Though this be madness, yet there is method in't. -Shakespeare's Hamlet, Act II, Scene ii.
To facilitate their replication, viruses utilize many host cell factors including receptors, enzymes, transcription factors, organelles, and membranes. In short, they commandeer the cellular environment. At the same time, viruses must circumvent cellular responses that have evolved to limit virus replication. Apoptosis, the genetically and biochemically controlled process of cell death, is one such cellular response that limits both the time and cellular machinery available for virus replication. Viruses employ a variety of strategies to evade apoptosis, and this is the subject of many good review articles (e.g., Cuconati and White, 2002, Everett and McFadden, 2002). The existence of multiple viral anti-apoptotic gene products across diverse groups of viruses suggests that apoptosis is something viruses should avoid at all costs. However, as is often the case in biological systems, the relationship between virus replication and apoptosis is actually much more complex. It is now recognized that some viruses directly utilize components of the apoptotic pathway to facilitate their replication. This novel and seemingly paradoxical strategy of virus replication, in the face of a very effective anti-viral response, is the subject of this review.
The caspases
The key effector proteins activated during apoptosis are the caspases, or cysteine-dependent aspartate-specific proteases (Alnemri et al., 1996). The primary function of caspases is to cleave cellular substrates, which results in amplification of the apoptotic process and the stepwise dismantling of the cell. To date, there are more than 280 known proteins targeted by caspases for cleavage (Fischer et al., 2003). For cleavage to occur, the target protein must possess a caspase cleavage site containing an aspartic acid (D) residue as well as be recognized by the enzyme's cysteine side chain which catalyzes the peptide-bond cleavage (Stennicke et al., 2002). Caspases exist in the cell as inactive pro-caspases or zymogens, which are themselves cleaved and activated in response to apoptotic stimuli. Apoptotic caspases are activated in a proteolytic cascade. Pro-caspases containing a longer prodomain (caspases 2, 8, 9, and 10) are activated first and are thus called initiator caspases. Initiator caspases then cleave and activate zymogens with a shorter prodomain called executioner caspases (caspases 3, 6, and 7). Caspase 3 cleaves the majority of cellular substrates during apoptosis and is also responsible for cleaving viral proteins. Caspases 7, 6, and 2 have also been implicated in the cleavage of viral protein substrates Al-Molawi et al., 2003, Eléouët et al., 2000, Grand et al., 2002.
The viruses
A prevailing view is that viruses encode inhibitors of caspase activity to escape apoptosis and thereby prolong their replication cycle. However, several viruses adopt a seemingly counterintuitive strategy and utilize caspases to cleave their own proteins. The evolutionary advantage to the virus of caspase-mediated cleavage of one of its own proteins is not always immediately apparent. For example, it is unclear how caspase cleavage of the nucleocapsid proteins of both Influenza A virus (Zhirnov et al., 1999) and transmissible gastroenteritis coronavirus (Eléouët et al., 2000), the capsid protein of feline calicivirus (Al-Molawi et al., 2003), and infected cell protein no.22 of herpes simplex virus 1 (Munger et al., 2003) benefits the virus. However, because mutation of a single D residue would eliminate the enzyme recognition site, conservation of these cleavage sites suggests a selection for caspase-mediated cleavage in virus replication or pathogenesis. In other cases, the cleavage of viral proteins has a more defined role in replication. Examples of these are discussed below and involve the cleavage of the viral nonstructural protein, which may function in the transcriptional regulation of virus or host-cell genes.
Caspases as facilitators of protein nuclear transport and transcriptional regulation
Aleutian mink disease parvovirus
Aleutian mink disease parvovirus (ADV) is a unique member of the genus parvovirus that exhibits two distinct patterns of virus replication. Infection of Crandell feline kidney (CrFK) cells in vitro or newborn mink kits in vivo is acute, with permissive and cytopathic virus replication. However, infection of adult mink leads to restricted, non-cytopathic infection of lymph node macrophages resulting in low levels of viral replication and viral persistence.
