Epidemiology is the study of determinants of health, disease, and productivity in populations of humans, plants, or animals. Epidemiologic studies often use field observations, laboratory tests for diagnosis of disease, measures of productivity, statistical or mathematic analyses, and other quantitative methods. Molecular methods are another set of tools that can be used in epidemiologic studies. Molecular epidemiology is the study of distribution and determinants of health and disease through the use of molecular biology methods [1]. The term molecular epidemiology is sometimes used for studies in molecular taxonomy and phylogeny. Although these scientific disciplines use molecular techniques and provide valuable tools for use in epidemiologic studies, they focus on the classification of organisms into naturally related groups and the study of the line of evolutionary descent rather than the study of determinants of disease and disease transmission [1]. Use of the misnomer “molecular epidemiology” for taxonomic and phylogenetic studies has given rise to the misconception that molecular epidemiology is not epidemiology in the true sense of the word, earning molecular epidemiology the dubious nickname “stamp collection.” The goal of molecular epidemiology, however, is not merely to classify organisms into taxonomic or phylogenetic groups but to “identify the microparasites responsible for infectious diseases and determine their physical sources, their biological relationships, and their route of transmission and those of the genes responsible for their virulence, vaccine-relevant antigens and drug resistance” [2].
Applications of molecular epidemiology in human health care, veterinary medicine, and food safety are manifold. Some applications and examples are listed in Table 1. Several reviews address applications of molecular epidemiology in specific disciplines or to specific organisms or virulence characteristics. Examples include reviews of molecular epidemiology as pertaining to foodborne pathogens [3], parasitology [4], virology [5], [6], mycobacteria [7], foot-and-mouth disease [8], Theileria parva [9], Giardia [10], antimicrobial resistance [11], and endemic infections [12]. This anthology is by no means exhaustive and is only meant to give the reader an idea of the range of fields in which molecular epidemiology is used and to give suggestions for additional reading. In the remainder of this article, most attention is given to the molecular epidemiology of bacterial infections, although examples from virology and other disciplines are included. First, a brief overview of terminology and molecular methodology is provided, followed by examples of applications of molecular epidemiology in veterinary medicine.
Table 1.
Examples of applications of molecular epidemiology in veterinary medicine
| Application | Example | Reference |
|---|---|---|
| Determination of the dynamics of disease transmission in geographically widespread areas | Global spread of foot-and-mouth disease; spread of Newcastle disease virus in Asia | [8], [38] |
| Distinction between pathovars and nonpathovars | Pathogenic and nonpathogenic Escherichia coli in petting zoos | [47] |
| Addressing hospital and institutional infectious disease problems | Methicillin-resistant Staphylococcus aureus in veterinary teaching hospitals | [120] |
| Identification of genetic determinants of disease and disease transmission | Lineage-specific pathogenicity of Listeria monocytogenes in humans and ruminants | [35] |
| Confirmation of epidemiologically suspected transmission | Transmission of Staphylococcus aureus mastitis by flies | [121] |
| Detection of epidemiologically unsuspected outbreaks | Multiresistant Salmonella in animals and humans | [122] |
| Support for mathematic modeling | Streptococcus uberis mastitis outbreak; local spread of Campylobacter spp | [63], [64], [67], [123] |
| Identification of risk factors and environments where transmission occurs | Mycobacterium bovis control schemes | [7] |
| Challenging of accepted dogmas | Origin of high bacteria counts in bulk tank milk | [55] |
| Identification of sources and reservoirs | Staphylococcus aureus in milk processing plants | [124] |
| Differentiation between persistence and reintroduction | Recurrent episodes of clinical E coli mastitis | [77] |
| Development of future control strategies | Identification of vaccine candidates | [113] |
| Host adaptation of strains | Human and bovine Streptococcus agalactiae | [92] |
| Differentiation between zoonotic, waterborne, and anthroponotic transmission | Cryptosporidium in cattle and humans; Giardia in humans, livestock, and pets | [10], [125] |
Molecular terminology
Internationally, attempts have been made to use standard definitions of a few key terms. These definitions are adhered to in this article and are explained in the following paragraphs. The reader should be aware that not all investigators and journals use the same definitions. In particular, the word “strain” is often used as a synonym for “isolate.” When using, discussing, or reading results from molecular epidemiologic studies, it is important that all parties have the same understanding of what is meant by these terms. The definitions of isolate, strain, and clone used in this article are in line with those used by the Molecular Typing Working Group of the Society for Healthcare Epidemiology of America [13], the European Study Group on Epidemiological Markers [14], and the recent book, Molecular Epidemiology of Infectious Diseases, published by the American Society for Microbiology [1].
An isolate is a population of microbial cells in pure culture derived from a single colony on an isolation plate and identified to the species level. For example, when Staphylococcus aureus is obtained from a milk sample, and a pure subculture from one colony of Staphylococcus aureus is used for storage or further study, this would be referred to as an isolate. A strain is an isolate or a group of isolates exhibiting characteristics that set it apart from other isolates belonging to the same species. For example, isolates belonging to the species Staphylococcus aureus can be subdivided into penicillin-sensitive and penicillin-resistant strains. This distinction can be made with phenotypic methods (expression of visible characteristics, such as growth on Mueller-Hinton agar with antibiotic-impregnated discs) or with genotypic methods (DNA-based methods, such as detection of a penicillin-resistance gene). Penicillin resistance is a determinant of health and disease because cows with mastitis caused by penicillin-resistant isolates are less likely to cure in response to antibiotic treatment than cows with penicillin-sensitive isolates, even when treatment choices are based on the antimicrobial sensitivity of the isolate [15]. A clone is the progeny of a common ancestor and the result of a direct chain of replication of that ancestor. Identification of clones is based on the monitoring of multiple genetic markers of sufficient discriminatory power. In human medicine, many infections with methicillin-resistant Staphylococcus aureus are caused by a limited number of clones that have spread internationally. For example, there is an Iberian clone and a New York clone of methicillin-resistant Staphylococcus aureus [16]. The terms isolate, strain, and clone form a hierarchic series (ie, our knowledge of the organism's characteristics is increasingly detailed at every step in the series) ( Fig. 1). For some organisms, descriptive groupings are used that are based on their geographic origin or pathogenicity. Examples of such descriptive groupings include topotypes (for groups of foot-and-mouth disease viruses that are genetically and geographically related) [8], and pathovars (pathogenic variants of an organism) [1].
Fig. 1.
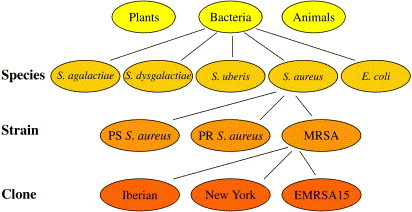
Hierarchic ordering of species, strains, and clones of bacteria. Note that an isolate can belong to a species, a strain, or a clone depending on how much is known about its phenotype and genotype. PS, penicillin sensitive; PR, penicillin resistant; MRSA, methicillin-resistant Staphylococcus aureus; EMRSA15, epidemic MRSA clone number 15.
When discussing molecular epidemiology or strain typing with producers or others, it can be helpful to compare the concept of strains within infectious organisms with the concept of breeds or cultivars within animal or plant species. Within the species “sheep,” we can distinguish breeds kept for milk production, for meat, or for fiber. The animals have enough in common to belong to the same species but they have enough differences to belong to different breeds. Similarly, bacteria may have enough in common to belong to the same species, say Escherichia coli, yet they may have enough differences to belong to different strains. Strains of E coli that produce shigalike toxins, such as E coli O157:H7, have very different characteristics than strains that do not produce these toxins. The toxins are a determinant of health and disease, and the response to detection of E coli O157:H7 in well water on a farm would be very different than the response to detection of a non–shiga toxin-producing strain of E coli. A major difference between the breed concept and the strain concept is that breeds are well defined through breed standards and breeding organizations, whereas no such standardization exists for the naming of microbial strains. Some naming systems allow for a degree of standardization, such as the identification of E coli by presence of shiga toxins, the identification of Salmonella DT104 by means of phage typing, or the use of DNA sequenced–based strain typing methods. Many strain typing systems, however, are not universally meaningful.
Typing methods that are not universally meaningful are called comparative typing methods. They can be used to study organisms within a defined context. An example of such a context is the comparison of Staphylococcus aureus isolates within herds (eg, from teat skin and from milk). This comparison allows us to determine whether skin and milk within these herds harbor the same or different strains of Staphylococcus aureus [17]. When a milk sample is submitted to a diagnostic laboratory and found to contain Staphylococcus aureus, however, there is no possibility of determining whether this particular isolate belongs to a skin strain or a milk strain using the same methodology. Do to so, a library typing method is needed. Library typing methods are methods that generate results with universal meaning, irrespective of when, where, or by whom the results were generated. Using a library typing method, it should be possible to identify an isolate from a single milk sample as belonging to a skin strain or a milk strain of Staphylococcus aureus. Indeed, it is now possible to do this using multilocus sequence typing (MLST), which is a library typing method based on DNA sequencing [18]. Comparative typing methods and library typing methods have a place in veterinary molecular epidemiology, and examples of applications of both types of methods are provided throughout this article.
Diagnostic tests are characterized by their sensitivity and specificity and by practical aspects such as cost, ease of use, and turn-around times. Cost, ease of use, and turn-around times also play a role in selection of suitable molecular tools in epidemiology. Other important characteristics of molecular methods are typeability, discriminatory power, reproducibility, and concordance [14], [19]. Typeability is the proportion of isolates that are assigned a type by the typing system [14]. Some techniques that were developed for typing of human pathogens do not work well for typing of animal isolates belonging to the same pathogen species. For example, serotyping is commonly used to classify Streptococcus agalactiae in humans, but it does not work well for bovine isolates of Streptococcus agalactiae [20]. Similarly, a proportion of bovine strains of Staphylococcus aureus are not typeable using phages developed for typing of human Staphylococcus aureus [21]. Reproducibility is the ability of the test to generate the same results every time that the test is applied to an isolate. Streptococci and enterococci can be speciated based on combinations of multiple phenotypic characteristics. Commercial test systems based on this principle are on the market and are used in veterinary diagnostic laboratories. Some of these systems produce results that are not reproducible. For example, an isolate that is identified as Enterococcus faecium the first time it is tested may be identified as Enterococcus faecalis at other times [22]. Discriminatory power is the ability of a method to differentiate between strains. The discriminatory power can be quantified using Simpson's Index of Discrimination, which is the probability that the typing system will assign a different type to two strains that are not related [23]. The outcome of molecular epidemiologic studies can be highly dependent on the discriminatory power of the typing method that is used. Comparisons of skin and milk isolates of Staphylococcus aureus were initially performed using phenotyping. Phenotyping did not differentiate between isolates from skin and milk, and it was concluded that bovine teat skin was an important reservoir of Staphylococcus aureus causing intramammary infections [24]. When the same isolates were re-examined with more discriminatory genotypic methods, it became apparent that the isolates did not belong to the same strains, and that teat skin is not as important as a source of intramammary Staphylococcus aureus as initially thought [17]. The discriminatory power of phenotypic methods can be improved by addition of more biochemical tests, phages, antibodies, and so forth, or through a change in interpretation criteria for test results. Such an increase in discriminatory power is often accompanied by a decrease in reproducibility of results [25], and a balance between the two characteristics may need to be struck, similar to the balance between sensitivity and specificity of diagnostic methods. Concordance can be assessed in two ways. Typing system concordance is a taxonomic interpretation of the concordance concept. It is the agreement between results of two independent typing systems. One could think of it as a kind of kappa-statistic for typing methods. Epidemiologic concordance is a purely epidemiologic concept and refers to the ability of a typing method to identify strains in agreement with the epidemiologic origin of the isolates. When a new typing method is developed, the epidemiologic origin of the isolates is the “gold standard” for evaluation of the typing system [14]. After the typing technique has been validated, it can subsequently be used to investigate epidemiologic questions.
