Abstract
Background
Although respiratory syncytial virus (RSV) lower respiratory tract infections (LRTIs) in early life are followed by later airway hyperreactivity, it is unclear whether there is a causal relationship between this and an atopic diathesis.
Objectives
To separate the effects of RSV LRTI and an atopic diathesis on subsequent recurrent wheezing, we examined the protective effect of previous palivizumab administration against subsequent recurrent wheeze in infants with and without a family history of atopy.
Methods
A prospective multicenter, matched, double cohort study was conducted in 27 centers in Europe and Canada. The rates of physician-diagnosed recurrent wheezing in premature infants <36 weeks gestation who had received palivizumab in the first year of life were compared to those of gestational age–matched controls.
Results
The relative protective effect of palivizumab on physician-diagnosed recurrent wheezing through the ages of 2 to 5 years was 68% in those with no family history of asthma (odds ratio, 0.32; (95% CI, 0.14-0.75; N = 146 palivizumab-treated, 171 untreated) and 80% in those with no family history of atopy or food allergies (odds ratio, 0.20; 95% CI, 0.07-0.59; N = 101 palivizumab-treated, 100 untreated). In contrast, there was no effect of palivizumab on subsequent recurrent wheezing in the 90 children with a family history of atopy or food allergies compared to 130 untreated infants with atopic families.
Conclusion
Respiratory syncytial virus prophylaxis in nonatopic children decreases by 80% the relative risk of recurrent wheezing but does not have any effect in infants with an atopic family history. This suggests that RSV predisposes to recurrent wheezing in an atopy-independent mechanism.
Key words: Respiratory syncytial virus, lower respiratory tract infection, asthma, recurrent wheezing, palivizumab
Abbreviations used: LRTI, Lower respiratory tract infection; RR, Relative risk; RSV, Respiratory syncytial virus
Asthma is the most important chronic disease of childhood.1 Prospective epidemiologic studies of respiratory syncytial virus (RSV) lower respiratory tract infections (LRTIs) in early life have demonstrated subsequent rates of asthma and airway hyperreactivity 25% to 80% greater than in uninfected controls up to 11 years later.2, 3, 4, 5 It is unclear whether most severe RSV LRTIs occur in predisposed patients with asthma or whether they are causal in subsequent asthma. We have used palivizumab (Synagis, MedImmune, Inc, Gaithersburg, Md; marketed outside the USA by Abbott, Abbott Park, Ill), a humanized mAb against the RSV fusion protein that prevents severe RSV LRTI, in a prospective matched double cohort multicenter study in premature infants to separate these effects.6 In a multivariate analysis, we demonstrated that by preventing severe RSV LRTI in infancy, later recurrent wheezing was reduced by 58% and physician-diagnosed recurrent wheezing by 65% compared to controls. However, a large study conducted in Wisconsin7 (the Childhood Origins of ASThma [COAST] study.) showed that in children with a strong family history of atopy, rhinovirus was the most important cause of development of children wheezing at 37 and 6 years.8 Finally, a study from Australia showed that both rhinovirus and RSV interact with atopy in infancy to promote later asthma, suggesting that both of these are required in disease pathogenesis.9 Other studies from Canada10 showed that exposure to parainfluenza virus and RSV in the first year of life was associated with possible asthma at 2 years of age. A Spanish study showed that human metapneumovirus and RSV in infancy were associated with asthma at age 5 years,11 and the COAST Study showed that human coronavirus NL63 but not human metapneumovirus produced asthma at age 6 years.12
Thus, the relationship between early RSV LRTI and allergic sensitization in the development of asthma at a later age is still unresolved. This controversy prompted us to examine the effect of the family history of atopy on the prevention of later recurrent wheezing in premature infants who had received palivizumab in the first year of life compared to controls.
Methods
Patient enrollment
This prospective multicenter, matched, double cohort study was conducted in 27 centers in Spain, Germany, The Netherlands, Canada, Poland, and Sweden as described earlier.6 Briefly, children ≤36 months of age who had received palivizumab in a previous respiratory season and were not hospitalized for RSV were matched on the basis of gestational age (<28 weeks, 28-32 weeks, 29-35 weeks) and age (±3 months) to control children who had never received palivizumab. There were 2 control groups: approximately one third had documented RSV hospitalization in the first year before enrollment (n = 76), and the second group (n = 154) was not hospitalized. All study participants were born prematurely (<36 weeks gestational age) without chronic lung disease and had never received vaccines to prevent RSV. Other exclusion criteria were mechanical ventilation at enrollment, a life expectancy of <6 months, or a known immunodeficiency.
