Highlights
-
•
We devise a novel system that allows M. haemolytica biofilms to form on epithelial cells.
-
•
Mucin significantly decreased biofilm formation.
-
•
Prior infection with several respiratory viruses does not alter biofilm formation.
-
•
Biofilms on epithelial cells were more resistant to some antibiotics than biofilms on polystyrene.
Keywords: Biofilm, Mannheimia haemolytica, Epithelial cells, Model system
Abstract
Mannheimia haemolytica is the most important bacterial agent associated with the bovine respiratory disease complex (BRDC), which causes worldwide economic losses to the cattle industry. M. haemolytica cells initially colonize the tonsillar crypts in the upper respiratory tract of cattle, from where they can subsequently descend into the lungs to cause disease. Many bacteria exist as biofilms inside their hosts. We hypothesize that M. haemolytica colonization of cattle during its commensal state may include biofilm formation. To begin to assess this possibility, we developed an in vitro system to study biofilm formation directly on bovine respiratory epithelial cells. Using fixed primary bovine bronchial epithelial cells, we observed M. haemolytica biofilm formation after a 48 h incubation period at 37 °C. Addition of mucin, the main component of mucus present in the upper respiratory tract, decreased M. haemolytica biofilm formation on bovine epithelial cells. We investigated the effects of prior viral infection of the epithelial cells on subsequent biofilm formation by M. haemolytica and found negligible effects. Utilization of this model system will provide new insights into the potential role of biofilm formation by M. haemolytica in the pathogenesis of BRDC.
1. Introduction
Bovine Respiratory Disease Complex (BRDC) causes hundreds of millions of dollars per year in losses to the cattle industry (Bowland and Shewen, 2000). The disease is associated with multiple bacterial and viral pathogens including Pasteurella multocida, Histophilus somni, Mycoplasma bovis, bovine viral diarrhea virus (BVDV), bovine respiratory syncytial virus (BRSV), and bovine herpes virus (BHV) (Caswell, 2014, Moore et al., 2015). The most important bacterial species associated with BRDC is Mannheimia haemolytica, a member of the family Pasteurellaceae. M. haemolytica exists as a commensal in the upper respiratory tract of healthy cattle, from where it can descend into the lungs to cause pneumonia (Frank and Smith, 1983, Frank et al., 1993). Previous studies have shown the ability of M. haemolytica to colonize bovine tonsils for extended periods (Frank et al., 1993). Severe BRDC is often preceded by infection of the respiratory epithelium with viruses like BVDV-1, BRSV, BHV-1, or bovine coronavirus (Frank et al., 1993). The mechanisms by which these viral infections predispose the host to M. haemolytica are unclear. One possibility is that viral infections alter the respiratory epithelial lining to favor bacterial colonization (Selinger et al., 1981).
Commensal bacteria often exist in their hosts as biofilms, which are microcolonies of bacteria encased in an extracellular polymeric substance (EPS) (Olson et al., 2002). These biofilms provide protection for the bacterial cells against the host immune response and antibiotic therapy. It has been shown that bacteria within a biofilm can be more resistant than planktonic cells to antibiotics (Olson et al., 2002). As a result, biofilms pose an added concern when treating infections with antibiotics.
A previous report provided scanning electron micrographs suggesting that microcolonies of M. haemolytica cells can be observed in the lungs of cattle with severe respiratory disease (Morck et al., 1989). Other members of the family Pasteurellaceae have been shown to produce biofilms in vitro. For example, the closely related porcine respiratory pathogen Actinobacillus pleuropneumoniae forms biofilms within 4 h, under static growth conditions in vitro (Tremblay et al., 2013). The bovine respiratory pathogen H. somni can form biofilms that are thought to allow this commensal to persist on the bovine mucosa (Sandal et al., 2011, Elswaifi et al., 2012). The EPS in H. somni biofilms was found to consist largely of polysaccharides, which contribute to persistence of the bacteria in its biofilm state during systemic infection (Elswaifi et al., 2012). In a previous study, we showed that M. haemolytica will form biofilm on plastic in vitro, and that protein is the major constituent in the EPS (Boukahil and Czuprynski, 2015).
To effectively study biofilm formation in the context of the host, it is necessary to examine their interactions with underlying epithelial cells (Tran et al., 2014). Some bacterial species require 48 h to form a biofilm (Boukahil and Czuprynski, 2015), which presents a technical challenge because the underlying host cells will detach as a result of bacterial overgrowth in a closed system. By fixing epithelial cells in tissue culture wells, we have devised a system to study biofilm formation directly on bovine respiratory epithelial cells.
We used variations of this model system to investigate how various conditions affect biofilm formation. We found that at concentrations similar to those found in the oral cavity (Kejriwal et al., 2014), mucin decreased M. haemolytica biofilm formation on bovine epithelial cells. In an effort to explain the link between viral infection and M. haemolytica pathogenesis, we infected the epithelial cells with several respiratory viruses to determine if biofilm formation was altered. Results obtained with our model system will help to elucidate how M. haemolytica persists as a commensal in the upper respiratory tract.
2. Materials and methods
2.1. Bacterial strains
M. haemolytica A1 (derived from a pneumonic bovine lung) (Gioia et al., 2006), was grown without shaking for 18 h in 10 ml of brain heart infusion broth (BHI broth, EMD Millipore, MA) at 37 °C to achieve a final concentration of 109 CFU/ml.
2.2. Primary cells
Primary bovine bronchial epithelial cells (BBEC) were kindly provided by Dr. D.S. Allen-Gipson (Omaha, NE) and maintained in DMEM-F12 media (Corning, VA) containing 10% fetal bovine serum, 2 mM L-glutamine, and 10 μg/ml of epidermal growth factor (Sigma, MO). These cells were harvested from lungs of a healthy cow obtained at an abattoir (Allen-Gipson et al., 2006) and have been used by our lab in previous studies (N’jai et al., 2013).
