Graphical abstract
Keywords: Acyclic nucleoside phosphonates, Open-ring, PMEO-DAPy, 5-Azacytosine, PME-azaC, HPMP-5-azaC, Prodrug, Phosphonate, Antivirals
Abstract
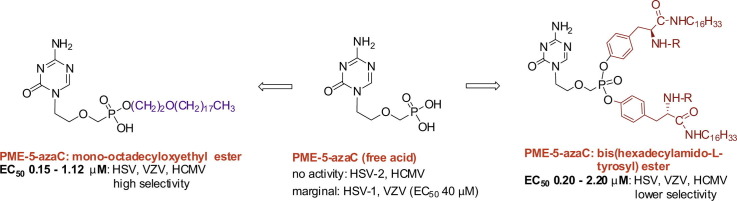
New 2,4-diamino-6-[2-(phosphonomethoxy)ethoxy]pyrimidine (PMEO-DAPy) and 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine (PME-5-azaC) prodrugs were prepared with a pro-moiety consisting of carbonyloxymethyl esters (POM, POC), alkoxyalkyl esters, amino acid phosphoramidates and/or tyrosine. The activity of the prodrugs was evaluated in vitro against different virus families. None of the synthesized prodrugs demonstrated activity against RNA viruses but some of them proved active against herpesviruses [including herpes simplex virus (HSV), varicella-zoster virus (VZV), and human cytomegalovirus (HCMV)]. The bis(POC) and the bis(amino acid) phosphoramidate prodrugs of PMEO-DAPy inhibited herpesvirus replication at lower doses than the parent compound although the selectivity against HSV and VZV was only slightly improved compared to PMEO-DAPy. The mono-octadecyl ester of PME-5-azaC emerged as the most potent and selective PME-5-azaC prodrug against HSV, VZV and HCMV with EC50’s of 0.15–1.12 µM while PME-5-azaC only had marginal anti-herpesvirus activity. Although the bis(hexadecylamido-l-tyrosyl) and the bis(POM) esters of PME-5-azaC were also very potent anti-herpesvirus drugs, these were less selective than the mono-octadecyl ester prodrug.
1. Introduction
Acyclic nucleoside phosphonates (ANPs) are compounds of great importance due to the broad spectrum of biological activities, especially antiviral but also cytostatic, immunomodulatory and antiparasitic.1, 2, 3, 4 Some of them have become already clinically available drugs: cidofovir for the treatment of human cytomegalovirus (CMV) retinitis in AIDS patients, adefovir in a prodrug form as adefovir dipivoxil for the treatment of hepatitis B (HBV) and tenofovir, either as tenofovir disoproxil fumarate, or newly (since 2015) also as a new prodrug form tenofovir alafenamide (TAF) for the treatment of HIV and HBV infections. On the other hand, it should be noted that over the last thirty years of systematic investigation of ANPs, there are dozens of other therapeutically attractive structures synthesized but never advanced to the stage of preclinical/clinical investigations.5 These structures are namely a) antiretroviral purine 3-fluoro-2-[(phosphonomethoxy)propyl] derivatives,6 b) acyclic nucleoside phosphonates with 5-azacytosine base moiety,7 c) 6-[2-(phosphonomethoxy)alkoxy]-2,4-diaminopyrimidines (“open-ring“ derivatives)8, 9, 10 and d) aza/deaza analogues of purine [(phosphonomethoxy)ethyl] derivatives.11
The common structural attribute of all ANPs is their highly polar character caused by the presence of the phosphonic acid residue which is responsible for their unfavourable pharmacological properties: low cell permeability and low oral bioavailability. To overcome this problem, transformation of free acyclic nucleoside phosphonates to appropriate prodrugs is often a solution.
In our work, we focus on two pharmacologically interesting ANP structures: 2,4-diamino-6-[2-(phosphonomethoxy)ethoxy]pyrimidine – an example of the so-called “open-ring” ANPs and on the group of 5-azacytosine derivatives, namely 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine, and their antiviral potential in diverse prodrug forms.
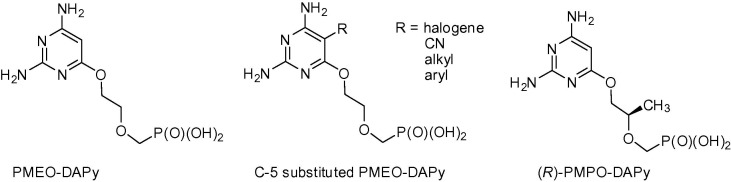
Open-ring ANPs are characterized by the phosphonomethoxy group containing an aliphatic part linked to the position 6 of 2,4-diaminopyrimidine via the oxygen atom. They are evidently mimics of the appropriate 2,6-diaminopurine derivatives with an open imidazole ring. Their antiviral activity is essentially identical to that of their parent compounds, including the enantiomeric specificity. Compounds as PMEO-DAPy, (R)-PMPO-DAPy and 5-substituted PMEO-DAPy (Fig. 1 ) are very efficient inhibitors of retroviruses8, 9, 10 and HBV. Despite the number of antiretroviral drugs currently available on the market, further investigation of new structures is advisable for several reasons: 1) the risk of emergence of resistance, 2) none of the known drugs is able to eradicate HIV infection completely, 3) no vaccination are yet available, 4) early development of new drug candidates is necessary due to the longtime process lasting from in vitro laboratory testing to clinical phases and final approval. Moreover, there is an additional reason to focus on open-ring analogs of the PMEO-DAPy type: they are incorporated more efficiently than (R)-PMPA (tenofovir) by the K65R HIV-1 reverse transcriptase (RT) mutant and they are not as efficiently excised as (R)-PMPA by the HIV-1 RT containing thymidine analog mutations.12 Additionally, PMEO-DAPy is active not only against retroviruses but also against DNA viruses, especially herpesviruses, which often affect immunocompromised patients, including those with HIV/AIDS.
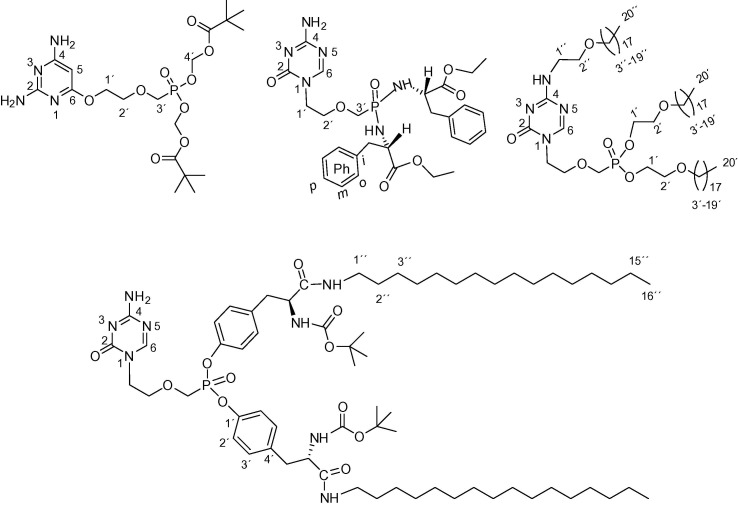
Fig. 1.
Structures of “open-ring” acyclic nucleoside phosphonates.
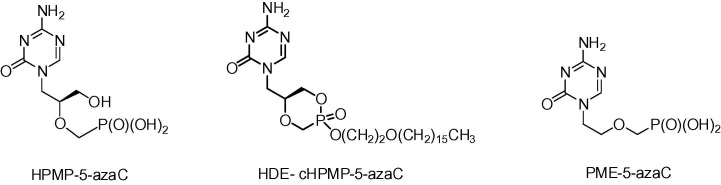
The development of 5-azacytosine ANPs was initiated in our laboratory in order to search for new demethylating (epigenetic) drugs similar to 5-azacytosine nucleosides.13 Although none of the new compounds fulfilled this criterion, we managed to find a new class of antiviral agents, 5-azacytosine analogue of cidofovir 1-(S)-[3-hydroxy-2-(phosphonomethoxy)propyl]-5-azacytosine (HPMP-5-azaC) and various ester prodrugs derived from its cyclic form (Fig. 2 ).7, 14 Compared to cidofovir, HPMP-5-azaC has improved selectivity. The prodrug hexadecyloxyethyl ester of its cyclic form (HDE-cHPMP-5-azaC) revealed the most potent anti-DNA virus activities and also the highest selectivity indices (ratio activity vs toxicity) in the order of thousands, e.g. 1160 for herpes simplex virus (HSV) ≥ 5800 for varicella zoster virus (VZV) and ≥24,600 for HCMV.14 The only disadvantage of HPMP-5-azaC is its complicated metabolic profile bound to instability of the 5-azacytosine ring in alkaline conditions including physiological pH. Studying the stability of various 5-azacytosine ANPs, we found much better stability for another 5-azacytosine derivative, i.e. 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine (PME-5-azaC).15 Despite the fact that its antiviral activity was only marginal in the free phosphonic acid form 7, we selected the compound for syntheses and further studies of its prodrug forms. We considered the fact that the activities of many ANPs were increased after transformation to appropriate prodrugs. Moreover, in some cases not only the activity was enhanced but also the spectrum of activity could be broaden by transformation to prodrugs. Typical examples are the anti-DNA viral agents 1-(S)-[3-hydroxy-2-(phosphonomethoxy)propyl]adenine (HPMPA) whose octadecyloxyethyl (ODE) ester is a potent and selective inhibitor of hepatitis C virus replication16 or cidofovir transformed to its hexadecyloxyethyl ester (brincidofovir) whose efficacy is also enlarged to some RNA viral infections including Ebola virus. In fact, the lipid moiety of brincidofovir was found to be required for in vitro antiviral activity against Ebola virus.17
Fig. 2.
Examples of acyclic nucleoside phosphonates with 5-azacytosine base.
