Abstract
Tea (Camellia sinensis) is one of the most popular nonalcoholic beverages, consumed by over two-thirds of the world's population because of its refreshing, mild stimulant and medicinal properties. It is processed in different ways in different parts of the world to give green, black, oolong, and pu-erh tea. Among all tea polyphenols, epigallocatechin-3-gallate has been responsible for much of the health promoting abilities of tea including anti-inflammatory, antimicrobial, antitumour, anti-oxidative, protection from cardiovascular disease, anti-obesity, and anti-aging properties. In the present review, the antibacterial, antiviral, and antifungal activities of different types of tea and their polyphenols are reported, highlighting their mechanisms of action and structure–activity relationship. Moreover, considering that the changing patterns of infectious diseases and the emergence of microbial strains resistant to current antibiotics, there is an urgent need to find out new potent antimicrobial agents as adjuvants to antibiotic therapy. The synergistic effect of tea polyphenols in combination with conventional antimicrobial agents against clinical multidrug-resistant microorganisms has also been discussed in this review.
Keywords: Camellia sinensis, Epigallocatechin-3-gallate, Polyphenols, MRSA, Synergism, Antibiotic
Highlights
► The antimicrobial activity of teas and their polyphenols was reviewed. ► EGCG was the most active tea polyphenol responsible for antimicrobial activity. ► EGCG directly binds to peptidoglycan and induces damage of the bacterial cell wall. ► The synergism of tea polyphenols and antibiotics was discussed. ► The low bioavailability is the main problem with tea polyphenols.
1. Introduction
Recently, there has been increased interest in polyphenolic compounds found in natural foods. Several of these plant foods may have beneficial effects in humans (Ferrazzano et al., 2011). Most antioxidants isolated from plants are polyphenols, which exhibit biological activities such as antibacterial, antiviral, anti-allergic, anti-inflammatory, anticancer, and immunostimulant (Scalbert, Manach, Morand, & Rémésy, 2005). The antioxidant activity of these compounds is due to their ability to stabilize or deactivate free radicals generated by a metabolic pathway within the body tissue, thereby reducing free radical-mediated degradation of cells and tissues in an organism (Staszewski, Pilosof, & Jagus, 2011). The main sources of the natural polyphenols in the daily human diet are tea, coffee, vegetables, legumes, whole-grain cereals, and fruits (Ferrazzano et al., 2011).
Tea is the most popular beverage next to water, consumed by over two-thirds of the world's population. In 2010, world tea production reached over 4.52 million tonnes. The largest producers of tea are the People's Republic of China, India, Kenya, Sri Lanka, and Turkey. Globally, India ranks second (991,180 tonnes) in tea production after China (1,467,467 tonnes) (Food and Agriculture Organization of the United Nations—Production FAOSTAT). The average estimated consumption of tea in the United Kingdom is 644.1 tonnes every day (http://www.statisticbrain.com/tea-drinking-statistics/). According to UK tea council, the British drink 165 million cups of tea daily or 60.2 billion cups of tea per year (http://www.tea.co.uk/page.php?id=237).
Tea is used as a beverage worldwide, although consumers vary their preferences for the degree of fermentation, taste, and color. Tea is generally consumed in the forms of green, oolong, pu-erh, and black tea, all of which originate from the leaves of the plant Camellia sinensis L. Oolong tea is very common in many countries however, China and Japan prefer green tea over other types. Black tea is popular among Western countries and dominates the market economically whereas pu-erh tea is consumed almost exclusively in Asia (Balentine, Wiseman, & Bouwens, 1997).
Polyphenols are the most important constituents of tea leaves. Fresh green tea leaves are rich in monomeric flavanols, known as catechins, among which (−)-epigallocatechin gallate (EGCG) is found to be the most abundant. Catechins are present at levels of 30–40% of the dry weight of fresh green tea leaves (Almajano, Carbó, Jiménez, & Gordon, 2008).
In particular, in vivo and in vitro biological activities of tea flavan-3-ols include preventing generation of free radicals (Frei & Higdon, 2003), inhibition of carcinogenesis (Otsuka et al., 1998, Sato, 1999), protection from the effects of radiation (Uchida, Ozaki, Suzuki, & Shikita, 1992), antimutagenic activity (Hayatsu et al., 1992), protection from cardiovascular diseases (Mukamal, Maclure, Muller, Sherwood, & Mittleman, 2002), lowering of plasma cholesterol levels (Ikeda et al., 1992), enhanced loss of body fat (Klaus, Pultz, Thone-Reineke, & Wolfram, 2005), improvement in type 2 diabetes (Shoji & Nakashima, 2006), increase of bone density (Devine, Hodqson, Dick, & Prince, 2007), protection from neurodegenerative diseases (Ramassamy, 2006), activation of leukocytes (Sakagami, Asano, Hara, & Shinamura, 1992), slowing the catabolism of catecholamines, and strengthening of capillaries (‘vitamin P effect’) (Min & Peigen, 1991). The potent antioxidant and antimicrobial activities of tea can be explored for its application in the food industry (Mo et al., 2008, Perumalla and Hettiarachchy, 2011).
In view of the various therapeutic activities associated with tea polyphenols, the antimicrobial activity has been much explored in recent times. The changing pattern of infectious diseases and the emergence of resistant microbial strains to current antibiotics have resulted in the need for fresh approaches to treatment of microbial infections (Taylor, Stapleton, & Luzio, 2002). Therefore, there has been an increased focus on the development of novel plant-derived antimicrobials, including polyphenols derived from tea leaves (Friedman, 2007).
This review focuses on recent findings about the antimicrobial activities of different types of tea and their polyphenols along with their synergistic effects with antibiotics. The main objective of this review is to unify and interpret widely scattered information of literature on inhibitory activities of polyphenols occurring in various types of tea leaves against bacteria, viruses, yeast, and filamentous fungi.
2. Types of tea and their manufacturing process
There are two major varieties used for tea, Chinese tea, Camellia sinensis var. sinensis, and Assam tea, Camellia sinensis var. assamica. Tea plants are widely cultivated in Southeast Asia, including China, India, Japan, Taiwan, Sri Lanka, and Indonesia, and in Central African countries. Tea is one of the most popular beverages in the world because of its attractive aroma, taste, and healthy effects (Lin, Lin, Liang, Lin-Shiau, & Juan, 1998). Depending on the manufacturing process, tea is classified into three major types: ‘non-fermented’ green tea (produced by drying and steaming the fresh leaves which inactivate the polyphenol oxidase and thus, oxidation is prevented); ‘semi-fermented’ oolong tea (produced when the fresh leaves are subjected to partial fermentation before drying); and ‘fermented’ black tea and pu-erh tea which undergo a post-harvest fermentation stage before drying and steaming. The fermentation of black tea is due to oxidation catalyzed by polyphenol oxidase while in pu-erh tea, it is attained by using microorganisms (Bancirova, 2010). The differences between the various processes of manufacture result in differences in the polyphenolic profile among green, oolong, black, and pu-erh teas. Fig. 1 outlines the processing of the various types of tea in detail (Karori et al., 2007, Wu et al., 2007).
Fig. 1.

Schematic diagram of the conventional manufacture process of green, oolong, black, and pu-erh tea.
3. Chemical composition of tea
The composition of tea varies with varieties, season, age of the leaf (plucking position), climate, and horticultural practices. The chemical composition of tea is complex and includes carbohydrates, amino acids, proteins, alkaloids (caffeine, theophylline and theobromine), volatile compounds, polyphenols, minerals, and trace elements. Among these, polyphenols particularly flavonoids are important for the biological activity in tea (Cabrera, Gimenez, & Lopez, 2003).
The common flavonoids in tea are the flavan-3-ols (flavanols or flavans), which are present in relatively large amounts compared to their levels in other foods. The flavan-3-ol subclasses are ranked by degree of polymerization. The monomers include catechins (Fig. 2 ) such as (−)-epigallocatechin-3-gallate (EGCG), (−)-epigallocatechin (EGC), (−)-epicatechin (EC), (−)-gallocatechin (GC), and (+)-catechin (C). The dimers include theaflavins (Fig. 3 ) such as theaflavin (TF), theaflavin-3-gallate, theaflavin-3′-gallate, and theaflavin-3,3′-digallate (TF3) and oligomers include derived tannins (thearubigins) of unknown structure. The other flavonoids including flavones (apigenin and luteolin) and flavonols (quercetin, kaempferol, and myricetin) (Fig. 4 ), are present in relatively lesser amount (Peterson et al., 2005). The flavonoid content of various types of tea is represented in Table 1 . EGCG is the most abundant and most bioactive catechin of green tea, representing 50–80% of the total catechin content (Bansal, Syan, Mathur, & Choudhary, 2011). In the manufacturing of black tea, the monomeric flavan-3-ols undergo polyphenol oxidase-dependent oxidative polymerization leading to the formation of bisflavanols, theaflavins, thearubigins, and other oligomers (Bansal, Singla, & Boparai, 2004).
Fig. 2.
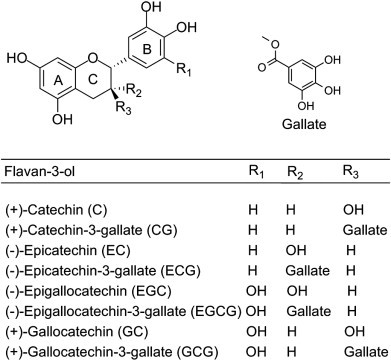
Chemical structure of flavan-3-ols (catechins and epicatechins).
Fig. 3.

Chemical structure of theaflavins.
Fig. 4.

Chemical structure of flavones and flavonols.
Table 1.
Flavonoid content of different types of tea (Peterson et al., 2005, USDA Database for the flavonoid content of selected foods release 2.1, 2007).
| Subclass Flavonoid | Concentration (mg/100 g dry tea) |
||||
|---|---|---|---|---|---|
| Green | Black | Oolong | Pu-erh | ||
| Flavan-3-ols | (−)-Epicatechin | 811.72 ± 21.10 | 255.19 ± 9.97 | 248.42 ± 16.30 | 81.00 ± 90.00 |
| (−)-Epicatechin-3-gallate | 1491.29 ± 112.42 | 688.27 ± 28.07 | 627.25 ± 45.40 | 40.00 ± 54.00 | |
| (−)-Epigallocatechin | 2057.98 ± 103.55 | 956.81 ± 57.09 | 750.80 ± 94.10 | 157.00 ± 126.00 | |
| (−)-Epigallocatechin-3-gallate | 7115.98 ± 632.06 | 1121.92 ± 64.31 | 3412.62 ± 360.53 | 120.00 ± 126.00 | |
| (+)-Catechin | 57.12 ± 3.40 | 137.82 ± 4.48 | 30.63 ± 4.01 | 0.00 | |
| (+)-Catechin-3-gallate | 7.07 ± 1.83 | 50.83 ± 0.00 | 19.89 ± 0.00 | No Data | |
| (+)-Gallocatechin | 258.11 ± 80.69 | 91.73 ± 28.84 | 305.69 ± 0.00 | No Data | |
| Theaflavin | 1.64 ± 0.74 | 159.20 ± 13.03 | 15.23 ± 0.00 | No Data | |
| Theaflavin-3,3′-digallate | 1.08 ± 0.63 | 170.77 ± 19.50 | No Data | No Data | |
| Theaflavin-3′-gallate | 0.44 ± 0.26 | 155.77 ± 16.10 | 18.62 ± 0.00 | No Data | |
| Theaflavin-3-gallate | 0.47 ± 0.32 | 132.25 ± 8.70 | No Data | No Data | |
| Thearubigins | 131.91 ± 131.91 | 5919.00 ± 563.00 | No Data | No Data | |
| Flavones | Apigenin | 12.03 ± 2.86 | 0.00 | 0.00 | 0.00 |
| Luteolin | 0.17 ± 0.17 | 0.00 | 0.00 | No Data | |
| Flavonols | Kaempferol | 147.55 ± 4.40 | 126.66 ± 4.99 | 62.40 ± 19.67 | 23.00 ± 0.00 |
| Myricetin | 104.76 ± 7.94 | 42.24 ± 1.23 | 61.85 ± 26.66 | 40.00 ± 0.00 | |
| Quercetin | 223.97 ± 9.60 | 199.75 ± 12.86 | 108.83 ± 40.94 | 52.00 ± 0.00 | |
4. Pharmacokinetics and bioavailability of tea and its polyphenols
Auger, Mullen, Hara, and Crozier (2008) and Yang, Chen, and Lee (1998) reported that the bioactive constituents of green tea are absorbed upon following oral administration in a dose dependent manner. The catechins are metabolized by the liver and excreted from the body chiefly by the kidneys. After administration of a single oral dose of decaffeinated green tea (20 mg tea solids/kg) or EGCG (2 mg/kg), the maximum plasma concentrations of EGCG, EGC, and EC were found to be 77.9 ± 22.2, 223.4 ± 35.2, and 124.03 ± 7.86 ng/mL and the elimination half-lives were 3.4 ± 0.3, 1.7 ± 0.4, and 2.0 ± 0.4 h, respectively (Lee et al., 2002). Phase I clinical trials involving pharmacokinetic studies of EGCG have shown that only a small percentage of the orally administered catechin appeared in the blood. Drinking 2 cups of green tea resulted in the mean peak plasma EGCG level of 0.17 μM after 1.5 h, and only 4% to 8% of the ingested EGCG was excreted in urine (Pisters et al., 2001).
