Abstract
Infectious bursal disease (IBD) continues to pose potential threat to poultry industry all over the world. The disease can spell disaster not only through its infection but also by break of immunity in chickens vaccinated for other diseases. l-Arginine, a ubiquitous, semi-essential amino acid has emerged as an imunostimulant from variety of human and animal studies. In the present study, we demonstrate the stimulatory effects of l-arginine on intestinal intraepithelial lymphocyte (iIELs) functions as well as on systemic immune response in chickens orally vaccinated with live intermediate plus (IP) strain of IBD vaccine. Challenge studies with virulent IBDV revealed complete (100%) protection in IP + l-arginine group compared with 80% protection recorded in IP strain vaccinated chickens. Functional activities of iIELs evaluated by cytotoxicity assay demonstrated significantly high percentage cytotoxicity in IP + l-arginine groups compared with IP group (P < 0.05). Proliferative response of iIELs against IBDV antigen and Con-A was also significantly higher in IP + l-arginine group. Similar results were obtained with peripheral blood mononuclear cell blastogenic response to IBDV and Con-A analyzed as an indicator of systemic cell-mediated immune response. Orally administered IP strain vaccine elicited good antibody titres in both the groups, IP and IP + l-arginine, however, the antibody titres were significantly higher in IP + l-arginine group compared with IP vaccinated group (P < 0.05). These results clearly demonstrate that l-arginine stimulates intestinal and systemic immune response against IBDV.
Keywords: l-Arginine, Chickens, Intestinal intraepithelial lymphocytes, Immunomodulation, Infectious bursal disease virus, Mucosal immunity, Vaccine
1. Introduction
Infectious bursal disease (IBD) or Gumboro disease caused by IBD virus is one of the most important immunosuppressive diseases in commercial poultry affecting mainly young chickens [1]. Eradication of IBD is difficult because of its high stability in the environment and the principal method like for other viral diseases of chickens is effective vaccination against IBD. The vaccines currently in use are either killed or cell culture adapted live, attenuated vaccines. Depending on the level of attenuation and virulence, these live vaccines are further categorized as hot or intermediate plus, intermediate and mild. The most effective strategies to control IBD includes vaccination of layer birds with inactivated oil-emulsified vaccines to provide maternal antibodies in chickens or immunization of young chickens with live attenuated vaccines followed with booster immunizations. After the emergence of very virulent IBDV, mild vaccines are generally ineffective and presence of maternal antibodies interferes with the efficacy of live vaccines. Another most important issue associated with live vaccines is immunosuppression and may cause lesions similar to natural infection in vaccinated birds [2]. There is definite requirement of a suitable immunomodulator that can minimize the suppressive effects of live vaccines and amplify specific protective response. l-arginine, a semi-essential dibasic amino acid has emerged as a regulator of many immunological and physiological processes. l-arginine attracted initial experimental attention in various animal tumor models [3] as a dietary supplement. The most promising immunostimulatory effects were observed in immunocompromised hosts after trauma, surgical stress or immunosuppression with HIV virus [4], [5]. Supplemental l-arginine stimulates the functional activities of different cell types including natural killer (NK) cells, macrophages, lymphokine activated killer cells, T and B cells [6], [7]. We recently demonstrated that l-arginine stimulates specific protective response against IBD in chickens [8]. Mucosal tissues constitute an enormous surface area and act as an interface between the external and internal milieu of the host. Among the mucosal surfaces, gut mucosa serves a primary portal of entry to most of the infectious agents and is provided with defense system, the gut associated mucosal tissue (GALT). During natural course of IBD infection, primary viral replication occurs in GALT followed by secondary invasion in bursa of fabricius [9]. Antigen specific activation of mucosal immune system is important for protection of chickens from variety of infections. Numbers of strategies are evolved to induce protective immunity by antigenic stimulation of mucosal surfaces [10]. One of these strategies is oral delivery of vaccines, which is one of the most convenient and cheaper immunization routes. While, majority of vaccination strategies with mucosal adjuvants and delivery systems have demonstrated promise for enhancing mucosal immune responses in mammalian species, there is no adequate information relating to their performance in chickens. Intestinal intraepithelial lymphocytes (iIELs) are a unique cell population found along the basement membrane of small intestine. Although, iIELs are strategically positioned to be the first line of cell defense against invading enteric pathogens, the true functions of iIELs are not clearly elaborated. There are several reports available regarding functional activities of iIELs such as cytotoxic [11], [12], [13], NK like [14], [15], antibacterial and mucosal immunosurveillance [16], [17]. There are either no or very few reports available regarding the role of iIELs and mucosal immunity in IBD infection. In the present study, we demonstrate that l-arginine stimulates the cytotoxicity and proliferation of iIELs against IBDV. Oral administration of live intermediate plus strain (IP) strain of IBD vaccine along with l-arginine elicited strong intestinal as well as systemic immune response.
