Abstract
Three new triterpenoids, triregelolides A, B (1, 2), and triregeloic acid (3), were isolated from the stems of Tripterygium regelii along with twenty known triterpene analogues (4–23). The structures of three new compounds were identified by analyzing their NMR spectroscopic and HRESIMS data. Compounds 4, 7, 8, 10, 13, 14, 17, 21–23 were isolated from T. regelii for the first time. Compounds 3, 5, 6, 8, 9, 10, 14 and 16 showed inhibitory effects on the proliferation of human breast cancer cells MCF-7 by 24.1%, 69.6%, 72.8%, 21.6%, 23.1%, 43.3%, 25.5% and 23.5% (p < 0.05) at a concentration of 10 μM, respectively.
Chemical compounds studied in this article: Celastrol (PubChem CID: 122,724); triptocalline A (PubChem CID: 44,559,634); polpunonic acid (PubChem CID: 343,427); demethylzeylasteral (PubChem CID: 10,322,911); wilforol A (PubChem CID: 10,096,097); regelin D (PubChem CID: 129,520); triptotriterpenic acid B (PubChem CID: 195,563); wilforlide A (PubChem CID: 158,477); wilforlide B (PubChem CID: 174,362); triptocallic acid A (PubChem CID: 44,575,704); regelinol (PubChem CID: 163,809); regelin (PubChem CID: 163,808); dulcioic acid (PubChem CID: 101,051,955); tripterygic acid A (PubChem CID: 21,672,627); demethylregelin (PubChem CID: 44,559,663)
Keywords: Tripterygium regelii Triterpenoids Cytotoxicity
Graphical abstract
1. Introduction
Triterpenoids naturally occurring in the plant kingdom are one of the largest groups of natural products [1]. Until now, it has been reported that some of them exhibited a wide spectrum of biological activities, such as antitumor, antiviral, antidiabetic, anti-inflammatory, antimicrobial, hepatoprotective, cardiotonic, gastroprotective and analgesic effects, etc. [2], [3], [4]. More importantly, some triterpenoids or their derivatives are promising candidates or lead compounds for the development of future drugs due to their therapeutic potential [3], [5], [6].
The plants in Tripterygium genus of family Celastraceae are well known for a rich source of triterpenoids. Many triterpenes isolated from this genus, showed various promising bioactivities. Celastrol, a quinone methide triterpene isolated from Tripterygium plants [7], [8], [9], exhibited potent anticancer activity against a variety of human cancer cell lines [4], anti-inflammatory [10] and neuroprotective effects [10], [11]. Recently, it has been reported to be used as a powerful anti-obesity agent [12]. In addition, celastrol, pristimerin, tingenone, iguesterin and dihydrocelastrol showed SARS-CoV 3CLpro inhibitory activity [13].
Tripterygium regelii, which is distributed throughout northeast China, Korea and Japan [14], has been used as a folk medicine in China for the treatment of rheumatoid arthritis, jaundice, swelling, etc. [15]. A few previous studies [9], [16], [17], [18], [19], [20] have shown that terpenoids were the principal constituents of T. regelii. Recently, we have reported the isolation of twelve new dihydro-β-agarofuran sesquiterpenoids from its stems [21] . As a part of our ongoing phytochemical investigation, three new triterpenoids and twenty known analogues were isolated and characterized from the stems of T. regelii. Herein, we reported the isolation and characterization of three novel triterpenoids (1–3) and twenty known compounds (4–23), as well as a cytotoxic evaluation of nine selected triterpenes against human breast cancer MCF-7 cells.
2. Experimental
2.1. General experimental procedures
Optical rotations and ultraviolet (UV) spectra were measured using a Rudolph Research Analytical Autopol I automatic polarimeter and a Beckman Coulter DU® 800 spectrophotometer (USA), respectively. HRMS spectra were performed on an Agilent 6230 electrospray ionization (ESI) time–of–flight (TOF) mass spectrometer (USA). Nuclear magnetic resonance (NMR) spectra were acquired with a Bruker Ascend 600 NMR spectrometer in CDCl3 and pyridine-d 5 using tetramethylsilane (TMS) as an internal reference. Chemical shifts were given in δ (ppm), and coupling constants (J) were expressed in hertz (Hz). Preparative HPLC was carried out on a Waters liquid chromatography system equipped with 1525 Binary HPLC Pump and 2489 UV/ Visible detector using a Waters Xbridge Prep C8 column (10 × 250 mm, 5 μm). Semi-preparative HPLC was conducted on an Agilent 1100 liquid chromatography system coupled with a quaternary pump and a diode array detector (DAD) using a Waters Xbridge Prep C18 column (10 × 250 mm, 5 μm). Silica gel (40–60 μm, Grace, USA) and Bondapak Waters ODS (40–63 μm, Waters, USA) were used for column chromatographies. Thin layer chromatography (TLC) used to monitor fractions was performed on precoated silica gel 60 F254 plates and TLC silica gel 60 RP-18 F254S plates (200 μm thick, Merck KGaA, Germany). Spots on the TLC were visualized by UV light (254 nm) or heating after spraying with 5% H2SO4 in ethanol.