2. Chemistry
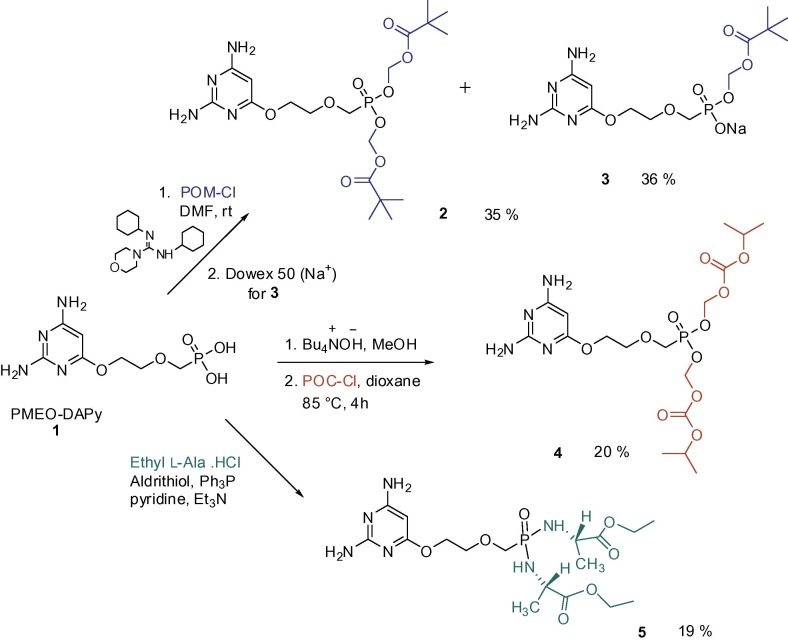
The starting compound 2,4-diamino-6-[2-(phosphonomethoxy)ethoxy]pyrimidine (1) was synthesized according to a procedure described in the literature.8 Briefly: base-catalysed alkylation of 2,4-diamino-6-hydroxypyrimidine with diisopropyl 2-(chloroethoxy)methylphosphonate gave a mixture of appropriate O- and N-diisopropyl (phosphonoethoxy)methyl derivatives where the N-isomer was separated and diisopropyl ester groups deprotected with bromotrimethylsilane. The synthesis of the prodrugs was rather complicated by a low solubility of the starting PMEO-DAPy; finally we managed to synthesize two biodegradable ester prodrugs – pivaloyloxymethyl (POM) and (isopropoxycarbonyl)oxymethyl (POC) esters and the amino acid phosphoramidate prodrug (Scheme 1 ). Pivaloyloxymethylation was performed by reaction of the starting phosphonic acid with chloromethyl pivalate using N,N′-dicyclohexyl-4-morpholinecarboxamidine as a base. The reaction proceeded very slowly to give a mixture of bis(POM) and mono(POM) esters 2 and 3. After chromatographic separation, both compounds were isolated in acceptable yields. Alternative reaction conditions (e.g. reaction in dioxane with DBU) completely failed. Also for the introduction of POC groups, different attempts have been examined (various solvents, DBU or diisopropylethylamine as bases). The only way that led to the bis(POC) derivative 4 consisted in transformation of free PMEO-DAPy to its tetrabutylammonium salt, followed by heating with POC-chloride in dioxane. The bis(amino acid) phosphoramidate prodrug 5 was prepared by the direct coupling of PMEO-DAPy with ethyl l-alaninate in pyridine and treatment with a premixed solution of triphenylphosphine and 2,2′-dipyridyl disulfide (Aldrithiol).
Scheme 1.
Synthesis of various prodrug structures derived from 2,4-diamino-6-[2-(phosphonomethoxy)ethoxy]pyrimidine (1).
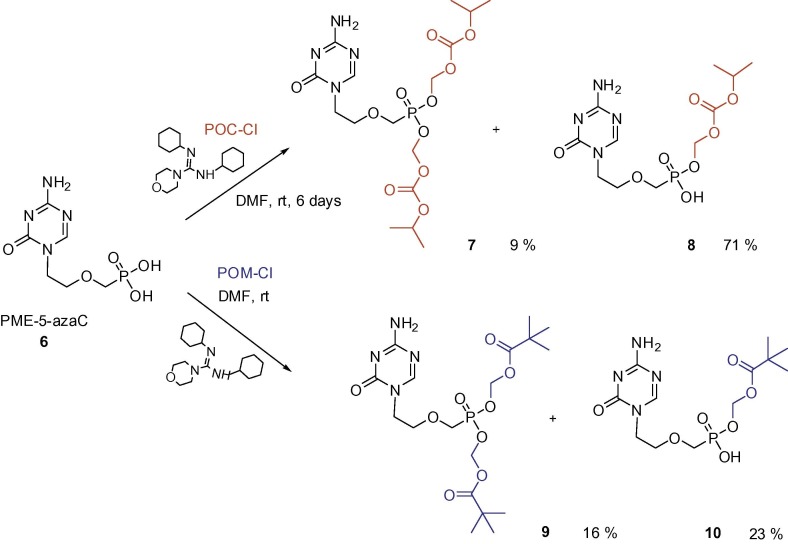
Synthetic efforts to obtain 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine prodrugs were targeted to carbonyloxymethyl esters (POM, POC), alkoxyalkyl esters, amino acid phosphoramidates and tyrosine based prodrugs. The introduction of POC and POM groups has been performed by the action of appropriate alkyl chloride and N,N′-dicyclohexyl-4-morpholinecarboxamidine in dimethylformamide, i.e. conditions described for transformation of adefovir to adefovir dipivoxil18 (Scheme 2 ). Unfortunately, and contrary to adefovir, reactivity and solubility of PME-5-azaC (6) was much lower giving the appropriate diesters 7 and 9 in modest yields only, accompanied by formation of monoesters. In pivaloyloxymethylation of PME-5-azaC, the mono-POM derivative 8 was even obtained as the main reaction product.
Scheme 2.
Synthesis of POM and POC esters derived from 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine (6).
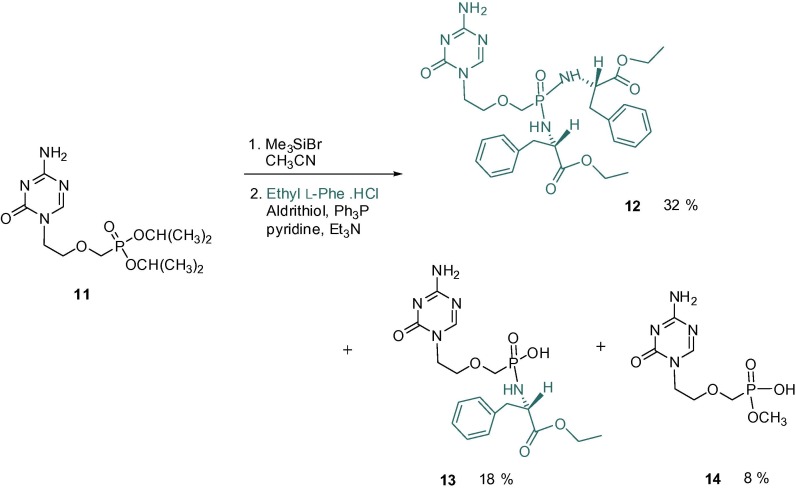
The amino acid phosphoramidates 12 and 13 were synthesized by the method developed by Jansa starting from the phosphonate dialkyl esters.19 Diisopropyl or diethyl esters are formed as common intermediates in syntheses of all ANPs; their deprotection with bromotrimethylsilane leads to the bis(trimethylsilyl) esters in situ which are further hydrolysed to the final phosphonic acids. The principle of this method is the utilization of the intermediary bis(trimethylsilyl) ester of ANP directly for reaction with 2,2′dithiodipyridine, triphenylphosphine and amino acid ester to obtain bis-amidates without the need of laborious procedures to isolate the free phosphonic acids. The selection of amino acid esters is directed especially to diethyl esters of l-alanine or l-phenylalanine leading to prodrugs with optimal pharmacokinetic profile.20 In our case, we selected the diethyl ester of l-phenylalanine (Scheme 3 ). Similarly as with the preparation of POM and POC esters, we obtained not only the desired symmetrical bis-amidate prodrug 12 but also mono-amidate 13 accompanied by a small amount of mono-methyl ester 14. Compound 14 was formed most probably during the chromatographic separation of the mono-amidate prodrug in a system containing high percentage of methanol.
Scheme 3.
Synthesis of phosphoramidate prodrugs of 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine (6).
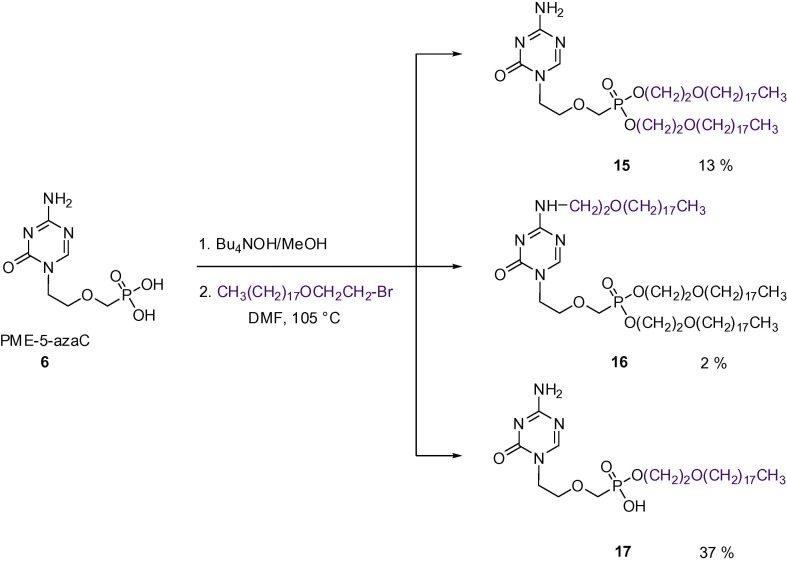
Another type of prodrugs selected for our studies include alkoxyalkylesters.21 The concept of these prodrugs is based on the mimicking of naturally occurring phospholipid lysophosphatidylcholine (LPC). The compounds use the LPC natural uptake pathway in the small intestine to reach the target tissue and achieve oral bioavailability. The most important compound of this family is the hexadecyloxypropyl ester of cidofovir (brincidofovir, CMX-001), currently in Phase III clinical trials for use in humans against HCMV and adenovirus infections. This compound is also being investigated as an experimental drug against smallpox and ebolavirus infections.22 In our previous studies, we have successfully applied the alkoxyalkylester approach to 9-(S)-[3-hydroxy-2-(phosphonomethoxy)propyl]-2,6-diaminopurine ((S)-HPMPDAP)23 within the search for new bioavailable anti-poxvirus agents and for the synthesis of the above mentioned HDE-cHPMP-5-azaC (Fig. 2).14 In our synthesis, the starting PME-azaC was transformed first to its tetrabutylammonium salt, which was subsequently treated with hexadecyloxypropyl bromide to give the mixture of diester and monoester (15, 17). The monoester 17 is predominant most probably for steric reasons. Besides, small amounts of N 4-alkylated product 16 were isolated (Scheme 4 ).
Scheme 4.
Synthesis of alkoxyalkyl esters of 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine (6).
