Abstract
The observation that HIV in vitro can infect CD4-and Gal-C-negative brain cell lines has stimulated this study to identify alternative gp120-binding proteins on brain cells. HIV-1 gp120 binding proteins of the CD4-negative and Gal-C-negative, non-productively infectable human glioblastoma cell line D54 were purified by affinity chromatography over a gp120-conjugated sepharose column and identified by peptide microsequencing. The binding capacity and specificity of this column was controlled using extracts of CD4-positive cells. Two of seven prominent proteins eluted from the gp120 affinity column specifically bound gp120 in Western blot overlay experiments and were identified by subsequent immunoblotting and microsequencing as ezrin and moesin, members of the ERM (ezrin, radixin, moesin) family of cellular structural membrane proteins. Antibodies to ezrin and moesin specifically recognized the eluted gp120 binding proteins confirming their identification. Ezrin and moesin are structural proteins binding to the cellular membrane and to several cytoskeletal and transmembrane proteins. Our results suggest that ezrin and moesin might play a role as gp160/gp120 binding proteins during the uptake, the assembly or the budding of HIV.
Keywords: HIV-1, HIV-1-gp120 binding proteins, Ezrin, Moesin, ERM-family
Infection with human immunodeficiency virus (HIV) results in immunological dysfunction and frequently leads to the development of a severe and progressive dementing neurologic disorder, commonly referred to as the AIDS dementia complex (ADC) (Epstein and Gendelman, 1993). Major pathological findings in the CNS are gliosis and white-matter pallor, multinucleated cell-encephalitis, vacuolar myelopathy and neuronal loss. Primary targets of HIV-1 infection in the adult human CNS are cells of the monocyte/macrophage lineage (including brain microglial cells), brain capillary endothelial cells and multinucleated giant cells, with very little evidence of detectable antigenic expression in neural cells of glial or neuronal origin (Wiley et al., 1986). The CD4 differentiation antigen, the major HIV receptor on the surface of T-lymphocytes and monocytes as well as on brain microglia is usually not detected in HIV-susceptible neural cell lines (Cheng-Mayer et al., 1987; Harouse et al., 1991; Jordan et al., 1991).
The envelope glycoprotein of HIV-1, gp120, mediates the attachment of the virion to the host cell. Its further role in fusion and uptake of the viral genome into the cytoplasm and its possible interaction with coreceptors is unclear. Several coreceptors acting in a specific manner for various strains of HIV have been identified recently as members of the TM7 family of transmembrane proteins (D'Souza and Harden, 1996). As a soluble protein shed from the surface of HIV-infected cells or from viral particles, gp120 might play a role in the induction of cytokines and exert neurotoxicity (Benos et al., 1994; Brenneman et al., 1988; Dreyer et al., 1990; Giulian et al., 1993; Levi et al., 1993; Lipton et al., 1991; Pulliam et al., 1990). Alternative receptors for gp120, such as galactosyl ceramide (GalC) (Harouse et al., 1991; Bhat et al., 1991; Fantini et al., 1993; Li et al., 1990; Moses et al., 1993; van der Berg et al., 1992), the recently identified coreceptors (D'Souza and Harden, 1996), and further unknown proteins binding gp160/gp120 (Ma et al., 1994; Schneider-Schaulies et al., 1992) may play an important role for the pathogenesis of the HIV infection.
The discrepancy between the severity of impairment and the low levels of detectable HIV-1 within the brain has led to an intense search for virus-and host-derived factors that might exert neurotoxicity. Soluble gp120 has the potential to be distributed throughout the brain and might interact with uninfected brain cells. There is evidence in vivo and in vitro that soluble gp120 plays a key role in HIV-1 associated nervous system impairment. (Toggas et al., 1994) demonstrated that expression of gp120 in the brains of transgenic mice is sufficient to induce pathological effects in neurons, astrocytes and microglia. In vitro it was found that picomolar concentrations of gp120 were toxic to rodent hippocampal neurons (Brenneman et al., 1988). Furthermore, there is evidence that gp120 could indirectly trigger a dramatic and potentially lethal rise in neuronal [Ca2+] by releasing toxic factors from activated macrophages/microglia and possibly astrocytes (Benos et al., 1994; Dreyer et al., 1990; Giulian et al., 1993; Lipton et al., 1991). These effects shown with recombinant gp120 (rgp120) require the specific binding of gp120 to either the receptors in the membrane or protein(s) closely associated with them.
