Graphical abstract
Keywords: Cost-effective elicitor, Plant in vitro cultures, Pharmacologically active flavonoids, Molecular mechanism, Antioxidant evaluation
Highlights
-
•
Chitosan elicitation was conducted for flavonoid enhancement in hairy root cultures.
-
•
Chitosan elicitation gave a 7.08-fold increase in flavonoid yield over control.
-
•
Chalcone synthase and flavonoid 3′-hydroxylase might be two crucial genes.
-
•
Chitosan elicitation can facilitate the commercialization of plant in vitro cultures.
Abstract
Elicitation for phytochemical enhancement via cost-effective elicitors can overcome the limitation of commercial application faced by plant cell and organ culture technology. Chitosan is a natural, low-cost, and nontoxic elicitor that can trigger plant defense responses with the concomitant enhancement in phytochemical biosynthesis. In this work, the elicitation of Isatis tinctoria L. hairy root cultures by chitosan was conducted to enhance the production of pharmacologically active flavonoids. In comparison with control (2.31 ± 0.29 mg/g DW), a 7.08-fold enhancement of total flavonoids (16.35 ± 0.88 mg/g DW) was achieved in 24 day-old I. tinctoria hairy root cultures elicited by 150 mg/L chitosan for 36 h. Interestingly, the multiple hydroxyl-substituted flavonoids (rutin, quercetin, isorhamnetin, and isoliquiritigenin) were noticed to increase significantly in chitosan-elicited I. tinctoria hairy root cultures. Moreover, the transcription of associated genes involved in flavonoid biosynthesis pathway was significantly up-regulated underlying chitosan elicitation, among which chalcone synthase and flavonoid 3′-hydroxylase might play an important role in flavonoid enhancement. Additionally, extracts from chitosan-elicited I. tinctoria hairy root cultures exhibited higher antioxidant activities with lower IC50 values as compared with control. Overall, a cost-effective strategy via the simple chitosan elicitation is provided here to enhance the production of high-added value flavonoids in I. tinctoria hairy root cultures, which paves the way toward the successful commercialization of this in vitro culture system in the future.
1. Introduction
Isatis tinctoria L. is an economically important crop widely cultivated in the northern region of China (Ni et al., 2012). Its dried root named “Banlangen” in Traditional Chinese Medicine (TCM), is one of the top-selling herbs in East Asian, due to the notable clinical treatment efficacy of influenza, such as severe acute respiratory syndrome (SARS) and novel swine-origin influenza A (H1N1) (Xiao et al., 2015). I. tinctoria root is commonly processed into two commercial pharmaceutical preparations, namely “Banlangen Keli” and “Banlangen Tangjiang”, which can be sold as over-the-counter (OTC) drugs at the pharmacy (Zhou and Zhang, 2013). Phenylpropanoids (flavonoids and lignans) are thought to be primarily related to the outstanding pharmacological activity of I. tinctoria (Nguyen et al., 2017). During the long-term evolution, plants can produce a huge number of secondary metabolites that play a crucial role in interaction with environmental factors and defense against unfavorable conditions (Ramirez-Estrada et al., 2016). Thus, the field cultivation of I. tinctoria is associated with an important challenge that the phytochemical profile is highly affected by geographical regions, climatic fluctuations, and soil conditions, which can significantly affect the therapeutic efficacy of I. tinctoria root (Chen et al., 2015). To overcome this issue, an in vitro culture system has been developed, i.e. I. tinctoria hairy root cultures (ITHRCs), as an alternative to the field cultivation for the sustainable and standard production of bioactive flavonoids (Gai et al., 2015).
Plant cell and organ cultures offer promising biotechnological platforms for the production of diverse phytochemicals of pharmaceutical and nutraceutical interest (Dias et al., 2016; Murthy et al., 2014). However, very few successful cases are commercially available due to the low phytochemical productivity not being sufficient to cover the culture cost (Davies and Deroles, 2014; Yue et al., 2016). In this context, research efforts need to be directed towards the enhancement of phytochemical productivity without significantly increasing production cost, which can make plant in vitro culture technology more commercially attractive.
Strategies including genetic modification, elicitation, permeabilization, immobilization, and two phase system, have been widely employed to enhance the production of valuable phytochemicals in various plant cell and organ cultures, among which elicitation via application of biotic/abiotic elicitors for activating plant defense reactions is implemented extensively owing to its simplicity of usage (Isah et al., 2018). Jasmonic acid, salicylic acid, and their analog derivatives are the most commonly used elicitors in plant cell and organ cultures (Nartop, 2018). Nevertheless, these elicitors are expensive, which presents an inevitable obstacle from the perspective of commercial applications (Giri and Zaheer, 2016). Therefore, search of cost-effective elicitors that can promote phytochemical production is an essential task (Giri and Zaheer, 2016; Narayani and Srivastava, 2017).
Notably, chitosan is a low-cost biopolymer mainly derived from the waste exoskeletons of edible crustaceans (Hadwiger, 2013). Moreover, chitosan is well documented as an effective “plant defense booster’’ that can trigger various physiological and biochemical reactions, in which the stimulation of phytoalexin biosynthesis is flagged as a characteristic event (Katiyar et al., 2015). More importantly, chitosan is a natural biopolymer with excellent biocompatibility and biodegradability (Sathiyabama et al., 2016). It is noteworthy that the toxicity of chitosan is very close to that of salt or sugar (Singla and Chawla, 2001). In view of costs and product safety, utilization of chitosan as a cost-effective and nontoxic elicitor for enhancing phytochemical production in plant in vitro cultures is strongly recommended (Jaisi and Panichayupakaranant, 2017; Kamalipourazad et al., 2016; Malayaman et al., 2017; Sivanandhan et al., 2012).
