Abstract
Although the risk of infection by blood transfusion is relatively low, breakthrough infections still occur, Transfusion-related fatalities caused by infections continue to be reported, and blood is not tested for many potentially dangerous pathogens. The current paradigm for increasing the safety of the blood supply is the development and implementation of laboratory screening methods and restrictive donor criteria. When considering the large number of known pathogens and the fact that pathogens continue to emerge, it is clear that the utility of new tests and donor restrictions will continue to be a challenge when considering the cost of developing and implementing new screening assays, the loss of potential donors, and the risk of testing errors. Despite improving the safety of blood components, testing remains a reactive approach to blood safety. The contaminating organisms must be identified before sensitive tests can be developed. In contrast, pathogen inactivation is a proactive strategy designed to inactivate a pathogen before it enters the blood supply. Almost all pathogen inactivation technologies target nucleic acids, allowing for the inactivation of a variety of nucleic acid–containing pathogens within plasma, platelets, or red blood cells thus providing the potential to reduce transfusion-transmitted diseases. However, widespread use of a pathogen inactivation technology can only be realized when proven safe and efficacious and not cost-prohibitive.
THE DEVELOPMENT OF increasingly sensitive laboratory screening methods and restrictive donor criteria has greatly decreased the risk of transmission of many pathogens through blood transfusion; however, transfusion is still not risk-free. Transfusion-related fatalities and infections continue to be reported,1., 2. and blood is currently not tested for many potentially dangerous known pathogens. In addition, the emergence of new agents such as West Nile virus (WNV) demonstrates that potential threats to the blood supply continue to emerge worldwide. The testing and donor deferral methods currently used to screen the blood supply may not offer complete protection against all of these emerging infectious agents.
The strategy of developing donor screening and testing methods as pathogens emerge has been successful in reducing the risk of transfusion transmission of many viruses, including human immunodeficiency virus (HIV), hepatitis C virus (HCV), and hepatitis B virus (HBV) (Fig 1 ). However, each of these interventions had a substantial delay before implementation. The blood banking system is already heavily burdened with multiple testing steps and stringent donor screening (Fig 2 ). In the United States, blood is tested for antibodies to HIV-1/2, human T-lymphotrophic virus (HTLV)-I/II, HBV, HCV, and Treponema pallidum, the spirochete that causes syphilis. Testing is also performed for the presence of hepatitis B surface antigen (HBsAg) and for HIV-1, HCV, and WNV nucleic acids (implemented under investigational new drug application process).3 Furthermore, it is likely that nucleic acid testing (NAT) for HBV, hepatitis A virus, and parvovirus B19 nucleic acids will be added to the requirements over the next several years.4., 5., 6. Moreover, the American Association of Blood Banks (AABB) recently issued a requirement for accreditation in 2003 mandating the testing of platelet components for bacterial contamination.3
Fig 1.
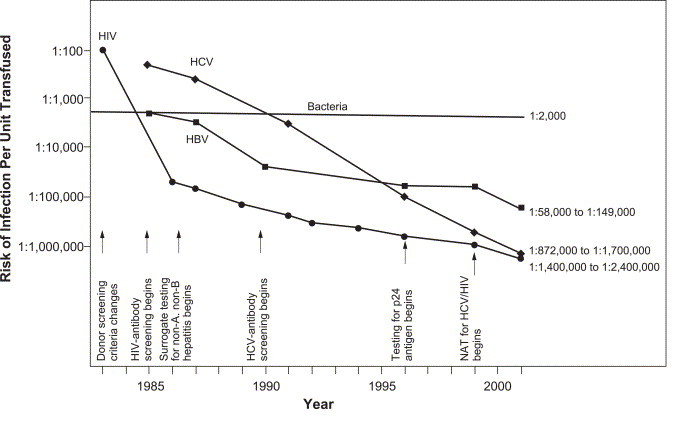
New test implementation and declining risk of viral infections from transfusion. Reprinted with permission from Lancet 361:161-169,2003 (Ref. [7]).
Fig 2.
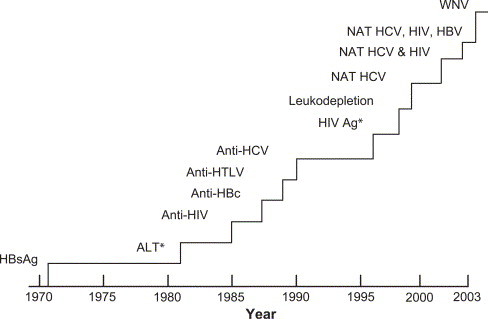
Approximate timeline (1970-2003) for the introduction of various interventions to improve transfusion safety. This timeline may not apply to all countries. The y-axis indicates the magnitude of the number of tests done annually. *No longer in use.
Although it is clear that there is a continual need to develop new technology to protect the safety of the blood supply, it is also clear that adding new tests and/or donor restrictions for each known and emerging pathogen is not feasible. Pathogen inactivation may be a promising alternative to expanded testing and donor deferrals. Such a technology, before its introduction, must be proven safe and efficacious and not be cost-prohibitive.
Pathogen inactivation is a proactive technology in which chemical compounds are added to blood resulting in multiple log reductions in infectivity (or viability) of a variety of pathogens, including viruses, bacteria, and protozoa. Several pathogen inactivation agents, including amotosalen, Inactine, methylene blue (MB), and solvent detergent, are currently in clinical development or clinical use. Pathogen inactivation technology offers the potential to improve the safety of the blood supply against most pathogens, even those that are currently unknown, because the technology is not specific to an individual pathogen.
This review will focus on the emerging viral, bacterial, and parasitic pathogens that threaten the blood supply and on the currently available detection methods. The dilemma of balancing the need to reduce the risk of transfusion-transmitted infections with the economic feasibility of requiring additional testing and the role of pathogen inactivation in improving the safety of the blood supply will also be explored.
Emerging Pathogens
Emerging pathogens are new, reemergent, migrating, or drug-resistant infectious agents whose incidence of infection has either increased within the past 2 decades or threatens to increase in the near future.8 This category includes completely new agents as well as agents that have evolved, in part, caused by the widespread environmental degradation, global warming, regional warfare and population displacement, economically induced migration of blood donors from rural to urban areas in less developed countries, and an ongoing loss of political and basic health infrastructure around the world. Other factors that contribute to the emergence of new pathogens include changing human demographics, evolving effects of new technology and industries, economic development and land use, international travel and commerce, and microbial adaptation and change (Table 1 ).8
Table 1.
Factors Contributing to the Emergence of Pathogens8
| Climate |
| Human demographics |
| Technology and industry practices |
| Economic development and land use |
| International travel and commerce |
| Microbial adaptation and change |
| Breakdown of public health measures |
Changes in technology and industry practices can contribute to the emergence of pathogens that are resistant to treatment. For example, the overprescription of antibiotics and their widespread use in livestock, soaps, and household cleaners can contribute to the resistance of many strains of bacteria. Human demographics, economic development and land use, international travel and commerce, and the breakdown of public health measures can result in the increase and spread of vectors, such as mosquitoes and ticks, which carry pathogens, as well as the spread of pathogens through human-to-human contact. In addition, pathogens that are not problematic in one country can become problematic when brought to another country. Pathogen adaptation and change can make testing less efficient because of genetic changes often related to antivirals, antibiotics, vaccines, and passive immunotherapy that put pressure on pathogens to escape through mutations. Because of the number of factors that can affect the patterns of pathogen emergence and the complexities of each, it is impossible to predict the next emerging pathogen or epidemic. An example of this lack of predictability is the recent worldwide severe acute respiratory syndrome (SARS) epidemic.
Viral Pathogens
Agents
There are at least 35 arboviruses, including the Flaviviruses WNV, DEN-1 to DEN-4, and close relatives of WNV such as St Louis encephalitis virus, western equine encephalitis virus, and eastern equine encephalitis virus.9., 10. All have the potential to become a threat to the blood supply if optimal conditions are present. Infection with arboviruses is often asymptomatic but can result in encephalitis and, in some cases, death.9 Encephalitis can have severe sequelae, such as neurological complications. Arboviruses pose a particular threat to the blood supply because most of arbovirus infections are asymptomatic. Asymptomatic infections in donors can result in transmission to recipients because viremia precedes clinical disease and antibody production, and most of infectious donors could donate without being detected.10
The WNV epidemic in the United States demonstrated that an arbovirus infection could emerge at any time and pose a significant threat to blood transfusion safety. The WNV epidemic that has established itself in the United States appears to have originated from an Egyptian strain of the virus. In 2002, there was a large increase in reported numbers of human WNV infection in North America, compared with previous years, with 4156 cases reported in the United States11 and 325 cases in Canada.12 In this same year, it was recognized that WNV could be transmitted through blood transfusion.13 Twenty-three confirmed cases of transfusion-transmitted WNV were reported in 2002 (transmitted via red blood cells [RBCs], platelets, and plasma).14 In November 2002, the blood bank industry met to address the problem of transfusion-transmitted WNV, and by July 1, 2003, routine NAT was implemented under the investigational new drug application process.
Four related but distinct Flaviviruses, DEN-1, DEN-2, DEN-3, and DEN-4, which are distant relatives of WNV, cause a disease known as dengue fever or dengue hemorrhagic fever. These Flaviviruses are another example of arboviruses that can threaten the safety of the blood supply. Dengue is reaching epidemic proportions in Asia and South America, and because of international travel, dengue has the potential to become an epidemic in the United States.15 Two cases of transfusion transmission of dengue through RBCs have been reported in Hong Kong.16
The hepatitis viruses are other examples of emerging viral pathogens. These viruses can cause liver disease that can result in cirrhosis of the liver and hepatocellular carcinoma.17 Several types of hepatitis viruses, A to E, have been identified.17 Hepatitis B and C can be readily transmitted by blood transfusion, and hepatitis A and E are rarely transmitted by transfusion. Although several hepatitis viruses have been identified, there is still a modest proportion of hepatitis that cannot be attributed to these known viruses. Hepatitis G virus and the TT virus (TTV) were candidates for non–A to E hepatitis, but extensive study failed to demonstrate that these agents were causative of posttransfusion hepatitis or any other disease. SEN viruses, a variant of TTV, may cause some posttransfusion hepatitis.18 SEN viruses have been shown to be prevalent in the donor pool, but a clear correlation between these viruses and hepatitis has not been shown. Transmission of viral hepatitis, particularly HBV and HCV, from blood donors to recipients through the transfusion of blood components and plasma derivatives has been well documented.19 The transfusion transmission of HBV and HCV has been dramatically reduced because of testing, but this has highlighted the residual risk of transfusion-transmitted hepatitis and the possible emergence of undiscovered hepatitis viruses.19
Human parvovirus B19 is a nonenveloped single-stranded DNA virus that is transmitted by blood.20 Infection is generally thought to be benign for people who are immunocompetent, but B19 can cause erythema infectiosum, arthralgia, aplastic crisis in patients with RBC defects, chronic anemia in immunocompromised patients, and fetal hydrops.21 Transfusion transmission of B19 has been reported primarily in recipients of plasma derivatives and in recipients of clotting factor concentrates manufactured from large plasma pools, but rare incidents of transmission via other blood components have also been reported.22., 23.