2. Materials and methods
2.1. Vaccine strain and virulent virus
2.1.1. Intermediate plus strain of IBDV
Live, cell culture adapted, intermediate plus (IP) strain of infectious bursal disease virus (IBDV) was obtained from the poultry vaccine testing laboratory, Indian Veterinary Research Institute (IVRI), Izatnagar and propogated in chicken embryo fibroblast (CEF) cell culture.
2.1.2. Virulent IBDV
Virulent IBDV (field strain) was obtained from Division of Avian Diseases, IVRI, Izatnagar.
2.2. Embryonated chicken eggs
Embryonated chicken eggs of 10 days old obtained from the Central Avian Research Institute (CARI), Izatnagar, were used for the preparation of CEF cell culture.
2.3. Chickens
Day old chicks were obtained from the hatchery of CARI, Izatnagar. These chicks were reared under standard conditions in the experimental facility of Division of Standardization, IVRI, Izatnagar. 3-week-old chickens were used for the experiments.
2.4. Lethal dose of virulent IBDV for 50% chickens (CLD50)
The CLD50 of the virulent virus was determined by the method of Reed and Muench [18] in 3-week-old chickens.
2.5. Tissue culture infective dose at 50% level (TCID50) of IP strain of IBDV
The TCID50 of IP strain was determined in CEF cell culture as per the method of Reed and Muench [18].
2.6. Purification of IBDV antigen for in vitro proliferation assay using MTT
For purification of IBDV antigen, sucrose gradient purification method was used as described previously [8]. Briefly, Bursas collected from infected chickens were homogenized and clarified at 1600 g for 30 min. The resultant supernatant was layered on 30% sucrose and ultracentrifuged at 110,000 × g for 4 h at 4 °C. The virus pellet was resuspended in TNE buffer (0.01 M Tris–HCl, 0.1 M NaCl, 0.001 M EDTA, pH 7.9). This virus suspension was layered on sucrose cushion (top layer 40% sucrose and bottom layer 60% sucrose) gradient, ultracentrifuged at 90,000 × g for 4 h at 4 °C. The virus band between the sucrose layers was collected and resuspended in TNE buffer that was again ultracentrifuged at 90,000 × g for 2 h at 4 °C. This purified IBDV antigen thus obtained was used at the concentration of 10 μg/ml for MTT assay.
2.7. Experimental design
Two groups of 3-week-old chickens comprising 60 in each were immunized orally with IP strain of IBDV (103.5 TCID50). Out of these, one group was supplemented with 2% l-arginine (Spectrochem, India) in feed during the entire experiment. Third group was supplemented with only 2% l-arginine in feed as an immunomodulator control and fourth group was kept as a unimmunized/untreated but challenged control. Fifth group was observed as an unimmunized and unchallenged control for the challenge experiment. The number of birds in each group was 60. Twenty-one days post-immunization (DPI), chickens of all the groups including controls were challenged each with virulent IBDV (103CLD50). After challenge, chickens were observed for 10 days for the appearance of clinical signs and mortality. All the chickens from different groups were euthanized 10 days post-challenge (DPC). Bursas and spleens were removed, weighed and the ratio of bursa to body weight and spleen to body weight were determined by the formula (organ weight in grams × 1000)/body weight in grams. The protection percentage was initially assessed on the basis of mortality, clinical signs, and gross bursal lesions and further confirmed by histopathological examination of bursa. For assessment of cytotoxicity and proliferation functions, intestinal intraepithelial lymphocytes (iIELs) were isolated from all the groups of chickens at 7th, 14th, 21st DPI and 3rd, 5th, and 10th days post-challenge (DPC). Blood was collected from all the groups on all the above DPI and DPC intervals. Serum samples were separated, heat inactivated and stored at −20 °C for further use in enzyme linked immunosorbent assay (ELISA). Parallel blood samples were collected on all the above DPI and DPC in tubes containing heparin (10 IU/ml of blood) for peripheral blood mononuclear cells (PBMCs) proliferation assays with purified IBDV antigen and Con-A using MTT dye.
2.8. Assessment of humoral immune response
2.8.1. Enzyme linked immunosorbent assay (ELISA)
A ready-made IBD ELISA kit from Kirkegaard and Perry laboratories, Maryland, USA was used for the test as per manufacturer's instructions. The kit was obtained from poultry vaccine testing laboratory, Division of Standardization, Izatnagar. The CEF passaged purified IBDV antigen was used for ELISA. Both positive (against IBDV) and negative control chicken sera were supplied with the kit. Standard ELISA procedure was followed with antigen coating for overnight at 4 °C. The plates were washed three times with wash buffer. Equal amounts (100 μl) of positive, negative controls and unknown serum samples were added to the previously marked wells and the plates were incubated for 30 min at room temperature. After 3 washings, 100 μl of conjugate provided with the kit was added to each well and incubated for 30 min at room temperature. The plates were again washed as described previously and 100 μl of substrate solution was added as per kit instructions and incubated for 15 min. Later, stop solution was added and plates were read at ELISA plate reader set at 405 nm (Anthos Labtech HTZ Salzburg, Austria).