Generally, plant secondary metabolism is very specific to the type of elicitor to which in vitro cell/organ cultures are exposed (Narayani and Srivastava, 2017). In this work, the effect of chitosan concentration and elicitation time on the total flavonoid yield in ITHRCs was initially studied. Afterwards, the profiles of eight flavonoids in ITHRCs with and without the appropriate chitosan elicitation were investigated. To clarify the molecular mechanism of flavonoid enhancement in ITHRCs underlying chitosan elicitation, the transcription of seven enzyme genes involved in flavonoid biosynthesis pathway was monitored. For human health, ROS is implicated in numerous chronic diseases including coronary heart disease, cancer, brain dysfunction, diabetes, and others (López-Alarcón and Denicola, 2013). Accordingly, antioxidant activities of extracts from ITHRCs following chitosan elicitation were evaluated. It is worth mentioning that there is no study regarding the application of chitosan to elevate flavonoid productivity, biosynthesis gene expression, and antioxidant activity in ITHRCs.
2. Material and methods
2.1. Establishment and maintenance of ITHRCs
Isatis tinctoria hairy roots were previously induced from petiole explants by Agrobacterium rhizogenes LBA9402 mediated transformation in the Key Laboratory of Forest Plant Ecology, Ministry of Education, Northeast Forestry University (Gai et al., 2015). A lead root line (ITHRL V) with high flavonoid productivity was adopted in this study for chitosan elicitation. The stock line was maintained on phytohormone-free MS/2-based solid medium supplemented with 30 g/L sucrose at 25 ± 1 °C in the dark. Additionally, ITHRCs were obtained by inoculating ITHRL V into 250 mL Erlenmeyer flasks containing 150 mL MS/2 liquid medium under the optimal culture conditions described in the previous study (Gai et al., 2015).
2.2. ITHRCs elicited by chitosan
In this work, ITHRCs undergone the aforementioned conditions for 24 days (the optimal harvest time) were subjected to chitosan elicitation, which aimed to further enhancing the flavonoid yield without affecting the root biomass. Chitosan (Sigma-Aldrich Chemical Co., USA) was dissolved in the acetic acid solution (pH = 5.5) to give a stock concentration of 10 mg/mL, and then the stock solution was sterilized by autoclaving before use. Prior to elicitation, the spent media of ITHRCs (24 day-old) were renewed by fresh ones (150 mL), in which the chitosan stock solution was aseptically added to make the final concentrations of 50, 100, 150, 200, and 400 mg/L. During elicitation, the chitosan-elicited ITHRCs were incubated on a rotary shaker (120 rpm) at 25 ± 1 °C in the dark, and harvested at a successive time points of 0, 6, 12, 18, 24, 30, 36, 48, 60, 72, and 96 h. Control cultures, i.e. adding the same volume of acetic acid solution (pH = 5.5) in ITHRCs, underwent the same conditions as the experimental groups. After elicitation, hairy roots were collected by filtration, and rinsed by distilled water. For flavonoid extraction, a part of roots were dried in a vacuum oven and ground into fine powders. For RNA extraction, the rest of roots were frozen immediately with liquid nitrogen and stored at −80 °C.
2.3. Total flavonoid determination
The powders of dried hairy roots were extracted with 80% ethanol solution using an ultrasonic-assisted method described by Gai et al. (2015). The crude extracts were obtained by concentrating the filtrates to dryness using a rotary evaporator under vacuum. Total flavonoid contents in the extracts were determined by a colorimetrical method reported by Yao et al. (2013). Total flavonoid contents were calculated by a calibration curve of rutin, and results were expressed as the microgram of rutin equivalents per gram of hairy roots (dry weight, DW).
2.4. Liquid chromatographic tandem mass spectrometry (LC–MS/MS) analysis
Eight target flavonoids (rutin, neohesperidin, buddleoside, liquiritigenin, quercetin, isorhamnetin, kaempferol, and isoliquiritigenin) in the extracts were determined using an Agilent 1100 series HPLC (Agilent Technologies, USA) coupled to an API 3000 triple tandem quadrupole MS (Applied Biosystems, Canada). Prior to LC–MS/MS analysis, samples were prepared by re-dissolving extracts in acetonitrile (20 mL) and filtering through a nylon filter (0.45 μm). The detailed operational procedures were carried out as reported previously (Gai et al., 2015). The MS selected reaction monitoring (SRM) mode with precursor ion/product ion combinations of m/z 609.1→300.0, m/z 609.5→301.4, m/z 591.4→283.1, m/z 255.9→119.0, m/z 301.0→151.0, m/z 315.0→300.1, m/z 285.3→183.1, and m/z 255.4→118.9 was conducted for the quali-quantitative analysis of rutin, neohesperidin, buddleoside, liquiritigenin, quercetin, isorhamnetin, kaempferol, and isoliquiritigenin, respectively. The analyte content was reported as the microgram per gram of hairy roots (DW).
2.5. Quantitative real-time PCR (qRT-PCR) analysis
To clarify the molecular mechanism of flavonoid enhancement in ITHRCs underlying chitosan elicitation, the transcription levels of genes encoding enzymes involved in flavonoid biosynthesis pathway were determined by qRT-PCR, i.e. phenylalanine ammonia lyase (PAL), cinnamate-4-hydroxylase (C4H), 4-coumarate coenzyme A ligase (4CL), chalcone synthase (CHS), chalcone isomerase (CHI), flavonol synthase (FS), and flavonoid 3′-hydroxylase (F3′H).
A MiniBEST Plant RNA Extraction Kit (TaKaRa, China) was used to extract total RNA from the frozen hairy roots (250 mg, fresh weight) following the manufacturer’s guidelines. After the quality and quantity assessment, a certain amount of RNA (500 ng) was reverse-transcribed to cDNA using a PrimeScript™ RT reagent Kit (TaKaRa, China) in accordance with the manufacturer’s protocols. The synthesized cDNA (20 μL) was diluted with sterile water to 200 μL, and stored at −20 °C till qRT-PCR assay.
