Abstract
Infections are a major cause of morbidity and mortality after lung transplantation. Pretransplant assessments for infection risk and immunization updates may help reduce posttransplant infections. In addition careful choice of posttransplant prophylaxis for cytomegalovirus and fungal infections is critical. Because of the potential association of infections such as respiratory viral infections and gram-negative bacterial infections with bronchiolitis obliterans syndrome, prompt attention to these pathogens is critical. Choice of antimicrobials for prophylaxis and treatment should take into consideration both adverse effects and drug interactions associated with antimicrobial choice.
Keywords: Lung transplant, Infection, Respiratory virus, Mycobacteria, Fungal infection, Gram-negative bacterial infection, Prophylaxis
Despite more than a quarter century of experience in lung transplantation, marked by advances in surgical techniques, immunosuppressive medications and prophylaxis strategies, survival among lung transplant recipients lags behind most other solid-organ transplant (SOT) recipients.1 In part, this situation may be attributed to infection-related complications, which continue to be a major source of morbidity and mortality in these patients.2, 3, 4 The lung allograft is unique in that it remains in constant exposure to the environment and potential respiratory pathogens. The nature of the transplant procedure adversely affects normal host defenses, including impairment of cough mechanics and mucociliary clearance. Additional contributors include the risk of cross-contamination of the transplanted lung by the native lung in single-lung-transplant patients, and the reduction of immune system function because of the high immunosuppression requirements in lung transplantation. By implementing a careful strategy of pretransplant screening of recipients and donors, pretransplantation and posttransplantation vaccination, antimicrobial prophylaxis, and microbial surveillance techniques, the incidence and severity of infectious complications after transplantation can be minimized. This review is an overview of some of the more common but increasingly challenging infections after lung transplantation, and provides guidance on current prevention and treatment strategies.
Recipient pretransplant screening and prevention
The prevention of infectious complications after SOT is paramount, and careful pretransplant evaluation for risks from infectious diseases should be conducted in all potential recipients. Comprehensive recommendations for the evaluation of SOT candidates and all other aspects of transplant-related infectious diseases have been recently summarized in guidelines from the American Society of Transplantation (AST) Infectious Diseases Community of Practice.5 Consultation with a specialist in infectious diseases in transplant should always be considered as part of the pretransplantation evaluation. A careful medical, social, and travel history should be performed to ascertain any potential exposures to problem pathogens (such as endemic mycoses or parasitic infections) so that appropriate risk stratification can occur, including consideration of specific posttransplantation prophylaxis. Vaccine histories should be reviewed, with careful attention paid to completion of childhood vaccination series (ie, measles/mumps/rubella), history of infection with pathogens such as varicella (chicken pox), and maintenance of adult vaccination schedules.6 In many cases, the patient’s primary disease process may render him or her functionally immunosuppressed, so initiation of missed or delayed vaccine series should begin as early in the transplant evaluation as possible, especially for live attenuated vaccines such as varicella vaccine (Varivax Merck, West Point, PA, USA), which are currently contraindicated after SOT. If vaccination must be delayed until after transplant, most centers defer administration until 3 to 6 months after transplantation or until baseline immunosuppression levels are achieved. The vaccine status of household contacts and family members should also be assessed for the optimal protection of the transplant recipient, and should be up to date before transplantation, if possible. Current recommendations for routine adult vaccines are summarized in Table 1 .6
Table 1.
Routine adult vaccines in transplantationa
| Vaccine | Before Transplant | After Transplant | Frequency |
|---|---|---|---|
| Hepatitis A | Y | Y | Follow titers |
| Hepatitis Bb | Y | Y | Follow titers |
| Human papilloma virus | Y | Y | Unknown |
| Influenza (including H1N1)b | Y | Y | Yearly |
| Neisseria meningiditis | Y | Y | Unknown |
| Pertussis/tetanus (Tdap)b | Y | Y | Every 10 years |
| Streptococcus pneumoniae (polysaccharide)b | Y | Y | Every 3–5 years or follow titers |
| Varicella zoster | Y | N | Follow titers |
Abbreviations: Y, yes; N, no.
These vaccines should be administered before transplantation to susceptible hosts following standard Advisory Committee on Immunization Practices (ACIP) guidelines for administration, and (with the exception of varicella zoster vaccine) are safe after transplantation.
These vaccines should be considered for all candidates before transplantation with readministration based on standard ACIP guidelines.
All transplant candidates should be screened for the presence of antibodies to CMV, varicella-zoster virus, Epstein-Barr virus, hepatitis B virus, hepatitis C virus, and human immunodeficiency virus to assess both suitability for transplantation and for appropriate risk stratification for prophylaxis (and vaccination when applicable) and monitoring after transplantation.5 Screening for tuberculosis (TB) should be performed in all transplant candidates, with either purified-protein derivative (or Mantoux) skin testing or an interferon γ release assay such as the Quantiferon TB Gold (Cellestis, Valencia, CA, USA) test.7 Tuberculin-test–positive patients should be evaluated for active TB; latent TB treatment (preferably with 9 months of isoniazid) is recommended for all transplant candidates if they have not received previous therapy. In lung transplantation in particular, pretransplant screening for highly resistant bacterial and fungal airway colonizers may be considered to help guide peritransplantation antimicrobial prophylaxis.
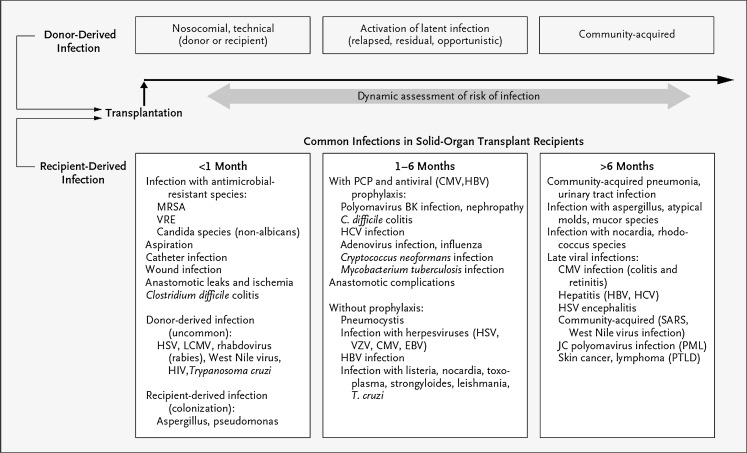
During the posttransplant period, the risk for particular infections varies depending on the time since the transplant surgery (Fig. 1 ). For example, the risks of surgical complications and nosocomial infections are highest in the first 30 days, whereas the risk of reactivation of latent, opportunistic infections is highest early (when the net state of immunosuppression is at its peak) and then declines with reduction of immunosuppression to maintenance levels.8 Similarly, the risk of community-acquired infections increases with exposure of the transplant recipient to the outpatient setting. Keeping this timeline in mind, as well as remaining cognizant of the individual patient’s pretransplant risk factors and exposures, can aid the clinician in formulating a more focused differential diagnosis for infectious complications that arise in the recipient.
Fig. 1.
Timeline of common infections in SOT recipients.
(Reprinted from Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med 2007;357(25):2606; with permission.)
Viruses
CMV
CMV remains a significant problem after lung transplantation despite advances in viral diagnostics, prophylaxis, and treatment strategies. A recent prospective multicenter cohort study by the Resistra group showed that despite 3 months of CMV-directed viral prophylaxis, 10% of postlung transplant pneumonias reported were caused by CMV.9 Lung transplant recipients have the highest risk of developing CMV disease among all SOT recipients, although the risk of CMV has decreased over time.10 This situation is likely because of the combination of the large CMV viral load that is transmitted via a CMV-positive lung allograft relative to other types of allografts and the more intensive immunosuppression often required in lung transplant recipients, as well as other common risk factors shared by other organ recipients.4 These risk factors include the serostatus of the donor and recipient, with a seronegative recipient of a seropositive organ at the highest risk, the presence of allograft rejection, the use of induction immunosuppression, and the presence of concurrent infections (primarily with other viruses). Areas under recent investigation include defects in CMV-specific cell-mediated immunity (CMI), polymorphisms in various components of the innate immune system, and other host factors such as renal dysfunction.11
Definitions for CMV infection and disease have been developed for use in both clinical and research settings.10, 12 CMV infection requires evidence of CMV viral replication via an accepted form of laboratory testing (see later discussion), whereas CMV disease requires evidence of CMV viral replication plus attributable symptoms, including fever, malaise, leukopenia, or thrombocytopenia, or evidence of tissue-invasive disease such as pneumonitis, colitis, hepatitis, or retinitis. Recent interest has also focused on the indirect effects of CMV, which include immunomodulatory effects of the virus that increase the risk of developing other opportunistic infections (human herpesvirus 6 [HHV-6], HHV-7, EBV, fungal, and bacterial), EBV-related posttransplant lymphoproliferative disease, and increased risk of both acute and chronic rejection of the allograft.13 The contribution of CMV to the development of bronchiolitis obliterans syndrome (BOS) is still being investigated, with conflicting results as reported in a recent systematic review and several single-center publications.14, 15, 16, 17, 18, 19 A more recent single-center study by Snyder and colleagues20 reported a 21% posttransplant incidence of CMV pneumonitis, and found an increased risk of subsequent BOS in both univariate and multivariate analyses (hazard ratio of 1.88 to 2.11 depending on time dependence).
