Abstract
Drinking water is a major source of microbial pathogens in developing regions, although poor sanitation and food sources are integral to enteric pathogen exposure. Gastrointestinal disease outcomes are also more severe, due to under-nutrition and lack of intervention strategies in these regions. Poor water quality, sanitation and hygiene account for some 1.7 million deaths a year world-wide (3.1% of all deaths and 3.7% of all DALY’s), mainly through infectious diarrhoea. Nine out of 10 such deaths are in children and virtually all of the deaths are in developing countries. Major enteric pathogens in these children include: rotavirus, Campylobacter jejuni, enterotoxigenic Escherichia coli, Shigella spp. and Vibrio cholerae O1, and possibly enteropathogenic E. coli, Aeromonas spp. V. cholerae O139, enterotoxigenic Bacteroides fragilis, Clostridium difficile and Cryptosporidium parvum. All except the latter are easily control by chlorination of water, but recontamination of treated water is a huge problem. Emerging environmental pathogens, such as Helicobacter pylori and Burkholderia pseudomallei, may well be of significance in some regions. In adults, much less is understood of various sequellae such as myocarditis, diabetes, reactive arthritis and cancers some months–years after initial infections. So in addition to the traditional pathogens (helminths, Entamoeba histolytica, Giardia lamblia hepatitis A and E) various enteroviruses, C. jejuni and H. pylori are emerging issues in adults.
Keywords: Waterborne pathogens, Enteric viruses, Cholera, Parasites
1. Introduction
Disease-causing organisms (pathogens) transmitted via drinking water are predominantly of faecal origin (and therefore known as enteric pathogens) (Ashbolt et al., 2001, Hunter et al., 2002). Since the pioneering epidemiology in the 1850’s, whereby the English physician John Snow established that cholera was waterborne (Paneth et al., 1998), we have amassed a sound understanding of the transmission of various pathogens that cause diarrhoea and other diseases via drinking water (Hunter et al., 2002). Furthermore, the efficacy of drinking water treatment (traditionally by filtration and chlorination) to remove the bacterial pathogens responsible for cholera (Vibrio cholerae) and typhoid fevers (Salmonella typhi and S. paratyphi), is well indexed by the common faecal indicator bacterium Escherichia coli (E. coli), which is excreted in the faeces of all warm-blooded animals and some reptiles (Edberg et al., 2000, Enriquez et al., 2001).
There are however, many enteric pathogens that behave differently to E. coli, particularly with respect to disinfection resistance and environmental persistence (Ashbolt et al., 2001). Of particular concern are the chlorine-resistant parasitic protozoa, such as the environmentally shed oocysts of Cryptosporidium parvum and various enteric viruses (Hambidge, 2001, Li et al., 2002). It is therefore important to match the appropriate indicator for the group of pathogen(s) of interest, noting that there is no universal indicator, as often assumed with thermo-tolerant (faecal) coliforms or E. coli.
This paper provides a brief review of our understanding of waterborne pathogens, with a focus on specific issues relevant to developing regions of the world. It begins by describing the significance of waterborne disease with respect to total global disease burden and summarises the importance of members of the four major pathogen groups—helminths, protozoa, bacteria and viruses. It concludes with a summary of emerging issues.
2. Disease burden via drinking water
The use of water treatment technology is not new, but dating back 6000 years when the Greeks used charcoal filters, boiling, straining and exposure to sunlight to improve the aesthetic quality of drinking water (WHO, 2003b). Yet the drinking water associated outbreak of cholera in Germany during 1892 was the foundation point of our modern understanding of waterborne pathogens. It was shown that residents of Hamburg suffered a very high mortality due to cholera, whilst people in neighbouring Altona served by the same-source water as in Hamburg, but a treatment by slow-sand filtration, helped them to escape the worst ravages of the outbreak. The German microbiologist, Robert Koch first isolated and described the causative agent, which he named Vibrio comma (later renamed to V. cholerae). This lead to sand filtration and taking John Snow’s demonstration (1845) of the efficacy of chlorine disinfection, both became the norm from 1897 for the treatment of piped water in the then developing regions of Europe, United Kingdom and North America. These late nineteenth century innovations resulted in the largest reduction in global disease burden of any intervention since. Similar improvements have been sought in various developing regions of the world, many of which have been reported by Saunders and Warford (1976) and subsequent World Bank funded developments.
Nonetheless, the World Health Organisation (WHO) estimates that about 1.1 billion people globally drink unsafe water (Kindhauser, 2003) and the vast majority of diarrhoeal disease in the world (88%) is attributable to unsafe water, sanitation and hygiene (WHO, 2003a). Approximately 3.1% of annual deaths (1.7 million) and 3.7% of the annual health burden (disability adjusted life years [DALYs]) world-wide (54.2 million) are attributable to unsafe water, sanitation and hygiene. Fig. 1 illustrates a typical rural village water supply system, which is prone to faecal contamination from domestic animals and human excreta.
Fig. 1.