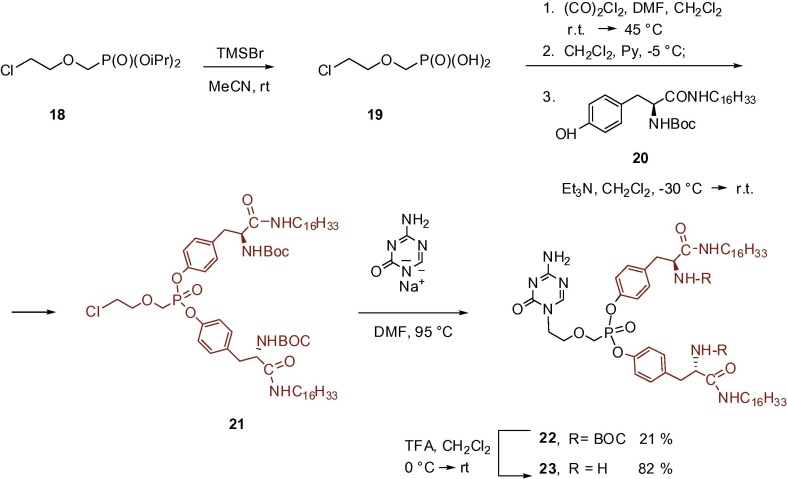
The last type of prodrugs included in this study are amino acid ester prodrugs where esterification of the phosphonic acid residue is performed via the free hydroxyl group of the appropriate hydroxy amino acid (serine, valine, tyrosine). This concept has been originally developed by McKenna’s group to improve the oral bioavailability of (S)-HPMPC and (S)-HPMPA.24, 25 Pharmacokinetic studies of various amino acid or dipeptide prodrugs of these ANPs finally revealed that the tyrosine single amino acid was the most favorable promoiety regarding prodrug plasma stability. It was also found that enzymatic stability of the tyrosine promoiety can be significantly increased by replacement of the carboxyl ester with an alkyl amide group. The highest increase in bioavailability and antiviral activity was observed when a long lipophilic alkyl chain was incorporated into the tyrosine amide group. A comprehensive review including synthesis, SAR studies and pharmacology of tyrosine N-alkyl amide ANP prodrugs has been published recently.26 Considering these findings, we decided to synthesize the symmetrical bis-tyrosine prodrug 23 whose carboxylic function is modified by the hexadecylamido group (Scheme 5 ). The compound can be prepared via alkylation of the 5-azacytosine sodium salt with the whole aliphatic moiety, i.e. BOC protected bis(hexadecylamido-l-tyrosyl) ester of 2-(phosphonomethoxy)ethyl chloride (21) prepared previously and followed by acidic removal of the protecting BOC groups. The tyrosine synthon 21 can be obtained by multistep synthesis from diisopropyl 2-(phosphonomethoxy)ethyl chloride (18) which is known as a common building block in the synthesis of diverse PME derivatives.27 Isopropyl groups in 18 were removed first by the action of bromotrimethylsilane followed by hydrolysis. The intermediary phosphonic acid 19 was then activated by reaction with oxalyl chloride in DMF forming a phosphorodichloridate28 whose further reaction with BOC-protected hexadecyl tyrosine amide 20 in a presence of triethylamine formed diester 21 in good preparative yield. Tyrosine amide precursor 20 was prepared from N-BOC-l-tyrosine by standard coupling procedure using EDC and N-hydroxybenzotriazole (HOBt) in CH2Cl2 (similarly as described for other alkylated l-tyrosine amides, e.g. octyl or octadecyl).26, 29
Scheme 5.
Synthesis of tyrosine-based prodrugs of 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine (6).
3. Antiviral activity
All the synthesized compounds were tested for their antiviral activities against: (a) human cytomegalovirus (HCMV), varicella-zoster virus (VZV) wild type and thymidine kinase deficient (TK−) strains, herpes simplex virus 1 (HSV-1) wild type and TK− strains, herpes simplex virus 2 (HSV-2), vaccinia virus and vesicular stomatitis in human embryonic lung (HEL) cells; Coxsackie virus B4, and respiratory syncytial virus in human cervix carcinoma (HeLa) cells; parainfluenza-3 virus, reovirus, sindbis virus, coxsackie B-4 virus, and punta toro virus in green monkey kidney (VERO) cells, feline corona virus in feline kidney (CRFK) cells; and influenza A H1N1, influenza B H3N2, and influenza B viruses in canine kidney (MDCK) cells. While none of the synthesized compounds displayed activity against RNA viruses, several of them proved active against herpesviruses (Table 1 ).
Table 1.
Antiviral and cytotoxic properties of the synthesized compounds in human embryonic lung (HEL) cells.
| Compound | EC50a (HEL) (µM) |
Cytotoxicity (µM) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HSV-1 (Kos) | HSV-2 (G) | HSV-1 TK− KOS ACVr | VZV TK+ (Oka) | VZV TK− (0 7 −1) | HCMV (AD-169) | HCMV (Davis) | Vaccinia virus | MCCb | CC50c | ||
| 1 | PMEO-DAPy | 45 ± 0 | 20 ± 0 | 54.0 ± 5.7 | 2.07 ± 0.39 | 4.72 ± 2.10 | >100 | >100 | >100 | >100 | 90.3 ± 13.8 |
| 2 | Bis(POM) | 10.0 ± 2.8 | 9.5 ± 7.8 | 14.5 ± 7.8 | 0.67 ± 0.49 | 1.26 ± 0.25 | 47.4 ± 22.2 | 36.5 ± 31.1 | ≥20 | ≥100 | 16.6 ± 1.3 |
| 3 | Mono(POM) | 39.5 ± 7.8 | 29 ± 12.7 | 54.0 ± 5.7 | 2.54 ± 0.20 | 5.91 ± 0.10 | >100 | >100 | >100 | >100 | ≥100 |
| 4 | Bis(POC) | 2.62 ± 1.43 | 1.01 ± 0.86 | 3.7 ± 3.0 | 0.32 ± 0.37 | 0.29 ± 0.19 | 90.1 ± 0 | 86.3 ± 5.4 | 160 ± 60 | >202 | 15.1 ± 10 |
| 5 | Bis(amino acid) phosphoramidate | 3.9 ± 0 | 3.46 ± 0.61 | 6.3 ± 3.4 | 0.56 ± 0 | 1.69 ± 1.04 | 28.4 ± 6.7 | 17.0 ± 8.1 | 157 ± 84 | 217 | 25.9 ± 10.9 |
| 6 | PME-5-azaCd | 39 | 103 | 68 | 100 | 40 | 195 | 147 | >398 | >398 | >200 |
| 7 | Bis(POC) | 22.0 ± 17.0 | 22.0 ± 17.0 | 22.0 ± 17.0 | 15.3 | 23.2 | 26.2 | 20 | >100 | >100 | ND |
| 8 | Mono(POC) | >100 | >100 | >100 | 48.9 | 48.9 | >100 | >100 | >100 | >100 | ND |
| 9 | Bis(POM) | 0.84 ± 0 | 1.26 ± 0.59 | 0.73 ± 0.15 | 0.65 ± 0.03 | 1.32 ± 0.50 | 3.24 ± 0.27 | 0.97 ± 0.19 | 152 ± 81 | 209 | 46.0 ± 5.9 |
| 10 | Mono(POM) sodium salt | 142 ± 25 | 142 ± 25 | 131 ± 10 | 40.7 ± 20.2 | 128 ± 12 | 123 ± 0 | 116 ± 40 | >275 | >275 | >275 |
| 12 | Bis-amidate | 32.5 ± 17.7 | 39.5 ± 7.8 | 42.0 ± 11.3 | 29.4 ± 26.0 | 26.8 ± 32.2 | 15.5 ± 6.4 | 7.5 ± 4.9 | >100 | >100 | >100 |
| 13 | Mono-amidate | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 14 | Mono-methyl ester | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 15 | Bis(octadecyloxyethyl) | >100 | >100 | >100 | >4 | >0.8 | >20 | >20 | >100 | 20 | ND |
| 16 | N4-Bis(octadecyloxyethyl) | >100 | >100 | >100 | 44.7 | 100 | >100 | >100 | >100 | 100 | ND |
| 17 | Mono-octadecyloxyethyl | 0.35 ± 0.21 | 0.15 ± 0.07 | 0.20 ± 0.14 | 0.32 ± 0.06 | 0.79 ± 0.38 | 1.12 ± 0.95 | 0.24 ± 0.11 | >100 | 100 ± 0 | 79.2 ± 7.1 |
| 23 | Bis(hexadecylamido-l-tyrosyl) | 0.60 0.28 | 0.40 0.28 | 0.40 0.28 | 0.58 ± 0.31 | 0.29 ± 0.22 | 2.16 ± 0.52 | 0.48 ± 0.08 | >100 | 60 ± 57 | 27.1 ± 6.3 |
| Acyclovir | 0.40 ± 0.26 | 0.18 0.11 | 54 ± 58 | 1.50 ± 0.96 | 38.9 ± 18.5 | ND | ND | >250 | >440 | >440 | |
| Brivudin | 0.04 ± 0.03 | 141 ± 27 | 45 ± 61 | 0.024 ± 0.020 | 58.8 ± 43.5 | ND | ND | 19.1 ± 15.3 | 300 | 242 ± 53 | |
| Ganciclovir | 0.04 ± 0.02 | 0.03 ± 0.01 | 0.10 ± 0.78 | ND | ND | 6.79 ± 2.71 | 2.96 ± 2.31 | >100 | >350 | ≥308 ± 99.4 | |
| Cidofovir | 2.93 ± 1.91 | 1.39 ± 0.47 | 2.66 ± 2.31 | ND | ND | 0.87 ± 0.31 | 0.76 ± 0.34 | 22.2 ± 2.9 | ≥300 | 216 ± 101 | |
50% effective concentration or compound concentration required reducing viral induced CPE or plaque formation by 50%.
Minimum cytotoxic concentration or compound concentration that caused a microscopically detectable alteration of cell morphology.
50% cytostatic concentration or compound concentration required reducing cell growth by 50%.
Data from Krecmerova et al. (Ref. 7).