There have been significant advances in CMV-specific diagnostic testing recently, and in many cases CMV infection or disease is initially diagnosed based on the detection of viremia. In the last decade, quantitative nucleic acid testing, primarily via polymerase chain reaction (PCR), has become the most widely accepted method of CMV viral load monitoring. The previous modality, CMV antigenemia detection via pp65 antigen detection in peripheral white blood cells, has fallen out of favor because of its relative insensitivity and labor intensity. However, the lack of standardization of CMV nucleic acid testing platforms and procedures across many laboratories produces significant interlaboratory variability.21 This situation can make interpretation of viral load results obtained from different laboratories problematic, and also poses problems for establishing uniform viral load cutoffs for positive and negative results. One promising area in CMV diagnostics is the measurement of CMV-specific CMI as a predictor of the development of CMV viremia and disease after the completion of primary prophylaxis. A recent study by Kumar and colleagues22 used a commercially available CD8+ CMV-specific interferon γ release assay (QuantiFERON-CMV, Cellestis, Valencia, CA, USA) to test serial CMV-specific CMI in SOT recipients during a 3-month posttransplant CMV prophylaxis period. Postprophylaxis CMV disease (within the first 6 months after transplant) occurred in 22.9% of patients with no detectable CMV-specific CMI, versus 5.3% in patients with a positive QuantiFERON-CMV result, suggesting a possible role in predicting late-onset CMV disease. CMV-specific enzyme-linked immunosorbent spot assays and intracellular cytokine staining followed by flow cytometry have also been studied as predictors of CMV viremia and disease, but these assays have not been standardized and may not be readily available at all transplant centers and are not currently recommended for routine patient monitoring.23
A 2008 Cochrane Database review of antiviral medications for preventing CMV disease in SOT recipients showed that CMV prophylaxis reduces the risk of CMV infection, disease, and all-cause mortality in heterogeneous groups of SOT recipients.24 Consensus guidelines for CMV prophylaxis have recently been updated by both the European and North American transplantation societies.10, 23 Screening of potential lung allograft donors and recipients with CMV IgG testing should be performed to allow for risk stratification and implementation of appropriate prophylaxis strategies. There are 2 generally accepted strategies for CMV prophylaxis in solid-organ transplantation: universal prophylaxis (administration of antivirals to all at-risk individuals) and preemptive therapy (administration of antivirals only to individuals with demonstrable viral replication). These strategies have not been compared in a randomized fashion in lung transplantation specifically, but the current expert opinion of both societies favors the use of universal prophylaxis in high-risk patients, including lung transplant recipients. Initial universal prophylaxis strategies used intravenous ganciclovir only, but with the development of the oral prodrug valganciclovir most centers have switched to oral valganciclovir exclusively, or oral valganciclovir after a short course of intravenous ganciclovir immediately after transplantation because similar efficacy has been shown with both medications.25 Some centers add CMV hyperimmune globulin to universal prophylaxis regimens, but this has not been evaluated in a randomized fashion.18, 26 Significant uncertainty remains regarding the appropriate duration of prophylaxis. Recent trials have suggested that extending prophylaxis to 1 year or longer after transplant may reduce CMV-related complications, but these trials had significant limitations.15, 27 Recently, the first prospective, randomized, placebo-controlled trial comparing short-course (3 months) with long-course (12 months) prophylaxis with valganciclovir in at-risk lung transplant recipients (positive donor or recipient serologic status) was reported by Palmer and colleagues.28 After completing an initial 3-month course of valganciclovir, patients were randomized to either placebo (short course) or continued prophylaxis (long course) and subsequently followed for the development of CMV disease until month 13 after transplantation. The investigators found 4% of long-course prophylaxis patients developed CMV disease during the trial, versus 32% of short-course patients. No significant differences were found in CMV resistance, adverse events, or acute rejection episodes between the groups during the observation period. Only a subset of study patients was followed for the development of CMV disease beyond the first 13 months after transplant. However, late CMV disease rates were low in both groups (only 3% in long-course and 2% in short-course subjects), suggesting that a longer duration of prophylaxis may not simply delay onset CMV disease but may also reduce the overall incidence.28 Whether additional studies examining varying durations of prophylaxis between 3 and 12 months or involving longer-term CMV surveillance after prophylaxis will confirm these findings is unknown. Reinitiation of CMV prophylaxis should be considered during the treatment of acute rejection if antilymphocyte antibody therapy or high-dose steroids are used.
Although preemptive approaches may result in reduced drug costs, avoidance of drug-related toxicity, and promotion of CMV-specific CMI via exposure to low-level viral replication, most centers favor universal prophylactic strategies, as do the consensus guidelines. Not only does the preemptive approach require frequent blood work with rapid access and reaction to results, but there is also concern about whether weekly testing might miss the onset of periods of rapid viral replication and thus risk the development of more severe CMV disease before treatment can be initiated, especially in higher-risk D+/R− and lung transplant recipients in general. It is also unknown whether low-level CMV viral replication may facilitate increased indirect effects of the virus, including rejection. There are limited data regarding the use of preemptive prophylaxis in the lung transplant setting specifically, with no large, randomized trials available. These studies have raised concerns about earlier onset of CMV disease and higher rates of ganciclovir resistance in D+/R− patients receiving preemptive therapy, and highlight the need for further research in this area.29, 30, 31
Treatment of CMV disease requires a coordinated reduction in maintenance immunosuppression in conjunction with antiviral medication, and is discussed in the recent consensus guidelines.10, 23 For CMV tissue-invasive disease, intravenous ganciclovir at 5 mg/kg every 12 hours (adjusted for renal function) is the preferred treatment, but recent studies have shown that for isolated CMV viremia, CMV syndrome, or mild to moderate CMV disease, oral valganciclovir 900 mg every 12 hours (adjusted for renal function) is noninferior to intravenous ganciclovir in a mixed population of SOT recipients.32, 33 However, caution should be exercised in patients in whom adequate absorption of oral medications is in question (eg, in CMV gastrointestinal disease), or in patients who may not be compliant with oral therapy. Treatment should be continued for a minimum of 2 to 3 weeks and until the cessation of viral replication has been verified and any CMV-attributable symptoms have resolved. Quantitative nucleic acid testing or antigenemia testing should be performed on a weekly basis during treatment to follow virologic response to therapy, and confirmation of resolution of viremia with consecutive negative tests at least 7 days apart is recommended before completion of therapy. Secondary prophylaxis with lower-dose valganciclovir (900 mg daily adjusted for renal function) can be considered for high-risk patients after completion of CMV treatment, especially those in whom reduction of immunosuppression is not possible. The addition of CMV hyperimmune globulin for more severe CMV disease, such as pneumonitis, may be considered. Patients who fail to respond to standard antiviral therapy should be suspected of having ganciclovir-resistant CMV disease and should be evaluated with genotypic resistance testing for mutations in the viral UL97 and UL54 gene products.10, 23 Additional therapy with the more toxic antiviral agents foscarnet or cidofovir may be required in these cases.34 Infectious diseases consultation should be considered for patients with suspected or documented CMV resistance.