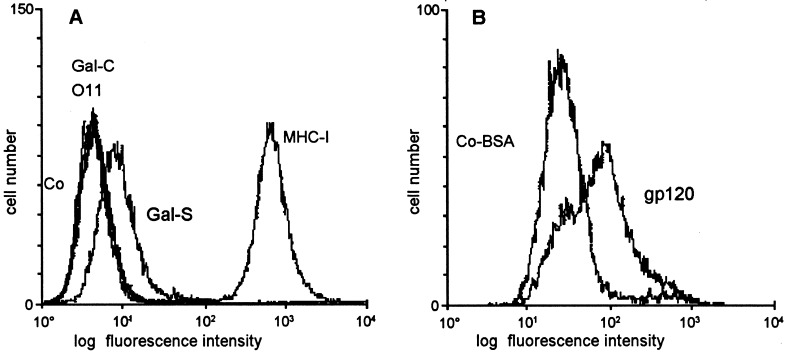
Our previous results showed that the CD4-negative and Gal-C-negative human glioblastoma cell line D54 (Bigner et al., 1981) could be non-productively infected with HIV-1 and that crosslinking experiments indicated the presence of a proteinaceous receptor for HIV-1 on the surface of these cells (Schneider-Schaulies et al., 1992). Gal-S, which was described as an alternative receptor for HIV (Bhat et al., 1991; Harouse et al., 1991; van der Berg et al., 1992) was expressed to a low degree by D54 cells (Fig. 1 ), whilst the oligodendrocyte cell surface antigen O11 (Ranscht et al., 1982) was not expressed. Interestingly, we were able to demonstrate specific binding of rgp120 to D54 cells (Fig. 1) indicating that this cell line expresses a binding partner for gp120 on its surface.
Fig. 1.
The expression of surface molecules and binding of rgp120 to the human glioblastoma cell line D54 were determined by flow. (a) D54 cells did not express galactosyl ceramide (GalC) or the lipid-specific O11 antigen, but are positive for galactosyl sulfatide (Gal-S). As negative control an anti-coronavirus S mAb (Co) and as positive control the anti-MHC class I mAb W6/32 was used (MHC-I). (b) Detection of rgp120 bound to D54 cells using a polyclonal anti-gp120 antiserum (gp120). As control BSA instead of rgp120 was incubated with the cells under the same conditions (Co-BSA).
In order to purify putative gp120-binding proteins, membrane vesicles were prepared by specific differential centrifugation and the proteins gently solubilized with the non-ionic detergent d-octylglucoside. To identify putative HIV-1 gp120 binding molecules the dialyzed protein fraction was circulated over an affinity column to which recombinant gp120 was covalently bound. After washing the column free of unbound proteins, which represented more than 99% of the total protein, bound proteins were eluted using a NaCl gradient. The bound proteins always showed a sharp elution peak between 300 and 500 mM NaCl and were dialyzed against distilled water prior to characterization. After preparation of larger amounts of cell membranes and subsequent chromatography the purified proteins were pooled and lyophilized. Eluted proteins were then analyzed by SDS-PAGE and Coomassie-staining (Fig. 2 ). For the exact determination of the MWs of the bound proteins, the stained gels were scanned and the relative mobility of proteins compared with marker proteins. The dominant proteins binding to the gp120 affinity matrix showed approximate MWs of 81, 76, 42 and 38 kDa. A Western blot analysis with an anti-actin mAb showed that the 42 kDa protein was not actin (not shown). Additional minor protein bands were detected with app. MWs of 220 (in some preparations), 110 and 57 kDa. To control the chromatography procedure, solubilized membrane proteins of the CD4-positive Molt4 cells were passed over the rgp120 column in a similar manner as described above. CD4 was shown by immunoblotting to be purified by the rgp120 affinity matrix and migrated as a dominant band of an app. MW of 58 kDa, eluting at 500 mM NaCl in TEO buffer (not shown).
Fig. 2.
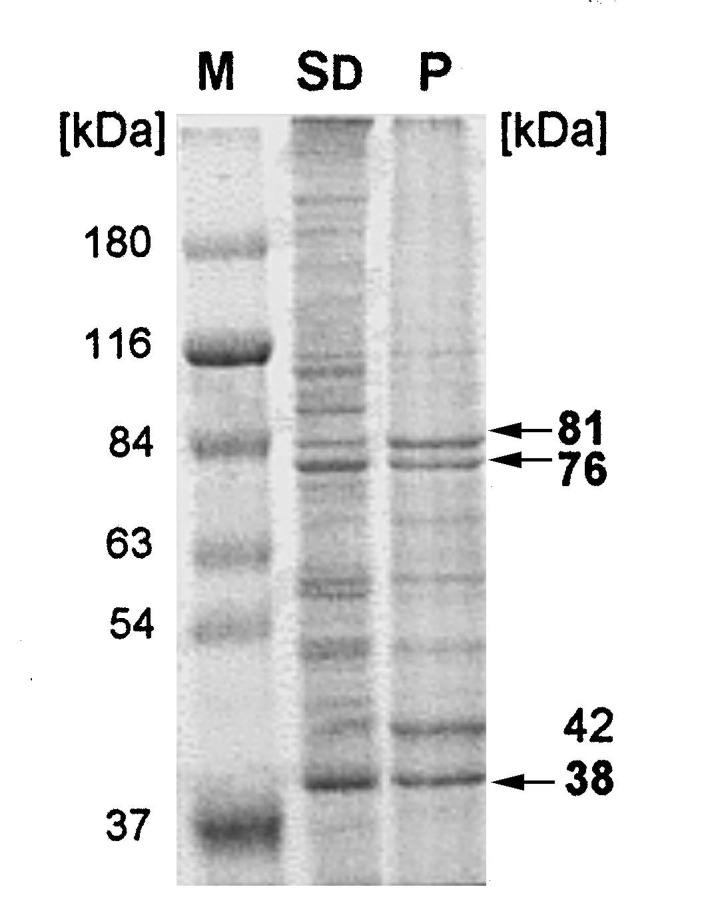
Purification of membrane proteins of D54 cells binding to rgp120 by affinity chromatography. A Coomassie stained SDS-PAGE of pooled protein fractions of three column runs which had bound to the rgp120 matrix is shown in lane P. In comparison, the fraction containing solubilised membrane proteins is shown in lane SD. D54 cells were resuspended in a small volume of BB (25 mM HEPES, pH 7.4; 150 mM NaCl) plus 10% sucrose containing protease inhibitors, DTT, EGTA and then hypotonically shocked by diluting in 10× the volume of ice cold HEPES, pH 7.4. The nuclei were removed by centrifuging for 1 min at 500×g after which the supernatant cytosolic and membrane proteins were separated by a high speed centrifugation step (2.5 h, 100 000×g). The resulting membrane pellet was resuspended in sucrose (10%)-containing BB and the proteins solubilized with 3% w/v octylglucoside. Membrane proteins were passed over an affinity matrix which was prepared by coupling recombinant HIV-1(IIIB)-gp120 (rgp120, AGMED) to CNBr-activated Sepharose 4B beads (Pharmacia). The proteins were passed over the affinity column in TEO buffer (10 mM Tris, pH 8.3, 1 mM EDTA, 0.1% OG) at 4°C and allowed to bind for at least 16 h. After washing the column in TEO to remove non-specifically attached proteins, bound proteins were eluted with a sodium chloride gradient.