A report by Chow et al. (2001) indicated that after ingestion of EGCG and polyphenon E (a tea polyphenol preparation) by human volunteers, plasma EGCG was mainly in the free (un-conjugated) form. The other catechins were highly conjugated with glucuronic acid and/or sulfate groups. The major circulating metabolites of epicatechin are epicatechin-3′-O-glucuronide, 4′-O-methylepicatechin-3′-O-glucuronide, 4′-O-methylepicatechin-5- or 7-O-glucuronide, 3′-O-methylepicatechin, and 3′-O-methylepicatechin-7-O-glucuronide (Natsume et al., 2003). Microbial metabolites, namely, 5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone, 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone, and 5-(3′,5′-dihydroxyphenyl)-γ-valerolactone, are found mostly in conjugated forms in plasma and urine of volunteers after ingestion of green tea (Meng et al., 2002). Catechins are rapidly eliminated, and galloylated catechins were never recovered in urine due to preferential excretion of these compounds in bile (Van et al., 2001). The low bioavailability of catechins may be perhaps because of relatively low concentration of circulating catechins in relation to ingested catechins or due to rapid degradation or uptake by other tissues (Yoda, Hu, Zhao, & Shimamura, 2004).
5. Antibacterial activity of tea and its polyphenols
EGCG and ECG are the most potent catechins showing antibacterial activity (Hamilton-Miller, 1995) due to the presence of galloyl moiety, which is not present in EC and EGC. Some hypotheses have been recently proposed for explaining the mechanism of antibacterial action of tea catechins. EGCG can directly bind to peptidoglycan and induce its precipitation. Therefore, the EGCG-induced damage of the cell wall and interference with its biosynthesis through direct binding with peptidoglycan are the major mechanisms for its antibacterial activity against Staphylococcus (Shimamura, Zhao, & Hu, 2007). Moreover, another study suggested that the bactericidal action of EGCG might also depend upon the generation of hydrogen peroxide by the reaction of EGCG with reactive oxygen species in the presence of superoxide dismutase (pro-oxidative activity) (Arakawa, Maeda, Okubo, & Shimamura, 2004). Different types of tea and their polyphenols were evaluated for antibacterial activity against various infectious agents.
5.1. Staphylococcus aureus and methicillin resistant S. aureus (MRSA)
S. aureus, a highly pathogenic, toxin-producing, food-borne organism, and MRSA is one of the most serious pathogens to have emerged in the past 20 years. Not only β-lactams, which act as selective antimicrobial agents for MRSA, but also other antibiotics such as macrolides, aminoglycosides, and fluoroquinolones have limited use in the treatment of MRSA (Klugman & Koornhof, 1989). Nongalloyl analogs like EC and EGC showed enhancement of binding to staphylococcal cells and significantly increased the capacity of ECG and EGCG to reduce levels of staphylococcal oxacillin resistance (Stapleton, Shah, Hara, & Taylor, 2006). Furthermore, Shin and Chung (2007) investigated the effect of major phenolic components from tea against several pathogenic microorganisms including gram-positive strains like S. aureus ATCC 29213 and Streptococcus pyogenes 308A; and gram-negative strains like Escherichia coli ATCC 25922, E. coli 078, Pseudomonas aeruginosa 9027, and Enterobacter cloacae 1321E. The minimum inhibitory concentration (MIC) values demonstrated that both EC and EGC were considerably toxic against S. aureus ATCC 29213 than the other two catechins like ECG and EGCG.
The effect of extraction conditions on total polyphenolic contents, antioxidant, and antibacterial activities of black tea has been reported. Black tea was extracted for 2, 8, and 18 h with absolute acetone, dimethylformamide (DMF), ethanol, methanol, and their 50% aqueous solutions. Aqueous acetone or DMF extracts displayed the highest polyphenol contents and antioxidant activity, while absolute acetone was the least efficient solvent. S. aureus was found to be the most sensitive to all tea extracts, except for the methanol extract (Turkmen, Velioglu, Sari, & Polat, 2007). Similarly, Sari, Turkmen, Polat, and Velioglu (2007) screened the extracts of black tea with different solvents (acetone, DMF, ethanol, and methanol) for polyphenol content, and antioxidant, and antibacterial activities. It was found that methanol was the least efficient solvent for polyphenol extraction from black tea. All the extracts showed antioxidant activity by 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay. The black tea extracts had strong antimicrobial activity against selected bacteria, except for E. coli O157:H7 while S. aureus was found to be the most sensitive to all the extracts. E. coli was the most resistant among bacterial strains. However, the instability of polyphenols is attributable to their oxidation; the authors examined the effects of antioxidants and inhibitors of polyphenol oxidation on the maintenance of polyphenol antibacterial activity towards MRSA. It was found that the antibacterial activity of EGCG was enhanced in the presence of ascorbic acid, and ascorbic acid was the most effective for retaining the concentration of stable EGCG (Hatano et al., 2008). Furthermore, Hua, Peng, Zeng, Yao, and Qian (2010) reported sub-minimum inhibitory concentrations (sub-MICs) of various types of tea and the main components of tea against MRSA. The MIC range of green tea was 1250–5000 μg/mL, oolong tea was 1250–5000 μg/mL, white tea was 625–2500 μg/mL, dark tea was 2500–5000 μg/mL, and black tea was 5000 μg/mL.
In another study, the relative affinity of EGCG to the cell surfaces of gram-positive and gram-negative bacteria has been studied. Highly EGCG-sensitive S. aureus and EGCG-resistant E. coli were treated with 0.5 mg/mL EGCG under pH 6.0. It was observed that attachment of EGCG was significantly lower to E. coli than S. aureus (Nakayama, Shigemune, Tsugukuni, Tokuda, & Miyamoto, 2011). Likewise, Liu et al. (2011) studied the antibacterial activity of catechins against the heterogeneous vancomycin resistant Staphylococcus (h-VRSA). The three catechins viz. ECG, EGCG, and EGC showed antibacterial activity against the origin (MIC = 128–512 μg/mL) and generation (MIC = 256–512 μg/mL) of heterogeneous vancomycin resistant Staphylococcus. The antibacterial effects of theaflavins were studied on four major bacterial strains, namely E. coli, S. aureus, S. mutans, and S. sobrinus 6715. The results indicated that theaflavins showed greater potency in the inhibition of E. coli and S. aureus, and also had an inhibitory effect on the growth and acid production of S. mutans and S. sobrinus 6715 (Jin, Wu, & Tu, 2011).
Nakayama et al. (2012) investigated the mechanism of the combined anti-bacterial effect of green tea extract (GTE) and NaCl against S. aureus NBRC 13276 and E. coli O157:H7. The authors observed that after treatment of 1 h, GTE was found to be more effective against S. aureus than E. coli O157:H7, and combined GTE/NaCl treatment caused greater cellular damage in S. aureus NBRC 13276 than E. coli O157:H7. More recently, Matsumoto et al. (2012) synthesized a series of new fatty acid esters of EGCG by lipase-catalyzed transesterification. These derivatives exhibited several-fold higher activities than EGCG, particularly against gram-positive organisms. Among the derivatives evaluated, sub-MICs of dioctanoate 1 and palmitate 2 (Fig. 5 ) for 17 strains of S. aureus were between 4 and 32 μg/mL, although MIC of EGCG for these 17 strains was ≥ 128 μg/mL. The enhanced activity of the palmitate derivative against S. aureus was supported by its increased membrane-permeabilizing activity. The palmitate derivative showed rapid bactericidal activity against MRSA ATCC43300 at ≥ 16 μg/mL. The finding suggested that the addition of long alkyl chains significantly enhanced the activity of EGCG against several bacteria, particularly against S. aureus and MRSA.
Fig. 5.

Chemical structure of EGCG derivatives.
5.2. Escherichia coli
E. coli is a food-borne, toxin-producing enteropathogen responsible for a hemorrhagic form of colitis, bloody diarrhea, and hemolytic uremic syndrome. Bruins et al. (2006) studied the effect of different tea types and subfractions on the intestinal fluid and electrolyte losses involved in enterotoxigenic E. coli (ETEC) diarrhea. Perfusion of the ETEC-infected segments with both 3 g/L green tea extract and black tea extract significantly inhibited the disturbances in fluid and electrolyte balance. Similarly, Neyestani, Khalaji, and Gharavi (2007) evaluated the microbiologic effects of black tea, compared to green tea, alone and in conjunction with selected antibiotics against E. coli. At a concentration of 25 mg/mL, both black tea and green tea completely inhibited E. coli growth after 5 and 7 h. Both tea extracts had either synergistic or antagonistic effects at different concentrations on selected antibiotics.
Cho, Schiller, Kahng, and Oh (2007) investigated the cellular responses and proteomic analysis of E. coli exposed to green tea polyphenols extracted from Korean green tea. The authors observed responses after exposure to tea polyphenols (TPP) like changes in cell-membrane fatty acids, presence of perforations, and irregular rod forms with wrinkled surfaces. During proteomic analysis, nine upregulated proteins were identified by exposure to TPP including chaperone protein HSP 60 and proteins involved in cellular defense mechanism, such as DNA gyrase subunit A, regulator ampC, RNA polymerase sigma factor, organic solvent tolerance protein, dihydrolipoamide acetyltransferase, superoxide dismutase, transcriptional regulator, and multidrug resistance protein K, whereas the expression of eight proteins was downregulated including chaperone protein HSP70, elongation factor EF-2, acyl-CoA dehydrogenase, enolase, succinate dehydrogenase/fumarate reductase, flavoprotein subunit, glycerophosphoryl diester phosphodiesterase, glutamate/aspartate transport system permease, and aromatic-amino acid aminotransferase. The above results indicated that E. coli cells stressed by exposure to TPP invest more energy in upregulating various defense mechanisms while simultaneously downregulating various metabolic and biosynthetic proteins, and provided relevant clues for understanding the mechanism of TPP-induced stress and cytotoxicity on E. coli. Furthermore, Lee et al. (2009) studied antipathogenic properties of green tea polyphenol EGCG at concentrations below the MIC (539 ± 22 μg/mL) against enterohemorrhagic E. coli O157:H7. At 25 μg/mL, the growth rate was not affected, but autoinducer 2 concentration, biofilm formation, and swarm motility decreased to 13.2, 11.8, and 50%, respectively. The black tea polyphenols reduced the expression of virulence traits of clinical isolates of Shigella dysenteriae and enteropathogenic E. coli (EPEC P2 1265) strains (Kiran et al., 2010). More recently, the antibacterial property and mechanism of a novel pu-erh tea nanofibrous membrane have been studied. In this study, pu-erh tea was used as a raw material for nanomaterial preparation and as an antibacterial agent. The results showed better antibacterial activity with smaller pu-erh tea powder (PTP) particles, the nano-sized particles had the best effects, and the MIC of nano-PTP (NPTP) was 13.5 mg/mL. Pu-erh tea in nanofibrous membranes damaged the E. coli cell membranes and caused leakage of potassium and enzymes (Su, Zhang, Wang, & Li, 2012).
5.3. Helicobacter pylori
H. pylori is a type 1 carcinogen and the most important risk factor for gastric cancer. Components found in green tea have been shown to inhibit bacterial growth, including the growth of Helicobacter (Stoicov, Saffari, & Houghton, 2009). Surh and co-workers found that green tea containing polyphenols exhibited antibacterial activity against H. pylori, which can be used for treating or preventing various stomach-related diseases including gastric cancer, gastric ulcer, or gastritis caused by H. pylori (Surh, Kang, Lee, & Park, 2006).
Stoicov et al. (2009) evaluated the effects of green tea on the development of Helicobacter-induced gastritis in an animal model. Furthermore, green tea consumption prevented gastric mucosal inflammation prior to exposure to Helicobacter infection. Recently, Ankolekar et al. (2011) investigated the effects of nine tea extracts—three different brands representing four different processed types (white, green, oolong, and black)—on the inhibition of H. pylori and also studied the influence of extraction time on H. pylori inhibition. All the 5 min extracts (extraction time—5 min) showed H. pylori inhibition, whereas 2 min extracts of only darjeeling black tea and tazo white tea inhibited the growth of H. pylori. None of the extracts inhibited the beneficial lactic acid bacteria. The authors concluded that tea could be potentially used as a low-cost dietary support to combat H. pylori-linked gastric diseases without affecting the beneficial intestinal bacteria.
5.4. Bacillus and Clostridium
The activity of tea polyphenols against the bacterial genera belonging to the Bacillaceae family (Bacillus and Clostridium) was extensively studied. Hara-Kudo et al. (2005) studied antibacterial effects of major green tea polyphenols using Clostridium and Bacillus spores. After incubation with crude catechins C. botulinum and C. butyricum spores were decreased in number while no effect was shown in Bacillus cereus spores. Out of six catechins investigated, ECG, EGC, EGCG, and GCG were more effective in decreasing C. botulinum and C. butyricum spore numbers. The authors observed that low concentrations of catechins, although requiring a long exposure time, inhibited the growth of bacterial spores.
The antimicrobial activities of tea catechins, theaflavins, and tea extracts have been evaluated against B. cereus (strain RM3190). The results indicated that GCG, EGCG, CG, ECG, TF3, theaflavin-3′-gallate, and theaflavin-3-gallate showed antimicrobial activities at nanomolar levels, and most of the compounds were more active than current antibiotics viz. tetracycline and vancomycin, at comparable concentrations (Friedman, Henika, Levin, Mandrell, & Kozukue, 2006). In a different study, Wu et al. (2007) reported antimutagenic and antimicrobial activities of pu-erh tea. The antimutagenic activity of the water extract of pu-erh tea (WEPT) against aflatoxin B1 (AFB1) and 4-nitroquinoline-N-oxide (NQNO) was weaker than other tea extracts (green, black, and oolong) because of the least amount of total catechin in WEPT. WEPT has a potential antimicrobial effect on gram-positive S. aureus and B. subtilis than that of gram-negative E. coli. Juneja, Bari, Inatsu, Kawamoto, and Friedman (2007) observed in C. perfringens that spore germination and outgrowth were inhibited by green tea extract during abusive cooling (54.4 to 7.2 °C) of cooked ground beef, chicken, and pork. Pu-erh tea extract could significantly inhibit the growth of Listeria monocytogenes, Salmonella typhimurium, Streptococcus faecalis, E. coli, and B. anthracis, and their MIC values were 0.07, 0.18, 0.50, 0.42, and 0.48 mg/mL, respectively while showing weak inhibition for S. aureus (Hu, Jia, Qiao, Ge, & Cao, 2010).
