Abstract
Nanotechnology has already started to significantly impact many industries and scientific fields including biotechnology, pharmaceutics, food technology and semiconductors. Nanotechnology-based tools and devices, including high-resolution imaging techniques, enable characterization and manipulation of materials at the nanolevel and further elucidate nanoscale phenomena and equip us with the ability to fabricate novel materials and structures. One of the most promising impacts of nanotechnology is in the area of nanotherapy. Employing nanosystems such as dendrimers, nanoliposomes, niosomes, nanotubes, emulsions and quantum dots, nanotherapy leads toward the concept of personalized medicine and the potential for early diagnoses coupled with efficient targeted therapy. The development of smart targeted nanocarriers that can deliver bioactives at a controlled rate directly to the designated cells and tissues will provide better efficacy and reduced side effects. Nanocarriers improve the solubility of bioactives and allow for the delivery of not only small-molecule drugs but also the delivery of nucleic acids and proteins. This review will focus on nanoscale bioactive delivery and targeting mechanisms and the role of high-resolution imaging techniques in the evaluation and development of nanocarriers.
Keywords: Nanotechnology, Nanotherapy, Heating method, Gene therapy, Nanoliposomes, Dendrimers, Electron microscopy, Scanning probe microscopy, Scanning tunneling microscopy, Atomic force microscopy
1. Introduction
Nanotechnology is a multidisciplinary approach that employs a vast and diverse array of tools and techniques derived from engineering, physics, chemistry, and biology (Sahoo et al., 2007). Within the past decade, there has been a flurry of new research, development and patent applications around nanoscaled technologies in the health area (Wagner et al., 2006). One of the principal areas of nanotechnology is “nanomedicine,” which, according to the National Institute of Health (NIH) Nanomedicine Roadmap Initiative, refers to highly specific medical intervention at the molecular scale for diagnosis, prevention and treatment of diseases (NIH Roadmap Initiatives, http://nihroadmap.nih.gov/initiatives.asp.). Nanomedicine has significant potential for revolutionizing the diagnostics and therapeutics under the premise of developing smart nanodevices. The overall objective of nanomedicine is the same as it has been in medicine: to diagnose as accurately and early as possible, to treat as effectively as possible without side effects, and to evaluate the efficacy of treatment noninvasively (Caruthers et al., 2007). Bioactive delivery nanosystems (nanocarriers) in general, and drug delivery in particular, constitute a significant domain of nanomedicine. Most drugs have been formulated for the oral or injection delivery routes, which are not always the most efficient routes for a particular therapy. New bioactive materials, such as nucleic acids and proteins, require novel delivery technologies that will minimize side effects and lead to better patient compliance (Hughes, 2005). On the other hand, reformulating old drugs can reduce side effects and increase patient compliance, thus saving money on health care system. Furthermore, drug candidates that did not pass through the trials phases can be reformulated to be used with new carrier systems.
Advancements in nanoscience and technology have made it possible to manufacture and characterize sub-micrometric bioactive carriers on routine basis. The delivery of bioactives to various sites within the body and their release behavior is directly affected by particle size. Compared to micrometer-sized carriers, nanocarriers provide more surface area and have the potential to increase solubility, enhance bioavailability, improve time-controlled release and enable precision targeting of the entrapped compounds to a greater extent (Mozafari, 2006). As a consequence of improved stability and targeting, the amount of material required for a specific effect when encapsulated in, or incorporated to, a nanocarrier is much less than the amount required when unencapsulated. This is particularly useful when dealing with expensive/rare bioactive materials. A timely and targeted release improves the effectiveness of bioactive compounds, broadens their application range and ensures optimal dosage, thereby improving cost-effectiveness of the product. In general, reactive or sensitive material, such as polynucleotides and polypeptides, can be turned into stable ingredients through encapsulation or entrapment by nanocarrier systems (Mozafari, 2006).
Innovative nanocarriers can make it possible to use certain chemicals or biologicals that were previously impractical because of toxicities or because they were impossible to administer. For example, bioactive targeting is enabling the delivery of chemotherapy agents directly to tumors, reducing systemic side effects (Hughes, 2005). Scientists are investigating new ways to deliver macromolecules that will facilitate the development of new biologic products such as bioblood proteins and biovaccines. Similarly, the success of DNA and RNA therapies will depend on innovative bioactive delivery techniques (El-Aneed, 2004). In many occasions, the success of a bioactive compound is dependent on the delivery method. This importance is exemplified by the presence of more than 300 companies based in the United States alone, which are involved with developing bioactive delivery platforms (D’Aquino, 2004).
It should be noted that effective bioactive carriers range from nanosystems (e.g., drug–polymer conjugates and polymeric micelles) to microparticles in the range of 100 μm. Both nano- and microscale systems have been extremely important in developing various clinically useful bioactive delivery systems. For instance, while microcarriers can be useful for bioactive targeting to certain parts of the pulmonary tract, for systemic targeting the tumors nanocarriers are more effectual. For practical reasons, in this perspective, “nanotechnology” includes “microtechnology” and “nanofabrication” or “nanomanufacturing” and its micro counterparts (Park, 2007). To describe what nanotechnology can do to manufacture nano-/microdrug delivery systems, the manufacturing of nano/micro particles (or capsules) can be taken as an example. Current methods of preparing nano-/microparticles are mainly based on double emulsion methods or solvent exchange technique (Freitas et al., 2005). The main problems with these methods are the low drug loading capacity, low loading efficiency, and poor ability to control the size distribution. Utilizing nanotechnologies, such as nanopatterning, could allow manufacturing of nano-/microparticles with high loading efficiency and monodisperse size distribution (Park, 2007).
This review focuses on the potential of nanotechnology in nanotherapy, including the recent status of nanocarriers for bioactive delivery and diagnostics and the role of high-resolution microscopies in this regard. These technologies will extend the limits of current molecular diagnostics and permit development of personalized medicine.
2. Nanotechnology in bioactive delivery
Nanotechnology is a relatively new discipline, and although the full scope of its contributions to the field of human health care remains unexplored, recent advances suggest that nanotechnology will have a profound impact on disease prevention, diagnosis, and treatment (Cheng et al., 2006, Emerich, 2005, Sahoo and Labhasetwar, 2003, Williams, 2004). Applications of nanotechnology in medicine are particularly promising and areas such as molecular imaging, disease diagnosis, bioactive encapsulation and targeted delivery at specific sites in the body are being intensively investigated and some products undergoing clinical trials (Moghimi et al., 2005, Shaffer, 2005, Wilkinson, 2003). Encapsulation and targeting the bioactive agents – including drugs, vaccines, nutrients and cosmetics – and their protection from degradation and inactivation have been investigated extensively using microencapsulation systems. However, to provide targeted controlled release is a key functionality that can be provided much more efficiently by employing nanocarrier technologies (Mozafari, 2006). Many of the current nanocarrier systems are in fact conventional drug delivery systems that happen to be in the nanometer range, such as nanoliposomes, polymeric micelles, nanoparticles, dendrimers, niosomes and nanocrystals (Park, 2007). In addition to reducing the frequency of drug administration and thus improving patient comfort, novel delivery systems would offer protection and improve the pharmacokinetics of easily degradable compounds, such as peptides and polynucleotides, which often have short half-lives in vivo (Orive et al., 2003). For the pharmaceutical industry the field of bioactive delivery represents a strategic tool for expanding drug markets, because new delivery technologies could reformulate/repackage classical drugs, offering a competitive edge after the upcoming patent expirations and avoiding competition from generics. It is noteworthy that 13% of the current global pharmaceutical market is related to the sale of products that include a bioactive delivery system (Mazzola, 2003).
The efficiency of bioactive delivery to various parts of the body is directly affected by particle size. Nanostructure-mediated bioactive delivery, a key technology for the realization of nanotherapy, has the potential to enhance bioavailability, improve the timed release of bioactive molecules, and enable precision targeting (Dass and Su, 2001, Dubin, 2004). Nanoscale delivery systems can be applied for pulmonary therapies (Courrier et al., 2002), as gene delivery vectors (Senior, 1998, Mozafari et al., 2007), and for stabilization of bioactives that would otherwise degrade rapidly (LaVan et al., 2002, LaVan et al., 2003). Additional benefits of using targeted nanocarriers are reduced toxicity and more efficient distribution of bioactive material (Ravi Kumar, 2000). Anatomic features such as the blood brain barrier, the branching pathways of the pulmonary tract, and the tight epithelial junctions of the skin make it difficult for bioactives to reach many desired physiologic targets. Nanocarriers will help to penetrate or overcome these barriers to bioactive delivery (Hughes, 2005).
Many bioactive materials are poorly water soluble, which results in a low bioavailability. Micelles are currently under investigation as carrier vehicles of poorly soluble, hydrophobic bioactives (Torchilin, 2004). Micelles solubilize these drugs by incorporating them into their hydrophobic core and thus increase the bioavailability. Microemulsions have also been investigated for their potential to serve as a drug carrier vehicle, since their oil phase can contain a high payload of hydrophobic drugs (Bagwe et al., 2001, Lawrence and Rees, 2000). Another possibility for the encapsulation and controlled release of lipid-soluble agents is the use of lipidic carriers with high lipid-phase to water-phase ratio, such as onion-shaped liposomes in the form of multilamellar vesicles (MLV).
Delivery of polynucleotides, as an important and new class of bioactives, is described below in a separate section.
2.1. Site-specific bioactive delivery
The anatomical changes and pathophysiological conditions of diseased or inflamed tissues offer opportunities for the delivery of various nanotechnological products. Bioactive targeting can be achieved by taking advantage of these specific characteristics of abnormal tissues (Vasir and Labhasetwar, 2005, Vasir et al., 2005). An ideal targeting system should have long circulation time, should be present at sufficient concentrations at the target site, and should not lose its activity or therapeutic efficacy while in circulation (Sahoo et al., 2007). The increased vascular permeability coupled with an impaired lymphatic drainage in tumors allows an enhanced permeability and retention effect of the nanosystems in the tumors or inflamed tissues (Hashizume et al., 2000, McDonald and Baluk, 2002, Maeda et al., 2000, Matsumura and Maeda, 1986). Therefore, this pathophysiological opportunity allows extravasation of the nanosystems and their selective localization in the inflamed tissues (Allen and Cullis, 2004, Hobbs et al., 1998) (Fig. 1 ). The tendency of nanosystems to specifically localize in the reticuloendothelial system also presents an excellent opportunity for passive targeting of therapeutic agents to the macrophages present in the liver and spleen. This natural system, therefore, can be used for bioactive targeting for intracellular infections such as candiasis, leishmaniasis and listeria. The macrophages of the infected individual play a role in these diseases; consequently, if the macrophages are destroyed then will be the disease as well (Daemen et al., 1995, Davis, 1997a).
Fig. 1.
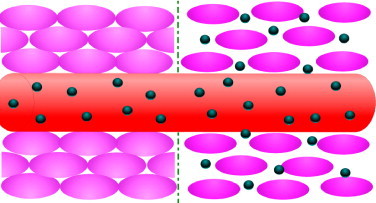
Nanocarrier accumulation within solid tumors—particle extravasation from the disorganized tumor vasculature (right part of the scheme) and nanocarrier particles in normal tissue (left part of the scheme) (from Mozafari and Mortazavi, 2005, with permission).