By and large, phenotypic tests have lower typeability, reproducibility, and discriminatory power than genotypic methods, although exceptions to that rule certainly exist [1]. The popularity of phenotypic methods stems from their low cost, ease of use, and short turn-around time, which makes them the method of choice for many diagnostic applications. The term molecular methods is commonly used to refer to techniques that rely on the characterization of an organism according to its genetic material (ie, genotypic method) [1]. In this article, the same interpretation of molecular methods is used and the focus is henceforth on genotypic characterization of microorganisms.
Molecular methods
It would require more than a whole textbook to introduce all of the genotypic methods currently in use in molecular epidemiology, and such a textbook would be outdated before it could be published. A short introduction to some widely used techniques and the “alphabet soup” of acronyms used for molecular methods is given here to facilitate reading of this and other texts. More comprehensive introductions to conventional and molecular techniques used in molecular epidemiology can be found elsewhere [1], [26].
Most comparative genotyping methods are based on modifications of two principles: the “cutting” (restriction) of specific points in the DNA by means of restriction enzymes or the amplification of specific parts of DNA by means of polymerase chain reaction (PCR). The DNA fragments that are obtained through amplification, restriction, or combinations thereof have a negative charge and will move through an electrical field, with small pieces of DNA moving faster than large pieces, allowing for separation of the fragments. The separated fragments appear as a banding pattern or “DNA fingerprint.” Because it is difficult to standardize sample processing and gel electrophoresis completely, slight variations in patterns between runs or even within runs are difficult to avoid. As a result, comparison of isolates that are run on the same gel or in the same laboratory is useful, giving rise to the name “comparative methods,” whereas comparison of results between laboratories, studies, or countries is difficult. Even within studies, pattern interpretation can be debatable. One article that claims environmental transmission of E coli O157:H7 from a cow to a pasture and then to children shows riboprinting results as evidence that the cow shed the strain with which the children became infected [27]. Although these investigators interpret banding patterns as being the same, the authors interpret them as being different and as evidence that the cow was not the source of infection. This example demonstrates the ambiguity of banding pattern based strain characterization and the potential for conflicting interpretations. To overcome difficulties with interpretation of banding patterns generated by comparative methods, standard protocols for strain characterization have been developed and implemented (eg, by the Centers for Disease Control and Prevention) [28].
Some commonly used comparative methods that are based on restriction (cutting) of DNA include restriction enzyme analysis (REA) or random fragment length polymorphism (RFLP), pulsed-field gel electrophoresis (PFGE), and ribotyping. In REA, the microbial genome is digested with a restriction enzyme, and DNA fragments of different lengths are visible on a gel after electrophoresis. The term RFLP refers to the fact that there is polymorphism (differences between isolates) in the size and number of fragments that are generated. The number of fragments generated by REA is often so large that it can be difficult to interpret results. This problem is overcome in PFGE by the use of restriction enzymes that cut less frequently. As a result, fewer and larger DNA fragments are generated ( Fig. 2). DNA fragments generated by PFGE are so large that they barely move through a gel unless their ability to move is enhanced by use of specialized equipment that uses changing electric fields or pulsed fields. The need for specialized equipment and training limits the availability of this method for diagnostic laboratories. Specialized equipment is also needed for automated ribotyping. Ribotyping is based on the enzymatic digestion of DNA, followed by capture of the digested fragments on a membrane, and detection of the fragments through hybridization of DNA probes to ribosomal genes in the fragments. The method can be used with or without automation. Automation and use of prefabricated reagents reduces run-to-run variability and contributes to ease of use and short turn-around times. Ribotyping increases standardization of typing results, allowing for comparison of typing results to libraries of banding patterns ( Fig. 3). It also increases the cost of strain typing. As a very rough rule of thumb, one could say that “you get what you pay for” with strain typing. Generally, higher discriminatory power, speed, standardization, and ease of use are associated with a higher cost. All of these factors, the availability of equipment and trained personnel, and most important, the epidemiologic question determine the suitability of a typing technique for any given situation [29], [30].
Fig. 2.
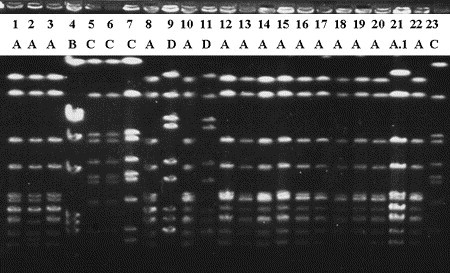
Example of PFGE typing results. Staphylococcus aureus from bulk tank milk (lanes 18 and 19) and quarter milk samples. Numbers and letters indicate sample and strain assignment, respectively. Samples 1 through 8 originate from herd I, samples 9 through 20 from herd II, and samples 21, 22, and 23 from herds III, IV, and V, respectively. Variability in intensity of banding patterns can be seen for strain A (eg, lane 18 is weaker than others) and for strain C (lane 7 is stronger than others). Imperfect standardization of band intensity may affect reproducibility of results. (Adapted from Zadoks R, van Leeuwen W, Barkema H, et al. Application of pulsed-field gel electrophoresis and binary typing as tools in veterinary clinical microbiology and molecular epidemiologic analysis of bovine and human Staphylococcus aureus isolates. J Clin Microbiol 2000;38(5):1933; with permission.)
Fig. 3.
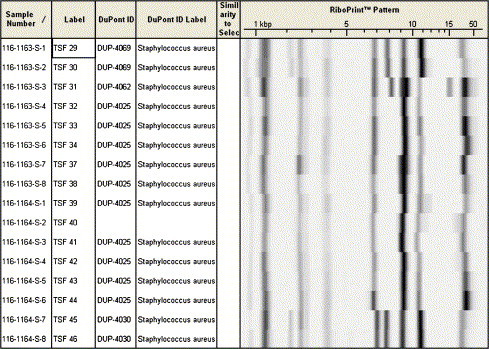
Example of ribotyping results. Staphylococcus aureus from dairy cows. Through comparison to the reference database of the Riboprinter Microbial Identification System (DuPont Qualicon, Wilmington, Delaware), species and strain identification is possible. Four strains were identified in this set of samples (DUP-4025, -4030, -4062, and -4069).
Many PCR-based methods are used in molecular epidemiology for DNA fingerprinting and in other applications. PCR can be used for diagnostic or epidemiologic applications and it is the goal and the context rather than the method that makes it a molecular diagnostic tool or a molecular epidemiologic tool. The primers used in PCR determine which complementary sequence in the target DNA is recognized and, hence, what characteristic is detected or which product is generated. The primers can be specific for bacterial species such as Salmonella or Listeria monocytogenes in milk or beef [31], [32]. Primers can also be selected to detect virulence genes or antimicrobial resistance genes, which may or may not be species specific. PCR can be used to generate amplicons (copies of DNA fragments) for subsequent DNA sequencing. This process is the starting point for MLST and can be used to track horizontal transmission of antimicrobial resistance genes [33] or other virulence genes [34]. A plethora of PCR-based strain typing methods exists, ranging from random amplified polymorphic DNA (RAPD) typing ( Fig. 4), which is highly versatile and can be used for nearly all known bacterial, fungal, viral, and parasitic pathogens, to insertion element PCR assays that are used only for typing of a specific bacterial subspecies (eg, IS900 typing of Mycobacterium avium subsp paratuberculosis [MAP]) [1]. PCR can be performed with one set of primers at a time, also known as uniplex PCR, or with multiple primer sets in one reaction vial, known as multiplex PCR. PCR can be followed by gel electrophoresis for detection of PCR products or take place in “real time,” that is, with detection of PCR products while they are being generated in the PCR machine, usually by means of a fluorescence-based method. Restriction methods can be combined with PCR methods. PCR can be the first step, followed by restriction of the amplified product, as in hemolysin typing of Listeria monocytogenes [35] or PCR-RFLP of Cryptosporidium parvum [36]. Alternatively, restriction can be followed by PCR, as in amplified fragment length polymorphism typing. In this last method, the ends of the fragments that are generated by restriction (similar to RFLP) are recognized by PCR primers that subsequently amplify the fragments, resulting in a higher sensitivity of detection [1].
Fig. 4.
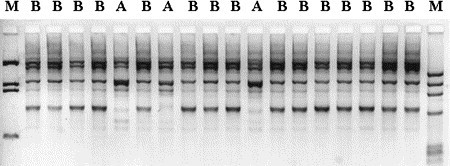
Example of RAPD PCR. Streptococcus uberis isolates from a bovine dairy herd collected during an outbreak of mastitis. Two strains (A and B) were identified among samples from 10 cows. M, molecular marker.
DNA sequencing has been used for more than a decade to study the molecular epidemiology of viral infections. For RNA viruses, use of reverse transcriptase PCR (RT-PCR; not to be confused with real-time PCR, which is also abbreviated RT-PCR) may be necessary. In this process, viral RNA is reverse transcribed into copy-DNA (cDNA), which is subsequently amplified by PCR. The method is used to trace the geographic origin of FMD outbreaks in cattle [37] and Newcastle disease (NCD) in poultry [38]; to determine the transmission potential of avian influenza viruses that jump the species barrier from poultry to humans, and to identify the origin of newly emerged or emerging viruses including HIV and severe acute respiratory syndrome [6]. In molecular epidemiology of bacterial diseases, the use of DNA sequencing was popularized by the introduction of MLST in the late 1990s. With MLST, isolates are identified by sequencing of multiple genes or loci in the organism's DNA ( Fig. 5). In a narrow sense, MLST has been defined as the sequencing of 450 to 500 base pair fragments of seven housekeeping genes [39]. Housekeeping genes encode essential cell functions. Their DNA sequence is mostly highly conserved because any change in DNA or in the proteins encoded by the gene's DNA might be detrimental to the cell's survival. In a broader sense, MLST comprises any method that identifies strains based on sequencing of any type or number of loci, including virulence genes [34], [40], hypervariable genes [41], and stress-response genes [42]. The DNA sequence of such genes is not highly conserved. On the contrary, changes in the DNA of virulence genes and in the proteins encoded by these genes may help an organism to adapt to adverse circumstances and may thus contribute to its survival. MLST is a library typing method, irrespective of the kind of gene that is included in the typing scheme. Databases with unambiguous strain typing results are accessible on-line (eg, http://www.mlst.net/ and http://pubmlst.org/) for a number of bacterial species [43]. The combination of MLST or other strain typing methods with the sequencing of virulence factor genes or antimicrobial resistance genes allows for the study of evolution of pathogenic strains (eg, shiga-toxin producing E coli [STEC]) [34] and the monitoring of the spread of antimicrobial resistance (eg, Salmonella) [33]. DNA sequencing of housekeeping genes can be used for species identification, especially when phenotypic methods for species identification are not fully reliable, as for example, in the case of enterococci of animal origin [44].
Fig. 5.
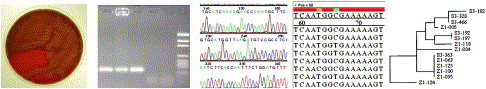
Producing and using DNA sequence data. Bacteria are grown in pure culture (left) to generate material for DNA isolation. Target DNA is amplified by PCR and detected (in this case) using gel electrophoresis (second from left). DNA is sequenced using a fluorescence-based detection system that generates electopherograms (center). Sequence data are aligned so that similarities (shown in red) and dissimilarities (shown in other colors) become visible (second from right). Dendrograms display the extent of similarity between sequences (right). This information can be used for species identification, MLST, and other applications.
Source tracing
Source tracking or tracing is no doubt one of the most common applications of molecular epidemiologic methods in veterinary medicine, food safety, and public health. Much of the current emphasis on on-farm food safety (eg, efforts to control E coli O157:H7 in feedlots) is due to the fact that molecular epidemiologic tools incriminated live animals as the primary sources of foodborne pathogens. Source tracing can be helpful at many levels, ranging from the detection of on-farm sources of infection or contamination to tracing of multistate or multinational outbreaks of foodborne or animal disease. It can be used to trace animal-to-human or animal-to-animal transmission of pathogens, to monitor spread of antimicrobial resistance determinants in pathogens and commensals of animals and people, to detect sources of pathogens or contaminants in animal products, and to detect products or environmental niches that may act as a source of infection for animals. It is beyond the scope of this article to summarize the wealth of studies on source tracing of foodborne pathogens and antimicrobial resistance determinants. Reviews of both topics have recently been published elsewhere [11], [45], [46], [47]. The authors focus on examples of source tracing that veterinarians may encounter in large animal practice.