Another example of a previously completely unknown virus that has recently emerged as a threat is the coronavirus that causes SARS.3 This virus was transmitted from animal to human beings in a region in China and then spread globally because of international travel. About 8000 cases of SARS were reported in 2003, and an estimated 700 deaths were attributed to SARS.24 Severe acute respiratory syndrome outbreaks were reported primarily in the Hong Kong Special Administrative Region, People's Republic of China, Taiwan, Singapore, Canada, Vietnam, and the United States, but smaller outbreaks have been reported in several other countries, including Australia, France, Germany, the Philippines, and Thailand.25 There has been no evidence of transfusion-transmitted SARS infection, but the virus is present in the blood of affected individuals, which could allow for transmission via transfusion of blood components.3
Avian influenza is another emerging pathogen that has recently received considerable media attention. Avian influenza is a contagious disease caused by viruses that normally infect only birds and, less commonly, pigs.26 When it crosses the species barrier and infects human beings, it can cause severe disease with high mortality. Since mid-December 2003, a growing number of Asian countries have reported outbreaks of avian influenza in chickens and ducks. A highly pathogenic strain known as H5N1 has been identified as the cause of most of the outbreaks. Outbreaks have been reported in Vietnam and Thailand.27., 28. It is not yet known if avian influenza is transmissible by transfusion.
Detection
Serological testing for viral antigens or antibodies has been the standard for the detection of most infectious disease agents in the blood supply since the identification of HBsAg in 1971.29 Today, blood is routinely screened for antibodies to HIV-1/2, HTLV-II/II, HBV, and HCV and for the presence of HBsAg and, in some countries, HIV p24 antigen. Although serology tests are effective and resulted in decreased risk of HIV and hepatitis B and C transfusion-transmitted infections, the presence of a “window period,” the period between infection and appearance of a detectable antigen or host antibody, necessitated the development of NAT.4 Nucleic acid testing for HIV and HCV has decreased the risk of transfusion transmitted infection with these agents from 1 in 1 468 000 to 1 in 2 135 000 donations for HIV and from 1 in 276 000 to 1 in 1 935 000 donations for HCV (Fig 1).30
Although the risk of transfusion transmission of HIV, HCV, and HBV has been greatly reduced by serological testing and NAT, breakthrough infections still occur. One case of breakthrough transmission of HIV was reported in Singapore1 and 3 cases by the American Red Cross.31., 32. One case of transfusion transmission of HCV has been reported.33 Breakthrough cases may be caused by viremia that is below the NAT sensitivity cutoff. For logistical and cost reasons, NAT is currently performed on minipools of plasma from 16 to 24 donations.4 Dilution of donor plasma in pools decreases the sensitivity of testing; however, single-donor testing is not cost-effective. Nucleic acid testing reduces the window period of these viruses but does not eliminate it.4 There is pressure to do single-unit NAT, but many feel that single-unit NAT is not feasible unless automation is available and multiple pathogens can be detected with a single NAT.
In the United States, routine NAT (under investigational new drug) and donor deferral are used to prevent transfusion transmission of WNV. The long-term benefit of these measures in reducing transfusion transmission of WNV is unknown at this time. It has been estimated that, although NAT is performed, up to 25% of potentially infectious donors are missed because of low viral load in the window phase (M.P. Busch, personal communication, 2004).34 Hemagglutination assays are available to detect parvovirus B19 in blood components, and polymerase chain reaction (PCR) assays have been developed and used in clinical studies to detect the virus in plasma, but blood is not routinely screened for the virus.6., 21. There are currently no tests to detect dengue or any other known arboviruses in the blood supply. Similarly, no test is available to detect hepatitis G, TTV, the SEN viruses, or the avian influenza virus. No test is available for the SARS coronavirus, but 3 donor deferral questions were implemented in the United States in 2003.
Although testing and donor deferral strategies have been very effective and have greatly reduced the risk of transfusion transmission of viruses such as HIV, HCV, and HBV, it is clear that these methods do not eliminate risk. It is not cost- or labor-effective to develop and implement serological testing or NAT for every known and emerging pathogen, and increasing donor deferrals can needlessly deplete the blood donor pool. Furthermore, all of these methods are reactive and are implemented after a pathogen has been identified and transfusion transmission has been reported.
Bacterial Pathogens
In developed countries, transfusion-transmitted bacterial contamination of platelets is the most common microbiological cause of fatalities related to transfusion.34., 35., 36., 37. Transfusion-transmitted bacterial infection can occur with RBCs as well as platelets but occurs more commonly with platelets, because platelets must be stored at room temperature to maintain viability and function. This, in combination with the biologic composition of platelets and their media, creates an ideal growth environment for bacteria. From 1983 to 2001, the risk of infection with HIV, HBV, and HCV per unit of platelets transfused has dramatically decreased. In contrast, the risk of transfusion-associated bacteremia has not changed during this same period (Fig 1). The risk of infection from bacterial contamination exceeds that from viral agents. Based on United States and European studies, the overall risk of contamination is similar in RBCs and in platelets, but because of the refrigerated storage, most bacteria will not proliferate in RBCs. The prevalence of bacterial contamination of platelets is approximately 1/2000 to 1/3000 U, whereas the highest reported risk of Yersinia contamination of RBCs leading to symptoms has been approximately 1 per 40 000.38., 39. In most countries, the incidence of severe septic episodes, leading to significant recipient morbidity and mortality, caused by the transfusion of contaminated blood products is not clearly established but may be as high as 1/50 000 U of apheresis platelets (higher for pooled platelets) and 1/500 000 U of RBCs transfused.38., 39.
Agents
The organisms that cause bacterial contamination of blood components include skin flora (the most common), enteric flora, and environmental flora. Contamination may occur because of asymptomatic donor bacteremia (rarely), during collection, or because of a faulty collection pack (leaky seal, damaged tubing, or micropuncture). The predominant organisms isolated from contaminated platelet units are skin commensals such as coagulase-negative staphylococci. Identified species include streptococci, Staphylococcus epidermidis, Bacillus cereus, Staphylococcus aureus, Pseudomonas fluorescens, Pseudomonas aeruginosa, Klebsiella pneumoniae, Serratia marcescens, and Serratia liquefaciens.40 Anaerobic bacteria, including Clostridium perfringens and Propionibacterium acnes, have also been isolated; however, the significance of anaerobic infection in the context of platelet transfusion is not known.41 Red blood cells associated with sepsis are usually contaminated with Enterobacteriaceae that will grow at refrigerator temperature; Yersinia enterocolitica and S liquefaciens are the 2 species of bacteria that are most commonly detected in RBCs.
Both gram-positive and gram-negative organisms have been shown to cause transfusion-associated sepsis. Data from reporting systems in the United States (BaCon), United Kingdom (SHOT), and France (Hémovigilance) suggest that more than 70% of cases of transfusion-related sepsis are caused by gram-positive organisms; however, more than 80% of fatalities caused by transfusion-related sepsis are caused by gram-negative organisms.42., 43., 44.
The bacterium Anaplasma phagocytophilum, the agent of human granulocytic ehrlichiosis, is an emerging threat to the blood supply.45., 46. Anaplasma phagocytophilum is found primarily in the United States and Europe and is transmitted primarily by Ixodes ticks. Seroprevalence studies in Connecticut demonstrate a seroprevalence rate between 3% and 4% when approximately 2000 donors were tested.45
Detection
The problem of bacterial contamination of blood products, particularly platelets (because they are stored at room temperature), has recently been addressed by the AABB. The AABB has mandated that, by March 1, 2004, blood banks and transfusion services should have a method to limit and detect bacterial contamination in all platelet components. However, no method has been endorsed by the AABB. Available strategies to reduce platelet transfusion-associated sepsis include better skin preparation, the use of single-donor apheresis platelets, diversion of the first 10 to 20 mL of collected blood, pretransfusion bacterial detection, and pathogen inactivation.
Improved skin disinfection of blood donors helps to reduce contamination somewhat. Isopropyl alcohol and iodine tincture have been found to be the most effective disinfectants, with chlorhexidine gluconate as the disinfectant of choice for those allergic to iodine.47 The use of single-donor apheresis platelets versus pooled donor platelets was shown in one study to reduce septic platelet transfusion reactions from 1 in 4818 transfusions to 1 in 15 098 transfusions with the increased use of single-donor platelets over 12 years.48 This decrease is likely because of the fact that there is only 1 donor (with only one chance of having a donor with an asymptomatic bacteremia) and 1 needle stick per draw unit of platelets, and therefore, there is less chance for contamination from skin. Diversion has also been shown to result in reduced bacterial contamination of blood products.49., 50. However, it is mostly gram-positive organisms that are reduced by diversion, but it is usually gram-negative organisms that cause fatalities.
Bacterial detection is a widely used method of minimizing the risk of transfusion-transmitted sepsis; however, most methods do not have a high level of sensitivity. Factors that affect the sensitivity and efficacy of bacterial detection methods include bacterial growth kinetics, size of initial inoculum, sampling time after platelet preparation, sample volume, assay sensitivity, and time to positivity.39 The primary difficulty stems from the fact that the initial inoculum of bacteria is often extremely low (≤1 colony-forming unit per milliliter), which cannot be detected by most methods. It may require 24 to 48 hours for bacteria to grow before a small sample can be used to detect the bacteria present. The amount of time required for bacteria to grow to a detectable level varies because it depends on the growth kinetics of the bacterium.