The qRT-PCR analysis of all target genes was performed as described in the previous study (Jiao et al., 2018). Briefly, the gene amplification reaction was carried out using SYBR Green detection chemistry on a Stratagene Mx3000 P Real-Time PCR system (Agilent Technologies, USA). The cycling procedure was determined as follows: initial denaturation step of 95 °C for 3 min, followed by 40 amplification cycles with denaturation at 95 °C for 15 s, annealing at 55 °C for 30 s, and extension at 72 °C for 20 s. The relative expression levels of all target genes were quantified based on ubiquntin (the internal reference gene) according to the ΔΔCT method (Livak and Schmittgen, 2001).
2.6. Antioxidant activity measurement
In this work, DPPH radical-scavenging assay and β-carotene/linoleic acid bleaching test were respectively used to measure anti-radical activities and anti-lipid peroxidation capacities of the root extracts, which aimed to provide the basis for the possible application in pharmaceutical and nutraceutical fields. The detailed experimental procedures of both methods were performed according to the colorimetrical methods reported previously (Yao et al., 2013). Two synthetic antioxidants, i.e. ascorbic acid (VC) and buthylated hydroxytoluene (BHT), were adopted as the positive controls. For analysis homogeneity, the absorbance of reaction solution of each sample either in DPPH radical-scavenging assay or β-carotene/linoleic acid bleaching test was determined by removing the background interference. And, DPPH radical scavenging activity or β-carotene bleaching inhibition ratio of each sample was calculated as percentage data (%) by transforming the absorbance data according to the corresponding equations described previously by Yao et al. (2013). Based on the simulation of experimental data by the logarithmic regression model, the curves of DPPH radical scavenging activity (%) or β-carotene bleaching inhibition ratio (%) versus the extract concentration (mg/mL) were illustrated. Antioxidant activity of the tested sample was expressed as IC50 value that represented the extract concentration necessary to cause 50% DPPH radical scavenging or 50% β-carotene bleaching inhibition. The lower IC50 value indicated the higher antioxidant activity.
2.7. Statistical analysis
All experiments were conducted in triplicate, and results were given as averages ± standard deviations. All statistical analyses were carried out using the SPSS statistical software 17.0 (SPSS Inc, Chicago, USA). One-way analysis of variance with Tukey’s test was used to determine significant differences between multiple groups of data at P < 0.05.
3. Results and discussion
3.1. Effect of chitosan concentration and exposure time on flavonoid yield in ITHRCs
Flavonoids, the second largest class of plant secondary metabolites, possess multiple physiological functions as diverse as UV protection, pathogen defense, insect attraction, rhizobium symbiosis, flower coloration, and auxin transport (Zhao and Dixon, 2010). Moreover, a popular concept assumes that flavonoids are phytoalexins that can be inducibly synthesized by plants in response to pathogen and insect challenges (Ahuja et al., 2012). Chitosan is known to trigger a variety of defense reactions in plants via the simulation of fungal pathogen attack, which can eventually lead to the enhanced accumulation of phytoalexins (Hadwiger, 2013). Accordingly, chitosan elicitation is likely to promote flavonoid biosynthesis in ITHRCs by inducing plant defense responses.
Elicitation for the improvement of desired secondary metabolites in a given plant in vitro cultures is very empirical. It is well documented that elicitor concentration and exposure time are of crucial importance for the success and efficiency of elicitation (Narayani and Srivastava, 2017). To achieve the maximal enhancement of flavonoids in ITHRCs, chitosan elicitation requires the careful optimization to find its appropriate concentration and exposure time. As reported previously, ITHRCs cultured at day 24 was capable of producing the highest productivity of flavonoids and biomasses (Gai et al., 2015). In an attempt to further enhance flavonoid level without affecting biomass yield, chitosan was supplemented in 24 day-old ITHRCs to evaluate its elicitation effect on flavonoid accumulation.
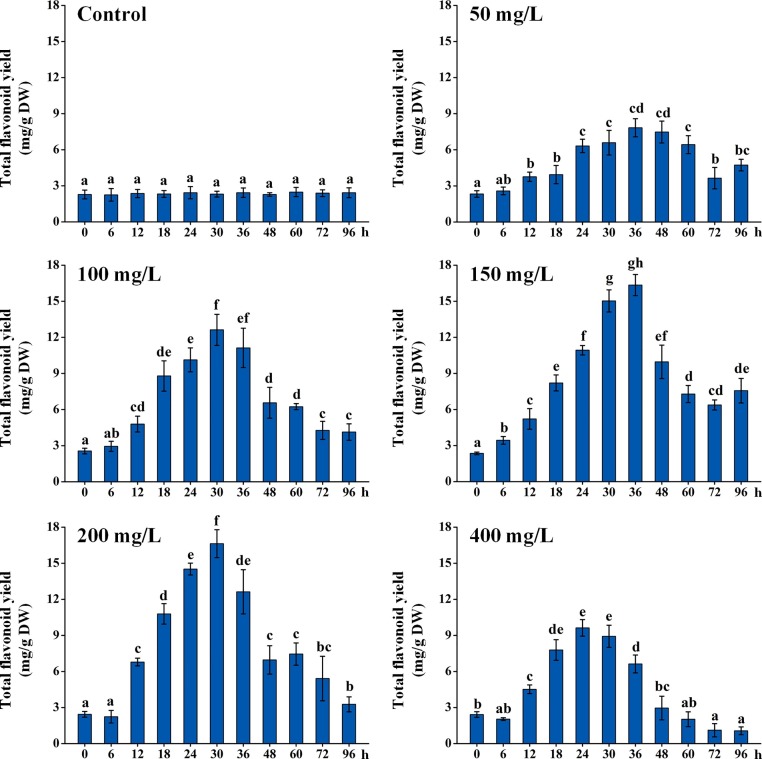
As shown in Fig. 1 , different chitosan concentrations (50, 100, 150, 200, and 400 mg/L) exhibited distinct influences on total flavonoid yields in ITHRCs along a time course from 0 to 96 h. As expected, chitosan elicitation was indeed feasible for promoting flavonoid accumulation in ITHRCs over control. Regardless of concentrations, chitosan could induce the significant enhancement of flavonoids in ITHRCs after 6 and 12 h. This phenomenon was consistent with the rapid stimulation effect of elicitors subjected to plant in vitro cultures, which can alter the secondary metabolite profile within a short time (Narayani and Srivastava, 2017). Taken as a whole, chitosan with moderate concentrations of 150 and 200 mg/L showed better elicitation efficiency at the time points of 36 and 30 h, where total flavonoid yields (16.35 ± 0.88 and 16.67 ± 1.16 mg/g DW) increased by 7.08- and 7.22-fold as compared with control (2.31 ± 0.29 mg/g DW), respectively. However, there is no significant difference (P > 0.05) in the flavonoid productivity between the above two chitosan elicitation treatments. In consideration of elicitor cost, chitosan concentration 150 mg/L along with exposure time 36 h were adopted as the appropriate elicitation conditions. Moreover, it is noted that the high chitosan concentration (400 mg/L) with the long exposure time (72–96 h) was not favorable for flavonoid enhancement in ITHRCs.
