Graphical abstract
.
Highlights
► Cu2+/TiO2-coated cordierite foam was developed. ► Cu2+/TiO2 cordierite foam has antiviral activity. ► High antiviral activity with Cu2+/TiO2-coated cordierite foam than TiO2-coated foam. ► Application of the methodology for the evaluation of antibacterial to antiviral.
Abbreviations: EDS, energy dispersive X-ray spectroscopy; SEM, scanning electron microscopy; TiO2, titanium dioxide; UV, ultraviolet
Keywords: Bacteriophages, Photocatalysis, Cu2+, TiO2, Air cleaning filters
Abstract
We investigated the antiviral activity of TiO2-coated cordierite foam used in air cleaners, as well as the evaluation methodology. Furthermore, we developed Cu2+/TiO2-coated cordierite foam and investigated the reduction in viral infection ratio. The method for evaluation of antibacterial activity of TiO2-coated cordierite foam could also be applied to evaluation of antiviral activity. We showed that Cu2+/TiO2-coated cordierite foam reduced the viral infection ratio to a greater extent than TiO2-coated cordierite foam. Our findings suggest that the infection risk by polluted air could be decreased using Cu2+/TiO2-coated cordierite foam in air cleaners.
1. Introduction
In 2009, a new type of influenza virus, pandemic H1N1, spread around the world [1]. Serious health problems due to other airborne diseases, such as severe acute respiratory syndrome, have also occurred in the past [2]. Furthermore, bacteria have become increasingly resistant to drug treatment, as in the examples of the multidrug-resistant bacteria Pseudomonas aeruginosa and Acinetobacter baumannii [3], [4]. These infectious diseases are a threat to human health, and indeed, outbreaks and serious clinical cases have occurred. Therefore, new antiviral and antibacterial materials or methods are urgently required.
Three infection pathways of viruses and bacteria have been defined: contact, droplets, and airborne transmission [5], [6], [7]. Contact transmission is caused by direct or indirect contact with polluted fomites. Droplet transmission occurs by direct sprays from coughing or sneezing by infected patients. Airborne transmission is spread across considerable distances in the form of polluted droplet nuclei. For the effective reduction of viral infection ratio, inhibition of these three pathways is needed.
Titanium dioxide (TiO2) is an attractive material for the reduction of viral and bacterial infection ratios. TiO2 undergoes strong oxidization under ultraviolet (UV) irradiation, [8] which can inactivate bacteria and viruses [9], [10], [11], [12], [13], [14], [15], [16]. Photocatalysis using TiO2 is effective in the elimination of toxic substances in water and air [17], [18], [19]. The photocatalysis of TiO2 combined with other metals or ions has been investigated and developed, and combined photocatalysts have been shown to have stronger photocatalytic, antiviral and antibacterial activities compared with those of TiO2 alone [15], [20]. Therefore, a photocatalytic reaction by TiO2 alone or in combination with a metal or ion is an attractive approach and could be applied to the elimination of bacteria, viruses, and toxic substances.
The potential for blocking the contact pathway described above through TiO2 photocatalysis has previously been reported, including demonstrations in practice [21], [22]. In contrast, the reduction of infection risk by droplet and airborne transmission pathways using a photocatalytic reaction has not been investigated thoroughly. Recently, we developed TiO2-coated cordierite foam for use in air cleaners [23]. This TiO2-coated cordierite foam has antibacterial activity and achieves the photocatalytic degradation of acetaldehyde. Furthermore, we developed a reliable methodology to confirm the antibacterial properties of TiO2-coated cordierite foam [23]. Therefore, we have been able to show that use of TiO2-coated cordierite foam in air cleaners reduces the risk of bacterial infection by airborne transmission. However, there are no experimental data to support the antiviral activity of TiO2-coated cordierite foam.