2.2. Plant material
The stems of T. regelii were collected in October 2012, from Changbai Mountain in Jilin province, People's Republic of China, and were identified by Dr. Liang Xu (Liaoning University of Traditional Chinese Medicine, Dalian, China). A voucher specimen (No. MUST − TR201210) has been deposited at State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology, Macau, China.
2.3. Extraction and isolation
The dried and ground stems of T. regelii (8.0 kg) were extracted with methanol (64 L × 3) under ultrasonic assistance at room temperature for 1 h. After evaporation of the solvent under reduced pressure, a dark brown residue was suspended in H2O, and then sequentially partitioned with n-hexane, ethyl acetate (EtOAc) and n-butanol. The EtOAc-soluble extract (150.0 g) was subjected to column chromatography over silica gel eluting with PE–acetone (100:0–35:65, v/v) to yield thirteen fractions (Fr.1–Fr.13).
The fraction Fr.5 (5.0 g) was separated by a silica gel column using a gradient of n-hexane–EtOAc (100:0–50:50, v/v) to produce eight fractions (Fr.5–1 − Fr.5–8). The fraction Fr.5–7 (71.1 mg) was chromatographed over an ODS column with a gradient of CH3OH − H2O (60:40–100:0, v/v) to afford compound 8 (5.0 mg). The fraction Fr.7 (5.4 g) was subjected to an ODS column using a gradient system of CH3OH − H2O (50:50–100:0, v/v) to afford nine fractions (Fr.7–1 − Fr.7–9). The fraction Fr.7–5 (120.5 mg) was further separated by preparative HPLC using an isocratic solvent system of CH3CN − H2O (70:30, v/v) as mobile phase to yield compound 7 (1.5 mg). The fraction Fr.8 (5.0 g) was subjected to an ODS column with a gradient condition of CH3OH − H2O (50:50–100:0) to product nine fractions (Fr.8–1–Fr.8–9). The fraction Fr.8–3 (264.9 mg) was purified by semi-preparative HPLC using CH3CN − H2O (68:32, v/v) as mobile phase to give compound 6 (4.3 mg). The fraction Fr.8–4 (500.9 mg) was isolated by preparative HPLC using CH3CN − H2O (70:30, v/v) as mobile phase to give compounds 19 (1.5 mg), 20 (2.1 mg) and 12 (2.0 mg). The fraction Fr.8–6 (425.1 mg) was subjected to a silica gel column with a solvent system of PE − EtOAc (90:10–65:35, v/v), and purified using an ODS column with a gradient of MeOH − H2O (70:30–100:0, v/v) to give compound 5 (60.0 mg). Compound 21 (28.3 mg) was obtained from fraction Fr.8–8 (80.5 mg) by using a silica gel column eluted sequentially with PE − EtOAc (90:10–60:40) solvent system. The fraction Fr.11 (5.5 g) was fractionated over an ODS column with a gradient system of CH3OH − H2O (40:60–90:10, v/v) to obtain fifteen fractions (Fr.11–1 − Fr.11–15). Fraction Fr.11–12 (261.0 mg) was separated by semi-preparative HPLC using CH3CN − H2O (60:40, v/v) as solvent system to furnish compound 11 (1.5 mg). The fraction Fr.11–13 (950.1 mg) was chromatographed on a silica gel column with a gradient of PE − EtOAc (70:30–0:100, v/v) to afford ten fractions (Fr.11–13-1 − Fr.11–13-10). The fraction Fr.11–13-1 (150.0 mg) was separated by semi-preparative HPLC using CH3CN − H2O (76:24, v/v) as solvent system to give compounds 1 (1.0 mg), 2 (0.6 mg) and 10 (30.0 mg). Compound 4 (2.0 mg) was purified by silica gel column using a gradient of CHCl3 − CH3OH (100:0–95:5, v/v) from fraction Fr.11–13-2 (100.0 mg). Then, the fraction Fr.11–13-6 (217.2 mg) was subjected to a silica gel column using a gradient elution of CHCl3 − CH3OH (100:0–90:10, v/v) to yield compound 13 (20.0 mg) and subfractions (Fr.11–13–6-1 − Fr.11–13–6-4). The subfractions Fr.11–13–6-2 and Fr.11–13–6-4 were isolated by semi-preparative HPLC using CH3CN − H2O (37:63 and 39:61, v/v, respectively) as mobile phase to afford compounds 18 (2.1 mg) and 23 (2.1 mg), respectively. The fraction Fr.11–13-9 (367.0 mg) was purified by semi-preparative HPLC using CH3CN − H2O (65:35, v/v) as eluting solvent to give compound 22 (2.0 mg). Compounds 14 (6.1 mg) and 17 (2.0 mg) were obtained by preparative HPLC using CH3CN − H2O (65:35, v/v) as mobile phase from fraction Fr.11–14 (200.0 mg). The fraction 11–15 (367.0 mg) was separated by a silica gel column using a PE-EtOAc (80:20–30:70 v/v) gradient solvent system to give compounds 15 (2.6 mg), 16 (9.3 mg), and six subfractions (Fr.11–15-3–Fr.11–15-8). Then, compounds 3 (3.0 mg) and 9 (5.0 mg) were isolated by an ODS columns with a gradient elution of CH3OH − H2O (40:60–100:0, v/v) from the subfractions Fr.11–15-7 (38.9 mg) and Fr.11–15-8 (40.0 mg), respectively.