Written informed consent was obtained from each subject's parent or legal guardian before performance of any study-related procedures. The study was conducted according to International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Good Clinical Practice Guidelines. At enrollment, a medical and sociodemographic history was taken, a physical examination was performed, and serum was obtained for RSV neutralizing antibody and IgE level.6
Patient follow-up
Monthly contact with the parents/caregivers was scheduled for 24 months after enrollment. Visits to the study site were conducted at 6-month intervals; all other monthly contacts were conducted by telephone. The patient's illnesses, medications, hospitalizations, and other medical events in the past month were recorded at each of the monthly follow-up contacts. At 6-month intervals, physician records were also reviewed for all intercurrent doctor visits, emergency visits, and hospitalizations for respiratory symptoms.
Respiratory assessment
An episode of wheezing was defined as 1 or more consecutive days of wheezing preceded and followed by a nonwheezing healthy period of at least 1 week. Physician-diagnosed recurrent wheezing was defined as 3 or more episodes of wheezing in the last 12 months verified by a physician at a physician's visit, emergency department visit, or hospitalization.
Statistical methods
This report includes all data collected through 24 months of follow-up. Three study groups were defined in the protocol (infants who received palivizumab prophylaxis, controls not prophylaxed with palivizumab with a history of RSV hospitalization, and unprophylaxed controls without RSV hospitalization). Analyses compared the prophylaxed group with the combined untreated groups and the nonhospitalized untreated group. Post hoc subgroups of infants were determined on the basis of whether the following were present at baseline: family history of asthma and family history of atopy (asthma, atopic dermatitis, or allergic rhinitis) or food allergies. Family was defined as anyone in the immediate family. Analyses were performed within these subgroups. A sample size of 200 infants per group would provide approximately 80% power for a 2-sided, .05-level test to detect as statistically significant a difference in physician-diagnosed recurrent wheezing rates when the true rates were 3% for the prophylaxed group and 10% for the untreated controls. This study was not powered to test for differences within the subgroups previously specified.
Demographics and baseline characteristics were analyzed for quantitative variables by using 1-way ANOVA and for categorical variables by using the Fisher exact test. Comparisons between the treated and untreated groups in incidence of physician-diagnosed recurrent wheezing were performed by using the Fisher exact test; the relative risk (RR) and 95% CI for the RR were calculated. The potential influence of baseline characteristics was explored with multiple logistic regression with covariates for RSV hospitalization history, sex, age at enrollment, gestational age at birth, birth weight, multiple birth status, baseline RSV-neutralizing antibody titers (log2 RSV antibody level), day care attendance, numbers of adults and siblings in the home, number of siblings in day care, presence of a wood-burning stove in the home, and family history of asthma, atopic dermatitis, allergic rhinitis, or food allergies. Time to third physician-diagnosed wheezing episode was analyzed with multivariable Cox proportional hazards models including the covariates listed. For each logistic regression and Cox proportional hazards analysis, a forward stepwise selection method was used to determine factors significant at the .15 level; these factors were included in the final model. Only factors significant at the .05 level in the final model were displayed. Comparisons between the treated and untreated groups in time to third physician-diagnosed wheezing episode were performed by using the log-rank test, and Kaplan-Meier estimates were computed and displayed graphically.
Results
There were 191 children in the palivizumab-treated group and 230 in the untreated group, among whom 154 were not hospitalized with RSV and 76 were hospitalized with RSV. Of the 27 centers that enrolled subjects, 13 had subjects discontinue on or before day 730 (2-year cutoff). Sixteen (8.4%) in the palivizumab-treated group versus 21 (9.1%) in the 230 children not receiving palivizumab were discontinued before day 730. This difference was not statistically significant (P = .864). Demographics and baseline characteristics of the groups stratified by absence or presence of family history of atopy or food allergies are presented in Table I, Table II , respectively. There were 201 children with no family history of atopy or food allergies and 220 children with a family history of atopy or food allergies who were enrolled in the study. In the subjects with no family history of atopy or food allergies, both untreated groups had significantly more children who were from multiple births, and accordingly there were more siblings than in the palivizumab-treated group (Table I). In those with family history of atopy or food allergies, there were more children in day care in the untreated groups compared to the treated group. In both of these subgroups, the untreated infants had greater mean gestational age and birth weight than the palivizumab-treated infants (Table I, Table II). This was expected because guidelines for palivizumab use favor treatment for smaller, more premature infants. The combined untreated group also had a statistically significantly lower proportion of infants with baseline RSV antibody less than 2log2 compared to the treated group, within the subgroup of infants with no family history of atopy or food allergies. Other demographic factors were comparable between the treated and untreated groups.