An important body organ considered for site-specific nanotherapy is the brain. However, the therapeutic value of many promising bioactives for the treatment of various neurological disorders is diminished by the presence of the blood-brain barrier (Calvo et al., 2001). The blood-brain barrier is a unique membrane that tightly segregates the brain from the circulating blood (Pardridge, 1999). As a result of this very efficient protection, bioactive delivery to the brain is a challenge. Nanotechnology offers a solution for using the numerous chemical entities for treating brain disorders that are otherwise not clinically useful because of the presence of the blood-brain barrier. Nanoparticles can be effectively used to deliver relevant therapeutics to the brain (Alyautdin et al., 1998, Garcia-Garcia et al., 2005). Encapsulation of bioactives into nanoparticles modifies cell and tissue distribution and leads to a more selective delivery of biologically active compounds to improve therapeutic efficacy and reduces toxicity (de Kozak et al., 2004, Feng et al., 2004, Kattan et al., 1992). Consequently, various nanosystems can be successfully used as new bioactive carriers for delivery to the brain. In a recent study, Visser et al. (2005) studied targeting of pegylated liposomes loaded with horse-radish peroxidase and tagged with transferrin to the blood-brain barrier in vitro. They obtained effective targeting of liposomes loaded with protein or peptide drugs to the brain capillary endothelial cells and suggested that the system is an attractive approach for drug delivery to the brain.
Enhanced uptake efficiency has also been shown for gastrointestinal absorption (Desai, 1996, Hussain et al., 2001) and transcutaneous permeation (Kohli and Alpar, 2004), with particles around 100 nm and 50 nm in size, respectively. However, such small particles traveling in the lungs may also have a greater chance of being exhaled. Larger, compartmental or multilayered bioactive carriers can help with delivery to the pulmonary extremities. For instance, the outer layers of the carrier architecture may be formulated to biodegrade as the carrier travels through the pulmonary tract. As the carrier penetrates further into the lung, additional shedding will allow the encapsulated material to be released. Biodegradable nanoparticles of gelatin and human serum albumin show promise for bioactive delivery to the lungs (Brzoska et al., 2004).
Another major site for bioactive transport and targeting is the skin. Skin acts as a key target as well as a principle barrier for topical/transdermal (TT) bioactive delivery. The topical/transdermal delivery route for drug administration has advantages over other pathways including avoiding the hepatic first pass effect, continuous drug delivery, fewer side effects and improved patient compliance (Barry, 2001). The interest of both the pharmaceutical and cosmetic industry for skin delivery has prompted the development and investigation of a wide variety of carrier systems with different physico-chemical characteristics. A major obstacle to TT drug delivery is low percutaneous penetration. The stratum corneum provides a principle barrier to TT delivery of applied bioactive and consists of corneocytes embedded in an inter-cellular lipid matrix composed of ceramides, free fatty acids, and cholesterol (Schurer and Elias, 1991). Several approaches have been used to weaken this skin barrier and to improve TT bioactive delivery (Choi and Maibach, 2005, Elias et al., 2002, Williams and Barry, 2004). Among the most efficient TT bioactive delivery have been the nanotechnological approaches employing elastic vesicles and ethosomes of nanometric size ranges (Elsayed et al., 2006, Lopez-Pinto et al., 2005). In a study on pig skin, Lopez et al. (2001) employed high-resolution, low-temperature scanning electron microscopy in order to detect the effect of nanoliposomes (ca. 200 nm average size) in the protection of stratum corneum (SC) against a nonionic surfactant. The imaging technique enabled visualization of native and treated SC (incubated with nanoliposomes and octyl glucoside) without causing damage to the SC during sample preparation for the microscopic investigations (Lopez et al., 2001).
3. Nanotechnology in polynucleotide delivery
Inadequacy of conventional drugs in the treatment of many of the existing health problems and emergence of new challenges, including acquired immunodeficiency syndrome (AIDS) and severe acute respiratory syndrome (SARS), make the requirement for potent therapeutic formulations a matter of urgency. A new class of bioactive therapeutic agents are based on the polynucleotide molecules and our increasing knowledge of genomics. These molecules, also known as nucleic acid drugs, have the potential to offer healing of human (and animal) diseases at their cause rather than only treating their symptoms. This is very important particularly in the case of hereditary diseases to make sure they are treated at the source and will not be passed to the next generations (Mozafari et al., 2005a). Polynucleotide-based therapeutics such as plasmids containing transgenes used in gene therapy, antisense and antigene oligonucleotides, ribozymes, DNAzymes, DNA and RNA aptamers and small interfering RNA (siRNA), have been developed over the past 20 years (Crooke, 1998, Mortazavi et al., 2007, Stull and Szoka, 1995, Ulrich et al., 2006). Although most polynucleotide-based bioactives are in the early stages of clinical trials, they have emerged during recent years as promising therapeutic candidates able to act in a large range of diseases such as hereditary disorders, cancer, neurological and cardiovascular disorders, AIDS and other viral infections (Mozafari et al., 2005a, Stull and Szoka, 1995, Ulrich et al., 2006).
During the past two decades, more than 400 clinical studies in gene therapy have been reported. Gene therapy is identified with the procedures used to insert the exogenous polynucleotides (DNA, mRNA, oligonucleotides) into cells or tissues to cure a disease or to improve the associated symptoms (Ruozi et al., 2007). Gene therapy starts with the choice of therapeutic gene, although the most critical objective is the success in the gene transfer to the target tissue for which nanotechnology can play a crucial role. Due to the limited ability of naked DNA to enter cells and its susceptibility to enzymatic degradation, gene transfer (transfection) has mainly been achieved using a delivery vector. Three main types of gene delivery systems have been described: viral vectors, nonviral vectors (in the form of nanoparticles, liposomes or dendrimers), and the direct injection of genetic materials into tissues using the so-called gene guns (Goverdhana et al., 2005, Labhasetwar, 2005, Mortazavi et al., 2007, Mozafari et al., 2005a). Viral vectors are attractive in terms of the scientific strategy exploiting the natural targeting mechanisms that viruses acquired during the course of evolution. As a result, viral vectors based on retroviruses, adenoviruses and other viruses are currently the most efficient method for DNA transfer into cells. However, these vectors could suffer from the serious difficulties of effective pharmaceutical processing and scale-up, and the possibility of the reversion of an engineered virus to the wild type (Sahoo et al., 2007). Furthermore, viral vectors have other drawbacks such as the risk of recombination, immunogenicity and carcinogenity (Crystal, 1995, Tripathy et al., 1996). Therefore, synthetic vectors have potential advantages for gene transfer even if they show a lower efficiency than viral systems.
Liposomes and nanoliposomes, in particular the cationic ones, have become one of the most studied synthetic nonviral vectors frequently used in human gene therapy (Audouy et al., 2002, Eastman and Scheule, 1999, Igarashi et al., 2006). The ability of cationic liposomes to mediate transfection was attributed to the intrinsic properties of these systems, namely spontaneous electrostatic interaction between the positively charged vesicles and the negatively charged DNA molecules that ensures an efficient condensation of the polynucleotides. By modifying the lipid composition the liposome–polynucleotide complex can exhibit an appropriate charge that enhances the possibility of cellular uptake. In the case of cationic liposomes both fusion and endocytosis have been proposed as mechanisms for the DNA or oligonucleotide uptake (de Lima et al., 2001). To efficaciously use these systems for in vivo gene transfer, the biological and the physicochemical properties of the liposomes/DNA complex must be elucidated. Microscopic techniques have proven to be useful in imaging and clarifying how the factors such as composition, carrier/DNA ratio, configuration, size and polydispersity can affect the assembly and stability of the vector and its gene transfer ability.
Recently anionic nanoliposomes are becoming more popular as polynucleotide delivery vehicles due to the toxicity and some other complications associated with the cationic agents (Mozafari et al., 2005a, Mozafari et al., 2007, Mortazavi et al., 2007). A method of incorporating polynucleotides to the similarly charged anionic liposomes, by the mediation of divalent cations, has been reported and is under development by Mozafari and coworkers since 1994 (Kahveci et al., 1994, Zhdanov et al., 1994, Mozafari, 1996, Zareie et al., 1997, Mozafari and Hasirci, 1998, Mozafari et al., 1998, Mozafari et al., 2002a, Mozafari et al., 2005a, Mozafari et al., 2007, Mortazavi et al., 2007). This group studied the structure of the ternary complexes of liposome–Ca2+–DNA morphologically using scanning probe and other microscopes (Zareie et al., 1997, Mozafari et al., 1998). In addition, the mechanism of calcium-induced DNA interaction with liposomes containing zwitterionic lipids, as well as those containing anionic lipids, has been studied using light scattering (Mozafari and Hasirci, 1998) and different microscopic techniques (Zareie et al., 1997, Mozafari et al., 1998). The problems of toxicity and scale-up to industrial levels have been addressed by a new technique, called the heating method, developed by Mozafari et al., 2005a, Mozafari et al., 2007 and Mortazavi et al. (2007), in which no potentially toxic solvent or deleterious procedure is involved.
Applications of nanotechnology in human gene therapy have been reviewed extensively by Davis (1997b), who described nonviral vectors based on nanoparticles (usually 50–500 nm in size) employed to transport plasmid DNA. He emphasized that nanotechnology in gene therapy would be applied to replace the currently used viral vectors by potentially less immunogenic nanosize polynucleotide carriers. Usefulness of high-resolution scanning probe imaging in the study of lipidic gene transfer vectors and the interaction between liposomes and DNA molecules have recently been reviewed by Mozafari et al. (2005b). Liposomes and other carrier systems used in bioactive transport and targeted nanotherapy are explained in the following sections.
4. Lipid-based nanocarriers
Lipid-based carrier systems, including liposomes and their nanoversions (nanoliposomes), are among the most promising encapsulation technologies employed in the rapidly developing field of nanotechnology. Compared with other encapsulation strategies, such as chitosan- and alginate-based carriers, lipid-based nanoencapsulation systems have unparalleled advantages, including the ability to entrap material with different solubilities, the possibility of being produced using natural ingredients on industrial scales, and targetability (Bummer, 2004, Mozafari et al., 2006, Yurdugul and Mozafari, 2004). Lipid-based carriers can shield an ingredient from free radicals, metal ions, pH and enzymes that might otherwise result in degradation of the bioactive compounds. They impart stability to water-soluble material, particularly in high water-activity applications (Gouin, 2004). They can accommodate not only water-soluble material but also lipid-soluble agents, if required, simultaneously, providing a synergistic effect (Suntres and Shek, 1996). Another unique property of lipid-based nanocarriers is the targeted delivery of their content to specific areas within the body as well as in nonliving systems. In addition, lipid based nanocarriers may be targeted to the required site inside the body via active (e.g., by incorporation of antibodies) and passive (e.g., targeting based on particle size) mechanisms (Mozafari and Mortazavi, 2005). The main lipid-based nanoencapsulation systems that can be used for the protection and delivery of various bioactive materials are explained below.
