Abstract
Pathologic and laboratory investigations are essential when identification of the specific cause of bovine respiratory disease is needed. Considerations for planning a diagnostic investigation include the goals of the inquiry, the potential impact of the diagnosis, the plausible causes based on the clinical and epidemiologic appearance, and the relative merits of the available diagnostic strategies. This review uses 4 cases to outline different approaches to laboratory diagnosis. The postmortem examination is described, along with the patterns and gross appearance of lesions, considerations for effective sampling from appropriately selected animals, and reasons for discrepant or negative laboratory test results.
Keywords: Postmortem examination, Laboratory diagnostic investigation, Bovine respiratory disease complex, Enzootic pneumonia, Bovine respiratory syncytial virus, Dictyocaulus viviparus, Acute phase proteins
Key Points.
-
•
A routine diagnostic investigation of bovine respiratory disease should be comprehensive and robust, allowing identification of common but also unexpected diseases. This may require examination of multiple animals, sampling for histopathology and microbiologic testing, and storing samples pending the outcome of initial tests.
-
•
An effective and concise clinical history is an essential element of any laboratory submission. In addition to the clinical diagnosis, it must include specific clinical observations that help the diagnostician to interpret the laboratory findings.
-
•
Postmortem examination reveals the distribution and texture of lesions, indicating one or more morphologic patterns of lung disease, and thereby suggesting causes of disease and providing tissues for confirmatory testing. To be relevant, the animals examined must be representative of the clinical problem, early in the course of disease, and untreated if possible; the multiple tissues analyzed should focus on primary lesions but represent the spectrum of changes observed. Important diagnoses will be overlooked if the examination does not include the upper respiratory tract, caudal bronchi, pulmonary arteries, and heart, and nonspecific changes in the lungs must be recognized.
-
•
Serology, although limited by the time needed for convalescent sampling, is an effective and sensitive method of establishing the cause of respiratory disease outbreaks.
-
•
The investigation is doomed if the animals sampled are not representative of the disease problem, if they are sampled too late in the disease course, if too few or inappropriate tissues are sampled, if specimens become autolyzed or undergo freeze-thaw damage, or if vaccination or maternal antibody interferes with detection of the pathogen.
-
•
Laboratory testing, such as measurement of serum haptoglobin, is useful as an indicator of inflammatory disease and a measure of disease severity.
Respiratory disease continues to be a major cause of clinical disease, mortality, production loss, and reduced carcass quality. Because the various causes of bovine respiratory disease (BRD) have overlapping clinical manifestations, pathologic and laboratory investigations are often required in those cases for which a specific diagnosis is required. A specific diagnosis is useful to direct antimicrobial or anthelmintic therapy, vaccination programs, and biosecurity practices and to satisfy the curiosity and concern of producers and veterinarians.
This review uses 4 cases to illustrate diagnostic approaches to the laboratory diagnosis of BRD, recognizing that the clinical, pathologic, and microbiologic aspects of the investigation each contribute key pieces of information. Some aspects are described in detail elsewhere, including descriptions of specific diseases,1, 2, 3, 4, 5, 6 neonatal respiratory distress syndrome,7, 8 necropsy technique, interpretation of necropsy findings,9, 10 sample submission to laboratories,11, 12 and interpretation of antimicrobial susceptibility testing.13, 14
Case 1: an outbreak of respiratory disease in dairy calves
An outbreak of respiratory disease occurred in an unvaccinated 350-cow dairy herd in December. Temperatures had been unseasonably warm, above freezing, then fell to –10°C overnight. Signs of disease appeared suddenly the following day. Adult cows were not affected. The outbreak involved 4- to 7-month-old calves kept as replacement stock. There was no history of recent introductions to the herd. At the time of examination, 30 calves of the group of 60 were affected, and 2 died as a result of respiratory distress. A necropsy examination performed by the practitioner revealed subcutaneous and pulmonary emphysema. The entire lung was firmer than normal. The cranioventral 70% of the lungs were darker red than the also-firm dorsocaudal lung.
Before embarking on an expensive diagnostic investigation, there is merit in reflecting on the objectives and the questions that might reasonably be answered. The goals vary considerably depending on the production system and the particular circumstances of each case. For respiratory disease involving beef cattle that have recently arrived to a feedlot, the important questions may be:
-
•
Does the problem involve one or several diseases?
-
•
Does the problem represent the usual occurrence of shipping fever pneumonia or something more unusual and unexpected?
-
•
Are the health protocols for processing calves at arrival and adaptation to the feedlot working adequately?
-
•
Is the age of the lesions consistent with the apparent timing of the clinical disease?
In contrast, the goal in this unexpected outbreak of disease in a group of dairy calves is to establish the exact cause to efficiently control the outbreak and institute changes to prevent future occurrences.
The major causes of respiratory diseases of cattle in North America, along with a summary of gross lesions and method of diagnosis, are listed in Table 1 . Neonatal respiratory distress syndrome is a clinically distinct entity with multiple causes and is described elsewhere.7, 8 In this case, an outbreak of viral respiratory disease was considered most likely, but bacterial pneumonia or lungworm should also be considered.
Table 1.
Causes of BRD
| Cause | Gross Lesions | Laboratory Diagnosis |
|---|---|---|
| Pasteurellaceae: M haemolytica, Histophilus somni, and Pasteurella multocida; Mannheimia varigena, and Bibersteinia trehalosi | Cranioventral reddening and firm to hard consolidation; may have irregularly shaped nonfriable foci of coagulation necrosis, interlobular edema (marbling), or fibrinous pleuritis | Histopathologic examination, bacterial culture of consolidated lung near the border with unaffected lung Live animal: clinical findings, response to treatment, ± culture of transtracheal wash |
| M bovis | Cranioventral reddening and collapse or consolidation, with round dry friable foci of caseous necrosis (see Fig. 1A, B). | Histopathology, IHC aCulture or RT-PCRb identifies M bovis but does not clarify the role in disease |
| Histophilus somni pleuritis | Fibrinous pleuritis, with or without consolidation of lung tissue (see Fig. 1G) | Culture of pleural exudate ± kidney, spleen, joints |
| Secondary or opportunistic bacterial pathogens: Arcanobacterium pyogenes, Streptococcus spp, etc | Cranioventral bronchopneumonia, abscesses, and/or bronchiectasis | Bacterial culture, but identifying the pathogen is not clinically useful |
| IBR (BHV-1) | Nasal cavity and trachea: multifocal to confluent erosions, covered with fibrin or necrotic debris Distinguish from expectorated lung exudate |
Acute stage: VI,b PCR, or IHC Live animal: serologic investigation (acute and convalescent); or VI or PCR from nasal swabs |
| Viral pneumonia: BRSV, BCV, BHV-1, BPI3V | Cranioventral lung is red-purple and slightly firm-rubbery; dorsocaudal lung is similar with edema ± emphysema (see Fig. 1D) Cranial lung may have bronchopneumonia if there is secondary bacterial infection | Acute stage: PCR, IHC, VI, or antigen-capture ELISA (BRSV is labile and difficult to isolate), and RT-PCR is more sensitive15 BCV isolation requires special HRT-18 cell lines1 Live animal: serology (acute and convalescent); or VI or PCR using nasal swabs |
| Infection with BVDV or the viruses listed as predisposing causes of bacterial pneumonia | Cranioventral lung is red and firm-to-hard as a result of bacterial bronchopneumonia; dorsocaudal lung is normal or slightly firm-rubbery | As given for respiratory viruses Samples for BVDV testing: skin or EDTA blood; or mucosal erosions, Peyer patch, spleen, lymph node |
| Dictyocaulus viviparus: acute prepatent disease as a result of larval migration, or chronic patent or postpatent infection | Acute: lungs are diffusely red, edematous, and firm (interstitial pneumonia) Chronic: adult worms in caudal bronchi (see Fig. 1H), lobular atelectasis, or consolidation of lung tissue, especially in dorsocaudal areas of lung |
Acute: histopathology to identify larvae/immature parasites in lung. Chronic: gross finding of worms in bronchi; histopathologic examination as given Live animals: Baermann test to identify larvae in chilled feces, but not in prepatent or postpatent infections Serologic tests may be useful for herd diagnosis16 |
| Ascaris suum larval migration | Generalized distribution of lobular atelectasis or consolidation | Histopathologic examination to identify larvae in lung Eggs or larvae are NOT present in feces |
| Heart disease causing pulmonary edema | Diffusely red-purple heavy lungs, interlobular edema, ooze fluid from the cut surface, abundant foam or fluid in trachea | Clinical, gross, or histologic evidence of heart disease |
| Anaphylaxis causing pulmonary edema and bronchoconstriction | Diffusely red-purple heavy lungs, interlobular edema, ooze fluid from the cut surface, abundant foam or fluid in trachea | Clinical history; rule out cardiac causes of edema; histopathology shows edema ± eosinophils depending on the timing |
| Tuberculosis (M bovis) | Single or multiple soft white raised granulomas, often with caseous necrosis and/or mineralization in the center | Histopathology and acid-fast stain ± special culture procedure Similar gross lesions can represent bacterial or fungal pyogranulomas, chronic abscesses, or hydatid cysts17 |
| Ingested toxins: l-tryptophan/3-methylindole (lush forage), 4-ipomeanol (moldy sweet potatoes), perilla ketone (purple mint) | Lungs are diffusely edematous and firm (interstitial pneumonia, see Fig. 1C) | Histopathologic examination confirms interstitial lung injury Diagnosis is based on clinical findings and identification of the source of toxin |
| Inhaled toxins: silo or pit gas, etc | As given | As given |
| Hypersensitivity pneumonitis | Diffusely firm and heavy lungs | Histopathologic and clinical findings |
| Contagious bovine pleuropneumonia (Mycoplasma mycoides ssp mycoides SC) | Often unilateral, caudal lung lobe consolidation with sequestrum formation, and fibrinous pleuritis | Culture of nasal swabs, pleural exudate, lung, lymph node Reportable OIE List A disease |
| Tracheal edema and hemorrhage (“honker”) syndrome | Tracheal mucosa is thickened by edema and hemorrhage, obstructing the lumen | Gross findings |
| Pulmonary emphysema, secondary to nonpulmonary disease | Interlobular septa distended by air bubbles, especially in dorsocaudal lung, with normal texture of lung lobules | Gross findings |
Abbreviations: BCV, bovine coronavirus; BHV, bovine herpesvirus 1; BPI3V, bovine parainfluenza virus 3; BRSV, bovine respiratory syncytial virus; BVDV, bovine viral diarrhea virus; ELISA, enzyme-linked immunosorbent assay; IHC, immunohistochemistry; OIE, world organization for animal health; PCR, polymerase chain reaction; PI, persistently infected; RT, reverse transcriptase; VI, virus isolation.