2.3. Biofilm assay
BBEC were seeded into wells of a flat bottom polystyrene 24 well plate (Corning, NY) and incubated at 37 °C with 5% CO2 until they reached confluency. The cells were then washed once with 1 ml sterile water, and incubated with 0.75 ml 0.1% glutaraldehyde (diluted in water) (Sigma) at room temperature for 10 min (Bollinger et al., 2003). The fixative was removed and the wells washed three times with 1 ml sterile water. M. haemolytica cells were suspended in RPMI to achieve a final concentration of 105 CFU/ml, and 0.8 ml of the bacterial suspension was added to each well. A control group of fixed BBEC incubated with RPMI that did not contain bacterial cells (Lonza, MD) was included as a negative control. The plate was incubated at 37 °C with 5% CO2 for 48 h. Every 12 h, the medium was removed from the wells, the wells rinsed with 1 ml warm PBS, and 0.8 ml of RPMI media dispensed into the wells. Following the 48 h incubation, the wells were rinsed twice with deionized water, and allowed to air dry for 1 h. The wells then were incubated with filtered crystal violet (Harleco, EMD Millipore) at room temperature for 15 min. After two washes with 1 ml of water, the plate was air dried for 1 h, the retained dye solubilized with 30% glacial acetic acid (Sigma) and absorbance at 595 nm determined using a plate reader (DTX 880 Multimode Detector, Beckman Coulter, CA). For scanning electron microscopy (SEM), biofilms were formed on fixed bovine respiratory epithelial cells in Lab-Tek slides (Thermo Scientific, MA), fixed overnight in 2.5% glutaraldehyde, and processed as previously described (Shippy et al., 2012). Images were taken at the Biological and Biophysical Preparation, Imaging and Characterization Laboratory at the University of Wisconsin-Madison on a Hitachi S900 microscope (Hitachi, Japan).
2.4. Adhesion assay
Confluent BBEC cells were seeded into wells of a 24 well plate, washed once with 1 ml sterile water, and either fixed with glutaraldehyde (as described in the biofilm assay above) or left unfixed. M. haemolytica cells (105 CFU/ml) in RPMI 1640 were added to each well and the cells incubated for 2 h at 37 °C with 5% CO2. Afterwards, the wells were rinsed twice with 1 ml PBS and adherent bacterial cells were removed by adding 1 ml PBS and disrupting the BBEC monolayer through scraping and vigorous pipetting. The bacteria were enumerated via serial dilution and plating onto blood agar plates. Each group was run in quintuplicate wells.
2.5. Mucin assay
Bovine submaxillary mucin (purified from the submaxillary mucin clot using the procedure described in Nisizawa and Pigman (1959) (Worthington Biochemical, Lakewood, NJ)) was prepared in PBS (10 mg/ml stock solution) and UV sterilized for six hours. The solution was diluted to the desired concentration in RPMI, combined with 105 M. haemolytica CFU/ml, and added to fixed BBEC as described above for the biofilm assay. The plates were incubated at 37 °C with 5% CO2 for 48 h. Every 12 h, the wells were rinsed and the media replaced with the appropriate mucin concentrations. As a control to mimic the viscosity of mucin, methyl cellulose of a similar concentration was added to one group of wells (Kavanaugh et al., 2014). After incubation, the wells were rinsed two times with deionized water and biofilm quantified with crystal violet as described in the biofilm assay.
To assess the effect of mucin on M. haemolytica adhesion, an adherence assay was performed similar to the conditions described above. Various concentrations of mucin were mixed with M. haemolytica cells (105 CFU/ml) and added to fixed BBEC as described above for the biofilm assay. The plates were incubated at 37 °C with 5% CO2 for 2 h, at which time samples were removed by adding 1 ml PBS and disrupting the BBEC monolayer through scraping and vigorous pipetting. Next, serial dilution and plating was used to estimate CFU of M. haemolytica adherent to the monolayers. To determine if mucin inhibits growth of M. haemolytica, 105 CFU/mL of M. haemolytica were combined with various concentrations of mucin (or methyl cellulose as a control) in RPMI. The solutions were dispensed into triplicate wells containing fixed BBEC cells and incubated at 37 °C with 5% CO2 for 24 h.
2.6. Effect of viral infection on biofilm formation
BRSV and BVDV-1 were kindly provided by Dr. Kathy Kurth (University of Wisconsin-Madison). BHV-1 was provided by Dr. Ronald Schultz (University of Wisconsin-Madison). To propagate the virus, confluent BBEC in a T75 flask were infected with virus and subsequently the viruses were concentrated using a procedure described previously (Eisfeld et al., 2014). To initiate viral infection, uninfected BBEC were seeded at a density of 105 per well in a 24 well plate. After incubation for 24 h at 37 °C with 5% CO2, the cells were infected with 105 PFU of virus (MOI of 1) in DMEM-F12 media containing 2% fetal bovine serum and 2 mM L-glutamine. Because viral infection of cells is based on a Poisson distribution we assume that at an MOI of 1 63% of the BBEC cells were virally infected (Knipe and Howley, 2013). A control group of uninfected BBEC was included as a control. BBEC were incubated at 37 °C with 5% CO2 until the first signs of CPE were observed (i.e. 12 h for BHV-1, 60 h incubation for BVDV-1, 72 h for BRSV). The cells were then fixed and used for the biofilm assay described above.
2.7. Antibiotic susceptibility assays
We assessed the effects of antibiotics on M. haemolytica using both minimum inhibitory concentration (MIC) and minimum biofilm eradication concentration (MBEC) assays. The MIC assay was performed by suspending 105 CFU/mL planktonic cells of M. haemolytica in RPMI. Next, two-fold dilutions (.0625 μg/ml to 64 μg/ml) of gentamicin (Sparhawk Laboratories Inc., KS), florfenicol (Hospira Inc., IL), chlortetracycline (MP Biomedicals, CA), erythromycin (Sigma), or tulathromycin (Pfizer, NJ) were added to the bacterial suspensions and the mixtures dispensed into duplicate wells (800 μl per well) of a 24 well plate. The plate was incubated at 37 °C with 5% CO2 for 24 h and the MIC determined to be the lowest dilution that showed no turbidity in the wells.