For clinicians, it is important to be aware of the possibility of on-farm animal-to-human transmission of disease, through direct or indirect contact. Veterinarians, family members, farm workers, and visitors to a farm may be at risk. When disease is diagnosed in farm animals, with a possibility of zoonotic pathogens playing a role, herd managers must be alerted so that preventive measures can be taken. Keep in mind that healthy animals may carry and shed foodborne pathogens too, posing a risk that is even harder to identify. Healthy goats and sheep in petting zoos have caused E coli O157:H7 disease in children on numerous occasions, as proved by epidemiologic investigations and PFGE typing of isolates [47], [48]. Healthy dairy cows have also caused infections in children. A 16-month-old girl on a dairy farm in Ontario contracted E coli O157:H7 although she had not been in contact with the cattle and had not consumed raw milk. Molecular investigations showed that cattle, well water, and the child were contaminated or infected with the same strain of E coli O157:H7. Subsequent hydrogeologic investigation revealed that the design and location of the well allowed manure-contaminated surface water to flow into the well [49]. In Pennsylvania, a class of school children developed diarrhea after a visit to a dairy and petting farm. Fifty-one patients were confirmed with or suspected of E coli O157:H7 and 8 developed hemolytic uremic syndrome [50]. The opening of farms to visitors may help people from nonagricultural communities to develop more appreciation for farming but when visits result in disease, the good intentions may backfire. Hemolytic uremic syndrome can also be associated with enterohemorrhagic E coli other than O157:H7 (eg, E coli O26:H−). Two children from different families came down with the disease. The families had stayed at the same hotel, and both children had consumed raw cow's milk. The strain that caused hemolytic uremic syndrome in the children was identified in cows on the farm that supplied the raw milk [51]. It is not just E coli that we need to worry about. Children attending a farm day camp in Minnesota contracted numerous animalborne infections, including Cryptosporidium parvum, a variety of STECs, Salmonella, and Campylobacter jejuni. PFGE of STECS and PCR-RFLP of Cryptosporidium parvum confirmed that calves that were bottle-fed by children shed the strains that infected the children. The risk of infection was increased for children that cared for a sick calf, failed to wash hands after calf contact, or had visible manure present on their hands [36].
Raw milk can be a source of numerous foodborne pathogens. Molecular epidemiologic studies have identified raw milk as the source of several outbreaks of disease, including disease due to Campylobacter jejuni in Wisconsin [52] and Salmonella enterica serotype Typhimurium in Illinois, Indiana, Ohio, and Tennessee [53]. Especially for a multistate outbreak, it would have been difficult to confirm a common origin without the use of molecular methods. The list gets much longer when raw milk cheeses or other raw milk products are included. A nice example (from an epidemiologic perspective) is an outbreak of Salmonella enterica serotype Typhimurium DT104 in Yakima County in Washington State, where investigations resulted in identification of the cause of the problem, and subsequent measures to prevent repeat occurrences were taken. This outbreak was associated with consumption of queso fresco (fresh cheese) made from raw milk, a traditional food in the Hispanic diet, and sparked an intervention in the human population. A pasteurized-milk queso fresco recipe with taste and texture acceptable to the Hispanic community was developed. Trained Hispanic volunteers conducted safe-cheese workshops, which were attended by more than 225 persons. Workshop participants' acceptance of the new recipe was excellent, and positive behavior changes were maintained over 6 months [54].
Strain typing is not only used to identify sources of foodborne pathogens. It can also be used to trace the source of nonpathogenic bacteria contaminating a product. Recently, the authors worked on a case of Lactobacillus contamination of a dairy product that compromised product quality. A specific farm was thought to be the source of the contaminant, and shipment of raw milk from the farm to the plant was suspended. A combination of selective culture methods, DNA sequencing (following the process outlined in Fig. 5) and automated ribotyping was used to determine the presence of Lactobacillus in raw milk from the suspected farm and other farms and to compare isolates from raw milk to those from processed product. Other farms were included because detection of the product strain in raw milk of the suspected farm would not mean much if that strain is commonly present in raw milk of most farms. Several raw milk samples contained Lactobacillus or related species, as shown by culture and DNA sequencing, but none of the DNA fingerprint patterns from raw milk isolates matched the fingerprint of an isolate from processed product. Based on results from the molecular investigation, herd inspections, and management changes, shipment of milk from the farm to the processing plant could be resumed.
Another example of the use of molecular epidemiologic methods to address on-farm milk quality issues concerns identification of sources of high bacteria counts in bulk tank milk (BTM). In New York State, streptococci are the most common group of bacteria identified in BTM, with 98% of BTM samples testing positive for streptococci. Streptococci also surpass staphylococci and coliform bacteria as contaminants of BTM in terms of the number of colony-forming units per milliliter of milk [55]. Streptococci other than Streptococcus agalactiae are commonly thought to be of environmental origin, and their presence in BTM is attributed to poor cow hygiene or poor milking-time hygiene [56]. Streptococcus uberis, in particular, which was the most common species of Streptococcus found in BTM [57], is thought to be of environmental origin. The environment harbors a wide variety of Streptococcus uberis strains. A few grams of soil may contain as many as five or more different strains [58]. Thus, if environmental contamination is the source of high Streptococcus uberis counts in milk, one would expect a large variety of strains to be present in the BTM sample. Comparison of multiple Streptococcus uberis isolates within BTM samples showed that the opposite was true: all samples tested (n = 5) contained one predominant strain of Streptococcus uberis, pointing to a single source rather than the environment as the source of contamination. In each herd, a cow shedding this predominant Streptococcus uberis strain was identified, showing that mastitic cows rather than poor cow hygiene or poor milking hygiene were the mostly likely cause of high Streptococcus uberis counts in BTM. The same approach (ie, the comparison of multiple isolates from a BTM sample to assess strain diversity and comparison to cow isolates to determine whether a cow could be the source of a predominant strain) has been used to troubleshoot a high E coli count problem. Contrary to prevailing paradigm, a cow was identified as the source of a high coliform count. The farm's BTM bacteria count problem was remedied by dry-off of the cow. No changes were made to milking routines, equipment cleaning, or BTM cooling, and yet BTM counts dropped from 37,000 colony-forming units per milliliter to below 10,000 colony-forming units per milliliter.
Molecular epidemiologic investigations may also be helpful when animals are the recipients rather than the sources of pathogens. Outbreaks of bovine mastitis due to Pseudomonas aeruginosa are uncommon but have been reported from Ireland and the Netherlands. In both countries, outbreaks occurred on multiple farms, often resulting in severe clinical disease or death [59], [60]. Epidemiologic findings suggested that the infection was associated with the use of certain teat wipes. Bacteriology confirmed presence of Pseudomonas aeruginosa in the wipes. The wipes had been provided free with the purchase of dry cow therapy as part of a sales promotion. Ironically, the purpose of the wipes was to clean and sterilize the teat end before the infusion of dry cow therapy antibiotic into the mammary gland by way of the teat opening. Molecular typing of isolates from the Irish herds confirmed that all outbreaks had been caused by the same Pseudomonas aeruginosa strain [58]. This example not only serves to show how preventive measures can go bad but also demonstrates that presence of the same strain in multiple animals does not necessarily prove contagious transmission. When multiple animals are infected with the same environmental strain, predominance of one strain is the result. It is easy to prove that infection in multiple animals is not the result of contagious transmission. Detection of different strains in each animal proves that. The opposite, proving that contagious transmission causes the spread of a disease, is much harder to do. Usually, a combination of molecular and epidemiologic data is needed to support the likelihood that infection of multiple animals with the same strain was due to common source exposure or contagious transmission, respectively.
Transmission dynamics
The distinction between transmission dynamics and source tracing is somewhat arbitrary. For the sake of this article, the authors consider the focus of transmission dynamics to be how organisms spread, as opposed to where they come from. Transmission dynamics can be studied at the international, regional, local, and farm level. International studies are often necessary to understand transmission dynamics of viral diseases such as classical swine fever [62], foot-and-mouth disease [8], avian influenza [61], or NCD [38]. Sequence analysis of NCD virus in Korea showed that five outbreaks of NCD, occurring in 1949, 1982 to 1984, 1988 to 1997, and 1995 to 2002 had been caused by five different strains of NCD virus that had replaced each other serially. It also showed that the strains causing the epidemics were closely related to those causing NCD outbreaks in other parts of the world. The early outbreaks were caused by strains that were related to a European NCD virus. From 1988 onward, outbreak strains resembled genotypes from Japan, Taiwan, and China. The increased trade of agricultural products and poultry among Far East Asian countries is likely to explain this shift in origin of outbreak strains [38]. Stricter sanitary measures and import controls are needed to prevent such transmission in the future.
An example at the regional level is provided by a multistate study of the transmission dynamics of Corynebacterium pseudotuberculosis in the United States [62]. Using RAPD typing, the origin of perceived epidemics of Corynebacterium pseudotuberculosis, which mostly affected horses, was shown to differ between states. All isolates from Utah belonged to one RAPD type of Corynebacterium pseudotuberculosis, consistent with a clonally expanding epidemic in that state. In contrast, the increased number of infections in Colorado, Kentucky, and California was caused by multiple strains of Corynebacterium pseudotuberculosis that were not derived from a common source. Possible causes for the perceived increase in Corynebacterium pseudotuberculosis incidence include reporting bias due to increased awareness of the disease, environmental factors facilitating infection, or host factors facilitating infection, such as greater herd susceptibility [62]. Although prevention of animal-to-animal transmission through biosecurity measures could halt the outbreak of the clonally expanding epidemic in Utah, different management measures would be needed to reduce the incidence of Corynebacterium pseudotuberculosis in the other states.
The transmission dynamics of Campylobacter have been studied at the local level in a rural area with a large number of dairy farms and outdoor recreational areas in the United Kingdom [63]. Samples were collected from water, soil, wildlife feces, and livestock feces, all of which could potentially play a role in exposure of humans to Campylobacter. Using model-based spatial statistics, the distribution of Campylobacter jejuni was shown to be consistent with very localized within-farm or within-field transmission. Thus, the risk of human exposure to Campylobacter jejuni is high in areas contaminated with cattle feces, but the risk of transmission from cattle feces to adjacent wildlife territories, watercourses, or other geographic features transcending field and farm boundaries is limited [63]. Results from the spatial analysis were confirmed by MLST. MLST showed that wildlife and water isolates largely belonged to sequence types that were different from those of bovine isolates. It also showed that many wildlife and water isolates belong to strains that have not been associated with human infections [64]. The combined results show how molecular data can support the results from mathematic or statistical analysis.
Poultry is another reservoir of Campylobacter. Litter from bird houses is thought to play a role in transmission of Campylobacter in poultry operations. Molecular typing was used to explore the role of litter as source of infection. In one study, a flock was raised in a broiler house. This flock, flock 1, had a high prevalence of Campylobacter jejuni in cecal droppings (60%). Most isolates from flock 1 belonged to one strain, RAPD type A. After flock 1 had been removed, part of the litter was transported to a different location and chicks were subsequently raised on this used litter. None of these chicks tested positive for Campylobacter jejuni during their 7-week growing period. The remainder of the litter from flock 1 stayed in the original broiler house and was subsequently used for flock 2. In flock 2, Campylobacter jejuni was detected but its prevalence was lower than in flock 1 (28%). Furthermore, almost none of the isolates from the second flock belonged to RAPD type A. The conclusion of both experiments was that litter does not play a large role in transmission of Campylobacter jejuni [65]. Studies like this can also be used to assess how well cleaning and disinfection of broiler houses prevent carryover between sequential flocks [66]. With collection of suitable samples and data, such farm-level studies could be performed in veterinary practice.
