Graphical abstract
Keywords: Emodin, Antiviral activity, HSV infection, Rhubarb
Abstract
Aim of the study
Herpes simplex viruses (HSV-1 and -2) are important pathogens for humans and the discovery of novel anti-HSV drugs with low toxicity deserves great efforts. Rhubarb is one of the oldest and best-known traditional Chinese medicines. We initiated this study to test if emodin is the active ingredients from Rheum tanguticum (R. tanguticum, one of the Chinese Rhubarb) against HSV infection and to investigate its antiviral activity on HSV infection in tissue culture cells and in a mouse model.
Materials and methods
Emodin (3-methyl-1,6,8-trihydroxyanthraquinone) was extracted and purified from R. tanguticum (cultivated at high mountainous area in Qinghai) and the purity was determined by high performance liquid chromatography. The antiviral experiments of emodin against HSV infection were performed in vitro and in vivo. In vivo, the HSV-infected mice were orally administered with emodin beginning at 24 h post-HSV exposures with dosages of 3.3 g/kg/day, 6.7 g/kg/day, and 11.3 g/kg/day, respectively, for 7 days.
Results
Emodin was found to inhibit the replication of HSV-1 and HSV-2 in cell culture at the concentration of 50 μg/ml with antiviral index of 2.07 and 3.53, respectively. The emodin treatment increased the survival rate of HSV-infected mice, prolonged survival time and showed higher efficacy of HSV elimination from brain, heart, liver and ganglion, compared to the viral controls. In addition, the antiviral activity of emodin was found to be equivalent to that of acyclovir in vivo.
Conclusions
Our results indicate that emodin has the anti-HSV activity in vitro and in vivo and is thus a promising agent in the clinical therapy of HSV infection.
1. Introduction
Herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) are common human pathogens of the family of Herpesviridae, and cause infections worldwide with an estimated 60–95% of human adults infected at least by one of them (Brady and Bernstein, 2004). Either type can establish lesions at any site of the human body with indistinguishable diseases (Patel et al., 2007). HSV is transmitted through direct contact of the infected secretions and presents the common biological features of herpesviruses, including latency and reactivation (Chakrabarty et al., 2004, Kleymann, 2005, Greco et al., 2007, Ramachandran and Kinchington, 2007). Clinical manifestations of HSV infection vary from asymptomatic infection to mucocutaneous lesions, life-threatening encephalitis, and fatal dissemination, depending on the portal of viral entry, host immune competence, and primary or secondary nature of the disease (Leflore et al., 2000, Nadelman and Newcomer, 2000, Brady and Bernstein, 2004)
To date, HSV infections are incurable and may persist during lifetime of the host, resulting in a series of psychosocial problems. Significant efforts have been made to develop vaccines and agents against HSV infections. However, no effective vaccine is currently available (Rajcani and Durmanova, 2006, Us, 2006, Ramachandran and Kinchington, 2007). Acyclovir (ACV) remained the reference treatment for more than thirty years after its discovery (Greco et al., 2007). Drug-resistant strains emerged due to the extensive clinical use of ACV and its analogues, such as valacyclovir, penciclovir and famciclovir. Although there are some novel treatments available, the uncertainty of their efficacies and the high costs limited their clinical use (Brady and Bernstein, 2004, Chakrabarty et al., 2004, Kleymann, 2005, Greco et al., 2007, Kovalchuk et al., 2007). Therefore, it remains a continuous need to discover antiviral agents against HSV with lower resistant rate, toxicity and cost.
Rhubarb is a group of plants that belong to the genus Rheum in the family Polygonaceae and is one of the oldest and best-known traditional Chinese medicines. Chinese Rhubarb includes Rheum tanguticum (R. tanguticum), Rheum palmatum, Rheum officinale, etc. The pharmaceutically relevant compounds in rhubarb are sennosides, anthraquinones, stilbenes, glucose gallates, naphthalenes, and catechins (Ye et al., 2007). Hsiang et al. (2001) observed that the extract of rhubarb prevented the process of HSV attachment and penetration. Wang et al. (2003) reported that the extract of rhubarb showed antiviral activity against HSV that was comparable to acyclovir in vivo. A number of anthraquinones and anthrones isolated from plants and lichens have been shown to exhibit virucidal activity and non-virucidal antiviral activity against enveloped viruses, including HSV (Meruelo et al., 1988, Schinazi et al., 1990, Tang et al., 1990, Andersen et al., 1991, Sydiskis et al., 1991, Barnard et al., 1992, Cohen et al., 1996, Hsiang et al., 2001, Semple et al., 2001). The anthraquinones in rhubarb are thus potentially the effective component against HSV. The anthraquinone derivatives in rhubarb include physcion, emodin, rhein, aloe-emodin, chrysophanol and their glucosides (Ye et al., 2007). Among these, emodin is the most abundant one (Huang et al., 2007). We initiated this study to test if emodin is the active ingredients from R. tanguticum against HSV infection and to investigate its antiviral activity HSV infection in tissue culture cells and in a mouse model.
2. Materials and methods
2.1. Cell culture, viruses, and animals
HEp-2 (human laryngeal carcinoma) cells were routinely grown in RPMI-1640 medium (HyClone) supplemented with 10% heat-inactivated fetal calf serum, 0.1% l-glutamine, 100 U/ml penicillin and 0.1 mg/ml streptomycin, at 37 °C in a humidified atmosphere containing 5% CO2. The test medium used for the cytotoxic assay and antiviral assays contained 2% of the appropriate serum. The overlay medium for the plaque assay consisted of RPMI-1640 medium plus 2% fetal calf serum, 2% agarose, and antibiotics as described above.