Table I.
Demographics and baseline characteristics—subjects with no family history of atopy (asthma, atopic dermatitis, or allergic rhinitis) or food allergies
| Palivizumab treated |
Palivizumab untreated |
Palivizumab untreated non-RSV hospitalized |
|
|---|---|---|---|
| Variable | (n = 101) | (n = 100) | (n = 75§) |
| Mean birth weight (kg) ± SD (range) | 1.36 ± 0.45 (0.7-2.5) | 1.60 ± 0.47‡ (0.6-2.7) | 1.55 ± 0.45† (0.6-2.7) |
| Mean gestational age (wk) ± SD (range) | 29.9 ± 2.36 (25-34) | 31.5 ± 2.58‡ (24-35) | 31.3 ± 2.63‡ (24-35) |
| Female sex (%) | 43.6 | 49.0 | 52.0 |
| White race (%) | 97.0 | 95.0 | 96.0 |
| Multiple birth (%) | 17.8 | 34.0† | 30.7∗ |
| Mean enrollment age (mo) ± SD (range) | 19.4 ± 7.46 (5-40) | 20.5 ± 9.45 (1-37) | 20.1 ± 9.63 (1-37) |
| Mean enrollment weight (kg) ± SD (range) | 10.26 ± 1.95 (4.9-16.2) | 10.23 ± 2.78 (3.1-16.5) | 10.00 ± 2.70 (3.1-16.5) |
| Breast-fed (%) | 55.4 | 51.0 | 52.0 |
| Mean no. of siblings ± SD (range) | 0.7 ± 0.78 (0-3) | 1.2 ± 1.04‡ (0-4) | 1.1 ± 1.01∗ (0-4) |
| Siblings in day care (%) | 28.7 | 37.0 | 38.7 |
| Subject in day care (%) | 24.8 | 17.0 | 18.7 |
| Smokers in home (%) | 49.5 | 57.0 | 60.0 |
| Caregiver smokes (%) | 27.7 | 28.0 | 33.3 |
| Pets in home (%) | 37.6 | 27.0 | 30.7 |
| Wood-burning stove (%) | 16.8 | 15.0 | 20.0 |
| RSV antibody (% <2log2) | 47.0 | 30.3∗ | 39.2 |
| IgE antibody (% <5 IU/mL) | 59.6 | 49.5 | 48.6 |
P ≤ .050.
P ≤ .010.
P ≤ .001 for comparison with palivizumab-treated group.
The 75 children were a subgroup of the 100 untreated subjects.
Table II.
Demographics and baseline characteristics—subjects with family history of atopy (asthma, allergic dermatitis, or allergic rhinitis) or food allergies
| Palivizumab treated |
Palivizumab untreated |
Palivizumab untreated non-RSV hospitalized |
|
|---|---|---|---|
| Variable | (n = 90) | (n = 130) | (n = 79§) |
| Mean birth weight (kg) ± SD (range) | 1.36 ± 0.44 (0.6-3.2) | 1.65 ± 0.54‡ (0.6-3.8) | 1.58 ± 0.45† (0.6-2.7) |
| Mean gestational age (wk) ± SD (range) | 29.9 ± 2.08 (25-35) | 31.3 ± 2.44‡ (25-35) | 31.0 ± 2.26† (27-35) |
| Female sex (%) | 53.3 | 42.3 | 40.5 |
| White race (%) | 93.3 | 95.4 | 96.2 |
| Multiple birth (%) | 33.3 | 39.2 | 38.0 |
| Mean enrollment age (mo) ± SD (range) | 17.9 ± 8.11 (4-36) | 19.5 ± 9.76 (1-38) | 18.9 ± 10.92 (1-38) |
| Mean enrollment weight (kg) ± SD (range) | 10.19 ± 2.59 (3.8-19.3) | 10.12 ± 2.81 (2.6-16.6) | 9.77 ± 2.94 (3.1-16.6) |
| Breast-fed (%) | 71.1 | 68.5 | 68.4 |
| Mean number of siblings ± SD (range) | 1.0 ± 1.01 (0-5) | 1.3 ± 1.02∗ (0-5) | 1.1 ± 0.93 (0-4) |
| Siblings in day care (%) | 40.0 | 52.3 | 48.1 |
| Subject in day care (%) | 20.0 | 33.1∗ | 36.7∗ |
| Smokers in home (%) | 43.3 | 36.9 | 39.2 |
| Caregiver smokes (%) | 27.8 | 21.5 | 22.8 |
| Pets in home (%) | 33.3 | 33.8 | 31.6 |