Among the synthesized PMEO-DAPy prodrugs, the bis(amino acid) phosphoramidate prodrug 5 emerged as the most active one against HSV, VZV and HCMV. The bis(POC) prodrug 4 was as active as compound 5 against HSV and VZV but proved less effective against HCMV. These prodrugs were 6–20-fold (HSV) and 3–16-fold (VZV) more active than the parental drug PMEO-DAPy and they gained activity against HCMV (EC50’s of 17–29 µM and 86–90 µM for, respectively, compounds 5 and 4 versus >100 µM for compound 1). Together with an increase in antiviral potency, an augmentation of the CC50 (50% cytostatic concentration) was seen for compounds 4 and 5 when compared to PMEO-DAPy. As a consequence, when calculating the selectivity index (SI, ratio: CC50/EC50) for the prodrugs 4 and 5 against HCMV, no selectivity was seen (SI’s ≤ 1). However, these two prodrugs selectively inhibited HSV and VZV replication with SI’s of, respectively, 4–15 and 15–52, which was slightly better than the SI’s for PMEO-DAPy, i.e. 1.7–4.5 (HSV) and 19–44 (VZV).
Pivaloyloxymethylation of PMEO-DAPy led to the synthesis of the bis(POM) and mono(POM) esters 2 and 3 but this strategy did not result in improvement of the anti-herpesvirus activity. Thus, the mono(POM) 3 prodrug had a potency and selectivity virtually identical to PMEO-DAPy while the bis(POM) 2 prodrug was 2 to 4.5-fold more active than PMEO-DAPy but 5.5-fold more cytostatic which resulted in decreased selectivity compared to the parental drug.
Among the carbonyloxymethyl esters (POM, POC) based prodrugs of PME-5-azaC, the bis(POM) compound 9 exhibited improved activity compared to the parent PME-5-azaC which had marginally activity against herpesviruses. The bis(POM) prodrug 9 inhibited the replication of HSV, VZV and HCMV with EC50′s in the range of, respectively, 0.7–1.3 µM, 0.7–1.3 µM and 1–3 µM and had substantial selectivity [i.e. SI’s of 37–63 (HSV), 35–71 (VZV) and 14–47 (HCMV)]. In contrast to the bis(POM) prodrug 9, the mono(POM) prodrug 8 and its sodium salt (i.e. compound 10), presented minimal or no anti-herpesvirus activities. The bis(POC) prodrug 7 demonstrated some anti-herpesvirus activity (EC50 values in the range of 15–22 µM) but was markedly less potent than the bis(POM) prodrug 9. Regarding the synthesized amino acid phosphoramidates prodrugs, the mono-amidate 13 and the mono-methyl ester 14 lacked activity against herpesviruses while the bis-amidate prodrug 12 inhibited herpesvirus replication with EC50’s of 32–42 µM (HSV), 27–29 µM (VZV) and 7.5–15.5 µM (HCMV). Notably, compound 12 was not toxic for cell growth and cell morphology up to the highest concentration tested (i.e. 100 µM).
Amongst the alkoxyalkyl esters, the bis(octadecyloxytheyl) ester 15 and the N4-alkylated prodrug 16 were deprived of anti-herpesvirus properties while the mono-octadecyloxyethyl ester 17 emerged as one of the most active prodrugs with EC50’s of 0.15–0.35 µM (HSV), 0.32–0.8 µM (VZV) and 0.24–1.12 µM (HCMV). Remarkably, compound 17 had very good selectivity against not only HSV (SI’s of 226–528) and VZV (SI’s of 100–248) but also against HCMV (SI’s of 71–330).
The bis(hexadecylamido-l-tyrosyl) ester 23 emerged as an interesting prodrug with significant activity against herpesviruses, i.e. EC50’s in the range of 0.2–0.6 µM (HSV and VZV) and 0.3–2.2 µM (HCMV), which was comparable to the potency demonstrated for the mono-octadecyloxyethyl ester 17. However, compound 23 appeared to be more cytostatic and less selective than compound 17 [CC50 = 79.2 µM for 17 versus CC50 = 27.1 µM for 23 and SI’s for 23 of 45–68 (HSV), 47–93 (VZV) and 13–56 (HCMV).
The bis(POM) ester 9 was the only PME-5-azaC prodrug showing weak activity against vaccinia virus (EC50 = 152 µM) although no selectivity was observed (Table 1). Similarly, two PMEO-DAPy prodrugs [i.e. the bis(POC) ester 4 and the bis(amino acid) phosphoramidate prodrug 5] demonstrated very low anti-vaccinia virus activity without selectivity.
4. Conclusions
We have successfully synthesized carbonyloxymethyl esters (POM, POC), alkoxyalkyl esters, amino acid phosphoramidates and tyrosine based prodrugs of PMEO-DAPy and/or PME-5-azaC that had weak or marginal activity against herpesviruses. The mono-octadecyloxyethyl ester of PME-5-azaC emerged as the most potent and selective inhibitor of herpesviruses (i.e. HSV, VZV and HCMV). The bis(hexadecylamido-l-tyrosyl) and the bis(POM) esters of PME-5-azaC also had potent anti-herpesvirus activity but less selectivity that the mono-octadecyloxyethyl prodrug. The bis(POC) and bis(amino acid) phosphoramidate prodrugs of PMEO-DAPy proved more active than the parental drug against HSV, VZV and HCMV, showing selectivity for HSV and VZV but not for HCMV.
5. Experimental
5.1. General
Unless stated otherwise, solvents were evaporated at 40 °C/2 kPa and compounds were dried at 13 Pa. Analytical TLC was performed on silica gel 60 F254 plates (Merck KGaA, Darmstadt, Germany); chromatographic systems are described in text. Column chromatography was performed on silica gel 60 μm (Fluka). 1H, 13C and 31P NMR spectra were measured on Bruker AVANCE III 500 and/or 600 spectrometers equipped with a cryoprobe and operating at 500.0 or 600.1 MHz (1H), and 125.7 or 150.9 MHz (13C). 31P NMR spectra were measured on Bruker AVANCE III 400 spectrometer operating at 202.3 MHz (31P). The assignment of NMR signals was based on a combination of 1D and 2D correlation experiments (H,H-COSY, H,C-HSQC, H,C-HMBC). The signals were referenced to residual solvent signals (DMSO δ = 2.50 and 39.7 ppm for 1H and 13C, respectively) or to an internal standard. Mass spectra were measured on a LTQ Orbitrap XL (Thermo Fisher Scientific) operated in the ESI mode. Most of chemicals were purchased from Sigma-Aldrich. Diisopropyl 2-chloroethoxymethylphosphonate (18) was prepared according to Ref. 27 General numbering scheme for assignment of NMR signals of selected structures is outlined in Fig. 3 .
Fig. 3.
General numbering scheme for assignment of NMR signals.
5.2. Pivaloyloxymethylation of 2,4-diamino-6-[2-(phosphonomethoxy)ethoxy]pyrimidine (PMEO-DAPy, 1)
A suspension of 1 (221 mg, 0.8 mmol) in DMF (15 mL) was stirred with N,N-dicyclohexyl-4-morpholinecarboxamidine (460 mg, 1.6 mmol) and chloromethyl pivalate (0.4 mL, 2.8 mmol) at room temperature for 6 days (dissolution occurred after 24 h). The solution was evaporated. The residue was chromatographed on a column of silica gel (100 mL) in ethyl acetate (elution of POM−Cl and its decomposition by-products), followed by system ethyl acetate–acetone–ethanol–water (15:3:4:3) to give bis(POM) derivative 2. Further elution of the column with methanol afforded mono-POM prodrug 3.
5.2.1. Bis(pivaloyloxymethyl) ester of 2,4-diamino-6-[2-(phosphonomethoxy)ethoxy]pyrimidine (2)
Yield 140 mg (35%) as a colorless syrup. 1H NMR (CDCl3, ppm) δ: 1.22 (s, 18 H, CH3), 3.86 (m, 2H, H-2′), 3.94 (d, 2H, J 3′,P = 7.6, H-3′), 4.35 (m, 2H, H-1′), 4.86 (bs, 2H, NH2), 4.98 (bs, 2H, NH2), 5.27 (s, 1H, H-5), 5.67–5.73 (m, 4H, H-4′). 13C NMR (CDCl3, ppm) δ: 26.80 (CH3), 38.70 (C(CH3)3), 64.67 (C-1′), 65.54 (d, J C,P = 165.6, C-3′), 71.62 (d, J C,P = 10.2, C-2′), 78.46 (C-5), 81.69 (d, J C,P = 6.3, C-4′), 161.69 and 164.47 (C-2, C-4), 170.86 (C-6), 176.84 (C O). ESIMS, m/z: 515.3 (M+Na)+ (76), 493.3 (100) (MH)+. HRMS (ESI): For C19H34N4O9P (MH)+ calculated: 493.20579; found: 493.20588.
5.2.2. Pivaloyloxymethyl ester of 2,4-diamino-6-[2-(phosphonomethoxy)ethoxy]-pyrimidine (3)
Yield 110 mg (36%) of white amorphous solid. 1H NMR (DMSO-d 6, ppm) δ: 1.13 (s, 9H, CH3), 3.41 (m, 2H, H-3′), 3.67 (m, 2H, H-2′), 4.17 (m, 2H, H-1′), 5.04 (s, 1H, H-5), 5.45 (d, 2H, J 4′, P = 11.2, H-4′), 5.96 (bs, 2H, NH2), 6.11 (bs, 2H, NH2). 13C NMR (DMSO-d 6, ppm) δ: 26.93 (CH3), 38.34 (C(CH3)3), 64.24 (C-1′), 68.63 (d, J C,P = 157.0, C-3′), 70.45 (d, J C,P = 9.9, C-2′), 76.36 (C-5), 83.40 (d, J C,P = 4.1, C-4′), 162.65 and 165.73 (C-2, C-4), 170.09 (C-6), 176.95 (C O). ESIMS, m/z: 377.2 (12) (M−H)−. HRMS (ESI): For C13H22N4O7P (M−H)− calculated: 377.12316; found: 377.12327.
