Abstract
The selective and potent aminopeptidase N inhibitor [125I]RB 129 has been used for the radioautographic localization of this enzyme in rat brain, spinal cord and intestine. Brain microvessels and intestine brush-border cells were shown to present a high concentration of aminopeptidase N. Moreover, a labeling of various brain structures was observed. A very high level of binding occurred in the meninges, choroid plexus, pineal gland, paraventricular nucleus and pituitary gland. Moderate to high labeling was also observed in the cortex, caudate–putamen, subthalamic nucleus, central periaqueductal gray, thalamus, as well as in the dorsal and ventral horn of the spinal cord, which are known to contain a high concentration of enkephalins, opioid receptors and neutral endopeptidase. This co-localization confirms the physiological implication of aminopeptidase N in the inactivation of enkephalins accounting for the requirement of dual inhibition of neutral endopeptidase and aminopeptidase N to observe highly significant morphine-like effects induced by the protected endogenous opioid peptides. Aminopeptidase N was also visualized in moderate to high levels in other brain structures such as the hippocampus, nucleus accumbens, substantia nigra, hypothalamus (dorsomedial and ventromedial nuclei), raphe nucleus, pontine nucleus, inferior olive, and in high concentration in the granular layer of cerebellum.
In summary, aminopeptidase N has been visualized for the first time in numerous brain areas using the selective inhibitor [125I]RB 129. This iodinated probe could allow the ex vivo and in vivo localization of aminopeptidase N in various tissues to be investigated and may also be used to evaluate quantitative changes in aminopeptidase N expression in pathological situations. Aminopeptidase N, which preferably removes NH2-terminal neutral amino acids from peptides, has probably a host of substrates. Nevertheless, a certain in vivo selectivity could be achieved by the presence of the enzyme in structures where the peptide effector and its receptors are also co-localized.
Keywords: enkephalins, neutral endopeptidase, intestine, aminopeptidase N inhibitor
Abbreviations: APN, aminopeptidase N; HPA, hypothalamo–pituitary–adrenal; NEP, neutral endopeptidase; PVN, paraventricular hypothalamic nucleus; RB 129, 2(S)-benzyl-3-[hydroxy(1′(R)-aminoethyl)phosphinyl]propanoyl-L-3-iodo-tyrosine
Aminopeptidase N (APN, EC 3.4.11.2), a 150 kDa glycoprotein with zinc-dependent metalloprotease activity (Luciani et al., 1998), has a wide distribution and numerous physiological functions. Thus, in hematopoietic and epithelial cells, the enzyme is believed to be involved in the control of growth and differentiation (Naquet et al., 1989). Moreover, APN was recently shown to be a target for inhibiting angiogenesis (Pasqualini et al., 2000). In the intestinal brush-border membranes it participates in the final steps of digestion by cleaving peptides into their constituent amino acids. APN is also found in reproductive organs (Fujiwara et al., 1999), and is very abundant in the kidney where it appears to cleave peptides, thereby preventing their reentry into the vasculature (Hersh et al., 1987). At the renal brush-border membrane level, APN has been suggested to act as anion exchanger. The peptidase has also been reported to serve as a receptor for transmissible gastroenteritis virus (Delmas et al., 1992), and simultaneously, Yeager et al. (1992) have reported that human APN is a target for coronavirus 229E, which plays a critical role in upper respiratory tract infection. Finally, APN has been shown to be identical to antigen p146 of type II alveolar epithelial cells of rat lung (Funkhouser et al., 1991) and to human lymphocyte surface cluster differentiation antigen CD13 (Look et al., 1989).
In the brain, peptidases play a key role in the metabolism of neuropeptides released from nerve endings, either in achieving their maturation from larger precursors or in carrying out their inactivation (Roques, 2000). APN, which preferably removes NH2-terminal neutral amino acids from peptide substrates, is a potential candidate to modulate signal transduction by hydrolyzing bioactive peptides such as the enkephalins in the CNS (review in Roques et al., 1993). One way to elucidate the functional role of APN activity is to study its regional distribution and to determine tissues where a co-localization with neuropeptide substrates occurs. A current approach to localize aminopeptidases is to use synthetic substrates, which give products easily detectable after hydrolysis by fluorimetry or high-performance liquid chromatography (Alba et al., 1993, Guyon et al., 1979). The limit of this methodology is the lack of sensitivity and resolution avoiding the discrete distribution of a given enzyme to be carried out. In the case of APN some studies on its localization have been done by using immunohistochemistry (Hersh et al., 1987, Solhonne et al., 1987). However, this procedure was not able to visualize APN in neuronal brain structures and the peptidase was reported to be essentially present in microvessels. Radioactive inhibitors which are usually small molecules have the advantage over antibodies to label the active site of the enzyme, quantitatively, in all tissues and species (review in Zajac and Roques, 1989). In contrast, antibodies are generated from a given animal, and although the primary sequences of zinc metallopeptidases were shown to be highly conserved, a lack of antibody recognition could occur due to various glycosylation levels in different tissues and/or species or due to differences in the tertiary structure and epitope presentation of the enzyme in various tissues, as already shown in the case of angiotensin converting enzyme (Strittmatter and Snyder, 1987). Therefore, the binding of a competitive and specific radioiodinated inhibitor of APN offered a quick, simple and reproducible method for visualization and quantitation of the peptidase in its intact membrane-anchored form, even in regions where the enzyme is in a very low concentration (Zajac and Roques, 1989).
Determination of the APN distribution in the brain was essential to evaluate the physiological relevance of this enzyme in peptide metabolism. The possible lack of selectivity due to the in vitro low specificity of such a widely distributed peptidase seems to be counterbalanced in vivo by the co-localization of the substrate and both its metabolizing enzyme(s) and receptor(s). Moreover, the involvement of APN in the physiological inactivation of neuropeptides could be confirmed by the use of specific antagonists capable of reversing the pharmacological responses induced either by administration of the endogenous effector or by an APN inhibitor. In this way, the role of APN, with the concomitant action of another metallopeptidase, neutral endopeptidase (NEP), has been firmly established (Bourgoin et al., 1986, Waksman et al., 1985). Due to the complementary role of NEP and APN, selective inhibition of only one of these peptidases does not increase the endogenous enkephalin concentration to a sufficient level, leading to weak antinociceptive effects, whereas strong antinociceptive responses can be obtained after complete inhibition of both enzymes (Bourgoin et al., 1986, Chen et al., 1998, Fournié-Zaluski et al., 1984, Noble et al., 1992). With the aim of visualizing APN in the CNS, a highly potent and selective APN inhibitor has been designed and then radioiodinated (Chen et al., 1999, Chen et al., 2000). This radioligand, [125I]RB 129 (2(S)-benzyl-3-[hydroxy(1′(R)-aminoethyl)phosphinyl]propanoyl-L-3-iodo-tyrosine), appears to be appropriate for autoradiography, as its affinity is in the nanomolar range (K d=3.4±0.3 nM in rat brain homogenates) and it is specific for APN (Chen et al., 1999, Noble et al., 2000). Moreover, [125I]RB 129 was found to interact with a single class of binding sites in rat brain homogenates, and the specific binding was high, being almost 95% at the K d value (Noble et al., 2000). In this paper, the localization of APN, in brain and spinal cord, was done for the first time by visualization of [125I]RB 129 binding, with intestine tissue used as control.