2.4. Spectroscopic data
Triregelolide A (1): white amorphous powder; [α]21 D + 157.8 (c 0.50, MeOH); UV (MeOH) λ max (log ε) 236 (3.65), 360 (3.88) nm; 1H (CDCl3, 600 MHz) and 13C (CDCl3, 150 MHz) NMR data, see Table 1 ; HRESIMS m/z 467.2812 [M − H]− (calcd for C29H39O5, 467.2803).
Table 1.
1H (600 MHz) and 13C (150 MHz) NMR spectroscopic data for 1–3.
| Position |
1 a |
2 a |
3 b |
|||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | |
| 1 | 5.81 (1H, d, 1.2) | 110.3, CH | 5.79 (1H, d, 1.8) | 110.2, CH | 1.23 (1H, dd, 12.0, 9.6) c | 47.0, CH2 |
| 2.24 (1H, dd, 12.0, 4.2) | ||||||
| 2 | 163.8, C | 163.8, C | 4.13 (1H, td, 9.6, 4.2) | 68.6, CH | ||
| 3 | 3.39 (1H, d, 9.6) | 83.8, CH | ||||
| 4 | 103.8, C | 105.6, C | 39.8, C | |||
| 5 | 125.9, C | 125.3, C | 0.98 (1H, dd, 12.1, 1.9) | 56.0, CH | ||
| 6 | 6.32 (1H, dd, 6.6, 1.2) | 126.6, CH | 6.29 (1H, dd, 6.6, 1.8) | 126.0, CH | 1.55 (1H, m) c | 17.9, CH2 |
| 1.71 (1H, m) c | ||||||
| 7 | 5.98 (1H, d, 6.6) | 115.7, CH | 6.35 (1H, d, 6.6) | 115.8, CH | 1.38 (1H, td, 12.6, 3.0) | 41.6, CH2 |
| 2.05 (1H, dt, 12.6, 2.4) | ||||||
| 8 | 162.4, C | 161.7, C | 39.3, C | |||
| 9 | 40.8, C | 40.5, C | 1.55 (1H, m) c | 49.5, CH | ||
| 10 | 165.5, C | 166.4, C | 39.3, C | |||
| 11 | 1.75 (1H, m) c | 33.2, CH2 | 1.78 (1H, m) c | 33.1, CH2 | 1.52 (1H, m) c | 19.2, CH2 |
| 1.94 (1H, m) c | 1.94 (1H, m) c | 1.65 (1H, m) c | ||||
| 12 | 1.59 (1H, m) c | 29.3, CH2 | 1.60 (1H, m) c | 29.4, CH2 | 1.57 (1H, m) c | 33.8, CH2 |
| 1.78 (1H, m) c | 1.78 (1H, m) c | 1.65 (1H, m) | ||||
| 13 | 38.4, C | 38.3, C | 38.0, C | |||
| 14 | 44.5, C | 44.5, C | 158.4, C | |||
| 15 | 1.47 (1H, m) c | 28.5, CH2 | 1.49 (1H, m) c | 28.5, CH2 | 5.62 (1H, dd, 8.0, 3.0) | 117.1, CH |
| 1.54 (1H, m) c | 1.59 (1H, m) c | |||||
| 16 | 1.44 (1H, m) c | 36.3, CH2 | 1.46 (1H, m) c | 36.3, CH2 | 1.74 (1H, m) c | 38.0, CH2 |
| 1.82 (1H, m) c | 1.85 (1H, m) c | 2.09 (1H, dd, 15.0, 3.0) | ||||
| 17 | 30.6, C | 30.6, C | 35.9, C | |||
| 18 | 1.53 (1H, m) c | 44.1, CH | 1.56 (1H, m) c | 44.1, CH | 1.16 (1H, dd, 13.2, 3.6) | 48.5, CH |
| 19 | 1.69 (1H, m) c | 30.7, CH2 | 1.72 (1H, m) c | 30.8, CH2 | 1.59 (1H, dd, 13.2, 3.6) | 32.4, CH2 |
| 2.41 (1H, d, 15.6) | 2.42 (1H, d, 15.6) | 2.32 (1H, t, 13.2) | ||||
| 20 | 40.2, C | 40.1, C | 41.0, C | |||
| 21 | 1.36 (1H, td, 14.4, 3.0) | 29.6, CH2 | 1.38 (1H, td, 13.8, 4.8) | 29.6, CH2 | 1.51 (1H, m) c | 29.8, CH2 |
| 2.15 (1H, br d, 14.4) | 2.16 (1H, br d, 13.8) | 2.54 (1H, dt, 14.4, 3.6) | ||||
| 22 | 0.95 (1H, br d, 14.4) | 34.6, CH2 | 0.96 (1H, br d, 13.8) | 34.5, CH2 | 1.20 (1H, m) c | 35.8, CH2 |
| 2.02 (1H, td, 14.4, 3.0) | 2.05 (1H, td, 13.8, 3.6) | 1.84 (1H, td, 14.4, 3.6) | ||||
| 23 | 1.73 (3H, s) | 24.8, CH3 | 1.63 (3H, s) | 28.2, CH3 | 1.28 (3H, s) | 29.2, CH3 |
| 24 | 1.13 (3H, s) | 17.6, CH3 | ||||
| 25 | 1.46 (3H, s) | 36.2, CH3 | 1.43 (3H, s) | 35.9, CH3 | 1.05 (3H, s) | 16.9, CH3 |
| 26 | 1.21 (3H, s) | 22.7, CH3 | 1.21 (3H, s) | 22.7, CH3 | 1.13 (3H, s) | 26.2, CH3 |
