Abstract
A review of common gastrointestinal disorders of donkeys and mules is presented. Clinically relevant aspects of donkey behavior, anatomy, and physiology are highlighted. Diagnosis, management, and treatment of conditions affecting the gastrointestinal tract from stomach to rectum, including liver and pancreas, are discussed.
Keywords: Donkey, Mule, Colic, Gastrointestinal, Parasite, Colitis, Hyperlipemia, Behavior
Key points
-
•
Donkeys with colic frequently show milder clinical signs than horses, despite the potential severity of the pathologic condition. Major presenting signs include dullness, inappetence, self-isolation, and recumbency.
-
•
Hyperlipemia is a frequent finding in donkeys with colic. Triglyceride concentrations should be measured regularly in inappetent animals and treatment instituted. The presence of hyperlipemia reduces the prognosis.
-
•
It is possible to perform a rectal examination in most donkeys; it is preferable to use spasmolytics before rectal examination. Ultrasound evaluation is useful if rectal examination is not possible and can be an adjunct to those cases whereby rectal examination alone has not been diagnostic.
-
•
The presence of dental disease and parasitism increases the risk of colic; donkeys and mules should be included in routine preventative dental care and anthelmintic use.
-
•
Because of their unique physiology, donkeys differ in their response to dehydration and drug metabolism compared with horses. Hematological and biochemical reference ranges are available for donkeys and mules.
Introduction
Donkeys are kept as companions or used as working or production animals, whereas mules are mainly used for working purposes. Gastrointestinal diseases are common in these animals worldwide. Awareness of their stoic behavior is paramount when interpreting the severity of clinical signs. Donkeys are desert-adapted animals with unique physiology and metabolism compared with horses. Knowledge of their particular behaviors and physiology is the key to successful handling and management of gastrointestinal disease.
Clinical examination
For many clinicians, the challenge when dealing with donkeys and mules is recognizing behavioral differences compared with horses. Donkeys have a highly developed prey species behavior and can mask low- to moderate-grade and chronic pain.1 Common clinical signs in association with gastrointestinal disease include dullness, behavior changes, lack of appetite or sham eating, recumbency, head and neck held below withers height, ears less mobile or backwards/sideways pointing and unresponsive to stimuli, self-isolation away from companions, and weight loss in chronic disease.
Donkeys show signs of acute colic, such as flank watching, kicking the abdomen, lying down, and rolling, which are less evident than in horses (Fig. 1 ). Mules show pain response characteristics of both parent species, being less evident than in horses, but more overt than in donkeys. Their physiologic parameters fall between the donkey and horse.
Fig. 1.
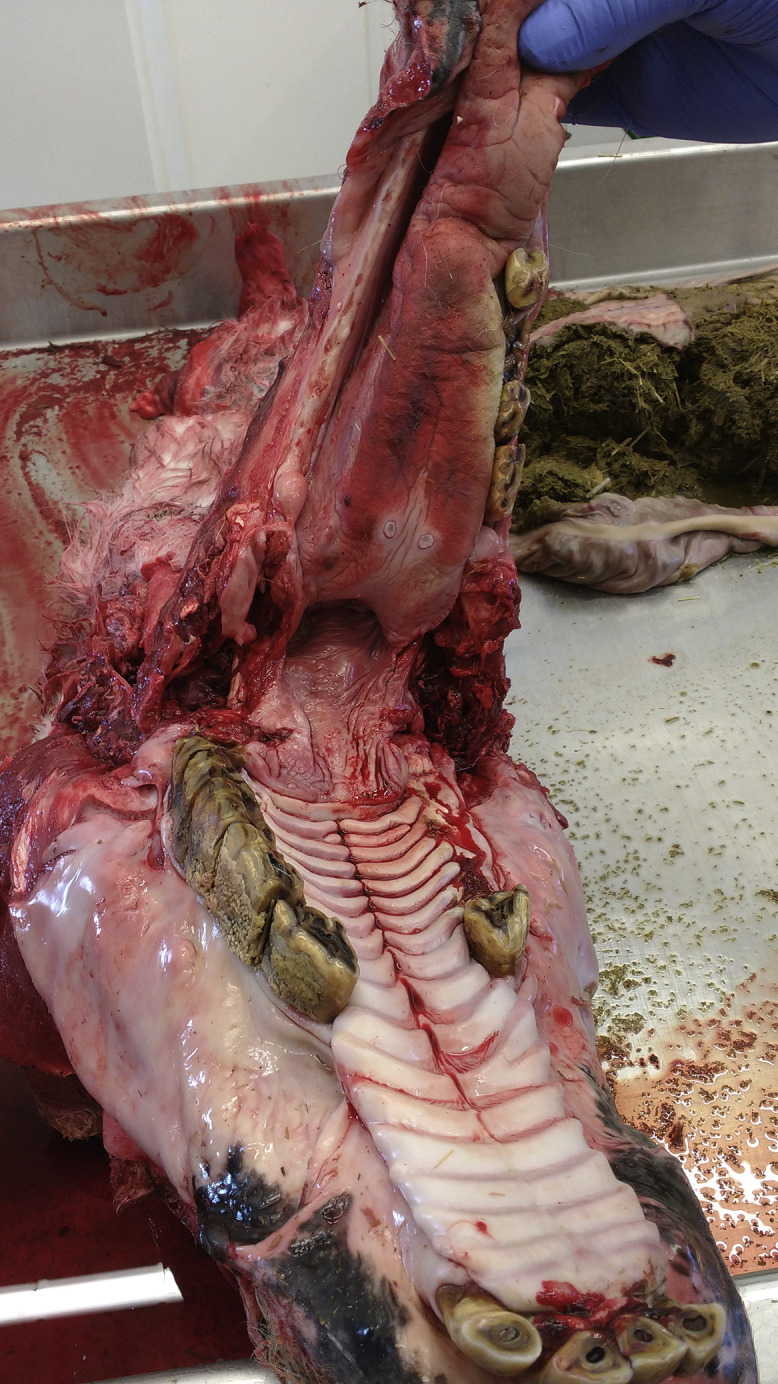
Postmortem view of donkey mouth showing nonfunctional molar arcades leading to impaction.