Tests performed on fixed tissues: histopathology and IHC (for test availability, see http://ihc.sdstate.org/). With the exception of BVDV IHC for detection of PI animals, IHC is rarely a stand-alone test. The decision to pursue IHC testing is typically based on histologic lesions present, and results are interpreted in the context of these lesions.
Tests performed on chilled or frozen tissues: VI, PCR, RT-PCR, and antigen-capture ELISA and other immunoassays.
The merits of the various diagnostic options are outlined in Table 2 . The diagnosis in this case was based on necropsy examination of the calf that died, with laboratory testing of samples obtained at necropsy. Serologic investigation would have been a reliable method to reach the same diagnosis, albeit with the limitations that the results would not be known for several weeks, and laboratory costs could be higher. A third option would involve laboratory testing of samples obtained from live animals, such as nasal swabs or transtracheal washes. Although this can achieve a rapid diagnosis and has the flexibility of being based on few or many samples, it is a riskier option because most respiratory viral infections are transient, and testing therefore requires collection of samples early in the course of disease. Practitioners may not examine the herd until this early stage of infection has passed, whereas in large or extensively managed groups it may not be known which animals have most recently developed disease.
Table 2.
Diagnostic approaches to determine the cause of an outbreak of BRD
| Diagnostic Approach | Advantages | Disadvantages |
|---|---|---|
| Detect an agent in clinical samples: nasal or nasopharyngeal swabs, transtracheal wash, bronchoalveolar lavage. | Rapid test results are possible. Multiple animals may be sampled, cases and controls. Identification of a virus may be highly significant, depending on the nature of the herd. |
Viral infections are transient. Identification is impaired by rising antibody titers. Bacterial isolates from nasal samples are of dubious significance. |
| Serology to detect rising antibody titers | High sensitivity: most respiratory pathogens of cattle induce a strong antibody response. Less time-dependent than other methods, and may be effective even relatively late in the course of disease. Multiple animals tested makes the result relevant to the herd problem. |
Requires convalescent serum, so results are not available for >3 wk. Testing of multiple samples can be expensive. Correlation of seroconversion with clinical disease may be impossible in situations when viral infections are expected to be common, such as recently arrived feedlot cattle. |
| Postmortem examination with subsequent laboratory testing | Gross and histopathologic examination usually suggests a cause and may be pathognomonic. The investigation is comprehensive and robust and leads to diseases that were not previously considered or recognized. Lung and trachea are easily sampled and are often the ideal samples for laboratory diagnosis. |
Diagnosis may be based on few animals. Animals that die may not be representative of the herd problem. Death may occur at a subacute stage of disease, when viral pathogens are no longer present. Cases are often treated with antibiotics before death, precluding isolation of bacterial pathogens and resulting in misleading antimicrobial sensitivity data. |
Key elements of the postmortem examination are outlined in Box 1 . Veterinarians are faced with too many respiratory diseases to remember the features of each, so classification of lung diseases into morphologic patterns is helpful for diagnostic recognition of diseases, for understanding the pathogenesis and relationships between cause and clinical signs, and for predicting the chronic sequelae that may be seen in survivors. Pathologists vary in their exact categorization of pulmonary lesions and in the terminology applied, but the following system is broadly used. The main morphologic patterns, gross appearances, and causes are summarized in Table 3 and illustrated in Fig. 1 A–H).
Box 1. Gross pathologic examination in cases of respiratory disease.
A. Lesions in other body systems
Postmortem examination in cases of respiratory disease must not only focus on the respiratory system but also search for clues in other body systems.
-
•
Bovine viral diarrhea virus (BVDV) is an important predisposing cause of bacterial pneumonia, so oral and esophageal erosions and Peyer patch necrosis should be actively sought at necropsy. However, many BVDV-infected calves have no gross lesions.
-
•
Heart disease causes pulmonary edema with dyspnea and hyperpnea, and the prosector should search for abscesses or infarcts in the left ventricular papillary muscle caused by Histophilus somni, for bacterial endocarditis of the valves, and for congenital anomalies.
-
•
Heart lesions may not be detected grossly and the diagnosis may depend on histologic evidence of myocarditis caused by BVDV or on myocardial necrosis resulting from toxin exposure or white muscle disease. In cases of respiratory distress in which a reliable diagnosis is not achieved by gross necropsy examination, formalin-fixed samples of heart (and other tissues) should always be included in the diagnostic investigation. For example, we recognize a fatal condition in feedlot cattle with mild bronchopneumonia as the only gross lesion, but lymphocytic necrotizing arteritis attributed to BVDV infection is obvious if multiple sections of lung, heart, and other organs are examined histologically.
-
•
When opening the pulmonic valve, continue the incision to open the main branches of the pulmonary arteries. Pulmonary emboli—a cause of either peracute or chronic disease—are routinely overlooked if this important step is neglected (see Fig. 1F).
B. Examination of the upper airways
-
•
The pluck—tongue, larynx, esophagus, trachea, lungs, and heart—should be removed at the time of necropsy. If the lungs are only examined in situ, unilateral lesions will be overlooked, and it is unlikely that the upper respiratory tract and heart will be effectively examined. Evaluation of the nasal cavity requires sectioning with a saw or axe. Examination of the upper respiratory tract may reveal miscellaneous causes of inspiratory dyspnea, including laryngeal lesions of diphtheria, focal masses obstructing airflow, or cellulitis of the head or neck.
-
•
In infectious bovine rhinotracheitis (IBR), the primary viral infection typically causes nonfatal febrile illness with respiratory distress; fatalities generally result from secondary bacterial bronchopneumonia. Lesions of tracheal erosion are essentially pathognomonic of IBR. In cases of bacterial bronchopneumonia, expectorated mucus, pus, or fibrin covers the tracheal mucosa and can easily be mistaken for lesions of IBR. However, this material is easily wiped from the tracheal mucosa, revealing a smooth intact mucosa, whereas cases of IBR have adherent exudate or multifocal pale areas of mucosal necrosis. Nevertheless, in autolyzed cases, this distinction can be problematic.
-
•
Examination of the large airways should not end at the tracheal bifurcation and must persevere to the bronchi in the caudal lung. Adult Dictyocaulus worms preferentially reside in these caudal bronchi (see Fig. 1H), and finding these worms provides an immediate and high-impact diagnosis that may forestall hundreds of dollars of laboratory testing.
C. Examination of the lungs
In many cases, the lungs hold the important clues that direct subsequent investigation, but some misleading changes must be recognized.
