Abstract
The avian coronavirus causes infectious bronchitis (IB), which is one of the most serious diseases affecting the avian industry worldwide. However, there are no effective strategies for controlling the IB virus (IBV) at present. Therefore, development of novel antiviral treatment strategies is urgently required. As reported, astragalus polysaccharides (APS) have potential antiviral effects against several viruses; however, the antiviral effect of APS against IBV remains unclear. In this study, we explored whether APS had the potential to inhibit IBV infectionby utilizing several in vitro experimental approaches. To this end, the effect of APS on the replication of IBV was examined in chicken embryo kidney (CEK) cells. Viral titers were calculated by using the plaque formation assay, and the cytotoxicity of APS was tested by utilizing a Cell Counting Kit-8 assay. The expression of viral mRNA and cytokine (IL-1β, IL-6, IL-8 and TNF-α) mRNA transcripts was determined by real-time quantitative RT-PCR(qRT-PCR). IBV titers in infected CEK cells treated with APS were significantly reduced in a dose-dependent manner, indicating that APS inhibited IBV replication in vitro. We also found that the decreased viral replication after APS treatment was associated with reduced mRNA levels of the cytokines IL-1B, IL-6, IL-8 and TNF-α. In conclusion, these results suggest that APS exhibit antiviral activities against IBV and it may represent a potential therapeutic agent for inhibiting the replication of IBV.
Keywords: Astragalus polysaccharides, Infectious bronchitis, Infectious bronchitis virus, Cytokines, Antiviral effects
Highlights
-
•
This study was the first to show that APS inhibit IBV infection, in vitro, in a dose-dependent manner.
-
•
Lower viral replication after APS treatment was associated with reduced mRNA levels of the pro-inflammatory cytokines IL-1β, IL-6, IL-8, and TNF-α.
-
•
APS might serve as a potential therapeutic agent for inhibiting the replication of IBV.
1. Introduction
Avian infectious bronchitis virus (IBV), a member of the Coronaviridae family, causes mild-to-acute respiratory disease in chickens and leads to huge economic lossesin the poultry industry worldwide [1], [2]. More than 50 serotypes of IBV have been documented since the first virus was isolated from birds exhibiting respiratory symptoms in the United States in 1931 [3]. Extensive genetic diversity of IBV strains worldwide renders vaccines largely ineffective, because of poor or no cross-protection between different IBV serotypes [4], [5]. Thus, finding an effective antiviral drug or agent is imperative for the prevention of IBV infection.
The Chinese government has prohibited the use of antiviral drugs in food animals in China; thus, utilization of traditional antiviral herbs remains a major focus. Several reports have confirmed that traditional Chinese herbs effectively inhibit the replication of various viruses [6], [7], [8]. Astragalus polysaccharides (APS), isolated from a traditional Chinese medicinal herb, Astragalus mongholicus, have been widely used immunopotentiators [9], [10], [11]. Recently, several studies have shown that supplementation with APS can inhibit replication of several animal viruses, including H9N2 avian influenza virus [12], foot and mouth disease virus [13], Newcastle disease virus [14], and infectious bursal disease virus [15]. However, the effect of APS on IBV replication remains unclear. Therefore, in this study, we investigated the antiviral effects ofAPS against IBV by utilizing several in vitro approaches.
2. Materials and methods
2.1. Virus, cells, and APS
The IBV strain M41 (China Institute of Veterinary Drug Control) was adapted and propagated in chicken embryo kidney (CEK) cells. The CEK cell monolayerswere maintained in Dulbecco's modified Eagle's medium (DMEM, Gibco, USA) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA), 100 units/mL penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine in a humidified chamber, supplemented with 5% CO2, at 37 °C.
APS (net content, 95.9%) brought from Sihai Plant Extracts Co., Ltd (Nantong, China)were dissolved in deionized water and diluted to 1,5,10, 20, 30, and 50 μg/ml. The APS solution was sterilized by heat treatment (100 °C for 30 min), and then stored at −4 °C until use.
