Abstract
Acute pharyngitis is one of the most common illnesses for which patients visit primary care physicians. Most cases are of viral origin, and with few exceptions these illnesses are both benign and self-limited. The most important bacterial cause is the beta-hemolytic group A streptococcus. There are other uncommon or rare types of pharyngitis. For some of these treatment is required or available, and some may be life threatening. Among those discussed in this article are diphtheria, gonorrhea, HIV infection, peritonsillar abscess, and epiglottitis.
Pharyngitis
Sore throat accounts for 1% to 2% of all patient visits to office-based primary care practitioners, hospital outpatient departments, and emergency departments in the United States [1]. Data from the National Medical Care Survey indicate approximately 7.3 million annual visits for children [2] and 6.7 such visits for adults [3]. Thus, familiarity with the principles of diagnosis and management of this disorder is essential for primary care physicians seeing patients of all ages.
Etiology
The hallmarks of acute pharyngitis are sore throat of varying degrees of clinical severity, pharyngeal erythema, and fever. Most cases of pharyngitis are of viral origin, and with few exceptions these illnesses are both benign and self-limited. A large proportion of cases of pharyngitis is associated with rhinovirus [4] and coronavirus colds or with influenza. The most important cause of bacterial pharyngitis is the beta-hemolytic group A streptococcus (Streptococcus pyogenes, GAS). There are other uncommon or rare types of pharyngitis, and for some of these treatment is also required or available.
The recognized microbial causes of pharyngitis are listed in Table 1, which shows the syndromes of respiratory illness caused by the various agents [5], [6], [7], [8], [9], [10]. It still is not possible to determine the cause in a sizable proportion of cases. A major task of the primary care physician is to identify those patients with acute pharyngitis who require specific antimicrobial therapy and to avoid unnecessary and potentially deleterious treatment in the great majority who suffer from a benign, self-limited, usually viral infection. In most cases this distinction can be made easily by attention to the epidemiologic setting, history, and physical findings augmented by performance of a few simple and readily available laboratory studies.
Table 1.
Microbial causes of acute pharyngitis
| Pathogen | Associated disorder(s) |
|---|---|
| Bacterial | |
| Streptococcus, group A | Pharyngitis, tonsillitis, scarlet fever |
| Streptococcus, groups C, G | Pharyngitis, tonsillitis |
| Mixed anaerobes | Vincent's angina |
| Neisseria gonorrhoeae | Pharyngitis, tonsillitis |
| Corynebacterium diphtheriae | Diphtheria |
| Arcanobacterium haemolyticum | Pharyngitis, scarlatiniform rash |
| Yersinia enterocolitica | Pharyngitis, enterocolitis |
| Yersinia pestis | Plague |
| Francisella tularensis | Tularemia, oropharyngeal form |
| Treponema pallidum | Secondary syphilis |
| Viral | |
| Rhinovirus | Common cold |
| Coronavirus | Common cold |
| Adenovirus | Pharyngoconjunctival fever |
| Herpes simplex type 1 & 2 | Pharyngitis, gingivostomatitis |
| Parainfluenza | Cold, croup |
| Coxsackie A | Herpangina, hand-foot-mouth disease |
| Epstein-Barr virus | Infectious mononucleosis |
| Cytomegalovirus | CMV mononucleosis |
| Human immunodeficiency virus | Primary HIV infection |
| Influenza A, B | Influenza |
| Mycoplasmal | |
| Mycoplasma pneumoniae | Pneumonia, bronchitis, pharyngitis |
| Chlamydophilal | |
| Chlamydophila psittaci | Acute respiratory disease, pneumonia |
| Chlamydophila pneumoniae | Pneumonia, pharyngitis |
Modified from Bisno AL. Pharyngitis. In: Mandell GL, Dolan R, Bennett JE, editors. Principles and practice of infectious diseases. 6th edition. New York: Churchill Livingstone; 2006. p. 752; with permission.
Epidemiology
The results of epidemiologic investigations are influenced by the season of the year, the age of the population, the severity of illness, and the diagnostic methods used to detect cases. Most cases of pharyngitis occur during the colder months of the year, during the respiratory disease season. Viral agents such as rhinoviruses tend to have annual periods of peak prevalence in the fall and spring; coronaviruses have been found most often in the winter. Influenza appears in epidemics, which in the United States usually occur between December and April. In military recruits, adenoviruses cause the syndrome of acute respiratory disease during the colder months. In civilians, acute respiratory disease occurs in the winter, and epidemics of pharyngoconjunctival fever occur in the summer. Streptococcal pharyngitis occurs during the respiratory disease season, with peak rates of infection in winter and early spring. Spread among family members in the home is a prominent feature of the epidemiologic behavior of most of these agents, with children being the major reservoir of infection [11].
Bacterial infections
Group A streptococci
As shown in Table 1, a number of bacteria may cause acute pharyngitis, but most of these are rare or unusual causes of the syndrome. Moreover, the benefit of antimicrobial therapy for some of these agents (ie, Arcanobacterium haemolyticum, non–group A beta hemolytic streptococci) is unclear. Thus, GAS is the only commonly occurring cause of sore throat for which antimicrobial therapy is definitely indicated.
GAS is estimated to be the cause of 15% to 30% of cases of acute tonsillopharyngitis occurring during the cooler months of the year in school-aged children [12] and of approximately 10% of cases in adults [13], [14], but there is considerable variability from study to study [15]. Nationally, however, approximately 53% of children [2] and 73% of adults [3] who have sore throat receive antimicrobial agents, and a substantial proportion receives antimicrobial agents not recommended as treatments of choice for GAS pharyngitis. The following discussion provides recommendations by which such unnecessary and/or inappropriate therapy may be minimized.
Clinical manifestations
The characteristic clinical findings are summarized in Table 2. The presence of marked odynophagia, exudative tonsillopharyngitis ( Fig. 1), anterior cervical adenitis, fever, and leukocytosis is highly suggestive of GAS pharyngitis. None of the signs and symptoms listed in the table is specific for “strep throat,” however. Moreover, patients vary widely in the severity of their symptoms. Many cases are milder and nonexudative. Only approximately half of children presenting with sore throats and positive throat cultures have tonsillar or pharyngeal exudates [12]. Patients who have had a tonsillectomy may have milder symptoms. Children less than 3 years of age may have coryza and crusting of the nares; exudative pharyngitis caused by GAS is rare in this age group. On the other hand, the presence of cough, coryza (in children older than 3 years), hoarseness, diarrhea, conjunctivitis, and/or anterior stomatitis is highly indicative of viral rather than streptococcal infection.
Table 2.
Clinical presentation of streptococcal tonsillopharyngitis
| Common findings in GAS infectiona | Findings not suggesting GAS infection |
|---|---|
| Symptoms | |
| Sudden onset sore throat | Coryza |
| Pain on swallowing | Hoarseness |
| Fever | Cough |
| Headache | Diarrhea |
| Abdominal pain | |
| Nausea and vomiting | |
| Signs | |
| Tonsillopharyngeal erythema | Conjunctivitis |
| Tonsillopharyngeal exudates | Anterior stomatitis |
| Soft palate petechiae (“doughnut” lesions) | Discrete ulcerative lesions |
| Beefy red, swollen uvula | |
| Anterior cervical adenitis | |
| Scarlatiniform rash | |
Abbreviation: GAS, group A streptococci.
From Dajani A, Taubert K, Ferrieri P, et al. Treatment of acute streptococcal pharyngitis and prevention of rheumatic fever: a statement for health professionals. Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, the American Heart Association. Pediatrics 1995;96:759; with permission.
These findings are noted primarily in children older than 3 years of age and adults. Symptoms and signs in younger children can be different and less specific.
Fig. 1.