2.9. Assessment of cytotoxic and proliferative response of chicken iIELs and proliferative response of PBMCs
2.9.1. Isolation of iIELs
Chicken iIELs were isolated as per the method described by Chai and Lillehoj, 1988 [19] and Agrawal and Reynolds, 1999 [20]. The intestinal tract was removed longitudinally from duodenal loop to iliocecal junction immediately after euthanasia. The fat and blood vessels on the serosal surface were removed, intestines cut into small pieces and washed several times with phosphate buffer saline (PBS) to remove detritus. The intestinal pieces were transferred to a beaker containing pre-warmed 2mM DTT (Sigma, USA) and incubated in a water bath at 39 °C for 15 min with occasional shaking to remove the intestinal mucus. The cloudy suspension was discarded and the DTT treatment was repeated with fresh solution. The tissue segments were washed with PBS and transferred to a fresh beaker containing 1 mM EDTA (Sigma, USA), stirred gently for 30 min on magnetic stirrer at room temperature. The supernatant was allowed to settle for 15 min to remove clumps of epithelial cells. The supernatant containing cells was filtered two times through pre-soaked nylon wool column. The filtrate was centrifuged at 1000 × g for 10 min. the pellet was suspended in RPMI-1640 medium. The cell suspension was further purified by density gradient centrifugation on histopaque (Sigma, USA). The cellular band at the interface between medium and histopaque was collected and washed with PBS at 1000 × g for 10 min. The pellet was suspended in RPMI medium. The cell count and viability was determined by trypan blue dye exclusion method. The final iIELs concentration was adjusted to 2 × 107 viable cells/ml.
2.9.2. In vitro cytotoxicity assay using MTT (3-4,5-dimethyl-thiazol 2,5-diphenyl tetrazolium bromide)
MTT colorimetrc assay was carried out to determine the cytotoxicity function of iIELs as per the method of Lillehoj [21] and Kumar et al. [22]. Confluent monolayer culture of Vero cells used as target cells was trpsinized, diluted to contain 2 × 105 cells/ml. Hundred microlitre of this cell suspension was added in a 96-well flat bottomed tissue culture plate (Greiner, USA) and incubated for 8–10 h at 37 °C in a incubator at 5% CO2 level. To these target cells, the chicken iIELs (effector cells) were added at the ratio of 50:1 effector: target ratio. Effector: target ratio was optimized in preliminary experiments. Effectors and target cell controls were kept by adding 100 μl RPMI medium to effector and target cell wells. Plates were incubated for 18 h at 37 °C in an incubator with 5% CO2 level. After incubation, 10 μl MTT solution (5 mg/ml, Sigma, USA) was added to each well and the plates were again incubated for 4 h. The plates were then centrifuged at 1500 × g for 15 min in a plate centrifuge. About 150 μl of DMSO (Sigma, USA) was added to each well and the contents of the well mixed thoroughly to dissolve formazon crystal completely. The optical density (OD) was measured in ELISA reader at 492 nm (Anthos labtech, HTZ Salzburg, Austria). The percentage cytotoxicity was calculated using the formula:
Percentage cytotoxicity = 1(−ODET−ODE/ODT) × 100, where ODET, mean OD of wells with effector and target cells; ODE, mean OD of effector cells alone and ODT, mean OD of target cells alone.
2.9.3. Proliferative response of chicken iIELs to Con-A and IBDV antigen using MTT colorimetric assay (3-4,5-dimethyl-thiazol 2,5-diphenyl tetrazolium bromide)
The MTT colorimetric assay described by Bonous et al. [23] and Agrawal and Reynolds [20] was followed with some modifications. Chicken iELs isolated as described above were used to determine the proliferative response to purified IBDV antigen and Con-A, which was assayed in 96 well flat bottom plates. Various concentrations of Con-A and IBDV antigen were optimized with 25 μg of Con-A/ml and 15 μg/ml of IBDV antigen was found to be optimal dose for blastogenesis of iIELs. Hundred microlitre of iIELs cell suspension (5 × 106 cells/ml) was added to three sets of quadruplicates. The first set received 100 μl of RPMI medium to serve as a negative control. The second and third set received 100 μl of Con-A and IBDV antigen, respectively. The plates were incubated at 37 °C in an incubator with 5% CO2 tension. After 72 h, 10 μl of MTT solution (5 mg/ml, Sigma, USA) was added and the plates were incubated for 4 h. MTT formazan was extracted using 150 μl of DMSO per well and optical density was measured at a test wavelength of 510 nm and a reference 650 nm. Stimulation index was calculated using the formula OD stimulated/OD of unstimulated control.