There are many methods of bacterial detection available; however, as is apparent from the length of the list, many of these methods are not very sensitive (Fig 3 ). Culture appears to be the most effective, with a sensitivity of approximately 10 colony-forming units per milliliter. Universal bacterial detection is complicated by amplification enzymes derived from bacteria (with nucleic acid “contamination”) and the fact that it is common to find small amounts of bacterial nucleic acid in healthy donor blood.51 Although culture is the most sensitive method, a drawback of the system is that bacterial contaminants might be detected after transfusion has taken place. If the initial inoculum is low or if the organism is a slow grower, it may take days before culture is positive.
Fig 3.
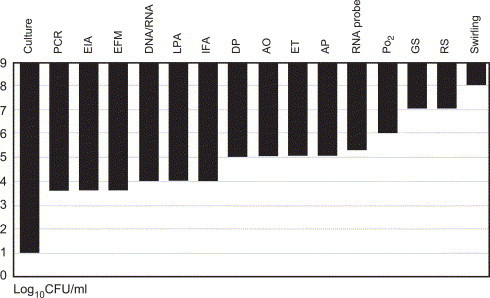
Sensitivity of bacterial detection methods. Abbreviations: AO, acridine orange stain; AP, antibiotic probe; DNA/RNA, DNA/RNA chemoluminescence; DP, dielectrophoresis; EIA, enzyme-linked immunoassay; EFM, epifluorescence microscopy; ET indicates endotoxin; GS, Gram stain; IFA, immunofluorescence assay; LPA, latex particle agglutination; Po2, PALL BDS Po2 method; RNA probe, ribosomal RNA probe; RS, reagent strips; Swirling, platelet swirling patterns. Reprinted with permission from Transfus Med Rev. 2004;18:11-24 (Ref. [52]). Values are based on data from several sources (Refs. 53., 54., 55., 56., 57., 58., 59.).
Bacterial detection has a role in reducing the risk of transfusion-associated sepsis; however, even if a method is 90% effective, risk will be reduced only 10-fold. It has been hypothesized that pathogen inactivation is the only method that will be able to reduce the risk of bacterial contamination to minuscule levels. However, to date, no method has been shown to reduce all organisms. For example, organisms capable of forming spores may elude pathogen reduction techniques.
Protozoan Pathogens
Agents
The 3 parasitic agents thought to constitute the greatest threat to the blood supply are the protozoans Trypanosoma cruzi, Plasmodium spp, and Babesia spp.60 Trypanosoma cruzi, the etiological agent of Chagas disease, is a small protozoan parasite primarily found in Latin America. This agent represents an emerging infection in the United States and Canada because of infected immigrants.60 Effective treatment is unavailable for infection with T cruzi. Clinical manifestations include potentially fatal cardiac and gastrointestinal disease. Signs and symptoms usually do not develop until several years or decades after infection. The incidence of T cruzi has increased in the United States and Canada in the last 30 years because of the emigration of infected and often asymptomatic people from T cruzi–endemic countries.61
During the last 16 years, 7 cases of transfusion-transmitted T cruzi have been reported in the United States (5 cases) and Canada (2 cases).62., 63., 64., 65., 66. Transmission occurred in some cases via platelets, and in others, the component could not be identified because the patient received multiple blood components and a donor could not be identified. Because studies demonstrate increasing numbers of seroprevalent donors and because Chagas disease is difficult to recognize, the reported incidence is thought to be an underestimate. A multiyear epidemiological study of T cruzi in Los Angeles blood donors demonstrated that seroprevalence rates in high-risk populations (comprised of people who were born in or who had spent a significant amount of time in a T cruzi–endemic country) increased significantly from 1996 to 1998.67 It has been estimated that 1 in 25 000 US blood donors are positive for T cruzi antibodies, and studies suggest that 63% of blood donors with antibodies to T cruzi have evidence of active parasitemia in their peripheral blood.67., 68.
Plasmodium is the etiological agent of human malaria. Four species of Plasmodium, Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale, are known to cause human malaria. Malaria is a mosquito-borne intracellular parasite of RBCs and liver cells that causes flulike symptoms with periodic episodes of fever and chills related to massive hemolysis of infected erythrocytes. In endemic areas, malaria is the main indication for transfusion and is a significant cause of morbidity in young children. Human malaria is found primarily in tropical and subtropical regions; however, approximately 1 to 2 cases of transfusion-acquired malaria are reported in the United States annually.69 Transfusion transmission is primarily via RBCs, but platelets have also been implicated, probably because of the presence of RBCs in platelet concentrates. The immigration of infected individuals who spread the parasite when bitten by local mosquitoes and a phenomenon called “airport malaria,” in which infected mosquitoes ride with airplanes and cause infections near airports, have contributed to the emergence of Plasmodium as a threat to the blood supply.70., 71., 72.
Babesia microti is one of the agents that cause zoonotic babesiosis—a mild self-limiting disease that causes flulike symptoms. A number of new divergent strains of Babesia, including WA-1, CA-1, and MO-1 (named after the states in which they were found), have recently been identified. Babesia are intracellular pathogens of RBCs and are transmitted by deer ticks. Human babesiosis has been reported primarily in North America and Europe, but cases have also occurred in Latin America, Africa, and Southeast Asia.69 Infection can be treated with common antibiotics; however, it can be fatal in the elderly, immunocompromised, or asplenic individuals.
More than 50 cases of transfusion-transmitted B microti have been reported since 1979.73 Most cases have been reported in the United States, but cases have also been reported in Japan and Canada.74., 75. Packed RBCs are the most frequently implicated, but cases have also been reported with frozen deglycerolized RBCs and platelets,76 Based on estimated seroprevalence rates, it has been suggested that transfusion transmission is underestimated. A recent study of seroprevalence in Connecticut, a highly endemic area for Lyme disease, which is carried by the same tick that carries Babesia, showed that over the past 5 years, 0.8% to 1.7% of approximately 2000 donors screened per year are seropositive for Babesia.45 Although the number varied from year to year, up to 56% of seropositive individuals were found to have positive PCR tests.
The threats that these parasites pose to blood safety have been known for years, but they have been largely ignored. As these agents become increasingly problematic, it is clear that steps must be taken to reduce the risk of transfusion transmission.
Detection
Trypanosoma cruzi in blood donors is usually detected by serological testing.60 In most Latin American countries, blood donations are screened for T cruzi antibodies using 2 or 3 serological tests (immunofluorescence assay, hemagglutination, or enzyme immunoassay). Blood in the United States and Canada is not screened for this parasite because licensed screening assays are unavailable. At a September 2002 meeting of the US Food and Drug Administration Blood Product Advisory Committee, the Food and Drug Administration issued a request for manufacturers to develop and submit for approval antibody screening tests for T cruzi. Nucleic acid testing for T cruzi is only cost-effective in areas in which it is highly endemic.60
Travel histories have generally been effective at preventing the transmission of malaria; however, questions are often misinterpreted by blood donors.60 In addition, travel histories needlessly defer thousands of blood donors who are unlikely to be infected with malaria and who, in most cases, never return as active donors after their deferral.60 Most cases of transfusion transmission of malaria come from donors who have been infected with Plasmodium in their home country and not from travel, and many donors are excluded unnecessarily. For some countries, serological and NAT testing for malaria may be useful for increasing the number of potential blood donors.
The diagnostic gold standard for detection of Babesia is the immunofluorescence assay; however, it is not licensed for blood screening and would be inefficient for testing large numbers of samples.45 A specific and sensitive enzyme immunoassay for B microti has been reported,77 and the identification of secreted antigens of B microti could lead to the development of a test to detect parasite antigens in serum.78 Sensitive PCR assays have been developed for a variety of Babesia DNA, suggesting that NAT may be used to detect early acute or window-period infections.79
Prion Agents
Variant Creutzfeldt-Jakob disease (vCJD) is a novel human prion disease caused by infection with the agent of bovine spongiform encephalopathy. Bovine spongiform encephalopathy contains no nucleic acids. Transmission is caused by the conversion of normal prion protein to the abnormal β-sheet amyloid structure. Recent reports suggested the risk of transmission of vCJD by blood transfusion.80., 81. There is also increased concern that chronic wasting disease of deer and elk–associated prions may enter the blood supply.82
Pathogen Inactivation
Although they have resulted in extensive reductions in risk, donor selection and testing have not eliminated pathogen transfusion transmission. With the continual discovery of more pathogens, the addition of a new test for each becomes difficult. Pathogen inactivation is an alternative to developing individual tests and donor criteria to address each pathogen individually. It is a proactive effective way to protect the blood supply from most known and emerging pathogens. The goal of pathogen inactivation is to increase the safety of the blood supply by targeting all pathogens, without compromising therapeutic efficacy of the blood product or causing adverse effects in the recipient.83 Two methods for the inactivation of pathogens during the preparation of fresh frozen plasma (FFP) and 1 method for the preparation of platelet concentrates have been introduced into clinical practice in the United States or Europe, and 2 others are in phase 3 clinical development (Table 2 ).84., 85.
Table 2.
Methods Developed for Pathogen Reduction of Blood Components in Clinical Practice or Phase 3 Clinical Trials84., 85.
| Component | Method | Phase (Europe) | Phase (United States) |
|---|---|---|---|
| Platelets | Amotosalen (S-59) | Clinical practice | Phase 3 |
| RBCs | FRALE (S-303) | Phase 3* | |
| RBCs | Inactine (PEN110) | Phase 3* | |
| FFP | Amotosalen (S-59) | Phase 3 | |
| FFP | Solvent detergent | Clinical practice | Clinical practice† |
| FFP | MB | Clinical practice |
Clinical trial halted.
Removed from the market.
Amotosalen
Amotosalen hydrochloride (S-59) intercalates between nucleic acid bases and, upon activation by UV-A light, forms covalent additions (monoadducts and diadducts) to pyrimidine bases in both DNA and RNA.86 These adducts then form interstrand or intrastrand cross-links within the genetic material, which block the replication and transcription machinery and cause cell death. Photochemical treatment of apheresis and pooled buffy coat platelets with amotosalen plus UV-A light has been shown to inactivate a broad range of viruses, bacteria, and protozoa.86., 87., 88., 89. It cannot be used for pathogen inactivation of RBCs, however, because of the light absorbance by hemoglobin and the viscosity of packed RBCs.