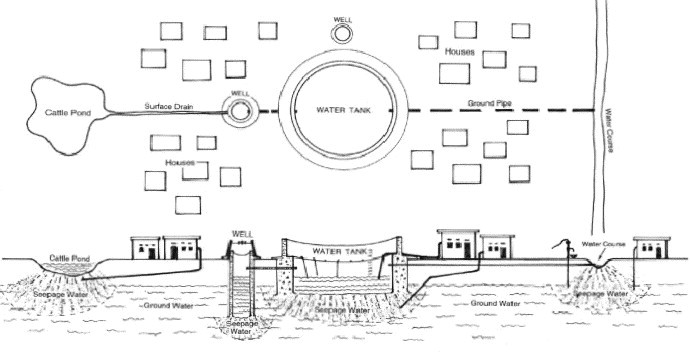
Water supply infrastructure of a typical rural village (from van der Hoek et al., 2003) © Eolss Publishers Co Ltd., (from van der Hoek et al., 2002, with permission from Eolss Publishers Co Ltd).
Due to the interactions between exposure to enteric pathogens via poor quality water, lack of sanitation and inadequate hygiene, data resolving the waterborne component is not generally available. Various estimates in North America suggest up to 15–30% of gastrointestinal disease may come via water (Payment et al., 1997) with a similar component via food (Tauxe, 2002), although ongoing studies of waterborne disease in developed regions have yet to supported the findings of Payment et al.
In developing regions, where there is a higher rate of endemic (background) gastrointestinal disease and pathogen concentrations in wastewater (Martins et al., 1983, Jimenez et al., 2002), the proportion specifically waterborne is rarely identified (as is also the case for most developed regions). Nonetheless, there are specific insights. In a random sample of 2355 Filipino infants over the first year of life, it has been suggested that improving drinking water quality would have no effect in the neighbourhoods with very poor environmental sanitation; yet in areas with better community sanitation, reducing the concentration of thermo-tolerant (faecal) coliforms by two orders of magnitude would lead to a 40% reduction in diarrhoea (Vanderslice and Briscoe, 1995). Further, providing private excreta disposal would be expected to reduce diarrhoea by 42%, while eliminating excreta around the house would lead to a 30% reduction in diarrhoea.
An alternate means of estimating waterborne disease is via a study of sensitive groups, such as human immunodeficiency virus (HIV)-infected patients in a developing world setting, as provided by Wuhib et al. (1994) for Sao Jose Hospital, Fortaleza, Brazil. Of the potential parasitic causes of diarrhoea, only Cryptosporidium parvum and microsporidia were significantly associated with diarrhoeal disease in HIV-AIDS patients (Wuhib et al., 1994). Of particular interest was the finding of C. parvum infections being associated with the rainy season (P<0.005), suggesting contaminated water may be important in its transmission (Wuhib et al., 1994).
Overall, water, sanitation and hygiene-related death (99.8%) occurs in developing countries and 90% are deaths of children (WHO, 2003a). Looking at the 20 leading risks factors for health burden in developing regions, unsafe water, sanitation and poor hygiene is third, behind being underweight or practising unsafe sex (WHO, 2003a). A factor not often considered results from the higher concentrations of pathogens in sewage from developing regions (discussed above); in that WHO guidelines based on the same level of E. coli (assuming the majority come from human excreta) will represent significantly higher risk in developing regions versus the developed regions. In addition, freeing up of food trade from developing to developed regions, pathogen guidelines may need to be tightened, given the practice of irrigated crops with faecally-contaminated waters.
3. Pathogens of concern
The better known waterborne pathogens of concern in developing regions are listed in Table 1 . All of these infectious agents are spread by the faecal–oral route, in which water may play an intermediate role (Table 2 ). Hence, environment, water, food, poor hygiene (poverty and nutritional status) are all factors of importance. In 2001, infectious diseases accounted for an estimated 26% of deaths world-wide (Kindhauser, 2003). Furthermore, social and environmental changes continue to result in new or re-emerging waterborne pathogen issues. For example, climate change was estimated to be responsible in 2000 for approximately 2.4% of world-wide diarrhoea, 6% of malaria in some middle-income countries and 7% of dengue fever in some industrialised countries. In total, the attributable mortality was 154 000 (0.3%) deaths and the attributable burden was 5.5 million (0.4%) DALYs. About 46% of this burden occurred in WHO-designated regions of SEAR-D (23% in AFR-E) and a further 14% in EMR-D (WHO, 2003a).
Table 1.