2.9.4. Assessment of peripheral blood mononuclear cell proliferation assay to Con-A and IBDV using MTT
Blood was aseptically collected in heparinized tubes and centrifuged at 1000 × g for 15 min. Buffy coat was carefully collected, layered over histopaque (1.077 g/ml, Sigma, USA) and centrifuged at 1400 × g for 20 min. After two washings in PBS, cells were adjusted to 2 × 106 viable cells in RPMI-1640 (Sigma, USA). Proliferation assay using MTT was performed as previously described [8] or in iIELs proliferation section.
2.10. Statistical analysis
Significance of differences was determined by analysis of variance (ANOVA) using Duncan's multiple range test. P < 0.05 value was considered significant.
3. Results
The intermediate plus strain of IBDV vaccine was prepared by propagating the virus in CEF cell culture. The TCID50 of the virus was found to be 103.5/0.1 ml and the CLD50 of the virulent IBDV determined in 3-week-old chickens was 103/0.1 ml. All the experimental birds used in the present studies were tested and found negative for the maternal antibodies at 3 weeks of age. In the control groups, all the pre challenge serum samples were negative for precipitin antibodies.
3.1. Assessment of protection percentage after challenge with virulent IBDV
At 21 DPI, chickens from all different groups were challenged with virulent IBDV and monitored for protection up to 10 days after the challenge. Oral vaccination with IP strain of IBDV resulted in 80% protection. Whereas 100% protection was achieved in groups of chickens vaccinated with IP strain and supplemented with l-arginine. All the unimmunized control birds succumbed to infection by virulent challenge virus. These chickens showed typical signs of IBD, some of them had gross bursal lesions characterized by pale color edema, point bleeding and significantly lower bursa to body weight ratio (P < 0.05) compared to other non-vaccinated and non-challenged chickens. Spleen enlargement was also significantly higher than the non-challenged control (not shown). These results are similar to our previous published work [8]. The protection in vaccinated chickens with or without l-arginine supplementation was further confirmed by histopathological examination of bursa.
3.2. Assessment of humoral immune response
The comparative mean antibody titres as detected by ELISA are presented in Fig. 1 . The increased antibody response from 7 to 21 DPI was observed in both the groups of IP strain immunized with or without l-arginine. Antibody titres were significantly higher at 14, 21 DPI and 5, 10 DPC in IP + l-arginine compared with IP group (P < 0.05).
Fig. 1.
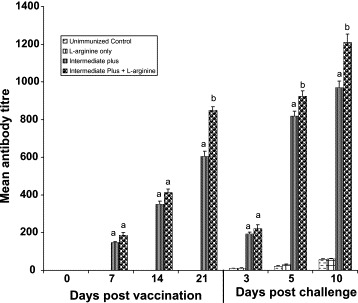
Antibody titres determined by ELISA in various groups of Chickens. Antibody titres in vaccinated chickens progressively increased from 7 to 21 days of immunization. Significantly elevated antibody titres were found in IP + l-arginine group (P < 0.05) at days 14 and 21 post-vaccination and 5,10 days post-challenge, suggesting potentiating effect of l-arginine on specific humoral immune response against IBDV. Means with no common superscript differ significantly (P < 0.05).
3.3. Assessment of iIELs cytotoxicity and proliferation functions
Isolation of iIELs using DTT and EDTA with subsequent passage through nylon wool column and density gradient purification resulted in high yields and relatively pure cell populations of iIELs with good viability. The iIELs preparations contained high percentage of cells with characteristic lymphocyte morphology with a few contaminated epithelial cells. The viability of iIELs was always more than 93% as revealed by trypan blue dye exclusion method.
3.3.1. Cytotoxicity of iIELs using MTT assay
To determine the cytotoxicity function of iIELs, mean percentage cytotoxicity of iIELs from different groups of chickens against Vero cells was assessed by MTT assay and is shown in Fig. 2 . Cytotoxicity of iIELs gradually increased from days 7 to 21 of immunization in IP and IP + l-arginine groups. However in IP + l-arginine group, significantly elevated cytotoxicity was found at 14, 21 DPI and 3, 5, 10 DPC compared with IP alone group (P < 0.05). In unimmunized controls and l-arginine groups of chickens, consistent baseline cytotoxicity was recorded during immunization. After challenge, cytotoxicity was reduced at 3 and 5 DPC in all the groups of chickens, which was restored in IP and IP + l-arginine groups of chickens at 10 DPC.
Fig. 2.
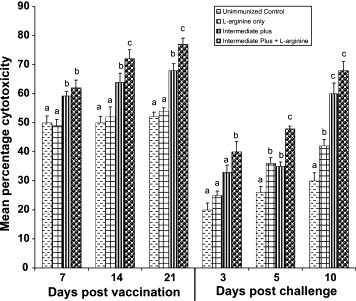
Mean percentage cytotoxicity of iIELs against Vero cells. The iIELs obtained from different groups of chickens immunized orally with intermediate plus strain of IBDV with or without l-arginine were used for the MTT cytotoxicity assay. Elevated iIELs cytotoxicity was found in IP + l-arginine group compared with IP. Means with no common superscript differ significantly (P < 0.05).