In the United Kingdom and United States, donkeys and mules are mainly considered companion animals and often reach advanced age, whereas in developing countries, these animals are used for work in agriculture, transport, pulling, and tourism, often lack owner and veterinary care, and have a shorter lifespan.2
The significance is that donkeys, and to a lesser extent, mules, often present with signs of gastrointestinal disease later than horses, tend to be more systemically ill when signs are noted, and often are afflicted with multiple comorbidities.
In addition to a complete history, a clinical examination should be performed in donkeys and mules with gastrointestinal disease. This clinical examination should include an assessment of mentation, general health and body condition, rectal temperature, pulse and respiration, evaluation of mucous membrane color and moisture (hydration status), auscultation of all quadrants for increased or decreased intestinal sounds, a rectal examination, nasogastric intubation in animals with abdominal pain, fecal evaluation for consistency, presence of endoparasites, and poorly digested foodstuffs. In animals with diarrhea, frequency, estimated volume, consistency, and appearance of feces, full dental examination with a mouth speculum, and development of a list of differentials that could lead to abdominal pain and dullness are recommended.
Normal parameters for adult donkeys include a rectal temperature of 36.5°C to 37.8°C (97.7°F to 100°F), a heart rate of 36 to 52 beats/min, a respiration rate of 20 breaths/min (range 12–28), and moist mucous membranes with a capillary refill time of less than 2 seconds. Interpretation of digital pulses in donkeys can be challenging.
General points to consider are as follows:
-
•
Because of their pair bonding behavior, donkeys being transported for evaluation ideally should be accompanied by a bonded animal to reduce stress.
-
•
Preventative care (anthelmintic use, dental care, regular vaccination) is often inadequate, in particular, in developing countries.
-
•
Weight loss may be the only indicator of chronic gastrointestinal disease in donkeys and mules.
-
•
Because of the large colon enhanced water absorption capacity, diarrhea is rarely seen with acute or chronic gastrointestinal disease, including colitis.
-
•
Rectal examination should be performed in all, except in miniature animals. Intravenous spasmolytics (hyoscine/scopolamine) at standard equine doses facilitate rectal examination. In unhandled donkeys and mules where rectal examination may be dangerous, heavy sedation and the use of stocks are indicated. Mules may require between one-third to one-half extra dose of sedatives compared with a similarly sized pony or horse.
-
•
If rectal examination is not possible, information about the nature of the feces and hydration status is useful.
-
•
Hyperlipemia is frequent in colicky and dull donkeys and can worsen the prognosis, and triglyceride measurement is indicated in these animals.
-
•
As donkeys evolved in dry and arid environments with limited water access, they can better tolerate dehydration and hemoconcentration than horses.
-
•
Transabdominal ultrasonography may be challenging in obese animals. Clipping their thick coat is recommended to facilitate image acquisition.
-
•
Abdominocentesis can be unrewarding in donkeys because of significant fat deposits on the linea alba; a teat cannula or catheter may be indicated, preferably after ultrasound evaluation.
-
•
A foal- or pony-sized tube can be used for evaluation of gastric reflux and nasogastric therapy.
-
•
In countries where rabies is endemic, the possibility of infection with this zoonosis must be considered, in particular when performing oral or rectal examination.
-
•
Donkeys without tetanus prophylaxis are susceptible to this condition and develop signs similar to other equids.
Anatomy and physiology
The donkey and mule have some anatomic differences from horses that are relevant to management and treatment. The head is larger in proportion to body size and supported ventrally by the strong cutaneous coli muscle. This muscle is highly developed in donkeys and can obscure the jugular vein in the midneck region, making jugular venipuncture more challenging. Donkeys have a narrower mandible and accentuated mandibular curve of Spee, which can lead to dental overcrowding, with rostral and caudal enamel overgrowths.
Recognition and treatment of dental problems are critical in the management and prevention of many gastrointestinal disorders (Fig. 2 ). See João B. Rodrigues and Gemma Lilly’s article, “Dental Disorders of Donkeys,” in this issue.
Fig. 2.
Typical presentation of donkey with impaction of pelvic flexure showing dullness, head down, and ears back.