Analysis of patterns of transmission in seemingly similar situations does not always yield similar conclusions. Different patterns of transmission (ie, contagious transmission of a specific pathogen strain and environmental transmission of a multitude of strains) can also be identified within one farm operation [67]. The authors have used molecular epidemiology to help dairy herds determine the origin of their mastitis problem and to identify management measures that could improve the udder health situation. One producer consulted the authors because he failed to earn quality premiums for his milk, which would have been paid if BTM somatic cell counts were below 200,000 cells per milliliter. Despite his best efforts, BTM somatic cell counts remained around 300,000 cells per milliliter. The milk inspector suggested that milking time hygiene might be insufficient, resulting in transmission of mastitis pathogens and subsequent elevated BTM somatic cell counts. The producer insisted that he did everything he could in the milking parlor to prevent mastitis in his animals. A herd visit, with inspection of the milking process, analysis of data stored in a management program, bacteriology of milk samples, and molecular identification of bacterial isolates, revealed (1) the milking routine was impeccable; (2) a scatter plot of linear scores (LS) for the most recent milk test day and the preceding test day showed that most animals had low LS on both test days (healthy cows) or high LS on both test days (chronic infections); (3) the number of cures (low LS on most recent test day, high LS on the preceding test days) was low; and (4) the number of new infections (high LS on most recent test day, low LS on preceding test day) was very low, as the parlor inspection suggested ( Fig. 6). Milk samples were taken from several animals with chronic high LS. Six animals tested positive for nonagalactiae streptococci. Using primers for identification of Streptococcus species [68], two animals were shown to be infected with Streptococcus dysgalactiae, whereas four others were infected with Streptococcus uberis. The presence of two species proves that not all infections had resulted from cow-to-cow transmission. Subsequent RAPD-based strain typing of the Streptococcus uberis isolates revealed that each cow was infected with her own strain of Streptococcus uberis, confirming again that these cases of mastitis were not due to contagious transmission or poor milking-time hygiene but to infections from environmental sources ( Fig. 7). With these data in hand, the producer felt confident that his milking routines were as good as he had thought. The problem was not in the milking parlor but in the close-up and fresh cow pens: animals became infected with streptococci around calving. In the LS graph, new infections around calving show up with a previous LS of zero and high LS for the most recent test day (see Fig. 6). In this herd, prevention of new infections through improved hygiene around calving and a reduction in mastitis prevalence through culling or treatment of chronically infected animals were the pathways to achieving the goal of a BTM somatic cell counts worthy of a quality premium.
Fig. 6.
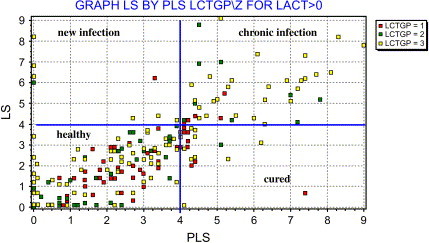
Scatter plot of most recent LS (vertical axis) and previous LS (horizontal axis) used to determine whether chronic or new infections were the main cause of elevated BTM somatic cell count. LS = 4 is used as cutoff value for elevated somatic cell count at the cow level. Cows from the upper right quadrant were selected for sampling and molecular follow-up.
Fig. 7.
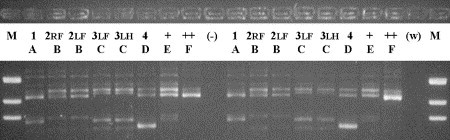
RAPD fingerprinting results for Streptococcus uberis isolates from six quarters of four cows in one herd. Isolates are fingerprinted in duplicate to assess reproducibility of fingerprint patterns. Each cow (identified by a number) has a unique pattern (identified by a letter), whereas multiple quarters within a cow (RF, LF, and LH) are infected with the same strain. Lanes marked + and ++ contain positive controls; lanes marked (−) and (w) contain negative controls. LF, left front; LH, left hind; M, marker; RF, right front.
Persistence, reintroduction, and reinfection
When repeated outbreaks of infection in a farming operation or repeated manifestations of clinical disease in an animal occur, the question arises whether the problem was ever solved or merely “went underground.” Molecular epidemiology can be used to distinguish between persistence and reintroduction of a pathogen or between failure to cure and cure followed by reinfection with a different organism.
Series of outbreaks of fowl cholera have been observed, raising the question whether such series were the result of persistence or repeat introductions of the causative agent, Pasteurella multocida. In Australia, two outbreaks of fowl cholera on a multiage free-range egg farm were investigated. The outbreaks occurred 8 years apart, in 1994 and 2002. In the 1994 outbreak, only acute fowl cholera was seen. The 2002 outbreak included acute and chronic cases of the disease. Despite the difference in time and manifestation, the same strain of Pasteurella multocida caused both outbreaks, as shown by REA typing [69]. In a Danish duck flock, outbreaks of fowl cholera occurred in 1996 and 1997, and asymptomatic carriers of Pasteurella multocida were detected in 1998 [70]. In this flock, a different REA type was found for each outbreak and each year. In the Australian example, repeated introduction of the same Pasteurella strain cannot be ruled out completely, but endemic persistence of the outbreak strain in healthy birds is a more likely explanation for repeated isolation of the same REA type. In the Danish flock, multiple introductions must have occurred, as evidenced by the diversity of REA types. The difference in epidemiology that was revealed by molecular methods could easily be explained by differences in management. The Danish duck farm had an annual clean-out period (ie, a period during which birds were not present on the farm). By contrast, the Australian poultry farm did not have any time period in which birds were completely absent from the property, not even in the 8 years separating the two observed outbreaks. It is not known why so many years went by without outbreaks, but it is thought that stress factors may precipitate fowl cholera [70]. For both outbreaks in the Australian farm, stress factors were identified. The first outbreak followed an attack on the hen house by dingos, and the second outbreak escalated while the flock owner was away [69]. It should be noted that repeated isolation of the same pathogen strain is not always the result of persistence of infection in the animal population. In feedlots, there is a high turnover of animals. Even so, pathogen strains may persist, as in the case for E coli O157:H7. Environmental survival of O157:H7 strains for 6 months and detection of the pathogen in other animal species could explain such persistence in feedlots [71]. The farm environment may thus be more important as a reservoir of E coli O157:H7 than the incoming animals [72].
To complicate matters, repeated isolation of the same pathogen strain does not need to be the result of persistence at all. As an example, consider two series of fowl cholera outbreaks in Hungary. One series of outbreaks occurred in goose flocks kept for eiderdown production and another series of outbreaks occurred in turkey farms. The strains of Pasteurella multocida that were isolated differed between host species; however, within each bird species, the outbreaks were caused by a predominant strain [73]. Analysis of epidemiologic data and contact structures indicated that the two series of outbreaks had different underlying causes. The goose flocks were all located in a village but belonged to different owners, and sharing of fodder or animals between flocks did not occur. The turkey flocks were geographically more dispersed but all belonged to the same large-scale breeder. Flock-to-flock transmission was deemed unlikely for the geese. Instead, the fact that multiple outbreaks were caused by the same strain was attributed to the presence of wildlife in the village. Wild animals were thought to have introduced the same strain to each farm. For the turkey flocks, distances and time intervals between outbreaks made transmission by wild animals unlikely. Here, repeated isolation of the same Pasteurella multocida strain due intracompany transmission was suspected. Both types of introduction (ie, by wildlife and through intracompany transmission) have also been described for Pasteurella multocida and Mycoplasma gallisepticum in turkeys in the United States [74], [75], [76].
For an animal-level example of distinction between persistence and reinfection, the authors return to mastitis in dairy cattle. In 1999, Döpfer and colleagues [77] described repeated cases of clinical E coli mastitis in dairy cows. Back then, infections with E coli were generally considered to be acute and of short duration, and repeat cases would be explained by repeat infections of the susceptible cows or quarters. Considering the large variety of E coli strains in the environment [78], each infection would be anticipated to be caused by a different strain, just like multiple infections within one dairy herd are caused by different strains [79]. Indeed, 13% of all clinical cases were repeat clinical cases caused by different strains. In those cases, recurrence of clinical episodes was the result of reinfection, and the fact that repeat cases were seen was probably associated with increased susceptibility of individual cows. Five percent of repeat clinical cases, however, were not caused by different strains but by one strain [77]. For those cases, intramammary persistence of the causative strain was the most likely explanation for observation of multiple clinical episodes, although this concept was highly disputed at the time. Since then, intramammary persistence has been proved with daily samplings, culture, and strain typing for cows with chronic E coli mastitis, and the existence of chronic E coli mastitis is now well accepted ( Fig. 8) [80], [81], [82]. Even single cases of clinical E coli mastitis during early lactation have been shown to be manifestations of pre-existing, chronic subclinical infections that originated in the dry period [83]. This finding implies that control measures to prevent clinical E coli mastitis may need to be taken in the dry period, rather than in lactation. In treatment trials, strain typing can be used to determine whether apparent failures to cure are true failures or cures followed by reinfection. Oliver and colleagues [84] showed that cows that test positive for Streptococcus uberis or Streptococcus dysgalactiae before and after the dry period can be persistently infected with the same strain (failure to cure) or infected with different strains at dry-off and at calving (cure and reinfection). If a dairy producer wants to assess the efficacy of the dry cow treatment used on a farm, strain typing is a tool that would allow him or her to do so. One caveat is that for strains that spread in a contagious manner, predominance of that contagious strain is expected, and repeat isolation of that strain from an animal could signify failure to cure or cure followed by reinfection with the predominant strain. It is only when different strains are isolated before and after treatment that one can be reasonably certain that reinfection occurred. Typing of multiple isolates from a sample may be necessary to assess strain diversity within samples and to identify mixed infections and partial cures [85]. Herd-level and animal-level examples of differentiation between persistence and reintroduction re-emphasize that molecular data cannot be interpreted in a meaningful manner without knowledge of the epidemiologic context in which the strain typing data were collected.
Fig. 8.

PFGE fingerprints of E coli isolates from seven sequential milk samples collected from one udder quarter at weekly intervals. All isolates have the same PFGE fingerprint, showing that recurrent clinical episodes were the result of persistent infection with one strain rather than the result of repeated cure and reinfection with E coli strains from the environment. E, E coli; M, molecular marker. (Courtesy of Dr. B. Dogan, Cornell University, Ithaca, New York).
Host specificity and niche adaptation
Molecular epidemiologic studies have provided a wealth of insight regarding niche adaptation of strains. Niche adaptation can be interpreted as adaptation to survival in a host (as opposed to the environment), as adaptation to a specific host species, or even as adaptation to an organ system of the host. Examples for all types of niche adaptation can be found among bovine mastitis pathogens.
In bovine mastitis, a distinction is commonly made between environmental and contagious mastitis. Environmental mastitis pathogens are adapted to environmental survival. They do not need a host for survival but can cause opportunistic infections in animals. When the immune system is compromised by negative energy balance, the risk of infection with environmental E coli is increased [86]. During the dry period, when udders are not “flushed out” by milking and the composition of mammary secretions changes, the risk of Streptococcus uberis mastitis is increased. There is a large variety of E coli and Streptococcus uberis strains in the environment [57], [78], and a large variety of strains may cause mastitis [79], [85]. In recent years though, several outbreaks of Streptococcus uberis mastitis have been described that were not caused by a variety of environmental strains but by a predominant strain (see Fig. 4) [67], [87]. Infection of multiple cows or quarters with the same strain has also been reported in nonoutbreak situations [84], [88]. In some cases, there is evidence to suggest that contagious transmission by way of the milking machine took place, similar to the transmission that is known to occur for Staphylococcus aureus [67]. In two herds, the strains that predominated caused infections that were more chronic than those caused by other strains. This observation gives rise to the ideas that certain strains may be more host adapted than others and that they may provide themselves with a longer window of opportunity for transmission to other animals in the population [67]. Differences in the ability of strains to spread within a herd have also been described for Staphylococcus aureus [89] and for Streptococcus agalactiae [90]. Between-cow transmission of E coli has not been documented, but adaptation of some pathogen strains to survival in the mammary gland, resulting in chronic infections and within-cow transmission, appears to occur [80].