The antibacterial activity of processed Nigerian lipton tea and South African five roses tea, extracted using distilled water, chloroform, and 70% ethanol, was determined against nine enteropathogenic bacteria—B. subtilis, Proteus species, Enterobacter species, Klebsiella pneumoniae, E. coli, S. typhi, S. paratyphi A, S. arizona, and S. aureus. The authors concluded that aqueous extract of lipton tea was found to be a more effective antibacterial agent than five roses tea (Ojo, Yunusa, Akindele, Vera, & Fowora, 2010). Moreover, Chan, Soh, Tie, and Law (2011) investigated the role of non-polymeric phenolic (NP) and polymeric tannin (PT) constituents in the antioxidant and antibacterial properties of six brands of green, black, and herbal teas of C. sinensis. Green tea inhibited the growth of all three screened gram-positive bacteria (Micrococcus luteus, S. aureus, and B. cereus) while black and herbal tea showed inhibition of M. luteus and B. cereus, but not S. aureus. Recently, Shigemune et al. (2012) investigated the mechanism of antibacterial action of EGCG against spore-forming bacteria (genus Bacillus). The spore count was independent of the presence of EGCG whereas, vegetative growth was suppressed by EGCG. The findings suggested that catechins did not suppress spore germination or inactivate spores since catechins were not adsorbed by spores, and thus could not act on them.
5.5. Streptococcus
Dental caries are caused by a group of acid-producing species of the genus Streptococcus, in particular S. mutans and S. sobrinus, which are reported to be the major infective agents of human dental plaque. A key role in such process is played by salivary amylase, which hydrolyses food starch to oligo- and monosaccharides (e.g. maltose, glucose). The fermentation of such carbohydrates by bacterial enzymes occurring in oral cavities provokes the formation of organic acids responsible to dental caries. Ooshima (2005) studied the anti-caries activity of various types of tea and their polyphenols. The fermentation process during processing of tea results in oxidation of simple polyphenols in green tea leaves to produce more complex structures due to polymerization. The IC50 of purified oolong tea polyphenol to inhibit insoluble glucan synthesis was found to be 2 μg/mL, 8 μg/mL with TF (purified from black tea leaves), 40 μg/mL with oolong tea extract, 250 μg/mL with a green tea extract, and much higher concentrations were needed with simple catechins. The author found that inhibition of glucosyltransferase activity of S. mutans and decreasing the surface hydrophobicity of oral Streptococci are responsible factors for anti-caries activity of tea polyphenols.
The inhibition of acid production in dental plaque bacteria (S. mutans) by EGCG was examined. After treatment with EGCG, the pH values of plaque samples from fifteen volunteers were significantly higher than those who were treated with water (Hirasawa, Takada, & Otake, 2006). Furthermore, Hassani et al. (2008) evaluated the efficacy of black and green tea extracts against S. mutans ATCC 25175, S. mitis ATCC 9811, and S. sanguis ATCC 10556 that are responsible for dental caries. The extracts were able to prevent the growth of oral Streptococci. A 1 mg/mL of black tea extract completely inhibited biofilm formation while green tea extract was unable to inhibit biofilm formation at the same concentration. The dry leaves of black tea contained more tannins than green tea and had stronger antibacterial activity (Stanczyk, Skolimowska, & Wedzisz, 2008).
The tea polyphenols extracted from Korean green tea were evaluated for their antimicrobial effects and inhibition of biofilm formation properties against twelve oral microorganisms. The authors observed various morphological changes, such as the presence of perforations, formation of cell aggregates, and leakage of cytoplasmic materials from cells treated with tea polyphenols, depending on the bacteria by scanning electron microscopy (SEM) analysis. The results concluded that tea polyphenols are effective against adherent cells of S. mutans and S. sanguis, and therefore, can be developed as a potential antimicrobial agent against oral microorganisms for the prevention and treatment of dental caries (Cho, Oh, & Oh, 2010).
Abd, Ibrahium, and Al-Atrouny (2011) evaluated the antimicrobial effect of black tea against S. mutans and Lactobacillus species in adult Egyptian citizens. The results showed that the black tea beverage had a highly significant effect on reducing the cariogenic bacterial counts. The antibacterial activity of Iranian green and black tea extracts on S. mutans was also studied at different concentrations (50, 100, 150, 200, 300, and 400 mg/mL). The MIC of Iranian green and black tea extracts was found to be 150 and 50 mg/mL, respectively (Naderi, Niakan, Kharazi, & Zardi, 2011). The suppression of cariogenic virulence factors of S. mutans by EGCG has been reported. Tea polyphenols, especially EGCG, were shown to inhibit glucosyltransferase activity and growth of S. mutans (Xu, Zhou, & Wu, 2011). Furthermore, Xu, Zhou, and Wu (2012) described the mechanism of inhibition of dental plaque accumulation by EGCG. They hypothesized that EGCG suppressed gtf genes in S. mutans at the transcriptional level disrupting the initial attachment of S. mutans and thus the formation of mature biofilms.
Recently, Cui et al. (2012) studied the comparison of morphological alterations in gram-positive and gram-negative bacteria induced by EGCG at sub-MICs. EGCG caused aggregates in the cell envelopes of gram-positive bacteria (S. aureus and Streptococcus mutans) (Fig. 6 ) while it caused microscale grooves in the cell envelopes of gram-negative bacteria (Pseudomonas aeruginosa and E. coli) (Fig. 7 ).
Fig. 6.

Topographical images of S. aureus treated with EGCG. Cells were: untreated (a–b); treated with 1/8 MIC (12.5 mg/L) EGCG for 1 h (c–d) and 2 h (e–f). White arrows indicate the lattice structures of the peptidoglycan layer.
Fig. 7.

Topographical images of E. coli O157:H7 treated with EGCG. Cells were: untreated (a–b); treated with 1/8 MIC (60 mg/L) EGCG for 1 h (c–d) and 2 h (e–f).
5.6. Other bacteria
Different types of tea and their polyphenols were also evaluated for antibacterial activity against other infectious agents. Representative agents include, Legionella pneumophila, Mycobacterium tuberculosis, Salmonella, P. aeruginosa, L. monocytogenes, and Vibrio cholera.
L. pneumophila causes Legionnaire disease, an infection of the lungs and other organs. Few authors found that EGCG inhibited interleukin-12 (IL-12) production while enhancing tumor necrosis factor-alpha (TNF-α) production in bone marrow-derived dendritic cells infected with L. pneumophila. These findings suggested that EGCG had a marked effect in modulating production of immunoregulatory cytokines (IL-12 and TNF-α) in stimulated dendritic cells, which are important for antimicrobial immunity, especially innate immunity (Rogers et al., 2005). Moreover, Kobayashi et al. (2006) found that growth inhibition of S. enteritidis was more pronounced in black tea, oolong tea, and green tea, which contained a larger amount of catechin with galloyl moiety such as EGCG and ECG than barley tea. Out of four kinds of tea, the bacteriostatic activity of black tea against S. enteritidis was at the maximum due to the presence of theaflavins.
The inhibitory activity of natural products has been evaluated against the growth of E. coli (ATCC 25922) and S. typhimurium (KCCM 11862). The results concluded that natural bioactives such as EGCG and chitosan may be used as antimicrobial agents for the improvement of food safety (Kim & Kim, 2007). Similarly, Cetojevic-Simin, Bogdanovic, Cvetkovic, and Velicanski (2008) investigated antimicrobial activity of kombucha beverages made from Camellia sinensis L. (black tea) and Satureja montana L. (winter savory tea). The authors found that both kombucha beverages had the most expressive antimicrobial activity against all investigated bacteria. Black tea kombucha showed higher activity than acetic acid against S. aureus and E. coli while both kombuchas had bacteriostatic activity on Salmonella enteritidis.
M. tuberculosis is a species that causes tuberculosis in humans. Lack of maturation of phagosomes containing pathogenic M. tuberculosis within macrophages has been widely recognized as a crucial factor for the persistence of mycobacterial pathogen. Anand, Kaul, and Sharma (2006) found that down-regulation of tryptophan-aspartate containing coat protein (TACO) gene expression by EGCG was responsible for the inhibition of M. tuberculosis survival. Furthermore, Sharma, Kumar, Kapoor, and Surolia (2008) reported that EGCG inhibited InhA, the enoyl acyl carrier protein reductase of M. tuberculosis with IC50 of 17.4 μM.
P. aeruginosa is an opportunistic pathogen responsible for a wide range of infections. The combined effect of the cell-surface damaging compounds (surfactants and preservatives) and GTE was investigated against P. aeruginosa. It was found that both surfactants and preservatives enhanced the antibacterial activity of GTE at pH 5.0, 6.5, and 8.0 (Nakayama et al., 2009). Production of virulence factors and biofilm formation by P. aeruginosa are partly regulated by cell-to-cell communication quorum-sensing systems. Identification of quorum-quenching reagents, which block the quorum-sensing process, can facilitate development of novel treatment strategies for P. aeruginosa infections. It was observed that EGCG has a higher binding affinity towards enoyl-acyl carrier protein reductase (ENR) of P. aeruginosa and is an efficient quorum-quenching reagent (Yang, Liu, Sternberg, & Molin, 2010).
Some additional examples of antibacterial activity of tea extracts or tea polyphenols are reported in Table 2 .
Table 2.
Some additional examples of antibacterial activity of tea extract or tea polyphenols.
| Bacterium | Tea extract or active compound (s) | Significant findings | References |
|---|---|---|---|
| Staphylococcus aureus | EGCG | Inhibition of staphylococcal superantigen (SsAg)-induced T-cell activation | Hisano et al. (2003) |
| MRSA | EGCG, ECG | EGCG (MIC = 32–64 μg/mL) more potent than ECG (MIC = 64–> 512 μg/mL) | Gibbons, Moser, and Kaatz (2004) |
| Helicobacter pylori | EGCG | Suppression of H. Pylori-induced gastric epithelial cytotoxicity via the blockage of toll-like receptor-4 (TLR-4) signaling | Lee et al. (2004) |
| Streptococcus mutans | Oolong tea extract | Synergistic antibacterial activity was shown by monomeric polyphenols. | Sasaki et al. (2004) |
| Legionella pneumophila | EGCG | Enhancement of in vitro resistance of alveolar macrophages to Legionella pneumophila infection | Yamamoto, Matsunaga, and Friedman (2004) |
| Listeria monocytogenes | EGCG, ECG | Inhibition of tested microorganism by complex of tea catechins (EGCG and ECG) | Patar, Nishikawa, Tanaka, Ishimaru, and Maeda (2003) |
| Vibrio cholera | Green tea extract | The MIC of 11 out of 23 strains of V. cholera was found to be 10 mg/mL, 4 had MIC at 20 mg/mL, 4 at 30 mg/mL, 1 at 40 mg/mL and 3 at 50 mg/mL. | Bandyopadhyay, Chatterjee, Dasgupta, Lourduraja, and Dastidar (2005) |
Kohda, Yanagawa, and Shimamura (2008) reported inhibition of intracellular survival of L. monocytogenes in macrophages by EGCG. Anti-L. monocytogenes activity of EGCG is through the inhibition of hemolytic and cholesterol-binding activity of listeriolysin O, which usually disrupts the phagosomal membrane in the escaping phase of L. monocytogenes. Podlipnik (2009) performed docking of selected natural polyphenols to ARF (ADP-ribosylation factor) activated A1 subunit of cholera toxin (CTA1). EGCG, TF3, and 1,2,3,6-tetra-O-galloyl-d-glucose were the best binder towards the active binding site of CTA1. Tea polyphenols have been also tested as antimicrobial drugs against food-borne pathogen and food-spoilage bacteria. In a study on major food-borne pathogens, Chinese green tea extract strongly inhibited these pathogens (E. coli O157:H7, S. Typhimurium DT104, L. monocytogenes, and S. aureus). A simple and efficient reversed-phase high-speed counter-current chromatography (HSCCC) method was developed for the separation and purification of four bioactive polyphenol compounds, ECG, EGCG, EC, and caffeine (CN). Among the four compounds, ECG and EGCG were the most active, particularly EGCG against S. aureus. EGCG had the lowest MIC90 values against S. aureus (58 μg/mL) and MRSA (37 μg/mL) (Si et al., 2006). Likewise, Osterburg, Gardner, Hyon, Neely, and Babcock (2009) reported that EGCG expressed bactericidal activity against multiresistant clinical isolates of Acinetobacter baumannii with sub-MICs ranging from 78 to 625 μg/mL, with MIC50 and MIC90 of 312 μg/mL and 625 μg/mL, respectively.
Tea extracts have been widely used to extend the shelf life of various foods such as fresh mutton (Kumudavally, Phanindrakumar, Tabassum, Radhakrishna, & Bawa, 2008), overnight pickled cucumber (Miyamoto et al., 2009), and Collichthys fish balls (Yi et al., 2011). Xu, Liu, Ren, and Sun (2006) reported significant bacteriostatic activities of a natural preservative (150 mg/kg nisin + 0.3% tea polyphenols) against Lactobacilli. Moreover, the combination of bacteriocin like nisin with GTE showed greater effectiveness than used alone against major foodborne pathogen like L. monocytogenes (Perumalla & Hettiarachchy, 2011).
6. Antiviral activity of tea and its polyphenols
The effects of tea and tea catechins on viruses have also been extensively studied. Some hypotheses have been recently proposed for explaining the mechanism of antiviral activity of tea catechins. EGCG inhibits viruses by direct binding to biological molecules and induces agglutination of the influenza virus thus preventing their adsorption to target cells. In addition, the direct binding of EGCG to viral receptors on cell surfaces may also interfere with viral infectivity, following the observation that EGCG binds with CD4 cells and interferes with binding to the HIV surface protein, gp120 (Tadakatsu, Wei-Hua, & Zhi-Qing, 2007).