4.1. Nanoliposomes
The word liposome derives from two Greek words, lipos (fat) and soma (body or structure), meaning a structure in which a fatty envelope encapsulates aqueous core(s) or compartment(s). A recent definition, proposed at a conference in the field of liposomology, describes liposomes as ‘closed, continuous bilayered structures made mainly of lipid and/or phospholipid molecules’ (Mozafari et al., 2002b, Mozafari and Mortazavi, 2005). They are under intensive investigation and development by the pharmaceutical, cosmetic and food industries as micro- and nanocarrier systems for the protection and delivery of bioactive agents. Recent studies suggest that liposomes are even naturally present in the very first food we take, breast milk (Keller et al., 2000, Keller, 2001).
Liposomes are composed of one or more lipid and/or phospholipid bilayers and can contain other molecules such as proteins or polymers in their structure. A liposome composed of a number of concentric bilayers is known as a multilamellar vesicle (MLV), while one composed of many small nonconcentric vesicles encapsulated within a single lipid bilayer is known as a multivesicular vesicle (MVV). Another type of liposome is known as a unilamellar vesicle (ULV), which contains a single lipidic bilayer (Fig. 2 ). Owing to the possession of both lipid and aqueous phases, liposomes can be utilized in the entrapment, delivery and release of both water-soluble and lipid-soluble material. The term nanoliposome has recently been introduced to exclusively refer to nanoscale lipid vesicles (Mozafari and Mortazavi, 2005), since liposome is a general word covering many classes of lipid vesicles whose diameter range from around 20 nm to several micrometers. Nanoliposomes possess the same physical, structural and thermodynamic properties as the liposomes described above. Manufacture of both liposomes and nanoliposomes requires input of energy to a dispersion of lipid/phospholipid molecules in an aqueous medium (Mozafari and Mortazavi, 2005). The underlying mechanism for the formation of liposomes and nanoliposomes is basically the hydrophilic–hydrophobic interaction between phospholipids and water molecules (Mozafari, 2005). Since liposomes are dynamic entities that tend to aggregate and/or fuse and as a result increase in size, vesicles prepared in nanometric size ranges may end up becoming micrometric particles upon storage. However, nanoliposomes should have sufficient stability to maintain their sizes and could be defined as ‘bilayer lipid vesicles possessing and maintaining nanometric size ranges during storage and application’ (Mozafari and Mortazavi, 2005). The unique properties of liposomes have triggered numerous applications in different fields of science and technology, from basic studies of membrane structure/function to bioactive agent delivery. Liposomes and nanoliposomes are particularly useful as efficient bioactive delivery devices because of their ability to pass through lipid bilayers and cell membranes. Targeted therapy can also be achieved efficiently via liposomes and nanoliposomes employing passive or active targeting mechanisms (Mozafari and Mortazavi, 2005, Mozafari, 2006). Active targeting is achieved by engineering carriers sensitive to different stimuli (e.g., pH, temperature, light, etc.) or conjugating the bioactive/carrier system to one or more targeting ligands such as tissue or cell-specific molecules. In a recent study, Zhang et al. (2003) showed that PEGylated (treated with polyethylene glycol) liposomes, linked to a monoclonal antibody for the human insulin receptor, led to widespread reporter expression in the brains of rhesus monkeys. Passive targeting, on the other hand, uses the natural course followed by the bioactive–carrier complex upon being introduced to the body as the method of site-specific delivery and release of the bioactive agent. It is therefore based on the physicochemical properties of bioactive–carrier complex and physio-anatomical conditions of the body. The clearance kinetics and in vivo biodistribution of carrier systems depend on the physicochemical factors like size, charge and hydrophobicity and can be manipulated to enable passive targeting.
Fig. 2.
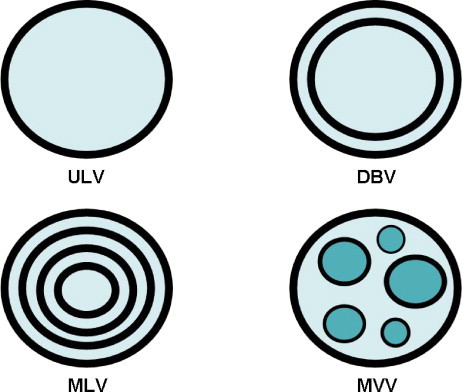
Schematic representation of a multilamellar vesicle (MLV), a multivesicular vesicle (MVV), a double-bilayer vesicle (DBV) and a unilamellar vesicle (ULV). Note that these vesicles vary in terms of lamellarity and lipid to aqueous phase proportion.
4.2. Archaeosomes
Archaeosomes are liposomes made from one or more of the polar ether lipids extracted from the domain Archaea (Archaeobacteria). Many Archaea live in environments including high salt concentrations, low pH values or high temperatures. Hence their membrane structure is unique and enables them to survive in such hostile conditions. The core lipids (polar head groups removed) of archaea consist of archaeols (diethers) and caldarchaeols (tetraethers), wherein the regularly branched, 5-carbon repeating units forming the isoprenoid chains (usually 20 carbons per chain in archaeols, and 40 carbons per chain in caldarchaeols) are attached via ether bonds at the sn-2,3 position of the glycerol carbons. In contrast to this, the core lipids found in Bacteria and Eucarya consist of unbranched (mostly) fatty acyl chains, often unsaturated, attached via ester bonds to the sn-1,2 glycerol carbons. The polar moieties (archaeols are monopolar and caldarchaeols are bipolar) are similar to those (phospho, glyco, polyol, amino, hydroxyl groups) encountered in ester lipids, but phosphatidylcholine is rarely present in archaeal lipids (Mozafari et al., 2006, Patel et al., 2000). Although archaeosomes are a recent technology, they have already proven to be a safe delivery system for bioactive agents including drugs and vaccines (Patel and Chen, 2006). Compared with liposomes (which are made from ester phospholipids), archaeosomes are relatively more thermostable, and more resistant to oxidation and chemical and enzymatic hydrolysis. They are also more resistant to low pH and bile salts that would be encountered in the gastrointestinal tract (Patel et al., 2000). Archaeosomes prepared from the total polar lipid extract or from individual purified polar lipids show promise as adjuvants that promote strong humoral and cytotoxic T-cell responses to encapsulated soluble antigens. Therefore, there is a great potential for using archaeosomes in drug, vaccine and other bioactive material delivery applications. As is the case with liposomes, it is possible to incorporate ligands such as polymers to archaeosomes. It has been shown that incorporation of polyethyleneglycol and coenzyme Q10 into archaeosomes can alter the tissue distribution profiles of intravenously administered vesicles (Omri et al., 2000). It has recently been reported that intravenous and oral delivery of nanometric-sized archaeosomes to an animal model was well tolerated with no apparent toxicity (Omri et al., 2003). The results of these studies are very promising for the utilization of archaeosomes in the encapsulation and delivery of different bioactive compounds.
4.3. Cochleates and nanocochleates
Cochleates are small-sized and stable lipid-based carriers comprised mainly of a negatively charged lipid (e.g., phosphatidylserine) and a divalent cation such as calcium (Zarif et al., 2000, Zarif, 2003). They have a cigar-shaped multilayered structure consisting of a continuous, solid, lipid bilayer sheet rolled up in a spiral shape with little or no internal aqueous space. Hydrophobic, amphiphilic, negatively or positively charged molecules can be delivered by cochleates. Cochleates and their sub-micron versions (i.e., nanocochleates) have been used to deliver proteins, peptides and DNA for vaccine and gene therapy applications and are able to cover unpleasant taste and smell of bioactive material intended for oral delivery (Mannino and Gould-Fogerite, 1997, Mozafari et al., 2006, Zarif and Mannino, 2000). Due to their nanometric size, stability and resistance to degradation in the gastrointestinal tract, nanocochleates have revealed great potential to deliver bioactive agents both orally and parenterally (Mannino and Gould-Fogerite, 1997, Zarif and Mannino, 2000, Zarif et al., 2000, Zarif, 2003). Cochleates containing amphotericin B (AmB) are now in development to enter Phase I clinical trials, for both the oral and parenteral treatment of fungal infections (Zarif, 2003). The unique structure and properties of cochleates make them an ideal candidate for oral and systemic delivery of sensitive material including peptide and nucleic acid drugs.
5. Polymeric nanocarriers
Polymer materials potentially possess several desirable properties to be used as nanocarriers including biocompatibility, biodegradability, and functionalization capability. Through functionalization and structural manipulation of polymer materials, bioactive molecules can be incorporated within the polymer. Entrapping or encapsulating the drug within a polymer allows for greater control of the pharmacokinetic behavior of the bioactive molecule (Hughes, 2005). The bioactive can be released with a more ideal, near zero-order kinetic profile, which establishes a more constant flow of the encapsulated substance out of the carrier. This pharmacokinetic behavior maintains more appropriate steady levels of the bioactive material at the site of delivery. In contrast, conventional oral delivery typically follows first-order release kinetics where the release rate is proportional to the amount of material remaining in the carrier. Landgraf et al. (2003) have compared the release kinetics of an anti-inflammatory agent taken orally by use of a macroporous copolymer carrier and a microporous copolymer carrier containing nanochannels. The macroporous bioactive carrier releases the encapsulant with an initial burst and follows first-order release kinetics. The microporous carrier structured with nanochannels steadily releases the biomaterial in near zero-order fashion.
Techniques that are used to couple the bioactive with the polymer include sequestering, conjugation, and micelle formation (Duncan, 2003). Ulrich et al. (1999) have reviewed the biodegradable polymeric materials that show promise for bioactive delivery applications. Biodegradable polymer nanoparticles, typically consisting of polylactic acid (PLA), polyglycolic acid (PGA), or a copolymer of PLA and PGA, are being investigated for the delivery of polynucleotides and polypeptides, vaccines, anticancer therapeutics, ocular drugs, and cytokines (Hughes, 2005). Other polymers being investigated as nanoscale bioactive carriers include polyalkylcyanoacrylate, poly(3-hydroxybutanoic acid) (PHB), poly(organophosphazene), poly(ethylene glycol) (PEG), poly(caprolactone) (PCL), poly(ethylene oxide) (PEO), and copolymers such as PLA-PEG. Synthetic polymers, such as PEG, can be used to encapsulate biologic materials to create a more stable nanocarrier (Hughes, 2005). One example of a hybrid drug carrier is a liposome coated with PEG, called a stealth liposome. Conventional liposomes and nanoliposomes are typically cleared rapidly from the blood circulation. Stealth liposomes, with PEG coatings, can have prolonged circulation times (Moghimi and Szebeni, 2003). The mechanisms behind this prolonged circulation are still being investigated. Furthermore, polymers are being used to enhance the release characteristics of other carrier systems; as is the case with the coating of tablets with hydroxypropyl methylcellulose phthalate (HPMCP) nanoparticles (Kim et al., 2003). The nanoparticle-coated tablets show a decrease in release rate and a migration towards zero-order release kinetics as the particle size is decreased.