We selected copper ion for deposition on TiO2-coated cordierite foam because copper is known to have antibacterial and antiviral activities [24], [25]. Furthermore, copper ion deposited on TiO2 enhances the photocatalytic activity [15], [20]. Antibacterial activity by a combination of Cu and TiO2 has been reported [20], [26], [27]. Sunada et al. [27] have shown that the first stage of bacterial inactivation under weak UV exposure is outer membrane degradation by photocatalytic reaction with Cu2+ ions infiltrating the cells as the second step. Furthermore, Cu2+/TiO2 shows strong decomposition of acetaldehyde [28]. The suggested photocatalytic reaction process is shown as in Fig. 1 [29]. Deposited Cu2+ works as an electron acceptor. Thus, enhanced photocatalytic reaction by deposited Cu2+ is beneficial for removal of many organic pollutants.
Fig. 1.
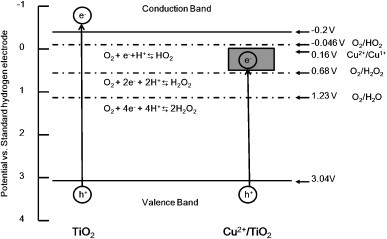
Schema of the suggested photocatalytic reaction process.
In the present study, we investigated the antiviral activity of previously developed TiO2-coated cordierite foam [23]. Furthermore, we developed Cu2+/TiO2-coated cordierite foam, which was expected to have stronger inactivation. As expected, our data showed that Cu2+/TiO2-coated cordierite foam had higher antiviral activity compared with that of TiO2-coated cordierite foam. We also evaluated the method for measuring the antiviral activity of coated cordierite foam.
2. Experimental
2.1. Bacteriophages and plaque assay
Qβ bacteriophage (NBRC 20012), T4 bacteriophage (NBRC 20004), and Escherichia coli (NBRC 13965 and NBRC 13168) were used. Nutrient broth (NB) and NB agar media were purchased from BD Biosciences (Franklin Lakes, NJ, USA). Bacteriophage stock was prepared according to the method reported in our previous study [9]. The titer of bacteriophage was calculated by the double agar layer method.
2.2. Legionella pneumophila and colony counting
L. pneumophila (GTC/GIFU 00296) was purchased from the Department of Microbiology, Regeneration and Advanced Medical Science, Graduate School of Medicine, Gifu University (Gifu, Japan). L. pneumophila was precultured on charcoal yeast extract medium agar with α-ketoglutarate (BD Biosciences) at 37 °C for 72 h. A single colony was selected from the precultured plate and cultured at 37 °C for 72 h. Cultured L. pneumophila was diluted in 1/500 NB to approximately 107 CFU ml−1 and used in experiments.
2.3. Preparation of TiO2-coated cordierite foam deposited with Cu2+ ion
TiO2-coated cordierite foam was prepared according to the method reported in our previous study [23]. Next, TiO2-coated or bare cordierite foam was immersed in 250 μM or 25 mM CuCl2 solution, washed with distilled water, and dried at 120 °C. The amount of Cu2+ coating was 0.8 mg/filter by 250 μM CuCl2 solution (about 1 wt%) and 80 mg/filter by 25 mM CuCl2 solution (about 10 wt%). Chemical elements on the surface of TiO2-coated and Cu2+/TiO2-coated cordierite foam were analyzed by energy dispersive X-ray spectroscopy (EDS). The structure of the surface of Cu2+/TiO2-coated cordierite foam was examined by scanning electron microscopy (SEM).
2.4. Photocatalytic reaction
Photocatalytic inactivation of L. pneumophila was applied as the test method for the evaluation of the antibacterial effect, as in our previous study [23]. For the experiments using bacteriophages, adsorption time and centrifugation conditions were examined using Qβ bacteriophage before the photocatalytic reaction. Cordierite foam samples were immersed in 1 × 109 PFU ml−1 Qβ bacteriophage solution in SM buffer (0.1 M NaCl, 8 mM MgSO4, 50 mM Tris–HCl pH 7.5, and 0.1% gelatin) for 6, 10 or 15 min. Each sample was centrifuged for 30 or 60 s at 500 or 3000 rpm. Bacteriophages in the samples were collected in 20 ml SM buffer by vortexing. The collected bacteriophages were diluted in SM buffer and evaluated by plaque assay using the double layer method.