Streptococcus agalactiae, also known as group B streptococci in human medicine, provides us with a nice example of adaptation to host species. Differences in the clinical manifestation and epidemiology of intramammary infections caused by Streptococcus agalactiae isolates of human and bovine origin were documented in the early 1980s [90], [91]. Comparison of human and bovine isolates of Streptococcus agalactiae of temporally and geographically matched origins in New York State showed that compared with mastitis in dairy cows, clinical disease in humans was caused by different strains of Streptococcus agalactiae [92]. On occasion, Streptococcus agalactiae has been isolated in the authors' diagnostic laboratory from milk samples originating from a closed herd thought to be free of Streptococcus agalactiae. Such isolates, which did not seem to spread in the herd or were found in BTM only, were shown to belong to a human type of Streptococcus agalactiae on a number of occasions. Opportunistic transmission to cattle and lack of spread in the bovine population has also been reported in the United Kingdom [93]. It is interesting to note that companion animals that carry Streptococcus agalactiae tend to harbor human rather than bovine strains of the organism [94]. Many humans are asymptomatic carriers of Streptococcus agalactiae, and several molecular epidemiologic studies have shown that most cases of severe clinical disease is associated with a limited number of specific strains only. MLST recently confirmed this finding. What is more, MLST showed that a neonatal invasive clone, which can be fatal to newborns, evolved from bovine rather than human subtypes of Streptococcus agalactiae [95]. Although evidence for direct bovine to human transmission had not been published at the time this article was written, the evolutionary relation between bovine and human Streptococcus agalactiae infections is seen by some as a reason to call for mandatory eradication of Streptococcus agalactiae from the dairy cattle population [96].
The third type of niche adaptation of interest is adaptation to organ systems within a host. Again, a mastitis pathogen can be used as an example. Several studies have shown that mammary isolates from different countries tend to belong to a limited number of strains [97], and that teat skin and milk harbor different strains [17]. MLST of Staphylococcus aureus isolates from North and South America and Europe confirmed the predominance of a limited number of clones—grouped together in a so-called “clonal complex”—as causative agents of mastitis, and differentiated this clonal complex from clones found on teat skin [18]. This organ specificity seems to hold across host species because mastitis isolates from cows, goats, and sheep have more in common with each other than skin and udder isolates within the bovine host species [98]. Existence of host-adapted or udder-specific strains as opposed to environmental strains has also been suggested based on clinical and epidemiologic observations. Precalving heifers in a dairy herd would not have been in contact with the milking machine and infected herd mates, so epidemiologic data would suggest that infections in heifers have a different origin. Strain typing confirmed this notion. In addition, the strains found in heifers were associated with very severe clinical disease, resulting in loss of life or loss of the affected udder quarters [99]. Loss of life or productivity removes the infected host or quarter from the dairy population that is milked and, hence, from the opportunity for contagious transmission. For a host-adapted pathogen, such severe damage to the host would not be a smart survival strategy.
The niche adaptation of mastitis pathogens implies that traditional classifications of pathogen species as contagious (host adapted) or environmental (non–host adapted) are too simplistic. Some species, for example Staphylococcus aureus, tend to be contagious, whereas other species such as Streptococcus uberis are commonly of environmental origin. Depending on management conditions and strains, however, environmental Staphylococcus aureus and contagious Streptococcus uberis may occur. Even Streptococcus agalactiae, which can be considered the prototype of contagious pathogens, can on rare occasions originate from environmental sources (human, companion animal). At the other end of the spectrum, E coli, the prototype of environmental pathogens, appears to be adapting to long-term survival in the bovine host. Thus, a black-and-white dichotomy does not do the epidemiology of mastitis justice and fails to provide dairy producers with adequate management advice in all circumstances. Rather, a sliding scale with Streptococcus agalactiae at the contagious end and E coli at the environmental end should be used to represent the epidemiology of mastitis ( Fig. 9).
Fig. 9.
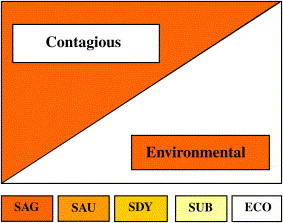
Sliding scale for contagious and environmental origin of mastitis pathogens, based on insights from molecular epidemiology. Vertical axis indicates to what extent species behave as contagious (orange) or as environmental (white) pathogens. The transmission pattern in a specific herd depends on bacterial species and strains and on management conditions. ECO, E coli; SAG, Streptococcus agalactiae; SAU, Staphylococcus aureus; SDY, Streptococcus dysgalactiae; SUB, Streptococcus uberis.
In veterinary practice, molecular typing data from milk isolates can be used to differentiate between contagious and environmental transmission, as discussed elsewhere in this article. In the absence of typing data, epidemiologic and clinical data on infected animals (new infections predominantly in heifers and dry cows versus new infections predominantly in lactating cows), herd hygiene (bedding, milking routine), and management information (implementation of postmilking teat disinfection, segregation of infected animals) can be used to assess the most likely mode of transmission. If people are reluctant to let go of the old mastitis paradigm, then molecular typing can be helpful to clarify the herd-specific situation. A last interesting twist to the mastitis story was revealed by the study on MLST of Staphylococcus aureus [18]. Among the isolates typed was the Newbould 305 strain. This strain was originally used to induce intramammary infections as part of a method to study intramammary treatments [100]. Strain Newbould 305 has since been used to study the effect of antibiotics [101], vaccines [102], and teat-dips [103] and to study the pathogenesis of Staphylococcus aureus mastitis [104], the role of minor pathogens [105], and host-level risk factors for infection [106]. MLST showed that Newbould 305 does not belong to the clonal complex of mammary Staphylococcus aureus strains. It is a skin strain. Thus, all the studies listed here (and many others) were done with a strain of Staphylococcus aureus that is not representative of most mastitis cases that occur naturally in our dairy herds.
An example of less clear-cut host adaptation is provided by MAP. Comparison of MAP isolates from sheep, goat, and cattle herds from Morocco, South Africa, the United States, and Germany showed that all sheep isolates grouped together in one cluster, whereas all cattle and goat isolates grouped together in a different cluster [107]. This finding means that sheep do not pose a risk of MAP infection for cattle. An Australian study also differentiated ovine MAP from bovine MAP and put MAP from goats, alpacas, a rhinoceros, and two humans into the same group as bovine MAP [108]. Later studies, however, showed that contact of calves with paratuberculous sheep can result in presence of ovine MAP in cattle. Under extensive grazing conditions, transmission of ovine MAP among cattle appeared uncommon [109]. Very different results were obtained in a study of MAP isolates from multiple host species, including wildlife, humans, sheep, and cattle in the United States [110]. In that study, MAP isolates from bovine and ovine sources from the same state were more closely associated with each other than isolates from the same host species but from different geographic regions. These results suggest that there is a lack of host adaptation and that strains are shared between ruminant species. A subsequent study confirmed that some strains of MAP are host specific, whereas others can be shared been domestic animals and wildlife [111].
Molecular epidemiology and vaccines
Molecular epidemiology is useful in the development of vaccines, in monitoring of intentional or unintentional spread of vaccine strains, and when predicting vaccine efficacy. Molecular methods are used to determine whether disease is predominantly caused by one strain of a pathogen or by a multitude of strains. In the case of bovine rotavirus diarrhea in France, most isolates were shown to belong to one genotype, G6. Consequently, a monovalent vaccine based on G6 antigen should be sufficient to elicit good protection [112]. For other diseases, a monovalent vaccine would not be useful. Multiple serotypes of bovine Staphylococcus aureus can cause mastitis, and a multivalent vaccine would be needed to protect against all main serotypes. To complicate matters, the distribution of serotypes differs between countries. A vaccine that could be useful in France would be only marginally effective in the United States because of differences in the distribution of the most common serotypes [113]. Even when vaccines do not induce antibodies against surface antigens of the infectious agent, an understanding of molecular epidemiology can be important. For several years, the possibility of a vaccine that targeted the plasminogen activator of Streptococcus uberis, PauA, was explored. The idea behind this vaccine target was that the immune response of the cow fails to clear the mammary gland of Streptococcus uberis, even though antibody levels can be boosted [114]. PauA is used by Streptococcus uberis to acquire nutrients when growing in milk. The aim of the vaccine was to prevent bacterial growth by depriving the bacteria of essential nutrients through inactivation of its nutrient-acquisition system with vaccine-induced antibodies against PauA [115]. Not all Streptococcus uberis isolates harbor pauA, the gene that codes for the protein PauA, and MLST showed that there is a pauA-negative subpopulation of Streptococcus uberis that also differs from most Streptococcus uberis isolates in presence and composition of several other genes [40]. A pauA-based vaccine would not provide protection against such strains. In the population of Streptococcus uberis isolates that does contain the pauA gene, the gene experiences positive selection, which implies that the gene may have the ability to change over time in response to selective pressures in the environment [40]. Other virulence genes and vaccine targets are currently sought after, and molecular tools facilitate that search dramatically [116]. While genomic and proteomic studies provide a rational basis for selection of vaccine candidates, MLST of the bacterial population provides a rational framework for selection of strains that represent relevant subgroups of the species that will be targeted with the vaccine.
Sometimes, administration of vaccines results in unintentional transmission of vaccine strains or contaminants. In Italy and the Netherlands, modified live-marker vaccine was used for active immunization of cattle against infectious bovine rhinotracheitis, caused by bovine herpesvirus 1 (BHV1). Ten to 15 days after vaccination, drop in milk yield, diarrhea, abortion, and death were reported from some farms. All case farms had been vaccinated with batches derived from the same stock materials. Serum neutralization tests and real-time PCR showed that the batches were contaminated with a highly virulent strain of bovine viral diarrhea virus type II. To prevent spread of this bovine viral diarrhea virus strain, which had not been detected in Europe before this outbreak, all contaminated product was recalled and all vaccinated cattle that had not yet died were slaughtered [117]. Vaccine contamination was also suspected when outbreaks of scrapie were observed in sheep and goats that had been vaccinated against Mycoplasma agalactiae in Italy. Iatrogenic scrapie in the vaccinated flocks was attributed to the presence of prions in mammary gland and brain homogenates used for vaccination [118]. Although prions do not have DNA, and molecular typing in the sense of DNA-based typing does not apply, the investigators described their study as a molecular analysis of scrapie strains. This example shows the risks involved in use of vaccines derived from animal tissues.
Spread of vaccine strains is not limited to vaccine contaminants. It can also result from the use of live vaccines. T parva is a tickborne protozoa that causes East Coast fever in cattle. To prevent the disease, which is often fatal, vaccination is used. Vaccines can be based on a so-called “local” strain approach or a “cocktail” approach. In Zambia, the local strain approach has been used on a large scale, whereas vaccination with a trivalent cocktail of exotic strains has been used on a limited geographic scale and for a limited time only. Comparison of RFLP-PCR data for T parva isolates from Kenya and Zambia showed that most Zambian isolates belonged to one stock, which contrasted with the variety of stocks found among Kenyan strains [9]. Zambian field isolates collected in 1996 to 1997 were subsequently compared with Zambian isolates from the prevaccination era and with vaccine stocks used for preparation of local and cocktail vaccines. The field data strongly suggested that one of the exotic vaccine stocks became widely disseminated in the country. For a full discussion of the interaction of protozoa, ticks, and bovine hosts in the epidemiology of this T parva strain, the reader is referred to the original publication [9]. Unintentional transmission of a viral vaccine strain was suspected in a Dutch herd, where replacement heifers on a dairy farm had been erroneously vaccinated with a live-virus infectious bovine rhinotracheitis vaccine. More than 18 months later, serology of the herd showed that more than 70% of the animals had developed an antibody response toward BHV1, interfering with the serology-based disease-free certification program. To determine whether the vaccine strain had caused seroconversion in the herd, two vaccinated and two unvaccinated animals (all seropositive) were treated with corticosteroids to reactivate latent BHV1. Virus isolates were obtained from the animals and analyzed by REA. At least one isolate was clearly distinct from the vaccine strain. It was concluded that there was no indication that the vaccine strain had circulated and that a BHV1 field virus had most likely been introduced into the farm despite the herd being closed and having biosecurity measures in place [119].
