Abstract
Introduction
Pulmonary infiltrates in immunosuppressed patients are common. Yields from bronchoscopy with bronchoalveolar lavage (BAL) has been reported to be between 31 and 65%. The clinical impact of pneumocystis and viral Polymerase chain reaction (PCR) testing on BAL has not been extensively evaluated in a mixed immunosuppressed patient population.
Methods
We performed a retrospective chart review of immunosuppressed adults with pulmonary infiltrates who underwent BAL at the University of Rochester Medical Center. Only one BAL per patient was included. We compared the rate of positive PCR testing to conventional testing. We then investigated factors associated with positive PCR testing. Finally, we assessed for changes in antimicrobial therapy after bronchoscopy.
Results
Three hundred and fifty-nine patients underwent BAL with 249 patients having pneumocystis PCR testing and 142 having viral PCR testing. Pneumocystis identification occurred in 43 patients and viral species identification occurred in 56 patients. PCR testing increased pneumocystis identification compared to microscopy, 14% vs. 5%, p = 0.01, and viral identification compared to culture, 25% vs. 6%, p = 0.0001. Of the patients with positive pneumocystis PCR testing 49% had antibiotics stopped, 66% were started on anti-pneumocystis therapy, and only 6% did not receive treatment. There was no difference in the number of patients with antibiotics stopped based on viral PCR testing results.
Discussion
PCR testing increases BAL yield in immunosuppressed patients compared to conventional testing. Pneumocystis identified by PCR only may cause a self-limited infection and may not require antimicrobial therapy. PCR testing should be included in the evaluation of pulmonary infiltrates in immunosuppressed patients.
Keywords: PCR, Pneumocystis, Viral, Immunosuppressed, BAL
Highlights
-
•
Polymerase chain reaction testing has increased bronchoscopy yield.
-
•
Pneumocystis is now being identified in non-HIV/AIDS with negative microscopy.
-
•
Viruses are identified during bronchoscopy that were missed during nasal testing.
-
•
Antimicrobial therapies are being changed based on testing results.
1. Introduction
Immunosuppressed patients are susceptible to the development of infectious and noninfectious pulmonary diseases. Flexible bronchoscopy with bronchoalveolar lavage (BAL) is an important tool utilized to diagnose pulmonary disease [1,2]. BAL has been reported to identify infection, malignancy, diffuse alveolar hemorrhage, or pulmonary alveolar proteinosis, in 65% of patients with mixed hematologic malignancy [3], between 35 and 55% of bone marrow transplant recipients [4,5], between 41 and 50% of heart and lung transplant recipients [6,7] and between 52 and 56% of patients with mixed immunosuppression [[8], [9], [10]]. The duration of symptoms, type and duration of immunosuppression, timing of bronchoscopy, use of prophylactic and empiric antimicrobial therapy, and the presence of endemic infections vary significantly across populations and likely impact reported yields.
In our prior study we observed a positive yield from BAL samples in 55.8% of immunosuppressed patients [9]. Since our reporting, there have been advances in microbiologic detection via PCR (polymerase chain reaction) with greater sensitivity for detection of Pneumocystis jiroveci and viral/atypical bacterial etiologies compared to conventional testing [[11], [12], [13], [14]] and serum (1,3)-Beta-D-Glucan testing for pneumocystis and fungal disease [15]. There are no guidelines recommending when these tests should be performed.
The clinical impact of PCR testing on immunosuppressed patients undergoing BAL has not been thoroughly evaluated. We investigated the impact of PCR testing performed on immunosuppressed patients with lower respiratory tract disease that underwent BAL testing. We hypothesized that PCR testing would lead to an increase in BAL pneumocystis and viral identification compared to conventional testing. We hypothesized the use or duration of antibiotics would not affect yield. We hypothesized viral PCR testing would lead to discontinuation of antibiotics more than pneumocystis PCR testing. We compared results and demographics to BAL performed on patients when PCR was not performed to determine whether PCR testing altered the demographics of patients undergoing bronchoscopy.
2. Methods
Immunosuppressed adults (>18 years old) who underwent bronchoscopy with BAL at the University of Rochester Medical Center between 2009 and 2016 were identified using an existing bronchoscopy database. Institutional review board approval was obtained prior to data collection. Only the first bronchoscopy per patient was included. Non-BAL bronchoscopies (i.e. bronchoalveolar washes) were excluded. Patients were categorized by type of immunosuppression: HIV/AIDS, any hematologic malignancy, solid organ transplantation (SOT), or other immunosuppression (solid organ malignancy on immunosuppressive chemotherapy, prednisone 40 mg daily, or two immunosuppressant agents). Clinical characteristics for each patient were documented and included; type of immunosuppression, duration and type of antimicrobial therapy, hypoxia, fever/hypothermia, complete blood counts, and presence of new CT abnormalities (nodules, consolidation, or ground glass opacities).