5.3. Bis[(isopropoxycarbonyl)oxymethyl] ester of 2,4-diamino-6-[2-(phosphonomethoxy)ethoxy]pyrimidine (4)
1 M methanolic solution of tetrabutylammonium hydroxide (1 mL) was added to a suspension of 1 (264 mg, 1 mmol) in absolute MeOH (75 mL) and the suspension stirred vigorously or treated in ultrasonic bath to dissolution. The clear solution was evaporated and the residue coevaporated with dioxane (2 × 25 mL). Dry dioxane (25 mL) was added, followed by POC chloride (2.6 mL, 20 mmol) and the mixture heated to 85 °C for 4 h. Reaction course was monitored by TLC in 15% methanol in chloroform. The mixture was set aside at room temperature overnight and evaporated. The residue was partitioned between water and chloroform (50 mL each), the organic layer dried over Na2SO4 and evaporated. The residue was chromatographed on silica gel in a gradient 5–15% methanol in chloroform. Yield 100 mg (20%) of a white amorphous solid. 1H NMR (CDCl3, ppm) δ: 1.318 (d, 6H, J CH3,CH = 6.3, CH3), 1.320 (d, 6H, J CH3,CH = 6.3, CH3), 3.88 (m, 2H, H-2′), 3.99 (d, 2H, J H-C-P = 7.4, H-3′), 4.36 (m, 2H, H-1′), 4.93 (sept, 2H, J CH,CH3 = 6.3, CH), 5.29 (s, 1H, H-5), 5.69–5.73 (m, 4H, O-CH2-O). 13C NMR (CDCl3, ppm) δ: 21.61 (CH3), 64.71 (C-1′), 65.50 (d, J C,P = 165.7, C-3′), 71.61 (d, J 2′,P = 10.0, C-2′), 73.36 (CH iPr), 78.41 (C-5), 84.29 (d, J C,P = 6.2, OCH2O), 153.11 (C O), 170.91 (C-6). C-2 and C-4 not found. ESIMS, m/z: 519.2 (M+Na)+ (12), 497.2 (12) (MH)+. HRMS (ESI): For C17H30N4O11P (MH)+ calculated: 497.16432; found: 497.16433.
5.4. Bis(l-alanine ethyl ester) prodrug of 2,4-diamino-6-[2-(phosphonomethoxy)ethoxy]pyrimidine (5)
A solution consisting of 1 (730 mg, 2.76 mmol), ethyl l-alaninate (1.7 g, 11 mmol), triethylamine (6 mL) and pyridine (25 mL) was heated to 60 °C for 5 min (solution A). Triphenylphosphine (4.3 g, 16 mmol) and Aldrithiol (3.5 g, 16 mmol) were dissolved in pyridine (16 mmol) and this solution added to the solution A. The whole reaction mixture was heated to 60 °C for 4 h, set aside at room temperature overnight and evaporated. The residue was coevaporated with toluene and chromatographed on a column of silica gel (700 mL), starting with system 5% methanol in chloroform (elution of triphenylphosphine oxide and mercaptopyridine), followed by the gradient 5–50% methanol in chloroform. The crude sirupy product 5 (1.25 g) still containing rests of ethyl l-alaninate was purified by further chromatography on silica gel in system ethyl acetate–acetone–ethanol–water (18:3.2.2). Yield 238 mg (19%) of a white foam. 1H NMR (CDCl3, ppm) δ: 1.26 (t, 3H, J CH3,CH2 = 7.1, CH3-CH2), 1.28 (t, 3H, J CH3,CH2 = 7.1, CH3CH2), 1.38 (d, 3H, J CH3,CH = 7.1, CH3-CH), 1.40 (d, 3H, J CH3,CH = 7.1, CH3-CH), 3.42 (t, 1H, J H-N-P = J NH-CH = 10.4, NH), 3.63 (dd, 1H, J H- N -P = 12.5, J NH-CH = 10.5, NH), 3.80–3.86 (m, 4H, H-2′, H-3′), 4.02–4.08 (m, 2H, CH-NH), 4.12–4.22 (m, 4H, CH2CH3), 4.36 (t, 2H, J 1′,2′ = 9.6, H-1′), 4.86 (bs, 2H, 4-NH2), 4.98 (bs, 2H, 2-NH2), 5.27 (s, 1H, H-5). 13C NMR (CDCl3, ppm) δ: 14.09 and 14.12 (CH3-CH2), 21.27–21.38 (m, CH3-CH), 48.31 and 48.86, (CH-NH), 61.28 and 61.30 (CH2-CH3), 64.55 (C-1′), 68.31 (d, J C,P = 134.2, C-3′), 71.62 (d, J C,P = 12.7, C-2′), 78.32 (C-5), 162.26 and 165.13 (C-2, C-4), 170.87 (C-6), 174.41 (d, J C-C- N -P = 4.3, C O), 174.58 (d, J C-C- N -P = 4.9, C O). ESIMS, m/z: 485.3 (M+Na)+ (100), 463.3 (80) (MH)+. HRMS (ESI): For C17H30N4O11P (MH)+ calculated: 463.20646; found: 463.20646.
5.5. Reaction of 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine (6) with (isopropoxycarbonyl)oxymethyl chloride
A mixture of 6 (500 mg, 2 mmol), N,N-dicyclohexyl-4-morpholinecarboxamidine (1.16 g, 4 mmol) and POC-Cl (0.8 mL, 6 mmol) in DMF (30 mL) was stirred at room temperature for 3 days. The mixture was evaporated, the residue coevaporated with toluene (2 × 50 mL) and chromatographed on a silica gel column starting with ethyl acetate (elution of the rest of POC-Cl), followed by system ethyl acetate–acetone–ethanol–water (15:3:4:3) to give diester 7 (RF 0.70) and monoester 8 (RF 0.20).
5.5.1. Bis[(isopropoxycarbonyl)oxymethyl] ester of 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine (7)
Yield 85 mg (9%) of a white solid. 1H NMR (DMSO-d 6, ppm) δ: 1.25 (d, 12H, J CH3,CH = 6.2, CH3), 3.69 (m, 2H, H-2′), 3.83 (m, 2H, H-1′), 3.98 (d, 2H, J P,CH2 = 7.8, H-3′), 4.83 (sept, 2H, J CH,CH3 = 6.2, CH(CH3)2), 5.57–5.61 (m, 4H, OCH2O), 7.37 and 7.39 (m, 2H, NH2), 8.14 (s, 1H, H-6). 13C NMR (DMSO-d 6, ppm) δ: 21.48 (CH3), 45.87 (C-1′), 64.26 (d, J P,C = 163.0, C-3′), 69.94 (d, J P,C = 11.1, C-2′), 73.07 (CH(CH3)2), 84.38 (d, J P,C = 6.0, OCH2O), 152.73 (C O), 153.93 (C-2), 159.47 (C-6), 166.60 (C-4). ESIMS, m/z: 505.1 (M+Na)+ (100), 483.1 (18) (MH)+. HRMS (ESI): For C16H27N4O11PNa (M+Na)+ calculated: 505.13062; found: 505.13040.
5.5.2. (Isopropoxycarbonyl)oxymethyl ester of 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine (8)
Yield 524 mg (71%) of a white foam. 1H NMR (DMSO-d 6, ppm) δ: 1.22 (d, 6H, J CH3,CH = 6.2, CH3), 3.32 (d, 2H, J 3′, P = 8.1, H-3′), 3.59 (m, 2H, H-2′), 3.82 (m, 2H, H-1′), 4.76 (sept, 1H, J CH,CH3 = 6.2, CH(CH3)2), 5.36 (d, 2H, J H-C-O-P = 11.8, OCH2O), 7.33 (bs, 2H, NH2), 8.26 (s, 1H, H-6). 13C NMR (DMSO-d 6, ppm) δ: 13C NMR (DMSO-d 6, ppm) δ: 21.63 (CH3), 46.07 (C-1′), 68.78 (d, J P,C = 153.8, C-3′), 69.01 (d, J P,C = 8.5, C-2′), 71.41 (CH(CH3)2), 85.82 (d, J P,C = 4.8, OCH2O), 153.51 (C O), 154.02 (C-2), 159.76 (C-6), 166.61 (C-4). ESIMS, m/z: 731.2 (10) (2 M−H)−, 365.1 (100) (M−H)−. HRMS (ESI): For C11H18N4O8P (M−H)− calculated: 365.08677; found: 365.08679.
5.6. Pivaloyloxymethylation of 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine
Reaction of PME-5-azaC 6 (450 mg, 1.8 mmol) with POM−Cl (0.9 mL, 6.5 mmol) was performed under the same conditions as described previously for 7 and 8. Chromatography in system ethyl acetate–acetone–ethanol–water (15:3:4:3) afforded bis(POM) ester 9 (RF 0.75) and mono(POM) ester 10 (RF 0.25).
5.6.1. Bis(pivaloyloxymethyl) ester of 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine (9)
Yield 135 mg (16%) of a colorless syrup crystallizing in refrigerator. 1H NMR (CDCl3, ppm) δ:1.23 (s, 18H, CH3), 3.83 (m, 2H, H-2′), 3.85 (d, 2H, J P,CH = 7.8, H-3′), 3.98 (m, 2H, H-1′), 5.65–5.71 (m, 4H, OCH2O), 5.77 (bs, 1H, NH), 6.56 (bs, 1H, NH), 8.02 (s, 1H, H-6). 13C NMR (CDCl3, ppm) δ: 26.80 (CH3), 38.70 (C(CH3)3), 47.26 (C-1′), 65.54 (d, J C,P = 166.8, C-3′), 70.33 (d, J C,P = 10.1, C-2′), 81.64 (d, J C-O-P = 6.3, OCH2O), 154.21 (C-2), 159.30 (C-6), 166.53 (C-4), 176.79 (COO). ESIMS, m/z: 501.3 (M+Na)+ (100), 479.3 (13) (MH)+. HRMS (ESI): For C18H31N4O9PNa (M+Na)+ calculated: 501.17209; found: 501.17187.
5.6.2. Pivaloyloxymethyl ester of 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine, sodium salt (10)
The crude product (620 mg, still in the form of dicyclohexylmorpholinocarboxamidine salt) was applied onto a column of Dowex 50 (Na+ form, 50 mL) and eluted with water. UV absorbing fraction was evaporated to give sodium salt of 10 in yield 150 mg (22%) as a white solid. 1H NMR (D2O, ppm) δ: 1.17 (s, 9H, CH3), 3.68 (d, 2H, J P,CH = 9.0, H-3′), 3.76 (m, 2H, H-2′), 4.03 (m, 2H, H-1′), 5.49 (d, 2H, J H-C-O-P = 12.8, OCH2O), 8.30 (s, 1H, H-6). 13C NMR (D2O, ppm) δ: 26.75 (CH3), 39.11 (C(CH3)3), 48.1 (C-1′), 67.41 (d, J C,P = 158.5, C-3′), 70.18 (d, J C,P = 13.3, C-2′), 83.55 (d, J C-O-P = 5.4, OCH2O), 157.08 (C-2), 161.28 (C-6), 166.75 (C-4), 180.92 (COO). ESIMS, m/z: 363.3 (100) (M−H)−. HRMS (ESI): For C12H20N4O7P (M−H)− calculated: 363.10751; found: 363.10735.
