Abstract
The thymus gland produces soluble molecules, which mediate significant immune functions. The first biologically active thymic extract was thymosin fraction V, the fractionation of which led to the isolation of a series of immunoactive polypeptides, including prothymosin alpha (proTα).
ProTα displays a dual role, intracellularly as a survival and proliferation mediator and extracellularly as a biological response modifier. Accordingly, inside the cell, proTα is implicated in crucial intracellular circuits and may serve as a surrogate tumor biomarker, but when found outside the cell, it could be used as a therapeutic agent for treating immune system deficiencies. In fact, proTα possesses pleiotropic adjuvant activity and a series of immunomodulatory effects (eg, anticancer, antiviral, neuroprotective, cardioprotective). Moreover, several reports suggest that the variable activity of proTα might be exerted through different parts of the molecule. We first reported that the main immunoactive region of proTα is the carboxy-terminal decapeptide proTα(100–109). In conjunction with data from others, we also revealed that proTα and proTα(100–109) signal through Toll-like receptor 4. Although their precise molecular mechanism of action is yet not fully elucidated, proTα and proTα(100–109) are viewed as candidate adjuvants for cancer immunotherapy.
Here, we present a historical overview on the discovery and isolation of thymosins with emphasis on proTα and data on some immune-related new activities of the polypeptide and smaller immunostimulatory peptides thereof. Finally, we propose a compiled scenario on proTα’s mode of action, which could eventually contribute to its clinical application.
Keywords: Thymic peptides, Prothymosin α, ProTα(100–109), Immune response, Alarmin, DAMP, Adjuvant, Immunoenhancing peptide, Cancer
1. Introduction
Biologic response modifiers (BRMs) are endogenous (ie, naturally produced in the body) or exogenous (administered together with a drug) agents that modulate immunity. BRMs regulate, among others, the type, duration, and intensity of immune responses and are characterized by pleiotropy and redundancy. The thymic polypeptide prothymosin alpha (proTα) has been incorporated in the large family of BRMs, mainly because of its modulating effects on several properties of immune effectors. Its wide distribution in cells, tissues, and organs, its broad phylogenetic dissemination and the lack of a mechanism supporting its secretion, questioned the initial characterization of proTα as “thymic hormone.” Now, it is widely acknowledged that proTα possesses an essential intracellular role related to cell survival and growth, and at the same time, extracellularly it enhances the functionalities of diverse subpopulations of the immune system. Several novel functions, beyond immunomodulation, have also been ascribed to proTα.
Accumulated data suggest that its immunopotentiating activity could be therapeutically exploited in various clinical conditions associated with immunodeficiency, immunosenescence, cancer, and autoimmune diseases. Herein, we present the most prominent effects of the polypeptide, as reported by various research teams for over 30 years, propose a compiled scenario on its mode of action, and provide means, which eventually could lead to its incorporation in clinical trials as an immunostimulant/adjuvant.
2. Historical Overview on ProTα Isolation and Properties
2.1. Thymosin Fraction V: The First Immunoactive Thymic Extract
The thymus had remained an enigmatic organ for centuries, as its clinical significance and true function were much disputed. Although its lymphopoietic role had been repeatedly demonstrated (Miller, 2002), the long-prevailing opinion claimed that the thymus was a redundant organ with no immunological function. It was not until the early 1960s that Miller, Good, and their colleagues tipped the balance in favor of a crucial and central role for thymus in establishing a competent immune system (Good et al., 1962, Miller, 1961). They were the first to show that neonatally thymectomized mice exhibited a marked deficiency of lymphocytes and impaired immune responses that were further associated with their inability to resist infections and reject histoincompatible transplants. However, even these studies could not fully elucidate the exact mechanism underlying the continued thymic control over lymphocytic function. A year later, it was unequivocally proven that the thymus is actually a gland mediating its function, at least partially, through humoral messages and not merely via cellular interactions (Aisenberg and Wilkes, 1965, Levey et al., 1963).
In the years to follow, Goldstein and his colleagues isolated and identified a series of hormonal-like agents with immune-restorative capacity (Goldstein, 2007). These initial efforts led to the isolation of a partially purified thymic extract that could enhance in vivo the incorporation of tritiated thymidine into DNA of mouse lymph nodes, indicative of increased lymphocytic proliferation (Klein, Goldstein, & White, 1965). With the invaluable aid of the newly developed in vitro proliferation assay, this soluble factor inducing lymphocytopoiesis was purified, characterized, and named “thymosin” (Goldstein, Slater, & White, 1966). However, the major breakthrough was the isolation of thymosin fraction V (TFV), a further purified preparation with immunoregulatory activity (Goldstein et al., 1981). Using a novel and complex process that enabled its purification in large amounts (Hooper et al., 1975), TFV's properties and activity were explored in several in vitro and in vivo studies. In vitro, TFV was shown to stimulate lymphocytes deriving from immunosuppressed humans and restore their functions to normal levels (Wara & Ammann, 1975). Additionally, TFV enhanced murine spleen cell responses in mixed lymphocyte reactions (MLRs) and in mixed lymphocyte-tumor cultures (MLTCs; Talmadge, Uithoven, Lenz, & Chirigos, 1984). In vivo, TFV increased the survival of neonatally thymectomized mice (Spangelo, Hall, & Goldstein, 1987), restored graft-vs-host reactivity (Spangelo et al., 1987), and “corrected” T-cell abnormalities in mice with autoimmune disease (Dauphinee, Tala, Goldstein, & White, 1974). Interestingly, TFV also possessed direct antiproliferative properties against malignant cells, as demonstrated in vitro against murine pituitary adenocarcinoma and glioma (Spangelo, Farrimond, Pompilius, & Bowman, 2000), and against human acute T lymphoblastic (Ho, Ma, Price, Hunstein, & HoVbrand, 1983) and promyelocytic leukemia cell lines (Spangelo et al., 2007). Finally, in vivo treatment with TFV conferred resistance to Dunning (Khaw & Rule, 1973) and murine lymphoblastic leukemias (Petro & Watson, 1982).
2.2. Dissecting TFV: Isolation of the First Immunoactive Thymosins
The immune-stimulating and restorative activities attributed to TFV triggered the need to further identify its several components. Thorough fractionation and analysis revealed that TFV consisted of at least 40 different molecules, of which 10–15 were principal and 20 or more were secondary components, and the majority of these molecules were acidic and of varying molecular weights (between 1000 and 15,000 Da). A nomenclature system based upon the isoelectric point (pI) of the peptides has resulted in TFV's subdivision into three different groups, each one identified by a Greek letter, ie, alpha (α), beta (β), and gamma (γ). Thus, peptides with pIs less than 5.0 were named α-peptides, these with pIs of 5.0–7.0 were termed β-peptides, and peptides with pIs greater than 7.0 were considered γ-peptides. A subscript number was used to identify the peptides from each group, indicative of the order in which they were isolated from TFV, eg, α1, α2, α3, etc. (Goldstein, 2007).
The first two peptides identified and fully characterized were thymosin α 1 (Tα1; Goldstein et al., 1966) and thymosin β 4 (Tβ4; Low, Hu, & Goldstein, 1981), and their immunopotentiating properties were extensively studied in the years to follow (Spangelo et al., 1987). Subsequently, a number of α- and β-peptides were isolated and sequenced from thymus and other tissues (Hannappel & Huff, 2003). Utilizing a newly developed method that reduced the effect of proteolysis during peptide isolation, Hannappel and colleagues demonstrated that Tβ4 was the major component of calf thymus extract and that Tα1 was absent or present in only trace amounts (Hannappel, Davoust, & Horecker, 1982a). The latter observation led the investigators to hypothesize that the presence of Tα1 in TFV was the result of proteolytic cleavage of a precursor peptide. This speculation was further fueled by evidence showing that a previously isolated peptide, Tβ8, was actually the proteolytic product of a longer molecule, Tβ9 (Hannappel, Davoust, & Horecker, 1982b). In addition, the same group isolated two peptides from TFV, whose sequence strongly resembled that of Tα1. When compared to Tα1, one peptide lacked four amino acids at its carboxy (C)-terminus and it was therefore named des-(25–28)-Tα1, and the second had an additional seven amino acids at its C-terminus, and was given the name Tα11 (Caldarella et al., 1983). Consequently, the search for a larger α-thymosin precursor molecule began.
In an effort to isolate the native polypeptide, Haritos and colleagues developed a radioimmunoassay based on an antibody raised against synthetic Tα1 (Haritos & Horecker, 1985). By coupling this assay with an isolation procedure designed to minimize any proteolytic activity in cell extracts, they eventually isolated, initially from rat thymus, a polypeptide of 112 amino acids long, which contained the Tα1 sequence (amino acid residues 1–28) at its amino (N)-terminus (Haritos, Goodall, & Horecker, 1984). They named this polypeptide proTα to indicate that it was the source of Tα1 and Tα1-related peptide fragments present in TFV preparations.
2.3. ProTα: Major Structural Characteristics and Properties
Human proTα is 109 amino acids long and is encoded by the PTMA gene located on chromosome 2 (Szabo et al., 1993). Nearly half of the total residues in proTα are accounted by glutamic and aspartic acid and, as a result, the molecule acquires a particularly low pI 3.55. Although it was initially referred to as a “thymic hormone,” proTα is not solely expressed in the thymus, but detected in all tissues (Haritos, Tsolas, & Horecker, 1984). It is a noticeably conserved polypeptide characterized by a high degree of sequence homology among mammals (Hannappel & Huff, 2003) and overexpressed in cells with increased physiological (eg, young thymus) or abnormal (eg, malignant) proliferative capacity (Haritos, 1987).
As proTα’s acidic residues are found primarily within the central segment of the polypeptide chain, the molecule has no specific secondary structure and, eventually, adopts a random coil conformation (Gast et al., 1995). However, the possibility that proTα acquires a secondary structure upon interaction with other proteins has not been ruled out (Piñeiro, Cordero, & Nogueira, 2000). In support of this, it has been demonstrated that under specific conditions (low pH and high concentration) the polypeptide forms amyloid fibrils (Pavlov, Cherny, Heim, Jovin, & Subramaniam, 2002). Although proTα is released by necrotic neurons via a unique nonclassical pathway (Halder et al., 2012, Matsunaga and Ueda, 2010), the peptide lacks a signal peptide sequence required for secretion. Instead, it bears a bipartite nuclear localization signal which consists of two blocks of basic residues (87KR89 and 100TKKQKT105), and is both necessary and sufficient for import of the protein to the nucleus, where it is predominantly located (Manrow et al., 1991, Rubtsov et al., 1997). In contradiction, many studies have also supported proTα’s cytoplasmic localization (Sburlati et al., 1990, Tsitsiloni et al., 1989). In an effort to address this antiphasis, Enkemann and coworkers demonstrated that proTα is in principle detected at active transcription sites in the nucleus, while a smaller fraction remains in the cytoplasm (Enkemann, Wang, Trumbore, & Berger, 2000). It was additionally suggested that, owing to its small size and its negative charge, proTα may facilitate the movement of other positively charged molecules (eg, of histones) into and within the nucleus, particularly in highly charged environments where there is a need to overcome electrostatic interactions. In terms of proTα’s biological role, a vast number of studies have attributed, among others, various and diverse immune-related properties to the polypeptide, the most important of which are analyzed later.