Minimum biofilm eradication concentration (MBEC) assays were performed as described previously (Boukahil and Czuprynski, 2015), with some modifications. BBEC were washed once with 1 ml of sterile water, fixed in wells of a 24 well plate, rinsed three times with 1 ml water, and M. haemolytica biofilms allowed to form as described above in the biofilm assay. Biofilms were rinsed once with 1 ml PBS, and 0.8 ml of RPMI containing serial two-fold dilutions (2–1024 μg/ml) of various antibiotics (florfenicol, gentamicin, chlortetracycline, erythromycin, or tulathromycin) were added to wells. The plates were incubated for 24 h at 37 °C with 5% CO2, rinsed once with 1 ml PBS, and replaced with 0.8 ml RPMI. The biofilms were dispersed from the well surface by scraping and vigorous pipetting and the plate incubated for an additional 24 h at 37 °C with 5% CO2. MBEC was defined as the lowest antibiotic concentration that did not result in visible turbidity or produce any colonies after plating (100 μl) onto blood agar.
2.8. Statistical analysis
Data were analyzed by either one way analysis of variance followed by the Tukey test for significance, or by a paired t-test using Prism 5 Software (GraphPad, CA). Statistical significance was set at P < 0.05.
3. Results
3.1. M. haemolytica forms biofilm on bovine bronchial epithelial cells
We previously reported that M. haemolytica produces the greatest amount of biofilm on polystyrene after a 48 h incubation in vitro (Boukahil and Czuprynski, 2015). The extended incubation period posed a challenge to our goal to form biofilm on epithelial cells because unfixed epithelial cells detached from the well when incubated with M. haemolytica for this amount of time (unpublished). To overcome this obstacle, we fixed the epithelial cells with 0.1% glutaraldehyde for 10 min prior to adding 105 CFU M. haemolytica cells. The plate then was incubated for 48 h at 37 °C with 5% CO2. During this time, the wells were washed once every 12 h to remove planktonic cells. After 48 h, the wells were stained with crystal violet and absorbance measured at 595 nm.
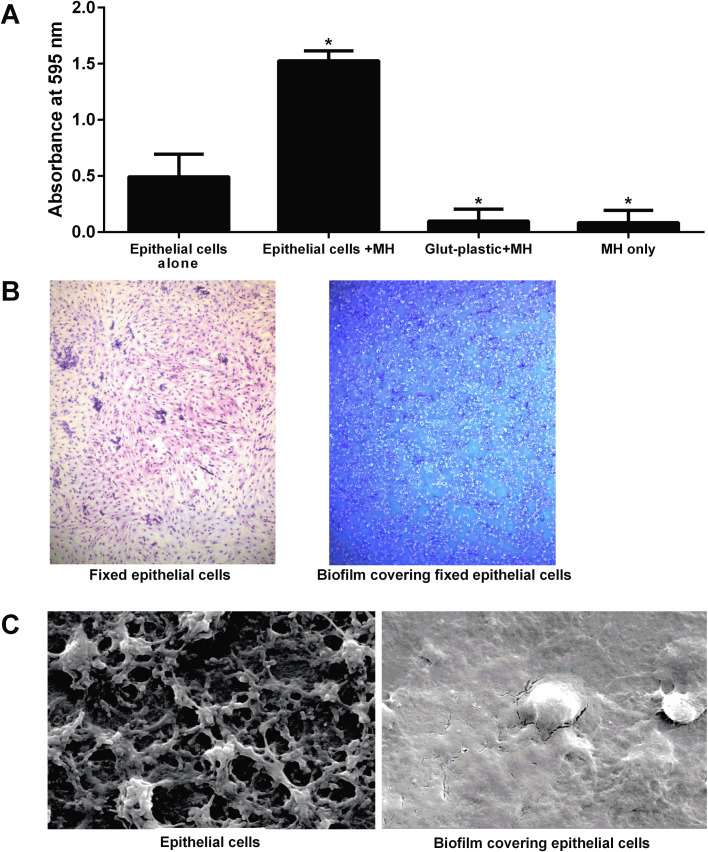
Using this method, we found that M. haemolytica formed biofilm on the fixed BBEC (Fig. 1 A). Representative examples of fixed epithelial cells and cells containing biofilms are illustrated in Fig. 1B. Fig. 1C shows scanning electron micrograph images of extensive EPS being produced by the bacteria compared to the epithelial cells alone. To ensure that the wash steps did not enhance biofilm formation we included a control group, without epithelial cells, that received M. haemolytica cells and was washed every 12 h. We found this treatment group produced less biofilm on plastic than a static culture of M. haemolytica. Another control group, glutaraldehyde-treated plastic, was tested to show that the glutaraldehyde did not somehow enhance biofilm formation on the plastic wells.
Fig. 1.
M. haemolytica can form biofilms on glutaraldehyde-fixed bovine respiratory epithelial cells. (A) Bovine respiratory epithelial cells grown in the wells of a 24 well plate were fixed with 0.1% glutaraldehyde, washed, and 105 CFU M. haemolytica (MH) in RPMI medium added to each well. Wells that contained fixed bovine respiratory epithelial cells but did not receive bacteria were used to determine background staining. The wells were then incubated at 37 °C with 5% CO2 for 48 h. Biofilm was stained with crystal violet and absorbance at 595 nm was determined. Data are the mean ± SEM of triplicate wells from three independent experiments. *, P < 0.05 compared to epithelial cells alone. (B) Fixed bovine respiratory epithelial cells without (left panel) and with (right panel) M. haemolytica biofilm were stained with Diff-Quik® and viewed by light microscopy (40×). Images are representative of three independent experiments. (C) Fixed epithelial cells alone (left panel) and cells supporting M. haemolytica biofilm (right panel) were imaged by scanning electron microscopy (2000×). Images are representative of those from three independent experiments that were performed.
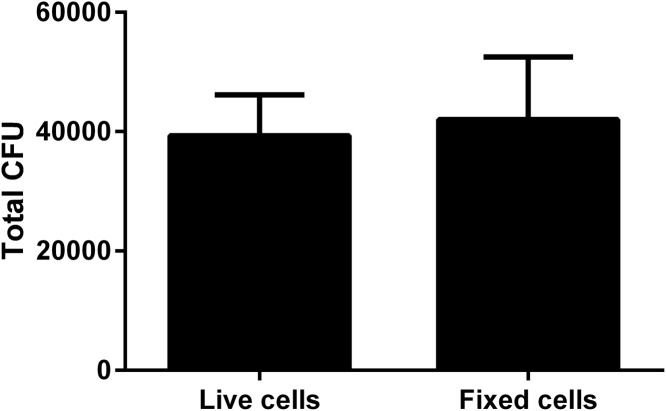
Bacterial adhesion is the critical first step in biofilm formation. To see if adhesion of bacteria was adversely affected by glutaraldehyde fixation of the BBEC, we examined the adhesion of M. haemolytica to both living and fixed epithelial cells. After incubating at 37 °C, unattached bacteria were washed away and the remaining adherent bacteria were quantified by plating onto agar. We recovered similar CFU of M. haemolytica from live and fixed cells (Fig. 2 ), suggesting that glutaraldehyde fixation does not alter bacterial adhesion to BBEC.