| 27 | 0.70 (3H, s) | 18.9, CH3 | 0.74 (3H, s) | 18.9, CH3 | 1.02 (3H, s) | 21.3, CH3 |
| 28 | 1.07 (3H, s) | 31.5, CH3 | 1.09 (3H, s) | 31.5, CH3 | 0.96 (3H, s) | 30.2, CH3 |
| 29 | 182.7, C | 181.7, C | 182.2, C | |||
| 30 | 1.20 (3H, s) | 32.7, CH3 | 1.22 (3H, s) | 32.7, CH3 | 1.53 (3H, s) | 26.1, CH3 |
| OMe | 3.30 (3H, s) | 50.5, CH3 | 3.30 (3H, s) | 50.9, CH3 | ||
a Measured in CDCl3. b Measured in pyridine-d5. c The overlapped signals were assigned from 1H − 1H COSY, HSQC, and HMBC spectra.
Triregelolide B (2): white amorphous powder; [α]21 D + 119.09 (c 0.50, MeOH); UV (MeOH) λ max (log ε) 236 (3.24), 360 (3.42) nm; 1H (CDCl3, 600 MHz) and 13C (CDCl3, 150 MHz) NMR data, see Table 1; HRESIMS m/z 467.2810 [M − H]− (calcd for C29H39O5, 467.2803).
Triregeloic acid (3): white amorphous powder; [α]21 D − 12.27 (c 0.125, MeOH); UV (MeOH) λ max (log ε) 255 (2.25) nm; 1H (pyridine-d 5, 600 MHz) and 13C (pyridine-d 5, 150 MHz) NMR data, see Table 1; HRESIMS m/z 471.3479 [M − H]− (calcd for C30H47O4, 471.3480).
2.5. Cytotoxicity on human breast cancer cells MCF-7
Human breast cancer cell lines (MCF-7) were purchased from American Type Culture Collection. The cells were cultured in Dulbecco's modified Eagle medium-F12 medium (DMEM) supplemented with 10% fetal bovine serum (FBS, Invitrogen) and 1% penicillin-streptomycin (Sigma) at 37 °C in humidified atmosphere containing 5% CO2. Exponentially growing cells were plated in a 96-well microplate at a density of 5 × 103 cells per well in 100 μL of culture medium and were allowed to adhere for 24 h before drug treatment. Then, the cells were treated with either fresh medium containing 0.1% DMSO or fresh medium containing 10 μM of triterpenes or paclitaxel (Taxol®), and incubated for another 24 h in a 5% CO2 humidified atmosphere at 37 °C. A volume of 10 μL MTT saline solution (5 mg/mL) was added into each well for further 4 h of incubation. Subsequently, 100 μL of lysing sodium dodecyl sulfate (SDS) were added into each well, and the 96-well microplate was kept overnight at room temperature. The absorbance of resulting solution in each well was colorimetrically determined at 570 nm by using a microplate reader (Infinite 200 PRO, Tecan). The inhibitory rate of compounds on cell proliferation was defined as (1 - absorbance of the drug-treated cells/ absorbance of the vehicle control cells) × 100%.
2.6. Statistical Analysis
Statistical analysis was performed using SPSS software, version 16.0 for Windows. The significance of difference among the experimental groups and controls was assessed by one-way ANOVA and Post hoc Bonferroni test. The results are presented as mean ± standard deviation (SD) from three independent experiments. Significance was accepted at a level of p < 0.05.
3. Results and discussion
The EtOAc-soluble fraction of methanolic extract of the stems of T. regelii was repeatedly subjected to silica gel and ODS columns, and then purified by preparative and/or semi-preparative HPLC to yield three new triterpenoids (1–3).