Donkeys have narrow nasal meati; hence, for nasogastric intubation, it is better to use a foal- or pony-sized tube with adequate lubrication and restraint to reduce the risk of hemorrhage.
The stomach of a 180-kg donkey can accommodate approximately 3 L of fluid. The length of the small intestine is around 6 m per 100 kg (vs 5 m for the horse); the transverse colon is short (10 cm), and the small colon is approximately 1 m. Other aspects of the internal anatomy of donkeys and mules are comparable to the horse.3
The spinal cord in the donkey terminates at the second sacral vertebra, and the dura may extend to the second coccygeal vertebrae. Epidural injections are best done in the second intercoccygeal space at an angle of 30° to the horizontal, 1 space further back and at a shallower angle than in the horse because of the lack of musculature over the rump and tail head.4 Correct placement of the epidural injection is relevant to manage conditions such as rectal prolapse, reproductive manipulation, or orthopedic pain.
The donkey, and to a lesser extent the mule, is highly efficient in water compartmentalization and conservation; it is reported that they can lose 20% to 30% of body weight and recover faster once fluid is provided. Plasma volume is maintained even with 20% dehydration. Under normal conditions, they have a lower urinary output than horses when water is unrestricted.5 These studies were done on a limited number of heat-adapted animals and should be viewed with caution when assessing companion donkeys in temperate climates.
Donkeys may show few clinical and hematologic signs of volume depletion until 12% to 15% dehydration is reached. Skin tenting is an unreliable sign of fluid loss in donkeys and mules (see Erin L. Goodrich and Erica Behling-Kelly’s article, “Clinical Pathology of Donkeys and Mules”; and Elena Barrio and colleagues’ article, “Clinical Evaluation and Preventative Care in Donkeys,” in this issue). The hindgut acts as a water reservoir similar to the forestomach in ruminants; donkeys have an enhanced cecal capacity for fluid retention when faced with dehydration, which is evident by the scant diarrhea with colitis.
Donkeys and mules have a slower gut transit time than horses and digest fiber more efficiently. Per body weight, they require less dry matter and energy intake than resting and working horses.6 Clinically, this is reflected by their ability to gain weight easily and develop large fat deposits on the neck, back, rump, around internal organs, and along the linea alba.
The donkey intestinal microbiome has not been extensively evaluated, but one can assume that there are differences with the horse and pony. Dysbiosis from different conditions (diet, antimicrobials, stress) can be a complication or potential cause of gastrointestinal disease.
Epidemiology and risk factors
The risk factors for gastrointestinal disease in donkeys and mules are similar to those in horses, influenced by the management and environment in which they are kept. In donkeys and mules in arid regions and marginal communities with poor access to preventative care, colic may occur because of inadequate water access, fibrous or indigestible feed provision, poor dentition, foreign object ingestion (eg, plastic bags), or heavy endoparasitism (Fig. 3 ). Parasites such as Strongylus vulgaris have higher prevalence in developing countries because of poor management and minimal access to anthelmintics. Ingestion of moldy feed or excess seasonal lush grass can result in tympanic colic, whereas grazing low in poor grasslands may contribute to sand colic.7
Fig. 3.
Donkey browsing on inappropriate plastic, which can lead to obstructive colic.
Donkey studies in the United Kingdom have identified impaction colic as having high morbidity and mortality.8 The pelvic flexure was the main site of impaction, but mortality was higher for cecal impactions. Colic impaction is also frequent with dental disease and aging. Multiple diastemata, painful periodontal disease, absent molars, and loss of masticatory function increase the incidence of colic. This can be reduced by dietary modifications, including a short chop, high-fiber feed, and provision of regular high-quality dental care. Note that diastemata and periodontal disease are not necessarily confined to geriatric patients, and younger animals should also have regular dental examinations.
Musculoskeletal disorders have also been associated with colic in geriatric donkeys, mainly due to reduced physical activity and water intake. In the United Kingdom, donkeys bedded on paper or cardboard have higher rates of impaction colic, because they regard the bedding as a fiber source and ingest large quantities. Donkeys are natural browsers on a wide range of feedstuffs, and this exploratory behavior, while advantageous in some environments, can cause problems.
Hyperlipemia can be a cause or consequence of gastrointestinal disease. It can induce ileus, hepatic failure, and gastric ulceration, whereas colic, enteritis, colitis, peritonitis, inappetence, and anorexia often lead to hyperlipemia.
Other causes of colic (eg, grass sickness, neoplasia, enteroliths, peritonitis) have been reported in a small number of donkeys and mules with similar signs and treatments to horses.
The etiology and epidemiology of colitis in donkeys have not been fully elucidated, but is likely influenced by geography and management. In the United Kingdom, individual cases and outbreaks of diarrhea have been documented, with parasites, infectious agents, and husbandry changes proposed as the main causes.
Role of parasites
An extensive discussion of donkey parasitology is provided elsewhere.7, 9 Relevant gastrointestinal parasites are listed in Table 1 .
Table 1.