Fig. 2.
Similar numbers of M. haemolytica bind to live and glutaraldehyde fixed epithelial cells. Bovine respiratory epithelial cells were seeded into wells of a 24 well plate and grown to confluence. One group was fixed with 0.1% glutaraldehyde for 10 min at room temperature and the other group remained unfixed. M. haemolytica (105 CFU/ml) in RPMI were then added to both groups and incubated at 37 °C with 5% CO2 for 2 h. Wells were washed with PBS, the adherent bacteria disrupted by vigorous scraping and pipetting, and the bacterial cells resuspended in 1 ml PBS. Bacteria were enumerated by serial dilution and plating onto blood agar plates. Data are the mean ± SEM from three independent experiments.
3.2. Mucin decreases M. haemolytica biofilm formation
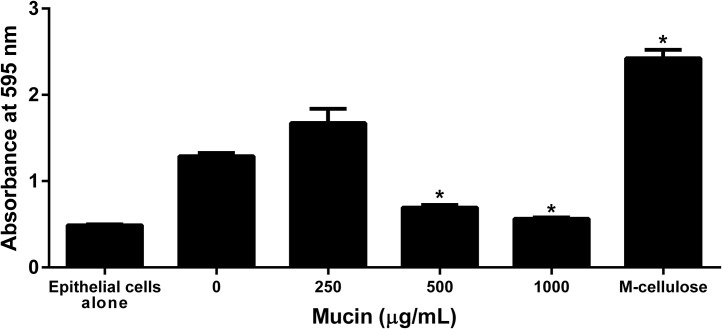
Mucin is present in the healthy respiratory tract, but it also plays a detrimental role in diseases like cystic fibrosis and periodontal disease (Landry et al., 2006). We tested whether mucin affects M. haemolytica biofilm formation on bovine epithelial cells by adding various concentrations of bovine mucin to the suspension of bacterial cells, prior to adding them to BBEC in the biofilm assay described above. We found that at 1 mg/ml, mucin led to less biofilm formation on BBEC than the control group without mucin (Fig. 3 ). As an additional control we also examined methyl cellulose, which has a similar viscosity to mucin, and found that it did not decrease biofilm formation. This finding suggests that the effect of mucin on biofilm formation is not simply because of increased viscosity of the medium.
Fig. 3.
Mucin decreases M. haemolytica biofilm formation on fixed bovine respiratory epithelial cells. 105 CFU M. haemolytica were incubated with either mucin (0–1000 μg/ml) or methyl cellulose (1000 μg/ml) and then added to fixed bovine respiratory epithelial cells. The plates were incubated at 37 °C with 5% CO2 for 48 h with washing every 12 h to remove planktonic cells. The resultant biofilms were stained with crystal violet and absorbance read at 595 nm. Data are the mean ± SEM from three independent experiments.*, P < 0.05 compared to control (0 μg/ml).
To determine if mucin affected adherence, we performed an adhesion assay as done earlier, in the presence of various concentrations of mucin. We found that mucin did not inhibit adhesion compared to no mucin or methyl cellulose controls (Fig. S1). We also examined mucin in a MIC assay and found it was not bacteriostatic or bactericidal to M. haemolytica at the concentrations used (data not shown).
Mucin does not inhibit M. haemolytica adhesion to bovine respiratory epithelial cells. M. haemolytica cells (3 × 105 CFU) were incubated on fixed epithelial cells in wells of a 24 well plate, in the presence of mucin (250–1000 μg/ml) or methyl cellulose (1000 μg/ml) for 2 h at 37 °C with 5% CO2. Adherent bacteria was disrupted by vigorous scraping and pipetting, resuspended in 1 ml PBS, and enumerated by serial dilution and plating onto blood agar plates. Total CFU per well of each group is shown. Data are the mean ± SEM from three independent experiments.
