Abstract
To develop a diagnostic trial enabling the selective examination for a target cystatin in human body fluids, we attempted to prepare monoclonal antibodies against human cystatin SA1 (originally cystatin SA) and its variant form (cystatin SA2). BALB/c mice were immunized with recombinant (r-) cystatins SA1 and SA2. Two monoclonal antibodies designated Cys3F11 and Cys2E5 were selected. By ELISA analyses, the Cys2E5 was shown to react with r-cystatin SA2 but also somewhat with r-cystatin SA1 (22% cross-reactivity) and with plasma cystatin C (18% cross-reactivity), indicating a high specificity for cystatin SA2. The Cys3F11 reacted not only with r-cystatin SA1 but also with r-cystatin SA2 (89% cross-reactivity) and plasma cystatin C (47% cross-reactivity). This finding was further emphasized by immunoblotting of human submandibular–sublingual saliva samples. ELISA additivity test suggests that the two monoclonal antibodies bind to distinct epitopes. In conclusion, we have succeeded in producing two antibodies that discriminate the structural differences between salivary cystatins S and SN, which share more than 90% identity in amino acid sequence with cystatin SA.
Keywords: Cystatin SA, Saliva, Monoclonal antibody, Western blot
1. Introduction
The animal cysteine protease inhibitors belonging to the cystatin superfamily and comprised of at least three families (family 1, family 2, and family 3) are believed to regulate endogenous papain-like cysteine proteases such as the lysosomal cathepsins in order to prevent inappropriate proteolysis, which could be harmful or lethal (Turk et al., 2002). In addition to modulating such protease activities, these cystatins should also be capable of controlling the cysteine proteases released from various microorganisms and inflammatory cells (Turk et al., 2002).
The human cystatins of family 2 have been shown to consist of at least 11 members (SN, SA, S, C, D, E/M, F/leukocystatin, 8, 9/testatin, 11, and cystatin like 1 precursor protein), 10 of which are produced by the genes CST1, CST2, CST3, CST4, CST5, CST7, CST8, CST9, CST11 and CSTL1 clustered on chromosome 20p11.21(Deloukas et al., 2001; GenBank no. NG000839)—the cystatin gene family (Saitoh et al., 1987). Cystatin E/M, however, is produced by the CST6 gene on chromosome 11p13 (Stenman et al., 1997). Cystatins SN, SA, and S are predominantly expressed in human submandibular gland and sublingual gland (Isemura et al., 1984, Isemura et al., 1986, Isemura et al., 1987, Isemura et al., 1991, Saitoh and Isemura, 1993); however, cystatin D is found in the parotid gland (Freije et al., 1993). Cystatin C and cystatin E/M are widely expressed ubiquitously in various human tissues (Abrahamson, 1994, Abrahamson et al., 2003, Sotiropoulou et al., 1997, Ni et al., 1997), while cystatin F (leukocystatin) is abundant in spleen and peripheral blood leukocytes (Ni et al., 1998, Halfon et al., 1998). Three recently discovered inhibitors (cystatins 8, 9, and 11) are predominantly expressed in the male reproductive tract (Cornwall et al., 1999, Eriksson et al., 2002, Hamil et al., 2002).
In human saliva, five cystatins (S, SA, SN, C, and D) have been identified (Saitoh and Isemura, 1993, Freije et al., 1993, Abrahamson, 1994). Cystatins in saliva have been shown to inhibit the growth of microorganisms such as Porphyromonas gingivalis and infectious viruses including coronavirus, poliovirus, and herpes simplex virus, suggesting that salivary cystatins may play a role as defense factors (Blankenvoorde et al., 1996, Abrahamson et al., 2003). Furthermore, cystatins of this class have been demonstrated not only to induce interleukin-6 production by human gingival fibroblast via its surface molecules (Kato et al., 2000, Kato et al., 2002) but also interferon gamma expression in CD4 positive T cells (Kato et al., 2004). Defining levels of a target cystatin in human body fluids and detecting a specific cystatin in tissues are helpful tools for investigating the physiological roles of each cystatin. In the course of studying the roles of family 2 cystatin, we conceived of producing highly specific monoclonal antibodies that could discriminate the structural differences between human salivary cystatins S, SN, and SA. These promise to provide a clinical trail for the cystatins.