Fig. 1.
Effect of different concentrations of chitosan (50, 100, 150, 200, and 400 mg/L) with successive exposure time (0, 6, 12, 18, 24, 30, 36, 48, 60, 72, and 96 h) on total flavonoid yield in 24 day-old ITHRCs. Mean ± SD values not sharing the same lowercase letters are significantly different (P < 0.05).
Similar to this investigation, many studies reported that chitosan elicitation could exert positive eff ;ect on enhancing the production of various secondary metabolites in intact plants or plant in vitro cultures, e.g. artemisinin in Artemisia annua L. (Lei et al., 2011), curcumin in Curcuma longa L. (Sathiyabama et al., 2016), echinacoside in cell suspension cultures of Scrophularia striata Boiss. (Kamalipourazad et al., 2016), hydrolysable tannin in cell suspension cultures of Phyllanthus debilis Klein ex Willd. (Malayaman et al., 2017), withanolides in adventitious root cultures of Withania somnifera (L.) Dunal (Sivanandhan et al., 2012), and plumbagin in adventitious root cultures of Plumbago indica L. (Jaisi and Panichayupakaranant, 2017). However, the chitosan elicitation conditions required for achieving the optimal enhancement of different target secondary metabolites are not the same in these reports and this work. Factually, the same elicitor possesses distinct abilities to induce the biosynthesis of different secondary metabolites, and there is complexity in the mechanism of action entailing variability of secondary metabolic responses to the stress mediated by elicitor due to thousands of intertwined events existing in plant cells (Giri and Zaheer, 2016; Narayani and Srivastava, 2017).
Generally, plants exposed to mild elicitation conditions can induce the eustress that contributes to the activation of plant defense secondary metabolisms for self-protection, and when the exposure exceeds the tolerance-limit level, plants will suffer from the distress that is characterized by metabolic damage or cell death, thus leading to the reduced productivity of secondary metabolites (Kranner et al., 2010). Moreover, plants have thresholds for specific secondary metabolites that can be synthesized by themselves. Once secondary metabolites are accumulated at high levels, plants will be stimulated to generate enzymes for product degradation through the feedback regulation (Malik et al., 2013). As inferred, the appropriate chitosan elicitation (150 mg/L and 36 h) might maintain the balance between eustress and distress, which is beneficial for inducing the biosynthesis and accumulation of flavonoids in the elicited ITHRCs.
3.2. Profiles of eight flavonoids in ITHRCs under the appropriate chitosan elicitation
Changes in profiles of all target compounds in ITHRCs with and without the appropriate chitosan elicitation can be appreciated by the comparison of LC–MS/MS chromatograms (Fig. 2 ). After calculation, yields of all target flavonoid derivatives are shown in Fig. 3 . In comparison with control, levels of rutin (812.28 ± 13.49 μg/g DW), neohesperidin (199.46 ± 6.96 μg/g DW), buddleoside (17.55 ± 1.14 μg/g DW), liquiritigenin (24.30 ± 1.28 μg/g DW), quercetin (733.02 ± 23.72 μg/g DW), isorhamnetin (618.49 ± 13.57 μg/g DW), kaempferol (285.09 ± 5.04 μg/g DW), and isoliquiritigenin (25.28 ± 3.92 μg/g DW) in chitosan-elicited ITHRCs (150 mg/L and 36 h) increased by 8.27-, 4.19-, 1.30-, 4.88-, 13.05-, 7.63-, 2.12-, and 8.52-fold, respectively. Interestingly, increments of the more hydroxylated flavonoids (rutin, quercetin, isorhamnetin, and isoliquiritigenin) were noticed to be much higher than other flavonoids.
Fig. 2.
Representative LC–MS/MS total ion chromatograms of eight flavonoids in extracts form control and ITHRCs elicited by 150 mg/L chitosan for 36 h. RUT, rutin; NEO, neohesperidin; BUD, buddleoside; LIQ, liquiritigenin; QUE, quercetin; ISR, isorhamnetin; KAE, kaempferol; ISL, isoliquiritigenin.
Fig. 3.
Yields of eight flavonoids in extracts form control and ITHRCs elicited by 150 mg/L chitosan for 36 h. Mean ± SD values not sharing the same lowercase letters are significantly different (P < 0.05). RUT, rutin; NEO, neohesperidin; BUD, buddleoside; LIQ, liquiritigenin; QUE, quercetin; ISR, isorhamnetin; KAE, kaempferol; ISL, isoliquiritigenin.