After an appropriate adsorption time and centrifuge conditions, bacteriophages or L. pneumophila on each cordierite foam were exposed to 0.1 or 0.25 mW cm−2 UV irradiation for 1, 2, 4, 8 and 24 h. After photocatalytic reaction, the bacteriophages were collected and the inactivation ratio was determined by plaque assay. All experiments were repeated more than three times. As a control, bare cordierite foam was used.
3. Results and discussion
3.1. Cu2+/TiO2-coated cordierite foam
We developed Cu2+/TiO2-coated cordierite foam, in which the presence of Cu2+ was confirmed by EDS (Fig. 2 ). Peaks corresponding to elements derived from bare cordierite foam (C, O, Mg, Al, and Si) were detected in TiO2-coated cordierite foam and Cu2+/TiO2-coated cordierite foam. A Ti peak was also detected in both cordierite foams. We speculate that the Ti peaks were due to the inclusion of a major anatase phase and a minor rutile phase, although this was not further investigated in this study. We have confirmed a Ti phase in TiO2-coated cordierite foam in our previous study [23]. Comparison between Fig. 2a and b clearly revealed one difference in the visible peaks: only Cu2+/TiO2-coated cordierite foam had a Cu2+ peak, which was not visible in TiO2-coated cordierite foam. Although we tried to analyze using X-ray diffraction analysis, it was impossible to detect Cu2+ because it was present in low amounts on the filter. Thus, we could confirm that we developed Cu2+/TiO2-coated cordierite foam.
Fig. 2.
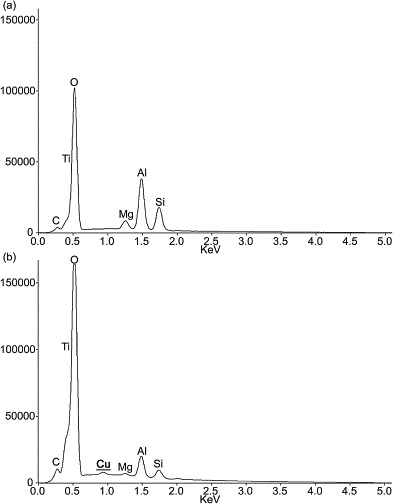
EDS analysis. (a) TiO2-coated cordierite foam; (b) Cu2+/TiO2-coated cordierite foam.
In our previous study, we showed that TiO2-coated cordierite foam has a predominantly smooth surface with some rough areas [23]. Fig. 3 illustrates the SEM images of the surface morphology of Cu2+/TiO2-coated cordierite foam. We observed a smooth surface with some cracks on the surface as seen in TiO2-coated cordierite foam. The photocatalytic reaction using TiO2 thin film results in virus inactivation; therefore, a decrease in viral infection ratio using TiO2-coated and Cu2+/TiO2-coated cordierite foam was also expected.
Fig. 3.

SEM images. Image of Cu2+/TiO2-coated cordierite foam (×500 magnification).
3.2. Antiviral and antibacterial activities by photocatalytic reaction
The cordierite foam has a complex three-dimensional structure; therefore, excess bacteriophage solution must be removed before the photocatalytic reaction. Using Qβ bacteriophages, we investigated the immersion time for bacteriophage adsorption and the centrifugation conditions for the removal of excess bacteriophage solution from the cordierite foam. As shown in Fig. 4a–c, respectively, adsorption time of Qβ bacteriophage (6, 10 and 15 min), centrifuge time (30 and 60 s), and centrifuge speed (500 and 3000 rpm) had no effect on Qβ bacteriophage concentration. Therefore, we established experimental conditions as 10 min for adsorption followed by centrifugation for 30 s at 500 rpm.
Fig. 4.
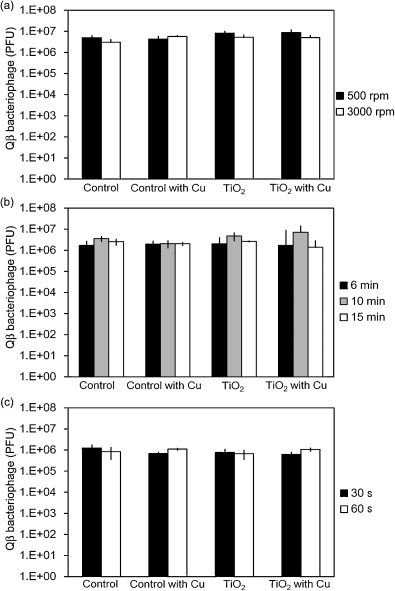
Determination of experimental conditions. (a) adsorption time; (b) centrifugation time; (c) centrifugation speed.
