Asthma is a highly variable disease, in terms of presentation, disease progression over time, and response to therapy. As such, the diagnosis and treatment of asthma in young children and adolescents is often challenging. The differential diagnosis has been described elsewhere1 and is beyond the scope of this article. At any age, recurrent lower respiratory symptoms associated with cough, wheezing, evidence of lower airway obstruction, and response to bronchodilator support a diagnosis of asthma; however, there is no single historical feature that confirms the asthma diagnosis. Comorbid allergic conditions such as allergic rhinitis and eczema are often present. Cough without wheezing is rarely associated with asthma in children.
In recent years, the National Asthma Education and Prevention Program’s Expert Panel Report 3 (EPR-3)2 and a number of studies have provided guidance to the clinician in diagnosing and treating this highly variable disease. In this article, we discuss various phenotypes of early childhood wheezing and asthma and review recent literature related to the treatment of preschool and school-aged children with asthma and wheezing disorders.
Intermittent Asthma
Intermittent asthma is, by definition, not characterized by frequent symptoms and is the classification of asthma severity for which no daily controller therapy is recommended. The EPR-3 changed the classification from “mild intermittent” to “intermittent” to emphasize that even patients who have intermittent asthma can have exacerbations of varying, and often substantial, severity.2
The heterogeneity of intermittent asthma in preschool children is exemplified by the use of several descriptors for this syndrome, including “episodic viral wheeze” and “severe intermittent wheezing.” Episodic viral wheeze is defined as wheeze in discrete episodes, usually in association with clinical evidence of a viral respiratory tract infection (typically rhinovirus, respiratory syncytial virus, coronavirus, human metapneumovirus, parainfluenza virus, and adenovirus3), with the child being well between episodes. Repeated episodes tend to occur seasonally. Episodic viral wheeze tends to occur in preschool-aged children and resolve by the age of 6 years.4 However, it may continue as episodic wheeze into school age and disappear later or change into persistent asthma.5
Children with severe intermittent wheezing often have significant morbidity in terms of symptom severity, need for oral corticosteroids, urgent care visits, and hospitalization, but lack chronic baseline symptoms consistent with the current description of persistent asthma.6 Atopic features are common in this phenotype: in one trial, 57.1% had eczema, hay fever, or food allergy, and 52.9% had at least one positive skin test to a food or aeroallergen.6
The Role of Inhaled Corticosteroids in the Management of Intermittent Asthma
It has long been appreciated that episodic viral wheeze responds poorly to prophylactic inhaled corticosteroids (ICSs).7, 8 However, the use of ICSs only during periods of respiratory illness may reduce exacerbation-related symptoms. The Acute Intervention Management Strategies (AIMS) trial included 238 children 1 to 4 years of age with moderate to severe intermittent wheezing who were randomly assigned to receive episodic therapy with either inhaled budesonide, oral montelukast, or placebo, started at the earliest signs of respiratory tract illness and used for 7 days. The budesonide and montelukast groups did not differ from the placebo group in terms of episode-free days over 1 year or in oral corticosteroid use but had statistically significant (albeit clinically modest) reductions in specific symptoms during illness, including difficulty in breathing and interference with activity.9
Ducharme et al10 examined the episodic use of high-dose inhaled fluticasone versus placebo in 129 children 1 to 6 years of age with recurrent viral wheezing. Study interventions were started at the first signs of a respiratory illness and continued until cough and wheeze subsided. Fluticasone therapy was associated with a 50% reduction in likelihood of oral corticosteroid use along with modest reductions in duration of symptoms and short-acting β-agonist (SABA) use. However, enthusiasm for these findings was tempered by weight gain and significant reductions in linear growth among children in the fluticasone group.10
It has been hypothesized that the intermittent use of ICSs during periods of respiratory illness may prevent or delay progression to persistent wheezing. The Prevention of Asthma in Childhood (PAC) Study included infants at risk of development of asthma due to maternal asthma who were randomly assigned, on a first episode of wheezing, to a 2-week treatment course with either inhaled budesonide or placebo. This treatment was repeated whenever wheezing episodes occurred, once symptoms had been present for 3 days, for 3 years thereafter. There was no significant difference between groups in any of the outcome measures, including long-term asthma prevention, nor was there any evidence of short-term reduction in wheezing episode severity.11