5.1. Dendrimers
Dendrimers are a unique class of polymers, which provide another avenue for nanodelivery of different bioactive compounds. The word dendrimer is derived from the Greek words dendri- (tree branch-like) and meros (part of) (Gardikis et al., 2006). Dendrimers are considered as highly branched macromolecules; they are small in size, while their low polydispersity can contribute to the reproducibility of their pharmacokinetic behavior (Cloninger, 2002). Dendrimers are fabricated from monomers using either convergent or divergent step-growth polymerization. The well-defined structure, monodispersity of size, surface functionalization capability and stability are properties of dendrimers that make them attractive nanocarrier candidates (Hughes, 2005). An ideal dendrimer as bioactive delivery system, however, must also be nontoxic, nonimmunogenic and biodegradable (for a review, see Aulenta et al., 2003).
The first complete dendrimer family which has been synthesized, characterized and commercialized is the poly(amidoamine) (PAMAM) dendrimers. They are characterized as safe and nonimmunogenic nanocarriers and have been used for the delivery of drugs and antisense nucleotides and in gene therapy, both in vitro and in vivo (Frechet and Tomalia, 2001). Bioactive molecules can be associated to dendrimers via either complexation or encapsulation as schematically shown in Fig. 3 . A conjugate carrier system composed of dendrimers and liposomes has recently been manufactured and characterized by Gardikis et al. (2006).
Fig. 3.
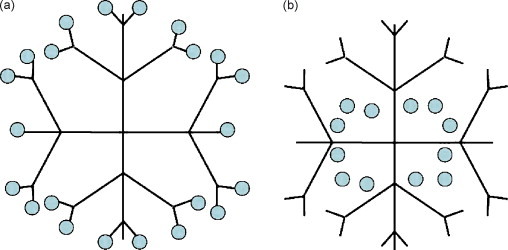
Representation of bioactive incorporation within the structure of a dendrimer. (a) Complexation: covalent attachment to end groups. (b) Encapsulation: bioactive entrapment inside the core of dendrimer.
6. Niosomes
Niosomes are vesicles made of nonionic surfactant molecules and have been developed as controlled delivery systems in order to overcome the problems associated with some other nanocarriers such as sterilization, large-scale production and stability. They are liposome-like vesicles formed from the hydrated mixtures of cholesterol, charge inducing molecules and nonionic surfactants such as monoalkyl or dialkyl polyoxyethylene ether. The assembly into closed bilayers, both in the case of liposomes/nanoliposomes (Mozafari and Mortazavi, 2005) and niosomes, is not spontaneous. Thermodynamically stable vesicles form only in the presence of proper mixtures of surfactants and charge inducing agents. The mechanism of vesicle formation upon use of nonionic surfactants is not completely clear. The most common theory is that nonionic surfactants form a closed bilayer in aqueous media based on their amphiphilic nature. Formation of this structure involves some input of energy, for instance by means of physical agitation (e.g., by using the hand-shaking method; see Baillie et al., 1985) or heat (e.g., by using the heating method; see Mozafari, 2005, Mozafari et al., 2002a, Mozafari et al., 2007). In this closed bilayer structure, hydrophobic parts of the molecule are oriented away from the aqueous solvent whereas the hydrophilic head comes in contact with the aqueous solvent. Niosomes resemble phospholipid vesicles (liposomes and nanoliposomes) and hence, enable entrapment of both hydrophilic and hydrophobic material.
The low cost, stability and resultant ease of storage of nonionic surfactants have led to the exploitation of these compounds as alternatives to phospholipids (Uchegbu and Vyas, 1998). Niosomes can entrap hydrophilic drugs and other bioactives upon encapsulation or hydrophobic material by partitioning of these molecules into their hydrophobic domains. These vesicles can be formulated either unilamellar or multilamellar in structure. Moreover, niosomes possess great stability, cost-effectiveness, and simple methodology for the routine and large-scale production without the use of hazardous solvents. Uchegbu et al., 1996a, Uchegbu et al., 1996b have studied the different phases and morphologies of niosomes (e.g., discomes and polyhedral niosomes) by employing confocal laser scanning microscopy. Microscopic examinations enabled observation and identification of the spherical, helical, tubular and polyhedral niosomes (for a review, see Uchegbu and Vyas, 1998).
7. Silicon-based nanocarriers
Along with the more conventional polymer- and liposome-based formulations, silicon-based nanocarriers are emerging in the field of bioactive encapsulation and targeting (Ferrari, 2005). The most commonly investigated silicon-based materials for bioactive delivery are porous silicon and silica, or silicon dioxide. Architectures include calcified nanopores, platinum-containing nanopores, porous nanoparticles, and nanoneedles (Hughes, 2005). Porous silicon was discovered more than 50 years ago and since then has attracted much interest after the demonstration of its photoluminescence at room temperature (Cullis et al., 1994, Vaccari et al., 2006). Two aspects of porous silicon are of particular relevance for in vivo applications; namely: (i) it can be used as a sensitive biosensor for proteins, antigens, and DNA, and (ii) it can be modified with a wide range of biological or organic molecules. These features should allow porous silicon to serve as a versatile biomaterial. Although efforts in this area are still in early developmental stages, combining the biocompatibility of the material with its highly bio-sensitive capabilities leads to new applications in tissue-based bioassays, bioactive delivery, and health-monitoring applications.
The demonstration in 1995 of porous silicon biodegradability in physiological environment (Canham, 1995) with a dissolution rate dependent on the medium acidity, porous silicon morphology, porosity, and on the chemical surface derivatization, opened the way for its applications in biomedicine (e.g., see Vaccari et al., 2006). In the most basic sense, porous silicon is a network of air holes within an interconnected crystalline silicon matrix. The free volume inside pores can be loaded with a bioactive that will be released in the body following the dissolution of the matrix. This idea has been the guideline followed for the design of an implantable microsystem prototype as an anticancer device for the release of doxorubicin in the treatment of tumors (Minotti et al., 2004, Vaccari et al., 2006).
Ordered mesoporous silica material with very high surface area and large pore volume appeared as a new member in the family of silica-based materials in 1992 (Beck et al., 1992). The pore size distribution of mesoporous silica is very narrow and can be modulated in the mesoporous region from 2 to several nanometers, which has expanded the available pore sizes of zeolites. Such properties would make these materials potentially very interesting as novel carriers for large drug molecules (≥2 nm). Recently, considerable investigations have been carried out for the applications of mesoporous silicas as nanocarrier systems for high bioactive loading capacity and sustained or controlled release (Xue and Shi, 2004, Tang et al., 2006).
Some examples of therapies being investigated for use with silicon-based delivery systems include porous silicon embedded with platinum as an antitumor agent, calcified porous silicon designed as an artificial growth factor, silicon nanopores for antibody delivery, and porous silica nanoparticles containing antibiotics, enzymes and DNA (for a review, see Hughes, 2005).
8. Carbon-based nanocarriers
The carbon nanostructures, which have received much attention in recent years are hollow, cage-like architectures known as nanotubes and fullerenes, also called buckyballs because of their spherical structure resembling the geodesic domes of Buckminster Fuller (Hughes, 2005). Single-wall nanotubes, multiwall nanotubes, and C60 fullerenes are common configurations. The size, geometry, and surface characteristics of these structures make them attractive for application as nanocarrier systems. Carbon nanotubes are on the light spot of nanoscience and nanotechnology owing to their exceptional physical properties (Zanella et al., 2007). The electronic properties of single-wall carbon nanotubes are remarkable insofar as they can be either metallic or semiconducting, depending on their (n, m) indices or chirality (Saito et al., 1998). Nanotubes are very stable molecules and their chemical inertness is due to the strong covalent sp2 bonds of the carbon atoms on their surface.
Surface-functionalized carbon nanotubes can be internalized within mammalian cells (Shi Kam et al., 2004). Much work with carbon nanotubes has involved composite materials. For example, temperature-stabilized hydrogels for bioactive delivery applications incorporate carbon nanotubes (Li et al., 2004). On the other hand, fullerenes have also shown bioactive targeting capability. Tissue-selective targeting as well as intracellular targeting of mitochondria have been shown with the use of fullerene structures. Furthermore, experiments with fullerenes have also shown that they exhibit antioxidant (Lin et al., 1999) and antimicrobial activities (Tsao et al., 2002).
Carbon nanostructures containing ferromagnetic material are another means of bioactive targeting. These nanocarriers can be injected intravenously and then directed using an external magnetic field upon which they will travel through the blood vessels to the region of interest for treatment (Berry and Curtis, 2003). The process of bioactive targeting using a magnetic delivery system is based on the competition between forces exerted on the nanocarrier by the blood flow and the magnetic forces generated by the externally applied magnetic field. When the magnetic forces exceed the linear blood flow rates in arteries (10 cm/s) or capillaries (0.05 cm/s), the nanocarriers are retained at the target site and internalized by the cells of the target tissue (Tartaj et al., 2003). The particles should be small enough to remain in the circulation after injection and to pass via the capillary systems of organs and tissues avoiding vessel embolism. The nanocarrier surface can be grafted by COOH groups after the acid treatment. Bioactive molecules and targeting ligands can be covalently attached to the nanocarriers via carboxyl groups (Fig. 4 ). The ligands attached to the nanocarriers recognize individual components characteristic for cell-surface antigens (Berry and Curtis, 2003, Wozniak et al., 2006). The morphology and cytotoxicity of magnetic carbon nanoparticles have recently been evaluated by Wozniak et al. (2006) using inverted microscope and atomic force microscopy (AFM).
Fig. 4.
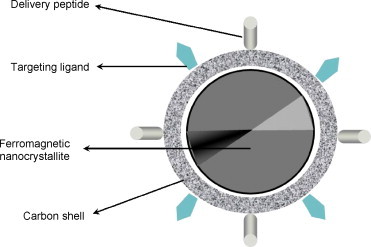
Schematic representation of a magnetic carbon nanocarrier with attached bioactive molecules and targeting ligands.
9. Role of imaging in nanocarrier studies
Modern nanocarrier systems such as nanoliposomes, niosomes, solid lipid nanoparticles (Saupe and Rades, 2006), as well as silicon-, carbon- and polymer-based nanocarriers play an important role in controlled delivery of the bioactive agents to the desired site of action, limiting the side effects at nontarget sites (Ruozi et al., 2007). Development of these nanosystems requires a rational characterization approach. Certain parameters pertained to each of the newly developed nanocarrier systems must be thoroughly assessed before being approved for clinical applications. In the case of liposomes, for instance, parameters having critical importance on their in vivo performance such as morphology, size, polydispersity index, number of lamellae, zeta potential, bilayer fluidity, lipid composition, encapsulation efficiency, carrier-bioactive interaction and chemical stability must be studied. For these, various analytical techniques are being applied. Dynamic light scattering (DLS, also known as photon correlation spectroscopy, PCS) is used in the determination of particle size distribution, while the nuclear magnetic resonance (NMR) and the electron paramagnetic resonance (EPR) are being applied to investigate the lamellarity, the permeability of the bilayer and the influence of particle size on the bioactive transport (for a recent review, see Ruozi et al., 2007).