| Wood-burning stove (%) | 13.3 | 13.8 | 15.2 |
| Family history of asthma (%) | 50.0 | 45.4 | 49.4 |
| Family history of atopic dermatitis %) | 16.7 | 19.2 | 16.5 |
| Family history of allergic rhinitis (%) | 56.7 | 54.6 | 54.4 |
| Family history of food allergies (%) | 14.4 | 16.2 | 17.7 |
| Family history of any atopy (%) | 94.4 | 90.8 | 88.6 |
| Family history of any atopy or food allergies (%) | 100 | 100 | 100 |
| RSV antibody (% <2log2) | 38.4 | 29.4 | 38.5 |
| IgE antibody (% <5 IU/ML) | 49.4 | 53.3 | 57.9 |
P ≤ .050.
P ≤ .010.
P ≤ .001 for comparison with palivizumab-treated group.
The 79 children were a subgroup of the 130 untreated subjects.
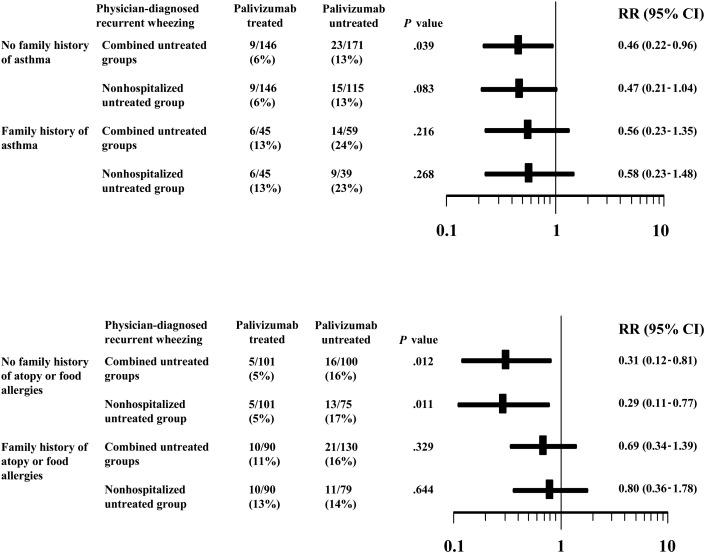
Significantly smaller proportions of palivizumab-treated children had physician-diagnosed recurrent wheezing during the first 24 months of follow-up compared to the combined untreated groups in the subgroups of children with (1) no family history of asthma (P = .039) and (2) no family history of atopy or food allergies (P = .012). Similar differences between the palivizumab-treated group and the nonhospitalized untreated group were also seen; however, the difference within the subgroup with no family history of asthma was not statistically significant (P = .083; Fig 1 ). The relative incidence rate reductions were not significant for the subgroups of infants with a family history of allergy or atopy/food allergy (Fig 1).
Fig 1.
Effect of palivizumab on physician-diagnosed recurrent wheezing in infants with or without a family history of asthma or atopy or food allergies. Results are expressed as frequencies of outcome in univariate analyses. The results are graphically represented as point estimates of the RRs and 95% CIs.
The potential influence of baseline characteristics on the incidence of respiratory outcomes was explored with multiple logistic regression (Table III ). Palivizumab treatment was associated with significant reductions in the risk of physician-diagnosed recurrent wheezing in infants without familial atopy/allergy histories. The reduction in the odds of an event was 68% in those with no family history of asthma and 80% in those with no history of atopy or food allergies. In contrast, in those subgroups with a family history of atopy or food allergies, palivizumab treatment did not have a significant effect on the occurrence of physician-diagnosed recurrent wheezing. Other significant factors in those with no family history of asthma or atopy/food allergies were birth weight and/or gestational age (with greater values reducing the risk of the event). In contrast, in children with a family history of asthma, being a member of a multiple birth and having more siblings in day care significantly increased the risk of physician-diagnosed recurrent wheezing, whereas having more siblings in the home decreased the risk.