-
•
Red discoloration of lung tissue commonly results from postmortem pooling of blood as veins dilate after death. Visible reddening indicates specific areas of the lung that should be carefully palpated and perhaps sampled histologically. However, diffuse or patchy red discoloration throughout the lung is usually a postmortem artifact, and localized discoloration with no change in texture should be interpreted with much caution. The dorsocaudal lung of cattle is normally white and opaque because the pleura in this area is thick and fibrous, but this must not be mistaken for an abnormality (see Fig. 1C, E). Interlobular and subpleural emphysema, appearing as lines of air-filled bubbles separating the lobules, arises commonly in cattle that are dyspneic for any reason (see Fig. 1C, D). Pulmonary emphysema occurs without other lung disease, for example in downer cows dying of toxic mastitis or hypocalcemia, but it is a clue to look for more diagnostically specific lesions such as firmness of the individual lung lobules that separate the emphysematous interlobular septa.
-
•Palpation—the key to effective examination of the lung—must be based on a cut section of lung because the thick pleura is a barrier to effective palpation. Superficial palpation is useless, and the fingers must push deeply, like microscopic probes assessing the texture of each individual lobule. It is useful to categorize the abnormal lung texture on a 5-point scale that often correlates with the morphologic pattern of lung disease:
-
○Hard and crisp, as a result of peracute fibrinous bronchopneumonia
-
○Firm or liver-like, as a result of acute or chronic bronchopneumonia
-
○Slightly firm or rubbery, as a result of bronchointerstitial or interstitial pneumonia
-
○Wet and heavy, but of essentially normal texture, as a result of edema
-
○Spongier than normal, as a result of air trapping from airway obstruction or emphysema
-
○
-
•
The distribution and the texture of the lung lesions are the basis for identifying the morphologic pattern of disease (see Table 3).
Table 3.
Morphologic patterns of lung disease
| Morphologic Pattern | Typical Gross Lesions: Distribution and Texture | Major Causes |
|---|---|---|
| 1. Bronchopneumonia | ||
| 1a. Bilateral | Cranioventral distribution, more or less bilaterally symmetric, hard and crisp or firm and liver-like (see Fig. 1A). | Opportunistic bacteria, usually Pasteurellaceae |
| 1b. Asymmetric or focal | Usually cranial or middle lung lobes, focal or asymmetrical Often necrotic, putrid, and green-brown |
Aspiration of rumen content, feed, or administered substances |
| 2. Interstitial and bronchointerstitial pneumoniaa | ||
| 2a. Generalized interstitial lung injury | Diffuse or lobular (“checkerboard”) lesions, generalized to all lung lobes (see Fig. 1C) Subtle firmness or rubbery texture, with alveolar and interlobular edema Lesions may be obscured by interlobular emphysema. |
Respiratory viruses, septicemia, endotoxemia, parasitic larval migration, idiopathic interstitial pneumonia of feedlot cattle, toxic lung disease, hypersensitivity pneumonitis |
| 2b. Cranioventral bronchointerstitial pneumonia | Cranioventral lung has slightly firm-rubbery texture, collapse, and reddening (see Fig. 1D). Dorsocaudal lung is heavy and edematous, often with interlobular emphysema. | Respiratory viruses, such as BRSV. Note that these viruses may also cause generalized interstitial lung injury. |
| 2c. Generalized interstitial lung injury plus cranioventral bronchopneumonia | Dorsocaudal regions are slightly firm-rubbery, like for generalized interstitial lung injury, but cranioventral areas are consolidated as a result of bacterial bronchopneumonia. | Viral pneumonia with secondary bacterial pneumonia. Idiopathic, perhaps acute lung injury secondary to bacterial pneumonia |
| 3. Embolic lung lesions | ||
| 3a. Multifocal embolic pneumonia | Multifocal lesions in all lobes (see Fig. 1E) Firm consolidation or abscesses |
Embolism or bacteremia from heart, liver, caudal vena cava, jugular veins, and uterus |
| 3b. Thromboembolism | Thrombi occluding large branches of the pulmonary arteries (see Fig. 1F) | Embolism, as given |
| 4. Pleuritis | ||
| Exudate on pleural surface, with or without consolidation of underlying lung tissue (see Fig. 1G) | H somni pleuritis, pleuropneumonia as a result of Pasteurellaceae bacteria, penetrating injury, Escherichia coli bacteremia in young calves, or ruptured abscess | |
The various gross appearances of interstitial/bronchointerstitial pneumonia may cause confusion. The term is based on the histologic appearance of the lesions, which remains constant whether the gross lesions are generalized, cranioventral, or complicated by bronchopneumonia.
Fig. 1.
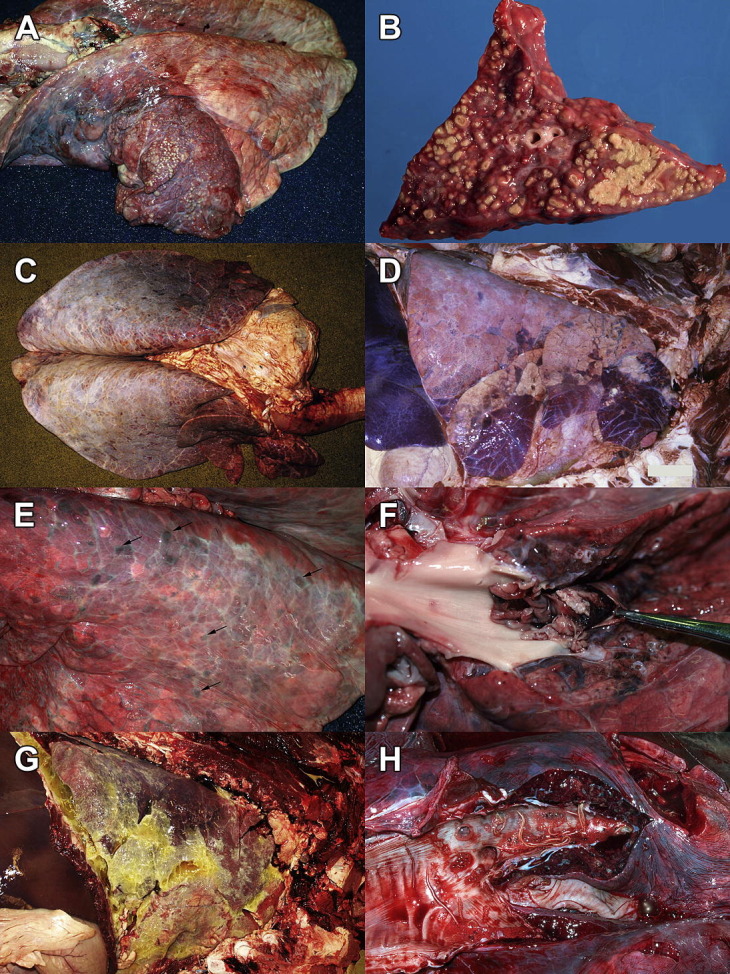
(A) Bronchopneumonia caused by M bovis. The cranioventral 40% of the lung is brick red and consolidated and contains innumerable pale, round approximately 3-mm-diameter nodules. Left lung: trachea is to the left. (B) M bovis bronchopneumonia. Section of lung with multiple coalescing nodules of caseous necrosis. (C) Generalized interstitial lung injury. The lung fails to collapse, is heavy as a result of edema, and has a generalized firm-rubbery texture. Interlobular septa contain innumerable tiny air bubbles (interlobular emphysema). A specific cause was not identified. Note the white opacity of the dorsocaudal pleura, which is normal in bovine lung. (D) Cranioventral bronchointerstitial pneumonia caused by BRSV. The cranioventral (lower right) 25% of the lung is collapsed and plum red and has a slightly firm-rubbery texture (but lacks the more obvious liver-like firmness that would be typical of bronchopneumonia). The dorsocaudal lung has a similar texture, with extensive interlobular emphysema. Right lung: trachea is to the right. (E) Embolic pneumonia. All lung lobes are peppered by 2- to 10-mm-diameter raised firm purple-red nodules (arrows), which had purulent exudate on cut section. The source of the infection was septic cellulitis and venous thrombosis, as a complication of a toggle surgery for displaced abomasum. Left lung: trachea is to the left. (F) Pulmonary embolism secondary to endocarditis. A ragged embolism fills the opened pulmonary artery. This lesion is easily overlooked if the arteries are not opened. Dorsal view of left lung: heart is to the left and caudal lung is to the right. (G) Fibrinous pleuritis caused by Histophilus somni. Yellow-white fibrin and fluid cover the pleural surface of the lung. The lung tissue is congested and edematous, but otherwise normal. Right lung: trachea is to the right. (H) Dictyocaulus viviparus. The caudal bronchi contain frothy fluid with a few adult nematodes. Dorsal view: trachea is to the left. Normal pink lobules contrasted with purple-red consolidated ones, in the adjacent lung tissue.