Similar to many other virus infections, permissive infection of ADV induces apoptosis and the addition of caspase inhibitors to infected cells blocks the apoptotic cascade. Surprisingly, inhibition of specific caspases results in a dramatic decrease in virus yield suggesting a requirement for caspase activity during replication (Best et al., 2002). It has recently been demonstrated that NS1, the major nonstructural protein of ADV, is cleaved by executioner caspases early in virus replication (Best et al., 2003). NS1 is responsible for many of the replication functions including viral and cellular gene transcription, viral DNA replication, and capsid assembly. NS1 is also highly cytotoxic to cells, causing apoptosis following infection or transfection. Caspase-mediated cleavage of NS1 occurs at two sites, immediately following residues D227 and D285 (Best et al., 2003). Mutation of these NS1 caspase cleavage sites abrogates virus replication by severely impairing translocation of NS1 to the nucleus of infected cells. However, the C-terminal caspase cleavage product translocates efficiently to the nucleus following expression, as does the full-length NS1 in the presence of the C-terminal product. As parvovirus NS1 molecules must oligomerize to translocate to the nucleus, it is likely that caspase cleavage of a portion of ADV NS1 generates an abbreviated NS1 molecule capable of binding the full-length NS1 and taxiing it into the nucleus (Fig. 1) . If caspase activation and apoptosis are limited after ADV infection in vivo, nuclear translocation and function of NS1 in DNA replication and control of viral gene expression may be restricted. The tight regulation of caspase activity by the cell or by ADV may therefore be a mechanism involved in the restriction of virus replication and may contribute to the persistent infection of adult mink.
Fig. 1.
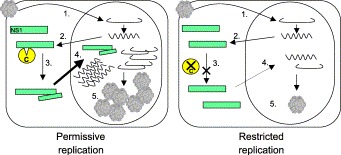
Caspase-mediated control of ADV replication. Following entry and uncoating of ADV into the host cell (1), early mRNA ( ) is transcribed and translated to produce NS1 (2). During permissive replication (left hand panel), NS1 is cleaved by activated caspases (C) (3) to produce an abbreviated NS1 fragment required for the efficient translocation of NS1 into the nucleus (solid arrow). Once in the nucleus, NS1 can efficiently function in its roles during viral DNA replication (
) is transcribed and translated to produce NS1 (2). During permissive replication (left hand panel), NS1 is cleaved by activated caspases (C) (3) to produce an abbreviated NS1 fragment required for the efficient translocation of NS1 into the nucleus (solid arrow). Once in the nucleus, NS1 can efficiently function in its roles during viral DNA replication ( ) and the control of gene expression (4), as well as the packaging of infectious virions (5). If caspase activation does not occur or is tightly controlled, lack of NS1 cleavage results in restricted virus replication (right hand panel) due to inefficient translocation of NS1 to the nucleus (dotted arrow), and subsequent low levels of viral DNA replication, gene expression, and infectious progeny.
) and the control of gene expression (4), as well as the packaging of infectious virions (5). If caspase activation does not occur or is tightly controlled, lack of NS1 cleavage results in restricted virus replication (right hand panel) due to inefficient translocation of NS1 to the nucleus (dotted arrow), and subsequent low levels of viral DNA replication, gene expression, and infectious progeny.
Hepatitis C virus
The flavivirus, hepatitis C virus (HCV), causes chronic hepatitis in 50–84% of infected individuals. Its single-stranded RNA genome is translated essentially as a single open reading frame (ORF), with the single large polyprotein cleaved by host and viral proteases to produce four structural and six nonstructural proteins. Of the nonstructural proteins, NS5A is a multifunctional protein of 447 amino acids (aa) involved in the enhancement of viral pathogenesis (reviewed in Reyes, 2002). NS5A is cleaved by caspases at two residues, D154 and D389, to yield NS5A:155–389, a central fragment with both the N- and C-terminal portions removed (Satoh et al., 2000). Uncleaved NS5A is a likely member of the HCV replicase complex located on the cytoplasmic side of the endoplasmic reticulum. However, cleavage to NS5A:155–389 unmasks a nuclear localization sequence (NLS) and removes a cytoplasmic retention sequence (CRS), enabling translocation to the cell nucleus. NS5A:155–389 also demonstrates transcriptional activation that can be regulated by protein kinase A (Satoh et al., 2000). The transcriptional activating domain of NS5A:155–389 maps to a region between aa 228 and 284, while full-length NS5A cannot function as a transcriptional activator (Fukuma et al., 1998). Together, these findings suggest that caspase-mediated cleavage of NS5A is required to enable its nuclear translocation and subsequent host-cell gene regulation.