2.2. Cytotoxicity assay
Cytotoxicity was determined by using a Cell Counting Kit-8 (CCK8; Donjindo, Japan) according to the manufacturer's instructions. Briefly, the CEK cells were seeded into96-well culture plates, at a density of 1 × 104 cells/well, and incubated at 37 °C in a 5% CO2 incubator for 24 h. After washing with PBS, three times, APS at various concentrations (1, 5, 10, 20, 30, or 50 μg/mL) were added to the wells. The cells were then cultured for a further 48 h. Mock-treated cells served as controls. After washing with PBS, the CEK cells were incubated with CCK8 solution at 37 °C for 4 h. Absorbance was measured at 450 nm by using a QuantUniversal Microplate Spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA). The relative cell viability rate was determined for each concentration based on the following formula: (OD450 drug)/(OD450 control) × 100%. APS concentrations below the 50% cytostatic concentration (CC50) were defined as non-toxic concentrations [16].
2.3. Virus titration and infection
To calculate viral titers (infectivity), a plaque-formation assay was performed. Briefly, 2 × 105 CEK cells seeded into 24 wells tissue culture dishes were grown until 100% confluence, and then inoculated with serially diluted IBV (10−1-10−6). Subsequently, overlay medium (1% low-melting-point agarose with DMEM containing 10% FBS) was added to each well and further incubated at 37 °C, 5% CO2, for 72 h. The cells were subsequently stained with gentian violet (1% crystal violet, 10% formaldehyde and 5% EtOH in PBS). The virus titer was determined by counting the number of plaques formed at a specific dilution, as described by Dove et al. [17].
2.4. Treatment of infected cells with APS
To analyze the effect of APS on infected cells, CEK cell monolayers were infected with IBV at 2 × 106 plaque-forming units/ml, and subsequently incubated at 37 °C for 1 h. Cell monolayers were then washed three times with PBS, and the infected cells were treated with various concentrations of APS (1,5,10,20, or 30 μg/mL). Mock cells and infected cells represented negative and positive controls, respectively. After 24 h, CEK cell lysates were prepared for subsequent plaque assays.
2.5. Real time quantitative RT- PCR (qRT-PCR)
Genomic and subgenomic RNA levels of IBV in mock and virus-infected CEK cells treated with different concentration of APS were quantified by TaqMan real-time RT-PCR as described previously [18].
To quantify the expression of cytokines (IL-1β, IL-6, IL-8 and TNF-α), total RNA was extracted from cultured cells using Trizol reagent (Takara Biotechnology, Dalian, China) according to the manufacturer's instructions. Total RNA purity and concentration were measured by using ultraviolet spectrophotometry (Life Technologies, Carlsbad, CA, USA). The isolated RNA was digested with DNase1 (Takara Biotechnology, Dalian, China) at 37 °C for 30 min cDNA was synthesized from total RNA using a PrimeScript RT Reagent Kit (TaKaRa). Amplifications were performed with 0.5 μL cDNA, ina total volume of 10 μL, using SYBR Green Real-Time PCR MasterMix (Roche, Mortlake, Australia), in a 7900HT Fast Real-Time PCR System (Applied Biosystems, Shanghai, China), according to the manufacturer's instruction. The primers for cytokine genes and GAPDH used in this study are listed in Table 1 . Cytokine gene expression was normalized to that of GAPDH using the2−ΔΔCt method.
Table 1.
Real time PCR primers used for mRNA expression analysis.
| Target gene | Prime (5′-3′) |
|---|---|
| IL-1β | F-GGGCATCAAGGGCTACAA R-CTGTCCAGGCGGTAGAAGAT |
| IL-6 | F-AGAAATCCCTCCTCGCCAAT R-AAATAGCGAACGGCCCTCA |
| IL-8 | F-GCCCTCCTCCTGGTTTCAG R-TGGCACCGCAGCTCATT |
| IFNa | F-GACAGCCAACGCCAAAGC R-GTCGCTGCTGTCCAAGCATT |
| GAPDH | F-TGCCAACGTGTCGGTTGT R-TGTCATCATATTTGGCAGGTTT |
Abbreviations: F, forward; mRNA, messenger RNA; PCR, polymerase chain reaction; R, reverse.
