Abstract
A novel approach involving the preparation of mannose-bearing chitosan microspheres with entrapping complexes of HBV DNA and PEI was developed to improve the delivery of DNA into antigen-presenting cells (APCs) after intramuscular (i.m.) injection. Compared with the traditional chitosan microspheres, the microspheres could quickly release intact and penetrative PEI/DNA complexes. What's more, chitosan was modified with mannose to target the primary APCs such as dendritic cells (DCs) owing to the high density of mannose receptors expressing on the surface of immature DCs. After i.m. immunization, the microspheres induced significantly enhanced serum antibody and cytotoxic T lymphocyte (CTL) responses in comparison to naked DNA.
Abbreviation: APCs, antigen-presenting cells; i.m., intramuscular; DCs, dendritic cells; CTL, cytotoxic T lymphocyte; HBV, hepatitis B virus; MΦs, macrophages; PEI, polyethylenimine; DS, degree of substitution; DLS, dynamic light scattering; TEM, transmission electron microscopy; AFM, atomic force microscopy; TA, anterior tibialis; LDH, lactate dehydrogenase.
Keywords: Mannose-bearing Chitosan microspheres, DNA vaccine, Target, PEI/DNA complexes, Immunogenicity
1. Introduction
The hepatitis B virus (HBV) is a noncytopathic DNA virus that causes both acute and chronic liver disease. Despite the availability of an effective vaccine for more than two decades, hepatitis B remains one of the 10 most common causes of death worldwide. Recovery from acute hepatitis B is the result of a combination of cellular and humoral immune responses. After recovery, HBV-specific T cells persist in the blood for decades [1]. In contrast, in chronic HBV infection, HBV-specific cellular immune responses are typically weak, narrowly focused, and rarely detectable in the peripheral blood [2], [3], [4], [5]. It has been suggested that therapeutic induction of HBV-specific cellular immune responses may lead to recovery from chronic hepatitis B [3]. However, currently traditional immuno-therapeutic approaches do not induce HBV-specific cellular immunity. Data demonstrating that cellular antiviral immunity can be induced following genetic immunization with plasmid DNA encoding HBV antigens have inspired novel approaches for vaccine construction [3], [4], [5]. The use of DNA vaccines in preclinical studies has been well established, with reports of both antibody and CTL responses in multiple independent studies [6]. Nevertheless, DNA vaccines have been met with limited success in both nonhuman primate models and human volunteers and often require multiple high dose immunizations [7]. For reasons such as potency, production, cost, and safety, there is a clear need to induce effective immunity in humans with lower and fewer doses of DNA, as well as to increase the magnitude of the immune response obtained.
There are a number of strategies available that have the potential to improve the potency of DNA vaccines. Several groups have attempted to improve the immunogenicity of DNA vaccines using cationic microparticles [8], chitosan [9], non-ionic block co-polymers [10] and cationic liposomes [11], [12] as gene vectors. Although such approaches have had some success, they are far from ideal for enhancing cellular immunity because activation of T-cell responses requires antigen presentation by professional APCs. Targeting phagocytic APCs has resulted in more efficient immune activation than direct injection of equivalent amounts of free antigen [13]. For example, biodegradable microspheres are efficiently phagocytosed by macrophages (MΦs) and capable of inducing a cytotoxic T cell response in vivo [14], [15], [16]. These spheres in the range of 1–10 μm are too large to enter cells via endocytosis, and therefore passively “target” phagocytic cells such as DCs by size exclusion. Goh et al demonstrated that MHC-I restricted antigen presentation can be enhanced by using spheres that rapidly degrade in the lysosome leading to disruption of the lysosome. This leads to IL-6 secretion and a response approximately 40-fold higher than that achieved with DNA alone [17].
These studies suggest that effective T-cell immunity can be induced at much lower doses of plasmid DNA if delivery systems can target primary APCs and induce DNA release from lysosomes. Base on these ideas, we have developed a novel approach to prepare mannose-bearing chitosan biodegradable microspheres with entrapping PEI/DNA complexes in order to enhance delivery of DNA to APCs after i.m. injection. The use of microsphere systems based on biodegradable polymers such as chitosan can provide sustained pharmacological activity of DNA vaccines. Chitosan is a polycationic, biodegradable natural polymer with an extensive record of safe use for gene delivery systems [18], [19]. However, previous research described the entrapment of naked DNA inside the unmodified chitosan microspheres. There might be some disadvantages to these systems, such as the following: (i) the low in vivo and in vitro expression levels suggest that chitosan is unable to mediate lysosomal escape possibly due to limited buffering capacity. (ii) Being insoluble in aqueous media due to its crystalline structure, chitosan forms a very viscous solution even at low concentrations, and is only soluble in water containing acetic acid which may induce cytotoxicity [20], [21]. (iii) Once entrapped in microspheres, the rate of release of DNA is slow, limiting the amount of DNA available for transfection of target cells and induction of immune responses. The limited life span of APCs requires the release of the encapsulated material in a reasonable time frame.