3.3. Viral infection does not enhance M. haemolytica biofilm formation
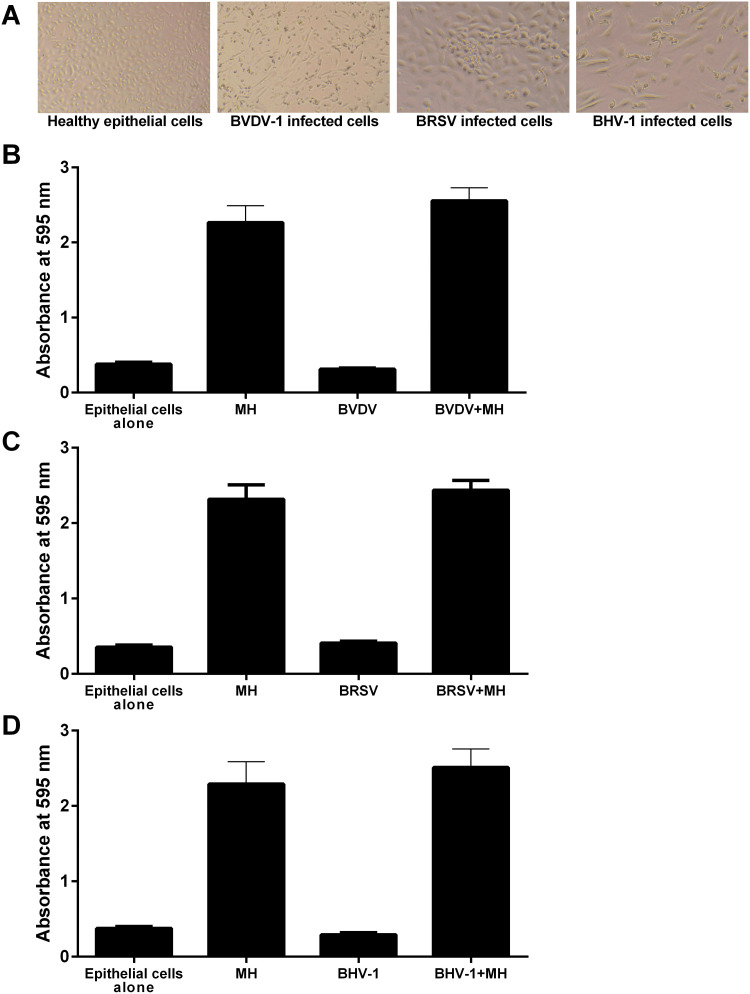
Viral infection can predispose cattle to M. haemolytica infection and cause severe BRDC (van der Sluijs et al., 2010, Moore et al., 2015). To test whether viral infection increases biofilm formation on epithelial cells, we used bovine viral diarrhea virus-1 (BVDV-1), bovine respiratory syncytial virus (BRSV), and bovine herpesvirus-1 (BHV-1), all of which have been shown to worsen M. haemolytica infection in vivo (Potgieter et al., 1984, van der Sluijs et al., 2010, Hodgson et al., 2012). We first infected BBEC with the indicated viruses at an MOI of 1 and incubated the cells until the first signs of cytopathic effects were evident (Fig. 4 A). We then fixed the cells with 0.1% glutaraldehyde, added 105 CFU M. haemolytica, and quantified biofilm formation as described earlier. We found that biofilm formation on virally infected cells did not differ from uninfected cells (Fig. 4B–D). When we quantify M. haemolytica biofilms forming on BBEC after 48 h absorbance values in the crystal violet assay can range up to 2.7. We considered the possibility that these relatively high values might confound our ability to detect inhibition by viral infection of the BBEC. To evaluate this possibility we quantified biofilm formation on BVDV infected fixed BBEC at 36 and 48 h after addition of M. haemolytica. As in Fig. 4, here too we did not observe any inhibitory effect of viral infection on biofilm formation at 36 h at these lower absorbance values (Fig. S2).
Virally infected cells do not enhance M. haemolytica biofilm formation during early biofilm development. Bovine epithelial cells were infected BVDV-1 and fixed with 0.1% glutaraldehyde. 105 CFU of M. haemolytica in RPMI medium was added to the monolayers and incubated for 48 h at 37 °C with 5% CO2. Biofilm was allowed to form for 36 (A) or 48 h (B), stained with crystal violet, and the absorbance at 595 nm was measured. Data are the mean ± SEM of triplicate wells from three independent experiments that were performed.
Fig. 4.
Viral infection of BBEC does not affect M. haemolytica biofilm formation. (A) Bovine respiratory epithelial cells were first infected with either BVDV-1, BRSV, or BHV-1 and incubated until the first signs of cytopathic effects were observed. A control was included that contained uninfected epithelial cells. Images were taken at 40× magnification. BBEC infected with BVDV (B), BRSV (C), or BHV-1 (D), were fixed and M. haemolytica suspended in RPMI added to the monolayers. The cells were incubated for 48 h at 37 °C with 5% CO2 and the resultant biofilms quantified by crystal violet staining. Data are the mean ± SEM of triplicate wells from three independent experiments.
3.4. Antibiotic resistance profile of M. haemolytica biofilms on epithelial cells
Biofilms pose a challenge to clinicians seeking to treat infections with antibiotics (da Costa Krewer et al., 2015). Biofilm cells generally have greater resistance to antibiotics than planktonic bacterial cells. We were interested in whether M. haemolytica biofilms on epithelial cells would have an even greater tolerance to antibiotics than biofilms formed on polystyrene. To answer this question, we performed a minimum biofilm eradication concentration (MBEC) assay to test the effects of chlortetracycline, erythromycin, gentamicin, tulathromycin, and florfenicol on M. haemolytica biofilms formed on plastic and BBEC. Chlortetracycline and erythromycin exhibited the biggest increases in MBEC on cells versus plastic (8 and 16 fold, respectively). We observed a four-fold increase in MBEC for gentamicin, and a two-fold increase for tulathromycin, for biofilms formed on epithelial cells compared to plastic (Table 1 ). Florfenicol showed no difference in MBEC for biofilms on polystyrene and BBEC, although the concentration needed was much greater than the other antibiotics tested. For comparative purposes we include MIC values for planktonic cells in Table 1. The MIC value for all antibiotics tested (planktonic state) were several-fold less than their respective MBEC values. This was particularly true for florfenicol where the difference was quite large.
Table 1.
Increased antibiotic resistance of M. haemolytica biofilms (MBEC) on bovine respiratory epithelial cells compared to polystyrene.
| Antibiotic | MIC (μg/ml)a | Polystyrene (μg/ml) | Epithelial cells (μg/ml) | Fold Increase |
|---|---|---|---|---|
| Chlortetracycline | 8 | 32 | 256 | 8 |
| Erythromycin | ≤0.0625 | 32 | 512 | 16 |
| Gentamicin | 1 | 16 | 64 | 4 |
| Tulathromycin | 0.0625 | 2 | 4 | 2 |
| Florfenicol | 1 | 1024 | 1024 | 0 |
MIC data for the same antibiotics used in the MBEC assays.
4. Discussion
In this study, we demonstrate that M. haemolytica can form biofilms on bovine respiratory epithelial cells. These findings are consistent with a previous report that M. haemolytica cells adhere and form a biofilm-like structure in vivo (Morck et al., 1989), and on plastic in vitro (Boukahil and Czuprynski, 2015). Other members of the Pasteurellaceae family, including A. pleuropneumoniae and H. somni, have been shown to form biofilms in vitro and in vivo (Sandal et al., 2011, Tremblay et al., 2013).