Gastrointestinal parasites of donkeys
| Organ | Parasites | Comments |
|---|---|---|
| Stomach |
Gasterophilus spp Habronema spp Draschia megastoma |
Their significance in relation to gastric ulcer syndrome is unclear. May be more relevant in working animals with malnutrition |
| Small intestine | Parascaris equorum | Adult donkeys do not seem to share the level of immunity demonstrated by horses and ponies, particularly in the case of immunocompromised working donkeys. Foals and heavily infested adult donkeys are at risk of ileal impaction and intestinal rupture in extreme cases |
| Ileocecal junction | Anoplocephala perfoliata | Rarely found among the UK herd at The Donkey Sanctuary, but higher infestation is found in working donkeys. Clinical signs of heavy burdens are similar to horses |
| Large intestine | Strongyle spp |
S vulgaris and other large strongyles are rarely found in donkeys in developed countries owing to management and anthelmintic use, but are highly prevalent in the developing world and should be a differential for chronic and acute colic Cyathostomins (small strongyles) are common in both nonworking and working donkey populations. Encysted cyathostomes are a major cause of acute and chronic disease in the United Kingdom |
| Liver | F hepatica | Liver fluke is emerging as a potential cause of liver pathologic condition in the United Kingdom |
| Rectum | Oxyuris equi | Pinworm infestations appear to be on the increase in areas of the United Kingdom and perhaps in other countries and may be a significant cause of perianal discomfort |
| Gasterophilus spp | Heavy infestations are associated with rectal prolapse in working donkey populations |
Gastric pathologic condition
Gastric Ulceration
Gastric ulcers have been identified during endoscopic evaluation10 and at postmortem examination,11 confirming that donkeys are at risk of this pathologic condition. Donkeys with gastric ulcer syndrome (DGUS) do not usually exhibit the clinical signs displayed by horses with equine gastric ulcer syndrome, likely because of their stoic nature. For example, 1 study of 39 donkeys without symptomatic evidence of gastric disease found lesions in the squamous gastric mucosa in 49% of animals, whereas only 2.6% had glandular disease.10 Postmortem data from The Donkey Sanctuary identified that most chronic gastric ulcers were located in the squamous area adjacent to the margo plicatus, whereas chronic active or acute ulcers affected both the glandular and the squamous part of the stomach in few animals (G. Paraschiou, personal communication, 2018). These findings are similar to those reported in Italy.10
Acute gastric ulcers are rarely documented in donkeys, but this may be a reflection of infrequent diagnostics rather than true pathologic condition. Concerns related to stress and risk of hyperlipemia from prolonged fasting for gastroscopic evaluation are valid. However, in 1 study where donkeys were fasted for 15 hours before the procedure, no adverse metabolic effects were noted.10 It is the authors’ opinion that donkeys undergoing gastroscopy should have triglyceride concentrations measured before fasting and after the procedure. If hyperlipidemia is identified, intravenous dextrose or oral administration of glucose or sugar-rich drenches is recommended to prevent clinical hyperlipemia.
Suggested risk factors for DGUS include high starch diets, chronic stress, hyperlipemia, long-term corticosteroid and nonsteroidal anti-inflammatory drug (NSAID) use. Prophylactic use of omeprazole is recommended when there are concerns.
Prophylaxis and treatment protocols for squamous and glandular ulcers with omeprazole have been extrapolated from horses. Pharmacologic information on injectable omeprazole is lacking in donkeys.
Other
Gastrointestinal transit time is longer in donkeys compared with horses and ponies, and there is suspicion that this could be in part due to delay gastric emptying, may have clinical relevance when planning gastroscopy as previously discussed or when withholding food for diagnostic testing purposes.
Gastric impaction occurs sporadically, primary or secondary to other conditions (liver disease, strictures, ileus). Gastric foreign bodies are more common in working donkeys because of their scavenging habits (eg, plastic bag ingestion). Affected donkeys are usually dull and inappetent and may display varying degrees of abdominal pain. Fecal output may be reduced. Diagnosis may be tentatively made on ultrasound examination; where ultrasound is not available, diagnosis may be made based on the presence of reflux or a marked discomfort on administration of enteral fluids.
Treatment of suspected gastric impaction is as for other equids.
Small intestine
Pathologic condition of the small intestine is similar to horses, although there appears to be a reduced incidence of strangulating lipomas. Small intestinal pathologic condition may be harder to identify in donkeys because of limitations in rectal examination.
Equine grass sickness is rarely reported in donkeys, but should be kept as a differential diagnosis of weight loss, inappetence, and ileus in the United Kingdom.12 Lack of clinical recognition may be the main reason for its apparent low incidence. Diagnosis and management are similar to other equids.
Ultrasonographic abdominal examination is recommended whereby acute or chronic small intestinal lesions are suspected from clinical signs or the results of other diagnostic tests.
The oral glucose absorption test may be used in donkeys suspected of small intestinal malabsorption, but careful consideration of the risks of prolonged food withholding should be made.
Cecum/colons/rectum
Impaction Colic
One of the most common causes of colic in donkeys and mules is pelvic flexure impaction from poorly masticated feedstuffs or foreign bodies (eg, plastic bags), followed by impactions of the transverse colon and cecum. Typically, these animals present with dullness but will continue to eat until the impaction is severe, when they show signs of abdominal pain or stand apart from the herd. There may be reduced intestinal motility.
Because of the delay in presentation for evaluation, there may be secondary hyperlipemia, which contributes to their higher mortality compared with large colon impaction in horses.