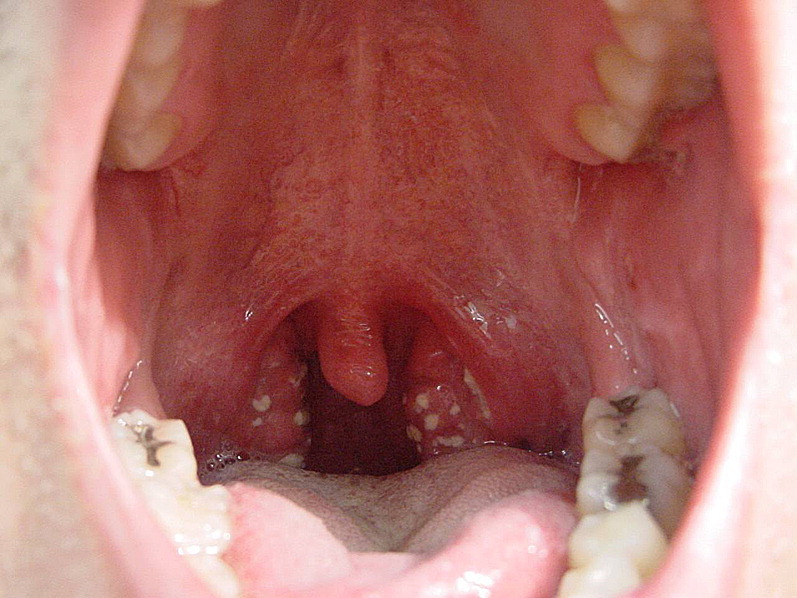
Streptococcal pharyngitis. Note white exudates on erythematous swollen tonsils. (From Nimishikavi S, Stead L. Images in clinical medicine. Streptococcal pharyngitis. N Engl J Med 2005;352(11):e10; with permission Copyright © 2005, Massachusetts Medical Society.)
Scarlet fever results from infection with a streptococcal strain that elaborates streptococcal pyrogenic exotoxins (erythrogenic toxins) to which the patient is not immune. Although this disease usually is associated with pharyngeal infections, it may follow streptococcal infections at other sites such as wound infections or puerperal sepsis. Nowadays, the clinical syndrome is similar in most respects to that associated with nontoxigenic strains, save for the scarlatinal rash. The latter must be differentiated from those of viral exanthems, drug eruptions, staphylococcal and streptococcal toxic shock syndrome, and Kawasaki disease.
Diagnosis
When confronted with a patient who has acute pharyngitis, the clinician must decide whether the likelihood of GAS infection is high enough to warrant a confirmatory diagnostic test. Patients lacking the suggestive clinical and epidemiologic findings or manifesting signs and symptoms indicative of viral pharyngitis (see Table 2) need not be tested or treated with antimicrobial agents. Attempts have been made to incorporate clinical and epidemiologic features of acute pharyngitis into scoring systems that attempt to predict the probability that a particular illness is caused by GAS [14], [16], [17], [18], [19]. These clinical scoring systems are helpful in identifying patients at such low risk of streptococcal infection that further testing is usually unnecessary. Selective use of diagnostic studies for GAS will increase the proportion of positive test results and also the percentage of patients with positive tests who are truly infected rather than merely streptococcal carriers. The signs and symptoms of streptococcal and nonstreptococcal pharyngitis overlap too broadly, however, to allow the requisite diagnostic precision on clinical grounds alone. Although some have suggested otherwise [1], [20], empiric antimicrobial treatment based on clinical and epidemiologic grounds alone has been found suboptimal in cost effectiveness [21] and by prospective analyses [18]. Therefore, if the clinician is unable to rule out strep throat on clinical and epidemiologic grounds, further testing is required (see later discussion) [22].
A properly performed and interpreted throat culture is the gold standard for the diagnosis of GAS pharyngitis. It has a sensitivity of 90% or higher, as judged by studies employing duplicate throat cultures. Obtaining definitive results of the throat culture takes 18 to 48 hours. In the minority of patients who are severely ill or toxic at presentation and in whom clinical and epidemiologic evidence leads to a high index of suspicion, oral antimicrobial therapy may be initiated while awaiting the results of the throat culture. If oral therapy is prescribed, the throat culture serves as a guide to the necessity of completion of a full antimicrobial course or, alternatively, of recalling the patient for an injection of penicillin G benzathine. Early initiation of antimicrobial therapy results in faster resolution of the signs and symptoms, but two facts should be kept in mind. First, GAS pharyngitis usually is a self-limited disease; fever and constitutional symptoms are markedly diminished within 3 or 4 days after onset even without antimicrobial therapy. Thus, antimicrobial therapy initiated within the first 48 hours of onset will hasten symptomatic improvement only modestly. Second, it has been shown that therapy can be postponed safely up to 9 days after the onset of symptoms and still prevent the occurrence of the major nonsuppurative sequela, acute rheumatic fever [23].
The issues alluded to in the previous discussion may be obviated in part by the use of rapid antigen detection tests (RADT), which can confirm the presence of GAS carbohydrate antigen on a throat swab in a matter of minutes. Currently available commercial test kits yield results that are highly specific for the presence of GAS. Thus, a positive RADT can be considered equivalent to a positive throat culture, and therapy may be initiated without further microbiologic confirmation. Unfortunately, the sensitivity of most of these tests ranges between 70% and 90% when compared with the blood agar plate culture [24]. For this reason it is necessary to back up negative RADTs with a conventional throat culture. One possible exception to the need for such backup relates to adults, in whom the prevalence of GAS pharyngitis is relatively low and the risk of a first attack of acute rheumatic fever in North America is minimal [25], [26].
A positive RADT or throat culture does not differentiate between the presence of acute streptococcal infection and chronic GAS carriage [27]. In the appropriate clinical setting, however, a positive test should be considered as confirmatory of strep throat. Follow-up throat cultures are not indicated routinely for asymptomatic patients who have received a complete course of therapy for GAS pharyngitis, because most such patients are streptococcal carriers. Exceptions include patients who have a history of rheumatic fever, patients who develop acute pharyngitis during outbreaks of either acute rheumatic fever or poststreptococcal acute glomerulonephritis, and outbreaks of GAS pharyngitis in closed or semiclosed communities. Follow-up throat cultures also may be indicated when “ping-pong” spread of GAS has been occurring within a family.
Antimicrobial therapy
Treatment of GAS pharyngitis is recommended to prevent acute rheumatic fever, prevent suppurative complications [28], shorten the clinical course (although only modestly) [28], and reduce transmission of the infection in family and school units. There is no definitive evidence that such therapy can prevent acute glomerulonephritis. A number of antibiotics have been shown to be effective in therapy of GAS pharyngitis. These include penicillin and its congeners (such as ampicillin and amoxicillin), numerous cephalosporins, macrolides, and clindamycin. Penicillin, however, remains the treatment of choice because of its proven efficacy, safety, narrow spectrum, and low cost. Amoxicillin often is used in place of oral penicillin V in young children; the efficacy appears equal, and this choice is related primarily to superior palatability of the suspension. Erythromycin is a suitable alternative for patients allergic to penicillin, although increases in GAS resistance to this agent have been reported in certain localized areas of the United States. First-generation cephalosporins also are acceptable for penicillin-allergic patients who do not manifest immediate-type hypersensitivity to beta-lactam antibiotics.
There is debate regarding the relative efficacy of cephalosporins vis-a-vis penicillin [29] in eradicating GAS from the pharynx and about the utility of shorter courses of certain antimicrobial agents in treating strep throat. For a further discussion of this topic, the reader is referred to the Infectious Diseases Society of America (IDSA) practice guideline [25]. Penicillin, however, remains the preferred therapy according to guidelines published by the American Heart Association [30], American Academy of Pediatrics [31], and IDSA ( Table 3) [25]. The IDSA guideline also offers guidance in management of patients who have recurrent episodes of culture- or RADT-positive acute GAS pharyngitis.
Table 3.
Recommendations for antimicrobial therapy of group A streptococcal pharyngitis
| Antimicrobial agents | Dosage | Durationa |
|---|---|---|
| Oral regimens | ||
| Penicillin Vb | Children: 250 mg b.i.d. or t.i.d. | 10 days |
| Adolescents and adults: 250 mg t.i.d. or q.i.d. or 500 mg b.i.d. | 10 days | |
| For patients allergic to penicillin | ||
| Erythromycin | Varies with formulatione | 10 days |
| First-generation cephalosporinsf | Varies with agent | 10 days |
| Intramuscular regimens | ||
| Benzathine penicillin G | 1.2 × 106 U | 1 dose |
| 6.0 × 105 Uc | 1 dose | |
| Mixtures of benzathine and procaine penicillin G | Varies with formulationd | 1 dose |
From Bisno AL, Gerber MA, Gwaltney JM Jr, et al. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Infectious Diseases Society of America. Clin Infect Dis 2002;35(2):120; with permission.