3. The Multifaceted Immune Activities of ProTα
3.1. The Thoroughly Studied Anticancer Activity of ProTα
The potent anticancer activity of proTα was studied in the early 1990s, when it was first reported that it regulates MHC class II expression on human and mouse antigen-presenting cells (APCs; Baxevanis et al., 1992). The significance of this observation was verified in three serial studies in a leukemic in vivo animal model, where mice inoculated with L1210 cells and therapeutically treated with proTα, survived for over 2 months (Papanastasiou, Baxevanis, & Papamichail, 1992). The immunological modifications caused by proTα included the in vivo generation of MHC-restricted tumor-specific CD8+ cytotoxic T lymphocytes (CTLs; Baxevanis, Gritzapis, Spanakos, Tsitsilonis, & Papamichail, 1995), concomitantly with the enhancement of tumor-reactive NK cell-mediated cytotoxicity (Baxevanis et al., 1994). Both effectors efficiently lysed the syngeneic L1210 tumor cells. The immunoenhancing effect of proTα was exerted upstream lymphocyte activation via an interleukin (IL)-2-dependent manner. Most importantly, proTα shifted antitumor-reactive immune responses toward the stimulation of the most suitable effectors, CTLs or NK cells, depending on the presence or absence of tumor-specific antigenic peptides, respectively.
Animal studies were followed by in vitro human studies in lymphocytes from cancer patients. Cancer induces severe immune dysfunctions, which are further intensified by anticancer therapies administered to patients. ProTα was shown to restore the deficiencies of peripheral blood lymphocytes deriving from patients with advanced solid tumors by enhancing: (1) the allogeneic cell-mediated lympholysis; (2) antigen presentation, as confirmed by the increased values recorded in MLR; and (3) the reduced NK and T-cell cytotoxic activity, by regulating the levels of prostaglandin E2 (PGE2) and IL-2 (Baxevanis, Reclos, & Papamichail, 1993). Two consecutive studies in melanoma and colon cancer patients showed that proTα could act beneficially at early-stage cancers, and when combined with low-dose interferon (IFN)-γ or IL-2, significantly enhanced monocyte, NK and LAK cell tumoristatic and tumorilytic activities (Eckert et al., 1995, Garbin et al., 1994). ProTα in conjunction with either IFN-γ or IL-2 increased the adhesion of monocytes to tumor targets and the expression of characteristic NK cell markers (eg, CD56, CD16), enhanced the lytic activity of LAK cells, in particular of CD16+CD2– cells, inhibited the secretion of TGF-β and PGE2, and induced the production and secretion of high levels of the proinflammatory cytokines IL-1β and tumor necrosis factor (TNF)-α (Cordero et al., 1995, Eckert, Grünberg, et al., 1997a, Eckert, Grünberg, et al., 1997b, Garbin et al., 1997, López-Rodríguez et al., 1994). Based on the aforementioned data, the first means of action of proTα was shaped, indicating that the polypeptide restores cancer patient lymphocyte deficiencies by selectively controlling the functions of monocytes. Monocytes, in turn, produce cytokines that generate a favorable cytokine milieu, facilitating lymphocyte activation. To optimize this effect, synergy between proTα and low concentrations of other BRMs was assessed. For example, proTα was combined with a monoclonal antibody to CD3 (anti-CD3), and this synergy further increased tumor cell-lysis by both MHC- and non-MHC-restricted PBMC effectors (Baxevanis et al., 1999).
All these results were complemented by the elegant study of Voutsas et al. (2000), where, using MLTCs, proTα in synergy with low-dose IL-2 led to expansion of tumor-reactive CD4+ T cells and the subsequent generation of MHC class I-restricted autologous tumor-specific CTLs. This was the first study showing that in order for proTα to fully exert its beneficial effect on CTLs, the concomitant presence of autologous CD4+ T cells and monocytes was required.
Combining all previous data, in an attempt to fill in the gaps, our research team used proteomics to elucidate the mechanism of action of proTα on healthy donor- and cancer patient-derived PBMCs. Based on proteins up- and downregulated upon PBMC treatment with proTα, we were the first to analytically describe the scenario underlying its immunoenhancing activity. We suggested that proTα ligates innate immunity receptor(s) on APCs, leading to formation of strong APC-T-cell synapses, proinflammatory cytokine secretion and thus, the indirect increase in the cytotoxicity of CTLs, NK cells, and other effectors (Skopeliti et al., 2009). We and others further confirmed the realism of this scenario, showing that proTα ligates Toll-like receptor 4 (TLR4) (Mosoian et al., 2010, Omotuyi et al., 2015) and matures monocyte-derived dendritic cells (DCs), which express costimulatory molecules (Skopeliti et al., 2009) and secrete proinflammatory cytokines. Moreover, proTα-matured DCs loaded with tumor antigens induced the polarization of TH1-type tumor-reactive immune responses, resulting in the generation of polyfunctional highly lytic tumor antigen-specific CTLs (Ioannou et al., 2013). Besides DCs, proTα could also activate other immune cell types expressing TLR4, eg, neutrophils to secrete and kill tumor cells in vitro (Samara, Ioannou, et al., 2013), and macrophages (Mosoian et al., 2010). Ex vivo experiments showed that proTα significantly restored the reduced cytotoxicity of immunosuppressed ascites-derived tumor-associated lymphocytes against ovarian tumor cells and inhibited ovarian tumor growth in SCID mice inoculated with human tumors (Voutsas et al., 2013).
3.2. Antiviral Activities of ProTα
Control of viral replication in humans requires the involvement of robust CTL responses, which in turn demand the presence of type I IFNs, that further support CD8+ T-cell development and function (Welsh, Bahl, Marshall, & Urban, 2012). Interestingly, proTα has been shown to be a regulator and enhancer of type I IFN secretion and as such, its antiviral properties were investigated. Qiu and colleagues provided the first evidence of proTα’s capacity to enhance IFN-α secretion by murine macrophages (Qiu et al., 2002). Later, proteomic analysis of human mononuclear cells treated with proTα revealed that the polypeptide enhanced the expression of mixovirus-resistance protein 2, an IFN-α-inducible protein that possesses significant antiviral activity (Skopeliti et al., 2007). Moreover, proTα was identified as a potent suppressor of human immunodeficiency virus (HIV)-1 replication in primary macrophages, when released by virus-infected CD8+ T cells. It was also shown that this HIV-1 inhibition occurs following viral integration, it is not virus specific (Mosoian et al., 2006), and is rather mediated by the upregulation of type I IFNs, following activation of TLR4 by proTα (Mosoian et al., 2010, Teixeira et al., 2015). Similarly, release of proTα upon infection of guinea pigs with an attenuated strain of Pichinde virus led to induction of potent antiviral immunity and consequent viral clearance (Bowick et al., 2010). In terms of in vivo applications, proTα has been used as a potent adjuvant for hepatitis B virus DNA vaccines, where it successfully enhanced both humoral and cellular immune responses (Jin et al., 2005). Impressively, a most recent report showed that proTα displays antiviral activity in other classes than mammals, specifically in fish (tongue sole) infected with megalocytivirus (Zhang & Sun, 2015).
3.3. ProTα Exerts Neuroprotective Functions
Neuronal death, induced by stroke or trauma, occurs via both necrosis and apoptosis. While apoptosis is a more regulated process that limits the damage in the brain, necrosis tends to expand and is therefore being targeted for stroke treatment (Ueda, 2009). Based on this premise, in an effort to identify soluble molecules that could inhibit necrosis, Ueda and colleagues isolated and identified proTα as a unique antineuronal necrosis factor in the conditioned medium of cortical neurons (Ueda, Fujita, Yoshida, Matsunaga, & Ueda, 2007). In more detail, proTα could reverse the rapid decrease in survival of cortical neurons, abolish the typical necrosis features in these cells, and switch the cell death mode from necrosis to apoptosis (Ueda et al., 2007). Later on, proTα’s potent neuroprotective functions were also demonstrated in in vivo experimentally induced cerebral and retinal ischemia (Fujita and Ueda, 2007, Fujita et al., 2009). As for the mechanism underlying proTα’s neuroprotective role, it has been shown that the polypeptide is released via a nonclassical manner under ischemic stress conditions and its extracellular release is facilitated by its interaction with a cargo molecule, namely S100A13 (Halder et al., 2012, Matsunaga and Ueda, 2010). Upon release, proTα activates the TLR4–TRIF-signaling pathway, inducing the expression of neuroprotective factors (Halder, Matsunaga, Ishii, & Ueda, 2015).
3.4. Miscellaneous Functions Reported for ProTα
Given its neuroprotective properties, the effect of proTα on cardiomyocytes during ischemic injury was also investigated (Cannavo et al., 2013). In vitro treatment of cardiomyocytes with recombinant proTα during simulated ischemia significantly decreased the apoptotic response and enhanced cell survival. Consistent with the in vitro findings, in vivo administration of proTα following myocardial infarction successfully reduced the infarct size in mice, when compared to untreated controls, an effect that was mediated by an Akt-dependent mechanism.
Li and colleagues had initially reported that proTα transgenic mice exhibited the polycystic kidney disease phenotype, as well as emphysema-like changes in the lung (Li, Shiau, Chiou, Yo, & Wu, 2005). Further studies by the same research group showed that proTα enhanced the acetylation of histones and nuclear factor-kappa B (NF-κB), or inhibited the TGF-β/Smad signaling, and thus contributed to the development of emphysema (Su et al., 2013, Su et al., 2016). Furthermore, high levels of proTα were positively correlated with the severity of emphysema, both in transgenic mice and in emphysema patients (Su et al., 2013).