([G] Courtesy of Dr Heindrich N. Snyman.)
Postmortem examination of the 2 calves that died revealed subcutaneous and pulmonary interlobular emphysema, increased weight of the lungs, and rubbery firmness of the cranioventral 70% of the lung (see Fig. 1D for similar lesions, from a different case). The emphysema, although dramatic, is a nonspecific consequence of respiratory distress, but the modestly increased texture of the cranioventral lung along with the clinical findings supports the possibility of viral bronchointerstitial pneumonia. Laboratory confirmation of the diagnosis was desirable in this case, based on the magnitude of the outbreak and the potential impact on future disease prevention strategies in the herd.
A concise but helpful clinical history is the foundation of the laboratory investigation. In the context of BRD, key features of the clinical history are summarized in Table 4 . The clinical features just described were critical in guiding the diagnostic investigation that followed. Conversely, it is depressingly apparent in other cases that a poor or missing history results in inappropriate testing, errors in interpretation, and failure to effectively relate the pathologic findings to the clinical picture.
Table 4.
Differentiating clinical and epidemiologic features of BRD that are of most importance to laboratory diagnosticiansa
| Clinical Features | Possible Interpretation or Cause |
|---|---|
| Basic epidemiologic information: time since arrival at a new facility or introduction of new animals, number at risk, proportion affected, case fatality rate and characteristics, age range of affected calves, attributes of affected and unaffected cattle, and duration of the disease. | |
| Outbreak or endemic disease | Viruses, parasites, and toxins are major considerations for outbreaks. |
| Pasture or housed | Dictyocaulus worms cause disease in pastured or formerly pastured animals. 3-Methylindole toxicity is associated with recent access to lush pasture. |
| Fever | Bacterial, viral, acute parasitism |
| Severity of depression relative to severity of dyspnea | Severe depression suggests bacterial pneumonia. Severe dyspnea suggests viral or parasitic pneumonia. |
| Inspiratory or expiratory dyspnea | Inspiratory dyspnea or stridor suggests upper respiratory tract obstruction, whereas expiratory dyspnea suggests pulmonary or cardiac disease. |
| Lameness | M bovis, H somni |
| Response to antibiotic therapy, vaccination history | Implications with respect to bacterial or viral infections |
This key information should be included with the diagnostic submission.
Boxes 2 and 3 outline considerations for tissue sampling from necropsy cases. Ideally, tissues should be collected from multiple animals that are reliably representative of the disease problem in the herd, early in the disease course, and not treated. This is often impossible for small herds of valuable animals, for which necropsy-based investigations must rely on the few animals that have died despite attempted therapy. In such cases, histopathologic examination often reveals lesions that suggest viral or bacterial pneumonia, yet the specific pathogen cannot be identified because it has been eliminated by the immune response or antimicrobial therapy. The lung is vast, and multiple (3–5) samples harvested from the various areas will increase the frequency of diagnosis. Foci of caseous necrosis are ideal for detection of Mycoplasma bovis, and granulomas are sites of M bovis infection, but abscesses are of no value in identifying the primary bacterial pathogen.
Box 2. Selection of animals for sampling.
-
•
Examine multiple animals.
-
•
Animals should be selected that are representative of the disease problem with respect to clinical signs, age, and pen grouping. Ensure that the expensive investigation is not conducted on those that have died of other causes.
-
•
Examine early in disease course, at the onset of clinical signs. Experimental studies suggest that animals in the first 1–4 days of clinical illness are most likely to shed virus (with virus detectable at 4–7 days after infection for bovine respiratory syncytial virus [BRSV] and other viruses).18, 19, 20 An absence of fever suggests that the animal is not likely to have active respiratory viral infection, but the converse is not true: animals may remain febrile after the pathogen has been cleared, for example, if there is secondary bacterial pneumonia. Serum haptoglobin and other acute phase proteins (APPs) remain elevated throughout the duration of the inflammatory response and thus do not necessarily indicate acute disease. Using APPs to select affected animals for testing is also currently constrained by the delay needed for laboratory testing.
-
•
Examine animals that were not treated with antibiotics, if identification of bacterial pathogens and their antimicrobial susceptibility is desirable.
Box 3. Tissue sampling at necropsy.
Selection of tissue samples
-
•
Unless there is considerable confidence in the diagnosis at the time of necropsy, it is recommended to submit nonrespiratory tissues as part of the respiratory disease investigation. Examples of significant microscopic lesions in nonrespiratory tissues include myocardial lesions causing heart failure and dyspnea, lymphocytic arteritis in the heart or depletion of lymphoid tissue as a result of BVDV infection, intestinal lesions as a result of bovine coronavirus (BCV) or BVDV, and various inflammatory lesions as the basis for febrile illnesses that may mimic bacterial pneumonia.
-
•Upper respiratory tract samples should be taken: nasal cavity, larynx, and trachea.
-
○Nasal swabs are effective for identification of respiratory viruses, including BRSV, bovine parainfluenza virus-1 (BPI3V), bovine herpesvirus-1 (BHV-1), and BCV.
-
○Bacteria isolated from nasal swabs are not well correlated with those from the lung, although there is some controversy in the literature on this point: isolates are the same species only 68% of the time and may be present in nasal swabs without causing disease, yet antimicrobial sensitivity of isolates seem to be similar in both locations.21, 22, 23
-
○
-
•Sampling of lung tissue
-
○Histopathology and immunohistochemistry: Sample at least 3–5 areas, including samples from the border between abnormal and normal lung and a sample from normal lung. Samples must cover the spectrum of lesions in the lung; for example, a single lung may have cranioventral consolidation, abscesses, dorsocaudal interstitial pneumonia, and pleuritis, and all should be examined histologically.
- ○
-
○Bacterial culture: Sample consolidated lung tissue near the border between abnormal and normal lung, avoiding abscesses. Bacterial pathogens are most reliably detected in consolidated lung tissue near the junction with normal lung. This represents the anatomic site that has most recently become infected, whereas the older lesions in the cranioventral area are more likely to be colonized by secondary pathogens. Samples of lung tissue should be ≥4 cm in diameter. Compared with swabs taken in the field, contamination of lung tissue samples may be less likely if a tissue block is submitted, because the surface is seared in the laboratory before sampling for culture. Conversely, swabs have the advantage of ease of use and improved preservation during transport.
-
○M bovis: Foci of caseous necrosis are ideal, or areas of consolidation.
-
○Tuberculosis: Caseating granulomas in lung (usually a single granuloma), in mediastinal, tracheabronchial, and retropharyngeal lymph nodes, and palatine tonsils.17
-
○
-
•Tissue preservation
-
○Samples for histopathology and immunohistochemistry are fixed in formalin. Small pieces, 1 cm thick, are ideal, with a 1:10 ratio of tissue to formalin. Large blocks of tissue autolyze during transport, because formalin does not penetrate to the center of the sample.
-
○Samples for bacterial culture, virus isolation, and molecular testing are chilled with freezer packs or refrigerated. Frozen samples are also useful, especially when tissues are held pending the outcome of initial tests.
-
○Swabs for bacterial culture or virus isolation are preserved in bacterial or viral transport medium, respectively, and chilled. The success of virus isolation is much reduced for swabs preserved in bacterial transport medium.
-
○
Histologically, in the present case, bronchiolar epithelium was thinned and irregular (bronchiolar necrosis); neutrophils, fibrin, and proteinaceous edema fluid packed the alveoli (bronchopneumonia), type II pneumocytes lined alveoli in a few areas (alveolar damage), and interlobular septa were emphysematous. These findings were interpreted as bronchointerstitial pneumonia suggestive of viral pneumonia and as bronchopneumonia typical of infection with Pasteurellaceae bacteria. There were multinucleated cells in alveoli, but these were more likely macrophage giant cells instead of the epithelial syncytia seen in BRSV infection. A single bronchiolar epithelial cell seemed to have a cytoplasmic inclusion body, suggestive of BRSV or BPI3V, but this was not considered conclusive.
Pathologists and practitioners sometimes find such suggestive but non-diagnostic lesions to be disappointing. Nevertheless, these findings are indispensible in 2 ways:
-
•
When laboratory testing identifies a pathogen, the presence of a compatible histologic lesion provides considerable confidence that the pathogen is indeed causing disease in that animal.
-
•
Histopathologic changes often indicate the disease process and the likely types of pathogens responsible, even if better samples, other animals, or more appropriate assays are required to identify the pathogen.