Pneumocystis jiroveci was identified using an in house real time PCR assay targeting DNA specific to pneumocystis. This is a qualitative test with a quantitative threshold. It measures DNA concentration and counts the number of DNA replications cycles (cycle time) needed to produce a detectable concentration of DNA. Positivity was determined based on reaching a certain DNA threshold and the number of replication cycles required. If positivity is reached prior to 40 replications cycles the sample was considered positive. If positivity is reached after 40 replication cycles the sample was considered negative. Fewer replication cycles (cycle time) needed until positivity suggest higher burden of starting organism, whereas higher replication cycles (cycle time) needed until positivity suggests lower burden of starting organism. Viruses were identified using multiplex PCR (FilmArray Biofire) which identifies: 15 viruses (adenovirus, coronavirus (4 strains), metapneumovirus, rhinovirus/enterovirus, influenza A (3 strains), influenza B, respiratory syncytial virus, and parainfluenza 1–4) and 3 atypical bacteria (Mycoplasma pneumonia, Chlamydophila pneumonia, and Bordetella pertussis). Pneumocystis jiroveci identification by microscopy consisted of Grocott-Gomori methenamine silver stain and Calcofluor white stain, and conventional identification of viruses utilized standard culture techniques. Cytomegalovirus in HIV/AIDS [16], herpes simplex virus without concomitant dissemination [17], and Candida, Saccharomyces, and Penicillium species identified by culture were not considered positive.
Antimicrobial therapy was documented before and after BAL. A change in therapy was assessed seven days after BAL [9]. Antibiotics were classified as none, community acquired pneumonia coverage, or hospital acquired coverage. Anti-pneumocystis treatment was not considered antibiotic therapy.
3. Statistics
The student t-test and ANOVA testing were used to assess for differences between groups. Linear and logistic regression analyses were used to determine associations between clinical variables and a positive yield. Database management and analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC) with statistical significance defined as p < 0.05.
4. Results
4.1. Characteristics
Between 2009 and 2016 359 patients underwent BAL testing. BAL PCR testing was performed on 254 patients (249 pneumocystis and 142 multiplex). Clinical characteristics are documented in Table 1 with the majority of the patients having hematologic malignancy (59%). There was a shift in the demographics of patients that underwent BAL testing compared to 2005–2008. The number of HIV/AIDS patients decreased 62%, p = 0.001, and the number of other immunosuppression patients increased 380%, p = 0.001 (Table 2 ).
Table 1.
Patient characteristics.
| Overall (n = 359) | |
|---|---|
| Demographics | |
| Age (Median, yrs) (Range) | 58 (19-84) |
| Any Smoking history | 162 (45%) |
| Male | 205 (57%) |
| White | 294 (82%) |
| Immunosuppression | |
| HIV/AIDS | 29 (8%) |
| Hematologic Malignancy | 213 (59%) |
| Allogeneic BMT | 74 (35%) |
| Other Immunosuppression | 81 (23%) |
| Connective Tissue Disease | 50 (62%) |
| Solid Organ Transplant | 36 (10%) |
| Lung | 14 (39%) |
| Heart | 10 (28%) |
| Bronchoscopy Indication | |
| Diffuse Infiltrate | 175 (49%) |
| Focal Infiltrate | 101 (28%) |
| Laboratory Values (Median) | |
| WBC (uL) | 6,150 (0.1 - 6,170,000) |
| Neutrophil (uL) | 5,700 (0 - 200,000) |
| Lymphocyte (uL) | 500 (0 - 147,000) |
| Clinical | |
| Fever (>38oC) | 69 (19%) |
| Hypothermia (<36oC) | 20 (6%) |
| Hypoxia (>1 L Supplemental Oxygen) | 171 (48%) |
| Intubated | 68 (19%) |
| Neutropenia (ANC <500/ul) | 80 (22%) |
| Radiographs | |
| Abnormal X-Ray (n= 327) | 271 (83%) |
| Computerized Tomography | 309 |
| Consolidation | 141 (46%) |
| Ground Glass Opacity | 166 (54%) |
| Nodules | 143 (46%) |
| Adenopathy | 88 (28%) |
Table 2.
Comparison of bronchoscopic yield by year.
| 2005–2008 | 2009–2016 | P-Value | |
|---|---|---|---|
| Number of Bronchoscopies | 215 | 359 | |
| HIV/AIDS | 46 (21%) | 29 (8%) | 0.001b |
| Hematologic Malignancy | 128 (60%) | 213 (59%) | 0.96 |
| Other Immunosuppression | 10 (5%) | 81 (23%) | 0.001† |
| Solid Organ Transplant | 31 (14%) | 36 (10%) | 0.11 |
| Total Infectious Yield | 81 (38%) | 144 (40%) | 0.8 |
| Gram Positive Bacteria | 22 (10%) | 28 (8%) | 0.22 |
| Gram Negative Bacteria | 14 (7%) | 26 (7%) | 0.28 |
| Mycobacterium | 4 (2%) | 8 (2%) | 0.76 |
| Total Bacteria | 37 (17%) | 57 (16%) | 0.68 |
| Pathologic Fungal | 6 (3%) | 16 (4%) | 0.36 |
| Pneumocystis | 15 (7%) | 43 (12%) | 0.01a |
| Pneumocystis PCRc | – | 35 (14.%) | 0.01a |
| Viral Pathogens | 35 (16%) | 56 (15%) | 0.74 |
| Viral PCRd | – | 35 (25%) | 0.03a |
P < 0.05 for the comparison between 2009-2016 and 2005–2008 data.
P < 0.01 for the comparison between 2009-2016 and 2005–2008 data.
n = 249 patients for pneumocystis PCR testing. P value represents the comparison of PJP PCR testing in 2009–2016 to the Pneumocystis identification rate in 2005–2008 (PCR testing was not performed during this period).
n = 142 patients for viral PCR testing. P value represents the comparison of Viral PCR testing in 2009–2016 to the Viral pathogen identification rate in 2005–2008 (PCR testing was not performed during this period). CMV in HIV/AIDS was not included.