6.1. Influenza virus
Nakayama and co-workers reported that tea polyphenols greatly reduce the infectivity of the influenza virus in cell culture (Nakayama et al., 1993). Following this report, tea polyphenols have received more attention as an alternative measure against seasonal as well as pandemic influenza. Recently, Furuta et al. (2007) synthesized di-deoxy-epigallocatechingallate 3 (Fig. 5), an analog of EGCG that was evaluated for anti-influenza virus activity. The compound showed potent anti-influenza virus activity, indicating that the hydroxyl substituents on the A-ring are not crucial for anti-influenza virus activity.
Similarly, few catechin derivatives with a different alkyl chain length and aromatic ring substitutions at the 3-hydroxyl group from EGC and (+)-catechin have been synthesized and evaluated for their anti-influenza viral activity. The derivatives carrying a chain length of 9–11 carbons such as compounds 4, 5, 6, and 7 (Fig. 8 ), showed pronounced antiviral activity compared to those with aromatic rings. These derivatives exerted inhibitory effects on all six influenza subtypes tested including three major types of currently circulating human influenza viruses (A/H1N1, A/H3N2, and B type), H2N2 and H9N2 avian influenza virus (Song et al., 2007).
Fig. 8.

Chemical structure of catechin derivatives.
The in vitro inhibitory effects of EGCG on influenza virus A-induced cytopathy were observed by cytopathic effect assay (CPE). Oral administration of EGCG reduced the mortality of influenza virus A H1N1 strain-infected mice and lessened the lesion degree of mice lung tissue (Xiao, Yang, Shi, Liu, & Chen, 2008). Furthermore, Mori et al. (2008) prepared a series of fatty acid monoester derivatives of EGCG by one-pot lipase-catalyzed transesterification. The EGCG-monoesters modified with butanoyl, octanoyl, lauroyl, palmitoyl, and eicosanoyl groups were represented as EGCG-C4, EGCG-C8, EGCG-C12, EGCG-C16, and EGCG-C20, respectively. The authors showed that the anti-influenza A/PR8/34 (H1N1) virus activities of EGCG-monoesters were enhanced in an alkyl chain length-dependent manner. EGCG-C16 8 (Fig. 5) was most potent (EC50 4 μM) among EGCG monoesters, and its inhibitory effect was found to be 24-fold higher than native EGCG.
Likewise, Tan, Shi, Zhu, and Tan (2009) investigated the anti-influenza virus activity of EGCG. The effect and potency of anti-influenza virus were examined in vitro in cell culture. There was a close relationship between dose and efficacy of EGCG on the effect of anti-influenza virus. EGCG possessed potent inhibitory effect on influenza virus. Another study established virus inhibition activity of EGCG and EGCG-monopalmitate on avian influenza A/Duck/Hong Kong/342/78 (H5N2) virus in 11 day old chicken embryonated eggs inoculated with compound-treated or -untreated viruses. EGCG-monopalmitate showed complete inhibition while EGCG exhibited a moderate viral inhibitory effect (Kaihatsu et al., 2009). Moreover, Huang et al. (2010) performed in vitro and in vivo study on anti-influenza virus effect of tea polyphenols. EGCG and ECG showed a marked antiviral effect against influenza virus infections in MDCK cells (Madin–Darby canine kidney cells) in all influenza virus subtypes tested, including H5N1, H1N1, and H9N2 viruses and also showed an inhibitory effect on neuraminidases (NAs) from H5N1, H1N1, and H9N2 viruses. At the concentration of 1000 mg/kg/day, tea extract possessed a potent inhibitory effect on BALB/c's pneumonia consolidation infected by influenza viruses.
A nasal inhalation containing EGCG and/or ECG has been prepared for treating common cold and influenza. The nasal preparation consisted of two parts: solid anti-influenza virus component EGCG or ECG, and liquid acetic acid–sodium acetate buffer. The inhalation containing 3.2 mg/L EGCG and 1.8 mg/L ECG showing significant inhibition against influenza virus. It has the advantages of good stability, long shelf life, rapid absorption, and good curative effect (Nie, 2010). More recently, in vitro anti-influenza virus and anti-inflammatory activities of TF derivatives have been reported. The results showed that all the derivatives of TF exerted significant inhibitory effects on the neuraminidase (NA) of three different subtypes of influenza virus strains (A/PR/8/34(H1N1), A/Sydney/5/97(H3N2), and B/Jiangsu/10/2003) with IC50 values ranging from 9.27 to 36.55 μg/mL, and they also displayed an inhibitory effect on hemagglutinin (HA) (Zu et al., 2012).
6.2. HIV
Among various viruses investigated for potential therapeutic targets, HIV has received the most attention. Several reports available in the literature have shown that tea polyphenols have a protective effect against HIV infection. The reports showed that green tea catechin, EGCG is the most effective compound against HIV infection. Hamza and Zhan (2006) reported a mechanism of inhibition of gp120–CD4 binding by EGCG. The authors performed extensive molecular docking, molecular dynamics simulations, and binding free-energy calculation studies to predict the most favorable structures of CD4–EGCG, gp120–CD4, and gp120–CD4–EGCG binding complexes in water. The results revealed that EGCG binds with CD4 in such a way that the calculated binding affinity of gp120 with the CD4–EGCG complex is negligible. Therefore, a favorable binding of EGCG with CD4 can effectively block gp120–CD4 binding. Similarly, Williamson, McCormick, Nance, and Shearer (2006) demonstrated binding of EGCG to the CD4 molecules at the gp120 attachment site and inhibition of gp120 binding. Molecular modeling studies suggested a binding site for EGCG in the D1 domain of CD4, the pocket that binds gp120. A 0.2 μM/L EGCG inhibited binding of gp120 to isolated human CD4 + T cells. This clearly demonstrated clear evidence of high-affinity binding of EGCG to the CD4 molecules with a Kd of approximately 10 nM and inhibition of gp120 binding to human CD4 + T cells.
A docking study was performed which concluded that when HIV-1 integrase did not combine with virus DNA, the four catechins with the galloyl moiety, including CG, EGCG, GCG, and ECG, were able to bind with Tyr143 and Gln148, altering the flexibility of the loop (Gly140–Gly149), and thereby leading to an inhibition of HIV-1 integrase activity (Jiang et al., 2010). The HIV fusion-inhibiting activity of peracetate ester of EGCG 9 (Fig. 9 ) was investigated by computer aided molecular docking to analyze the binding sites of peracetate ester of EGCG on gp41. The stability of EGCG was significantly enhanced after introduction of acetate protection groups on the reactive hydroxyls of EGCG. After hydrolysis, peracetate ester of EGCG inhibited HIV fusion by targeting gp41 (Yang, Li, et al., 2010).
Fig. 9.
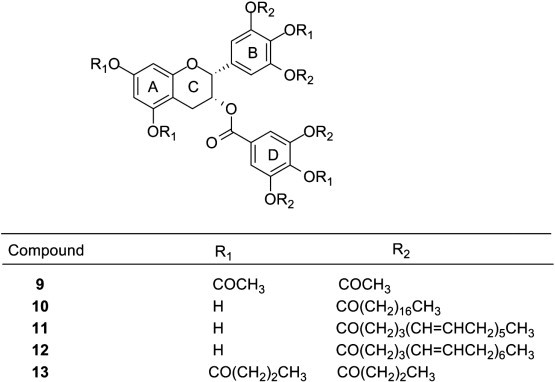
Chemical structure of EGCG esters.
Li, Hattori, and Kodama (2011) found that EGCG appeared to act mainly as an allosteric reverse transcriptase inhibitor with mechanisms different from those of currently approved non-nucleoside reverse transcriptase inhibitors (NNRTIs) that directly interact with the NNRTI binding pocket. It also showed synergistic inhibition with 3′-azido-3′-deoxythymidine (AZT). Liu et al. (2005) reported anti-HIV activity of TF derivatives in black tea and catechin derivatives in green tea. The authors found that TF derivatives had more potent anti-HIV-1 activity than the catechin derivatives. They inhibited HIV-1 entry into target cells by blocking HIV-1 envelope glycoprotein-mediated membrane fusion. Computer-aided molecular docking analysis indicated that these tea polyphenols, TF3 as an example, may bind to the highly conserved hydrophobic pocket on the surface of the central trimeric coiled coil formed by the N-terminal heptad repeats of gp41. The inhibition of HIV-1 infection by natural theaflavins preparation has been reported. TFmix is an economic natural product preparation containing high content of theaflavins with potent anti-HIV-1 activity by targeting the viral entry step through the disruption of gp41 6–HB core structure (Yang et al., 2012).
6.3. Epstein–Barr virus
Epstein–Barr virus (EBV) is a DNA virus belonging to the Herpesviridae family and is associated with several human malignancies, such as Burkitt's lymphoma and nasopharyngeal carcinoma. However, the expression of Epstein–Barr nuclear antigen 1 (EBNA1) is prevalent in all EBV-associated tumors and has become one of the most attractive drug targets for the discovery of anti-EBV compounds. Recently, Chen, Tsai, and Peng (2012) found that the treatment of cells with 50 μM EGCG effectively blocked the binding of EBNA1 to oriP-DNA both in vivo and in vitro, leading to the abrogation of EBNA1-dependent episome maintenance and transcriptional enhancement. The anti-EBNA1 effects caused by EGCG ultimately impaired the persistence of EBV latent infection.
6.4. Hepatitis B virus
Hepatitis B virus (HBV) infection is endemic in Asia and is a major public health concern worldwide. Present treatment strategies for HBV infections are not satisfactory and the clinical limitation of current antiviral drugs for HBV, such as lamivudine, has caused rapid emergence of drug-resistant viral strains during the prolonged therapeutic treatment. The green tea extract and primary active ingredient EGCG inhibited the expression of hepatitis B antigens and the replication of HBV DNA and could be used as drugs or adjuvants for treating hepatitis B infection (Wang, Xu, Deng, & Hu, 2007). Similarly, the efficacy of a natural green tea extract (GTE) was examined against HBV in a stably expressed HBV cell line HepG2-N10. The results indicated that EC50 values of GTE on HBsAg, HBeAg, extracellular HBV DNA, and intracellular HBV DNA were 5.02, 5.681, 19.81, and 10.76 μg/mL, respectively (Xu, Wang, Deng, Hu, & Wang, 2008).
The antiviral mechanism of EGCG has been analyzed against HBV replicating cell line HepG2.117. EGCG inhibited HBV replication by impairing HBV replicative intermediates of DNA synthesis which resulted in reduced production of HBV covalently closed circular DNA (He, Li, Liao, Liu, & Chen, 2011). Recently, Pei, Zhang, Xu, Chen, and Chen (2011) studied the role of pu-erh tea extract (PTE) against HBV by using a stably HBV-transfected cell line HepG2 2.2.15. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay showed that PTE and its active components (tea polyphenols, theaflavins, and theanine) had a low cytotoxicity profile against HBV. More recently, four lipophilic ester derivatives of EGCG, namely EGCG-O-tetrastearate 10, EGCG-O-tetraeicosapentaenoate 11, EGCG-O-tetradocosahexaenoate 12, and EGCG-O-octabutyrate 13 (Fig. 9) were prepared and evaluated for their in vitro antioxidant and antiviral activities. Incorporation of long chain polyunsaturated fatty acids (PUFA), into EGCG resulted in increased peroxyl radical scavenging activity and metal chelation capacity. The EGCG–PUFA esters were found to be 1700-fold more effective in inhibiting hepatitis C virus (HCV) protease than embelin which serves as a positive control. The derivatives were also found to be α-glucosidase inhibitors, suggesting their potential role as an anti-HIV agent (Zhong, Ma, & Shahidi, 2012).
6.5. Herpes simplex virus
Herpes simplex virus 1 and 2 (HSV-1 and HSV-2), also known as Human herpes virus 1 and 2 (HHV-1 and HHV-2), are belonging to family, Herpesviridae, that infect humans. Both HSV-1 (which is responsible for cold sores) and HSV-2 (which causes most genital herpes) are ubiquitous and contagious. Savi, Barardi, and Simoes (2006) evaluated antiherpetic activity and genotoxicity of tea catechins and their derivatives using the MTT colorimetric and comet assays. The study showed that all compounds have antiviral activity with selective indexes varying from 1.3 to 13, depending on the tested HSV-1 strain.
Isaacs et al. (2008) observed that binding of EGCG to HSV-1 envelope glycoproteins gB and gD was responsible for anti-HSV activity. Furthermore, the in vitro antiviral activity and therapeutic efficacy of catechin polyphenols have been evaluated in mice infected with herpes simplex virus (HSV) and influenza virus (IFV). The in vitro and in vivo studies demonstrated that green tea polyphenols were active against HSV while failing to show any activity against IFV (Daikoku et al., 2011). EGCG inactivates HSV-1 and HSV-2 at pH 8.0 by 3 log10 to 4 log10 but is ineffective at pH 5.7. Recently, Isaacs et al. (2011) noticed that the EGCG digallate dimers such as theasinensin A, P2 (Fig. 10 ), and TF3 inactivated HSV-1 and HSV-2 viruses by 3 log10 to 4 log10 at pH 5.7 and as much as 5 log10 at pH 8.0 while TF3 inactivated both viruses by 4 log10 to 5 log10 in the pH range of 4.0 to 5.7. Dimers with one gallate moiety had antiviral activity intermediate between the activities of EGCG and digallate dimers. The results concluded that digallate dimers of EGCG had excellent potential as microbicidal agents against HSV at both acidic and neutral pH.
Fig. 10.

Chemical structures of EGCG digallate dimers.
6.6. Other viruses
Different types of tea and their polyphenols were evaluated for antiviral activity against other infectious agents, which include bovine coronavirus, adenovirus, tobacco mosaic virus, and cucumber mosaic virus.