Experimental procedures
Animals
Male Wistar rats, 200–220 g (Charles River, France) were used. Care of the animals was in accordance with standard ethical guidelines and under the control of Ethical Committee of the Faculty. Only the number of rats necessary to produce reliable scientific data were used (n=5).
Autoradiography
Animals were deeply anaesthetized with pentobarbital and perfused intracardially with 100 ml of saline (0.9% NaCl). After rat decapitation, the brain, spinal cord and intestine were rapidly removed and frozen in isopentane at −45°C, and stored at −80°C until use. Sections (20 μm thick) were cut on a Bright cryostat at −17°C, thaw-mounted onto Superfrost Plus slides, and stored at −20°C. All sections were warmed to room temperature just prior to incubation with ligands. The slides were incubated with 0.5 nM [125I]RB 129 in 50 mM Tris–HCl (pH 7.4)–5 mM MgCl2–0.2% bovine serum albumin for 120 min at room temperature. For non-specific binding, the slices were incubated as above but in the presence of 1 μM of unlabeled RB 129. At the end of the incubation, the slices were washed four times in ice-cold buffer (50 mM Tris–HCl (pH 7.4)–0.2% bovine serum albumin) for 5 min, followed by a rapid rinse in ice-cold water and then dried under a cold airstream. Then, the films were exposed for 2 days at room temperature and developed.
Materials
The synthesis of RB 129 and of its 125I-radioiodinated analogue was performed as previously described (Chen et al., 1999, Chen et al., 2000). The Superfrost Plus slides were purchased from Menzel-Gläser, and β-max films from Amersham (France).
Analysis of binding sites by densitometry
PhosphoImager screens were laid on the top of the slide to begin the exposure. Five hours later, the images on the screen were acquired by Molecular Dynamics scanners and analyzed using ImageQuant software, converting into arbitrary values the relative optical densities.
Results
Radioautograms were obtained in a series of coronal and sagittal sections of rat brain, spinal cord and intestine. In each case, the high levels of specific binding observed in the different tissue sections are reflected by well-defined total binding in each section while non-specific labeling remained very weak and homogenous (Fig. 1 ).
Fig. 1.
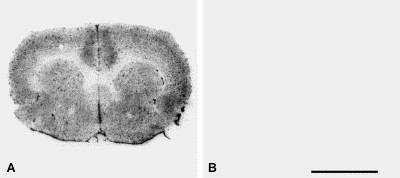
Specific binding of the radiolabeled APN inhibitor [125I]RB 129. Radioautography of coronal sections at the level of the striatum in rat brain with 0.5 nM [125I]RB 129. (A) Total binding of [125I]RB 129. (B) [125I]RB 129 binding in the presence of 1 μM RB 129 (non-specific binding). Scale bar ∼5 mm.
In the brain, [125I]RB 129 labeling was very high in the meninges, choroid plexus (Fig. 3F–H) and blood vessels, which appear as dark points on the slices (Table 1 ).
Fig. 3.
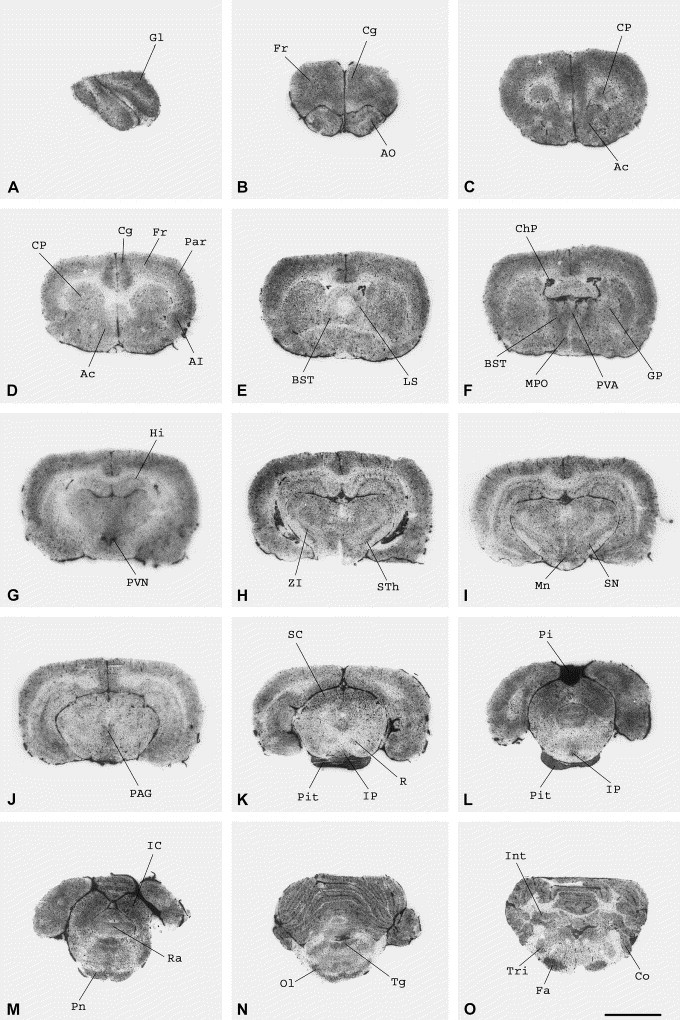
Distribution of APN in the rat brain labeled with the selective inhibitor [125I]RB 129. Radioautography of coronal sections showing the distribution of 0.5 nM [125I]RB 129 total binding at different levels of the rat brain. (A) Olfactory bulb. (B) Frontal (Fr) and cingulate cortex (Cg), anterior olfactory nucleus (AO). (C,D) Cortex, caudate–putamen (CP), nucleus accumbens (Ac). (E) Cortex, caudate–putamen, lateral septal nucleus (LS), bed nucleus stria terminal (BST). (F) Cortex, striatum, globus pallidus (GP), bed nucleus stria terminal, paraventricular thalamic nucleus (PVA), medial preoptic nucleus (MPo), choroid plexus (ChP). (G) Hippocampus (Hi), paraventricular hypothalamic nucleus (PVN). (H) Zona incerta (ZI), subthalamic nucleus (STh). (I) Zona incerta, substantia nigra (SN), mamillary nucleus (Mn). (J) Periaqueductal gray matter level (PAG). (K) Superior colliculus (SC), periaqueductal gray matter, red nucleus (R), interpeduncular nucleus (IP), pituitary gland (Pit). (L) Pineal gland (Pi), interpeduncular nucleus, pituitary gland. (M) Inferior colliculus (IC), raphe nucleus (Ra), pontine nucleus (Pn). (N) Cerebellum, tegmental nuclei (Tg), olivary region (Ol). (O) Interposed cerebellar nuclei (Int), cochlear nuclei (Co), trigeminal nuclei (Tri), facial nucleus (Fa). The slides were incubated for 120 min at room temperature as described in Experimental procedures. For non-specific binding, the slices were incubated in the presence of 1 μM of unlabeled RB 129. Scale bar ∼3 mm.