The specificity of the gp120-binding activity of the proteins eluted from the affinity column was confirmed by an immunoblot overlay assay. The affinity purified proteins were separated on SDS-PAGE, electroblotted onto nitrocellulose (NC) and each lane was incubated with 250 ng rgp120 for 4 h at RT. The immobilized protein-gp120 complexes were detected by a polyclonal anti-gp120 serum and HRP-coupled secondary antibodies. The gp120-overlay was performed in TEO-buffer, which was also used for binding the proteins to the affinity matrix. Recombinant gp120 bound to proteins of apparent MW of 81 and 76 kDa, but not to the 42 and 38 kDa proteins (Fig. 3 ). In addition we tested whether gp120 might bind to a p81 and/or p76 associated lipid or glycolipid as for example Gal-S. We performed an immunoblot assay after having subjected these proteins to lipid extraction. No difference between the blots was observed (not shown). Therefore, a lipid-based interaction between gp120 and the proteins p81 and p76 can be excluded. These results suggest that gp120 interacts directly with the 81 and 76 kDa proteins.
Fig. 3.
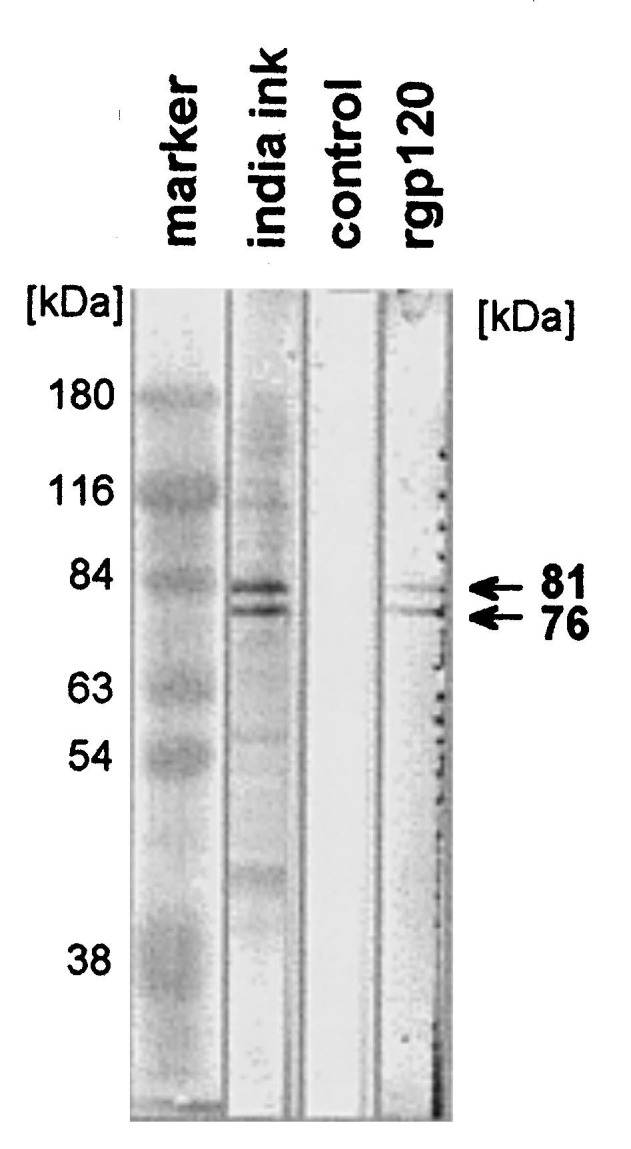
Immunoblot-overlay assay of the affinity purified protein fraction with rgp120. The affinity purified proteins were separated by SDS-PAGE and transferred to nitrocellulose. Staining of total proteins (lane 2: india ink). Strips of nitrocellulose were incubated with gp120 binding (250 μg/lane) in TEO (10 mM Tris, pH 8.3, 1 mM EDTA, 0.1% OG) at 4°C overnight. After incubation with an anti-gp120 serum (1:250 in TBST/BSA) at RT for 4 h, and HRP-conjugated anti-rabbit serum, bands were visualized with chloronaphthol (lane 4: rgp120). As control the rgp120 was omitted (lane 3: control).