Although shorter courses of azithromycin and some cephalosporins have been reported to be effective for treating group A streptococcal upper respiratory tract infections, evidence is not sufficient to recommend these shorter courses for routine therapy at this time.
Amoxicillin often is used in place of oral penicillin V for young children; efficacy seems to be equal. The choice is related primarily to acceptance of the taste of the suspension.
For patients who weigh < 27 kg.
Dose should be determined on basis of the benzathine component. For example, mixtures of 9 × 105 U of benzathine penicillin G and 3 × 105 U of procaine penicillin G contain less benzathine penicillin G than is recommended for treatment of adolescents or adults.
Available as stearate, ethyl succinate, estolate, or base. Cholestatic hepatitis, rarely, may occur in patients, primarily adults, receiving erythromycin estolate; the incidence is greater among pregnant women, who should not receive this formulation.
These agents should not be used to treat patients with immediate-type hypersensitivity to beta-lactam antibiotics.
Preliminary investigations have demonstrated that once-daily amoxicillin therapy is effective in the treatment of group A beta hemolytic streptococcus pharyngitis [32], [33], [34]. In the most careful of these studies, Feder and colleagues [32] randomly assigned 152 children to receive amoxicillin, 750 mg once daily, or penicillin V, 250 mg three times daily. Compliance was monitored by urine antimicrobial activity, and serotyping was performed to distinguish treatment failures from new acquisitions. The two regimens were equally effective in eradicating GAS from the pharynx. If additional investigations confirm these results, once-daily amoxicillin therapy, because of its low cost and relatively narrow spectrum, could become an alternative regimen for the treatment of group A beta-hemolytic streptococcal pharyngitis.
Suppurative complications of GAS pharyngitis include peritonsillar infection, retropharyngeal abscess, cervical lymphadenitis, mastoiditis, Lemierre syndrome, sinusitis, and otitis media. Such complications have become relatively rare since the advent of effective chemotherapy.
Peritonsillar infection may take the form of cellulitis or abscess (quinsy) and is the most common form of deep oropharyngeal infection. Although peritonsillar abscesses may occur as complications of GAS pharyngitis, the abscesses themselves frequently contain a variety of other oral flora including anaerobes, with or without GAS [35], [36], [37]. They occur more frequently in adolescents and adults than in young children. Pharyngeal pain is usually severe, and dysphagia is common. On examination, there is inflammation and swelling of the peritonsillar area with medial displacement of the tonsil, and patients speak with a “hot potato” voice. Trismus may be present. Peritonsillar cellulitis may be treated with antibiotics alone, but abscesses require drainage by direct aspiration or by incision and drainage. Given the polymicrobial nature of these infections, parenteral antibiotics such as clindamycin, penicillin with metronidazole, or ampicillin-sulbactam are frequently employed. Their superiority over penicillin has not been demonstrated definitively, however.
Lemierre syndrome (postanginal septicemia) is caused by an acute oropharyngeal infection with secondary septic thrombophlebitis of the internal jugular vein and, often, bacteremic spread to lungs or elsewhere [38]. The most common causative organism is Fusobacterium necrophorum. The clinical findings include acute presentation with fever, swelling and tenderness at the angle of the jaw, and neck rigidity. Dysphagia and dysphonia can occur. The peritonsillar area is inflamed only early in the disease. Emergency medical treatment with intravenous antibiotics such as clindamycin, penicillin with metronidazole or ampicillin-sulbactam is usually sufficient, but at times ligation of the internal jugular vein is required.
Acute rheumatic fever and poststreptococcal acute glomerulonephritis are the delayed nonsuppurative sequelae of GAS pharyngitis. Although the former occurs only after GAS upper respiratory infection, the latter may occur after infection of the throat or skin (streptococcal pyoderma). Discussion of these entities is beyond the scope of this article.
Streptococci other than group A
Non–group A beta-hemolytic streptococci are classified biochemically by species. For clinical purposes, however, they usually are identified by their expression of the Lancefield cell-wall antigens. Certain of these strains clearly are capable of causing acute pharyngitis when ingested in high inocula. Streptococci of serogroup G have been linked to common-source outbreaks of pharyngitis, usually related to a food product. One unusual species of group C organisms, Streptococcus equi subspecies zooepidemicus, has given rise to common-source epidemics of pharyngitis, usually caused by consumption of unpasteurized dairy products. Several of these outbreaks have been associated with poststreptococcal acute glomerulonephritis [39].
Groups C and G streptococci are common commensals of the human pharynx. They form large colonies similar to those of GAS and belong to the species Streptococcus dysgalactiae subspecies equisimilis. Physicians who do not back up negative RADTs with throat cultures will not identify these organisms.
The extent to which they cause sporadically occurring episodes of true acute pharyngitis is unclear, but several careful studies suggest that group C streptococci may cause episodes of pharyngitis that mimic GAS infection [40], [41]. Moreover, a community-wide outbreak of group G streptococcal pharyngitis occurred in Connecticut during the winter of 1986–1987 [42]. Because of the difficulty in differentiating benign colonization from true infection with groups C or G streptococci, the benefit, if any, of antimicrobial therapy in sporadically occurring cases is unknown. Should therapy be elected, agents listed in Table 3 would be appropriate, but probably for a shorter duration, because non–group A streptococci never have been shown to cause acute rheumatic fever.
Arcanobacterium haemolyticum
Arcanobacterium (formerly Corynebacterium) haemolyticum is a gram-positive bacillus that is a relatively uncommon cause of acute pharyngitis and tonsillitis. Symptomatic infection with this organism closely mimics acute streptococcal pharyngitis. Rarely, A haemolyticum produces a membranous pharyngitis that can be confused with diphtheria. Two features of pharyngeal infection with this organism are notable. It has a predilection for adolescents and young adults, and it frequently provokes a generalized rash that may resemble that of scarlet fever [43], [44], [45]. The organism is detected more readily on human- than sheep-blood agar plates and thus may not be identified in the routine throat culture. Thus, the clinician should suspect A haemolyticum infection in a teenager or young adult who has an acute pharyngitis and scarlatiniform rash but a negative culture or RADT for GAS. Should antimicrobial therapy of a patient who has A haemolyticum pharyngitis be elected, a macrolide or, alternatively, a beta-lactam antimicrobial agent may be prescribed.
Neisseria gonorrhoeae
Neisseria gonorrhoeae is an uncommon, sexually transmitted cause of acute pharyngitis seen in persons who practice receptive oral sex. The risk of acquisition by this means is highest in men who have sex with men (MSM). Rates of pharyngeal gonorrhea in MSM have been reported as being as high as 15%. A recent study in San Francisco found N gonorrhoeae in the pharynx of 5.5% of MSM and concluded that the pharynx was the most common site of infection in this population [46]. Because of this high prevalence, the United States Centers for Disease Control and Prevention (CDC) guidelines for sexually transmitted diseases recommend a yearly pharyngeal test for N gonorrhoeae in MSM who receive oral intercourse [47]. Although the presence of gonorrhea in the pharynx is common, the occurrence of symptomatic pharyngitis is rare. In the above-mentioned San Francisco study, there was no association between the presence of N gonorrhoeae and pharyngeal symptoms.
When symptomatic oral infection does occur, pharyngitis and tonsillitis are the most common manifestations. Sore throat with an erythematous pharynx, bilateral tonsillar enlargement, at times with grayish-yellowish exudate, and occasionally with cervical lymphadenopathy have been reported [48]. Gingivitis and glossitis also have been described. The diagnosis should be confirmed by culture on Thayer-Martin medium. Currently, ceftriaxone (125 mg in a singular intramuscular dose) is the only CDC recommended therapy for uncomplicated pharyngeal gonorrhea. Concomitant therapy for chlamydia is recommended if this infection has not been ruled out.