The caspase-cleaved NS5A:155–389 contains multiple regions that may be involved in pathogenesis through interactions with cellular proteins in the nucleus. For example, NS5A is able to directly interact with and negatively regulate the transcriptional regulator p53 tumor suppressor protein resulting in attenuated interferon and apoptotic responses to virus infection (reviewed in Reyes, 2002). Nuclear NS5A also induces expression of IL-8, an inhibitor of interferon-α activity. The region of NS5A generated via caspase-mediated cleavage also contains two of the three Bcl-2 homology (BH) domains of NS5A. These domains are involved in the inhibition of apoptosis and may function by interacting with the pro-apoptotic molecule Bax in the nucleus (Chung et al., 2003). Hence, caspase-mediated cleavage of NS5A may serve as a ‘sensor’ for caspase activity and the activation of the viral anti-apoptotic and perhaps anti-interferon responses that occur in the nucleus of infected cells.
Adenoviruses
The adenoviruses (Ad) are a large group of DNA viruses capable of causing both acute and persistent infections in humans and other animals, depending on the serotype. The relatively large genome (36 kb) is organized into early and late transcription units. The early transcription units E1A and E1B encode proteins involved in cellular transformation and transactivation of both cellular and viral transcription. Translation of E1A results in the production of two major proteins (12S and 13S) that are both strong inducers of apoptosis in mammalian cells via multiple pathways. However, the pro-apoptotic effect of E1A is modulated during infection by apoptosis-inhibitory effects of E1B proteins (reviewed in Frisch and Mymryk, 2002). Apoptosis has been shown to positively affect virus release (Chiou and White, 1998), but it is unclear if apoptosis has a more direct role in virus replication. Following transfection of the E1A proteins from Ad2 or Ad12, both 12S and 13S are cleaved by caspases at multiple sites, resulting in progressive cleavage from the N-termini (Grand et al., 2002). This cleavage disrupts the interactions of E1A with cellular transcriptional-regulating proteins that bind to its N-terminus but not to the C-terminus (Grand et al., 2002), suggesting a potential regulatory role of caspases in E1A-mediated gene expression.
Following infection with Ad5, E1A is also proteolytically cleaved by caspases. However, cleavage of both the E1A proteins, as well as cellular proteins normally cleaved during apoptosis, is limited and is cell-type dependent (Grand et al., 2002). This type of limited caspase activity affecting some proteins while leaving other vital cellular proteins intact has been observed during non-apoptotic events such as cell cycle control, cellular proliferation, and differentiation (Fischer et al., 2003). While the mechanisms for this limited caspase activity remain to be elucidated, the possibility that caspase-mediated cleavage of Ad E1A has a function in virus replication, cellular transformation, or viral persistence is intriguing. Studies on the molecular relationships between Ad E1A and the infected cell may lead to further insight on the role of caspase activity in non-apoptotic events.
Influenza A virus
The orthomyxovirus Influenza A is yet another virus that utilizes caspases for virus replication. Infection with influenza A can induce apoptosis both in vitro and in vivo and two influenza proteins, NS1 and PB1-F2, promote apoptosis induction. However, the consequences of apoptosis for virus replication and pathogenesis have not been well understood. Similar to our results for ADV, the addition of peptide inhibitors of caspases to influenza A-infected cell cultures not only inhibited virus-induced apoptosis, but also impaired virus replication (Wurzer et al., 2003). The requirement for caspase activity operated at late steps in virus replication, facilitating the export of viral ribonucleoprotein complexes from the nucleus for packaging into progeny virions in the cytoplasm of infected cells. The most likely explanation for the role of caspase activation in this case was in the caspase-dependent increase in the diffusion limit of nuclear pores (Wurzer et al., 2003). Although this is not a direct utilization of caspases for the cleavage of viral proteins and subsequent replication, activation of specific caspases is an absolute requirement in this case and is not simply a byproduct of cell stress and apoptosis.