Table 1.
Distribution of APN in rat brain
| Brain structure | [125I]RB 129 specific binding (arbitrary values) |
| Olfactory bulb | |
| Glomerular layer | 380±15 |
| Telencephalon | |
| Anterior olfactory nucleus | |
| Anterior olfactory nucleus, dorsal | 208±10 |
| Anterior olfactory nucleus, lateral | 215±20 |
| Anterior olfactory nucleus, medial | 211±19 |
| Anterior olfactory nucleus, ventral | 187±17 |
| Anterior olfactory nucleus, posterior | 230±5 |
| Cortex | |
| Cingulate cortex | 330±8 |
| Frontal cortex | 233±7 |
| Parietal cortex, area 1 | 240±14 |
| Agranular insular cortex | 255±18 |
| Lateral orbital cortex | 245±12 |
| Infralimbic cortex | 270±22 |
| Caudate–putamen | 166±4 |
| Nucleus accumbens | |
| Anterior | 230±6 |
| Posterior | 207±6 |
| Globus pallidus | 110±3 |
| Hippocampus | 112±6 |
| Lateral septal nucleus | 150±2 |
| Bed nucleus stria terminal | 235±21 |
| Diencephalon | |
| Thalamus | |
| Paraventricular thalamic nucleus, anterior | 238±3 |
| Zona incerta | 165±8 |
| Subthalamic nucleus | 208±7 |
| Medial preoptic nucleus | 245±25 |
| Hypothalamus | |
| PVN | 380±14 |
| Dorsomedial nucleus | 189±10 |
| Ventromedial nucleus | 171±19 |
| Mesencephalon | |
| Substantia nigra | 203±10 |
| Mamillary nucleus | 260±8 |
| Inferior colliculus | 305±18 |
| Superior colliculus | 269±12 |
| Periaqueductal gray matter | |
| Darkschewitsch nucleus | 215±3 |
| Edinger–Westphal nucleus | 210±5 |
| Red nucleus | 140±8 |
| Interpeduncular nucleus | 207±7 |
| Raphe nucleus | 267±11 |
| Pontine nucleus | 280±10 |
| Metencephalon | |
| Cerebellum | |
| Granular layer | 298±16 |
| Molecular layer | 167±10 |
| Interposed cerebellar nuclei | 261±22 |
| Tegmental nuclei | 295±10 |
| Cochlear nuclei | 274±18 |
| Inferior olive | 275±21 |
| Trigeminal nucleus | 170±18 |
| Facial nuclei | 306±17 |
| Choroid plexus | |
| Dorsal third ventricle | 495±9 |
| Lateral ventricle | 471±25 |
| Spinal cord | |
| Spinal gray matter | |
| Dorsal horn | 310±6 |
| Ventral horn | 315±5 |
| Meninges | |
| Brain | 525±23 |
| Spinal cord | 523±22 |
| Pituitary gland | |
| Anterior pituitary | 492±38 |
| Posterior pituitary | 423±25 |
| Pineal gland | 1517±98 |
Olfactory bulb
In the glomerular layer of the olfactory bulb the labeling of APN was also very dense (Table 1, Fig. 3A).
Telencephalon
A strong labeling was observed in the different parts of the anterior olfactory nucleus (Table 1; Fig. 2, Fig. 3 ). High levels of binding were also found in the cortex (cingulate cortex, frontal cortex, parietal cortex, agranular insular cortex, lateral orbital cortex and infralimbic cortex) (Fig. 3B–G). This was also the case for the anterior part of the nucleus accumbens (Fig. 3C). Contrastingly, moderate to low labeling was found in the caudate–putamen, posterior part of the nucleus accumbens, globus pallidus, hippocampus, lateral septal nucleus and bed nucleus stria terminal (Fig. 2, Fig. 3).
Fig. 2.
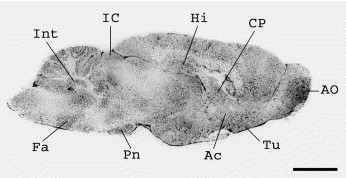
Distribution of APN in the rat brain labeled with the selective inhibitor [125I]RB 129. Radioautography of sagittal section showing the distribution of 0.5 nM [125I]RB 129 total binding in the rat brain. Labeling is particularly dense at the level of the meninges. Olfactory tubercle (Tu), pontine nucleus (Pn), inferior colliculus (IC) contain a moderate level of binding. The slides were incubated for 120 min at room temperature as described in Experimental procedures. For non-specific binding, the slices were incubated in the presence of 1 μM of unlabeled RB 129. Scale bar ∼5 mm.
Diencephalon
In the thalamus, the different nuclei exhibited moderate labeling (Table 1; Fig. 2, Fig. 3), as the medial preoptic nucleus (Fig. 3F) and zona incerta (Fig. 3H,I). The labeling was moderate in the ventromedial and dorsomedial nuclei of the hypothalamus (Table 1), but higher in the paraventricular nucleus (PVN) (Fig. 3G).
Mesencephalon
Moderate binding was found in the substantia nigra (Fig. 3I), interpeduncular nucleus (Fig. 3K,L), raphe nucleus (Fig. 3M) and pontine nuclei (Fig. 2, Fig. 3). In the periaqueductal gray matter the [125I]RB 129 labeling was also moderate, in the Darkschewitsch and Edinger–Westphal nuclei (Fig. 3J,K). A high binding was observed in the inferior (Fig. 3M) and superior colliculus (Fig. 3K) and in the mamillary nucleus (Fig. 3I), whereas the labeling was low in the red nucleus (Fig. 3K).
Metencephalon
The labeling was moderate in the trigeminal nucleus, and high in the tegmental nuclei, cochlear nuclei, olivary region, and facial nuclei (Table 1; Fig. 2, Fig. 3). In the granular layer of the cerebellum and the anterior and posterior parts of interposed cerebellar nucleus (Fig. 2, Fig. 3) the [125I]RB 129 binding sites were present at relatively high levels, whereas the labeling was weaker in the molecular layer of the cerebellum.