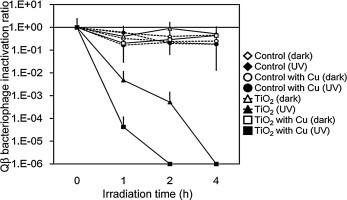
Cordierite foams treated with bacteriophages were exposed to UV irradiation at 0.25 mW cm−2 for 4 h. Bacteriophages were collected and the inactivation ratio was measured (Fig. 5 ). Qβ and T4 bacteriophage (on bare cordierite foam) with or without UV irradiation had no inactivation ratio (white and black diamonds). The bare cordierite foam loaded with Cu2+ only also had no inactivation ratio for both bacteriophages with or without UV irradiation (white triangle and black diamond). TiO2-coated cordierite foam with UV irradiation had an effective time-dependent inactivation ratio for both bacteriophages (black triangles). The inactivation ratio differed between the Qβ and T4 bacteriophages. At 4 h, the infectious activity of Qβ bacteriophage could not be detected, while that of T4 phage could be. Cu2+/TiO2-coated cordierite foam under UV irradiation also inactivated both bacteriophages (black squares). In particular, greater bacteriophage inactivation was observed than that with TiO2-coated cordierite foam under UV irradiation. The Qβ bacteriophages were inactivated to an undetectable level after 2 h irradiation. The T4 bacteriophages were also inactivated to an undetectable level after 4 h irradiation. Using TiO2-coated and Cu2+/TiO2-coated cordierite foam under UV irradiation, the inactivation ratio of the T4 bacteriophages was less than that of the Qβ bacteriophages. We suggest that differences in size and structure between the two bacteriophages were responsible for this difference. Supporting our suggestion, experiments in bacteriophage inactivation using thin films have revealed differences in inactivation ratio between these two bacteriophages [9]. Therefore, photocatalytic inactivation by Cu2+/TiO2-coated cordierite foam also depends on the size and structure of the target bacteriophage.
Fig. 5.

Changes in inactivation ratio of bacteriophages with UV exposure at 0.25 mW cm−2. (a) Qβ bacteriophage; (b) T4 bacteriophage. Points indicate the mean value and standard deviation of three replicate experiments. Concentration of bacteriophage at 0 h under dark conditions was set at 100%.
Bacteriophage inactivation was further confirmed by weak UV irradiation at 0.1 mW cm−2 for up to 8 h. As shown in Fig. 6 , TiO2-coated and Cu2+/TiO2-coated cordierite foam inactivated the Qβ bacteriophages. When the inactivation ratio was compared between UV irradiation at 0.1 and 0.25 mW cm−2 for 4 h, a difference in inactivation by TiO2-coated cordierite foam was observed. The infection activity of Qβ bacteriophages on TiO2-coated cordierite foam could be detected after UV irradiation at 0.1 mW cm−2 but not after UV irradiation at 0.25 mW cm−2. In contrast, the infection activity of Qβ bacteriophages on Cu2+/TiO2-coated cordierite foam could not be detected after UV irradiation at 0.1 mW cm−2 for 4 h. However, we also tested the difference between the amounts of 250 μM and 25 mM Cu2+, but there were no differences between them (Fig. 7 ). This suggested that the deposited range from 1 to 10 wt% Cu2+ was sufficient to inactivate the viruses. Thus, photocatalytic reaction by the deposited Cu2+ has a stronger photocatalytic activation, and therefore, viruses could be inactivated more effectively compared with TiO2-coated cordierite foam.
Fig. 6.