Community-acquired Respiratory Viruses
Community-acquired respiratory viruses (CARVs) are common among lung transplant recipients especially in the ambulatory setting, with a recent prospective study detecting viral pathogens in the bronchoalveolar lavage (BAL) of 53% of lung transplant recipients enrolled during the 3-year study period.35 These viruses can cause severe lower respiratory tract infections in these patients, resulting in significant morbidity and mortality. Several studies have also suggested that CARV infection is an independent risk factor for both acute and chronic rejection in this population, although this remains controversial.16, 36, 37, 38, 39 This group of viruses includes such notable pathogens as influenza A, B, and novel H1N1, respiratory syncytial virus (RSV), adenovirus, parainfluenza, and coronaviruses such as the severe acute respiratory syndrome virus, as well as rhinovirus, enteroviruses, human metapneumovirus, bocavirus, and polyomaviruses such as KI and WU viruses. The diagnosis of respiratory viruses has been aided by the development of PCR-based quantitative nucleic acid testing for most of the known clinically relevant viruses, but there is considerable center-to-center variability in the particular panel of viruses routinely tested. Clinicians should be aware of viruses circulating in the community to ensure that adequate testing is performed. Rapid antigen testing is available for a limited number of CARVs, but their sensitivity in immunocompromised patients is unclear, thus a negative test does not definitively exclude viral infection.40 Direct fluorescence antibody testing is also available for some CARVs. Appropriate samples for viral diagnostics include nasopharyngeal swabs, aspirates, washes, or BAL specimens; however, assay sensitivity may vary with the sample collected.
Effective antiviral therapies for most CARVs are not available, with the notable exception of influenza virus and perhaps RSV, thus appropriate infection control strategies including hand hygiene and droplet precautions are mandatory to prevent the spread of disease. This finding is especially important in the health care setting, because transplant patients seem to have prolonged viral shedding after infection. Supportive care and reduction of immunosuppression when possible remain the mainstays of CARV treatment. Consensus guidelines for the management of most common CARVs and novel H1N1 influenza have recently been published, and current recommendations for influenza and RSV are briefly summarized in the next sections.40, 41
Influenza
All transplant recipients and their household contacts should receive yearly influenza vaccination with inactivated influenza vaccine for the prevention of disease. Suspected cases of influenza should be treated ideally within 48 hours of symptom onset, but transplant patients in particular may still receive benefit if therapy is initiated outside this window, and symptomatic patients should be treated regardless of the duration of symptoms.40, 41 Every effort should be made to establish the diagnosis of influenza, including the type, because specific therapy depends on the resistance pattern of the current circulating viruses. In most cases, a neuraminidase inhibitor such as oseltamivir or zanamivir taken twice daily (adjusted for renal function) is recommended for either influenza A or B; alternatively an M2 inhibitor (ie, amantidine or rimantidine) could be considered for influenza A strains. Treatment should be continued for at least 5 to 10 days, and there may be a benefit in extending therapy beyond this period for patients slow to clinically respond or who have evidence of continued viral shedding on repeated testing.40, 41 Unvaccinated patients with a suspected exposure to an individual with influenza should receive prophylaxis with oseltamivir or zanamivir given once daily (adjusted for renal function) for 5 to 10 days after the last known exposure contact. Seasonal or extended prophylaxis is not recommended because of concerns about emerging viral resistance.
RSV
Although RSV is primarily considered a significant pathogen of young children, lung transplant recipients are at risk of developing severe lower respiratory tract infections from RSV. No vaccine is licensed for the prevention of RSV, and antiviral treatment strategies are controversial. Supportive care with the reduction of immunosuppression if possible is universally recommended.40 The use of ribavirin in transplant recipients remains controversial because of the lack of controlled studies in this population. Aerosolized ribavirin is commonly used in the pediatric population for seasonal RSV bronchiolitis, and limited data suggest a benefit in lower tract disease in the stem cell transplant population.42 The addition of high-dose steroids and adjunctive antibody-based therapies such as palivizumab, RSV immunoglobulin (Ig), or intravenous immunoglobulin is controversial. Two small case series suggest a role for parenteral or oral ribavirin in lung transplant patients specifically.43, 44 Both studies treated patients with ribavirin (1 with an oral formulation, 1 with intravenous) plus high-dose oral or parenteral corticosteroids until repeated nasopharyngeal swabs were negative. After a median follow-up of greater than 300 days in both studies, all subjects had full recovery of FEV1 (forced expiratory volume after 1 second) after resolution, and only 1 case of late BOS was identified out of 23 total subjects. The oral ribavirin study reported no adverse events,44 and the intravenous study reported a mild but reversible hemolytic anemia.43 Randomized studies are needed to fully assess the usefulness of ribavirin in this population. There is no consensus recommendation for prophylaxis with palivizumab or RSV Ig in transplant recipients.
Bacteria
Gram-negative Bacterial Infections
Bacterial pathogens remain the most common cause of pneumonia after lung transplantation, with gram-negative bacteria responsible for the bulk of disease.9, 45, 46 Of these, Pseudomonas aeruginosa is the most commonly isolated species, but other gram-negatives such as Acinetobacter spp, Enterobacteriaceae, Stenotrophomonas spp, and Burkholderia spp are also frequent causes of posttransplant pneumonia. Colonization of the airway before transplantation, especially in patients with cystic fibrosis (CF), is an important consideration for determining both suitability for transplantation and postoperative care, and posttransplant colonization may be an important factor for the development of BOS.
Colonization with most resistant gram-negative bacteria before transplantation does not seem to affect survival after transplant, with the notable exception of certain Burkholderia species.47, 48 It is recommended that the resistance patterns of known pretransplant colonizing bacteria be taken into account when peritransplant antimicrobial prophylaxis is used. Historically, patients with CF who were colonized with B cepacia before transplant had poorer outcomes than those who were not colonized, leading to the exclusion of these patients from consideration for transplantation. However, recent studies have revealed that 9 genetically distinct species (genomovars) make up the B cepacia complex (BCC), with B cenocepacia (genomovar III) and B multivorans (genomovar II) causing the bulk of disease in patients with CF.49 This finding has allowed for more precise study of specific BCC genomovars in the context of lung transplantation, and several recent studies have suggested that infection with B cenocepacia specifically is a risk factor for poor outcome after transplantation.50, 51, 52 These studies all found an increased risk of death among patients infected with B cenocepacia, whereas those infected with non-cenocepacia species did not have significantly worse outcomes than uninfected controls. In addition, in the study by Murray and colleagues,52 the subset of patients infected with nonepidemic strains of B cenocepacia had a higher risk of death compared with transmissible or epidemic strains, which were not significantly different from uninfected patients. These investigators’ data also suggested that infection with B gladioli before transplantation was associated with an increased risk of death. Taken together, these new data suggest that more specific selection criteria might be established based on the specific BCC genomovar isolated (ie, B cenocepacia vs B multivorans), the presence of nonepidemic versus epidemic strains of B cenocepacia, or the presence of B gladioli. This strategy may allow access to transplantation for patients with CF with BCC who are at lower/standard risk for posttransplant complications.
The relevance of positive donor bacterial cultures has been addressed by several recent studies. Historically, the presence of purulent secretions or numerous white blood cells or bacteria on sputum Gram stain precluded consideration of the donor lung for transplantation.53 A single-center retrospective study by Avlonitis and colleagues54 suggested longer stay in the intensive care unit, longer duration of mechanical ventilation, and poorer survival in recipients who received a donor lung with a positive Gram stain. However, several recent studies have suggested that a positive donor Gram stain does not lead to poorer posttransplant outcomes if targeted, aggressive antimicrobial prophylaxis is given initially.45, 55, 56 Weill and colleagues55 reported no difference in the incidence of posttransplant pneumonia at 30 days or duration of mechanical ventilation between recipients of donor lungs with positive or negative Gram stains in patients treated with standard postoperative antibiotic prophylaxis (vancomycin plus a third-generation cephalosporin for 7 days). Currently suggested lung donor acceptability criteria state that a positive Gram stain of the donor tracheal aspirate does not preclude lung donation, but the amount of purulent secretions is of probable, but unproven importance.53
The association of posttransplantation bacterial colonization and the risk of developing BOS has become more clear in recent years, with several studies suggesting that posttransplantation colonization with gram-negative rods (GNR), particularly Pseudomonas aeruginosa, is associated with reduced BOS-free survival.57, 58, 59 Vos and colleagues57 described a retrospective study of 92 lung transplant recipients in whom colonization by GNR was associated with lower BOS-free survival by univariate analysis, and trended toward statistical significance in multivariable analysis. Gottlieb and colleagues58 examined a prospective cohort of 59 patients with CF who were colonized with GNR before transplant. These subjects were followed for a median of 966 days, and those subjects who remained chronically colonized with GNR had lower rates of BOS-free survival. Botha and colleagues59 reported that de novo colonization with P aeruginosa after transplantation was associated with a higher incidence of BOS and a shorter period of BOS-free survival. Why GNR colonization of the airways leads to BOS is still unclear, but the report by Vos and colleagues60 of an association in lung transplant recipients between P aeruginosa colonization and the presence of bile acid in BAL samples suggests that aspiration may be the underlying mechanism.