Summary
The advent of molecular or DNA-based typing methods for microorganisms has opened up a new world of possibilities in veterinary epidemiology. Molecular epidemiology deals with the detection of sources and transmission dynamics of microorganisms using molecular methods and with the identification of determinants of health, disease, spread, and control. Examples of such determinants include virulence genes, antimicrobial resistance genes, and potential vaccine targets. Using molecular epidemiologic methods, it is possible to monitor global spread of pathogens, to identify highly virulent or highly contagious strains of pathogens, to differentiate between persistence and reintroduction of infectious agents in the farm environment, to distinguish between chronic and recurrent infections at the animal level, to detect transmission of vaccine strains, and to classify the epidemiology of an infectious disease as contagious or environmental. The use of molecular tools provides a level of detail and insight that is not available with traditional culture methods or species-level identification of viruses, bacteria, protozoa, or parasites. Molecular epidemiology can pinpoint not only sources of infection or contamination in humans, animals, farm environments, and animal products but also environmental or host factors that contribute to the introduction and spread of microorganisms. As a result, old paradigms can be reassessed, specific targets for intervention and control can be identified, and the effectiveness of control measures can be monitored. Molecular epidemiologic methods have been used with incredible success in global, national, regional, local, farm-level, and animal-level studies. Now that techniques are becoming increasingly user friendly and affordable, they have started to make their way into veterinary diagnostic laboratories. Although their routine implementation still faces challenges (eg, in terms of cost recovery and turn-around times), it is inevitable that in another few years, molecular methods will be among the routine tools that are used in veterinary epidemiology to promote veterinary public health and to improve production-animal health management.
Acknowledgments
The authors thank Dr. Linda L. Tikofsky and Dr. Frank L. Welcome for helpful discussions and critical reading of the manuscript and Dr. Belgin Dogan for providing Fig. 8.
References
- 1.Riley L.W. ASM Press; Washington, DC: 2004. Molecular epidemiology of infectious diseases. Principles and practices. [Google Scholar]
- 2.Levin B.R., Lipsitch M., Bonhoeffer S. Population biology, evolution, and infectious disease: convergence and synthesis. Science. 1999;283(5403):806–809. doi: 10.1126/science.283.5403.806. [DOI] [PubMed] [Google Scholar]
- 3.Lukinmaa S., Nakari U.M., Eklund M. Application of molecular genetic methods in diagnostics and epidemiology of food-borne bacterial pathogens. APMIS. 2004;112(11–12):908–929. doi: 10.1111/j.1600-0463.2004.apm11211-1213.x. [DOI] [PubMed] [Google Scholar]
- 4.Thompson R.C., Constantine C.C., Morgan U.M. Overview and significance of molecular methods: what role for molecular epidemiology? Parasitology. 1998;117(Suppl):S161–S175. doi: 10.1017/s0031182099004151. [DOI] [PubMed] [Google Scholar]
- 5.Haas L. Molecular epidemiology of animal virus diseases. Zentralbl Veterinarmed B. 1997;44(5):257–272. doi: 10.1111/j.1439-0450.1997.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 6.Hungnes O., Jonassen T.O., Jonassen C.M. Molecular epidemiology of viral infections. How sequence information helps us understand the evolution and dissemination of viruses. APMIS. 2000;108(2):81–97. doi: 10.1034/j.1600-0463.2000.d01-31.x. [DOI] [PubMed] [Google Scholar]
- 7.Skuce R.A., Neill S.D. Molecular epidemiology of Mycobacterium bovis: exploiting molecular data. Tuberculosis (Edinb) 2001;81(1–2):169–175. doi: 10.1054/tube.2000.0270. [DOI] [PubMed] [Google Scholar]
- 8.Knowles N.J., Samuel A.R. Molecular epidemiology of foot-and-mouth disease virus. Virus Res. 2003;91(1):65–80. doi: 10.1016/s0168-1702(02)00260-5. [DOI] [PubMed] [Google Scholar]
- 9.Geysen D., Bishop R., Skilton R. Molecular epidemiology of Theileria parva in the field. Trop Med Int Health. 1999;4(9):A21–A27. doi: 10.1046/j.1365-3156.1999.00447.x. [DOI] [PubMed] [Google Scholar]
- 10.Thompson R.C. The zoonotic significance and molecular epidemiology of Giardia and giardiasis. Vet Parasitol. 2004;126(1–2):15–35. doi: 10.1016/j.vetpar.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Boerlin P. Molecular epidemiology of antimicrobial resistance in veterinary medicine: where do we go? Anim Health Res Rev. 2004;5(1):95–102. doi: 10.1079/ahr200465. [DOI] [PubMed] [Google Scholar]
- 12.Blanc D.S. The use of molecular typing for epidemiological surveillance and investigation of endemic nosocomial infections. Infect Genet Evol. 2004;4(3):193–197. doi: 10.1016/j.meegid.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Tenover F.C., Arbeit R.D., Goering R.V. How to select and interpret molecular strain typing methods for epidemiological studies of bacterial infections: a review for healthcare epidemiologists. Molecular Typing Working Group of the Society for Healthcare Epidemiology of America. Infect Control Hosp Epidemiol. 1997;18(6):426–439. doi: 10.1086/647644. [DOI] [PubMed] [Google Scholar]
- 14.Struelens M. Members of the European Study Group on Epidemiological Markers (ESGEM) of the European Society for Clinical Microbiology and Infectious Diseases (ESCMID). Consensus guidelines for appropriate use and evaluation of microbial epidemiologic typing systems. Clin Microbiol Infect. 1996;2:2–11. doi: 10.1111/j.1469-0691.1996.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 15.Sol J., Sampimon O.C., Barkema H.W. Factors associated with cure after therapy of clinical mastitis caused by Staphylococcus aureus. J Dairy Sci. 2000;83(2):278–284. doi: 10.3168/jds.S0022-0302(00)74875-2. [DOI] [PubMed] [Google Scholar]
- 16.Crisostomo M.I., Westh H., Tomasz A. The evolution of methicillin resistance in Staphylococcus aureus: similarity of genetic backgrounds in historically early methicillin-susceptible and -resistant isolates and contemporary epidemic clones. Proc Natl Acad Sci U S A. 2001;98(17):9865–9870. doi: 10.1073/pnas.161272898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zadoks R.N., van Leeuwen W.B., Kreft D. Comparison of Staphylococcus aureus isolates from bovine and human skin, milking equipment, and bovine milk by phage typing, pulsed-field gel electrophoresis, and binary typing. J Clin Microbiol. 2002;40(11):3894–3902. doi: 10.1128/JCM.40.11.3894-3902.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith E.M., Green L.E., Medley G.F. Multilocus sequence typing of intercontinental bovine Staphylococcus aureus isolates. J Clin Microbiol. 2005;43:4737–4743. doi: 10.1128/JCM.43.9.4737-4743.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maslow J.N., Mulligan M.E., Arbeit R.D. Molecular epidemiology: application of contemporary techniques to the typing of microorganisms. Clin Infect Dis. 1993;17(2):153–162. doi: 10.1093/clinids/17.2.153. [DOI] [PubMed] [Google Scholar]
- 20.Dogan B., Schukken Y.H., Santisteban C. Distribution of serotypes and antimicrobial resistance genes among Streptococcus agalactiae isolates from bovine and human hosts. J Clin Microbiol. 2005;43:5899–5906. doi: 10.1128/JCM.43.12.5899-5906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aarestrup F.M., Wegener H.C., Jensen N.E. A study of phage- and ribotype patterns of Staphylococcus aureus isolated from bovine mastitis in the Nordic countries. Acta Vet Scand. 1997;38(3):243–252. doi: 10.1186/BF03548487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudson C.R., Fedorka-Cray P.J., Jackson-Hall M.C. Anomalies in species identification of enterococci from veterinary sources using a commercial biochemical identification system. Lett Appl Microbiol. 2003;36(4):245–250. doi: 10.1046/j.1472-765x.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 23.Hunter P.R., Gaston M.A. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J Clin Microbiol. 1988;26(11):2465–2466. doi: 10.1128/jcm.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox L.K., Gershman M., Hancock D.D. Fomites and reservoirs of Staphylococcus aureus causing intramammary infections as determined by phage typing: the effect of milking time hygiene practices. Cornell Vet. 1991;81(2):183–193. [PubMed] [Google Scholar]
- 25.Hunter P.R. Reproducibility and indices of discriminatory power of microbial typing methods. J Clin Microbiol. 1990;28(9):1903–1905. doi: 10.1128/jcm.28.9.1903-1905.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Belkum A. High-throughput epidemiologic typing in clinical microbiology. Clin Microbiol Infect. 2003;9(2):86–100. doi: 10.1046/j.1469-0691.2003.00549.x. [DOI] [PubMed] [Google Scholar]
- 27.Grif K., Orth D., Lederer I. Importance of environmental transmission in cases of EHEC O157 causing hemolytic uremic syndrome. Eur J Clin Microbiol Infect Dis. 2005;24(4):268–271. doi: 10.1007/s10096-005-1320-z. [DOI] [PubMed] [Google Scholar]
- 28.Swaminathan B., Barrett T.J., Hunter S.B. PulseNet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerg Infect Dis. 2001;7(3):382–389. doi: 10.3201/eid0703.010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olive D.M., Bean P. Principles and applications of methods for DNA-based typing of microbial organisms. J Clin Microbiol. 1999;37(6):1661–1669. doi: 10.1128/jcm.37.6.1661-1669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Belkum A., Struelens M., de Visser A. Role of genomic typing in taxonomy, evolutionary genetics, and microbial epidemiology. Clin Microbiol Rev. 2001;14(3):547–560. doi: 10.1128/CMR.14.3.547-560.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Kessel J.S., Karns J.S., Perdue M.L. Using a portable real-time PCR assay to detect Salmonella in raw milk. J Food Prot. 2003;66(10):1762–1767. [PubMed] [Google Scholar]
- 32.Nguyen L.T., Gillespie B.E., Nam H.M. Detection of Escherichia coli O157:H7 and Listeria monocytogenes in beef products by real-time polymerase chain reaction. Foodborne Pathog Dis. 2004;1(4):231–240. doi: 10.1089/fpd.2004.1.231. [DOI] [PubMed] [Google Scholar]
- 33.Alcaine S.D., Sukhnanand S.S., Warnick L.D. Ceftiofur-resistant Salmonella strains isolated from dairy farms represent multiple widely distributed subtypes that evolved by independent horizontal gene transfer. Antimicrob Agents Chemother. 2005;49(10):4061–4067. doi: 10.1128/AAC.49.10.4061-4067.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reid S.D., Herbelin C.J., Bumbaugh A.C. Parallel evolution of virulence in pathogenic Escherichia coli. Nature. 2000;406(6791):64–67. doi: 10.1038/35017546. [DOI] [PubMed] [Google Scholar]
- 35.Wiedmann M., Bruce J.L., Keating C. Ribotypes and virulence gene polymorphisms suggest three distinct Listeria monocytogenes lineages with differences in pathogenic potential. Infect Immun. 1997;65(7):2707–2716. doi: 10.1128/iai.65.7.2707-2716.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith K.E., Stenzel S.A., Bender J.B. Outbreaks of enteric infections caused by multiple pathogens associated with calves at a farm day camp. Pediatr Infect Dis J. 2004;23(12):1098–1104. [PubMed] [Google Scholar]
