Highlights
-
•
Microglia are highly plastic cells controlled by their environment.
-
•
Low baseline levels of type I IFN are detectable in CNS and sensed by microglia.
-
•
Microglial type I IFN limits viral spread.
-
•
IFNβ release mediates microglial phagocytosis of myelin debris in CNS autoimmunity.
-
•
Dysregulated microglia are involved in the pathogenesis of certain interferonopathies.
Abstract
Type I interferons (IFN) are pleiotropic cytokines originally described as molecules used for communication between cells to trigger the protective defenses against viral infections. Upon activation, type I IFN can be produced locally in the central nervous system (CNS) from a number of different cell types including microglia, the CNS-resident macrophages. Increased type I IFN production and signaling in microglia are critically important to limit viral infection and disease progression in multiple sclerosis. However, recent findings suggest that even baseline levels of constitutive IFN expression and secretion are important for homeostasis of the CNS. In fact, in the absence of viral particles chronic elevation of IFN I may be tremendously harmful for the CNS, as assumed for patients suffering from Aicardi-Goutières syndrome, Cree encephalitis or other type I interferonopathies. The highly diverse nature of type I IFN for brain homeostasis during health and disease will be discussed in this report.
Current Opinion in Neurobiology 2016, 36:38–42
This review comes from a themed issue on Neurobiology of disease
Edited by Dennis J Selkoe and Daniel R Weinberger
For a complete overview see the Issue and the Editorial
Available online 20th September 2015
http://dx.doi.org/10.1016/j.conb.2015.09.003
0959-4388/© 2015 Elsevier Ltd. All rights reserved.
Introduction
As resident immune competent cells of the CNS, microglia are engaged in a multitude of processes including pathogen defense, inflammatory responses and phagocytosis of damaged endogenous tissue [1••]. Microglial phagocytosis is not restricted to pathogenic events but also occurs in the course of synaptic pruning during developmental stages and even adulthood. These characteristics make microglia essential for normal CNS development, tissue homeostasis and recovery from acute injury. With this review we want to highlight the most recent achievements in the study of type I interferons, which are pleiotropic cytokines that allow microglia to exert a multifactorial role. Type I IFN can be released by microglia to control the microenvironment including, among other cells, even microglia. In part, depending on the IFN type I levels and exposure times, microglia elicit detrimental or beneficial effects.
Origin & function of microglia
Microglia belong to the group of mononuclear phagocytes and are the tissue-resident macrophages within the CNS. Unlike neurons and macroglia (astrocytes and oligodendrocytes), microglia cells arise from the primitive hematopoiesis as lineage-negative (Lin−)-erythromyeloid precursors in the extraembryonic yolk sac and colonize the early developing brain [2, 3, 4]. A physiological contribution from monocytes or bone marrow-derived precursors, derived from hematopoietic stem cells during the definitive hematopoiesis, could be ruled out by different experimental setups [5, 6, 7]. Shortly after birth, the ultimate pool of microglia is set and virtually maintained throughout the lifetime [3, 4, 8]. Initially microglia were thought to be in a silent, resting state until disturbances of the CNS homeostasis occur, which in turn activate microglia in a specific manner. However, more and more evidence accumulates that these cells do not only contribute to pathophysiological processes associated with neurodegeneration or neuroinflammation, but are also mandatory for normal brain homeostasis [9, 10]. Thus, dysregulated microglia might be responsible for a group of neuropsychiatric, neurodegenerative and neuroinflammatory diseases [1••, 11, 12]. Under non-pathological conditions, microglia can directly interact with synapse-associated elements, thus shaping brain architecture and eventually cognitive processes [13, 14]. These cells are constantly active and involved in normal brain physiology (extensively reviewed in [1••, 4]). Recent data suggest that macrophages are not in a certain stage but rather change their responsive program specifically by reacting to incoming stimuli (e.g. various cytokine combinations) [15]. One can assume that microglia cells possess a versatile repertoire to react to a certain stimulus or to a combination of stimuli depending on the prevailing settings. This implies that even the status of a microglia cell is highly plastic and depends on the local microenvironment within the parenchyma, which again might depend on signals from the periphery. As an example, short-chain fatty acids produced by gut microbiota are able to modulate microglia maturation, morphology and function [16••]. Furthermore, peripheral injection of Toll-like receptor agonists can induce type I IFN production by microglia, one of the main sources of IFNβ within the CNS during inflammatory conditions [17].