3.3.2. Proliferative response of iIELs to specific IBDV antigen and nonspecific Con-A stimulation
Proliferative response of iIELs after stimulation with specific IBDV antigen was used as a measure to determine more specific effects of IBDV on mucosal cell-mediated immune response (Fig. 3 ). Proliferative response of iIELs to IBDV was severely reduced in unimmunized and l-arginine control groups (P < 0.05). In IP group, proliferative response gradually increased from 7 to 21 DPI but was significantly lower than IP + l-arginine group (P < 0.05). Increased proliferation in IP immunized chickens indicates a specific cell-mediated immune response against the vaccine virus. After challenge, proliferation was suppressed in IP group compared with IP + l-arginine. Interestingly, at 10 DPC significantly higher (P < 0.05) stimulatory response was recorded in IP + l-arginine, indicating that specific cell-mediated immune response was mounted against the challenge virus. Results of nonspecific stimulation of iIELs with Con-A are shown in Fig. 4 . The mitogenic response of iIELs to Con-A was significantly lower in IP group compared to unimmunized and l-arginine control groups at 7, 14 and 21 DPI (P < 0.05). Comparison between IP and IP + l-arginine groups revealed that IP strain immunized and supplemented with l-arginine group had significantly higher (P < 0.05) mitogenic response than IP vaccinated group. After challenge with virulent IBDV on 21 DPI, the mitogenic response of iIELs was significantly reduced in all the groups. In unimmunized and l-arginine control groups, significant reduction (P < 0.05) in the mitogenic response was observed after challenge, indicating the immunosuppressive effects of virulent IBDV. At day 10 DPC, the mitogenic response reached normal levels in IP + l-arginine group and to a lesser extent in IP group.
Fig. 3.
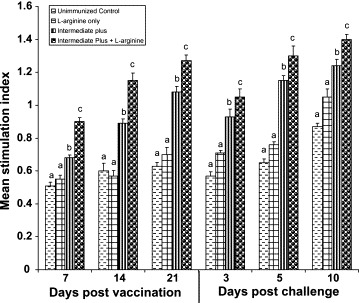
Proliferative response of iIELs to specific stimulation with purified IBDV antigen assayed by MTT colorimetric assay. Means with no common superscript differ significantly (P < 0.05).
Fig. 4.
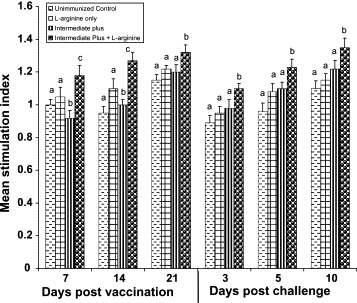
Proliferative response of iIELs to Con-A mitogen. Means with no common superscript differ significantly (P < 0.05).
3.4. Assessment of systemic cell-mediated immune response
3.4.1. Mitogenic response of peripheral blood mononuclear cells to specific IBDV antigen and nonspecific Con-A stimulation
The PBMCs mitogenic response to specific IBDV antigen and nonspecific Con-A stimulation was evaluated as an indicator of the effect of orally administered IP strain of IBDV vaccine with or without l-arginine on systemic cellular immune response. Results of specific IBDV stimulation revealed gradual increase in proliferative response in IP + l-arginine group compared with IP group from 7 to 21 DPI. After challenge, proliferation was suppressed in IP group compared with IP + l-arginine. Results of PBMCs non-specifically stimulated with Con-A revealed that IP group had significantly lower mitogenic response compared with IP + l-arginine group. These results are in accordance with our previous published report [8] hence not shown. The effect on systemic cellular response was further confirmed by inoculation of lymphokines obtained from sensitized lymphocytes from all the groups in the wattles of adult chickens. Significantly higher wattle thickness indicative of hypersensitivity reaction was found in IP + l-arginine group at 24 h compared with all the other groups. The results of production of skin reactive factor were similar to our results described previously [8], hence not shown.