In addition to formalin-fixed tissue, lung samples from this case had been individually bagged, chilled on freezer packs, and transported overnight to the laboratory. Available tests and preferred diagnostic methods vary considerably between diagnostic laboratories, so test selection must be based on the user’s guide for each laboratory or consultation with laboratory personnel. In this case, results of initial testing of lung samples were:
-
•
Immunohistochemistry: negative for BRSV and BHV-1
-
•
Bacterial culture: no pathogens identified
-
•
Fluorescent antibody test: negative for BPI3V, BRSV, and BHV-1
These results raise a consideration of the reasons for discrepant or unexpectedly negative test results. General considerations are outlined in Box 4 . Histologic findings in this case of bronchointerstitial pneumonia fit well with the clinical picture of viral pneumonia and motivated further investigation of the cause. Subsequently, immunohistochemical testing for BVDV, BCV, and BPI3V was performed: BVDV infection does not cause primary lung disease but is an important predisposing cause of bacterial pneumonia and should be considered in any herd problem of respiratory disease, whereas BCV is an uncommon but underrecognized cause of viral pneumonia in cattle. These tests were negative, but a quantitative (“real-time”) reverse transcriptase–polymerase chain reaction (RT-PCR) test for BRSV was performed subsequently and the results were positive.
Box 4. Interpretation of negative diagnostic laboratory data.
When laboratory tests to detect an antigen, nucleic acid, culturable pathogen, or antibody unexpectedly fail, general considerations include the following:
-
A.
Incorrect clinical diagnosis: The suspected pathogen is not the cause, and alternative possibilities should be considered.
-
B.Disease without active infection: The pathogen is the cause of the disease, but it is no longer present in the animal.
- •
-
•Bacteria may be eliminated or fail to grow in culture as a result of antibiotic treatment.
-
•Dictyocaulus: During the acute phase of larval migration through the lung (7–25 days after infection), adults are not yet present in bronchi nor are there larvae in feces. Conversely, chronic or postpatent infections can still elicit clinical signs, despite the absence of adult worms in the lung or larvae in the feces. Finally, partially immune animals that are reinfected can develop severe disease, yet the infection never becomes patent. The results of the Baermann test on feces are negative in all 3 of these situations: acute prepatent, postpatent, and partially immune nonpatent infections.
-
C.Inadequate samples: The pathogen is the cause of the disease, but the samples are inadequate. See sampling strategies in Boxes 2 and 3.
- •
-
•Sampling from abscesses instead of actively infected tissue is likely to yield secondary pathogens.
-
•Sample autolysis as a result of inappropriate storage or transport of chilled specimens results in degradation of antigens and loss of viability of culturable pathogens. Immunohistochemical tests fail if tissues are autolyzed or underfixed, if the volume of formalin is too small, or if excessively large samples of tissue are used (1-cm-thick pieces are adequate for histopathologic examination).
-
•Freeze-thaw cycles are harmful, and they may occur from storage of samples in frost-free freezers or from thawing during transport.
-
•Prolonged fixation may interfere with antigen detection by immunohistochemistry: fixing for <1 week is generally recommended, although improved methods allow antigen retrieval with up to 7 weeks of fixation.26 Bouin solution or other alternatives to formalin fixation may affect immunohistochemical detection of some antigens,27, 28 but the addition of ethanol to prevent freezing (to a final concentration of 10%) is considered safe.
-
D.Test failure: The pathogen is present in the sample but was not detected.
-
•Antibody interference: Antibody in the diagnostic specimen interferes with the ability of the test to detect the pathogen. The antibody may result from colostral transfer of maternal antibody, from vaccination, or from development of an immune response following infection. Such antibody may not protect against disease, yet may interfere with the ability of an immunoassay to detect viral antigen (eg, antigen-capture enzyme-linked immunosorbent assay) or inhibit growth of virus in tissue culture (virus isolation).
-
•“Inhibitors” are substances in the sample that interfere with molecular detection of pathogens. This problem should be detected by routine quality control procedures in the laboratory.
-
•It is possible that genetic variants are not detected by routine diagnostic tests, particularly for RNA viruses. Although assays are tested during development against a broad collection of archived and contemporary isolates, it is possible that emerging strains of a pathogen are not detected by current tests.29
-
•Practitioners are dependent on a quality assurance program in the diagnostic laboratory, including test validation, appropriate use of positive and negative control samples, and valid interpretation of test data. Test results that conflict with clinical findings might represent laboratory error, and discussion with laboratory personnel can occasionally be fruitful in these circumstances.
-
•
Findings supporting the diagnosis of BRSV in this case were the clinical picture, the histologic diagnosis of bronchointerstitial pneumonia with a single equivocal inclusion body, and the quantitative RT-PCR result. These contrasted with the negative results of the immunohistochemistry and fluorescent antibody tests and the absence of histologically evident syncytia. Reasons for the discrepancy in this case are speculative. The animal may have been subacutely infected, with little viral antigen remaining in the lung. Test sensitivity is a likely contributor: immunohistochemistry has the advantage that the best histologic lesions are selected for testing, and the test results are easily interpreted in conjunction with the histologic findings, yet prior studies have identified that quantitative RT-PCR testing for BRSV is more sensitive than other available tests.15, 24, 30 Further, because the 2 tests in this case were performed on different pieces of tissue, uneven distribution of antigen may have randomly resulted in one test being positive and the other negative. Laboratory quality control and overfixation of tissues were not thought to contribute to the negative tests in this case, based on appropriate results in control samples. The failure to detect bacterial pathogens despite histologic evidence of bronchopneumonia was presumed to have resulted from antibiotic treatment before death or perhaps from incorrect sample selection.
False-positive tests may also occur, and causes include laboratory error, contamination between specimens from different cases, and vaccination in the past 20 days.31 For herpesvirus infections, stress- or glucocorticoid-induced reactivation of latent infection may be detected by diagnostic testing, even if the herpesvirus played no role in causing the clinical disease. Thus, when discrepancies occur, a positive test should not automatically be considered more reliable than a negative test.
This case illustrates the laboratory investigation of BRD using samples obtained at necropsy and followed a somewhat standardized protocol. The initial testing provided clues but was nondiagnostic and somewhat conflicting, and an inadequate laboratory submission may have ended with this unsatisfactory outcome. However, the accurate clinical history and the availability of lung samples permitted—and indeed motivated—the subsequent testing that led to the diagnosis. This illustrates a major virtue of the necropsy: the breadth of the examination and the variety of samples collected permit a comprehensive and robust diagnostic investigation that can be amended according to interim test results.
All calves in the pen were treated with antibiotics at the time the group was initially examined, and further deaths were not noted. BRSV was considered the cause of the outbreak and the producer was advised on prevention of future recurrences, but the herd remains unvaccinated.
Case 2: ongoing problems with respiratory disease in a beef herd
A small 30-head beef herd was examined in late October because of respiratory disease in 20 of the cows and calves. Half of the cases involved the mature animals. A cow and a bull had been purchased in September, and the herd was usually vaccinated and dewormed in the autumn but this had not yet been done this year. Clinical signs included fever, cough, hyperpnea, dyspnea, frothing at the mouth, and weight loss. A field necropsy of 3 animals revealed consolidation of the lungs, interpreted as bacterial bronchopneumonia.
A 4-year-old cow from the herd died after more than 2 weeks of weight loss and respiratory disease, which had been nonresponsive to sequential therapy with florfenicol, tilmicosin, and penicillin. The cow was submitted to the diagnostic laboratory, and necropsy revealed numerous lungworms and catarrhal exudate in bronchi of all lobes (see Fig. 1H for a different case, albeit with fewer worms). The cranial and middle lobes were reddened and consolidated, all lobes had multifocal 2- to 8-mm-diameter firm red-purple lesions, and interlobular emphysema was most prominent in the caudal lobes. Klebsiella pneumoniae was isolated in large number from both samples of lung tested. Histopathology did not reveal evidence of bronchopneumonia, but the nodular and consolidated lesions corresponded to pyogranulomatous inflammation centered on caseous foci containing aspirated eggs and larvae, and chronic parasitic bronchitis and bronchiolitis were present.
The diagnosis of lungworm was verified on one additional animal that died on the farm. Testing fecal samples from multiple animals, to detect larvae using the Baermann technique, would be an alternative method to establish the extent of infection in the group. This technique is highly sensitive and is reported to be capable of detecting a single egg-producing worm, at those stages when the infection is patent.32 The herd problem was reported to have resolved quickly after treatment of the herd with ivermectin.
The case is notable because diagnoses of Dictyocaulus spp are uncommon in this herd’s geographic area, as in many areas of North America. However, it does occur in most areas and is of particular diagnostic significance because the therapy and control strategies are so different from those of other BRDs. The case is an effective illustration of the value of a thorough necropsy examination, the ease with which diagnostically important lesions can be overlooked, and the importance of dogged persistence and examination of multiple animals in conducting a diagnostic investigation. The cause of a disease problem can usually be identified by a systematic, insightful, and tenacious herd-level investigation, even if the initial attempts have failed.