Preclinical Data
Treatment of platelets has been shown to result in high levels of log reductions of enveloped single-stranded and double-stranded RNA and DNA viruses: HIV-1 (proviral, cell-free, and cell-associated; single-stranded RNA virus), duck HBV (DHBV, a model for HBV; single-and double-stranded DNA virus), human HBV (MS-2 strain), bovine viral diarrhea virus (BVDV, a model for HCV; single-stranded RNA virus), human HCV (Hutchinson strain), and cytomegalovirus (CMV, double-stranded DNA virus; Table 3 ).86., 89., 90.
Table 3.
| Virus | Infectivity Log Reduction |
|||
|---|---|---|---|---|
| Platelets | Plasma | RBC | ||
| Enveloped | HIV-1, cell-free | >6.2 | >5.9 | >6.5 |
| HIV-1, cell-assoc. | >6.1 | 6.4 | >6.2 | |
| HBV | >5.5 | >4.5 | ||
| HCV | >4.5 | >4.5 | ||
| HTLV-I, cell assoc. | 4.7 | 4.2 | ||
| HTLV-II, cell assoc. | 5.1 | 5.1 | ||
| CMV, cell-assoc. | >5.9 | |||
| DHBV (model for HBV) | >6.2 | >5.1 | >6.3 | |
| BVDV (model for HCV) | >6.0 | >6.0 | >7.3 | |
| Nonenveloped | Blue tongue virus | 6.1-6.4 | 6.0 | |
| Calicivirus | 1.7-2.4 | |||
| Sindbis virus-15 | 0.7-2.3 | |||
| B19 | ∼4* | 3.5* | >3.4* | |
| Human adenovirus 5 | >5.7* | |||
NOTES. Apheresis platelets were inoculated with high levels of virus, then treated with 150 μmol/L Amotosalen and 3 J/cm2 UV-A light. The infectivity of the viruses was measured using established biologic assays.
Preliminary results.
Preclinical studies also showed that gram-positive and gram-negative bacteria in platelets were sensitive to inactivation (Table 4 ).86., 87., 91.
Table 4.
| Log Reduction |
Log Reduction |
||||
|---|---|---|---|---|---|
| Gram-Positive (Aerobes and Anaerobes) | Platelets | RBC | Gram-Negative (Aerobes) | Platelets | RBC |
| S epidermidis | >6.6 | >6.9 | Escherichia coli | >6.4 | 7.4* |
| S aureus | 6.6 | >5.1 | S marcescens | >6.7 | 4.1 |
| Streptococcus pyogenes | >6.8 | K pneumoniae | >5.6 | ||
| Listeria monocytogenes | >6.3 | >7.1 | P aeruginosa | 4.5 | 4.5 |
| Corynebacterium minutissimum | >6.3 | P fluorescens | 5.7 | ||
| B cereus (vegetative) | >6.0 | >6.3 | Salmonella choleraesuis | >6.2 | 4.8 |
| Deinococcus radiodurans | >6.0 | Salmonella typhimurium | 4.8 | ||
| Lactobacillus sp† | >6.9 | Y enterocolitica | >5.9 | 7.4 | |
| Bifidobacterium adolescentis | >6.5 | Enterobacter cloacae | 5.9 | ||
| P acnes† | >6.7 | Spirochetes | |||
| C perfringens | >7.0 | T pallidum | 6.8-7.0 | ||
NOTES. Apheresis platelets were inoculated with high levels of bacteria and treated with 150 μmol/L amotosalen plus 3 J/cm2 UV-A light. The viability of bacteria was measured with established biologic assays.
Minimum plasma.
Facultative anaerobes.
One preclinical study also demonstrated that the protozoan T cruzi was inactivated in both platelets and plasma with greater than 5.4-log reduction in platelet concentrates and greater than 5.0-log reduction in plasma.88
A comprehensive, pharmaceutical-grade, preclinical safety program failed to find any specific target organ toxicity, reproductive toxicity, or carcinogenicity associated with amotosalen.92 Preclinical studies also demonstrated that treated plasma and platelets have acceptable functional characteristics.93., 94., 95.
Clinical Data
Clinical studies have demonstrated that amotosalen is nontoxic and that treated plasma and platelets have acceptable functional characteristics.92., 93., 94., 95. Two randomized controlled phase 3 clinical studies, the euroSPRITE and SPRINT trials, demonstrated that photochemically treated (PCT) buffy coat and apheresis (collected on the Amicus Separator) platelets have therapeutic efficacy that is comparable to untreated platelets in thrombocytopenic patients, primarily in patients with hematologic malignancies.94., 96. Despite hemostatic efficacy, patients in the PCT group had lower platelet count increments and shorter transfusion intervals and required more platelet transfusions than the reference group. However, subsequent analysis suggested that this outcome was at least in part caused by the inconsistent doses transfused. Patients in the PCT group received significantly high proportion of platelet doses of <3 × 1011. When PCT and reference patients were supported with comparable platelet doses, more transfusions of PCT platelets were not required.97
S-303
S-303 is a compound that belongs to a class of compounds called frangible anchor-linked effectors (FRALEs). It was developed for inactivation of pathogens in RBCs.84., 85. The FRALE compounds contain a nucleic acid–targeted intercalator group, an effector group for covalent addition to nucleic acid, and a central frangible bond facilitating compound degradation. Frangible anchor-linked effectors are activated by a pH shift when added to packed red cells suspended in residual plasma and a red cell additive solution at neutral pH. Clinical trial results with S-303 in healthy volunteers and patients demonstrated that transfusions of S-303–treated RBCs were well tolerated and showed comparable recovery and survival to control RBC.98., 99. Although no apparent clinical consequence was observed, phase 3 clinical trials for this compound were halted in September 2003, because 2 study patients developed antibodies against RBCs treated with S-303.100
Inactine
Inactine is a pathogen inactivation technology developed for use in RBCs. It is a low–molecular-weight compound chemically related to binary ethyleneimine that is highly selective for nucleic acids.101 It covalently binds to nucleic acids, resulting in the inhibition of nucleic acid replication or translation of a pathogen's genome.
Preclinical Data
Preclinical studies have demonstrated that Inactine can inactivate a broad range of viruses and bacteria, as well as mycoplasma in RBCs.102., 103., 104., 105. In several studies, various viruses, including enveloped, nonenveloped, and cell-associated viruses, were found to be sensitive to Inactine treatment (Table 5 ).102., 103., 104., 105.
Table 5.
| Viruses | Log Reductions (PFU/mL) |
|---|---|
| Enveloped | |
| WNV | 5-7 |
| BVDV | 4.2-7.5 |
| Pseudorabies virus | 4.2-7.5 |
| VSV | 4.2-7.5 |
| Sindbis virus | 4.2-7.5 |
| Nonenveloped | |
| PPV | 4.2-7.5 |
| Human adenovirus 2 | 4.2-7.5 |
| Reovirus 3 | 4.2-7.5 |
| Vesicular exanthema of swine virus | 4.2-7.5 |
| Blue tongue virus | 4.2-7.5 |
| Foot-and-mouth disease virus | 4.2-7.5 |
| Cell-associated | |
| HIV-1 | 4.2-7.5 |
NOTES. Red blood cells were spiked with viruses, then treated with 0.1% Inactine (vol/vol) for up to 22 hours. Virus inactivation was measured using established biologic assays.
Abbreviations: PFU indicates plaque-forming unit; VSV, vesicular stomatitis virus; PPV, porcine parvovirus.
Preclinical studies also demonstrated the inactivation of bacteria by Inactine.106 Treatment of RBCs spiked with Y enterocolitica, P fluorescens, or Pseudomonas putida with Inactine resulted in the inhibition of bacterial growth in any RBC unit throughout 6 weeks of storage. Inactine also inactivated mycoplasma in whole blood and RBCs.107
Reproductive toxicology studies in rats and rabbits also demonstrated that Inactine was not associated with reproductive toxicity.108
Clinical Data
Results of a phase 1 clinical study of RBCs treated with Inactine demonstrated that Inactine-treated RBCs obtained from healthy individuals and stored for 28 days met the in vivo criteria requirements for therapeutically useful units.101 Recently, it was reported that 1 of 2 phase 3 studies of Inactine was halted by a data safety monitoring committee because of concern with antibody responses to Inactine.109 The antibody responses did not appear to be associated with any clinical consequences.
Methylene Blue
Methylene blue (MB) is a phenothiazine dye that has been used in Europe to inactivate pathogens in FFP for several years.110 The virucidal properties of phenothiazine dyes have been recognized since the early 1930s. Methylene blue is ineffective against intracellular viruses, and it has been suggested that factor VIII and fibrinogen are sensitive to MB.111 Several studies have reported on the virucidal activity of another phenothiazine dye, dimethylmethylene blue (DMMB) in RBCs. Dimethylmethylene blue is a photoactive phenothiazine dye with a greater affinity for nucleic acids than MB.112., 113., 114. Dimethylmethylene blue plus visible light has been shown to inactivate RNA and DNA model viruses, including vesicular stomatitis virus and DHBV, and leukocytes in RBCs.113., 114., 115., 116. Clinical studies have not yet been initiated with DMMB.