Nontuberculous Mycobacteria
The incidence of nontuberculous mycobacterial (NTM) infections in SOT is largely unknown, but several recent reports suggest that it may be an underrecognized cause of posttransplant complications. Two single-center case series from the late 1990s found 9% (22 patients) and 3% (6 patients) of lung transplant patients respectively had an NTM infection over a 12-year to 13-year period in the late 1980s to mid-1990s.61, 62 However, more recent studies suggest an increase in the incidence of NTM infection, particularly Mycobacterium abscessus, and patients with CF may be at particular risk for colonization, if not infection, with this organism.63 Factors most strongly linked to invasive infection in CF were pretransplant colonization with nontuberculous mycobacteria and isolation of M abscessus in particular.63
M abscessus seems to be emerging as a pathogen of special interest in lung transplantation. Multiple case reports and small case series have suggested that M abscessus may result in worse outcomes after transplantation, with several patients experiencing disseminated disease and death as a result of M abscessus infection.64, 65, 66, 67, 68 Although an international survey of lung transplant centers conducted in 2006 found an overall low incidence of M abscessus infection (0.33%), most affected patients had pleuropulmonary infection requiring treatment, and 2 of 17 died as a result of infection.69 A recent review and several meeting abstracts have focused on the increased recognition of M abscessus in SOT recipients, including lung recipients, noting increased risk of dissemination and mortality.70, 71
The effect of other NTM infections has been variable with regard to lung transplant outcomes. Although M avium complex has long been recognized as both a colonizer and cause of pulmonary infection in patients with underlying lung disease, including chronic obstructive pulmonary disease, its effect on lung transplantation is less notable.72 Similarly, other NTM infections have been reported in lung transplant candidates and recipients, but these organisms have not been commonly described or notable for causing the excessive morbidity and mortality seen with M abscessus. Candidates for lung transplantation should be screened for NTM infections with respiratory cultures if radiographic or clinical findings are suggestive, and considered for treatment before transplantation when possible.
The diagnosis and treatment of NTM infections is summarized in a recent guideline from the American Thoracic Society and the Infectious Diseases Society of America (IDSA).73 These criteria are applicable to transplant recipients and are reflected in the current AST Infectious Diseases Guidelines.74 Susceptibility testing should be performed for all clinically relevant isolates because of the evolving resistance patterns of many NTM isolates (eg, M abscessus) and because of potential drug interaction issues (eg, the use of rifamycins in M avium disease). In additional, repeat susceptibility testing should be performed if disease recurs after treatment because of inducible mechanisms of drug resistance in some isolates.
Fungal infections
Lung transplant recipients are at exceptionally high risk for fungal infections, and recent estimates place the incidence of fungal infection between 15% and 35%, with an overall mortality of 80%.75, 76 Most of these infections are caused by Candida and Aspergillus species, but lung transplant recipients may also be infected with Zygomycetes, Scedosporium, Fusarium, Cryptococcus species, and endemic mycoses such as histoplasmosis and coccidiodomycosis. Pneumocystis jirovecii is uncommon now that universal prophylaxis (most commonly with trimethoprim-sulfamethoxazole) is the standard of care for all lung transplant recipients. It seems that most cases of cryptococcosis and the endemic mycoses are caused by reactivation of latent disease, so a careful pretransplant evaluation is critical to determine a history of disease before transplantation. Some situations of previous or recent disease caused by endemic mycoses may warrant specific antifungal prophylaxis, and current recommendations for SOT recipients have been recently published.77, 78
Aspergillus
Aspergillus infection can manifest in a variety of ways during the course of lung transplantation, including pretransplant airway colonization, posttransplant tracheobronchitis, invasive pulmonary aspergillosis, and disseminated disease. Working definitions of these entities have been previously published.79 The incidence of these complications varies depending on the literature cited, but recent literature reviews found airway colonization with Aspergillus without disease was most commonly seen (23% of patients), and a minority of patients (less than 10%) developed either tracheobronchitis or invasive aspergillosis (IA).75, 80 Two recent fungal surveillance networks, the PATH alliance registry and TRANSNET (Transplant-Associated Infection Surveillance Network), noted that Aspergillus accounted for most fungal infections in SOT patients (44%–59.7%); Candida was the second most common fungus seen (approximately 23% in both series).81, 82
The data regarding the role of pretransplantation Aspergillus colonization in the development of posttransplant fungal complications is limited. Helmi and colleagues83 performed a retrospective single-center study comparing colonization in patients with CF with transplant recipients who did not have CF. Patients with CF were more likely to be colonized with Aspergillus and to have tracheobronchial infection after transplantation; nevertheless, IA and disseminated infection did not occur in the patients with CF and was uncommon in recipients who did not have CF. In addition, a literature review comprising studies from 1996 to 1999 found no cases of transplant recipients with CF colonized with Aspergillus before transplant progressing to IA.84 A recent single-center retrospective analysis of mold infections in explanted lungs reported 5% of explants with invasive fungal infections, and pretransplant diagnosis of these infections occurred less than 50% of the time.85 There was an association of unrecognized pretransplant mold infection with invasive fungal infections after transplant, regardless of voriconazole prophylaxis, and attributable mortality was 29% in these patients.85 Earlier studies suggested posttransplant Aspergillus colonization was associated with an 11-fold higher risk of the development of IA,86 but a subsequent single-center series combined with a pooled data analysis from the published literature by Mehrad and colleagues84 suggests that the risk may be substantially lower. Only 1 of 38 colonized subjects progressed to IA in the single-center series, and only 3 of 97 patients (3%) in the pooled literature review progressed to IA.