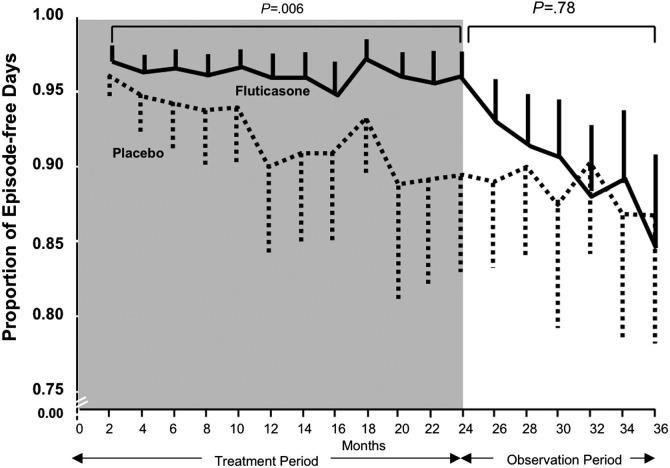
The Prevention of Early Asthma in Kids study investigated the early use of ICSs in 285 patients ages 2 to 3 years with recurrent wheezing (but not yet persistent asthma) and asthma risk factors as reflected by a positive modified Asthma Predictive Index (mAPI) (Figure 1 ). Participants were randomly assigned to receive 2 years of treatment with either fluticasone or placebo, followed by a third observation year, during which no daily study medication was given. During the 2-year treatment period, daily fluticasone therapy significantly reduced symptom frequency, exacerbations requiring oral corticosteroids, and need for additional controller medication compared with placebo (Figure 2 ). However, despite the reduction in symptom frequency and exacerbations, there was no long-term effect on symptoms after ICS discontinuation, as the two groups had comparable proportions of episode-free days during the observation year. Furthermore, ICS treatment was associated with a 1.1-cm decrease in linear growth.12 These results suggest that ICSs are effective in reducing daily symptoms and preventing exacerbations in preschool children with recurrent wheeze and a positive mAPI, but should not be used in an attempt to prevent subsequent persistent asthma in such children.
Figure 1.
The modified Asthma Predictive Index. The presence of one major criterion or two minor criteria indicates a high likelihood that the infant or child will have persistent asthma.
Adapted from The Journal of Clinical Immunology; Volume 114; Guilbert TW et al; Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma; pgs 1282-7; © 2004 with permission from Elsevier Inc.
Figure 2.
Bimonthly proportion of episode-free days during the 2-year treatment period and the observation period in the Prevention of Early Asthma in Kids (PEAK) study. Fluticasone treatment, as compared with placebo, did not increase the proportion of episode-free days during the observation year, but during the 2-year treatment period (shaded area) it significantly increased the proportion of episode-free days.
Adapted with permission from Guilbert et al; © 2006 New England Journal of Medicine.12
At the present time, there are no current data comparing the efficacy of daily versus episodic ICS therapy in preschool children with recurrent wheeze, so it is not known if one is more effective than the other. However, an ongoing trial by the Childhood Asthma Research and Education Network (clinicaltrials.gov #NCT00675584) is examining the use of daily versus episodic ICS in children with recurrent wheeze, risk factors for asthma persistence, and oral corticosteroid use in the previous year.
The Role of Systemic Corticosteroids in the Management of Acute Viral Wheezing
The EPR-3 recommends the use of oral corticosteroids for preschool children with virus-induced wheezing who present to a hospital.2 However, unlike in older children, trials specifically studying the efficacy of systemic corticosteroids in young children with acute wheezing have produced contradictory results.13, 14, 15, 16, 17 A recent study assessed the efficacy of a short course of oral prednisolone in 700 preschool age children hospitalized with virus-induced wheezing. There was no significant difference between oral prednisolone and placebo in the duration of hospitalization (the primary outcome), any of the secondary outcomes, or the number of adverse events.18 The results, along with an editorial accompanying the article,19 suggest that oral prednisolone should not be routinely given to preschool children presenting to the hospital with acute, mild-to-moderate viral wheezing. However, interpretation of these findings is complicated by a substantial proportion of children in the study who were having their first episode of wheezing because it has been convincingly demonstrated that such episodes (typically diagnosed as bronchiolitis) are poorly responsive to systemic corticosteroid therapy.20 The heterogeneity of the patient population, the relatively mild severity of the wheezing episodes and relatively low dose of prednisolone, and the short duration of hospitalization in the placebo group lend caution to the widespread and complete abandonment of oral corticosteroid use during all acute episodes of viral wheezing. Furthermore, recent studies have raised the possibility of a differential and delayed response to systemic corticosteroids as a function of the infecting virus. In two recent studies, prednisolone reduced recurrent wheezing after episodes triggered by rhinovirus but not respiratory syncytial virus,15, 21 suggesting that rhinovirus-induced early wheezing may be a criterion for the selection of young children who are likely to respond to systemic corticosteroids. The clinical utility of this information will remain to be seen, as the infecting virus is not routinely identified in clinical practice.