The two 81 kDa and 76 kDa proteins binding gp120 and the protein with the app. MW of 38 kDa were identified by amino acid sequence analysis. For this purpose the proteins were digested with trypsin in the gel matrix, the resulting fragments extracted and separated by RP-HPLC prior to Edman degradation. The sequence data were confirmed by mass spectrometry (Table 1 ). The 81 kDa protein was unequivocally identified as ezrin following analysis of two peptide sequences. For the 76 kDa protein, a sequence was determined which identified it as a member of the ERM (ezrin-radixin-moesin; Sato et al., 1992) family of proteins although conclusive identification was made using methods described below. In the case of the 38 kDa protein, four peptide sequences were identified which were derived from the glyceraldehyde-3-phosphate-dehydrogenase (GAPDH, EC 1.2.1.12).
Table 1.
Analysis of HPLC-purified tryptic peptides derived from p81, p76 and p38
| Protein sequence | Identified as | Peptide position | Calc. mass | Exp. mass | |
|---|---|---|---|---|---|
| p81 | SGYLSSER | Ezrin, human | 142–150 | 897.94 | 897.7 |
| LIPQR | Ezrin, human | 151–155 | 625.77 | 625.6 | |
| p76 | LFFLQVK | Ezrin, human | 100–106 | 894.12 | n.d. |
| Moesin, human | 100–106 | ||||
| Radixin, human | 101–107 | ||||
| Merlin, human | 117–123 | ||||
| p38 | FHGTVK | GAPDH, human | 055–060 | 687.80 | 687.4 |
| LTGMAFR | GAPDH, human | 227–233 | 810.98a | 810.5 | |
| LEKPAKYDDIKK | GAPDH, human | 248–259 | 1447.69 | 1447.7 | |
| VVDLMAHMASKE | GAPDH, human | 323–334 | 1362.59a | 1362.3 | |
n.d., not determined.
Using the SWISS-PROT protein sequence database p38 was identified as glyceraldehyde-3-phosphate-dehydrogenase (G3P1-HUMAN or G3P2_HUMAN), p76 as a member of the ERM-family (see text), and p81 as human ezrin (EZRI_HUMAN). For N-terminal amino acid sequencing up to ten Coomassie-blue stained protein bands were excised from Lämmli slab gels and the protein was digested in the gel matrix with trypsin (1 μg for the 81 and 76 kDa proteins and 2 μg for the 38 kDa protein) as described by Eckerskorn and Lottspeich (Eckerskorn and Lottspeich, 1989). The resulting peptides were eluted from the gel and seperated by reverse-phase HPLC using a C18 column (Vydac, 2.1×250 mm) with a Waters 600 HPLC (Millipore). As solvent system was used 0.1% trifluoroacetic acid in H2O (aqueous phase A) and 0.085% trifluoroacetic acid in acetonitrile (organic phase B). Peptides were identified by Edman sequencing and mass spectrometry. Automated sequence analysis of the purified peptides (2/3) was performed using a type 473A protein sequencer (Applied Biosystems). Electrospray ionisation mass spectrometry of the peptides (1/3) was performed using a Finnigan TSQ MAT 700 mass spectrometer.
a Met oxidized.
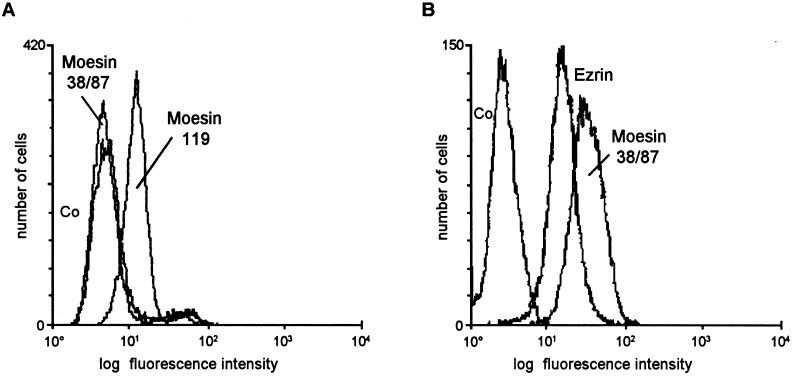
Nitrocellulose membranes with the immobilized affinity purified gp120-binding proteins were probed with antibodies for ezrin and moesin (Fig. 4 ). The anti-ezrin antibodies specifically identified a protein of 81 kDa. Higher concentrations of the anti-ezrin mAb also crossreacted with a 76 kDa protein. The anti-moesin mAb 38/87 strongly bound to the 76 kDa band. In addition, this antibody recognized a band which migrated slightly faster than the 81kDa ezrin protein band. According to published data concerning the mobility of ERM proteins in SDS-PAGE, the 76 kDa protein is moesin, the 81 kDa protein is ezrin, and the slightly faster migrating protein is likely to be radixin (Sato et al., 1992; Tsukita et al., 1989). Since the ERM family proteins are highly homologous, anti-moesin antibodies usually crossreact with radixin. On the surface of D54 cells, the mAb 38/87 against moesin did not detect its epitope, whereas mAb 119 against moesin showed a clear signal (Fig. 5 (a)). The anti-ezrin mAb did not recognize its epitope on the surface of D54 cells (not shown). Using permeabilized cells, anti-moesin and anti-ezrin antibodies recognized high levels of these molecules (Fig. 5(b)).