Riboflavin
The naturally occurring vitamin B2, riboflavin, has also been developed as a nucleic acid–binding agent to be used for pathogen inactivation.118 After irradiation with UV-A or visible light, intercalated riboflavin molecules form cross-links with DNA and RNA which results in inactivation of nucleic acid–containing pathogens.117., 118. Riboflavin-based pathogen inactivation systems for plasma, platelets, and RBCs are currently in development. Preclinical studies have demonstrated reduction in infectivity of several viruses, including HIV-1, BVDV, and pseudorabies virus, as well as bacteria in blood products.117., 118., 119. The data indicate that the riboflavin system successfully reduced the number of selected pathogens in platelet concentrates. Despite the fact that significant differences exist between treated and control in vitro variables, it is speculated that the clinical effectiveness of both products will not be significantly different, based on comparison with historical data for products in routine clinical use today.120
Solvent-Detergent
The solvent-detergent (SD) process has been developed for use in FFP and has been in clinical practice in both North America and Europe.121 This process has been shown to inactivate several enveloped viruses but is ineffective against nonenveloped viruses. Furthermore, SD-FFP has reduced levels of the antithrombotic protein antiplasmin and antitrypsin.122 This may be responsible for the occurrence of unexpected venous thrombotic events after large-volume exposure.123 Because of this concern and commercial factors, SD-FFP is no longer used in the United States; however, it is still used in Europe and other countries.84
Challenges for Pathogen Inactivation
The current pathogen inactivation methods reviewed here have in common the addition of a compound to the blood component. Most compounds target the genomic nucleic acids by forming stable bonds with the nucleic acid, thus preventing replication. One method, the SD process for FFP, targets the membrane by disrupting it. Laboratory studies have shown that these methods are effective in inactivating a broad range of viruses, bacteria, protozoa, and nucleated cells, although the effectiveness varies among each approach. For example, SD is not effective against nonenveloped viruses, and MB is not effective in inactivating nucleated cells and, correspondingly, cell-associated virus. Although pathogen inactivation is considered an important development for blood safety and has the potential to shift the current paradigm of testing, there are limitations that need to be considered.
Nucleic acid targeting pathogen inactivation methods will not impact the risk because of prions that cause vCJD and possibly chronic wasting disease. These risks will need to continue to be managed by donor deferral, removal of contaminating agents, and potentially testing strategies.
Certain nonenveloped viruses with tight capsids appear to be relatively resistant to inactivation. Making it even more challenging, for example, parvovirus B19 can exist in donor blood in titers as high as 1012 genome equivalence per milliliter (GEq/mL) during the window period.124 The conservative approach would be to retain the most sensitive current tests to assure that levels of viremia are well below the documented pathogen inactivation effectiveness threshold. Some new tests may be required, even in the setting of universal pathogen inactivation, to safeguard against various high-titer pathogens that could potentially “break through” pathogen inactivation.
However, it should be noted that very high viral titers, such as those reported for B19, are expressed as GEq measured by quantitative PCR of a small fragment (100-200 bases) of the viral genome. In contrast, all viral, bacterial, and protozoan inactivation experiments have been performed using infectivity assays, which detect the presence of intact viral genomes required for replication and disease transmission. Whether 1 GEq corresponds to 1 infectious unit of a virus has not been unequivocally demonstrated. Published ratios of GEq to infectivity are in the range of 103 to 106 for parvovirus B19.124., 125. Assays are now being developed based on inhibition of nucleic acid amplification that may be able to extend the dynamic range of pathogen inactivation studies.126., 127. Although these assays cannot answer the question of whether 1 GEq equals an infectious virion, they will be able to extend the dynamic range of pathogen inactivation experiments beyond the dynamic range of infectivity assays. It should also be noted that the pathogen inactivation compounds are used at concentrations that are in excess of the amount required for inactivation of the highest reported GEq viral titers.
For the pathogen inactivation systems that inactivate bacteria, spores, for example, B cereus, represent a problem as they are resistant to inactivation.87 However, because blood components are nutrient-rich media, spores will enter the vegetative phase. In contrast to spores, the vegetative form of B cereus is highly sensitive to treatment.91 In the context of blood components, data need to be generated to show whether conditions exist in which bacteria would form spores. This may also have to be done for parasites, particularly plasmodia, because they are present in the human host in various forms, which may have varying degrees of accessibility to pathogen inactivation compounds. Only parasites obtained in culture have been inactivated experimentally.
Each pathogen inactivation method reviewed here involves the addition of a compound to the blood component, which is activated either by light or a pH shift. Compound absorption devices or washing procedures are used to remove or substantially reduce the quantity of the residual compounds before transfusion to provide the highest possible safety margins. Despite reduction in the level of residual compounds, traces of the compounds remain in the treated product; this raises concerns on the potential long-term toxicity to recipients, processing personnel, and environment. Therefore, to obtain regulatory approval and licensure of a pathogen inactivation system, each method/compound needs to be examined independently. For example, the amotosalen system for platelets was evaluated in a comprehensive toxicology program, including a carcinogenicity study in animals, in conformance of that required for approval of a pharmaceutical. There were no toxicological relevant findings in any tissues or organs found. Multiple phase III clinical trials as well as postmarketing phase IV experience on INTERCEPT platelets further provided the efficacy and safety data for the pathogen inactivation process.94., 96.
There are limited data on cost-effectiveness of the pathogen inactivation systems. The economics of an intervention, such as pathogen inactivation, can be evaluated in 2 ways: by the direct net cost consequences and by the more traditional cost-effectiveness analysis.128 Net cost analysis assesses the direct cost on the blood center for adoption of a new intervention. For example, pathogen inactivation offers the potential to avoid other costs such as bacterial testing, γ-irradiation for leukocyte inactivation, and CMV serological testing, thus directly impacting the net cost of the new technology. Traditional cost-effectiveness analysis generally uses a quality-adjusted life-year threshold of US$100 000 to discriminate cost-effective from cost-ineffective interventions.128 However, blood safety interventions, such as NAT, with higher quality-adjusted life-year thresholds have been adopted. From a practical standpoint, the cost and logistics of implementing pathogen inactivation systems need to be considered, but in the long run, pathogen inactivation systems offer a potential insurance against the threat of emerging pathogens.
Finally, the impact of pathogen inactivation on reduced donor selection and testing will only be possible when all major components derived from blood donations are subjected to licensed pathogen inactivation techniques.
Conclusions
The foundation for the prevention of transfusion-transmitted infections has been donor screening and testing, including serological testing and NAT. This approach has led to significant advances in the safety of the blood supply with respect to certain pathogens, including HIV, HCV, and HBV. However, the blood supply continues to be vulnerable. For example, the bacterial contamination of platelets remains the number one microbiological cause of transfusion-related mortality worldwide and is responsible for many deaths each year.
The blood supply is also vulnerable to emerging and reemerging pathogens. The list of viral, parasitic, and bacterial pathogens that could potentially threaten the blood supply is long and continues to grow (Table 6 ). As was illustrated with HIV, WNV, and SARS, a new pathogen can emerge rapidly, spread globally, and possibly cause serious morbidity and mortality to recipients of infected blood products. Although testing has been successful in reducing the risk of infection with a few high-profile viruses, it remains a reactive strategy, and continuing to add tests for each of the many known and yet to be identified pathogens will continue to be a challenge when considering the cost of developing and implementing new screening assays and the risk of testing errors. Furthermore, increasingly stringent donor criteria often result in the unnecessary loss of many viable donors. Additional measures including pathogen inactivation have been proposed as alternate approaches to reducing the risk of transfusion-transmitted infection.
Table 6.
Emerging Pathogens That Threaten the Blood Supply
| Emerging Pathogens | ||
|---|---|---|
| Viruses | Bacteria | Protozoa |
| Arboviruses | S epidermidis | T cruzi |
| WNV | B cereus | Plasmodium spp. |
| Dengue | S aureus | Babesia spp. |
| Coronovirus that causes SARS | C perfringens | |
| Hepatitis viruses | P acnes | Other |
| Parvovirus B19 | Y enterocolitica | A phagocytophilum |
| Avian influenza | S liquefaciens | |
| P fluorescence | ||
| P aeruginosa | ||
| K pneumoniae | ||
| S marcescens | ||
Pathogen inactivation is a proactive alternative to the current paradigm of developing a new test for each pathogen that threatens the safety of the blood supply. This report reviewed the efficacy of several pathogen inactivation systems for use in FFP, platelets, or RBCs currently in various stages of clinical development. Preclinical studies have demonstrated that pathogen inactivation technology can target a broad range of nucleic acid–containing pathogens while maintaining the in vitro function of treated plasma and platelets. The availability of these systems, once proven safe and efficacious, and implementation worldwide will have the potential to significantly impact the safety of blood supply.