2.6. Western blots
Total protein was extracted from cultured CEK cells using a modified radioimmunoprecipitation assay buffer supplemented with protease inhibitor cocktail (Beyotime, Shanghai, China). Protein concentrations were determined using a BCA protein assay kit (Pierce, Rockford, IL,USA). Equal amounts of protein were separated on a 10% SDS polyacrylamide gel and electro-transferred from the gel to a polyvinylidene fluoride (PVDF) membrane (Amersham Bioscience, USA). After blocking with 5% non-fat milk in PBS, the membrane was probed with chicken anti-nucleocapsid polyclonal antibody (diluted 1:1000) and chicken anti-GAPDH polyclonal antibody (diluted 1:5000) overnight, followed by incubation with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. GAPDH was used as the internal loading control. The protein bands were detected using a chemiluminescent substrate kit (Millipore Company, Bedford, MA, USA), according to the manufacturer's instructions.
2.7. Statistical analyses
All results are represented as mean ± standard deviation (SD) from at least three independent experiments. All statistical analysis was performed using SPSS 19 software package (SPSS Inc; Chicago, IL, USA). One-way ANOVA with Bonferroni'spost-hoc tests were performed to compare the differences among three or more groups. Differences were considered significant at ??< 0.05.
3. Results
3.1. The cytotoxic effect of APS on CEK cell proliferation
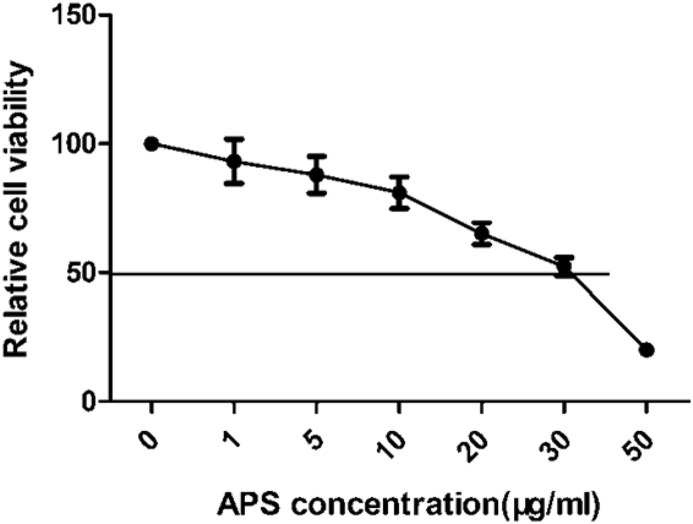
To investigate whether APS treatment affects cell viability, the toxicity of APS onCEK cells was determined using the CCK8 method. At concentrations of 5 μg/ml and 10 μg/ml, only 12.1% and 18.3% of CEK cells were killed after 48 h, respectively (Fig. 1 ). At 30 μg/ml, APS killed 47.6% of cells, whereas the viability was below 50% after treatment with APS at 50 μg/ml. These results indicate that APS did not influence cell viability at concentrations below 30 μg/ml, and therefore this concentration was chosen as the maximum concentration of APS for the antiviral assays.
Fig. 1.
The cytotoxic effect of APS treatment on CEK cells. CEK cells were treated with 0,1, 5, 10, 20, 30, or 50 μg/mL of APS for 48 h. Relative cell viability was determined by the CCK8 assay and normalized to the value of the 0 group (set at 100%).
3.2. APS inhibit IBV replication in vitro
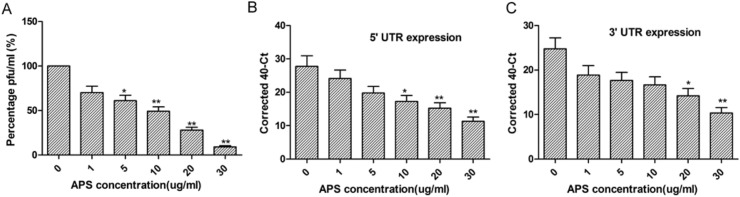
The antiviral activity of APS against IBV was determined by plaque formation assay. As showed in Fig. 2 A, virus titers significantly decreased in infected CEK cells treated with APS, in a dose-dependent manner.
Fig. 2.
APS inhibits IBV production. A. CEK cells were infected with IBV before treatment with different concentrations of APS(0,1, 5, 10, 20, or 30 μg/mL) for 24 h. Viral titers in supernatants were determined by the plaque formation assay and normalized to the value of the 0 group (set at 100%). B,C. Real-time qRT-PCR analysis of the levels of IBV genomic RNA as well as genomic and subgenomic mRNAs, asdetermined by analysis of the IBV 5′ UTR (B) and 3′UTR (C) in infected CEK cells following APS treatment. The differences between means were considered significant at *P < 0.05 and highly significant at **P < 0.01 when compared with the control groups (i.e. APS concentration, 0).