Management of Intermittent Asthma
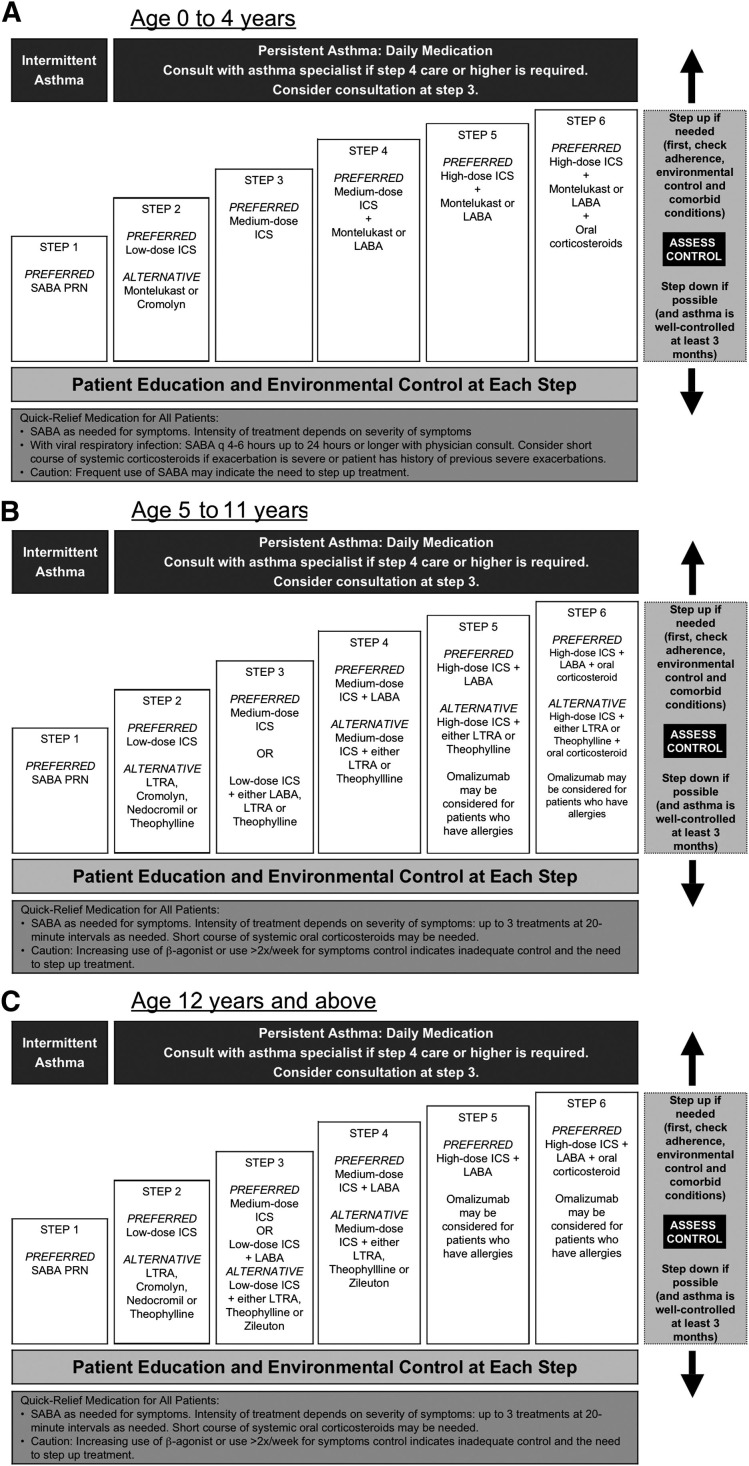
For children with intermittent asthma who have infrequent exacerbations separated by periods of no symptoms and normal pulmonary function, the goal of management should be to reduce the risk of recurrent exacerbations while minimizing adverse medication effects. As shown in step 1 of the EPR-3 (Figure 3 ), recommendations for management of intermittent asthma include therapy with a SABA as needed for symptoms and as pretreatment for patients who have exercise-induced bronchospasm. Though not included in the 2007 EPR-3 recommendations, recent evidence suggests that episodic use of ICS may be considered to decrease episode severity.
Figure 3.
The EPR-3 recommends a stepwise approach for managing asthma long-term in children A, ages 0 to 4 years B, 5 to 11 years C, and 12 years and older. PRN indicates as needed.