HSV-1 and HSV-2 used in this study were HSV-1F stain and HSV-2 333 stain. The viruses were propagated in HEp-2 cells. The virus titer was estimated from cytopathic effect (CPE) induced by viral infection and expressed as 50% tissue culture infectious doses/ml (TCID50/ml) by Reed–Muench method.
In vivo experiments were carried out with specific-pathogen-free BALB/c mice, 5–7 weeks old, obtained from Animal Center of Wuhan University. The virus titer for inoculation was expressed as median lethal dose (LD50/0.1 mL) and the titers of isolated virus were expressed as PFU/ml determined by plaque assay. All the animal research was conducted in accordance with the internationally accepted principles and guidelines for Care and Use of Laboratory Animals of Wuhan University.
2.2. Preparation and purification of emodin
R. tanguticum were cultivated and collected from at high mountainous area in Qinghai, China. The extract was produced and purified by Department of Plant Chemistry, Hubei College of Traditional Chinese Medicine, Hubei, China. Fifty grams of the dried powdered roots of R. tanguticum was extracted by 8-fold of 85% ethanol under reflux three times (1.5 h each time). Concrete was obtained from the ethanol solution and dissolved in 500 mL H2O overnight. The solution was mixed with 500 mL 80% ethanol: acetone (1:1, v/v, pH = 4.0) and followed by ultrasonication for 30 min. The final product was obtained after filtering and drying. The main component is emodin (3-methyl-1,6,8-trihydroxyanthraquinone). The quantity of emodin was observed and determined by high performance liquid chromatography (HPLC). The content of emodin monomer is 84% and has a little impurities (Hou et al., 2003). Acyclovir (ACV, 9-(2-hydroxyethoxymethyl)guanine) was synthesized by Keyi Pharmaceutical Co. Ltd., Hubei, China.
2.3. In vitro experiments
2.3.1. Cytotoxicity assay
HEp-2 cells were seeded at 3.5 × 104 cells per well in 96-well plates and grown at subconfluence. After removal of the growth medium, cells were incubated with various concentrations of emodin (5, 10, 100, 200, 300, 500, 1000, and 1500 μg/ml, dissolved in 200 μl test medium) for 72 h at 37 °C in a humidified atmosphere containing 5% CO2. The cytotoxicity of emodin was evaluated on the basis of the morphological changes of the cells under microscope.
2.3.2. Indirect immunofluorescence (IF) assay
The infected cells were mounted on 10-well slides, air-dried and fixed with ice-cold methanol at room temperature for 10 min. The slides were blocked with 5% BSA in PBS for 1 h at 37 °C followed by incubation with rabbit serum reactive against HSV for 1 h at 37 °C. The slides were then washed with PBS for three times and incubated with fluorescein isothiocyanate (FITC)-conjugated goat immunoglobulin against rabbit IgG (Santa Cruz, 1:6000) for 1 h at 37 °C. Results were observed under fluorescence microscope.
2.3.3. Drug treatment before virus infection
HEp-2 cells were preincubated with test mediums containing different concentrations of emodin (100, 200, 300 μg/ml) at 37 °C in a humidified atmosphere containing 5% CO2 for 8 h and 12 h, respectively. The cells were then washed with PBS for twice and challenged with 100 TCID50/ml of HSV-1 and HSV-2, respectively. After 1 h incubation for virus adsorption, the cells were rinsed twice with PBS and further incubated with test medium for about 72 h until typical CPE was visible. The inhibition of virus-induced CPE was scored by observation under microscopy and the virus titration was measured by indirect IF assay. Four untreated virus controls and four uninfected cell controls were included in all assays. The antiviral index was calculated using the following formula: antiviral index = viral titersdrug therapy group /viral titersviral control group. All data presented are results of experiments performed in triplicate.
2.3.4. Virucidal assay
Viral suspensions containing 100 TCID50/ml of virus were incubated with an equal volume of medium containing different concentrations of emodin (5, 10, 20, 50, 100, 200, 300 μg/ml) at 37 °C in a humidified atmosphere containing 5% CO2 for 6 h, 12 h, and 24 h, respectively. One hundred microliters of the mixed suspensions were then added to subconfluent monolayers of HEp-2 cells. After 1 h incubation for virus adsorption, the mixed suspensions were removed; the cell monolayers were rinsed carefully with PBS and maintained in test medium at 37 °C in a humidified atmosphere containing 5% CO2 for 72 h. The virucidal effect was determined using indirect IF assay following the procedures described above.
2.3.5. Drug treatment after virus infection
The experiment was carried out as stated above with the following difference: monolayers of subconfluent HEp-2 cells were challenged with 100 TCID50/ml HSV-1 and -2 for 1 h. The cells were washed with PBS and overlaid with 200 μl test medium containing different concentrations of emodin (5, 15, 25, 50, 100, 200, 300 μg/ml). The antiviral effect was determined using indirect IF assay following the procedures described above.