To further confirm the inhibitory effect of APS, real-time qRT-PCR was performed to measure IBV genomic and subgenomic RNA levels in mock and virus-infected CEK cells treated with different concentrations of APS. Genomic RNA (5′ UTR sets) and subgenomic mRNA (3′ UTR sets) expression was downregulated in infected CEK cells treated with APS in a dose-dependent manner (Fig. 2B and C). These data indicate that infected CEK cells treated with APS exhibit an overall reduction in viral RNA levels.
3.3. APS treatment decreases N protein in infected CEK cells in a dose-dependent manner
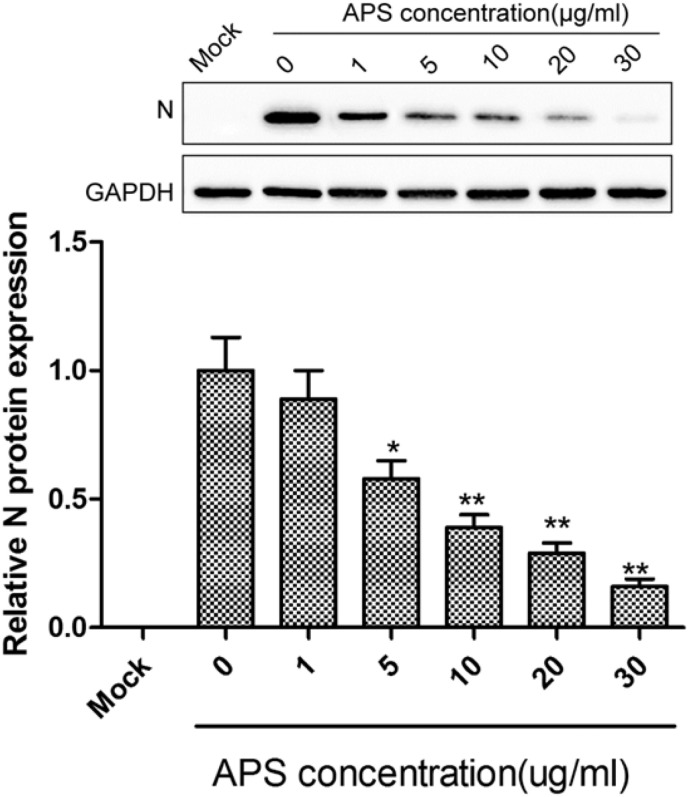
To determine the effect of APS on virus protein production, the amount of nucleocapsid (N) protein of IBV was determined by western blot. N protein is one of the most abundantly expressed viralproteins in an IBV-infected cell and has a high affinity for viral RNA [19]. Quantitation of N protein levels is a sensitive marker for viral protein production [19]. In this study, we found that N protein expression decreased in infected CEK cells treated with APS in a dose-dependent manner (Fig. 3 ), suggesting that APS can inhibit IBV replication.
Fig. 3.
APS treatment reduces N protein expression in IBV-infected CEK cells in a dose-dependent manner. Western blots were utilized to analyze the levels of N protein in infected CEK cells treated with different concentrations of APS. The differences between means were considered significant at *P < 0.05 and highly significant at **P < 0.01 when compared with the control groups (i.e. APS concentration, 0).