Fig. 4.
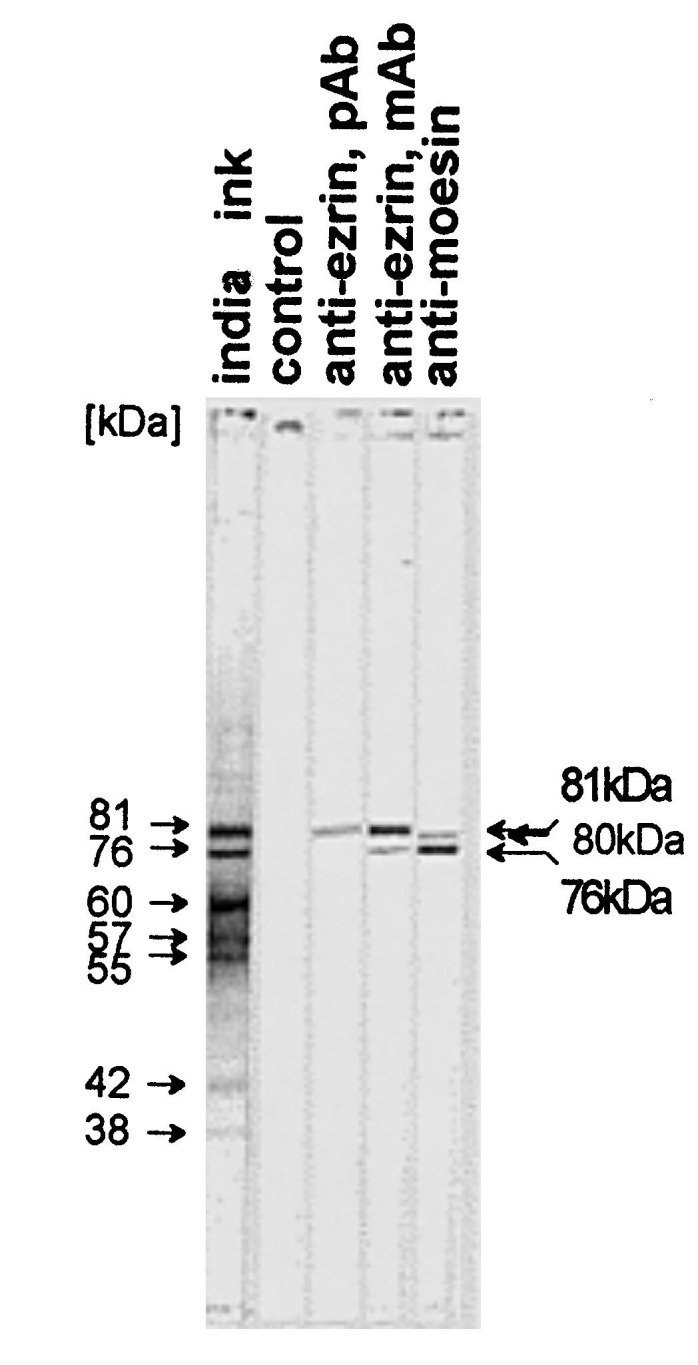
Identification of the 76 and 81 kDa proteins by Western blot. The affinity purified proteins were separated by 10% SDS-PAGE, immobilized on nitrocellulose and strips probed as follows: lane 1: india ink control; lane 2: control without primary antibody; lane 3: polyclonal anti-ezrin serum (1:500); lane 4: monoclonal anti-ezrin antibody (1:250); lane 5: monoclonal anti-moesin antibody 38/87 (1:250). Proteins were visualized with HRP-conjugated secondary antibodies and 4-chloro-1-naphtol.
Fig. 5.
Expression of ezrin and moesin by D54 cells. (a) Flow cytometric analysis of the moesin expression on the surface of D54 cells using mAb 38/87 and 119. (b) Analysis of the ezrin (mAb EZ1) and moesin (mAb 38/87) expression by permeabilized D54 cells. To quantify the staining of permeabilized cells, cells were first fixed with 3.5% paraformaldehyde for 25 min, permeabilized for 10 min in 0.25% Triton X-100 or 0.5% octylglucoside (Calbiochem) and non-specific binding sites blocked with 10% horse serum for 15 min on ice. As negative control for this staining, an anti-Coronavirus S protein mAb was used (Co).