5.7. Synthesis of amidate prodrugs of 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine
Diisopropyl ester 11 (1.1 g, 3.29 mmol) in acetonitrile (30 mL) was stirred with bromotrimethylsilane (2.2 mL, 16.5 mmol) at room temperature for 24 h. The mixture was evaporated and the residue coevaporated with toluene (2 × 50 mL). A mixture consisting of: diethyl (l)-phenylalanine hydrochloride (3.0 g, 13.1 mmol), pyridine (26 mL) and triethylamine (6.5 mL) was added under argon. The resulting mixture was heated to 60 °C for 5 min. A solution of Ph3P (5.2 g, 19.74 mmol) and Aldrithiol (4.35 g, 19.7 mmol) in pyridine (20 mL) was added, the reaction mixture heated to 50 °C for 3 h, then set aside at room temperature overnight and evaporated. The residue was coevaporated with toluene (3 × 40 ml), applied onto column of silica gel and subjected to flash chromatography starting with chloroform (elution of Ph3P). Additional elution was performed with a gradient 1–20% MeOH in chloroform (elution of Ph3P-O, followed by pyridine-thiol). Bis-amidate 12 was eluted subsequently, in system 20% MeOH in chloroform. Product containing fractions were evaporated and dried in vacuo.
5.7.1. Bis(l-phenylalanine ethyl ester) prodrug of 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine (12)
Yield: 248 mg (18%) of a white foam. 1H NMR (DMSO-d 6, ppm) δ: 1.06 (t, 3H, J CH3CH2 = 7.1, CH3), 1.12 (t, 3H, J CH3CH2 = 7.1, CH3), 2.74–2.91 (m, 4H, Ph-CH2), 3.15 (dd, 1H, J 3′a, P = 8.3, J gem = 13.2, H-3′a), 3.26 (bddd, 1H, J 3′b, P = 7.9, J gem = 13.2, H-3′b), 3.47 (m, 2H, H-2′), 3.77 (m, 2H, H-1′), 3.90 (m, 1H, CH), 3.94–4.07 (m, 5H, CH2CH3, CH), 4.19 (dd, 1H, J NH,P = 12.3, J NH,CH = 10.7, NH), 4.51 (dd, 1H, J NH,P = 12.3, J NH,CH = 10.9, NH), 7.13 (m, 2H, o-Ph), 7.18 (m, 2H, o-Ph), 7.19–7.29 (m, 6H, p-Ph, m-Ph), 7.38 and 7.41 (2x bs, 2H, NH2), 8.16 (bs, 1H, H-6). 13C NMR (DMSO-d 6, ppm) δ: 14.08 and 14.14 (CH3), 40.15 (m, CH2-Ph), 46.13 (C-1′), 53.98 (CH), 54.32 (CH), 60.50 (CH2CH3), 60.61 (CH2CH3), 67.45 (d, J C,P = 135.1, C-3′), 69.61 (d, J C,P = 11.6, C-2′), 126.64 and 126.69 (p-Ph), 128.29 and 128.33 (m-Ph), 129.62 and 129.65 (o-Ph), 137.34 and 137.37 (i-Ph), 154.11 (C-2), 159.66 (C-6), 166.67 (C-4), 172.93 (d, J C,P = 4.6, C O), 173.00 (d, J C,P = 2.8, C O). ESIMS, m/z: 1123.5 (2 M+Na)+ (3), 623.2 (M+Na)+ (100), 601.3 (8) (MH)+. HRMS (ESI): For C28H37N6O7PNa (M+Na)+ calculated: 623.23536; found: 623.23523. Anal. Calcd. for C28H37N6O7P. ½ H2O: C, 55.20; H, 6.29; N, 13.79; P, 5.08. Found: C, 55.29; H, 6.28; N, 13.46; P, 5.01.
5.7.2. (l-Phenylalanine ethyl ester) prodrug of 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine (13)
After elution of bis(amidate) 12, the column was eluted with methanol. The UV absorbing eluate was concentrated, adsorbed to a small amount of silica gel (20–30 mL) and applied onto a column of silica gel (broad and short column, 150 mL). Elution with system ethyl acetate–acetone–ethanol–water (15:3:4:3) afforded monoamidate prodrug 13 (RF 0.2) in yield 248 mg (18%) as a white solid. 1H NMR (CD3OD, ppm) δ: 1.14 (t, 3H, J CH3,CH2 = 7.2, CH3), 2.92 (dd, 1H, J P,CHa = 7.0, J gem = 13.3, CHa-Ph), 2.98 (dd, 1H, J P,CHb = 6.3, J gem = 13.3, CHb-Ph), 3.39 (d, 2H, J 3′,P = 8.6, H-3′), 3.62 (m, 2H, H-2′), 3.94 (m, 2H, H-1′), 4.05 (m, 2H, CH2CH3), 4.11 (m, 1H, CH), 7.18–7.30 (m, 5H, H-arom.), 8.26 (s, 1H, H-6).
13C NMR (CD3OD, ppm) δ: 14.44 (CH3), 42.64 (m, CH2-Ph), 48.26 (C-1′), 57.35 (CH), 61.90 (CH2CH3), 70.46 (d, J C,P = 146.9, C-3′), 70.94 (d, J C,P = 13.1, C-2′), 127.67 (p-Ph), 129.32 (m-Ph), 130.63 (o-Ph), 138.58 (i-Ph), 157.28 (C-2), 161.29 (C-6), 168.31 (C-4), 175.78 (C O). ESIMS, m/z: 424.2 (100) (M−H)−. HRMS (ESI): For C17H25N5O6P (M−H)− calculated: 426.15370; found: 426.15355.
5.7.3. Methyl ester of [2-(phosphonomethoxy)ethyl]-5-azacytosine (14)
After elution of monoamidate 13, the column was eluted with methanol to give compound 14, RF 0.1 (ethyl acetate–acetone–ethanol–water, 15:3:4:3). Yield: 70 mg (8%) as a white solid. 1H NMR (CD3OD, ppm) δ: 3.58 (d, 3H, J CH3,P = 10.3, CH3), 3.64 (d, 2H, J P,CH2 = 8.9, H-3′), 3.74 (m, 2H, H-2′), 3.99 (m, 2H, H-1′), 8.25 (s, 1H, H-6). 13C NMR (CD3OD, ppm) δ: 48.33 (C-1′), 52.51 (d, J C,P = 5.8, CH3), 67.58 (d, J C,P = 160.4, C-3′), 70.80 (d, J C,P = 12.0, C-2′), 157.03 (C-2), 161.35 (C-6), 168.26 (C-4). ESIMS, m/z: 265.1 (100) (MH)+, 287.1 (M+Na)+ (23), 529.2 (2M+H)+ (50), 551.2 (2 M+Na)+ (15).
5.8. Octadecyloxyethyl (ODE) esters of PME-azaC
1 M solution Bu4N+OH− in THF (12 mL, 12 mmol) was added to a stirred suspension of 6 (1.5 g, 6 mmol) in absolute MeOH (50 mL). After 5 min, the resulting solution was evaporated and the residue coevaporated with DMF. Dry DMF (35 mL) and ODE-Br (5.6 g, 15 mmol) were added and the reaction mixture heated to 105 °C for 5 h. The mixture was evaporated, the residue partitioned between chloroform and water, an organic layer dried over magnesium sulphate and evaporated. The residue was chromatographed on a column of silica gel (800 mL) in system 7.5% methanol in CHCl3 to give crude products 16 (RF 0.48) and 15 (RF 0.28). Both compounds required further purification (see below). Further elution of the column with methanol afforded the crude monoester 17 (its further purification described below).
5.8.1. Bis(octadecyloxyethyl) ester of 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine (15)
1.3 g of the crude compound 15 still containing UV non-absorbing impurities was chromatographed on silica gel (400 mL) in system ethyl acetate–acetone–ethanol–water (18:3:2:2) with UV detection, followed by spraying of TLC plate with a solution of phosphomolybdenic acid and heating. Pure fractions were collected and evaporated to give 15 as a white solid. Yield: 678 mg (13.4%). 1H NMR (CDCl3, ppm) δ: 0.88 (t, 6H, J H-20′-H-19′ = 7.0, H-20′), 1.21–1.33 (m, 60H, H-5′- H-19′), 1.56 (m, 4H, H-4′), 3.45 (t, 4H, J 3′,4′ = 6.8, H-3′), 3.61 (t, 4H, J 2′,1′ = 4.8, H-2′), 3.82 (m, 2H, NCH2 CH2), 3.86 (d, 2H, J P,CH2 = 8.3, PCH2), 3.99 (m, 2H, NCH2), 4.16–4.27 (m, 4H, H-1′), 5.66 bs and 6.37bs (2H, NH2), 8.06 (s, 1H, H-6). 13C NMR (CDCl3, ppm) δ:14.10 (C-20′), 22.67 (C-19′), 26.05 (C-5′), 29.34–29.69 (m, C-4′, C-6′- C-17′), 31.90 (C-18′), 47.25 (N-CH2), 65.17 (d, J P,C = 167.6, P-C), 65.33 (d, J C,P = 6.8, C-1′), 69.60 (d, J C,P = 5.6, C-2′), 70.10 (d, J C,P = 10.2, NCH2 CH2), 71.46 (C-3′), 154.32 (C-2), 159.47 (C-6), 166.52 (C-4). MALDI MS, m/z: 843.7 (5) (MH)+, 865.7 (M+Na)+ (26). HRMS (MALDI): For C46H91N4O7PNa (M+Na)+ calculated: 865.6518; found: 865.6510; for C46H92N4O7P (MH)+ calculated: 843.6698; found: 843.6688.
5.8.2. Bis(octadecyloxyethyl) ester of N4-octadecyloxyethyl-1-[2-(phosphonomethoxy)ethyl]-5-azacytosine (16)
3.5 g of the crude product 16 was purified on a column of silica gel (400 mL) in system ethyl acetate–acetone–ethanol–water (36:6:1:1) analogously as described above. Yield: 110 mg (1.6%) of a white solid. 1H NMR (CDCl3, ppm) δ: 0.88 (t, 9H, J CH3,CH2 = 7.0, CH3), 1.22–1.33 (m, 90H, H-5′–H-19′, H-5″–H-19″), 1.56 (m, 6H, H-4′, H-4″), 3.42–3.48 (m, 6H, H-3′, H-3″), 3.56 (m, 2H, H-2″), 3.60–3.65 (m, 6H, H-2′, H-1″), 3.81 (m, N1-CH2 CH2), 3.84 (d, J P,CH2 = 8.3, PCH2), 3.97 (m, 2H, N1-CH2), 4.14–4.31 (m, 4H, H-1′), 5.83 (bt, 1H, J NH,1″ = 5.6, NH), 7.97 (s, 1H, H-6). 13C NMR (CDCl3, ppm) δ: 14.11 (C-20′, C-20″), 22.68 (C-19′, C-19″), 26.07 (C-5′, C-5″), 29.35–29.71 (m, C-4′, C-6′-C-17′, C-4″, C-6″-C-17″), 31.91 (C-18, C-18″), 41.06 (C-1″), 47.18 (N1-CH2), 65.21 (d, J C,P = 167.5, P-C), 65.32 (d, J C,P = 6.8, C-1′), 68.59 (C-2″), 69.62 (d, J C,P = 5.7, C-2′), 70.36 (d, J C,P = 10.4, N1-CH2 CH2), 71.38 (C-3″), 71.48 (C-3′), 154.61 (C-2), 158.30 (C-6), 164.57 (C-4). MALDI MS, m/z: 1140.0 (6) (MH)+, 1162.0 (M+Na)+ (12). HRMS (MALDI): For C66H131N4O8PNa (M+Na)+ calculated: 1161.9597; found: 1161.9617; for C66H132N4O8P (MH)+ calculated: 1139.9777; found: 1139.9779.