The inhibitory effect of EGCG on bovine coronavirus (BCV) propagation has been investigated in Madin–Darby bovine kidney (MDBK) cells (Matsumoto, Mukai, Furukawa, & Ohori, 2005). The authors observed that EGCG possessed a distinct anti-BCV activity and interfered with the adsorption of BCV to MDBK cells by the interaction of EGCG with BCV particles. Moreover, Furukawa, Kawabe, Ohori, Mukai, and Matsumoto (2005) developed a composition containing reduced glutathione and catechin (EGCG) against viruses belonging to the Coronaviridae and Flaviviridae family.
Some additional examples of antiviral activity of tea extract or tea polyphenols are reported in Table 3 .
Table 3.
Some additional examples of antiviral activity of tea extract or tea polyphenols.
| Virus | Tea extract or active compound (s) | Significant findings | References |
|---|---|---|---|
| HIV | EGCG | Direct binding of EGCG to CD4 and inhibition of gp120–CD4 binding. | Kawai et al. (2003) |
| Epstein–Barr virus | EGCG | Interference with EBV-encoding activator protein-1 (AP-1) signal transduction pathway. | Zhao et al. (2004) |
| Hepatitis B virus | Tea catechin | Protection of liver functions and reduction of pathological changes of the liver tissue. | Li, Zhou, and Zhang (2001) |
| Adenovirus | EGCG | EGCG inactivated purified adenovirions with IC50 of 250 μM. | Weber, Ruzindana-Umunyana, Imbeault, and Sircar (2003) |
| Rotavirus | Tea polyphenol | Reduction of infectivity of rotavirus by combination of anti-retroviral agent with tea polyphenol. | Apostolides and Selematsela (2003) |
The importance of catechins as antiviral drugs for prevention and treatment of respiratory viral infection, avian influenza infection, respiratory syncytial infection, coxsackie infection, and adenovirus infection has been reported by authors (Yang & Shi, 2007). Recently, Zhou et al. (2008) reported the isolation and characterization of ZH14 with antiviral activity against tobacco mosaic virus. The bacterium ZH14, which was isolated from Chinese oolong tea, secreted the antiviral substances, having 94.2% virus inhibition.
7. Antifungal activity of tea and its polyphenols
However, not enough studies have been carried out so far on the antifungal activity of tea polyphenols. Among tea polyphenols, EGCG was the most active against Candida albicans.
7.1. Candida albicans
C. albicans is a diploid fungus that grows both as yeast and filamentous cells. It is a causal agent for opportunistic oral and genital infections in humans. In vitro antimycotic activity of some plant extracts have been studied towards yeast and yeast-like strains. Green tea extract exhibited broad antimycotic activity towards Candida glabrata, Clavispora lusitatiae, Cryptococcus laurentii, Filobasidiella neoformans, Issatchenkia orientalis, Saccharomyces cerevisiae, and Prototheca wickerhamii strains. The compounds responsible for antimycotic activity were ECG and EGCG (Turchetti et al., 2005).
Navarro-Martinez, Garcia-Canovas, and Rodriguez-Lopez (2006) elucidated the mechanism of action of EGCG against C. albicans. The authors demonstrated that by disturbing the folate metabolism, EGCG could inhibit ergosterol production. Furthermore, the antifungal susceptibility of 21 clinical isolates of seven Candida species to EGCG has been investigated. Among the tested species, C. glabrata exhibited the highest susceptibility to EGCG (MIC50, 0.5–1 μg/mL and MIC90, 1–2 μg/mL) compared favorably with fluconazole. Moreover, the susceptibility of Candida krusei strains (MIC50, 2 μg/mL and MIC90, 4–8 μg/mL) to EGCG was found to be approximately 2- to 8-fold higher than flucytosin and fluconazole (Park et al., 2006). It has been reported that EGCG treatment inhibited the hyphal formation of the yeast form of C. albicans, causing growth-inhibition of the candidal cells in a murine model of disseminated candidiasis. The authors also observed a synergistic effect between amphotericin B and EGCG (Han, 2007).
Few topical antifungal compositions containing polyphenols mainly EGCG with ≥ 98% purity have been developed. Teavigo (high-purity EGCG) showed MIC50 values of 8, 0.5, and 0.125 μg/mL against C. albicans NBRC 0583, C. glabrata NBRC 0005, and C. parapsilosis NBRC 0840, respectively (Sugai, Park, Han, & Hyeon, 2008). Tea polyphenols showed inhibition of biofilm formation and proteasome inactivation of C. albicans. Cultures treated with 1.0 μM EGCG displayed a 75% reduction of viable cells during biofilm formation. Established biofilms treated with EGCG were also reduced, by 80%, as determined through XTT (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) colorimetric assays (Evensen & Braun, 2009). Likewise, Sitheeque et al. (2009) studied antifungal activity of black tea polyphenols (catechins and theaflavins) against Candida species. The polyphenols showed anti-candidal activity against all tested Candida species and demonstrated a MIC of 6.25 mg/mL for C. albicans. C. glabrata was found to be the most sensitive species followed by C. parapsilosis, C. albicans, C. krusei, and C. tropicalis.
7.2. Other fungi
The tea catechin mainly EGCG and caffeine showed antifungal activity against Trichophyton tonsurans, T. violaceum, T. rubrum, and T. mentagrophytes (Matsuura, Ogawa, Goto, & Hara, 2007). Few oral cavity care compositions containing EGCG from green tea polyphenol and ε-poly-l-lysine were tested for antifungal activity. All compositions containing EGCG had significant antifungal activity in combination with ε-poly-l-lysine (Matsumura, Okazaki, Xuan, Komasa, & Sugai, 2009). Similarly, the antifungal activity of EGCG and other antifungal agents has been investigated against thirty-five clinical isolates of dermatophytes. All isolates exhibited good susceptibility to EGCG (MIC50, 2–4 μg/mL, MIC90, 4–8 μg/mL) than those of fluconazole (MIC50, 2–16 μg/mL, MIC90, 4–32 μg/mL) and flucytosin (MIC50, MIC90 > 64 μg/mL) (Park et al., 2011).
8. Synergism between tea polyphenols and antibiotics
Current literature indicates that combined use of antibiotics and green tea polyphenols can increase the antimicrobial activity of the former through specific synergistic interactions. Tea catechin, EGCG synergized the activity of β-lactams against MRSA because of the fact that both EGCG and β-lactams directly or indirectly attack peptidoglycan synthesis (Zhao, Hu, Okubo, Hara, & Shimamura, 2001). Direct binding of EGCG with penicillinase inhibits enzymatic activity and protects the antibacterial activity of penicillin. EGCG also enhanced the activity of tetracycline in resistant staphylococcal isolates by inhibiting its efflux from bacterial cells (Roccaro, Blanco, Giuliano, Rusciano, & Enea, 2004).
8.1. β-lactam antibiotics
The mechanism of synergistic antibacterial action has been hypothesized between ECG/EGCG and β-lactam antibiotics against MRSA. ECG/EGCG showed synergistic effect with β-lactams against MRSA due to down-regulation of PBP2a expression and up-regulation of LytM and LgrA expression, which resulted in increased secretion of autolytic enzymes, increased mucopeptide hydrolysis, and synergism with β-lactam antibiotics in inhibiting bacterial cell wall synthesis (Hua, Peng, Huang, Yao, & Qian, 2010). The synergic anticandidal effect of EGCG has been investigated in a murine model of disseminated candidiasis caused by C. albicans. EGCG treatment inhibited the hyphal formation from the yeast form of C. albicans, causing growth-inhibition of the candidal cells. A group of mice administered with combination of amphotericin B (0.5 mg/kg) and EGCG (2 mg/kg) had a mean survival time (MST) of 42.1 days, which was approximately 30 days longer than the group receiving amphotericin B alone-received mice groups (Han, 2007).
Combination of green tea polyphenols mainly ECG/EGCG and various antibiotics exhibited synergistic antibacterial effect against MRSA. Inhibition zones of penicillin, oxacillin, ampicillin, ceftazidime, ceftezole, minocycline, and tetracycline in combination with green tea against MRSA were larger than the single antibiotics (Hua, Peng, Zeng, et al., 2010). Recently, Cho, Oh, and Oh (2011) explored the synergistic antibacterial and proteomic effects of imipenem alone and in combination with EGCG on clinical isolates of imipenem-resistant Klebsiella pneumoniae (IRKP). The MIC of imipenem and EGCG for 12 clinical isolated IRKP strains ranged from 8–32 μg/mL and 300–650 μg/mL, respectively. Each of the 12 IRKP strains experienced a 4- to 64-fold reduction in the MIC of imipenem upon co-incubation with 0.25 × MIC level of EGCG. Compared to imipenem alone, combination of EGCG with imipenem demonstrated enhanced bactericidal activity. Two-dimensional polyacrylamide gel electrophoresis identified eight down-regulated proteins including proteins involved in energy metabolism (glyceraldehyde-3-phosphate dehydrogenase and pyruvate kinase), biosynthesis, biosynthesis of cofactor, protein synthesis (elongation factor Tu, acetyl-coA carboxylase, molybdenum cofactor biosynthesis protein A, and 50S ribosomal protein L9), cell envelope (outer membrane protein) and DNA metabolism (single stranded DNA binding protein), and four up-regulated proteins including molecular chaperone DnaK, chaperonin GroEL, alkyl hydroperoxide reductase, and superoxide dismutase in the IRKP strain upon exposure to 1 × MIC of EGCG. Analysis of the outer membrane protein profiles of IRKP cultures treated with EGCG revealed unique changes in outer membrane proteins. These outcomes demonstrated that sub-MIC exposure of EGCG dramatically affected the expression of several important IRKP proteins.
8.2. Other antibiotics
Other groups of antibiotics have been studied for their synergistic interactions with green tea catechins. Lee et al. (2005) observed that a combination of catechins with fluoroquinolone antibiotic ciprofloxacin acted synergistically to alleviate chronic bacterial prostatitis in rats. Hirasawa and Takada (2004) evaluated the susceptibility of C. albicans to green tea catechin and the synergism of the combination of catechin and antimycotics. The authors found that the combined treatment with 3.12–12.5 mg/L EGCG and amphotericin B 0.5 mg/L (below MIC) markedly decreased the growth of amphotericin B-resistant C. albicans. Likewise, the combined use of 12.5 mg/L EGCG and fluconazole 10–50 mg/L (below MIC) effectively inhibited the growth of fluconazole-resistant C. albicans by 98.5%–99.7%. These results indicated that EGCG enhanced the antifungal effect of amphotericin B or fluconazole against antimycotic-susceptible and -resistant C. albicans. The authors also reported synergistic effect with concomitant use of teavigo and amphotericin B, fluconazole, flucytosine, itraconazole, micafungin, or miconazole (Sugai et al., 2008).
9. Conclusion
In this review, we have discussed various studies exploring antimicrobial profile of different types of tea and their polyphenols. These findings could be attributed to direct action against bacteria, viruses, and fungi, as well as suppression of microbial virulence factors. However, the detailed mechanism of antimicrobial activity of tea polyphenols still remains to be explored. Based on literature, EGCG was found the most potent tea polyphenol against bacteria, viruses, and fungi. Gram-positive bacteria were more susceptible towards EGCG compare to gram-negative strains. EGCG derivatives possessing hydrophobic alkyl side chain exhibited greater potency against parent molecule (EGCG) because of increased membrane-permeability. Therefore, structural modifications could improve its activity. In addition, there is growing evidence that EGCG acts synergistically with various conventional antibiotics against multidrug-resistant microorganisms. However, the in vivo efficacy of EGCG appears hindered by limited absorption, presystemic metabolism, and non-specific binding with other biological macromolecules. Recently, a successful attempt was made to improve the bioavailability by nanoemulsification of green tea extract (Kim et al., 2012). The following findings therefore suggest that future investigations should be carried out to study their in vivo activity, toxicity, and bioavailability of tea polyphenols (specially EGCG) to determine the actual relevance in the treatment of human and animal infections.
References
- Abd A.A.A., Ibrahium M.I., Al-Atrouny A.M. Effect of black tea on some cariogenic bacteria. World Applied Sciences Journal. 2011;12:552–558. [Google Scholar]
- Almajano M.P., Carbó R., Jiménez J.A.L., Gordon M.H. Antioxidant and antimicrobial activities of tea infusions. Food Chemistry. 2008;108:55–63. [Google Scholar]
- Anand P.K., Kaul D., Sharma M. Green tea polyphenol inhibits Mycobacterium tuberculosis survival within human macrophages. The International Journal of Biochemistry & Cell Biology. 2006;38:600–609. doi: 10.1016/j.biocel.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Ankolekar C., Johnson D., Pinto M.D.S., Johnson K., Labbe R., Shetty K. Inhibitory potential of tea polyphenolics and influence of extraction time against Helicobacter pylori and lack of inhibition of beneficial lactic acid bacteria. Journal of Medicinal Food. 2011;14:1321–1329. doi: 10.1089/jmf.2010.0237. [DOI] [PubMed] [Google Scholar]
- Apostolides, Z. & Selematsela, M. (2003). Anti-retroviral agent in combination with tea polyphenol for the treatment of viral infections. PCT International Application, WO 2003002126 A1 20030109.