Transabdominal ultrasonography can be helpful in patients too small for rectal examination and can be also used to measure intestinal wall thickness and edema.
Impactions due to dehydrated feces or fibrous material can be managed via enteral fluids and osmotic laxatives (eg, magnesium sulfate or sodium sulfate), whereas impactions due to plastic bags or foreign matter may respond better to mineral oil as a nonosmotic lubricant.
Nutritional support via nasogastric or intravenous routes, and small amounts of grass or short chop, can be used to stimulate appetite and encourage gastrointestinal motility, but also to prevent hyperlipemia.
In dehydrated working donkeys and mules, NSAIDs should be administered at half the indicated dose to reduce the risk of renal toxicity.
Geriatric donkeys with dental disease, especially with absent molars, are more prone to large colon impaction and require short chop diets and grazing to reduce risk.
Colitis
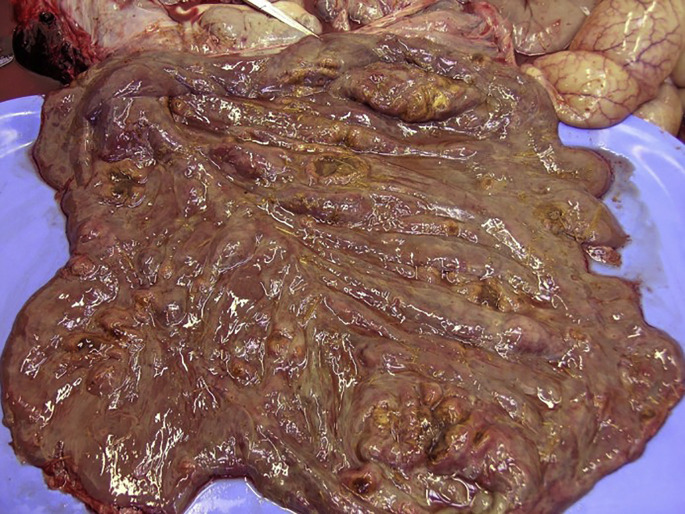
Typhlitis and colitis in donkeys can be challenging to diagnose. Animals could be found dead from peracute colitis, whereas most cases show signs of intestinal inflammation and systemic inflammatory response syndrome, including dullness, fever, and occasionally diarrhea. Ventral edema and weight loss are common findings in chronic cases associated with protein-losing enteropathy and hypoalbuminemia.13 As with colitis in other equids, achieving a definite diagnosis can be challenging, but may be linked to emerging cyathostomins and infectious agents (Fig. 4 ). Salmonella spp and Clostridium spp are occasionally isolated. Information on coronavirus and gastrointestinal disease in donkeys is limited. Feed contamination with mycotoxins has been implicated. Rapid dietary changes that alter the microbiota could be an aggravating or risk factor. Again, hyperlipemia is a frequent finding in donkeys with colitis. Therapeutic protocols for colitis in donkeys and mules are similar to horses.
Fig. 4.
Severe inflammation of colon: colitis, postmortem specimen.
Rectal Injuries
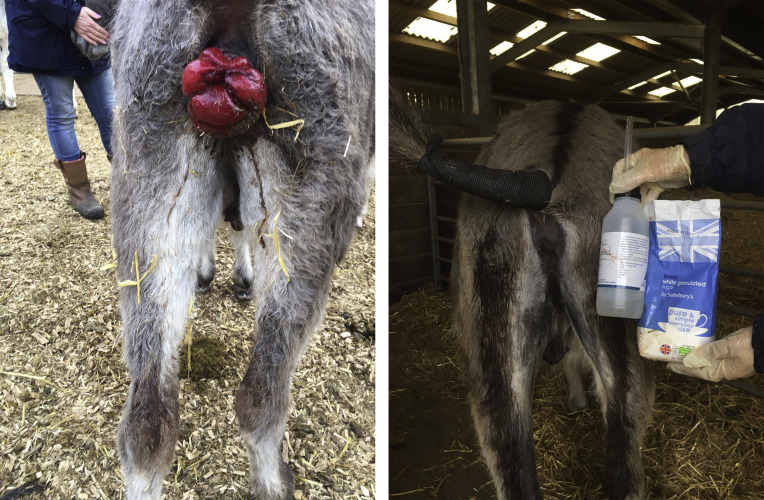
Rectal prolapse is seen more commonly in working donkeys, where exhaustion and parasitism are common. It has been associated with Gasterophilus nasalis in Ethiopia, which causes irritation and constant straining when present on the rectal mucosa.14 Rectal prolapse has been reported secondary to rectal tumors and penetrating injuries (Fig. 5 ). Rectal prolapse from acute colitis is unusual compared with horses. Treatment will depend on the size of prolapsed tissue and the severity of the damage. Simple cases can respond to osmotic reduction using sugar and lubrication to replace the prolapse, combined with anti-inflammatory drugs (see Fig. 5). Animals with severe lesions or that do not respond to medical management may require a purse-string suture or surgical resection. Sedation, epidural anesthesia, and local anaesthetics are required. The purse-string suture using thick suture material or sterile umbilical tape is often successful. The string must be loose enough to allow defecation and should be removed within 72 hours. Depending on the inciting cause, a soft diet or a laxative may be helpful.