Adapted from the National Asthma Education and Prevention Program, Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma.2
As with all other asthma phenotypes, management plans should include the identification of triggers and events during which the child is more susceptible to an asthma flare-up (such as vigorous exercise, school opening, viral season, or specific allergen exposure) so that triggers may be avoided when possible. Parental education should focus on the early recognition of symptoms that are likely to be followed by wheezing. If used, episodic ICS treatment should begin as soon as the parent or caregiver believes the child is showing the earliest signs of an impending wheezing episode; starting ICS therapy once wheezing is established is unlikely to provide significant benefit. It is essential to treat all “colds” as asthma episodes, using asthma medications and not over-the-counter cough and cold products. Should the above strategy not prevent symptom progression, a short course of systemic corticosteroids should be considered, realizing that recent evidence provides some uncertainty as to the efficacy of their use in preschool wheezing illnesses, particularly those not requiring hospital-based care. For patients who have a history of severe exacerbations with viral respiratory tract infections, systemic corticosteroids may be considered at the first (or early) sign of infection and may be available at home. If the strategy of episodic therapy does not help the patient achieve better episode control over time, then consider a daily controller regimen.
Persistent Asthma
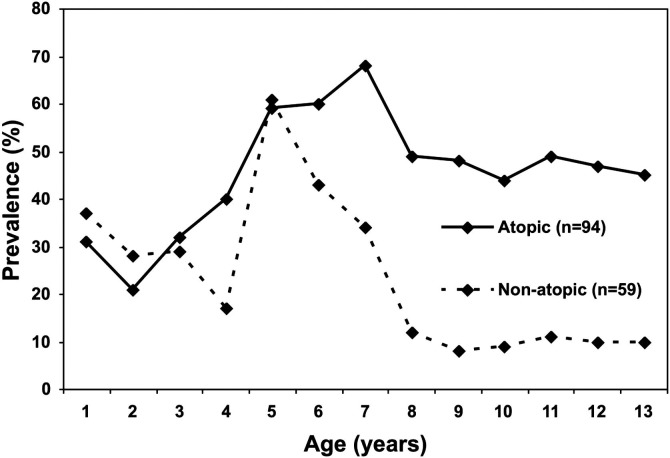
Many children diagnosed with asthma at school age will have exhibited their first symptoms during the preschool years, but the majority of children who wheeze or have symptoms of asthma before 3 years of age do not have symptoms after 6 years of age.5 Determining who will go on to have persistent asthma after early childhood wheezing is a challenge. Epidemiologic studies have demonstrated that factors determining the development of persistent asthma include early exposure and sensitization to indoor allergens, environmental tobacco smoke, residence near a heavily traveled road, exposure to elevated ozone levels, and sex (male prepuberty, female postpuberty)22, 23 (Figure 4; available at www.jpeds.com). In the clinical setting, the use of the mAPI (Figure 1) may be helpful in predicting which patients will or will not go on to have persistent disease.24
The Childhood Asthma Management Program (CAMP) and Inhaled Steroid Treatment as Regular Therapy in Early Asthma (START) studies assessed the clinical benefit of regular treatment with low-dose ICS in children diagnosed with mild persistent asthma. In the CAMP study,25 children 5 to 12 years of age were treated with either inhaled budesonide, nedocromil, or placebo for 4 to 6 years. There was no difference between groups in the change from baseline in postbronchodilator forced expiratory volume in 1 second percent (FEV1 %) of predicted. However, treatment with budesonide significantly improved airways responsiveness to methacholine, as well as resulting in fewer hospitalizations, fewer urgent visits to a caregiver, fewer courses of prednisone, and a smaller percentage of days on which SABAs or other asthma medications were needed. Furthermore, the use of budesonide had a small effect on growth (1.1 cm over 4 to 6 years), confirming the efficacy and relative safety of ICSs. However, the beneficial effect of budesonide on airways responsiveness to methacholine was lost after study medication washout, and there was no difference between groups in postbronchodilator FEV1. The CAMP data regarding the failure of asthma controller therapy to modify disease progression has prompted a shift in the field, with the most recent guidelines focusing on maximizing symptom control and quality of life rather than long-term disease remission.26
Although the CAMP study included patients with an average asthma duration of 5 years, the START study enrolled patients with asthma of more recent onset to assess early intervention with budesonide on long-term asthma control. The study consisted of 3 years of treatment with either budesonide or placebo, in addition to other usual asthma therapy, followed by a 2-year open-label period during which all participants received budesonide. In the double-blind phase, patients who received budesonide had a significant reduction in asthma exacerbations (OR, 0.57; P < .001), a longer time to first severe asthma-related event, and less of a decrease in prebronchodilator and postbronchodilator FEV1 values.27 During the open-label period, the original placebo group effectively “caught up” to the budesonide group, so that there were no differences between the two groups in terms of asthma-related events, symptoms, activity restrictions, or sleep disturbances. However, patients in the placebo group required significantly more asthma medication than the budesonide group to reach the same level of asthma control during the open-label phase, indicating a potentially diminished response to ICSs when treatment is delayed. Results from the START study suggest that early intervention with ICSs will not affect lung function but may improve overall treatment effectiveness and reduce the need for additional medication to maintain asthma control.28
The results of the CAMP and START studies have been supplemented by a recent meta-analysis of the literature regarding the efficacy of ICSs in infants and preschoolers with recurrent wheezing or asthma.29 ICS therapy was associated with a significant reduction in wheezing/asthma exacerbations, less albuterol use, and more clinical and functional improvement when compared with placebo. The benefits of ICSs were found to be independent of age, atopic condition, type of ICS, and mode of delivery but were more robust in children with a diagnosis of asthma.