4. Discussion
We assessed safety and efficacy of orally administered live IP strain of IBD vaccine in 3-week-old chickens. The main objective of the study was to evaluate l-arginine as an immunomodulator of intestinal and systemic immunity that can reduce the immunosuppressive effects of live hot vaccine and amplify specific protective immune response against IBD. 2% l-arginine supplementation in feed was safe and free from any side effects [8]. Complete protection was recorded in IP + l-arginine group, where as in IP vaccine group 80% protection was achieved. Unimmunized and l-arginine control showed high mortality after challenge indicating that the challenge experiment was successful. The systemic humoral immune response was assessed by commercially available standard IBD ELISA kit. The levels of neutralizing antibodies determine the protection against IBDV in chickens [24]. Strong antibody response was observed in both the vaccinated groups, however antibody titres were significantly higher in IP + l-arginine group. These Results confirm that orally administered vaccine elicited good systemic antibody response. The elevated antibody response and protection in IP vaccinated and l-arginine supplemented group is attributed to the number of immunoregulatory functions of l-arginine on the immune system. l-Arginine is required for the differentiation of pro B to pre B cells and is also involved in the release of these cells from bone marrow [7]. After challenge with virulent IBDV, sudden fall in antibody titres indicates the antigen antibody interactions in vivo. Preformed antibodies specific to IBDV might be involved in neutralizing virus and the resultant immune complexes removal by complement-mediated lysis. The rise in antibody titres at 10 DPC indicates the booster effect of challenge virus on immune system mediated by clonal proliferation and increased numbers of plasma cells leading to enhance antibody production [25]. Both humoral and cellular immune responses mediated by T cells play an important role in the control of IBD infection [26]. The functions of iIELs were assessed by cytotoxicity and proliferative response to specific stimulation with IBDV and nonspecific stimulation with Con-A mitogen. The iIELs are anatomically positioned to serve as a first line of cellular defense against variety of enteric pathogens. In chickens, iIELs constitute two major subsets as NK and T cell types, which are phenotypically and functionally distinct IEL subpopulations [14] but their role in IBD infection remained unexplored. We speculate that iIELs are activated during natural course of IBD infection and may be involved in restricting the spread of IBDV to other lymphoid tissues. Results of cytotoxicity revealed increased activity from 7 to 21 DPI in IP and IP + l-arginine groups. The cytotoxicity of iIELs was drastically reduced at 3 and 5 DPC in all the groups, however the reduction was less severe in IP + l-arginine group compared with IP (P < 0.05). The iIELs are capable of wide range of effector functions known to T and NK cells. Recent evidences suggest that both antigen specific and nonspecific cytotoxic cells are integral component of mucosal immunity [27]. The cytotoxicity of iIELs is seen against chicken tumor cell lines, rotavirus in chickens [19], Salmonella typhimirium in mice [28] and corona virus infection in mice [29]. The enhanced iIELs cytotoxicity in IP + l-arginine group may be attributed to immunostimulatory activities of l-arginine on GALT. It has been shown that supplemental l-arginine increased specific lymphocyte subsets in GALT [30]. We used purified IBDV antigen and Con-A to measure specific and nonspecific proliferative response of iIELs and PBMCs. Although both iIELs and PBMCs responded to IBDV and Con-A stimulation, optical densities of PBMCs were always higher than iIELs indicating that the cellular activity was higher in PBMCs. There are mixed reports regarding proliferative ability of iIELs to mitogens, our results shows that iIELs respond well to mitogenic stimuli and are in agreement with other published studies [20], [31]. The iIELs and lymphocyte proliferation response was depressed in unimmunized and l-arginine controls after challenge with virulent IBDV indicates a possible damage to T cells by the challenge virus. Suppression of lymphocyte proliferation to Con-A compared to controls was observed at 7 DPI. Proliferation was severely depressed when iIELs and PBMCs were specifically stimulated with IBDV. Several authors reported that IBDV causes hyporesponsiveness of the PBMCs [32], [33], [34]. Administration of relatively pathogenic vaccine caused transitory depression of mitogenic response of peripheral lymphocytes [35]. The direct cytolysis of T lymphocytes by IBDV may be a possible factor responsible for mitogenic hyporensponsiveness [34]. The data of depressed blastogenic response were consistent with other reports [34], [35], where detectable depression was noticed a week after infection. Subsequently hyporesponsiveness returned to normal or near normal levels. After challenge with virulent virus, the proliferative response to Con-A and IBDV was severely depressed indicating the immunosuppressive effects of challenge virus. Both iIELs and PBMCs obtained from chickens immunized with IP strain and supplemented with l-arginine showed significantly elevated proliferative response to IBDV and Con-A and the suppression was rapidly cleared compared to IP group and controls. l-arginine acts on different components of immune system. Supplementation of l-arginine enhanced proliferative response of T cells to mitogens [36] through the release of IL-2. l-Arginine also enhances the cytotoxicity of LAK and NK cells [6]. In summary, our results clearly demonstrate that complete protection was achieved against virulent IBDV challenge in chickens immunized orally with IP strain of IBDV and supplemented with l-arginine. l-Arginine is strong immunoregulator of the IP strain of IBDV vaccine and is safe, inexpensive, easy to administer in feed. l-Arginine not only enhanced the specific intestinal immune response as demonstrated by functional activities of iIELs against IBDV but also amplified the systemic immunity. l-Arginine, a newly emerged mucosal immunostimulant should prove valuable not only for IBD but also for several other diseases of poultry.
Acknowledgements
The authors are thankful to the Director, Indian Veterinary Research Institute for providing necessary facilities to carry out this research.