In summary, we found that two proteins, ezrin and moesin, of the CD4-negative/GalC-negative human glioblastoma cell line D54 specifically bind rgp120. Other proteins specifically binding rgp120 could not be detected. In particular an approximately 220 kDa protein, a protein in the approximate molecular weight range of a putative alternative receptor for gp120 as found earlier on D54 cells (larger than 180 kDa; Schneider-Schaulies et al., 1992), and on human fetal astrocytes (260 kDa; Ma et al., 1994), could not reproducibly be eluted from the gp120 affinity matrix. Our data do not exclude the potential interaction of this high molecular weight protein with gp120. Both gp120-binding proteins, ezrin and moesin, are members of the ERM (ezrin, radixin, moesin) family of proteins. The ERM family consists of three closely related proteins with nearly 75% amino acid identity between each of the proteins (Sato et al., 1992; Funayama et al., 1991; Gould et al., 1989; Lankes and Furthmayr, 1991). The proteins comprising this family were identified independently in various tissues and cells, including the brain (Bretscher, 1983; Schwartz-Albiez et al., 1995). Whereas radixin is predominantly expressed at adherens junctions (Tsukita et al., 1989), moesin, initially described as a heparin binding protein (Lankes et al., 1988), is predominantly present in microvilli of cells (Lankes and Furthmayr, 1991; Franck et al., 1993). All three ERM proteins are mainly localized just beneath the plasma membrane as part of the cortical cytoskeleton and are thought to be directly involved in actin filament-plasma membrane interactions. Although these proteins do not have a typical transmembrane domain, small amounts are accessable at the outer cell surface by antibody staining and by iodination and subsequent immunoprecipitation (Dunster et al., 1994; Lankes et al., 1988; Schneider-Schaulies et al., 1995) or are shed by the cell (Lankes and Furthmayr, 1991).
ERM-proteins have the tendency to associate with other transmembrane proteins as the H+/K+-ATPase (Hanzel et al., 1991), CD43 (Yonemura et al., 1993), and CD44 (Tsukita et al., 1994), and are easily separated from the actin cytoskeleton during purification (Algrain et al., 1993; Hanzel et al., 1991). Moesin was found to form a complex with CD46 (Dunster et al., 1994; Schneider-Schaulies et al., 1995), a complement regulatory protein which was identified as the measles virus receptor (Dörig et al., 1993; Naniche et al., 1993). In addition, ERM proteins have been found to be incorporated in small percentages into enveloped viral particles such as rabies virus (Sagara et al., 1995) and HIV (Ott et al., 1996). Because of this association of moesin and ezrin to transmembrane proteins, these proteins could play a role during the uptake of HIV particles in an post-adsorption step acting as link between transmembrane and viral proteins. In addition, moesin and ezrin could interact intracellularly with gp160/gp120 and influence the transport of the viral envelope proteins to the plasma membrane. An adhesion induced polar secretion of HIV and an association of HIV-proteins with the cytoskeleton has already been suggested (Pearce-Pratt et al., 1994). Ezrin and moesin are incorporated in HIV particles at concentrations of approximately 2% of the gag protein (Ott et al., 1996). Ott et al. suggest that these ERM proteins, which are involved in the formation of microvilli, may generally be necessary for the budding of enveloped viruses. The findings that ezrin and moesin specifically bind to HIV-1 gp120, that ERM proteins are incorporated into virions of enveloped viruses (Ott et al., 1996; Sagara et al., 1995), and that moesin is linked to the susceptibility of cell with measles virus (Dunster et al., 1994), support the hypothesis that ERM proteins are involved in the uptake or budding of viruses.
Acknowledgements
We thank Dr J.H. Nuske for helpful discussions, Dr L.M. Dunster for critical reading of the manuscript, Dr R. Schwartz-Albiez for the anti-moesin antibody Professor Dr D. Drenckhahn for providing us with the anti-ezrin antiserum and the Deutsche Forschungsgemeinschaft, Fonds der Chemischen Industrie and Wilhelm Sander Stiftung for financial support. Mass spectrometric analysis was performed in the department of neurochemistry of the Free University Berlin (Professor Hucho) by Dr Peter Franke.