4.2. Yield
A positive BAL yield occurred in 154 patients (43%), with 94% being infectious (Table 2). Non-BAL testing (i.e. Blood or urine culture) was positive in 82 patients. Pneumocystis was identified in 43 patients (12%) (Table 3 ). 56 patients (16%) had a virus identified, 57 patients (16%) had a bacteria identified, and 16 patients (4%) had a fungus identified.
Table 3.
Results of Pneumocystis Jiroveci Pneumonia (PJP) testing in specific patient populations.
| PJP Microscopy (+)b | PJP PCRc (+) | PJP PCR (+) and Microscopy (+) | PJP PCR (+) and Microscopy (−) | Total PJP Identified | |
|---|---|---|---|---|---|
| HIV/AIDS (n = 29) | 13 | 8 | 8 | 0 | 13 |
| Heme (n = 213) | 4 | 14 | 2 | 12 | 16 |
| Other (n = 81) | 1 | 12 | 0 | 12 | 13 |
| SOTa (n = 36) | 0 | 1 | 0 | 1 | 1 |
| Total | 18 | 35 | 10 | 25 | 43 |
SOT = Solid Organ Transplant.
n = 359 patients for pneumocystis microscopy testing.
n = 249 patients for pneumocystis PCR testing.
4.3. Pneumocystis yield 2009–2016
Pneumocystis identification occurred in 43 patients (Table 3). Identification was higher via PCR testing compared to microscopy, 35/249 patients (14%) vs 18/359 patients (5%) p = 0.01. This remained true when compared to 2005–2008, 14% vs 7% respectively, p = 0.01 (Table 2). Ten patients (28.5%), 8 being HIV/AIDS, were positive via both microscopy and PCR testing from BAL sample (Table 3). Positive sputum and BAL PCR testing occurred in four patients, two patients had negative sputum PCR testing but positive BAL PCR testing, and 20 patients had both negative sputum and BAL PCR testing. Compared to BAL testing sputum testing has a positive predictive value of 100%, negative predictive value of 91%, sensitivity of 67%, and specificity of 100%. In patients with paired positive sputum and BAL PCR testing we found cycle time varied. In one patient the sputum had a lower cycle time compared to BAL (34 vs 37) while another patient had a significantly higher cycle time in the sputum compared to BAL (37 vs 24). Five patients (14.2%) with positive PCR testing had multiple pathogens requiring treatment identified on BAL testing.
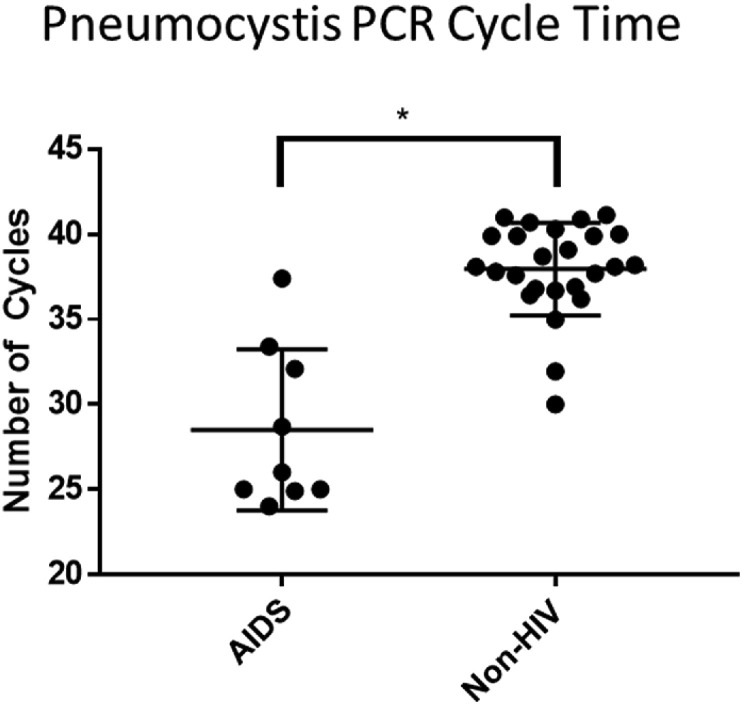
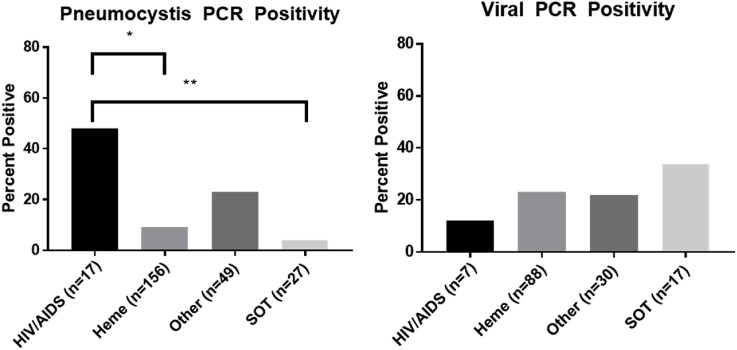
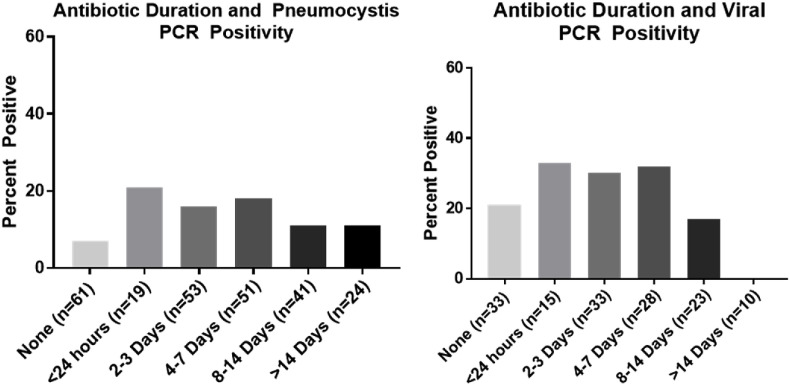
Pneumocystis identified in HIV/AIDS patients occurred at a lower PCR cycle time compared to non-HIV tested patients, 29 vs 38, p < 0.0001 (Fig. 1 ). When microscopy was positive, PCR cycle times were lower, 26 vs 38, p < 0.0001. PCR positivity was more likely to be found in HIV/AIDS and other immunosuppression, p < 0.0001 (Fig. 2 ). PCR testing increased identification compared to microscopy in patients with hematologic malignancy [14 (9%) vs 4 (2%), p = 0.001] and other immunosuppression [12 (24%) vs 1 (1%), p < 0.0001] but did not alter the rate of identification in patients with HIV/AIDS [8 (47%) vs 13 (45%)] or in patients that had undergone SOT [1 (4%) vs 0]. Duration of non-prophylactic antibiotics prior to bronchoscopy did not affect pneumocystis identification (p = 0.47) (Fig. 3 ). Fifty-four patients were on pneumocystis prophylaxis (15%) prior to bronchoscopy with 4 testing PCR positive (p = 0.32). No patients had recurrent pneumocystis pneumonia. Six patients had repeat bronchoscopies (6 weeks–3 years later) and repeat PCR testing was negative. 13/18 (72%) non-HIV/AIDS patients had positive (1,3)-Beta-D-Glucan testing with only five BAL PCR positive. Four received treatment for pneumocystis despite PCR negativity. No patients had a positive PCR test and negative (1,3)-Beta-D-Glucan test.