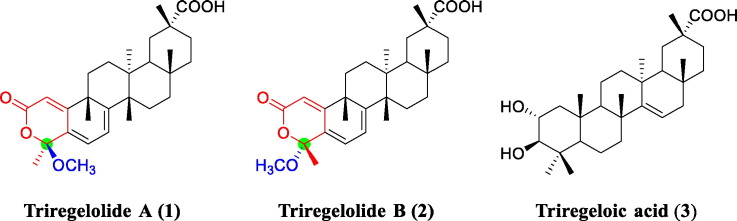
Compound 1 was obtained as white amorphous powder. Its HRESIMS showed a deprotonated molecular ion at m/z 467.2812 [M − H]− (calcd for C29H39O5, 467.2803), corresponding to the molecular formula of C29H40O5 with ten degrees of unsaturation. Its UV spectrum exhibited absorption bands at 236 and 360 nm, suggesting the presence of a conjugated system in 1. The 1H NMR data (Table 1) showed characteristic signals for three olefinic protons [δH 6.32 (1H, dd, J = 6.6, 1.2 Hz, H-6), 5.98 (1H, d, J = 6.6 Hz, H-7), 5.81 (1H, d, J = 1.2 Hz, H-1)], a methoxyl group [δH 3.30 (3H, s)], and six tertiary methyl groups [δH 1.73 (3H, s, H3–23), 1.46 (3H, s, H3–25), 1.21 (3H, s, H3–26), 1.20 (3H, s, H3–30), 1.07 (3H, s, H3–28), 0.70 (3H, s, H3–27)]. The 13C NMR spectroscopic data (Table 1) displayed 29 carbon signals, including signals of a carboxylic carbon (δc 182.7, C-29), a conjugated ester carbonyl carbon (δc 163.8, C-2), three double bonds, six quaternary carbons (including a ketal one), one methine, seven methylenes, and seven methyl groups (including an oxygenated one), which were accounted for five degrees of unsaturation. These characteristic data suggested 1 to be a dinor-friedelene derivative with five rings [22], [23], [24]. Comparison of the 1H and 13C NMR data of 1 with those of dzununcanone [22], [24], a 3,24-dinor-2,4-seco-friedelene triterpene, indicated both compounds bearing similar B-, C-, D- and E-rings. The downfield shift of C-29 (δc 182.7) in 1 relative to that (δc 179.1) in dzununcanone [22], [24] implied a carboxylic acid group present at C-29 in 1 instead of the methyl ester in dzununcanone. The remaining signals of 1 showed a olefinic carbon (δc 110.3, C-1), a conjugated carbonyl carbon (δc 163.8, C-2), a ketal carbon (δc 103.8, C-4), a tertiary methyl (δc 24.8, C-23) and a methoxyl (δc 50.5) groups. The upfield shift of the C-2 carbonyl (δc 163.8) in 1 relative to that (δc 166.1) in dzununcanone revealed that an α,β-unsaturated δ-lactone ring was formed between C-2 and C-4 in 1, accounting for the remaining one degree of unsaturation. Both the methoxyl and the tertiary methyl groups were assigned at C-4, as deduced from HMBC correlations from their protons to C-4 (δ C 103.8) in 1. Thus, the planar structure of 1 was established as a 2, 4-esterified derivative of dzununcanone, which was further confirmed by 1H − 1H COSY and HMBC correlations (Fig. 2A).
Fig. 2.
The 1H − 1H COSY, key HMBC (A) and selected ROESY (B) correlations of 1.
The relative configuration of 1 was determined by analysis of ROESY spectrum (Fig. 2B). The observed key correlation between C-4 methoxy protons and H3–25β indicated that the methoxyl group at C-4 was β-oriented. The remaining stereogenic centers in 1 were assigned as the same relative configurations with those in dzununcanone, as evidenced from the important ROESY correlations of H3–25/H3–26, H3–26/H3–28, and H3–28/H3–30. Therefore, the structure of 1 was characterized as shown in Fig. 1 , and given a trivial name of triregelolide A.
Fig. 1.
The chemical structures of compounds 1–23, dzununcanone and esculentoic acid A.
Compound 2 has the same molecular formula of C29H40O5 as that of 1 based on HRESIMS data (m/z 467.2810 [M − H]−, calcd for C29H39O5, 467.2803). The 1H and 13C NMR spectroscopic data (Table 1) of 2 are closely similar to those of 1 except for the downfield shifts of C-4 and C-23 (δ C 105.6 and 28.2) relative to those (δ C 103.8 and 24.8) in 1. These data were indicative of different configuration of the C-4 stereogenic center of both compounds. Moreover, a key ROESY correlation between H3–23 and H3–25β was observed, revealing an α-orientation of the methoxy group at C-4. Therefore, the structure of 2 was elucidated as a 4-epimer of 1, and given a trivial name of triregelolide B.