Presence of IFN signaling in the healthy CNS
IFN are a family of cytokines involved as effector molecules in adaptive immunity during viral resistance [18, 19]. Furthermore, IFN contributes to and modulates autoimmune diseases and cancer, thereby having an impact on proliferation, cell cycle and cell survival [18, 19, 20•]. IFNs consist of three different types, namely I, II (IFNγ), and III (IFNλ). Type I IFNs are further subdivided into α, β, ω, κ, and ɛ. All of these type I IFN subtypes, but neither type II nor type III IFNs, are capable of binding to the IFNα/β receptor, which itself consists of two different subunits (IFNAR1 and IFNAR2) [21•]. Upon binding to IFNAR, the two associated kinases, tyrosine kinase 2 (TYK2) and Janus kinase 1 (JAK1), phosphorylate signal transducers and activators of transcription (STAT) family members 1–6. This, in turn, leads to a specific regulation via induction and/or termination of a multitude of different genes, so called IFN-stimulated genes (ISGs) [18, 21•]. Interestingly, the affinity of the IFNAR receptor varies between the different type I IFN ligands, which again has an impact on the compilation of genes expressed. This diversity is achieved by the activation of different regulatory elements, providing the potential activation of varying signaling pathways in the same cell [21•, 22]. Finally, the IFN-induced signaling is terminated via suppressor of cytokine signaling (SOCS) proteins or ubiquitin specific protease (USP)18, which compete with STAT proteins or bind to IFNAR2, respectively [19, 23].
Typically, type I IFNs are induced by ssRNA, dsRNA, and cytosolic DNA from viruses or bacteria [18]. However, also under normal physiological conditions a constant type I IFN expression and secretion is present in healthy individuals, and these rather low levels are thought to maintain expression of STAT1 (Figure 1a). Since STAT1 is a key factor for immune cell responses to type II IFN, type I IFN primes cells, through providing baseline expression of STAT1, to respond to type II IFN signals [24, 25]. Consequently, the absence of IFN-signaling leads to a disturbance of the myeloid compartment in bone marrow and blood of IFNAR−/− mice [26]. Low baseline levels of type I IFN were also detected in the CNS by ELISA [26], or by crossing a fluorescent reporter line to MX1Cre mice, which express Cre recombinase under the control of a type I interferon inducible promoter (Mx1) [27]. Of note, these mice were raised under specific pathogen free conditions. Consequently, the lack of the negative regulator of type I IFN, ubiquitin-specific protease (USP)18, results in hyperactivated microglia in the white matter of the CNS [27]. During aging, increased expression of typical type I IFN induced genes is present in CNS, which is responsible for a reduction of hippocampal neurogenesis [28]. Interfering with the increased amount of type I IFN using neutralizing α-IFNAR antibodies counteracted aged-dependent memory decline [28]. However, the origin and the actual stimulus for constitutive type I IFN production as well as the necessity of type I IFN to maintain homeostasis within the CNS still remain to be solved.
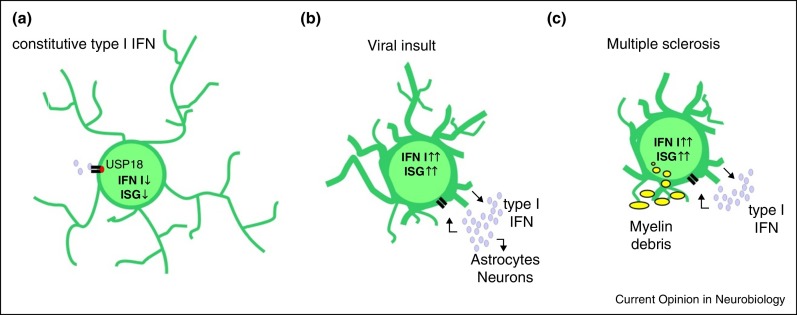
Figure 1.