5.8.3. Octadecyloxyethyl ester of 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine (17)
The crude compound 17 was absorbed to a small amount of silica (20–30 mL) from a mixture methanol–chloroform (1:1), applied to a silica gel column (250 mL) and chromatographed in system ethyl acetate–acetone–ethanol–water (15:3:4:3). Yield: 1.2 g (36.6%) of a white solid. 1H NMR (CD3OD, ppm) δ: 0.90 (t, 3H, J H-20′- H-19′ = 7.0, H-20′), 1.24–1.36 (m, 30H, H-5′-′ H-19′), 1.55 (m, 2H, H-4′), 3.46 (t, 2H, J 3′,4′ = 6.7, H-3′), 3.56 (m, 2H, H-2′), 3.65 (d, 2H, J P,CH2 = 9.0, PCH2), 3.73 (m, 2H, N-CH2 CH2), 3.97–4.02 (m, 4H, N-CH2, H-1′), 8.28 (s, 1H, H-6). 13C NMR (CD3OD, ppm) δ: 14.45 (CH3), 23.75 (C-19′), 27.27 (C-5′), 30.49–30.84 (m, C-4′, C-6′-C17′), 33.09 (C-18′), 48.37 (N-CH2), 65.26 (d, J C,P = 5.7, C-1′), 68.20 (d, J C,P = 159.7, P-C), 70.86 (d, J C,P = 12.3, N-CH2 CH2), 71.80 (d, J C,P = 6.6, C-2′), 72.37 (C-3′), 157.12 (C-2), 161.39 (C-6), 168.34 (C-4). ESIMS, m/z: 545.3 (100) (M−H)−. HRMS (ESI): For C26H50N4O6P (M−H)− calculated: 545.34734; found: 545.34706.
5.9. [(2-Chloroethoxy)methyl]phosphonic acid (19)
A mixture of 18 (3 g, 10.6 mmol) and bromotrimethylsilane (15 mL, 119.8 mmol) in acetonitrile (65 mL) was stirred overnight at room temperature, then evaporated and coevaporated with water (2 × 20 mL). The mixture was dissolved in water (50 mL) and extracted with diethyl ether (3 × 10 mL). The aqueous layer was evaporated, the residue coevaporated with toluene (2 × 20 mL) and dried under reduced pressure to dryness. Yield: 2.1 g (56.9%) of a colorless oil. 1H NMR (500.0 MHz, D2O, ref(dioxane) = 3.75 ppm): 3.75 (m, 2H, CH2Cl); 3.83 (d, 2H, J H,P = 8.6, CH2P); 3.90 (m, 2H, CH2O). 13C NMR (125.7 MHz, D2O, ref(dioxane) = 69.30 ppm): 45.62 (CH2Cl); 68.36 (d, J C,P = 159.3, CH2P); 75.55 (d, J C,P = 10.9, CH2O). 31P{1H} NMR (202.3 MHz, D2O): 20.73. HRMS (ESI): for C8H8ClO4P (MH)+ calculated: 174.9849; found: 174.9922. Anal. Calcd. for C3H8ClO4P: C, 20.65; H, 4.62. Found: C, 20.52; H, 4.77.
5.10. Di-tert-butyl ((2S,2′S)-(({[(2-chloroethoxy)methyl]phosphoryl}bis(oxy))bis(4,1-phenylene))bis(3-(hexadecylamino)-3-oxopropane-1,2-diyl))dicarbamate (21)
A mixture of 18 (3.6 g, 13.8 mmol) and bromotrimethylsilane (19 mL, 151.8 mmol) in acetonitrile (80 mL) was stirred overnight at room temperature, evaporated and coevaporated with water (2 x 20 mL), followed by toluene (2 x 20 mL). The residue was dissolved in dry dichloromethane (60 mL) and DMF (0.7 mL, 9.2 µmol) under argon, followed by a dropwise addition of oxalyl chloride (3.7 mL, 41.0 mmol, reflux occurs). The mixture was stirred for 30 min at room temperature, then 30 min at 45–50 °C and evaporated. The residue was dissolved in dichloromethane (70 mL) under argon. Pyridine (5.7 mL, 71.3 mmol) was added dropwise at −5 °C and the mixture stirred for 25 min (Solution A). In another flask, tyrosine amide precursor 20 (13.9 g, 27.6 mmol) was suspended in dichloromethane (520 mL) at room temperature, followed by addition of dioxane (80 mL). After that, triethylamine was added at −30 °C (Solution B). Solution A was added via cannulation to a solution B at −30 °C. The resulting reaction mixture was allowed to warm to room temperature, stirred overnight and then heated to 45–55 °C till conversion proceeded. The mixture was evaporated and the residue chromatographed on a column of silica gel in a gradient of hexane-ethyl acetate (6:4) to (1:9). Yield: 3.8 g (24.2 %) of a white amorphous solid. Rf = 0.27 (hexane-ethyl acetate 4:6). 1H NMR (CDCl3, ppm) δ: 0.88 (m, 6H, H-16′′′); 1.19-1.32 (m, 52H, H-3′′′-15′′′); 1.40 (bm, 4H, H-2′′′); 1.419, 1.421 (2 × s, 2 × 9H, CH3 (Boc)); 2.92, 2.98 (2 × bm, 2 × 2H, H-5′′); 3.17 (bm, 4H, H-1′′′); 3.67 (t, 2H, J 1′-2′ = 5.6, H-1′); 3.94 (t, 2H, J 2′-1′ = 5.6, H-2′); 4.15 (d, 2H, J 3′-P = 7.3, H-3′); 4.22 (bq, 2H, J 6′′-NH = J 6′′-5′′ = 7.3, H-6′′); 5.06 (bs, 2H, NH-6′′); 6.27 (bs, 2H, NH-1′′′); 7.03 (bm, 4H, H-2′′); 7.12 (m, 4H, H-3′′). 13C NMR (CDCl3, ppm) δ: 14.12 (C-16′′′); 22.68, 26.84 (C-3′′′-15′′′); 28.28 (Me (Boc)); 29.25, 29.35, 29.45, 29.53, 29.61, 29.65, 29.69, 31.91 (C-2′′′-15′′′); 37.92 (C-5′′); 39.52 (C-1′′′); 42.40 (C-1′); 56.19 (C-6′′); 64.66 (d, J 3′-P = 169.3, C-3′); 73.31 (d, J 2′-P = 10.6, C-2′); 80.30 (CMe3 (Boc)); 120.49, 120.88 (C-2′′); 130.59 (C-3′′), 134.29, 134.43 (C-4′′); 148.83, 149.13 (2 × d, J 1′′-P = 9.0, C-1′′); 155.69 (CO (Boc)); 171.10 (C-7′′). ESIMS: 1169 (M+Na)+ (100). HRMS (ESI): For C63H108O10N4ClNaP (M+Na)+ calculated: 1169.7392; found: 1169.7384. Anal Calcd for C63H108O10N4ClP: C, 65.91; H, 9.48; N, 4.88; P, 2.70 Cl, 3.09. Found: C, 65.90; H, 9.50; N, 4.78; P, 2.73; Cl, 2.90.
5.11. Bis(Hexadecylamido-N-tert-Butoxycarbonyl-l-tyrosyl) ester of 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine (22)
A mixture of 5-azacytosine sodium salt (320 mg, 2.4 mmol) and tyrosine synthon 21 (2.3 g, 2.0 mmol) in dimethylsulfoxide (40 mL) was heated at 95 °C for 16 h (dissolution occurs). The solution was evaporated, the residue coevaporated with toluene (2 × 50 mL) and applied onto a column of silica gel (400 mL). Elution was started with ethyl acetate (elution of the rest of 21 and nonpolar by-products), followed by system ethyl acetate–acetone–ethanol–water (18:3:2:2, RF of the product 22: 0.50). Nevertheless, 22 is bound to silica gel very strongly and its elution from the column proceeds after further addition of methanol to ratio: ethyl acetate–acetone–ethanol–water (18:3:2:2)/MeOH (1:1). Yield: 600 mg (21%) of a glassy amorphous solid. 1H NMR (CDCl3, ppm) δ: 0.88 (t, 6H, J 16″,15″ = 7.0, H-16″), 1.20–1.31 (m, 52H, H-3″- H-15″), 1.37–1.44 (m, 22H, H-2″, C(CH3)3), 2.96 (m, 4H, 4′-CH2), 3.15 (m, 4H, H-1″), 3.71–3.78 (m, 2H, N1-CHa, N1-CH2-CHa), 3.84 (m, 1H, N1-CH2-CHb), 4.02–4.09 (m, 2H, PCHa, N1-CHb), 4.17 (m, 1H, PCHb), 4.45 (m, 2H, 4′-CH2-CH), 5.47 (m, 2H, NH-COO), 6.14 (bs, 1H, 4-NH), 6.63 (bs, 1H, 1″-NH), 6.72 (bs, 1H, 1″-NH), 7.01–7.23 (m, 9H, H-2′arom., H-3′arom., 4-NH), 7.82 (bs, 1H, H-6). 13C NMR (CDCl3, ppm) δ: 14.10 (C-16″), 22.66 (C-15″), 26.87 (C-3″), 28.30 (C(CH3)3), 29.26–29.68 (m, C-2″, C-4″- C13″), 31.89 (C-14″), 38.40 (4′-CH2), 38.61 (4′-CH2), 39.52 (C-1″), 47.75 (N1-CH2), 55.86 (4′-CH2-CH), 65.20 (d, J C,P = 166.5, PCH2), 70.31 (d, J C,P = 11.0, N1-CH2-CH2), 80.12 (C(CH3)3), 80.17 (C(CH3)3), 119.78–120.03 (m, C-2′arom.), 130.74 (C-3′arom.), 134.30 and 134.41 (C-4′arom.), 148.69–148.97 (m, C-1′arom.), 154.33 (2 C, N- COO), 155.59 (C-2), 158.95 (C-6), 166.27 (C-4), 171.04 (CON). ESIMS, m/z: 1245.6 (M+Na)+ (100), 1223.6 (15) (MH)+. HRMS (ESI): For C66H111N8O11PNa (M+Na)+ calculated: 1245.80021; found: 1245.80030. For C66H112N8O11P (MH)+ calculated: 1223.81827; found: 1223.81864.