Table III.
Factors associated with occurrence of physician-diagnosed recurrent wheezing by multiple logistic regression for subgroups based on family history
| Odds ratio (95% CI) |
||||
|---|---|---|---|---|
| No history |
History |
|||
| Variable | vs combined untreated groups | vs untreated non-RSV hospitalized group | vs combined untreated groups | vs untreated non-RSV hospitalized group |
| Asthma | ||||
| Palivizumab treatment | 0.32 (0.14-0.75)† | 0.32 (0.13-0.80)∗ | NA | NA |
| Member of multiple birth | NA | NA | 6.63 (1.64-26.74)† | NA |
| Siblings in household | NA | NA | 0.30 (0.09-0.98)∗ | NA |
| Siblings in day care | NA | NA | 5.40 (1.47-19.86)∗ | 2.02 (1.07-3.82)∗ |
| Birth weight | 0.35 (0.15-0.81)∗ | NA | NA | NA |
| Gestational age (per 1-wk increase) | NA | 0.78 (0.66-0.93)† | NA | NA |
| Atopy (asthma, atopic dermatitis, or allergic rhinitis) or food allergies | ||||
| Palivizumab treatment | 0.20 (0.07-0.59)† | 0.18 (0.06-0.55)† | NA | NA |
| Family history of asthma | NA | NA | NA | 3.10 (1.12-8.62)∗ |
| Gestational age (per 1-wk increase) | 0.82 (0.68-0.98)∗ | 0.79 (0.65-0.96)∗ | NA | 0.80 (0.64-1.00)∗ |
NA, not applicable, variable was not included in the final model.
Statistically significant at the .050 level.
Statistically significant at the .010 level.
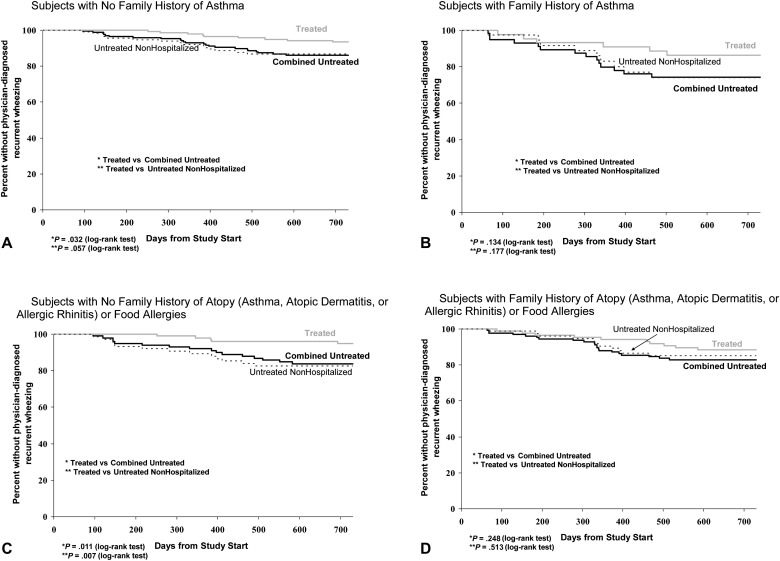
When we examined the time to the third episode of physician-diagnosed wheezing by using Kaplan-Meier methodology (Fig 2 ), the palivizumab-treated group demonstrated significantly longer times to the third episode in subgroups of infants with no family history of asthma or atopy/food allergies. In contrast, in the subgroups of infants with family histories of asthma or atopy/food allergies, the differences in time to third episode between the treated and untreated groups were not statistically significant. The potential influence of baseline characteristics on time to the third episode of physician-diagnosed wheezing was explored by using a multivariate Cox proportional hazards regression model. Palivizumab treatment remained associated with a significantly longer time to the third episode in both of the subgroups of infants with no family history of asthma and those with no family atopy/food allergy histories (Table IV ). Greater gestational age was also associated with a longer time to the third episode in these subgroups. In contrast, palivizumab did not appear to have a significant effect on those with family histories of asthma or atopy/food allergies. Interestingly, in the subgroup of infants with a family history of atopy or food allergy, a family history of asthma was the most significant risk factor for the time to the third episode of physician-diagnosed wheezing.
Fig 2.