Corynebacterium diphtheriae
Pharyngeal diphtheria is caused by Corynebacterium diphtheriae. Humans are the only reservoir of the organism. Asymptomatic carriers account for 3% to 5% of the population in endemic areas, and transmission occurs through respiratory secretions. Although diphtheria has become extremely rare in the United States and other developed countries with effective childhood immunization programs, there are reasons for the primary care physician and infectious disease specialist to be familiar with this disease. First, there have been a few indigenous cases in the United States in the last 10 years in unimmunized or underimmunized individuals in the lower socioeconomic groups [49]. Second, a large proportion of adults in North America and Western Europe lack protective serum levels of antitoxic immunity and are at risk for acquiring the infection when traveling to endemic areas. In 2003, a fatal case occurred in a Pennsylvania resident who traveled to Haiti to assist in building a church [50]. Epidemics of diphtheria involving thousands of cases occurred in the 1990s among residents of the newly independent countries of the former Soviet Union. Third, early diagnosis and treatment are important predictors of ultimate prognosis.
Respiratory diphtheria is typically caused by toxin-producing (“toxigenic”) strains of C diphtheriae and rarely by toxigenic strains of Corynebacterium ulcerans. The main pathogenic factor is an exotoxin capable of producing a severe local reaction of the respiratory mucosa with the formation of a dense necrotic coagulum and pseudomembranes. These pseudomembranes can lead to airway obstruction resulting in suffocation and death. The toxin is also responsible for severe cardiac and neurologic complications [51].
The incubation period of 2 to 4 days is followed by malaise, sore throat, and low-grade fever. The most notable physical finding is the grayish-brown diphtheritic pseudomembrane that may involve one or both tonsils or may extend widely to involve the nares, uvula, soft palate, pharynx, larynx, and tracheobronchial tree. It is firmly adherent to the mucosa, and its removal provokes bleeding. In severe cases, there is swelling of the soft tissues of the neck (“bull neck”), cervical adenopathy, profound malaise, prostration, and stridor. Cardiac complications manifested as myocarditis with cardiac dysfunction occur in 10% to 25% of cases, usually when the pharyngeal manifestations are improving. Neurologic complications can occur also and are related directly to the severity of the primary infection, the immunization history, and the time between the onset of symptoms and institution of treatment.
The diagnosis of diphtheria requires a high index of suspicion and specific laboratory techniques. Diagnosis should be suspected on epidemiologic grounds and in the presence of pharyngitis with pseudomembranes, especially if extending to the uvula and soft palate and bleeding when dislodged. Plating on Loeffler's or Tindale's selective media can identify black colonies with metachromatic granules, but definitive diagnosis requires demonstration of toxin production by immunoprecipitation, polymerase chain reaction (PCR), or immunochromatography. Because successful treatment is inversely related to the duration of the disease, therapy should be started once the diagnosis seems likely on clinical grounds and while awaiting laboratory confirmation.
Treatment of diphtheria includes diphtheria antitoxin and antibiotics. Equine diphtheria antitoxin is only available through the National Immunization Program of the CDC. The therapeutic dose and mode of administration are recommended by the American Academy of Pediatrics according to the duration and extension of the disease [52]. Antibiotics are effective in decreasing local infection, decreasing toxin production, and decreasing spread. Intramuscular penicillin G, switched to oral penicillin V once the patient is able to swallow, and erythromycin are the antibiotics of choice. Respiratory diphtheria (in contrast to cutaneous diphtheria) does not induce protective immunity, so diphtheria toxoid should be administered to patients during convalescence. Prevention of transmission is crucial and is accomplished by strict isolation. Close contacts should be cultured and started on prophylactic antibiotics while awaiting culture results; if not fully immunized, they should receive diptheria toxoid. Both penicillin and erythromycin are efficacious in eradicating the carrier state, but erythromycin has been shown to be superior in some reports.
C ulcerans is an animal pathogen that causes bovine mastitis but can be transmitted to humans through the consumption of raw milk. It can produce diphtheritic toxin and a clinical disease undistinguishable from C diphtheriae.
Atypical bacteria
Mycoplasma pneumoniae and Chlamydophila pneumoniae are known causes of lower respiratory tract infections; they also can be found in the throats of patients who have symptomatic pharyngitis and of asymptomatic carriers. Although these agents probably cause some cases of acute pharyngitis, either as primary pathogens or copathogens, the frequency with which this occurs is still unclear. The pharyngeal manifestations that have been described include erythema, tonsillar enlargement, and, less often, exudate with cervical lymphadenopathy. In a recent Italian study, 133 children who had acute tonsillopharyngitis were tested for M pneumoniae and C pneumoniae with acute- and convalescent-phase titers and PCR on nasopharyngeal aspirates. Thirty-six of the children (27%) had serologically confirmed acute M pneumoniae infection, and 10 (7.5%) had serologically confirmed C pneumoniae infection. Five of the latter also had a positive nasopharyngeal PCR for this organism [53]. The children were assigned randomly to receive azithromycin plus symptomatic treatment or symptomatic treatment alone and were followed for 6 months. In the short term, there was no difference in the outcomes of children with or without atypical infection. A significantly decreased rate of recurrent upper and lower respiratory infections occurred in patients with atypical infections who were randomized to azithromycin, however. These results require confirmation.
Viral infections
Infectious mononucleosis
Clinical manifestations
Infectious mononucleosis (IM) or “glandular fever” is caused by the Epstein-Barr virus (EBV). The virus is present in the oropharyngeal secretions of patients who have IM and is spread by person-to-person contact. Infection with EBV is frequent in childhood but usually is asymptomatic. Clinical manifestations are more common when the infection is acquired in adolescence or young adulthood. Thus, most cases of IM occur between ages 15 and 24 years. Symptoms develop after an incubation period of 4 to 7 weeks. Following a 2- to 5-day prodromal period of chills, sweats, feverishness, and malaise, the disease presents with the classic triad of severe sore throat accompanied by fever as high as 38°C to 40°C and lymphadenopathy. Pharyngitis with associated tonsillitis occurs in 70% to 90% of the patients. Tonsillar exudates are present in approximately one third of cases, and palatal petechiae may also be present Lymphadenopathy is bilateral, particularly posterior cervical, but can involve axillary and inguinal areas. About 10% of patients have a rash of variable morphology, but administration of ampicillin or amoxicillin provokes a pruritic maculopapular eruption in 90% of patients. Hepatomegaly is present in 10% to 15% of patients who have IM, and splenomegaly occurs in almost half of the patients. This classic clinical presentation of IM occurs in most of the children and young adults. Older adults may not exhibit pharyngitis or lymphadenopathy, and disease can be manifested only with fever and more prominent hepatic abnormalities (typhoidal presentation) [54]. EBV infection can be complicated by a variety of neurologic and oncologic conditions that are beyond the scope of this article.
The hematologic findings include leukocytosis with 60% to 70% lymphocytosis and thrombocytopenia that usually is mild but occasionally may be severe. The lymphocytosis usually is found at presentation and peaks 2 to 3 weeks after onset of the disease. The presence of more than 10% atypical lymphocytes in peripheral blood is one of the characteristic features of IM and supports the diagnosis.
The differential diagnosis at initial presentation includes GAS pharyngitis, other respiratory viral infections (see Table 1), cytomegalovirus infection, and, if suggested by epidemiologic history, the acute retroviral syndrome (see later discussion). Rarely, entities such as toxoplasmosis, hepatitis A, human herpesvirus 6, and rubella must be considered. In most cases, the diagnosis is readily confirmed if suspected. Initial diagnostic studies should include throat culture or RADT for GAS and a serologic test for the presence of heterophile antibodies. The latter are antibodies directed against antigens in erythrocytes from different animal species. They are present in approximately 90% of affected adolescents and adults within the first 1 to 3 weeks of illness and may persist for up to 1 year. Spot and slide tests that use horse or purified bovine erythrocytes and allow rapid screening are commercially available. When combined with a compatible clinical presentation, a positive rapid test for heterophile antibodies can be considered diagnostic of IM. False-negative tests can occur in up to 10% of patients, however, especially in children and older adults and in the early stages of the disease.
Approximately 10% of patients who have a classic mononucleosis syndrome have negative tests for heterophile antibodies. In such patients, EBV-specific antibodies should be assayed. The most useful of these for general clinical purposes is the IgM antibody to viral capsid antigen. This test is present at clinical presentation and persists for 4 to 8 weeks. Antibody to Epstein-Barr nuclear antigen first appears 3 to 4 weeks after onset and persists for life [55].