3.4. APS regulate the mRNA expression of cytokines
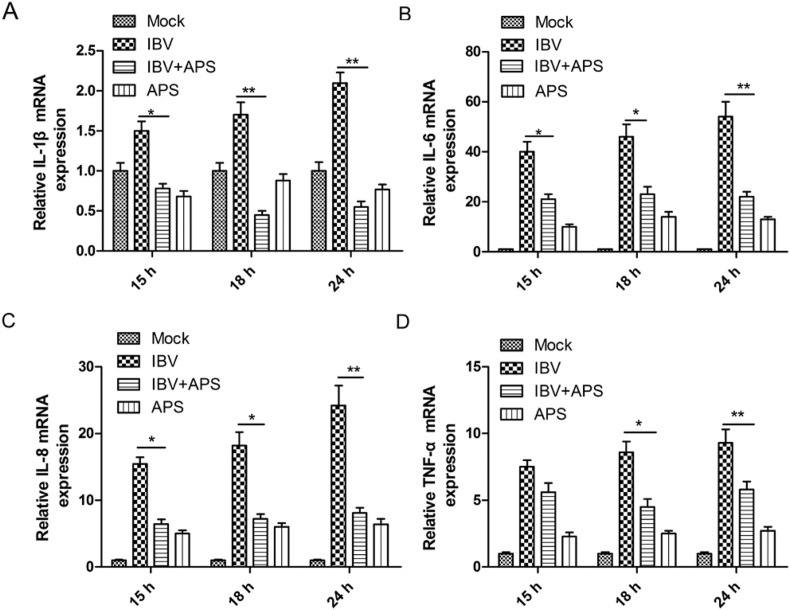
We next determined whether treatment of CEK cells with 30 μg/mL of APS affected the expression of cytokines induced by IBV infection. APS treatment significantly reduced the mRNA levels of IL-1β, IL-6, IL-8, and TNF-α, which were upregulated by IBV infection at 15, 18, and 24 h, compared to those of mock-treated groups (Fig. 4 A–D). These data demonstrate that APS inhibited inflammatory responses in IBV-infected CEK cells by decreasing the expression of pro-inflammatory cytokines.
Fig. 4.
APS reduces pro-inflammatory cytokine mRNA levels in IBV-infected CEK cells. CEK cells were infected with IBV and incubated in the presence or absence of 30 μg/ml APS. Total RNA was subsequently extracted from cell lysates at 15, 18, and 24 h after treatment. The relative mRNA expression ofIL-1β (A), IL-6(B), IL-8(C), and TNF-α (D) were assessed by using qRT-PCR. The differences between means were considered significant at *P < 0.05 and highly significant at**P < 0.01when compared with the IBV-infected control group.
4. Discussion
At present, multiple serotypes of IBV exist, and new variants regularly emerge due to frequent point mutations and recombination events in the viral genome [20], which ultimately leads to vaccine failure. Therefore, development of an effective antiviral therapy is a crucial strategy for treating IBV infection. Here, we showed that treatment of CEK cells with APS at 30 μg/mL or less did not induce significant toxicity. Furthermore, inhibition of IBV replication in CEK cells by APS occurred in a dose-dependent manner in vitro. Hence, APS have potential utility as antiviral agents against IBV.
APS, active ingredients extracted from Astragalus, possess a wide range of medicinal benefits, such as immunomodulatory [9], [10], [11], antioxidant [21], antidiabetic [22], antitumor [23], and anti-inflammatory effects [24]. Recent studies have shown that APS have an antiviral effect on several viruses [9], [10], [11], [12], [13], [14], [15], suggesting that APS have the potential to be developed and used as antiviral drugs. Our group recently reported that APS could be used as an adjuvants in IBV vaccine preparations, and provide better protection against IBV by stimulating both humoral andcellular immunity [25]. However, it remains unclear whether APS had a direct inhibitory effect on IBV. In this study, we found that APS could inhibit IBV replication, in vitro, in a dose-dependent manner. In addition, APS also regulated cytokine expression during IBV infection. These results suggested that APS could inhibit IBV replication.
It is well known that pro-inflammatory cytokines play a crucial role in avian respiratory disease progression, by coordinating and activating the adaptive immune response, which enables the host to eliminate pathogens [26]. Nii et al. reported that the expression of pro-inflammatory cytokines was higher in the IBV-infected group than in the uninfected control group, suggesting that pro-inflammatory cytokines were involved in IBV progression [27]. Several reports have shown that APS could regulate cytokine expression in various diseases. For example, Lv et al. found that treatment with APS significantly reduced mRNA expression of TNF-α, IL-6 and IL-1β in colon tissues of mice with colitis [28]. Wang et al. reported that administration of APS significantly downregulated the expression of TNF-α, IL-1β, and IL-8 (P < 0.05) in LPS-treated Caco2 cells [24]. In our study, we also found that TNF-α, IL-1β, IL-6, and IL-8 mRNA expression was significantly downregulated following treatment of IBV-infected cells with APS, suggesting that APS moderate IBV-induced inflammatory responses associated with viralreplication.
In summary, the present study was the first to show that APS inhibit IBV infection, in vitro, in a dose-dependent manner. Furthermore, lower viral replication after APS treatment was associated with reduced mRNA levels of the pro-inflammatory cytokines IL-1β, IL-6, IL-8, and TNF-α. These data suggest the potential use of APS as antiviral agents against IBV; however, further studies are required to elucidate their mechanism of action, which remains unclear.
Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Acknowledgements
This work was supported by the development program of science and technology of Jilin Province (20160411001XH, 20170204037NY), the development program of science and technology of Songyuan City (Ny2015002).
Contributor Information
Xintao Li, Email: lixintao2005@126.com.
Xinghong Wu, Email: wxhcajss@126.com.
References
- 1.Cavanagh D. Coronaviruses in poultry and other birds. Avian pathology J. WVPA. 2005;34:439–448. doi: 10.1080/03079450500367682. [DOI] [PubMed] [Google Scholar]
- 2.Raj G.D., Jones R.C. Infectious bronchitis virus: immunopathogenesis of infection in the chicken. Avian pathology J. WVPA. 1997;26:677–706. doi: 10.1080/03079459708419246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabricant J. The early history of infectious bronchitis. Avian Dis. 1998;42:648–650. [PubMed] [Google Scholar]
- 4.Cavanagh D. Coronaviruses in poultry and other birds. Avian pathology J. WVPA. 2005;34:439–448. doi: 10.1080/03079450500367682. [DOI] [PubMed] [Google Scholar]
- 5.Cook J.K., Jackwood M., Jones R.C. The long view: 40 years of infectious bronchitis research. Avian pathology J. WVPA. 2012;41:239–250. doi: 10.1080/03079457.2012.680432. [DOI] [PubMed] [Google Scholar]
- 6.Yamasaki K., Otake T., Mori H., Morimoto M., Ueba N., Kurokawa Y. Screening test of crude drug extract on anti-HIV activity. Yakugaku zasshi J. Pharm. Soc. Jpn. 1993;113:818–824. doi: 10.1248/yakushi1947.113.11_818. [DOI] [PubMed] [Google Scholar]
- 7.Chen X., Yang L., Zhang N., Turpin J.A., Buckheit R.W., Osterling C. Shikonin, a component of Chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1. Antimicrob. agents Chemother. 2003;47:2810–2816. doi: 10.1128/AAC.47.9.2810-2816.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J., Yin J., Sui X., Li G., Ren X. Comparative analysis of the effect of glycyrrhizin diammonium and lithium chloride on infectious bronchitis virus infection in vitro. Avian pathology J. WVPA. 2009;38:215–221. doi: 10.1080/03079450902912184. [DOI] [PubMed] [Google Scholar]
- 9.Dang S.S., Jia X.L., Song P., Cheng Y.A., Zhang X., Sun M.Z. Inhibitory effect of emodin and Astragalus polysaccharide on the replication of HBV. World J. gastroenterology. 2009;15:5669–5673. doi: 10.3748/wjg.15.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Chen Y., Du H., Yang J., Ming K., Song M. Comparison of the anti-duck hepatitis A virus activities of phosphorylated and sulfated Astragalus polysaccharides. Exp. Biol. Med. 2017;242:344–353. doi: 10.1177/1535370216672750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue H., Gan F., Zhang Z., Hu J., Chen X., Huang K. Astragalus polysaccharides inhibits PCV2 replication by inhibiting oxidative stress and blocking NF-kappaB pathway. Int. J. Biol. Macromol. 2015;81:22–30. doi: 10.1016/j.ijbiomac.2015.07.050. [DOI] [PubMed] [Google Scholar]
- 12.Kallon S., Li X., Ji J., Chen C., Xi Q., Chang S. Astragalus polysaccharide enhances immunity and inhibits H9N2 avian influenza virus in vitro and in vivo. J. animal Sci. Biotechnol. 2013;4:22. doi: 10.1186/2049-1891-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., Zhong Y., Li H., Zhang N., Ma W., Cheng G. Enhancement of Astragalus polysaccharide on the immune responses in pigs inoculated with foot-and-mouth disease virus vaccine. Int. J. Biol. Macromol. 2011;49:362–368. doi: 10.1016/j.ijbiomac.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Guo L., Liu J., Hu Y., Wang D., Li Z., Zhang J. Astragalus polysaccharide and sulfated epimedium polysaccharide synergistically resist the immunosuppression. Carbohydr. Polym. 2012;90:1055–1060. doi: 10.1016/j.carbpol.2012.06.042. [DOI] [PubMed] [Google Scholar]
- 15.Huang X., Wang D., Hu Y., Lu Y., Guo Z., Kong X. Effect of sulfated astragalus polysaccharide on cellular infectivity of infectious bursal disease virus. Int. J. Biol. Macromol. 2008;42:166–171. doi: 10.1016/j.ijbiomac.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Boubaker-Elandalousi R., Mekni-Toujani M., Kaabi B., Larbi I., Diouani M.F., Gharbi M. Non-cytotoxic Thymus capitata extracts prevent Bovine herpesvirus-1 infection in cell cultures. BMC veterinary Res. 2014;10:231. doi: 10.1186/s12917-014-0231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dove B., Brooks G., Bicknell K., Wurm T., Hiscox J.A. Cell cycle perturbations induced by infection with the coronavirus infectious bronchitis virus and their effect on virus replication. J. virology. 2006;80:4147–4156. doi: 10.1128/JVI.80.8.4147-4156.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison S.M., Tarpey I., Rothwell L., Kaiser P., Hiscox J.A. Lithium chloride inhibits the coronavirus infectious bronchitis virus in cell culture. Avian pathology J. WVPA. 2007;36:109–114. doi: 10.1080/03079450601156083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H., Gill A., Dove B.K., Emmett S.R., Kemp C.F., Ritchie M.A. Mass spectroscopic characterization of the coronavirus infectious bronchitis virus nucleoprotein and elucidation of the role of phosphorylation in RNA binding by using surface plasmon resonance. J. virology. 2005;79:1164–1179. doi: 10.1128/JVI.79.2.1164-1179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai M.M., Cavanagh D. The molecular biology of coronaviruses. Adv. virus Res. 1997;48:1–100. doi: 10.1016/S0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pu X., Ma X., Liu L., Ren J., Li H., Li X. Structural characterization and antioxidant activity in vitro of polysaccharides from angelica and astragalus. Carbohydr. Polym. 2016;137:154–164. doi: 10.1016/j.carbpol.2015.10.053. [DOI] [PubMed] [Google Scholar]
- 22.Jin M., Zhao K., Huang Q., Shang P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int. J. Biol. Macromol. 2014;64:257–266. doi: 10.1016/j.ijbiomac.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Tian Q.E., De Li H., Yan M., Cai H.L., Tan Q.Y., Zhang W.Y. Effects of Astragalus polysaccharides on P-glycoprotein efflux pump function and protein expression in H22 hepatoma cells in vitro. BMC complementary Altern. Med. 2012;12:94. doi: 10.1186/1472-6882-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X., Li Y., Yang X., Yao J. Astragalus polysaccharide reduces inflammatory response by decreasing permeability of LPS-infected Caco2 cells. Int. J. Biol. Macromol. 2013;61:347–352. doi: 10.1016/j.ijbiomac.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Zhang P., Wang J., Wang W., Liu X., Liu H., Li X. Astragalus polysaccharides enhance the immune response to avian infectious bronchitis virus vaccination in chickens. Microb. Pathog. 2017;111:81–85. doi: 10.1016/j.micpath.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 26.Jiang J., Kong F., Li N., Zhang D., Yan C., Lv H. Purification, structural characterization and in vitro antioxidant activity of a novel polysaccharide from Boshuzhi. Carbohydr. Polym. 2016;147:365–371. doi: 10.1016/j.carbpol.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Nii T., Isobe N., Yoshimura Y. Effects of avian infectious bronchitis virus antigen on eggshell formation and immunoreaction in hen oviduct. Theriogenology. 2014;81:1129–1138. doi: 10.1016/j.theriogenology.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Lv J., Zhang Y., Tian Z., Liu F., Shi Y., Liu Y. Astragalus polysaccharides protect against dextran sulfate sodium-induced colitis by inhibiting NF-kappacapital VE, Cyrillic activation. Int. J. Biol. Macromol. 2017;98:723–729. doi: 10.1016/j.ijbiomac.2017.02.024. [DOI] [PubMed] [Google Scholar]