Kaplan-Meier plots (A-D) showing the time to the third episode of physician-diagnosed wheezing in palivizumab-treated group (solid gray line) versus combined untreated groups (solid black line) and palivizumab-treated group versus nonhospitalized untreated group (dashed line).
Table IV.
Factors associated with time to onset of physician-diagnosed recurrent wheezing by multivariable Cox proportional hazards regression for subgroups based on family history
| Hazard Ratio (95% CI) |
||||
|---|---|---|---|---|
| No history |
History |
|||
| Variable | vs combined untreated groups | vs untreated non-RSV hospitalized group | vs combined untreated groups | vs untreated non-RSV hospitalized group |
| Asthma | ||||
| Palivizumab treatment | 0.33 (1.51-0.74)† | 0.34 (0.15-0.79)∗ | NA | NA |
| Member of multiple birth | NA | NA | 11.09 (2.14,57.45)† | NA |
| Siblings in household | NA | NA | 0.27 (0.09-0.81)∗ | NA |
| Siblings in day care | NA | NA | 6.36 (1.76-22.99)† | 1.84 (1.10-3.09)∗ |
| Gestational age (per 1-wk increase) | 0.84 (0.73-0.96)† | 0.79 (0.67-0.92)† | NA | NA |
| Atopy (asthma, atopic dermatitis, or allergic rhinitis) or food allergies | ||||
| Palivizumab treatment | 0.21 (0.08-0.59)† | 0.20 (0.07-0.56)† | NA | NA |
| Family history of asthma | NA | NA | 3.77 (1.60-8.92)† | 2.94 (1.14-7.61)∗ |
| Gestational age (per 1-wk increase) | 0.82 (0.70-0.96)∗ | 0.80 (0.67-0.95)∗ | NA | 0.81 (0.66-0.98)∗ |
NA, not applicable, variable was not included in the final model.
Statistically significant at the .050 level.
Statistically significant at the .010 level.
Discussion
The results of the COAST Study7, 8, 12 and the subsequent study from Australia9 appear to be in contrast to our initial findings that the administration of palivizumab in infancy protects premature subjects against subsequent recurrent wheezing in the first 3 to 4 years of life.6 This prompted us to examine the potential for a differential effect of RSV prevention in those with and without an atopic diathesis. Our finding of a significant protection in children with no family history of asthma or atopy does suggest that the differences may be explained by an atopic diathesis. Thus, whereas our study was conducted in preterm children, and whereas we recruited almost equivalent numbers of children with an atopic background and a nonatopic background, the COAST Study consisted almost entirely of infants with an atopic background.7 Because we did not examine the effects of rhinovirus infection in early infancy in children in the atopic group, we could not comment on the relationship between early rhinovirus infection and later subsequent asthma. However, the apparent lack of an effect of prevention of RSV in atopic subjects fits in well with the apparent lack of an independent effect of early life RSV seen in the COAST Study.7 The relatively low number of children in our study with a family history of asthma may in part explain the nonsignificant reduction in that group. In the group of children with an atopic family history, however, there was almost the same number of children as with no atopic history. The consistency of our findings in all the subgroups on the basis of the absence of family atopy/allergy history and the increasing protection (at least for the point estimates) as successive atopic backgrounds are removed (from a family history of asthma to family history of atopy or food allergies), despite a lower number of children with no family history of atopy or food allergies, suggest that our observation is real.
The subjects in this trial were recruited at the mean age of 19 months (range, 1-40 months). It is possible that some of these children already had recurrent wheezing (3 or more episodes of physician-diagnosed recurrent wheezing) before that age. When we examined the subgroup of children with no family history of atopy or food allergy with multiple logistic regression, palivizumab treatment reduced the risk of physician-diagnosed recurrent wheezing by 80%; this was in contrast with palivizumab not having a significant effect in those with such a history. Children with recurrent wheezing in this group may have had recurrent wheezing for several reasons. A minority would have asthma starting early in life, and some would be those with recurrent wheezing after RSV, occurring before enrollment. These would be few, because our definition was 3 or more episodes of recurrent wheezing (in this case, before enrollment). Because subjects were between 2 and 5 years of age at the completion of the study, this may be too early for hereditary allergic factors to exert their effect on wheezing. However, allergic factors may have exerted an effect, because the influence of palivizumab was less apparent in these subjects. The children within the group with the most protection thus probably had no atopic diathesis and would probably not go on to develop asthma in later life. It is precisely within this group that the maximum protection was seen.