5.12. Bis(Hexadecylamido-l-tyrosyl) ester of 1-[2-(phosphonomethoxy)ethyl]-5-azacytosine (23)
A solution of 22 (200 mg, 0.16 mmol) in mixture CH2Cl2–TFA (1:1, 4 mL) was set aside at room temperature for 12 h. The mixture was evaporated, the residue coevaporated with toluene (2 × 20 mL) and chromatographed on silica gel (30 mL) in gradient MeOH/CH2Cl2 (2:7) to pure MeOH. RF 0.30 (MeOH/CH2Cl2 2:7). Yield: 150 mg (82%) of a white foam. The product was isolated as bis(trifluoroacetate) salt. 1H NMR (CD3OD, ppm) δ: 0.90 (t, 6H, J 16″,15″ = 7.0, H-16″), 1.22–1.35 (m, 52 H, H-3″- H-15″), 1.42 (m, 4H, H-2″), 3.00–3.21 (m, 8H, H-1″, 4′-CH2), 3.83 (t, 2H, J CH2,CH2 = 4.8, N1-CH2-CH2), 3.93–4.02 (m, 4H, N1-CH2, CH-NH2), 4.24 (m, 2H, PCH2), 7.14–7.17 (m, 4H, H-2′arom.), 7.26–7.29 (m, 4H, H-3′arom.), 8.07 (s, 1H, H-6). 13C NMR (CD3OD, ppm) δ: 14.45 (C-16″), 23.73 (C-15″), 27.97 (C-3″), 30.22–30.80 (m, C-2″, C-4″- C13″), 33.07 (C-14″), 38.70 and 38.73 (4′-CH2), 40.58 (C-1″), 48.27 (N1-CH2), 56.02 (CH-NH2), 65.11 (d, J C,P = 165.2, P-C), 71.27 (d, J C,P = 11.4, N1-CH2-CH2), 121.84–121.89 (m, C-2′), 132.25 (C-3′), 134.02 and 134.06 (C-4′), 150.57 and 150.64 (C-1′), 156.87 (C-2), 161.00 (C-6), 168.10 (C-4), 170.62 and 170.67 (CON). ESIMS, m/z: 1045.6 (M+Na)+ (80), 1023.7 (48) (MH)+. HRMS (ESI): For C56H95N8O7PNa (M+Na)+ calculated: 1045.69535; found: 1045.69577. For C56H96N8O7P (MH)+ calculated: 1023.71341; found: 1023.71398.
6. Biological assays
The compounds were evaluated against the following viruses: herpes simplex virus type 1 (HSV-1) strain KOS, thymidine kinase-deficient (TK−) HSV-1 KOS strain resistant to ACV (ACVr), herpes simplex virus type 2 (HSV-2) strains Lyons and G, varicella-zoster virus (VZV) strain Oka, TK− VZV strain 07–1, human cytomegalovirus (HCMV) strains AD-169 and Davis, vaccinia virus Lederle strain, respiratory syncytial virus (RSV) strain Long, vesicular stomatitis virus (VSV), Coxsackie B4, Parainfluenza 3, Influenza virus A (subtypes H1N1, H3N2), influenza virus B, Reovirus-1, Sindbis, Reovirus-1 and Punta Toro. The antiviral, assays were based on inhibition of virus-induced cytopathicity or plaque formation in human embryonic lung (HEL) fibroblasts, African green monkey cells (Vero), human epithelial cells (HeLa) or Madin-Darby canine kidney cells (MDCK). Confluent cell cultures in microtiter 96-well plates were inoculated with 100 CCID50 of virus (1 CCID50 being the virus dose to infect 50% of the cell cultures) or with 20 plaque forming units (PFU) (VZV) and further incubated in the presence of varying concentrations of the test compounds. Viral cytopathicity or plaque formation was recorded as soon as it reached completion in the control virus-infected cell cultures that were not treated with the test compounds. Antiviral activity was expressed as the EC50 or compound concentration required to reduce virus-induced cytopathogenicity or viral plaque formation by 50%.
The cytostatic activity measurements were based on the inhibition of cell growth. HEL cells were seeded at a rate of 5 × 103 cells/well into 96-well microtiter plates and allowed to proliferate for 24 h. Then, medium containing different concentrations of the test compounds was added. After 3 days of incubation at 37 °C, the cell number was determined with a Coulter counter. The cytostatic concentration was calculated as the CC50, or the compound concentration required to reduce cell proliferation by 50% relative to the number of cells in the untreated controls. CC50 values were estimated from graphic plots of the number of cells (percentage of control) as a function of the concentration of the test compounds. The selectivity index for each compound was calculated as the CC50/EC50 ratio.
Alternatively, cytotoxicity of the test compounds was expressed as the minimum cytotoxic concentration (MCC) or the compound concentration that caused a microscopically detectable alteration of cell morphology.
Acknowledgements
This work was supported by the Subvention for development of research organization RVO 61388963 and the grant 14-00522S by the Czech Science Foundation (GAČR). The authors wish also to express their gratitude to Mrs. Ellen De Waegenaere, Mr. Seppe Kelchtermans, Mrs. Leentje Persoons and Mrs. Lies Van Den Heurck for excellent technical assistance.
Contributor Information
Marcela Krečmerová, Email: marcela@uochb.cas.cz.
Graciela Andrei, Email: graciela.andrei@kuleuven.ac.be.
References
- 1.Holý A. Curr Pharm Des. 2003;9:2567. doi: 10.2174/1381612033453668. [DOI] [PubMed] [Google Scholar]
- 2.De Clercq E., Holý A. Nat Rev Drug Disc. 2005;4:928. doi: 10.1038/nrd1877. [DOI] [PubMed] [Google Scholar]
- 3.De Clercq E. Biochem Pharmacol. 2011;82:99. doi: 10.1016/j.bcp.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 4.De Clercq E. Med Res Rev. 2012;32:765. doi: 10.1002/med.21267. [DOI] [PubMed] [Google Scholar]
- 5.De Clercq E. Med Res Rev. 2009;29:571. doi: 10.1002/med.20149. [DOI] [PubMed] [Google Scholar]
- 6.Balzarini J., Holý A., Jindřich J., et al. Proc Nat Acad Sci USA. 1991;88:4961. doi: 10.1073/pnas.88.11.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krečmerová M., Holý A., Pískala A., et al. J Med Chem. 2007;1069:50. doi: 10.1021/jm061281+. [DOI] [PubMed] [Google Scholar]
- 8.Holý A., Votruba I., Masojídková M., et al. J Med Chem. 2002;45:1918. doi: 10.1021/jm011095y. [DOI] [PubMed] [Google Scholar]
- 9.Balzarini J., Pannecouque C., De Clercq E., et al. Antimicrob Agents Chemother. 2002;46:2185. doi: 10.1128/AAC.46.7.2185-2193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hocková D., Holý A., Masojídková M., et al. J Med Chem. 2003;46:5064. doi: 10.1021/jm030932o. [DOI] [PubMed] [Google Scholar]
- 11.Dvořáková H., Holý A. Collect Czech Chem Commun. 1993;58:1419. [Google Scholar]
- 12.Herman B.D., Votruba I., Holý A., Sluis-Cremer N., Balzarini J. J Biol Chem. 2010;285:12101. doi: 10.1074/jbc.M109.096529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krečmerová M., Otmar M. Future Med Chem. 2012;4:991. doi: 10.4155/fmc.12.36. [DOI] [PubMed] [Google Scholar]
- 14.Krečmerová M., Holý A., Pohl R., et al. J Med Chem. 2007;50:5765. doi: 10.1021/jm0707166. [DOI] [PubMed] [Google Scholar]
- 15.Dračínský M., Krečmerová M., Holý A. Bioorg Med Chem. 2008;16:6778. doi: 10.1016/j.bmc.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 16.Wyles D.L., Kaihara K.A., Korba B.E., Schooley R.T., Beadle J.R., Hostetler K.Y. Antimicrob Agents Chemother. 2009;53:2660. doi: 10.1128/AAC.01546-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMullan L.K., Flint M., Dyall J., et al. Antiviral Res. 2016;125:71. doi: 10.1016/j.antiviral.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Starrett J.E., Jr., Tortolani D.R., Russell J., et al. J Med Chem. 1994;37:4857. doi: 10.1021/jm00038a015. [DOI] [PubMed] [Google Scholar]
- 19.Jansa P., Baszczyňski O., Dračínský M., et al. Eur J Med Chem. 2011;46:3748. doi: 10.1016/j.ejmech.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 20.Mehellou Y., Balzarini J., McGuigan C. ChemMedChem. 2009;4:1779. doi: 10.1002/cmdc.200900289. [DOI] [PubMed] [Google Scholar]
- 21.Hostetler K.Y. Antiviral Res. 2009;82:A84. doi: 10.1016/j.antiviral.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.http://www.chimerix.com/research-development/clinical-trials/.
- 23.Krečmerová M., Holý A., Andrei G., et al. J Med Chem. 2010;53:6825. doi: 10.1021/jm901828c. [DOI] [PubMed] [Google Scholar]
- 24.Peterson L.W., Kim J.S., Kijek P., et al. Bioorg Med Chem Lett. 2011;21:4045. doi: 10.1016/j.bmcl.2011.04.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zakharova V.M., Serpi M., Krylov I.S., et al. J Med Chem. 2011;54:5680. doi: 10.1021/jm2001426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krylov I.S., Kashemirov B.A., Hilfinger J.M., McKenna C.E. Mol Pharm. 2013;10:445–458. doi: 10.1021/mp300663j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holý A., Günter J., Dvořáková H., et al. J Med Chem. 1999;42:2064. doi: 10.1021/jm9811256. [DOI] [PubMed] [Google Scholar]
- 28.Vrbková S., Dračinský M., Holý A. Tetrahedron. 2007;63:11391. [Google Scholar]
- 29.Gruber B., Balk S., Stadlbauer S., König S. Angew Chem Int Ed. 2012;124:10207. doi: 10.1002/anie.201205701. [DOI] [PubMed] [Google Scholar]