- 37.Stram Y., Chai D., Fawzy H.E. Molecular epidemiology of foot-and-mouth disease (FMD) in Israel in 1994 and in other Middle-Eastern countries in the years 1992–1994. Arch Virol. 1995;140(10):1791–1797. doi: 10.1007/BF01384342. [DOI] [PubMed] [Google Scholar]
- 38.Lee Y.J., Sung H.W., Choi J.G. Molecular epidemiology of Newcastle disease viruses isolated in South Korea using sequencing of the fusion protein cleavage site region and phylogenetic relationships. Avian Pathol. 2004;33(5):482–491. doi: 10.1080/03079450400003700. [DOI] [PubMed] [Google Scholar]
- 39.Enright M.C., Spratt B.G. Multilocus sequence typing. Trends Microbiol. 1999;7(12):482–487. doi: 10.1016/s0966-842x(99)01609-1. [DOI] [PubMed] [Google Scholar]
- 40.Zadoks R.N., Schukken Y.H., Wiedmann M. Multilocus sequence typing of Streptococcus uberis provides sensitive and epidemiologically relevant subtype information and reveals positive selection in the virulence gene pauA. J Clin Microbiol. 2005;43(5):2407–2417. doi: 10.1128/JCM.43.5.2407-2417.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meinersmann R.J., Phillips R.W., Wiedmann M. Multilocus sequence typing of Listeria monocytogenes by use of hypervariable genes reveals clonal and recombination histories of three lineages. Appl Environ Microbiol. 2004;70(4):2193–2203. doi: 10.1128/AEM.70.4.2193-2203.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nightingale K.K., Windham K., Wiedmann M. Evolution and molecular phylogeny of Listeria monocytogenes isolated from human and animal listeriosis cases and foods. J Bacteriol. 2005;187:5537–5551. doi: 10.1128/JB.187.16.5537-5551.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aanensen D.M., Spratt B.G. The multilocus sequence typing network: mlst.net. Nucleic Acids Res. 2005;1(33):W728–W733. doi: 10.1093/nar/gki415. [Web server issue] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loch I.M., Glenn K., Zadoks R.N. Macrolide and lincosamide resistance genes of environmental streptococci from bovine milk. Vet Microbiol. 2005;111(1–2):133–138. doi: 10.1016/j.vetmic.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Guardabassi L., Schwarz S., Lloyd D.H. Pet animals as reservoirs of antimicrobial-resistant bacteria. J Antimicrob Chemother. 2004;54(2):321–332. doi: 10.1093/jac/dkh332. [DOI] [PubMed] [Google Scholar]
- 46.Torrence M.E. Epidemiology and food safety. Foodborne Pathog Dis. 2005;2(1):2–11. doi: 10.1089/fpd.2005.2.2. [DOI] [PubMed] [Google Scholar]
- 47.Heuvelink A.E., van H.C., Zwartkruis-Nahuis J.T. Escherichia coli O157 infection associated with a petting zoo. Epidemiol Infect. 2002;129(2):295–302. doi: 10.1017/s095026880200732x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.David S.T., MacDougall L., Louie K. Petting zoo-associated Escherichia coli 0157:h7—secondary transmission, asymptomatic infection, and prolonged shedding in the classroom. Can Commun Dis Rep. 2004;30(20):173–180. [PubMed] [Google Scholar]
- 49.Jackson S.G., Goodbrand R.B., Johnson R.P. Escherichia coli O157:H7 diarrhoea associated with well water and infected cattle on an Ontario farm. Epidemiol Infect. 1998;120(1):17–20. doi: 10.1017/s0950268897008479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crump J.A., Sulka A.C., Langer A.J. An outbreak of Escherichia coli O157:H7 infections among visitors to a dairy farm. N Engl J Med. 2002;347(8):555–560. doi: 10.1056/NEJMoa020524. [DOI] [PubMed] [Google Scholar]
- 51.Allerberger F., Friedrich A.W., Grif K. Hemolytic-uremic syndrome associated with enterohemorrhagic Escherichia coli O26:H infection and consumption of unpasteurized cow's milk. Int J Infect Dis. 2003;7(1):42–45. doi: 10.1016/s1201-9712(03)90041-5. [DOI] [PubMed] [Google Scholar]
- 52.Anonymous Outbreak of Campylobacter jejuni infections associated with drinking unpasteurized milk procured through a cow-leasing program—Wisconsin, 2001. MMWR Morb Mortal Wkly Rep. 2002;51(25):548–549. [PubMed] [Google Scholar]
- 53.Mazurek J., Salehi E., Propes D. A multistate outbreak of Salmonella enterica serotype typhimurium infection linked to raw milk consumption—Ohio, 2003. J Food Prot. 2004;67(10):2165–2170. doi: 10.4315/0362-028x-67.10.2165. [DOI] [PubMed] [Google Scholar]
- 54.Bell R.A., Hillers V.N., Thomas T.A. The Abuela Project: safe cheese workshops to reduce the incidence of Salmonella typhimurium from consumption of raw-milk fresh cheese. Am J Public Health. 1999;89(9):1421–1424. doi: 10.2105/ajph.89.9.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zadoks R.N., Gonzalez R.N., Boor K.J. Mastitis-causing streptococci are important contributors to bacterial counts in raw bulk tank milk. J Food Prot. 2004;67(12):2644–2650. doi: 10.4315/0362-028x-67.12.2644. [DOI] [PubMed] [Google Scholar]
- 56.Farnsworth R.J. Microbiologic examination of bulk tank milk. Vet Clin North Am Food Anim Pract. 1993;9(3):469–474. doi: 10.1016/s0749-0720(15)30614-9. [DOI] [PubMed] [Google Scholar]
- 57.Zadoks R.N., Tikofsky L.L., Boor K.J. Ribotyping of Streptococcus uberis from a dairy's environment, bovine feces and milk. Vet Microbiol. 2005;109:257–265. doi: 10.1016/j.vetmic.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 58.Daly M., Power E., Bjorkroth J. Molecular analysis of Pseudomonas aeruginosa: epidemiological investigation of mastitis outbreaks in Irish dairy herds. Appl Environ Microbiol. 1999;65(6):2723–2729. doi: 10.1128/aem.65.6.2723-2729.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sol J., Barkema H.W., Berghege I.M. [Mastitis following drying up associated with teat wipes contaminated with Pseudomonas aeruginosa.] Tijdschr Diergeneeskd. 1998;123(4):112–113. [PubMed] [Google Scholar]
- 60.Greiser-Wilke I., Fritzemeier J., Koenen F. Molecular epidemiology of a large classical swine fever epidemic in the European Union in 1997–1998. Vet Microbiol. 2000;77(1–2):17–27. doi: 10.1016/s0378-1135(00)00253-4. [DOI] [PubMed] [Google Scholar]
- 61.Suarez D.L., Spackman E., Senne D.A. Update on molecular epidemiology of H1, H5, and H7 influenza virus infections in poultry in North America. Avian Dis. 2003;47(3 Suppl):888–897. doi: 10.1637/0005-2086-47.s3.888. [DOI] [PubMed] [Google Scholar]
- 62.Foley J.E., Spier S.J., Mihalyi J. Molecular epidemiologic features of Corynebacterium pseudotuberculosis isolated from horses. Am J Vet Res. 2004;65(12):1734–1737. doi: 10.2460/ajvr.2004.65.1734. [DOI] [PubMed] [Google Scholar]
- 63.Brown P.E., Christensen O.F., Clough H.E. Frequency and spatial distribution of environmental Campylobacter spp. Appl Environ Microbiol. 2004;70(11):6501–6511. doi: 10.1128/AEM.70.11.6501-6511.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.French N.P., Barrigas M., Brown P. Spatial epidemiology and natural population structure of Campylobacter jejuni colonizing a farmland ecosystem. Environ Microbiol. 2005;7:1116–1126. doi: 10.1111/j.1462-2920.2005.00782.x. [DOI] [PubMed] [Google Scholar]
- 65.Payne R.E., Lee M.D., Dreesen D.W. Molecular epidemiology of Campylobacter jejuni in broiler flocks using randomly amplified polymorphic DNA-PCR and 23S rRNA-PCR and role of litter in its transmission. Appl Environ Microbiol. 1999;65(1):260–263. doi: 10.1128/aem.65.1.260-263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shreeve J.E., Toszeghy M., Ridley A. The carry-over of Campylobacter isolates between sequential poultry flocks. Avian Dis. 2002;46(2):378–385. doi: 10.1637/0005-2086(2002)046[0378:TCOOCI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 67.Zadoks R.N., Gillespie B.E., Barkema H.W. Clinical, epidemiological and molecular characteristics of Streptococcus uberis infections in dairy herds. Epidemiol Infect. 2003;130(2):335–349. doi: 10.1017/s0950268802008221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Phuektes P., Mansell P.D., Browning G.F. Multiplex polymerase chain reaction assay for simultaneous detection of Staphylococcus aureus and streptococcal causes of bovine mastitis. J Dairy Sci. 2001;84(5):1140–1148. doi: 10.3168/jds.S0022-0302(01)74574-2. [DOI] [PubMed] [Google Scholar]
- 69.Zhang P., Fegan N., Fraser I. Molecular epidemiology of two fowl cholera outbreaks on a free-range chicken layer farm. J Vet Diagn Invest. 2004;16(5):458–460. doi: 10.1177/104063870401600517. [DOI] [PubMed] [Google Scholar]
- 70.Muhairwa A.P., Christensen J.P., Bisgaard M. Investigations on the carrier rate of Pasteurella multocida in healthy commercial poultry flocks and flocks affected by fowl cholera. Avian Pathol. 2000;29:133–142. doi: 10.1080/03079450094162. [DOI] [PubMed] [Google Scholar]
- 71.Hancock D., Besser T., Lejeune J. The control of VTEC in the animal reservoir. Int J Food Microbiol. 2001;66(1–2):71–78. doi: 10.1016/s0168-1605(00)00487-6. [DOI] [PubMed] [Google Scholar]
- 72.Lejeune J.T., Besser T.E., Rice D.H. Longitudinal study of fecal shedding of Escherichia coli O157:H7 in feedlot cattle: predominance and persistence of specific clonal types despite massive cattle population turnover. Appl Environ Microbiol. 2004;70(1):377–384. doi: 10.1128/AEM.70.1.377-384.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kardos G., Kiss I. Molecular epidemiology investigation of outbreaks of fowl cholera in geographically related poultry flocks. J Clin Microbiol. 2005;43(6):2959–2961. doi: 10.1128/JCM.43.6.2959-2961.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carpenter T.E., Snipes K.P., Kasten R.W. Molecular epidemiology of Pasteurella multocida in turkeys. Am J Vet Res. 1991;52(8):1345–1349. [PubMed] [Google Scholar]
- 75.Snipes K.P., Hirsh D.C., Kasten R.W. Use of an rRNA probe and restriction endonuclease analysis to fingerprint Pasteurella multocida isolated from turkeys and wildlife. J Clin Microbiol. 1989;27(8):1847–1853. doi: 10.1128/jcm.27.8.1847-1853.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Charlton B.R., Bickford A.A., Chin R.P. Randomly amplified polymorphic DNA (RAPD) analysis of Mycoplasma gallisepticum isolates from turkeys from the central valley of California. J Vet Diagn Invest. 1999;11(5):408–415. doi: 10.1177/104063879901100504. [DOI] [PubMed] [Google Scholar]
- 77.Döpfer D., Barkema H.W., Lam T.J. Recurrent clinical mastitis caused by Escherichia coli in dairy cows. J Dairy Sci. 1999;82(1):80–85. doi: 10.3168/jds.S0022-0302(99)75211-2. [DOI] [PubMed] [Google Scholar]
- 78.Nemeth J., Muckle C.A., Gyles C.L. In vitro comparison of bovine mastitis and fecal Escherichia coli isolates. Vet Microbiol. 1994;40(3–4):231–238. doi: 10.1016/0378-1135(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 79.Lam T.J., Lipman L.J., Schukken Y.H. Epidemiological characteristics of bovine clinical mastitis caused by Staphylococcus aureus and Escherichia coli studied by DNA fingerprinting. Am J Vet Res. 1996;57(1):39–42. [PubMed] [Google Scholar]
- 80.Bradley A.J., Green M.J. Adaptation of Escherichia coli to the bovine mammary gland. J Clin Microbiol. 2001;39(5):1845–1849. doi: 10.1128/JCM.39.5.1845-1849.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Döpfer D., Almeida R.A., Lam T.J. Adhesion and invasion of Escherichia coli from single and recurrent clinical cases of bovine mastitis in vitro. Vet Microbiol. 2000;74(4):331–343. doi: 10.1016/s0378-1135(00)00191-7. [DOI] [PubMed] [Google Scholar]
- 82.Schukken YH, Dogan B, Klaessig S, et al. Chronic and recurrent coliforms: implications for lactation therapy. Proceedings of the 43rd Annual Meeting of the National Mastitis Council. Verona (WI): National Mastitis Council; 2004. p. 35–40.