Fig. 5.
Rectal prolapse in a donkey before (left) and after osmotic reduction using sugar and lubrication (right).
Rectal tears are a serious complication of rectal examination, and antispasmodic drugs with good restraint are recommended for rectal examination. Rectal tears have been reported when stallions attempt to cover geldings.
Liver
Background
Liver disease has major morbidity and mortality in the UK donkey population. Clinical signs are often insidious, and liver pathologic condition may only be found because of investigation into other diseases. Clinical signs of liver disease include colic (dysmotility, gastric impaction), weight loss, fever, depression, photosensitization and other skin diseases, icterus, and neurologic signs (including behavioral changes). Weight loss is the most consistent finding but is not pathognomonic. Hepatic disease is frequently subclinical and often detected based on serum biochemistry evaluation. Liver dysfunction from excessive fat infiltration from a negative energy balance, stress, or insulin insensitivity may occur with severe hyperlipemia. Liver disease has been found in donkeys with elevated adreno cortico trophic hormone (ACTH) and assumed poorly controlled pars pituitary intermedia disorder (PPID), but firm data on any association are lacking at present.
Diagnosis and Treatments
Evaluation of serum chemistry should be an early diagnostic step to investigate liver function. Reference values for liver specific parameters are available in Erin L. Goodrich and Erica Behling-Kelly’s article, “Clinical Pathology of Donkeys and Mules,” in this issue.
Ultrasonography is useful to assess size, echogenicity, architecture, and the presence of focal or diffuse lesions, if there is suspicion of severe acute or chronic liver pathologic condition. A biopsy may provide the most meaningful diagnostic and prognostic information. There is no validated hepatic histopathology scoring system for donkeys, and the grading system for horses is the one used.15 Hemosiderin accumulation is a common histopathological finding in donkey hepatic tissue, but its significance is unknown. It is important to note that liver biopsy carries risks, in particular, bleeding, which can be exacerbated from hepatic disease.
It is highly indicated to have another donkey available as a companion, but also as a potential blood donor. Close monitoring for signs of colic and bleeding for the next 24 hours after the procedure is recommended.
Treatment of liver pathologic condition will be dictated by the clinical and diagnostic findings. Liver fibrosis is frequently found in the UK donkey population. Use of corticosteroids and nutritional management are the mainstays of therapy. Repeat biopsies would be ideal to monitor response to treatment, but it is often impractical, costly, or hard to justify. Serial blood evaluation of markers of liver disease and function may be the most practical method. In animals with minor serum abnormalities of liver function, monthly chemistry evaluations are indicated.
Pyrrolizidine alkaloid toxicity has been documented in donkeys, with similar histopathological findings and prognosis as horses.
Antimicrobials should be reserved for cases where there is evidence of a bacterial hepatitis or cholangiohepatitis.
Hydatid cysts are occasionally found as an incidental finding on ultrasound examination. Treatment or monitoring is advised as for other equines.
The incidence of liver fluke (Fasciola hepatica) is increasing among the UK donkey population. This parasite should be considered in donkeys inhabiting wetter environments where the presence of lymnaeid intermediate hosts (Lymnaea spp; Galba spp) has been documented.
Flukes may increase liver enzymes because of pathologic condition of the bile ducts. Some animals will present with weight loss and marked elevation in liver enzymes from severe cholangiohepatitis.
Treatment of all animals in the herd is advised. There are no licensed fluke treatments for donkeys, and therapy is prescribed using the cascade system. The Donkey Sanctuary uses triclabendazole (18 mg/kg orally) with follow-up fecal egg analysis 14 to 28 days after treatment to assess efficacy. When triclabendazole is ineffective, closantel (20 mg/kg orally) may be considered, keeping in mind that it is only effective against adult flukes and redosing is required 8 to 10 weeks later. Closantel can cause anorexia, ataxia, and blindness if overdosed. Fencing off wet, marshy environments is advised to reduce exposure, and cattle or sheep screened and treated for liver flukes if they graze the same pasture as donkeys.
Pancreatitis
Measurement of amylase and lipase activities in dull donkeys with nonspecific abdominal pain may be suggestive of pancreatitis, but a definitive diagnosis is rare, and very few cases have been confirmed at postmortem examination. Many veterinary laboratories do not measure amylase and lipase as routine tests, and results should be interpreted with caution. In horses, migrating Strongylus spp and Parascaris spp larvae have been proposed as a potential etiology,16 but their role in donkey pancreatic disease remains to be determined. Hyperlipemia may further the risk of pancreatitis in donkeys.
Surgical considerations and pharmacology
Indications for surgery in donkeys are similar to those in horses, with the caveat that their demeanor and physiology may mask deteriorating parameters. Pain level and dehydration are harder to assess in donkeys. Before embarking on exploratory laparotomy, a thorough clinical examination is recommended to ascertain that factors that could compromise a successful outcome (eg, dental disease, malnutrition, laminitis, osteoarthritis) are taken into consideration. Again, routine rectal examination and nasogastric intubation should be performed when feasible. Blood and peritoneal fluid lactate values can be used in a similar way as for horses. Normal blood and peritoneal fluid lactate values for donkeys have not been established; however, rising lactate concentrations suggest the presence of a surgical lesion or the presence of bacteria in the peritoneal fluid.