- Arakawa H., Maeda M., Okubo S., Shimamura T. Role of hydrogen peroxide in bactericidal action of catechin. Biological & Pharmaceutical Bulletin. 2004;27:277–281. doi: 10.1248/bpb.27.277. [DOI] [PubMed] [Google Scholar]
- Auger C., Mullen W., Hara Y., Crozier A. Bioavailability of polyphenon E flavan-3-ols in humans with an ileostomy. Journal of Nutrition. 2008;138:1535S–1542S. doi: 10.1093/jn/138.8.1535S. [DOI] [PubMed] [Google Scholar]
- Balentine D.A., Wiseman S.A., Bouwens L.C.M. The chemistry of tea flavonoids. CRC Reviews in Food Sciences and Nutrition. 1997;37:693–704. doi: 10.1080/10408399709527797. [DOI] [PubMed] [Google Scholar]
- Bancirova M. Comparison of the antioxidant capacity and the antimicrobial activity of black and green tea. Food Research International. 2010;43:1379–1382. [Google Scholar]
- Bandyopadhyay D., Chatterjee T.K., Dasgupta A., Lourduraja J., Dastidar S.G. In vitro and in vivo antimicrobial action of tea: The commonest beverage of Asia. Biological & Pharmaceutical Bulletin. 2005;28:2125–2127. doi: 10.1248/bpb.28.2125. [DOI] [PubMed] [Google Scholar]
- Bansal D.D., Singla A., Boparai R. TeaóRole in health and diseases. Natural Product Radiance. 2004;3:156–166. [Google Scholar]
- Bansal S., Syan N., Mathur P., Choudhary S. Pharmacological profile of green tea and its polyphenols: a review. Medicinal Chemistry Research. 2011 [Google Scholar]
- Bruins M.J., Cermak R., Kiers J.L., Van D.M.J., Van A.J.M.M., Van K.B.J.W. In vivo and in vitro effects of tea extracts on enterotoxigenic Escherichia coli-induced intestinal fluid loss in animal models. Journal of Pediatric Gastroenterology and Nutrition. 2006;43:459–469. doi: 10.1097/01.mpg.0000239992.12646.df. [DOI] [PubMed] [Google Scholar]
- Cabrera C., Gimenez R., Lopez C.M. Determination of tea components with antioxidant activity. Journal of Agricultural and Food Chemistry. 2003;51:4427–4435. doi: 10.1021/jf0300801. [DOI] [PubMed] [Google Scholar]
- Cetojevic-Simin D.D., Bogdanovic G.M., Cvetkovic D.D., Velicanski A.S. Antiproliferative and antimicrobial activity of traditional Kombucha and Satureja montana L. Kombucha. Journal of Balkan Union of Oncology. 2008;13:395–401. [PubMed] [Google Scholar]
- Chan E.W.C., Soh E.Y., Tie P.P., Law Y.P. Antioxidant and antibacterial properties of green, black, and herbal teas of Camellia sinensis. Pharmacognosy Research. 2011;3:266–272. doi: 10.4103/0974-8490.89748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.-L., Tsai H.-L., Peng C.-W. EGCG debilitates the persistence of EBV latency by reducing the DNA binding potency of nuclear antigen 1. Biochemical and Biophysical Research Communications. 2012;417:1093–1099. doi: 10.1016/j.bbrc.2011.12.104. [DOI] [PubMed] [Google Scholar]
- Cho Y.-S., Oh J.-J., Oh K.-H. Antimicrobial activity and biofilm formation inhibition of green tea polyphenols on human teeth. Biotechnology and Bioprocess Engineering. 2010;15:359–364. [Google Scholar]
- Cho Y.-S., Oh J.J., Oh K.-H. Synergistic anti-bacterial and proteomic effects of epigallocatechin gallate on clinical isolates of imipenem-resistant Klebsiella pneumonia. Phytomedicine. 2011;18:941–946. doi: 10.1016/j.phymed.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Cho Y.S., Schiller N.L., Kahng H.Y., Oh K.H. Cellular responses and proteomic analysis of Escherichia coli exposed to green tea polyphenols. Current Microbiology. 2007;55:501–506. doi: 10.1007/s00284-007-9021-8. [DOI] [PubMed] [Google Scholar]
- Chow H.H., Cai Y., Alberts D.S., Hakim I., Dorr R., Shahi F. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiology, Biomarkers & Prevention. 2001;10:53–58. [PubMed] [Google Scholar]
- Cui Y., Oh Y.J., Lim J., Youn M., Lee I., Pak H.K. AFM study of the differential inhibitory effects of the green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) against gram-positive and gram-negative bacteria. Food Microbiology. 2012;29:80–87. doi: 10.1016/j.fm.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Daikoku T., Horiba K., Miyata K., Takemoto M., Okuda T., Yoshida Y. Polyphenols including catechin from green tea with in vitro antiviral activity exhibited anti-herpes simplex virus activity but not anti-influenza virus activity in mice. Journal of Traditional Medicines. 2011;28:63–72. [Google Scholar]
- Devine A., Hodqson J.M., Dick I.M., Prince R.L. Tea drinking is associated with benefits on bone density in older women. The American Journal of Clinical Nutrition. 2007;86:1243–1247. doi: 10.1093/ajcn/86.4.1243. [DOI] [PubMed] [Google Scholar]
- Evensen N.A., Braun P.C. The effects of tea polyphenols on Candida albicans: Inhibition of biofilm formation and proteasome inactivation. Canadian Journal of Microbiology. 2009;55:1033–1039. doi: 10.1139/w09-058. [DOI] [PubMed] [Google Scholar]
- Ferrazzano G.F., Roberto L., Amato I., Cantile T., Sangianantoni G., Ingenito A. Antimicrobial properties of green tea extract against cariogenic microflora: an in vivo study. Journal of Medicinal Food. 2011;14:907–911. doi: 10.1089/jmf.2010.0196. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations—Production FAOSTAT http://faostat.fao.org/DesktopDefault.aspx?PageID=567&lang=en#ancor Available from: (accessed on 25.07.2012)
- Frei B., Higdon J.V. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. Journal of Nutrition. 2003;133:3275S–3284S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- Friedman M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Molecular Nutrition & Food Research. 2007;51:116–134. doi: 10.1002/mnfr.200600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M., Henika P.R., Levin C.E., Mandrell R.E., Kozukue N. Antimicrobial activities of tea catechins and theaflavins and tea extracts against Bacillus cereus. Journal of Food Protection. 2006;69:354–361. doi: 10.4315/0362-028x-69.2.354. [DOI] [PubMed] [Google Scholar]
- Furukawa, S., Kawabe, H., Ohori, H., Mukai, T., & Matsumoto, M. (2005). Preventive or therapeutic composition containing glutathione and/or catechin for viral infectious disease. PCT International Application, WO 2005007640 A1 20050127.
- Furuta T., Hirooka Y., Abe A., Sugata Y., Ueda M., Murakami K. Concise synthesis of dideoxy-epigallocatechingallate (DO-EGCG) and evaluation of its anti-influenza virus activity. Bioorganic & Medicinal Chemistry Letters. 2007;17:3095–3098. doi: 10.1016/j.bmcl.2007.03.041. [DOI] [PubMed] [Google Scholar]
- Gibbons S., Moser E., Kaatz G.W. Catechin gallates inhibit multidrug resistance (MDR) in Staphylococcus aureus. Planta Medica. 2004;70:1240–1242. doi: 10.1055/s-2004-835860. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J.M.T. Antimicrobial properties of tea (Camellia sinensis L.) Antimicrobial Agents and Chemotherapy. 1995;39:2375–2377. doi: 10.1128/aac.39.11.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza A., Zhan C.-G. How can (−)-epigallocatechin gallate from green tea prevent HIV-1 infection? Mechanistic insights from computational modeling and the implication for rational design of anti-HIV-1 entry inhibitors. The Journal of Physical Chemistry. B. 2006;110:2910–2917. doi: 10.1021/jp0550762. [DOI] [PubMed] [Google Scholar]
- Han Y. Synergic anticandidal effect of epigallocatechin-O-gallate combined with amphotericin B in a murine model of disseminated candidiasis and its anticandidal mechanism. Biological & Pharmaceutical Bulletin. 2007;30:1693–1696. doi: 10.1248/bpb.30.1693. [DOI] [PubMed] [Google Scholar]
- Hara-Kudo Y., Yamasaki A., Sasaki M., Okubo T., Minai Y., Haga M. Antibacterial action on pathogenic bacterial spore by green tea catechins. Journal of the Science of Food and Agriculture. 2005;85:2354–2361. [Google Scholar]
- Hassani A.S., Amirmozafari N., Ordouzadeh N., Hamdi K., Nazari R., Ghaemi A. Volatile components of Camellia sinensis inhibit growth and biofilm formation of oral streptococci in vitro. Pakistan Journal of Biological Sciences. 2008;11:1336–1341. doi: 10.3923/pjbs.2008.1336.1341. [DOI] [PubMed] [Google Scholar]
- Hatano T., Tsugawa M., Kusuda M., Taniguchi S., Yoshida T., Shiota S. Enhancement of antibacterial effects of epigallocatechin gallate, using ascorbic acid. Phytochemistry. 2008;69:3111–3116. doi: 10.1016/j.phytochem.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Hayatsu H., Inada N., Kakutani T., Arimoto S., Negishi T., Mori K. Suppression of genotoxicity of carcinogens by epigallocatechin gallate. Preventive Medicine. 1992;21:370–376. doi: 10.1016/0091-7435(92)90044-i. [DOI] [PubMed] [Google Scholar]
- He W., Li L.-X., Liao Q.-J., Liu C.-L., Chen X.-L. Epigallocatechin gallate inhibits HBV DNA synthesis in a viral replication-inducible cell line. World Journal of Gastroenterology. 2011;17:1507–1514. doi: 10.3748/wjg.v17.i11.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa M., Takada K. Multiple effects of green tea catechin on the antifungal activity of antimycotics against Candida albicans. Journal of Antimicrobial Chemotherapy. 2004;53:225–229. doi: 10.1093/jac/dkh046. [DOI] [PubMed] [Google Scholar]
- Hirasawa M., Takada K., Otake S. Inhibition of acid production in dental plaque bacteria by green tea catechins. Caries Research. 2006;40:265–270. doi: 10.1159/000092236. [DOI] [PubMed] [Google Scholar]
- Hisano M., Yamaguchi K., Inoue Y., Ikeda Y., Iijima M., Adachi M. Inhibitory effect of catechin against the superantigen staphylococcal enterotoxin B (SEB) Archives of Dermatological Research. 2003;295:183–189. doi: 10.1007/s00403-003-0411-x. [DOI] [PubMed] [Google Scholar]
- http://www.statisticbrain.com/tea-drinking-statistics/ (accessed on 30.07.2012)
- http://www.tea.co.uk/page.php?id=237 (accessed on 31.07.2012)
- Hu Y., Jia J., Qiao J., Ge C., Cao Z. Antimicrobial activity of pu-erh tea extracts in vitro and its effects on the preservation of cooled mutton. Journal of Food Safety. 2010;30:177–195. [Google Scholar]
- Hua D., Peng Q., Huang Y., Yao F., Qian Y. The mechanism of synergistic antibacterial action between ECG/EGCG and β-lactam antibiotics against MRSA. Zhongguo Ganran Yu Hualiao Zazhi. 2010;10:123–126. [Google Scholar]
- Hua D., Peng Q., Zeng X., Yao F., Qian Y. Antibacterial activity of green tea and its extracts against MRSA. Zhongguo Kangshengsu Zazhi. 2010;35:228–233. [Google Scholar]
- Huang S.-H., Tang Y.-Z., Zhou X.-M., Xie G., Kurihara H., He Z.-H. Study on anti-influenza virus effect of tea polyphenols in vitro and in vivo. Chaye Kexue. 2010;30:302–308. [Google Scholar]
- Ikeda I., Imasato Y., Sasaki E., Nakayama M., Nagao H., Takeo T. Tea catechins decrease micellar solubility and intestinal absorption of cholesterol in rats. Biochimica et Biophysica Acta. 1992;1127:141–146. doi: 10.1016/0005-2760(92)90269-2. [DOI] [PubMed] [Google Scholar]