Waterborne pathogens of concern in developing regions
| Name of micro-organisms | Major diseases | Major reservoirs and primary sources | ||
| Bacteria | ||||
| Salmonella typhi | Typhoid fever | Human faeces | ||
| Salmonella paratyphi | Paratyphoid fever | Human faeces | ||
| Other Salmonella | Salmonellosis | Human and animal faeces | ||
| Shigella spp. | Bacillary dysentery | Human faeces | ||
| Vibrio cholera | Cholera | Human faeces and freshwater zooplankton | ||
| Enteropathogenic E. coli | Gastroenteritis | Human faeces | ||
| Yersinia enterocolitica | Gastroenteritis | Human and animal faeces | ||
| Campylobacter jejuni | Gastroenteritis | Human and animal faeces | ||
| Legionella pneumophila and related bacteria | Acute respiratory illness (legionellosis) | Thermally enriched water | ||
| Leptospira spp. | Leptospirosis | Animal and human urine | ||
| Various mycobacteria | Pulmonary illness | Soil and water | ||
| Opportunistic bacteria | Variable | Natural waters | ||
| Enteric viruses | ||||
| Enteroviruses | ||||
| Polio viruses | Poliomyelities | Human faeces | ||
| Coxsackie viruses A | Aseptic meningitis | Human faeces | ||
| Coxsackie viruses B | Aseptic meningitis | Human faeces | ||
| Echo viruses | Aseptic meningitis | Human faeces | ||
| Other enteroviruses | Encephalities | Human faeces | ||
| Rotaviruses | Gastroenteritis | Human faeces | ||
| Adenoviruses | Upper respiratory and gastrointestinal illness | Human faeces | ||
| Hepatitis A virus | Infectious hepatitis | Human faeces | ||
| Hepatitis E virus | Infectious hepatitis; miscarriage and death | Human faeces | ||
| Norovirus | Gastroenteritis | Fomites and water | ||
| Protozoa | ||||
| Acanthamocba castellani | Amoebic meningoencephalitis | Human faeces | ||
| Balantidium coli | Balantidosis (dysentery) | Human and animal faeces | ||
| Cryptosporidium homonis, C. parvum | Cryptosporidiosis (gastroenteritis) | Water, human and other mammal faeces | ||
| Entamoeba histolytica | Amoebic dysentery | Human and animal faeces | ||
| Giardia lamblia | Giardiasis (gastroenteritis) | Water and animal faeces | ||
| Naegleria fowleri | Primary amoebic meningoencephalitis | Warm water | ||
| Helminths | ||||
| Ascaris lumbricoides | ascariosis | Animal and human faeces | ||
Table 2.
Water supply related diseases
| Group | Diseases |
| Water-borne diseases: diseases spread through water in which water acts as a passive carrier for the infecting pathogens. These diseases depend also on sanitation | Cholera, Typhoid, Bacillary dysentry, Infectious hepatitis, Leptospirosis, Giardiasis, Gastroenteriris etc. |
| Water-related diseases: diseases spread by vectors and insects that live in or close to water. Stagnant ponds of water provides the breeding place for the disease spreading vectors such as mosquitoes, flies and insects. | Yellow fever, Dengue fever, Encephalitis, Malaria, Filariasis (all by mosquitoes), Sleeping sickness (Tsetse fly), Onchocerciasis (Simulium fly) etc. |
| Water-based diseases: diseases caused by infecting agents spread by contact with or ingestion of water. Water supports an essential part of the life cycle of infecting agents such as aquatic snails. | Schistosomiasis, Dracunculosis, Bilharziosis, Philariosis, Oncholersosis, Treadworm and other helminths |
| Water-washed diseases: diseases caused by the lack of adequate quantity of water for proper maintenance of personal hygiene. Some are also depended on poor sanitation. | Scabies, Trachoma (eye-infection), Leprosy, Conjuctivitis, Salmonellosis, Ascariasis, Trichuriasis, Hookworm, Amoebic dysentery, Paratyphoid fever etc. |
Modified from White et al. (1972).
By 2001, a total of 1415 species of infectious organisms known to be pathogenic to humans had been recorded (WHO, 2003b). Some of the more important ones in developing regions are now described.
3.1. Cholera and typhoid
In addition to the enormous endemic disease burden in developing regions, WHO verified 578 infectious disease outbreaks in 132 countries from July 1998 until August 2001 and cholera was the most frequent, with acute diarrhoea as the fourth (WHO, 2002). Further behind in importance were typhoid and paratyphoid fevers (caused by Salmonella typhi and S. paratyphi, respectively), resulting in an annual incidence of about 17 million cases world-wide (Kindhauser, 2003). Both typhoid pathogens are passed in the faeces and urine and people become infected after eating food or drinking beverages that have been handled by a person who is infected or by drinking water that has been contaminated by sewage containing the bacteria. Once the bacteria enter the person’s body they multiply and spread from the intestines, into the bloodstream. Even after recovery from typhoid or paratyphoid, a small number of individuals (called carriers) continue to carry the bacteria.
Cholera behaves slightly differently. In warm regions of the world, the serogroups that cause epidemic cholera (Vibrio cholerae O1 and O139) are endemic in freshwater zooplankton (Colwell et al., 2003) and outbreaks occur in a regular seasonal pattern in developing regions in association with poverty and poor sanitation. The disease is characterised by devastating watery diarrhoea which leads to rapid dehydration and death occurs in 50–70% of untreated patients (Faruque et al., 1998). Cholera toxin (CT), which is responsible for the profuse diarrhoea, is encoded by a lysogenic bacteriophage designated CTX Phi, which probably results in a continual emergence of new epidemic clones. Hence, the ecosystem comprising V. cholerae, CTX Phi in the aquatic environment and the mammalian host offers a complex relationship between pathogenesis and the natural selection of a pathogen (Faruque et al., 1998), wherever there continues to be a lack of adequate water filtration and/or disinfection (Table 3 ).
Table 3.
Selected cholera pandemics since 1817 and principal outcomes
| Period | Principal outcomes |
| 1817–1823 | Possible emergence of a more virulent strain of Vibrio cholerae (V. cholerae). Global trade with the Indian sub-continent carried the cholera vibrio around the world. |
| 1829–1851 | Waterborne transmission of cholerae suspected. |
| 1852–1859 | First isolation of cholera bacterium. Fear of cholera stimulated international co-operation in health. |
| 1881–1896 | Conclusive demonstration that cholera was caused by a bacterium. |
| 1961 | Emergence of V. cholerae O1, biotype El Tor (sixth pandemic) |
| 1992 | Emergence of V. cholerae O139 (seventh pandemic) |
From WHO (2003b).