2. Materials and methods
2.1. Cystatins
Recombinant cystatin (r-cystatin) SA1 (originally cystatin SA), r-cystatin SA2 (a variant of cystatin SA harboring two amino acid substitutions: 59Gly→Asp, 120Glu→Asp) (Shintani et al., 1994, Haga and Minaguchi, 1999), and r-cystatin S were produced as described (Saitoh et al., 1998, Saitoh and Isemura, 1994). Cystatin A purified from human placenta and cystatin C from human plasma were purchased from BioPur AG, Bubendorf, Switzerland. Recombinant cystatin D and r-cystatin E/M were obtained from R & D Systems, Inc., Minneapolis, MN, USA. Chicken egg white cystatin was purchased from Sigma Chemical Co., St. Louis, MO, USA.
2.2. Preparation of murine monoclonal antibodies
Female BALB/c mice, 5 weeks of age, were immunized with r-cystatin SA1 or r-cystatin SA2 as the immunogen. For the first immunization, they were subcutaneously administered 0.3 ml of either immunogen (0.4 mg/ml) emulsified with an equal volume of Freund's incomplete adjuvant (Difco Laboratories, Detroit, MI, USA). Thirty days later, the mice were given the same immunogen intraperitoneally; in all, five booster administrations were given. Three days after the final immunization, the mice were bled, and the sera were separated by centrifugation. The reactivities and titers of antisera to cystatin SA1 or SA2 were confirmed by enzyme-linked immunosorbent assay (ELISA). Hybridomas were produced by the polyethylene glycol (PEG 4000; Sigma Chemical) fusion of SP2 murine myeloma cells with splenocytes from the immunized mice as described previously (Kato et al., 1989). To produce antibody against cystatins SA1 and/or SA2, viable hybridomas were screened by ELISA and then cloned twice by the limiting dilution method. Monoclonal antibody isotypes were determined by using a monoclonal subisotyping kit (American Qualex Company, San Clemente, CA, USA). The reactivities of the monoclonal antibodies were confirmed by an ELISA system. Large quantities of the monoclonal antibodies were produced by intraperitoneal injection of hybridoma cells into pristane-treated BALB/c mice. After 7–14 days, the ascites containing high concentrations of antibodies, were harvested. All of this study followed “A Guideline for the Treatment of Experimental Animals in Tokyo Dental College”.
2.3. Enzyme-linked immunosorbent assay (ELISA)
To elucidate the cross-reactivity of polyclonal and monoclonal antibodies against r-cystatins SA1 or SA2, the recombinant and authentic cystatins were used as coating antigens. These cystatins were diluted in phosphate-buffered saline (PBS; pH 7.2) to a final concentration of 10 μg/ml. The wells of 96-well microtiter plates (Corning Glass Works, Corning, NY, USA) were coated with 50 μl of antigen solution and incubated overnight at 4 °C. Plates were washed twice with PBS 0.05% Tween 20 (PBS-T), blocked for 1 h with 3% skim milk (Difco Laboratories) at 37 °C, and washed. Antiserum serially diluted with PBS or monoclonal antibody was added to each well; plates were incubated for 1 h at 37 °C and washed with PBS-T. The plates were incubated for 1 h with peroxidase-conjugated anti-mouse immunoglobulins (Cappel Laboratories, Cochranville, PA, USA), washed, and developed with 200 μl of o-phenylenediamine dihydrochloride (Wako Pure Chemical Industries, Ltd., Osaka, Japan) substrate solution containing hydrogen peroxide. After the reaction was stopped by the addition of 50 μl of 3 M H2SO4, the optical densities (OD) were measured at 490 nm using a microplate reader (model 3550, Bio-Rad Laboratories, Hercules, CA, USA).