Microglia during health, viral insult and multiple sclerosis. (a) Low levels of constitutive type I IFN are present under healthy conditions. The IFN signal is controlled by the negative regulator USP18, limiting the expression of interferon-stimulated genes (ISGs). (b) Most viruses are sensed by microglia, which in turn induce the release of type I IFN as well as the expression of diverse ISGs. Secreted IFNs act on microglia, astrocytes and neurons to limit viral spread. (c) During multiple sclerosis, microglia express, release and respond to type I IFN. The presence of IFNβ is critical for the clearance of myelin debris.
Microglia: IFN responsive and producing cells
As the IFN system plays an essential role in the viral defense, it seems obvious that this signaling cascade is critical in microglia, as they are thought to be the immune competent cells of CNS [29]. For example, intracerebral infection with lymphatic choriomeningitis virus (LCMV) induces a tight and quick IFN response, including the expression of IFN by most likely all cells of the CNS and subsequent activation of microglia, which limits the viral spread [30, 31•] (Figure 1b). Upon infection with La Cross virus or coronavirus mouse hepatitis virus, either microglia together with astrocytes or microglia only were identified as type I IFN producing cells [32, 33]. As a consequence of viral infection of the CNS and subsequent type I IFN release, expression of interferon-stimulated genes (ISGs) and interferon regulatory factors (IRFs) are induced in microglia and astrocytes as well as in neurons, ependymal and choroid plexus cells [34, 35, 36]. While an overactivation of the type I IFN signaling, present in mice deficient for the negative regulator USP18, leads to a resistance to intracranial LCMV infections, mice lacking the IFN receptor IFNAR fail to activate microglia and other myeloid cells of the CNS and massively increase their LCMV viral load [31•, 34]. Higher disease burden, increased viral replication and reduced amounts of type I IFN were also present in IFN-induced proteins with tetratricopeptide repeats (IFIT) 2 knockout mice infected intracranially with mouse hepatitis virus strain A59 [37]. Besides the above mentioned examples, there are many more viruses which elicit a similar type I IFN response within the CNS, highlighting the importance of this signaling cascade for anti-viral defense (for reviews see [29, 38]).
Microglial type I IFN signaling also contributes to the resolution of sterile injury [39]. In this lesion model, induction of IRF7 expression is restricted to microglial cells and this limits leukocyte recruitment [39]. Entry of leukocytes (monocytes, T-cell and B-cell) into the CNS is a key feature of multiple sclerosis (MS), the most prominent form of CNS autoimmune diseases [1••, 12]. Besides the infiltration of mononuclear cells, MS is characterized by a disruption of the blood–brain-barrier and by activation of microglia, which, in the end, leads to demyelination and death of neurons. Increased expression of type I IFN, measured by ELISA and qPCR, was found in the spinal cord of an experimental autoimmune encephalitis (EAE) mouse model, the most used animal model for MS, resulting in a robust expression of IFN target genes [27, 40•, 41•]. On a cellular level, the expression of IFNs was assigned to microglia/macrophages and astrocytes either by immunohistochemical detection of IFNα, IFNβ and IFNγ in human tissue samples [42] or to microglia only with the help of an IFNβ/YFP reporter mouse line [41•]. Furthermore, phosphorylation of STAT1 in CD68+ microglia/macrophages indicates the activation of the IFN pathway, which might be responsible for the expression of diverse ISGs [27, 40•, 41•]. The induction of the type I IFN system in the CNS is critical for limiting EAE as IFNβ-deficient and IFNAR-deficient mice suffer from a higher disease course, increased macrophage, T-cell and B-cell infiltration and greater demyelination [40•, 43]. CNS endogenous microglia-derived IFNβ induces microglia-mediated phagocytosis of myelin debris via Tir domain-containing adapter inducing interferon beta (TRIF), which is suggested to reduce disease burden in EAE [41•, 44] (Figure 1c).