The most intense labeling of [125I]RB 129 was observed in the pineal gland (Table 1; Fig. 3L), and to a lesser extent in the anterior and posterior part of the pituitary gland (Table 1; Fig. 3K,L).
In the spinal cord, a rather homogenous [125I]RB 129 labeling, restricted to the meninges and spinal gray matter (ventral and dorsal horn), was observed whatever the spinal level considered (cervical enlargement, thoracic level, lumbar enlargement, sacral level). A very poor binding was found scattered over the rest of the spinal cord (Fig. 4 ).
Fig. 4.
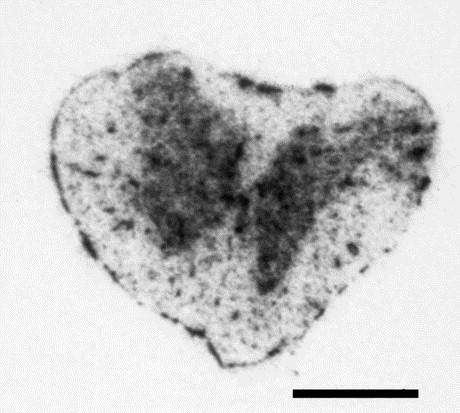
Distribution of APN in the rat spinal cord labeled with the selective inhibitor [125I]RB 129. Radioautography of coronal sections showing the distribution of 0.5 nM [125I]RB 129 total binding in the cervical spinal cord. The binding sites are highly concentrated in the substantia gelatinosa. The slides were incubated for 120 min at room temperature as described in Experimental procedures. For non-specific binding, the slices were incubated in the presence of 1 μM of unlabeled RB 129. Scale bar ∼12 mm.
In the intestine, the APN is visualized by [125I]RB 129 binding at the level of brush-border cells (Fig. 5 ).
Fig. 5.
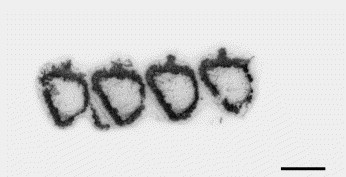
Distribution of APN in the rat intestine labeled with the selective inhibitor [125I]RB 129. Radioautography of rat intestine showing distribution of 0.5 nM [125I]RB 129 total binding in the intestinal membranes. The slides were incubated for 120 min at room temperature as described in Experimental procedures. For non-specific binding, the slices were incubated in the presence of 1 μM of unlabeled RB 129. Scale bar ∼4 mm.
Discussion
A detailed mapping of APN in the rat brain has been carried out for the first time using the highly selective radiolabeled APN inhibitor, [125I]RB 129 (K d=3.4±0.3 nM in rat brain homogenates). The high selectivity of RB 129 for APN is supported by the non-specific labeling determined in the presence of an excess of unlabeled inhibitor which is almost undetectable in all regions studied, in good agreement with the results obtained in enzymatic and binding studies (Chen et al., 1999, Noble et al., 2000).
In the brain, the pattern of labeling with this inhibitor shows clearly the presence of the peptidase on the brain microvessels, in agreement with previous studies using immunohistochemical detection (Hersh et al., 1987, Solhonne et al., 1987). Moreover, molecular and cellular studies have shown that APN is localized to the abluminal membrane of vessels, and more precisely at the level of astrocytic feet and pericytes which surround the microvessels (Barnes et al., 1994, Kunz et al., 1994). A dense labeling was also observed at the level of meninges in brain and spinal cord, which form a barrier protecting both organs. The occurrence of an aminopeptidase-like activity in rat meninges was already reported (Zajac et al., 1987) and confirmed by the specific APN labeling observed in the present study. Enzymatic activities at the levels of microvessels and meninges could represent a clearing system for regulatory peptides, and the presence of APN with other peptidases such as angiotensin converting enzyme and NEP (Zajac et al., 1987) seems to support this hypothesis. Moreover, these enzymes could prevent the access of potentially harmful peptides to the brain or spinal cord. The aminopeptidase activity found in microvessels may also have physiological functions. Thus, it has been shown that APN rapidly hydrolyzes a number of vasoactive peptides at physiological pH (Palmieri et al., 1985), and may play a role in modulating their levels in the circulation and/or within the wall of the blood vessel. For instance, endogenous opioid peptides enkephalins are present in the human plasma (Pert et al., 1976) where they are probably degraded by both endothelial NEP (Soleilhac et al., 1996) and APN (review in Roques et al., 1993). Moreover, in the microvessels APN may play an important role in immune functions, as opioid receptors are localized on immune cells (review in Bhargava, 1990, Sharp et al., 1998).
However, the most interesting result of this study is the autoradiographic data showing that APN is widely distributed throughout the CNS. In the brain, APN has been found to be involved in the degradation of neuropeptides, particularly the endogenous opioid peptides, enkephalins (Waksman et al., 1985) in association with NEP, another zinc metallopeptidase (review in Roques et al., 1993). A moderate APN concentration was observed in the caudate–putamen where a dense labeling of NEP was shown to be present (Waksman et al., 1986). Thus, the distribution of both enzymes and enkephalin peptides (Mansour and Watson, 1993) occurred in this structure. This co-localization of APN, NEP and enkephalins is in agreement with the well-admitted interruption of the messages conveyed by these peptides (Daugé et al., 1996, review in Roques et al., 1993, Waksman et al., 1985). In addition to enkephalin fibers, the caudate–putamen of the rat contains scattered dynorphin-positive fibers, another opioid peptide degraded by APN (Safavi and Hersh, 1995), which may thus play a role in the regulatory mechanisms controlling the activity of this neuropeptide.
On the other hand, the pattern of [125I]RB 129 labeling clearly indicates the presence of APN in the central periaqueductal gray, several nuclei of the thalamus, and the dorsal horn of the spinal cord, where NEP and enkephalins are co-localized. These structures play a key role in control of nociceptive messages (reviews in Basbaum and Fields, 1984, Besson and Chaouch, 1987) and it has been shown using dual NEP/APN inhibitors that enkephalins interact with opioid receptors in these areas to produce analgesia (Valverde et al., 1996).
A dense labeling of the selective APN inhibitor was observed in the inferior colliculus which receives axons from most brainstem auditory nuclei. Unilateral lesions of the inferior colliculus produced sound localization deficits (Jenkins and Masterton, 1982). The presence of APN in this region has not been associated until now with any functional effect. Nevertheless, NEP and enkephalins have previously been visualized in this brain area (review in Roques et al., 1993). Co-localization of these peptides and their metabolizing enzymes, NEP and APN, suggests that enkephalins could have several roles, in addition to nociception, such as the regulation of auditory functions. Thus, APN has been shown to be involved in the metabolism of a heptadecapeptide, nociceptin (Montiel et al., 1997, Noble and Roques, 1997), which is abundant in the inferior colliculus (Houtani et al., 1996), where this peptide was reported to participate in the control of the auditory system. Accordingly, Nishi et al. (1997) have found defects in hearing recovery in mice lacking the nociceptin receptor and submitted to intense sound.