Many patients who have heterophile-negative IM are found by the aforementioned tests to be infected with EBV. In the majority of the remainder, serologic studies confirm infection with cytomegalovirus, which can produce a syndrome closely mimicking that induced by EBV.
IM is predominantly a self-limited disease, and studies have failed to detect any benefit of using antiviral agents. Most symptoms resolve within 3 weeks of onset. Physical activity is tailored to patient tolerance. Because of the risk of splenic rupture, contact sports and heavy lifting should be avoided until the spleen returns to normal size, usually in approximately 3 to 4 weeks. The use of corticosteroids has been studied in clinical trials, but no clear benefit has been demonstrated [56], [57]. Steroids, however, may be useful in the management of severe complications such as airway obstruction, hemolytic anemia, severe thrombocytopenia, and aplastic anemia.
HIV
Within days to weeks after initial infection with HIV type 1, 50% to 90% of patients develop a constellation of symptoms known as the “acute retroviral syndrome.” Fever, sore throat, lymphadenopathy, maculopapular rash, myalgia, arthralgias, and mucocutaneous ulcerations are the landmarks of the syndrome [58], [59], [60], [61]. A nonexudative pharyngitis is present in 50% to 70% of patients. Other oropharyngeal findings include ulcers and thrush. Oral ulcers, which occur in 10% to 20% of the patients, appear in the first days of the illness and last for approximately 1 week. Their distribution is usually in the inner lips and in the floor of the mouth, but tonsils, soft palate, and uvula also can be involved.
Fever occurs in almost 100% of patients who have acute retroviral syndrome, and it is usually high. Lymphadenopathy occurs in 40% to 70% of the patients, is nontender, usually develops after 1 week of illness, and involves cervical, axillary, and inguinal regions. Skin rash, which occurs in 40% to 50% of patients, usually is maculopapular, disseminated, and almost invariably involves the neck and upper trunk. The rash usually spares the distal extremities, although palms and soles can be affected.
The clinical findings of fever, pharyngitis, and lymphadenopathy may simulate IM. Atypical lymphocytosis, although infrequent, also could lead to a misdiagnosis of mononucleosis. Acute retroviral syndrome can be differentiated from mononucleosis, however, by its more acute onset, the absence of exudate or prominent tonsillar hypertrophy, the frequent occurrence of rash (rare in mononucleosis except after treatment with ampicillin or amoxicillin), and the presence of oral ulcerations ( Table 4) [62].
Table 4.
Differentiating clinical features of infectious mononucleosis and acute retroviral infection
| Infectious mononucleosis | Acute HIV infection | |
|---|---|---|
| Onset | Insidious | Acute |
| Exudate | Often present | Absent |
| Maculopapular rash | Rare, unless provoked by antibiotics | 40%–50% |
| Oral ulcerations | Absent | 10%–20% |
| Diarrhea | Rare | Common |
It is important for the clinician to recognize that the ELISA commonly used to diagnose HIV-1 infection are negative in the first 3 to 4 weeks after infection and therefore are not useful in this setting. Tests for p24 antigen or, preferably, quantitative assays for plasma HIV RNA by branched chain DNA or PCR should be performed. The viral load can be anticipated to be very high during this acute phase of infection. An HIV antibody test always should be performed later in time to confirm the diagnosis.
Treatment of acute retroviral syndrome with highly active antiretroviral medication has been controversial, current recommendations consider the use of antiretroviral medications in the setting of acute HIV infection to be optional [63]. Potential benefits of antiretroviral therapy in acute HIV would be to decrease the severity of acute disease and to decrease viral replication. Studies have demonstrated improvement of laboratory markers of disease progression when highly active antiretroviral therapy is used in acute HIV infection [64], [65]. These results would suggest a decrease in progression and transmission of the disease. Treating acute HIV infection, however, also can have potential risks, including known side effects to medications, possible development of resistance, and adverse effects on quality of life. If the patient and clinician elect to start antiretroviral medications, the goal should be suppression of viral replication.
Other viral infections
Whereas most respiratory viruses can cause symptoms indistinguishable from the common cold or acute pharyngitis, some viruses may produce more distinctive clinical syndromes.
Adenovirus
Adenovirus is a common cause of viral pharyngitis. It is manifested clinically as an upper respiratory infection with fever, cough, rhinorrhea, and sore throat, usually more pronounced than in the common cold. The pharynx is erythematous and frequently may have exudates that mimic streptococcal pharyngitis [66]. A distinctive syndrome associated with adenovirus infection in children is pharyngoconjunctival fever. The disease occurs in outbreaks and is characterized by conjunctivitis, pharyngitis, rhinitis, cervical adenitis, and high fever. Although adenoviral infections commonly occur in winter months, pharyngoconjuntival fever has been implicated in outbreaks in summer camps [67] and associated with contaminated swimming pools and ponds. Several types of adenovirus also have been implicated in outbreaks of influenza-like illnesses with sore throat, rhinorrhea, and tracheobronchitis, known as acute respiratory disease of army recruits [68], [69]. These infections are self limited, and symptomatic treatment alone is recommended.
Coxsackie virus
Most enteroviral infections occur in the summer and fall and present as febrile illnesses with sore throat, cough, or coryza. Distinctive manifestations of enteroviral infection are herpangina and hand-foot-and-mouth disease. Herpangina most often is caused by coxsackie A and is most frequent in infants or young children. It usually presents acutely with fever, sore throat, odynophagia, diffuse pharyngeal erythema, and a vesicular enanthem. Headache and vomiting can be preceding symptoms. The oral lesions consist of 1- to 2-mm gray-white papulovesicles that progress to ulcers on an erythematous base and may be present on the soft palate, uvula, and anterior tonsillar pillars [70]. They are moderately painful and usually number less than a dozen. Hand-foot-and-mouth disease also is caused by coxsackie A. It is characterized by a febrile vesicular stomatitis with associated exanthema. The oral findings include small, painful vesicles in the buccal mucosa and tongue that can coalesce and form ulcerative bullae. The lesions are similar to those seen in herpangina, but there is an associated peripheral rash involving hands and feet that can extend proximally. These coxsackie diseases are self limited, and treatment is symptomatic.
Herpes simplex virus
Several studies have documented primary human herpes simplex (HSV) type 1 infection as a cause of pharyngitis in college students [71], [72]. HSV2 occasionally can cause a similar illness as a consequence of oral–genital contact [73]. This form of pharyngitis represents primary infection in immunocompetent patients but can occur as a reactivation of latent virus in immunocompromised hosts. Although the characteristic presentation consists of small vesicles and ulcerations in the posterior pharynx and tonsils, HSV also may produce pharyngeal erythema and exudates at times indistinguishable from strep throat. Lesions may extend to the palate, gingiva, tongue, lip, and face. General symptoms include fever, malaise, inability to eat, and cervical lymphadenopathy. Treatment of HSV oral infection with antiherpetic medication is efficacious in reducing the duration of signs and symptoms as well as viral shedding. Acyclovir, valcyclovir, and famciclovir are all useful in treating HSV infection.
Symptomatic treatment of pharyngitis
Acetaminophen or nonsteroidal anti-inflammatory drugs are effective analgesics and antipyretics. Treatment should be started as soon as symptoms develop and is useful in both viral and bacterial diseases. Oral hydration and gargles with salt water may help alleviate the pharyngeal complaints. Oral cough suppressants, decongestants, and antihistamines are helpful, depending on the symptoms present. Lozenges containing local anesthetics are widely available over the counter and seem to provide temporary relief of sore throat [74], [75]. Several authors have reported that adjuvant therapy with dexamethasone decreases the duration of throat pain in patients who have severe odynophagia [76], [77], [78]. The regimens used and the magnitude of the effect varied among the studies. No adverse effects have been reported, but duration of follow-up often has been limited. One randomized, double-blind, placebo-controlled trial of adjuvant dexamethasone therapy in children found efficacy only in patients who had RADT-positive pharyngitis, and the benefit was judged to be only “of marginal clinical importance” [79]. The authors do not recommend use of corticosteroids in the therapy of GAS pharyngitis.