Fig. 1.
Pneumocystis identified in HIV/AIDS patients occurred at a lower PCR cycle time compared to non-HIV tested patients,28.5 vs 37.9,p = 0.0001.
Fig. 2.
Pneumocystis PCR identification varied based on immunosuppression, ANOVA p < 0.0001. post hoc testing showed pneumocystis was more likely to be found in HIV/AIDS compared to hematologic malignacy, p = 0.001, and solid organ transplant, p = 0.002. Viral PCR identification did vary based on immunosuppression, p = 0.74.
Fig. 3.
Antibiotic duration did not influence pneumocystis, p = 0.47, or viral PCR, p = 0.27, positivity.
4.4. Viral PCR and culture yield 2009–2016
Multiplex viral PCR from BAL testing increased detection of respiratory viruses in BAL samples compared to viral culture, 35/142 patients (25%) vs. 26/359 patients (6%), p < 0.001, specifically Influenza A/B (6 vs 1, p < 0.001) and respiratory syncytial virus (4 vs 2, p = 0.03). There was no difference in BAL viral PCR yield based on the type of immunosuppression, p = 0.74 (Fig. 2) or duration of antibiotics, p = 0.27 (Fig. 3). Viral culture identified cytomegalovirus in eight non-HIV patients. Eight patients (23%) with positive viral PCR testing from BAL had multiple pathogens identified (seven bacteria and aspergillus twice). Five out of the six patients diagnosed with influenza had received the influenza vaccine. Two patients diagnosed with influenza only had BAL testing. Nasopharyngeal viral PCR testing without BAL viral PCR testing occurred in 29 patients with five being positive. As we previously reported nasopharyngeal multiplex viral PCR testing had a positive and negative predictive value of 88% and 89%, respectively, when compared to BAL viral PCR testing. Influenza was missed twice on nasopharyngeal multiplex viral PCR testing but detected on BAL PCR testing [18].
4.5. Associations with positive pneumocystis and viral BAL PCR yield 2009–2016
Univariate analysis identified that HIV/AIDS (OR 6.7, 2.4–19.0, p < 0.001), other immunosuppression (OR 2.5, 1.14–5.46, p = 0.02), and ground glass opacities on CT imaging (OR 3.55, 1.39–9.04, p = 0.008) were associated with detection of pneumocystis by PCR. Multivariate analysis confirmed that HIV/AIDS (OR 13.2, 3.43–50.51, p=<0.001), other immunosuppression (OR 4.02, 1.62–9.99, p = 0.003) and ground glass opacities on CT imaging (OR 4.55, 1.64–12.65, p = 0.004) were associated with positive pneumocystis PCR testing (Table 4 ). Univariate analysis also showed that hematologic malignancy (0.34, 0.16–0.70, p = 0.003), CT imaging with primarily nodular disease (OR 0.32, 0.14–0.75, p = 0.008), and outpatient bronchoscopy (0.19, 0.04–0.81, p = 0.02) were associated with negative pneumocystis PCR testing. Multivariate analysis confirmed that hematologic malignancy (0.29, 0.13–0.67, p = 0.003), CT imaging demonstrating primarily nodular disease (OR 0.42, 0.16–0.98, p = 0.04), and outpatient bronchoscopy (0.20, 0.04–0.91, p = 0.04) were all independently associated with negative pneumocystis PCR testing (Table 4). Testing performed during winter months was associated with positive viral PCR testing (OR 2.44, 1.12–5.32, p = 0.02). Allogeneic bone marrow transplant (OR 2.84, 1.53–5.26, p=<0.001) and intubation (OR 1.93, 1.01–3.71, p = 0.04) were associated with positive viral PCR or viral culture on univariate analysis. Multivariate analysis confirmed allogeneic bone marrow transplant (OR 2.97, 1.59–5.55, p= <0.001) and intubation (OR 2.08, 1.06–4.07, p = 0.03) as being independently associated with positive viral PCR or viral culture (Table 4).