References
- 1.Ling A.E., Robbins K.E., Brown T.M. Failure of routine HIV-1 tests in a case involving transmission with preseroconversion blood components during the infectious window period. JAMA. 2000;284:210–214. doi: 10.1001/jama.284.2.210. [DOI] [PubMed] [Google Scholar]
- 2.Glynn S.A., Kleinman S.H., Schreiber G.B. Trends in incidence and prevalence of major transfusion-transmissible viral infections in US blood donors, 1991 to 1996. Retrovirus Epidemiology Donor Study (REDS) JAMA. 2000;284:229–235. doi: 10.1001/jama.284.2.229. [DOI] [PubMed] [Google Scholar]
- 3.American Association of Blood Banks Facts about blood and blood banking. AABB. http://www.aabb.org/All_About_Blood/FAQs/aabb_faqs.htm Available at: [Accessed October 17, 2003]
- 4.Busch M.P., Kleinman S.H., Nemo G.J. Current and emerging infectious risks of blood transfusions. JAMA. 2003;289:959–962. doi: 10.1001/jama.289.8.959. [DOI] [PubMed] [Google Scholar]
- 5.Kleinman S.H., Busch M.P. HBV: Amplified and back in the blood safety spotlight. Transfusion. 2001;41:1081–1085. doi: 10.1046/j.1537-2995.2001.41091081.x. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt I., Blumel J., Seitz H. Parvovirus B19 DNA in plasma pools and plasma derivatives. Vox Sang. 2001;81:228–235. doi: 10.1046/j.1423-0410.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 7.Goodnough L.T., Shander A., Brecher M.E. Transfusion medicine: Looking to the future. Lancet. 2003;361:161–169. doi: 10.1016/S0140-6736(03)12195-2. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine . Factors in emergence. In: Lederberg J., Shope R., Oaks S., editors. Emerging infections: Microbial threats to health in the United States. National Academy Press; Washington, DC: 1992. pp. 34–112. [PubMed] [Google Scholar]
- 9.Calisher C.H. Medically important arboviruses of the United States and Canada. Clin. Microbiol. Rev. 1994;7:89–116. doi: 10.1128/cmr.7.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero J.R., Newland J.G. Viral meningitis and encephalitis: Traditional and emerging viral agents. Semin. Pediatr. Infect. Dis. 2003;14:72–82. doi: 10.1053/spid.2003.127223. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Statistics, surveillance, and control. West Nile virus 2003 human cases as of August 12, 2003. CDC. http://www.cdc.gov/ncidod/dvbid/westnile/surv&controlCaseCount03.htm Available at: [Accessed March 17, 2004]
- 12.Health Canada and the Canadian Cooperative Wildlife Health Centre West Nile virus: Canada. Results of 2002 surveillance program, 23 April 2003. http://www.hc-sc.gc.ca/pphb-dgspsp/wnv-vwn/pdf_sr-rs/2003/situation_report_042303_hm.pdf Available at: [Accessed March 17, 2004]
- 13.Centers for Disease Control and Prevention Investigations of West Nile virus infections in recipients of blood transfusions. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5139a5.htm Available at: [Accessed March 17, 2004] [DOI] [PubMed]
- 14.Pealer L.N., Marfin A.A., Petersen L.R. Transmission of West Nile virus through blood transfusion in the United States in 2002. N. Engl. J. Med. 2003;349:1236–1245. doi: 10.1056/NEJMoa030969. [DOI] [PubMed] [Google Scholar]
- 15.Dove A. Dengue fever on the increase. Nat. Med. 1998;4:543. doi: 10.1038/nm0598-543b. [DOI] [PubMed] [Google Scholar]
- 16.Dengue virus, transfusion transmission—China (Hong Kong). Promed-mail. http://www.promedmail.org/pls/askus/f?p=2400:1202:2378434143043139813::NO::F2400_P1202_CHECK_DISPLAY,F2400_P1202_PUB_MAIL_ID:X,19530 Available at: [Accessed February 2, 2004]
- 17.Chen D.S. Viral hepatitis: From A to E, and beyond? J. Formos. Med. Assoc. 2003;102:671–679. [PubMed] [Google Scholar]
- 18.Umemura T., Yeo A.E., Sottini A. SEN virus infection and its relationship to transfusion-associated hepatitis. Hepatology. 2001;33:1303–1311. doi: 10.1053/jhep.2001.24268. [DOI] [PubMed] [Google Scholar]
- 19.Prati D. Transmission of viral hepatitis by blood and blood derivatives: Current risks, past heritage. Dig. Liver Dis. 2002;34:812–817. doi: 10.1016/s1590-8658(02)80076-7. [DOI] [PubMed] [Google Scholar]
- 20.Kerr J.R. Parvovirus B19 infection. Eur. J. Clin. Microbiol. Infect. Dis. 1996;15:10–29. doi: 10.1007/BF01586181. [DOI] [PubMed] [Google Scholar]
- 21.Jordan J., Tiangco B., Kiss J. Human parvovirus B19: Prevalence of viral DNA in volunteer blood donors and clinical outcomes of transfusion recipients. Vox Sang. 1998;75:97–102. [PubMed] [Google Scholar]
- 22.Allain J.P., Thomas I., Sauleda S. Nucleic acid testing for emerging viral infections. Transfus. Med. 2002;12:275–283. doi: 10.1046/j.1365-3148.2002.00386.x. [DOI] [PubMed] [Google Scholar]
- 23.Cohen B.J., Beard S., Knowles W.A. Chronic anemia due to parvovirus B19 infection in a bone marrow transplant patient after platelet transfusion. Transfusion. 1997;37:947–952. doi: 10.1046/j.1537-2995.1997.37997454023.x. [DOI] [PubMed] [Google Scholar]
- 24.Berger A., Drosten C., Doerr H.W. Severe acute respiratory syndrome (SARS)—paradigm of an emerging viral infection. J. Clin. Virol. 2004;29:13–22. doi: 10.1016/j.jcv.2003.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization Summary table of SARS cases by country, 1 November 2002 to 7 August 2003. WHO. http://www.who.int/csr/sars/country/en/country2003_08_15.pdf Available at: [Accessed April 7, 2004]
- 26.Capua I., Alexander D.J. Avian influenza and human health. Acta Trop. 2002;83:1–6. doi: 10.1016/s0001-706x(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization Avian influenza frequently asked questions. WHO. http://www.who.int/csr/disease/avian_influenza/avian_faqs/en/print.html Available at: [Accessed February 2, 2004] [PubMed]
- 28.World Health Organization Confirmed human cases of avian influenza A (H5N1). WHO. http://www.who.int/csr/disease/avian_influenza/country/cases_table_2004_02_02/en/print.htm Available at: [Accessed February 2, 2004]
- 29.Dodd R.Y., Westphal R.G. Emerging infections and the safety of the blood supply. Blood Ther. Med. 2002;2:48–54. [Google Scholar]
- 30.Dodd R.Y., Notari E.P., Stramer S.L. Current prevalence and incidence of infectious disease markers and estimated window-period risk in the American Red Cross blood donor population. Transfusion. 2002;42:975–979. doi: 10.1046/j.1537-2995.2002.00174.x. [DOI] [PubMed] [Google Scholar]
- 31.Delwart E.L., Kalmin N.D., Jones T.S. First report of human immunodeficiency virus transmission via an RNA-screened blood donation. Vox Sang. 2004;86:171–177. doi: 10.1111/j.0042-9007.2004.00416.x. [DOI] [PubMed] [Google Scholar]
- 32.Phelps R., Robbins K., Liberti T. Window-period human immunodeficiency virus transmission to two recipients by an adolescent blood donor. Transfusion. 2004;44:929–933. doi: 10.1111/j.1537-2995.2004.03364.x. [DOI] [PubMed] [Google Scholar]
- 33.Schuttler C.G., Caspari G., Jursch C.A. Hepatitis C virus transmission by a blood donation negative in nucleic acid amplification tests for viral RNA. Lancet. 2000;355:41–42. doi: 10.1016/S0140-6736(99)04719-4. [DOI] [PubMed] [Google Scholar]
- 34.Kleinman S., Chan P., Robillard P. Risks associated with transfusion of cellular blood components in Canada. Transfus. Med. Rev. 2003;17:120–162. doi: 10.1053/tmrv.2003.50009. [DOI] [PubMed] [Google Scholar]
- 35.Stainsby D., Cohen H., Jones H. Summary of annual report 2001-2002, serious hazards of transfusion (SHOT) http://www.shot-uk.org Available at: [Accessed March 25, 2004]
- 36.Andreu G., Morel P., Forestier F. Hemovigilance network in France: Organization and analysis of immediate transfusion incident reports from 1994 to 1998. Transfusion. 2002;42:1356–1364. doi: 10.1046/j.1537-2995.2002.00202.x. [DOI] [PubMed] [Google Scholar]
- 37.FDA/CBER Annual report FY 2002. FDA/CBER. http://www.fda.gov/CBER/inside/annrptpart3.htm Available at: [Accessed March 17, 2004]
- 38.Blajchman M.A. Incidence and significance of the bacterial contamination of blood components. Dev. Biol. (Basel) 2002;108:59–67. [PubMed] [Google Scholar]
- 39.Blajchman M.A., Goldman M. Bacterial contamination of platelet concentrates: Incidence, significance, and prevention. Semin. Hematol. 2001;38:20–26. doi: 10.1016/s0037-1963(01)90120-9. [DOI] [PubMed] [Google Scholar]
- 40.Yomtovian R., Lazarus H.M., Goodnough L.T. A prospective microbiologic surveillance program to detect and prevent the transfusion of bacterially contaminated platelets. Transfusion. 1993;33:902–909. doi: 10.1046/j.1537-2995.1993.331194082380.x. [DOI] [PubMed] [Google Scholar]
- 41.McDonald C.P., Hartley S., Orchard K. Fatal Clostridium perfringens sepsis from a pooled platelet transfusion. Transfus. Med. 1998;8:19–22. doi: 10.1046/j.1365-3148.1998.00120.x. [DOI] [PubMed] [Google Scholar]
- 42.Roth V.R., Kuehnert M.J., Haley N.R. Evaluation of a reporting system for bacterial contamination of blood components in the United States. Transfusion. 2001;41:1486–1492. doi: 10.1046/j.1537-2995.2001.41121486.x. [DOI] [PubMed] [Google Scholar]
- 43.Williamson L.M., Lowe S., Love E.M. Serious hazards of transfusion (SHOT) initiative: Analysis of the first two annual reports. BMJ. 1999;319:16–19. doi: 10.1136/bmj.319.7201.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perez P., Salmi L.R., Follea G. Determinants of transfusion-associated bacterial contamination: Results of the French BACTHEM case-control study. Transfusion. 2001;41:862–872. doi: 10.1046/j.1537-2995.2001.41070862.x. [DOI] [PubMed] [Google Scholar]