2.4. ELISA additivity test
In order to test the monoclonal antibodies recognize different epitopes, an ELISA additivity test was carried out as described by Friguet et al. (1983). The r-cystatin SA2 was first coated onto a 96-well microtiter plate (Corning Glass Works). Two monoclonal antibodies were then added either separately or simultaneously, and the amount of bound antibody was quantitatively measured. Additivity of the bound activity is observed when the monoclonal antibodies bind to distinct epitopes. To quantitate the experimental results of the additivity test, an additivity index (AI) has been defined for a pair of antibodies as:
where A 1, A 2 and A 1+2 are the OD in the ELISA, with the first monoclonal antibody alone, the second monoclonal antibody alone, and the two antibodies together. When the two antibodies bind randomly at the same site, AI will be equal to zero.
2.5. Western blotting analysis
For the examination of the cross-reactivity of the polyclonal and monoclonal antibodies with S-type cystatins by Western blotting analysis, basic slab polyacrylamide gel electrophoresis was employed as described (Shintani et al., 1994). Human submandibular–sublingual saliva was collected by means of a universal cup (Shintani et al., 1994). The saliva samples were dissolved in the loading buffer and applied onto the gels. Following the gel electrophoresis, the separated proteins were transferred to nylon membranes (Millipore Co., Bedford, MA, USA) using Trans-Blot SD (Bio-Rad Laboratories). Each membrane was washed with PBS-T three times for 15 min and then placed in a blocking solution of PBS-3% skim milk and incubated for 1 h at room temperature. The membrane was incubated with the murine monoclonal antibodies or antisera overnight. After being probed with the antibody and washed with PBS-T, the membrane was incubated with horseradish peroxidase-conjugated anti-mouse immunoglobulins (Cappel Laboratories). Finally, the membrane was developed.
3. Results and discussion
The anti-r-cystatin SA1 mouse antiserum (polyclonal) showed a strong cross-reactivity with cystatins of family 2 such as r-cystatin SA2, r-cystatin S, and plasma cystatin C or egg white cystatin, but not with cystatin A from human placenta (family 1 cystatin), as shown in Fig. 1 . The antiserum against r-cystatin SA2 (polyclonal) revealed almost the same cross-reactivity as the antiserum mentioned above. To obtain highly specific antibodies for human cystatin SA1 or its variant (SA2), we attempted to produce monoclonal antibodies using r-cystatin SA1 or r-cystatin SA2. Eventually, we were able to select two hybridomas harboring the monoclonal antibodies for r-cystatin SA1 and r-cystatin SA2, which are designated, respectively, as Cys3F11and Cys2E5 (see Table 1 ). By ELISA, the antibody Cys2E5 (IgG1 isotype) was found to show a strong reactivity with r-cystatin SA2 and a weak cross-reactivity with r-cystatin SA1 (22% cross-reactivity) and plasma cystatin C (18% cross-reactivity). The Cys3F11 (IgG1 isotype) was demonstrated to react not only with r-cystatin SA1 but also with r-cystatin SA2 (89% cross-reactivity) and plasma cystatin C (47% cross-reactivity). Neither monoclonal antibody reacted with any other cystatin tested, including r-cystatins S, SN, D, and E/M or egg white cystatin, as shown in Table 1. The epitope specificity of the two monoclonal antibodies was then analyzed by ELISA additivity test. This assay requires that the antigen be saturated with each antibody tested. Accordingly, we determined the lowest concentration of each antibody at which saturation was achieved and used concentrations to perform ELISA with single and with mixed pair of antibodies. As shown in Table 2 , AI for Cys3F11 and Cys2E5 was 64%. This result shows that the bindings of the two monoclonal antibodies are additive, suggesting that these antibodies bind to distinct epitopes.
Fig. 1.
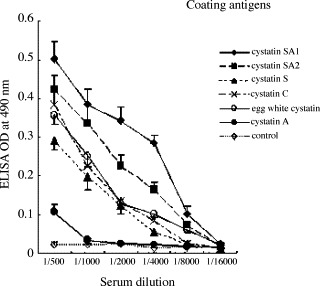
ELISA reactivities of mouse antiserum against r-cystatin SA1. The recombinant and native cystatins were used as coating antigens. The wells of 96-well microtiter plates were coated with 10 μg of either antigen. Antiserum was serially diluted with PBS.