The microscopical approach is commonly used to characterize the structure/morphology/geometry of the nanocarriers. Electron microscopy techniques have been widely used to measure the size and the size distribution of particles. Several electron microscopy techniques can be employed for nanocarrier research. These include: (i) scanning electron microscopy (SEM); (ii) transmission electron microscope (TEM); (iii) negative stain electron microscopy (NSEM); (iv) freeze fracture transmission electron microscopy (FFTEM). In particular, TEM provides information on the size distribution and shape of nanocarrier systems. In addition to the configuration of nanocarriers, electron microscopy can also provide information on the interaction between particles (e.g., in the form of aggregation or fusion), different types of each nanocarrier (e.g., in the case of liposomes, MLV, ULV and MVV types; see Fig. 2), different phases of each carrier (e.g., discomes and polyhedral niosomes), location of the bioactive with respect to the carrier (e.g., internalized or attached to the surface) and the stability of the carrier systems in time. Compared with other electron microscopes, SEM is a less frequently used imaging technique, particularly in liposome research. However, several SEM micrographs showing cells with absorbed liposomes have been published, which are very useful in determining mechanisms of cell-liposome interactions (e.g., see Vinay et al., 1996). Unfortunately, nanocarriers may suffer structural perturbations as a result of the high vacuum conditions and the staining process required by some of the electron microscopes (see Fig. 5 ).
Fig. 5.
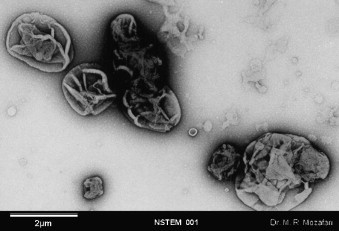
A representative micrograph of structural perturbations of lipidic bioactive carriers possibly incurred as a result of sample preparation for negative stain electron microscopy examination (see text for details).
In the previous years, besides the progress in sample preparation, other microscopical techniques have been developed. Atomic force microscopy (AFM), one of the techniques belonging to the family of scanning probe microscopes (SPM) with dimensional resolution approaching 1 Å, has revolutionized imaging of the nanosamples (Binning et al., 1986, Santos and Castanho, 2004). The most attractive characteristics of SPM are the potential to image samples with subnanometer spatial resolution under physiological conditions and provide information on their physical and mechanical properties. The other SPM technique is scanning tunneling microscope (STM) that was invented in 1982 by Binnig and coworkers (Binnig et al., 1982a, Binnig et al., 1982b) and has since become established as a powerful tool for the study of micro- and nanoscale structures. Compared with the other types of microscopy, STM has unique characteristics that include: (i) ultra-high resolution down to atomic dimensions; (ii) three-dimensional images with very high resolution especially in the vertical direction; (iii) a variety of operating conditions, such as vacuum, air, and liquids; (iv) observation range from micrometer to angstrom; (v) the ability to do tunneling spectroscopy (Feng et al., 1989). Fig. 6 depicts three-dimensional STM images of double-stranded DNA molecules. Major and minor grooves of DNA are visible in Fig. 6B. Representative STM images of two-months old nanoliposomes are presented in Fig. 7 . These nanoscale images enabled characterization of the nanocarriers including evaluation of their size and stability; as indicated by their size and morphology variation in time (e.g., see Mozafari et al., 2002a). AFM, on the other hand, was developed in 1986 and has since been applied for imaging surfaces of different material including ceramics, metals, in addition to biological and pharmaceutical samples (Binning et al., 1986, Mozafari et al., 2005b, Ruozi et al., 2007, Santos and Castanho, 2004). The AFM ability to explore samples under variety of environmental conditions, including biological specimens in an aqueous/physiological environment, or in air at different temperatures, or under controlled humidity, make it a very versatile characterization technique. In addition, it is possible to study samples at low temperatures using cryo-AFM (Prater et al., 1991, Shao et al., 2000). Unlike the electron microscopical methods, which often require sophisticated sample preparation procedures, the sample preparation for AFM is easy and fast and it allows the material to be preserved in its native state (Mozafari et al., 2005b).
Fig. 6.
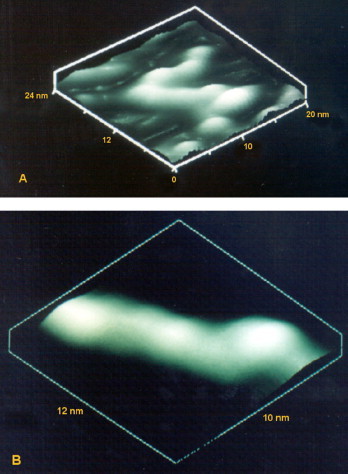
Three-dimensional scanning tunneling microscopy images of double-stranded DNA molecules. Image dimensions: (A) 24 nm × 20 nm and (B) 12 nm × 10 nm.
Fig. 7.
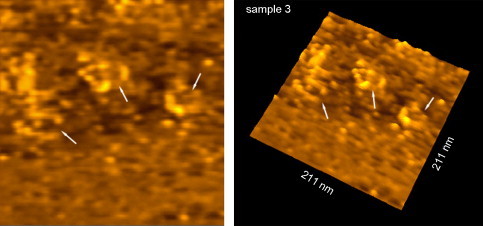
Two and three-dimensional scanning tunneling micrographs of nanoliposomes deposited on HOPG (highly oriented pyrolytic graphite) and dried at room temperature under atmospheric pressure. Nanoliposomes (indicated by arrows) were prepared by the heating method two months prior to imaging.
AFM can be operated in a number of different imaging modes depending on the nature of the interaction between its tip and sample surface. When the scanning is carried out keeping an approximately constant distance between probe and sample, AFM operation is in the “contact mode.” New generations of AFM also use another scanning mode called “tapping mode” or “MacMode” (Santos and Castanho, 2004). Tapping mode AFM provides high-resolution topography images with minimal damage to the sample surface. Application of the additional measurement modes such as lateral force mode (LFM) and force modulation mode (FMM) are possible with AFM (Ruozi et al., 2007). In addition to topographic imaging, and especially considering the versatility of the operation modes, AFM can also provide information regarding micromechanical properties such as surface and adhesive forces (Ruozi et al., 2007). SPM studies of phospholipid bilayers, for example, have been providing valuable insights into the micromechanical properties of biomembranes (Liang et al., 2004) as well as biological processes such as formation of pili and bacterial conjugation (Maeda et al., 2002). As is the case with the electron microscopes, SPM techniques also enable investigation of particle-particle interactions and the aggregation and fusion processes characteristics of certain nanocarriers (Uvarov et al., 1996). Furthermore, STM in particular is very useful in determining the bilayer thickness of liposomes, nanoliposomes, niosomes and archaeosomes by its analytical ability in the vertical axis (Zareie et al., 1997). Another useful application of the microscopical techniques in general, and SPM in particular (due to their high resolution), is that they can identify both the density and the spatial distribution of ligands such as polymers, peptides, and antibody molecules anchored to the surface of the nanocarriers. As explained above, these ligands equip the nanocarriers with specific targeting mechanisms.
For a rational development of nanocarriers as bioactive delivery systems, it is essential to characterize these systems as particles. The evaluation of nanocarrier morphology includes the characterization of shape, structure, surface morphology and size measurement of these particles. Evaluation of size distribution of nanocarriers is important not only to study the physico-chemical properties and the stability of the preparations but also to identify the in vivo kinetics of these systems and in particular their ability to cross vessel walls and to be accumulated in target tissues (e.g., tumors or infected sites) in order to exert the desired effect. Determination of nanocarrier size distribution is an obligatory quality control assay due to the following reasons: (i) The size distribution of bioactive delivery formulations is an important parameter with respect to the physical properties and stability (Goren et al., 1990); (ii) size distribution, along with composition, defines plasma pharmacokinetics, biodistribution, and stability of nanocarriers and their associated/entrapped substances in plasma and other organs (Barenholz and Amselem, 1993); (iii) nanocarrier size is a major factor in their permeation through tumor microvessels and their local residence in tumor tissue (Nagayasu et al., 1999); (iv) in pulmonary applications the deposition region of bioactive carriers depends mainly on density, shape, and size of the particles (Mozafari et al., 2005b).
Each of the currently used particle size determination techniques has its own advantages and limitations. Light scattering, for example, provides cumulative average information of the size of a large number of particles simultaneously. However, it does not provide information on the shape of the nanosystem and it assumes any aggregation of more than one particle as one single particle. SPM and other microscopic techniques, on the other hand, make direct observation possible, and hence provide information on the shape of the nanocarriers as well as presence/absence of any aggregation/fusion (Mozafari et al., 2005b). The drawback of the microscopic investigations is that the number of particles that can be studied at any certain time is limited. With respect to a statistically meaningful analysis of size distribution of the nanocarriers, methods such as light scattering, which measure the average size of large number of particles, are more appropriate than microscopic techniques (Mozafari and Mortazavi, 2005). It should be noted that SPM techniques can assess samples in liquid or as adsorbed on a solid surface (partially or fully dehydrated) while the light scattering method evaluates particles in suspension. The general approach for the determination of size distribution of nanocarrier formulations should hence be to use as many different techniques as possible, or at least combine high-resolution imaging and particle sizing techniques together.
10. Conclusions
As we gain more knowledge with respect to disease pathophysiology and cellular mechanisms, more specific bioactive materials are being developed. To use the specificity and potency of these bioactives, new carrier systems must be exploited. Nanostructured delivery systems are promising candidates that will enable efficient and targeted delivery of novel bioactive compounds. Systematic characterization of the nanocarriers is one of the main steps in the evaluation of their present and coming applications. In this respect, the microscopical approach enables direct visualization and provides valuable information about the geometry and morphology, size distribution and the superficial properties of the nanocarriers affecting their interaction with the bioactive material and target cells. The microscopical techniques in general, and SPM in particular, enable us to identify both the density and the spatial distribution of targeting ligands such as polymers, peptides, and antibody molecules anchored to the surface of the nanocarriers. The localization and the way by which nanocarriers interact with the bioactive materials are very important to obtain a good in vivo applicability. Moreover, force measurement is another interesting property of high-resolution microscopes such as SPM, which provides significant information about the elastic, chemical and adhesion properties of nanocarriers. The information obtained through high-resolution imaging techniques are crucial in the rational design and formulation of optimal nanocarrier systems to be used in the modern field of nanotherapy.
Acknowledgement
Valuable assistance of Professor E. Piskin, Dr. C. Kocum, and Dr. H. Zareie in the STM studies is highly acknowledged.