Compound 3 was obtained as amorphous powder. The molecular formula was determined as C30H48O4, based on its HRESIMS data (m/z 471.3479 [M − H] −, calcd for C30H47O4, 471.3480). The 1H NMR data (Table 1) displayed the presence of an olefinic proton [δ H 5.62 (1H, dd, J = 8.0, 3.0 Hz, H-15)], two oxygenated methine protons [δ H 4.13 (1H, td, J = 9.6, 4.2 Hz, H-2) and 3.39 (1H, d, J = 9.6 Hz, H-3)], and seven tertiary methyl groups [δ H 1.53 (3H, s, H3–30), 1.28 (3H, s, H3–23), 1.13 (6H, s, H3–24 and H3–26), 1.05 (3H, s, H3–25), 1.02 (3H, s, H3–27) and 0.96 (3H, s, H3–28)]. The 13C NMR data (Table 1) of 3 showed 30 carbon signals, which were ascribed to a carboxylic group (δ C 182.2, C-29), two olefinic carbons [δ C 158.4 (C-14) and 117.1 (C-15)], five methines (including two oxygenated ones), six quaternary carbons, nine methylenes, and seven methyl carbons by DEPT and HMBC experiments. These characteristic signals indicated that 3 was a taraxerene-type triterpene [25], [26], [27]. The 1H and 13C NMR data of 3 were very similar to those of esculentoic acid A [26] isolated from Manihot esculenta, except for the presence of an additional oxygenated methine [δ H 4.13 (1H, td, J = 9.6, 4.2 Hz, H-2); δ C 68.6] and the absence of a methylene signals (δ H 1.78 and 1.60; δ C 25.3) in esculentoic acid A [26]. The downfield shift of H-2 at δ H 4.13 suggested hydroxylation of C-2 in 3 compared to those (δ H 1.78 and 1.60) in esculentoic acid A [26]. The coupling constant of 9.6 Hz between H-2 and H-3 (J 2, 3) indicated a 2,3-trans diaxial relationship of the above two protons in 3, whereas the H-3 was in a equatorial bond in esculentoic acid due to a smaller J 2, 3 of 2.8 Hz. The key NOESY correlations of H-2/H3–25β, and H-3/H-5α suggested an α-oriented hydroxyl group at C-2 and a β-oriented hydroxyl group at C-3 in 3. Thus, the structure of 3 was determined as 2α, 3β-dihydroxytaraxer-14-ene-29-oic acid, and named triregeloic acid.
In addition, twenty known triterpenoids were also isolated and identified as NST6A (4) [28], celastrol (5) [29], [30], 22β-hydroxy-tingenone (6) [31], triptocalline A (7) [32], polpunonic acid (8) [33], orthophenic acid (9) [31], demethylzeylasteral (10) [30], wilforol A (11) [31], regelin D (12) [19], triptotriterpenic acid B (13) [34], [35], abrusgenic acid (14) [34], [36], wilforlide A (15) [31], [37], wilforlide B (16) [37], triptocallic acid A (17) [38], regelinol (18) [16], regelin C (19) [19] , regelin (20) [19], [39], dulcioic acid (21) [31], tripterygic acid A (22) [40], demethylregelin (23) [31], based on analyses of their NMR spectroscopic data and comparisons with those in the literatures. Ten triterpenes (4, 7, 8, 10, 13, 14, 17, 21–23) were isolated from T. regelii for the first time.
Cytotoxic effects of nine selected triterpenes (3, 5, 6, 8–10, 14, 16, 21) were evaluated against human breast cancer MCF-7 cells at a concentration of 10 μM (Table 2 ), the other triterpenes were not conducted cytotoxic bioassay due to a limited amount obtained from T. regelii. Taxol was used as a positive control drug. As a result, triterpenes 3, 5, 6, 8, 9, 10, 14 and 16 showed inhibitory effects on the proliferation of MCF-7 cells by 24.1%, 69.6%, 72.8%, 21.6%, 23.1%, 43.3%, 25.5% and 23.5% (p < 0.05) at a concentration of 10 μM, respectively. Triterpenes 5 and 6 exhibited more potent (p < 0.001) cytotoxic effects than taxol (with an inhibitory rate of 35.0%) at 10 μM drug concentration.
Table 2.
Cytotoxic effects of nine triterpenes and taxol on human breast cancer MCF-7 cells.
| Compounds a | Inhibitory rate (%) b |
|---|---|
| Taxol | 35.0 ± 5.06 |
| 3 | 24.1 ± 2.21⁎ |
| 5 | 69.6 ± 0.75⁎ |
| 6 | 72.8 ± 0.53⁎ |
| 8 | 21.6 ± 1.66⁎ |
| 9 | 23.1 ± 1.31⁎ |
| 10 | 43.3 ± 2.21⁎ |
| 14 | 25.5 ± 1.45⁎ |
| 16 | 23.5 ± 3.12⁎ |
| 21 | 7.2 ± 5.07 |
All compounds except for 21, showed significant (p < 0.05) inhibitory effects on the proliferation of MCF-7 cells compared to the vehicle control group. The value of p < 0.001 when statistical comparison was conducted between either compound 5 or 6 with taxol.
Other triterpenes were not evaluated for cytotoxicity due to the limited amount obtained.
The inhibitory rate (%) on MCF-7 cells was determined at a drug concentration of 10 μM.
Conflict of interest
All the authors declare that there is no conflict of interest concerning this work.
Acknowledgments
This research work was financially supported by Macao Science and Technology Development Fund, MSAR (Grant no. 056/2013/A2 and 063/2011/A3).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.fitote.2016.07.006.
Appendix A. Supplementary data
Supplementary material.