In our system, we have designed novel chitosan microspheres with entrapped PEI/DNA complexes to specially address these concerns. PEI was introduced to condense negatively charged DNA into nanomeric particles and imparted the microsphere system with strong buffer capacity over a broad pH range. Covalent attachment of mannose to the primary amine of chitosan improved hydrophilicity and biodegradation by the mannonyl group. This increased the hydrogen bonding between the mannonyl group and solvent and disturbed the hydrogen bonding between amino groups and N-acetyl groups of chitosan. Furthermore, microspheres attached with mannose can target the DCs and be internalized via recognition by the mannose receptor on the DC surface. After preparation and characterization, the microspheres were administered to experimental animals and immune responses were characterized.
2. Materials and methods
2.1. Materials
The 25 kDa PEI, NaBH(CN)3, Mannobiose, Chitosan (MW 102 kDa; 85% deacetylated) were purchased from Sigma-Aldrich. All reagents used were of the highest purity available. The HBV S/V1012 plasmid, pCMV Luc plasmid were constructed by our Lab. The plasmids were purified by using a Qiagen Plasmid Giga kit, and the final product was endotoxin free (< 2.5 units/mg).
2.2. Methods
2.2.1. Mannose-bearing chitosan synthesis and characterization
Mannose-bearing chitosan was obtained by reductive amination with mannobiose (Scheme 1 ) described in detail elsewhere [22]. Briefly, chitosan dissolved with stirring in 1% aqueous acetic acid (pH = 5.5). With vigorous stirring, a solution of the mannobiose (mol/GlcN = 1) and sodium cyanoborohydride were added to the resulting viscous solution. The reaction mixture was left stirring at room temperature for 36 h. The products were dialyzed for 4–6 days and lyophilized. The degree of substitution (DS) of mannonsyl units on the chitosan was obtained from elementary microanalysis. Water solubility of mannose-bearing chitosan was estimated using the transmittance of the chitosan derivatives solution [23]. The in vitro biodegradation of chitosan derivative was estimated by a decrease in the viscosity of a polymer solution in presence of lysozyme [23].
Scheme 1.
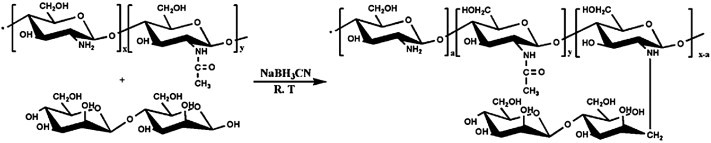
Synthetic route of mannose-bearing chitosan derivatives.
2.2.2. Mannose-bearing chitosan microsphere preparation and characterization
Mannose-bearing chitosan (m-chitosan) microspheres were prepared following the report of Aral et. al [24] with some modification. Briefly, PEI/DNA complexes were added to 50 mM sodium sulphate solution, and this mixture was quickly dropped into the mannose-bearing chitosan solution (0.2% w/v in 0.5% Tween 80 and 0.5% acetic acid) and vortexed for 15–30 s. Formed microspheres were washed with deionized water and separated by centrifugation at 8000 g then freeze-dried. The unmodified chitosan/PEI/DNA (u-chitosan) microspheres were prepared by a same method.
The size distribution of the microspheres was determined by dynamic light scattering (DLS) with a Zetasizer 3000 HS (Malvern Instruments, U.K.) and the value was calculated by volume measurement. The loading level of the DNA in the microspheres was determined by assaying both the supernatant after centrifugation and by hydrolyzing the microspheres and measuring DNA by absorbance at 260 nm. The encapsulation efficiency of microspheres was calculated. The Zeta potential of the microspheres was measured on the same apparatus as DLS. Selected batches of microspheres were evaluated by transmission electron microscopy (TEM) and atomic force microscopy (AFM) for size and shape. AFM images of the microspheres were taken with a Nanoscope IIIa AFM Multimode (Digital Instruments, Santa Barbara, CA) under ambient conditions. AFM was operated in the tapping mode with an optical readout using Si3 N4 cantilevers (Nanoprobes, Digital Instruments).
2.2.3. In vitro release study
In vitro release studies of DNA from m-chitosan microspheres were carried out by suspending 10 mg of microspheres in 1 ml of PBS at pH 7.4 (120 mM NaCl, 2.7 mM KCl, 10 mM PBS) at 37 °C under stirring. At predetermined time intervals, the suspension was centrifuged and replaced with the same volume of fresh medium. The DNA concentration in the supernatant was determined in triplicate by UV absorbance at 260 nm. Release profiles of u-chitosan microspheres were performed as a control according to the same operation.
2.2.4. In vitro transfection and cytotoxicity
For transfection in vitro, RAW264.7 cells (murine monocyte-macrophage cells) were cultured in DMEM medium containing Gln supplemented with 10% heated-inactivated FCS and antibiotics. Cells were grown at 37 °C in humidified air containing 5% CO2 and passaged every 2–3 days. The cells (1 × 105 per well) were plated on twelve-well tissue-culture plates 24 h before transfection. Immediately before the initiation of transfection experiments, the medium was removed from each well, and the cells were washed once with DMEM without serum and antibiotics and treated with a serial dilution of m-chitosan or u-chitosan microspheres. Transfection with m-chitosan/DNA or PEI/DNA complexes was performed as control. Luciferase gene expression was monitored 48 h later by using a commercial kit (Promega). Each transfection experiment was done in triplicate and is expressed as mean light units per mg of cell protein ± SD.
Simultaneously, in vitro cytotoxicity of PEI/DNA complexes, m-chitosan/DNA complexes, m-chitosan microspheres and u-chitosan microspheres were assessed by MTT assay (1 × 104 cells/well, 4 h incubation). As reference, PBS (negative, 100%) and 0.1% (w/w) Triton X-100 solution (positive, 100%) were applied.
2.2.5. Gene expression: in vivo
Three groups of female BALB/c mice (n = 6) were injected with either 50 μg of pCMVLuc DNA or u-chitosan, m-chitosan microspheres containing 50 μg of pCMVLuc DNA. Each group of mice was injected i.m. in the anterior tibialis (TA) muscle on either leg. The TA muscle from each mouse in three groups were harvested either at day 1, 7, or day 14 and stored in a − 80 °C freezer. The muscles were ground up with a mortar and pestle on dry ice. The powdered muscles were collected in Eppendorf tubes with 0.5 ml of 1 × reporter lysis buffer. After freeze/thawing three times, the samples were spun at 14,000 rpm for 10 min. The supernatants of the TA muscle of each mouse at each time point were pooled, and 20 μl of the samples was assayed by using the luciferase assay and normalized to the total volume content in the sample.
2.2.6. Mice
BALB/c mice were bred and cared in the animal facilities of the vaccine research center of Jilin University, Changchun. Only female mice 6 to 8 weeks old were chosen for the first vaccination.
2.2.7. Humoral immune response
Female BALB/c mice in groups of 8 were immunized with the m-chitosan microspheres, control DNA or PBS at different doses. The immunization protocol was performed at weeks 0, 2, 4 and 6. Sera samples were collected from all mice by tail bleeding at different times and HBV specific serum IgG titers were confirmed by ELISA.
2.2.8. Cellular immune response
2.2.8.1. CTL
Spleens from immunized mice were harvested 2 weeks after the fourth immunization and used as pools of five. Spleen cells were cultured with RMPI 1640 phenol red-free medium. Approximately 1 × 106 P815/BALB target cells were sensitized with synthetic epitope peptide at a concentration of 1 mM for 2 h at 37 °C. Cytotoxicity was measured by a standard LDH (lactate dehydrogenase) — release assay with a CTL kit (Promega).
2.2.8.2. IFN-γ production assays
The HBsAg-specific T cells in the spleen were also indirectly determined using cytokine ELISPOT by measuring HBsAg induced IFN-γ production, which was performed according to the instruction of the murine IFN-γ ELISPOT kit (BD).
2.2.9. Statistical analysis
Differences were compared for significance using post hoc t-test or one-way ANOVA. Data were analyzed with SPSS 15.0 for windows. “p” value less than 0.05 was considered statistically significant.
3. Results and discussion
3.1. Mannose-bearing chitosan synthesis and characterization
To improve water solubility and biodegradability, chitosan was modified with hydrophilic mannose, followed by reductive alkylation using sodium cyanoborohydride. Mannose was covalently attached to the primary amino groups of chitosan through Schiff base formation. The reactions of mannose and chitosan yielded products with high degrees of substitution (DS) as determined by microanalysis. The water solubility of u-chitosan and m-chitosan was evaluated in various pH solutions (Table 1 ). Chitosan and its derivatives, synthesized in this study, were soluble in an aqueous acidic solution below pH 6.5. This was caused by the protonation of primary amino groups, thus indicating that a significant amount of d-glucosamine residues still remained for the unique solubility of chitosan. In our case, it was evident that m-chitosan derivatives were soluble in a slightly broader range of pH than u-chitosan. What's more, the pH range was extended as the DS increased, indicating that the enhanced hydrophilicity was attributed to the mannonyl group (Data not shown). This was expected because, while the d-glucosamines were consumed, the random distribution of mannonyl groups is considered to inhibit the formation of the ordered structure and increase the hydrogen bonding between mannonyl group and solvent.
Table 1.
Chitosan and N-alkylation of chitosan with mannobiose
| Time (h) | dsa | Formula | Solubilityb |
|---|---|---|---|
| 0 | 0 | [(C8H13NO5)0.15(C6H13NO4)0.85] · 0.23H2O | 6.5 ± 0.5 |
| 36 | 0.3 | [(C8H13NO5)0.05(C6H11NO4)0.65(C18H33NO14)0.3] · 0.72H2O | 9.0 ± 0.2 |
Obtained from microanalysis.
The highest pH that can dissolve the polymer; the pH of the polymer solution in 0.1 N HCl was adjusted by adding stepwise 6 M NaOH, by which the solubility in aqueous solutions was determined at pH 1.0–12.0. The polymer was considered as an insoluble one when the transmittance of the polymer solution was lower than 50%, compared to that of a control solution (0.1 M HCl). All data were expressed as mean ± SD (n = 3).
Chitosan is considered a biodegradable polymer because of its susceptibility to various enzymes such as chitinase, chitosanase, lysozyme, β-glucosidase, and protease [25], [26]. Lysozyme has been widely used to estimate the in vitro degradation behavior of chitosan since it is known to be ubiquitous in the body and to produce results correlated with in vivo degradation [27], [28]. In this study, u-chitosan and m-chitosan were incubated with lysozyme-containing acetate buffer (pH = 5.0) at 37 °C. A decrease in the viscosity of the polymer solution, resulting from the enzymatic degradation, was investigated as a function of time (Fig. 1 ). No significant decrease in viscosity was observed in the u-chitosan solution within 90 min (< 10%), whereas the solution of m-chitosan exhibited the rapid decrease in the viscosity just after 10 min due to hydrolysis in the presence of lysozyme (p < 0.001). The preservation of the viscosity of the u-chitosan solution can be attributed to the fact that u-chitosan has a small amount of N-acetylglucosamine residues that are susceptible to lysozyme. It was of interest that the m-chitosan derivatives with a higher DS showed a higher degradation rate, indicating that the mannonyl moieties in the chitosan enhanced the susceptibility to lysozyme (Data not shown). Lee et al [29] also reported that the chitosans with bulky side groups could be degraded readily in the presence of lysozyme.
Fig. 1.
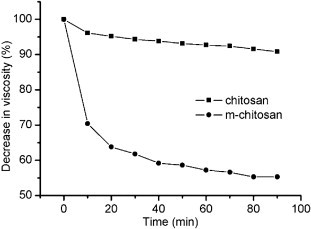
Enzymatic degradation of u-chitosan and m-chitosan. Each polymer solution (0.2 w/v% in 0.1 M acetate buffer) was exposed to lysozyme (18 μg/mL), and the viscosity of 1 ml aliquots was measured as a function of time (p < 0.001). All data were expressed as mean ± SD (n = 3).
3.2. M-chitosan microspheres preparation and characterization
The m-chitosan microspheres formed as a result of complex coacervation (Table 2 ). Sodium sulfate was included as a desolvating reagent to facilitate the phase separation, as with a greater affinity for water, it facilitates the removal of the associated water layer from around the dissolved colloidal chains. Compared with 19.44 ± 2.78 mV of u-chitosan microspheres and 36.64 ± 1.77 mV of PEI/DNA complexes, the zeta potential of m-chitosan microspheres at the physiological pH was approximately 10.19 ± 3.23 mV, which indicated that the mannonyl groups could shield the potential of chitosan and PEI. The low surface potential of the m-chitosan microspheres is fit for use in vivo due to the low reaction with components of the in vivo milieu. The m-chitosan microspheres obtained under these conditions showed ellipsoidal and polydisperse nature as revealed by TEM and AFM imaging (Fig. 2 ).
Table 2.
U-chitosan and m-chitosan microspheres encapsuled PEI/DNA within it
| Formulation | Mean size (nm)a | Encapsulation efficiency (%)b | Zeta potential (mV) |
|---|---|---|---|
| U-chitosan | 415.2 ± 44.2 | (94 ± 5.4)% | 19.44 ± 2.78 |
| M-chitosan | 326.3 ± 31.9 | (89 ± 2.6)% | 10.19 ± 3.23 |
| PEI/DNA (N/P = 6) | 86.6 ± 30.2 | 36.64 ± 1.77 |
All data were expressed as mean ± SD (n = 3).
Obtained from DLS.
The encapsulation efficiency was determined by assaying the supernatant after centrifugation and measuring DNA by absorbance at 260 nm.
Fig. 2.
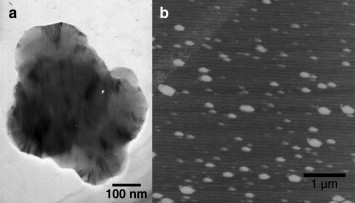
Appearance and size of m-chitosan microspheres. (a) Transmission electron micrograph of m-chitosan microspheres. Scale bar represents 100 nm. (b) Atomic force micrograph of m-chitosan microspheres. Scale bar represents 1 μm.
3.3. In vitro release study
The release of PEI/DNA complexes from the u-chitosan and m-chitosan microspheres showed no initial burst release. In the first seven days, 5.1% and 22.4% of the PEI/DNA complexes were released from u-chitosan and m-chitosan microspheres respectively. The biodegradability of chitosan and m-chitosan significantly affected the drug release. It was indicated that m-chitosan microspheres release faster than u-chitosan microspheres (p < 0.001) (Fig. 3 ). The faster release rate adapts to the limited life span of APCs.
Fig. 3.
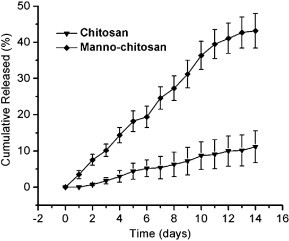
Release profiles of u-chitosan and m-chitosan microspheres. The microspheres (10 mg) were suspended in 1 ml of PBS at pH 7.4 (120 mM NaCl, 2.7 mM KCl, 10 mM PBS) at 37 °C under stirring. At predetermined time intervals, the suspension was centrifuged and replaced with the same volume of fresh medium. The DNA concentration in the supernatant was determined in triplicate by UV absorbance at 260 nm. All data were expressed as mean ± SD (n = 3) (p < 0.001).
3.4. In vitro transfection and cytotoxicity
The in vitro transfection efficiency of m-chitosan microspheres was evaluated in RAW264.7 cells using the pCMV luciferase plasmid (Fig. 4a). The expressed luciferase activity of m-chitosan was about one order of magnitude higher than u-chitosan microspheres (p < 0.05). The decreased transfection activity (p < 0.05) by the addition of superfluous mannose, demonstrated that m-chitosan microspheres might be internalized by endocytosis or phagocytosis via recognizing the mannose receptor on the surface of macrophages (Fig. 4a). What's more, the ternary complexes microspheres significantly enhanced the gene expression over m-chitosan/DNA complexes (p < 0.001), were only 150–300-fold less than that observed with PEI/DNA complexes. Simultaneously, the cell viability was assessed by MTT assay. No effect on cell viability for m-chitosan or u-chitosan microspheres was observed in RAW264.7 cells. However, PEI, which is most commonly used in gene delivery, was used for comparison and cell viability around 30–50% was observed at 5–25 μg (p < 0.05). The low cytotoxicity of m-chitosan microspheres, having superior transfection ability, may be suitable for both large dose and repeated administration of gene (Fig. 4b).
Fig. 4.
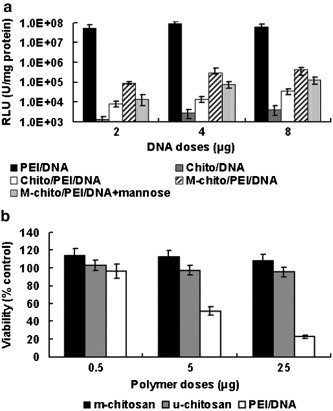
In vitro transfection and cytotoxicity. (a) In vitro gene expression of m-chitosan microspheres in RAW264.7 cells compared with PEI/DNA, m-chitosan/DNA complexes and u-chitosan microspheres, and the luciferase values have been normalized to total protein content, all data were expressed as mean ± SD (n = 3). (p < 0.05); (b) Cytotoxicity of u-chitosan, m-chitosan microspheres and PEI/DNA complexes in COS-7 cells by MTT assay. Cell viability was calculated as 100 × [(A570 of treated cells-A570 of blank)/(A570 of control cells-A570 of blank)]. The data points represent the averages of triplicates ± standard deviations (p < 0.05).
3.5. Gene expression after intramuscular injection
The m-chitosan microspheres induced expression of luciferase in vivo after injection into TA muscle in BALB/c mice. The level of in vivo expression of luciferase was higher for m-chitosan microspheres than for u-chitosan microspheres and naked DNA at days 7 and14 as shown in Fig. 5 (p < 0.05).
Fig. 5.
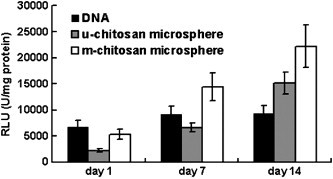
In vivo gene expression of luciferase was performed in mice at days 1, 7 and 14 with either naked DNA, u-chitosan or m-chitosan microspheres. Mean average values ± standard deviation of triplicates are given in relative light units (RLU), correlated to the total volume content (p < 0.05).
The uptake and expression of naked plasmid in muscle cells may be related to muscle physiology and function, most likely via a receptor-mediated event involving the T tubules and caveolae. For naked DNA, the peak level of gene expression in muscle was found 1.7–2.5-fold lower than u-chitosan or m-chitosan microspheres (Fig. 5). Manthope et al [30] have demonstrated the rapid loss of plasmid from muscle after injection of plasmid. Their studies showed that greater than 95% of the plasmid appeared to be degraded by 90 min post-injection, as determined by Southern blotting. Thus, the very low transfection efficiency coupled with the rapid degradation and elimination of DNA from the muscle suggests that nuclease mediated degradation is a rate-limiting step for gene delivery into muscle cells. For chitosan microspheres, the hydrophobic nature of the chitosan may offer protection to the encapsulated DNA from nuclease attack. This increased stability may explain the increased levels of gene expression observed at days 7 and 14.
After dosing in muscle, the overall expression levels achieved using the u-chitosan microspheres were 1.5–2.5-fold lower than those achieved with m-chitosan microspheres. The release of plasmid DNA is also an essential step for expression to be achieved in muscle. M-chitosan showed a faster dissociation and release of plasmid DNA, resulting in a faster onset of action and a higher gene expression (Fig. 5) (p < 0.05).
3.6. Evaluation of immune responses
The m-chitosan microspheres that were effective in vitro and in vivo without cytotoxicity were subsequently chosen for immunization. In the current studies, the m-chitosan microspheres were immunized by the i.m. route, which is optimal for immunization with naked DNA, producing a direct comparison of immune induction.
3.6.1. Enhanced HBsAg-specific humoral responses
The formulated m-chitosan microspheres significantly enhanced antibody responses over naked DNA at two time points and at two doses evaluated (Fig. 6a) (p < 0.05). In an extensive dose-response titration assessed by ELISA, naked DNA primed HBsAg-specific antibody response after four doses of DNA as high as 10 μg. In contrast, m-chitosan microspheres were effective at 1 μg, indicating a > 10 fold increase in DNA vaccine potency, based on the lowest dose of DNA required to prime a measurable response. The m-chitosan microspheres may increase the humoral response of naked DNA by extending its longevity in release kinetics and half-time. In addition, m-chitosan microspheres accelerated the antibody responses (data not shown). The detectable antibody responses against HBsAg were achieved within 5 weeks after injection of naked DNA at a 10 μg dose, whereas only 3 weeks were required for m-chitosan microspheres at the same dose. This acceleration by 2 weeks is statistically significant, and is of clinical interest whenever a rapid immune response is critical. In the past years, outbreaks of Ebola virus, severe acute respiratory syndrome and influenza A have increased demands for vaccination strategies that rapidly induce immunity [31], [32]. The vaccine strategy described here enhances the humoral immune response to DNA vaccines, and increases the speed of immune induction that is of clinical interest.
Fig. 6.
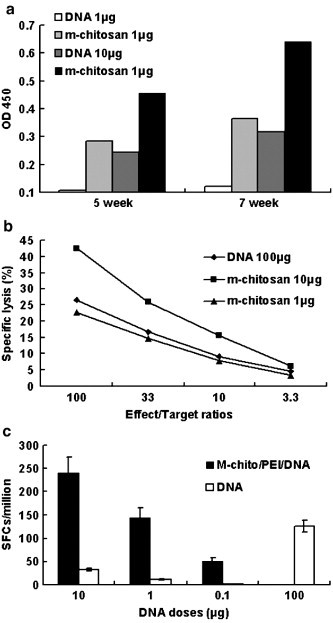
(a) HBsAg serum antibody assay in groups of BALB/c mice immunized either m-chitosan microspheres or naked DNA alone. The immunization protocol was performed at weeks 0, 2, 4 and 6. At the different time points of 5 and 7 weeks, HBsAg-specific antibody was measured in serum from each group of mice (as pools of five) (p < 0.05). OD450≥0.2 represents positive value. (b) Analysis of cytolytic activities of splenic mononuclear cells harvested from mice immunized. P815 mastocytoma cells pulsed with epitope peptides were used as targets. Percent specific lysis was determined by a 4-h LDH-release assay (p < 0.05). (c) The HBsAg-specific, IFN-γ producing splenocytes were generated in mice by HBsAg DNA vaccine after intramuscular injection. Each bar represents the average number of cells secreting IFN-γ per 106 cells (p < 0.05).
3.6.2. Enhanced HBsAg-specific cellular responses
3.6.2.1. Facilitated HBsAg-specific CTL responses
HBsAg-specific CD8+ T cells were measured in vitro by assessing the P815/BALB target cell killings, which were sensitized with HBsAg CTL epitope peptide. Naked DNA primed mild HBsAg-specific CD8+ T cells at the dose of DNA as high as 100 μg. In contrast, m-chitosan microspheres were effective at 100 ng, indicating a 100–1000-fold increase in DNA vaccine potency (p < 0.05), as judged by reduction of DNA vaccine dose (Fig. 6b).
3.6.2.2. Enhanced HBsAg-specific IFN-γ production
During natural infection, HBV-specific Th1 cells, CTLs and the associated antiviral cytokines (IFN-γ, TNF-a, IL-2) may play key roles in virus resolution [33], [34], [35]. HBsAg-specific CD8+ T cells were measured in vitro by determining IFN-γ production in spleen cells in response to brief restimulation with CTL epitope, as measured by ELISPOT (Fig. 6c). M-chitosan microsphere immunization at 1 μg clearly corresponds the response induced by 100 μg naked DNA, which indicated a ∼ 100-fold increase in DNA vaccine potency, as judged by reduction of DNA vaccine dose (p < 0.01).
M-chitosan microspheres were 100–1000-fold more potent than naked DNA for CD8+ T-cell and 10-fold more potent for antibody responses. This result is not surprising since other reports have observed no correlation between humoral and cellular immune responses. It is evident that the plentiful antigen presentation through intracellular DNA expression by APCs has polarized the immune response toward its cellular arm. Endogenous synthesis of foreign protein antigens is known to elicit class I-restricted CD8+ CTL responses. Successful induction of broad HBsAg-specific cellular immunity is expected to control HBV replication in infected individuals and lead to recovery from chronic hepatitis B.
4. Conclusion
The increased immunogenicity caused by m-chitosan microspheres may be due to the controlled release of DNA, endosome escape and targeted delivery to APCs. This augmented immunogenicity may allow for the dose of DNA to be minimized, making the DNA vaccine safer. Subsequent studies have confirmed the induction of Th1-skewed immunity at low dose of DNA in big animals such as guinea pigs and rhesus macaques, and ultimately this technology holds promise for use in human-beings.
Acknowledgment
We thank Prof. Xiao-Fang Yu for advice and Elana S. Ehrlich for editorial assistance (Department of Molecular Microbiology and Immunology, Bloomberg School of Public Health, John Hopkins University). We also thank Dr. Qi-Feng Wang at Jilin University for his help with AFM measurements. This work was supported by a grant from NSFC (20674029 and 30371317) to Wei Kong.
Reference
- 1.Rehermann B., Lau D., Hoofnagle J.H., Chisari F.V. Cytotoxic T lymphocyte responsiveness after resolution of chronic hepatitis B virus infection. J. Clin. Invest. 1996;97(7):1655–1665. doi: 10.1172/JCI118592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rehermann B., Fowler P., Sidney J., Person J., Redeker A., Brown M., Moss B., Sette A., Chisari F.V. The Cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during the after acute viral hepatitis. J. Exp. Med. 1995;181(3):1047–1058. doi: 10.1084/jem.181.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrari C., Penna A., Bertoletti A., Valli A., Antoni A.D., Giuberti T., Cavalli A., Petit M.A., Fiaccadori F. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J. Immunol. 1990;145(10):3442–3449. [PubMed] [Google Scholar]
- 4.Missale G., Redeker A., Person J., Fowler P., Guilhot S., Schlicht H.J., Ferrari C., Chisari F.V. HLA-A31-and HLA-Aw68-restricted cytotoxic T cell responses to a single hepatitis B virus nucleocapsid epitope during acute viral hepatitis. J. Exp. Med. 1993;177(3):751–762. doi: 10.1084/jem.177.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nayersina R., Fowler P., Guilhot S., Missale G., Cerny A., Schlicht H.J., Vitiello A., Chesnut R., Person J.L., Redeker A.G., Chisari F.V. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J. Immunol. 1993;150(10):4659–4671. [PubMed] [Google Scholar]
- 6.Letvin N.L., Montefiori D.C., Yasutomi Y., Perry H.C., Davies M.E., Lekutis C., Alroy M., Freed D.C., Lord C.I., Handt L.K., Liu M.A., Shiver J.W. Proc. Natl. Acad. Sci. U. S. A. 1997;94(17):9378–9383. doi: 10.1073/pnas.94.17.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McConkey S.J., Reece W.H., Moorthy V.S., Webster D., Dunachie S., Butcher G., Vuola J.M., Blanchard T.J., Gothard P. Nat. Med. 2003;9(6):729–735. doi: 10.1038/nm881. [DOI] [PubMed] [Google Scholar]
- 8.Singh M., Briones M., Ott G., O'Hagan D. Cationic microparticles: a potent delivery system for DNA vaccines. Proc. Natl. Acad. Sci. U. S. A. 2000;97(2):811–816. doi: 10.1073/pnas.97.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Illum L., Jabbal-Gill I., Hinchcliffe M., Ficsher A.N., Davis S.S. Chitosan as a novel nasal delivery system for vaccines. Adv. Drug Deliv. Rev. 2001;51(1-3):81–96. doi: 10.1016/s0169-409x(01)00171-5. [DOI] [PubMed] [Google Scholar]
- 10.Shiver J.W., Fu T.M., Chen L., Casimiro D.R., Davies M.E., Evans R.K. Replication-incompletent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415(6869):331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 11.Felgner J.H., Kumar R., Sridhar C.N., Wheeler C.J., Tsai Y.J., Border R., Ramsey P., Martin M., Felgner P.L. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J. Biol. Chem. 1994;269(4):2550–2561. [PubMed] [Google Scholar]
- 12.Gregoriadis G., McCormack B., Obrenovic M., Saffie R., Zadi B., Perrie Y. Vaccine entrapment in liposomes. Methods. 1999;19(1):156–162. doi: 10.1006/meth.1999.0841. [DOI] [PubMed] [Google Scholar]
- 13.Yewdell W.J., Norbury C.C., Bennink J.R. Mechanisms of exogenous antigen presentation by MHC class I molecules in vitro and in vivo: implications for generating CD8+ T cell responses to infectious agents, tumor, transplants, and vaccines. Adv. Immunol. 1999;73:1–77. doi: 10.1016/s0065-2776(08)60785-3. [DOI] [PubMed] [Google Scholar]
- 14.Men Y., Audran R., Thomasin C., Eberl G., Demotz S., Merkle H.P., Gander B., Corradin G. MHC class I-and class II-restricted processing and presentation of microencapsulated antigens. Vaccine. 1999;17:1047–1056. doi: 10.1016/s0264-410x(98)00321-1. [DOI] [PubMed] [Google Scholar]
- 15.Kanke M., Morlier E., Geissler R., Powell D., Kaplan A., DeLuca P.P. Interaction of microspheres with blood constituents II: Uptake of biodegradable particles by macrophages. J. Parenter. Sci. Technol. 1986;40(4):114–118. [PubMed] [Google Scholar]
- 16.Raychaudhuri S., Rock K.L. Fully mobilizing host defense: building better vaccines. Nat. Biotechnol. 1998;16:1025–1031. doi: 10.1038/3469. [DOI] [PubMed] [Google Scholar]
- 17.Goh S.L., Murthy N., Xu M., Frechet J.M. Cross-linked microparticles as carriers for the delivery of plasmid DNA for vaccine development. Bioconjug. Chem. 2004;15(3):467–474. doi: 10.1021/bc034159n. [DOI] [PubMed] [Google Scholar]
- 18.Katas H., Alpar H.O. Development and characterization of chitosan nanoparticles for siRNA delivery. J. Control. Release. 2006;115(2):216–225. doi: 10.1016/j.jconrel.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Mao H.Q., Roy K., Troung-Le V.L., Janes K.A., Lin K.Y., Wang Y., August J.T., Leong K.W. Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J. Control. Release. 2001;70(3):399–421. doi: 10.1016/s0168-3659(00)00361-8. [DOI] [PubMed] [Google Scholar]
- 20.Geng X., Kwon O.H., Jang J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials. 2005;26(27):5427–5432. doi: 10.1016/j.biomaterials.2005.01.066. [DOI] [PubMed] [Google Scholar]
- 21.Qin C., Du Y., Xiao L., Li Z., Gao X. Enzymic preparation of water-soluble chitosan and their antitumor activity. Int. J. Biol. Macromol. 2002;31(1-3):111–117. doi: 10.1016/s0141-8130(02)00064-8. [DOI] [PubMed] [Google Scholar]
- 22.Mansur Y., Laurance D.H. Some chemical and analytical aspects of polysaccharide modifications. 3. Formation of branch-chain, soluble chitosan derivatives. Macromolecules. 1984;17:272–281. [Google Scholar]
- 23.Park J.H., Cho Y.W., Chung H., Kwon I.C., Leong S.Y. Synthesis and characterization of sugar-bearing chitosan derivatives: aqueous solubility and biodegradability. Biomacromolecules. 2003;4:1087–1091. doi: 10.1021/bm034094r. [DOI] [PubMed] [Google Scholar]
- 24.Aral C., Akbuga J. Preparation and in vitro transfection efficiency of chitosan microspheres containing plasmid DNA: poly(L-lysine) complexes. J. Pharm. Pharm. Sci. 2003;6(3):321–326. [PubMed] [Google Scholar]
- 25.Varum K.M., Myhr M.M., Hjerde R.J., Smidsrod O. In vitro degradation rates of partially N-acetylated chitosans in human serum. Carbohydr. Res. 1997;299(1-2):99–101. doi: 10.1016/s0008-6215(96)00332-1. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H., Neau S.H. In vitro degradation of chitosan by a commercial enzyme preparation: effect of molecular weight and degree of deacetylation. Biomaterials. 2001;22(12):1653–1658. doi: 10.1016/s0142-9612(00)00326-4. [DOI] [PubMed] [Google Scholar]
- 27.Cho Y.W., Cho Y.N., Chung S.H., Yoo G., Ko S.W. Water-soluble chitin as a wound healing accelerator. Biomaterials. 1999;20(22):2139–2145. doi: 10.1016/s0142-9612(99)00116-7. [DOI] [PubMed] [Google Scholar]
- 28.Tomihata K., Ikada Y. In vitro and in vivo degradation of films of chitin and its deacetylated derivatives. Biomaterials. 1997;18(7):567–575. doi: 10.1016/s0142-9612(96)00167-6. [DOI] [PubMed] [Google Scholar]
- 29.Lee K.Y., Ha W.S., Park W.H. Blood compatibility and biodegradability of partially N-acylated chitosan derivatives. Biomaterials. 1995;16(16):1211–1216. doi: 10.1016/0142-9612(95)98126-y. [DOI] [PubMed] [Google Scholar]
- 30.Manthorpe M., Cornefert-Jensen F., Hartikka J., Felgner J., Rundell A., Margalith M., Dwarki V. Gene therapy by intramuscular injection of plasmid DNA: studies on firefly luciferase gene expression in mice. Hum. Gene Ther. 1993;4(4):419–431. doi: 10.1089/hum.1993.4.4-419. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan N.J., Sanchez A., Rollin P.E., Yang Z.Y., Nabel G.J. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408(6812):605–609. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan N.J., Geisbert T.W., Geisbert J.B., Xu L., Yang Z.Y., Roederer M., Koup R.A., Jahrling P.B., Nabel G.J. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature. 2003;424(6949):681–684. doi: 10.1038/nature01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deml L., Schirmbeck R., Reimann J., Wolf H., Wagner R. Purification and characterization of hepatitis B virus surface antigen particles produced in Drosophila Schneider-2 cells. J. Virol. Methods. 1999;79(2):205–217. doi: 10.1016/s0166-0934(99)00022-1. [DOI] [PubMed] [Google Scholar]
- 34.Rico M.A., Quiroga J.A., Subira D., Castanon S., Esteban J.M., Pardo M., Carreno V. Hepatitis B virus-specific T-cell proliferation and cytokine secretion in chronic hepatitis B e antibody-positive patients treated with ribavirin and interferon alpha. Hepatology. 2001;33(1):295–300. doi: 10.1053/jhep.2001.21147. [DOI] [PubMed] [Google Scholar]
- 35.Seder R.A., Hill A.V. Vaccines against intracellular infections requiring cellular immunity. Nature. 2000;406(6797):793–798. doi: 10.1038/35021239. [DOI] [PubMed] [Google Scholar]