Table 1.
Comparison of the percent cross-reactivity of monoclonal antibodies for S-type cystatins determined by ELISA
| Antibodies | Cystatins (coating antigens) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| SA1 | SA2 | S | SN | C | D | E/M | EWC | A | |
| Cys2E5/SA2a | 22 | 100 | – | – | 18 | – | – | – | – |
| Cys3F11/SA1a | 100 | 89 | – | – | 47 | – | – | – | – |
| MAB1285/SNb | – | N | 50 | 100 | – | – | – | N | – |
| MAB1201/SAb | 100 | N | 100 | 100 | – | – | – | N | – |
| MAB1296/Sb | 100 | N | 100 | – | – | – | – | N | – |
Data are from two duplicate experiments. EWC, egg white cystatin; (–), does not show cross-reactivity; N, not shown.
Present study.
Monoclonal antibodies produced by R & D Systems, Inc.
Table 2.
Additivity index for Cys2E5 and Cys3F11 monoclonal antibodies
| Ascites and dilutions | ELISA OD | Additivity index (AI) |
|---|---|---|
| Cys2E5 1:100 | 0.421 | – |
| Cys3F11 1:200 | 0.534 | – |
| Cys2E5 1:100 + Cys3F11 1:200 | 0.785 | 64 |
Cystatin SA2 was used as a coating antigen.
Ascites dilutions correspond to the lowest concentrations at which saturation of antigen was achieved.
The additivity index (AI) is shown in %.
AI = [{2A1+2/(A1 + A2)}−1] × 100, where A1 and A2 are the OD of each antibody alone and A1+2 is the OD obtained with the two antibodies in the same reaction.
Generally, the immunoblots of saliva samples by native gel with anti S-type cystatin antisera give five positive bands (for homozygotes of cystatin SA1 or SA2) or six bands (for the heterozygote of SA1 and SA2), as shown in Fig. 2 . Fig. 2 (lanes A and B) clearly demonstrated that immunoblots with monoclonal antibodies do not provide positive signals for non-phosphorylated cystatin S, mono-phosphorylated cystatin S, di-phosphorylated cystatin S, or cystatin SN. The immunoblot with Cys3F11 showed two positive bands for cystatins SA1 and SA2 with almost the equal intensity; however, Cys2E5 formed a strongly reacting band with cystatin SA2 and a faint band (indicated by the arrowhead) with cystatin SA1, as seen in Fig. 2. These observations emphasize that the monoclonal antibody, Cys2E5, can discriminate the structural differences between cystatins SA1 and SA2, both of which are encoded by the CST2 locus. Neither monoclonal antibody showed a positive reaction with cystatin D in the immunoblot by acidic polyacrylamide gel electrophoresis (data not shown).
Fig. 2.
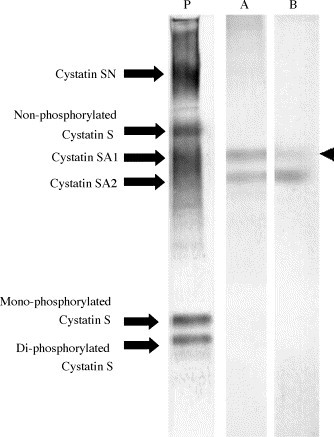
Detection of S-type cystatins by Western blot. Western blot of human submandibular–sublingual saliva was performed as described by Shintani et al. (1994).
The first antibodies used are: P; anti-r-cystatin SA1 antiserum, A; Cys3F11, B; Cys2E5.
Recently, monoclonal antibodies have been produced from a hybridoma resulting from the fusion of a mouse immunized with r-human S-type cystatins expressed with a carboxyl terminal 10× His-Tag in a mouse myeloma cell line, NS0 (R & D Systems, Inc., catalog nos. MAB1296 for r-cystatin S, MAB1285 for r-cystatin SN, and MAB1201 for cystatin SA). As compared in Table 1, the commercially available antibodies do not show the ability to select r-human S-type cystatins. However, all the commercial monoclonal antibodies do discriminate the structural differences between r-human cystatin C and r-human S-type cystatins. It is noteworthy that the cross-reactivity of two monoclonal antibodies, Cys2E5 and MAB1201, is quite different.