References
- 1.Thimmappa R., Geisler K., Louveau T., O'Maille P., Osbourn A. Triterpene biosynthesis in plants. Annu. Rev. Plant Biol. 2014;65:225–257. doi: 10.1146/annurev-arplant-050312-120229. [DOI] [PubMed] [Google Scholar]
- 2.Sun H., Fang W.S., Wang W.Z., Hu C. Structure-activity relationships of oleanane-and ursane-type triterpenoids. Bot. Stud. 2006;47:339–368. [Google Scholar]
- 3.Sheng H., Sun H. Synthesis, biology and clinical significance of pentacyclic triterpenes: a multi-target approach to prevention and treatment of metabolic and vascular diseases. Nat. Prod. Rep. 2011;28:543–593. doi: 10.1039/c0np00059k. [DOI] [PubMed] [Google Scholar]
- 4.Salvador J.A.R., Santos R.C., Figueiredo S.A.C., Jing Y. Antitumor Effects of Celastrol and Semi-Synthetic Derivatives. Mini -Rev. Org. Chem. 2014;11:400–407. [Google Scholar]
- 5.Safayhi H., Sailer E. Anti-inflammatory actions of pentacyclic triterpenes. Planta Med. 1997;63:487–493. doi: 10.1055/s-2006-957748. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y.Y., Zhe H., Zhao R. Preclinical evidences toward the use of triterpenoid CDDO-Me for solid cancer prevention and treatment. Mol. Cancer. 2014;13:1–8. doi: 10.1186/1476-4598-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schechter M.S., Haller H. Identity of the red pigment in the roots of Tripterygium wilfordii and Celastrus scandens. J. Am. Chem. Soc. 1942;64:182–183. [Google Scholar]
- 8.Duan H., Kawazoe K., Bando M., Kido M., Takaishi Y. Di-and triterpenoids from Tripterygium hypoglaucum. Phytochemistry. 1997;46:535–543. [Google Scholar]
- 9.Shen J.H., Zhou B.N. Study on the Triterpenoids of Tripterygium regelii. Acta Bot. Sin. 1992;34:475–479. [Google Scholar]
- 10.Allison A.C., Cacabelos R., Lombardi V.R., Álvarez X.A., Vigo C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer's disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2001;25:1341–1357. doi: 10.1016/s0278-5846(01)00192-0. [DOI] [PubMed] [Google Scholar]
- 11.Deng Y.N., Shi J., Liu J., Qu Q.M. Celastrol protects human neuroblastoma SH-SY5Y cells from rotenone-induced injury through induction of autophagy. Neurochem. Int. 2013;63:1–9. doi: 10.1016/j.neuint.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Liu J., Lee J., Hernandez M.A.S., Mazitschek R., Ozcan U. Treatment of obesity with celastrol. Cell. 2015;161:999–1011. doi: 10.1016/j.cell.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryu Y.B., Park S.J., Kim Y.M., Lee J.Y., Seo W.D., Chang J.S. SARS-CoV 3CL pro inhibitory effects of quinone-methide triterpenes from Tripterygium regelii. Bioorg. Med. Chem. Lett. 2010;20:1873–1876. doi: 10.1016/j.bmcl.2010.01.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flora of China Editorial Committee of Chinese Academy of Sciences . Beijing Science and Technology Press; Beijing: 1999. Flora of China; p. 181. [Google Scholar]
- 15.Editorial Board of Zhonghua Bencao . Shanghai Science and Technology Press; Shanghai: 1999. Zhonghua Bencao; pp. 205–206. [Google Scholar]
- 16.Hori H., Pang G.M., Harimaya K., Iitaka Y., Inayama S. Isolation and structure of regelin and regelinol, new antitumor ursene-type triterpenoids from Tripterygium regelii. Chem. Pharm. Bull. 1987;35:2125–2128. doi: 10.1248/cpb.35.2125. [DOI] [PubMed] [Google Scholar]
- 17.Shen J.H., Zhou B.N. Studies on diterpene-quinones of Tripterygium regelii Sprague. Chin. Chem. Lett. 1992;3:113–116. [Google Scholar]
- 18.Lee B.W., Seo W.D., Gal S.W., Yang M.S., Park K.H. Quinone methide triterpenes from Tripterygium regelii. Agric. Chem. Biotechnol. 2004;47:77–80. [Google Scholar]
- 19.Pang G.M., Zhao C.J., Hori H., Inayama S. Studies on new triterpenoids of Tripterygium regelii. Acta Pharm. Sin. 1989;24:75–79. [PubMed] [Google Scholar]
- 20.Harada R., Kakisawa H., Kobayashi S., Musya M., Nakanishi K., Takahashi Y. Structure of pristimerin, a quinonoid triterpene. Tetrahedron Lett. 1962;3:603–607. [Google Scholar]
- 21.Fan D., Zhu G.Y., Chen M., Xie L.M., Jiang Z.H., Xu L. Dihydro-β-agarofuran sesquiterpene polyesters isolated from the stems of Tripterygium regelii. Fitoterapia. 2016;112:1–8. doi: 10.1016/j.fitote.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Mena-Rejón G.J., Pérez-Espadas A.R., Moo-Puc R.E., Cedillo-Rivera R., Bazzocchi I., Jiménez-Diaz I. Antigiardial Activity of Triterpenoids from Root Bark of Hippocratea excelsa. J. Nat. Prod. 2007;70:863–865. doi: 10.1021/np060559y. [DOI] [PubMed] [Google Scholar]