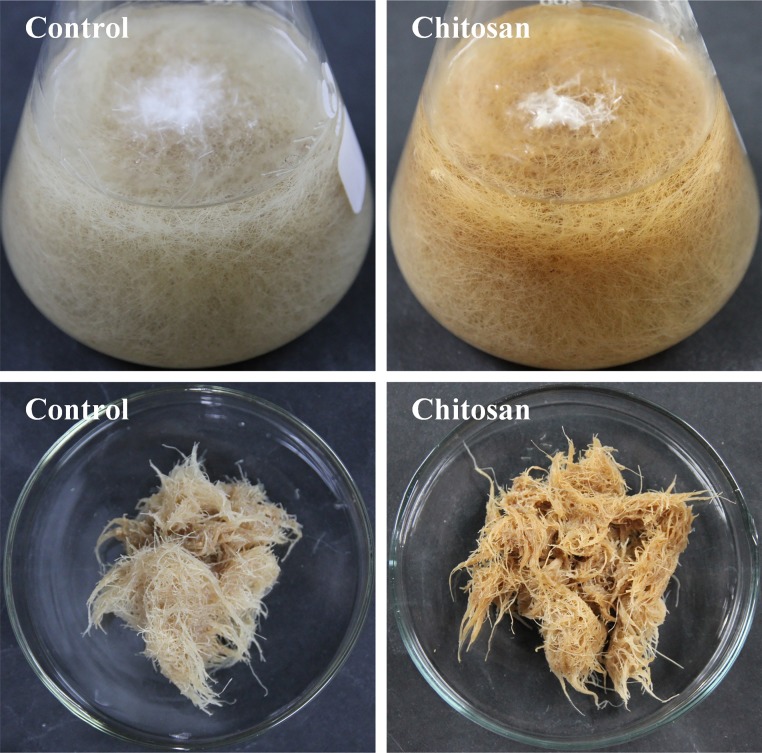
Generally, oxidative burst is a common response of plants to elicitor treatments, which is characterize by the excessive generation of reactive oxygen species (ROS) that can cause oxidative damages to various functional macromolecules (nucleic acids, lipids, proteins, carbohydrates, etc.) (Ramirez-Estrada et al., 2016). It is reported that chitosan elicitation can induce ROS overproduction, perturb the intracellular redox equilibrium and ultimately result in oxidative stress in S. striata cell cultures (Kamalipourazad et al., 2016). In this work, chitosan-elicited root tissues exhibited browning color as compared with control (Fig. 4 ), providing a significant indication of oxidative stress following chitosan elicitation. It is well known that the phenolic hydroxyl-substituted flavonoids possess reduction potentials that can effectively neutralize active oxygen radicals (Agati et al., 2012). In view of this, the significant enhancements of rutin, quercetin, isorhamnetin, and isoliquiritigenin in chitosan-elicited ITHRCs might be ascribed to their multiple hydroxyl groups with efficient ROS scavenging ability.
Fig. 4.
Phenotype comparison of hairy root tissues from control and ITHRCs elicited by 150 mg/L chitosan for 36 h.
3.3. Biosynthesis gene expression in ITHRCs underlying chitosan elicitation
As reported previously, chitosan can be recognized by immune receptors localized on plant cell membranes as the attack of fungal pathogens, trigger diverse signaling cascades, and lead to a variety of defense responses in which the activation of genes associated with phytoalexin biosynthesis is flagged as a characteristic even (Bueter et al., 2013; Hadwiger, 2013). Accordingly, the enhanced accumulation of flavonoids in chitosan-elicited ITHRCs is likely to be attributed to the transcriptional activation of genes involved in flavonoid biosynthesis pathway.
For the accurate determination of gene expression pattern, samples collected from ITHRCs elicited by 150 mg/L chitosan and control at a successive time points (6, 12, 24, 36, 48, 60, 72, and 96 h) were taken for qRT-PCR analysis in this work. The relative expression level of each tested gene was normalized using ubiquntin (the internal reference gene), and the expression value of individual gene in control was set as 1.
As shown in Fig. 5 , different degrees of increase in the expression levels of all investigated genes were noticed in chitosan-elicited ITHRCs along a time course from 6 to 48 h, which indicated that the enhanced flavonoid yield was a result of the up-regulated expression of these biosynthetic genes. Additionally, all targeted genes responded sensitively to chitosan elicitation, and the expression levels were found to be highest at 12 or 24 h, which were earlier than the time point (36 h) necessary for the maximal enhancement of flavonoids. Factually, this was a typical metabolic phenomenon that the synthesis of downstream products lags behind the expression of upstream genes (Expósito et al., 2009). Moreover, most of genes exhibited a significant decline trend in the transcription levels after 60 h, among which C4H, 4CL, and F3′H were observed to revert to the control level gradually. However, the expression levels of PAL and CHI genes were significantly repressed from 72 to 96 h, which proved that metabolic damages occurred after the prolonged elicitation duration. Taken as a whole, CHS and F3’H genes showed much higher transcriptional abundances (45.13- and 41.04-fold) at 12 h than other investigated genes, which suggested that the both genes were more sensitive to chitosan elicitation for inducing flavonoid biosynthesis in ITHRCs.
Fig. 5.
Transcriptional profiles of flavonoid biosynthesis genes in ITHRCs elicited by 150 mg/L chitosan for different durations (6, 12, 24, 36, 48, 60, 72, and 96 h). PAL, phenylalanine ammonia lyase; C4H, cinnamate-4-hydroxylase; 4CL, 4-coumarate coenzyme A ligase; CHS, chalcone synthase; CHI, chalcone isomerase; F3′H, flavonoid 3′-hydroxylase; FS, flavonol synthase. Mean ± SD values not sharing the same lowercase letters are significantly different (P < 0.05).
It is well acknowledged that CHS is the gatekeeper in flavonoid biosynthesis pathway that can catalyze the sequential addition of three units of malonyl-coenzyme A (CoA) to one unit of p-coumaroyl-CoA, yielding naringenin chalcone as the basic C6-C3-C6 skeleton for the subsequent flavonoid metabolism (Han et al., 2014). Generally, the regulation of flavonoid biosynthesis can be effectively achieved via controlling CHS gene expression. Schijlen et al. (2007) found that the RNA interference silencing of CHS gene in tomatoes could cause the reduction of total flavonoid levels to a few percent of those in wild-type ones. Rahnama et al. (2013) reported that the overexpression of CHS gene could result in the transgenic Silybum marianum hairy roots containing a 10-fold increase in silybin (an active flavonolignan) content. Moreover, CHS gene expression can be induced by environmental stresses such as fungal pathogen attack, wounding, or UV irradiation, which is capable of triggering the accumulation of flavonoid phytoalexins to improve plant resistance (Dao et al., 2011). Factually, chitosan is the derivative of chitin (fungal cell wall component) can induce plant systemic resistance by mimicking fungal pathogen (Zhang et al., 2015). In this study, the significant sensitivity of CHS gene expression to chitosan elicitation possibly contributed to enhance ITHRCs resistance by heightening flavonoid accumulation. Additionally, F3′H gene plays an essential role in the regulation of rutin and quercetin biosynthesis because its encoding enzyme can catalyze the hydroxylation of flavonoid B-ring at the 3′ position (Liu et al., 2014). Accordingly, the remarkable enhancement in the yields of rutin (8.27-fold increase) and quercetin (13.05-fold increase) might be attributed to the significant induction of F3'H transcription underlying chitosan elicitation.
3.4. Antioxidant activities of extracts from ITHRCs following chitosan elicitation
Plant-derived antioxidants have been considered as effective ROS scavengers in preventing human chronic diseases (Shahidi and Ambigaipalan, 2015). Moreover, there is a growing evidence that ROS plays a vital role in viral infections and contributes to pathogenesis for many viruses, such as respiratory syncytial virus (RSV), H5N1 influenza A virus, coxsackievirus B3, and Japanese encephalitis virus (Panchal et al., 2012). Treatment with natural antioxidants, therefore, is beneficial for the prevention of viral infections. In view of this, it is inferred that the notable antiviral efficacy of I. tinctoria root extracts is likely related to the antioxidant activities.
It is clearly observed from Fig. 6 that chitosan-elicited samples exhibited superiority in antioxidant activities determined by the both methods. To be exact, extracts from chitosan-elicited ITHRCs showed better DPPH radical scavenging property and β-carotene bleaching inhibition capacity with lower IC50 values (0.23 and 0.28 mg/mL) as against those in control (0.42 and 0.39 mg/mL), respectively. As mentioned above, the increased contents of multiple hydroxyl-substituted flavonoids (rutin, quercetin, isorhamnetin, and isoliquiritigenin) in chitosan-elicited ITHRCs is likely to be responsible for scavenging the excess ROS induced by chitosan elicitation. Anyway, the objective existence of these polyhydroxylated flavonoids might contribute to the higher antioxidant activities of extracts from chitosan-elicited ITHRCs. Additionally, stress conditions can cause plant cells to overproduce other antioxidants, such as, glutathione, proline, ascorbic acid, tocopherol, and carotenoid (Gill and Tuteja, 2010), which might also contribute to the increase in antioxidant activities of extracts from chitosan-elicited ITHRCs. Overall, chitosan-elicited ITHRCs could offer higher antioxidant extracts that would potentially improve the antiviral therapeutic benefits, and possess promising prospects for the development of antioxidant drugs or nutraceuticals.
Fig. 6.
Antioxidant activities of extracts from control and ITHRCs elicited by 150 mg/L chitosan for 36 h in DPPH radical scavenging assay (A) and β-carotene/linoleic acid bleaching test (B).
4. Conclusions
This work provided a feasible and cost-effective elicitation practice using the nontoxic and biocompatibility chitosan for improving the productive of pharmacologically active flavonoids in ITHRCs. Results showed that 24 day-old ITHRCs elicited by 150 mg/L chitosan for 36 h gave a 7.08-fold increase in total flavonoid yield as compared with control. The price range of chitosan is $1–2/Kg in China. Accordingly, the cost range of chitosan used in this work for the aforementioned enhancement in flavonoid productivity per 100 L of ITHRCs is only $0.015–0.03. Especially, the significant increase in the contents of polyhydroxylated flavonoids was found in ITHRCs following chitosan elicitation. Moreover, the transcriptional activation of associated genes involved in flavonoid biosynthetic pathway, especially CHS and F3’H genes, might account for the elevated level of flavonoids in chitosan-elicited ITHRCs. Furthermore, antioxidant activity enhancement was noticed in the extracts from chitosan-elicited ITHRCs, which would expand the applicability in pharmaceutical and nutraceutical fields. Overall, the chitosan elicitation protocol proposed in this work will make ITHRCs more feasible for the large-scale production of pharmacologically active flavonoids on commercial scale in the future.
Acknowledgements
The authors gratefully acknowledge the financial supports by National Key R&D Program of China (2017YFD0600205), Fundamental Research Funds for the Central Universities (2572017DA04), Heilongjiang Province Science Foundation for Youths (QC2017012), Scientific Research Start-up Funds for Talents Introduction of Northeast Forestry University (1020160010 and YQ2017-03), and Double First-rate Special Funds (41112432 and 41112460).
Contributor Information
Jiao Jiao, Email: jj_nefu@163.com.
Yu-Jie Fu, Email: yujie_fu@163.com.
References
- Agati G., Azzarello E., Pollastri S., Tattini M. Flavonoids as antioxidants in plants: location and functional significance. Plant Sci. 2012;196:67–76. doi: 10.1016/j.plantsci.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Ahuja I., Kissen R., Bones A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012;17:73–90. doi: 10.1016/j.tplants.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Bueter C.L., Specht C.A., Levitz S.M. Innate sensing of chitin and chitosan. PLoS Pathog. 2013;9:e1003080. doi: 10.1371/journal.ppat.1003080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wang S., Huang X., Hong J., Du L., Zhang L., Ye L. Environmental factors affecting growth and development of Banlangen (Radix Isatidis) in China. Afr. J. Plant Sci. 2015;9:421–426. [Google Scholar]
- Dao T.T.H., Linthorst H.J.M., Verpoorte R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev. 2011;10:397–412. doi: 10.1007/s11101-011-9211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K.M., Deroles S.C. Prospects for the use of plant cell cultures in food biotechnology. Curr. Opin. Biotechnol. 2014;26:133–140. doi: 10.1016/j.copbio.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Dias M.I., Sousa M.J., Alves R.C., Ferreira I.C. Exploring plant tissue culture to improve the production of phenolic compounds: a review. Ind. Crops Prod. 2016;82:9–22. [Google Scholar]
- Expósito O., Bonfill M., Onrubia M., Jané A., Moyano E., Cusidó R.M., Palazón J., Piñol M.T. Effect of taxol feeding on taxol and related taxane production in Taxus baccata suspension cultures. New Biotechnol. 2009;25:252–259. doi: 10.1016/j.nbt.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Gai Q.Y., Jiao J., Luo M., Wei Z.F., Zu Y.G., Ma W., Fu Y.J. Establishment of hairy root cultures by Agrobacterium rhizogenes mediated transformation of Isatis tinctoria L. for the efficient production of flavonoids and evaluation of antioxidant activities. PLoS One. 2015;10:e0119022. doi: 10.1371/journal.pone.0119022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S.S., Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Giri C.C., Zaheer M. Chemical elicitors versus secondary metabolite production in vitro using plant cell, tissue and organ cultures: recent trends and a sky eye view appraisal. Plant Cell Tissue Organ Cult. 2016;126:1–18. [Google Scholar]
- Hadwiger L.A. Multiple effects of chitosan on plant systems: solid science or hype. Plant Sci. 2013;208:42–49. doi: 10.1016/j.plantsci.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Han Y., Zhao W., Wang Z., Zhu J., Liu Q. Molecular evolution and sequence divergence of plant chalcone synthase and chalcone synthase-Like genes. Genetica. 2014;142:215–225. doi: 10.1007/s10709-014-9768-3. [DOI] [PubMed] [Google Scholar]
- Isah T., Umar S., Mujib A., Sharma M.P., Rajasekharan P.E., Zafar N., Frukh A. Secondary metabolism of pharmaceuticals in the plant in vitro cultures: strategies, approaches, and limitations to achieving higher yield. Plant Cell Tissue Organ Cult. 2018;132:239–265. [Google Scholar]
- Jaisi A., Panichayupakaranant P. Chitosan elicitation and sequential Diaion® HP-20 addition a powerful approach for enhanced plumbagin production in Plumbago indica root cultures. Process Biochem. 2017;53:210–215. [Google Scholar]
- Jiao J., Gai Q.Y., Wang W., Zang Y.P., Niu L.L., Fu Y.J., Wang X. Remarkable enhancement of flavonoid production in a co-cultivation system of Isatis tinctoria L. hairy root cultures and immobilized Aspergillus niger. Ind. Crops Prod. 2018;112:252–261. doi: 10.1016/j.indcrop.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamalipourazad M., Sharifi M., Maivan H.Z., Behmanesh M., Chashmi N.A. Induction of aromatic amino acids and phenylpropanoid compounds in Scrophularia striata Boiss. cell culture in response to chitosan-induced oxidative stress. Plant Physiol. Biochem. 2016;107:374–384. doi: 10.1016/j.plaphy.2016.06.034. [DOI] [PubMed] [Google Scholar]
- Katiyar D., Hemantaranjan A., Singh B. Chitosan as a promising natural compound to enhance potential physiological responses in plant: a review. Indian J. Plant Physiol. 2015;20:1–9. [Google Scholar]
- Kranner I., Minibayeva F.V., Beckett R.P., Seal C.E. What is stress? Concepts, definitions and applications in seed science. New Phytol. 2010;188:655–673. doi: 10.1111/j.1469-8137.2010.03461.x. [DOI] [PubMed] [Google Scholar]
- Lei C., Ma D., Pu G., Qiu X., Du Z., Wang H., Li G., Ye H., Liu B. Foliar application of chitosan activates artemisinin biosynthesis in Artemisia annua L. Ind. Crops Prod. 2011;33:176–182. [Google Scholar]
- Liu S., Ju J., Xia G. Identification of the flavonoid 3′-hydroxylase and flavonoid 3′, 5′-hydroxylase genes from Antarctic moss and their regulation during abiotic stress. Gene. 2014;543:145–152. doi: 10.1016/j.gene.2014.03.026. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- López-Alarcón C., Denicola A. Evaluating the antioxidant capacity of natural products: a review on chemical and cellular-based assays. Anal. Chim. Acta. 2013;763:1–10. doi: 10.1016/j.aca.2012.11.051. [DOI] [PubMed] [Google Scholar]