- 45.Leiby D.A., Chung A.P., Cable R.G. Relationship between tick bites and the seroprevalence of Babesia microti and Anaplasma phagocytophila (previously Ehrlichia sp.) in blood donors. Transfusion. 2002;42:1585–1591. doi: 10.1046/j.1537-2995.2002.00251.x. [DOI] [PubMed] [Google Scholar]
- 46.Wells G.M., Woodward T.E., Fiset P. Rocky mountain spotted fever caused by blood transfusion. JAMA. 1978;239:2763–2765. doi: 10.1001/jama.239.26.2763. [DOI] [PubMed] [Google Scholar]
- 47.Goldman M., Roy G., Frechette N. Evaluation of donor skin disinfection methods. Transfusion. 1997;37:309–312. doi: 10.1046/j.1537-2995.1997.37397240214.x. [DOI] [PubMed] [Google Scholar]
- 48.Ness P., Braine H., King K. Single-donor platelets reduce the risk of septic platelet transfusion reactions. Transfusion. 2001;41:857–861. doi: 10.1046/j.1537-2995.2001.41070857.x. [DOI] [PubMed] [Google Scholar]
- 49.de Korte D., Marcelis J.H., Verhoeven A.J. Diversion of first blood volume results in a reduction of bacterial contamination for whole-blood collections. Vox Sang. 2002;83:13–16. doi: 10.1046/j.1423-0410.2002.00189.x. [DOI] [PubMed] [Google Scholar]
- 50.Bruneau C., Perez P., Chassaigne M. Efficacy of a new collection procedure for preventing bacterial contamination of whole-blood donations. Transfusion. 2001;41:74–81. doi: 10.1046/j.1537-2995.2001.41010074.x. [DOI] [PubMed] [Google Scholar]
- 51.Brecher M.E., Hay S.N. Improving platelet safety: Bacterial contamination of platelets. Curr. Hematol. Rep. 2004;3:121–127. [PubMed] [Google Scholar]
- 52.Blajchman M.A., Goldman M., Baeza F. Improving the bacteriological safety of platelet transfusions. Transfus. Med. Rev. 2004;18:11–24. doi: 10.1016/j.tmrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Rider J., Newton A. Electrochemiluminescent detection of bacteria in blood components. Transfus. Med. 2002;12:115–123. doi: 10.1046/j.1365-3148.2002.00364.x. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell K.M., Brecher M.E. Approaches to the detection of bacterial contamination in cellular blood products. Transfus. Med. Rev. 1999;13:132–144. doi: 10.1016/s0887-7963(99)80008-x. [DOI] [PubMed] [Google Scholar]
- 55.Ortolano G.A., Freundlich L.F., Holme S. Detection of bacteria in WBC-reduced PLT concentrates using percent oxygen as a marker for bacteria growth. Transfusion. 2003;43:1276–1285. doi: 10.1046/j.1537-2995.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 56.Depcik-Smith N.D., Hay S.N., Brecher M.E. Bacterial contamination of blood products: Factors, options, and insights. J. Clin. Apheresis. 2001;16:192–201. doi: 10.1002/jca.10004. [DOI] [PubMed] [Google Scholar]
- 57.Brecher M.E., Boothe G., Kerr A. The use of a chemiluminescence-linked universal bacterial ribosomal RNA gene probe and blood gas analysis for the rapid detection of bacterial contamination in white cell–reduced and nonreduced platelets. Transfusion. 1993;33:450–457. doi: 10.1046/j.1537-2995.1993.33693296805.x. [DOI] [PubMed] [Google Scholar]
- 58.Burstain J.M., Brecher M.E., Workman K. Rapid identification of bacterially contaminated platelets using reagent strips: Glucose and pH analysis as markers of bacterial metabolism. Transfusion. 1997;37:255–258. doi: 10.1046/j.1537-2995.1997.37397240205.x. [DOI] [PubMed] [Google Scholar]
- 59.Seaver M., Crookston J.C., Roselle D.C. First results using automated epifluorescence microscopy to detect Escherichia coli and Staphylococcus epidermidis in WBC-reduced platelet concentrates. Transfusion. 2001;41:1351–1355. doi: 10.1046/j.1537-2995.2001.41111351.x. [DOI] [PubMed] [Google Scholar]
- 60.Leiby D.A. Parasites: An emerging threat to blood safety. Blood Ther. Med. 2003;4:5–10. [Google Scholar]
- 61.Dodd R.Y. Transmission of parasites by blood transfusion. Vox Sang. 1998;74:161–163. doi: 10.1111/j.1423-0410.1998.tb05415.x. (suppl. 2) [DOI] [PubMed] [Google Scholar]
- 62.Nickerson P., Orr P., Schroeder M.L. Transfusion-associated Trypanosoma cruzi infection in a non-endemic area. Ann. Intern. Med. 1989;111:851–853. doi: 10.7326/0003-4819-111-10-851. [DOI] [PubMed] [Google Scholar]
- 63.Leiby D.A., Lenes B.A., Tibbals M.A. Prospective evaluation of a patient with Trypanosoma cruzi infection transmitted by transfusion. N. Engl. J. Med. 1999;341:1237–1239. doi: 10.1056/NEJM199910143411615. [DOI] [PubMed] [Google Scholar]
- 64.Grant I.H., Gold J.W., Wittner M. Transfusion-associated acute Chagas disease acquired in the United States. Ann. Intern. Med. 1989;111:849–851. doi: 10.7326/0003-4819-111-10-849. [DOI] [PubMed] [Google Scholar]
- 65.Cimo P.L., Luper W.E., Scouros M.A. Transfusion-associated Chagas' disease in Texas: Report of a case. Tex. Med. 1993;89:48–50. [PubMed] [Google Scholar]
- 66.Lane D., Sher G., Ward B. Investigation of the second case of transfusion transmitted Chagas disease in Canada. Blood. 2000;96:60a. (part 61) [Google Scholar]
- 67.Leiby D.A., Read E.J., Lenes B.A. Seroepidemiology of Trypanosoma cruzi, etiologic agent of Chagas' disease, in US blood donors. J. Infect. Dis. 1997;176:1047–1052. doi: 10.1086/516534. [DOI] [PubMed] [Google Scholar]
- 68.Smith T., Leiby D., Herron R. Transfusion-transmitted Trypanosoma cruzi: Are seropositive blood donors at-risk for transmitting Chagas' disease? Transfusion. 2000;40:42S. (suppl.) [Google Scholar]
- 69.Kjemtrup A.M., Conrad P.A. Human babesiosis: An emerging tick-borne disease. Int. J. Parasitol. 2000;30:1323–1337. doi: 10.1016/s0020-7519(00)00137-5. [DOI] [PubMed] [Google Scholar]
- 70.Thang H.D., Elsas R.M., Veenstra J. Airport malaria: Report of a case and a brief review of the literature. Neth. J. Med. 2002;60:441–443. [PubMed] [Google Scholar]
- 71.Centers for Disease Control and Prevention Local transmission of Plasmodium vivax malaria—Virginia 2002. JAMA. 2002;288:2113–2114. [PubMed] [Google Scholar]
- 72.Centers for Disease Control and Prevention Local transmission of Plasmodium vivax malaria—Palm Beach County, Florida, 2003. MMWR Morb. Mortal. Wkly. Rep. 2003;52:908–911. [PubMed] [Google Scholar]
- 73.Lux J.Z., Weiss D., Linden J.V. Transfusion-associated babesiosis after heart transplant. Emerg. Infect. Dis. 2003;9:116–119. doi: 10.3201/eid0901.020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsui T., Inoue R., Kajimoto K. First documentation of transfusion-associated babesiosis in Japan. Rinsho Ketsueki. 2000;41:628–634. (in Japanese) [PubMed] [Google Scholar]
- 75.Bu Jassoum S., Fong I.W., Hannach B. Transfusion-transmitted babesiosis in Ontario: First reported case in Canada. Can. Commun. Dis. Rep. 2000;26:9–13. [PubMed] [Google Scholar]
- 76.McQuiston J.H., Childs J.E., Chamberland M.E. Transmission of tick-borne agents of disease by blood transfusion: A review of known and potential risks in the United States. Transfusion. 2000;40:274–284. doi: 10.1046/j.1537-2995.2000.40030274.x. [DOI] [PubMed] [Google Scholar]
- 77.Houghton R.L., Homer M.J., Reynolds L.D. Identification of Babesia microti–specific immunodominant epitopes and development of a peptide EIA for detection of antibodies in serum. Transfusion. 2002;42:1488–1496. doi: 10.1046/j.1537-2995.2002.00215.x. [DOI] [PubMed] [Google Scholar]
- 78.Homer M.J., Lodes M.J., Reynolds L.D. Identification and characterization of putative secreted antigens from Babesia microti. J. Clin. Microbiol. 2003;41:723–729. doi: 10.1128/JCM.41.2.723-729.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Persing D.H., Mathiesen D., Marshall W.F. Detection of Babesia microti by polymerase chain reaction. J. Clin. Microbiol. 1992;30:2097–2103. doi: 10.1128/jcm.30.8.2097-2103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Llewelyn C.A., Hewitt P.E., Knight R.S. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet. 2004;363:417–421. doi: 10.1016/S0140-6736(04)15486-X. [DOI] [PubMed] [Google Scholar]
- 81.Peden A.H., Head M.W., Ritchie D.L. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet. 2004;364:527–529. doi: 10.1016/S0140-6736(04)16811-6. [DOI] [PubMed] [Google Scholar]
- 82.Belay E.D., Maddox R.A., Williams E.S. Chronic wasting disease and potential transmission to humans. Emerg. Infect. Dis. 2004;10:977–984. doi: 10.3201/eid1006.031082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kluter H. The struggle for safer blood: Pathogen inactivation of cellular blood preparations. Blood Ther. Med. 2002;2:42–47. [Google Scholar]
- 84.Corash L. Pathogen reduction technology: Methods, status of clinical trials, and future prospects. Curr. Hematol. Rep. 2003;2:495–502. [PubMed] [Google Scholar]
- 85.Wu Y.Y., Snyder E.L. Safety of the blood supply: Role of pathogen reduction. Blood Rev. 2003;17:111–122. doi: 10.1016/s0268-960x(02)00063-2. [DOI] [PubMed] [Google Scholar]
- 86.Lin L., Cook D.N., Wiesehahn G.P. Photochemical inactivation of viruses and bacteria in platelet concentrates by use of a novel psoralen and long-wavelength ultraviolet light. Transfusion. 1997;37:423–435. doi: 10.1046/j.1537-2995.1997.37497265344.x. [DOI] [PubMed] [Google Scholar]
- 87.Knutson F., Alfonso R., Dupuis K. Photochemical inactivation of bacteria and HIV in buffy-coat–derived platelet concentrates under conditions that preserve in vitro platelet function. Vox Sang. 2000;78:209–216. doi: 10.1159/000031183. [DOI] [PubMed] [Google Scholar]