References
- Algrain M., Turunen O., Vaheri A., Louvard D., Arpin M. Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J. Cell Biol. 1993;120:129–139. doi: 10.1083/jcb.120.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benos D.J., Hahn B.H., Bubien J.K., Ghosh S.K., Mashburn N.A., Chaikin M.A., Shaw G.M., Benveniste E.N. Envelope glycoprotein gp120 of human immunodeficiency virus type 1 alters ion transport in astrocytes: implications for AIDS dementia complex. Proc. Natl. Acad. Sci. USA. 1994;91:494–498. doi: 10.1073/pnas.91.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat S., Mettus R.V., Reddy E.P., Ugen K.E., Srikanthan V., Williams W.V., Weiner D.B. The galactosyl ceramide/sulfatide receptor binding region of HIV-1 gp120 maps to amino acids 206–275. AIDS Res. Hum. Retrovir. 1991;9:175–181. doi: 10.1089/aid.1993.9.175. [DOI] [PubMed] [Google Scholar]
- Bigner D.D., Bigner S.H., PontÈn J., Westermark B., Mahaley Jr M.S., Ruoslahti E., Herschman H., Eng L.F., Wikstrand C.J. Heterogenity of genotypic and phenotypic characteristics of fifteen permanent cell lines derived from human gliomas. J. Neuropath. Exp. Neurol. 1981;XL:201–229. doi: 10.1097/00005072-198105000-00001. [DOI] [PubMed] [Google Scholar]
- Brenneman D.E., Westbrook G.L., Fitzgerald S.P., Ennist D.L., Elkins K.L., Ruff M.R., Pert C.B. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature. 1988;335:639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- Bretscher A. Purification of an 80 000-dalton protein that is a component of the isolated microvillus cytoskeleton, and its localization in nonmuscle cells. J. Cell Biol. 1983;97:425–432. doi: 10.1083/jcb.97.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Rutka J.T., Rosenblum M.L., McHugh T. Human immunodeficiency virus can productively infect cultured human glial cells. Proc. Natl. Acad. Sci. USA. 1987;84:3526–3530. doi: 10.1073/pnas.84.10.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza M.P., Harden V.A. Chemokines and HIV-1 second receptors. Nature Med. 1996;2:1293–1300. doi: 10.1038/nm1296-1293. [DOI] [PubMed] [Google Scholar]
- Dörig R.E., Marcil A., Chopra A., Richardson C.D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- Dreyer E.B., Kaiser P.K., Offermann J.T., Lipton S.A. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990;248:364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- Dunster L.M., Schneider-Schaulies J., Löffler S., Lankes W., Schwartz-Albiez R., Lottspeich F., ter Meulen V. Moesin: a cell membrane protein linked with susceptibility to measles virus infection. Virology. 1994;198:265–274. doi: 10.1006/viro.1994.1029. [DOI] [PubMed] [Google Scholar]
- Eckerskorn C., Lottspeich F. Internal amino acid sequence analysis of proteins separeated by gel electrophoresis after tryptic digestion in polyacrylamide matrix. Chromatographia. 1989;28:92–94. [Google Scholar]
- Epstein L.G., Gendelman H.E. Human immunodeficiency virus type 1 infection of the nervous system: pathogenic mechanisms. Ann. Neurol. 1993;33:429–436. doi: 10.1002/ana.410330502. [DOI] [PubMed] [Google Scholar]
- Fantini J., Cook D.G., Nathanson N., Spitalnik S.L., Gonzalez-Scarano F. Infection of colonic epithelial cell lines by type 1 human immunodeficiency virus is associated with cell surface expression of galactosylceramide, a potential alternative gp120 receptor. Proc. Natl. Acad. Sci. USA. 1993;90:2700–2704. doi: 10.1073/pnas.90.7.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck Z., Gary R., Bretscher A. Moesin, like ezrin, colocalizes with actin in the cortical cytoskeleton in cultured cells, but its expression is more variable. J. Cell Sci. 1993;105:219–231. doi: 10.1242/jcs.105.1.219. [DOI] [PubMed] [Google Scholar]
- Funayama N., Nagafuchi A., Sato N., Tsukita Sh. Radixin is a novel member of the band 4.1 family. J. Cell Biol. 1991;115:1039–1048. doi: 10.1083/jcb.115.4.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D., Wendt E., Vaca K., Noonan C.A. The envelope glycoprotein of human immunideficiency virus type 1 stimulates release of neurotoxins from monocytes. Proc. Natl. Acad. Sci. USA. 1993;90:2769–2773. doi: 10.1073/pnas.90.7.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K.L., Bretscher A., Esch F.S., Hunter T. cDNA cloning and sequencing of a protein-tyrosine kinase substrate, ezrin, reveals homology to band 4.1. EMBO J. 1989;8:4133–4142. doi: 10.1002/j.1460-2075.1989.tb08598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzel D., Reggio H., Bretscher A., Forte J.G., Mangeat P. The secretion-stimulated 80K phosphoprotein of parietal cells is ezrin, and has properties of a membrane cytoskeletal linker in the induced apical microvilli. EMBO J. 1991;10:2363–2373. doi: 10.1002/j.1460-2075.1991.tb07775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harouse J.M., Laughlin M.A., Pletcher C., Friedman H.M., Gonzalez-Scarano F. Entry of human immunodeficiency virus-1 into glial cells proceeds via an alternative, efficient pathway. J. Leukocyte Biol. 1991;49:605–609. doi: 10.1002/jlb.49.6.605. [DOI] [PubMed] [Google Scholar]