Dysregulation of microglial type I IFNs in the CNS
While type I IFN levels were helpful to restrict viral infections, chronically increased levels of IFN have the opposite effect and cause various forms of diseases. This can be achieved by reduced or less-functional expression of USP18, a potent negative regulator of IFN-I signaling that is highly expressed in white matter microglia, where USP18 closely controls their activation status [27]. From this point of view one might speculate that dysregulated microglia might contribute to pathogenesis of white matter diseases. Interestingly, in patients harboring mutations in ISG15, the presence of calcifications of the cerebral basal ganglia as well as an increased type I IFN signature due to reduced protein stability of USP18 have been reported [45•]. Intracranial calcification is also a feature of a group of autoinflammatory diseases with upregulated type I IFN signaling, such as Aicardi-Goutières syndrome (AGS) [46, 47]. The hallmark of AGS and Cree encephalitis, which are allelic hereditary disorders, is markedly increased typeI IFN in the CSF together with a progressive inflammatory encephalopathy [46]. AGS phenotypically also overlaps with systemic lupus erythematosus (SLE), which is likewise associated with elevated IFN I levels in the CNS [46, 47]. Chronic elevation of IFN I in the CNS is also linked to encephalopathies resulting from congenital or persistent viral infections. Alterations in the CNS histology include characteristic microglial nodules and multinucleated macrophages [48, 49]. Although highly speculative, it seems worthwhile to determine the exact role of activated microglia in diseases such as AGS, Cree encephalitis or SLE.
Conclusion
Within the CNS, microglial cells are one of the main type I IFN producing cells but at the same time, are also highly responsive to IFN type I. Even though type I IFNs have a central role in immune defense against injury or infection, the actual IFN levels must be tightly controlled so as to reduce potential damage to the CNS. A persistent IFN I level, which is either excessively elevated or possibly decreased, seems to induce neurotoxic changes. It is remarkable to note that already in the healthy brain, baseline levels of IFN I are present to contribute to brain homeostasis. While in a variety of neurological disorders increased type I IFN-signatures are detectable in the brain, it is still under debate whether these elevated IFN I levels are causative for the disorder or a simple consequence. The exact mechanisms and the regulating factors important for proper microglia function in health and disease are subjects of ongoing research. Our recent data, using mice lacking microglial USP18, indicates that pronounced IFN I signaling in microglia and their concomitant activation are primary to white matter disease pathogenesis in the CNS. The availability of different new mouse lines, which specifically target microglia cells, has great potential to clarify how far IFN I signaling and microglia are liable for the aforementioned neurological disorders [8, 14, 50].
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
TB is supported by a DFG grant BL 1153/1-1 and a Marie Curie Reintegration grant, project no. 248033 — MBfUSEDIT. MP is supported by BMBF-funded competence network of multiple sclerosis, the Sobek Foundation, the DFG (SFB 992, FOR1336, PR 577/8-1), Fritz-Thyssen Foundation (no. 10.13.1.191) and Gemeinnützige Hertie Foundation (GHST, no. P1130078 (Multiple sklerose)).
References
- 1••.Prinz M., Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]; A comprehensive review that covers all aspects of microglia such as origin, function in health and in different disease conditions.