- 88.Van Voorhis W.C., Barrett L.K., Eastman R.T. Trypanosoma cruzi inactivation in human platelet concentrates and plasma by a psoralen (amotosalen HCl) and long-wavelength UV. Antimicrob. Agents Chemother. 2003;47:475–479. doi: 10.1128/AAC.47.2.475-479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin L. Inactivation of cytomegalovirus in platelet concentrates using Helinx technology. Semin. Hematol. 2001;38:27–33. doi: 10.1016/s0037-1963(01)90121-0. (suppl. 11) [DOI] [PubMed] [Google Scholar]
- 90.Lin L., Alfonso R., Behrman B. Photochemical treatment of platelet concentrates with a novel psoralen and UVA to enhance the safety of platelet transfusions. Infus. Ther. Transfus. Med. 1998;25:39–48. [Google Scholar]
- 91.Lin L., Dikeman R., Molini B. Photochemical treatment of platelet concentrates with amotosalen and UVA inactivates a broad spectrum of pathogenic bacteria. Transfusion. 2004;44:1496–1504. doi: 10.1111/j.1537-2995.2004.04125.x. [DOI] [PubMed] [Google Scholar]
- 92.Ciaravino V. Preclinical safety of a nucleic acid-targeted Helinx compound: A clinical perspective. Semin. Hematol. 2001;38:12–19. doi: 10.1016/s0037-1963(01)90119-2. [DOI] [PubMed] [Google Scholar]
- 93.van Rhenen D.J., Vermeij J., Mayaudon V. Functional characteristics of S-59 photochemically treated platelet concentrates derived from buffy coats. Vox Sang. 2000;79:206–214. doi: 10.1159/000056732. [DOI] [PubMed] [Google Scholar]
- 94.van Rhenen D., Gulliksson H., Cazenave J.P. Transfusion of pooled buffy coat platelet components prepared with photochemical pathogen inactivation treatment: The euroSPRITE trial. Blood. 2003;101:2426–2433. doi: 10.1182/blood-2002-03-0932. [DOI] [PubMed] [Google Scholar]
- 95.Hambleton J., Wages D., Radu-Radulescu L. Pharmacokinetic study of FFP photochemically treated with amotosalen (S-59) and UV light compared to FFP in healthy volunteers anticoagulated with warfarin. Transfusion. 2002;42:1302–1307. doi: 10.1046/j.1537-2995.2002.00220.x. [DOI] [PubMed] [Google Scholar]
- 96.McCullough J., Vesole D.H., Benjamin R.J. Therapeutic efficacy and safety of platelets treated with a photochemical process for pathogen inactivation: The SPRINT trial. Blood. 2004;104:1534–1541. doi: 10.1182/blood-2003-12-4443. [DOI] [PubMed] [Google Scholar]
- 97.Murphy S., Snyder E., Cable R. Transfusion of INTERCEPT platelets vs. reference platelets at doses ≥3 × 1011 results in comparable hemostasis and platelet and RBC transfusion requirements. Results of the SPRINT trial. Blood. 2003;102:815a. [Google Scholar]
- 98.Rios J., Hambleton J., Viele M. Helinxa treated RBC transfusions are well tolerated and show comparable recovery and survival to control RBCs. Transfusion. 2001;41:38s. [Google Scholar]
- 99.Wages D., Stassinopoulous A., Corash L. RBCs treated with Helinx™ pathogen inactivation have recovery and half-life comparable to conventional RBCs in a randomized crossover trial. Hematol. J. 2002;e:171. [Google Scholar]
- 100.Cerus Corporation Baxter and Cerus halt red blood cell clinical trials for investigational pathogen inactivation system. Cerus Corporation. http://www.cers.com/pages/PR/2003/PR100403.html Available at: [Accessed April 13, 2004]
- 101.AuBuchon J.P., Pickard C.A., Herschel L.H. Production of pathogen-inactivated RBC concentrates using PEN110 chemistry: A phase I clinical study. Transfusion. 2002;42:146–152. doi: 10.1046/j.1537-2995.2002.00021.x. [DOI] [PubMed] [Google Scholar]
- 102.Lazo A., Tassello J., Jayarama V. Broad-spectrum virus reduction in red cell concentrates using Inactine PEN110 chemistry. Vox Sang. 2002;83:313–323. doi: 10.1046/j.1423-0410.2002.00234.x. [DOI] [PubMed] [Google Scholar]
- 103.Purmal A., Valeri C.R., Dzik W. Process for the preparation of pathogen-inactivated RBC concentrates by using PEN110 chemistry: Preclinical studies. Transfusion. 2002;42:139–145. doi: 10.1046/j.1537-2995.2002.00020.x. [DOI] [PubMed] [Google Scholar]
- 104.Ohagen A., Gibaja V., Aytay S. Inactivation of HIV in blood. Transfusion. 2002;42:1308–1317. doi: 10.1046/j.1537-2995.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- 105.Mather T., Takeda T., Tassello J. West Nile virus in blood: Stability, distribution, and susceptibility to PEN110 inactivation. Transfusion. 2003;43:1029–1037. doi: 10.1046/j.1537-2995.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- 106.Zavizion B., Serebryanik D., Serebryanik I. Prevention of Yersinia enterocolitica, Pseudomonas fluorescens, and Pseudomonas putida outgrowth in deliberately inoculated blood by a novel pathogen-reduction process. Transfusion. 2003;43:135–142. doi: 10.1046/j.1537-2995.2003.00294.x. [DOI] [PubMed] [Google Scholar]
- 107.Zavizion B., Purmal A., Chapman J. Inactivation of mycoplasma species in blood by Inactine PEN110 process. Transfusion. 2004;44:286–293. doi: 10.1111/j.1537-2995.2004.00647.x. [DOI] [PubMed] [Google Scholar]
- 108.Chapman J.R., Moore K., Butterworth B.E. Pathogen inactivation of RBCs: PEN110 reproductive toxicology studies. Transfusion. 2003;43:1386–1393. doi: 10.1046/j.1537-2995.2003.00497.x. [DOI] [PubMed] [Google Scholar]
- 109.Vitex halts final-stage test of Inactine. Boston Business Journal. November 18, 2003. http://boston.bizjournals.com/boston/stories/2003/11/17/daily11.html?t=printable Available at: [Accessed January 12, 2004]
- 110.Mohr H., Lambrecht B., Knuever-Hopf J. Virus inactivated single-donor fresh plasma preparations. Infus.ther. Transfus.med. 1992;19:79–83. doi: 10.1159/000222588. [DOI] [PubMed] [Google Scholar]
- 111.Aznar J.A., Molina R., Montoro J.M. Factor VIII/von Willebrand factor complex in methylene blue–treated fresh plasma. Transfusion. 1999;39:748–750. doi: 10.1046/j.1537-2995.1999.39070748.x. [DOI] [PubMed] [Google Scholar]
- 112.Wagner S.J. Virus inactivation in blood components by photoactive phenothiazine dyes. Transfus. Med. Rev. 2002;16:61–66. doi: 10.1053/tmrv.2002.29405. [DOI] [PubMed] [Google Scholar]
- 113.Wagner S.J., Skripchenko A., Robinette D. The use of dimethylmethylene blue for virus photoinactivation of red cell suspensions. Dev. Biol. Stand. 2000;102:125–129. [PubMed] [Google Scholar]
- 114.Skripchenko A.A., Wagner S.J. Inactivation of WBCs in RBC suspensions by photoactive phenothiazine dyes: Comparison of dimethylmethylene blue and MB. Transfusion. 2000;40:968–975. doi: 10.1046/j.1537-2995.2000.40080968.x. [DOI] [PubMed] [Google Scholar]
- 115.Wagner S.J., Skripchenko A., Pugh J.C. Duck hepatitis B photoinactivation by dimethylmethylene blue in RBC suspensions. Transfusion. 2001;41:1154–1158. doi: 10.1046/j.1537-2995.2001.41091154.x. [DOI] [PubMed] [Google Scholar]
- 116.Hirayama J., Wagner S.J., Gomez C. Virus photoinactivation in stroma-free hemoglobin with methylene blue or 1,9-dimethylmethylene blue. Photochem. Photobiol. 2000;71:90–93. doi: 10.1562/0031-8655(2000)071<0090:vpisfh>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 117.Goodrich R.P. The use of riboflavin for the inactivation of pathogens in blood products. Vox Sang. 2000;78:211–215. (suppl. 2) [PubMed] [Google Scholar]
- 118.Schuyler R. Use of riboflavin for photoinactivation of pathogens in blood components. Transfus. Apher. Sci. 2001;25:189–190. doi: 10.1016/s1473-0502(01)00119-7. [DOI] [PubMed] [Google Scholar]
- 119.Corbin F., III Pathogen inactivation of blood components: Current status and introduction of an approach using riboflavin as a photosensitizer. Int. J. Hematol. 2002;76:253–257. doi: 10.1007/BF03165125. (suppl. 2) [DOI] [PubMed] [Google Scholar]
- 120.Ruane P.H., Edrich R., Gampp D. Photochemical inactivation of selected viruses and bacteria in platelet concentrates using riboflavin and light. Transfusion. 2004;44:877–885. doi: 10.1111/j.1537-2995.2004.03355.x. [DOI] [PubMed] [Google Scholar]
- 121.Horowitz B., Bonomo R., Prince A.M. Solvent/detergent–treated plasma: A virus-inactivated substitute for fresh frozen plasma. Blood. 1992;79:826–831. [PubMed] [Google Scholar]
- 122.Mast A.E., Stadanlick J.E., Lockett J.M. Solvent/detergent–treated plasma has decreased antitrypsin activity and absent antiplasmin activity. Blood. 1999;94:3922–3927. [PubMed] [Google Scholar]
- 123.Yarranton H., Cohen H., Pavord S.R. Venous thromboembolism associated with the management of acute thrombotic thrombocytopenic purpura. Br. J. Haematol. 2003;121:778–785. doi: 10.1046/j.1365-2141.2003.04360.x. [DOI] [PubMed] [Google Scholar]
- 124.Bluel J., Schmidt I., Willkommen H. Inactivation of parvovirus B19 during pasteurization of human serum albumin. Transfusion. 2002;42:1011–1018. doi: 10.1046/j.1537-2995.2002.00158.x. [DOI] [PubMed] [Google Scholar]
- 125.Lazo A., Tassello J., Ohagen A. Inactivation of human parvovirus B19 by Inactine PEN110. Transfusion. 2003;43:86A. [Google Scholar]
- 126.Bruchmüller I., Janetzko K., Bugert P. Use of a PCR inhibition assay to monitor the photochemical pathogen inactivation process by Helinx technology is feasible. Blood. 2003;102:816a. [Google Scholar]
- 127.Aytay S., Ohagen A., Busch M.R. Development of a sensitive PCR inhibition method to demonstrate HBV nucleic acid inactivation. Transfusion. 2004;44:476–484. doi: 10.1111/j.1537-2995.2003.03306.x. [DOI] [PubMed] [Google Scholar]
- 128.Bell C.F., Botteman M.F., Gao X. Cost-effectiveness of transfusion of platelet component prepared with pathogen inactivation treatment in the United States. Clin. Ther. 2003;25:2464–2486. doi: 10.1016/S0149-2918(03)80288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