2.4. In vivo experiments
BALB/c mice were infected intracerebrally with 20 μl viral suspension containing 100 LD50/0.1 ml of HSV-1 or -2. The mice infected with virus were randomly divided into 6 groups; emodin at a dose of 3.3, 6.7, 11.3 g/kg/day, and acyclovir at a dose of 0.1 g/kg/day were orally administered three times daily to the mice (at 8 h intervals) for 7 days beginning 24 h post-virus exposure. The placebo controls received 0.9% sodium chloride; the virus controls and the normal controls without viral infection received no treatment. The mice were observed daily for 40 days after infection. Blood and organs were collected for virus isolation and detection in each group on 2, 4, 6, 8, 10, 12, 16, 20, 30, and 40 days after infection (three mice per group). The tissue samples were homogenized to ∼10% (w/v) suspensions in test medium and the sera were isolated from the blood samples by centrifugation. The homogenates and sera were inoculated into the monolayer HEp-2 cells in 24-well plates in quadruplicate at 37 °C in a humidified atmosphere containing 5% CO2. The virus antigen was detected by indirect IF assay after CPE were observed. The titers of isolated virus were determined by plaque assay. Serial dilution (1:10) of viral supernatant mixture from positive wells was inoculated into the monolayer HEp-2 cells in 24-well plates in quadruplicate. Excess virus medium was removed after 2 h incubation and replaced with overlay medium containing 2% agarose. The agarose overlay was removed after incubation for 72 h. The cells were fixed by 10% formalin and stained with 0.5% crystal violet. Plaques were counted and viral titers were determined.
2.5. Statistical analysis
The data were analyzed by SPSS 13.0 software. The data of in vitro experiments was analyzed using Student's t-test, and that of in vivo experiments was analyzed using Wilcoxon test for survival rates, analysis of variance (ANOVA) for mean time to death (MTD) and Kaplan–Meier method for survival analysis.
3. Results
3.1. In vitro experiment
3.1.1. Cytotoxicities of emodin on HEp-2 cells
The cytotoxicites of emodin for HEp-2 cells were evaluated. Subconfluent monolayers treated with emodin at concentrations under 1000 μg/ml did not show any visible changes in cell morphology or cell density. Cells treated with emodin at concentrations of 1000 μg/ml and 1500 μg/ml grew tardily, and showed alterations in cell morphology.
3.1.2. Drug treatment before infection
Cells were treated with emodin and subsequently infected with HSV-1 or HSV-2 as described in Section 2. Emodin at concentrations of 100, 200, and 300 μg/ml did not show any inhibitory activity against HSV-1 or HSV-2. The titers were between 5.8 ± 0.24–6.1 ± 0.18, and the antiviral indexes were 0.92 ± 0.1–1.02 ± 0.12. There was no significant difference between these groups (p > 0.05). These indicated that emodin was not able to block viral adsorption to HEp-2 cells.
3.1.3. Virucidal activity
To investigate the direct inactivating effect of emodin, viruses were treated for 6 h, 12 h, and 24 h with concentrations of emodin ranging from 5 to 300 μg/ml, respectively. No virucidal effect of emodin was observed. The mixed suspension could still infect cells and propagate. The infective titer of the suspension was not influenced by either interaction time or doses of emodin. The titers were over 5.7 and the antiviral indexes were under 1.03 in emodin-treated groups. There was no significant difference between emodin-treated groups and the untreated virus control group (p > 0.05). These indicated that emodin was not able to directly inactivate viruses and it showed no virucidal effect against HSV-1 and HSV-2.
3.1.4. Drug treatment after infection
Subconfluent cells were infected with virus and then incubated with the drugs as described in Section 2. As shown in Table 1 , the effective dose of emodin on HSV-1 started from 50 μg/ml, and 25 μg/ml on HSV-2. The virus titers at these dosages decreased 2-fold compared with the virus control group (p < 0.01). The antiviral effect enhanced correspondingly with the increase of the dosage. The antiviral index of emodin on HSV-2 was 3.4 times as that on HSV-1 when the concentration reached 300 μg/ml. The antiviral activity of emodin against HSV-2 was stronger than that against HSV-1 at the same dosages according to the antiviral index. The antiviral effects of emodin and ACV are equivalent at concentrations of 5, 15, 25, and 50 μg/ml regarding to the antiviral index (p > 0.05).
Table 1.
The antiviral effect of emodin and ACV on the replication of HSV-1 and HSV-2.
| Compound | Dosage (μg/ml) | Viral titera (log10TCID50)/antiviral index |
|
|---|---|---|---|
| HSV-1 | HSV-2 | ||
| Emodin | 5 | 5.8 ± 0.24 (1.03) | 6.0 ± 0.27 (1.00) |
| 15 | 5.4 ± 0.25 (1.11) | 4.4 ± 0.21 (1.50) | |
| 25 | 4.1 ± 0.18 (1.46) | 3.1 ± 0.17 (1.94) | |
| 50 | 2.9 ± 0.21 (2.07) | 1.7 ± 0.24 (3.53) | |
| 100 | 1.9 ± 0.21 (3.21) | 1.1 ± 0.14 (5.35) | |
| 200 | 1.1 ± 0.09 (5.55) | 0.6 ± 0.11 (10.0) | |
| 300 | 0.7 ± 0.11 (8.71) | 0.2 ± 0.14 (30.0) | |
| Acyclovir | 5 | 5.7 ± 0.25 (1.05) | 5.8 ± 0.21 (1.03) |
| 15 | 5.6 ± 0.21 (1.07) | 5.6 ± 0.24 (1.07) | |
| 25 | 4.5 ± 0.20 (1.20) | 3.6 ± 0.21 (1.67) | |
| 50 | 2.5 ± 0.22 (2.40) | 2.1 ± 0.23 (2.86) | |
| Control | – | 6.1 ± 0.31 | 6.0 ± 0.34 |
Viral titer represent geometric mean ± SD of three independent experiments.