- Jordan C.A., Watkins B.A., Kufta C., Dubois-Dalcq M. Infection of brain microglial cells by human immunodeficiency virus type 1 is CD4 dependent. J. Virol. 1991;65:2736–2742. doi: 10.1128/jvi.65.2.736-742.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankes W., Griesmacher A., Gruenwald J., Schwartz-Albiez R. A heparin-binding protein involved in inhibition of smooth-muscle cell proliferation. Biochem. J. 1988;251:831–842. doi: 10.1042/bj2510831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankes W., Furthmayr H. Moesin: a member of the protein 4.1-talin-ezrin family. Proc. Natl. Acad. Sci. USA. 1991;88:8297–8301. doi: 10.1073/pnas.88.19.8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi G., Patrizio M., Bernardo A., Petrucci T.C. Human immunodeficiency virus coat protein gp120 inhibits the fl-adrenergic regulation of astroglial and microglial function. Proc. Natl. Acad. Sci. USA. 1993;90:1541–1545. doi: 10.1073/pnas.90.4.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.L., Moudgil T., Vinters H.V., Ho D.D. CD4-independent, productive infection of a neuronal cell line by human immunodeficiency virus type 1. J. Virol. 1990;64:1383–1387. doi: 10.1128/jvi.64.3.1383-1387.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton S.A., Sucher N.J., Kaiser P.K., Dreyer E.B. Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron. 1991;7:111–118. doi: 10.1016/0896-6273(91)90079-f. [DOI] [PubMed] [Google Scholar]
- Ma M., Geiger J.D., Nath A. Characterization of a novel binding site for the human immunodeficiency virus type 1 envelope protein gp120 on human fetal astrocytes. J. Virol. 1994;68:6824–6828. doi: 10.1128/jvi.68.10.6824-6828.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses A.V., Bloom F.E., Pauza C.D., Nelson J.A. Human immunodefiviency virus infection of human brain capuillary endothelial cells occurs via a CD4/galactosylceramide-independent mechanism. Proc. Natl. Acad. Sci. USA. 1993;90:10474–10478. doi: 10.1073/pnas.90.22.10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naniche D., Varior-Krishnan G., Cervoni F., Wild T.F., Rossi B., Rabourdin-combe C., Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott D.E., Coren L.V., Kane B.P., Busch L.K., Johnson D.G., Sowder R.C., Chertova E.N., Arthur L.O., Henderson L.E. Cytoskeletal proteins inside human immunodeficiency virus type 1 virions. J. Virol. 1996;70:7734–7743. doi: 10.1128/jvi.70.11.7734-7743.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce-Pratt R., Malamud D., Phillips D.M. Role of the cytoskeleton in cell-to-cell transmission of human immunodeficiency virus. J. Virol. 1994;68:2898–2905. doi: 10.1128/jvi.68.5.2898-2905.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam L., Herndier B.G., Tang N.M., McGrath M.S. Human immunodeficiency virus-infected macrophages produce soluble factors that cause histological and chemical alterations in cultured human brains. J. Clin. Invest. 1990;87:503–512. doi: 10.1172/JCI115024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranscht B., Clapshaw P.A., Price J., Noble M., Seifert W. Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proc. Natl. Acad. Sci. USA. 1982;79:2709–2713. doi: 10.1073/pnas.79.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara J., Tsukita S., Yonemura S., Tsukita S., Kawai A. Cellular actin-binding ezrin-radixin-moesin (ERM) family proteins are incorporated into the rabies virion and closely associated with viral envelope proteins in the cell. Virology. 1995;206:485–494. doi: 10.1016/s0042-6822(95)80064-6. [DOI] [PubMed] [Google Scholar]
- Sato N., Funayama N., Nagafuchi A., Yonemura S., Tsukita S., Tsukita S. A gene family consisting of ezrin, radixin and moesin. J. Cell Sci. 1992;103:131–143. doi: 10.1242/jcs.103.1.131. [DOI] [PubMed] [Google Scholar]
- Schneider-Schaulies J., Schneider-Schaulies S., Brinkmann R., Tas P., Halbrügge M., Walter U., Holmes H.C., ter Meulen V. HIV-1 gp120 receptor on CD4-negative brain cells activates a tyrosine kinase. Virology. 1992;191:765–772. doi: 10.1016/0042-6822(92)90252-k. [DOI] [PubMed] [Google Scholar]
- Schneider-Schaulies J., Dunster L.M., Schwartz-Albiez R., Krohne G., ter Meulen V. Physical association of moesin and CD46 as a receptor complex for measles virus. J. Virol. 1995;69:2248–2256. doi: 10.1128/jvi.69.4.2248-2256.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz-Albiez R., Merling A., Spring H., Moeller P., Koretz K. Differential expression of the microspike-associated protein moesin in human tissues. Eur. J. Cell Biol. 1995;67:189–198. [PubMed] [Google Scholar]
- Toggas S.M., Masliah E., Rockenstein E.M., Rall G.F., Abraham C.R., Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Tsukita Sa., Hieda Y., Tsukita Sh. A new 82-kD barbed end-capping protein (radixin) localized in the cell-to-cell adherens junction: purification and characterization. J. Cell Biol. 1989;113:321–330. doi: 10.1083/jcb.108.6.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S., Oishi K., Sato N., Sagara J., Kawai A., Tsukita S. ERM family members as molecular linkers bewteen the cell surface glycoprotein CD44 and actin-based cytoskeletons. J. Cell Biol. 1994;126:391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Berg L.H., Sadiq S.A., Lederman S., Latov N. The gp120 glycoprotein of HIV-1 binds to sulfatide and to the myelin associated glycoprotein. J. Neurosci. Res. 1992;33:513–518. doi: 10.1002/jnr.490330403. [DOI] [PubMed] [Google Scholar]
- Wiley C.A., Schrier R.D., Nelson J.A., Lampert P.W., Oldstone M.B.A. Cellular localization of human immunodeficiency virus infection within the brains of aquired immune deficiency syndrome patients. Proc. Natl. Acad. Sci. USA. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S., Nagafuchi A., Sato N., Tsukita S. Concentration of an integral membrane protein, CD43 (Leukosialin, sialophorin) in the cleavage furrow through interaction of its cytoplasmic domain with actin-based cytoskeletons. J. Cell Biol. 1993;120:437–449. doi: 10.1083/jcb.120.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]