References
- Allen T.M., Cullis P.R. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- Alyautdin R.N., Tezikov E.B., Ramge P., Kharkevich D.A., Begley D.J., Kreuter J. Significant entry of tubocurarine into the brain of rats by adsorption to polysorbate 80-coated polybutylcyanoacrylate nanoparticles: an in situ brain perfusion study. J. Microencapsul. 1998;15:67–74. doi: 10.3109/02652049809006836. [DOI] [PubMed] [Google Scholar]
- Audouy S.A., de Leij L.F., Hoekstra D., Molema G. In vivo characteristics of cationic liposomes as delivery vectors for gene therapy. Pharm. Res. 2002;19:1599–1605. doi: 10.1023/a:1020989709019. [DOI] [PubMed] [Google Scholar]
- Aulenta F., Hayes W., Rannard S. Dendrimers a new class of nanoscopic containers and delivery devices. Eur. Polym. J. 2003;39:1741–1771. [Google Scholar]
- Bagwe R.P., Kanicky J.R., Palla B.J., Patanjali P.K., Shah D.O. Improved drug delivery using microemulsions: rationale, recent progress and new horizons. Crit. Rev. Ther. Drug Carrier Syst. 2001;18:77–140. [PubMed] [Google Scholar]
- Baillie A.J., Florence A.T., Hume L.R., Muirhead G.T., Rogerson A. The preparation and properties of niosomes non-ionic surfactant vesicles. J. Pharm. Pharmacol. 1985;37:863–868. doi: 10.1111/j.2042-7158.1985.tb04990.x. [DOI] [PubMed] [Google Scholar]
- Barenholz Y., Amselem S. Quality control assays in the development and clinical use of liposome-based formulations. In: Gregoriadis G., editor. second ed. vol. 1. CRC Press; Boca Raton, FL: 1993. pp. 527–616. (Liposome Technology. Liposome Preparation and Related Techniques). [Google Scholar]
- Barry B.W. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. 2001;14:101–114. doi: 10.1016/s0928-0987(01)00167-1. [DOI] [PubMed] [Google Scholar]
- Beck J.S., Vartuli J.C., Roth W.J., Leonowicz M.E., Kresge C.T., Schmitt K.D., Chu C.T.-W., Olson D.H., Sheppard E.W., McCullen S.B., Higgins J.B., Schlenker J.L. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992;114(27):10834–10843. [Google Scholar]
- Berry C.C., Curtis A.S.G. Functionalisation of magnetic nanoparticles for applications in biomedicine. J. Phys. D: Appl. Phys. 2003;36:R198–R206. [Google Scholar]
- Binnig G., Rohrer H., Gerber C., Weibel E. Surface studies by scanning tunneling microscopy. Phys. Rev. Lett. 1982;49:57–61. [Google Scholar]
- Binnig G., Rohrer H., Gerber C., Weibel E. Tunneling through a controllable vacuum gap. Appl. Phys. Lett. 1982;40:178–180. [Google Scholar]
- Binning G., Quate C.F., Gerber C. Atomic force microscope. Phys. Rev. Lett. 1986;56:930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Brzoska M., Langer K., Coester C., Loitsch S., Wagner T.O.F., Mallinckrodt C.V. Incorporation of biodegradable nanoparticles into human airway epithelial cells—in vitro study of the suitability as a vehicle for drug or gene delivery in pulmonary diseases. Biochem. Biophys. Res. Commun. 2004;318:562–570. doi: 10.1016/j.bbrc.2004.04.067. [DOI] [PubMed] [Google Scholar]
- Bummer P.M. Physical chemical considerations of lipid-based oral drug delivery—solid lipid nanoparticles. Crit. Rev. Ther. Drug Carrier Syst. 2004;21:1–20. [PubMed] [Google Scholar]
- Calvo P., Gouritin B., Chacun H., Desmaele D., D’Angelo J., Noel J.P. Long-circulating PEGylated polycyanoacrylate nanoparticles as new drug carrier for brain delivery. Pharm. Res. 2001;18:1157–1166. doi: 10.1023/a:1010931127745. [DOI] [PubMed] [Google Scholar]
- Canham L.T. Bioactive silicon structure fabrication through nanoetching techniques. Adv. Mater. 1995;7:1033. [Google Scholar]
- Caruthers S.D., Wickline S.A., Lanza G.M. Nanotechnological applications in medicine. Curr. Opin. Biotech. 2007;18:26–30. doi: 10.1016/j.copbio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Cheng M.M., Cuda G., Bunimovich Y.L., Gaspari M., Heath J.R., Hill H.D. Nanotechnologies for biomolecular detection and medical diagnostics. Curr. Opin. Chem. Biol. 2006;10:11–19. doi: 10.1016/j.cbpa.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Choi M.J., Maibach H.I. Elastic vesicles as topical/transdermal drug delivery systems. Int. J. Cosmet. Sci. 2005;27:211–221. doi: 10.1111/j.1467-2494.2005.00264.x. [DOI] [PubMed] [Google Scholar]
- Cloninger M. Biological application of dendrimers. Curr. Opin. Chem. Biol. 2002;6:742–748. doi: 10.1016/s1367-5931(02)00400-3. [DOI] [PubMed] [Google Scholar]
- Courrier H.M., Butz N., Vandamme T.F. Pulmonary drug delivery systems: recent developments and prospects. Crit. Rev. Ther. Drug Carrier Syst. 2002;19:425–498. doi: 10.1615/critrevtherdrugcarriersyst.v19.i45.40. [DOI] [PubMed] [Google Scholar]
- Crooke S.T. An overview of progress in antisense therapeutics. Antisense Nucl. Acid Drug Dev. 1998;8:115–122. doi: 10.1089/oli.1.1998.8.115. [DOI] [PubMed] [Google Scholar]
- Crystal R.G. Transfer of genes to humans-early lessons and obstacles to success. Science. 1995;270:404–410. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- Cullis A.G., Canham L.T., Williams G.M., Smith P.W., Dosser O.D. Correlation of the structural and optical-properties of luminescent, highly oxidized porous silicon. J. Appl. Phys. 1994;75(1):493–501. [Google Scholar]
- Daemen T., Hofstede G., Ten Kate M.T., Bakker-Woudenberg I.A., Scherphof G.L. Liposomal doxorubicin-induced toxicity: depletion and impairment of phagocytic activity of liver macrophages. Int. J. Cancer. 1995;61(5):716–721. doi: 10.1002/ijc.2910610520. [DOI] [PubMed] [Google Scholar]
- D’Aquino R. Good drug therapy: it's not just the molecule—it's the delivery. CEP Magazine. 2004;100:15S–17S. [Google Scholar]
- Dass C.R., Su T. Particle-mediated intravascular delivery of oligonucleotides to tumors: associated biology and lessons from genotherapy. Drug Deliv. 2001;8:191–213. doi: 10.1080/107175401317245886. [DOI] [PubMed] [Google Scholar]
- Davis H.L. Plasmid DNA expression systems for the purpose of immunization. Curr. Opin. Biotech. 1997;8:635–646. doi: 10.1016/s0958-1669(97)80041-9. [DOI] [PubMed] [Google Scholar]
- Davis S.S. Biomedical applications of nanotechnology-implications for drug targeting and gene therapy. Trends Biotechnol. 1997;15:217–224. doi: 10.1016/S0167-7799(97)01036-6. [DOI] [PubMed] [Google Scholar]
- de Kozak Y., Andrieux K., Villarroya H., Klein C., Thillaye-Goldenberg B., Naud M.C. Intraocular injection of tamoxifen-loaded nanoparticles: a new treatment of experimental autoimmune uveoretinitis. Eur. J. Immunol. 2004;34:3702–3712. doi: 10.1002/eji.200425022. [DOI] [PubMed] [Google Scholar]
- de Lima M.C.P., Simoes S., Pires P., Faneca H., Duzgunes N. Cationic lipid–DNA complexes in gene delivery: from biophysics to biological applications. Adv. Drug Del. Rev. 2001;47:277–294. doi: 10.1016/s0169-409x(01)00110-7. [DOI] [PubMed] [Google Scholar]
- Desai M.P. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm. Res. 1996;13:1838–1845. doi: 10.1023/a:1016085108889. [DOI] [PubMed] [Google Scholar]
- Dubin C.H. Special delivery: pharmaceutical companies aim to target their drugs with nano precision. Mech. Eng. Nanotechnol. 2004;126(Suppl):10–12. [Google Scholar]
- Duncan R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- Eastman S.J., Scheule R.K. Cationic lipid: pDNA complexes for the treatment of cystic fibrosis. Curr. Opin. Mol. Ther. 1999;1:186–196. [PubMed] [Google Scholar]
- El-Aneed A. An overview of current delivery systems in cancer gene therapy. J. Control. Release. 2004;94:1–14. doi: 10.1016/j.jconrel.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Elias P.M., Tsai J., Menon G.K., Hollern W.M., Feingold K.R. The potential of metabolic interventions to enhance transdermal drug delivery. J. Invest. Dermatol. Sym. Proc. 2002;7:79–85. doi: 10.1046/j.1523-1747.2002.19632.x. [DOI] [PubMed] [Google Scholar]
- Elsayed M.M.A., Abdallah O.Y., Naggar V.F., Khalafallah N.M. Deformable liposomes and ethosomes: Mechanism of enhanced skin delivery. Int. J. Pharm. 2006;322:60–66. doi: 10.1016/j.ijpharm.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Emerich D.F. Nanomedicine—prospective therapeutic and diagnostic applications. Expert Opin. Biol. Ther. 2005;5:1–5. doi: 10.1517/14712598.5.1.1. [DOI] [PubMed] [Google Scholar]
- Feng L., Andrade J.D., Hu C.Z. Scanning tunneling microscopy of proteins on graphite surfaces. Scann. Microsc. 1989;3:399–410. [Google Scholar]
- Feng S.S., Mu L., Win K.Y. Nanoparticles of biodegradable polymers for clinical administration of paclitaxel. Curr. Med. Chem. 2004;11:413–424. doi: 10.2174/0929867043455909. [DOI] [PubMed] [Google Scholar]
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- Frechet J., Tomalia D., editors. Dendrimers and other Dendritic Polymers. J. Wiley & Sons; Chisester: 2001. [Google Scholar]
- Freitas S., Merkle H.P., Gander B. Microencapsulation by solvent extraction/evaporation: reviewing the state of the art of microsphere preparation process technology. J. Control. Release. 2005;102:313–332. doi: 10.1016/j.jconrel.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia E., Gil S., Andrieux K. A relevant in vitro rat model for the evaluation of blood-brain barrier translocation of nanoparticles. Cell. Mol. Life Sci. 2005;62:1400–1408. doi: 10.1007/s00018-005-5094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardikis K., Hatziantoniou S., Viras K., Wagner M., Demetzos C. Interactions of dendrimers with model lipid membranes assessed by DSC and Raman spectroscopy. In: Mozafari M.R., editor. Nanocarrier Technologies: Frontiers of Nanotherapy. Springer; The Netherlands: 2006. pp. 207–220. [Google Scholar]
- Goren D., Gabizon A., Barenholz Y. Correlation between physical characteristics and pharmacological behavior of doxorubicin-containing liposomes. Biochim. Biophys. Acta. 1990;1029:285–294. doi: 10.1016/0005-2736(90)90165-k. [DOI] [PubMed] [Google Scholar]
- Gouin S. Micro-encapsulation: industrial appraisal of existing technologies and trends. Trends Food Sci. Technol. 2004;15:330–347. [Google Scholar]