Case 3: serologic investigation of upper respiratory disease in a dairy herd
A Holstein dairy herd with 40 milking cows experienced a respiratory disease outbreak in 20% of the cows. The herd had had no outside introductions for years, and artificial insemination was used. Annual vaccination with killed vaccines for BHV, BVDV, BPI3V and BRSV was last done 11 months previously. The facility was a bank barn, with milking cows in 2 rows of tie stalls. The producer noted significant amounts of blood and fibrin in the manger. Affected cows had obvious dyspnea, until they dislodged a fibrin plug from their nasal cavity, when they seemed more normal and would eat. Only 3 cows were treated with antibiotics, and none died.
Pens containing 8 young calves were adjacent to one of the rows of milking cows, and all of these had clinical signs of pneumonia that developed a few days after the disease outbreak in the cows. Calves were treated with antibiotics, and 1 died. Pens of older (∼1 year of age) calves elsewhere in the same barn had no clinical signs.
This is an unusual clinical presentation, and a diagnosis was not immediately apparent. A field postmortem examination of the dead calf revealed fibrinous bronchopneumonia. Although laboratory testing of tissues from this animal might have been useful, it was uncertain whether the problem in the calves was the same as the more perplexing problem in the cows. Instead, the diagnostic investigation focused on the cows, in an effort to ensure that the results were applicable to the problem of most concern.
A diagnostic option, which was not pursued in this case, would have been to attempt to isolate a virus from nasal swabs or samples of the dislodged fibrin plugs. With this method, identifying a viral pathogen would be of reliable significance in a closed herd with compatible clinical signs. However, because the practitioner was not involved at the onset of the outbreak in the cows and was only later called to the farm when the calves became ill, there would be a risk that active viral infection might no longer be detected.
Instead, the diagnostic quest in this case was based on serologic examination. Samples were collected at the time of the initial visit and 3 weeks later, from 5 affected cows and 5 of the affected young calves. Data are shown in Table 5 .
Table 5.
Serology data from an outbreak of upper respiratory disease in a dairy herd
| BAV | BCV | BRSV | BVDV | BHV-1 | BPI3V | |
|---|---|---|---|---|---|---|
| Cow 1 | 3 | 256 | 3072 | 32 | 48 | 1536 |
| 3 | 256 | 2048 | 24 | 48 | >4096 | |
| Cow 2 | 48 | 256 | 4096 | 768 | 48 | 1536 |
| 48 | 512 | 4096 | 768 | 48 | 4096 | |
| Cow 3 | 3 | 512 | 3072 | 4 | 6 | 768 |
| 2 | 512 | 2048 | 2 | 12 | >4096 | |
| Cow 4 | 2 | 512 | 1536 | 384 | 16 | 1024 |
| 3 | 512 | 1536 | 256 | 32 | >4096 | |
| Cow 5 | 32 | 32 | 2048 | 256 | 32 | 1024 |
| 24 | 24 | 3072 | 256 | 64 | >4096 | |
| Calf 1 (oldest) | 4 | 32 | 2048 | 12 | <2 | 96 |
| 3 | 32 | 2048 | 6 | <2 | 96 | |
| Calf 2 | <2 | 32 | 2048 | 6 | 2 | 64 |
| <2 | 32 | 2048 | 6 | 2 | 96 | |
| Calf 3 | 4 | 128 | 768 | <2 | 2 | 384 |
| 2 | 128 | 512 | <2 | 2 | 128 | |
| Calf 4 | 24 | 128 | 384 | 128 | 32 | 512 |
| 16 | 128 | 384 | 96 | 16 | 768 | |
| Calf 5 (youngest) | 24 | 2048 | 1024 | 32 | 12 | 3072 |
| 12 | 2048 | 768 | 16 | 16 | 1024 |
Data are the antibody titers in serum samples, for acute (upper rows) and convalescent (lower rows, bold font) samples, reported as the mean of the reciprocal log titers measured in duplicate.
Abbreviation: BAV, bovine adenovirus.
Observations and interpretation of these data are as follows:
-
1.
Four of the 5 cows tested had a greater than 4-fold increase in titers to BPI3V, in the convalescent compared to the acute serum samples. In a closed herd in which active parainfluenza virus infection is expected to be uncommon and in the presence of compatible clinical signs, the authors interpret this to be the cause of the outbreak. Others have described a comparable outbreak of upper respiratory disease characterized by fever, nasal discharge with erosions, and submandibular swelling in vaccinated adult cows that was associated with seroconversion to this virus.33 The authors are not aware of any description of this condition in the peer-reviewed literature.
-
2.
None of the calves seroconverted to BPI3V, confirming the just-noted suspicion that the disease in the calves differed from that in the cows.
-
3.
There was a consistent trend of higher titers in young calves and lower titers in older calves, for all viruses tested except BRSV. This is interpreted to represent decay of maternal antibody as the calves age.
-
4.
Titers to BRSV were considerably higher in older than in younger calves, but none seroconverted. We would not rule out the possibility that this virus caused the disease in the calves, but this remains uncertain.
The laboratory fees for measuring a single serum antibody titer are low compared with the costs of histopathologic examination, virus isolation, or molecular testing. However, it is of limited value and sometimes highly misleading to evaluate titers in single serum samples, so acute and convalescent sera from several animals are required to properly assess the relationship of seroconversion with disease. The total cost of such a diagnostic investigation can be startling.
This case illustrates the value of serologic investigation as a reliable method of establishing a diagnosis, even some time after the beginning of the outbreak. None of the veterinarians were aware that BPI3V could cause this form of respiratory disease, and an unusual manifestation of IBR was considered likely at the time. A well-planned diagnostic investigation allowed the authors to stumble upon diagnoses of which they were not aware. This presents a notable contrast with case 1, in which the clinical findings suggestive of respiratory disease served to direct the laboratory investigation.
Case 4: laboratory testing to identify cattle with respiratory disease
Tromping through a beef feedlot in Alberta were the experienced operator of the large Western feedlot and a veterinary pathologist and a veterinary student from small-farm Ontario. For each calf in the pen, questions were raised: Is it sick? Does it have bacterial pneumonia? Is it affected severely enough to require antibiotic treatment? One calf was obviously sick and later determined to have a fever. But another calf had been under observation by the producer since the previous day, with a subtle tendency to stay apart from its mates and away from the feed bunk. The authors decided, with considerable debate and uncertainty, that it was just homesick, and the decision was perhaps justified because it never did require antibiotic therapy.
Clinical examination from a distance—the art of pen checking—is an efficient screening test for disease in all production systems from large pens of beef steers to small groups of pastured dairy heifers, and most animals that require therapy to prevent death or chronic disease are identified with this method. However, even well-trained and experienced producers, staff, or veterinarians are imperfect and uncertain when it comes to distinguishing calves with disease from those experiencing distress as a result of recent weaning, transportation, processing, and disruption of social groups. Further distinction between respiratory disease and diseases of other body systems and between antibiotic-responsive pneumonia and nonbacterial causes of respiratory disease are even more imprecise based on clinical features alone. In a study to assess diagnosis of BRD by feedlot personnel, the sensitivity of BRD diagnosis by pen checkers was 94% and specificity was 77%.34 Similarly, there was poor correlation between pulling a calf for suspected BRD and the presence of lung lesions at slaughter.35 Further, these challenges only cover the need to identify those calves with severe bacterial pneumonia that without treatment would die or progress to chronic disease: it is likely that early treatment of more mildly affected calves would have a beneficial effect on retreatment rates, feed efficiency, growth, and carcass quality, but our ability to accurately identify these calves is limited.36, 37, 38, 39 In this scenario, if experienced feedlot operators and veterinarians are imprecise in making these determinations, what can the laboratory diagnostician from Ontario contribute?
Laboratory analysis of clinical samples can be a highly effective means of refining the clinical diagnosis in situations where the benefits of diagnosis outweigh the costs of acquiring and analyzing blood, nasal fluid, transtracheal wash, bronchoalveolar lavage, exhaled breath condensate, or lung biopsy samples.40, 41 Box 5 outlines the methods and uses of laboratory analysis in this context.
Box 5. Laboratory tests to detect subclinical disease and to refine the clinical diagnosis.