Epiglottitis
Acute epiglottitis or supraglottitis is an inflammatory process of the epiglottis and adjacent structures that can lead to life-threatening acute respiratory obstruction. In the past, epiglottitis occurred most frequently in children between 2 and 4 years of age and was associated mainly with Haemophilus influenzae type B (Hib) infection. Since the initiation of the childhood vaccination programs, epiglottitis caused by this organism is much less common. Nevertheless, there are still cases of Hib epiglottitis in both immunized and nonimmunized children, and the possibility of an infection with this pathogen cannot be excluded completely in vaccinated patients [80], [81]. Bacteria associated with epiglottitis nowadays include Streptococcus pneumoniae, Staphylococcus aureus, and beta hemolytic streptococci. Multiple agents including other bacteria, viruses, and fungi have been implicated in rare cases. The typical presentation in children includes fever, irritability, sore throat, and rapidly progressive stridor with respiratory distress. The affected child adopts a forward-leaning position, drooling oral secretions while trying to breathe. Adults usually present with sore throat and a milder disease, although airway compromise can occur also. Physical examination of patients suspected of having epiglottitis requires careful inspection of the oropharyngeal and suprapharyngeal area. The diagnosis requires direct visualization of an erythematous and swollen epiglottis under laryngoscopy. Because of the risk of airway obstruction, this procedure should be performed in children only when skilled personnel and equipment to secure the airway are available [82]. Once the airway has been secured, culture of the surface of the epiglottis along with blood cultures should be obtained to guide antibiotic therapy. Management focuses on two important aspects: close monitoring of the airway with intubation if necessary and treatment with intravenous antibiotics. Because of the aforementioned possibility of failure of vaccination, antibiotics should be directed against Hib in every patient regardless of immunization status. Cefotaxime, ceftriaxone, or ampicillin/sulbactam are appropriate choices. Steroids are used commonly in the management of acute epiglottitis although no randomized trial has been done to support this practice. When a case of Hib epiglottitis is diagnosed, the American Academy of Pediatrics recommends that postexposure prophylaxis with rifampin be given to household contacts when there is at least one child in the household younger than 4 years of age, a child in the household younger than 12 months of age who has not received the primary series of Hib vaccine, or an immunosuppressed child, regardless of that child's Hib immunization status [83].
Summary
Acute pharyngitis is an extremely common disorder that usually runs a benign course. In almost all cases, the primary care physician must discriminate between a viral sore throat, which requires only symptomatic management, and GAS pharyngitis, which requires specific antimicrobial therapy. This distinction is important so that GAS pharyngitis can be treated appropriately to minimize the risk of suppurative and nonsuppurative complications. Equally important is minimizing unnecessary and potentially deleterious overtreatment of viral infections with antimicrobial agents. This article has outlined the epidemiologic, clinical, and laboratory findings that assist in decision making. The clinician also must be alert to the occurrence of rare but serious upper respiratory infections that may be life threatening and require special forms of therapy (eg, diphtheria, parapharyngeal suppurative processes, acute epiglottitis).
References
- 1.Snow V., Mottur-Pilson C., Cooper R.J. Principles of appropriate antibiotic use of acute pharyngitis in adults. Ann Intern Med. 2001;134:506–508. doi: 10.7326/0003-4819-134-6-200103200-00018. [DOI] [PubMed] [Google Scholar]
- 2.Linder J.A., Bates D.W., Lee G.M. Antibiotic treatment of children with sore throat. JAMA. 2005;294(18):2315–2322. doi: 10.1001/jama.294.18.2315. [DOI] [PubMed] [Google Scholar]
- 3.Linder J.A., Stafford R.S. Antibiotic treatment of adults with sore throat by community primary care physicians: a national survey, 1989–1999. JAMA. 2001;286(10):1181–1186. doi: 10.1001/jama.286.10.1181. [DOI] [PubMed] [Google Scholar]
- 4.Gwaltney J.M. Clinical significance and pathogenesis of viral respiratory infections. Am J Med. 2002;112(Suppl 6A):13S–18S. doi: 10.1016/s0002-9343(01)01059-2. [DOI] [PubMed] [Google Scholar]
- 5.Banham T.M. A collaborative study of the aetiology of acute respiratory infection in Britain 1961-4. A report of the Medical Research Council working party on acute respiratory virus infections. BMJ. 1965;2(5457):319–326. doi: 10.1136/bmj.2.5457.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gwaltney J.M., Jr. Virology of middle ear. Ann Otol Rhinol Laryngol. 1971;80(3):365–370. doi: 10.1177/000348947108000310. [DOI] [PubMed] [Google Scholar]
- 7.Hamre D., Connelly A.P., Jr., Procknow J.J. Virologic studies of acute respiratory disease in young adults. IV. Virus isolations during four years of surveillance. Am J Epidemiol. 1966;83(2):238–249. doi: 10.1093/oxfordjournals.aje.a120579. [DOI] [PubMed] [Google Scholar]
- 8.Monto A.S., Ullman B.M. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164–169. [PubMed] [Google Scholar]
- 9.Evans A.S., Dick E.C. Acute pharyngitis and tonsillitis in University of Wisconsin students. JAMA. 1964;190:699–708. doi: 10.1001/jama.1964.03070210005001. [DOI] [PubMed] [Google Scholar]
- 10.Glezen W.P., Clyde W.A.J., Senior R.J. Group A streptococci, mycoplasmas, and viruses associated with acute pharyngitis. JAMA. 1967;202:455–460. [PubMed] [Google Scholar]
- 11.Gwaltney J.M.J., Bisno A.L. Pharyngitis. In: Mandell G.L., Bennett J.E., Dolin R., editors. Principles and practice of infectious diseases. Churchill Livingstone; Philadelphia: 2000. pp. 656–662. [Google Scholar]
- 12.Kaplan E.L., Top F.H., Jr., Dudding B.A. Diagnosis of streptococcal pharyngitis: differentiation of active infection from the carrier state in the symptomatic child. J Infect Dis. 1971;123:490–501. doi: 10.1093/infdis/123.5.490. [DOI] [PubMed] [Google Scholar]
- 13.McIsaac W.J., White D., Tannenbaum D. A clinical score to reduce unnecessary antibiotic use in patients with sore throat. Can Med Assoc J. 1998;158(1):75–83. [PMC free article] [PubMed] [Google Scholar]
- 14.Komaroff A.L., Pass T.M., Aronson M.D. The prediction of streptococcal pharyngitis in adults. J Gen Intern Med. 1986;1:1–7. doi: 10.1007/BF02596317. [DOI] [PubMed] [Google Scholar]
- 15.Peter G.S., Bisno A.L. Group A streptococcal pharyngitis in adults. In: Pechere J.C., Kaplan E.L., editors. Streptococcal pharyngitis. Karger; Basel (Switzerland): 2004. [Google Scholar]
- 16.Centor R.M., Witherspoon J.M., Dalton H.P. The diagnosis of strep throat in adults in the emergency room. Med Decis Making. 1981;1(3):239–246. doi: 10.1177/0272989X8100100304. [DOI] [PubMed] [Google Scholar]
- 17.Wald E.R., Green M.D., Schwartz B. A streptococcal score card revisited. Pediatr Emerg Care. 1998;14(2):109–111. doi: 10.1097/00006565-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 18.McIsaac W.J., Kellner J.D., Aufricht P. Empirical validation of guidelines for the management of pharyngitis in children and adults. JAMA. 2004;291(13):1587–1595. doi: 10.1001/jama.291.13.1587. [DOI] [PubMed] [Google Scholar]