Because none of the patients in the palivizumab group and 33% of the control group were hospitalized with RSV, it is possible that the results reflect the more severe previous RSV disease in the control group.13 Hence, we compared the outcomes in the treated and control groups that were not hospitalized for RSV. There were still significant differences between the palivizumab-treated and the nonhospitalized untreated group in those subgroups with no family histories of atopy or food allergies. The logistic regression analysis once again showed that the major factor associated with a reduction in physician-diagnosed recurrent wheezing was treatment with palivizumab in those without an atopic diathesis.
A family history of atopy or asthma may be a risk factor for severe LRTI in early life14, 15 or RSV hospitalization.16, 17 In patients with a family history of asthma, the effect of palivizumab appeared to be marginal, suggesting that the atopic or asthmatic background is a severe enough predisposing factor for the development of later occurring wheezing, and perhaps RSV infection does not increase that risk very much. This thesis is supported in part by our observation in the Cox regression model clearly showing that in those with an atopic diathesis or food allergies, the family history of asthma is still the strongest predictor of recurrent wheezing later, and prevention by palivizumab of RSV does not appear to have any significant effect in this population. This study shows a protective effect of 65% in the overall population and 80% in those with no background of atopy or food allergies, and the consistency of the effect appears to be a strong indicator that RSV may lead to recurrent wheezing in this population. Whether this effect persists into later childhood or into adult life is a question that remains to be answered.
Studies from Australia seem to suggest that early-life respiratory infections with either RSV or rhinovirus in conjunction with atopic sensitization may contribute to the risk for subsequent development of persistent asthma.9 At the same time, studies from Tucson18 predict that children with persistent wheezing at the age of 6 years in conjunction with a family history of asthma predict the onset of later asthma in adulthood.19 Our study clearly cannot answer questions about the genesis of later childhood asthma or adult-onset asthma, but the observation does lend itself to the intriguing possibility that perhaps preventing RSV infection early in life prevents sensitization and potentially the development of later asthma. This hypothesis was suggested by data from a mouse model of infection and supported by a study of RSV prophylaxis in human beings. In the mouse model of RSV infection, it has been shown that respiratory infections of young mice sensitize the mice to aeroallergens to which they were exposed during infection.20 However, a more recent study suggests that remodeling after RSV in the mouse model occurs without sensitization.21 In a previous study using RSV immunoglobulin,22 it was suggested that the combined immunosuppressive effect of high doses of monthly prophylactic immunoglobulin and the anti-RSV activity of the RSV immunoglobulin given to children with chronic lung disease of prematurity abrogated the development of abnormal lung function at 7 to 9 years of age. Future studies (randomized control trials) of RSV infection using mAbs in children with and without an atopic background are being planned to address the role of RSV in the development of subsequent asthma.
In premature infants, the prevention of RSV by using palivizumab in children without an atopic background appears to decrease by 80% the relative risk of recurrent wheezing from 2 to 5 years of age, an effect that is not seen in those with an atopic background.
Clinical implications.
RSV prophylaxis with palivizumab significantly reduced the relative risk of subsequent recurrent wheezing in nonatopic premature infants.
Footnotes
Supported by Abbott.
Disclosure of potential conflict of interest: E. A. F. Simoes has received grants, honoraria, and research support from Abbott International and MedImmune, Inc. X. Carbonell-Estrany has given lectures sponsored by Abbott. I. Mitchell is a lecturer for Abbott Canada and has received research support from Abbott Canada and the Alberta Law Foundation. J. R. Groothuis is employed by MedImmune and has received research support from Abbott. The rest of the authors have declared that they have no conflict of interest.