Our model system was driven by a desire to investigate the ability of M. haemolytica to form biofilms on respiratory epithelial cells. As with other bacterial species, M. haemolytica requires 48 h to form a biofilm in vitro. This incubation period poses technical challenges associated with controlling numbers of planktonic bacterial cells and maintaining the integrity of the underlying epithelial cells. One group of investigators used a transwell membrane barrier between the biofilm being formed and the underlying epithelial cells (Tran et al., 2014). Although this approach allowed the epithelial cells to survive, the lack of direct contact between the biofilm cells and epithelial cells offers limited insights into the likely process of biofilm formation in vivo. Other investigators have reported model systems that allow for direct contact between the bacterial and epithelial cells, however these studies used bacterial species that rapidly formed biofilms (i.e. 9 h) (Anderson et al., 2008). Incubation beyond 12 h resulted in detachment of the underlying eukaryotic cells due to the toxicity of bacterial products in the culture system. Our model is the first to report biofilm formation directly on host cells during incubation periods of up to 72 h (Supplementary Fig. S3). To allow the underlying epithelial cells to support the growing biofilm, we gently fixed the epithelial cells with glutaraldehyde, which is reported to have limited effects on the tertiary structures of proteins (Chui and Wan, 1997). Indeed, others have shown that the protein trypsin retains catalytic activity after being similarly treated with glutaraldehyde (Chui and Wan, 1997). We confirmed fixation had limited effects on the epithelial cells by showing equivalent adhesion of M. haemolytica cells to fixed and living epithelial cells (Fig. 2).
M. haemolytica forms biofilms on fixed bovine respiratory epithelial cells for an extended period. Bovine respiratory epithelial cells in the wells of a 24 well plate were first fixed with 0.1% glutaraldehyde and 105 CFU of M. haemolytica in RPMI medium were then added to each well. The wells were then incubated at 37°C with 5% CO2 for up to 72 h. Biofilm was stained with crystal violet and the absorbance at 595 nm was determined. Data are the mean ± SEM of triplicate wells from three independent experiments that were performed. *, P < 0.05 compared to epithelial cells alone.
Our finding that mucin decreases biofilm formation is consistent with studies, which showed that mucin significantly decreased biofilm formation by Streptococcus mutans and P. aeruginosa (Haley et al., 2014, Frenkel and Ribbeck, 2015). It has been proposed that mucin, the main glycoprotein of mucus, acts as an anti-biofouling agent that impairs pathogen colonization of epithelial cells lining the respiratory tract (Hollingsworth and Swanson, 2004, Linden et al., 2008). We found that at mucin concentrations similar to those reported in vivo, biofilm formation was nearly eliminated (Fig. 3). There are several possible mechanisms by which mucin protects the epithelium from bacterial colonization. Bergstrom et al. showed that mucin-deficient mice had a greater commensal bacterial load in the intestinal epithelium when infected with the pathogen Citrobacter rodentium than did wildtype mice (Bergstrom et al., 2010). Mucin prevented over-colonization of commensal and pathogenic organisms alike, by enabling cilia to sweep away the bacterial cells (Bergstrom et al., 2010). A similar mechanism of bacterial clearance is believed to occur in the upper respiratory tract (Thornton et al., 2008). In humans, chronic oral disease leads to increased mucin levels, which in turn are thought to contribute to inflammation during infection (Hollingsworth and Swanson, 2004). As infection proceeds, the resulting inflammation stimulates overproduction of mucin (Thornton et al., 2008). Consistent with this finding, one of the characteristic signs of severe M. haemolytica respiratory infection is excess mucus emanating from the nostrils of cattle (Poulsen and McGuirk, 2009). If mucin has antimicrobial properties, then the question remains as to why commensal bacteria can colonize the respiratory tract in humans and animals. This apparent paradox may be explained by evidence that mucin helps to maintain consistent levels of commensal bacteria while suppressing colonization by pathogenic bacteria (Frenkel and Ribbeck, 2015). Because M. haemolytica is regarded as part of the microflora in the upper respiratory tract of healthy cattle, perhaps mucin allows M. haemolytica to colonize at low levels, as a commensal biofilm.
The link between viral infection and increased susceptibility to M. haemolytica infection is well-documented (van der Sluijs et al., 2010, Caswell, 2014). We found that viral infection of the bovine respiratory epithelial cells did not have an effect on biofilm formation. While our findings do not provide evidence for a direct link between viral infection and biofilm formation, perhaps viruses may affect M. haemolytica colonization of host epithelia through indirect means such as by weakening the host’s innate immune system’s ability to remove the bacterial cells.
Growth as a biofilm can alter resistance to antibiotics. Standard antibiotic susceptibility assays may not reflect the susceptibility of bacterial cells enmeshed in a biofilm (Olson et al., 2002). Furthermore, bacterial cells in biofilms may have an increased mutation rate, and the close proximity of bacterial cells can facilitate transfer of antibiotic resistance, allowing resistant cells to emerge (Driffield et al., 2008). Some research laboratories have investigated antibiotic resistance by biofilms formed on abiotic surfaces to predict antibiotic doses that are effective in vivo (Hengzhuang et al., 2011). Here we showed that biofilm formation by M. haemolytica on epithelial cells enhanced antibiotic resistance relative to biofilm formation on polystyrene. One simple explanation for the enhanced resistance is that M. haemolytica cells in the extracellular matrix exuded by both biofilm cells and BBEC are physically shielded from the antibiotic. Hence, a greater amount of antibiotic is needed to kill these embedded bacterial cells.
Future work will examine the ability of M. haemolytica to form co-biofilms with other bacterial species on BBEC. M. haemolytica has been shown to share its niche in the upper respiratory tract with other bacterial species, such as P. multocida and H. somni, that are also associated with BRDC (Moore et al., 2015), and have been show to form biofilms (Olson et al., 2002, Sandal et al., 2011, Elswaifi et al., 2012). These studies may elucidate interactions among these bacterial species when they are in close proximity on the respiratory epithelium.
5. Conclusion
We developed a novel in vitro model system that allows M. haemolytica to form biofilms on fixed bovine respiratory epithelial cells in vitro. We confirm that adhesion of the bacteria cells to living and fixed epithelial cells was similar. Addition of mucin (0.5 to 1.0 mg/ml), the main glycoprotein of mucus, significantly reduced biofilm formation. Prior viral infection of the epithelial cells did not alter subsequent biofilm levels, suggesting that BRDC viruses enhance bacterial infection by other mechanisms. Finally, M. haemolytica biofilms formed on epithelial cells had greater resistance to several antibiotics than biofilms formed on polystyrene. Taken together, this model system provides a new tool for understanding the relationship of M. haemolytica with its host.