Table 4.
Clinical Variable associated with positive Pneumocystis and Viral Testing.
| Pneumocystis PCR | Point Estimate | Confidence Interval | P value |
|---|---|---|---|
| Pneumocystis Prophylaxis | 0.58 | 0.19, 1.74 | 0.32 |
| Bactrim Prophylaxis | 0.55 | 0.16, 1.92 | 0.35 |
| Hematologic Malignancy | 0.34 | 0.16, 0.7 | 0.003 |
| HIV/AIDS | 6.75 | 2.4, 18.97 | 0.0003 |
| Other Immunosuppression | 2.50 | 1.14, 5.46 | 0.02 |
| Solid Organ Transplant | 0.21 | 0.03, 1.62 | 0.13 |
| CT Imaging With Consolidation | 0.52 | 0.22, 1.23 | 0.13 |
| CT Imaging With Nodules | 0.32 | 0.14, 0.75 | 0.01 |
| CT Imaging With Ground Glass Opacities | 3.55 | 1.39, 9.05 | 0.01 |
| Outpatient | 0.19 | 0.04, 0.81 | 0.02 |
| Viral Identification (PCR or Culture) | |||
| HIV/AIDS | 0.18 | 0.02, 1.35 | 0.09 |
| Heme | 1.56 | 0.85, 2.85 | 0.15 |
| Allo BMT | 2.84 | 1.53, 5.27 | 0.00 |
| Other | 0.62 | 0.29, 1.32 | 0.21 |
| SOT | 1.64 | 0.71, 3.82 | 0.25 |
| Winter | 1.40 | 0.78, 2.48 | 0.25 |
| Summer | 0.71 | 0.39, 1.32 | 0.28 |
| Intubation | 1.93 | 1.01, 3.71 | 0.04 |
| CT Imaging With Consolidation | 0.93 | 0.49, 1.79 | 0.83 |
| CT Imaging with Ground Glass Opacities | 1.82 | 0.93, 3.55 | 0.07 |
| CT Imaging With Nodules | 0.86 | 0.45, 1.63 | 0.64 |
4.6. Management post bronchoscopy 2009–2016
Changes in antimicrobial therapy after BAL testing occurred in 272 patients (76%). Negative BAL testing prevented 57 (16%) patients from receiving antibiotics and 85 (24%) patients had antibiotics stopped. Antibiotics for culture negative infiltrates occurred in 131 patients (37%). Seventeen patients (49%) with positive pneumocystis PCR testing had antibiotics stopped. Three patients died in the hospital from progression of their underlying disease and the other 14 patients improved on anti-pneumocystis therapy only. Twenty-three patients (66%) started anti-pneumocystis therapy after positive PCR testing. Ten patients (29%), 5 with negative microscopy, continued on anti-pneumocystis treatment after positive PCR testing. Two patients (6%) received no treatment for pneumocystis despite having positive PCR testing. One patient died in the hospital unrelated to pneumocystis and the other patient had no follow up after discharge. Eleven patients (31%) received concomitant antibiotics along with anti-pneumocystis treatment. Seven patients (3%) had treatment stopped after negative PCR testing. In patients with negative pneumocystis PCR testing but positive (1,3)-Beta-D-Glucan testing three had anti-pneumocystis treatment continued, three patients had anti-pneumocystis treatment stopped, one patient had anti-pneumocystis treatment started, and one patient did not receive anti-pneumocystis treatment.
Sixty-three patients (44%) that underwent viral PCR testing were not on antibiotics 7 days later. There was no difference in antibiotics being stopped based on viral PCR testing results, 11 patients (73%) with positive testing and 32 patients (67%) with negative testing (p = 0.63). After positive BAL viral PCR testing 7/8 patients had antiviral therapy started (oseltamivir x 5, cidofovir, and ribavirin). After negative BAL viral testing one patient had oseltamivir discontinued.
5. Discussion
Our study evaluated the clinical impact of PCR testing on immunosuppressed patients undergoing BAL and the influence PCR testing had on antimicrobial changes in immunosuppressed patients. We found the addition of PCR testing of BAL fluid increased yield by 33% and likely identified pneumocystis and viruses previously missed through conventional testing. Importantly, empiric treatment with antibiotic or anti-pneumocystis therapy did not affect PCR yield. Pneumocystis PCR testing also helped to limit empiric antibiotic use in some patients with positive testing and decreased anti-pneumocystis therapy in patients with negative testing. Multiplex viral PCR had the same effect with antibiotic stewardship, and lead to the addition of specific antiviral therapies after BAL.
PCR testing has improved management of pulmonary infiltrates in immunosuppressed patient. Compared to BAL PCR testing noninvasive viral testing occurred less often, 62% of patients and pneumocystis testing occurred in 10% of patients. Multiple factors contributed to that including infectious concern, bronchoscopy setting, and timing of bronchoscopy. Multiplex PCR testing has the advantage of identifying viruses not easily cultured (i.e. coronavirus and metapneumovirus) and faster analysis compared to viral culture [[12], [13], [14],18]. Unlike pneumocystis PCR testing multiplex PCR cannot measure cycle time and determining viral burden is not possible. We recently observed discordance between nasopharyngeal and BAL samples, making BAL testing a reasonable option if clinical suspicion is high for viral infection despite negative noninvasive testing [18]. A delay in starting oseltamavir occurred in two patients because non-BAL testing did not occur. While non-BAL PCR testing can be helpful in making a diagnosis, it is less helpful excluding infections as 19% of patients with positive non-BAL PCR testing had multiple organisms identified on BAL.