The gold standard for the diagnosis of IA remains biopsy with culture, accompanied by compatible clinical and radiographic abnormalities. However, the development of rapid, minimally invasive diagnostic testing modalities for aspergillosis remains an area of urgent clinical need. Galactomannan is a polysaccharide cell wall component of Aspergillus, and antigen detection in body fluids can be performed using an enzyme immunoassay technique (Platelia Galactomannan EIA, BioRad Laboratories, Hercules, CA, USA). The galactomannan assay has been studied primarily in the hematopoietic stem cell transplant population, in whom it is currently approved for use as a twice-weekly serum-based screening test for the development of IA. Several studies have assessed its usefulness in the diagnosis of IA infection in lung transplant recipients, with mixed results. Prospective testing of serum specimens had poor sensitivity for IA (30%), and did not detect any cases of Aspergillus tracheobronchitis.87 Testing of BAL was more promising, with sensitivities ranging from 60% to 82% and a specificity of 95% in 2 studies.88, 89 False-positive test results can be seen with the use of β-lactam antibiotics (especially piperacillin-tazobactam, ticarcillin-clavulanate, and amoxicillin), and the test may cross-react in the presence of other molds and endemic fungi. The (1→3)-β-d-glucan assay tests for a cell wall component present in most fungi, thus it is not specific for aspergillosis. The test also has a high false-positive rate in critically ill patients, and does not seem to have better sensitivity than the galactomannan test.90 PCR-based methods for detection of Aspergillus are in development but are not yet standardized for clinical use.91
Prophylaxis of fungal infections in lung transplant recipients is widely variable from center to center and there are no large-scale multicenter studies to guide prophylactic choices. Guidelines suggest stratifying patients based on their individual risk factors, including pretransplant colonization with Aspergillus, or acquiring colonizing organisms in the first 12 months after transplantation. Other theorized risk factors include early airway ischemia, placement of a bronchial stent, single lung transplantation, hypogammaglobulinemia, CMV infection, use of alemtuzumab or thymoglobulin induction therapies, and acute rejection requiring augmentation of immunosuppression. Both inhaled amphotericin compounds and oral itraconazole and voriconazole prophylaxis strategies have been used, but no randomized controlled trials have assessed these approaches (for a summary of recent study results see Ref.92). A recent single-center, retrospective, sequential study compared universal voriconazole prophylaxis for a minimum of 4 months against 4 months of targeted prophylaxis with itraconazole plus or minus inhaled amphotericin in patients with pretransplant or posttransplant Aspergillus colonization and reported reduced IA in the voriconazole arm.93 However, several recent studies have reported unanticipated adverse events in lung transplant patients given prolonged voriconazole therapy for prophylaxis or treatment of IA, including disabling neuromuscular disorders and periostitis resembling hypertrophic osteoarthropathy.94, 95 Because of the numerous drug interactions of azole antifungals with calcineurin inhibitors and mTOR (mammalian target of rapamycin) inhibitors, close attention to the dosing of these immunosuppressive agents is imperative.96
Treatment recommendations for Aspergillus tracheobronchitis have recently been summarized in 2 guidelines.97, 98 Voriconazole is recommended as first-line therapy for biopsy-confirmed Aspergillus tracheobronchitis along with reduction of immunosuppression, with attention paid to drug interactions with calcineurin inhibitors and to potential side effects including hepatotoxicity and visual hallucinations.97, 98 Duration of treatment is typically guided by bronchoscopic surveillance for resolution. Parenteral lipid formulations of amphotericin B deoxycholate remain an alternative in patients who cannot tolerate voriconazole. There has been limited experience using echinocandins (ie, caspofungin) or posaconazole in this setting. Inhaled amphotericin B deoxycholate or lipid formulations of amphotericin have been used in Aspergillus tracheobronchitis, but this strategy remains investigational because no standardized approach has been rigorously studied.97, 98 A single case report describing weekly topical instillation of liposomal amphotericin B via bronchoscopy in combination with standard antifungal therapy has also been recently described.99
Treatment of IA also relies on voriconazole as a first-line agent, with consideration of parenteral lipid formulations of amphotericin B deoxycholate, echinocandins, posaconazole, or itraconazole as alternatives. Reduction of immunosuppression is again an important component of treatment. Combination therapy with 2 or more agents is not routinely recommended as an initial therapeutic approach because of the lack of evidence for improved outcomes. Recent guidelines also suggest monitoring of voriconazole levels because serum concentrations are highly variable among patient populations, especially in patients with CF.98, 100 Trough levels between 1 and 5 μg/μL are recommended for optimal efficacy and prevention of toxicity.101 Surgical intervention, including debridement and resection, may be necessary for life-threatening hemoptysis, lesions in close proximity to the great vessels or pericardium, sinonasal infections, and intracranial lesions.97 Surgical assessment is also warranted in cases of progressive or refractory disease when optimal antifungal therapy has failed. Duration of treatment is typically guided by clinical and radiographic resolution of attributable abnormalities, with 12 weeks recommended as a minimum course of therapy. The role of immunomodulatory agents such as interferon γ remains investigational, with anecdotal evidence in renal transplant recipients supporting its use.102 This approach needs to be further studied and validated in a controlled manner before it can be incorporated into clinical practice.
Candida
As noted earlier, Candida is the second most common cause of invasive fungal infections in lung transplant recipients. Risk factors for candidal infections in lung transplant recipients are often related to postoperative complications including prolonged stays in the intensive care unit, prolonged indwelling catheters and broad-spectrum antibiotic therapy and parenteral nutrition, as well as heavy growth of Candida from the donor lung.79 Manifestations of candidal disease typically occur in the first 30 days after transplantation, and include bloodstream infections, empyema, mediastinitis, bronchial anastomotic breakdown, and infection of vascular anastomoses with mycotic aneurysm formation. No cases of invasive pulmonary candidiasis have been reported in the adult lung transplant literature, although in 1 pediatric transplantation series 5% of proven pulmonary invasive fungal infections were caused by Candida species.92 Despite relatively good antifungal therapies targeting Candida species, the prognosis for patients with most invasive candidal disease (excluding bronchial anastomotic disease) is grim, with mortality greater than 50%.79 A clinical suspicion of invasive fungal disease in addition to an appropriate positive culture should be the criteria used to begin antifungal therapy.
Culture remains the gold standard for the diagnosis of candidal infections, because other modalities such as β-D-glucan detection and Candida mannan antigenemia testing do not yet have sufficient sensitivity or specificity to be useful in the clinical setting.90 In addition, the identification to the species level for any clinically significant Candida isolate is critical. In this regard, several species have intrinsic resistance to certain antifungals, such as C krusei resistance to fluconazole, whereas other isolates may have dose-dependent susceptibility, such as C glabrata to fluconazole and C lusitaniae to amphotericin.103 Real-time PCR techniques are in development that may aid in the rapid determination of a specific Candida species from a clinical specimen, but these assays are currently experimental only.91 Suggested treatment of invasive candidal infections has been summarized recently in both the 2009 AST guidelines and the 2009 IDSA guidelines.103, 104 Culture data are essential in all cases to determine the identification of the isolate and subsequent susceptibility information. In nonneutropenic patients with mild to moderate illness, no previous significant azole exposure, and at low risk for C glabrata infection, high-dose fluconazole may be used initially. However, given the increasing use of azole prophylaxis (both fluconazole and voriconazole) in lung transplant patients and their higher risk level for developing severe illness, many experts recommend empiric therapy with an echinocandin or liposomal amphotericin B (depending on renal tolerability). Ongoing treatment should be adjusted based on susceptibility data. The duration of treatment depends on the extent and severity of disease, and ranges from a minimum of 2 weeks after negative blood cultures for uncomplicated candidemia to prolonged courses of treatment with the potential for life-long suppression for endocarditis or similar conditions.
The treatment of Candida species isolated from recipient airway secretions is less straightforward, because Candida spp are a common colonizer of the oropharynx, and the incidence of invasive candidal disease in lung transplant recipients colonized with Candida spp seems to be rare.92 Many centers routinely use posttransplant antifungal prophylaxis, but the agent used and the duration of therapy are highly variable, as noted earlier. However, most experts agree that if antifungal prophylaxis is used the agent should target both Candida and Aspergillus species. In the case of Candida tracheobronchitis, a visual inspection of the anastomosis should be performed with both cultures and histologic confirmation. It is critical that cultures be obtained in this setting, because Aspergillus is another common cause of necrotizing anastomotic infections, and the choice of antifungal therapy may be different from those commonly used for candidal infections. The choice of therapy should be guided by the culture results, as discussed earlier, and the duration of therapy should be guided by bronchoscopically confirmed resolution of infection.103
Summary
Infections continue to be a major source of morbidity and mortality in lung transplant recipients. Careful pretransplant assessments, selection of appropriate prophylactic regimens, and prompt diagnosis and treatment of posttransplant infections may improve posttransplant outcomes. Nevertheless, the unique exposure of the transplanted organ to the external environment and the intensive immunosuppression required to prevent rejection continue to provide unique challenges in the management of infections in lung transplantation.