Integration of the hypothalamo–pituitary–adrenal (HPA) stress response occurs by means of interactions between stress-sensitive brain circuitry and neuroendocrine neurons of the hypothalamic PVN (Herman and Cullinan, 1997). The very high concentration of APN in both the pituitary gland and PVN suggests that this peptidase may have an important role in the HPA axis regulation. APN may regulate the endogenous enkephalins levels in these brain areas which exert a negative control on the HPA axis. Thus, it has been shown that blockade of opioidergic inhibitory input to corticotropin-releasing hormone-containing neurons of the PVN by the opioid antagonist, naloxone, induced an increase in plasma adrenocorticotropic hormone and cortisol (Wand and Schumann, 1998). A functional deficiency of endogenous opioidergic inhibitory tone may be involved in depression, a disease marked by hyperactivity of the HPA axis (reviews in Arborelius et al., 1999, Kathol et al., 1989), or in the pathophysiology of drug addiction (Culpepper-Morgan and Kreek, 1997, Gonzalvez et al., 1991, Schluger et al., 1998).
The highest concentration of APN labeled by [125I]RB 129 was found in the pineal gland. This structure contains multiple afferent peptidergic nerve fibers, with the presence of neuropeptide Y, norepinephrine, vasoactive intestinal peptide, substance P, calcitonin gene-related peptide, somatostatin or opioid peptides (Aloyo, 1991; review in Moller et al., 1996). APN may be involved in the regulation of different endogenous peptide levels which may directly modulate pineal function.
A large [125I]RB 129 binding was also observed in the cerebellum, where high amounts of NEP have also been visualized (Waksman et al., 1986), while the distribution of both enkephalins and opioid receptors is sparce. The low concentration of the two opioid pentapeptides and their receptors contrasting with the relatively high levels of peptidases suggests that NEP and APN could be used to hydrolyze other still unknown peptide substrates in this structure.
APN is also visualized in other brain areas with a moderate to high labeling in the anterior olfactory nucleus, hippocampus, cortex, thalamus nuclei, nucleus accumbens and substantia nigra, suggesting that APN in these structures plays a role in the interruption of the enkephalinergic transmission involved in several functions such as locomotor activity, emotional responses, rewarding effects and memory processes (Roques et al., 1993). The rat substantia nigra contains the highest CNS concentration of immunoreactive substance P (Kanazawa and Jessell, 1976), and relatively high levels of Leu-enkephalin, supplied by striatonigral axons and generated from the precursor dynorphin (Zamir et al., 1984). The physiological function of APN in this brain area has not been determined but the peptidase is probably involved in the regulation of enkephalin and dynorphin pathways.
As a control, distribution of APN has also been studied in the rat intestine. Binding of the radioiodinated inhibitor [125I]RB 129 was visualized in the brush border in agreement with previous studies using immunohistochemistry (Sjöström et al., 1978, Terashima et al., 1992). APN is anchored to the microvillar membrane via an uncleaved signal for membrane insertion. The physiological function of APN at this level could be to participate in the final steps of digestion, by cleaving peptides into their constituent amino acids. Moreover, the tonic participation of endogenous enkephalins at different levels of the gastrointestinal tract has been demonstrated using NEP inhibitor (reviews in Checler, 1991, Roques et al., 1993). Thus, a naloxone-antagonized antidiarrheal effect of the NEP inhibitor thiorphan has been reported (Marçais-Collado et al., 1987). It is due to the antisecretory action resulting from the stimulation of peripheral delta opioid receptors by the endogenous enkephalins tonically released from the submucosal plexus neurons (Kachur et al., 1980). Given these results and because both NEP and APN are present in the gastrointestinal tract, it would be interesting to test the effects of orally administered mixed inhibitor (Roques et al., 1993).
In conclusion, using the highly potent and selective inhibitor [125I]RB 129, the presence of APN has been visualized for the first time in brain areas where it was suspected to be physiologically active. For instance, the present study demonstrates the co-localization of APN, NEP, enkephalins and opioid receptors in several regions, a result which was expected from numerous studies carried out with dual inhibitors, but not demonstrated until now. Nevertheless, APN, which preferably removes NH2-terminal neutral amino acids from peptides, has certainly other biologically active peptides as substrates. However, the possible physiological relevance of the degradation of these peptides, which remain to be characterized in vivo, is critically dependent on the extent of their tonic release. This might be investigated through appropriate behavioral experiments, provided that the receptor(s) of the peptide substrate should be characterized and the responses blocked by selective antagonists, as shown in the case of opioid system. Moreover, despite the relative broad specificities of APN, an in vivo selectivity is certainly achieved by the presence of the enzyme in brain areas where both the peptide and its receptors are co-localized.
On the other hand, the iodinated inhibitor could afford to study the localization of APN in various peripheral tissues, and to evaluate quantitatively its regulation in pathological situations (Hwang et al., 1993, Pasqualini et al., 2000). Moreover, as previously demonstrated in the case of NEP (Dutriez et al., 1992) the distribution of APN could be monitored during pre- and post-natal development, to evaluate whether APN may have a modulatory role in the ontogeny of several organs. This radioiodinated compound could also be used to study the in vivo distribution of APN in various parts of the body after systemic administration, as already shown for NEP (Sales et al., 1991). Finally, the possibility to follow directly by competition the bioavailability of selective or dual NEP/APN inhibitors administered by different routes as already shown in the case of NEP and angiotensin converting enzyme (Fournié-Zaluski et al., 1994, Jackson et al., 1986) is important considering the potential clinical application of these molecules (review in Roques et al., 1993).
Abbreviations used in the figures
- Ac
accumbens
- AI
agranular insular cortex
- AO
anterior olfactory nucleus
- BST
bed nucleus stria terminal
- Cg
cingulate cortex
- ChP
choroid plexus
- Co
cochlear nuclei
- CP
caudate–putamen
- Fa
facial nucleus
- Fr
frontal cortex
- Gl
glomerular layer
- GP
globus pallidus
- Hi
hippocampus
- IC
inferior colliculus
- Int
interposed cerebellar nuclei
- IP
interpeduncular nucleus
- LS
lateral septal nucleus
- Mn
mamillary nucleus
- MPO
medial preoptic nucleus
- Ol
olivary region
- PAG
periaqueductal gray matter
- Pa
parietal cortex
- Pit
pituitary gland
- Pn
pontine nucleus
- PVA
paraventricular thalamic nucleus
- R
red nucleus
- Ra
raphe nucleus
- SC
superior colliculus
- SN
substantia nigra
- STh
subthalamic nucleus
- Tg
tegmental nucleus
- Tri
trigeminal nuclei
- Tu
olfactory tubercle
- ZI
zona incerta
Acknowledgements
This work was supported by institutional grants from CNRS and INSERM, grants from the European Community (BMH4 CT98 2267), and grants from Synthelabo Recherche. The authors would like to thank Dr. W. Rostène for his helpful comments on the manuscript.