Hence, given the combinations of poverty, poor water treatment and presence of V. cholerae, the re-entry of cholera into Africa in 1970 and Peru in 1991, although devastating, should not have been a total surprise. Cholera in Africa, after an absence for over a 100 years, accounted for 94% of the total global cholera cases reported to WHO in 2001 (WHO, 2002). In the first half of 2002, outbreaks involving thousands of cases occurred in the Dominican Republic of the Congo, Malawi and Mozambique. A similar story has been seen in South America, with the seventh pandemic reaching Peru in 1991, after an absence of over 100 years in Latin America. Within a year, 400 000 cases and 4000 deaths were reported from 11 South American countries (WHO, 2002).
3.2. Other enteric bacterial pathogens
As children are the most at-risk group, it is of interest to see what types of pathogens result in their diarrhoea. Studies in Dhaka, Bangladesh have demonstrated that some 75% of diarrhoeal children and 44% of control children have an enteric pathogen in their stools. The major pathogens associated with diarrhoea being rotavirus, Cryptosporidium parvum and the following bacterial pathogens: Campylobacter jejuni, enterotoxigenic Escherichia coli [ETEC], enteropathogenic E. coli [EPEC], Shigella spp. and Vibrio cholerae O1 or O139 and to a lesser degree Aeromonas spp., Bacteroides fragilis and Clostridium difficile (Albert et al., 1999). Other potential bacterial pathogens, Plesiomonas shigelloides, Salmonella spp. diffusely adherent E. coli and enteroaggregative E. coil, along with the parasitic protozoa Entamoeba histolytica and Giardia lamblia were not significantly associated with diarrhoea and enteroinvasive E. coli, enterohemorrhagic E. coli [EHEC] and Cyclospora cayetanensis were not detected in any of the children in the Dhaka studies. Viral and parasite pathogens are discussed below, but the two other leading enteric pathogens EPEC and Campylobacter spp. are briefly discussed next.
Typical enteropathogenic E. coli (EPEC) strains are a leading cause of infantile diarrhoea in developing countries, whereas they are rare in industrialized countries, where atypical EPEC seems to be a more important cause of diarrhoea (Trabulsi et al., 2002). What is interesting about typical EPEC (and the closely-related Shigella spp.) is that the only reservoir is thought to be humans, suggesting poor handling of human excreta/water quality as the major problem. For atypical EPEC, both animals and humans can be reservoirs (Trabulsi et al., 2002).
The Campylobacters (Campylobacter jejuni and C. coli) are generally regarded as one of the most common bacterial cause of gastroenteritis world-wide (and 5–14% of all diarrhoea world-wide). In both developed and developing countries, they cause more cases of diarrhoea than Salmonella bacteria. In developed countries, the disease is found mainly in children under the age of 5 years and in young adults. In developing countries, children under 2 are most affected. It is also a frequent cause of traveller’s diarrhoea (WHO, 2003a, WHO, 2003b http://www.who.int/water_sanitation_health/diseases/diseasefact/en/). What is of particular concern is that in some individuals a reactive arthritis (painful inflammation of the joints) can occur, or in rare complications, seizures due to high fever or neurological disorders such as Guillain–Barré syndrome or meningitis (Havelaar et al., 2000).
3.3. Environmental bacterial pathogens
In addition to the zooplankton-found cholera bacteria, there are several aquatic species of bacteria that are opportunistic pathogens of humans (Ashbolt, 2003). Best known are the Legionellae that cause legionnaires disease and Pontiac fever (Atlas, 1999). Several species of Legionella may be transported in drinking waters; however, it is the growth of certain serogroups (sub-types) in warm waters/biofilms that results in the high numbers necessary to be aerosolised and inhaled into human lungs, where the target (phagocyte) cells reside. Environmental growth is most commonly seen in cooling towers and institutional hot water systems (deliberately maintained below 50 °C).
Environmental pathogens that may well be transmitted directly by drinking water in developing regions are fast-growing atypical mycobacteria, Burkholderia pseudomallei and Helicobacter pylori. In tropical regions, Mycobacterium ulcerans is found in aquatic environments and M. avium complex and M. intracellulare bacteria appear to grow in piped (and chlorinated) water biofilms and are a major concern to immuno-suppressed individuals (Falkinham et al., 2001). Burkholderia pseudomallei causes melioidosis, which is hyperendemic in the top end of the Northern Territory of Australia and in parts of south-east Asia. It is the commonest cause of fatal community-acquired septicemic pneumonia (Currie et al., 2000) and has been shown to be associated with drinking water (Inglis et al., 2000). Helicobacter pylori causes up to 95% of duodenal ulcers and 80% of stomach ulcers and between 50 and 90% of all stomach cancers (Rupnow et al., 2000). In developing countries, 70 to 90% of the population carries H. pylori (Dunn and Cohen, 1997). Epidemiological studies in Peru and other developing regions strongly support the transmission of H. pylori via drinking water (Hulten et al., 1996).