References
- 1.Christie J.D., Edwards L.B., Kucheryavaya A.Y. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report–2010. J Heart Lung Transplant. 2010;29(10):1104–1118. doi: 10.1016/j.healun.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Kotloff R.M., Ahya V.N., Crawford S.W. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2004;170(1):22–48. doi: 10.1164/rccm.200309-1322SO. [DOI] [PubMed] [Google Scholar]
- 3.Kotloff R.M., Ahya V.N. Medical complications of lung transplantation. Eur Respir J. 2004;23(2):334–342. doi: 10.1183/09031936.03.00043403. [DOI] [PubMed] [Google Scholar]
- 4.Remund K.F., Best M., Egan J.J. Infections relevant to lung transplantation. Proc Am Thorac Soc. 2009;6(1):94–100. doi: 10.1513/pats.200809-113GO. [DOI] [PubMed] [Google Scholar]
- 5.Fischer S.A., Avery R.K. Screening of donor and recipient prior to solid organ transplantation. Am J Transplant. 2009;9(Suppl 4):S7–S18. doi: 10.1111/j.1600-6143.2009.02888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danzinger-Isakov L., Kumar D. Guidelines for vaccination of solid organ transplant candidates and recipients. Am J Transplant. 2009;9(Suppl 4):S258–S262. doi: 10.1111/j.1600-6143.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 7.Subramanian A., Dorman S. Mycobacterium tuberculosis in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S57–S62. doi: 10.1111/j.1600-6143.2009.02894.x. [DOI] [PubMed] [Google Scholar]
- 8.Fishman J.A. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 9.Aguilar-Guisado M., Givaldá J., Ussetti P. Pneumonia after lung transplantation in the RESITRA Cohort: a multicenter prospective study. Am J Transplant. 2007;7(8):1989–1996. doi: 10.1111/j.1600-6143.2007.01882.x. [DOI] [PubMed] [Google Scholar]
- 10.Humar A., Snydman D. Cytomegalovirus in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S78–S86. doi: 10.1111/j.1600-6143.2009.02897.x. [DOI] [PubMed] [Google Scholar]
- 11.Eid A.J., Razonable R.R. New developments in the management of cytomegalovirus infection after solid organ transplantation. Drugs. 2010;70(8):965–981. doi: 10.2165/10898540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Humar A., Michaels M. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant. 2006;6(2):262–274. doi: 10.1111/j.1600-6143.2005.01207.x. [DOI] [PubMed] [Google Scholar]
- 13.Rubin R.H. The pathogenesis and clinical management of cytomegalovirus infection in the organ transplant recipient: the end of the ‘silo hypothesis’. Curr Opin Infect Dis. 2007;20(4):399–407. doi: 10.1097/QCO.0b013e328285a358. [DOI] [PubMed] [Google Scholar]
- 14.Glanville A.R., Valentine V.G., Aboyoun C.L. CMV mismatch is not a risk factor for survival or severe bronchiolitis obliterans syndrome after lung transplantation. Immunol Cell Biol. 2004;82(2):A7. [Google Scholar]
- 15.Chmiel C., Speich R., Hofer M. Ganciclovir/valganciclovir prophylaxis decreases cytomegalovirus-related events and bronchiolitis obliterans syndrome after lung transplantation. Clin Infect Dis. 2008;46(6):831–839. doi: 10.1086/528689. [DOI] [PubMed] [Google Scholar]
- 16.Manuel O., Kumar D., Moussa G. Lack of association between beta-herpesvirus infection and bronchiolitis obliterans syndrome in lung transplant recipients in the era of antiviral prophylaxis. Transplantation. 2009;87(5):719–725. doi: 10.1097/TP.0b013e3181963262. [DOI] [PubMed] [Google Scholar]
- 17.Johanssson I., Martensson G., Andersson R. Cytomegalovirus and long-term outcome after lung transplantation in Gothenburg, Sweden. Scand J Infect Dis. 2010;42(2):129–136. doi: 10.3109/00365540903341828. [DOI] [PubMed] [Google Scholar]
- 18.Ruttmann E., Geltner C., Bucher B. Combined CMV prophylaxis improves outcome and reduces the risk for bronchiolitis obliterans syndrome (BOS) after lung transplantation. Transplantation. 2006;81(10):1415–1420. doi: 10.1097/01.tp.0000209439.27719.ed. [DOI] [PubMed] [Google Scholar]
- 19.Tamm M., Aboyoun C.L., Chhajed P.N. Treated cytomegalovirus pneumonia is not associated with bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2004;170(10):1120–1123. doi: 10.1164/rccm.200310-1405OC. [DOI] [PubMed] [Google Scholar]
- 20.Snyder L.D., Finlen-Copeland C.A., Turbyfill W.J. Cytomegalovirus pneumonitis is a risk for bronchiolitis obliterans syndrome in lung transplantation. Am J Respir Crit Care Med. 2010;181(12):1391–1396. doi: 10.1164/rccm.200911-1786OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pang X.L., Fox J.D., Fenton J.M. Interlaboratory comparison of cytomegalovirus viral load assays. Am J Transplant. 2009;9(2):258–268. doi: 10.1111/j.1600-6143.2008.02513.x. [DOI] [PubMed] [Google Scholar]
- 22.Kumar D., Chernenko S., Moussa G. Cell-mediated immunity to predict cytomegalovirus disease in high-risk solid organ transplant recipients. Am J Transplant. 2009;9(5):1214–1222. doi: 10.1111/j.1600-6143.2009.02618.x. [DOI] [PubMed] [Google Scholar]
- 23.Kotton C.N., Kumar D., Caliendo A.M. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89(7):779–795. doi: 10.1097/TP.0b013e3181cee42f. [DOI] [PubMed] [Google Scholar]
- 24.Hodson E.M., Craig J.C., Strippoli G.F. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2008;2 doi: 10.1002/14651858.CD003774.pub3. CD003774. [DOI] [PubMed] [Google Scholar]
- 25.Zamora M.R. Cytomegalovirus and lung transplantation. Am J Transplant. 2004;4(8):1219–1226. doi: 10.1111/j.1600-6143.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- 26.Zamora M.R., Nicolls M.R., Hodges T.N. Following universal prophylaxis with intravenous ganciclovir and cytomegalovirus immune globulin, valganciclovir is safe and effective for prevention of CMV infection following lung transplantation. Am J Transplant. 2004;4(10):1635–1642. doi: 10.1111/j.1600-6143.2004.00571.x. [DOI] [PubMed] [Google Scholar]
- 27.Valentine V.G., Weill D., Gupta M.R. Ganciclovir for cytomegalovirus: a call for indefinite prophylaxis in lung transplantation. J Heart Lung Transplant. 2008;27(8):875–881. doi: 10.1016/j.healun.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Palmer S.M., Limaye A.P., Banks M. Extended valganciclovir prophylaxis to prevent cytomegalovirus after lung transplantation: a randomized, controlled trial. Ann Intern Med. 2010;152(12):761–769. doi: 10.7326/0003-4819-152-12-201006150-00003. [DOI] [PubMed] [Google Scholar]
- 29.Kelly J., Hurley D., Raghu G. Comparison of the efficacy and cost effectiveness of pre-emptive therapy as directed by CMV antigenemia and prophylaxis with ganciclovir in lung transplant recipients. J Heart Lung Transplant. 2000;19(4):355–359. doi: 10.1016/s1053-2498(00)00070-x. [DOI] [PubMed] [Google Scholar]
- 30.Limaye A.P., Raghu G., Koelle D.M. High incidence of ganciclovir-resistant cytomegalovirus infection among lung transplant recipients receiving preemptive therapy. J Infect Dis. 2002;185(1):20–27. doi: 10.1086/338143. [DOI] [PubMed] [Google Scholar]
- 31.Monforte V., Román A., Gavaldà J. Preemptive therapy with intravenous ganciclovir for the prevention of cytomegalovirus disease in lung transplant recipients. Transplant Proc. 2005;37(9):4039–4042. doi: 10.1016/j.transproceed.2005.09.141. [DOI] [PubMed] [Google Scholar]
- 32.Asberg A., Humar A., Rollag H. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2007;7(9):2106–2113. doi: 10.1111/j.1600-6143.2007.01910.x. [DOI] [PubMed] [Google Scholar]
- 33.Asberg A., Humar A., Jardine A.G. Long-term outcomes of CMV disease treatment with valganciclovir versus IV ganciclovir in solid organ transplant recipients. Am J Transplant. 2009;9(5):1205–1213. doi: 10.1111/j.1600-6143.2009.02617.x. [DOI] [PubMed] [Google Scholar]
- 34.Mylonakis E., Kallas W.M., Fishman J.A. Combination antiviral therapy for ganciclovir-resistant cytomegalovirus infection in solid-organ transplant recipients. Clin Infect Dis. 2002;34(10):1337–1341. doi: 10.1086/340101. [DOI] [PubMed] [Google Scholar]