Success rates for abdominal surgery in donkeys tend to be lower than values reported for horses17 for several reasons, including lack of medical and surgical experience with the species, presentation at an advanced stage of disease, delayed referral due to reduced veterinary experience, lack of knowledge regarding appropriate analgesia and donkey-specific pharmacology, and finances.
Hospital stall accommodation may need to be adapted for the donkey; stall doors that are too high to permit visibility may need to be replaced with a hurdle or gate. It is vital that the clinician has knowledge of how to estimate and measure donkey weight and condition score before giving any medications.18
Few drugs are licensed for use in donkeys and must be selected on the basis of the legislation of each country. In the United Kingdom, prescribing guidance to avoid animal suffering where no approved drug is available is based on the cascade system, whereas in the United States, the Animal Medicinal Drug Use Clarification Act permits the extralabel use of certain approved animal drugs under some conditions.
It is important to mention that for several drugs, there are pharmacologic differences between donkeys and mules with horses, and clinicians should use caution when extrapolating doses (see Francisco J. Mendoza and colleagues’ article, “Clinical Pharmacology in Donkeys and Mules,” in this issue).19
It is essential to monitor the response to analgesia given for gastrointestinal pain and be prepared to dose at more frequent intervals. Phenylbutazone and flunixin meglumine can be given twice daily at standard equine doses.19 Multimodal pain relief using opioids and other drugs should be considered, as for other equines. Further information on sedation and general anesthesia in donkeys is described elsewhere (see Nora Matthews and Johannes P.A.M. van Loon article’s, “Anesthesia, Sedation and Pain Management of Donkeys and Mules,” in this issue).20
Abdominal surgery increases the risk of hyperlipemia, and preemptive measures to prevent and manage this complication should be in place. Postoperative food withholding should be weighed against the risk of hyperlipemia and other complications. Triglycerides should be monitored regularly, and parenteral support may be required to maintain normal levels (see Francisco J. Mendoza and colleagues’ article, “Metabolic and Endocrine Disorders in Donkeys,” in this issue).21
Postsurgical monitoring is essential to reduce stress. In donkeys that require prolonged stall rest, enrichment methods may be necessary. The following link provides information on enrichment for donkeys (https://www.thedonkeysanctuary.org.uk/what-we-do/knowledge-and-advice/for-owners/environment-enrichment).
Fluid therapy
There are no known published guidelines on fluid therapy for donkeys and mules, and recommendations are based on equine principles, taking into consideration that there are differences in body water partitioning.
Enteral fluid therapy is suitable for gastrointestinal disorders,22 such as large colon impaction and mild dehydration, as long as there is no gastric distension or ileus, and when resources are limited. Nasogastric intubation might be the only option for working donkeys.
Commercially available isotonic fluids should be the first option before considering homemade recipes. Supplementation with dextrose should be done in most cases. For enteral fluids, do not exceed 3 L of fluid for a 180-kg donkey. The frequency of enteral fluid administration will depend on the metabolic status, the animal response to therapy, and feasibility. An indwelling nasogastric tube may be a more practical method for intermittent or continuous fluid administration.
Enteral fluids should not be used in animals in which reduced intestinal perfusion is suspected.
Intravenous fluid therapy is indicated in animals with evidence of volume depletion, when electrolyte and metabolic abnormalities are present, for those in which enteral fluid therapy is not an option, or in surgical cases. The approach follows the equine guidelines. When administering as a bolus, be aware that the advised crystalloid rate of 20 to 40 mL/kg over 1 to 2 hours22 equates to 3.6 to 7.2 L for a 180-kg donkey. In animals with colitis, high intravenous fluid rates have been associated with mural edema and a poorer prognosis (A. Thiemann, personal communication). To reduce complications from overhydration, it is recommended to monitor urine output, respiratory rate, peripheral edema, and body weight. A full discussion of intravenous fluid therapy is beyond the scope of this article, and the reader is referred to equine fluid therapy reviews.23
Use of rectal fluids has been investigated,24 but, in general, has been considered impractical and risky.
Summary
Gastrointestinal disorders are common in donkeys and mules. Knowledge of these conditions, including their etiology, pathophysiology, and epidemiology can assist in their successful diagnosis and management. Awareness of donkey- and mule-specific behavior, anatomy, and physiology will improve the prognosis. Adequate pain management to reduce stress and understanding metabolic complications from gastrointestinal disease are essential for success.
Footnotes
Disclosure Statement: None.