3.4. Enteric viruses
Viral gastroenteritis occurs with two epidemiologic patterns, diarrhoea that is endemic in children and outbreaks that affect people of all ages. Viral diarrhoea in children is caused by group A rotaviruses, enteric adenoviruses (largely types 40, 41 and subgenus F), astroviruses (three serogroups) and the human caliciviruses (predominantly Noroviruses); the illness affects all children world-wide in the first few years of life regardless of their level of hygiene, quality of water, food or sanitation, or type of behaviour (Glass et al., 2001).
Rotaviruses represent 80% of recognised viral etiologies and 140 million cases of diarrhoea per year (Albert et al., 1999). They strike young children with similar frequency throughout the world, but the mortality rate is high in developing countries only, with some 870 000 deaths per year (WHO, 1997). It is of interest to note that while UV disinfection is much more effective disinfectant than chlorination for some pathogens (Cryptosporidium, enteroviruses), adenoviruses are very resistant to UV disinfection (Meng and Gerba, 1996).
For most of the enteric viruses infections early in life provide immunity from severe disease upon re-infection. In contrast, epidemic viral diarrhoea is caused primarily by the Norovirus genus of the human caliciviruses. These viruses affect people of all ages, are often transmitted by fecally contaminated food or water and probably represent the most important cause of diarrhoea in developed countries (Lopmam et al., 2003). The tremendous antigenic diversity of caliciviruses and short-lived immunity to infection permit repeated episodes throughout life (Glass et al., 2001), but infection appears to be blood-group related (C. Moe, personal communication). In addition to childhood rotavirus disease, adults in developing regions also suffer large outbreaks from rotaviruses (Hung et al., 1984).
Other waterborne enteric viruses of importance that cause non-diarrhoeal disease include Hepatitis A and E, enterovirus 71 and various enteroviruses (Polio, Coxsachie and ECHO viruses). Despite world-wide immunisation and near eradication of polio virus, there have been recent outbreaks in large metropolitan cities in India due to high population density and the presence of large urban slums (Deshpande et al., 2003). Of further worry have been the outbreaks in the Dominican Republic and Haiti due to derivatives from an attenuated polio virus vaccine (OPV) in use during 1998–1999 (Kew et al., 2002).
Hepatitis A (HAV) and hepatitis E (HEV) viruses are associated with inadequate water supplies and poor sanitation and hygiene, leading to infection and inflammation of the liver. Poor sanitation in developing regions, however, results in early infection of HAV and lifelong protection from the severe ill effects seen in unexposed people (in developed regions) of 50 years or older (Kindhauser, 2003). In the case of HEV, although the mortality rate is usually low (0.07–0.6%), the illness may be particularly severe among pregnant women, with mortality rates reaching as high as 25% (Aggarwal and Krawczynski, 2000), as seen in large outbreaks in China and sporadic outbreaks in the Indian subcontinent, southeast and central Asia, the Middle East, parts of Africa and Mexico (Naik et al., 1992). Recent isolation of a swine virus resembling human HEV has also opened the possibility of zoonotic HEV infection (Halbur et al., 2001). A study in India on hepatitis E infections indicated that 70% of the cases were due to contaminated water and 20% due to food (Gerba and Rose, 2003).
Of particular recent concern in developing regions have been the possibly water-related outbreaks of enterovirus 71, which causes hand-foot-and-mouth disease associated with severe neurological sequellae and death in a small proportion of cases (McMinn, 2002).
3.5. Parasitic protozoa
Waterborne and foodborne parasitic protozoa in developing countries include; Cryptosporidium parvum, Giardia lamblia and Toxoplasma gondii, all of considerable concern in immuno-compromised people, along with Entamoeba histolytica, Cyclospora cayetanensis and Sarcocystis spp. Of these parasitic protozoa, persistent diarrhoea, which is defined as an episode that begins acutely and lasts for at least 14 days, is caused by Cryptosporidium parvum, Giardia lamblia and Entamoeba histolytica (Black, 1993).
In developed regions, C. cayetanensis and Sarcocystis spp. have emerged as causes of traveller’s diarrhoea acquired overseas and for Cyclospora a foodborne pathogen in foods (irrigated with sewage-impacted waters) imported from South America and southern Europe (CDC, 1997, Doller et al., 2002). Hence, along with Cryptosporidium parvum and Giardia lamblia these protozoa seem to be endemic to developing regions. Studies in a peri-urban shanty town in Lima, Peru, suggest that Giardia lamblia is hyperendemic in children (<10 years old) and despite treatment, 98% of the children became re-infected with Giardia lamblia within 6 months (and excreted for a mean of 3.2 months). Hence, treatment of all symptomless Giardia lamblia infections in a developing country hyperendemic for the disease is of questionable value because of rapid re-infection (Gilman et al., 1988).
3.6. Helminths
Ascariasis is an infection of the small intestine caused by Ascaris lumbricoides, a large roundworm (nematode). The eggs of the worm are found in soil contaminated by human faeces or in uncooked food contaminated by soil containing eggs of the worm. Ascariasis occurs with greatest frequency in tropical and subtropical regions and in any areas with inadequate sanitation. While a major cause of morbidity and mortality (up to 10% of the population of the developing world is infected with intestinal worms – a large percentage of which is caused by Ascaris; and world-wide severe Ascaris infections cause approximately 60 000 deaths per year, mainly in children [Kindhauser, 2003]) it is largely a disease of people exposed to untreated wastewater or food grown on it. The 85 000 hectares in the Mezquital Valley of central Mexico is a classic example where raw sewage is used to irrigated food crops and causes significant diarrhoea and Ascariasis (Cifuentes et al., 1993). WHO have long recognised the importance of wastewater associated Ascariasis and set a guideline of <1 ova per litre (in 1989), which is likely to remain in future guidelines (Blumenthal and Peasey, 2002).