Conflict of interest
None.
Acknowledgments
We thank Kathy Kurth (University of Wisconsin-Madison) for providing the bovine respiratory syncytial virus and bovine viral diarrhea virus. This work was supported by Wisconsin Agricultural Experiment Station [grant WIS01607]; Agriculture and Food Research Initiative competitive grant from the USDA National Institute of Food and Agriculture [grant number 2011-67015-30171]; and the Walter and Martha Renk Endowed Laboratory for Food Safety.
References
- Allen-Gipson D.S., Wong J., Spurzem J.R., Sisson J.H., Wyatt T.A. Adenosine A2A receptors promote adenosine-stimulated wound healing in bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290:L849–L855. doi: 10.1152/ajplung.00373.2005. [DOI] [PubMed] [Google Scholar]
- Anderson G.G., Moreau-Marquis S., Stanton B.A., O’Toole G.A. In vitro analysis of tobramycin-treated Pseudomonas aeruginosa biofilms on cystic fibrosis-derived airway epithelial cells. Infect. Immun. 2008;76:1423–1433. doi: 10.1128/IAI. 01373-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom K.S., Kissoon-Singh V., Gibson D.L., Ma C., Montero M., Sham H.P., Ryz N., Huang T., Velcich A., Finlay B.B., Chadee K., Vallance B.A. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6:e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger R.R., Everett M.L., Palestrant D., Love S.D., Lin S.S., Parker W. Human secretory immunoglobulin A may contribute to biofilm formation in the gut. Immunology. 2003;4:580–587. doi: 10.1046/j.1365-2567.2003.01700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukahil I., Czuprynski C.J. Characterization of Mannheimia haemolytica biofilm formation in vitro. Vet. Microbiol. 2015;175:114–122. doi: 10.1016/j.vetmic.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Bowland S.L., Shewen P.E. Bovine respiratory disease: commercial vaccines currently available in Canada. Can. Vet. J. 2000;41:33–48. [PMC free article] [PubMed] [Google Scholar]
- Caswell J.L. Failure of respiratory defenses in the pathogenesis of bacterial pneumonia of cattle. Vet. Pathol. 2014;51:393–409. doi: 10.1177/0300985813502821. [DOI] [PubMed] [Google Scholar]
- Chui W.K., Wan L.S. Prolonged retention of cross-linked trypsin in calcium alginate microspheres. J. Microencapsul. 1997;14:51–61. doi: 10.3109/02652049709056467. [DOI] [PubMed] [Google Scholar]
- Driffield K., Miller K., Bostock M., O’Neill A.J., Chopra I. Increased mutability of Pseudomonas aeruginosa in biofilms. J. Antimicrob. Chemother. 2008;61:1053–1056. doi: 10.1093/jac/dkn044. [DOI] [PubMed] [Google Scholar]
- Eisfeld A.J., Neumann G., Kawaoka Y. Influenza A virus isolation, culture and identification. Nat. Protoc. 2014;9:2663–2681. doi: 10.1038/nprot.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elswaifi S.F., Scarratt W.K., Inzana T.J. The role of lipooligosaccharide phosphorylcholine in colonization and pathogenesis of Histophilus somni in cattle. Vet. Res. 2012;43:49. doi: 10.1186/1297-9716-43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G.H., Smith P.C. Prevalence of Pasteurella haemolytica in transported calves. Am. J. Vet. Res. 1983;44:981–985. [PubMed] [Google Scholar]
- Frank G.H., Briggs R.E., DeBey B.M. Bovine tonsils as reservoirs for Pasteurella haemolytica: colonisation, immune response, and infection of the nasopharynx. Aciar Proc. 1993;43:83–88. [Google Scholar]
- Frenkel E.S., Ribbeck K. Salivary mucins protect surfaces from colonization by cariogenic bacteria. Appl. Environ. Microbiol. 2015;81:332–338. doi: 10.1128/AEM. 02573-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia J., Qin X., Jiang H., Clinkenbeard K., Lo R., Liu Y., Fox G.E., Yerrapragada S., McLeod M.P., McNeill T.Z., Hemphill L., Sodergren E., Wang Q., Muzny D.M., Homsi F.J., Weinstock G.M., Highlander S.K. The genome sequence of Mannheimia haemolytica A1: insights into virulence, natural competence, and Pasteurellaceae phylogeny. J. Bacteriol. 2006;188:7257–7266. doi: 10.1128/jb. 00675-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley C.L., Kruczek C., Qaisar U., Colmer-Hamood J.A., Hamood A.N. Mucin inhibits Pseudomonas aeruginosa biofilm formation by significantly enhancing twitching motility. Can. J. Microbiol. 2014;60:155–166. doi: 10.1139/cjm-2013-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengzhuang W., Wu H., Ciofu O., Song Z., Høiby N. Pharmacokinetics/pharmacodynamics of colistin and imipenem on mucoid and nonmucoid Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2011;55:4469–4474. doi: 10.1128/AAC. 00126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson P.D., Aich P., Stookey J., Popowych Y., Potter A., Babiuk L., Griebel P.J. Stress significantly increases mortality following a secondary bacterial respiratory infection. Vet. Res. 2012;43:21. doi: 10.1186/1297-9716-43-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth M.A., Swanson B.J. Mucins in cancer: protection and control of the cell surface. Nat. Rev. Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- Kavanaugh N.L., Zhang A.Q., Nobile C.J., Johnson A.D., Ribbeck K. Mucins suppress virulence traits of Candida albicans. MBio. 2014;5:e01911. doi: 10.1128/mBio. 01911-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kejriwal S., Bhandary R., Thomas B., Kumari S. Estimation of levels of salivary mucin, amylase and total protein in gingivitis and chronic periodontitis patients. J. Clin. Diagn. Res. 2014;8:ZC56–ZC60. doi: 10.7860/jcdr/2014/8239.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D.M., Howley P. Lippincott Williams & Wilkins; Philadelphia: 2013. Fields Virology; pp. 42–50. [Google Scholar]
- Landry R.M., An D., Hupp J.T., Singh P.K., Parsek M.R. Mucin-Pseudomonas aeruginosa interactions promote biofilm formation and antibiotic resistance. Mol. Microbiol. 2006;59:142–151. doi: 10.1111/j.1365-2958.2005.04941.x. [DOI] [PubMed] [Google Scholar]