References
- Alba F, Arenas J.C, Iribar C, Ramirez M. Regional distribution of soluble and membrane-bound aminopeptidase activities in rat brain. Brain Res. Bull. 1993;31:393–396. doi: 10.1016/0361-9230(93)90232-z. [DOI] [PubMed] [Google Scholar]
- Aloyo V.J. Preproenkephalin A gene expression in rat pineal. Neuroendocrinology. 1991;54:594–598. doi: 10.1159/000125965. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Owens M.J, Plotsky P.M, Nemeroff C.B. The role of corticotropin-releasing factor in depression and anxiety disorders. J. Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Barnes K, Kenny A.J, Turner A.J. Localization of aminopeptidase N and dipeptidyl peptidase IV in pig striatum and in neuronal and glial cell cultures. Eur. J. Neurosci. 1994;6:531–537. doi: 10.1111/j.1460-9568.1994.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Basbaum A.I, Fields H.L. Endogenous opioid pain control systems: brainstem spinal pathways and endorphin circuitry. Annu. Rev. Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Besson J.M, Chaouch A. Peripheral and spinal mechanisms of nociception. Physiol. Res. 1987;64:67–186. doi: 10.1152/physrev.1987.67.1.67. [DOI] [PubMed] [Google Scholar]
- Bhargava H.N. Opioid peptides, receptors, and immune function. NIDA Res. Monogr. 1990;96:220–233. [PubMed] [Google Scholar]
- Bourgoin S, Le Bars D, Artaud F, Clot A.M, Bouboutou R, Fournié-Zaluski M.C, Roques B.P, Hamon M, Cesselin F. Effects of kelatorphan and other peptidase inhibitors on the in vitro and in vivo release of Met-enkephalin-like material from the rat spinal cord. J. Pharmacol. Exp. Ther. 1986;238:360–366. [PubMed] [Google Scholar]
- Checler, F., 1991. Peptidases and neuropeptide-inactivating mechanisms in the circulation and in the gastrointestinal tract. In: Daniel, E.E. (Ed.), Neuropeptide Function in the Gastrointestinal Tract. CRC Press, Boston, MA, pp. 274–307.
- Chen H, Noble F, Coric P, Fournié-Zaluski M.C, Roques B.P. Aminophosphinic inhibitors as transition state analogues of enkephalin-degrading enzymes: A new class of central analgesics. Proc. Natl. Acad. Sci. USA. 1998;95:12028–12033. doi: 10.1073/pnas.95.20.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Roques B.P, Fournié-Zaluski M.C. Design of the first highly potent and selective aminopeptidase N (EC 3.4.11.2) inhibitor. Bioorg. Med. Chem. Lett. 1999;9:1511–1516. doi: 10.1016/S0960-894X(99)00219-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Bischoff L, Fournié-Zaluski M.C, Roques B.P. Synthesis of 2(S)-benzyl-3-[hydroxy(1′(R)-amino ethyl)phosphinyl]propanoyl-L-3-[125I]-iodotyrosine: a radiolabelled inhibitor of aminopeptidase N. J. Lab. Comp. Radiopharm. 2000;43:103–111. [Google Scholar]
- Culpepper-Morgan J.A, Kreek M.J. Hypothalamic–pituitary–adrenal axis hypersensitivity to naloxone in opioid dependence: a case of naloxone-induced withdrawal. Metabolism. 1997;46:130–134. doi: 10.1016/s0026-0495(97)90289-4. [DOI] [PubMed] [Google Scholar]
- Daugé V, Mauborgne A, Cesselin F, Fournié-Zaluski M.C, Roques B.P. The dual peptidase inhibitor RB 101 induces a long lasting increase in the extracellular level of Met-enkephalin in the nucleus accumbens of freely moving rats. J. Neurochem. 1996;67:1301–1308. doi: 10.1046/j.1471-4159.1996.67031301.x. [DOI] [PubMed] [Google Scholar]
- Delmas B, Gelfi J, L’Haridon R, Vogel L.K, Sjöström H, Norén O, Laude H. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature. 1992;357:417–419. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutriez I, Sales N, Fournié-Zaluski M.C, Roques B.P. Pre- and post-natal ontogeny of neutral endopeptidase 24.11 (‘enkephalinase’) studied by in vitro autoradiography in the rat. Experientia. 1992;48:290–300. doi: 10.1007/BF01930479. [DOI] [PubMed] [Google Scholar]
- Fournié-Zaluski M.C, Chaillet P, Bouboutou R, Coulaud A, Chérot P, Waksman G, Costentin J, Roques B.P. Analgesic effects of kelatorphan, a new highly potent inhibitor of multiple enkephalin degrading enzymes. Eur. J. Pharmacol. 1984;102:525–528. doi: 10.1016/0014-2999(84)90575-2. [DOI] [PubMed] [Google Scholar]
- Fournié-Zaluski M.C, Coric P, Turcaud S, Rousselet N, Gonzalez W, Barbe B, Pham I, Jullian N, Michel J.B, Roques B.P. New dual inhibitors of neutral endopeptidase and angiotensin converting enzyme: rational design, bioavailability and pharmacological responses on experimental hypertension. J. Med. Chem. 1994;37:1070–1083. doi: 10.1021/jm00034a005. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Imai K, Inoue T, Maeda M, Fujii S. Membrane-bound cell surface peptidases in reproductive organs. Endocr. J. 1999;46:11–25. doi: 10.1507/endocrj.46.11. [DOI] [PubMed] [Google Scholar]
- Funkhouser J.D, Tangada S.D, Peterson R.D. Ectopeptidases of alveolar epithelium: candidates for roles in alveolar regulatory mechanisms. Am. J. Physiol. 1991;260:L381–L385. doi: 10.1152/ajplung.1991.260.6.L381. [DOI] [PubMed] [Google Scholar]
- Gonzalvez M.L, Milanes M.V, Vargas M.L. Effects of acute and chronic administration of mu- and delta-opioid agonists on the hypothalamic–pituitary–adrenocortical (HPA) axis in the rat. Eur. J. Pharmacol. 1991;200:155–158. doi: 10.1016/0014-2999(91)90678-j. [DOI] [PubMed] [Google Scholar]
- Guyon A, Barbet J, Roques B.P, Swerts J.P, Malfroy B, Schwartz J.C. Disappearance of energy-transfer as a tool for fluorimetric study of the degradation of enkephalins by aminopeptidase activity from mouse brain. Biochem. Biophys. Res. Commun. 1979;88:919–926. doi: 10.1016/0006-291x(79)91496-7. [DOI] [PubMed] [Google Scholar]