- Goverdhana S., Puntel M., Xiong W., Zirger J.M., Barcia C., Curtin J.F. Regulatable gene expression systems for gene therapy applications: progress and future challenges. Mol. Ther. 2005;12:189–211. doi: 10.1016/j.ymthe.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume H., Baluk P., Morikawa S., McLean J.W., Thurston G., Roberge S., Jain R.K., McDonald D.M. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol. 2000;156:1363–1380. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs S.K., Monsky W.L., Yuan F., Roberts W.G., Griffith L., Torchilin V.P., Jain R.K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Nat. Acad. Sci. U.S.A. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes G.A. Nanostructure-mediated drug delivery. Nanomed. Nanotech. Biol. Med. 2005;1:22–30. doi: 10.1016/j.nano.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Hussain N., Jaitley V., Florence A.T. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv. Drug Deliv. Rev. 2001;50:107–142. doi: 10.1016/s0169-409x(01)00152-1. [DOI] [PubMed] [Google Scholar]
- Igarashi S., Hattori Y., Maitani Y. Biosurfactant MEL-A enhances cellular association and gene transfection by cationic liposome. J. Control. Release. 2006;112:362–368. doi: 10.1016/j.jconrel.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Kahveci G., Mozafari M.R., Rouhvand F., Altay G., Zhdanov R.I. Importance of nucleic acid-lipid interactions in functioning of the initial cell; Istanbul; 1994. p. C-338. [Google Scholar]
- Kattan J., Droz J.P., Couvreur P. Phase I clinical trial and pharmacokinetic evaluation of doxorubicin carried by polyisohexylcyanoacrylate nanoparticles. Invest. New Drugs. 1992;10:191–199. doi: 10.1007/BF00877245. [DOI] [PubMed] [Google Scholar]
- Keller B.C. Liposomes in nutrition. Trends Food Sci. Technol. 2001;12:25–31. [Google Scholar]
- Keller B.C., Faulkner G., Lasic D.D. Liposomes in breastmilk. Agro. Food Ind. HiTech. 2000;11:6–8. [Google Scholar]
- Kim I.H., Park J.H., Cheong I.W., Kim J.H. Swelling and drug release behavior of tablets coated with aqueous hydroxypropyl methylcellulose phthalate (HPMCP) nanoparticles. J. Control. Release. 2003;89:225–233. doi: 10.1016/s0168-3659(03)00089-0. [DOI] [PubMed] [Google Scholar]
- Kohli A.K., Alpar H.O. Potential use of nanoparticles for transcutaneous vaccine delivery: effect of particle size and charge. Int. J. Pharm. 2004;275:13–17. doi: 10.1016/j.ijpharm.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Labhasetwar V. Nanotechnology for drug and gene therapy: the importance of understanding molecular mechanisms of delivery. Curr. Opin. Biotechnol. 2005;16:674–680. doi: 10.1016/j.copbio.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Landgraf W., Li N.H., Benson J.R. Polymer microcarrier exhibiting zero-order release. Drug Deliv. Technol. 2003;3(1):56–63. [Google Scholar]
- LaVan D.A., Lynn D.M., Langer R. Moving smaller in drug discovery and delivery. Nat. Rev. Drug Discov. 2002;1:77–84. doi: 10.1038/nrd707. [DOI] [PubMed] [Google Scholar]
- LaVan D.A., McGuire T., Langer R. Small-scale systems for in vivo drug delivery. Nat. Biotechnol. 2003;21:1184–1191. doi: 10.1038/nbt876. [DOI] [PubMed] [Google Scholar]
- Lawrence M.J., Rees G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2000;45:89–121. doi: 10.1016/s0169-409x(00)00103-4. [DOI] [PubMed] [Google Scholar]
- Li H., Wang D.Q., Liu B.L., Gao L.Z. Synthesis of a novel gelatincarbon nanotubes hybrid hydrogel. Colloids Surf. B Biointerfaces. 2004;33:85–88. [Google Scholar]
- Liang X., Mao G., Simon Ng K.Y. Probing small unilamellar Egg PC vesicles on mica surface by atomic force microscopy. Colloids Surf. 2004;34:41–51. doi: 10.1016/j.colsurfb.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Lin A.M.Y., Chyi B.Y., Wang S.D., Yu H.H., Kanakamma P.P., Lu T.Y. Carboxyfullerene prevents iron-induced oxidative stress in rat brain. J. Neurochem. 1999;72:1634–1640. doi: 10.1046/j.1471-4159.1999.721634.x. [DOI] [PubMed] [Google Scholar]
- Lopez O., Cocera M., Walther P., Wehrli E., Coderch L., Parra J.L., de la Maza A. Liposomes as protective agents of stratum corneum against octyl glucoside: a study based on high-resolution, low-temperature scanning electron microscopy. Micron. 2001;32:201–205. doi: 10.1016/s0968-4328(99)00146-8. [DOI] [PubMed] [Google Scholar]
- Lopez-Pinto J.M., Gonzalez-Rodriguez M.L., Rabasco A.M. Effect of cholesterol and ethanol on dermal delivery from DPPC liposomes. Int. J. Pharm. 2005;298:1–12. doi: 10.1016/j.ijpharm.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Maeda H., Wu J., Sawa T., Matsumura Y., Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- Maeda N., Senden T.J., di Meglio J.M. Micromanipulation of phospholipid bilayers by atomic force microscopy. Biochim. Biophys. Acta. 2002;1564:165–172. doi: 10.1016/s0005-2736(02)00443-1. [DOI] [PubMed] [Google Scholar]
- Mannino R.J., Gould-Fogerite S. Antigen cochleate formulations for oral and systemic vaccination in new generation vaccines. In: Levine M.M., editor. New Generation Vaccines. Marcel Dekker; New York, NY: 1997. pp. 1–9. [Google Scholar]
- Matsumura Y., Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- Mazzola L. Commercializing nanotechnology. Nat. Biotechnol. 2003;21:1137–1143. doi: 10.1038/nbt1003-1137. [DOI] [PubMed] [Google Scholar]
- McDonald D.M., Baluk P. Significance of blood vessel leakiness in cancer. Cancer Res. 2002;62(18):5381–5385. [PubMed] [Google Scholar]
- Minotti G., Menna P., Salvatorelli E., Cairo G., Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- Moghimi S.M., Szebeni J. Stealth liposomes and long circulation nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog. Lipid Res. 2003;42:463–478. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- Moghimi S.M., Hunter A.C., Murray J.C. Nanomedicine: current status and future prospects. FASEB J. 2005;19:311–330. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- Mortazavi S.M., Mohammadabadi M.R., Khosravi-Darani K., Mozafari M.R. Preparation of liposomal gene therapy vectors by a scalable method without using volatile solvents or detergents. J. Biotechnol. 2007;129:604–613. doi: 10.1016/j.jbiotec.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Mozafari M.R. Liposomes: an overview of manufacturing techniques. Cell Mol. Biol. Lett. 2005;10:711–719. [PubMed] [Google Scholar]
- Mozafari, M.R., 1996. Liposome-derived model DNA delivery system. M.Sc. Thesis. Middle East Technical University, Ankara.
- Mozafari M.R. Bioactive entrapment and targeting using nanocarrier technologies: an introduction. In: Mozafari M.R., editor. Nanocarrier Technologies: Frontiers of Nanotherapy. Springer; The Netherlands: 2006. pp. 1–16. [Google Scholar]
- Mozafari M.R., Hasirci V. Mechanism of calcium ion induced multilamellar vesicle-DNA interaction. J. Microencapsul. 1998;15(1):55–65. doi: 10.3109/02652049809006835. [DOI] [PubMed] [Google Scholar]
- Mozafari M.R., Mortazavi S.M., editors. Nanoliposomes: From Fundamentals to Recent Developments. Trafford Publishing Ltd.; Oxford, UK: 2005. [Google Scholar]
- Mozafari M.R., Zareie M.H., Piskin E., Hasirci V. Formation of supramolecular structures by negatively charged liposomes in the presence of nucleic acids and divalent cations. Drug Deliv. 1998;5:135–141. doi: 10.3109/10717549809031389. [DOI] [PubMed] [Google Scholar]
- Mozafari M.R., Reed C.J., Rostron C., Kocum C., Piskin E. Construction of stable anionic liposome-plasmid particles using the heating method: a preliminary investigation. Cell Mol. Biol. Lett. 2002;7:923–927. [PubMed] [Google Scholar]
- Mozafari M.R., Reed C.J., Rostron C., Kocum C., Piskin E. Formation and characterisation of non-toxic anionic liposomes for delivery of therapeutic agents to the pulmonary airways. Cell Mol. Biol. Lett. 2002;7:243–244. [PubMed] [Google Scholar]
- Mozafari M.R., Reed C.J., Rostron C., Hasirci V. Construction of a gene delivery vector using anionic liposomes. In: Mozafari M.R., Mortazavi S.M., editors. Nanoliposomes: From Fundamentals to Recent Developments. Trafford Publishing Ltd.; Oxford, UK: 2005. pp. 101–111. [Google Scholar]
- Mozafari M.R., Reed C.J., Rostron C., Hasirci V. A review of scanning probe microscopy investigations of liposome–DNA complexes. J. Liposome Res. 2005;15(1–2):93–107. doi: 10.1081/lpr-64965. [DOI] [PubMed] [Google Scholar]
- Mozafari M.R., Flanagan J., Matia-Merino L., Awati A., Omri A., Suntres Z.E., Singh H. Recent trends in the lipid-based nanoencapsulation of antioxidants and their role in foods. J. Sci. Food Agric. 2006;86:2038–2045. [Google Scholar]
- Mozafari M.R., Reed C.J., Rostron C. Cytotoxicity evaluation of anionic nanoliposomes and nanolipoplexes prepared by the heating method without employing volatile solvents and detergents. Pharmazie. 2007;62:205–209. [PubMed] [Google Scholar]
- Nagayasu A., Uchiyama K., Kiwada H. The size of liposomes: a factor which affects their targeting efficiency to tumors and therapeutic activity of liposomal antitumor drugs. Adv. Drug Deliv. Rev. 1999;40:75–87. doi: 10.1016/s0169-409x(99)00041-1. [DOI] [PubMed] [Google Scholar]
- Omri A., Makabi-Panzu B., Agnew B.J., Sprott G.D., Patel G.B. Influence of coenzyme Q10 on tissue distribution of archaeosomes and pegylated archaeosomes administered to mice by oral and intravenous routes. J. Drug Target. 2000;7:383–392. doi: 10.3109/10611869909085521. [DOI] [PubMed] [Google Scholar]
- Omri A., Agnew B.J., Patel G.B. Short-term repeated-dose toxicity profile of archaeosomes administered to mice via intravenous and oral routes. Int. J. Toxicol. 2003;22:9–23. doi: 10.1080/10915810305080. [DOI] [PubMed] [Google Scholar]
- Orive G., Hernandez R.M., Rodriguez Gascon A., Dominguez-Gil A., Pedraz J.L. Drug delivery in biotechnology: present and future. Curr. Opin. Biotechnol. 2003;14:659–664. doi: 10.1016/j.copbio.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Pardridge W.M. Vector-mediated drug delivery to the brain. Adv. Drug Deliv. Rev. 1999;36:299–321. doi: 10.1016/s0169-409x(98)00087-8. [DOI] [PubMed] [Google Scholar]