- 19.Stillerman M., Bernstein S.H. Streptococcal pharyngitis: evaluation of clinical syndromes in diagnosis. Am J Dis Child. 1961;101:476–489. [Google Scholar]
- 20.Cooper R.J., Hoffman J.R., Bartlett J.G. Principles of appropriate antibiotic use for acute pharyngitis in adults: background. Ann Emerg Med. 2001;37(6):711–719. doi: 10.1067/s0196-0644(01)70090-x. [DOI] [PubMed] [Google Scholar]
- 21.Neuner J.M., Hamel M.B., Phillips R.S. A cost-effectiveness analysis of diagnosis and management of adults with pharyngitis. Ann Intern Med. 2003;139:113–122. doi: 10.7326/0003-4819-139-2-200307150-00011. [DOI] [PubMed] [Google Scholar]
- 22.Bisno A.L. Diagnosing strep throat in the adult patient: do clinical criteria really suffice? Ann Intern Med. 2003;139(2):150–151. doi: 10.7326/0003-4819-139-2-200307150-00015. [DOI] [PubMed] [Google Scholar]
- 23.Catanzaro F.J., Stetson C.A., Morris A.J. The role of streptococcus in the pathogenesis of rheumatic fever. Am J Med. 1954;17:749–756. doi: 10.1016/0002-9343(54)90219-3. [DOI] [PubMed] [Google Scholar]
- 24.Gerber M.A., Shulman S.T. Rapid diagnosis of pharyngitis caused by group A streptococci. Clin Microbiol Rev. 2004;17(3):571–580. doi: 10.1128/CMR.17.3.571-580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bisno A.L., Gerber M.A., Gwaltney J.M., Jr. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Infectious Diseases Society of America. Clin Infect Dis. 2002;35(2):113–125. doi: 10.1086/340949. [DOI] [PubMed] [Google Scholar]
- 26.Humair J.P., Revaz S.A., Bovier P. Management of acute pharyngitis in adults: reliability of rapid streptococcal tests and clinical findings. Arch Intern Med. 2006;166(6):640–644. doi: 10.1001/archinte.166.6.640. [DOI] [PubMed] [Google Scholar]
- 27.Gerber M.A. Treatment failures and carriers: perception or problems? Pediatr Infect Dis J. 1994;13:576–579. doi: 10.1097/00006454-199406000-00036. [DOI] [PubMed] [Google Scholar]
- 28.Del Mar C.B., Glasziou P.P., Spinks A.B. Antibiotics for sore throat. Cochrane Database Syst Rev. 2006;(4) doi: 10.1002/14651858.CD000023.pub3. CD000023. [DOI] [PubMed] [Google Scholar]
- 29.Bisno A.L. Are cephalosporins superior to penicillin for treatment of acute streptococcal pharyngitis? Clin Infect Dis. 2004;38(11):1535–1537. doi: 10.1086/392520. [DOI] [PubMed] [Google Scholar]
- 30.Dajani A., Taubert K., Ferrieri P. Treatment of acute streptococcal pharyngitis and prevention of rheumatic fever: a statement for health professionals. Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, the American Heart Association. Pediatrics. 1995;96(4 Pt 1):758–764. [PubMed] [Google Scholar]
- 31.Committee on Infectious Diseases . Group A streptococcal infections. In: Pickering L.K., editor. 2003 red book. American Academy of Pediatrics; Elk Grove Village (IL): 2003. pp. 573–584. [Google Scholar]
- 32.Feder H.M.J., Gerber M.A., Randolph M.F. Once-daily therapy for streptococcal pharyngitis with amoxicillin. Pediatrics. 1999;103(1):47–51. doi: 10.1542/peds.103.1.47. [DOI] [PubMed] [Google Scholar]
- 33.Gopichand I., Williams G.D., Medendorp S.V. Randomized, single-blinded comparative study of the efficacy of amoxicillin (40 mg/kg/day) versus standard-dose penicillin V in the treatment of group A streptococcal pharyngitis in children. Clin Pediatr (Phila) 1998;37(6):341–346. doi: 10.1177/000992289803700602. [DOI] [PubMed] [Google Scholar]
- 34.Shvartzman P., Tabenkin H., Rosentzwaig A. Treatment of streptococcal pharyngitis with amoxycillin once a day. BMJ. 1993;306:1170–1172. doi: 10.1136/bmj.306.6886.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brook I. Microbiology and management of peritonsillar, retropharyngeal, and parapharyngeal abscesses. J Oral Maxillofac Surg. 2004;62(12):1545–1550. doi: 10.1016/j.joms.2003.12.043. [DOI] [PubMed] [Google Scholar]
- 36.Shoemaker M., Lampe R.M., Weir M.R. Peritonsillitis: abscess of cellulitis? Pediatr Infect Dis J. 1986;5:435–439. [PubMed] [Google Scholar]
- 37.Snow D.G., Campbell J.B., Morgan D.W. The microbiology of peritonsillar sepsis. J Laryngol Otol. 1991;105(7):553–555. doi: 10.1017/s0022215100116585. [DOI] [PubMed] [Google Scholar]
- 38.Chirinos J.A., Lichtstein D.M., Garcia J. The evolution of Lemierre syndrome: report of 2 cases and review of the literature. Medicine (Baltimore) 2002;81(6):458–465. doi: 10.1097/00005792-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Baracco G.J., Bisno A.L. Group C and group G streptococcal infections: epidemiologic and clinical aspects. In: Fischetti V.A., Novick R.P., Ferreti J.J., editors. Gram positive pathogens. ASM Press; Washington, DC: 2006. pp. 222–229. [Google Scholar]
- 40.Turner J.C., Hayden F.G., Lobo M.C. Epidemiologic evidence for Lancefield group C beta-hemolytic streptococci as a cause of exudative pharyngitis in college students. J Clin Microbiol. 1997;35(1):1–4. doi: 10.1128/jcm.35.1.1-4.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meier F.A., Centor R.M., Graham L., Jr. Clinical and microbiological evidence for endemic pharyngitis among adults due to group C streptococci. Arch Intern Med. 1990;150:825–829. [PubMed] [Google Scholar]
- 42.Gerber M.A., Randolph M.F., Martin N.J. Community-wide outbreak of group G streptococcal pharyngitis. Pediatrics. 1991;87(5):598–603. [PubMed] [Google Scholar]
- 43.Karpathios T., Drakonaki S., Zervoudaki A. Arcanobacterium haemolyticum in children with presumed streptococcal pharyngotonsillitis or scarlet fever. J Pediatr. 1992;121:735–737. doi: 10.1016/s0022-3476(05)81903-1. [DOI] [PubMed] [Google Scholar]
- 44.Mackenzie A., Fuite L.A., Chan F.T. Incidence and pathogenicity of Arcanobacterium haemolyticum during a 2-year study in Ottawa. Clin Infect Dis. 1995;21:177–181. doi: 10.1093/clinids/21.1.177. [DOI] [PubMed] [Google Scholar]
- 45.Miller R.A., Brancato F., Holmes K.K. Corynebacterium hemolyticum as a cause of pharyngitis and scarlatiniform rash in young adults. Ann Intern Med. 1986;105:867–872. doi: 10.7326/0003-4819-105-6-867. [DOI] [PubMed] [Google Scholar]
- 46.Morris S.R., Klausner J.D., Buchbinder S.P. Prevalence and incidence of pharyngeal gonorrhea in a longitudinal sample of men who have sex with men: the EXPLORE study. Clin Infect Dis. 2006;43(10):1284–1289. doi: 10.1086/508460. [DOI] [PubMed] [Google Scholar]
- 47.Workowski K.A., Berman S.M. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1–94. [PubMed] [Google Scholar]
- 48.Balmelli C., Gunthard H.F. Gonococcal tonsillar infection–a case report and literature review. Infection. 2003;31(5):362–365. doi: 10.1007/s15010-003-4003-7. [DOI] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention (CDC) Status report on the Childhood Immunization Initiative: reported cases of selected vaccine-preventable diseases–United States, 1996. MMWR Morb Mortal Wkly Rep. 1997;46(29):665–671. [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention (CDC) Fatal respiratory diphtheria in a U.S. traveler to Haiti–Pennsylvania, 2003. MMWR Morb Mortal Wkly Rep. 2004;52(53):1285–1286. [PubMed] [Google Scholar]
- 51.MacGregor R.R. Corynebacteria diphtheriae. In: Mandell G.L., Bennett J.E., Dolan R., editors. Principles and practice of infectious diseases. 6th edition. Elsevier Churchill Livingstone; Philadelphia: 2005. pp. 2457–2465. [Google Scholar]