Microscopy has identified pneumocystis primarily in the AIDS population [19] with PCR testing increasing pneumocystis identification in other immunosuppressed groups [11,20]. PCR Positivity rates vary between 0 and 20% in non-BAL tested asymptomatic healthy adults [[21], [22], [23], [24]] and 14–68% in BAL tested samples from immunosuppressed adults with lower respiratory tract disease [20,25,26]. We found a 12-fold increase in pneumocystis identification using PCR in our other immunosuppressed group (connective tissues disease or solid organ transplant) and no change in our HIV/AIDS identification rate (Table 3). The observed increase may be due to immunosuppressive therapies predisposing patients to infection with a lower burden of organism [27], and may explain why microscopy is typically negative. The increase may also be due to a combination of the lack of specific prophylaxis recommendations in connective tissue disease treatment [28,29] (only 9% of our patients in this group was on prophylaxis), the increased sensitivity of PCR testing, and the detection of reactivated latent [30] or self-limited pneumocystis infections. Based on our data PCR testing was equivalent to microscopy in diagnosing or excluding pneumocystis in HIV/AIDS patients. As a potential cost savings measure either test could be performed during BAL to diagnose or exclude pneumocystis in HIV/AIDS.
Guidelines on using PCR to determine when treatment is necessary after detection of pneumocystis are currently lacking. Both Robert-Gangneux et al. (2014) and Maillet et al. (2014) found HIV/AIDS with pneumocystis pneumonia had lower PCR thresholds indicating a higher burden of organisms compared to non-HIV/AIDS patients they deemed colonized [20,31]. We observed that HIV/AIDS and microscopy positivity had lower cycle time compared to non-HIV/AIDS and microscopy negative patients (Fig. 1). We had six patients with PCR positivity only that had negative pneumocystis PCR testing on subsequent BAL testing. We would have expected persistent positivity if colonization occurred. We also had one patient with a cycle time difference of 13 when comparing non-BAL and BAL testing performed at the same time. This large difference makes using a single cycle time difficult in distinguishing when treatment is necessary. We think pneumocystis infection may occur on a spectrum, with high pneumocystis burden (low cycle time) requiring therapy while lower burden (high cycle time) being a self-limited process that may not requiring treatment. Compared to microscopy PCR testing is excellent at excluding disease as both Durand-Joly et al.(2005) [32] and our results found PCR testing to have 100% negative predictive value. (1,3)-Beta-D-Glucan testing calls into question the negative predictive value as we had four patients with positive testing and negative PCR testing. Sampling error could have contributed to these findings. Further studies are needed evaluating PCR cycle time in guiding treatment and comparing (1,3)-Beta-D-Glucan with negative PCR testing.
As this was retrospective, interpretation of management decisions is limited. Our study included two patients (5%) not treated, 11 patients (31%) treated with concomitant antibiotics along with anti-pneumocystis therapy (four with bacteria on BAL), and 17 patients (48.5%) had antibiotics stopped and treated with anti-pneumocystis therapy only after positive PCR testing. PCR testing led to the initiation of anti-pneumocystis therapy in 2/3 of patients after positive testing results returned. This approach limits potential toxicities [33] from empiric pneumocystis treatment. We do not think the treatment decisions made based on PCR testing affected morbidity or mortality. Importantly, in instances when BAL cannot be performed, pneumocystis therapy does not appear to decrease yield, as 28% of our patients were on pneumocystis anti-therapy prior to testing (1–4 days) and still had a positive PCR test.
There are limitations to this study. First, this was an observational study relying on data entered into the electronic medical record subject to data entry errors. Second, provider discretion influenced whether patients underwent bronchoscopy as not every patient with pulmonary infiltrates was evaluated with bronchoscopy. Third, some patients had multiple infections (pulmonary and non-pulmonary) and these other infections may have affected antimicrobial management. We could not routinely ascertain what type of infection was of greatest concern at the time of bronchoscopy. Lastly, management decisions post-bronchoscopy were often made by multiple different providers, adding to the variability in management. Further prospective evaluations are needed to better define infection rates with standardized microbiologic evaluation and the impact on management decisions.
Pulmonary infiltrates in immunosuppressed patients are a common problem, with etiologies varying depending on of the degree and type of underlying immunosuppression. Guidelines are currently lacking on microbial evaluation based on type of immunosuppression and radiographic abnormality. PCR testing on BAL samples is increasing identification of infections that previously went undiagnosed through conventional testing and allows for tailored therapy based on results. Further studies are needed to help guide when non-BAL and BAL testing should be pursued. Until further studies are completed, thoughtful microbial evaluation by clinicians remains the strongest tool to interpret the significance of testing results. Based on our findings, we propose that pneumocystis and viral PCR testing (both noninvasive and invasive) should be included in the evaluation of all immunosuppressed patients with ground glass opacities on imaging. Furthermore we propose that patients with positive pneumocystis PCR testing may actually have self-limiting infection and that pneumocystis infections may exist as a spectrum of disease. Further studies of this hypothesis are needed. Combined with clinical suspicion PCR testing can be a helpful tool in the managing infection in immunosuppressed patients.
Funding
This work was supported by the National Institutes of Health T-32 training grant.
Declarations of interest
None.
Footnotes
This work is not under consideration for publication elsewhere, has not been presented or published prior, and is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder.