- 35.Kumar D., Husain S., Chen M.H. A prospective molecular surveillance study evaluating the clinical impact of community-acquired respiratory viruses in lung transplant recipients. Transplantation. 2010;89(8):1028–1033. doi: 10.1097/TP.0b013e3181d05a71. [DOI] [PubMed] [Google Scholar]
- 36.Billings J.L., Hertz M.I., Savik K. Respiratory viruses and chronic rejection in lung transplant recipients. J Heart Lung Transplant. 2002;21(5):559–566. doi: 10.1016/s1053-2498(01)00405-3. [DOI] [PubMed] [Google Scholar]
- 37.Khalifah A.P., Hachem R.R., Chakinala M.M. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170(2):181–187. doi: 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]
- 38.Kumar D., Erdman D., Keshavjee S. Clinical impact of community-acquired respiratory viruses on bronchiolitis obliterans after lung transplant. Am J Transplant. 2005;5(8):2031–2036. doi: 10.1111/j.1600-6143.2005.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milstone A.P., Brumble L.M., Barnes J. A single-season prospective study of respiratory viral infections in lung transplant recipients. Eur Respir J. 2006;28(1):131–137. doi: 10.1183/09031936.06.00105505. [DOI] [PubMed] [Google Scholar]
- 40.Ison M.G., Michaels M.G. RNA respiratory viral infections in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S166–S172. doi: 10.1111/j.1600-6143.2009.02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar D., Morris M.I., Kotton C.N. Guidance on novel influenza A/H1N1 in solid organ transplant recipients. Am J Transplant. 2010;10(1):18–25. doi: 10.1111/j.1600-6143.2009.02960.x. [DOI] [PubMed] [Google Scholar]
- 42.Boeckh M., Englund J., Li Y. Randomized controlled multicenter trial of aerosolized ribavirin for respiratory syncytial virus upper respiratory tract infection in hematopoietic cell transplant recipients. Clin Infect Dis. 2007;44(2):245–249. doi: 10.1086/509930. [DOI] [PubMed] [Google Scholar]
- 43.Glanville A.R., Scott A.I., Morton J.M. Intravenous ribavirin is a safe and cost-effective treatment for respiratory syncytial virus infection after lung transplantation. J Heart Lung Transplant. 2005;24(12):2114–2119. doi: 10.1016/j.healun.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 44.Pelaez A., Lyon G.M., Force S.D. Efficacy of oral ribavirin in lung transplant patients with respiratory syncytial virus lower respiratory tract infection. J Heart Lung Transplant. 2009;28(1):67–71. doi: 10.1016/j.healun.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campos S., Caramori M., Teixeira R. Bacterial and fungal pneumonias after lung transplantation. Transplant Proc. 2008;40(3):822–824. doi: 10.1016/j.transproceed.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 46.Valentine V.G., Bonvillain R.W., Gupta M.R. Infections in lung allograft recipients: ganciclovir era. J Heart Lung Transplant. 2008;27(5):528–535. doi: 10.1016/j.healun.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 47.Dobbin C., Maley M., Harkness J. The impact of pan-resistant bacterial pathogens on survival after lung transplantation in cystic fibrosis: results from a single large referral centre. J Hosp Infect. 2004;56(4):277–282. doi: 10.1016/j.jhin.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Hadjiliadis D., Steele M.P., Chaparro C. Survival of lung transplant patients with cystic fibrosis harboring panresistant bacteria other than Burkholderia cepacia, compared with patients harboring sensitive bacteria. J Heart Lung Transplant. 2007;26(8):834–838. doi: 10.1016/j.healun.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 49.Lynch J.P., 3rd Burkholderia cepacia complex: impact on the cystic fibrosis lung lesion. Semin Respir Crit Care Med. 2009;30(5):596–610. doi: 10.1055/s-0029-1238918. [DOI] [PubMed] [Google Scholar]
- 50.Alexander B.D., Petzold E.W., Reller L.B. Survival after lung transplantation of cystic fibrosis patients infected with Burkholderia cepacia complex. Am J Transplant. 2008;8(5):1025–1030. doi: 10.1111/j.1600-6143.2008.02186.x. [DOI] [PubMed] [Google Scholar]
- 51.Boussaud V., Guillemain R., Grenet D. Clinical outcome following lung transplantation in patients with cystic fibrosis colonised with Burkholderia cepacia complex: results from two French centres. Thorax. 2008;63(8):732–737. doi: 10.1136/thx.2007.089458. [DOI] [PubMed] [Google Scholar]
- 52.Murray S., Charbeneau J., Marshall B.C. Impact of burkholderia infection on lung transplantation in cystic fibrosis. Am J Respir Crit Care Med. 2008;178(4):363–371. doi: 10.1164/rccm.200712-1834OC. [DOI] [PubMed] [Google Scholar]
- 53.Orens J.B., Boehler A., de Perrot M. A review of lung transplant donor acceptability criteria. J Heart Lung Transplant. 2003;22(11):1183–1200. doi: 10.1016/s1053-2498(03)00096-2. [DOI] [PubMed] [Google Scholar]
- 54.Avlonitis V.S., Krause A., Luzzi L. Bacterial colonization of the donor lower airways is a predictor of poor outcome in lung transplantation. Eur J Cardiothorac Surg. 2003;24(4):601–607. doi: 10.1016/s1010-7940(03)00454-8. [DOI] [PubMed] [Google Scholar]
- 55.Weill D., Dey G.C., Hicks R.A. A positive donor gram stain does not predict outcome following lung transplantation. J Heart Lung Transplant. 2002;21(5):555–558. doi: 10.1016/s1053-2498(01)00415-6. [DOI] [PubMed] [Google Scholar]
- 56.Bonde P.N., Patel N.D., Borja M.C. Impact of donor lung organisms on post-lung transplant pneumonia. J Heart Lung Transplant. 2006;25(1):99–105. doi: 10.1016/j.healun.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 57.Vos R., Vanaudenaerde B.M., Geudens N. Pseudomonal airway colonisation: risk factor for bronchiolitis obliterans syndrome after lung transplantation? Eur Respir J. 2008;31(5):1037–1045. doi: 10.1183/09031936.00128607. [DOI] [PubMed] [Google Scholar]
- 58.Gottlieb J., Mattner F., Weissbrodt H. Impact of graft colonization with gram-negative bacteria after lung transplantation on the development of bronchiolitis obliterans syndrome in recipients with cystic fibrosis. Respir Med. 2009;103(5):743–749. doi: 10.1016/j.rmed.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 59.Botha P., Archer L., Anderson R.L. Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation. 2008;85(5):771–774. doi: 10.1097/TP.0b013e31816651de. [DOI] [PubMed] [Google Scholar]
- 60.Vos R., Blondeau K., Vanaudenaerde B.M. Airway colonization and gastric aspiration after lung transplantation: do birds of a feather flock together? J Heart Lung Transplant. 2008;27(8):843–849. doi: 10.1016/j.healun.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 61.Malouf M.A., Glanville A.R. The spectrum of mycobacterial infection after lung transplantation. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1611–1616. doi: 10.1164/ajrccm.160.5.9808113. [DOI] [PubMed] [Google Scholar]
- 62.Kesten S., Chaparro C. Mycobacterial infections in lung transplant recipients. Chest. 1999;115(3):741–745. doi: 10.1378/chest.115.3.741. [DOI] [PubMed] [Google Scholar]
- 63.Chalermskulrat W., Sood N., Neuringer I.P. Non-tuberculous mycobacteria in end stage cystic fibrosis: implications for lung transplantation. Thorax. 2006;61(6):507–513. doi: 10.1136/thx.2005.049247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanguinetti M., Ardito F., Fiscarelli E. Fatal pulmonary infection due to multidrug-resistant Mycobacterium abscessus in a patient with cystic fibrosis. J Clin Microbiol. 2001;39(2):816–819. doi: 10.1128/JCM.39.2.816-819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fairhurst R.M., Kubak B.M., Shpiner R.B. Mycobacterium abscessus empyema in a lung transplant recipient. J Heart Lung Transplant. 2002;21(3):391–394. doi: 10.1016/s1053-2498(01)00339-4. [DOI] [PubMed] [Google Scholar]
- 66.Taylor J.L., Palmer S.M. Mycobacterium abscessus chest wall and pulmonary infection in a cystic fibrosis lung transplant recipient. J Heart Lung Transplant. 2006;25(8):985–988. doi: 10.1016/j.healun.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 67.Gilljam M., Scherstén H., Silverborn M. Lung transplantation in patients with cystic fibrosis and Mycobacterium abscessus infection. J Cyst Fibros. 2010;9(4):272–276. doi: 10.1016/j.jcf.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 68.Zaidi S., Elidemir O., Heinle J.S. Mycobacterium abscessus in cystic fibrosis lung transplant recipients: report of 2 cases and risk for recurrence. Transpl Infect Dis. 2009;11(3):243–248. doi: 10.1111/j.1399-3062.2009.00378.x. [DOI] [PubMed] [Google Scholar]