- Isaacs C.E., Wen G.Y., Xu W., Jia J.H., Rohan L., Corbo C. Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. Antimicrobial Agents and Chemotherapy. 2008;52:962–970. doi: 10.1128/AAC.00825-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs C.E., Xu W., Merz G., Hillier S., Rohan L., Wen G.Y. Digallate dimers of (−)-epigallocatechin gallate inactivate herpes simplex virus. Antimicrobial Agents and Chemotherapy. 2011;55:5646–5653. doi: 10.1128/AAC.05531-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F., Chen W., Yi K., Wu Z., Si Y., Han W. The evaluation of catechins that contain a galloyl moiety as potential HIV-1 integrase inhibitors. Clinical Immunology. 2010;137:347–356. doi: 10.1016/j.clim.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Jin E., Wu Y., Tu Y. Study on antibacterial effect of theaflavins. Zhongguo Shipin Xuebao. 2011;11:108–112. [Google Scholar]
- Juneja V.K., Bari M.L., Inatsu Y., Kawamoto S., Friedman M. Control of Clostridium perfringens spores by green tea leaf extracts during cooling of cooked ground beef, chicken, and pork. Journal of Food Protection. 2007;70:1429–1433. doi: 10.4315/0362-028x-70.6.1429. [DOI] [PubMed] [Google Scholar]
- Kaihatsu K., Mori S., Matsumura H., Daidoji T., Kawakami C., Kurata H. Broad and potent anti-influenza virus spectrum of epigallocatechin-3-O-gallate-monopalmitate. Journal of Molecular and Genetic Medicine. 2009;3:195–197. doi: 10.4172/1747-0862.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karori S.M., Wachira F.N., Wanyoko J.K., Ngure R.M. Antioxidant capacity of different types of tea products. African Journal of Biotechnology. 2007;6:2287–2296. [Google Scholar]
- Kawai K., Tsuno N.H., Kitayama J., Okaji Y., Yazawa K., Asakage M. Epigallocatechin gallate, the main component of tea polyphenol, binds to CD4 and interferes with gp120 binding. The Journal of Allergy and Clinical Immunology. 2003;112:951–957. doi: 10.1016/s0091-6749(03)02007-4. [DOI] [PubMed] [Google Scholar]
- Kim Y.J., Houng S.-J., Kim J.H., Kim Y.-R., Ji H.G., Lee S.-J. Nanoemulsified green tea extract shows improved hypocholesterolemic effects in C57BL/6 mice. The Journal of Nutritional Biochemistry. 2012;23:186–191. doi: 10.1016/j.jnutbio.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Kim J.-S., Kim Y. The inhibitory effect of natural bioactives on the growth of pathogenic bacteria. Nutrition Research and Practice. 2007;1:273–278. doi: 10.4162/nrp.2007.1.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran S., Ratho R.K., Sharma P., Harjai K., Capalash N., Tiwari R.P. Effect of black tea (Camellia sinensis) on virulence traits of clinical isolates of Shigella dysenteriae and Escherichia coli EPEC P2 1265 strain. European Food Research and Technology. 2010;231:763–770. [Google Scholar]
- Klaus S., Pultz S., Thone-Reineke C., Wolfram S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. International Journal of Obesity. 2005;29:615–623. doi: 10.1038/sj.ijo.0802926. [DOI] [PubMed] [Google Scholar]
- Klugman K.P., Koornhof H.J. Worldwide increase in pneumococcal antibiotic resistance. Lancet. 1989;2:444. doi: 10.1016/s0140-6736(89)90617-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi N., Nishikawa T., Okayasu T., Yamada R., Isogai E., Isogai H. Inhibitory effects of catechin-containing drinks on the growth of Salmonella enteritidis. Shikoku Igaku Zasshi. 2006;62:43–48. [Google Scholar]
- Kohda C., Yanagawa Y., Shimamura T. Epigallocatechin gallate inhibits intracellular survival of Listeria monocytogenes in macrophages. Biochemical and Biophysical Research Communications. 2008;365:310–315. doi: 10.1016/j.bbrc.2007.10.190. [DOI] [PubMed] [Google Scholar]
- Kumudavally K.V., Phanindrakumar H.S., Tabassum A., Radhakrishna K., Bawa A.S. Green tea — A potential preservative for extending the shelf life of fresh mutton at ambient temperature (25 ± 2 °C) Food Chemistry. 2008;107:426–433. [Google Scholar]
- Lee Y.S., Han C.H., Kang S.H., Lee S.J., Kim S.W., Shin O.R. Synergistic effect between catechin and ciprofloxacin on chronic bacterial prostatitis rat model. International Journal of Urology. 2005;12:383–389. doi: 10.1111/j.1442-2042.2005.01052.x. [DOI] [PubMed] [Google Scholar]
- Lee K.-M., Kim W.-S., Lim J., Nam S., Youn M., Nam S.-W. Antipathogenic properties of green tea polyphenol epigallocatechin gallate at concentrations below the MIC against enterohemorrhagic Escherichia coli O157:H7. Journal of Food Protection. 2009;72:325–331. doi: 10.4315/0362-028x-72.2.325. [DOI] [PubMed] [Google Scholar]
- Lee M.J., Maliakal P., Chen L., Meng X., Bondoc F.Y., Prabhu S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiology, Biomarkers & Prevention. 2002;11:1025–1032. [PubMed] [Google Scholar]
- Lee K.-M., Yeo M., Choue J.-S., Jin J.-H., Park S.J., Cheong J.-Y. Protective mechanism of epigallocatechin-3-gallate against Helicobacter pylori-induced gastric epithelial cytotoxicity via the blockage of TLR-4 signaling. Helicobacter. 2004;9:632–642. doi: 10.1111/j.1083-4389.2004.00281.x. [DOI] [PubMed] [Google Scholar]
- Li S., Hattori T., Kodama E.N. Epigallocatechin gallate inhibits the HIV reverse transcription step. Antiviral Chemistry & Chemotherapy. 2011;21:239–243. doi: 10.3851/IMP1774. [DOI] [PubMed] [Google Scholar]
- Li J., Zhou L., Zhang Y. Studies on the effects of tea catechins against hepatitis B virus infection. Zhonghua Yu Fang Yi Xue Za Zhi. 2001;35:404–407. [PubMed] [Google Scholar]
- Lin J.-K., Lin C.-L., Liang Y.-C., Lin-Shiau S.-Y., Juan I.-M. Survey of catechins, gallic acid, and methylxanthines in green, oolong, pu-erh, and black teas. Journal of Agricultural and Food Chemistry. 1998;46:3635–3642. [Google Scholar]
- Liu S., Lu H., Zhao Q., He Y., Niu J., Debnath A.K. Theaflavin derivatives in black tea and catechin derivatives in green tea inhibit HIV-1 entry by targeting gp41. Biochimica et Biophysica Acta, General Subjects. 2005;1723:270–281. doi: 10.1016/j.bbagen.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Liu Q., Wang Y., Xu P., Zhang H., Zhao Y., Lu Y. Antibacterial activity of catechins against heterogeneous vancomycin resistant Staphylococcus. Zhongguo Kangshengsu Zazhi. 2011;36:557–560. [Google Scholar]
- Matsumoto Y., Kaihatsu K., Nishino K., Ogawa M., Kato N., Yamaguchi A. Antibacterial and antifungal activities of new acylated derivatives of epigallocatechin gallate. Frontiers in Microbiology. 2012;3(53):1–10. doi: 10.3389/fmicb.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Mukai T., Furukawa S., Ohori H. Inhibitory effects of epigallocatechin gallate on the propagation of bovine coronavirus in Madin–Darby bovine kidney cells. Animal Science Journal. 2005;76:507–512. [Google Scholar]
- Matsumura, K., Okazaki, S., Xuan, C. X., Komasa, H., & Sugai, H. (2009). Epigallocatechin gallate from green tea polyphenol and ε-poly-l-lysine as antifungal oral cavity care compositions. Japanese Kokai Tokkyo Koho, JP 2009269899 A 20091119.
- Matsuura, K., Ogawa, H., Goto, K., & Hara, M. (2007). Tea catechins and caffeine as antifungal agents against tiena. Japanese Kokai Tokkyo Koho, JP 2007176884 A 20070712.
- Meng X., Sang S., Zhu N., Lu H., Sheng S., Lee M.J. Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice, and rats. Chemical Research in Toxicology. 2002;15:1042–1050. doi: 10.1021/tx010184a. [DOI] [PubMed] [Google Scholar]
- Min Z., Peigen X. Quantitative analysis of the active constituents in green tea. Phytotherapy Research. 1991;5:239–240. [Google Scholar]
- Miyamoto T., Nakayama M., Shigemune N., Tokuda H., Matsushita T., Furuta K. Application of green tea extract as food preservative to improve shelf life of overnight pickled cucumber. Nippon Shokuhin Kagaku Kogaku Kaishi. 2009;56:660–664. [Google Scholar]
- Mo H., Zhu Y., Chen Z. Microbial fermented tea — A potential source of natural food preservatives. Trends in Food Science & Technology. 2008;19:124–130. [Google Scholar]
- Mori S., Miyake S., Kobe T., Nakaya T., Fuller S.D., Kato N. Enhanced anti-influenza A virus activity of (−)-epigallocatechin-3-O-gallate fatty acid monoester derivatives: Effect of alkyl chain length. Bioorganic & Medicinal Chemistry Letters. 2008;18:4249–4252. doi: 10.1016/j.bmcl.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Mukamal K.J., Maclure M., Muller J.E., Sherwood J.B., Mittleman M.A. Tea consumption and mortality after acute myocardial infarction. Circulation. 2002;105:2476–2481. doi: 10.1161/01.cir.0000017201.88994.f7. [DOI] [PubMed] [Google Scholar]
- Naderi N.J., Niakan M., Kharazi F.M.J., Zardi S. Antibacterial activity of Iranian green and black tea on Streptococcus mutans: an in vitro study. Journal of Dentistry. 2011;8:55–59. [PMC free article] [PubMed] [Google Scholar]
- Nakayama M., Shigemune N., Tokuda H., Furuta K., Matsushita T., Yoshizawa C. Combined effects of surfactants and preservatives on the antibacterial activity of green tea extracts. Bokin Bobai. 2009;37:169–179. [Google Scholar]
- Nakayama M., Shigemune N., Tsugukuni T., Jun H., Matsushita T., Mekada Y. Mechanism of the combined anti-bacterial effect of green tea extract and NaCl against Staphylococcus aureus and Escherichia coli O157:H7. Food Control. 2012;25:225–232. [Google Scholar]
- Nakayama M., Shigemune N., Tsugukuni T., Tokuda H., Miyamoto T. Difference of EGCG adhesion on cell surface between Staphylococcus aureus and Escherichia coli visualized by electron microscopy after novel indirect staining with cerium chloride. Journal of Microbiological Methods. 2011;86:97–103. doi: 10.1016/j.mimet.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Nakayama M., Suzuki K., Toda M., Okubo S., Hara Y., Shimamura T. Inhibition of the infectivity of the influenza virus by tea polyphenols. Antiviral Resistance. 1993;21:289–299. doi: 10.1016/0166-3542(93)90008-7. [DOI] [PubMed] [Google Scholar]
- Natsume M., Osakabe N., Oyama M., Sasaki M., Baba S., Nakamura Y. Structures of (−)-epicatechin glucuronide identified from plasma and urine after oral ingestion of (−)-epicatechin: differences between human and rat. Free Radical Biology & Medicine. 2003;34:840–849. doi: 10.1016/s0891-5849(02)01434-x. [DOI] [PubMed] [Google Scholar]
- Navarro-Martinez M.D., Garcia-Canovas F., Rodriguez-Lopez J.N. Tea polyphenol epigallocatechin-3-gallate inhibits ergosterol synthesis by disturbing folic acid metabolism in Candida albicans. Journal of Antimicrobial Chemotherapy. 2006;57:1083–1092. doi: 10.1093/jac/dkl124. [DOI] [PubMed] [Google Scholar]
- Neyestani T.R., Khalaji N., Gharavi A. Selective microbiologic effects of tea extract on certain antibiotics against Escherichia coli in vitro. Journal of Alternative and Complementary Medicine. 2007;13:1119–1124. doi: 10.1089/acm.2007.7033. [DOI] [PubMed] [Google Scholar]
- Nie, J. (2010). Preparation of pharmaceutical formulations containing epigallocatechin-3-O-gallate and/or epicatechin-3-O-gallate for nasal inhalation drug delivery for treating common cold and influenza. Faming Zhuanli Shenqing, CN 101816645 A 20100901.