-
•
Total white blood cell count and differential cell count are poor predictors of respiratory disease in feedlot cattle and do not significantly differentiate clinically sick from clinically normal animals in the first week after arrival. Similarly, these parameters, when measured in acutely sick calves, were not useful predictors of the severity of lung lesions at necropsy.42, 43
-
•
Arterial blood gas analysis and blood lactate levels do not reliably predict disease in challenged calves with mild lung lesions.42 However, in cattle with severe respiratory disease, a plasma lactate concentration of 4 mmol/L predicted death within 24 hours.44
-
•
Serum haptoglobin concentrations in serum or plasma increase following experimental Mannheimia haemolytica challenge of BHV-1–infected calves, and the degree of haptoglobin elevation correlated with disease severity, treatment failure, and greater likelihood of death.45, 46, 47 Compared with the rapid (24 hours) increase in serum haptoglobin within 24 hours of the induction of pneumonic pasteurellosis, the response to BRSV or BVDV infection is more delayed, occurring during a period of 4–8 days and corresponding with the onset of a febrile response to the viral infection.45, 48, 49, 50 The findings of these experimental studies extend to the naturally occurring disease, in which increased serum haptoglobin level has been correlated with the presence of fever and clinical diagnosis of BRD.46, 47, 51, 52
-
•
Tests to measure other APPs, including lipopolysaccharide-binding protein and serum amyloid A, are not routinely available but may have advantages of earlier peak concentration.50, 53 In other studies, measuring fibrinogen, serum amyloid A, or α1-acid glycoprotein, have not effectively distinguished between healthy cattle and those with respiratory disease under field conditions.46, 47, 51, 52
-
•
Animals need to be sampled early in the course of disease, at the onset of clinical signs, for haptoglobin and other APPs to have good diagnostic sensitivity and specificity. The sensitivity and specificity of detecting BRD are further improved by testing multiple APPs in parallel instead of using a single protein.47 A peril in using APPs to detect BRD is that an increase in APPs reflects inflammation that is not specific to the respiratory system. Haptoglobin values were elevated to a similar degree as with BRD-affected calves when measured in dairy calves affected by diarrhea, umbilical infections, and oral abscesses.51 Thus, a clinical assessment of the animal assists in the interpretation of elevated APP concentrations.
Summary
Pathologic and laboratory investigations are necessary in situations in which a specific diagnosis or identification of the cause is required. The 4 cases presented outline different approaches to laboratory diagnosis, including the microbiological analysis of samples obtained from animals that have died, the postmortem examination as a method of specific diagnosis, the investigation of a clinical mystery using serologic examination, and the use of laboratory testing to identify subclinical disease or refine the clinical diagnosis.
Elements of each diagnostic approach may hold merit in evaluating respiratory disease in cattle, and the diagnostic path will vary depending on the goals of the producer and veterinarian. Underscoring all approaches is the need to consider the herd history and evolution of clinical disease when trying to align clinical findings with the results of clinicopathologic, necropsy, and microbiologic testing. Open and ongoing discussion with the laboratory is key to optimizing test selection and interpretation.
Acknowledgments
Dr Dave Douglas, Navan Veterinary Services; Dr Craig DeGroot, Tavistock Veterinary Service; Drs Susy Carman, Jan Shapiro, Beverly McEwen, Murray Hazlett. and Hugh Cai, Animal Health Laboratory, University of Guelph; and Drs David Sandals and Andrew Peregrine, Ontario Veterinary College, University of Guelph made considerable contributions to these cases and the article.
Footnotes
The authors are supported by the Natural Sciences and Engineering Research Council of Canada (NSERC), the Ontario Cattleman's Association, the Agricultural Adaptation Council, and the Ontario Ministry of Agriculture, Food, and Rural Affairs.
The authors have nothing to disclose.
References
- 1.Saif L.J. Bovine respiratory coronavirus. Vet Clin North Am Food Anim Pract. 2010;26(2):349–364. doi: 10.1016/j.cvfa.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellis J.A. Bovine parainfluenza-3 virus. Vet Clin North Am Food Anim Pract. 2010;26(3):575–593. doi: 10.1016/j.cvfa.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Ridpath J.F. The contribution of infections with bovine viral diarrhea viruses to bovine respiratory disease. Vet Clin North Am Food Anim Pract. 2010;26(2):335–348. doi: 10.1016/j.cvfa.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Griffin D. Bovine pasteurellosis and other bacterial infections of the respiratory tract. Vet Clin North Am Food Anim Pract. 2010;26(1):57–71. doi: 10.1016/j.cvfa.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Brodersen B.W. Bovine respiratory syncytial virus. Vet Clin North Am Food Anim Pract. 2010;26(2):323–333. doi: 10.1016/j.cvfa.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Doster A.R. Bovine atypical interstitial pneumonia. Vet Clin North Am Food Anim Pract. 2010;26(2):395–407. doi: 10.1016/j.cvfa.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Bleul U. Respiratory distress syndrome in calves. Vet Clin North Am Food Anim Pract. 2009;25(1):179–193. doi: 10.1016/j.cvfa.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Poulsen K.P., McGuirk S.M. Respiratory disease of the bovine neonate. Vet Clin North Am Food Anim Pract. 2009;25(1):121–137. doi: 10.1016/j.cvfa.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Panciera R.J., Confer A.W. Pathogenesis and pathology of bovine pneumonia. Vet Clin North Am Food Anim Pract. 2010;26(2):191–214. doi: 10.1016/j.cvfa.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caswell J.L., Williams K. The respiratory system. In: Maxie M., editor. 5th edition. vol. 2. Saunders; New York: 2007. pp. 523–653. (Pathology of domestic animals). [Google Scholar]
- 11.Cooper V.L., Brodersen B.W. Respiratory disease diagnostics of cattle. Vet Clin North Am Food Anim Pract. 2010;26(2):409–416. doi: 10.1016/j.cvfa.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Nietfeld J.C. Field necropsy techniques and proper specimen submission for investigation of emerging infectious diseases of food animals. Vet Clin North Am Food Anim Pract. 2010;26(1):1–13. doi: 10.1016/j.cvfa.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Apley M. Antimicrobials and BRD. Anim Health Res Rev. 2009;10(2):159–161. doi: 10.1017/S1466252309990223. [DOI] [PubMed] [Google Scholar]
- 14.Lamm C.G., Love B.C., Krehbiel C.R. Comparison of antemortem antimicrobial treatment regimens to antimicrobial susceptibility patterns of postmortem lung isolates from feedlot cattle with bronchopneumonia. J Vet Diagn Invest. 2012;24(2):277–282. doi: 10.1177/1040638711428149. [DOI] [PubMed] [Google Scholar]
- 15.Timsit E., Maingourd C., Dréan E.L. Evaluation of a commercial real-time reverse transcription polymerase chain reaction kit for the diagnosis of bovine respiratory syncytial virus infection. J Vet Diagn Invest. 2010;22(2):238–241. doi: 10.1177/104063871002200211. [DOI] [PubMed] [Google Scholar]
- 16.Klewer A., Forbes A., Schnieder T. A survey on Dictyocaulus viviparus antibodies in bulk milk of dairy herds in northern Germany. Prev Vet Med. 2012;103(2–3):243–245. doi: 10.1016/j.prevetmed.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Liebana E., Johnson L., Gough J. Pathology of naturally occurring bovine tuberculosis in England and Wales. Vet J. 2008;176(3):354–360. doi: 10.1016/j.tvjl.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Bryson D.G., McNulty M.S., Logan E.F. Respiratory syncytial virus pneumonia in young calves: clinical and pathologic findings. Am J Vet Res. 1983;44(9):1648–1655. [PubMed] [Google Scholar]
- 19.McNulty M.S., Bryson D.G., Allan G.M. Experimental respiratory syncytial virus pneumonia in young calves: microbiologic and immunofluorescent findings. Am J Vet Res. 1983;44(9):1656–1659. [PubMed] [Google Scholar]
- 20.Castleman W.L., Lay J.C., Dubovi E.J. Experimental bovine respiratory syncytial virus infection in conventional calves: light microscopic lesions, microbiology, and studies on lavaged lung cells. Am J Vet Res. 1985;46(3):547–553. [PubMed] [Google Scholar]
- 21.Allen J.W., Viel L., Bateman K.G. The microbial flora of the respiratory tract in feedlot calves: associations between nasopharyngeal and bronchoalveolar lavage cultures. Can J Vet Res. 1991;55(4):341–346. [PMC free article] [PubMed] [Google Scholar]
