inhibition of infection of macrophages with Ehrlichia risticii by cytochalasins, monodansylcadaverine, and taxol. Infect. Immun. 1994;62:5126–5132. doi: 10.1128/iai.62.11.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Detilleux P.G., et al. Effect of endocytic and metabolic inhibitors on the internalization and intracellular growth of Brucella abortus in Vero cells. Am. J. Vet. Res. 1991;52:1658–1664. [PubMed] [Google Scholar]
- 49.Oelschlaeger T.A., Tall B.D. Invasion of cultured human epithelial cells by Klebsiella pneumoniae isolated from the urinary tract. Infect. Immun. 1997;65:2950–2958. doi: 10.1128/iai.65.7.2950-2958.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maruta K., et al. Entry and intracellular localization of Legionella dumoffii in Vero cells. Microb. Pathog. 1998;24:65–73. doi: 10.1006/mpat.1997.0171. [DOI] [PubMed] [Google Scholar]
- 51.Oelschlaeger T.A., et al. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc. Natl. Acad. Sci. U. S. A. 1993;90:6884–6888. doi: 10.1073/pnas.90.14.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Porat N., et al. Neisseria gonorrhoeae utilizes and enhances the biosynthesis of the asialoglycoprotein receptor expressed on the surface of the hepatic HepG2 cell line. Infect. Immun. 1995;63:1498–1506. doi: 10.1128/iai.63.4.1498-1506.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harvey H.A., et al. Receptor-mediated endocytosis of Neisseria gonorrhoeae into primary human urethral epithelial cells: the role of the asialoglycoprotein receptor. Mol. Microbiol. 2001;42:659–672. doi: 10.1046/j.1365-2958.2001.02666.x. [DOI] [PubMed] [Google Scholar]
- 54.Grassme H.U., et al. Gonococcal opacity protein promotes bacterial entry-associated rearrangements of the epithelial cell actin cytoskeleton. Infect. Immun. 1996;64:1621–1630. doi: 10.1128/iai.64.5.1621-1630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hodinka R.L., Wyrick P.B. Ultrastructural study of mode of entry of Chlamydia psittaci into L-929 cells. Infect. Immun. 1986;54:855–863. doi: 10.1128/iai.54.3.855-863.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hodinka R.L., et al. Ultrastructural study of endocytosis of Chlamydia trachomatis by McCoy cells. Infect. Immun. 1988;56:1456–1463. doi: 10.1128/iai.56.6.1456-1463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wyrick P.B., et al. Entry of genital Chlamydia trachomatis into polarized human epithelial cells. Infect. Immun. 1989;57:2378–2389. doi: 10.1128/iai.57.8.2378-2389.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ward M.E., Murray A. Control mechanisms governing the infectivity of Chlamydia trachomatis for HeLa cells: mechanisms of endocytosis. J. Gen. Microbiol. 1984;130:1765–1780. doi: 10.1099/00221287-130-7-1765. [DOI] [PubMed] [Google Scholar]
- 59.Boleti H., et al. Chlamydia infection of epithelial cells expressing dynamin and Eps15 mutants: clathrin-independent entry into cells and dynamin-dependent productive growth. J. Cell Sci. 1999;112:1487–1496. doi: 10.1242/jcs.112.10.1487. [DOI] [PubMed] [Google Scholar]
- 60.Benmerah A., et al. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 1999;112:1303–1311. doi: 10.1242/jcs.112.9.1303. [DOI] [PubMed] [Google Scholar]
- 61.Damke H., et al. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parker J.S., Parrish C.R. Cellular uptake and infection by canine parvovirus involves rapid dynamin-regulated clathrin-mediated endocytosis, followed by slower intracellular trafficking. J. Virol. 2000;74:1919–1930. doi: 10.1128/jvi.74.4.1919-1930.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matlin K.S., et al. Infectious entry pathway of influenza virus in a canine kidney cell line. J. Cell Biol. 1981;91:601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matlin K.S., et al. Pathway of vesicular stomatitis virus entry leading to infection. J. Mol. Biol. 1982;156:609–631. doi: 10.1016/0022-2836(82)90269-8. [DOI] [PubMed] [Google Scholar]
- 65.Aggeler J., Werb Z. Initial events during phagocytosis by macrophages viewed from outside and inside the cell: membrane-particle interactions and clathrin. J. Cell Biol. 1982;94:613–623. doi: 10.1083/jcb.94.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aggeler J., et al. High-resolution three-dimensional views of membrane-associated clathrin and cytoskeleton in critical-point-dried macrophages. J. Cell Biol. 1983;97:1452–1458. doi: 10.1083/jcb.97.5.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tse S.M., et al. Differential role of actin, clathrin, and dynamin in Fc gamma receptor-mediated endocytosis and phagocytosis. J. Biol. Chem. 2003;278:3331–3338. doi: 10.1074/jbc.M207966200. [DOI] [PubMed] [Google Scholar]
- 68.Champion J.A., Mitragotri S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ehrlich M., et al. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 70.Sachse M., et al. Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol. Biol. Cell. 2002;13:1313–1328. doi: 10.1091/mbc.01-10-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raiborg C., et al. Flat clathrin coats on endosomes mediate degradative protein sorting by scaffolding Hrs in dynamic microdomains. J. Cell Sci. 2006;119:2414–2424. doi: 10.1242/jcs.02978. [DOI] [PubMed] [Google Scholar]
- 72.Meyerholz A., et al. Effect of clathrin assembly lymphoid myeloid leukemia protein depletion on clathrin coat formation. Traffic. 2005;6:1225–1234. doi: 10.1111/j.1600-0854.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- 73.Mengaud J., et al. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 74.Nhieu G.T., et al. Tyrosine kinase signaling and type III effectors orchestrating Shigella invasion. Curr. Opin. Microbiol. 2005;8:16–20. doi: 10.1016/j.mib.2004.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