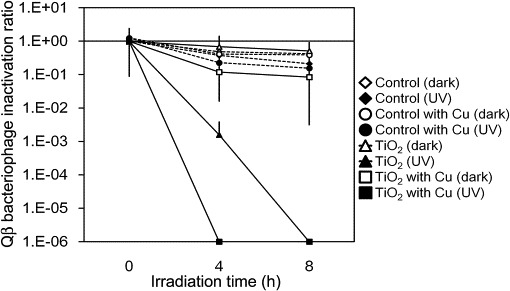
Changes in inactivation ratio of bacteriophages with UV exposure at 0.1 mW cm−2. Points indicate the mean value and standard deviation of three replicate experiments. Concentration of Qβ bacteriophages at 0 h under dark conditions was set at 100%.
Fig. 7.
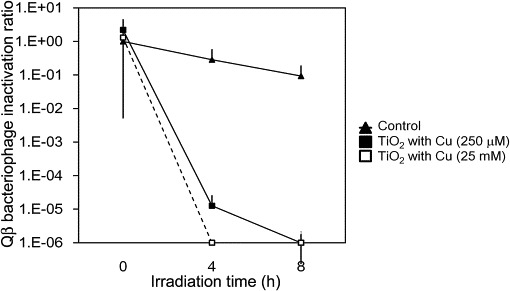
Changes in inactivation ratio of bacteriophages by different Cu2+ concentration. Points indicate the mean value and standard deviation of three replicate experiments. Concentration of Qβ bacteriophages at 0 h under dark conditions was set at 100%.
Finally, we tested antibacterial activity of Cu2+/TiO2-coated cordierite foam using L. pneumophila as a model, and demonstrated antibacterial activity (Fig. 8 ). After 24 h, L. pneumophila was inactivated to an undetectable level. Photocatalytic reactions decompose the outer membrane and cell walls of bacteria; therefore, we suggest that Cu2+/TiO2-coated cordierite foam could also inactivate other bacteria.
Fig. 8.
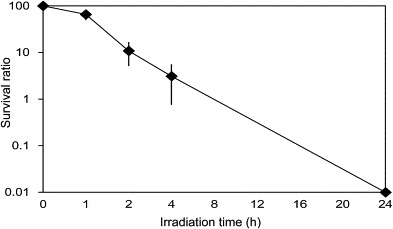
Changes in inactivation of L. pneumophila with UV exposure at 0.25 mW cm−2. Points indicate the mean value and standard deviation of three replicate experiments. Concentration of L. pneumophila at 0 h was set at 100%.
4. Conclusion
We have developed a Cu2+/TiO2-coated cordierite foam that has higher antiviral and antibacterial activities compared with those of TiO2-coated cordierite foam. This foam could therefore be beneficial in decreasing the risk of viral and bacterial infection by use in air and water purification devices.
Acknowledgments
We thank Mr. H. Ando, Mr. N. Kuriyano (Seiwa Kogyo Co. Ltd.), Dr. Y. Hosogi, and Dr. Y. Kuroda (Showa Titanium Co. Ltd.) for assistance in preparation of the foam samples and Ms. T. Sasaki and Ms. Y. Shimizu for help with the experiments. This work was supported by the New Energy and Industrial Technology Development Organization (NEDO) of Japan.