Finally, the polypeptide's involvement in the induction of insulin resistance was investigated, as proTα reportedly regulated some inflammatory responses and oxidative stress, features that are associated with diabetes (Su et al., 2015). Patients with type 2 diabetes had significantly higher levels of serum proTα compared to normal individuals and proTα transgenic mice exhibited an insulin-resistant phenotype. Exploitation of the underlying mechanism revealed that proTα induces insulin resistance through a TLR4–NF-κB-dependent pathway.
4. The Immunostimulatory Activity of ProTα-Derived Peptides
Since Tα1 was the first thymosin isolated and identified from TFV and proTα was the natural precursor of Tα1 and other smaller α-thymosins (Haritos, Goodall, et al., 1984), Tα1 was initially considered as the major immunoactive fragment of the polypeptide. However, data accrued in particular over the last years revealed that proTα exerts its immunomodulating role through diverse fragments, such as the C-terminal fragment spanning residues 100–109 (Skopeliti et al., 2009) and the central, negatively charged region 50–89 (Mosoian et al., 2010). Two points that we consider very important are: (1) when compared to its fragments, intact proTα seems to perform better at least in most immune-based assays; and (2) different areas of the molecule seem to be responsible for proTα’s diverse activities (Table 1 ).
Table 1.
Prothymosin Alpha Fragments with Distinct Activities
| ProTα Fragment | Type of Activity | Reported in: |
|---|---|---|
| ProTα(1–28), referred to as “Tα1” | Immunomodulatory; DC activation; anticancer; antiviral; antifungal; vaccine enhancement | Camerini and Garaci (2015) |
| ProTα(1–35), referred to as “Tα11” | Antifungal | Hannappel and Huff (2003) |
| ProTα(49–78) (P30) ProTα(52–60) (P9) |
Neuroprotective | Halder, Sugimoto, Matsunaga, and Ueda (2013) |
| ProTα(50–89), referred to as “Mosoian domain” | Anti-HIV-1 | Mosoian et al. (2010) |
| ProTα variants (p7 [proTα(32–49)] and isoB [proTα(32–41), proTα(51–55), proTα(56–61), proTα(65–71)]) | Anti-HIV-1 | Teixeira et al. (2015) |
| ProTα(100–109), referred to as “Skopelitian domain” | Immunomodulatory; anticancer; DC maturation; enhancement of phagocytosis, respiratory burst, and cytotoxicity of human neutrophils | Skopeliti et al. (2009), Voutsas et al. (2013), Ioannou et al. (2013), and Samara, Ioannou, et al. (2013) |
| ProTα(1–100) | Cardioprotective | Cannavo et al. (2013) |
4.1. The Amino-Terminal Peptide Tα1 Has Shown Some Immune Activity
The 28-amino acid-long N-terminal proTα fragment Tα1 was reported to enhance human cell-mediated immunity and stimulate endothelial cell migration, angiogenesis, and wound healing (Malinda et al., 1998). Being the only α-thymosin tested in man, Tα1 has been used in a broad range of clinical applications, both as a single agent and in combination with other standard treatments (eg, chemotherapeutics), showing an excellent safety profile (Romani et al., 2012). Among other clinical cases, Tα1 has been used for treating infectious diseases including viral (chronic hepatitis B and C, AIDS), fungal (aspergillosis in bone marrow-transplanted patients) and bacterial (Pseudomonas aeruginosa) sepsis, severe lung pathologies (eg, chronic obstructive pulmonary disease, acute respiratory distress syndrome, and severe acute respiratory syndrome), age-related deficiencies, cancer, and as a vaccine enhancer (Camerini & Garaci, 2015). However, despite the promising data generated so far, the clinical benefit of Tα1 administration in these specific pathologies is still disputed and needs to be verified in larger well-designed randomized clinical trials.
4.2. The Carboxy-Terminal ProTα Peptides Exhibit Improved Immune Functions
The C-terminal decapeptide proTα(100–109) (TKKQKTDEDD) was identified by our research team as the immunoactive area of the polypeptide and a potent lymphocyte stimulator (Skopeliti et al., 2006). ProTα(100–109) has been shown to stimulate PBMC proliferation and cytotoxicity and to promote the phenotypic maturation of DCs (Skopeliti et al., 2009), and, consequently, it improved the functionality of immunogenic peptide-pulsed DCs, induced TH1-type immune response polarization (Ioannou et al., 2013), augmented basic properties of human neutrophils (Samara, Ioannou, et al., 2013), enhanced the depressed cytotoxicity of tumor-associated lymphocytes against autologous tumor cells in vitro, and retarded tumor growth in vivo (Voutsas et al., 2013). Using as control a scrambled decapeptide with the same amino acid composition but a different primary structure, the immunoenhancing activity of proTα(100–109) was shown to be sequence-specific and comparable to that of intact proTα. Most recently, we reported that proTα(100–109) radiolabeled with (99m)Tc binds on the surface of human neutrophils via a complex involving TLR4 (Karachaliou et al., 2015) and selectively accumulates in sites of experimentally induced inflammation (C.E. Karachaliou et al., personal communication).
Based on the fact that the decapeptide proTα(100–109) is in vivo generated upon caspase cleavage of proTα during apoptosis (Enkemann et al., 2000, Evstafieva et al., 2003), we developed a highly sensitive and specific competitive ELISA for proTα(100–109), using high affinity-purified polyclonal antibodies (Samara, Kalbacher, et al., 2013). The decapeptide was quantified in the serum of healthy humans, where it was detected at very low concentrations (≤ 0.5 ng/mL; P. Samara et al., unpublished data), whereas higher levels were recorded in the serum of mice infected with the bacterium Streptococcus pyogenes, suggesting a correlation between proTα(100–109) levels and the progress of bacterial infection.
Two more C-terminal immunomodulatory peptide fragments of proTα have been reported. In an earlier study, our research group identified a slightly smaller segment, namely proTα(103–109), which was also effective in vitro in restoring the immune functions of PBMCs derived from cancer patients (Skopeliti et al., 2006). Most recently, gravimetric assays and molecular dynamics simulation revealed that proTα, via its C-terminal segment (91–111) and similar to LPS, biophysically interacts in vitro with the TLR4/MD-2 complex at overlapping LPS-binding positions (Omotuyi et al., 2015).
4.3. The Immune Activity of ProTα Middle Segment Peptides
The research group of Mosoian introduced the synthetic peptide spanning amino acids 50–89 of proTα, designated proTα(50–89), in an attempt to comprehend how proTα mediates its anti-HIV-1 activity and induces type I IFN production. Incubation of human macrophages and myeloid DCs with proTα or proTα(50–89) stimulated IFN-α1 and IFN-β production, as shown by the increase in type I IFN mRNA (Mosoian et al., 2010). Furthermore, the same research team recently reported that proTα variants (p7 and isoB), spanning middle segment sequences of the polypeptide (amino acids 32–41, 32–49, 51–55, 56–61, and 65–71), also induced mRNA expression of type I and type III IFNs in human macrophages, suggesting that these peptides possess strong antiviral activities, responsible for the registered suppression of HIV-1 replication in this cell type (Teixeira et al., 2015).
In addition, Ueda and colleagues reported that a 30-amino acid middle peptide sequence of proTα [termed P30; proTα(49–78)] exerts substantial neuroprotective activity in vitro and in vivo and inhibits cerebral blood vessel damage caused by ischemic stress in retina and brain. The minimum neuroprotective sequence encompasses the 9-amino acid peptide 52–60 (P9), which was shown to comprise the full neuroprotective effect of proTα in cultured cortical neurons under ischemic conditions (Halder et al., 2013).
5. Evidence Supporting a Dual—Intracellular and Extracellular—Role for ProTα
It has long been disputed whether a strictly intracellular, in principle nuclear molecule like proTα, could modulate immune responses and several researchers initially rejected the idea that the polypeptide could exert an extracellular role. The wide distribution of proTα in tissues and cells strongly supported the sole implication of the polypeptide in crucial intracellular processes.
5.1. The Intracellular Role of ProTα
Indeed, in normal cells, proTα was shown to be important for survival and proliferation. High levels of proTα mRNA were detected in lymphocytes (Eschenfeldt and Berger, 1986, Szabo et al., 1992) and in NIH3T3 cells stimulated to divide (Wu, Shiau, & Lin, 1997), whereas proliferation of myeloma cells was hindered in the presence of proTα antisense oligomers (Sburlati, Manrow, & Berger, 1991). Over the years, research on the intracellular activity of proTα revealed its implication in: (1) gene transcription; proTα was reported to localize in nuclear sites of active transcription, physically interact with the CREB-binding protein (CBP), bind to histone H1, and stimulate transcription (Karetsou, Kretsovali, Murphy, Tsolas, & Papamarcaki, 2002); (2) DNA remodeling; during proliferation proTα increased the accessibility of micrococcal nuclease to chromatin (Gomez-Marquez & Rodriguez, 1998); (3) inhibition of apoptosis; proTα was shown to bind Apaf-1 and inhibit apoptosome formation (Jiang et al., 2003); and (4) protection against oxidative stress; proTα interacted with INrf2 leading to its nuclear translocation, where it mediated the degradation of Nrf2 and promoted cell survival and growth (Niture, Kaspar, Shen, & Jaiswal, 2010) (Fig. 1 ).
Fig. 1.
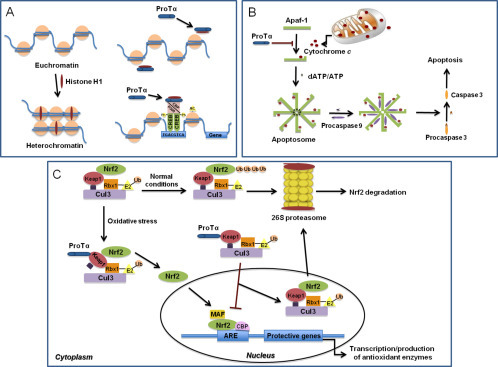
The intracellular role of proTα. (A) In the absence of proTα, histone H1 binds to nucleosomes and induces condensation of euchromatin to heterochromatin. ProTα interacts with histone H1, mediates its transfer from and to chromatin, and leads to the formation of CREB–CBP–p300 complex, chromatin remodeling, and gene transcription. (B) Apoptotic stimuli induce the release of cytochrome c, which binds to Apaf-1 and forms the apoptosome. The subsequent activation of caspase 9 results in conversion of procaspase 3 to caspase 3, leading to apoptosis. ProTα hinders binding of cytochrome c to Apaf-1, and the apoptotic cascade is inhibited. (C) Normally, the Nrf2–Keap1–Cul3–Rbx1 complex is ubiquitinated and degraded by proteasomes. Under oxidative stress, proTα binds to the complex via Keap1, and Nrf2 is released, migrates to the nucleus, and promotes gene transcription and antioxidant enzyme production.