References
- 1.Rano A., Agusti C., Benito N., Rovira M., Angrill J., Pumarola T. Prognostic factors of non-HIV immunocompromised patients with pulmonary infiltrates. Chest. 2002;122(1):253–261. doi: 10.1378/chest.122.1.253. [DOI] [PubMed] [Google Scholar]
- 2.Harris B., Lowy F.D., Stover D.E., Arcasoy S.M. Diagnostic bronchoscopy in solid-organ and hematopoietic stem cell transplantation. Ann. Am. Thoracic Soc. 2013;10(1):39–49. doi: 10.1513/AnnalsATS.201212-114FR. [DOI] [PubMed] [Google Scholar]
- 3.Kim S.W., Rhee C.K., Kang H.S., Lee H.Y., Kang J.Y., Kim S.J. Diagnostic value of bronchoscopy in patients with hematologic malignancy and pulmonary infiltrates. Ann. Hematol. 2015;94(1):153–159. doi: 10.1007/s00277-014-2172-3. [DOI] [PubMed] [Google Scholar]
- 4.Shannon V.R., Andersson B.S., Lei X., Champlin R.E., Kontoyiannis D.P. Utility of early versus late fiberoptic bronchoscopy in the evaluation of new pulmonary infiltrates following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45(4):647–655. doi: 10.1038/bmt.2009.203. [DOI] [PubMed] [Google Scholar]
- 5.White P., Bonacum J.T., Miller C.B. Utility of fiberoptic bronchoscopy in bone marrow transplant patients. Bone Marrow Transplant. 1997;20(8):681–687. doi: 10.1038/sj.bmt.1700957. [DOI] [PubMed] [Google Scholar]
- 6.Lehto J.T., Anttila V.-J., Lommi J., Nieminen M.S., Harjula A., Taskinen E. Clinical usefulness of bronchoalveolar lavage in heart transplant recipients with suspected lower respiratory tract infection. J. Heart Lung Transplant. 2004;23(5):570–576. doi: 10.1016/S1053-2498(03)00228-6. [DOI] [PubMed] [Google Scholar]
- 7.Lehto J.T., Koskinen P.K., Anttila V.-J., Lautenschlager I., Lemström K., Sipponen J. Bronchoscopy in the diagnosis and surveillance of respiratory infections in lung and heart–lung transplant recipients. Transpl. Int. 2005;18(5):562–571. doi: 10.1111/j.1432-2277.2005.00089.x. [DOI] [PubMed] [Google Scholar]
- 8.Jain P., Sandur S., Meli Y., Arroliga A.C., Stoller J.K., Mehta A.C. Role of flexible bronchoscopy in immunocompromised patients with lung infiltrates. Chest. 2004;125(2):712–722. doi: 10.1378/chest.125.2.712. [DOI] [PubMed] [Google Scholar]
- 9.Kottmann R.M., Kelly J., Lyda E., Gurell M., Stalica J., Ormsby W. Bronchoscopy with bronchoalveolar lavage: determinants of yield and impact on management in immunosuppressed patients. Thorax. 2011;66(9):823. doi: 10.1136/thx.2010.145540. [DOI] [PubMed] [Google Scholar]
- 10.Brownback K.R., Simpson S.Q. Association of bronchoalveolar lavage yield with chest computed tomography findings and symptoms in immunocompromised patients. Ann. Thorac. Med. 2013;8(3):153–159. doi: 10.4103/1817-1737.114302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azoulay É., Bergeron A., Chevret S., Bele N., Schlemmer B., Menotti J. POlymerase chain reaction for diagnosing pneumocystis pneumonia in non-hiv immunocompromised patients with pulmonary infiltrates. Chest. 2009;135(3):655–661. doi: 10.1378/chest.08-1309. [DOI] [PubMed] [Google Scholar]
- 12.Azadeh N., Sakata K.K., Brighton A.M., Vikram H.R., Grys T.E. FilmArray respiratory panel assay: comparison of nasopharyngeal swabs and bronchoalveolar lavage samples. J. Clin. Microbiol. 2015;53(12):3784–3787. doi: 10.1128/JCM.01516-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hakki M., Strasfeld L.M., Townes J.M. Predictive value of testing nasopharyngeal samples for respiratory viruses in the setting of lower respiratory tract disease. J. Clin. Microbiol. 2014;52(11):4020–4022. doi: 10.1128/JCM.01944-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammond S.P., Gagne L.S., Stock S.R., Marty F.M., Gelman R.S., Marasco W.A. Respiratory virus detection in immunocompromised patients with FilmArray respiratory panel compared to conventional methods. J. Clin. Microbiol. 2012;50(10):3216–3221. doi: 10.1128/JCM.00538-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salerno D., Mushatt D., Myers L., Zhuang Y., de la Rua N., Calderon E.J. Serum and bal beta-D-glucan for the diagnosis of Pneumocystis pneumonia in HIV positive patients. Respir. Med. 2014;108(11):1688–1695. doi: 10.1016/j.rmed.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mann M., Shelhamer J.H., Masur H., Gill V.J., Travis W., Solomon D. Lack of clinical utility of bronchoalveolar lavage cultures for cytomegalovirus in HIV infection. Am. J. Respir. Crit. Care Med. 1997;155(5):1723–1728. doi: 10.1164/ajrccm.155.5.9154883. [DOI] [PubMed] [Google Scholar]