References
- 1.Lukert P.D., Saif Y.M. Infectious bursal disease. In: calnek B.W., Barnes H., Beard C.W., Mcdougald L.R., Saif Y.M., editors. Diseases of poultry. Iowa State University Press; Ames: 1997. pp. 721–738. [Google Scholar]
- 2.Muller H., Islam M.R., Raue R. Research on infectious bursal disease – the past, the present and the future. Vet Microbiol. 2003;97(1–2):153–165. doi: 10.1016/j.vetmic.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Barbul A., Wasserkrug H.L., Sisto D.A., Seifter E., Rettura G., Levenson S.M. Thymic stimulatory actions of arginine. JPEN J Parenter Enteral Nutr. 1980;4(5):446–449. doi: 10.1177/014860718000400502. [DOI] [PubMed] [Google Scholar]
- 4.Daly J.M., Reynolds J., Thom A., Kinsley L., Dietrick-Gallagher M., Shou J. Immune and metabolic effects of arginine in the surgical patient. Ann Surg. 1988;208(4):512–523. doi: 10.1097/00000658-198810000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wrightham M.N., Cann A.J., Sewell H.F. l-Arginine: a therapeutic option for AIDS/HIV infection? Med Hypotheses. 1992;38(3):236–239. doi: 10.1016/0306-9877(92)90101-h. [DOI] [PubMed] [Google Scholar]
- 6.Park K.G., Hayes P.D., Garlick P.J., Sewell H., Eremin O. Stimulation of lymphocyte natural cytotoxicity by l-arginine. Lancet. 1991 16;337(8742):645–646. doi: 10.1016/0140-6736(91)92456-c. [DOI] [PubMed] [Google Scholar]
- 7.de Jonge W.J., Kwikkers K.L., te Velde A.A., van Deventer S.J., Nolte M.A., Mebius R.E. Arginine deficiency affects early B cell maturation and lymphoid organ development in transgenic mice. J Clin Invest. 2002;110(10):1539–1548. doi: 10.1172/JCI16143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tayade C., Jaiswal T.N., Mishra S.C., Koti M. l-Argininine stimulates immune response in chickens imunized with intermediate plus strain of infectious bursal disease virus. Vaccine. 2006;24(5):552–560. doi: 10.1016/j.vaccine.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 9.Kibenge F.S., Dhillon A.S., Russell R.G. Biochemistry and immunology of infectious bursal disease virus. J Gen Virol. 1988;69(8):1757–1775. doi: 10.1099/0022-1317-69-8-1757. [DOI] [PubMed] [Google Scholar]
- 10.Bergmann K.C., Waldman R.H. Enhanced murine respiratory tract IgA antibody response to oral influenza vaccine when combined with a lipoidal amine (avridine) Int Arch Allergy Appl Immunol. 1988;87(3):334–335. doi: 10.1159/000234695. [DOI] [PubMed] [Google Scholar]
- 11.Dillon S.B., MacDonald T.T. Functional properties of lymphocytes isolated from murine small intestinal epithelium. Immunology. 1984;52(3):501–509. [PMC free article] [PubMed] [Google Scholar]
- 12.Fujihashi K., Taguchi T., McGhee J.R., Eldridge J.H., Bruce M.G., Green D.R. Regulatory function for murine intraepithelial lymphocytes. Two subsets of CD3+, T cell receptor-1+ intraepithelial lymphocyte T cells abrogate oral tolerance. J Immunol. 1990;145(7):2010–2019. [PubMed] [Google Scholar]
- 13.Tufveson G., Alm G.V. Effects of mitogens for mouse B lymphocytes on chicken lymphoid cells. Immunology. 1975;29(4):697–707. [PMC free article] [PubMed] [Google Scholar]
- 14.Gobel T.W., Kaspers B., Stangassinger M. NK and T cells constitute two major, functionally distinct intestinal epithelial lymphocyte subsets in the chicken. Int Immunol. 2001;13(6):757–762. doi: 10.1093/intimm/13.6.757. [DOI] [PubMed] [Google Scholar]
- 15.Myers T.J., Schat K.A. Natural killer cell activity of chicken intraepithelial leukocytes against rotavirus-infected target cells. Vet Immunol Immunopathol. 1990;26(2):157–170. doi: 10.1016/0165-2427(90)90064-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lassila O., Eskola J., Toivanen P. A micromethod for stimulation of chicken lymphocytes in vitro using whole blood. Clin Exp Immunol. 1976;26(3):641–646. [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa H., Li Y., Abeliovich A., Yamamoto S., Kaufmann S.H., Tonegawa S. Cytotoxic and interferon gamma-producing activities of gamma delta T cells in the mouse intestinal epithelium are strain dependent. Proc Natl Acad Sci USA. 1993;90(17):8204–8208. doi: 10.1073/pnas.90.17.8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed L.J., Muench H. A simple method of estimating 50% end point. Am J Hyg. 1938;27:493. [Google Scholar]