References
- 1.Ashley F.H., Waterman-Pearson A.E., Whay H.R. Behavioural assessment of pain in horses and donkeys: application to clinical practice and future studies. Equine Vet J. 2005;37(6):565–575. doi: 10.2746/042516405775314826. [DOI] [PubMed] [Google Scholar]
- 2.Burns C.C., Dennison T.L., Whay H.R. Relationships between behaviour and health in working horses, donkeys and mules in developing countries. Appl Anim Behav Sci. 2010;126:109–118. [Google Scholar]
- 3.Jerbi H., Rejeb A., Erdogan S., et al. Anatomical and morphometric study of gastrointestinal tract of donkey (Equus africanus asinus) J Morphol Sci. 2014;31(1):18–22. [Google Scholar]
- 4.Evans L., Crane M. In: Clinical companion of the donkey. 1st edition. Evans L., Crane M., editors. Troubador Publishing Ltd; Leicestershire: 2018. Sedation, anaesthesia and analgesia; p. 225. [Google Scholar]
- 5.Yousef M.K., Dill D.B., Mayes M.G. Shifts in body fluid during dehydration in the burro, Equus asinus. J Appl Physiol. 1970;29(3):345–349. doi: 10.1152/jappl.1970.29.3.345. [DOI] [PubMed] [Google Scholar]
- 6.Pearson R.A., Merritt J.B. Intake, digestion and gastrointestinal transit time in resting donkeys and ponies and exercised donkeys given ad libitum hay and straw diets. Equine Vet J. 1991;23(5):339–343. doi: 10.1111/j.2042-3306.1991.tb03734.x. [DOI] [PubMed] [Google Scholar]
- 7.Thiemann A.K., Rickards K.J., Getachew M., et al. In: The equine acute abdomen. 3rd edition. Blikslager A.T., White N.A. II, Moore J.N., et al., editors. John Wiley and Sons Inc; Hoboken (NJ): 2017. Colic in the donkey; pp. 469–487. [Google Scholar]
- 8.Cox R., Proudman C.J., Trawford A.F., et al. Epidemiology of impaction colic in donkeys in the UK. BMC Vet Res. 2007;3:1–11. doi: 10.1186/1746-6148-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews J.B., Burden F.A. Common helminth infections of donkeys and their control in temperate regions. Equine Vet Educ. 2013;25:461–467. [Google Scholar]
- 10.Sgorbini M., Bonelli F., Papini R., et al. Equine gastric ulcer syndrome in adult donkeys: investigation on prevalence, anatomical distribution and severity. Equine Vet J. 2018;30(4):206–210. [Google Scholar]
- 11.Burden F.A., Gallagher J., Thiemann A.K., et al. Necropsy survey of gastric ulcers in a population of aged donkeys: prevalence, lesion description and risk factors. Animal. 2009;3(2):287–293. doi: 10.1017/S1751731108003480. [DOI] [PubMed] [Google Scholar]
- 12.Mellor N.E., Bladon B., Foote A.K., et al. Successful treatment of chronic grass sickness in a donkey. Equine Vet Educ. 2013;25(12):628–632. [Google Scholar]
- 13.McGorum B.C., Pirie R.S. Asinine typhlocolitis; 'scouring' the literature for diagnostic and aetiological clues. Equine Vet Educ. 2010;22(2):58–59. [Google Scholar]
- 14.Getachew A.M., Innocent G., Trawford A.F., et al. Gasterophilosis: a major cause of rectal prolapse in working donkeys in Ethiopia. Trop Anim Health Prod. 2012;44:757–762. doi: 10.1007/s11250-011-9961-7. [DOI] [PubMed] [Google Scholar]
- 15.Durham A.E., Smith K.C., Newton J.R., et al. Development and application of a scoring system for prognostic evaluation of equine liver biopsies. Equine Vet J. 2003;35(6):534–540. doi: 10.2746/042516403775467171. [DOI] [PubMed] [Google Scholar]
- 16.Edery N., Rosenbaum A., Busnach A., et al. Acute pancreatitis in a horse–a case report. Israel J Vet Med. 2015;70(1):49–52. [Google Scholar]
- 17.Merridale-Punter MS, Parker RA, Prutton JSW, et al. Outcome following exploratory laparotomy in 24 donkeys: a retrospective multicentre study. BEVA Conference Proceedings 2017. Liverpool, United Kingdom, 13–16 September, 2017.
- 18.Evans L., Crane M. Clinical companion of the donkey. 1st edition. Troubador Publishing Ltd; Leicestershire: 2018. Appendix 2, body weight estimator; p. 257. [Google Scholar]
- 19.Grosenbaugh D.A., Reinmeyer C.R., Figueiredo D.A. Pharmacology and therapeutics in donkeys. Equine Vet Educ. 2011;23(10):523–530. [Google Scholar]
- 20.Matthews N., van Loon J.P.A.M. Anaesthesia and analgesia of the donkey and mule. Equine Vet Educ. 2013;25(1):47–51. [Google Scholar]
- 21.Durham A.E., Thiemann A.K. Nutritional management of hyperlipaemia. Equine Vet Educ. 2015;27(9):482–488. [Google Scholar]
- 22.Fielding L.C. Practical fluid therapy and treatment modalities for field conditions for horses and foals with gastrointestinal problems. Vet Clin North Am Equine Pract. 2018;34(1):155–168. doi: 10.1016/j.cveq.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fielding L.C. Crystalloid and colloid therapy. Vet Clin North Am Equine Pract. 2014;30(2):415–425. doi: 10.1016/j.cveq.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Khan A., Hallowell G., Underwood C., et al. Evaluation of the rectal route of fluid administration in horses. ECEIM conference proceedings, Helsinki 2016. J Vet Intern Med. 2017;31(2):604–618. [Google Scholar]