- 17.van den Brink J.W., Simoons-Smit A.M., Beishuizen A., Girbes A.R., Strack van Schijndel R.J., Groeneveld A.B. Respiratory herpes simplex virus type 1 infection/colonisation in the critically ill: marker or mediator? J. Clin. Virol. : Offic. Publ. Pan Am. Soc. Clin. Virol. 2004;30(1):68–72. doi: 10.1016/j.jcv.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Lachant D.J., Croft D.P., McGrane Minton H., Prasad P., Kottmann R.M. Respirology (Carlton, Vic); 2017. Nasopharyngeal Viral PCR in Immunosuppressed Patients and its Association with Virus Detection in Bronchoalveolar Lavage by PCR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas C.F.J., Limper A.H. Pneumocystis pneumonia. N. Engl. J. Med. 2004;350(24):2487–2498. doi: 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 20.Maillet M., Maubon D., Brion J.P., Francois P., Molina L., Stahl J.P. Pneumocystis jirovecii (Pj) quantitative PCR to differentiate Pj pneumonia from Pj colonization in immunocompromised patients. Eur. J. Clin. Microbiol. Infect. Dis.: Offic. Publ. Eur. Soc. Clin. Microbiol. 2014;33(3):331–336. doi: 10.1007/s10096-013-1960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leigh T.R., Kangro H.O., Gazzard B.G., Jeffries D.J., Collins J.V. DNA amplification by the polymerase chain reaction to detect sub-clinical Pneumocystis carinii colonization in HIV-positive and HIV-negative male homosexuals with and without respiratory symptoms. Respir. Med. 1993;87(7):525–529. doi: 10.1016/0954-6111(93)90008-n. [DOI] [PubMed] [Google Scholar]
- 22.Nevez G., Magois E., Duwat H., Gouilleux V., Jounieaux V., Totet A. Apparent absence of Pneumocystis jirovecii in healthy subjects. Clin. Infect. Dis. : Offic. Publ. Infect. Dis. Soc. Am. 2006;42(11):e99–101. doi: 10.1086/503908. [DOI] [PubMed] [Google Scholar]
- 23.Nevez G., Jounieaux V., Linas M.D., Guyot K., Leophonte P., Massip P. High frequency of Pneumocystis carinii sp.f. hominis colonization in HIV-negative patients. J. Eukaryot. Microbiol. 1997;44(6):36s. doi: 10.1111/j.1550-7408.1997.tb05760.x. [DOI] [PubMed] [Google Scholar]
- 24.Medrano F.J., Montes-Cano M., Conde M., de la Horra C., Respaldiza N., Gasch A. Pneumocystis jirovecii in general population. Emerg. Infect. Dis. 2005;11(2):245–250. doi: 10.3201/eid1102.040487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nevez G., Raccurt C., Vincent P., Jounieaux V., Dei-Cas E. Pulmonary colonization with pneumocystis cariniiin human immunodeficiency virus-negative patients: assessing risk with blood CD4+ T cell counts. Clin. Infect. Dis. 1999;29(5):1331–1332. doi: 10.1086/313478. [DOI] [PubMed] [Google Scholar]
- 26.Davis J.L., Welsh D.A., Beard C.B., Jones J.L., Lawrence G.G., Fox M.R. Pneumocystis colonisation is common among hospitalised HIV infected patients with non-Pneumocystis pneumonia. Thorax. 2008;63(4):329–334. doi: 10.1136/thx.2007.088104. [DOI] [PubMed] [Google Scholar]
- 27.Limper A.H., Offord K.P., Smith T.F., Martin W.J., 2nd Pneumocystis carinii pneumonia. Differences in lung parasite number and inflammation in patients with and without AIDS. Am. Rev. Respir. Dis. 1989;140(5):1204–1209. doi: 10.1164/ajrccm/140.5.1204. [DOI] [PubMed] [Google Scholar]
- 28.Suryaprasad A., Stone J.H. When is it safe to stop Pneumocystis jiroveci pneumonia prophylaxis? Insights from three cases complicating autoimmune diseases. Arthritis Rheum. 2008;59(7):1034–1039. doi: 10.1002/art.23822. [DOI] [PubMed] [Google Scholar]
- 29.Green H., Paul M., Vidal L., Leibovici L. Prophylaxis of Pneumocystis pneumonia in immunocompromised non-HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin. Proc. 2007;82(9):1052–1059. doi: 10.4065/82.9.1052. [DOI] [PubMed] [Google Scholar]
- 30.Morris A., Norris K.A. Colonization by Pneumocystis jirovecii and its role in disease. Clin. Microbiol. Rev. 2012;25(2):297–317. doi: 10.1128/CMR.00013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robert-Gangneux F., Belaz S., Revest M., Tattevin P., Jouneau S., Decaux O. Diagnosis of pneumocystis jirovecii pneumonia in immunocompromised patients by real-time PCR: a 4-year prospective study. J. Clin. Microbiol. 2014;52(9):3370–3376. doi: 10.1128/JCM.01480-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durand-Joly I., Chabe M., Soula F., Delhaes L., Camus D., Dei-Cas E. Molecular diagnosis of Pneumocystis pneumonia. FEMS Immunol. Med. Microbiol. 2005;45(3):405–410. doi: 10.1016/j.femsim.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Safrin S., Finkelstein D.M., Feinberg J., Frame P., Simpson G., Wu A. Comparison of three regimens for treatment of mild to moderate Pneumocystis carinii pneumonia in patients with AIDS. A double-blind, randomized, trial of oral trimethoprim-sulfamethoxazole, dapsone-trimethoprim, and clindamycin-primaquine. ACTG 108 Study Group. Ann. Intern. Med. 1996;124(9):792–802. doi: 10.7326/0003-4819-124-9-199605010-00003. [DOI] [PubMed] [Google Scholar]