- Park K. Nanotechnology: what it can do for drug delivery. J. Control. Release. 2007;120:1–3. doi: 10.1016/j.jconrel.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel G.B., Agnew B.J., Deschatelets L., Fleming L.P., Sprott G.D. In vitro assessment of archaeosome stability for developing oral delivery systems. Int. J. Pharm. 2000;194:39–49. doi: 10.1016/s0378-5173(99)00331-2. [DOI] [PubMed] [Google Scholar]
- Patel G.B., Chen W. Archaeosomes as drug and vaccine nanodelivery systems. In: Mozafari M.R., editor. Nanocarrier Technologies: Frontiers of Nanotherapy. Springer; The Netherlands: 2006. pp. 17–40. [Google Scholar]
- Prater C.B., Wilson M.R., Garnaes J., Massie J., Elings V.B., Hansma P.K. Atomic force microscopy of biological samples at low temperature. J. Vac. Sci. Technol. B. 1991;14:1399–1404. [Google Scholar]
- Ravi Kumar M.N. Nano and microparticles as controlled drug delivery devices. J. Pharm. Pharm. Sci. 2000;3:234–258. [PubMed] [Google Scholar]
- Ruozi B., Tosi G., Leo E., Vandelli M.A. Application of atomic force microscopy to characterize liposomes as drug and gene carriers. Talanta. 2007;73:12–22. doi: 10.1016/j.talanta.2007.03.031. [DOI] [PubMed] [Google Scholar]
- Sahoo S.K., Labhasetwar V. Nanotech. approaches to drug delivery and imaging. Drug Discov. Today. 2003;8:1112–1120. doi: 10.1016/s1359-6446(03)02903-9. [DOI] [PubMed] [Google Scholar]
- Sahoo S.K., Parveen S., Panda J.J. The present and future of nanotechnology in human health care. Nanomed. Nanotech. Biol. Med. 2007;3:20–31. doi: 10.1016/j.nano.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Saito R., Dresselhaus G., Dresselhaus M.S. Imperial College Press; London: 1998. Physical Properties of Carbon Nanotubes. [Google Scholar]
- Santos N.C., Castanho M.A.R.B. An overview of the biophysical applications of atomic force microscopy. Biophys. Chem. 2004;107:133–149. doi: 10.1016/j.bpc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Saupe A., Rades T. Solid lipid nanoparticles. In: Mozafari M.R., editor. Nanocarrier Technologies: Frontiers of Nanotherapy. Springer; The Netherlands: 2006. pp. 41–50. [Google Scholar]
- Schurer N.Y., Elias P.M. The biochemistry and function of stratum corneum lipids. Adv. Lipid Res. 1991;24:27–56. doi: 10.1016/b978-0-12-024924-4.50006-7. [DOI] [PubMed] [Google Scholar]
- Senior K. Nano-dumpling with drug delivery potential. Mol. Med. Today. 1998;4:321. doi: 10.1016/s1357-4310(98)01308-2. [DOI] [PubMed] [Google Scholar]
- Shaffer C. Nanomedicine transforms drug delivery. Drug Discov. Today. 2005;10:1581–1582. doi: 10.1016/S1359-6446(05)03654-8. [DOI] [PubMed] [Google Scholar]
- Shao Z., Shi D., Somlyo A.V. Cryoatomic force microscopy of filamentous actin. Biophys. J. 2000;78:950–958. doi: 10.1016/S0006-3495(00)76652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Kam N.W., Jessop T.C., Wender P.A., Dai H. Nanotube molecular transporters: internalization of carbon nanotube-protein conjugates into mammalian cells. J. Am. Chem. Soc. 2004;126:6850–6851. doi: 10.1021/ja0486059. [DOI] [PubMed] [Google Scholar]
- Stull R.A., Szoka F.C., Jr Antigene, ribozyme and aptamer nucleic acid drugs: progress and prospects. Pharm. Res. 1995;12:465–483. doi: 10.1023/a:1016281324761. [DOI] [PubMed] [Google Scholar]
- Suntres Z.E., Shek P.N. Alleviation of paraquat-induced lung injury by pretreatment with bifunctional liposomes containing α-tocopherol and glutathione. Biochem. Pharmacol. 1996;52:1515–1520. doi: 10.1016/s0006-2952(96)89626-2. [DOI] [PubMed] [Google Scholar]
- Tang Q., Xu Y., Wu D., Sun Y., Wang J., Xu J., Deng F. Studies on a new carrier of trimethylsilyl-modified mesoporous material for controlled drug delivery. J. Control. Release. 2006;114:41–46. doi: 10.1016/j.jconrel.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Tartaj P., del Puerto Morales M., Veintemillas-Verdaguer S., Gonzales-Cerreno T., Serena C.J. The preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D: Appl. Phys. 2003;36:R182–R197. [Google Scholar]
- Torchilin V.P. Targeted polymeric micelles for delivery of poorly soluble drugs. Cell. Mol. Life Sci. 2004;61:2549–2559. doi: 10.1007/s00018-004-4153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy S.K., Black H.B., Goldwasser E., Leiden J.M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat. Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- Tsao N., Luh T.Y., Chou C.K., Chang T.Y., Wu J.J., Liu C.C. In vitro action of carboxyfullerene. J. Antimicrob. Chemother. 2002;49:641–649. doi: 10.1093/jac/49.4.641. [DOI] [PubMed] [Google Scholar]
- Uchegbu I.F., Vyas S.P. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int. J. Pharm. 1998;172:33–70. [Google Scholar]
- Uchegbu I.F., Double J.A., Kelland L.R., Turton J.A., Florence A.T. The activity of doxorubicin niosomes against an ovarian-cancer cell-line and 3 in vivo mouse-tumor models. J. Drug Target. 1996;3:399–409. doi: 10.3109/10611869608996831. [DOI] [PubMed] [Google Scholar]
- Uchegbu I.F., McCarthy D., Schatzlein A., Florence A.T. Phase-transitions in aqueous dispersions of the hexadecyl diglycerol ether (c(16)g(2)) non-ionic surfactant, cholesterol and cholesteryl poly-24-oxyethylene ether-vesicles, tubules, discomes and micelles. STP Pharm. Sci. 1996;6:33–43. [Google Scholar]
- Ulrich K.E., Cannizzaro S.M., Langer R.S., Shakeshelf K.M. Polymeric systems for controlled drug release. Chem. Rev. 1999;99:3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- Ulrich H., Trujillo C.A., Nery A.A. DNA and RNA aptamers: from tools for basic research towards therapeutic applications. Comb. Chem. High Throughput Screen. 2006;9:619–632. doi: 10.2174/138620706778249695. [DOI] [PubMed] [Google Scholar]
- Uvarov V.Y., Ivanov Y.D., Romanov A.N., Gallyamov M.O., Kiselyova O.I., Yaminsky I.V. Scanning tunneling microscopy study of cytochrome P450 2B4 incorporated in proteoliposomes. Biochimie. 1996;78:780–784. doi: 10.1016/s0300-9084(97)82536-9. [DOI] [PubMed] [Google Scholar]
- Vaccari L., Canton D., Zaffaroni N., Villa R., Tormen M., di Fabrizio E. Porous silicon as drug carrier for controlled delivery of doxorubicin anticancer agent. Microelectron. Eng. 2006;83:1598–1601. [Google Scholar]
- Vasir J.K., Labhasetwar V. Targeted drug delivery in cancer therapy. Technol. Cancer Res. Treat. 2005;4:363–374. doi: 10.1177/153303460500400405. [DOI] [PubMed] [Google Scholar]
- Vasir J.K., Reddy M.K., Labhasetwar V. Nanosystems in drug targeting: opportunities and challenges. Curr. Nanosci. 2005;1:47–64. [Google Scholar]
- Vinay D.S., Raje M., Mishra G.C. Characterization of a novel co-stimulatory molecule: a 155–160 kD B cell surface protein provides accessory help to CD4+ T cells to proliferate and differentiate. Mol. Immun. 1996;33(1):1–14. doi: 10.1016/0161-5890(95)00133-6. [DOI] [PubMed] [Google Scholar]
- Visser C.C., Stevanovic S., Voorwinden L.H., Bloois L.V., Gaillard P.J., Danhof M., Crommelin D.J.A., Boer A.G. Targeting liposomes with protein drugs to the blood–brain barrier in vitro. Eur. J. Pharm. Sci. 2005;25:299–305. doi: 10.1016/j.ejps.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Wagner V., Dullaart A., Bock A.K., Zweck A. The emerging nanomedicine landscape. Nat. Biotechnol. 2006;24:1211–1217. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- Wilkinson J.M. Nanotechnology applications in medicine. Med. Device Technol. 2003;14:29–31. [PubMed] [Google Scholar]
- Williams D. Nanotechnology: a new look. Med. Device Technol. 2004;15:9–10. [PubMed] [Google Scholar]
- Williams A.C., Barry B.W. Penetration enhancers. Adv. Drug Del. Rev. 2004;56:603–618. doi: 10.1016/j.addr.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Wozniak M.J., Wozniak P., Bystrzejewski M., Cudzilo S., Huczko A., Jelen P., Kaszuwara W., Kozubowski J.A., Lange H., Leonowicz M., Lewandowska-Szumiel M. Magnetic nanoparticles of Fe and Nd-Fe-B alloy encapsulated in carbon shells for drug delivery systems: study of the structure and interaction with the living cells. J. Alloys Compounds. 2006;423:87–91. [Google Scholar]
- Xue J.M., Shi M. PLGA/mesoporous silica hybrid structure for controlled drug release. J. Control. Release. 2004;98:209–217. doi: 10.1016/j.jconrel.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Yurdugul S., Mozafari M.R. Recent advances in micro- and nano-encapsulation of food ingredients. Cell. Mol. Biol. Lett. 2004;9(Suppl. 2):64–65. [Google Scholar]
- Zanella I., Fagan S.B., Motac R., Fazzio A. Ab initio study of pristine and Si-doped capped carbon nanotubes interacting with nimesulide molecules. Chem. Phys. Lett. 2007;439:348–353. [Google Scholar]
- Zareie M.H., Mozafari M.R., Hasirci V., Piskin E. Scanning tunnelling microscopy investigation of liposome–DNA–Ca2+ complexes. J. Liposome Res. 1997;7:491–502. [Google Scholar]
- Zarif L., Graybill J.R., Perlin D., Mannino R.J. Cochleates: new lipid-based drug delivery system. J. Liposome Res. 2000;10:523–538. [Google Scholar]
- Zarif L., Mannino R.J. Cochleates: lipid-based vehicles for gene delivery — concept, achievements and future development. In: Habib N., editor. Cancer Gene Therapy: Past Achievements and Future Challenges. Plenum; London: 2000. pp. 83–94. [DOI] [PubMed] [Google Scholar]
- Zarif L. Nanocochleate cylinders for oral and parenteral delivery of drugs. J. Liposome Res. 2003;13:109–110. [Google Scholar]
- Zhang Y., Schlachetzki F., Li J.Y., Boado R.J., Pardridge W.M. Organ-specific gene expression in the rhesus monkey eye following intravenous nonviral gene transfer. Mol. Vis. 2003;9:465–472. [PubMed] [Google Scholar]
- Zhdanov R.I., Volkova L.A., Artemova L.G., Rodin V.V. Lipid spin labeling and n.m.r. study of the interaction between polyadenylic acid:polyuridilic acid duplex and egg phosphatidylcholine vesicles. Evidence for involvement of surface groups of bilayer, phosphoryl groups and bivalent metal cations. Appl. Magn. Res. 1994;7:131–145. [Google Scholar]