Beyond cell death
As mentioned previously, caspase activation is involved in many cellular processes, apoptotic as well as non-apoptotic. The latter includes inflammation, cell cycle control, cell differentiation, and hematopoiesis. The activation of caspases in these non-apoptotic processes must be tightly regulated so that it does not culminate in the death of the cell. This level of control is just now beginning to be appreciated. The degree of regulation is also likely to be host cell-type dependent. Many viruses are dependent on the stage of cell cycle or cellular differentiation for precise temporal expression of host proteins required for replication. It is now being realized that caspases are important host cell proteases utilized by viruses. This direct requirement renders caspase activation a possible point of control or restriction of virus replication, both in apoptotic and non-apoptotic cells.
In the literature to date, the caspase targets for ADV, HCV, and Ad are nonstructural proteins involved in the transcriptional regulation of viral and cellular genes (Fig. 2) . Thus, a common theme for the restriction or regulation of virus replication may be caspase-mediated cleavage of viral transcriptional regulators. The replication of a potentially lytic virus (e.g., ADV) that requires caspases for efficient gene transcription may be restricted if, due to tight control at the cellular level, caspase activation does not occur following infection. In turn, this restriction of replication may enable the establishment of virus persistence. If such cleavage of viral proteins during replication is a wider phenomenon among virus families, it may have escaped observation due to the subsequent inhibition of apoptosis by the cleaved protein itself (e.g., HCV NS5A, Satoh et al., 2000), by additional virally encoded proteins (e.g., Ad5), or by cellular inhibition of caspase activity. Nevertheless, the direct facilitation of virus replication by caspases may be an important and novel mechanism that contributes to the replication strategies of other important viruses. Though initially counterintuitive, the employment of the anti-viral apoptotic response is yet another clever strategy adapted by viruses to thrive in the host environment.
Fig. 2.
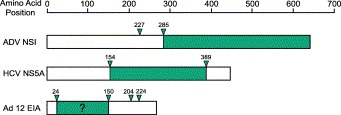
A common theme for caspase-mediated cleavage of viral nonstructural proteins? Activated caspases cleave ADV NS1 and HCV NS5A at two sites, and Ad 12 E1A at multiple sites (amino acid positions indicated by arrows), to yield cleavage fragments capable of localizing to the nucleus (indicated in green) and interacting with the transcriptional machinery of cells. Caspase-mediated cleavage of viral nonstructural proteins may be an important mechanism involved in transcriptional regulation. The question mark indicates that the actual cellular localization of the caspase cleaved product is not yet known.
References
- Al-Molawi N., Beardmore V.A., Carter M.J., Kass G.E.N., Roberts L.O. Caspase-mediated cleavage of the feline calicivirus capsid protein. J. Gen. Virol. 2003;84:1237–1244. doi: 10.1099/vir.0.18840-0. [DOI] [PubMed] [Google Scholar]
- Alnemri E.S., Livingston D.J., Nicholson D.W., Salvesen G., Thornberry N.A., Wong W.W., Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- Best S.M., Wolfinbarger J.B., Bloom M.E. Caspase activation is required for permissive replication of Aleutian mink disease parvovirus in vitro. Virology. 2002;292:224–234. doi: 10.1006/viro.2001.1238. [DOI] [PubMed] [Google Scholar]