In cancer cells, which are highly proliferative and divide constantly, increased proTα content was also reported. High proTα levels were detected in human colon (Mori et al., 1993), colorectal (Zhang et al., 2014), lung (Sasaki, Nonaka, et al., 2001), bladder (Tsai et al., 2014), and liver cancers (Wu, Habib, et al., 1997); and in neuroblastoma (Sasaki, Sato, et al., 2001), rhabdomyosarcoma (Carey et al., 2006), thyroid carcinomas (Kashat et al., 2010, Letsas et al., 2007), pituitary tumors (Pawlikowski et al., 2014), head and neck cancers (Tripathi et al., 2011), gastric adenocarcinomas (Leys et al., 2007), hepatocellular (Ha, Song, Hwang, Cho, & Park, 2015), and urinary tract transitional cell carcinomas (Jou et al., 2009). We and others reported that proTα was elevated in breast tumors (Tsitsiloni et al., 1993) and tumor proTα levels correlated with the disease outcome (Dominguez et al., 1993), the metastatic potential of the tumor (Tsitsilonis et al., 1998), and the risk of recurrence and death (Magdalena, Dominguez, Loidi, & Puente, 2000). It is worth mentioning that high proTα levels detected in prostate cancer samples (Klimentzou et al., 2008) were shown to progressively increase as prostate tissue progressed from normal epithelium, through prostatic intraepithelial neoplasia to carcinoma, and correlated with Gleason's score (Suzuki et al., 2006). Therefore, following the appropriate validation, proTα tumor content and/or its serum levels may be used as a surrogate tumor biomarker for cancer development, prognosis, and follow-up.
5.2. The Extracellular Role of ProTα
Nevertheless, evidence supporting the extracellular activity of proTα was also reported by in vivo studies in animals, which showed that proTα can protect mice against opportunistic infections, in particular with Candida albicans (Pan et al., 1986). Numerous in vitro studies further confirmed the extracellular role of the polypeptide. As already aforementioned, peripheral blood T cells stimulated with proTα produced high amounts of IL-2 and increased the expression of IL-2 receptor on their surface (Baxevanis et al., 1990, Cordero et al., 1991), while APCs and DCs activated with proTα upregulated MHC class II (Baxevanis et al., 1992) and costimulatory molecules (CD11b, CD80, CD83, CD86, and CD40; Skopeliti et al., 2009), respectively. NK and LAK cells cultured in the presence of proTα augmented their cytotoxicity (Cordero et al., 1992, López-Rodríguez et al., 1994), and proTα-activated neutrophils showed increased chemotaxis, produced high amounts of ROS, and became cytotoxic against cancer cell targets (Heidecke et al., 1997, Samara, Ioannou, et al., 2013). All these multiple immunological responses suggested that proTα acts pleiotropically, especially in immunosuppressed environments.
At that time, it was obvious that the cytokine-like activities of proTα would be justified if specific-binding sites (receptors?) on immune cell surface were discovered. In an initial attempt, Cordero and colleagues radiolabeled proTα and searched for binding sites on the surface of lymphoblasts. Two binding sites, of low and high affinity, were identified (Cordero, Sarandeses, & Nogueira, 1996). Five years later, the same researchers demonstrated, by affinity cross-linking and chromatography, the existence of three binding partners for proTα on the membrane of PHA-activated lymphoblasts, which were associated with lipid rafts (Piñeiro et al., 2001, Salgado et al., 2005). However, a specific proTα receptor was never cloned. In 2007, based on the detailed proteomic analysis of protein changes induced by proTα on immune cells and on the upregulation of the signaling protein IRAK4, we proposed that proTα ligates TLRs (Skopeliti et al., 2007). In 2010, Mosoian and colleagues confirmed that proTα ligates TLR4 and signals through both the TRIF-dependent and the MyD88 pathways for induction of IFN-β and proinflammatory cytokines, respectively (Mosoian et al., 2010). Most recently, proTα was shown to bind to the TLR4/MD-2 complex and activate the TRIF–IRF3-signaling pathway downstream TLR4 (Halder et al., 2015, Omotuyi et al., 2015).
5.3. Evidence Suggesting That ProTα May Act as an Alarmin
Although more data need to be generated to specifically define the pathways activated in response to proTα, it is more than tempting to speculate that the polypeptide probably belongs to the vague family of molecules termed damage-associated molecular patterns (DAMPs) or else “alarmins” (Bianchi, 2007), and as such, can simultaneously and distinctively exert both an intracellular and an extracellular role.
With the term “alarmins” we practically refer to endogenous pathogen-associated molecular patterns (PAMPs), or in more detail, to multifunctional endogenous constitutively available molecules, passively released from damaged cells or rapidly secreted by stimulated leukocytes and epithelial cells following tissue destruction. Alarmins activate both innate and adaptive immune responses. Uncontrolled and excessive release of alarmins extracellularly induces concomitantly the recruitment and activation of APCs through pattern recognition receptors, such as TLRs. In the absence of such stimuli, alarmins exert their vital intracellular roles.
The alarmin family is rapidly growing and, as for now, its best-characterized members are high-mobility group protein B1 (HMGB1) and some heat-shock proteins (Bianchi, 2007). Thymosins were suggested as candidate alarmins, although, to our knowledge, direct comparison of their characteristics with known alarmins has not been performed. Herein, we compile evidence that proTα possesses several attributes to be considered an alarmin (Table 2 ). Among others and in comparison to HMGB1, proTα is a ubiquitously expressed, nonhistone nuclear protein with a marked intracellular physiological role in regulating transcription. It is released via a nonclassical pathway upon ischemic stress, and when localized extracellularly, proTα can recruit and activate innate immune cells, promoting inflammation and cytokine secretion, similar to HMGB1. In addition, proTα signals through TLR4, activates leukocytes and orchestrates immune responses (Ioannou et al., 2012). Finally, it is implicated in neuro- and cardio-tissue repair, suggesting the regenerative potential of the polypeptide (Cannavo et al., 2013, Halder et al., 2012).
Table 2.
Characteristics Ascribed to Alarmins (HMGB1) and Comparison with Reported Properties of Prothymosin Alpha
| Characteristic | HMGB1 (Chan et al., 2012, Schiller et al., 2013, Telusma et al., 2006) | ProTα (Cannavo et al., 2013, Halder et al., 2013, Ioannou et al., 2012) |
|---|---|---|
| Origin | Nonhistone nuclear protein | Nonhistone nuclear protein |
| Expression | Expressed in all cells | Expressed in all cells |
| Physiological intracellular role | DNA organization, transcriptional regulator | DNA organization, transcriptional regulator, antiapoptotic and oxidative stress regulator |
| Extracellular role | Cytokine/inflammatory mediator | Cytokine/inflammatory mediator |
| Release mechanism | Passive release and active secretion | Release upon ischemic stress |
| Receptors | TLR2, TLR4, TLR9, TIM3, and RAGE | TLR4 |
| Regenerative potential | Cardiac and nervous cell regeneration, skin wound healing, bone repair, skeletal muscle repair | Cardiac and nervous cell regeneration |
| Implication in diseases | Cancer, rheumatoid arthritis stroke, atherosclerosis, sepsis | Cancer, autoimmune diseases, ischemic stroke, viral infections |
| Additional similarities | ||
| Immunoactive peptides | Hp-106, Hp-31, Hp-91, and Hp-16 | ProTα(100–109), proTα(50–89), Tα1 |
| Intracellular mobility | Translocation from nucleus to cytoplasm; during apoptosis, translocation into apoptotic cell-derived membranous vesicles | Translocation from nucleus to cytoplasm; during apoptosis, NLS cleavage by caspases and generation of proTα(100–109) |
6. Proposed Scenario for the Mechanism of Action of ProTα
Taking all the above into consideration, we propose a scenario depicting the mode of action of proTα. In normal healthy cells, proTα is localized in the nucleus (Eschenfeldt & Berger, 1986), albeit a fraction of it is assigned to remain in the cytoplasm (Tsitsiloni et al., 1989). In the nucleus, proTα regulates gene transcription (Karetsou et al., 2002), shapes DNA remodeling, and promotes cell proliferation (Gomez-Marquez & Rodriguez, 1998). In the cytoplasm, proTα protects cells from apoptosis (Jiang et al., 2003) and, due to its negative charge, facilitates the transportation of molecules in the nucleus, protecting the cell from insults, eg, oxidative stress (Niture et al., 2010). In hyperproliferative cells, like cancer cells, proTα’s gene expression and protein content are increased and this contributes to uncontrolled proliferation and induction of resistance to apoptosis and oxidative stress. Cells receiving death stimuli die violently by necrosis or undergo programmed apoptotic death. During necrosis, cell swelling and membrane disruption lead to abrupt uncontrolled release of internal cell components, including proTα, which acts as alarmin, alerts cells of the innate arm of immunity expressing TLR4, and initiates immune responses (Ioannou et al., 2012). During apoptosis, the major proportion of proTα is transferred to the cytoplasm, where activated caspases cleave the molecule at its C-terminus, generating among other fragments, the decapeptide proTα(100–109). The negatively charged fragment proTα(1–99) remains in the cytoplasm, where most probably is degraded or, as suggested, is exposed on apoptotic cell surface where it acts as an “eat-me” signal (Evstafieva et al., 2003). The decapeptide proTα(100–109), or a proportion of it, is protected from degradation as it polymerizes adopting a β-sheet conformation (Skopeliti et al., 2009) and, consequently, is excreted from dying cells via an unknown mechanism. In both necrosis and apoptosis, exocytosed proTα and proTα(100–109) are sensed as DAMPs by innate immune cells and ligate TLR4 (Ioannou et al., 2013, Mosoian et al., 2010). Stimulated innate immune cells, such as macrophages, neutrophils, monocytes, and DCs, respond by initiating molecular signaling pathways leading to NF-κB activation, chemokine, IFN, and proinflammatory cytokine secretion. Additionally, stimulated macrophages and neutrophils increase their phagocytosis and produce TNF-α and , respectively (Mosoian et al., 2010, Samara, Ioannou, et al., 2013). Stimulation of APCs (DCs and monocytes) increases antigen (eg, shed from dying cancer cells) uptake and presentation through MHC class I molecules directly to CD8+ cytotoxic T cells. Upregulation of MHC class II expression by the thymic peptides on monocytes and DCs increases antigen presentation and their synapse with CD4+ helper T cells, which produce TH1-type cytokines (eg, IL-2, IFN-γ), providing a favorable environment for enhancing specific and nonspecific cytotoxicity. CTLs and NK cells produce lytic molecules (perforin), express adhesion molecules (CD2), secrete TNF-α and IFN-γ, and kill cell targets (eg, cancer cells) (Fig. 2 ).