- 83.Bradley A.J., Green M.J. A study of the incidence and significance of intramammary enterobacterial infections acquired during the dry period. J Dairy Sci. 2000;83(9):1957–1965. doi: 10.3168/jds.S0022-0302(00)75072-7. [DOI] [PubMed] [Google Scholar]
- 84.Oliver S.P., Gillespie B.E., Jayarao B.M. Detection of new and persistent Streptococcus uberis and Streptococcus dysgalactiae intramammary infections by polymerase chain reaction-based DNA fingerprinting. FEMS Microbiol Lett. 1998;160(1):69–73. doi: 10.1111/j.1574-6968.1998.tb12892.x. [DOI] [PubMed] [Google Scholar]
- 85.McDougall S., Parkinson T.J., Leyland M. Duration of infection and strain variation in Streptococcus uberis isolated from cows' milk. J Dairy Sci. 2004;87(7):2062–2072. doi: 10.3168/jds.S0022-0302(04)70024-7. [DOI] [PubMed] [Google Scholar]
- 86.Suriyasathaporn W., Heuer C., Noordhuizen-Stassen E.N. Hyperketonemia and the impairment of udder defense: a review. Vet Res. 2000;31(4):397–412. doi: 10.1051/vetres:2000128. [DOI] [PubMed] [Google Scholar]
- 87.VanWorth C, Rossitto PV, Wood S, et al. PFGE analysis of Streptococcus uberis recovered from two San Joaquin Valley dairy herds with elevated levels of environmental streptococcus in California. Proceedings of the 44th Annual Meeting of the National Mastitis Council. Verona (WI): National Mastitis Council; 2005. p. 295–6.
- 88.Phuektes P., Mansell P.D., Dyson R.S. Molecular epidemiology of Streptococcus uberis isolates from dairy cows with mastitis. J Clin Microbiol. 2001;39(4):1460–1466. doi: 10.1128/JCM.39.4.1460-1466.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith T.H., Fox L.K., Middleton J.R. Outbreak of mastitis caused by one strain of Staphylococcus aureus in a closed dairy herd. J Am Vet Med Assoc. 1998;212(4):553–556. [PubMed] [Google Scholar]
- 90.Jensen N.E. Herd types of group-B streptococci. Their prevalence among herds in four Danish mastitis control areas and the relation of type to the spread within herds. Acta Vet Scand. 1980;21(4):633–639. doi: 10.1186/BF03546851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jensen N.E. Experimental bovine group-B streptococcal mastitis induced by strains of human and bovine origin. Nord Vet Med. 1982;34(12):441–450. [PubMed] [Google Scholar]
- 92.Sukhnanand S., Dogan B., Ayodele M.O. Molecular subtyping and characterization of bovine and human Streptococcus agalactiae isolates. J Clin Microbiol. 2005;43(3):1177–1186. doi: 10.1128/JCM.43.3.1177-1186.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leigh JA. Are bovine Streptococcus agalactiae (GBS) a leading cause of neonatal death? Proceedings of the 44th Annual Meeting of the National Mastitis Council. Verona (WI): National Mastitis Council; 2005. p. 41–51.
- 94.Yildirim A.O., Lammler C., Weiss R. Pheno- and genotypic properties of streptococci of serological group B of canine and feline origin. FEMS Microbiol Lett. 2002;212(2):187–192. doi: 10.1111/j.1574-6968.2002.tb11265.x. [DOI] [PubMed] [Google Scholar]
- 95.Bisharat N., Crook D.W., Leigh J. Hyperinvasive neonatal group B streptococcus has arisen from a bovine ancestor. J Clin Microbiol. 2004;42(5):2161–2167. doi: 10.1128/JCM.42.5.2161-2167.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hillerton J.E., Leigh J.A., Ward P.N. Streptococcus agalactiae infection in dairy cows. Vet Rec. 2004;154(21):671–672. [PubMed] [Google Scholar]
- 97.Fitzgerald J.R., Meaney W.J., Hartigan P.J. Fine-structure molecular epidemiological analysis of Staphylococcus aureus recovered from cows. Epidemiol Infect. 1997;119(2):261–269. doi: 10.1017/s0950268897007802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Leeuwen W.B., Melles D.C., Alaidan A. Host- and tissue-specific pathogenic traits of Staphylococcus aureus. J Bacteriol. 2005;187(13):4584–4591. doi: 10.1128/JB.187.13.4584-4591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zadoks R., van Leeuwen W., Barkema H. Application of pulsed-field gel electrophoresis and binary typing as tools in veterinary clinical microbiology and molecular epidemiologic analysis of bovine and human Staphylococcus aureus isolates. J Clin Microbiol. 2000;38(5):1931–1939. doi: 10.1128/jcm.38.5.1931-1939.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Newbould F.H. The use of induced mammary infections for evaluating dry cow treatment products. I. Development of a method. Can J Comp Med. 1979;43(4):426–429. [PMC free article] [PubMed] [Google Scholar]
- 101.Owens W.E., Washburn P.J., Ray C.H. The postantibiotic effect of selected antibiotics on Staphylococcus aureus Newbould 305 from bovine intramammary infection. Zentralbl Veterinarmed B. 1993;40(9–10):603–608. doi: 10.1111/j.1439-0450.1993.tb00182.x. [DOI] [PubMed] [Google Scholar]
- 102.Shkreta L., Talbot B.G., Diarra M.S. Immune responses to a DNA/protein vaccination strategy against Staphylococcus aureus induced mastitis in dairy cows. Vaccine. 2004;23(1):114–126. doi: 10.1016/j.vaccine.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 103.Watts J.L., Boddie R.L., Owens W.E. Determination of teat dip germicidal activity using the excised teat model. J Dairy Sci. 1988;71(1):261–265. doi: 10.3168/jds.S0022-0302(88)79551-X. [DOI] [PubMed] [Google Scholar]
- 104.Hensen S.M., Pavicic M.J., Lohuis J.A. Location of Staphylococcus aureus within the experimentally infected bovine udder and the expression of capsular polysaccharide type 5 in situ. J Dairy Sci. 2000;83(9):1966–1975. doi: 10.3168/jds.S0022-0302(00)75073-9. [DOI] [PubMed] [Google Scholar]
- 105.Pankey J.W., Nickerson S.C., Boddie R.L. Effects of Corynebacterium bovis infection on susceptibility to major mastitis pathogens. J Dairy Sci. 1985;68(10):2684–2693. doi: 10.3168/jds.S0022-0302(85)81153-X. [DOI] [PubMed] [Google Scholar]
- 106.Schukken Y.H., Leslie K.E., Barnum D.A. Experimental Staphylococcus aureus intramammary challenge in late lactation dairy cows: quarter and cow effects determining the probability of infection. J Dairy Sci. 1999;82(11):2393–2401. doi: 10.3168/jds.S0022-0302(99)75490-1. [DOI] [PubMed] [Google Scholar]
- 107.Bauerfeind R., Benazzi S., Weiss R. Molecular characterization of Mycobacterium paratuberculosis isolates from sheep, goats, and cattle by hybridization with a DNA probe to insertion element IS900. J Clin Microbiol. 1996;34(7):1617–1621. doi: 10.1128/jcm.34.7.1617-1621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Whittington R.J., Hope A.F., Marshall D.J. Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis: IS900 restriction fragment length polymorphism and IS1311 polymorphism analyses of isolates from animals and a human in Australia. J Clin Microbiol. 2000;38(9):3240–3248. doi: 10.1128/jcm.38.9.3240-3248.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Whittington R.J., Taragel C.A., Ottaway S. Molecular epidemiological confirmation and circumstances of occurrence of sheep (S) strains of Mycobacterium avium subsp. paratuberculosis in cases of paratuberculosis in cattle in Australia and sheep and cattle in Iceland. Vet Microbiol. 2001;79(4):311–322. doi: 10.1016/s0378-1135(00)00364-3. [DOI] [PubMed] [Google Scholar]
- 110.Motiwala A.S., Strother M., Amonsin A. Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis: evidence for limited strain diversity, strain sharing, and identification of unique targets for diagnosis. J Clin Microbiol. 2003;41(5):2015–2026. doi: 10.1128/JCM.41.5.2015-2026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Motiwala A.S., Amonsin A., Strother M. Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis isolates recovered from wild animal species. J Clin Microbiol. 2004;42(4):1703–1712. doi: 10.1128/JCM.42.4.1703-1712.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vende P., Karoum R., Manet G. Molecular epidemiology or bovine rotaviruses from the Charolais area. Vet Res. 1999;30(5):451–456. [PubMed] [Google Scholar]
- 113.Guidry A., Fattom A., Patel A. Prevalence of capsular serotypes among Staphylococcus aureus isolates from cows with mastitis in the United States. Vet Microbiol. 1997;59(1):53–58. doi: 10.1016/s0378-1135(97)00172-7. [DOI] [PubMed] [Google Scholar]
- 114.Leigh J.A., Field T.R. Streptococcus uberis resists the bactericidal action of bovine neutrophils despite the presence of bound immunoglobulin. Infect Immun. 1994;62(5):1854–1859. doi: 10.1128/iai.62.5.1854-1859.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Leigh J.A., Finch J.M., Field T.R. Vaccination with the plasminogen activator from Streptococcus uberis induces an inhibitory response and protects against experimental infection in the dairy cow. Vaccine. 1999;17(7–8):851–857. doi: 10.1016/s0264-410x(98)00270-9. [DOI] [PubMed] [Google Scholar]
- 116.Leigh J.A., Ward P.N., Field T.R. The exploitation of the genome in the search for determinants of virulence in Streptococcus uberis. Vet Immunol Immunopathol. 2004;100(3–4):145–149. doi: 10.1016/j.vetimm.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 117.Falcone E., Tollis M., Conti G. Bovine viral diarrhea disease associated with a contaminated vaccine. Vaccine. 1999;18(5–6):387–388. doi: 10.1016/s0264-410x(99)00244-3. [DOI] [PubMed] [Google Scholar]
- 118.Zanusso G., Casalone C., Acutis P. Molecular analysis of iatrogenic scrapie in Italy. J Gen Virol. 2003;84(4):1047–1052. doi: 10.1099/vir.0.18774-0. [DOI] [PubMed] [Google Scholar]
- 119.van der Poel W.H., Rijsewijk F.A., Meijer F.A. [Identification of an introduced bovine herpesvirus type 1 strain in a closed dairy herd by experimental virus reactivation followed by DNA restriction enzyme analysis.] Tijdschr Diergeneeskd. 2000;125(23):714–717. [PubMed] [Google Scholar]
- 120.Middleton J.R., Fales W.H., Luby C.D. Surveillance of Staphylococcus aureus in veterinary teaching hospitals. J Clin Microbiol. 2005;43(6):2916–2919. doi: 10.1128/JCM.43.6.2916-2919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gillespie B.E., Owens W.E., Nickerson S.C. Deoxyribonucleic acid fingerprinting of Staphylococcus aureus from heifer mammary secretions and from horn flies. J Dairy Sci. 1999;82(7):1581–1585. doi: 10.3168/jds.S0022-0302(99)75386-5. [DOI] [PubMed] [Google Scholar]
- 122.O'Brien T.F., Hopkins J.D., Gilleece E.S. Molecular epidemiology of antibiotic resistance in salmonella from animals and human beings in the United States. N Engl J Med. 1982;307(1):1–6. doi: 10.1056/NEJM198207013070101. [DOI] [PubMed] [Google Scholar]
- 123.Zadoks R.N., Allore H.G., Barkema H.W. Analysis of an outbreak of Streptococcus uberis mastitis. J Dairy Sci. 2001;84(3):590–599. doi: 10.3168/jds.S0022-0302(01)74512-2. [DOI] [PubMed] [Google Scholar]
- 124.Tondo E.C., Guimaraes M.C., Henriques J.A. Assessing and analysing contamination of a dairy products processing plant by Staphylococcus aureus using antibiotic resistance and PFGE. Can J Microbiol. 2000;46(12):1108–1114. doi: 10.1139/w00-111. [DOI] [PubMed] [Google Scholar]
- 125.Xiao L., Ryan U.M. Cryptosporidiosis: an update in molecular epidemiology. Curr Opin Infect Dis. 2004;17(5):483–490. doi: 10.1097/00001432-200410000-00014. [DOI] [PubMed] [Google Scholar]