- Herman J.P, Cullinan W.E. Neurocircuitry of stress: central control of the hypothalamo–pituitary–adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hersh L.B, Aboukhair N, Watson S. Immunohistochemical localization of aminopeptidase M in rat brain and periphery: relationship of enzyme localization and enkephalin metabolism. Peptides. 1987;8:523–532. doi: 10.1016/0196-9781(87)90019-2. [DOI] [PubMed] [Google Scholar]
- Houtani T, Nishi M, Takeshima H, Nakada T, Sugimoto T. Structure and regional distribution of nociceptin/orphanin FQ precursor. Biochem. Biophys. Res. Commun. 1996;219:714–719. doi: 10.1006/bbrc.1996.0300. [DOI] [PubMed] [Google Scholar]
- Hwang L, Leichter R, Okamoto A, Payan D, Collins S.M, Bunnett N.W. Downregulation of neutral endopeptidase (EC 3.4.24.11) in the inflamed rat intestine. Am. J. Physiol. 1993;264:G735–G743. doi: 10.1152/ajpgi.1993.264.4.G735. [DOI] [PubMed] [Google Scholar]
- Jackson B, Cubela R, Johnston C.I. Angiotensin-converting enzyme (ACE) measurement in human serum using radioinhibitor ligand binding. Aust. J. Exp. Biol. Med. Sci. 1986;64:149–155. doi: 10.1038/icb.1986.16. [DOI] [PubMed] [Google Scholar]
- Jenkins W.M, Masterton R.B. Sound localization: effects of unilateral lesions in central auditory system. J. Neurophysiol. 1982;47:987–1016. doi: 10.1152/jn.1982.47.6.987. [DOI] [PubMed] [Google Scholar]
- Kachur J.F, Miller R.J, Field M. Control of guinea-pig intestinal electrolyte secretion by a delta opioid receptor. Proc. Natl. Acad. Sci. USA. 1980;77:2753–2756. doi: 10.1073/pnas.77.5.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa I, Jessell T. Post mortem changes and regional distribution of substance P in the rat and mouse nervous system. Brain Res. 1976;117:362–367. doi: 10.1016/0006-8993(76)90748-4. [DOI] [PubMed] [Google Scholar]
- Kathol R.G, Jaeckle R.S, Lopez J.F, Meller W.H. Pathophysiology of HPA axis abnormalities in patients with major depression: an update. Am. J. Psychiatry. 1989;146:311–317. doi: 10.1176/ajp.146.3.311. [DOI] [PubMed] [Google Scholar]
- Kunz J, Krause D, Kremer M, Dermietzel R. The 140-kDa protein of blood–brain barrier-associated pericytes is identical to aminopeptidase N. J. Neurochem. 1994;62:2375–2386. doi: 10.1046/j.1471-4159.1994.62062375.x. [DOI] [PubMed] [Google Scholar]
- Look A.T, Ashmun R.A, Shapiro L.H, Peiper S.C. Human myeloid plasma membrane glycoprotein CD13 (gp150) is identical to aminopeptidase N. J. Clin. Invest. 1989;83:1299–1307. doi: 10.1172/JCI114015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciani N, Marie-Claire C, Ruffet E, Beaumont A, Roques B.P, Fournié-Zaluski M.C. Characterization of Glu350 as a critical residue involved in the N-terminal amine binding site of aminopeptidase N (EC 3.4.11.2): Insights into its mechanism of action. Biochemistry. 1998;37:686–692. doi: 10.1021/bi971705p. [DOI] [PubMed] [Google Scholar]
- Mansour, A., Watson, S.J., 1993. Anatomical distribution of opioid receptors in mammalians: an overview. In: Herz, A., Akil, H., Simon, A.J. (Eds.), Handbook of Experimental Pharmacology. Opioids I. Springer-Verlag, Berlin, pp. 79–105.
- Marçais-Collado H, Uchida G, Costentin J, Schwartz J.C, Lecomte J.M. Naloxone reversible antidiarrheal effects of enkephalinase inhibitors. Eur. J. Pharmacol. 1987;144:125–132. doi: 10.1016/0014-2999(87)90510-3. [DOI] [PubMed] [Google Scholar]
- Moller M, Ravault J.P, Cozzi B. The chemical neuroanatomy of the mammalian pineal gland: neuropeptides. Neurochem. Int. 1996;28:23–33. doi: 10.1016/0197-0186(95)00046-b. [DOI] [PubMed] [Google Scholar]
- Montiel J.L, Cornille F, Roques B.P, Noble F. Nociceptin/orphanin FQ metabolism. Role of aminopeptidase and endopeptidase 24.15. J. Neurochem. 1997;68:354–361. doi: 10.1046/j.1471-4159.1997.68010354.x. [DOI] [PubMed] [Google Scholar]
- Naquet P, Vivier I, Gorvel J.P, Brekelmans P, Barad M, Bernard A.M, Pierres M. Activation of mouse T lymphocytes by a monoclonal antibody to a developmentally regulated surface aminopeptidase (THAM) Immunol. Rev. 1989;1989:177–193. doi: 10.1111/j.1600-065x.1989.tb00546.x. [DOI] [PubMed] [Google Scholar]
- Nishi M, Houtani T, Noda Y, Mamiya T, Sato K, Doi T, Kuno J, Takeshima H, Nukada T, Nabeshima T, Yamashita T, Noda T, Sugimoto T. Unrestrained nociceptive response and disregulation of hearing ability in mice lacking the nociceptin/orphanin FQ receptor. EMBO J. 1997;16:1858–1864. doi: 10.1093/emboj/16.8.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble F, Soleilhac J.M, Soroca-Lucas E, Turcaud S, Fournié-Zaluski M.C, Roques B.P. Inhibition of the enkephalin-metabolizing enzymes by the first systemically active mixed inhibitor prodrug RB 101 induces potent analgesic responses in mice and rats. J. Pharmacol. Exp. Ther. 1992;261:181–190. [PubMed] [Google Scholar]
- Noble F, Roques B.P. Association of aminopeptidase N and endopeptidase 24.15 inhibitors potentiate behavioral effects mediated by nociceptin/orphanin FQ in mice. FEBS Lett. 1997;401:227–229. doi: 10.1016/s0014-5793(96)01476-7. [DOI] [PubMed] [Google Scholar]
- Noble F, Luciani N, Da Nascimento S, Laï-Kuen R, Bischoff L, Chen H, Fournié-Zaluski M.C, Roques B.P. Binding properties of a highly potent and selective iodinated aminopeptidase N inhibitor appropriate for radioautography. FEBS Lett. 2000;467:81–86. doi: 10.1016/S0014-5793(99)01645-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri F.E, Petrelli J.J, Ward P.E. Vascular, plasma membrane aminopeptidase M. Metabolism of vasoactive peptides. Biochem. Pharmacol. 1985;34:2309–2317. doi: 10.1016/0006-2952(85)90787-7. [DOI] [PubMed] [Google Scholar]