- 2.Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M.F., Conway S.J., Ng L.G., Stanley E.R. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kierdorf K., Erny D., Goldmann T., Sander V., Schulz C., Perdiguero E.G., Wieghofer P., Heinrich A., Riemke P., Holscher C. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci. 2013;16:273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- 4.Gomez Perdiguero E., Klapproth K., Schulz C., Busch K., Azzoni E., Crozet L., Garner H., Trouillet C., de Bruijn M.F., Geissmann F. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulz C., Gomez Perdiguero E., Chorro L., Szabo-Rogers H., Cagnard N., Kierdorf K., Prinz M., Wu B., Jacobsen S.E., Pollard J.W. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 6.Mildner A., Schmidt H., Nitsche M., Merkler D., Hanisch U.K., Mack M., Heikenwalder M., Bruck W., Priller J., Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 7.Ajami B., Bennett J.L., Krieger C., Tetzlaff W., Rossi F.M. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 8.Goldmann T., Wieghofer P., Muller P.F., Wolf Y., Varol D., Yona S., Brendecke S.M., Kierdorf K., Staszewski O., Datta M. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat Neurosci. 2013;16:1618–1626. doi: 10.1038/nn.3531. [DOI] [PubMed] [Google Scholar]
- 9.Bruttger J., Karram K., Wörtge S., Regen T., Marini F., Hoppmann N., Klein M., Blank T., Yona S., Wolf Y., Mack M., Pinteaux E., Müller W., Zipp F., Binder H., Bopp T., Prinz M., Jung S., Waisman A. Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity. 2015;43:92–106. doi: 10.1016/j.immuni.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Ransohoff R.M., Perry V.H. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 11.Blank T., Prinz M. Microglia as modulators of cognition and neuropsychiatric disorders. Glia. 2013;61:62–70. doi: 10.1002/glia.22372. [DOI] [PubMed] [Google Scholar]
- 12.Goldmann T., Prinz M. Role of microglia in CNS autoimmunity. Clin Dev Immunol. 2013;2013:208093. doi: 10.1155/2013/208093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tremblay M.E. The role of microglia at synapses in the healthy CNS: novel insights from recent imaging studies. Neuron Glia Biol. 2011;7:67–76. doi: 10.1017/S1740925X12000038. [DOI] [PubMed] [Google Scholar]
- 14.Parkhurst C.N., Yang G., Ninan I., Savas J.N., Yates J.R., 3rd, Lafaille J.J., Hempstead B.L., Littman D.R., Gan W.B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue J., Schmidt S.V., Sander J., Draffehn A., Krebs W., Quester I., De Nardo D., Gohel T.D., Emde M., Schmidleithner L. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40:274–288. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Erny D., Hrabe de Angelis A.L., Jaitin D., Wieghofer P., Staszewski O., David E., Keren-Shaul H., Mahlakoiv T., Jakobshagen K., Buch T. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article is the first to show a link between gut microbiota and microglia morphology and function.
- 17.Costello D.A., Lynch M.A. Toll-like receptor 3 activation modulates hippocampal network excitability, via glial production of interferon-beta. Hippocampus. 2013;23:696–707. doi: 10.1002/hipo.22129. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Navajas J.M., Lee J., David M., Raz E. Immunomodulatory functions of type I interferons. Nat Rev Immunol. 2012;12:125–135. doi: 10.1038/nri3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivashkiv L.B., Donlin L.T. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Gough D.J., Messina N.L., Clarke C.J., Johnstone R.W., Levy D.E. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity. 2012;36:166–174. doi: 10.1016/j.immuni.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors of this review highlight the importance of constitutive type I IFN production without viral insult and how defects in this process impact disease.
- 21•.Schreiber G., Piehler J. The molecular basis for functional plasticity in type I interferon signaling. Trends Immunol. 2015;36:139–149. doi: 10.1016/j.it.2015.01.002. [DOI] [PubMed] [Google Scholar]; This review summarizes how similar type I IFN-IFNAR promotes a multitude of biological responses induced by different signaling cascades.