- 52.Pickering L.K., editor. Red book: 2006 report of the Committee on Infectious Diseases. American Academy of Pediatrics; Elk Grove (IL): 2006. Committee on Infectious Diseases. Diphtheria; pp. 263–266. [Google Scholar]
- 53.Esposito S., Bosis S., Begliatti E. Acute tonsillopharyngitis associated with atypical bacterial infection in children: natural history and impact of macrolide therapy. Clin Infect Dis. 2006;43(2):206–209. doi: 10.1086/505120. [DOI] [PubMed] [Google Scholar]
- 54.Auwaerter P.G. Infectious mononucleosis in middle age. JAMA. 1999;281(5):454–459. doi: 10.1001/jama.281.5.454. [DOI] [PubMed] [Google Scholar]
- 55.Johannsen E.C., Schooley R.T., Kaye K.M. Epstein-Barr Virus (infectious mononucleosis) In: Mandell G.L., Bennett J.E., Dolin R., editors. Principles and practice of infectious diseases. 6th edition. Elsevier Churchill Livingstone; Philadelphia: 2005. pp. 1801–1820. [Google Scholar]
- 56.Straus S.E., Cohen J.I., Tosato G. NIH conference. Epstein-Barr virus infections: biology, pathogenesis, and management. Ann Intern Med. 1993;118(1):45–58. doi: 10.7326/0003-4819-118-1-199301010-00009. [DOI] [PubMed] [Google Scholar]
- 57.Candy B., Hotopf M. Steroids for symptom control in infectious mononucleosis. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD004402.pub2. CD004402. [DOI] [PubMed] [Google Scholar]
- 58.Kahn J.O., Walker B.D. Acute human immunodeficiency virus type 1 infection. N Engl J Med. 1998;339(1):33–39. doi: 10.1056/NEJM199807023390107. [DOI] [PubMed] [Google Scholar]
- 59.Cooper D.A., Gold J., Maclean P. Acute AIDS retrovirus infection. Definition of a clinical illness associated with seroconversion. Lancet. 1985;1(8428):537–540. doi: 10.1016/s0140-6736(85)91205-x. [DOI] [PubMed] [Google Scholar]
- 60.Hare C., Kahn J. Primary HIV infection. Curr Infect Dis Rep. 2004;6:65–71. doi: 10.1007/s11908-004-0026-1. [DOI] [PubMed] [Google Scholar]
- 61.Sun H.Y., Chen M.J., Hung C.C. Clinical presentations and virologic characteristics of primary human immunodeficiency virus type-1 infection in a university hospital in Taiwan. J Microbiol Immunol Infect. 2004;37(5):271–275. [PubMed] [Google Scholar]
- 62.Gaines H., von Sydow M., Pehrson P. Clinical picture of HIV infection presenting as a glandular-fever-like illness. BMJ. 1988;297:1363–1368. doi: 10.1136/bmj.297.6660.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith D.E., Walker B.D., Cooper D.A. Is antiretroviral treatment of primary HIV infection clinically justified on the basis of current evidence? AIDS. 2004;18(5):709–718. doi: 10.1097/00002030-200403260-00001. [DOI] [PubMed] [Google Scholar]
- 64.Hoen B., Dumon B., Harzic M. Highly active antiretroviral treatment initiated early in the course of symptomatic primary HIV-1 infection: results of the ANRS 053 trial. J Infect Dis. 1999;180(4):1342–1346. doi: 10.1086/315002. [DOI] [PubMed] [Google Scholar]
- 65.Malhotra U., Berrey M.M., Huang Y. Effect of combination antiretroviral therapy on T-cell immunity in acute human immunodeficiency virus type 1 infection. J Infect Dis. 2000;181(1):121–131. doi: 10.1086/315202. [DOI] [PubMed] [Google Scholar]
- 66.Dominguez O., Rojo P., de Las H.S. Clinical presentation and characteristics of pharyngeal adenovirus infections. Pediatr Infect Dis J. 2005;24(8):733–734. doi: 10.1097/01.inf.0000172942.96436.2d. [DOI] [PubMed] [Google Scholar]
- 67.Centers for Disease Control and Prevention (CDC) Outbreak of pharyngoconjunctival fever at a summer camp–North Carolina, 1991. MMWR Morb Mortal Wkly Rep. 1992;41(19):342–344. [PubMed] [Google Scholar]
- 68.Hendrix R.M., Lindner J.L., Benton F.R. Large, persistent epidemic of adenovirus type 4-associated acute respiratory disease in U.S. army trainees. Emerg Infect Dis. 1999;5(6):798–801. doi: 10.3201/eid0506.990609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kolavic-Gray S.A., Binn L.N., Sanchez J.L. Large epidemic of adenovirus type 4 infection among military trainees: epidemiological, clinical, and laboratory studies. Clin Infect Dis. 2002;35(7):808–818. doi: 10.1086/342573. [DOI] [PubMed] [Google Scholar]
- 70.Scott L.A., Stone M.S. Viral exanthems. Dermatol Online J. 2003;9(3):4. [PubMed] [Google Scholar]
- 71.McMillan J.A., Weiner L.B., Higgins A.M. Pharyngitis associated with herpes simplex virus in college students. Pediatr Infect Dis J. 1993;12:280–284. doi: 10.1097/00006454-199304000-00004. [DOI] [PubMed] [Google Scholar]
- 72.Glezen W.P., Fernald G.W., Lohr J.A. Acute respiratory disease of university students with special reference to the etiologic role of Herpesvirus hominis. Am J Epidemiol. 1975;101:111–121. doi: 10.1093/oxfordjournals.aje.a112077. [DOI] [PubMed] [Google Scholar]
- 73.Young E.J., Vainrub B., Musher D.M. Acute pharyngotonsillitis caused by herpesvirus type 2. JAMA. 1978;239:1885–1886. [PubMed] [Google Scholar]
- 74.Fischer J., Pschorn U., Vix J.M. Efficacy and tolerability of ambroxol hydrochloride lozenges in sore throat. Randomised, double-blind, placebo-controlled trials regarding the local anaesthetic properties. Arzneimittelforschung. 2002;52(4):256–263. doi: 10.1055/s-0031-1299889. [DOI] [PubMed] [Google Scholar]
- 75.Schachtel B.P., Homan H.D., Gibb I.A. Demonstration of dose response of flurbiprofen lozenges with the sore throat pain model. Clin Pharmacol Ther. 2002;71(5):375–380. doi: 10.1067/mcp.2002.124079. [DOI] [PubMed] [Google Scholar]
- 76.Wei J.L., Kasperbauer J.L., Weaver A.L. Efficacy of single-dose dexamethasone as adjuvant therapy for acute pharyngitis. Laryngoscope. 2002;112(1):87–93. doi: 10.1097/00005537-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 77.Olympia R.P., Khine H., Avner J.R. Effectiveness of oral dexamethasone in the treatment of moderate to severe pharyngitis in children. Arch Pediatr Adolesc Med. 2005;159(3):278–282. doi: 10.1001/archpedi.159.3.278. [DOI] [PubMed] [Google Scholar]
- 78.Niland M.L., Bonsu B.K., Nuss K.E. A pilot study of 1 versus 3 days of dexamethasone as add-on therapy in children with streptococcal pharyngitis. Pediatr Infect Dis J. 2006;25(6):477–481. doi: 10.1097/01.inf.0000219469.95772.3f. [DOI] [PubMed] [Google Scholar]
- 79.Bulloch B., Kabani A., Tenenbein M. Oral dexamethasone for the treatment of pain in children with acute pharyngitis: a randomized, double-blind, placebo-controlled trial. Ann Emerg Med. 2003;41(5):601–608. doi: 10.1067/mem.2003.136. [DOI] [PubMed] [Google Scholar]
- 80.Gonzalez V.H., Wald E.R., Rose E. Epiglottitis and Haemophilus influenzae immunization: the Pittsburgh experience–a five-year review. Pediatrics. 1995;96(3 Pt 1):424–427. [PubMed] [Google Scholar]
- 81.McEwan J., Giridharan W., Clarke R.W. Paediatric acute epiglottitis: not a disappearing entity. Int J Pediatr Otorhinolaryngol. 2003;67(4):317–321. doi: 10.1016/s0165-5876(02)00393-2. [DOI] [PubMed] [Google Scholar]
- 82.Rafei K., Lichenstein R. Airway infectious disease emergencies. Pediatr Clin North Am. 2006;53(2):215–242. doi: 10.1016/j.pcl.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 83.Committee on Infectious Diseases . Haemophilus influenzae. In: Pickering L.K., editor. Red book: 2006 report of the committee on infectious diseases. American Academy of Pediatrics; Elk Grove Village (IL): 2006. pp. 310–313. [Google Scholar]