- Pasqualini R, Koivunen E, Kain R, Lahdenranta J, Sakamoto M, Stryhn A, Ashmun R.A, Shapiro L.H, Arap W, Ruoslahti E. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000;60:722–727. [PMC free article] [PubMed] [Google Scholar]
- Pert C.B, Pert A, Tallman J.F. Isolation of a novel endogenous opiate analgesic from human blood. Proc. Natl. Acad. Sci. USA. 1976;73:2226–2230. doi: 10.1073/pnas.73.7.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roques B.P, Noble F, Daugé V, Fournié-Zaluski M.C, Beaumont A. Neutral endopeptidase 24.11. Structure, inhibition, and experimental and clinical pharmacology. Pharmacol. Rev. 1993;45:87–146. [PubMed] [Google Scholar]
- Roques B.P. Novel approaches to targeting neuropeptide systems. Trends Pharmacol. Sci. 2000;21:475–483. doi: 10.1016/s0165-6147(00)01571-6. [DOI] [PubMed] [Google Scholar]
- Safavi A, Hersh L.B. Degradation of dynorphin-related peptides by the puromycin-sensitive aminopeptidase and aminopeptidase M. J. Neurochem. 1995;65:389–395. doi: 10.1046/j.1471-4159.1995.65010389.x. [DOI] [PubMed] [Google Scholar]
- Sales N, Dutriez I, Mazière B, Ottaviani M, Roques B.P. Neutral endopeptidase 24.11 in rat peripheral tissues: Comparative localization by ex vivo and in vitro autoradiography. Regul. Pept. 1991;33:209–222. doi: 10.1016/0167-0115(91)90215-3. [DOI] [PubMed] [Google Scholar]
- Schluger J.H, Ho A, Borg L, Porter M, Maniar S, Gunduz M, Perret G, King A, Kreek M.J. Nalmefene causes greater hypothalamic–pituitary adrenal axis activation than naloxone volunteers: implication for the treatment of alcoholism. Alcohol Clin. Exp. Res. 1998;22:1430–1436. doi: 10.1111/j.1530-0277.1998.tb03931.x. [DOI] [PubMed] [Google Scholar]
- Sharp B.M, Roy S, Bidlack J.M. Evidence for opioid receptors on cells involved in host defense and the immune system. J. Neuroimmunol. 1998;83:45–56. [PubMed] [Google Scholar]
- Sjöström N, Noren O, Jeppesen L, Staun M, Svensson B, Christiansen L. Purification of different amphiphilic forms of a microvillus aminopeptidase from pig small intestine using immunoadsorbent chromatography. J. Biochem. 1978;88:503–511. doi: 10.1111/j.1432-1033.1978.tb12476.x. [DOI] [PubMed] [Google Scholar]
- Soleilhac J.M, Lafuma C, Porcher J.M, Aubertin G, Roques B.P. Characterization of a soluble form of neutral endopeptidase-24.11 (EC 3.4.24.11) in human serum: enhancement of its activity in serum of underground miners exposed to coal dust particles. Eur. J. Clin. Invest. 1996;26:1011–1017. doi: 10.1046/j.1365-2362.1996.2420580.x. [DOI] [PubMed] [Google Scholar]
- Solhonne B, Gros C, Pollard H, Schwartz J.C. Major localization of aminopeptidase M in rat brain microvessels. Neuroscience. 1987;22:225–232. doi: 10.1016/0306-4522(87)90212-0. [DOI] [PubMed] [Google Scholar]
- Strittmatter S.M, Snyder S.H. Angiotensin converting enzyme immunohistochemistry in rat brain and pituitary gland: correlation of isoenzyme type with cellular localization. Neuroscience. 1987;21:407–420. doi: 10.1016/0306-4522(87)90131-x. [DOI] [PubMed] [Google Scholar]
- Terashima H, Wong H, Kobayashi R, Bunnett N.W. Immunochemical localization of aminopeptidase M in the alimentary tract of the guinea pig and rat. Gastroenterology. 1992;102:1867–1876. doi: 10.1016/0016-5085(92)90307-k. [DOI] [PubMed] [Google Scholar]
- Valverde O, Fournié-Zaluski M.C, Roques B.P, Maldonado R. Similar involvement of several brain areas in the antinociception of endogenous and exogenous opioids. Eur. J. Pharmacol. 1996;312:15–25. doi: 10.1016/0014-2999(96)00437-2. [DOI] [PubMed] [Google Scholar]
- Waksman G, Bouboutou R, Devin J, Bourgoin S, Cesselin F, Hamon M, Fournié-Zaluski M.C, Roques B.P. In vitro and in vivo effects of kelatorphan on enkephalin metabolism in rodent brain. Eur. J. Pharmacol. 1985;117:233–243. doi: 10.1016/0014-2999(85)90608-9. [DOI] [PubMed] [Google Scholar]
- Waksman G, Hamel E, Fournié-Zaluski M.C, Roques B.P. Autoradiographic comparison of the distribution of the neutral endopeptidase ‘enkephalinase’ and of mu and delta opioid receptors in rat brain. Proc. Natl. Acad. Sci. USA. 1986;83:1523–1527. doi: 10.1073/pnas.83.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand G.S, Schumann H. Relationship between adrenocorticotropin, hypothalamic opioid tone, and plasma leptin. J. Clin. Endocrinol. Metab. 1998;83:2138–2142. doi: 10.1210/jcem.83.6.4900. [DOI] [PubMed] [Google Scholar]
- Yeager C.L, Ashmun R.A, Williams R.K, Cardellichio C.B, Shapiro L.H, Look A.T, Holmes K.V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357:420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac, J.M., Roques, B.P., 1989. Properties required for reversible and irreversible radiolabelled probes for selective characterization of brain receptors and peptidases by autoradiography. In: Sharif, N.A., Lewis, M.E. (Eds.), Brain Imaging, Techniques and Applications. Ellis Horwood, John Wiley and Sons, pp. 18–35.
- Zajac J.M, Charnay Y, Soleilhac J.M, Sales N, Roques B.P. Enkephalin-degrading enzymes and angiotensin-converting enzyme in human and rat meninges. FEBS Lett. 1987;216:118–122. doi: 10.1016/0014-5793(87)80768-8. [DOI] [PubMed] [Google Scholar]
- Zamir N, Palkovits M, Weber E, Mezey E, Brownstein M.J. A dynorphinergic pathway of Leu-enkephalin production in rat substantia nigra. Nature. 1984;307:643–645. doi: 10.1038/307643a0. [DOI] [PubMed] [Google Scholar]