- 23.Li G.P., yang L.J., Zhao J.F., Yang X., Li L. Studies on lipophilic chemical constituents from Passiflora wilsonii Hems. Chem. Ind. For. Prod. 2007;27:27–30. [Google Scholar]
- 24.Connolly J.D., Hill R.A. Triterpenoids. Nat. Prod. Rep. 2010;27:79–132. doi: 10.1039/b808530g. [DOI] [PubMed] [Google Scholar]
- 25.Pengsuparp T., Cai L., Fong H.H., Kinghorn A.D., Pezzuto J.M., Wani M.C. Pentacyclic triterpenes derived from Maprounea africana are potent inhibitors of HIV-1 reverse transcriptase. J. Nat. Prod. 1994;57:415–418. doi: 10.1021/np50105a017. [DOI] [PubMed] [Google Scholar]
- 26.Chaturvedula V., Schilling J.K., Malone S., Wisse J.H., Werkhoven M.C., Kingston D.G. New cytotoxic triterpene acids from aboveground parts of Manihot esculenta from the Suriname rainforest. Planta Med. 2003;69:271–274. doi: 10.1055/s-2003-38488. [DOI] [PubMed] [Google Scholar]
- 27.Cao T.W., Geng C.A., Jiang F.Q., Ma Y.B., He K., Zhou N.J. Chemical constituents of Swertia yunnanensis and their anti-hepatitis B virus activity. Fitoterapia. 2013;89:175–182. doi: 10.1016/j.fitote.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 28.Tang K., Huang Q., Zeng J., Wu G., Huang J., Pan J. Design, Synthesis and Biological Evaluation of C (6)-Modified Celastrol Derivatives as Potential Antitumor Agents. Molecules. 2014;19:10177–10188. doi: 10.3390/molecules190710177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miao K., Zhang X., Dong Y. Studies on triterpenoids constituents of Tripterygium wilfordii Hook. F, Natural Product Research and. Development. 1999;12:1–7. [Google Scholar]
- 30.Morota T., Yang C.X., Ogino T., Qin W.Z., Katsuhara T., Xu L.H. D: A-friedo-24-noroleanane triterpenoids from Tripterigium wilfordii. Phytochemistry. 1995;39:1159–1163. [Google Scholar]
- 31.Takaishi Y., Wariishi N., Tateishi H., Kawazoe K., Nakano K., Ono Y. Triterpenoid inhibitors of interleukin-1 secretion and tumour-promotion from Tripterygium wilfordii var. regelii. Phytochemistry. 1997;45:969–974. [Google Scholar]
- 32.Nakano K., Oose Y., Takaishi Y. A novel epoxy-triterpene and nortriterpene from callus cultures of Tripterygium wilfordii. Phytochemistry. 1997;46:1179–1182. [Google Scholar]
- 33.Nozaki H., Matsuura Y., Hirono S., Kasai R., Chang J.J., Lee K.H. Antitumor agents, 116. Cytotoxic triterpenes from Maytenus diversifolia. J. Nat. Prod. 1990;53:1039–1041. doi: 10.1021/np50070a050. [DOI] [PubMed] [Google Scholar]
- 34.Kutney J.P., Hewitt G.M., Lee G., Piotrowska K., Roberts M., Rettig S.J. Studies with tissue cultures of the Chinese herbal plant, Tripterygium wilfordii. Isolation of metabolites of interest in rheumatoid arthritis, immunosuppression, and male contraceptive activity. Can. J. Chem. 1992;70:1455–1480. [Google Scholar]
- 35.Zhang C.P., Zhang Y.G., Lv X.Y., Chen Y., Ma P.C., He C.H. Studes on triterpenoids of total glucosides of Tripterygium wilfordii (TΙΙ) Acta Acad. Med. Sinicae. 1989;5:322–325. [PubMed] [Google Scholar]
- 36.Chiang T.C., Chang H.M., Mak T.C. New oleanene-type triterpenes from Abrus precatorius and X-ray crystal structure of abrusgenic acid-methanol 1: 1 solvate. Planta Med. 1983;49:165–169. doi: 10.1055/s-2007-969840. [DOI] [PubMed] [Google Scholar]
- 37.Wang F., Zhang Y., Zhao Y. Chemical constituents of Tripterygium hypoglaucum. Chin. Tradit. Herb. Drugs. 2011;42:46–49. [Google Scholar]
- 38.Nakano K., Oose Y., Masuda Y., Kamada H., Takaishi Y. A diterpenoid and triterpenes from tissue cultures of Tripterygium wilfordii. Phytochemistry. 1997;45:293–296. [Google Scholar]
- 39.Duan H., Takaishi Y., Momota H., Ohmoto Y., Taki T., Tori M. Immunosuppressive terpenoids from extracts of Tripterygium wilfordii. Tetrahedron. 2001;57:8413–8424. [Google Scholar]
- 40.Zhang D.M., Yu D.Q. Structure of Tripterygic Acid A: A New Triterpene of Tripterygium wilfordii. Planta Med. 1990;56:98–100. doi: 10.1055/s-2006-960896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.