- Linden S.K., Sutton P., Karlsson N.G., Korolik V., McGuckin M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008;1:183–197. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S.J., O’Dea M.A., Perkins N., O’Hara A.J. Estimation of nasal shedding and seroprevalence of organisms known to be associated with bovine respiratory disease in Australian live export cattle. J. Vet. Diagn. Invest. 2015;27:6–17. doi: 10.1177/1040638714559741. [DOI] [PubMed] [Google Scholar]
- Morck D., Olson M., Acres S., Daoust P., Costerton J. Presence of bacterial glycocalyx and fimbriae on Pasteurella haemolytica in feedlot cattle with pneumonic pasteurellosis. Can. J. Vet. Res. 1989;53:167–171. [PMC free article] [PubMed] [Google Scholar]
- N’jai A.U., Rivera J., Atapattu D.N., Owusu-Ofori K., Czuprynski C.J. Gene expression profiling of bovine bronchial epithelial cells exposed in vitro to bovine herpesvirus 1 and Mannheimia haemolytica. Vet. Immunol. Immunopathol. 2013;155:182–189. doi: 10.1016/j.vetimm.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisizawa K., Pigman W. The composition and properties of the mucin clot from cattle submaxillary glands. Arch. Oral Biol. 1959;1:161–170. doi: 10.1016/0003-9969(59)90008-1. [DOI] [PubMed] [Google Scholar]
- Olson M.E., Ceri H., Morck D.W., Buret A.G., Read R.R. Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can. J. Vet. Res. 2002;66:86–92. [PMC free article] [PubMed] [Google Scholar]
- Potgieter L.N., McCracken M.D., Hopkins F.M., Walker R.D., Guy J.S. Experimental production of bovine respiratory tract disease with bovine viral diarrhea virus. Am. J. Vet. Res. 1984;45:1582–1585. [PubMed] [Google Scholar]
- Poulsen K.P., McGuirk S.M. Respiratory disease of the bovine neonate. Vet. Clin. North Am. Food Anim. Pract. 2009;25:121–137. doi: 10.1016/j.cvfa.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Sandal I., Inzana T.J., Molinaro A., De Castro C., Shao J.Q., Apicella M.A., Cox A.D., St Michael F., Berg G. Identification, structure, and characterization of an exopolysaccharide produced by Histophilus somni during biofilm formation. BMC Microbiol. 2011;11:186. doi: 10.1016/j.micinf.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger D.S., Reed W.P., McLaren L.C. Model for studying bacterial adherence to epithelial cells infected with viruses. Infect. Immun. 1981;32:941–944. doi: 10.1128/iai.32.2.941-944.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippy D.C., Heintz J.A., Albrecht R.M., Eakley N.M., Chopra A.K., Fadl A.A. Deletion of glucose-inhibited division (gidA) gene alters the morphological and replication characteristics of Salmonella enterica Serovar typhimurium. Arch. Microbiol. 2012;194:405–412. doi: 10.1007/s00203-011-0769-7. [DOI] [PubMed] [Google Scholar]
- Thornton D.J., Rousseau K., McGuckin M.A. Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- Tran C.S., Rangel S.M., Almblad H., Kierbel A., Givskov M., Tolker-Nielsen T., Hauser A.R., Engel J.N. The Pseudomonas aeruginosa type III translocon is required for biofilm formation at the epithelial barrier. PLoS Pathog. 2014;10:e1004479. doi: 10.1371/journal.ppat.1004479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay Y.D., Deslandes V., Jacques M. Actinobacillus pleuropneumoniae genes expression in biofilms cultured under static conditions and in a drip-flow apparatus. BMC Genom. 2013;14:364. doi: 10.1186/1471-2164-14-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa Krewer C., Santos Amanso E., Veneroni Gouveia G., de Lima Souza R., da Costa M.M., Aparecido Mota R. Resistance to antimicrobials and biofilm formation in Staphylococcus spp. isolated from bovine mastitis in the Northeast of Brazil. Trop. Anim. Health Prod. 2015;47:511–518. doi: 10.1007/s11250-014-0752-9. [DOI] [PubMed] [Google Scholar]
- van der Sluijs M.T., Kuhn E.M., Makoschey B. A single vaccination with an inactivated bovine respiratory syncytial virus vaccine primes the cellular immune response in calves with maternal antibody. BMC Vet. Res. 2010;6:2. doi: 10.1186/1746-6148-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mucin does not inhibit M. haemolytica adhesion to bovine respiratory epithelial cells. M. haemolytica cells (3 × 105 CFU) were incubated on fixed epithelial cells in wells of a 24 well plate, in the presence of mucin (250–1000 μg/ml) or methyl cellulose (1000 μg/ml) for 2 h at 37 °C with 5% CO2. Adherent bacteria was disrupted by vigorous scraping and pipetting, resuspended in 1 ml PBS, and enumerated by serial dilution and plating onto blood agar plates. Total CFU per well of each group is shown. Data are the mean ± SEM from three independent experiments.
Virally infected cells do not enhance M. haemolytica biofilm formation during early biofilm development. Bovine epithelial cells were infected BVDV-1 and fixed with 0.1% glutaraldehyde. 105 CFU of M. haemolytica in RPMI medium was added to the monolayers and incubated for 48 h at 37 °C with 5% CO2. Biofilm was allowed to form for 36 (A) or 48 h (B), stained with crystal violet, and the absorbance at 595 nm was measured. Data are the mean ± SEM of triplicate wells from three independent experiments that were performed.
M. haemolytica forms biofilms on fixed bovine respiratory epithelial cells for an extended period. Bovine respiratory epithelial cells in the wells of a 24 well plate were first fixed with 0.1% glutaraldehyde and 105 CFU of M. haemolytica in RPMI medium were then added to each well. The wells were then incubated at 37°C with 5% CO2 for up to 72 h. Biofilm was stained with crystal violet and the absorbance at 595 nm was determined. Data are the mean ± SEM of triplicate wells from three independent experiments that were performed. *, P < 0.05 compared to epithelial cells alone.