Taken together, it can be concluded that there may be common epitopes and specific epitopes for the cystatins of this family.
Acknowledgement
This study was supported by Oral Health Science Center Grant 5A04 from Tokyo Dental College.
References
- Abrahamson M. Cystatins. Methods Enzymol. 1994;244:685–700. doi: 10.1016/0076-6879(94)44051-4. [DOI] [PubMed] [Google Scholar]
- Abrahamson M., Alvarez-Fernadez M., Nathanson C.M. Cystatins. Biochem. Soc. Symp. 2003;70:179–199. doi: 10.1042/bss0700179. [DOI] [PubMed] [Google Scholar]
- Blankenvoorde M.F.J., Henskens Y.M.C., Van’t Hof W., Veerman E.C.I., Nieuw Amerongen A.V. Inhibition of the growth and cysteine proteinase activity of Porphyromonas gingivalis by human salivary cystatin S and chicken cystatin. Biol. Chem. 1996;377:847–850. [PubMed] [Google Scholar]
- Cornwall G.A., Hsia N., Sutton G. Structure, alternative splicing and chromosomal localization of the cystatin-related epididymal spermatogenic gene. Biochem. J. 1999;340(Pt. 1):85–93. [PMC free article] [PubMed] [Google Scholar]
- Deloukas P., Matthews L.H., Ashurst J., Burton J.G.R. The DNA sequence and comparative analysis of chromosome 20. Nature. 2001;414:865–871. doi: 10.1038/414865a. [DOI] [PubMed] [Google Scholar]
- Eriksson A., Töhönen V., Wedell A., Nordqvist K. Isolation of the human testatin gene and analysis in patients with abnormal gonadal development. Mol. Hum. Reprod. 2002;8:8–15. doi: 10.1093/molehr/8.1.8. [DOI] [PubMed] [Google Scholar]
- Freije J.P., Balbín M., Abrahamson M., Verasco G., Dalboge H., Grubb A., Lópenz-Otín C. Human cystatin D. cDNA cloning, characterization of the Escherichia coli expressed inhibitor, and identification of the native protein in saliva. J. Biol. Chem. 1993;268:15,737–15,744. [PubMed] [Google Scholar]
- Friguet B., Djavadi-Ohaniance L., Pages J., Bussard A., Goldberg M. A convenient enzyme-linked immunosorbent assay for testing whether monoclonal antibodies recognize the same antigenic site, application to hybridomas specific for the ß2-subunit of Escherichia coli tryptophan synthase. J. Immunol. Methods. 1983;60:351–358. doi: 10.1016/0022-1759(83)90292-2. [DOI] [PubMed] [Google Scholar]
- Haga T., Minaguchi K. Sequence variations of the CST2 gene related to the polymorphism of salivary cystatin SA. J. Dent. Res. 1999;78:835–839. doi: 10.1177/00220345990780040301. [DOI] [PubMed] [Google Scholar]
- Halfon S., Ford J., Foster J., Dowling L., Lucian L., Sterling M., Xu Y., Weiss M., Ikeda M., Liggett D., Helms A., Caux C., Lebecque S., Hannum C., Menon S., McClanahan T., Gorman D., Zurawski G. Leukocystatin, a new class II cystatin expressed selectively by hematopoietic cells. J. Biol. Chem. 1998;273:16,400–16,408. doi: 10.1074/jbc.273.26.16400. [DOI] [PubMed] [Google Scholar]
- Hamil G.K., Liu Q., Sivashanmugam P., Yenugu S., Soundararajan R., Grossman G., Richardson R.T., Zhang Y.-L., O’rand M.G., Petrusz P., French F.S., Hall S.H. Cystatin 11: a new member of the cystatin type 2 family. Endcrinology. 2002;143:2787–2796. doi: 10.1210/endo.143.7.8925. [DOI] [PubMed] [Google Scholar]
- Isemura S., Saitoh E., