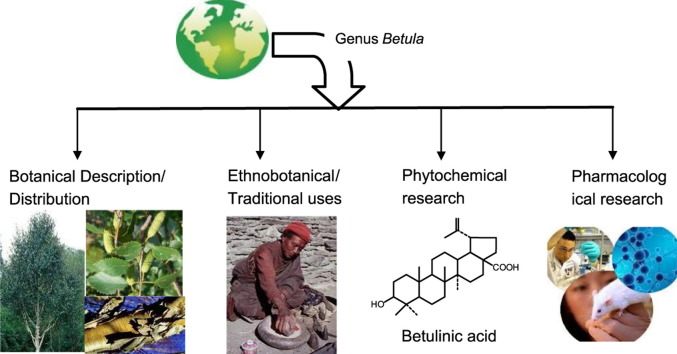
Abstract
Ethnopharmacological relevance
Trees and shrubs of the genus Betula (Betulaceae) inhabit various ecosystems in temperate and boreal climate zones of the northern hemisphere. The healing properties of Betula bark and bark extracts have been known for a long time in traditional medicine in different parts of the world. Several species of Betula have traditionally been used for the treatment of various inflammatory diseases including arthritis. The purpose of this review is to provide updated, comprehensive and categorized information on the botany, traditional uses, phytochemistry and pharmacological and toxicological research of Betula species in order to explore their therapeutic potential and evaluate future research opportunities.
Materials and methods
All the available information on various species belonging to the genus Betula was collected via electronic search (using Pubmed, SciFinder, Scirus, Google Scholar, JCCC@INSTIRC and Web of Science) and a library search for articles published in peer-reviewed journals.
Results
Although over a hundred Betula species are found distributed globally, about 7 different species of Betula have been documented for their traditional uses. Phytochemical research on Betula species has led to the isolation of triterpenoids, diarylheptanoids, phenylbutanoids, lignans, phenolics and flavonoids. Crude extracts, fractions and phytochemical constituents isolated from Betula showed a wide spectrum of in vitro and in vivo pharmacological activities like immunomodulatory, anti-inflammatory, antimicrobial, antiviral, antioxidant, antidiabetic, dermatological, gastroprotective and hepatoprotective. Antiarthritic and anticancer are the two major areas of research conducted on these species. The anti-carcinogenic effects of Betula bark, betulin as well as betulinic acid have been extensively studied.
Conclusions
Several species belonging to the genus Betula are widely used in traditional medicine. Betula platyphylla and Betula pendula have specifically been found to be potentially useful in the treatment of degenerative joint disease. There is convincing evidence in experimental animal models in support of their anti-carcinogenic effects. However, it would be worthwhile to investigate the biochemical and physiological mechanisms as well as detailed preclinical toxicity, bioavailability, pharmacokinetics and pharmacodynamics of the different biologically active extracts as well as molecules in sufficient detail. An integrated and holistic approach is required for tapping the full potentials of this important genus.
Abbreviations: AD, atopic dermatitis; ATPase, adenosinetriphosphatase; AWB, Asian white birch; BE, Betula pendula leaves ethanolic extract; Bet-APEs, Betula alba aqueous pollen extracts; BFBP, n-butanol fraction from the bark of Betula platyphylla; BHA, tert-butyl-4-hydroxyanisole; BLE, methanolic extract of Betula pendula leaves; BPE, Betula pendula leaf extract; CCl4, carbon tetrachloride; CFSE, carboxyfluorescein diacetate succinimidyl ester; CD, cluster of differentiation; CIA, collagen-induced arthritis; CinnAc, hydroxycinnamic acids; COX, cyclooxygenase; DBBEE, dried Betula bark ethanolic extract; DC, dendritic cell; D-GalN, D-galactosamine; DNCB, 2,4-dinitrochlrobenzene; DOPA, dihydroxyphenylalanine; DPPH, 2,2-diphenyl-1-picrylhydrazyl; ELISA, enzyme-linked immunosorbent assay; EtOAc, ethyl acetate; GAGs, glycosaminoglycans; H2O2, hydrogen peroxide; HIV, human immunodeficiency virus; HMC-1, human mast cells; HSV-1, herpes simplex virus type 1; IBMX, 3-isobutyl-1-methylxanthine; IFN, interferon; IgE, immunoglobulin E; IL, interleukin; iNOS, inducible nitric oxide synthase; LDCsA, low-dose cyclosporine A; LPS, lipopolysaccharides; MDA, malondialdehyde; MDR, multidrug resistance; MMP, matrix metalloproteinase; MTT, 3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide; NF-κB, nuclear factor—kappa B; OA, osteoarthritis; OVA, ovalbumin; PAF, platelet activating factor; PARP, poly (ADP-ribose) polymerase; PAs, proanthocyanidins; PC, picryl chloride; PDE, phosphodiesterase; PGE2, prostaglandin E2; P-gp, permeability glycoprotein; PMACI, phorbol 12-myristate 13-acetate plus calcium ionophore A23187; RA-FLS, fibroblast-like synoviocytes from rheumatoid arthritis patients; RBC, red blood cell; RPMCs, rat peritoneal mast cells; RT-PCR, reverse transcription-polymerase chain reaction; SPA, scintillation proximity assays; T(H)2, T helper cell type 2; TBDE, trypan blue dye exclusion; TEWL, transepidermal water loss; TIMPs, tissue inhibitors of metalloproteinases; TNF, tumor necrosis factor; TPA, 12-O-tetradecanoylphorbol-13-acetate; X/XO, xanthine/xanthine oxidase; YBMac, yellow birch extract obtained by maceration
Chemical compounds studied in this article: Betulin (PubChem CID: 72326), Betulinic acid (PubChem CID: 64971), Platyphylloside (PubChem CID: 9826264), Papyriferic acid (PubChem CID: 441683)
Keywords: Betula, Betulin, Betulinic acid, Ethnomedicine, Phytochemical constituents, Pharmacology
Graphical abstract
1. Introduction
Betulaceae (birch family) is an important group of the Angiosperms comprising of 6 plant genera viz. Alnus (Alders), Betula (Birches), Carpinus (Hornbeams), Corylus (Iron-wood), Ostrya (Hazel) and Ostryopsis (Hazel-hornbeam). It is most common in the northern hemisphere, but can occasionally be found in the southern hemisphere especially South America. Betula is the largest genus of this family and includes 119 accepted names, till date, based on The Plant List (http://www.theplantlist.org/).
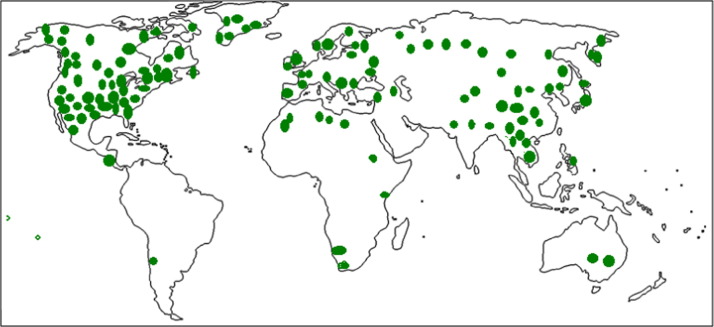
Trees and shrubs of this genus inhabit various ecosystems in temperate and boreal climate zones of the northern hemisphere. Distribution of different Betula species around the world is depicted in Fig. 1. Birches are among the most attractive trees known for their autumn color and contribute to the fall color in eastern North America. Various birches have yielded sugar, vinegar, a tea from the leaves, and a birch beer from the sap. The sweet, birch (Betula lenta L.) is now the chief source of oil of wintergreen (Ashburner and McAllister, 2013, http://encyclopedia2.thefreedictionary.com,). Betula utilis D. Don, found in the Himalayan region of India, is used in religious ceremonies in north region (Rawat and Pangtey, 1987).
Fig. 1.
Distribution areas of Betula species.
The healing properties of Betula bark and bark extracts have been known for a long time in traditional medicine in different parts of the world. Different Betula species find mention in several pharmacopoeias (Menković et al., 2011, Shikov et al., 2014) including the Russian, French, European, Deutsches Pharmacopoeias, the Ayuevedic Pharmacopoeia of India and Pharmacopoeia Jugoslavica. Several classical texts as well as modern books also contain monographs that describe the botanical, chemical, as well as pharmacological properties and uses of Betula species (Tschirch, 1910, Fuchs, 1964, Marzell, 1967, Hänsel et al., 1992–1994, Quer, 1995, EMEA/HMPC, 2008a, EMEA/HMPC, 2008b, Wichtl, 2009).
The Betula species exhibit various pharmacological properties. Their chemistry is complex and they are known to contain molecules of therapeutic importance. In the last few decades, several studies have been carried out that also provide evidence in favor of their conventional uses. The purpose of this review is to provide comprehensive information on the botany, traditional uses, phytochemistry, pharmacological and toxicological research of Betula species in order to explore their therapeutic potential, highlight the lacunae in our present knowledge and evaluate future research opportunities. All the available information on various species belonging to the genus Betula was collected via electronic search (using Pubmed, SciFinder, Scirus, Google Scholar, JCCC@INSTIRC and Web of Science) and a library search for articles published in peer-reviewed journals. This review thus provides the scientific basis for future research on species belonging to this genus.
2. Distribution and botanical description
Betula is a genus of tree and shrubs, monecious, leaves simple alternate, deciduous penninerved, toothed or serrate. Male flower in pendulous spikes; bracts peltate; with 3 bi-bracteolate flowers; sepals 2–4; stamens 2, filaments forked separating the anther cells. Female flower in erect or pendulum spikes; bracts imbricate, bracteoles 2 adnate to the bract which thus appears 3-lobbed; perianth 0; ovary compressed, 2-celled, cells 1-ovuled; styles 2, slender, stigmas terminal. Fruit a spike of lenticular winged or margined nuts; cotyledons flat. The genus Betula is widely distributed from North temperate and arctic regions, circle to, the Himalayas, Afghanistan, China, Japan, Kazakhstan, Korea, Kyrgyzstan, Mongolia, Nepal, Russia, Sikkim, southern Europe, and extending to the North and South America (Hooker, 1890, Anonymous, 1948, Gaur, 1999).
Betula pendula Roth (silver birch) and Betula pubescens Ehrh. (downy birch) both have wide distribution in Europe and are also found in northern parts of Asia (Ha¨met-Ahti et al., 1989; Niemistö et al., 2008). Betula alnoides is found widely distributed in E. Asia—Himalayas to S.W. China. Betula alleghaniensis Britton (yellow birch), Betula lenta L. (sweet birch), Betula papyrifera Marshall (paper birch) Betula populifolia Marsh. (gray birch) and Betula nigra L. (river birch) are species typical for North America (Ha¨met-Ahti et al., 1989). In Scandinavia and northern Europe Betula pendula is an important tree species for forest industry, but also used as amenity trees in parks, alleys and in gardens. Betula alleghaniensis, Betula lenta and Betula papyrifera are also valuable for forest industry. Birches are cold tolerant pioneer species and in southern Europe they are found mainly on higher altitudes. Many Betula species such as Betula nana L. (dwarf birch), Betula pubescens subsp. czerepanovii (Orlova) Hämet-Ahti (arctic moor birch) and Betula utilis D. Don (Himalayan birch) are typical for treeline. Betula nana and its subspecies are shrubs native to arctic and cool temperate regions of northern Europe, northern Asia and northern North America. They are also present in Greenland as well as in mountains in Scotland and the Alps. Betula utilis is growing as a shrub or tree native to the Himalayas (Ha¨met-Ahti et al., 1989, http://www.discoverlife.org,).
However, the taxonomy of the European members of the genus has long been in dispute because of their high morphological variability and frequent hybridization (Atkinson, 1992). Difficulties in distinguishing between closely related birch species have prompted numerous biometric and chemotaxonomic studies. The former have been based mainly on morphological characteristics of birch leaves and fruits (reviewed in Atkinson (1992)), and the latter on the composition of phenolics (particularly flavonoids) and terpenoids in birch leaves, buds, bark and stems (reviewed in Keinänen et al. (1999)). Besides these, studies have also been conducted to determine the phylogenetic relationships in Betula based on amplified fragment length polymorphisms (AFLP) markers (Schenk et al., 2008), nuclear alcohol dehydrogenase genes (ADH) and chloroplast MaturaseK (MatK) gene sequences (Ja¨ Rvinen et al., 2004). Hynynen et al. (2010) studied the important ecological characteristics and typical growth and yield patterns of two commercially important treelike birch species (Betula pendula and Betula pubescens) that occur naturally in Europe.
3. Traditional uses and ethnopharmacology
The available literature and information show that several Betula species have traditionally been used as medicine in different parts of the world. The most widespread use has been in the treatment of bone related problems including arthritis, rheumatism and gout as well as renal ailments. Birch sap has also been recommended against hepatitis, rash, intestinal worms and scurvy. Besides the medicinal uses, cosmetic applications have also been reported, mainly for hair growth and against freckles. Tea infusion of Betula pendula has also been frequently used in Europe, specially the Czech Republic, in herbal teas (Dadáková et al., 2010).
Table 1 presents the various ethnobotanical uses of those Betula species that have been widely used in the different parts of the world and documented. The local names as well as the methods of administration and the induced effects that have been reported have also been mentioned.
Table 1.
Ethnomedicinal uses of Betula species.
| Plant name | Vernacular name (Locality) | Part used | Ethnobotanical uses | Ref. |
|---|---|---|---|---|
| Betula alnoides Buch.-Ham. ex D. Don | Paiyun (Jajarkot district, Nepal) | Bark | A decoction of the bark is boiled to a gelatinous mass which is applied to treat micro-fracture or dislocated bone. | Manandhar, 1995, Manandhar, 2002 |
| Bark | Bark is boiled with water and the liquid mass is applied to dislocated bone and injury. Bark is chewed orally to treat sore throat and to check excessive menstruation. | Rajbhandari (2001) | ||
| Betula papyrifera Marsh. | Paper birch (USA) | Whole plant | Used as a preservative | Moerman (1998) |
| Betula pendula Roth | Breza (Bosnia and Herzegovina) | Leaf, Bark | Fluid unction with Arctium lappa for increased growth of hair and dandruff (Arnica, Betula) | Broza et al. (2010) |
| Juice for renal gravel. | ||||
| Tea for renal ailments, rheumatism and blood purification | ||||
| Tea for urinary tract infections (Betula, Althaea officinalis, Equisetum, Salvia, Uva), (Betula, Althaea officinalis, Equisetum, Salvia) | ||||
| Tea for renal ailments (Betula, Salix), (Betula, Ocimum, Solidago) | ||||
| Decoct for asthma (Betula, Pimpinella major, Pimpinella saxifraga, Potentilla, Ruta) | ||||
| Decoct for hindered diuresis (Betula, Saponaria, Solidago) | ||||
| Decoct for rheumatism (Betula, Populus alba, Sambucus nigra, Tilia, Urtica), (Betula, Sambucus nigra, Tilia, Urtica) | ||||
| Decoct for enlarged spleen (Betula, Coriandrum) | ||||
| Breza Silver birch (Bosnia and Herzegovina; Western Balkan Peninsula; Southeast Europe) | Bark, Leaves | Used for renal diseases and ague | Broza et al. (2011) | |
| As a mixture with other drugs, used for urogenital tract ailments: urinary bladder infections, urinary tract infections, purification of urinary bladder, renal inflammations, renal stones and hindered diuresis; for arrhythmia, blood purification, purification of lungs, rheumatism, arthritis, common cold and fever | ||||
| Urogenital tract ailments, rheumatism, skin problems, blood system disorders, respiratory tract ailments and influenzal infections. | ||||
| Breza (Prokletije Mountains; Montenegro) | Leaves | Bacterial and inflammatory disease of the urinary tract and for kidney stones | Menković et al. (2011) | |
| Externally for hair loss and dandruff | ||||
| Breza (Bulgaria) | Bark | Infusion, decoction; diuretic, cholagogue | Leporatti and Ivancheva (2003) | |
| Oqkayın (Toshkent, Djizzax, and Samarqand provinces; Uzbekistan, Central Asia) | Resin | Against rheumatic pain; ingested 3 times a day for 3 weeks | Sezik et al. (2004) | |
| Batoula (Lebanon) | Leaves | Arthritis-sleep in a sack filled with leaves | Marc et al. (2008) | |
| Arthritis and rheumatism-decoction for bathing | ||||
| Bedoll (Pallars; Pyrenees, Catalonia, Iberian Peninsula) | Bark, Leaves | Oral; Tisane (infusion or decoction), direct ingestion is antiarthrosic, useful against hypercholesterolemia, anticephalalgic, anticholagogue, antihelminthic, salutiferous | Agelet and Vallès (2003) | |
| Bidollo betulla Silver birch (Marches region; Central-Eastern Italy) | Bark | Decoction, in external washes; to prevent hair loss | Pieroni et al. (2004) | |
| Buds and leaves | Decoction, in external application; cicatrizing | |||
| Betulla (Italy) | Bark | Infusion, decoction; antipyretic, diuretic, cholagogue, diaphoretic | Leporatti and Ivancheva (2003) | |
| Infusion, decoction; in skin diseases | ||||
| Betulla (Lucca Province, north-west Tuscany, central Italy) | Bark and sap | Against cold | Pieroni (2000) | |
| Against alopecia (decoction of the bark, then adding sap) | ||||
| Symida (Thessaloniki (N Greece) | Leaves | Infusion, decoction; for metabolic diseases (urea, uric acid), systematic diseases (arthritis, rheumatisms), skin diseases (cellulites) and urogenital system (diuretic, renal disorders) | Hanlidou et al. (2004) | |
| Silver Birch (Transylvania, East and Central Romania) | Leaves | As a foment for cold; | Papp et al. (2014) | |
| As a bath and foment for rheumatism and arthritis; | ||||
| For kidney stones as a tea with Alnus glutinosa, Origanum vulgare and Equisetum arvense; | ||||
| For heart and liver disease, flatulence and renal pain, for gall stones | ||||
| Bark | For wounds. | |||
| Sap | For kidney disease; as an appetite stimulant, for stomach and liver disease; for colds; for chilblain as a foment. | |||
| Betula platyphylla Sukat. var. japonica (Miq.) Hara | Jajaknamu (Southern mountainous region of Korea) | Stem | Decoction given orally; bone diseases | Kim and Song (2011) |
| Betula pubescens Ehrh. Syn-Betula alba L | Batoula (Morocco: Tafilalet) | Aerial part | Cardiac disease; hypertension | Eddouks et al. (2002) |
| Abedul, bidueiro (El Caurel; Galicia, northwest Spain) | Sap fresh plant | Bath, as a vulnerary | Blanco et al. (1999) | |
| Inflorescences | Decoction is used against gout | |||
| Vidoeiro Vido (Tras-os-Montes; northern of Portugal) | Flowers, leaves, bark and resin (in Spring). | Bile stimulant; diuretic; soporific and anti-edema; against cholesterol and urea; against gout; calculus | Neves et al. (2009) | |
| Anti-edema; anti-podagric; cholagogue; complexion; diaphoretic; diuretic; hypocholesterolemia; lithiasis treatment; vulnerary | ||||
| (West Azerbaijan; Iran) | Leaves | Sambucus nigra L. flowers in combination with Arctium lappa L. leaves, Malva sylvestris L. leaves, Betula alba L. leaves; all the ingredients are boiled in 1 l of water and then clean clay is added in order to produce a cream to be applied to epidermis | Miraldi et al. (2001) | |
| Betula pumila L. var. glandulifera Regel | Bog birch (USA) | Flower | Smoke inhalation used for respiratory tract diseases | Moerman (1998) |
| Betula utilis D.Don | Joonsh, Zhoonsh (Bulashbar Nullah, Astore; Northern Pakistan) | Bark | Local people cover Desi ghee in its bark and burry in the soil; as the time passes (10–20 years), the taste of Ghee becomes pleasant. This ghee is more valuable than normal Desi Ghee | Shinwari and Gilani (2003) |
| Due to the water proof nature of the paper, they spread this paper on the roofs of their houses like sheets during construction as well as cover the potatoes and wheat which are present in small digs made in the fields | ||||
| Towa (Western Ladakh, India) | Bark, Root | Jaundice, burns, leprosy and bronchitis | Angmo et al. (2012) | |
| Bhuj (Humla district; Western Nepal) | Bark | Wounds are covered by papery barks for antiseptic purpose | Rokaya et al. (2010) | |
| For the storage of food grains, the hole is dug in the ground and all sides of hole are covered by papery barks supported by young branches of Pinus wallichiana. The hole is filled with food grains, covered by soil and stored for futher use | ||||
| Birch (Leepa valley; Azad Jammu and Kashmir, Pakistan) | Bark | Powder taken orally; used for leprosy and convulsion; tonic | Mahmood et al. (2012) | |
| Bhojpattra (India) | Stem bark | Abortifacient | Malhi and Trivedi (1972) | |
| Bhojpattra (Johari tribals; Uttarakhand, India) | Stem bark | Antiseptic, for ear complaint, hysteria, jaundice | Malhotra and Balodi (1984) and Chopra et al. (1986) | |
| Bhojpattra (Garhwal Himalayan region of Uttarakhand, India) | Stem bark | For wounds | Negi et al. (1985) | |
| Bhojpattra (North India) | Resin | For cuts and burns | Shah (1982) and Shah and Joshi (1971) | |
| Contraceptive | ||||
| Bhuj (Nepal) | Bark | A paste made from the bark is used as a poultice on cuts, wounds and burns. | Manandhar (2002) | |
| Bark | Decoction is used for sores. | Rajbhandari (2001) | ||
| Bhuj (Dolpa, Nepal) | Bark | Poultice used in wounds, swellings etc. | Ghimire et al. (2008) | |
| Resin | Used in bile and phlegm disorders. | |||
| Buspath (Manang, Nepal) | Bark and leaves | Mixture of bark and leaves with other herbs is used to treat fever. | Ghimire et al. (2008) and Gewali and Awale (2008) | |
| Bark and resin | Antiseptic, carminative. Bark decoction is useful for sore throat. Bark is used for bacterial infections, skin diseases, bronchitis cough. | Baral and Kurmi (2006) | ||
| Betula species (Betula pendula, and Betula pubescens) | Birch (Northern, Central and Eastern Europe) | Tree sap | Lung diseases, gout, skin diseases, infertility, revitalization, stomach diseases, kidney stones, jaundice, diuretic, rheumatism, arthritis, liver disease, pneumonia, cholera. | Svanberg et al. (2012) |
4. Chemical constituents
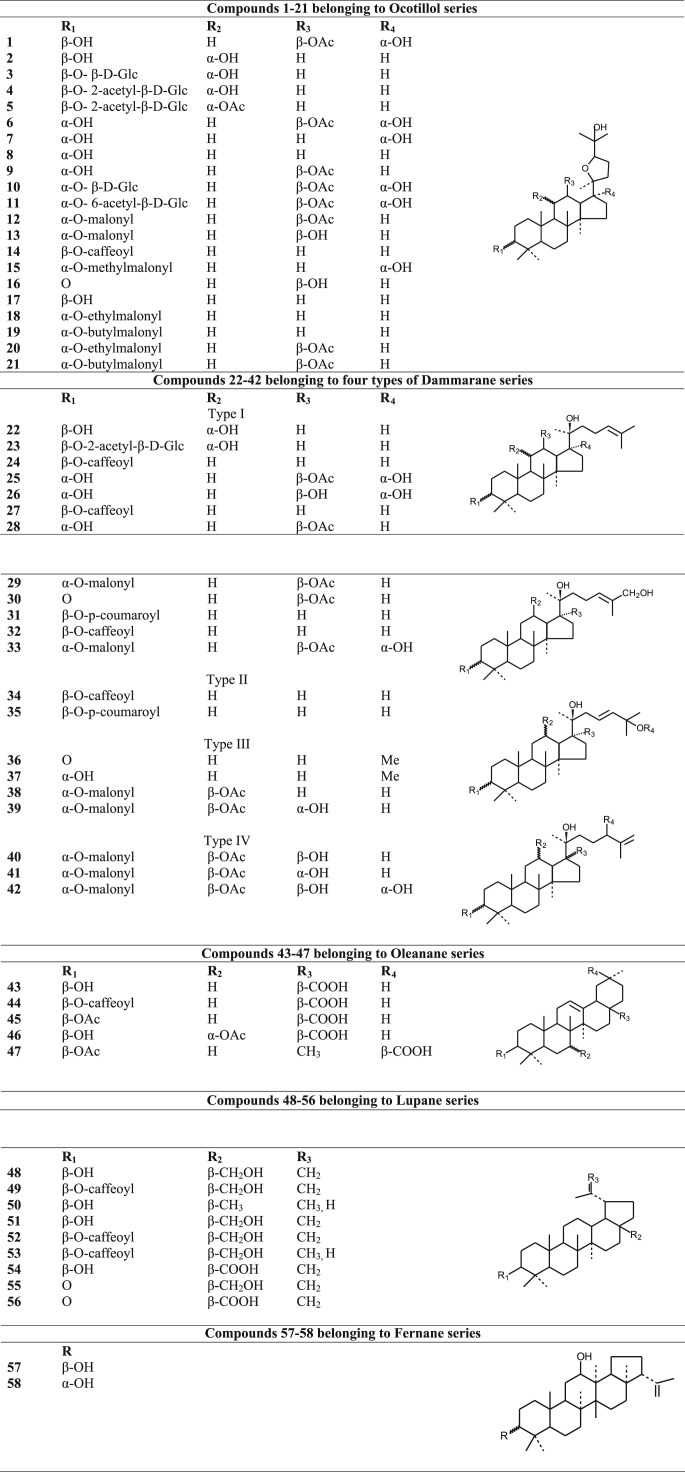
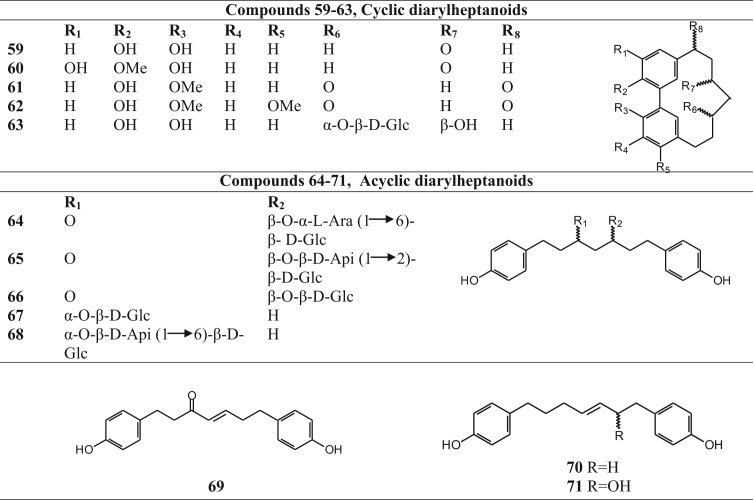
Detailed and extensive phytochemical investigations are necessary for understanding the pharmacological activity of the species, to gain an understanding of the mechanisms of action and for quality control purposes. Chemical investigations of the different species of this genus have led to the isolation and identification of several groups of chemical constituents. Basic phytochemical groups as well as the structures of the correspomding compounds that have been isolated and reported from Betula species are shown in Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8 whereas their related information is shown in Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8.
Fig. 2.
Structures of triterpenoids of Betula species.
Fig. 3.
Structures of diarylheptanoids of Betula species.
Fig. 4.
Structures of phenylbutanoids of Betula species.
Fig. 5.
Structures of phenolics of Betula species.
Fig. 6.
Structures of flavonoids, flavones and flavanones of Betula species.
Fig. 7.
Structures of catechins and lignans of Betula species.
Fig. 8.
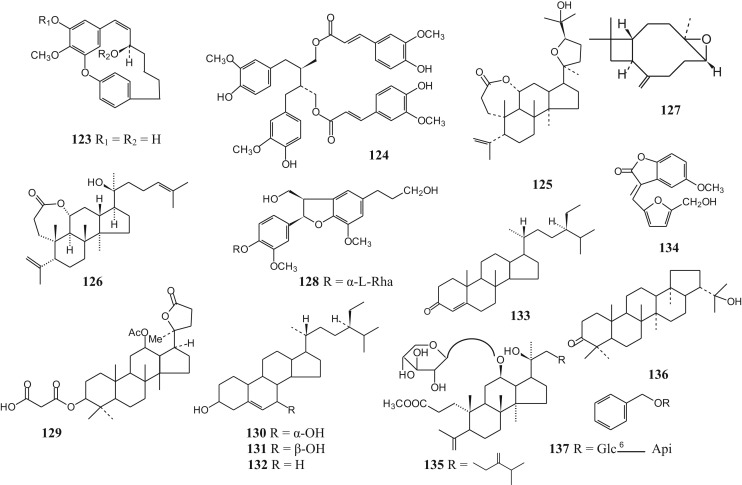
Structures of steroids and miscellaneous compounds 123–137.
Table 2.
Triterpenoids isolated from Betula species.
| Name | Specie from which isolated | Reference | |
|---|---|---|---|
| Ocotillol series | |||
| 1 | 12-O-acetylbetulafolienetetraol oxide | Betula dahurica | Fuchino et al. (1998b) |
| 2 | 20(S),24(R)-epoxydammaran-3β | Betula ermanii | Fuchino et al. (1995) |
| 3 | 11α,25-triol, 3-O-β-d-glucopyranoside of 2 | Betula ermanii | Fuchino et al. (1995) |
| 4 | 2′-acetate of 3 | Betula ermanii | Fuchino et al. (1995) |
| 5 | 11,2′-diacetate of 3 | Betula ermanii | Fuchino et al. (1995) |
| 6 | 12β-acetoxy-20(S),24(R)-epoxy-3α,17 α,25-trihydroxydammarane | Betula maximowicziana | Fuchino et al. (1996b) |
| 7 | 20(S),24(R)-epoxy-3α,17 α,25-trihydroxydammarane | Betula maximowicziana | Fuchino et al. (1996b) and Xiong et al. (2011) |
| 8 | 3-epi-ocotillol II | Betula maximowicziana | Fuchino et al. (1996b) |
| 9 | 12β-acetoxy-20(S),24(R)-epoxy-3α, 25-dihydroxydammarane | Betula maximowicziana | Fuchino et al. (1996b) |
| 10 | 12β-acetoxy-20(S),24(R)-epoxy-3α,17 α,25-trihydroxydammarane 3-O-β-d-glucopyranoside (=betulamaximoside A) | Betula maximowicziana | Fuchino et al. (1996b) |
| 11 | 12β-acetoxy-20(S),24(R)-epoxy-3α,17 α,25-trihydroxydammarane 3-O-β-d-(6-O-acetyl)-glucopyranoside (=betulamaximoside B) | Betula maximowicziana | Fuchino et al. (1996b) |
| 12 | 12-O-acetyl-20,24-epoxy-3α,12β,20(S),24(R),25-pentahydroxydammar-3-yl hydrogen propanedioate (=papyriferic acid) | Betula neoalaskana | McLean et al. (2009), Tegelberg et al. (2002), Fuchino et al. (1996a), and Hilpisch et al. (1997) |
| Betula pendula | |||
| Betula pendula | |||
| Betula platyphylla | |||
| 13 | Deacetylpapyriferic acid | Betula pendula | Tegelberg et al. (2002) |
| 14 | Ocotillol II 3-O-caffeate | Betula platyphylla | Fuchino et al. (1996a) |
| 15 | 3-O-methylmalonyl-3α,17α,25-trihydroxy-20(S),24(R)-epoxydammarane | Betula platyphylla | Xiong et al. (2011) |
| 16 | 12β-acetoxyl-ocotillone | Betula platyphylla | Xiong et al. (2011) |
| 17 | Ocotillol | Betula platyphylla | Xiong et al. (2011) |
| 18 | 3-O-ethylmalonylepiocotillol II | Betula platyphylla | Xiong et al. (2011) |
| 19 | 3-O-butylmalonylepiocotillol II | Betula platyphylla | Xiong et al. (2011) |
| 20 | Ethyl papyriferate | Betula platyphylla | Xiong et al. (2011) |
| 21 | Butyl papyriferate | Betula platyphylla | Xiong et al. (2011) |
| Dammarane series | |||
| Type I | |||
| 22 | Dammar-24-en-3β,11α,20(S)-triol | Betula ermanii | Fuchino et al. (1995) |
| 23 | 3-O-β-d-2-O-acetylglucopyranoside of 22 | Betula ermanii | Fuchino et al. (1995) |
| 24 | Dammarendiol II 3-caffeate | Betula ermanii | Fuchino et al. (1995) |
| 25 | 12-O-acetylbetulafolienetetraol | Betula maximowicziana | Fuchino et al. (1996b) |
| 26 | Betulafolienetetraol | Betula maximowicziana | Fuchino et al. (1996b) |
| 27 | Dammarenediol II 3-O-caffeate | Betula maximowicziana | Fuchino et al. (1996b) |
| 28 | 12-O-acetylbetulafolienetriol | Betula platyphylla | Fuchino et al. (1996a) |
| 29 | 12-O-acetyl-3-O-malonylbetulafolienetriol | Betula platyphylla | Fuchino et al. (1996a) |
| 30 | 12-O-acetylbetulafolienediolone | Betula platyphylla | Fuchino et al. (1996a) |
| 31 | Dammarenediol II 3-O-p-coumarate | Betula platyphylla | Fuchino et al. (1996a) |
| 32 | Dammarenediol II 3-O-caffeate | Betula platyphylla | Fuchino et al. (1996a) |
| 33 | 12-O-acetyl-3α,12β,17α,20(S)-tetrahydroxydammar-24-en-3-yl hydrogen propanedioate | Betula pendula | Hilpisch et al. (1997) |
| Type II | |||
| 34 | Dammar-24-ene-3β, 20(S),26-triol 3-O-caffeate | Betula maximowicziana | Fuchino et al. (1996b) |
| 35 | Dammar-24-ene-3β, 20(S),26-triol 3-O-p-coumarate | Betula maximowicziana | Fuchino et al. (1996b) |
| Type III | |||
| 36 | 20(S)-hydroxy-25-methoxy-dammar-23-en-3-one | Betula platyphylla | Xiong et al. (2011) |
| 37 | 3α,20(S)-dihydroxy-25-methoxy-dammar-23-ene | Betula platyphylla | Xiong et al. (2011) |
| 38 | 12-O-acetyl-3α,12β,20(S),25-tetrahydroxydammar-23(E)-en-3-yl hydrogen propanedioate | Betula pendula | Hilpisch et al. (1997) |
| 39 | 12-O-acetyl-3α,12β,17α,20(S),25-pentahydroxydammar-23(E)-en-3-yl hydrogen propanedioate | Betula pendula | Hilpisch et al. (1997) |
| Type IV | |||
| 40 | 12-O-acetyl-3 α,12 β,20(S),24(R)-tetrahydroxydammar-25-en-3-yl hydrogen propanedioate | Betula pendula | Hilpisch et al. (1997) |
| 41 | 12-O-acetyl-3 α,12 β,20(S),24(S)-tetrahydroxydammar-25-en-3-yl hydrogen propanedionate | Betula pendula | Hilpisch et al. (1997) |
| 42 | 12-O-acetyl-3 α,12 β,17 α,20(S),24(R)-pentahydroxydammar-25-en-3-yl hydrogen propanedioate | Betula pendula | Hilpisch et al. (1997) |
| Oleanane series | |||
| 43 | Oleanolic acid | Betula dahurica | Kovac-Besović et al. (2009), Yin et al. (2012), Fuchino et al., 1995, Fuchino et al., 1996a, Fuchino et al., 1998a, Fuchino et al., 1998b, and Khan et al. (2012) |
| Betula ermanii | |||
| Betula pendula | |||
| Betula platyphylla | |||
| Betula schmidtii | |||
| Betula utilis | |||
| 44 | Oleanolic acid 3-O-caffeate | Betula dahurica | Fuchino et al., 1995, Fuchino et al., 1996b, Fuchino et al., 1998a, Fuchino et al., 1998b |
| Betula ermanii | |||
| Betula maximowicziana | |||
| Betula schmidtii | |||
| 45 | Acetyl-oleanolic acid | Betula maximowicziana | Fuchino et al., 1996a, Fuchino et al., 1996b and Khan et al. (2012) |
| Betula platyphylla | |||
| Betula utilis | |||
| 46 | Karachic acid | Betula utilis | Khan (1975) |
| 47 | Betuloleanolic acid acetate | Betula pendula | Mukhtar et al. (2003) |
| Lupane series | |||
| 48 | Betulin | Betula dahurica | Fuchino et al., 1995, Fuchino et al., 1996a, Fuchino et al., 1996b, Fuchino et al., 1998a, Fuchino et al., 1998b, Fuchino et al., 1998c, Kovac-Besović et al. (2009), Yin et al. (2012), Khan et al. (2012), and Prince et al. (2010) |
| Betula ermanii | |||
| Betula maximowicziana | |||
| Betula ovalifolia | |||
| Betula pendula | |||
| Betula platyphylla | |||
| Betula schmidtii | |||
| Betula utilis | |||
| 49 | Betulin 3-caffeate | Betula dahurica | Fuchino et al., 1995, Fuchino et al., 1996a, Fuchino et al., 1996b, Fuchino et al., 1998b, Fuchino et al., 1998c |
| Betula ermanii | |||
| Betula maximowicziana | |||
| Betula ovalifolia | |||
| Betula platyphylla | |||
| 50 | Monogynol A | Betula dahurica | Fuchino et al., 1995, Fuchino et al., 1996b, Fuchino et al., 1998b |
| Betula ermanii | |||
| Betula maximowicziana | |||
| 51 | Lupeol | Betula ermanii | Fuchino et al., 1995, Fuchino et al., 1996a, Fuchino et al., 1996b, Fuchino et al., 1998a, Kovac-Besović et al. (2009), and Khan et al. (2012) |
| Betula maximowicziana | |||
| Betula pendula | |||
| Betula platyphylla | |||
| Betula schmidtii | |||
| Betula utilis | |||
| 52 | Lupeol caffeate | Betula ermanii | Fuchino et al. (1995) |
| 53 | Lupane-3β,20,28-triol 3-O-caffeate | Betula maximowicziana | Fuchino et al. (1996b) |
| 54 | Betulinic acid | Betula pendula | Kovac-Besović et al. (2009), Fuchino et al. (1996a), and Prince et al. (2010) |
| Betula platyphylla | |||
| Betula utilis | |||
| 55 | Betulone | Betula schmidtii | Fuchino et al. (1998a) |
| 56 | Betulonic acid | Betula schmidtii | Fuchino et al. (1998a) |
| Fernane series | |||
| 57 | Betufernanediol A | Betula pendula | Mukhtar et al. (2003) |
| 58 | Betufernanediol B | Betula pendula | Mukhtar et al. (2003) |
Table 3.
Diarylheptanoids isolated from Betula species.
| Name | Specie from which isolated | Reference | |
|---|---|---|---|
| Cyclic diarylheptanoids | |||
| 59 | Acerogenin E | Betula dahurica | Fuchino et al., 1995, Fuchino et al., 1996a, Fuchino et al., 1996b, Fuchino et al., 1998b |
| Betula ermanii | |||
| Betula maximowicziana | |||
| Betula platyphylla | |||
| 60 | 16-hydroxy-17-O-methylacerogenin E | Betula maximowicziana | Fuchino et al. (1996b) |
| 61 | 17-O-methyl-7-oxoacerogenin E | Betula dahurica | Fuchino et al. (1998b) |
| 62 | 15-methoxy-17-O-methyl-7-oxoacerogenin E | Betula dahurica | Fuchino et al. (1998b) |
| 63 | Alnusdiol β-d-glucopyranoside | Betula maximowicziana | Fuchino et al. (1996b) |
| Acyclic diarylheptanoids | |||
| 64 | Papyriferoside A | Betula papyrifera | Mshvildadze et al. (2007) |
| 65 | 5-O-β-d-apiofuranosyl-(1→2)-β-d-glucopyranosyl-1,7-bis-(4-hydroxyphenyl)-heptan-3-one | Betula papyrifera | Mshvildadze et al. (2007) |
| 66 | Platyphylloside | Betula papyrifera | Mshvildadze et al. (2007) |
| 67 | Aceroside VII | Betula papyrifera | Mshvildadze et al. (2007) and Fuchino et al. (1996a) |
| Betula platyphylla | |||
| 68 | Aceroside VIII | Betula platyphylla | Fuchino et al. (1996a) |
| 69 | 1,7-bis-(4-hydroxyphenyl)-4-hepten-3-one | Betula papyrifera | Mshvildadze et al. (2007) and Fuchino et al. (1996a) |
| Betula platyphylla | |||
| 70 | 1,7-bis[4-hydroxyphenyl]-3-hepten-5-one | Betula platyphylla | Fuchino et al. (1996a) |
| 71 | 2-hydroxy-1,7-bis[4-hydroxyphenyl]-3-hepten-5-one | Betula platyphylla | Fuchino et al. (1996a) |
Table 4.
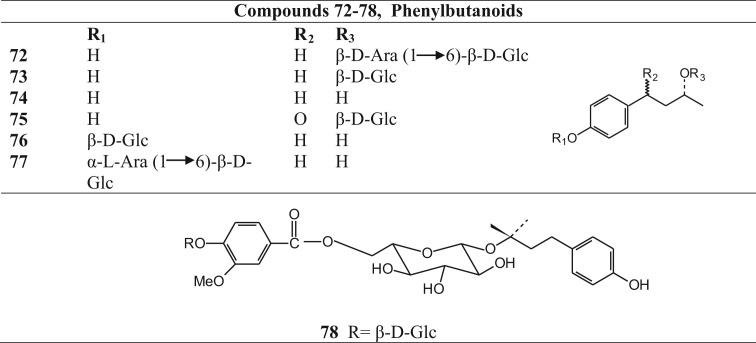
Phenylbutanoids isolated from Betula species.
| Name | Specie from which isolated | Reference | |
|---|---|---|---|
| 72 | 4-(4-hydroxyphenyl)-2-butanol 2-O-β-d-apiofuranosyl-(1→6)-β-d-glucopyranoside | Betula ermanii | Fuchino et al. (1995) |
| 73 | Rhododendrin(=betuloside) | Betula ovalifolia | Fuchino et al., 1996a, Fuchino et al., 1998c |
| Betula platyphylla | |||
| 74 | Rhododendol (=betuligenol) | Betula platyphylla | Fuchino et al. (1996a) |
| 75 | 3-β-glucopyranosyloxy-1-(4-hydroxyphenyl)-butanone | Betula pendula | Liimatainen et al. (2008) |
| 76 | (-)-rhododendrol 4′-O-β-d-glucopyranoside | Betula schmidtii | Fuchino et al. (1998a) |
| 77 | (-)-rhododendrol 4′-O-α-l-arabinofuranosyl-(1→6)-β-d-glucopyranoside | Betula schmidtii | Fuchino et al. (1998a) |
| 78 | 7-{3R-[(4-hydroxyphenyl)butyl] β-glucopyranosid-O-6-yl} 4-O-β-glucopyranosylvanillin | Betula pendula | Liimatainen et al. (2008) |
Table 5.
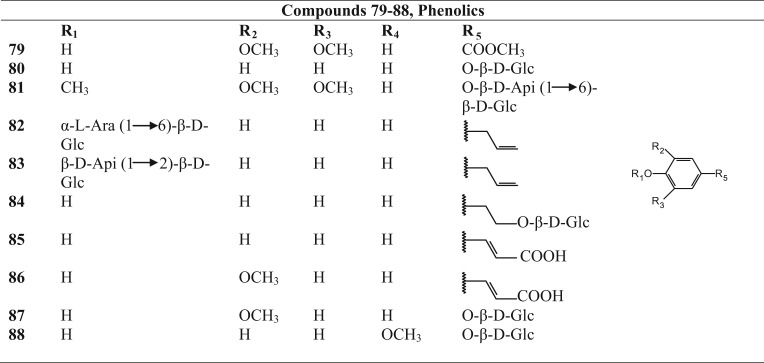
Phenolics isolated from Betula species.
| Name | Specie from which isolated | Reference | |
|---|---|---|---|
| 79 | Methyl syringate | Betula alba | Jermnak et al. (2012) |
| 80 | Arbutin | Betula alnoides | Thongchai et al. (2007) |
| 81 | 3,4,5-trimethoxyphenol β-d-apiofuranosyl-(1→6)-β-d-glucopyranoside | Betula dahurica | Fuchino et al., 1995, Fuchino et al., 1996b, Fuchino et al., 1998b |
| Betula ermanii | |||
| Betula maximowicziana | |||
| 82 | Chavicol 4-O-α-l-arabinofuranosyl-(1→6)-β-d-glucopyranoside | Betula papyrifera | Mshvildadze et al. (2007) |
| 83 | Chavicol 4-O-β-d-apiofuranoside-(1→6)-β-d-glucopyranoside | Betula papyrifera | Mshvildadze et al. (2007) |
| 84 | Salidroside | Betula pendula | Tegelberg et al. (2002) and Smite et al. (1995) |
| 85 | p-coumaric acid | Betula pendula | Rozema et al. (2001) |
| 86 | Ferulic acid | Betula pendula | Rozema et al. (2001) |
| 87 | 4-hydroxy-3-methoxyphenyl β-d-glucopyranoside (=tachioside) | Betula pendula | Smite et al. (1995) |
| 88 | 4-hydroxy-2-methoxyphenyl β-d-glucopyranoside (=isotachioside) | Betula pendula | Smite et al. (1995) |
Table 6.
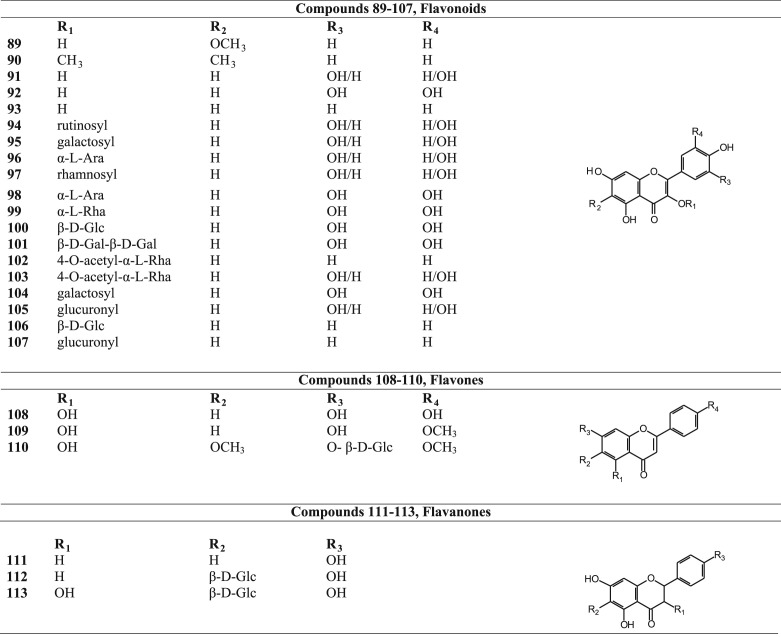
Flavonoids, flavones and flavanones isolated from Betula species.
| Name | Specie from which isolated | Reference | |
|---|---|---|---|
| Flavonoids | |||
| 89 | 6-methoxykaempferol | Betula maximowicziana | Fuchino et al. (1996b) |
| 90 | 6-methoxy-3-O-methylkaempferol | Betula maximowicziana | Fuchino et al. (1996b) |
| 91 | Quercetin | Betula pendula | Olszewska (2012) and Stark et al. (2008) |
| Betula pubescens | |||
| 92 | Myricetin | Betula pendula | Olszewska (2012) |
| 93 | Kaempferol | Betula pendula | Olszewska (2012) and Keinanen and Tiitto (1998) |
| Betula pubescens | |||
| 94 | Rutin | Betula dahurica | Fuchino et al., 1998b, Fuchino et al., 1998c and Horhammer et al. (1953) |
| Betula ovalifolia | |||
| Betula humilis | |||
| 95 | Hyperoside (quercetin-3-O-galactoside) | Betula pendula | Carnat et al. (1996) and Germano et al. (2012a) |
| 96 | Avicularin (quercetin-3-O-arabinoside) | Betula pendula | Carnat et al. (1996) and Germano et al. (2012a) |
| 97 | Quercitrin (quercetin-3-O-rhamnoside) | Betula pendula | Carnat et al. (1996) |
| 98 | Myricetin 3-O-α-l-arabinofuranoside | Betula schmidtii | Fuchino et al. (1998a) |
| 99 | Myricetin 3-O-α-l-rhamnopyranoside | Betula schmidtii | Fuchino et al. (1998a) |
| 100 | Myricetin 3-O-β-d-galactopyranoside | Betula schmidtii | Fuchino et al. (1998a) |
| 101 | Myricetin-3-digalactoside | Betula verrucosa | Horhammer et al. (1957) |
| Betula pubescens | |||
| 102 | Kaempferol 3-O-(4-O-acetyl)-α-l-rhamnopyranoside | Betula platyphylla | Fuchino et al. (1996a) |
| 103 | Quercetin 3-O-(4-O-acetyl)-α-l-rhamnopyranoside | Betula platyphylla | Fuchino et al. (1996a) |
| 104 | Myricetin-3-O-galactoside | Betula pendula | Carnat et al. (1996) and Germano et al. (2012a) |
| 105 | Quercetin-3-O-glucuronide | Betula pendula | Carnat et al. (1996) and Germano et al. (2012a) |
| 106 | Kaempfereol-3-O-glucoside | Betula pendula | Germano et al. (2012a) |
| 107 | Kaempfereol-3-O-glucuronide | Betula pendula | Germano et al. (2012a) |
| Flavones | |||
| 108 | Apigenin | Betula pendula | Olszewska (2012) |
| 109 | Acacetin | Betula pendula | Olszewska (2012) |
| 110 | 4′,6-Dimethoxy-5-hydroxyflavone-7-O-β-d-glucoside | Betula platyphylla | Lee (1994) |
| Flavanones | |||
| 111 | Naringenin | Betula maximowicziana | Fuchino et al. (1996b) |
| 112 | 6-C-glucosylnaringenin | Betula platyphylla | Lee (1994) |
| 113 | 6-C-glucosylaromadendrin | Betula platyphylla | Lee (1994) |
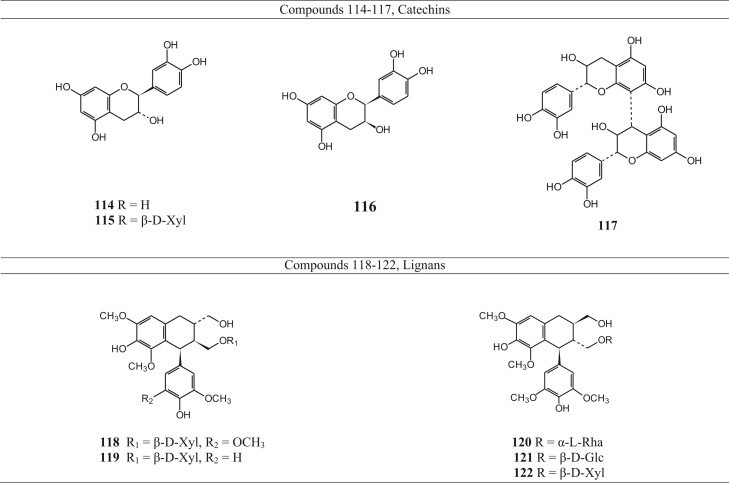
Table 7.
Catechins and lignana isolated from Betula species.
| Name | Specie from which isolated | Reference | |
|---|---|---|---|
| Catechins | |||
| 114 | (+) catechin | Betula dahurica | Tegelberg et al. (2002), Smite et al. (1995), Fuchino et al., 1996a, Fuchino et al., 1998a, Fuchino et al., 1998b, Fuchino et al., 1998c, Mshvildadze et al. (2007), and Li et al. (1998) |
| Betula ovalifolia | |||
| Betula papyrifera | |||
| Betula pendula | |||
| Betula platyphylla | |||
| Betula schmidtii | |||
| 115 | (+) catechin 7-O-β-d-xylopyranoside | Betula dahurica | Fuchino et al., 1995, Fuchino et al., 1996a, Fuchino et al., 1996b, Fuchino et al., 1998b, Fuchino et al., 1998c, Smite et al. (1995), and Mshvildadze et al. (2007) |
| Betula ermanii | |||
| Betula maximowicziana | |||
| Betula ovalifolia | |||
| Betula papyrifera | |||
| Betula pendula | |||
| Betula platyphylla | |||
| 116 | Epicatechin | Betula platyphylla | Li et al. (1998) |
| 117 | Procyanidin B-3 | Betula ovalifolia | Fuchino et al. (1998c) |
| Lignans | |||
| 118 | (-)-lyoniresinol 3α-O-β-d-xylopyranoside (=nudiposide) | Betula dahurica | Fuchino et al., 1995, Fuchino et al., 1998a, Fuchino et al., 1998b and Mshvildadze et al. (2007) |
| Betula ermanii | |||
| Betula papyrifera | |||
| Betula schmidtii | |||
| 119 | (-)-isolarisiresinol 3α-O-β-d-xylopyranoside | Betula pendula | Smite et al. (1995) |
| 120 | (+)-lyoniresinol 3α-O-α-l-rhamnopyranoside | Betula ermanii | Fuchino et al., 1995, Fuchino et al., 1996b |
| Betula maximowicziana | |||
| 121 | (+)-lyoniresinol 3α-O-β-d-glucopyranoside | Betula schmidtii | Fuchino et al. (1998a) |
| 122 | (+)-lyoniresinol 3α-O-β-d-xylopyranoside (=lyoniside) | Betula pendula | Smite et al. (1995) |
Table 8.
Steroids and miscellaneous compounds isolated from Betula species.
| Name | Specie from which isolated | Reference | |
|---|---|---|---|
| 123 | (3R)-3,5′-dihydroxy-4′-methoxy-3′,4″-oxo-1,7-diphenyl-1-heptene | Betula dahurica | Fuchino et al., 1996a, Fuchino et al., 1998a, Fuchino et al., 1998b, Fuchino et al., 1998c |
| Betula ovalifolia | |||
| Betula platyphylla | |||
| Betula schmidtii | |||
| 124 | 9,9′-di-O-feruloyl-(-)-secoisolariciresinol | Betula ermanii | Fuchino et al. (1995) |
| 125 | Ovalifoliolide A | Betula ovalifolia | Fuchino et al. (1998c) |
| 126 | Ovalifoliolide B | Betula ovalifolia | Fuchino et al. (1998c) |
| 127 | Caryophyllene oxide | Betula platyphylla | Fuchino et al. (1996a) |
| 128 | (2R,3R)-2,3-dihydro-3-hydroxymethyl-7-methoxy-2-(3′-methoxy-4′-α-l-rhamnopyranosyloxyphenyl)-5-benzofuranpropanol | Betula pendula | Smite et al. (1995) |
| 129 | [12β-acetoxy-4,4,8,10,14-pentamethyl-17-(2-methyl-5-oxotetrahydrofuran2(S)-yl)-hexadecahydrocyclopenta[a]phenanthren-3α-yl] hydrogen propanedioate | Betula pendula | Hilpisch et al. (1997) |
| 130 | 7α-hydroxy-β-sitosterol | Betula platyphylla | Fuchino et al. (1996a) |
| 131 | 7β-hydroxy-β-sitosterol | Betula platyphylla | Fuchino et al. (1996a) |
| 132 | β-sitosterol | Betula utilis | Khan et al. (2012) |
| 133 | Stigmast-4-ene-3-one | Betula platyphylla | Fuchino et al. (1996a) |
| 134 | Platyphyllin A | Betula platyphylla | Wang et al. (2001) |
| 135 | Methyl (12R,20S)-20-hydroxy-12-β-d-xylopyranosyloxy-3,4-secodammara-4(28),24-dien-3-oate (betula-schmidtoside A) | Betula schmidtii | Fuchino et al. (1998a) |
| 136 | Hydroxyhopanone | Betula platyphylla | Fuchino et al. (1996a) |
| 137 | Benzyl alcohol β-d-apiofuranosyl-(1→6)-β-d-glucopyranoside | Betula maximowicziana | Fuchino et al. (1996b) |
Phytochemical investigations revealed that the triterpenoids, generally present in the leaves and bark of the Betula species, belong mainly to the ocotillol and dammarane type of triterpenoids (Fig. 2, Table 2). Several of them are esterified with malonic acid and caffeic acid at C-3 position. Other reperted triterpenoids belong mainly to the oleanane, lupane and fernane series. Betulin (48) and betulinic acid (54), belonging to the lupane series, are two important triterpenoids present in Betula species. Diarylheptanoids, both cyclic and acyclic (Fig. 3, Table 3), phenylbutanoids (Fig. 4, Table 4) and other phenolics (Fig. 5, Table 5) form the other major groups of compounds present and are found to occur mainly in the leaves. Platyphylloside (66) is a diarylheptanoid glycoside that has been isolated and identified. Presence of flavonoids (Fig. 6, Table 6) and catechins and lignans (Fig. 7, Table 7) is a regular feature. A few steroids and other compounds (Fig. 8, Table 8) have also been reported.
Besides these, Demirci et al. (2004) analyzed the essential oil of Betula pendula buds and identified more than 50 compounds. The main components found were α-copaene, germacrene D and δ-cadinene. The major components of the volatile oil from the inner bark of Betula pendula were trans α-bergamotene and α-santalene (Weston and Smith, 2012). Tea infusion of Betula pendula, which is used in herbal teas in Europe, was analyzed for the occurrence and content of quercetin and rutin and other minor biologically active compounds (Dadáková et al., 2010).
Procyanidin glycosides, that are still rarely found in nature, were isolated from the bark of Betula pendula by Liimatainen et al. (2012a). The polymeric proanthocyanidins in the birch leaves were determined by Karonen et al. (2006). Klejdus et al. (2008) determined the amino acid composition of Betula pendula leaves. Salminen et al., 1999, Salminen et al., 2001, Salminen et al., 2002 have worked extensively on the hydrolysable tannins of Betula. Rimpler et al. (1966) made a comparative study of the triterpenes of Betula pendula and Betula pubescens. Rickling and Glombitza (1993) have reported hemolytic dammarane triterpenoid esters from Betula pendula. Smite et al. (1993) have reported the presence of several arylbutanoid and diarylheptanoid glycosides in Betula pendula. Cˆınt˘a-Pˆınzaru et al. (2012) employed modern techniques such as FT-Raman and FT-IR spectroscopy in conjunction with GC–MS to characterize the raw bark and natural extract products obtained from the Betula species and to evaluate their potential to directly identify the main active compounds from birch bark natural extract products.
Chemotaxonomic and ecological studies have been carried out using rhododendrin, platyphylloside, phenolics and flavonoids (Santamour and Lennart, 1996, Santamour and Lennart, 1997, Mämmelä, 2001, Valkama et al., 2003, Valkama et al., 2004, Shults et al., 2005, Lahtinen et al., 2006, Stark et al., 2008, Millet et al., 2010, Muilenburg et al., 2011, Liimatainen et al., 2012b).
5. Pharmacological activity
The varied ethnomedicinal uses of the different species of Betula have led to the initiation of many pharmacological investigations. Previous research demonstrates that the Betula species exhibit a wide range of biological activities, such as anticancer, anti-inflammatory and immunomodulatory and others like antioxidant, antidiabetic, antiviral and antiarthritic activities. An overview of the modern pharmacological evaluations carried out on these species has been described in greater detail in the following sections.
5.1. Anticancer
Different Betula species were subjected to varied experiments and assays in order to determine their anticancer activity. The different human as well as murine cell lines against which the cytotoxicity studies have been conducted have been listed in Table 9.
Table 9.
Cell lines used for cytotoxicity studies.
| Cell line | Origin tissue | Reference |
|---|---|---|
| A2780 | Human ovarian carcinoma | Dehelean et al. (2012) |
| A431 | Human skin epidermoid carcinoma | Dehelean et al. (2012) and Şoica et al. (2012) |
| A-549 | Human lung carcinoma | Mshvildadze et al. (2007) |
| DLD-1 | Human colorectal adenocarcinoma | Mshvildadze et al. (2007) |
| HeLa | Human cervix adenocarcinoma | Dehelean et al. (2012) and Şoica et al. (2012) |
| HL-60 | Human promyelocytic leukemia cells | Ju et al. (2004) |
| K562/Adr | Human multidrug-resistant cancer cells, resistant to adriamycin | Kashiwada et al. (2007) |
| KB-C2 | Human multidrug-resistant cancer cells | Kashiwada et al. (2007) and Xiong et al. (2010) |
| MCF7 | Human breast adenocarcinoma | Dehelean et al. (2012) and Şoica et al. (2012) |
| ATCC L1210 | Mouse lymphocytic leukemia cells | Goun et al. (2002) |
| HSC-T6 | Rat liver stellate cells | Lee et al. (2012) |
| V79-4 | Chinese hamster lung fibroblast cells | Ju et al. (2004) |
The leaves and buds of Betula pubescens (Betula alba) have been reported to be used for the treatment of cancer of the uterus (Balitski and Vorontsova, 1980).
The methylene chloride as well as the methanol extracts of Betula pendula were evaluated for the activity against leukemia and thrombin (Goun et al., 2002). A cytotoxicity assay was used with L1210 as target cells. This assay determines the inhibitory effect of test samples on the growth of mouse leukemia cells (ATCC L1210). The extracts exhibited 99% and 91% activity respectively at 10 mg per well concentration of the extract. They however exhibited only 15% and 7% antithrombin activity respectively when compared with the blank solution. Antiproliferative activity of Betula pendula was also studied on B16 melanoma cells (Calliste et al., 2001). Şoica et al. (2012) studied the antiproliferative effects of Betula pendula bark and its two major compounds betulin (48) and betulinic acid (54) in vitro on three human cell lines: HeLa (cervix adenocarcinoma), MCF7 (breast adenocarcinoma) and A431 (skin epidermoid carcinoma), using the MTT ([3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]) assay. They were found to possess antiproliferative activities, as is also suggested in the literature against other tumoural cell lines (Fulda, 2008, Drag et al., 2009). Although the birch bark extract presented antiproliferative activity, it was not very different to the main compound in its composition, betulin. They could both be considered important therapeutic compounds for skin pathology but also for other types of hyperproliferative pathologies, including other solid cancers. Although the exact IC50 values were not determined, the results revealed considerable efficacies of both the extract and its active components against A431 and HeLa cell lines. The concentration range in which the proliferation of skin cancer cells was substantially inhibited (approximately 70–80%) may present practical significance, especially in the case of local administration. The in vitro data suggested the high efficiency of betulinic acid and also of the related compound betulin as anticancerous agents, but further that the exploitation of their natural source, birch bark extract, could prove to be very useful. Betulin enriched extracts of the bark of Betula pendula, containing over 90% betulin, were also tested for their growth inhibiting effects in vitro on four malignant human cell lines: A431 (skin epidermoid carcinoma), A2780 (ovarian carcinoma), HeLa (cervix adenocarcinoma) and MCF7 (breast adenocarcinoma), by means of MTT assay (Dehelean et al., 2012). All of the prepared bark extracts exerted a pronounced antiproliferative effect against human cancer cell lines. The goal of the MTT assay was a direct comparison of the extracts, concluding that the IC50 values of all the samples was between 1 and 5 μg/mL. The substantial differences in betulin and betulinic acid content of the extracts were not reflected in the antiproliferative activities. The reason of this contradiction could be the presence of other active natural compounds in birch bark, including flavonoids.
Mshvildadze et al. (2007) carried out in vitro investigations on the methanolic extract of the inner bark of Betula papyrifera and found it to exert a cytotoxic activity against human lung carcinoma (A-549; IC50, 104±3 µg/mL) and colorectal adenocarcinoma (DLD-1; IC50, 79±2 µg/mL) cell lines. The bioassay-guided fractionation of the crude extract led to the isolation of 10 phenolic compounds including diarylheptanoid glycosides, a diarylheptanoid, a lignan and flavonoids. In vitro cytotoxic activities of the isolated compounds were assessed against lung carcinoma (A-549) and colorectal adenocarcinoma (DLD-1) human cell lines, as well as against human normal skin fibroblasts (WS1) using the resazurin reduction test. Among the isolated compounds, platyphylloside (66), a diarylheptanoid glycoside, exerted the most potent cytotoxic activity (IC50, 10.3–13.8 µM), showing stronger activity than 5-fluorouracil towards the DLD-1 cell line. In addition, a diarylheptanoid and 2 other diarylheptanoid glycosides displayed moderate cytotoxicities against all tested cell lines. The other compounds did not exhibit any significant in vitro cytotoxicity against tested cancer cell lines.
Extracts of birch bark were also tested in actinic keratosis. The most important pathogenetic factor in the development of actinic keratoses is ultraviolet B (UVB) radiation. Mutations in keratinocytes caused by UVB radiation lead to abnormal cell proliferation and disturbed repair of gene defects and also interfere with apoptosis by inactivating protein p53. Actinic keratoses are considered an initial, still non-invasive form of squamous cell carcinoma. A pilot study using a standardized birch bark ointment was performed. Treatment response was assessed clinically after 2 months. The standardized birch bark extract was found to be effective in the treatment of actinic keratoses and had no side effects. This birch bark ointment may be a new therapeutic option for actinic keratoses (Huyke et al., 2006).
Studies were carried out to investigate whether Betula platyphylla var. japonica inhibits H2O2-induced oxidative stress in Chinese hamster lung fibroblast (V79-4) cells and to characterize the mechanism of its anticancer effects in human promyelocytic leukemia (HL-60) cells. It was observed that the total methanol extract of Betula platyphylla had protective effects against hydrogen peroxide (H2O2) in the Chinese hamster lung fibroblast (V79-4) cell line and induced apoptotic cell death in human promyelocytic leukemia (HL-60) cell line. It significantly increased cell viability against H2O2 induced oxidative stress. The extract reduced the number of V79-4 cells arrested in G2/M in response to H2O2 treatment. Treatment with the extract induced cytotoxicity and apoptosis in HL-60 cells, as shown by nucleosomal DNA fragmentation, increases in the subdiploid cell population, and fluorescence microscopy. It gradually increased the expression of pro-apoptotic Bax and led to the activation of caspase-3 and cleavage of PARP. The findings suggested that Betula platyphylla var. japonica exhibits potential anticancer properties (Ju et al., 2004).
Bioassay-guided fractionation and repeated chromatography of the methanol extract of the floral spikes of Betula platyphylla var. japonica led to the isolation of 20 triterpenoids. The cytotoxicity of the isolated triterpenes against human cancer cell lines as well as multidrug-resistant cancer cell lines was evaluated. Most of the isolated triterpenes showed very weak cellular toxicities. Although no discernible differences were found in the cytotoxicities for the tested compounds against sensitive and resistant cell lines, the cytotoxicities for several triterpenes against multidrug-resistant cancer cell lines (KB-C2 or K562/Adr) were enhanced in the presence of nontoxic concentrations of colchicine or doxorubicin (Kashiwada et al., 2007). Since one of the major causes of multidrug resistance (MDR) in cancer cells is over-expression of P-glycoprotein (P-gp), the MDR reversing activity might be involved in inhibition of P-gp ATPase (Sekiya et al., 2007).
5.2. Antiinflammatory
A number of extracts from Betula species have shown anti-inflammatory activity in different test models. Tunon et al. (1995) tested the aqueous extract of the leaves of Betula pendula for anti-inflammatory activity using isolated cells and enzymatic tests in vitro. It was evaluated for inhibitory activity on prostaglandin biosynthesis and platelet activating factor (PAF)-induced exocytosis in vitro at a concentration of 0.2 mg/mL and 0.25 mg/mL respectively. The leaves exhibited 23±2% prostaglandin synthesis inhibition and 76+4% PAF-exocytosis inhibition. The effects shown by the extracts of the leaves of Betula pendula might be explained by the high contents of tannins and other kinds of polyphenols. Several hemolytic compounds of dammarane type that have been isolated from the leaves of Betula pendula might affect the neutrophils in the PAF-test and thus influence the result. Later, Wacker et al. (2012) demonstrated the effect of Betula pendula leaf extract (BPE) on corneal inflammation following keratoplasty in the rat model. T cells were stimulated in vitro in the presence of BPE. Proliferation, activation phenotype and the number of apoptotic/necrotic cells in cell culture were analyzed by flow cytometry. Corneal transplantation was performed between Fisher and Lewis rats. Recipient rats were either treated with cyclosporine A at a low dosage (Low-dose CsA=LDCsA) or received LDCsA in combination with BPE (2×1 ml/day). Clinical signs for corneal inflammation and rejection time points were determined. Infiltrating leukocytes were analyzed histologically. BPE specifically inhibited T cell proliferation in vitro by inducing apoptosis. The phenotype was not affected. In vivo, BPE significantly delayed the onset of corneal opacification (p<0.05). The amount of infiltrating CD45(+) leukocytes and CD4(+) T cells (p<0.001) was significantly reduced by BPE, whereas infiltration of CD163(+) macrophages was not significantly different between the two groups. BPE selectively induces apoptosis of activated T cells. Accordingly, BPE treatment significantly reduces infiltrating T cells and subsequent corneal opacification following keratoplasty. The findings suggest BPE as a promising anti-inflammatory drug to treat corneal inflammation. Dehelean et al. (2012) conducted in vivo studies involving the anti-inflammatory effect of Betula pendula extracts on 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced model of inflammation in mice. It is well known that in a few hours application of 12-O-tetradecanoylphorbol-13-acetate (TPA) can induce an inflammatory process to mouse ears by increasing vascular permeability, producing edema and swelling inside dermis. Indometacin was used as reference. Both betulin and ethanolic extracts (1 pp and 3 pp) were used to determine the reduction of edema. Betulin was found to be comparable with indometacin; however, the extracts revealed the most intense anti-inflammatory potential probably because of the aggregation of effects of other triterpenes, also present in the extract composition, even in small concentrations (betulinic acid, lupeol etc.).
The anti-inflammatory activity of Betula alnoides extract was evaluated in acute and subacute inflammation models. The extract was also evaluated for antiinflammatory activity in sheep RBC induced sensitivity and in membrane stabilization models (Sur et al., 2002). The inhibitory percentage of the extract was high 40.7% at a dose of 100 mg/kg and increased in a dose dependent manner, on carrageenan induced paw edema in mice. The inhibitory percentage of the extract on granuloma tissue formation was 29.7% at a dose of 100 mg/kg and 47.63% at a dose of 200 mg/kg. Although the extract was not statistically significant on sheep RBC-induced sensitivity, the inhibitory percentage on the rabbit membrane lysis was high; 56.25+2.29 at a concentration of 250 µg/mL and increased to 72.19+3.18 at a concentration of 500 µg/mL.
The inhibitors of prostaglandin biosynthesis and nitric oxide production have been considered as potential anti-inflammatory and cancer chemopreventive agents (Hong et al., 2002). Methanolic extract of the cork of Betula platyphylla was screened for its inhibitory effect on prostaglandin biosynthesis and nitric oxide production in LPS-stimulated RAW264.7 cell, a murine macrophage cell line at the test concentration of 10 µg/mL. However only 25.9% inhibition of COX-2 activity was observed.
5.3. Arthritis and rheumatism
One of the prominent uses of Betula species in traditional medicine is against arthritis and rheumatism. Several studies have been carried out to support these claims. In vitro xanthine oxidase inhibitory properties of plants traditionally used in Czech Republic and Central-East Europe region for gout, arthritis or rheumatism treatment were studied by Havlik et al. (2010). Since gouty arthritis or uric acid nephrolithiasis is a result of marked hyperuricemia, leading to the deposition of monosodium urate crystals in joints or kidneys, the enzyme xanthine oxidase is a possible target for urate-lowering drugs in humans and is used predominately in hypouricemic therapy. Betula pendula and Populus nigra were identified as species with the highest xanthine oxidase inhibitory potential in the study. The 80% ethanolic and methylene chloride–methanolic extracts of Betula pendula exhibited IC50 values of 39.4 and 25.9 µg/mL, respectively. Betula pendula was among the most active supposedly because of the content of salicylates or other phenolics. In another study (Gründemann et al., 2011), in vitro experiments were conducted to investigate the influence of the extract of Betula pendula on primary human lymphocytes in comparison to the synthetic anti-arthritis drug methotrexate. Lymphocyte proliferation and cell division were measured by the activity of mitochondrial dehydrogenases and by using the membrane-permeable dye carboxyfluorescein diacetate succinimidyl ester (CFSE), respectively. Apoptosis was analyzed by surface staining of phosphatidylserine and intracellular activation of effector caspases 3 and 7 in comparison to the drug methotrexate using flow cytometric and photometrical analysis. In addition, the impact of the extract on cell cycle distribution was investigated by propidium iodide staining of DNA. For the bioassays BPE concentrations of 10–160 µg/mL were investigated. Leaf extracts of Betula pendula inhibited the growth and cell division (CD8+: 40 µg/mL: 45%; 80 µg/mL: 60%; 160 µg/mL: 87%) (CD+: 40 µg/mL: 33%; 80 µg/mL: 54%; 160 µg/mL: 79%) of activated, but not of resting T lymphocytes in a significant dose-dependent manner. The inhibition of lymphocyte proliferation due to apoptosis induction (compared to untreated control: 40 µg/mL: 163%; 80 µg/mL: 240%; 160 µg/mL: 348%) and cell cycle arrest was comparable to that of methotrexate. It is known that peripheral blood lymphocytes play an important role in the perpetuation of the autoimmune processes in rheumatoid arthritis and the maintenance of these cells might be caused by the dysregulation of proliferation and apoptosis. The study, thus, gave a rational basis for the use of Betula pendula leaf extract for the treatment of immune disorders, like rheumatoid arthritis, by diminishing proliferating inflammatory lymphocytes
Osteoarthritis is a degenerative joint disease characterized by the progressive loss of articular cartilage, subchondral bond remodeling, spur formation, synovial inflammation and the degradation of proteoglycan and collagen. The integrity of these macromolecules is vital to cartilage and joint function. Cho et al. (2006) investigated the cartilage protective effects and mechanism of Betula platyphylla on rabbit articular cartilage. It was observed that the bark extract of Betula platyphylla inhibited the degradation of proteoglycan and collagen through the down regulation of MMP-3 and MMP-13 expressions and activities without affecting the viability or morphology of IL-1α-stimulated rabbit articular cartilage explants. The n-butanol fraction from the bark of Betula platyphylla (BFBP) was identified as the most potent cartilage protective fraction. It was shown to have protective effects against cartilage degradation in a collagenase-induced osteoarthritis rabbit model (Huh et al., 2009). Oral administration of BFBP dose-dependently suppressed the stiffness and global histologic score. The proteoglycan content was considerably increased in a dose-dependent manner in the BFBP treated group. The mRNA expression of MMP-1 and MMP-3 was also decreased. On the contrary, the level of TIMP-1 in the synovial fluids was significantly increased in the BFBP treated group. The pathologic inflammatory molecules such as PGE2 and COX-2 were inhibited by BFBP, but COX-1 expression was not affected. It was, therefore, suggested that BFBP showed the protective effect on cartilage alternations through balance of MMPs/TIMP-1. BFBP is a COX-2 inhibitor through suppressing the production of PGE2 and inhibiting of expression of COX-2 in CIA. It regulated inflammatory-related molecules in in vivo model of OA. Huh et al. (2011) also investigated the inhibitory effects of Betula platyphylla in IL-1β-stimulated rheumatoid arthritis fibroblast-like synoviocytes derived from patients with rheumatoid arthritis. The anti-nociceptive and anti-inflammatory efficacy was compared to celecoxib, a selective COX-2 inhibitor, in animal models of arthritis. Betula platyphylla significantly inhibited proliferation of IL-1β-induced synoviocytes. It reduced the levels of inflammatory mediators, such as IL-6, TNF-α, MMP-1, MMP13, and PGE2. The release of nitrites and iNOS, as well as release of NF-κB, into the nucleus of IL-1β-treated synoviocytes was significantly inhibited, even at concentrations as low as 1 µg/mL. Oral administrant of Betula platyphylla at 400 mg/kg significantly decreased about 27.8% of tail flick withdrawal and inhibited the number of paw flinches in both phases 1 and 2 of the formalin test. In the carrageenan-induced acute pain and arthritis model, Betula platyphylla dose dependently reduced the nociceptive threshold and the arthritic symptoms at day 8, respectively. At 400 mg/kg, it markedly reduced the inflammatory area about 48% in the ankle joints. This capacity of Betula platyphylla at 400 mg/kg was similar to that of the celecoxib-2 inhibitor in carrageenan-induced nociceptive and inflammatory arthritis model. These results suggested that Betula platyphylla has anti-nociceptive and anti-inflammatory effects in IL-1β-stimulated RA FLS and in an animal model of arthritis.
5.4. Antioxidant
Various Betula extracts were thus investigated for their free radical scavenging activities. Betula pendula was sequentially percolated with five solvents of increasing polarities (hexane, chloroform, ethyl acetate, methanol, and water). These were screened for antioxidant activity. The free radical scavenging activities were examined in different systems using electron spin resonance (ESR) spectroscopy. These assays were based on the stable free radical DPPH, the hydroxyl radicals generated by a Fenton reaction, and the superoxide radicals generated by the X/XO system. It possessed high antioxidant activities for the most polar fractions (Calliste et al., 2001).
The total methanol extract of Betula platyphylla var. japonica exhibited protective effects against hydrogen peroxide (H2O2) in the Chinese hamster lung fibroblast (V79-4) cell line. The extract also showed high DPPH radical scavenging activity (IC50 2.4 µg/ml) and lipid peroxidation inhibitory activity (IC50 below 4.0 µg/mL). Furthermore, the extract increased the activities of several cellular antioxidant enzymes, including superoxide dismutase, catalase and glutathione peroxidase (Ju et al., 2004).
Ghimire et al. (2012) investigated the 80% methanolic extract of Betula alnoides and its sub-fractions for antioxidant activities by using antioxidant tests, including electron donation ability test, reducing power, and metal-chelating activity assay. The results showed that 80% methanolic extracts exhibited high DPPH scavenging activity (80.68%). In addition, both the 80% methanolic extract and EtOAc fraction exhibited more potent reducing activity than did butylated hydroxyanisole (BHA) and trolox. The aqueous fraction had higher metal-chelating activity than other fractions. The EtOAc fraction had the highest phenolic and flavonoid content (217.73±1.02 mg GAE/g extract, and 38.42±1.87 mg QE/g extract, respectively).
5.5. Antimicrobial and antiviral
A number of extracts from Betula species have demonstrated antibacterial, antifungal and antiviral activities against numerous pathogenic strains. Jones et al. (2000) noted that the ethanolic extract of Betula allegheniensis showed activity against Saccharomyces cerevisiae while Webster et al. (2008) observed that Betula alleghaniensis showed a significant degree of activity against many of the yeast isolates. It had the strongest activity against Saccharomyces cerevisiae, with activity observed at 200 mg/mL at 72 h. White birch (Betula pubescens) was found to be active against gram-positive Staphylococcus aureus (Rauha et al., 2000). The 80% methanolic extract of Betula alnoides and its sub-fractions were investigated for antioxidant and antimicrobial activities. The 80% methanolic extract and EtOAc fraction showed higher levels of antimicrobial activity than did other fractions (Ghimire et al., 2012).
The ethanolic extract of the wood and bark of Betula papyrifera along with 13 other eastern North American hardwood tree species was screened for antimicrobial activity against eight strains of bacteria and six strains of fungi (Omar et al., 2000). Bacterial strains tested included the gram positive strains: Staphylococcus aureus methicillin-sensitive, Enterococcus faecalis, Mycobacterium phlei, Bacillus subtilus, and the gram negative strains: Eschericia coli wild strain, Pseudomonas aeruginosa 187 (wild). Staphylococcus aureus causes serious food intoxication; Enterococcus faecalis, Salmonella typhimurium, Eschericia coli, Bacillus subtilus, Pseudomonas aeruginosa 187 (wild), and Mycobacterium phlei cause food spoilage and human infection whereas the toxin from Klebsiella pneumonia is known for fish poisoning. The fungal strains used in this study were opportunistic pathogens of humans except for Saccharomyces cerevisiae. Cryptococcus neoformans causes generalized mycosis with a predilection for the central nervous system, Candida albicans causes oral thrush and systemic infections, Aspergillus fumigatus may be associated with respiratory infections and Microsporum gypseum and Trichophyton mentagrophytes are dermatophytes. The bark extract was found to be active against Staphylococcus aureus (methicillin-sensitive). When tested against Bacillus subtilus, Enterococcu faecalis, Mycobacterium phlei, the bark extract showed zones of growth inhibition.
The methanolic extracts obtained from external and internal bark, flowers, leaves and buds of Betula pendula were also evaluated for their antibacterial activity. Triterpene compounds, betulin, betulinic acid, oleanolic acid and lupeol, isolated from the external parts of birch bark, were also investigated for their antibacterial activity against selected Gram-positive bacteria, Bacillus subtilis, Staphylococcus aureus and Gram-negative bacteria, Escherichia coli and Pseudomonas aeruginosa. The most prominent antibacterial activity was shown by oleanolic acid against bacterial species Staphylococcus aureus and Bacillus subtilis while Escherichia coli showed resistance on all investigated samples (Duric et al., 2013).
Antibacterial activity of aqueous extracts and solvent extracts of Betula utilis bark was determined by a cup diffusion method on nutrient agar medium against 14 important human pathogenic bacterial cultures. The methanol and ethanol extracts recorded antibacterial activity against all the test pathogens (Kumaraswamy et al., 2008).
Methanolic plant extract of Betula papyrifera was screened for antiviral activity against seven viruses viz. bovine coronavirus (BCV, Coronaviridae), bovine herpesvirus type 1 (BHVl, Herpesviridae), bovine parainfluenza virus type 3 (BP13, Paramyxoviridae), bovine rotavirus (BRV, Reoviridae), bovine respiratory syncytial virus (BRSV, Paramyxoviriaize), vaccinia virus (Poxviridae) and vesicular stomatitis virus (VSV, Rhabdoviridae). However no significant activity was observed (McCutcheon et al., 1995).
5.6. Dermatological uses
Kim et al. (2008) investigated if and how the extract of the bark of Betula platyphylla Sukat. var. japonica Hara (Asian white birch, AWB) inhibited the development of atopic dermatitis (AD) like skin lesions in NC/Nga mice, which is a recently recognized murine model of AD. The skin symptom severity, itching behavior, serum IgE level and mRNA expression of the cytokines at lymph node in the mice were examined. The hapten-induced dermatitis model was used in this study since repeated hapten (picryl chloride; PC) treatment causes apparent dermatitis in 100% of NC/Nga mice. Oral administration of AWB extracts (25, 100 and 250 mg/kg) to the PC-treated mice inhibited the development of AD-like skin lesions as exemplified by a significant decrease in the total skin severity scores, itching behavior and a decrease in hypertrophy and infiltration of inflammatory cells into dermis. The serum IgE level was also significantly reduced by AWB extract. In the RT-PCR results, the expression of interleukin-4 mRNA was reduced by AWB extract, whereas the expression of interferon-γ mRNA was not changed. These results suggest that AWB inhibits the development of AD-like skin lesions in NC/Nga mice through the suppression of the T(H)2 cell response. Besides, Oh et al. (2012) carried out in vitro and in vivo studies to elucidate whether and how Betula platyphylla modulated the mast cell-mediated allergy inflammation. Pharmacological effects of Betula platyphylla on both compound 48/80 or histamine-induced scratching behaviors and 2,4-dinitrochlrobenzene (DNCB)-induced atopic dermatitis in mice were ascertained. Additionally, to find a possible explanation for the anti-inflammatory effects of Betula platyphylla, the effects of Betula platyphylla on the release of histamine in compound 48/80-induced rat peritoneal mast cells (RPMCs), production of inflammatory mediators and activation of nuclear factor-κB (NF-κB) and caspase-1 in phorbol 12-myristate 13-acetate plus calcium ionophore A23187 (PMACI)-stimulated human mast cells (HMC-1) were also evaluated. The finding of this study demonstrated that Betula platyphylla reduced compound 48/80 or histamine-induced scratching behaviors and DNFB-induced atopic dermatitis in mice. Additionally, it also inhibited the release of histamine in RPMC and production of inflammatory cytokines as well as the activation of NF-κB and caspase-1 in stimulated HMC-1. Collectively, the findings of this study provided novel insights into the pharmacological actions of Betula platyphylla as a potential molecule for use in the treatment of allergic inflammatory diseases.
Reports show that North American natives made extensive use of barks and resins from trees for treating dermatological conditions. Studies were thus carried out to evaluate the antioxidant activities of ethanolic and hot water extracts from barks of Canadian wood species, their toxicological effects on normal human keratinocytes and the antiproliferative properties of these extracts on the growth of normal, lesional and non-lesional psoriatic keratinocytes. These properties of crude extracts from barks of Canadian species were also compared with those of the standardized French maritime pine bark extract (Oligopin®) (García-Pérez et al., 2010). The results showed that yellow birch (Betula alleghalensis) extract obtained by maceration had high antioxidant capacity. Also, that after 24 h the initial toxicities of YBMac and Oligopin® determined by the TBDE method are comparable. After 48 h, the initial toxicity of YBMac did not significantly vary and Oligopin® showed to be less toxic. IC90 values determined by the TBDE method after 24 h showed an inverse correlation with the content of CinnAc (r=−0.73; p=0.0246) and PAs (r=−0.66; p=0.049) whereas after 48 h no correlations could be determined between IC90 and the different classes of phenolic compounds. Toxicity of extracts on keratinocyte plasma membrane and mitochondria after 24 h was attributed to their content of hydroxycinnamic acids and proanthocyanidins. The mean degree of polymerization of proanthocyanidins (DPm) of crude extract from barks of Betula spp. is reported to be 6.4. Thus, it could be hypothesized that the higher degree of polymerization of YBMac proanthocyanidines and the presence of terpenic compounds in this extract, which can be extracted with ethanol at high concentrations (Diouf et al., 2009), could influence on the higher initial toxicity observed on keratinocytes plasma membrane after 24 h. The crude extracts from bark of Canadian wood species and Oligopin® presented a modest inhibition on the growth of normal and psoriatic keratinocytes, but were not selective for lesional psoriatic cells after exposure during 48 h. YBMac at 90 µg/mL was shown to inhibit by 26% the NHK growth, but it failed to induce any change in lesional and nonlesional PK growth. These studies demonstrated the toxicological and antiproliferative properties of polyphenolic extracts from barks of yellow birch as well as other Canadian species on normal and psoriatic keratinocytes.
It has been observed that with a recently developed triterpene extract from the outer bark of Betula pubescens syn. Betula alba, with over 80% betulin, a cream can be produced without the aid of emulsifiers. It only consists of 4.5% (v/v) birch bark extract, vegetable oil and water. In an artificially damaged skin barrier, a comparable or even superior effect of the birch bark cream with respect to improvement in stratum corneum hydration, reduction of TEWL and skin erythema as opposed to ‘hydrophilic cream NRF’ (consisting of nonionic emulsifying alcohols, 2-ethylhexyllauromyristate, glycerol, potassium sorbate, citric acid and water) was seen. In vitro, birch bark extract increased calcium influx into primary keratinocytes and upregulated various differentiation markers including keratin-10 and involucrin. Topical treatment with an oleogel containing 10% (v/v) birch bark extract of actinic keratosis, which represent in situ squamous cell carcinomas with disturbed epithelial differentiation, resulted in upregulation of keratin-10 in situ. Thus, bark displays skin-barrier-reinforcing properties that may be used in dermocosmetics for dry skin (Casetti et al., 2011).
Birch leaves extracts are included in many skin cosmetic products. The potential ability of Betula pendula leaves ethanolic extract (BE) for the development of skin whitening agents was evaluated on mushroom tyrosinase activity (Germano et al., 2012b). Results showed that BE was capable of inhibiting, dose-dependently, l-DOPA oxidation catalyzed by tyrosinase. The inhibition kinetics, analyzed by Lineweaver-Burk plots, showed a noncompetitive inhibition of BE towards the enzyme, using l-DOPA as substrate. The inhibitory mechanism of BE as studied by spectrophotometric analysis, demonstrated its ability to chelate copper ion in the active site of tyrosinase. In addition, BE exhibited Fe(2+)-chelating ability (IC50 614.12±2.14 μg/mL), reducing power and radical-scavenging properties (IC50 137.22±1.98 μg/mL). These results suggest the usefulness of birch leaves extracts in cosmetic and pharmaceutical industries for their skin-whitening and antioxidant effects.
5.7. Immunoregulatory
Studies have shown that birch bark extracts can have immunoregulatory effects. The effects of birch bark from Betula pubescens on immune system were studied by Freysdottir et al. (2011). Human monocyte-derived dendritic cells (DCs) were matured with or without dried Betula bark ethanolic extract (DBBEE) or its fractions I–V at several concentrations. The effects of the extract and fractions on DC maturation were determined by measuring cytokine secretion by ELISA and expression of surface molecules by flow cytometry. DBBEE and fractions III and IV reduced DC secretion of IL-6, IL-10 and IL-12p40 and expression of CD83, CD86, CCR7 and DC-SIGN compared with control DCs. Proliferation of allogeneic CD4(+) T cells co-cultured with DCs matured with fraction IV, as measured by (3)H-thymidine incorporation, was similar to proliferation of allogeneic CD4(+) T cells co-cultured with control DCs. However, IFN-γ secretion was reduced and IL-10 and IL-17 secretion was increased, a cytokine profile consistent with a Th17 regulatory phenotype. These results indicated that bark from Betula pubescens contained compound(s) that could modulate DCs so that their interaction with T cells leads to an immunoregulatory response.
Betula pubescens (syn Betula alba) aqueous pollen extracts (Bet-APEs) and pollen-associated phytoprostanes were also analyzed for their immunomodulatory capacities in the murine system, both, in vitro and in vivo (Gutermuth et al., 2007). It has been demonstrated that the pollens modulate human dendritic cell (DC) function in a way that results in an enhanced T(H)2 polarization in vitro. DC function was analyzed in vitro by using BALB/c bone marrow-derived DCs. T-cell responses were analyzed with DO11.10 peptide 323-339 from chicken ovalbumin (OVA)-specific CD4 T cells as responder cells. For in vivo studies, OVA-specific CD4 T cells were adoptively transferred into BALB/c mice. 24 h later, mice were challenged by means of intranasal application of OVA in the absence or presence of Bet-APEs or phytoprostanes. Polarization of T-cell responses in vivo was analyzed in draining lymph node cells. In vitro Bet-APEs and E(1)-phytoprostanes dose-dependently inhibited LPS-induced IL-12p70 of DCs. In addition, Bet-APEs induced a T(H)2 polarization in vitro. Similarly, intranasal instillation of Bet-APEs in vivo, together with the antigen, leads to increased IL-4, IL-5, and IL-13 secretion and decreased IFN-gamma secretion from antigen-specific T cells in the draining lymph nodes. In contrast, intranasal E1- and F1-phytoprostanes downregulated both T(H)1 and T(H)2 cytokine production in vivo. Thus, Betula alba pollen releases water-soluble factors that display T(H)2-polarizing capacities in vivo independently of E(1)- and F(1)-phytoprostanes.
5.8. Hepatoprotective
Matsuda et al. (1998) showed the 50% aqueous methanolic extract from the bark of Betula platyphylla Sukat. var. japonica (MIQ). Hara possessed potent inhibitory activity on the liver-injury induced by CCl4 or d-galactosamine (d-GalN)/lipopolysaccharide as well as O2-scavenging and antioxidative activities. From the 50% aqueous methanolic extract, 20 compounds were isolated. Four of these compounds showed protective activity against d-GalN-induced cytotoxicity in primary cultured rat hepatocytes. Furthermore, several aromatic constituents exhibited potent O2-scavenging and antioxidative activities.
In another study, the antifibrotic effects of seven diarylhepanoids, isolated from the n-butanol fraction of the inner bark of Betula platyphylla, were evaluated with HSC-T6 cells by assessing cell proliferation (Lee et al., 2012). Among them, four compounds significantly inhibited the proliferation of HSCs in a dose-dependent manner at concentrations from 10 µM to 100 µM. One compound in particular dramatically decreased the collagen content and increased the Caspase-3/7 activity. The results suggested that the antifibrotic activity of Betula platyphylla and its constituents might possess therapeutic potential against liver fibrosis.
The ethanolic and aqueous extracts of Betula utilis bark were also subjected to in vitro and in vivo hepatoprotective activity against d-GalN induced hepatic damage. Since all the biochemical parameters were restored to normal significantly, the findings suggested that Betula utilis extracts protect the liver from severe damage caused by d-galN (Duraiswamy et al., 2012).
Shikov et al. (2011) carried out in vivo studies on the hepatoprotective effect of birch (Betula pubescens syn. Betula alba) bark extract (BBE) in patients with chronic hepatitis C (CHC). After treatment for 12 weeks with 160 mg standardized BBE per day, outcome parameters like alanine aminotransferase (ALT), aspartate aminotransferase (AST) levels, quantitative HCV RNA levels, subjective symptoms associated with CHC (fatigue, abdominal discomfort, depression, and dyspepsia), safety and compliance were monitored and promising results were observed.
5.9. Gastroprotective
Methanolic extract of Betula pendula leaves (BLE) was evaluated for its gastroprotective effects in vivo and the inhibitory activity on lipid peroxidation in vitro (Germano et al., 2012a). Its gastroprotective effects were studied against 90% ethanol-induced ulcer in rats. Oral pretreatment of rats with BLE (100, 200 and 400 mg kg−1) significantly reduced the incidence of gastric lesions induced by ethanol administration as compared with misoprostol (0.50 mg kg−1). Furthermore, BLE inhibited the increase in malondialdehyde (MDA) and prevented depletion of total sulhydryl and non-protein sulhydryl groups in rat stomach homogenate when compared with ethanol group. With regard to the effect of lipid peroxidation in vitro, BLE showed the ability to reduce methyl linoleate autoxidation. Chemical characterization showed the presence of myricetin-3-O-galactoside, quercetin glycosides, and kaempferol glycosides.
5.10. Miscellaneous
Antidiabetic: Ghimire et al. (2012) studied the α-glucosidase inhibitory activity of 80% methanolic extract of Betula alnoides and its sub-fractions. The 80% methanolic extract had the most powerful α-glucosidase inhibitory effect (98.46%) at a concentration of 40 µg/mL.
Ahmad et al. (2008) reported that the ethanolic extract of the stem wood of Betula utilis mildly helped in the decline of bloodglucose of streptozotocin-induced diabetic rats and possessed antihyperglycaemic potential, although it was found to cause respiratory allergy.
Thromboplastic: It was determined that the thromboplastic agents from the inflorescence of the Betula pendula provoke protective reaction of the animal׳s anticoagulation system, though weaker expressed than the reaction of thromboplastin from brain. The mechanisms of action of thromboplastic agents of plant origin are similar to the mechanism of action of tissue thromboplastin (Kudriashov et al., 1986).
Phosphodiesterase inhibitory activity: PDE inhibitors have been used for treatment of many indications such as cardiovascular diseases, chronic obstructive pulmonary diseases, erectile dysfunction and pulmonary hypertension. Some Thai medicinal plants used as aphrodisiac and neurotonic agents were screened for PDE inhibitory activity using a radioassay. 3-isobutyl-1-methylxanthine (IBMX) was used as the standard inhibitor (Temkitthawon et al., 2008). IC50 values of ethanolic extract of Betula alnoides stem bark against PDEs using the SPA radioassay were 3.79±0.98 µg/mL while IBMX standard showed an IC50 value of 0.68±0.13 µg/mL. At 0.1 mg/mL, Betula alnoides showed complete inhibitory effect against PDEs.
Aflatoxin production inhibitor: The essential oil of Betula pubescens syn. Betula alba yielded methyl syringate which was found to be an inhibitor of aflatoxin production. It inhibited aflatoxin production of Aspergillus parasiticus and Aspergillus flavus with IC50 values of 0.9 and 0.8 mM, respectively, without significantly inhibiting fungal growth. It reduced mRNA levels of genes (aflR, pksA, and omtA) encoding proteins required for aflatoxin biosynthesis. Aflatoxin production inhibitory activities of some related compounds were also studied. However it was found that methyl gallate, methyl 3,4,5-trimethoxybenzoate, and methyl 3-O-methylgallate inhibited both aflatoxin production as well as fungal growth of Aspergillus parasiticus and Aspergillus flavus (Jermnak et al., 2012).
Other studies suggest that extracts from Betula pendula exhibit mild diuretic activity (Schilcher and Rau, 1988, Major, 2002) and anti-inflammatory capacity (Tunon et al., 1995, Trouillas et al., 2003). They were also used for the supportive treatment of rheumatic diseases in anthroposophic medicine (Pieroni and Gray, 2008, Girke, 2010).
6. Some biologically active triterpenoids from Betula species
6.1. Betulin and betulinic acid
Betulin (lup-20(29)-ene-3β,28-diol) also known as betulinol, betuline and betulinic alcohol, and betulinic acid (3β-hydroxy-lup-20(29)-en-28-oic acid) are two pentacyclic lupane-type triterpenoids that are widely distributed throughout the plant kingdom. Although betulin can also be isolated from other sources in small amounts, the birch bark could be a large and feasible source of raw material for its isolation on an industrial scale. It can be isolated up to 30% dry weight from the birch bark. The birch tree (Betula spp.) is one of the most widely reported sources of betulinic acid and betulin which can be obtained in considerable quantities. In recent years, anti-cancer, anti-microbial, anti-inflammatory, differentiation-promoting effects and wound-healing properties of betulin have been described. Betulin can be used as such or after chemical modification as a starting compound for other useful materials and compounds, which possess various interesting pharmacological properties. Betulinic acid has been reported to display a wide range of biological and medicinal properties such as inhibition of human immunodeficiency virus (HIV), anti-bacterial, anti-malarial, anti-inflammatory, anthelmintic, antinociceptive, anti-HSV-1, and anti-cancer activities. Because of its selective cytotoxicity against tumor cells and favorable therapeutic index, even at doses up to 500 mg/kg body weight, betulinic acid is a very promising new chemotherapeutic agent for the treatment of HIV infection and cancer. In a major step toward realizing clinical chemotherapeutic applications for this class of compounds, the University of Illinois at Chicago had licensed the worldwide rights to develop betulinic acid to Advanced Life Sciences, a company specializing in the development of potential new drug candidates in disease categories including viral infections, cancer, and inflammation.
In light of the tremendous interest generated with respect to the chemistry and pharmacological properties of betulinic acid and its analogs, several reviews concerning betulin and particularly its biologically more active derivatives, such as betulinic acid have been published (Baglin et al., 2003, Cichewicz and Kouzi, 2004, Eiznhamer and Xu, 2004, Aiken and Chen, 2005) in an effort to summarize the available literature about these promising bioactive natural products and identify areas for further research. (Sami et al., 2006, Moghaddam et al., 2012).
6.2. Papyriferic acid
Another biologically active triterpene isolated from birch trees is Papyriferic acid that is secreted by glands on twigs of the juvenile ontogenetic phase of resin producing tree birches. It deters the browsing by mammals such as the snowshoe hare (Lepus americanus) on the juvenile developmental stage of the birch tree (McLean et al., 2009, Forbey et al., 2011). Since methyl papyriferate was found to exhibit potent reversing effect on cytotoxicity of colchicine against multidrug resistance (MDR) human cancer cells (KB-C2), several papyriferic acid derivatives were prepared and evaluated for their cytotoxicity and effect on reversing P-gp-mediated MDR against KB-C2 cells. It was observed that 3-O-(Morpholino-β-oxopropanoyl)-12β-acetoxy-3α,25-dihydroxy-(20S,24R)-epoxydammarane significantly increased the sensitivity of colchicine against KB-C2 cells by 185-fold at 5 µ/mL (7.4 µM), and the cytotoxicity of colchicine was recovered to nearly that of sensitive (KB) cells. The other several new amide derivatives also exhibited potent reversal activity comparable to or more potent than methyl papyriferate and verapamil (Xiong et al., 2010).
7. Toxicity studies
Huh et al., 2009, Huh et al., 2011 carried out experiments to evaluate the acute oral toxicity of the 50% hydroalchoholic extract as well as its n-butanol fraction (BFBP) obtained from the bark of Betula platyphylla. Their single dose toxicities were determined in both sexes of rats at dose of 5.0 g/kg of body weight. After a single oral dose, rats were assigned randomly to two experimental groups, the vehicle (distilled water) group and the treated (5 g/kg) group and monitored during the 14 days after treatment. At this dose the 50% hydroalchoholic extract as well as its BFBP had no effect on mortality, body weight changes, gross findings and clinical signs by biochemical analysis in either sex. Also, Betula platyphylla did not cause gastric irritation, erosion, or ulceration up to the orally administered dose of 5 g/kg, thus confirming them to be nontoxic at this dose.
A triterpene rich dry extract extracted from the outer bark of Betula pubescens syn. Betula alba containing pentacyclic triterpenes, mainly betulin, but also betulinic acid, oleanolic acid, lupeol and erythrodiol was subjected to subchronic toxicity studies. With rats, daily i.p. doses of triterpene rich dry extract up to 540 mg/kg for 28 days produced no toxic symptoms or mortality and no histopathological changes were seen (Jager et al., 2008).
Studies have also shown betulin, like other pentacyclic triterpenes, to be non-toxic (Jager et al., 2008). Betulinic acid was also found to be non-toxic at doses up to 500 mg/kg body weight in mice (Pisha et al., 1995).
8. Conclusion
The healing properties of birch bark and birch bark extracts have been known for a long time in traditional medicine. Recent studies have shown it to display different biological activities some of which justify their ethnopharmacological uses. Several species of Betula have extensively been used for the treatment of osteoarthritis, rheumatism and other bone related problems in different parts of the world, traditionally. Pharmacological studies carried out have supported their traditional use in bone related problems. Studies have shown Betula platyphylla and Betula pendula to be potentially useful in the treatment of degenerative joint disease (Cho et al., 2006, Gründemann et al., 2011, Huh et al., 2011). Experiments have shown that Betula species also exhibit anti-inflammatory, immunomodulatory as well as antioxidant activities, all of which contribute towards the protection of joints. Traditional use in bile disorders has also been documented. These find support from the different pharmacological experiments carried out to determine the effect on liver and hepatoprotective action.
Cancer, after cardiovascular disease, is the second leading cause of death. There is a growing importance of triterpenoids as a source of medication for various chronic diseases and several evidences suggest that both natural as well as synthetic triterpenoids have potential anti-cancer activities (Patlolla and Rao, 2012). Although Betula species are currently at the focus of a variety of projects on novel anticancer agents, no direct ethnobotanical link seems to exist between the traditional uses (i.e., as an anticancer agent) and modern biomedical research. This is not surprising, because only few species have recorded uses as anticancer agents. However, many of these uses imply that the extract will modulate the cell cycle, a property that is explored in the development of novel anticancer agents (Heinrich, 2010). There is convincing evidence in experimental animal models in support of anti-carcinogenic effects of Betula bark, betulin as well as betulinic acid. This is in synchronization with the fact that Betula is a rich source of triterpenoids especially betulin.
A number of studies have also proven the other biological properties of betulin and betulinic acid: antiviral (antiflu, anti-HIV), anti-inflammatory, antiallergenic, antihypoxic, liver protectant, hypolipemic and antituberculosis. Derivatives of betulinic acid have showed cytotoxicity and anti-HIV activity at micromolar or even at nanomolar concentrations, which are comparable to some clinically used drugs (Sami et al., 2006, Şoica et al., 2012).
Thus, although the present situation on Betula offers a lot of promising prospects, one should also be aware of the shortcomings, possible drawbacks and pitfalls. a) Although betulin and betulinic acid have been the subject of extensive research related to anticancer activity, the other less explored biological activities of Betula species, especially their usefulness in the treatment of osteoarthritis, need to be studied in much more detail. b) There is need to study biochemical and physiological mechanisms as well as detailed preclinical toxicity, bioavailability, pharmacokinetics and pharmacodynamics in sufficient detail. c) Controlled clinical trials in the treatment of different conditions would offer fundamental basis for the claims made on them. d) Given the morphological similarities between some species, it is highly likely that species are often substituted with each other for different medicinal uses. Thus, proper quality control protocols, lack of which could lead to misidentification and possible adulteration of the required species, are necessary. e) Finally, an integrated and holistic approach is required for tapping the full potentials of Betula species.
References
- Agelet A., Vallès J. Studies on pharmaceutical ethnobotany in the region of Pallars (Pyrenees, Catalonia, Iberian Peninsula). Part II. New or very rare uses of previously known medicinal plants. Journal of Ethnopharmacology. 2003;84:211–227. doi: 10.1016/s0378-8741(02)00319-7. [DOI] [PubMed] [Google Scholar]
- Ahmad R., Srivastava S.P., Maurya R., Rajendran S.M., Arya K.R., Srivastava A.K. Mild antihyperglycaemic activity in Eclipta alba, Berberis aristata, Betula utilis, Cedrus deodara, Myristica fragrans and Terminalia chebula. Indian Journal of Science and Technology. 2008;1:1–6. [Google Scholar]
- Aiken C., Chen C.H. Betulinic acid derivatives as HIV-1 antivirals. Trends in Molecular Medicine. 2005;11:31–36. doi: 10.1016/j.molmed.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Angmo K., Adhikari B.S., Rawat G.S. Changing aspects of traditional healthcare system in Western Ladakh, India. Journal of Ethnopharmacology. 2012;143:621–630. doi: 10.1016/j.jep.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Anonymous . Council of Scientific and Industrial Research; New Delhi: 1948. The Wealth of India, Raw Materials Vol I. [Google Scholar]
- Ashburner K., McAllister H. Kew Publishing; Kew: 2013. The Genus Betula: A taxonomic Revision of Birches. [Google Scholar]
- Atkinson M.D. Betula pendula Roth (B. verrucosa Ehrh.) and B. pubescens Ehrh. Journal of Ecology. 1992;80:837–870. [Google Scholar]
- Baglin I., Mitaine-Offer A.C., Nour M., Tan K., Cave C., Lacaille-Dubois M.A. A review of natural and modified betulinic, ursolic and echinocystic acid derivatives as potential antitumor and anti-HIV agents. Mini Reviews in Medicinal Chemistry. 2003;3:525–539. doi: 10.2174/1389557033487917. [DOI] [PubMed] [Google Scholar]
- Balitski K.P., Vorontsova A.L. 3rd ed. Rostovskoe knizhnoe izdatel'stvo, USSR; 1980. Medical Plants for Therapy of Malignant Tumors; p. 296. [Google Scholar]
- Baral S.R., Kurmi P.P. A Compendium of Medicinal Plants in Nepal. Mrs Rachana Sharma; Kathmandu, Nepal: 2006. [Google Scholar]
- Blanco E., Macía M.J., Morales R. Medicinal and veterinary plants of El Caurel (Galicia, northwest Spain) Journal of Ethnopharmacology. 1999;65:113–124. doi: 10.1016/s0378-8741(98)00178-0. [DOI] [PubMed] [Google Scholar]
- Broza S.K., Christoph D., Valerie K.A., Johannes S. Ethnobotanical study on medicinal use of wild and cultivated plants in middle, south and west Bosnia and Herzegovina. Journal of Ethnopharmacology. 2010;131:33–55. doi: 10.1016/j.jep.2010.05.061. [DOI] [PubMed] [Google Scholar]
- Broza S.K., Christoph D., Valerie K.A., Johannes S. Ethnobotanical survey of traditionally used plants in human therapy of east, north and north-east Bosnia and Herzegovina. Journal of Ethnopharmacology. 2011;133:1051–1076. doi: 10.1016/j.jep.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Cˆınt˘a-Pˆınzaru S., Dehelean C.A., Soica C., Culea M., Borcan F. Evaluation and differentiation of the Betulaceae birch bark species and their bioactive triterpene content using analytical FT-vibrational spectroscopy and GC–MS. Chemistry Central Journal. 2012;6:67. doi: 10.1186/1752-153X-6-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calliste C.A., Trouillas P., Allais D.P., Simon A., Duroux J.L. Free radical scavenging activities measured by electron spin resonance spectroscopy and B16 cell antiproliferative behaviors of seven plants. Journal of Agriculture and Food Chemistry. 2001;49:3321–3327. doi: 10.1021/jf010086v. [DOI] [PubMed] [Google Scholar]
- Carnat A., Lacouture I., Fraisse D., Lamaison J.L. Standardization of the birch leaf. Annales Pharmaceutiques Françaises. 1996;54:231–235. [PubMed] [Google Scholar]
- Casetti F., Wölfle U., Gehring W., Schempp C.M. Dermocosmetics for dry skin: a new role for botanical extracts. Skin Pharmacology and Physiology. 2011;24:289–293. doi: 10.1159/000329214. [DOI] [PubMed] [Google Scholar]
- Cho Y.J., Huh J.E., Kim D.Y., Kim N.J., Lee J.D., Baek Y.H., Cho E.M., Yang H.R., Choi D.Y., Park D.S. Effect of Betula platyphylla var. japonica on proteoglycan release, type II collagen degradation, and matrix metalloproteinase expresstion in rabbit articular cartilage explants. Biological and Pharmaceutical Bulletin. 2006;29:1408–1413. doi: 10.1248/bpb.29.1408. [DOI] [PubMed] [Google Scholar]
- Chopra R.N., Nayar S.L., Chopra I.C. Council of Scientific and Industrial Research; New Delhi: 1986. Glossary of Indian Medicinal Plants (Including the Supplement) [Google Scholar]
- Cichewicz R.H., Kouzi S.A. Chemistry, biological activity and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Medicinal Research Reviews. 2004;24:90–114. doi: 10.1002/med.10053. [DOI] [PubMed] [Google Scholar]
- Dadáková E., Vrchotová N., Tˇríska J. Content of selected biologically active compounds in tea infusions of widely used European medicinal plants. Journal of Agrobiology. 2010;27:27–34. [Google Scholar]
- Dehelean C.A., Şoica C., Ledeţi I., Aluaş M., Zupko I., Gǎluşcan A., Cinta-Pinzaru S., Munteanu M. Study of the betulin enriched birch bark extracts effects on human carcinoma cells and ear inflammation. Chemistry Central Journal. 2012;6:137. doi: 10.1186/1752-153X-6-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirci B., Paper D.H., Demirci F., Can Başer K.H., Franz G. Essential oil of Betula pendula Roth. buds. Evidence Based Complementary and Alternative Medicine. 2004;1:301–303. doi: 10.1093/ecam/neh041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diouf P.N., Stevanovic T., Boutin Y. The effect of extraction process on polyphenol content, triterpene composition and bioactivity of yellow birch (Betula alleghaniensis Britton) extracts. Industrial Crops and Products. 2009;30:297–303. [Google Scholar]
- Drag M., Surowiak P., Drag-Zalesinka M., Dietel M., Lage H., Oleksyszyn J. Comparison of the cytotoxic effects of birch bark extract, betulin and betulinic acid towards human gastric carcinoma and pancreatic carcinoma drug-sensitive and drugresistant cell lines. Molecules. 2009;14:1639–1651. doi: 10.3390/molecules14041639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraiswamy B., Satishkumar M.N., Gupta S., Rawat M., Porwal O., Murugan R. Hepatoprotective activity of Betula utilis bark on d-galactosamine induced hepatic insult. World Journal of Pharmacy and Pharmaceutical Sciences. 2012;1:456–471. [Google Scholar]
- Duric K., Kovac-Besovic E., Niksic H., Sofic E. Antibacterial activity of methanolic extracts, decoction and isolated triterpene products from different parts of birch, Betula pendula, Roth. Journal of Plant Studies. 2013;2:61–70. [Google Scholar]
- Eddouks M., Maghrani M., Lemhadri A., Ouahidi M.L., Jouad H. Ethnopharmacological survey of medicinal plants used for the treatment of diabetes mellitus, hypertension and cardiac diseases in the south-east region of Morocco (Tafilalet) Journal of Ethnopharmacology. 2002;82:97–103. doi: 10.1016/s0378-8741(02)00164-2. [DOI] [PubMed] [Google Scholar]
- Eiznhamer D.A., Xu Z. Betulinic acid: a promising anticancer candidate. IDrugs: The Investigative Drugs Journal. 2004;7:359–373. [PubMed] [Google Scholar]
- EMEA/HMPC, 2008a. Betula pendula Roth.; Betula pubescens Ehrh., folium. Assessment report for the development of community monographs and for inclusion of herbal substance(s), preparation(s), or combinations thereof in the list. Doc. Ref. EMEA/HMPC /260018/2006. 〈http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC _assessment_report/2010/01/WC500069013.pdf〉.
- EMEA/HMPC, 2008b. Community herbal monograph on Betula pendula Roth.; Betula pubescens Ehrh., folium. Doc. Ref. EMEA/HMPC/260019/2006. 〈http://www.ema.europa/eu/docs/en_GB/document_library/Herbal_-_Community_herbal_monograph/2009/12 /WC500018091.pdf〉.
- Quer P. Font. Plantas medicinales. El Dioscorides renovado. 15 a ed. Editorial labor; Barcelona: 1995. [Google Scholar]
- Forbey J.S., Pu X., Xu D., Kielland K., Bryant J. Inhibition of Snowshoe hare succinate dehydrogenase activity as a mechanism of deterrence for papyriferic acid in birch. Journal of Chemical Ecology. 2011;37:1285–1293. doi: 10.1007/s10886-011-0039-9. [DOI] [PubMed] [Google Scholar]
- Freysdottir J., Sigurpalsson M.B., Omarsdottir S., Olafsdottir E.S., Vikingsson A., Hardardottir I. Ethanol extract from birch bark (Betula pubescens) suppresses human dendritic cell mediated Th1 responses and directs it towards a Th17 regulatory response in vitro. Immunology Letters. 2011;136:90–96. doi: 10.1016/j.imlet.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Fuchino H., Konishi S., Satoh T., Yagi A., Saitsu K., Tatsumi T., Tanaka N. Chemical evaluation of Betula species in Japan. II. Constituents of Betula platyphylla var. japonica. Chemical and Pharmaceutical Bulletin. 1996;44:1033–1038. [Google Scholar]
- Fuchino H., Satoh T., Hida J., Terada M., Tanaka N. Chemical evaluation of Betula species in Japan. VI. Constituents of Betula schmidtii. Chemical and Pharmaceutical Bulletin. 1998;46:1051–1053. [Google Scholar]
- Fuchino H., Satoh T., Shimizu M., Tanaka N. Chemical evaluation of Betula species in Japan. IV. Constituents of Betula davurica. Chemical and Pharmaceutical Bulletin. 1998;46:166–168. [Google Scholar]
- Fuchino H., Satoh T., Tanaka N. Chemical evaluation of Betula species in Japan. I. Constituents of Betula ermanii. Chemical and Pharmaceutical Bulletin. 1995;43:1937–1942. [Google Scholar]
- Fuchino H., Satoh T., Tanaka N. Chemical evaluation of Betula species in Japan III. Constituents of Betula maximowicziana. Chemical and Pharmaceutical Bulletin. 1996;44:1748–1753. [Google Scholar]
- Fuchino H., Satoh T., Yokochi M., Tanaka N. Chemical evaluation of Betula species in Japan. V. Constituents of Betula ovalifolia. Chemical and Pharmaceutical Bulletin. 1998;46:169–170. [Google Scholar]
- Fuchs L. Basel; Isingrin: 1964. New Kreüterbuch. München. Kölbl. Reprograph. Nachdr. der Ausg; p. 1543. [Google Scholar]
- Fulda S. Betulinic acid for cancer treatment and prevention. International Journal of Molecular Sciences. 2008;9:1096–1107. doi: 10.3390/ijms9061096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Pérez M.E., Royer M., Duque-Fernandez A., Diouf P.N., Stevanovic T., Pouliot R. Antioxidant, toxicological and antiproliferative properties of Canadian polyphenolic extracts on normal and psoriatic keratinocytes. Journal of Ethnopharmacology. 2010;132:251–258. doi: 10.1016/j.jep.2010.08.030. [DOI] [PubMed] [Google Scholar]
- Gaur R.D. Transmedia publication; Srinagar Garhwal, Uttarakhand, India: 1999. Flora of the District Garhwal—North West Himalaya: With Ethnobotanical Notes. [Google Scholar]
- Germano M.P., Cacciola F., Donato P., Dugo P., Certo G., D’Angelo V., Mondello L., Rapisarda A. Betula pendula Roth leaves: gastroprotective effects of an HPLC-fingerprinted methanolic extract. Natural Product Research. 2012;1:1–7. doi: 10.1080/14786419.2012.740036. [DOI] [PubMed] [Google Scholar]
- Germano M.P., Cacciola F., Donato P., Dugo P., Certo G., D’Angelo V., Mondello L., Rapisarda A. Betula pendula leaves: polyphenolic characterization and potential innovative use in skin whitening products. Fitoterapia. 2012;83:877–882. doi: 10.1016/j.fitote.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Gewali M.B., Awale S. Institute of Natural Medicine, University of Toyama; Japan: 2008. Aspects of Traditional Medicine in Nepal. [Google Scholar]
- Ghimire S.K. WWF Nepal; Kathmandu, Nepal: 2008. Non-Timber Forest Products of Nepal Himalaya: Database of Some Important Species Found in the Mountain Protected Areas and Surrounding Regions. [Google Scholar]
- Ghimire B.K., Tamang J.P., Yu C.Y., Jung S.J., Chung I.M. Antioxidant, antimicrobial activity and inhibition of α-glucosidase activity by Betula alnoides Buch. bark extract and their relationship with polyphenolic compounds concentration. Immunopharmacology and Immunotoxicology. 2012;34:824–831. doi: 10.3109/08923973.2012.661739. [DOI] [PubMed] [Google Scholar]
- Girke M. 1st ed. Salumed Verlag; Berlin: 2010. Innere Medizin. [Google Scholar]
- Goun E.A., Petrichenko V.M., Solodnikov S.U., Suhinina T.V., Kline M.A., Cunningham G., Nguyen C., Miles H. Anticancer and antithrombin activity of Russian plants. Journal of Ethnopharmacology. 2002;81:337–342. doi: 10.1016/s0378-8741(02)00116-2. [DOI] [PubMed] [Google Scholar]
- Gründemann C., Gruber C.W., Hertrampf A., Zehl M., Kopp B., Huber R. An aqueous birch leaf extract of Betula pendula inhibits the growth and cell division of inflammatory lymphocytes. Journal of Ethnopharmacology. 2011;136:444–451. doi: 10.1016/j.jep.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Gutermuth J., Bewersdorff M., Traidl-Hoffmann C., Ring J., Mueller M.J., Behrendt H., Jakob T. Immunomodulatory effects of aqueous birch pollen extracts and phytoprostanes on primary immune responses in vivo. Journal of Allergy and Clinical Immunology. 2007;120:293–299. doi: 10.1016/j.jaci.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Ha¨met-Ahti L., Palmen A., Alanko P., Tigerstedt P.M.A. Dendrologian seura; Helsinki: 1989. Suomen Puu-Ja Pensaskasvio; pp. 83–87. (In Finnish) [Google Scholar]
- Hanlidou E., Karousou R., Kleftoyanni V., Kokkini S. The herbal market of Thessaloniki (N Greece) and its relation to the ethnobotanical tradition. Journal of Ethnopharmacology. 2004;91:281–299. doi: 10.1016/j.jep.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Hänsel R., Keller K., Rimpler H., Schneider G. Springer Verlag; Heidelberg: 1992–1994. Hagers Handbuch der Pharmazeutischen Praxis. Drogen. 3 Bände. [Google Scholar]
- Havlik J., Gonzalez d e la Huebra R., Hejtmankova K., Fernandez J., Simonova J., Melich M., Rada V. Xanthine oxidase inhibitory properties of Czech medicinal plants. Journal of Ethnopharmacology. 2010;132:461–465. doi: 10.1016/j.jep.2010.08.044. [DOI] [PubMed] [Google Scholar]
- Heinrich M. Ethnopharmacology and drug discovery. In: Verpoorte R., editor. Vol. 3. Elsevier; Oxford: 2010. pp. 351–381. (Comprehensive Natural Products II: Chemistry and Biology, Development & Modification of Bioactivity). [Google Scholar]
- Hilpisch U., Hartmann R., Glombitza K.W. New dammaranes, esterified with malonic acid, from leaves of Betula pendula. Planta Medica. 1997;63:347–351. doi: 10.1055/s-2006-957698. [DOI] [PubMed] [Google Scholar]
- Hong C.H., Hur S.K., Oh O.J., Kim S.S., Nam K.A., Lee S.K. Evaluation of natural products on inhibition of inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) in cultured mouse macrophage cells. Journal of Ethnopharmacology. 2002;83:153–159. doi: 10.1016/s0378-8741(02)00205-2. [DOI] [PubMed] [Google Scholar]
- Hooker J.D. L Relve & Co.; London: 1890. Flora of British India. [Google Scholar]
- Horhammer L., Hansel R., Frank P. Archiv der Pharmazie und Berichte der Deutschen Pharmazeutischen Gesellschaft. 1953;286(10):481–482. doi: 10.1002/ardp.19532861002. ([Isolation of rutin from Betula humilis]) [DOI] [PubMed] [Google Scholar]
- Horhammer L., Wagner H., Luck R. Isolation of a myricetin-3-digalactoside from Betula verrucosa and Betula pubescens. Archiv der Pharmazie und Berichte der Deutschen Pharmazeutischen Gesellschaft. 1957;290/62(7):338–341. doi: 10.1002/ardp.19572900709. [DOI] [PubMed] [Google Scholar]
- http://encyclopedia2.thefreedictionary.com
- http://www.discoverlife.org
- http://www.theplantlist.org/
- Huh J.E., Baek Y.H., Kim Y.J., Lee J.D., Choi D.Y., Park D.S. Protective effects of butanol fraction from Betula platyphyla var. japonica on cartilage alterations in a rabbit collagenase-induced osteoarthritis. Journal of Ethnopharmacology. 2009;123:515–521. doi: 10.1016/j.jep.2008.08.028. [DOI] [PubMed] [Google Scholar]
- Huh J.E., Hong J.M., Baek Y.H., Lee J.D., Choi D.Y., Park D.S. Anti-inflammatory and anti-nociceptive effect of Betula platyphylla var. japonica in human interleukin-1β-stimulated fibroblast-like synoviocytes and in experimental animal models. Journal of Ethnopharmacology. 2011;135:126–134. doi: 10.1016/j.jep.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Huyke C., Laszczyk M., Scheffler A., Ernst R., Schempp C.M. Treatment of actinic keratoses with birch bark extract, a pilot study. Journal der Deutschen Dermatologischen Gesellschaft. 2006;4:132–137. doi: 10.1111/j.1610-0387.2006.05906.x. [DOI] [PubMed] [Google Scholar]
- Hynynen J., Niemistö P., Viherä-Aarnio A., Brunner A., Hein S., Velling P. Silviculture of birch (Betula pendula Roth and Betula pubescens Ehrh.) in northern Europe. Forestry. 2010;83(1):103–119. [Google Scholar]
- Ja¨ Rvinen P., Palme A., Morales L.O., La Nnenpa M.A., Nen M.K., Sopanen T., Lascoux M. Phylogenetic relationships of Betula species (betulaceae) based on nuclear ADH and chloroplast MatK sequences. American Journal of Botany. 2004;91:1834–1845. doi: 10.3732/ajb.91.11.1834. [DOI] [PubMed] [Google Scholar]
- Jager S., Laszczyk M.N., Scheffler A. A preliminary pharmacokinetic study of betulin, the main pentacyclic triterpene from extract of outer bark of birch (Betulae alba cortex) Molecules. 2008;13:3224–3235. doi: 10.3390/molecules13123224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jermnak U., Yoshinari T, Sugiyama Y., Tsuyuki R., Nagasawa H., Sakuda S. Isolation of methyl syringate as a specific aflatoxin production inhibitor from the essential oil of Betula alba and aflatoxin production inhibitory activities of its related compounds. International Journal of Food Microbiology. 2012;153:339–344. doi: 10.1016/j.ijfoodmicro.2011.11.023. [DOI] [PubMed] [Google Scholar]
- Jones N.P., Arnason J.T., Abou-Zaid M., Akpagana K., Sanchez-Vindas P., Smith M.L. Antifungal activity of extracts from medicinal plants used by First Nations Peoples of eastern Canada. Journal of Ethnopharmacology. 2000;73:191–198. doi: 10.1016/s0378-8741(00)00306-8. [DOI] [PubMed] [Google Scholar]
- Ju E.M., Lee S.E., Hwang H.J., Kim J.H. Antioxidant and anticancer activity of extract from Betula platyphylla var. japonica. Life Sciences. 2004;74:1013–1026. doi: 10.1016/j.lfs.2003.07.025. [DOI] [PubMed] [Google Scholar]
- Karonen M., Ossipov V., Sinkkonen J., Loponen J., Haukioja E., Pihlaja K. Quantitative analysis of polymeric proanthocyanidins in birch leaves with normal-phase HPLC. Phytochemical Analysis. 2006;17:149–156. doi: 10.1002/pca.898. [DOI] [PubMed] [Google Scholar]
- Kashiwada Y., Sekiya M., Yamazaki K., Ikeshiro Y., Fujioka T., Yamagishi T., Kitagawa S., Takaishi Y. Triterpenoids from the floral spikes of Betula platyphylla var. japonica and their reversing activity against multidrug-resistant cancer cells. Journal of Natural Products. 2007;70:623–627. doi: 10.1021/np060631s. [DOI] [PubMed] [Google Scholar]
- Keinanen M., Tiitto R.J. High-performance liquid chromatographic determination of flavonoids in Betula pendula and Betula pubescens leaves. Journal of Chromatography A. 1998;793:370–377. [Google Scholar]
- Keinänen M.J., Tiitto R., Rousi M., Tahvanainen J. Taxonomic implications of phenolic variation in leaves of birch (Betula L.) species. Biochemical Systematics and Ecology. 1999;27:243–254. [Google Scholar]
- Khan I., Sangwan P.L., Dhar J.K., Koul S. Simultaneous quantification of five marker compounds of Betula utilis stem bark using a validated high-performance thin-layer chromatography method. Journal of Separation Science. 2012;35:392–399. doi: 10.1002/jssc.201100647. [DOI] [PubMed] [Google Scholar]
- Khan M.A., Atta-ur-Rahman Karachic acid: a new triterpenoid from Betula utilis. Phytochemistry. 1975;14:789–791. [Google Scholar]
- Kim E.C., Lee H.S., Kim S.K., Choi M.S., Lee S., Han J.B., Um H.J., Kim J.Y., Lee H.M., Bae N.Y., Min, B.I. H. The bark of Betula platyphylla var. japonica inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. Journal of Ethnopharmacology. 2008;116:270–278. doi: 10.1016/j.jep.2007.11.042. [DOI] [PubMed] [Google Scholar]
- Kim H., Song M.J. Analysis and recordings of orally transmitted knowledge about medicinal plants in the southern mountainous region of Korea. Journal of Ethnopharmacology. 2011;134:676–696. doi: 10.1016/j.jep.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Klejdus B., Lojková L., Kula E., Buchta I., Hrdlicka P., Kubán V. Supercritical fluid extraction of amino acids from birch (Betula pendula Roth) leaves and their liquid chromatographic determination with fluorimetric detection. Journal of Separation Science. 2008;31:1363–1373. doi: 10.1002/jssc.200700560. [DOI] [PubMed] [Google Scholar]
- Kovac-Besović E., Durić K., Kalodera Z., Sofić E. Identification and isolation of pharmacologically active triterpenes in Betuale cortex, Betula pendula Roth., Betulaceae. Bosnian Journal of Basic Medical Sciences. 2009;9:31–38. doi: 10.17305/bjbms.2009.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudriashov B.A., Azieva L.D., Liapina L.A. Hemostatic system function as affected by thromboplastic agents from higher plants. Nauchnye Doki Vyss Shkoly Biol Nauki. 1986;4:58–61. [PubMed] [Google Scholar]
- Kumaraswamy M.V., Kavitha H.U., Satish S. Antibacterial evaluation and phytochemical analysis of Betula utilis D. Don against some human pathogenic bacteria. Advances in Biological Research. 2008;2:21–25. [Google Scholar]
- Lahtinen M., Lempa K., Salminen J.P., Pihlaja K. HPLC analysis of leaf surface flavonoids for the preliminary classification of birch species. Phytochemical Analysis. 2006;17:197–203. doi: 10.1002/pca.906. [DOI] [PubMed] [Google Scholar]
- Lee M., Park J.H., Min D.S., Yoo H., Park J.H., Kim Y.C., Sung S.H. Antifibrotic activity of diarylheptanoids from Betula platyphylla toward HSC-T6 cells. Bioscience, Biotechnology and Biochemistry. 2012;76:1616–1620. doi: 10.1271/bbb.110887. [DOI] [PubMed] [Google Scholar]
- Lee M.W. Determination of the structure for polysubstituted flavonoid and 6-C-glucosyl flavonoids using 13C-1H long range couplings. Archives of Pharmacal Research. 1994;7:487–489. doi: 10.1007/BF02979132. [DOI] [PubMed] [Google Scholar]
- Leporatti M.L., Ivancheva S. Preliminary comparative analysis of medicinal plants used in the traditional medicine of Bulgaria and Italy. Journal of Ethnopharmacology. 2003;87:123–142. doi: 10.1016/s0378-8741(03)00047-3. [DOI] [PubMed] [Google Scholar]
- Li J., Wang X., Zhou S. Studies on the chemical constituents of the bark of Betula platyphylla. Zhong Yao Cai. 1998;21:83–85. [PubMed] [Google Scholar]
- Liimatainen J., Sinkkonen J., Karonen M., Pihlaja K. Two new phenylbutanoids from inner bark of Betula pendula. Magnetic Resonance in Chemistry. 2008;46:195–198. doi: 10.1002/mrc.2163. [DOI] [PubMed] [Google Scholar]
- Liimatainen J., Karonen M., Sinkkonen J. Procyanidin xylosides from the bark of Betula pendula. Phytochemistry. 2012;76:178–183. doi: 10.1016/j.phytochem.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Liimatainen J., Karonen M., Sinkkonen J., Helander M., Salminen J.P. Phenolic compounds of the inner bark of Betula pendula: seasonal and genetic variation and induction by wounding. Journal of Chemical Ecology. 2012;38:1410–1418. doi: 10.1007/s10886-012-0199-2. [DOI] [PubMed] [Google Scholar]
- Mahmood A., Mahmood A., Malik R.N. Indigenous knowledge of medicinal plants from Leepa valley, Azad Jammu and Kashmir, Pakistan. Journal of Ethnopharmacology. 2012;143:338–346. doi: 10.1016/j.jep.2012.06.046. [DOI] [PubMed] [Google Scholar]
- Major H. Humboldt University; Berlin, Germany: 2002. Untersuchungen zur Wirkungsweise von Birkenblattern (Betulae folium) und phenolischer Verbindungen, unter besonderer Berucksichtigung der Beeinflussung von Metallopeptidasen. [Google Scholar]
- Malhi B.S., Trivedi V.P. Vegetable anti-fertility drugs of India. Quarterly Journal of Crude Drug Research. 1972;12:1922–1928. doi: 10.3109/13880207209068244. [DOI] [PubMed] [Google Scholar]
- Malhotra C.L., Balodi B. Wild medicinal plants in the use of Johari tribals. Journal of Economic and Taxonomic Botany. 1984;5:841–843. [Google Scholar]
- Mämmelä P. Phenolics in selected European hardwood species by liquid chromatography-electrospray ionisation mass spectrometry. Analyst. 2001;126:1535–1538. doi: 10.1039/b104584a. [DOI] [PubMed] [Google Scholar]
- Manandhar N.P. A survey of medicinal plants of Jajarkot district, Nepal. Journal of Ethnopharmacology. 1995;48:1–6. doi: 10.1016/0378-8741(95)01269-j. [DOI] [PubMed] [Google Scholar]
- Manandhar N.P. Plants and People of Nepal. Timber Press; Oregon: 2002. [Google Scholar]
- Marc E.B., Nelly A., Annick D.D., Frederic D. Plants used as remedies antirheumatic and antineuralgic in the traditional medicine of Lebanon. Journal of Ethnopharmacology. 2008;120:315–334. doi: 10.1016/j.jep.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Marzell H. Geschichte und Volkskunde der deutschen Heilpflanzen. Wissenschaftliche Buchgesellschaft; Darmstadt: 1967. (2. verm. u. verb. Aufl. von “Unsere Heilpflanzen”) [Google Scholar]
- Matsuda H., Ishikado A., Nishida N., Ninomiya K., Yoshikawa M. Hepatoprotective, superoxide scavenging, and antioxidative activities of aromatic constituents from the bark of Betula platyphylla var. japonica. Bioorganic and Medicinal Chemistry. 1998;8:2939–2944. doi: 10.1016/S0960-894X(98)00528-9. [DOI] [PubMed] [Google Scholar]
- McCutcheon A.R., Roberts T.E., Gibbons E., Ellis S.M., Babiuk L.A., Hancock R.E.W., Towers G.H.N. Antiviral screening of British Columbian medicinal plants. Journal of Ethnopharmacology. 1995;49:101–110. doi: 10.1016/0378-8741(95)90037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean S., Richards S.M., Cover S.L., Brandon S., Davies N.W., Bryant J.P., Clausen T.P. Papyriferic acid, an antifeedant triterpene from birch trees, inhibits succinate dehydrogenase from liver mitochondria. Journal of Chemical Ecology. 2009;35:1252–1261. doi: 10.1007/s10886-009-9702-9. [DOI] [PubMed] [Google Scholar]
- Menković N., Šavikin K., Tasić S., Zdunić G., Stešević D., Milosavljević S., Vincek D. Ethnobotanical study on traditional uses of wild medicinal plants in Prokletije Mountains (Montenegro) Journal of Ethnopharmacology. 2011;133:97–107. doi: 10.1016/j.jep.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Millet A., Stintzing F., Merfort I. Flavonol quantification and stability of phenolics in fermented extracts from fresh Betula pendula leaves. Journal of Pharmaceutical and Biomedical Analysis. 2010;53:37–44. doi: 10.1016/j.jpba.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Miraldi E., Ferri S., Mostaghimi V. Botanical drugs and preparations in the traditional medicine of West Azerbaijan (Iran) Journal of Ethnopharmacology. 2001;75:77–87. doi: 10.1016/s0378-8741(00)00381-0. [DOI] [PubMed] [Google Scholar]
- Moerman D.E. Timber Press; Portland: 1998. Native American Ethnobotany. [Google Scholar]
- Moghaddam M.G., Ahmad F.B.H., Kermani A.S. Biological activity of betulinic acid: a review. Pharmacology & Pharmacy. 2012;3:119–123. [Google Scholar]
- Mshvildadze V., Legault J., Lavoie S., Gauthier C., Pichette A. Anticancer diarylheptanoid glycosides from the inner bark of Betula papyrifera. Phytochemistry. 2007;68:2531–2536. doi: 10.1016/j.phytochem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Muilenburg V.L., Phelan P.L., Bonello P., Herms D.A. Inter- and intra-specific variation in stem phloem phenolics of paper birch (Betula papyrifera) and European white birch (Betula pendula) Journal of Chemical Ecology. 2011;37:1193–1202. doi: 10.1007/s10886-011-0028-z. [DOI] [PubMed] [Google Scholar]
- Mukhtar H.M., Ansari S.H., Ali M., Naved T. New oleanene and fernane-type triterpenes from the stem bark of Betula pendula roth. Pharmazie. 2003;58:671–673. [PubMed] [Google Scholar]
- Negi K.S., Tiwari J.K., Gaur R.D. Economic importance of some common trees in Garhwal Himalaya: an ethnobotanical study. Indian Journal of Forestry. 1985;8:276–289. [Google Scholar]
- Neves J.M., Matos C., Moutinho C., Queiroz G., Gomes L.R. Ethnopharmacological notes about ancient uses of medicinal plants in Trás-os-Montes (northern of Portugal) Journal of Ethnopharmacology. 2009;124:270–283. doi: 10.1016/j.jep.2009.04.041. [DOI] [PubMed] [Google Scholar]
- Niemistö P., Viherä-Aarnio A., Velling P., Heräjärvi H., Verkasalo E. Karisto Oy, Hämeenlinna; 2008. Koivun kasvatus ja käyttö. Metsäkustannus Oy. p. 254. ISBN 978-952-5694-12-3. [Google Scholar]
- Oh S.R., Um J.Y., Choi H.J., Im C.K., Kim K.J., Jung J.W., Jeong G.S., Hong S.H., Kim S.J. Betula platyphylla attenuated mast cell-mediated allergic inflammation in vivo and in vitro. Life Science. 2012;91:20–28. doi: 10.1016/j.lfs.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Olszewska M.A. New validated high-performance liquid chromatographic method for simultaneous analysis of ten flavonoid aglycones in plant extracts using a C18 fused-core column and acetonitrile-tetrahydrofuran gradient. Journal of Separation Science. 2012;35:2174–2183. doi: 10.1002/jssc.201200287. [DOI] [PubMed] [Google Scholar]
- Omar S., Lemonnier B., Jones N., Ficker C., Smith M.L., Neema C., Towers G.H.N., Goel K., Arnason J.T. Antimicrobial activity of extracts of eastern North American hardwood trees and relation to traditional medicine. Journal of Ethnopharmacology. 2000;73:161–170. doi: 10.1016/s0378-8741(00)00294-4. [DOI] [PubMed] [Google Scholar]
- Papp N., Czégényi D., Hegedűs A., Morschhauser T., Quave C.L., Cianfaglione K., Pieroni A. The uses of Betula pendula Roth among Hungarian Csángós and Székelys in Transylvania, Romania. Acta Societatis Botanicorum Poloniae. 2014;83:113–122. [Google Scholar]
- Patlolla J.M.R., Rao C.V. Triterpenoids for cancer prevention and treatment: current status and future prospects. Current Pharmaceutical Biotechnology. 2012;13:147–155. doi: 10.2174/138920112798868719. [DOI] [PubMed] [Google Scholar]
- Pieroni A. Medicinal plants and food medicines in the folk traditions of the upper Lucca Province, Italy. Journal of Ethnopharmacology. 2000;70:235–273. doi: 10.1016/s0378-8741(99)00207-x. [DOI] [PubMed] [Google Scholar]
- Pieroni A., Gray C. Herbal and food folk medicines of the Russlanddeutschen living in Kunzelsau/Talacker South-Western Germany. Phytotherapy Research. 2008;22:889–901. doi: 10.1002/ptr.2410. [DOI] [PubMed] [Google Scholar]
- Pieroni A., Quave C.L., Villanelli M.L., Mangino P., Sabbatini G., Santini L., Boccetti T., Profili M., Ciccioli T., Rampa L.G., Antonini G., Girolamini C., Cecchi M., Tomasi M. Ethnopharmacognostic survey on the natural ingredients used in folk cosmetics, cosmeceuticals and remedies for healing skin diseases in the inland Marches, Central-Eastern Italy. Journal of Ethnopharmacology. 2004;91:331–344. doi: 10.1016/j.jep.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Pisha E., Chai H., Lee I.S., Chagwedera T.E., Farnsworth N.R., Cordell G.A., Beecher C.W.W., Fong H.H.S., Kinghorn A.D., Brown D.M., Wani M.C., Wall M.E., Hieken T.J., Das Gupta T.K., Pezzuto J.M. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nature Medicine. 1995;1:1046–1051. doi: 10.1038/nm1095-1046. [DOI] [PubMed] [Google Scholar]
- Prince P., Sharma R.K., Roy A., Gupta D. Pentacyclic triterpinoids from Betula utilis and Hyptis suaveolens. International Journal of Pharm Tech Research. 2010;2:1558. (-1532) [Google Scholar]
- Rajbhandari K.R. Ethnobotanical Society of Nepal; Kathmandu, Nepal: 2001. Ethnobotany of Nepal; p. 189. [Google Scholar]
- Rauha J.P., Remes S., Heinonen M., Hopia A., Kähkönen M., Kujala T., Pihlaja K., Vuorela H., Vuorela P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. International Journal of Food Microbiology. 2000;56:3–12. doi: 10.1016/s0168-1605(00)00218-x. [DOI] [PubMed] [Google Scholar]
- Rawat G.S., Pangtey Y.P.S. A contribution to the ethnobotany of Alpine regions of Kumaon. Journal of Economic and Taxonomic Botany. 1987;11:139–148. [Google Scholar]
- Rickling B., Glombitza K.W. Saponins in the leaves of birch? Hemolytic dammarane triterpenoid esters of Betula pendula. Planta Medica. 1993;59:76–79. doi: 10.1055/s-2006-959609. [DOI] [PubMed] [Google Scholar]
- Rimpler H., Kuhn H., Leuckert C. Triterpenes of Betula pendula Roth and Betula pubescens Ehrh. Comparative study of the bark. Archiv der Pharmazie und Berichte der Deutschen Pharmazeutischen Gesellschaft. 1966;299:422–428. doi: 10.1002/ardp.19662990508. [DOI] [PubMed] [Google Scholar]
- Rokaya M.B., Münzbergová Z., Timsina B. Ethnobotanical study of medicinal plants from the Humla district of western Nepal. Journal of Ethnopharmacology. 2010;130:485–504. doi: 10.1016/j.jep.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Rozema J., Broekman R.A., Blokker P., Meijkamp B.B., de Bakker N., van de Staaij J., van Beem A., Ariese F., Kars S.M. UV-B absorbance and UV-B absorbing compounds (para-coumaric acid) in pollen and sporopollenin: the perspective to track historic UV-B levels. Journal of Photochemistry and Photobiology B. 2001;62:108–117. doi: 10.1016/s1011-1344(01)00155-5. [DOI] [PubMed] [Google Scholar]
- Salminen J.P., Ossipov V., Haukioja E., Pihlaja K. Seasonal variation in the content of hydrolysable tannins in leaves of Betula pubescens. Phytochemistry. 2001;57:15–22. doi: 10.1016/s0031-9422(00)00502-1. [DOI] [PubMed] [Google Scholar]
- Salminen J.P., Ossipov V., Loponen J., Haukioja E., Pihlaja K. Characterisation of hydrolysable tannins from leaves of Betula pubescens by high-performance liquid chromatography-mass spectrometry. Journal of Chromatography A. 1999;864:283–291. doi: 10.1016/s0021-9673(99)01036-5. [DOI] [PubMed] [Google Scholar]
- Salminen J.P., Ossipov V., Pihlaja K. Distribution of hydrolysable tannins in the foliage of Finnish birch species. Zeitschrift für Naturforschung C. 2002;57:248–256. doi: 10.1515/znc-2002-3-409. [DOI] [PubMed] [Google Scholar]
- Sami A., Taru M., Salme K., Jari Y.K. Pharmacological properties of the ubiquitous natural product betulin. European Journal of Pharmaceutical Sciences. 2006;29:1–13. doi: 10.1016/j.ejps.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Santamour F.S., Lennart N.L. Distribution and inheritance of platyphylloside in Betula. Biochemical Systematics and Ecology. 1996;24:145–156. [Google Scholar]
- Santamour F.S., Lennart N.L. Rhododendrin in Betula: a reappraisal. Biochemical Systematics and Ecology. 1997;25:335–341. [Google Scholar]
- Schenk M.F., Thienpont C.N., Koopman W.J.M., Gilissen L.J.W.J., Smulders M.J.M. Phylogenetic relationships in Betula (Betulaceae) based on AFLP markers. Tree Genetics & Genomes. 2008;4:911–924. [Google Scholar]
- Schilcher H., Rau H. Nachweis der aquaretischen Wirkung von Birkenblattern- und Goldrutenkauauszugen im Tierversuch. Urologe B. 1988;28:274–280. [Google Scholar]
- Sekiya M., Kashiwada Y., Nabekura T., Kitagawa S., Yamagishi T., Yokozawa T., Ichiyanagi T., Ikeshiro Y., Takaishi Y. Effect of triterpenoids isolated from the floral spikes of Betula platyphylla var. japonica on P-glycoprotein function. Planta Medica. 2007;73:1558–1562. doi: 10.1055/s-2007-993745. [DOI] [PubMed] [Google Scholar]
- Sezik E., Yesilada E., Shadidoyatov H., Kulivey Z., Nigmatullaev A.M., Aripov H.N., Takaishi Y., Takeda Y., Honda G. Folk medicine in Uzbekistan: I. Toshkent, Djizzax, and Samarqand provinces. Journal of Ethnopharmacology. 2004;92:197–207. doi: 10.1016/j.jep.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Shah N.C. Herbal folk medicines in north India. Journal of Ethnopharmacology. 1982;12:223–230. doi: 10.1016/0378-8741(82)90052-6. [DOI] [PubMed] [Google Scholar]
- Shah N.C., Joshi M.C. An ethnobotanical study of Kumaon region of India. Economic Botany. 1971;25:414–422. [Google Scholar]
- Shikov A.N., Pozharitskaya O.N., Makarov V.G., Wagner H., Verpoorte R., Heinrich M. Medicinal plants of the Russian Pharmacopoeia; their history and applications. Journal of Ethnopharmacology. 2014;153:481–536. doi: 10.1016/j.jep.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Shikov A.N., Djachuk G.I, Sergeev D.V., Pozharitskaya O.N., Esaulenko E.V., Kosman V.M., Makarov V.G. Birch bark extract as therapy for chronic hepatitis C—a pilot study. Phytomedicine. 2011;18:807–810. doi: 10.1016/j.phymed.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Shinwari Z.K., Gilani S.S. Sustainable harvest of medicinal plants at Bulashbar Nullah, Astore (Northern Pakistan) Journal of Ethnopharmacology. 2003;84:289–298. doi: 10.1016/s0378-8741(02)00333-1. [DOI] [PubMed] [Google Scholar]
- Shults E.E., Bakhvalov S.A., Martem’ianov V.V., Petrova T.N., Syromiatnikova I.N., Shakirov M.M., Tolstikov G.A. Effects of natural and artificial defoliations on the content and composition of extractive substances in birch leaves. Prikladnaya Biokhimiya i Microbiologiya. 2005;41:107–112. [PubMed] [Google Scholar]
- Smite E., Lundgren L.N., Anderson R. Arylbutanoid and diarylheptanoid glycosides from inner bark of Betula pendula. Phytochemistry. 1993;32:365–369. [Google Scholar]
- Smite E., Pan H., Lundgren L.N. Lignan glycosides from inner bark of Betula pendula. Phytochemistry. 1995;40:341–343. [Google Scholar]
- Şoica C.M., Dehelean C.A., Peev C., Aluas M., Zupkó I., Kása P., Jr., Alexa E. Physico-chemical comparison of betulinic acid, betulin and birch bark extract and in vitro investigation of their cytotoxic effects towards skin epidermoid carcinoma (A431), breast carcinoma (MCF7) and cervix adenocarcinoma (HeLa) cell lines. Natural Product Research: Formerly Natural Product Letters. 2012;26:968–974. doi: 10.1080/14786419.2010.545352. [DOI] [PubMed] [Google Scholar]
- Stark S., Julkunen-Tiitto R., Holappa E., Mikkola K., Nikula A. Concentrations of foliar quercetin in natural populations of white birch (Betula pubescens) increase with latitude. Journal of Chemical Ecology. 2008;34:1382–1391. doi: 10.1007/s10886-008-9554-8. [DOI] [PubMed] [Google Scholar]
- Sur T.K., Pandit S., Battacharyya D., Kumar C.K.A., Lakshmi S.M., Chatttopadhyay D., Mandal S.C. Studies on the antiinflammatory activity of Betula alnoides bark. Phytotherapy Research. 2002;16:669–671. doi: 10.1002/ptr.942. [DOI] [PubMed] [Google Scholar]
- Svanberg I., Sõukand R., Łuczaj Ł., Kalle R., Zyryanova O., Dénes A., Papp N., Nedelcheva A., Šeškauskaitė D., Kołodziejska-Degórska I., Kolosova V. Uses of tree saps in northern and eastern parts of Europe. Acta Societatis Botanicorum Poloniae. 2012;81:343–357. [Google Scholar]
- Tegelberg R., Aphalo P.J., Julkunen-Tiitto R. Effects of long-term, elevated ultraviolet-B radiation on phytochemicals in the bark of silver birch (Betula pendula) Tree Physiology. 2002;22:1257–1263. doi: 10.1093/treephys/22.17.1257. [DOI] [PubMed] [Google Scholar]
- Temkitthawon P., Viyoch J., Limpeanchob N., Pongamornkul W., Sirikul C., Kumpila A., Suwanborirux K., Ingkaninan K. Screening for phosphodiesterase inhibitory activity of Thai medicinal plants. Journal of Ethnopharmacology. 2008;119:214–217. doi: 10.1016/j.jep.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Thongchai W., Liawruangrath B., Liawruangrath S. High-performance liquid chromatographic determination of arbutin in skin-whitening creams and medicinal plant extracts. Journal of Cosmetic Science. 2007;58:35–44. [PubMed] [Google Scholar]
- Trouillas P., Calliste C.A., Allais D.P., Simon A., Marfak A., Delage C., Duroux J.L. Antioxidant, anti-inflammatory and antiproliferative properties of sixteen water plant extracts used in the Limousin countryside as herbal teas. Food Chemistry. 2003;80:399–407. [Google Scholar]
- Tschirch, A., 1910. Handbuch der Pharmakognosie. 2. Abteilung (Die Hilfswissenschaften der Pharmakognosie). Leipzig. C.H. Tachnitz. 1. Auflage.
- Tunon H., Olavsdotter C., Bohlin L. Evaluation of anti-inflammatory activity of some Swedish medicinal plants. Inhibition of prostaglandin biosynthesis and PAF-induced exocytosis. Journal of Ethnopharmacology. 1995;48:61–76. doi: 10.1016/0378-8741(95)01285-l. [DOI] [PubMed] [Google Scholar]
- Valkama E., Salminen J.P., Koricheva J., Pihlaja K. Comparative analysis of leaf trichome structure and composition of epicuticular flavonoids in Finnish birch species. Annals of Botany. 2003;91:643–655. doi: 10.1093/aob/mcg070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkama E., Salminen J.P., Koricheva J., Pihlaja K. Changes in leaf trichomes and epicuticular flavonoids during leaf development in three birch taxa. Annals of Botany. 2004;94:233–242. doi: 10.1093/aob/mch131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker K., Gründemann C., Kern Y., Bredow L., Huber R., Reinhard T., Schwartzkopff J. Inhibition of corneal inflammation following keratoplasty by birch leaf extract. Experimental Eye Research. 2012;97:24–30. doi: 10.1016/j.exer.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Wang S.J., Pei Y.H., Hua H.M. Platyphyllin A, a novel coumarone from the leaves of Betula platyphylla Suk. Journal of Asian Natural Products Research. 2001;3:157–160. doi: 10.1080/10286020108041384. [DOI] [PubMed] [Google Scholar]
- Webster D., Taschereau P., Belland R.J., Sand C., Rennie R.P. Antifungal activity of medicinal plant extracts; preliminary screening studies. Journal of Ethnopharmacology. 2008;115:140–146. doi: 10.1016/j.jep.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Weston R.J., Smith G.J. Sesquiterpenes from the inner bark of the silver birch and the paper birch. Natural Product Communications. 2012;7:145–148. [PubMed] [Google Scholar]
- Wichtl Max. Teedrogen & Phytopharmaka. Wissenschaftliche Verlagsgesellschaft; Stuttgart: 2009. (Hrsg.) [Google Scholar]
- Xiong J., Taniguchi M., Kashiwada Y., Yamagishi T., Takaishi Y. Seven new dammarane triterpenes from the floral spikes of Betula platyphylla var. japonica. Journal of Natural Medicine. 2011;65:217–223. doi: 10.1007/s11418-010-0462-1. [DOI] [PubMed] [Google Scholar]
- Xiong J., Taniguchi M., Kashiwada Y., Sekiya M., Yamagishi T., Takaishi Y. Papyriferic acid derivatives as reversal agents of multidrug resistance in cancer cells. Bioorganic and Medicinal Chemistry. 2010;18:2964–2975. doi: 10.1016/j.bmc.2010.02.046. [DOI] [PubMed] [Google Scholar]
- Yin J., Ren C.L., Zhan Y.G., Li C.X., Xiao J.L., Qiu W., Li X.Y., Peng H.M. Distribution and expression characteristics of triterpenoids and OSC genes in white birch (Betula platyphylla Suk.) Molecular Biology Reports. 2012;39:2321–2328. doi: 10.1007/s11033-011-0982-0. [DOI] [PubMed] [Google Scholar]