- Ojo O.A., Yunusa A.O., Akindele S.K., Vera V.N., Fowora N. The antimicrobial activities of processed Nigerian and South African black tea. African Journal of Medicine and Medical Sciences. 2010;39:145–151. [PubMed] [Google Scholar]
- Ooshima T. Anti-caries activity of tea polyphenols. Foods & Food Ingredients Journal of Japan. 2005;210:325–330. [Google Scholar]
- Osterburg A., Gardner J., Hyon S.H., Neely A., Babcock G. Highly antibiotic-resistant Acinetobacter baumannii clinical isolates are killed by the green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) Clinical Microbiology and Infection. 2009;15:341–346. doi: 10.1111/j.1469-0691.2009.02710.x. [DOI] [PubMed] [Google Scholar]
- Otsuka T., Ogo T., Eto T., Asano Y., Suganuma M., Niho Y. Growth inhibition of leukemic cells by (−)-epigallocatechin gallate, the main constituent of green tea. Life Sciences. 1998;63:1397–1403. doi: 10.1016/s0024-3205(98)00406-8. [DOI] [PubMed] [Google Scholar]
- Park B.J., Park J.-C., Taguchi H., Fukushima K., Hyon S.-H., Takatori K. Antifungal susceptibility of epigallocatechin 3-O-gallate on clinical isolates of pathogenic yeasts. Biochemical and Biophysical Research Communications. 2006;347:401–405. doi: 10.1016/j.bbrc.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Park B.J., Taguchi H., Kamei K., Matsuzawa T., Hyon S.-H., Park J.-C. In vitro antifungal activity of epigallocatechin-3-O-gallate against clinical isolates of dermatophytes. Yonsei Medical Journal. 2011;52:535–538. doi: 10.3349/ymj.2011.52.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patar A., Nishikawa K., Tanaka N., Ishimaru K., Maeda H. Antibacterial activities of extracts and constituents in the complex of tea catechins and soybean protein. Nippon Shokuhin Kagaku Gakkaishi. 2003;10:161–164. [Google Scholar]
- Pei S., Zhang Y., Xu H., Chen X., Chen S. Inhibition of the replication of hepatitis B Virus in vitro by pu-erh tea extracts. Journal of Agricultural and Food Chemistry. 2011;59:9927–9934. doi: 10.1021/jf202376u. [DOI] [PubMed] [Google Scholar]
- Perumalla A.V.S., Hettiarachchy N.S. Green tea and grape seed extracts — Potential applications in food safety and quality. Food Research International. 2011;44:827–839. [Google Scholar]
- Peterson J., Dwyera J., Bhagwat S., Haytowitz D., Holden J., Eldridge A.L. Major flavonoids in dry tea. Journal of Food Composition and Analysis. 2005;18:487–501. [Google Scholar]
- Pisters K.M., Newman R.A., Coldman B., Shin D.M., Khuri F.R., Hong W.K. Phase I trial of oral green tea extract in adult patients with solid tumors. Journal of Clinical Oncology. 2001;19:1830–1838. doi: 10.1200/JCO.2001.19.6.1830. [DOI] [PubMed] [Google Scholar]
- Podlipnik C. Docking of selected natural polyphenols to ARF activated A1 subunit of cholera toxin. Acta Chimica Slovenica. 2009;56:156–165. [Google Scholar]
- Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. European Journal of Pharmacology. 2006;545:51–64. doi: 10.1016/j.ejphar.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Roccaro A.S., Blanco A.R., Giuliano F., Rusciano D., Enea V. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrobial Agents and Chemotherapy. 2004;48:1968–1973. doi: 10.1128/AAC.48.6.1968-1973.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Perkins I., Van O.A., Burdash N., Klein T.W., Friedman H. Epigallocatechin gallate modulates cytokine production by bone marrow-derived dendritic cells stimulated with lipopolysaccharide or muramyldipeptide, or infected with Legionella pneumophila. Experimental Biology and Medicine. 2005;230:645–651. doi: 10.1177/153537020523000906. [DOI] [PubMed] [Google Scholar]
- Sakagami H., Asano K., Hara Y., Shinamura T. Stimulation of human monocyte and polymorphonuclear cell iodination and interleukin-1 production by epigallocatechin gallate. Journal of Leukocyte Biology. 1992;51:478–483. doi: 10.1002/jlb.51.5.478. [DOI] [PubMed] [Google Scholar]
- Sari F., Turkmen N., Polat G., Velioglu Y.S. Total polyphenol, antioxidant and antibacterial activities of black mate tea. Food Science and Technology Research. 2007;13:265–269. doi: 10.3390/12030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H., Matsumoto M., Tanaka T., Maeda M., Nakai M., Hamada S. Antibacterial activity of polyphenol components in oolong tea extract against Streptococcus mutans. Caries Research. 2004;38:2–8. doi: 10.1159/000073913. [DOI] [PubMed] [Google Scholar]
- Sato D. Inhibition of urinary bladder tumors induced by N-butyl-N-(4-hydroxybutyl)-nitrosamine in rats by green tea. International Journal of Urology. 1999;6:93–99. doi: 10.1046/j.1442-2042.1999.06239.x. [DOI] [PubMed] [Google Scholar]
- Savi L.A., Barardi C.R.M., Simoes C.M.O. Evaluation of antiherpetic activity and genotoxic effects of tea catechin derivatives. Journal of Agricultural and Food Chemistry. 2006;54:2552–2557. doi: 10.1021/jf052940e. [DOI] [PubMed] [Google Scholar]
- Scalbert A., Manach C., Morand C., Rémésy C. Dietary polyphenols and the prevention of diseases. Critical Reviews in Food Science and Nutrition. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- Sharma S.K., Kumar G., Kapoor M., Surolia A. Combined effect of epigallocatechin gallate and triclosan on enoyl-ACP reductase of Mycobacterium tuberculosis. Biochemical and Biophysical Research Communications. 2008;368:12–17. doi: 10.1016/j.bbrc.2007.10.191. [DOI] [PubMed] [Google Scholar]
- Shigemune N., Nakayama M., Tsugukuni T., Hitomi J., Yoshizawa C., Mekada Y. The mechanisms and effect of epigallocatechin gallate (EGCG) on the germination and proliferation of bacterial spores. Food Control. 2012;27:269–274. [Google Scholar]
- Shimamura T., Zhao W.-H., Hu Z.-Q. Mechanism of action and potential for use of tea catechin as an antiinfective agent. Anti-Infective Agents in Medicinal Chemistry. 2007;6:57–62. [Google Scholar]
- Shin J.-S., Chung H.-S. Antibacterial activities of phenolic components from Camellia sinensis L. on pathogenic microorganisms. Journal of Food Sciences and Nutrition. 2007;12:135–140. [Google Scholar]
- Shoji Y., Nakashima H. Glucose-lowering effect of powder formulation of African black tea extract in KK-A(y)/TaJcl diabetic mouse. Archives of Pharmacal Research. 2006;29:786–794. doi: 10.1007/BF02974080. [DOI] [PubMed] [Google Scholar]
- Si W., Gong J., Tsao R., Kalab M., Yang R., Yin Y. Bioassay-guided purification and identification of antimicrobial components in Chinese green tea extract. Journal of Chromatography. A. 2006;1125:204–210. doi: 10.1016/j.chroma.2006.05.061. [DOI] [PubMed] [Google Scholar]
- Sitheeque M.A.M., Panagoda G.J., Yau J., Amarakoon A.M.T., Udagama U.R.N., Samaranayake L.P. Antifungal activity of black tea polyphenols (catechins and theaflavins) against Candida species. Chemotherapy. 2009;55:189–196. doi: 10.1159/000216836. [DOI] [PubMed] [Google Scholar]
- Song J.M., Park K.D., Lee K.H., Byun Y.H., Park J.H., Kim S.H. Biological evaluation of anti-influenza viral activity of semi-synthetic catechin derivatives. Antiviral Research. 2007;76:178–185. doi: 10.1016/j.antiviral.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Stanczyk A., Skolimowska U., Wedzisz A. Contents of tannins in green and black teas and antibactericidal activity of methanolic extracts. Bromatologia i Chemia Toksykologiczna. 2008;41:976–980. [Google Scholar]
- Stapleton P.D., Shah S., Hara Y., Taylor P.W. Potentiation of catechin gallate-mediated sensitization of Staphylococcus aureus to oxacillin by nongalloylated catechins. Antimicrobial Agents and Chemotherapy. 2006;50:752–755. doi: 10.1128/AAC.50.2.752-755.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staszewski M.V., Pilosof A.M.R., Jagus R.J. Antioxidant and antimicrobial performance of different argentinean green tea varieties as affected by whey proteins. Food Chemistry. 2011;125:186–192. [Google Scholar]
- Stoicov C., Saffari R., Houghton J. Green tea inhibits Helicobacter growth in vivo and in vitro. International Journal of Antimicrobial Agents. 2009;33:473–478. doi: 10.1016/j.ijantimicag.2008.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y., Zhang C., Wang Y., Li P. Antibacterial property and mechanism of a novel pu-erh tea nanofibrous membrane. Applied Microbiology and Biotechnology. 2012;93:1663–1671. doi: 10.1007/s00253-011-3501-2. [DOI] [PubMed] [Google Scholar]
- Sugai, H., Park, J. C., Han, D. W., & Hyeon, S. G. (2008). Topical antifungal compositions containing polyphenol. Japanese Kokai Tokkyo Koho, JP 2008007464 A 20080117.
- Surh, Y. J., Kang, D. K., Lee, J. S., & Park, C. S. (2006). Green tea extract comprising polyphenol ingredient of green tea having antibacterial activity of Helicobacter pylori and inhibitory effect to urease activity and health food using the same. Republic Korean Kongkae Taeho Kongbo, KR 2006122439 A 20061130.
- Tadakatsu S., Wei-Hua Z., Zhi-Qing H. Mechanism of action and potential for use of tea catechin as an antiinfective agent. Anti-Infective Agents in Medicinal Chemistry. 2007;6:57–62. [Google Scholar]
- Tan W., Shi L., Zhu W., Tan J. Effect of anti-influenza virus of EGCG. Zhongguo Yiyuan Yaoxue Zazhi. 2009;29:376–378. [Google Scholar]
- Taylor P.W., Stapleton P.D., Luzio J.P. New ways to treat bacterial infections. Drug Discovery Today. 2002;7:1086–1091. doi: 10.1016/s1359-6446(02)02498-4. [DOI] [PubMed] [Google Scholar]
- Turchetti B., Pinelli P., Buzzini P., Romani A., Heimler D., Franconi F. In vitro antimycotic activity of some plant extracts towards yeast and yeast-like strains. Phytotherapy Research. 2005;19:44–49. doi: 10.1002/ptr.1622. [DOI] [PubMed] [Google Scholar]
- Turkmen N., Velioglu Y.S., Sari F., Polat G. Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules. 2007;12:484–496. doi: 10.3390/12030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Ozaki M., Suzuki K., Shikita M. Radioprotective effects of epigallocatechin 3-O-gallate (green tea tannin) in mice. Life Sciences. 1992;50:147–152. doi: 10.1016/0024-3205(92)90296-2. [DOI] [PubMed] [Google Scholar]
- USDA Database for the flavonoid content of selected foods release 2.1. 2007. http://www.nal.usda.gov/fnic/foodcomp/Data/Flav/Flav02-1.pdf Available from: (accessed on 02.08.2012)
- Van A.J.M., Van H.K.H., Mathot J.N., Mulder T.P., Wiersma A., Tijburg L.B. Plasma concentrations of individual tea catechins after a single oral dose in humans. Xenobiotica. 2001;31:891–901. doi: 10.1080/00498250110079149. [DOI] [PubMed] [Google Scholar]
- Wang, H., Xu, J., Deng, F., & Hu, Z. (2007). Natural extract of green tea for inhibiting hepatitis B virus and its primary active ingredient. Faming Zhuanli Shenqing, CN 101028382 A 20070905.
- Weber J.M., Ruzindana-Umunyana A., Imbeault L., Sircar S. Inhibition of adenovirus infection and adenain by green tea catechins. Antiviral Research. 2003;58:167–173. doi: 10.1016/s0166-3542(02)00212-7. [DOI] [PubMed] [Google Scholar]
- Williamson M.P., McCormick T.G., Nance C.L., Shearer W.T. Epigallocatechin gallate, the main polyphenol in green tea, binds to the T-cell receptor, CD4: potential for HIV-1 therapy. The Journal of Allergy and Clinical Immunology. 2006;118:1369–1374. doi: 10.1016/j.jaci.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Wu S.-C., Yen G.-C., Wang Z.-S., Chiu C.-K., Yen W.-J., Chang L.-W. Antimutagenic and antimicrobial activities of pu-erh tea. LWT—Food Science and Technology. 2007;40:506–512. [Google Scholar]
- Xiao X., Yang Z., Shi L., Liu J., Chen W. Study on anti-influenza virus effects of epigallocatechingallate. Zhongguo Zhongyao Zazhi. 2008;33:2678–2681. [PubMed] [Google Scholar]
- Xu B., Liu Z., Ren F., Sun Y. Effects of natural preservative and modified atmosphere packaging on shelf-life of sliced beef ham. Nongye Gongcheng Xuebao. 2006;22:143–147. [Google Scholar]
- Xu J., Wang J., Deng F., Hu Z., Wang H. Green tea extract and its major component epigallocatechin gallate inhibits hepatitis B virus in vitro. Antiviral Research. 2008;78:242–249. doi: 10.1016/j.antiviral.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Xu X., Zhou X.D., Wu C.D. The tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans. Antimicrobial Agents and Chemotherapy. 2011;55:1229–1236. doi: 10.1128/AAC.01016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Zhou X.D., Wu C.D. Tea catechin epigallocatechin gallate inhibits Streptococcus mutans biofilm formation by suppressing gtf genes. Archives of Oral Biology. 2012;57:678–683. doi: 10.1016/j.archoralbio.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Matsunaga K., Friedman H. Protective effects of green tea catechins on alveolar macrophages against bacterial infections. BioFactors. 2004;21:119–121. doi: 10.1002/biof.552210123. [DOI] [PubMed] [Google Scholar]
- Yang C.S., Chen L., Lee M.J. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiology, Biomarkers & Prevention. 1998;7:351–354. [PubMed] [Google Scholar]
- Yang J., Li R.-M., Mao Q.-C., Chen Z.-P., Jiang S.-B., Jiang Z.-H. HIV fusion-inhibiting activity of peracetate ester of (−)-epigallocatechin gallate and the mechanism. Zhongguo Xinyao Zazhi. 2010;19:1967–1972. [Google Scholar]
- Yang J., Li L., Tan S., Jin H., Qiu J., Mao Q. A natural theaflavins preparation inhibits HIV-1 infection by targeting the entry step: Potential applications for preventing HIV-1 infection. Fitoterapia. 2012;83:348–355. doi: 10.1016/j.fitote.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Yang L., Liu Y., Sternberg C., Molin S. Evaluation of enoyl-acyl carrier protein reductase inhibitors as Pseudomonas aeruginosa quorum-quenching reagents. Molecules. 2010;15:780–792. doi: 10.3390/molecules15020780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. & Shi, L. (2007). Application of plant extract catechins in preparing antiviral drugs. Faming Zhuanli Shenqing, CN 1994294 A 20070711.
- Yi S., Li J., Zhu J., Lin Yi, Fu L., Chen W. Effect of tea polyphenols on microbiological and biochemical quality of Collichthys fish ball. Journal of the Science of Food and Agriculture. 2011;91:1591–1597. doi: 10.1002/jsfa.4352. [DOI] [PubMed] [Google Scholar]
- Yoda Y., Hu Z.-Q., Zhao W.-H., Shimamura T. Different susceptibilities of Staphylococcus and gram-negative rods to epigallocatechin gallate. Journal of Infection and Chemotherapy. 2004;10:55–58. doi: 10.1007/s10156-003-0284-0. [DOI] [PubMed] [Google Scholar]
- Zhao W.-H., Hu Z.-Q., Okubo S., Hara Y., Shimamura T. Mechanism of synergy between epigallocatechin gallate and β-lactams against methicillin-resistant Staphylococcus aureus. Antimicrobial Agents and Chemotherapy. 2001;45:1737–1742. doi: 10.1128/AAC.45.6.1737-1742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Wang H., Zhao X.-R., Luo F.-J., Tang M., Cao Y. Epigallocatechin-3-gallate interferes with EBV-encoding AP-1 signal transduction pathway. Zhonghua Zhong Liu Za Zhi. 2004;26:393–397. [PubMed] [Google Scholar]
- Zhong Y., Ma C.-M., Shahidi F. Antioxidant and antiviral activities of lipophilic epigallocatechin gallate (EGCG) derivatives. Journal of Functional Foods. 2012;4:87–93. doi: 10.1016/j.jff.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W.-W., Zhang L.-X., Zhang B., Wang F., Liang Z.-H., Niu T.-G. Isolation and characterization of ZH14 with antiviral activity against tobacco mosaic virus. Canadian Journal of Microbiology. 2008;54:441–449. doi: 10.1139/w08-026. [DOI] [PubMed] [Google Scholar]
- Zu M., Yang F., Zhou W., Liu A., Du G., Zheng L. In vitro anti-influenza virus and anti-inflammatory activities of theaflavin derivatives. Antiviral Research. 2012;94:217–224. doi: 10.1016/j.antiviral.2012.04.001. [DOI] [PubMed] [Google Scholar]