- Malayaman V., Sisubalan N., Senthilkumar R.P., Ranjithkumar R., Basha M.J. Chitosan mediated enhancement of hydrolysable tannin in Phyllanthus debilis Klein ex Willd via plant cell suspension culture. Int. J. Biol. Macromol. 2017;104:1656–1663. doi: 10.1016/j.ijbiomac.2017.03.138. [DOI] [PubMed] [Google Scholar]
- Malik S., Hossein Mirjalili M., Fett-Neto A.G., Mazzafera P., Bonfill M. Living between two worlds: two-phase culture systems for producing plant secondary metabolites. Crit. Rev. Biotechnol. 2013;33:1–22. doi: 10.3109/07388551.2012.659173. [DOI] [PubMed] [Google Scholar]
- Murthy H.N., Lee E.J., Paek K.Y. Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult. 2014;118:1–16. [Google Scholar]
- Narayani M., Srivastava S. Elicitation: a stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem. Rev. 2017;16:1227–1252. [Google Scholar]
- Nartop P. Engineering of biomass accumulation and secondary metabolite production in plant cell and tissue cultures. In: Ahmad P., Ahanger M.A., Singh V.P., Tripathi D.K., Alam P., Alyemeni M.N., editors. Plant Metabolites and Regulation Under Environmental Stress. Elsevier Inc.; London: 2018. pp. 169–194. [Google Scholar]
- Nguyen T., Jamali A., Grand E., Morreel K., Marcelo P., Gontier E., Dauwe R. Phenylpropanoid profiling reveals a class of hydroxycinnamoyl glucaric acid conjugates in Isatis tinctoria leaves. Phytochemistry. 2017;144:127–140. doi: 10.1016/j.phytochem.2017.09.007. [DOI] [PubMed] [Google Scholar]
- Ni Y., Song R., Kokot S. Discrimination of Radix Isatidis and Rhizoma et Radix Baphicacanthis Cusiasamples by near infrared spectroscopy with the aid of chemometrics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012;96:252–258. doi: 10.1016/j.saa.2012.05.031. [DOI] [PubMed] [Google Scholar]
- Panchal R.G., Tran J.P., Bergeron A.A., Wells J., Kota K.P., Aman J., Bavari S. Identification of an antioxidant small-molecule with broad-spectrum antiviral activity. Antivir. Res. 2012;93:23–29. doi: 10.1016/j.antiviral.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Rahnama H., Razi Z., Dadgar M.N., Hasanloo T. Enhanced production of flavonolignans in hairy root cultures of Silybum marianum by over-expression of chalcone synthase gene. J. Plant Biochem. Biotechnol. 2013;22:138–143. [Google Scholar]
- Ramirez-Estrada K., Vidal-Limon H., Hidalgo D., Moyano E., Golenioswki M., Cusidó R.M., Palazon J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules. 2016;21:182. doi: 10.3390/molecules21020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathiyabama M., Bernstein N., Anusuya S. Chitosan elicitation for increased curcumin production and stimulation of defence response in turmeric (Curcuma longa L.) Ind. Crops Prod. 2016;89:87–94. [Google Scholar]
- Schijlen E.G., de Vos C.R., Martens S., Jonker H.H., Rosin F.M., Molthoff J.W., Tikunov Y.M., Angenent G.C., van Tunen A.J., Bovy A.G. RNA interference silencing of chalcone synthase, the first step in the flavonoid biosynthesis pathway, leads to parthenocarpic tomato fruits. Plant Physiol. 2007;144:1520–1530. doi: 10.1104/pp.107.100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi F., Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects–a review. J. Funct. Foods. 2015;18:820–897. [Google Scholar]
- Singla A.K., Chawla M. Chitosan: some pharmaceutical and biological aspects-an update. J. Pharm. Pharmacol. 2001;53:1047–1067. doi: 10.1211/0022357011776441. [DOI] [PubMed] [Google Scholar]
- Sivanandhan G., Arun M., Mayavan S., Rajesh M., Mariashibu T.S., Manickavasagam M., Selvaraj N., Ganapathi A. Chitosan enhances withanolides production in adventitious root cultures of Withania somnifera (L.) Dunal. Ind. Crops Prod. 2012;37:124–129. [Google Scholar]
- Xiao Y., Ji Q., Gao S., Tan H., Chen R., Li Q., Chen J., Yang Y., Zhang L., Wang Z., Chen W., Hu Z. Combined transcriptome and metabolite profiling reveals that Ii PLR1 plays an important role in lariciresinol accumulation in Isatis indigotica. J. Exp. Bot. 2015;66:6259–6271. doi: 10.1093/jxb/erv333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X.H., Zhang D.Y., Zu Y.G., Fu Y.J., Luo M., Gu C.B., Li C.Y., Mu F.S., Efferth T. Free radical scavenging capability, antioxidant activity and chemical constituents of Pyrola incarnata Fisch. leaves. Ind. Crops Prod. 2013;49:247–255. [Google Scholar]
- Yue W., Ming Q.L., Lin B., Rahman K., Zheng C.J., Han T., Qin L.P. Medicinal plant cell suspension cultures: pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit. Rev. Biotechnol. 2016;36:215–232. doi: 10.3109/07388551.2014.923986. [DOI] [PubMed] [Google Scholar]
- Zhang D., Wang H., Hu Y., Liu Y. Chitosan controls postharvest decay on cherry tomato fruit possibly via the mitogen-activated protein kinase signaling pathway. J. Agric. Food Chem. 2015;63:7399–7404. doi: 10.1021/acs.jafc.5b01566. [DOI] [PubMed] [Google Scholar]
- Zhao J., Dixon R.A. The ‘ins’ and ‘outs’ of flavonoid transport. Trends Plant Sci. 2010;15:72–80. doi: 10.1016/j.tplants.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Zhou W., Zhang X.Y. Research progress of Chinese herbal medicine Radix isatidis (banlangen) Am. J. Chin. Med. 2013;41:743–764. doi: 10.1142/S0192415X1350050X. [DOI] [PubMed] [Google Scholar]