3.2. In vivo experiment
3.2.1. Characteristics of HSV infection on mice
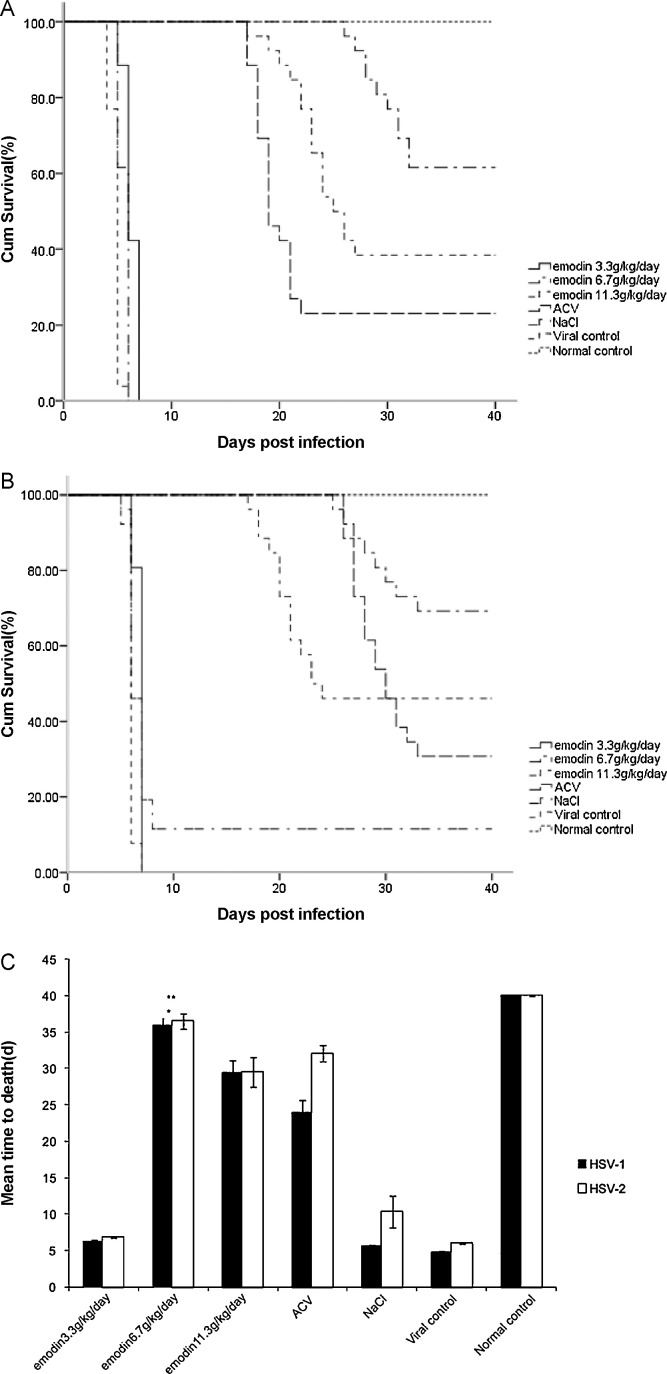
The signs of viral infection were observed in the mice at Day 4 after inoculation, such as weight-loss, tendencies to huddle, ruffled fur, paralysis of limbs, spasm and diminished vitality. The clinical manifestations showed no difference between the mice infected with HSV-1 and HSV-2. However, HSV-2 infected mice showed 1 day delay at the onset of the disease compared to HSV-1 infected mice. As shown in Fig. 1 , mice in virus control group (n = 20) and mice in placebo control group (n = 26) died within 40 days because of the disease. Survival rate increased and MTD prolonged in the treatment groups of the infected mice. The 6.7 g/kg/day emodin groups showed better survival rate (16/26, 61.5%) and prolonged MTD (29.4 ± 2.1 days) compared to those in 3.3 g/kg/day (0/26 (0%), 6.3 ± 0.7 days) and 11.3 g/kg/day (10/26 (38.5%), 22.9 ± 2.7 days) groups (p < 0.01). Toxicities appeared when the dosage reached 11.3 g/kg/day. The 6.7 g/kg/day emodin group showed favorable survival rates in both HSV subtype infected mice and longer MTD in HSV-1 infected mice (4.8 ± 0.3 days) compared to ACV treatment (19.0 ± 1.5) group (p< 0.01).
Fig. 1.
Effects of orally adminstered emodin on lethal HSV challenge model. Emodin at 3.3, 6.7 and 11.3 g/kg/day was orally administered 24 h post viral infection with 100 LD50 of HSV. (A) Survival curves for BALB/c mice after HSV-1 infection with emodin treatment. (B) Survival curves for BALB/c mice after HSV-2 infection with emodin treatment. (C) Mean time death (MTD) of BALB/c mice from different experimental groups. *The 6.7 g/kg/day emodin groups showed prolonged MTD compared to those in 3.3 g/kg/day and 11.3 g/kg/day groups (p < 0.01). **The 6.7 g/kg/day emodin group showed favorable survival rates in both HSV subtype infected mice and longer MTD in HSV-1 infected mice compared to ACV treatment group (p < 0.01).