Several recent studies have sought to compare controller medications and determine whether any patient factors may predict response to a particular agent. In the Pediatric Asthma Controller Trial (PACT), which included 285 children with mild to moderate persistent asthma, ICS monotherapy was superior to both ICS/long-acting β-agonist (LABA) combination and montelukast over 48 weeks.30 A post hoc multivariate analysis of the PACT data found that factors associated with the best long-term outcomes with ICS therapy included a parental history of asthma, increased exhaled nitric oxide levels, marked airways hyperresponsiveness, or a history of ICS use. The investigators were unable to identify any factor that predicted a better response to montelukast compared with fluticasone.31 Similarly, in the Characterizing Response to Leukotriene Receptor Antagonist and Inhaled Corticosteroids (CLIC) study, which also compared ICS with leukotriene receptor antagonist (LTRA), 144 children ages 6 to 17 years with mild to moderate persistent asthma using only as-needed bronchodilators took fluticasone and montelukast, each for 8 weeks, in a crossover fashion. Fluticasone was superior to montelukast in asthma control days, Asthma Control Questionnaire scores, albuterol use, and pulmonary function measures. Baseline factors predicting a more favorable response to fluticasone included fewer asthma control days, higher exhaled nitric oxide levels, greater albuterol use, and more positive aeroallergen skin test responses (potentially reflective of a greater inflammatory component). Younger age and shorter duration of disease predicted a preferential response to montelukast.32
Even though no pharmacological agent has yet been demonstrated to modify the disease process, the results of these studies suggest that ICSs are the most effective single agent for preschool and school-age children with persistent asthma, forming the basis of the EPR-3 recommendations (Figure 3). ICSs improve lung function and reduce the incidence of exacerbations, oral corticosteroid use, unscheduled provider visits, and hospitalization. Young children who are unable to use inhalers properly may benefit from budesonide aerosol administered via nebulizer, although there are no data comparing the efficacy of ICS delivery by metered-dose inhaler versus nebulization in the preschool age group. Because the benefits of ICS therapy disappear on discontinuation, compliance should be regularly assessed and reinforced.
Severe Persistent Asthma
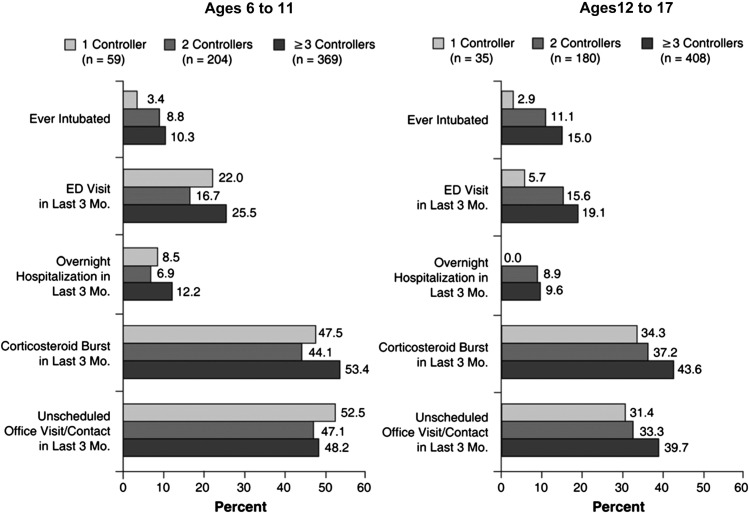
Children and adolescents with severe or difficult-to-treat asthma are an understudied population, despite the fact that most of the economic burden of asthma is related to severe disease.33 The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens observational study followed 1261 patients (ages 6 to 17 years) with severe or difficult-to-treat asthma from 2001 to 2004 in an attempt to characterize these patients and the treatment patterns and outcomes related to their asthma. Health care utilization in these patients was high, even though almost two-thirds of patients were using three or more long-term controller medications (Figure 5; available at www.jpeds.com). Assessment of age-associated and sex-associated differences revealed that height for age decreased across age groups, especially in females. In general, TENOR children and adolescents were shorter and heavier compared with national averages. Lung function (as FEV1 % predicted) decreased with increasing age. In boys, baseline prebronchodilator FEV1 % of predicted was 91 in the youngest age group (6 to 8 years) and 82 in the oldest age group (15 to 17 years); corresponding values in girls were 94 and 84, respectively.34