- 69.Chernenko S.M., Humar A., Hutcheon M. Mycobacterium abscessus infections in lung transplant recipients: the international experience. J Heart Lung Transplant. 2006;25(12):1447–1455. doi: 10.1016/j.healun.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Garrison A.P., Morris M.I., Doblecki L.S. Mycobacterium abscessus infection in solid organ transplant recipients: report of three cases and review of the literature. Transpl Infect Dis. 2009;11(6):541–548. doi: 10.1111/j.1399-3062.2009.00434.x. [DOI] [PubMed] [Google Scholar]
- 71.Longworth S.A., Vinnard C., Lee I. Risk factors for non-tuberculous mycobacterial infections in heart and lung transplant recipients. J Heart Lung Transplant. 2010;29(2S):S82. [Google Scholar]
- 72.Doucette K., Fishman J.A. Nontuberculous mycobacterial infection in hematopoietic stem cell and solid organ transplant recipients. Clin Infect Dis. 2004;38(10):1428–1439. doi: 10.1086/420746. [DOI] [PubMed] [Google Scholar]
- 73.Griffith D.E., Aksamit T., Brown-Elliott B.A. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 74.Dorman S., Subramanian A. Nontuberculous mycobacteria in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S63–S69. doi: 10.1111/j.1600-6143.2009.02895.x. [DOI] [PubMed] [Google Scholar]
- 75.Kubak B.M. Fungal infection in lung transplantation. Transpl Infect Dis. 2002;4(Suppl 3):24–31. doi: 10.1034/j.1399-3062.4.s3.4.x. [DOI] [PubMed] [Google Scholar]
- 76.Sole A., Salavert M. Fungal infections after lung transplantation. Curr Opin Pulm Med. 2009;15(3):243–253. doi: 10.1097/MCP.0b013e328326f410. [DOI] [PubMed] [Google Scholar]
- 77.Proia L., Miller R. Endemic fungal infections in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S199–S207. doi: 10.1111/j.1600-6143.2009.02912.x. [DOI] [PubMed] [Google Scholar]
- 78.Singh N., Forrest G. Cryptococcosis in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S192–S198. doi: 10.1111/j.1600-6143.2009.02911.x. [DOI] [PubMed] [Google Scholar]
- 79.Sole A., Salavert M. Fungal infections after lung transplantation. Transplant Rev (Orlando) 2008;22(2):89–104. doi: 10.1016/j.trre.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 80.Singh N. Antifungal prophylaxis for solid organ transplant recipients: seeking clarity amidst controversy. Clin Infect Dis. 2000;31(2):545–553. doi: 10.1086/313943. [DOI] [PubMed] [Google Scholar]
- 81.Neofytos D., Fishman J.A., Horn D. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl Infect Dis. 2010;12(3):220–229. doi: 10.1111/j.1399-3062.2010.00492.x. [DOI] [PubMed] [Google Scholar]
- 82.Pappas P.G., Alexander B.D., Andes D.R. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin Infect Dis. 2010;50(8):1101–1111. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 83.Helmi M., Lover R.B., Welter D. Aspergillus infection in lung transplant recipients with cystic fibrosis: risk factors and outcomes comparison to other types of transplant recipients. Chest. 2003;123(3):800–808. doi: 10.1378/chest.123.3.800. [DOI] [PubMed] [Google Scholar]
- 84.Mehrad B., Paciocco G., Martinez F.J. Spectrum of Aspergillus infection in lung transplant recipients: case series and review of the literature. Chest. 2001;119(1):169–175. doi: 10.1378/chest.119.1.169. [DOI] [PubMed] [Google Scholar]
- 85.Vadnerkar A., Clancy C.J., Celik U. Impact of mold infections in explanted lungs on outcomes of lung transplantation. Transplantation. 2010;89(2):253–260. doi: 10.1097/TP.0b013e3181c3c417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cahill B.C., Hibbs J.R., Savik K. Aspergillus airway colonization and invasive disease after lung transplantation. Chest. 1997;112(5):1160–1164. doi: 10.1378/chest.112.5.1160. [DOI] [PubMed] [Google Scholar]
- 87.Husain S., Kwak E.J., Obman A. Prospective assessment of Platelia Aspergillus galactomannan antigen for the diagnosis of invasive aspergillosis in lung transplant recipients. Am J Transplant. 2004;4(5):796–802. doi: 10.1111/j.1600-6143.2004.00415.x. [DOI] [PubMed] [Google Scholar]
- 88.Clancy C.J., Jaber R.A., Leather H.L. Bronchoalveolar lavage galactomannan in diagnosis of invasive pulmonary aspergillosis among solid-organ transplant recipients. J Clin Microbiol. 2007;45(6):1759–1765. doi: 10.1128/JCM.00077-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Husain S., Paterson D.L., Studer S.M. Aspergillus galactomannan antigen in the bronchoalveolar lavage fluid for the diagnosis of invasive aspergillosis in lung transplant recipients. Transplantation. 2007;83(10):1330–1336. doi: 10.1097/01.tp.0000263992.41003.33. [DOI] [PubMed] [Google Scholar]
- 90.Wheat L.J. Approach to the diagnosis of invasive aspergillosis and candidiasis. Clin Chest Med. 2009;30(2):367–377. doi: 10.1016/j.ccm.2009.02.012. viii. [DOI] [PubMed] [Google Scholar]
- 91.Wengenack N.L., Binnicker M.J. Fungal molecular diagnostics. Clin Chest Med. 2009;30(2):391–408. doi: 10.1016/j.ccm.2009.02.014. viii. [DOI] [PubMed] [Google Scholar]
- 92.Hosseini-Moghaddam S.M., Husain S. Fungi and molds following lung transplantation. Semin Respir Crit Care Med. 2010;31(2):222–233. doi: 10.1055/s-0030-1249118. [DOI] [PubMed] [Google Scholar]
- 93.Husain S., Paterson D.L., Studer S. Voriconazole prophylaxis in lung transplant recipients. Am J Transplant. 2006;6(12):3008–3016. doi: 10.1111/j.1600-6143.2006.01548.x. [DOI] [PubMed] [Google Scholar]
- 94.Boussaud V., Daudet N., Billaud E.M. Neuromuscular painful disorders: a rare side effect of voriconazole in lung transplant patients under tacrolimus. J Heart Lung Transplant. 2008;27(2):229–232. doi: 10.1016/j.healun.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 95.Wang T.F., Wang T., Altman R. Periostitis secondary to prolonged voriconazole therapy in lung transplant recipients. Am J Transplant. 2009;9(12):2845–2850. doi: 10.1111/j.1600-6143.2009.02837.x. [DOI] [PubMed] [Google Scholar]
- 96.Thomas L.D., Miller G.G. Interactions between antiinfective agents and immunosuppressants. Am J Transplant. 2009;9(Suppl 4):S263–S266. doi: 10.1111/j.1600-6143.2009.02918.x. [DOI] [PubMed] [Google Scholar]
- 97.Singh N., Husain S. Invasive aspergillosis in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S180–S191. doi: 10.1111/j.1600-6143.2009.02910.x. [DOI] [PubMed] [Google Scholar]
- 98.Walsh T.J., Anaissie E.J., Denning D.W. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(3):327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 99.Morales P., Galán G., Sanmartín E. Intrabronchial instillation of amphotericin B lipid complex: a case report. Transplant Proc. 2009;41(6):2223–2224. doi: 10.1016/j.transproceed.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 100.Berge M., Guillemain R., Boussaud V. Voriconazole pharmacokinetic variability in cystic fibrosis lung transplant patients. Transpl Infect Dis. 2009;11(3):211–219. doi: 10.1111/j.1399-3062.2009.00384.x. [DOI] [PubMed] [Google Scholar]
- 101.Howard A., Hoffman J., Sheth A. Clinical application of voriconazole concentrations in the treatment of invasive aspergillosis. Ann Pharmacother. 2008;42(12):1859–1864. doi: 10.1345/aph.1L243. [DOI] [PubMed] [Google Scholar]
- 102.Armstrong-James D., Teo I.A., Shrivastava S. Exogenous interferon-gamma immunotherapy for invasive fungal infections in kidney transplant patients. Am J Transplant. 2010;10(8):1796–1803. doi: 10.1111/j.1600-6143.2010.03094.x. [DOI] [PubMed] [Google Scholar]
- 103.Pappas P.G., Silveira F.P. Candida in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S173–S179. doi: 10.1111/j.1600-6143.2009.02909.x. [DOI] [PubMed] [Google Scholar]
- 104.Pappas P.G., Kauffman C.A., Andes D. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(5):503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]