Other important enteric helminths in developing regions include, Strongyloides spp. and Trichuris trichiura (Hodges, 1993). Hence there are a range of helminths potentially transmitted by water, but due to their large size (ova >40 μm dia) they readily settle out in treatment ponds and are easily removed from drinking water by filtration. Hence, helminths are generally less of a problem via drinking water than the smaller microbial pathogens discussed above.
4. Conclusions
The current major obstacles to human health in developing regions are well understood and a large component relates to unsafe water, poor sanitation and inappropriate hygiene (WHO, 2003a). There are, however, several emerging waterborne issues. Foremost is the rapid urbanisation of humans in developing regions and the further stress that places on inadequate water supply and sanitation. Associated with increased human activity is the eutrophication of waterways and the resultant increases in diseases. For example, cholera outbreaks are well known to be associated with phytoplankton blooms in nutrient-rich coastal waters. Climate change too is now seen as a reality, with not only a change in the distribution of rainfall, but one also of greater extremes in global weather patterns. Major waterborne outbreaks typically follow large storm events in developing regions. Yet perhaps the greatest threat of all lies with the nature of microbial evolution, significantly increases with high density living and close association with animals. SARS has been a recent messenger to remind us of the significance small genetic changes can have in what was regarded as a relatively benign Coronavirus (Kuiken et al., 2003). Not only do we have to contend with continual evolution of new pathogens, but decline of the ozone layer also has microbial health implications. For example, various diseases have been shown to increase due to UV light suppression of human defence systems (Norval, 2003). In conclusion, despite our efforts, pathogens will always be a major issue for human health, and particularly so in developing regions.
References
- Aggarwal R, Krawczynski K. Hepatitis E: an overview and recent advances in clinical and laboratory research. J. Gastroenterol. Hepatol. 2000;15(1):9–20. doi: 10.1046/j.1440-1746.2000.02006.x. [DOI] [PubMed] [Google Scholar]
- Albert M.J, Faruque A.S.G, Faruque S.M, Sack R.B, Mahalanabis D. Case-control study of enteropathogens associated with childhood diarrhoea in Dhaka, Bangladesh. J. Clin. Microbiol. 1999;37(11):3458–3464. doi: 10.1128/jcm.37.11.3458-3464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbolt, N.J., 2003. Methods to identify and enumerate frank and opportunistic bacterial pathogens in water and biofilms. In: Bartram, J., Cotruvo, J., Exner, M., Fricker, C., Glasmacher, A. (Eds.), Heterotrophic Plate Counts and Drinking-water Safety. The Significance of HPCs for Water Quality and Human Health, IWA Publishing, London (Chapter 9), pp. 146–176.
- Ashbolt, N.J., Grabow, W.O.K., Snozzi, M., 2001. Indicators of microbial water quality. In: Fewtrell, L., Bartram, J. (Eds.), Water Quality: Guidelines, Standards and Health. Risk assessment and management for water-related infectious disease. IWA Publishing, London (Chapter 13), pp. 289–315.
- Atlas R.M. Legionella: from environmental habitats to disease pathology, detection and control (Review) Environ. Microbiol. 1999;1(4):283–293. doi: 10.1046/j.1462-2920.1999.00046.x. [DOI] [PubMed] [Google Scholar]
- Black R.E. Persistent diarrhoea in children of developing countries. Pediatr. Infect. Dis. J. 1993;12(9):751–761. doi: 10.1097/00006454-199309000-00010. [DOI] [PubMed] [Google Scholar]
- Blumenthal, U., Peasey, A., 2002. Critical Review of Epidemiological Evidence of the Health Effects of Wastewater and Excreta Use in Agriculture. London School of Tropical Medicine, London.