- 22.Rusinova I., Forster S., Yu S., Kannan A., Masse M., Cumming H., Chapman R., Hertzog P.J. Interferome v2.0: an updated database of annotated interferon-regulated genes. Nucleic Acids Res. 2013;41:D1040–D1046. doi: 10.1093/nar/gks1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malakhova O.A., Kim K.I., Luo J.K., Zou W., Kumar K.G., Fuchs S.Y., Shuai K., Zhang D.E. UBP43 is a novel regulator of interferon signaling independent of its ISG15 isopeptidase activity. EMBO J. 2006;25:2358–2367. doi: 10.1038/sj.emboj.7601149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tovey M.G., Streuli M., Gresser I., Gugenheim J., Blanchard B., Guymarho J., Vignaux F., Gigou M. Interferon messenger RNA is produced constitutively in the organs of normal individuals. Proc Natl Acad Sci U S A. 1987;84:5038–5042. doi: 10.1073/pnas.84.14.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gough D.J., Messina N.L., Hii L., Gould J.A., Sabapathy K., Robertson A.P., Trapani J.A., Levy D.E., Hertzog P.J., Clarke C.J. Functional crosstalk between type I and II interferon through the regulated expression of STAT1. PLoS Biol. 2010;8:e1000361. doi: 10.1371/journal.pbio.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang S.Y., Hertzog P.J., Holland K.A., Sumarsono S.H., Tymms M.J., Hamilton J.A., Whitty G., Bertoncello I., Kola I. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc Natl Acad Sci U S A. 1995;92:11284–11288. doi: 10.1073/pnas.92.24.11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldmann T., Zeller N., Raasch J., Kierdorf K., Frenzel K., Ketscher L., Basters A., Staszewski O., Brendecke S.M., Spiess A. USP18 lack in microglia causes destructive interferonopathy of the mouse brain. EMBO J. 2015;34:1612–1629. doi: 10.15252/embj.201490791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baruch K., Deczkowska A., David E., Castellano J.M., Miller O., Kertser A., Berkutzki T., Barnett-Itzhaki Z., Bezalel D., Wyss-Coray T. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science. 2014;346:89–93. doi: 10.1126/science.1252945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nayak D., Roth T.L., McGavern D.B. Microglia development and function. Annu Rev Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merigan T.C., Oldstone M.B., Welsh R.M. Interferon production during lymphocytic choriomeningitis virus infection of nude and normal mice. Nature. 1977;268:67–68. doi: 10.1038/268067a0. [DOI] [PubMed] [Google Scholar]
- 31•.Nayak D., Johnson K.R., Heydari S., Roth T.L., Zinselmeyer B.H., McGavern D.B. Type I interferon programs innate myeloid dynamics and gene expression in the virally infected nervous system. PLoS Pathog. 2013;9:e1003395. doi: 10.1371/journal.ppat.1003395. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a combination of genomic and imaging approaches, the authors revealed that the brain induces a pronounced and dynamic type I IFN response to limit to LCMV spread.
- 32.Roth-Cross J.K., Bender S.J., Weiss S.R. Murine coronavirus mouse hepatitis virus is recognized by MDA5 and induces type I interferon in brain macrophages/microglia. J Virol. 2008;82:9829–9838. doi: 10.1128/JVI.01199-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kallfass C., Ackerman A., Lienenklaus S., Weiss S., Heimrich B., Staeheli P. Visualizing production of beta interferon by astrocytes and microglia in brain of La Crosse virus-infected mice. J Virol. 2012;86:11223–11230. doi: 10.1128/JVI.01093-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ritchie K.J., Hahn C.S., Kim K.I., Yan M., Rosario D., Li L., de la Torre J.C., Zhang D.E. Role of ISG15 protease UBP43 (USP18) in innate immunity to viral infection. Nat Med. 2004;10:1374–1378. doi: 10.1038/nm1133. [DOI] [PubMed] [Google Scholar]
- 35.Ousman S.S., Wang J., Campbell I.L. Differential regulation of interferon regulatory factor (IRF)-7 and IRF-9 gene expression in the central nervous system during viral infection. J Virol. 2005;79:7514–7527. doi: 10.1128/JVI.79.12.7514-7527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wacher C., Muller M., Hofer M.J., Getts D.R., Zabaras R., Ousman S.S., Terenzi F., Sen G.C., King N.J., Campbell I.L. Coordinated regulation and widespread cellular expression of interferon-stimulated genes (ISG) ISG-49, ISG-54, and ISG-56 in the central nervous system after infection with distinct viruses. J Virol. 2007;81:860–871. doi: 10.1128/JVI.01167-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butchi N.B., Hinton D.R., Stohlman S.A., Kapil P., Fensterl V., Sen G.C., Bergmann C.C. Ifit2 deficiency results in uncontrolled neurotropic coronavirus replication and enhanced encephalitis via impaired alpha/beta interferon induction in macrophages. J Virol. 2014;88:1051–1064. doi: 10.1128/JVI.02272-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swanson P.A., 2nd, McGavern D.B. Viral diseases of the central nervous system. Curr Opin Virol. 2015;11C:44–54. doi: 10.1016/j.coviro.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khorooshi R., Owens T. Injury-induced type I IFN signaling regulates inflammatory responses in the central nervous system. J Immunol. 2010;185:1258–1264. doi: 10.4049/jimmunol.0901753. [DOI] [PubMed] [Google Scholar]
- 40•.Prinz M., Schmidt H., Mildner A., Knobeloch K.P., Hanisch U.K., Raasch J., Merkler D., Detje C., Gutcher I., Mages J. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity. 2008;28:675–686. doi: 10.1016/j.immuni.2008.03.011. [DOI] [PubMed] [Google Scholar]; This study shows that mice lacking IFNAR in myeloid cells show a more severe EAE phenotype, suggesting a distinct protective function of type I IFNs during CNS autoimmunity.