For patients with severe persistent asthma that is not controlled despite use of low-dose ICS, the EPR-3 currently recommends a step up to medium-dose ICS, then to medium-dose ICS plus either a LABA or montelukast. The recently published Best Add-on Therapy Giving Effective Responses (BADGER) triple-crossover study assessed 182 children with uncontrolled asthma despite treatment with low-dose ICS (fluticasone 100 μg twice daily). The study compared three different step-up therapies: the addition of salmeterol 50 μg twice daily (LABA step-up), the addition of montelukast 5 to 10 mg daily (LTRA step-up), or an increase of fluticasone to 250 μg twice daily (ICS step-up). A differential response occurred in 98% of patients, with the best response occurring significantly more frequently with LABA step-up than with LTRA or ICS step-up. Better asthma control at baseline and lack of eczema were associated with a higher probability that LABA would be the best add-on therapy. In a secondary analysis, black patients were equally likely to have a best response to LABA or ICS step-up and were least likely to have a best response to LTRA step-up, whereas white patients were most likely to have a best response to LABA. The authors caution that the best overall performance of LABA therapy should be weighed against the potential increased risks of such therapy.35
The anti-IgE monoclonal antibody omalizumab has recently been studied as add-on therapy in patients 6 to 12 years of age with persistent asthma who are inadequately controlled on medium-dose inhaled corticosteroids (>200 μg/d fluticasone propionate). A total of 627 patients with allergic asthma (all patients had at least one positive prick test to a perennial allergen and serum IgE levels ranging from 30 to 1300 IU/mL) were randomly assigned to receive either omalizumab or placebo for 52 weeks, which included a 24-week fixed ICS dose phase followed by a 28-week ICS reduction phase. In the omalizumab group, there was a 31% reduction in exacerbations during the first 24 weeks of the study and a 43% reduction over the entire 52 weeks. Omalizumab was well tolerated with no significant adverse effects.36 Omalizumab is currently FDA-approved for use in patients 12 years of age and older, in whom similar results have been seen in clinical trials.
Conclusion
Viral upper respiratory tract infections are the most common cause of wheezing in children. Many children with intermittent wheezing in the preschool years will outgrow their symptoms, and young children with a negative mAPI are most likely to have remission of symptoms by school age. Although multiple factors determine disease persistence, the primary predictor of persistent symptoms is the presence or development of an allergic diathesis.
Patients with intermittent wheezing should usually be treated with as-needed SABAs, with consideration given to short courses of inhaled or oral corticosteroids, depending on symptom severity. Controller therapy is not indicated unless episodic therapy is required 2 or more times per year or otherwise provides insufficient episode control.
For patients with persistent asthma requiring controller therapy, ICSs are the treatment of choice, with the addition of LABAs and/or LTRAs considered in selected patients. ICSs do not alter disease progression and are only beneficial when used regularly and continually.
Footnotes
B.C. is a consultant for Alcon, Sanofi-Aventis, Genentech, AstraZeneca, GlaxoSmithKline, Meda, Novartis, Sepracor, Merck-Schering, ISTA, Quintiles, and Dey; he received grant support from Alcon, Sanofi-Aventis, Genentech, AstraZeneca, GlaxoSmithKline, Novartis, Sepracor, and Merck-Schering; he is on the speaker’s bureau for Alcon, Sanofi-Aventis, Genentech, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Meda, Novartis, Pfizer, Sepracor, Merck-Schering, and ISTA; and served as an expert witness in mold litigation. L.B. received honoraria from AstraZeneca, Genentech, GlaxoSmithKline, Merck, and Aerocrine; he served on the advisory board for AstraZeneca, Merck, and GlaxoSmithKline; and received research support from the National Institutes of Health/National Heart, Lung, and Blood Institute.
Appendix
Figure 4.
Prevalence of current wheeze from birth to age 13 years in children with any wheezing episode at school age (5 to 7 years), stratified by atopy at school age.
Adapted from The Lancet; Volume 368; Illi et al; Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study; pgs 763-70; © 2006 with permission from Elsevier Inc.23
Figure 5.
Health care utilization by number of long-term controller medications in patients ages 6 to 11 years (left panel) and 12 to 17 years (right panel) in the TENOR study. Long-term controllers include inhaled corticosteroids, LABA, leukotriene modifiers, methylxanthines, and cromolyn sodium or nedocromil. ED, emergency department.