- CDC, 1997. Update: outbreaks of cyclosporiasis: United States and Canada, 1997. Morb. Mort. Weekly Report 46, 521–523. [PubMed]
- Cifuentes E, Blumenthal U, Ruizpalacios G, Bennett S, Quigley M, Peasey A, Toerozlvarez H. Health problems related to irrigation with waste water in Mexico (Spanish) Salud Publica de Mexico. 1993;35(6):614–619. [PubMed] [Google Scholar]
- Colwell R.R, Huq A, Islam S.M, Aziz K.M.A, Yunus M, Khan H.N, Mahmud A, Sack R.B, Nair G.B, Chakraborty J, Sack D.A, Russek-Cohen E. Reduction of cholera in Bangladeshi villages by simple filtration. Proc. Natl Acad. Sci. 2003;100(3):1051–1055. doi: 10.1073/pnas.0237386100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie B.J, Fisher D.A, Howard D.M, Burrow J.N.C, Selvanayagam S, Snelling P.L, Anstey N.M, Mayo M.J. The epidemiology of melioidosis in Australia and Papua New Guinea. Acta Tropica. 2000;74(2–3):121–127. doi: 10.1016/s0001-706x(99)00060-1. [DOI] [PubMed] [Google Scholar]
- Deshpande J.M, Shetty S.J, Siddiqui Z.A. Environmental surveillance system to track wild polio virus transmission. Appl. Environ. Microbiol. 2003;69(5):2919–2927. doi: 10.1128/AEM.69.5.2919-2927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doller P.C, Dietrich K, Filipp N, Brockmann S, Dreweck C, Vonthein R, Wagner-Wiening C, Wiedenmann A. Cyclosporiasis outbreak in Germany associated with the consumption of salad. Emerging Infect. Dis. 2002;8(9):992–994. doi: 10.3201/eid0809.10.3201/eid0809.010517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B.E, Cohen H. Helicobacter pylori. Clin. Microbiol. Rev. 1997;10(4):720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edberg S.C, Rice E.W, Karlin R.J, Allen M.J. Escherichia coli: the best biological drinking water indicator for public health protection. J. Appl. Microbiol. Symp. Suppl. 2000;88(Suppl. S):106S–116S. doi: 10.1111/j.1365-2672.2000.tb05338.x. [DOI] [PubMed] [Google Scholar]
- Enriquez C, Nwachuku N, Gerba C.P. Direct exposure to animal enteric pathogens. Rev. Environ. Health. 2001;16(2):117–131. doi: 10.1515/reveh.2001.16.2.117. [DOI] [PubMed] [Google Scholar]
- Falkinham J.O, Norton C.D, LeChevallier M.W. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other mycobacteria in drinking water distribution systems. Appl. Environ. Microbiol. 2001;67(3):1225–1231. doi: 10.1128/AEM.67.3.1225-1231.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faruque S.M, Albert M.J, Mekalanos J.J. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae (Review) Microbiol. Mol. Biol. Rev. 1998;62(4):1301. doi: 10.1128/mmbr.62.4.1301-1314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C.P, Rose J.B. International guidelines for water recycling: microbiological considerations. Water Sci. Tech. Water. Supply. 2003;3(4):311–316. [Google Scholar]
- Gilman R.H, Marquis G.S, Miranda E, Vestegui M, Martinez H. Rapid reinfection by Giardia lamblia after treatment in a hyperendemic Third World community. Lancet. 1988;1(8581):343–345. doi: 10.1016/s0140-6736(88)91131-2. [DOI] [PubMed] [Google Scholar]
- Glass, R.I., Bresee, J., Jiang, B., Gentsch, J., Ando, T., Fankhauser, R., Noel, J., Parashar, U., Rosen, B., Monroe, S.S., 2001. Gastroenteritis viruses: an overview. Novartis Found. Symp. 238, 5–19/19–25 (discussion). [DOI] [PubMed]
- Halbur P, Kasorndorkbua C, Gilbert C, Guenette D, Potters M, Purcell R, Emerson S, Toth T, Meng X.J. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J. Clin. Microbiol. 2001;39:918–923. doi: 10.1128/JCM.39.3.918-923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambidge A. Reviewing efficacy of alternative water treatment techniques. Health Estate. 2001;55(6):23–25. [PubMed] [Google Scholar]
- Havelaar A.H, de Wit M.A, van Koningsveld R, van Kempen E. Health burden in The Netherlands due to infection with thermophilic Campylobacter spp. Epidemiol. Infect. 2000;125:505–522. doi: 10.1017/s0950268800004933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges M. Diarrhoeal disease in early childhood: experiences from Sierra Leone. Parasite. 1993;107(73):S37–S51. doi: 10.1017/s0031182000075491. [DOI] [PubMed] [Google Scholar]
- Hulten K, Han S.W, Enroth H, Klein P.D, Opekun A.R, Gilman R.H, Evans D.G, Engstrand L, Graham D.Y, El-Zaatari F.A.K. Helicobacter pylori in the drinking water in Peru. Gastroenterology. 1996;110(4):1031–1035. doi: 10.1053/gast.1996.v110.pm8612990. [DOI] [PubMed] [Google Scholar]
- Hung T, Chen G.M, Wang C.G, Yoa H.L, Fang Z.Y, Chao T.X, Chou Z.Y, Ye W, Chang X.J, Den S.S. Waterborne outbreak of rotavirus diarrhoea in adults in China caused by a novel rotavirus. Lancet. 1984;1(8387):1139–1142. [PubMed] [Google Scholar]
- Hunter, P.R., Waite, M., Ronchi, E., 2002. Drinking Water and Infectious Disease: Establishing the Links. IWA Publishing, London.
- Inglis, T.J.J., Garrow, S.C., Henderson, M., Clair, A., Sampson, J., O’Reilly, L., Cameron, B., 2000. Outbreak strain of Burkholderia pseudomallei traced to water treatment plant. Emerging Infect. Dis. 6 (1), 56–59. [DOI] [PMC free article] [PubMed]
- Jimenez B, Maya C, Sanchez E, Romero A, Lira L, Barrios J.A. Comparison of the quantity and quality of the microbiological content of sludge in countries with low and high content of pathogens. Water Sci. Tech. 2002;46(10):17–24. [PubMed] [Google Scholar]
- Kew, O., Morris-Glasgow, V., Landaverde, M., Burns, C., Shaw, J., Garib, Z., André, J., Blackman, E., Freeman, C.J., Jorba, J., Sutter, R., Tambini, G., Venczel, L., Pedreira, C., Laender, F., Shimizu, H., Yoneyama, T., Miyamura, T., van der Avoort, H., Oberste, M.S., Kilpatrick, D., Cochi, S., Pallansch, M., de Quadros, C., 2002. Outbreak of poliomyelitis in Hispaniola associated with circulating type I vaccine-derived polio virus. Science Express Reports published online 14 March 2002 10.1126/science.1068284
- Kindhauser, M.K., 2003. Global defence against the infectious disease threat. Communicable Diseases 2002. World Health Organization, Geneva.
- Kuiken T, Fouchier R.A.M, Schutten M, Rimmelzwaan G.F, van Amerongen G, van Riel D, Laman J.D, de Jong T, van Doornum G, Lim W, Ling A.E, Chan P.K.S, Tam J.S, Zambon M.C, Gopal R, Drosten C, van der Werf S, Escriou N, Manuguerra J.C, Stöhr K, Peiris J.S.M, Osterhaus A.D.M.E. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362(9280):263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.W, Xin Z.T, Wang X.W, Zheng J.L, Chao F.H. Mechanisms of inactivation of hepatitis A virus by chlorine. Appl. Environ. Microbiol. 2002;68(10):4951–4955. doi: 10.1128/AEM.68.10.4951-4955.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopmam B.A, Reacher M.H, van Duijnhoven Y, Hanon F.X, Brown D, Koopmans M. Viral gastroenteritis outbreaks in Europe, 1995–2000. Emerging Infect. Dis. 2003;9(1):90–96. doi: 10.3201/eid0901.020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins M.T, Soares L.A, Marques E, Molina A.G. Human enteric viruses isolated from influents of sewage treatment plants in Sao Paulo, Brazil. Water Sci. Tech. 1983;15(5):69–73. [Google Scholar]
- McMinn P.C. An overview of the evolution of enterovirus 71 and its clinical and public health significance (Review) FEMS Microbiol. Rev. 2002;26(1):91–107. doi: 10.1111/j.1574-6976.2002.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Meng Q.S, Gerba C.P. Comparative inactivation of enteric adenoviruses, polio virus and coliphages by ultraviolet irradiation. Water Res. 1996;30(11):2665–2668. [Google Scholar]
- Naik S.R, Aggarwal R, Salunke P.N, Mehoratra N.N. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull. World Health Org. 1992;70:597–604. [PMC free article] [PubMed] [Google Scholar]
- Norval M. The consequences of sunlight exposure for human viral infections. J. Appl. Environ. Sci. Pub. Health. 2003;1(1):23–32. [Google Scholar]
- Paneth N, Viten-Johansen P, Brody H, Al E. A rivalry of foulness: official and unofficial investigations of the London cholera epidemic of 1854. Am. J. Pub. Health. 1998;88(10):1545–1553. doi: 10.2105/ajph.88.10.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payment P, Siemiatychi J, Richardson L, Renaud G, Franco E, Prévost M. A prospective epidemiological study of gastrointestinal health effects due to the consumption of drinking water. Int. J. Environ. Health Res. 1997;7:5–31. [Google Scholar]
- Rupnow M.F.T, Shachter R.D, Owens D.K, Parsonnet J. A dynamic transmission model for predicting trends in Helicobacter pylori and associated diseases in the United States. Emerging Infect. Dis. 2000;6(3):228–237. doi: 10.3201/eid0603.000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders, R.J., Warford, J.J., 1976. Village Water Supply. The John Hopkins University Press, Baltimore.
- Tauxe R.V. Emerging foodborne pathogens. Int. J. Food Microbiol. 2002;78(1–2):31–41. doi: 10.1016/s0168-1605(02)00232-5. [DOI] [PubMed] [Google Scholar]
- Trabulsi L.R, Keller R, Tardelli Gromes T.A. Typical and atypical enteropathogenic Escherichia coli. Emerging Infect. Dis. 2002;8(5):508–513. doi: 10.3201/eid0805.010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoek, W., Boelee, E., Konradsen, K., 2003. Irrigation, domestic water supply and human health. In, on–line version of Encyclopedia of Life Support Systems, http://www.greenplanet.eolss.net/cgi-bin/.
- Vanderslice J, Briscoe J. Environmental interventions in developing countries—interactions and their implications. Am. J. Epidemiol. 1995;141(2):135–144. doi: 10.1093/oxfordjournals.aje.a117401. [DOI] [PubMed] [Google Scholar]
- White, G.F., Bradley, D.J., White, A.U., 1972. Drawers of water domestic water use in East Africa. University of Chicago Press, Chicago, IL.
- WHO 2002. Emerging and epidemic-prone diseases. In: Global Defence Against the Infectious Disease Threat. World Health Organization, Geneva (Chapter 4).
- WHO 2003a. Quantifying selected major risks to health. The World Health Report 2002. World Health Organization, Geneva (Chapter 4).
- WHO 2003b. Emerging Issues in Water and Infectious Disease. World Health Organization, Geneva.
- Wuhib T, Silva T.M.J, Newman R.D, Garcia L.S, Pereira M.L.D, Chaves C.S, Wahlquist S.P, Bryan R.T, Guerrant R.L, Sousa A.D, Dequeiroz T, Sears C.L. Cryptosporidial and microsporidial infections in human immunodeficiency virus-infected patients in Northeastern Brazil. J. Infect. Dis. 1994;170(2):494–497. doi: 10.1093/infdis/170.2.494. [DOI] [PubMed] [Google Scholar]