3.2.2. Elimination of viral antigens in the HSV infected mice
The viral antigens in the organs of the infected mice (brain, heart, liver, and ganglion) were detected by indirect IF assay. Viral antigens were positive in all of the organs at the early stage of infection (within 1 week after infection). The viral antigens disappeared over different lengths of time corresponding to various dosages of the treatment medicines (Table 2 ).
Table 2.
The effect of emodin on viral antigen changes in mice organs.
| Virus type | Compound and dosage (g/kg/day) | The time of the viral antigen disappearing (days after treatment) |
|||
|---|---|---|---|---|---|
| Brain | Heart | Liver | Ganglion | ||
| HSV-1 | Emodin group | ||||
| 3.3 | NO | NO | NO | NO | |
| 6.7 | 16 | 16 | 10 | 20 | |
| 11.3 | 12 | 12 | 10 | 12 | |
| ACV group (0.1) | 12 | 16 | 10 | 20 | |
| 0.9% NaCl | NO | NO | NO | NO | |
| HSV-2 | Emodin group | ||||
| 3.3 | NO | NO | NO | NO | |
| 6.7 | 12 | 12 | 8 | 16 | |
| 11.3 | 12 | 16 | 8 | 20 | |
| ACV group (0.1) | 12 | 10 | 10 | 16 | |
| 0.9% NaCl | NO | NO | NO | NO | |
Note: NO means “not disappeared”.
3.2.3. Changes of virus titers in HSV infected mice
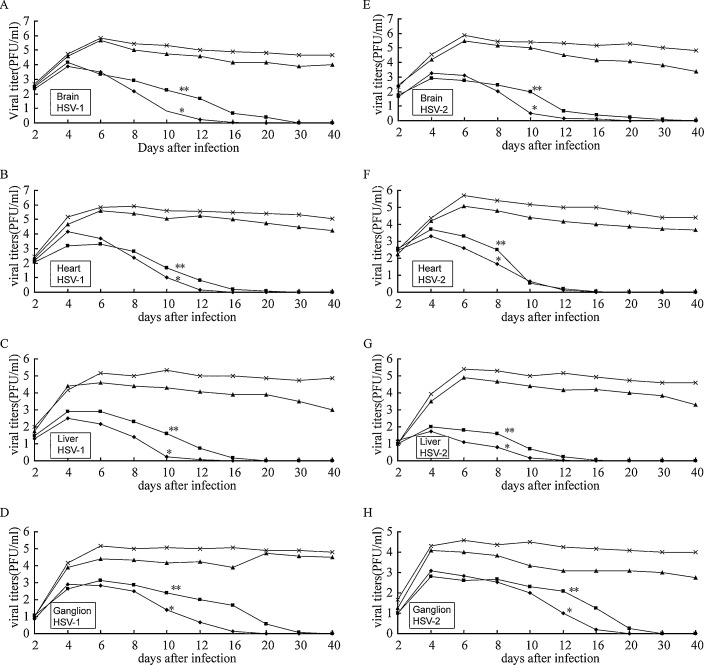
The changes of the virus titers in brain, heart, liver and ganglion of the HSV-1 and HSV-2 infected mice from different groups are shown in Fig. 2 . The administration of emodin (6.7 g/kg/day and 11.3 g/kg/day groups) significantly reduced the virus titers of mice organ homogenates compared to 0.9% NaCl control group (p < 0.05), and the time needed for virus elimination was dose-dependent. The virus titers in brains, hearts and livers of the emodin groups began to decrease at day 5 after treatment (day 6 after infection). The reduction of virus titers in the ganglion was observed a 2-day delay compared to other organs. The 6.7 g/kg/day and 11.3 g/kg/day emodin group showed faster virus elimination than 3.3 g/kg/day group (p < 0.05). These results suggest that emodin is effective for treatment of HSV infection.
Fig. 2.
Effects of emodin on viral titers change on HSV-infected mice. (A) Viral titer in brains from HSV-1 infected mice, (B) viral titer in hearts from HSV-1 infected mice, (C) viral titer in livers from HSV-1 infected mice, (D) viral titer in ganglions from HSV-1 infected mice, (E) viral titer in brains from HSV-2 infected mice, (F) viral titer in hearts from HSV-2 infected mice, (G) viral titer in livers from HSV-2 infected mice, (H) viral titer in ganglions from HSV-2 infected mice; mice were infected with HSV-1 and HSV-2 as described in Section 2. The mice were treated with different doses of emodin of 3.3 (▴), 6.7 (◆), or 11.3 g/kg/day (■) or with 0.9% NaCl as a control (×) for 7 days beginning 24 h after infection. Viral titers represent geometric mean ± SD per organ of the mice in each group. The *6.7 g/kg/day and **11.3 g/kg/day emodin group started to show significantly reduced virus titers of mice organ homogenates at day 6 after infection compared to 0.9% NaCl group (p < 0.05).