Reprinted from The Journal Allergy and Clinical Immunology; Volume 119; Chipps BE et al; Demographic and clinical characteristics of children and adolescents with severe or difficult-to-treat asthma; pgs 1156-63; © 2007 with permission from Elsevier Inc.34
References
- 1.Chipps B.E. Evaluation of infants and children with refractory lower respiratory tract symptoms. Ann Allergy Asthma Immunol. 2010;104:279–283. doi: 10.1016/j.anai.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 2.National Asthma Education and Prevention Program . National Heart, Lung, and Blood Institute; Bethesda, MD: August 2007. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Full Report 2007.www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm Available at: Accessed June 25, 2010. [Google Scholar]
- 3.Papadopoulos N.G., Kalobatsou A. Respiratory viruses in childhood asthma. Curr Opin Allergy Clin Immunol. 2007;7:91–95. doi: 10.1097/ACI.0b013e328013d501. [DOI] [PubMed] [Google Scholar]
- 4.Kurukulaaratchy R.J., Fenn M.H., Waterhouse L.M., Matthews S.M., Holgate S.T., Arshad S.H. Characterization of wheezing phenotypes in the first 10 years of life. Clin Exp Allergy. 2003;33:573–578. doi: 10.1046/j.1365-2222.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- 5.Martinez F.D., Wright A.L., Taussig L.M., Holberg C.J., Halonen M., Morgan W.J. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 6.Bacharier L.B., Phillips B.R., Bloomberg G.R., Zeiger R.S., Paul I.M., Krawiec M. Severe intermittent wheezing in preschool children: a distinct phenotype. J Allergy Clin Immunol. 2007;119:604–610. doi: 10.1016/j.jaci.2006.12.607. [DOI] [PubMed] [Google Scholar]
- 7.Kaditis A.G., Winnie G., Syrogiannopoulos G.A. Anti-inflammatory pharmacotherapy for wheezing in preschool children. Pediatr Pulmonol. 2007;42:407–420. doi: 10.1002/ppul.20591. [DOI] [PubMed] [Google Scholar]
- 8.McKean M., Ducharme F. Inhaled steroids for episodic viral wheeze of childhood. Cochrane Database Syst Rev. 2000;1 doi: 10.1002/14651858.CD001107. CD001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bacharier L.B., Phillips B.R., Zeiger R.S., Szefler S.J., Martinez F.D., Lemanske R.F., Jr. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate to severe intermittent wheezing. J Allergy Clin Immunol. 2008;122:1127–1135. doi: 10.1016/j.jaci.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ducharme F.M., Lemire C., Noya F.J., Davis G.M., Alos N., Leblond H. Pre-emptive use of high-dose fluticasone for virus-induced wheezing in young children. N Engl J Med. 2009;360:409–410. doi: 10.1056/NEJMoa0808907. [DOI] [PubMed] [Google Scholar]
- 11.Bisgaard H., Hermansen M.N., Loland L., Halkjaer L.B., Buchvald F. Intermittent inhaled corticosteroids in infants with episodic wheezing. N Engl J Med. 2006;354:1998–2005. doi: 10.1056/NEJMoa054692. [DOI] [PubMed] [Google Scholar]
- 12.Guilbert T.W., Morgan W.J., Zeiger R.S., Mauger D.T., Boehmer S.J., Szefler S.J. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 13.Csonka P., Kaila M., Laippala P., Iso-Mustajarvi M., Vesikari T., Ashorn P. Oral prednisolone in the acute management of children age 6 to 35 months with viral respiratory infection-induced lower airway disease: a randomized, placebo-controlled trial. J Pediatr. 2003;143:725–730. doi: 10.1067/S0022-3476(03)00498-0. [DOI] [PubMed] [Google Scholar]
- 14.Tal A., Levy N., Bearman J.E. Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics. 1990;86:350–356. [PubMed] [Google Scholar]
- 15.Jartti T., Lehtinen P., Vanto T., Hartiala J., Vuorinen T., Mäkelä M.J. Evaluation of the efficacy of prednisolone in early wheezing induced by rhinovirus or respiratory syncytial virus. Pediatr Infect Dis J. 2006;25:482–488. doi: 10.1097/01.inf.0000215226.69696.0c. [DOI] [PubMed] [Google Scholar]
- 16.Jartti T., Lehtinen P., Vanto T., Vuorinen T., Hiekkanen H., Hartiala J. Atopic characteristics of wheezing children and responses to prednisolone. Pediatr Pulmonol. 2007;42:1125–1133. doi: 10.1002/ppul.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oommen A., Lambert P.C., Grigg J. Efficacy of a short course of parent-initiated oral prednisolone for viral wheeze in children aged 1-5 years: randomised controlled trial. Lancet. 2003;362:1433–1438. doi: 10.1016/S0140-6736(03)14685-5. [DOI] [PubMed] [Google Scholar]