- 41•.Kocur M., Schneider R., Pulm A.K., Bauer J., Kropp S., Gliem M., Ingwersen J., Goebels N., Alferink J., Prozorovski T. IFNbeta secreted by microglia mediates clearance of myelin debris in CNS autoimmunity. Acta Neuropathol Commun. 2015;3:20. doi: 10.1186/s40478-015-0192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; The presented data show that during the course of EAE, microglia produce IFNβ, which is mandatory for an efficient clearance of myelin debris.
- 42.Traugott U., Lebon P. Demonstration of alpha, beta, and gamma interferon in active chronic multiple sclerosis lesions. Ann N Y Acad Sci. 1988;540:309–311. doi: 10.1111/j.1749-6632.1988.tb27083.x. [DOI] [PubMed] [Google Scholar]
- 43.Teige I., Treschow A., Teige A., Mattsson R., Navikas V., Leanderson T., Holmdahl R., Issazadeh-Navikas S. IFN-beta gene deletion leads to augmented and chronic demyelinating experimental autoimmune encephalomyelitis. J Immunol. 2003;170:4776–4784. doi: 10.4049/jimmunol.170.9.4776. [DOI] [PubMed] [Google Scholar]
- 44.Hosmane S., Tegenge M.A., Rajbhandari L., Uapinyoying P., Kumar N.G., Thakor N., Venkatesan A. Toll/interleukin-1 receptor domain-containing adapter inducing interferon-beta mediates microglial phagocytosis of degenerating axons. J Neurosci. 2012;32:7745–7757. doi: 10.1523/JNEUROSCI.0203-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Zhang X., Bogunovic D., Payelle-Brogard B., Francois-Newton V., Speer S.D., Yuan C., Volpi S., Li Z., Sanal O., Mansouri D. Human intracellular ISG15 prevents interferon-alpha/beta over-amplification and auto-inflammation. Nature. 2015;517:89–93. doi: 10.1038/nature13801. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this publication, the authors provide evidence, that ISG15-deficient patients suffer from enhanced IFNα/β immunity, resembling AGS.
- 46.Crow Y.J., Rehwinkel J. Aicardi-Goutieres syndrome and related phenotypes: linking nucleic acid metabolism with autoimmunity. Hum Mol Genet. 2009;18:R130–R136. doi: 10.1093/hmg/ddp293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goutieres F. Aicardi-Goutieres syndrome. Brain Dev. 2005;27:201–206. doi: 10.1016/j.braindev.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 48.Gray F., Scaravilli F., Everall I., Chretien F., An S., Boche D., Adle-Biassette H., Wingertsmann L., Durigon M., Hurtrel B. Neuropathology of early HIV-1 infection. Brain Pathol. 1996;6:1–15. doi: 10.1111/j.1750-3639.1996.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 49.Petito C.K., Adkins B., McCarthy M., Roberts B., Khamis I. CD4+ and CD8+ cells accumulate in the brains of acquired immunodeficiency syndrome patients with human immunodeficiency virus encephalitis. J Neurovirol. 2003;9:36–44. doi: 10.1080/13550280390173391. [DOI] [PubMed] [Google Scholar]
- 50.Yona S., Kim K.W., Wolf Y., Mildner A., Varol D., Breker M., Strauss-Ayali D., Viukov S., Guilliams M., Misharin A. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]