- Best S.M., Shelton J.F., Pompey J.M., Wolfinbarger J.B., Bloom M.E. Caspase cleavage of the nonstructural protein NS1 mediates replication of Aleutian mink disease parvovirus. J. Virol. 2003;77:5305–5312. doi: 10.1128/JVI.77.9.5305-5312.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou S.-K., White E. Inhibition of ICE-like proteases inhibits apoptosis and increases virus production during adenovirus infection. Virology. 1998;244:108–118. doi: 10.1006/viro.1998.9077. [DOI] [PubMed] [Google Scholar]
- Chung Y.-L., Sheu M.-L., Yen S.-H. Hepatitis C virus NS5A as a potential viral Bcl-2 homologue interacts with Bax and inhibits apoptosis in hepatocellular carcinoma. Int. J. Cancer. 2003;107:63–73. doi: 10.1002/ijc.11303. [DOI] [PubMed] [Google Scholar]
- Cuconati A., White E. Viral homologs of Bcl-2: role of apoptosis in the regulation of virus infection. Genes Dev. 2002;16:2465–2478. doi: 10.1101/gad.1012702. [DOI] [PubMed] [Google Scholar]
- Eléouët J.-F., Slee E.A., Saurini F., Castagné N., Poncet D., Garrido C., Solary E., Martin S.J. The viral nucleocapsid protein of transmissible gastroenteritis coronavirus (TGEV) is cleaved by caspase-6 and -7 during TGEV-induced apoptosis. J. Virol. 2000;74:3975–3983. doi: 10.1128/jvi.74.9.3975-3983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett H., McFadden G. Poxviruses and apoptosis: a time to die. Curr. Opin. Microbiol. 2002;5:395–402. doi: 10.1016/s1369-5274(02)00340-5. [DOI] [PubMed] [Google Scholar]
- Fischer U., Janicke R.U., Schulze-Osthoff K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ. 2003;10:76–100. doi: 10.1038/sj.cdd.4401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch S.M., Mymryk J.S. Adenovirus-5 E1A: paradox and paradigm. Nat. Rev., Mol. Cell Biol. 2002;3:441–452. doi: 10.1038/nrm827. [DOI] [PubMed] [Google Scholar]
- Fukuma T., Enomoto N., Marumo F., Satoh C. Mutations in the interferon-sensitivity determining region of hepatitis C virus and transcriptional activity of the nonstructural region 5A protein. Hepatology. 1998;28:1147–1153. doi: 10.1002/hep.510280433. [DOI] [PubMed] [Google Scholar]
- Grand R.J.A., Schmeiser K., Gordon E.M., Zhang X., Gallimore P.H., Turnell A.S. Caspase-mediated cleavage of adenovirus early region 1A proteins. Virology. 2002;301:255–271. doi: 10.1006/viro.2002.1586. [DOI] [PubMed] [Google Scholar]
- Munger J., Hagglund R., Roizman B. Infected cell protein no.22 is subject to proteolytic cleavage by caspases activated by a mutant that induces apoptosis. Virology. 2003;305:364–370. doi: 10.1006/viro.2002.1728. [DOI] [PubMed] [Google Scholar]
- Reyes G.R. The nonstructural NS5A protein of hepatitis C virus: an expanding, multifunctional role in enhancing hepatitis C virus pathogenesis. J. Biomed. Sci. 2002;9:187–197. doi: 10.1007/BF02256065. [DOI] [PubMed] [Google Scholar]
- Satoh S., Hirota M., Noguchi T., Hijikata M., Handa H., Shimotonot K. Cleavage of hepatitis C virus nonstructural protein 5A by a caspase-like protease(s) in mammalian cells. Virology. 2000;270:476–487. doi: 10.1006/viro.2000.0287. [DOI] [PubMed] [Google Scholar]
- Stennicke H.R., Ryan C.A., Salvesen G.S. Reprival from execution: the molecular basis of caspase inhibition. Trends Biochem. Sci. 2002;27:94–101. doi: 10.1016/s0968-0004(01)02045-x. [DOI] [PubMed] [Google Scholar]
- Wurzer W.J., Planz O., Ehrhardt C., Giner M., Silberzahn T., Pleschka S., Ludwig S. Caspase 3 activation is essential for efficient influenza virus propagation. EMBO J. 2003;22:2717–2728. doi: 10.1093/emboj/cdg279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhirnov O.P., Konakova T.E., Garten W., Klenk H.-D. Caspase-dependent N-terminal cleavage of influenza nucleocapsid protein in infected cells. J. Virol. 1999;73:10158–10163. doi: 10.1128/jvi.73.12.10158-10163.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