References
- 1.Masoli M., Fabian D., Holt S., Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Bont L., Steijn M., Van Aalderen W.M., Brus F., Th Draaisma J.M., Van Diemen-Steenvoorde R.A. Seasonality of long term wheezing following respiratory syncytial virus lower respiratory tract infection. Thorax. 2004;59:512–516. doi: 10.1136/thx.2003.013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigurs N., Bjarnason R., Sigurbergsson F., Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 4.Sigurs N., Bjarnason R., Sigurbergsson F., Kjellman B., Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–505. [PubMed] [Google Scholar]
- 5.Stein R.T., Sherrill D., Morgan W.J., Holberg C.J., Halonen M., Taussig L.M. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 6.Simoes E.A., Groothuis J.R., Carbonell-Estrany X., Rieger C.H., Mitchell I., Fredrick L.M. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151:34–42. doi: 10.1016/j.jpeds.2007.02.032. e1. [DOI] [PubMed] [Google Scholar]
- 7.Lemanske R.F., Jr., Jackson D.J., Gangnon R.E., Evans M.D., Li Z., Shult P.A. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Jackson D.J., Gangnon R.E., Evans M.D., Roberg K.A., Anderson E.L., Pappas T.E. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kusel M.M., de Klerk N.H., Kebadze T., Vohma V., Holt P.G., Johnston S.L. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee K.K., Hegele R.G., Manfreda J., Wooldrage K., Becker A.B., Ferguson A.C. Relationship of early childhood viral exposures to respiratory symptoms, onset of possible asthma and atopy in high risk children: the Canadian Asthma Primary Prevention Study. Pediatr Pulmonol. 2007;42:290–297. doi: 10.1002/ppul.20578. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Garcia M.L., Calvo C., Casas I., Bracamonte T., Rellan A., Gozalo F. Human metapneumovirus bronchiolitis in infancy is an important risk factor for asthma at age 5. Pediatr Pulmonol. 2007;42:458–464. doi: 10.1002/ppul.20597. [DOI] [PubMed] [Google Scholar]
- 12.Pappas T.E., Sullivan Dille K.T., Lee W., Grindle K.A., Roberg K.A., Da Silva D.F. Coronavirus NL63 illnesses in infancy are a risk factor for asthma at age six. J Allergy Clin Immunol. 2007;S146:577. [Google Scholar]
- 13.Carroll K.N., Wu P., Gebretsadik T., Griffin M.R., Dupong W.D., Mitchel E.F. J Allergy Clin Immunol. 2009;123:1055–1061. doi: 10.1016/j.jaci.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosken C.H., Hunt W.C., Lambert W.E., Samet J.M. A parental history of asthma is a risk factor for wheezing and nonwheezing respiratory illnesses in infants younger than 18 months of age. Am J Respir Crit Care Med. 2000;161:1810–1815. doi: 10.1164/ajrccm.161.6.9903030. [DOI] [PubMed] [Google Scholar]
- 15.Goetghebuer T., Kwiatkowski D., Thomson A., Hull J. Familial susceptibility to severe respiratory infection in early life. Pediatr Pulmonol. 2004;38:321–328. doi: 10.1002/ppul.20069. [DOI] [PubMed] [Google Scholar]
- 16.Stensballe L.G., Kristensen K., Simoes E.A., Jensen H., Nielsen J., Benn C.S. Atopic disposition, wheezing, and subsequent respiratory syncytial virus hospitalization in Danish children younger than 18 months: a nested case-control study. Pediatrics. 2006;118:e1360–e1368. doi: 10.1542/peds.2006-0907. [DOI] [PubMed] [Google Scholar]
- 17.Trefny P., Stricker T., Baerlocher C., Sennhauser F.H. Family history of atopy and clinical course of RSV infection in ambulatory and hospitalized infants. Pediatr Pulmonol. 2000;30:302–306. doi: 10.1002/1099-0496(200010)30:4<302::aid-ppul5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 18.Martinez F.D., Wright A.L., Taussig L.M., Holberg C.J., Halonen M., Morgan W.J. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 19.Stern D.A., Morgan W.J., Halonen M., Wright A.L., Martinez F.D. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372:1058–1064. doi: 10.1016/S0140-6736(08)61447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Donnell D.R., Openshaw P.J. Anaphylactic sensitization to aeroantigen during respiratory virus infection. Clin Exp Allergy. 1998;28:1501–1508. doi: 10.1046/j.1365-2222.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- 21.Ostler T., Hussell T., Surh C.D., Openshaw P., Ehl S. Long-term persistence and reactivation of T cell memory in the lung of mice infected with respiratory syncytial virus. Eur J Immunol. 2001;31:2574–2582. doi: 10.1002/1521-4141(200109)31:9<2574::aid-immu2574>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 22.Wenzel S.E., Gibbs R.L., Lehr M.V., Simoes E.A. Respiratory outcomes in high-risk children 7 to 10 years after prophylaxis with respiratory syncytial virus immune globulin. Am J Med. 2002;112:627–633. doi: 10.1016/s0002-9343(02)01095-1. [DOI] [PubMed] [Google Scholar]