- 22.DeRosa D.C., Mechor G.D., Staats J.J. Comparison of Pasteurella spp. simultaneously isolated from nasal and transtracheal swabs from cattle with clinical signs of bovine respiratory disease. J Clin Microbiol. 2000;38(1):327–332. doi: 10.1128/jcm.38.1.327-332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheehan M., Markey B., Cassidy J. New transtracheal bronchoalveolar lavage technique for the diagnosis of respiratory disease in sheep. Vet Rec. 2005;157(11):309–313. doi: 10.1136/vr.157.11.309. [DOI] [PubMed] [Google Scholar]
- 24.Larsen L.E., Tjornehoj K., Viuff B. Diagnosis of enzootic pneumonia in Danish cattle: reverse transcription-polymerase chain reaction assay for detection of bovine respiratory syncytial virus in naturally and experimentally infected cattle. J Vet Diagn Invest. 1999;11(5):416–422. doi: 10.1177/104063879901100505. [DOI] [PubMed] [Google Scholar]
- 25.Kimman T.G., Terpstra G.K., Daha M.R. Pathogenesis of naturally acquired bovine respiratory syncytial virus infection in calves: evidence for the involvement of complement and mast cell mediators. Am J Vet Res. 1989;50(5):694–700. [PubMed] [Google Scholar]
- 26.Webster J.D., Miller M.A., DuSold D. Effects of prolonged formalin fixation on the immunohistochemical detection of infectious agents in formalin-fixed, paraffin-embedded tissues. Vet Pathol. 2010;47(3):529–535. doi: 10.1177/0300985809359607. [DOI] [PubMed] [Google Scholar]
- 27.Benavides J., Garcia-Pariente C., Gelmetti D. Effects of fixative type and fixation time on the detection of maedi visna virus by PCR and immunohistochemistry in paraffin-embedded ovine lung samples. J Virol Methods. 2006;137(2):317–324. doi: 10.1016/j.jviromet.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Ramos-Vara J.A. Technical aspects of immunohistochemistry. Vet Pathol. 2005;42(4):405–426. doi: 10.1354/vp.42-4-405. [DOI] [PubMed] [Google Scholar]
- 29.Decaro N., Buonavoglia C. Canine parvovirus: a review of epidemiological and diagnostic aspects, with emphasis on type 2c. Vet Microbiol. 2012;155(1):1–12. doi: 10.1016/j.vetmic.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willoughby K., Thomson K., Maley M. Development of a real time reverse transcriptase polymerase chain reaction for the detection of bovine respiratory syncytial virus in clinical samples and its comparison with immunohistochemistry and immunofluorescence antibody testing. Vet Microbiol. 2008;126(1–3):264–270. doi: 10.1016/j.vetmic.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Timsit E., Le Drean E., Maingourd C. Detection by real-time RT-PCR of a bovine respiratory syncytial virus vaccine in calves vaccinated intranasally. Vet Rec. 2009;165(8):230–233. doi: 10.1136/vr.165.8.230. [DOI] [PubMed] [Google Scholar]
- 32.Eysker M. The sensitivity of the Baermann method for the diagnosis of primary Dictyocaulus viviparus infections in calves. Vet Parasitol. 1997;69(1–2):89–93. doi: 10.1016/s0304-4017(96)01099-0. [DOI] [PubMed] [Google Scholar]
- 33.Wieringa L., van Dreumel T., Godkin A. University of Guelph; 2005. Unusual clinical signs in cattle associated with viral infection. Newsletter of the Animal Health Laboratory. [Google Scholar]
- 34.Salman M.D., Frank G.R., MacVean D.W. Validation of disease diagnoses reported to the National Animal Health Monitoring System from a large Colorado beef feedlot. J Am Vet Med Assoc. 1988;192(8):1069–1073. [PubMed] [Google Scholar]
- 35.Buhman M.J., Perino L.J., Galyean M.L. Association between changes in eating and drinking behaviors and respiratory tract disease in newly arrived calves at a feedlot. Am J Vet Res. 2000;61(10):1163–1168. doi: 10.2460/ajvr.2000.61.1163. [DOI] [PubMed] [Google Scholar]
- 36.Wittum T.E., Woollen N.E., Perino L.J. Relationships among treatment for respiratory tract disease, pulmonary lesions evident at slaughter, and rate of weight gain in feedlot cattle. J Am Vet Med Assoc. 1996;209(4):814–818. [PubMed] [Google Scholar]
- 37.White B.J., Renter D.G. Bayesian estimation of the performance of using clinical observations and harvest lung lesions for diagnosing bovine respiratory disease in post-weaned beef calves. J Vet Diagn Invest. 2009;21(4):446–453. doi: 10.1177/104063870902100405. [DOI] [PubMed] [Google Scholar]
- 38.Gardner B.A., Dolezal H.G., Bryant L.K. Health of finishing steers: effects on performance, carcass traits, and meat tenderness. J Anim Sci. 1999;77(12):3168–3175. doi: 10.2527/1999.77123168x. [DOI] [PubMed] [Google Scholar]
- 39.Thompson P.N., Stone A., Schultheiss W.A. Use of treatment records and lung lesion scoring to estimate the effect of respiratory disease on growth during early and late finishing periods in South African feedlot cattle. J Anim Sci. 2006;84(2):488–498. doi: 10.2527/2006.842488x. [DOI] [PubMed] [Google Scholar]
- 40.Reinhold P., Rabeling B., Gunther H. Comparative evaluation of ultrasonography and lung function testing with the clinical signs and pathology of calves inoculated experimentally with Pasteurella multocida. Vet Rec. 2002;150(4):109–114. doi: 10.1136/vr.150.4.109. [DOI] [PubMed] [Google Scholar]
- 41.Ollivett TL, Burton AJ, Bicalho RC, et al. Use of rapid thoracic ultrasonography for detection of subclinical and clinical pneumonia in dairy calves. Proceedings of the American association of bovine practitioners 44th annual meeting. St Louis (MO); 2011. p. 148.
- 42.Hanzlicek G.A., White B.J., Mosier D. Serial evaluation of physiologic, pathological, and behavioral changes related to disease progression of experimentally induced Mannheimia haemolytica pneumonia in postweaned calves. Am J Vet Res. 2010;71(3):359–369. doi: 10.2460/ajvr.71.3.359. [DOI] [PubMed] [Google Scholar]
- 43.Thomson R.G., Chander S., Savan M. Investigation of factors of probable significance in the pathogenesis of pneumonic pasteurellosis in cattle. Can J Comp Med. 1975;39(2):194–207. [PMC free article] [PubMed] [Google Scholar]
- 44.Coghe J., Uystepruyst C.H., Bureau F. Validation and prognostic value of plasma lactate measurement in bovine respiratory disease. Vet J. 2000;160(2):139–146. doi: 10.1053/tvjl.2000.0487. [DOI] [PubMed] [Google Scholar]
- 45.Godson D.L., Campos M., Attah-Poku S.K. Serum haptoglobin as an indicator of the acute phase response in bovine respiratory disease. Vet Immunol Immunopathol. 1996;51(3–4):277–292. doi: 10.1016/0165-2427(95)05520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carter J.N., Meredith G.L., Montelongo M. Relationship of vitamin E supplementation and antimicrobial treatment with acute-phase protein responses in cattle affected by naturally acquired respiratory tract disease. Am J Vet Res. 2002;63(8):1111–1117. doi: 10.2460/ajvr.2002.63.1111. [DOI] [PubMed] [Google Scholar]
- 47.Humblet M.F., Coghe J., Lekeux P. Acute phase proteins assessment for an early selection of treatments in growing calves suffering from bronchopneumonia under field conditions. Res Vet Sci. 2004;77(1):41–47. doi: 10.1016/j.rvsc.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 48.Ganheim C., Hulten C., Carlsson U. The acute phase response in calves experimentally infected with bovine viral diarrhoea virus and/or Mannheimia haemolytica. J Vet Med B Infect Dis Vet Public Health. 2003;50(4):183–190. doi: 10.1046/j.1439-0450.2003.00658.x. [DOI] [PubMed] [Google Scholar]
- 49.Heegaard P.M., Godson D.L., Toussaint M.J. The acute phase response of haptoglobin and serum amyloid A (SAA) in cattle undergoing experimental infection with bovine respiratory syncytial virus. Vet Immunol Immunopathol. 2000;77(1–2):151–159. doi: 10.1016/S0165-2427(00)00226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knobloch H., Schroedl W., Turner C. Electronic nose responses and acute phase proteins correlate in blood using a bovine model of respiratory infection. Sens Actuators B Chem. 2010;144(1):81–87. [Google Scholar]
- 51.Svensson C., Liberg P., Hultgren J. Evaluating the efficacy of serum haptoglobin concentration as an indicator of respiratory-tract disease in dairy calves. Vet J. 2007;174(2):288–294. doi: 10.1016/j.tvjl.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Angen O., Thomsen J., Larsen L.E. Respiratory disease in calves: microbiological investigations on trans-tracheally aspirated bronchoalveolar fluid and acute phase protein response. Vet Microbiol. 2009;137(1–2):165–171. doi: 10.1016/j.vetmic.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orro T., Pohjanvirta T., Rikula U. Acute phase protein changes in calves during an outbreak of respiratory disease caused by bovine respiratory syncytial virus. Comp Immunol Microbiol Infect Dis. 2011;34(1):23–29. doi: 10.1016/j.cimid.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