References
- 1.Fraser C., Donnelly C.A., Cauchemez S. Science. 2009;324:1557–1561. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes J.M. Transactions of the American Clinical and Climatological Association. 2004;115:361–372. [PMC free article] [PubMed] [Google Scholar]
- 3.Falagas M.E., Kopterides P. Journal of Hospital Infection. 2006;64:7–15. doi: 10.1016/j.jhin.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Gupta V. Expert Opinion on Investigational Drugs. 2008;17:131–143. doi: 10.1517/13543784.17.2.131. [DOI] [PubMed] [Google Scholar]
- 5.Gralton J., Tovey E., Mclaws M.L., Rawlinson W.D. Journal of Infection. 2011;62:1–13. doi: 10.1016/j.jinf.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber T.P., Stilianakis N.I. Journal of Infection. 2008;57:361–373. doi: 10.1016/j.jinf.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rheinbaben F., Schünemann S., Gross T., Wolff M.H. Journal of Hospital Infection. 2000;46:61–66. doi: 10.1053/jhin.2000.0794. [DOI] [PubMed] [Google Scholar]
- 8.Fujishima A., Zhang X., Tryk D.A. Surface Science Reports. 2008;63:515–582. [Google Scholar]
- 9.Ishiguro H., Nakano R., Yao Y., Kajioka J., Fujishima A., Kubota Y. Photochemical and Photobiological Sciences. 2011;10:1825–1829. doi: 10.1039/c1pp05192j. [DOI] [PubMed] [Google Scholar]
- 10.Matsunaga T., Tomoda R., Nakajima T., Wake H. FEMS Microbiology Letters. 1985;29:211–214. [Google Scholar]
- 11.Matsunaga T., Tomoda R., Nakajima T., Nakamura N., Komine T. Applied and Environment Microbiology. 1988;54:1330–1333. doi: 10.1128/aem.54.6.1330-1333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ireland J.C., Klostermann P., Rice E.W., Clark R.M. Applied and Environment Microbiology. 1993;59:1668–1670. doi: 10.1128/aem.59.5.1668-1670.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sunada K., Kikuchi Y., Hashimoto K., Fujishima A. Environmental Science and Technology. 1998;32:726–728. [Google Scholar]
- 14.Cho M., Chung H., Choi W., Yoon J. Applied and Environment Microbiology. 2005;71:270–275. doi: 10.1128/AEM.71.1.270-275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster H.A., Ditta I.B., Varghese S., Steele A. Applied Microbiology and Biotechnology. 2011;90:1847–1868. doi: 10.1007/s00253-011-3213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei C., Lin W.Y., Zaianl Z., Williams N.E., Zhu K., Kruzic A.P., Smith R.L., Rajeshwar K. Environmental Science and Technology. 1994;28:934–938. doi: 10.1021/es00054a027. [DOI] [PubMed] [Google Scholar]
- 17.Ryu H., Gerrity D., Crittenden J.C., Abbaszadegan M. Water Research. 2008;42:1523–1530. doi: 10.1016/j.watres.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 18.Noguchi T., Fujishima A. Environmental Science and Technology. 1998;32:3831–3833. [Google Scholar]
- 19.Nakashima T., Ohko Y., Kubota Y., Fujishima A. Journal of Photochemistry and Photobiology A: Chemistry. 2003;160:115–120. [Google Scholar]
- 20.Kumar S.G., Devi L.G. Journal of Physical Chemistry A. 2011;115:13211–13241. doi: 10.1021/jp204364a. [DOI] [PubMed] [Google Scholar]
- 21.Yao Y., Ohko Y., Sekiguchi Y., Fujishima A., Kubota Y. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2008;85B:453–460. doi: 10.1002/jbm.b.30965. [DOI] [PubMed] [Google Scholar]
- 22.Ohko Y., Utsumi Y., Niwa C., Tatsuma T., Kobayakawa K., Satoh Y., Kubota Y., Fujishima A. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2001;58:97–101. doi: 10.1002/1097-4636(2001)58:1<97::aid-jbm140>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Yao Y., Ochiai T., Ishiguro H., Nakano R., Kubota Y. Applied Catalysis B: Environmental. 2011;106:592–599. doi: 10.1016/j.apcatb.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grass G., Rensing C., Solioz M. Applied and Environment Microbiology. 2011;77:1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J., Dennehy J.J. Applied and Environment Microbiology. 2011;77:6878–6883. doi: 10.1128/AEM.05661-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ditta I.B., Steele A., Liptrot C., Tobin J., Tyler H., Yates H.M., Sheel D.W., Foster H.A. Applied Microbial and Cell Physiology. 2008;79:127–133. doi: 10.1007/s00253-008-1411-8. [DOI] [PubMed] [Google Scholar]
- 27.Sunada K., Watanabe T., Hashimoto K. Environmental Science and Technology. 2003;37:4785–4789. doi: 10.1021/es034106g. [DOI] [PubMed] [Google Scholar]
- 28.Murakami N., Chiyoya T., Tsubota T., Ohno T. Applied Catalysis A-General. 2008;348:148–152. [Google Scholar]
- 29.Irie H., Kamiya H.K., Shibanuma T., Miura S., Tryk D.A., Yokoyama T., Hashimoto K. Journal of Physical Chemistry C. 2009;113:10761–10766. [Google Scholar]