4. Discussion
In the current study, we demonstrated the antiviral activity of emodin, extracted from R. tanguticum, against HSV-1 and HSV-2 both in vitro and in vivo. In vitro, emodin could not prevent HEp-2 cells from HSV infection in the pre-treatment assay, nor could it show virucidal effect against HSV-1 and HSV-2. However, it presented antiviral activities after HSV infection. Our results revealed that the antiviral activity of emodin was more potent against HSV-2 than HSV-1. However, the decrease of CPE and viral titers in culture media was dose-dependent in both HSV types. These were equivalent to the antiviral activities of ACV against both HSV types. Our results imply that emodin may inhibit HSV biological synthesis rather than directly inactivating the viruses or blocking their absorption to the susceptible cells in vitro.
We employed an HSV-infected BALB/c mice mouse model which can be used to screen and evaluate the anti-HSV agents. The emodin-treated mice showed better survival rate, prolonged MTD, decreased viral titers and faster elimination of virus in tissues compared to the virus control group. The most effective dose was 6.7 g/kg/day which was equivalent to ACV at a dose of 0.1 g/kg/day. Mice died in 6.7 g/kg/day emodin groups after 20 days due to the remnant virus existing in the organs (brain and ganglion, other than heart and liver).
Hsiang and Ho (2008) found that emodin specifically inhibited HSV-1 UL 12 activity, which is a viral protein involved in viral DNA processing and capsid egression, and then exerted its antiviral activity by accumulating nucleocapsids in the nucleus and reducing the HSV-1 yields. This can explain why emodin decreased HSV replication in our experiment. Several other studies demonstrated that emodin inhibited casein kinase 2 (CK2), which involves in the phosphorylation of many viral proteins that are essential to their life cycle (Yim et al., 1999, Battistutta et al., 2000). The potent antiviral capacity of emodin could also be based on its ability to disrupt the lipid bilayer, resulting in the effective inactivation of envelope virus (Alves et al., 2004).
HSV-2 infection is currently the most common cause for genital ulcerative disease, which is one of the risk factors for human immunodeficiency virus (HIV) acquisition (Celum et al., 2004, Anuradha et al., 2008). Studies have shown that HIV-infected patients have a significant seroprevalence of HSV-2 (Chen et al., 2000, Mbopi-Keou et al., 2000, Mostad et al., 2000, Anuradha et al., 2008), and the latter alters the host immunity (Sheth et al., 2008) and facilitates HIV transmission and acquisition (Celum, 2004). Anti-HSV therapy should therefore be evaluated as a strategy for HIV prevention (Celum et al., 2005) and may benefit HIV-infected patients as well (Abu-Raddad et al., 2008, Mayaud et al., 2008). In our study, emodin showed potent anti-HSV-2 activity both in vitro and in vivo. It may serve as a promising agent in combating HSV-2 and simultaneously prevent the high risk population from acquiring HIV.
In summary, our results indicated that emodin was an effective antiviral agent against both HSV-1 and HSV-2 infection. It was observed to be more potent in inhibiting HSV-2 than HSV-1. Other studies have also demonstrated antiviral activities of emodin against some enveloped viruses, such as hepatitis B virus, human cytomegalovirus, and severe acute respiratory syndrome-corona virus; and non-enveloped viruses, such as poliovirus (Barnard et al., 1992, Cohen et al., 1996, Semple et al., 2001, Shuangsuo et al., 2006, Ho et al., 2007, Hsiang and Ho, 2008). Considering these findings, emodin may potentially play a significant role in anti-HSV management with broad spectrum of antiviral activities.
Acknowledgements
We thank Keli Chen, Department of Plant Chemistry, Hubei College of Traditional Chinese Medicine for assistance in emodin extraction. We would like to thank Dr. Ying Lin, Second Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China for editing assistance. This work was supported by the National Mega Project on Major Drug Development (2009ZX09301-014-1), National Natural Science Foundation of China (NSFC Project No. 30873104) and the Fundamental Research Funds for the Central Universities (4101045).
References
- Abu-Raddad L.J., Magaret A.S., Celum C., Wald A., Longini I.M., Jr., Self S.G., Corey L. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS One. 2008;3:e2230. doi: 10.1371/journal.pone.0002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves D.S., Perez-Fons L., Estepa A., Micol V. Membrane-related effects underlying the biological activity of the anthraquinones emodin and barbaloin. Biochemical Pharmacology. 2004;68:549–561. doi: 10.1016/j.bcp.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Andersen D.O., Weber N.D., Wood S.G., Hughes B.G., Murray B.K., North J.A. In vitro virucidal activity of selected anthraquinones and anthraquinone derivatives. Antiviral Research. 1991;16:185–196. doi: 10.1016/0166-3542(91)90024-l. [DOI] [PubMed] [Google Scholar]
- Anuradha K., Singh H.M., Gopal K.V., Rama Rao G.R., Ramani T.V., Padmaja J. Herpes simplex virus 2 infection: a risk factor for HIV infection in heterosexuals. Indian Journal of Dermatology, Venereology and Leprology. 2008;74:230–233. doi: 10.4103/0378-6323.41367. [DOI] [PubMed] [Google Scholar]
- Barnard D.L., Huffman J.H., Morris J.L., Wood S.G., Hughes B.G., Sidwell R.W. Evaluation of the antiviral activity of anthraquinones, anthrones and anthraquinone derivatives against human cytomegalovirus. Antiviral Research. 1992;17:63–77. doi: 10.1016/0166-3542(92)90091-i. [DOI] [PubMed] [Google Scholar]
- Battistutta R., Sarno S., De Moliner E., Papinutto E., Zanotti G., Pinna L.A. The replacement of ATP by the competitive inhibitor emodin induces conformational modifications in the catalytic site of protein kinase CK2. The Journal of Biological Chemistry. 2000;275:29618–29622. doi: 10.1074/jbc.M004257200. [DOI] [PubMed] [Google Scholar]
- Brady R.C., Bernstein D.I. Treatment of herpes simplex virus infections. Antiviral Research. 2004;61:73–81. doi: 10.1016/j.antiviral.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Celum C.L. The interaction between herpes simplex virus and human immunodeficiency virus. Herpes. 2004;11(Suppl. 1):36A–45A. [PubMed] [Google Scholar]
- Celum C., Levine R., Weaver M., Wald A. Genital herpes and human immunodeficiency virus: double trouble. Bulletin of the World Health Organization. 2004;82:447–453. [PMC free article] [PubMed] [Google Scholar]
- Celum C.L., Robinson N.J., Cohen M.S. Potential effect of HIV type 1 antiretroviral and herpes simplex virus type 2 antiviral therapy on transmission and acquisition of HIV type 1 infection. The Journal of Infectious Diseases. 2005;191(Suppl. 1):S107–S114. doi: 10.1086/425272. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A., Pang K.R., Wu J.J., Narvaez J., Rauser M., Huang D.B., Beutner K.R., Tyring S.K. Emerging therapies for herpes viral infections (types 1–8) Expert Opinion on Emerging Drugs. 2004;9:237–256. doi: 10.1517/14728214.9.2.237. [DOI] [PubMed] [Google Scholar]
- Chen C.Y., Ballard R.C., Beck-Sague C.M., Dangor Y., Radebe F., Schmid S., Weiss J.B., Tshabalala V., Fehler G., Htun Y., Morse S.A. Human immunodeficiency virus infection and genital ulcer disease in South Africa: the herpetic connection. Sexually Transmitted Diseases. 2000;27:21–29. doi: 10.1097/00007435-200001000-00005. [DOI] [PubMed] [Google Scholar]
- Cohen P.A., Hudson J.B., Towers G.H. Antiviral activities of anthraquinones, bianthrones and hypericin derivatives from lichens. Experientia. 1996;52:180–183. doi: 10.1007/BF01923366. [DOI] [PubMed] [Google Scholar]
- Greco A., Diaz J.J., Thouvenot D., Morfin F. Novel targets for the development of anti-herpes compounds. Infectious Disorders Drug Targets. 2007;7:11–18. doi: 10.2174/187152607780090766. [DOI] [PubMed] [Google Scholar]
- Ho T.Y., Wu S.L., Chen J.C., Li C.C., Hsiang C.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Research. 2007;74:92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W., Yang Z.Q., Chen K.L., Yang J.J., Wang W.H., Xiao H., Cheng L. Study of emodin against herpes simplex virus in vitro. Chinese Journal of Pharmaceutical Analysis. 2003;23:259–262. [Google Scholar]
- Hsiang C.Y., Ho T.Y. Emodin is a novel alkaline nuclease inhibitor that suppresses herpes simplex virus type 1 yields in cell cultures. British Journal of Pharmacology. 2008;155:227–235. doi: 10.1038/bjp.2008.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiang C.Y., Hsieh C.L., Wu S.L., Lai I.L., Ho T.Y. Inhibitory effect of anti-pyretic and anti-inflammatory herbs on herpes simplex virus replication. The American Journal of Chinese Medicine. 2001;29:459–467. doi: 10.1142/S0192415X01000472. [DOI] [PubMed] [Google Scholar]
- Huang Q., Lu G., Shen H.M., Chung M.C., Ong C.N. Anti-cancer properties of anthraquinones from rhubarb. Medicinal Research Reviews. 2007;27:609–630. doi: 10.1002/med.20094. [DOI] [PubMed] [Google Scholar]
- Kleymann G. Agents and strategies in development for improved management of herpes simplex virus infection and disease. Expert Opinion on Investigational Drugs. 2005;14:135–161. doi: 10.1517/13543784.14.2.135. [DOI] [PubMed] [Google Scholar]
- Kovalchuk L.V., Gankovskaya L.V., Gankovskaya O.A., Lavrov V.F. Herpes simplex virus: treatment with antimicrobial peptides. Advances in Experimental Medicine and Biology. 2007;601:369–376. doi: 10.1007/978-0-387-72005-0_39. [DOI] [PubMed] [Google Scholar]
- Leflore S., Anderson P.L., Fletcher C.V. A risk-benefit evaluation of acyclovir for the treatment and prophylaxis of herpes simplex virus infections. Drug Safety: An International Journal of Medical Toxicology and Drug Experience. 2000;23:131–142. doi: 10.2165/00002018-200023020-00004. [DOI] [PubMed] [Google Scholar]
- Mayaud P., Nagot N., Konate I., Ouedraogo A., Weiss H.A., Foulongne V., Defer M.C., Sawadogo A., Segondy M., Van de Perre P. Effect of HIV-1 and antiretroviral therapy on herpes simplex virus type 2: a prospective study in African women. Sexually Transmitted Infections. 2008;84:332–337. doi: 10.1136/sti.2008.030692. [DOI] [PubMed] [Google Scholar]
- Mbopi-Keou F.X., Gresenguet G., Mayaud P., Weiss H.A., Gopal R., Matta M., Paul J.L., Brown D.W., Hayes R.J., Mabey D.C., Belec L. Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: opportunities for intervention. The Journal of Infectious Diseases. 2000;182:1090–1096. doi: 10.1086/315836. [DOI] [PubMed] [Google Scholar]
- Meruelo D., Lavie G., Lavie D. Therapeutic agents with dramatic antiretroviral activity and little toxicity at effective doses: aromatic polycyclic diones hypericin and pseudohypericin. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5230–5234. doi: 10.1073/pnas.85.14.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostad S.B., Kreiss J.K., Ryncarz A.J., Mandaliya K., Chohan B., Ndinya-Achola J., Bwayo J.J., Corey L. Cervical shedding of herpes simplex virus in human immunodeficiency virus-infected women: effects of hormonal contraception, pregnancy, and vitamin A deficiency. The Journal of Infectious Diseases. 2000;181:58–63. doi: 10.1086/315188. [DOI] [PubMed] [Google Scholar]
- Nadelman C.M., Newcomer V.D. Herpes simplex virus infections. New treatment approaches make early diagnosis even more important. Postgraduate Medicine. 2000;107:189–195. doi: 10.3810/pgm.2000.03.948. 199–200. [DOI] [PubMed] [Google Scholar]
- Patel A.R., Romanelli P., Roberts B., Kirsner R.S. Treatment of herpes simplex virus infection: rationale for occlusion. Advances in Skin & Wound Care. 2007;20:408–412. doi: 10.1097/01.ASW.0000280199.58260.62. [DOI] [PubMed] [Google Scholar]
- Rajcani J., Durmanova V. Developments in herpes simplex virus vaccines: old problems and new challenges. Folia Microbiologica. 2006;51:67–85. doi: 10.1007/BF02932160. [DOI] [PubMed] [Google Scholar]
- Ramachandran S., Kinchington P.R. Potential prophylactic and therapeutic vaccines for HSV infections. Current Pharmaceutical Design. 2007;13:1965–1973. doi: 10.2174/138161207781039760. [DOI] [PubMed] [Google Scholar]
- Schinazi R.F., Chu C.K., Babu J.R., Oswald B.J., Saalmann V., Cannon D.L., Eriksson B.F., Nasr M. Anthraquinones as a new class of antiviral agents against human immunodeficiency virus. Antiviral Research. 1990;13:265–272. doi: 10.1016/0166-3542(90)90071-e. [DOI] [PubMed] [Google Scholar]
- Semple S.J., Pyke S.M., Reynolds G.D., Flower R.L. In vitro antiviral activity of the anthraquinone chrysophanic acid against poliovirus. Antiviral Research. 2001;49:169–178. doi: 10.1016/s0166-3542(01)00125-5. [DOI] [PubMed] [Google Scholar]
- Sheth P.M., Sunderji S., Shin L.Y., Rebbapragada A., Huibner S., Kimani J., Macdonald K.S., Ngugi E., Bwayo J.J., Moses S., Kovacs C., Loutfy M., Kaul R. Coinfection with herpes simplex virus type 2 is associated with reduced HIV-specific T cell responses and systemic immune activation. The Journal of Infectious Diseases. 2008;197:1394–1401. doi: 10.1086/587697. [DOI] [PubMed] [Google Scholar]
- Shuangsuo D., Zhengguo Z., Yunru C., Xin Z., Baofeng W., Lichao Y., Yan’an C. Inhibition of the replication of hepatitis B virus in vitro by emodin. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research. 2006;12:BR302–BR306. [PubMed] [Google Scholar]
- Sydiskis R.J., Owen D.G., Lohr J.L., Rosler K.H., Blomster R.N. Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrobial Agents and Chemotherapy. 1991;35:2463–2466. doi: 10.1128/aac.35.12.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Colacino J.M., Larsen S.H., Spitzer W. Virucidal activity of hypericin against enveloped and non-enveloped DNA and RNA viruses. Antiviral Research. 1990;13:313–325. doi: 10.1016/0166-3542(90)90015-y. [DOI] [PubMed] [Google Scholar]
- Us D. Herpes simplex virus vaccine studies: from past to present. Mikrobiyoloji Bulteni. 2006;40:413–433. [PubMed] [Google Scholar]
- Wang Z.Y., Xu B., Song Y.Y., Wang G.T., Xu H.Z., Wang X.F., Xue Y.L., Wang Z.Y., Yu X.P. Inhibition effects of rhubarb ethanol extract on herpes simplex virus infection in vivo. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2003;17:169–173. [PubMed] [Google Scholar]
- Ye M., Han J., Chen H., Zheng J., Guo D. Analysis of phenolic compounds in rhubarbs using liquid chromatography coupled with electrospray ionization mass spectrometry. Journal of the American Society for Mass Spectrometry. 2007;18:82–91. doi: 10.1016/j.jasms.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Yim H., Lee Y.H., Lee C.H., Lee S.K. Emodin, an anthraquinone derivative isolated from the rhizomes of Rheum palmatum, selectively inhibits the activity of casein kinase II as a competitive inhibitor. Planta Medica. 1999;65:9–13. doi: 10.1055/s-1999-13953. [DOI] [PubMed] [Google Scholar]