- 18.Panickar J., Lakhanpaul M., Lambert P.C., Kenia P., Stephenson T., Smyth A. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009;360:329–338. doi: 10.1056/NEJMoa0804897. [DOI] [PubMed] [Google Scholar]
- 19.Bush A. Practice imperfect: treatment for wheezing in preschoolers. N Engl J Med. 2009;360:4. doi: 10.1056/NEJMe0808951. [DOI] [PubMed] [Google Scholar]
- 20.Zorc J.J., Hall C.B. Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010;125:342–349. doi: 10.1542/peds.2009-2092. [DOI] [PubMed] [Google Scholar]
- 21.Lehtinen P., Ruohola A., Vanto T., Vuorinen T., Ruuskanen O., Jartti T. Prednisolone reduces recurrent wheezing after a first wheezing episode associated with rhinovirus or eczema. J Allergy Clin Immunol. 2007;119:570–575. doi: 10.1016/j.jaci.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chipps B.E. Determinants of asthma and its clinical course. Ann Allergy Asthma Immunol. 2004;93:309–315. doi: 10.1016/S1081-1206(10)61388-9. [DOI] [PubMed] [Google Scholar]
- 23.Illi S., von Mutius E., Lau S., Niggemann B., Gruber C., Wahn U., Multicentre Allergy Study (MAS) Group Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–770. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 24.Castro-Rodriguez J.A., Holberg C.J., Wright A.L., Martinez F.D. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403–1406. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 25.The Childhood Asthma Management Program Research Group Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 26.Strunk R.C. Childhood Asthma Management Program: lessons learned. J Allergy Clin Immunol. 2007;119:36–42. doi: 10.1016/j.jaci.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 27.Pauwels R.A., Pedersen S., Busse W.W., Tan W.C., Chen Y.-Z., START Investigators Group Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet. 2003;361:1071–1076. doi: 10.1016/S0140-6736(03)12891-7. [DOI] [PubMed] [Google Scholar]
- 28.Busse W.W., Pedersen S., Pauwels R.A., Tan W.C., Chen Y.-Z., Lamm C.J., the START Investigators Group The Inhaled Steroid Treatment as Regular Therapy in Early Asthma (START) study 5-year follow-up: effectiveness of early intervention with budesonide in mild persistent asthma. J Allergy Clin Immunol. 2008;121:1167–1174. doi: 10.1016/j.jaci.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 29.Castro-Rodriguez J.A., Rodrigo G.J. Efficacy of inhaled corticosteroids in infants and preschoolers with recurrent wheezing and asthma: a systematic review with metaanalysis. Pediatrics. 2009;123:e519–e525. doi: 10.1542/peds.2008-2867. [DOI] [PubMed] [Google Scholar]
- 30.Sorkness C.A., Lemanske R.F., Mauger D.T., Boehmer S.J., Chinchilli V.M., Martinez F.D. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. J Allergy Clin Immunol. 2007;119:64–72. doi: 10.1016/j.jaci.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 31.Knuffman J.E., Sorkness C.A., Lemanske R.F., Mauger D.T., Boehmer S.J., Martinez F.D. Phenotypic predictors of long-term response to inhaled corticosteroid and leukotriene modifier therapies in pediatric asthma. J Allergy Clin Immunol. 2009;123:411–416. doi: 10.1016/j.jaci.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeiger R.S., Szefler S.J., Phillips B.R., Schatz M., Martinez F.D., Chinchilli V.M. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol. 2006;117:45–52. doi: 10.1016/j.jaci.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Beasley R. The burden of asthma with specific reference to the United States. J Allergy Clin Immunol. 2002;109:5482–5489. doi: 10.1067/mai.2002.122716. [DOI] [PubMed] [Google Scholar]
- 34.Chipps B.E., Szefler S.J., Simons E.R., Haselkorn T., Mink D.R., Deniz Y. Demographic and clinical characteristics of children and adolescents with severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2007;119:1156–1163. doi: 10.1016/j.jaci.2006.12.668. [DOI] [PubMed] [Google Scholar]
- 35.Lemanske R.F., Mauger D.T., Sorkness C.A., Jackson D.J., Boehmer S.J., Martinez F.D. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med. 2010;362:975–985. doi: 10.1056/NEJMoa1001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lanier B., Bridges T., Kulus M., Taylor A.F., Berhane I., Vidaurre C.F. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. 2009;124:1210–1216. doi: 10.1016/j.jaci.2009.09.021. [DOI] [PubMed] [Google Scholar]