Fig. 2.
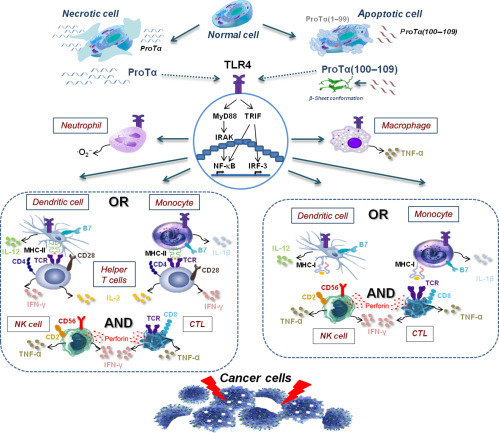
Proposed scenario on proTα’s dual role. In normal cells, proTα regulates gene expression and cell proliferation in the nucleus. Under abnormal conditions, cells die via necrosis or apoptosis. During necrosis, cell membrane is ruptured and intact proTα is released out of the cell. During apoptosis, proTα is relocated to the cytoplasm and is cleaved at its C-terminus by activated caspases, and proTα(100–109) is generated. The decapeptide adopts a β-sheet conformation and is excreted. Extracellularly, both proTα and proTα(100–109) activate innate immune cells expressing TLR4 (macrophages, neutrophils, DCs, and monocytes) and signal through the MyD88 and TRIF pathways. Cytotoxic responses are enhanced through antigen presentation with MHC class II molecules and synapsis with helper T cells which secrete TH1-type cytokines, and/or with MHC class I molecules, leading to activation of cytotoxic effectors (NK cells and CTLs). In both cases, effector cells upregulate adhesion molecule expression (eg, CD2) and produce lytic molecules (eg, perforin), mediating cell binding and cell destruction, respectively (eg, cancer cell targets, as shown here).
7. Conclusions and Future Directions
ProTα, although initially characterized as a thymic hormone, is a ubiquitously expressed polypeptide that exerts pleiotropic immunostimulatory adjuvant effects. Nevertheless, and despite extensive and intensive research on its mode of action, the efficacy of proTα has not yet been tested in humans. In our opinion, the main reason stands in our lack of understanding of its dual role and in elucidating how the same molecule promotes cell proliferation and mediates immunity. This paradox is most surprising and contradictory in the case of cancer. We know that increased proTα gene transcription correlates with carcinogenesis, whereas proTα ligation to TLR4 promotes anticancer-reactive immune responses. In view of recent progress in elucidating the complex machinery of immune responses and the concept that the immune system responds to both pathogens (PAMPs) and danger signals (DAMPs), the adjuvant mode of action of proTα points toward its integration in the family of alarmins. As such, under specific conditions related to danger (resulting from cell damage, destruction, or death), proTα and/or smaller fragments of the molecule [eg, proTα(100–109)] are relocalized extracellularly, ie, at a different site than inside the cell, where they physiologically function. Innate immune cells like DCs that are programmed to sense danger respond to proTα, and the cascade of an immune response is initiated.
Recent evidence additionally suggests that proTα is a TLR agonist. At present, driving closer to optimal orchestration of the immune machinery, several TLR agonists have reached the clinical setting. We believe that proTα has adequately proven its potent immunostimulating capacity in vitro and in animals in vivo, by generating the appropriate cytokine milieu promoting the activation of effector cells. Although it should be studied in more detail, it is also known that the polypeptide when administered in animals at relatively high concentrations does not induce toxicity or severe adverse effects. Therefore, the next step should be to test the adjuvant effectiveness of proTα or smaller immunoactive fragments thereof in clinical studies, aiming to strengthen deficient immune responses.
Acknowledgments
The authors thank all members of the laboratory for their excellent work, as well as Lilian Williams, Niki Kappa, and Sotirios Fortis for their assistance in preparation of the figures. Research was supported by IKY Fellowships of Excellence for Postgraduate Studies in Greece-Siemens Program to PS, European Union FP7 Capacities Grant REGPOT-CT-2011-284460 (INsPiRE), NATO SfP Project 982838, IKYDA 61/2003, IKYDA 165/2010, Empeirikion Foundation of Athens, and John S. Latsis Public Benefit Foundation. Funding sources had no involvement in manuscript writing.
References
- Aisenberg A.C., Wilkes B. Partial immunological restoration of neonatally thymectomized rats with thymus-containing diffusion chambers. Nature. 1965;205:716–717. doi: 10.1038/205716a0. [DOI] [PubMed] [Google Scholar]
- Baxevanis C.N., Frillingos S., Seferiadis K., Reclos G.J., Arsenis P., Katsiyiannis A. Enhancement of human T lymphocyte function by prothymosin alpha: Increased production of interleukin-2 and expression of interleukin-2 receptors in normal human peripheral blood T lymphocytes. Immunopharmacology and Immunotoxicology. 1990;12:595–617. doi: 10.3109/08923979009019679. [DOI] [PubMed] [Google Scholar]
- Baxevanis C.N., Gritzapis A.D., Dedoussis G.V., Papadopoulos N.G., Tsolas O., Papamichail M. Induction of lymphokine-activated killer activity in mice by prothymosin alpha. Cancer Immunology, Immunotherapy. 1994;38:281–286. doi: 10.1007/BF01533521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxevanis C.N., Gritzapis A.D., Spanakos G., Tsitsilonis O.E., Papamichail M. Induction of tumor-specific T lymphocyte responses in vivo by prothymosin alpha. Cancer Immunology, Immunotherapy. 1995;40:410–418. doi: 10.1007/BF01525392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxevanis C.N., Reclos G.J., Papamichail M. Prothymosin alpha restores depressed allogeneic cell-mediated lympholysis and natural-killer-cell activity in patients with cancer. International Journal of Cancer. 1993;53:264–268. doi: 10.1002/ijc.2910530216. [DOI] [PubMed] [Google Scholar]
- Baxevanis C.N., Spanakos G., Voutsas I.F., Gritzapis A.D., Tsitsilonis O.E., Mamalaki A. Increased generation of autologous tumor-reactive lymphocytes by anti-CD3 monoclonal antibody and prothymosin alpha. Cancer Immunology, Immunotherapy. 1999;48:71–84. doi: 10.1007/s002620050550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxevanis C.N., Thanos D., Reclos G.J., Anastasopoulos E., Tsokos G.C., Papamatheakis J. Prothymosin alpha enhances human and murine MHC class II surface antigen expression and messenger RNA accumulation. Journal of Immunology. 1992;148:1979–1984. [PubMed] [Google Scholar]
- Bianchi M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. Journal of Leukocyte Biology. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- Bowick G.C., Soman K.V., Wang H. Proteomic analysis of Pichindé virus infection identifies differential expression of prothymosin-α. Journal of Biomedicine and Biotechnology. 2010;2010 doi: 10.1155/2010/956823. Article ID: 956823, 9 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldarella J., Goodall G.J., Felix A.M., Heimer E.P., Salvin S.B., Horecker B.L. Thymosin alpha 11: A peptide related to thymosin alpha 1 isolated from calf thymosin fraction 5. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:7424–7427. doi: 10.1073/pnas.80.24.7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerini R., Garaci E. Historical review of thymosin α1 in infectious diseases. Expert Opinion on Biological Therapy. 2015;1:S117–S127. doi: 10.1517/14712598.2015.1033393. [DOI] [PubMed] [Google Scholar]
- Cannavo A., Rengo G., Liccardo D., Pironti G., Scimia M.C., Scudiero L. Prothymosin alpha protects cardiomyocytes against ischemia-induced apoptosis via preservation of Akt activation. Apoptosis. 2013;18:1252–1261. doi: 10.1007/s10495-013-0876-9. [DOI] [PubMed] [Google Scholar]
- Carey K.A., Segal D., Klein R., Sanigorski A., Walder K., Collier G.R. Identification of novel genes expressed during rhabdomyosarcoma differentiation using cDNA microarrays. Pathology International. 2006;56:246–255. doi: 10.1111/j.1440-1827.2006.01958.x. [DOI] [PubMed] [Google Scholar]
- Chan J.K., Roth J., Oppenheim J.J., Tracey K.J., Vogl T., Feldmann M. Alarmins: Awaiting a clinical response. The Journal of Clinical Investigation. 2012;122:2711–2719. doi: 10.1172/JCI62423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero O.J., Sarandeses C.S., López J.L., Cancio E., Regueiro B.J., Nogueira M. Prothymosin alpha enhances interleukin 2 receptor expression in normal human T-lymphocytes. International Journal of Immunopharmacology. 1991;13:1059–1065. doi: 10.1016/0192-0561(91)90156-2. [DOI] [PubMed] [Google Scholar]
- Cordero O.J., Sarandeses C.S., López J.L., Nogueira M. Prothymosin alpha enhances human natural killer cell cytotoxicity: Role in mediating signals for NK activity. Lymphokine and Cytokine Research. 1992;11:277–285. [PubMed] [Google Scholar]
- Cordero O.J., Sarandeses C., López-Rodríguez J.L., Nogueira M. The presence and cytotoxicity of CD16 + CD2- subset from PBL and NK cells in long-term IL-2 cultures enhanced by Prothymosin-alpha. Immunopharmacology. 1995;29:215–223. doi: 10.1016/0162-3109(95)00057-z. [DOI] [PubMed] [Google Scholar]
- Cordero O.J., Sarandeses C.S., Nogueira M. Binding of 125I-prothymosin alpha to lymphoblasts through the non-thymosin alpha 1 sequence. Life Sciences. 1996;58:1757–1770. doi: 10.1016/0024-3205(96)00157-9. [DOI] [PubMed] [Google Scholar]
- Dauphinee M.J., Tala N., Goldstein A.L., White A. Thymosin corrects the abnormal DNA synthetic response of NZB mouse thymocytes. Proceedings of the National Academy of Sciences of the United States of America. 1974;71:2637–2641. doi: 10.1073/pnas.71.7.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez F., Magdalena C., Cancio E., Roson E., Paredes J., Loidi L. Tissue concentrations of prothymosin alpha: A novel proliferation index of primary breast cancer. European Journal of Cancer. 1993;29A:893–897. doi: 10.1016/s0959-8049(05)80433-2. [DOI] [PubMed] [Google Scholar]
- Eckert K., Garbin F., Maurer H.R., Büttner P., Garbe C., Czarnecki J. Prothymosin alpha 1 modulates lymphokine-activated killer cell activity and IL-2 production by peripheral blood lymphocytes from melanoma patients in vitro. International Journal of Immunopharmacology. 1995;17:555–561. doi: 10.1016/0192-0561(95)00040-9. [DOI] [PubMed] [Google Scholar]
- Eckert K., Grünberg E., Garbin F., Maurer H.R. Preclinical studies with prothymosin alpha1 on mononuclear cells from tumor patients. International Journal of Immunopharmacology. 1997;19:493–500. doi: 10.1016/s0192-0561(97)00079-9. [DOI] [PubMed] [Google Scholar]
- Eckert K., Grünberg E., Immenschuh P., Garbin F., Kreuser E.D., Maurer H.R. Interleukin-2-activated killer cell activity in colorectal tumor patients: Evaluation of in vitro effects by prothymosin alpha1. Journal of Cancer Research and Clinical Oncology. 1997;123:420–428. doi: 10.1007/BF01372545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkemann S.A., Wang R.H., Trumbore M.W., Berger S.L. Functional discontinuities in prothymosin α caused by caspase cleavage in apoptotic cells. Journal of Cell Physiology. 2000;182:256–268. doi: 10.1002/(SICI)1097-4652(200002)182:2<256::AID-JCP15>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Eschenfeldt W.H., Berger S.L. The human prothymosin alpha gene is polymorphic and induced upon growth stimulation: Evidence using a cloned cDNA. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:9403–9407. doi: 10.1073/pnas.83.24.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evstafieva A.G., Belov G.A., Rubtsov Y.P., Kalkum M., Joseph B., Chichkova N.V. Apoptosis-related fragmentation, translocation, and properties of human prothymosin alpha. Experimental Cell Research. 2003;284:211–223. doi: 10.1016/s0014-4827(02)00047-2. [DOI] [PubMed] [Google Scholar]
- Fujita R., Ueda H. Prothymosin-alpha prevents necrosis and apoptosis following stroke. Cell Death and Differentiation. 2007;14:1839–1842. doi: 10.1038/sj.cdd.4402189. [DOI] [PubMed] [Google Scholar]
- Fujita R., Ueda M., Fujiwara K., Ueda H. Prothymosin-alpha plays a defensive role in retinal ischemia through necrosis and apoptosis inhibition. Cell Death and Differentiation. 2009;16:349–358. doi: 10.1038/cdd.2008.159. [DOI] [PubMed] [Google Scholar]
- Garbin F., Eckert K., Büttner P., Garbe C., Maurer H.R. Prothymosin alpha augments deficient antitumor activity of monocytes from melanoma patients in vitro. Anticancer Research. 1994;14:2405–2411. [PubMed] [Google Scholar]
- Garbin F., Eckert K., Immenschuh P., Kreuser E.D., Maurer H.R. Prothymosin alpha 1 effects, in vitro, on the antitumor activity and cytokine production of blood monocytes from colorectal tumor patients. International Journal of Immunopharmacology. 1997;19:323–332. doi: 10.1016/s0192-0561(97)00024-6. [DOI] [PubMed] [Google Scholar]
- Gast K., Damaschun H., Eckert K., Schulze-Forster K., Maurer H.R., Müller-Frohne M. Prothymosin alpha: A biologically active protein with random coil conformation. Biochemistry. 1995;34:13211–13218. doi: 10.1021/bi00040a037. [DOI] [PubMed] [Google Scholar]
- Goldstein A.L. History of the discovery of the thymosins. Annals of the New York Academy of Sciences. 2007;1112:1–13. doi: 10.1196/annals.1415.045. [DOI] [PubMed] [Google Scholar]
- Goldstein A.L., Low T.L., Thurman G.B., Zatz M.M., Hall N., Chen J. Current status of thymosin and other hormones of the thymus gland. Recent Progress in Hormone Research. 1981;37:369–415. doi: 10.1016/b978-0-12-571137-1.50012-8. [DOI] [PubMed] [Google Scholar]
- Goldstein A.L., Slater F.D., White A. Preparation, assay, and partial purification of a thymic lymphocytopoietic factor (thymosin) Proceedings of the National Academy of Sciences of the United States of America. 1966;56:1010–1017. doi: 10.1073/pnas.56.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Marquez J., Rodriguez P. Prothymosin alpha is a chromatin-remodeling protein in mammalian cells. Biochemical Journal. 1998;333:1–3. doi: 10.1042/bj3330001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good R.A., Dalmasso A.P., Martinez C., Archer O.K., Pierce J.C., Papermaster B.W. The role of the thymus in development of immunologic capacity in rabbits and mice. Journal of Experimental Medicine. 1962;116:773–796. doi: 10.1084/jem.116.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S.Y., Song D.H., Hwang S.H., Cho S.Y., Park C.K. Expression of prothymosin alpha predicts early recurrence and poor prognosis of hepatocellular carcinoma. Hepatobiliary & Pancreatic Diseases International. 2015;14:171–177. doi: 10.1016/s1499-3872(14)60326-x. [DOI] [PubMed] [Google Scholar]
- Halder S.K., Matsunaga H., Ishii K.J., Ueda H. Prothymosin-alpha preconditioning activates TLR4-TRIF signaling to induce protection of ischemic retina. Journal of Neurochemistry. 2015;135:1161–1177. doi: 10.1111/jnc.13356. [DOI] [PubMed] [Google Scholar]
- Halder S.K., Matsunaga H., Ueda H. Neuron-specific non-classical release of prothymosin alpha: A novel neuroprotective damage-associated molecular patterns. Journal of Neurochemistry. 2012;123:262–275. doi: 10.1111/j.1471-4159.2012.07897.x. [DOI] [PubMed] [Google Scholar]
- Halder S.K., Sugimoto J., Matsunaga H., Ueda H. Therapeutic benefits of 9-amino acid peptide derived from prothymosin alpha against ischemic damages. Peptides. 2013;43:68–75. doi: 10.1016/j.peptides.2013.02.022. [DOI] [PubMed] [Google Scholar]
- Hannappel E., Davoust S., Horecker B.L. Isolation of peptides from calf thymus. Biochemical and Biophysical Research Communications. 1982;104:266–271. doi: 10.1016/0006-291x(82)91969-6. [DOI] [PubMed] [Google Scholar]
- Hannappel E., Davoust S., Horecker B.L. Thymosins beta 8 and beta 9: Two new peptides isolated from calf thymus homologous to thymosin beta 4. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:1708–1711. doi: 10.1073/pnas.79.6.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannappel E., Huff T. The thymosins. Prothymosin alpha, parathymosin, and beta-thymosins: Structure and function. Vitamins and Hormones. 2003;66:257–296. doi: 10.1016/s0083-6729(03)01007-0. [DOI] [PubMed] [Google Scholar]
- Haritos A.A. Alpha-thymosins: Relationships in structure, distribution, and function. Isozymes Current Top Biological & Medicinal Research. 1987;14:123–152. [PubMed] [Google Scholar]
- Haritos A.A., Goodall G.J., Horecker B.L. Prothymosin alpha: Isolation and properties of the major immunoreactive form of thymosin alpha 1 in rat thymus. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:1008–1011. doi: 10.1073/pnas.81.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haritos A.A., Horecker B.L. A radioimmunoassay for thymosin alpha 1 that detects the native polypeptide, prothymosin alpha. Journal of Immunological Methods. 1985;81:199–205. doi: 10.1016/0022-1759(85)90204-2. [DOI] [PubMed] [Google Scholar]
- Haritos A.A., Tsolas O., Horecker B.L. Distribution of prothymosin alpha in rat tissues. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:1391–1393. doi: 10.1073/pnas.81.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidecke H., Eckert K., Schulze-Forster K., Maurer H.R. Prothymosin alpha 1 effects in vitro on chemotaxis, cytotoxicity and oxidative response of neutrophils from melanoma, colorectal and breast tumor patients. International Journal of Immunopharmacology. 1997;19:413–420. doi: 10.1016/s0192-0561(97)00089-1. [DOI] [PubMed] [Google Scholar]
- Ho A.D., Ma D.D., Price G., Hunstein W., HoVbrand A.V. Biochemical and immunological differentiation of human thymocytes induced by thymic hormones. Immunology. 1983;50:471–476. [PMC free article] [PubMed] [Google Scholar]
- Hooper J.A., McDaniel M.C., Thurman G.B., Cohen G.H., Schulof R.S., Goldstein A.L. Purification and properties of bovine thymosin. Annals of the New York Academy of Sciences. 1975;249:125–144. doi: 10.1111/j.1749-6632.1975.tb29063.x. [DOI] [PubMed] [Google Scholar]
- Ioannou K., Derhovanessian E., Tsakiri E., Samara P., Kalbacher H., Voelter W. Prothymosin α and a prothymosin α-derived peptide enhance T(H)1-type immune responses against defined HER-2/neu epitopes. BMC Immunology. 2013;14:43. doi: 10.1186/1471-2172-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou K., Samara P., Livaniou E., Derhovanessian E., Tsitsilonis O.E. Prothymosin alpha: A ubiquitous polypeptide with potential use in cancer diagnosis and therapy. Cancer Immunology, Immunotherapy. 2012;61:599–614. doi: 10.1007/s00262-012-1222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Kim H.E., Shu H., Zhao Y., Zhang H., Kofron J. Distinctive roles of PHAP proteins and prothymosin-alpha in a death regulatory pathway. Science. 2003;299:223–226. doi: 10.1126/science.1076807. [DOI] [PubMed] [Google Scholar]
- Jin Y., Cao C., Li P., Liu X., Huang W., Li C. Boosting immune response to hepatitis B DNA vaccine by coadministration of Prothymosin alpha-expressing plasmid. Clinical and Diagnostic Laboratory Immunology. 2005;12:1364–1369. doi: 10.1128/CDLI.12.12.1364-1369.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou Y.C., Tung C.L., Tsai Y.S., Shen C.H., Syue-Yi C., Shiau A.L. Prognostic relevance of prothymosin-alpha expression in human upper urinary tract transitional cell carcinoma. Urology. 2009;74:951–957. doi: 10.1016/j.urology.2008.11.060. [DOI] [PubMed] [Google Scholar]
- Karachaliou C.E., Liolios C., Triantis C., Zikos C., Samara P., Tsitsilonis O.E. Specific in vitro binding of a new (99m)Tc-radiolabeled derivative of the C-terminal decapeptide of prothymosin alpha on human neutrophils. International Journal of Pharmaceutics. 2015;486:1–12. doi: 10.1016/j.ijpharm.2015.03.031. [DOI] [PubMed] [Google Scholar]
- Karetsou Z., Kretsovali A., Murphy C., Tsolas O., Papamarcaki T. Prothymosin alpha interacts with the CREB-binding protein and potentiates transcription. EMBO Reports. 2002;3:361–366. doi: 10.1093/embo-reports/kvf071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashat L., So A.K., Masui O., Wang X.S., Cao J., Meng X. Secretome-based identification and characterization of potential biomarkers in thyroid cancer. Journal of Proteome Research. 2010;9:5757–5769. doi: 10.1021/pr100529t. [DOI] [PubMed] [Google Scholar]
- Khaw B.A., Rule A.H. Immunotherapy of the Dunning leukemia with thymic extracts. British Journal of Cancer. 1973;28:288–292. doi: 10.1038/bjc.1973.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J.J., Goldstein A.L., White A. Enhancement of in vivo incorporation of labeled precursors into DNA and total protein of mouse lymph nodes after administration of thymic extracts. Proceedings of the National Academy of Sciences of the United States of America. 1965;53:812–817. doi: 10.1073/pnas.53.4.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimentzou P., Drougou A., Fehrenbacher B., Schalle M., Voelter W., Barbatis C. Immunocytological and preliminary immunohistochemical studies of prothymosin alpha, a human cancer-associated polypeptide, with a well-characterized polyclonal antibody. The Journal of Histochemistry and Cytochemistry. 2008;56:1023–1031. doi: 10.1369/jhc.2008.950956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letsas K.P., Vartholomatos G., Tsepi C., Tsatsoulis A., Frangou-Lazaridis M. Fine-needle aspiration biopsy-RT-PCR expression analysis of prothymosin alpha and parathymosin in thyroid: Novel proliferation markers? Neoplasma. 2007;54:57–62. [PubMed] [Google Scholar]
- Levey R.H., Trainin N., Law L.W. Evidence for function of thymic tissue in diffusion chambers implanted in neonatally thymectomized mice. Preliminary report. Journal of the National Cancer Institute. 1963;31:199–217. [PubMed] [Google Scholar]
- Leys C.M., Nomura S., LaFleur B.J., Ferrone S., Kaminishi M., Montgomery E. Expression and prognostic significance of prothymosin-alpha and ERp57 in human gastric cancer. Surgery. 2007;141:41–50. doi: 10.1016/j.surg.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Li K.J., Shiau A.L., Chiou Y.Y., Yo Y.T., Wu C.L. Transgenic overexpression of prothymosin alpha induces development of polycystic kidney disease. Kidney International. 2005;67:1710–1722. doi: 10.1111/j.1523-1755.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- López-Rodríguez J.L., Cordero O.J., Sarandeses C., Viñuela J., Nogueira M. Interleukin-2 killer cells: In vitro evaluation of combination with prothymosin alpha. Lymphokine and Cytokine Research. 1994;13:175–182. [PubMed] [Google Scholar]
- Low T.L., Hu S.K., Goldstein A.L. Complete amino acid sequence of bovine thymosin beta 4: A thymic hormone that induces terminal deoxynucleotidyl transferase activity in thymocyte populations. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:1162–1166. doi: 10.1073/pnas.78.2.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdalena C., Dominguez F., Loidi L., Puente J.L. Tumour prothymosin alpha content, a potential prognostic marker for primary breast cancer. British Journal of Cancer. 2000;82:584–590. doi: 10.1054/bjoc.1999.0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinda K.M., Sidhu G.S., Banaudha K.K., Gaddipati J.P., Maheshwari R.K., Goldstein A.L. Thymosin alpha 1 stimulates endothelial cell migration, angiogenesis, and wound healing. Journal of Immunology. 1998;160:1001–1006. [PubMed] [Google Scholar]
- Manrow R.E., Sburlati A.R., Hanover J.A., Berger S.L. Nuclear targeting of prothymosin alpha. Journal of Biological Chemistry. 1991;266:3916–3924. [PubMed] [Google Scholar]
- Matsunaga H., Ueda H. Stress-induced non-vesicular release of prothymosin-α initiated by an interaction with S100A13, and its blockade by caspase-3 cleavage. Cell Death and Differentiation. 2010;17:1760–1772. doi: 10.1038/cdd.2010.52. [DOI] [PubMed] [Google Scholar]
- Miller J.F. Immunological function of the thymus. Lancet. 1961;2:748–749. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- Miller J.F. The discovery of thymus function and of thymus-derived lymphocytes. Immunology Reviews. 2002;185:7–14. doi: 10.1034/j.1600-065x.2002.18502.x. [DOI] [PubMed] [Google Scholar]
- Mori M., Barnard G.F., Staniunas R.J., Jessup J.M., Steele G.D., Jr., Chen L.B. Prothymosin-alpha mRNA expression correlates with that of c-myc in human colon cancer. Oncogene. 1993;8:2821–2826. [PubMed] [Google Scholar]
- Mosoian A., Teixeira A., Burns C.S., Sander L.E., Gusella G.L., He C. Prothymosin-alpha inhibits HIV-1 via Toll-like receptor 4-mediated type I interferon induction. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10178–10183. doi: 10.1073/pnas.0914870107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosoian A., Teixeira A., High A.A., Christian R.E., Hunt D.F., Shabanowitz J. Novel function of prothymosin alpha as a potent inhibitor of human immunodeficiency virus type 1 gene expression in primary macrophages. Journal of Virology. 2006;80:9200–9206. doi: 10.1128/JVI.00589-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niture S.K., Kaspar J.W., Shen J., Jaiswal A.K. Nrf2 signaling and cell survival. Toxicology and Applied Pharmacology. 2010;244:37–42. doi: 10.1016/j.taap.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omotuyi O., Matsunaga H., Ueda H. Evidence for ProTα-TLR4/MD-2 binding: Molecular dynamics and gravimetric assay studies. Expert Opinion on Biological Therapy. 2015;1:S223–S229. doi: 10.1517/14712598.2015.1005597. [DOI] [PubMed] [Google Scholar]
- Pan L.X., Haritos A.A., Wideman J., Komiyama T., Chang M., Stein S. Human prothymosin alpha: Amino acid sequence and immunologic properties. Archives of Biochemistry and Biophysics. 1986;250:197–201. doi: 10.1016/0003-9861(86)90717-4. [DOI] [PubMed] [Google Scholar]
- Papanastasiou M., Baxevanis C.N., Papamichail M. Promotion of murine antitumor activity by prothymosin alpha treatment: I. Induction of tumoricidal peritoneal cells producing high levels of tumour necrosis factor alpha. Cancer Immunology, Immunotherapy. 1992;35:145–150. doi: 10.1007/BF01741862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov N.A., Cherny D.I., Heim G., Jovin T.M., Subramaniam V. Amyloid fibrils from the mammalian protein prothymosin alpha. FEBS Letters. 2002;517:37–40. doi: 10.1016/s0014-5793(02)02572-3. [DOI] [PubMed] [Google Scholar]
- Pawlikowski M., Radek M., Kunert-Radek J., Jaranowska M., Świętosławski J., Winczyk K. Overexpression of prothymosin alpha is related to pituitary adenoma recurrence but not to adenoma invasiveness and proliferation. Endokrynologia Polska. 2014;65:382–386. doi: 10.5603/EP.2014.0053. [DOI] [PubMed] [Google Scholar]
- Petro T.M., Watson R.R. Resistance to L1210 mouse leukemia cells in moderately protein-malnourished BALB/c mice treated in vivo with thymosin fraction V. Cancer Research. 1982;42:2139–2145. [PubMed] [Google Scholar]
- Piñeiro A., Begoña Bugia M., Pilar Arias M., Cordero O.J., Nogueira M. Identification of receptors for prothymosin alpha on human lymphocytes. Biological Chemistry. 2001;382:1473–1482. doi: 10.1515/BC.2001.181. [DOI] [PubMed] [Google Scholar]
- Piñeiro A., Cordero O.J., Nogueira M. Fifteen years of prothymosin alpha: Contradictory past and new horizons. Peptides. 2000;21:1433–1446. doi: 10.1016/s0196-9781(00)00288-6. [DOI] [PubMed] [Google Scholar]
- Qiu L., Guo B.Y., Miao H., Dao S.Y., Zhang R., Yuan P.Q. Effect of recombinant prothymosin alpha on secretion of IFN-gamma, IFN-alpha and TNF-alpha in vitro. Yao Xue Xue Bao. 2002;37:326–328. [PubMed] [Google Scholar]
- Romani L., Moretti S., Fallarino F., Bozza S., Ruggeri L., Casagrande A. Jack of all trades: Thymosin α1 and its pleiotropy. Annals of the New York Academy of Sciences. 2012;1269:1–6. doi: 10.1111/j.1749-6632.2012.06716.x. [DOI] [PubMed] [Google Scholar]
- Rubtsov Y.P., Zolotukhin A.S., Vorobjev I.A., Chichkova N.V., Pavlov N.A., Karger E.M. Mutational analysis of human prothymosin alpha reveals a bipartite nuclear localization signal. FEBS Letters. 1997;413:135–141. doi: 10.1016/s0014-5793(97)00824-7. [DOI] [PubMed] [Google Scholar]
- Salgado F.J., Piñeiro A., Canda-Sánchez A., Lojo J., Nogueira M. Prothymosin alpha-receptor associates with lipid rafts in PHA-stimulated lymphocytes. Molecular Membrane Biology. 2005;22:163–176. doi: 10.1080/09687860500063506. [DOI] [PubMed] [Google Scholar]
- Samara P., Ioannou K., Neagu M., Arnogiannaki N., Ardavanis A., Voelter W. The C-terminal decapeptide of prothymosin α is responsible for its stimulatory effect on the functions of human neutrophils in vitro. International Immunopharmacology. 2013;15:50–57. doi: 10.1016/j.intimp.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Samara P., Kalbacher H., Ioannou K., Radu D.L., Livaniou E., Promponas V.J. Development of an ELISA for the quantification of the C-terminal decapeptide prothymosin α(100-109) in sera of mice infected with bacteria. Journal of Immunological Methods. 2013;395:54–62. doi: 10.1016/j.jim.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Sasaki H., Nonaka M., Fujii Y., Yamakawa Y., Fukai I., Kiriyama M. Expression of the prothymosin-alpha gene as a prognostic factor in lung cancer. Surgery Today. 2001;31:936–938. doi: 10.1007/s005950170040. [DOI] [PubMed] [Google Scholar]
- Sasaki H., Sato Y., Kondo S., Fukai I., Kiriyama M., Yamakawa Y. Expression of the prothymosin alpha mRNA correlated with that of N-myc in neuroblastoma. Cancer Letters. 2001;168:191–195. doi: 10.1016/s0304-3835(01)00540-7. [DOI] [PubMed] [Google Scholar]
- Sburlati A.R., Manrow R.E., Berger S.L. Human prothymosin alpha: Purification of a highly acidic nuclear protein by means of a phenol extraction. Protein Expression and Purification. 1990;1:184–190. doi: 10.1016/1046-5928(90)90014-p. [DOI] [PubMed] [Google Scholar]
- Sburlati A.R., Manrow R.E., Berger S.L. Prothymosin alpha antisense oligomers inhibit myeloma cell division. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:253–257. doi: 10.1073/pnas.88.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller M., Heyder P., Ziegler S., Niessen A., Claßen L., Lauffer A. During apoptosis HMGB1 is translocated into apoptotic cell-derived membranous vesicles. Autoimmunity. 2013;46:342–346. doi: 10.3109/08916934.2012.750302. [DOI] [PubMed] [Google Scholar]
- Skopeliti M., Iconomidou V.A., Derhovanessian E., Pawelec G., Voelter W., Kalbacher H. Prothymosin alpha immunoactive carboxyl-terminal peptide TKKQKTDEDD stimulates lymphocyte reactions, induces dendritic cell maturation and adopts a beta-sheet conformation in a sequence-specific manner. Molecular Immunology. 2009;46:784–792. doi: 10.1016/j.molimm.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Skopeliti M., Kratzer U., Altenberend F., Panayotou G., Kalbacher H., Stevanovic S. Proteomic exploitation on prothymosin alpha-induced mononuclear cell activation. Proteomics. 2007;7:1814–1824. doi: 10.1002/pmic.200600870. [DOI] [PubMed] [Google Scholar]
- Skopeliti M., Voutsas I.F., Klimentzou P., Tsiatas M.L., Beck A., Bamias A. The immunologically active site of prothymosin alpha is located at the carboxy-terminus of the polypeptide. Evaluation of its in vitro effects in cancer patients. Cancer Immunology, Immunotherapy. 2006;55:1247–1257. doi: 10.1007/s00262-005-0108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangelo B.L., Farrimond D.D., Pompilius M., Bowman K.L. Interleukin-1β and thymic peptide regulation of pituitary and glial cell cytokine expression and cellular proliferation. Annals of the New York Academy of Sciences. 2000;917:597–607. doi: 10.1111/j.1749-6632.2000.tb05425.x. [DOI] [PubMed] [Google Scholar]
- Spangelo B.L., Hall N.R., Goldstein A.L. Biology and chemistry of thymosin peptides. Modulators of immunity and neuroendocrine circuits. Annals of the New York Academy of Sciences. 1987;496:196–204. doi: 10.1111/j.1749-6632.1987.tb35766.x. [DOI] [PubMed] [Google Scholar]
- Spangelo B.L., Roach J.D., Hadi F., Damavandy A.A., Plieskatt J., Badamchian M. Thymosin fraction-5 possesses antiproliferative properties in HL-60 human promyelocytic leukemia cells: Characterization of an active peptide. Annals of the New York Academy of Sciences. 2007;1112:305–316. doi: 10.1196/annals.1415.022. [DOI] [PubMed] [Google Scholar]
- Su Y.C., Ou H.Y., Wu H.T., Wu P., Chen Y.C., Su B.H. Prothymosin-α overexpression contributes to the development of insulin resistance. The Journal of Clinical Endocrinology and Metabolism. 2015;100:4114–4123. doi: 10.1210/jc.2015-2277. [DOI] [PubMed] [Google Scholar]
- Su B.H., Tseng Y.L., Shieh G.S., Chen Y.C., Shiang Y.C., Wu P. Prothymosin α overexpression contributes to the development of pulmonary emphysema. Nature Communications. 2013;4 doi: 10.1038/ncomms2906. Article number: 1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B.-H., Tseng Y.-L., Shieh G.-S., Chen Y.-C., Wu P., Shiau A.-L. Over-expression of prothymosin-α antagonizes TGFβ signalling to promote the development of emphysema. Journal of Pathology. 2016;238:412–422. doi: 10.1002/path.4664. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Takahashi S., Takahashi S., Takeshita K., Hikosaka A., Wakita T. Expression of prothymosin alpha is correlated with development and progression in human prostate cancers. Prostate. 2006;66:463–469. doi: 10.1002/pros.20385. [DOI] [PubMed] [Google Scholar]
- Szabo P., Ehleiter D., Whittington E., Weksler M.E. Prothymosin alpha expression occurs during G1 in proliferating B or T lymphocytes. Biochemical and Biophysical Research Communications. 1992;185:953–959. doi: 10.1016/0006-291x(92)91719-7. [DOI] [PubMed] [Google Scholar]
- Szabo P., Panneerselvam C., Clinton M., Frangou-Lazaridis M., Weksler D., Whittington E. Prothymosin alpha gene in humans: Organization of its promoter region and localization to chromosome 2. Human Genetics. 1993;90:629–634. doi: 10.1007/BF00202480. [DOI] [PubMed] [Google Scholar]
- Talmadge J.E., Uithoven K.A., Lenz B.F., Chirigos M. Immunomodulation and therapeutic characterization of thymosin fraction five. Cancer Immunology, Immunotherapy. 1984;18:185–194. doi: 10.1007/BF00205510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira A., Yen B., Gusella G.L., Thomas A.G., Mullen M.P., Aberg J. Prothymosin α variants isolated from CD8 + T cells and cervicovaginal fluid suppress HIV-1 replication through type I interferon induction. Journal of Infectious Diseases. 2015;211:1467–1475. doi: 10.1093/infdis/jiu643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telusma G., Datta S., Mihajlov I., Ma W., Li J., Yang H. Dendritic cell activating peptides induce distinct cytokine profiles. International Immunology. 2006;18:1563–1573. doi: 10.1093/intimm/dxl089. [DOI] [PubMed] [Google Scholar]
- Tripathi S.C., Matta A., Kaur J., Grigull J., Chauhan S.S., Thakar A. Overexpression of prothymosin alpha predicts poor disease outcome in head and neck cancer. PLoS One. 2011;6:e19213. doi: 10.1371/journal.pone.0019213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y.S., Jou Y.C., Tung C.L., Lin C.T., Shen C.H., Chen S.Y. Loss of nuclear prothymosin-α expression is associated with disease progression in human superficial bladder cancer. Virchows Archiv. 2014;464:717–724. doi: 10.1007/s00428-014-1578-6. [DOI] [PubMed] [Google Scholar]
- Tsitsiloni O.E., Stiakakis J., Koutselinis A., Gogas J., Markopoulos C., Yialouris P. Expression of alpha-thymosins in human tissues in normal and abnormal growth. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:9504–9507. doi: 10.1073/pnas.90.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsitsiloni O.E., Yialouris P.P., Sekeri-Pataryas K., Haritos A.A. Prothymosin alpha is not a nuclear polypeptide. Experientia. 1989;45:332–334. doi: 10.1007/BF01957467. [DOI] [PubMed] [Google Scholar]
- Tsitsilonis O.E., Bekris E., Voutsas I.F., Baxevanis C.N., Markopoulos C., Papadopoulos S.A. The prognostic value of alpha-thymosins in breast cancer. Anticancer Research. 1998;18:1501–1508. [PubMed] [Google Scholar]
- Ueda H. Prothymosin alpha and cell death mode switch, a novel target for the prevention of cerebral ischemia-induced damage. Pharmacology & Therapeutics. 2009;123:323–333. doi: 10.1016/j.pharmthera.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Ueda H., Fujita R., Yoshida A., Matsunaga H., Ueda M. Identification of prothymosin-alpha1, the necrosis-apoptosis switch molecule in cortical neuronal cultures. Journal of Cell Biology. 2007;176:853–862. doi: 10.1083/jcb.200608022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutsas I.F., Baxevanis C.N., Gritzapis A.D., Missitzis I., Stathopoulos G.P., Archodakis G. Synergy between interleukin-2 and prothymosin alpha for the increased generation of cytotoxic T lymphocytes against autologous human carcinomas. Cancer Immunology, Immunotherapy. 2000;49:449–458. doi: 10.1007/s002620000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutsas I.F., Pistamaltzian N., Tsiatas M.L., Skopeliti M., Katsila T., Mavrothalassiti I. Ovarian malignant ascites-derived lymphocytes stimulated with prothymosin α or its immunoactive decapeptide lyse autologous tumour cells in vitro and retard tumour growth in SCID mice. European Journal of Cancer. 2013;49:1706–1714. doi: 10.1016/j.ejca.2012.11.037. [DOI] [PubMed] [Google Scholar]
- Wara D.W., Ammann A.J. Activation of T-cell rosettes in immunodeficient patients by thymosin. Annals of the New York Academy of Sciences. 1975;249:308–315. doi: 10.1111/j.1749-6632.1975.tb29078.x. [DOI] [PubMed] [Google Scholar]
- Welsh R.M., Bahl K., Marshall H.D., Urban S.L. Type 1 interferons and antiviral CD8 T-cell responses. PLoS Pathogen. 2012;8:e1002352. doi: 10.1371/journal.ppat.1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.G., Habib N.A., Mitry R.R., Reitsma P.H., van Deventer S.J., Chamulueau R.A. Overexpression of hepatic prothymosin alpha, a novel marker for hepatocellular carcinoma. British Journal of Cancer. 1997;76:1199–1204. doi: 10.1038/bjc.1997.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.L., Shiau A.L., Lin C.S. Prothymosin alpha promotes cell proliferation in NIH3T3 cells. Life Sciences. 1997;61:2091–2101. doi: 10.1016/s0024-3205(97)00882-5. [DOI] [PubMed] [Google Scholar]
- Zhang M., Cu F., Lu S., Lu H., Jiang T., Chen J. Increased expression of prothymosin-α, independently or combined with TP53, correlates with poor prognosis in colorectal cancer. International Journal of Clinical and Experimental Pathology. 2014;7:4867–4876. [PMC free article] [PubMed] [Google Scholar]
- Zhang B.C., Sun L. Tongue sole (Cynoglossus semilaevis) prothymosin alpha: Cytokine-like activities associated with the intact protein and the C-terminal region that lead to antiviral immunity via Myd88-dependent and -independent pathways respectively. Developmental & Comparative Immunology. 2015;53:96–104. doi: 10.1016/j.dci.2015.07.004. [DOI] [PubMed] [Google Scholar]