- 19.Chai J.Y., Lillehoj H.S. Isolation and functional characterization of chicken intestinal intra-epithelial lymphocytes showing natural killer cell activity against tumour target cells. Immunology. 1988;63(1):111–117. [PMC free article] [PubMed] [Google Scholar]
- 20.Agrawal P.K., Reynolds D.L. An evaluation of the mitogenic reactivity of intestinal intraepithelial lymphocytes of chickens. Avian Dis. 1999;43(2):172–181. [PubMed] [Google Scholar]
- 21.Lillehoj H.S. Intestinal intraepithelial and splenic natural killer cell responses to Eimerian infections in inbred chickens. Infect Immun. 1989;57(7):1879–1884. doi: 10.1128/iai.57.7.1879-1884.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar P.A., Das S.K., Rao J.R. Effect of immunostimulation on cytotoxic activity of intestinal intraepithelial lymphocytes of chickens in infectious bursal disease and Eimeria tenella infections. Acta Vet Hung. 1998;46(1):1–11. [PubMed] [Google Scholar]
- 23.Bounous D.I., Campagnoli R.P., Brown J. Comparison of MTT colorimetric assay and tritiated thymidine uptake for lymphocyte proliferation assays using chicken splenocytes. Avian Dis. 1992;36(4):1022–1027. [PubMed] [Google Scholar]
- 24.Van den berg T.P. Acute infectious bursal disease in poultry: a review. Avian Pathol. 2000;29(3):175–194. doi: 10.1080/03079450050045431. [DOI] [PubMed] [Google Scholar]
- 25.Tizard I. W.B. Saunders Company; Philadelphia: 2000. Veterinary immunology an introduction. [Google Scholar]
- 26.Rautenschlein S., Yeh H.Y., Sharma J.M. The role of T cells in protection by an inactivated infectious bursal disease virus vaccine. Vet Immunol Immunopathol. 2002;89(3–4):159–167. doi: 10.1016/s0165-2427(02)00202-7. [DOI] [PubMed] [Google Scholar]
- 27.Muir W.I., Bryden W.L., Husband A.J. Immunity, vaccination and the avian intestinal tract. Dev Comp Immunol. 2000;24:325–342. doi: 10.1016/s0145-305x(99)00081-6. [DOI] [PubMed] [Google Scholar]
- 28.Nencioni L., Villa L., Boraschi D., Berti B., Tagliabue A. Natural and antibody-dependent cell-mediated activity against Salmonella typhimurium by peripheral and intestinal lymphoid cells in mice. J Immunol. 1983;130(2):903–907. [PubMed] [Google Scholar]
- 29.Carman P.S., Ernst P.B., Rosenthal K.L., Clark D.A., Befus A.D., Bienenstock J. Intraepithelial leukocytes contain a unique subpopulation of NK-like cytotoxic cells active in the defense of gut epithelium to enteric murine coronavirus. J Immunol. 1986;136(5):1548–1553. [PubMed] [Google Scholar]
- 30.Fukatsu K., Ueno C., Maeshima Y., Hara E., Nagayoshi H., Omata J. l-arginine-enriched parenteral nutrition affects lymphocyte phenotypes of gut-associated lymphoid tissue. JPEN J Parenter Enteral Nutr. 2004;28(4):246–250. doi: 10.1177/0148607104028004246. [DOI] [PubMed] [Google Scholar]
- 31.Agrawal P.K., Reynolds D.L. A comparative analysis of the mitogenic response of intestinal intraepithelial lymphocytes in various age groups of turkeys. Avian Dis. 1999;43(3):376–383. [PubMed] [Google Scholar]
- 32.Sivanandan V., Maheswaran S.K. Immune profile of infectious bursal disease (IBD). II. Effect of IBD virus on pokeweed-mitogen-stimulated peripheral blood lymphocytes of chickens. Avian Dis. 1980;24(3):734–742. [PubMed] [Google Scholar]
- 33.Confer A.W., Springer W.T., Shane S.M., Donovan J.F. Sequential mitogen stimulation of peripheral blood lymphocytes from chickens inoculated with infectious bursal disease virus. Am J Vet Res. 1981;42(12):2109–2113. [PubMed] [Google Scholar]
- 34.Sharma J.M., Lee L.F. Effect of infectious bursal disease on natural killer cell activity and mitogenic response of chicken lymphoid cells: role of adherent cells in cellular immune suppression. Infect Immun. 1983 Nov;42(2):747–754. doi: 10.1128/iai.42.2.747-754.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sivanandan V., Maheswaran S.K. Immune profile of infectious bursal disease: I. Effect of infectious bursal disease virus on peripheral blood T and B lymphocytes of chickens. Avian Dis. 1980;24(3):715–725. [PubMed] [Google Scholar]
- 36.Barbul A., Sisto D.A., Wasserkrug H.L., Efron G. Arginine stimulates lymphocyte immune response in healthy human beings. Surgery. 1981;90(2):244–251. [PubMed] [Google Scholar]


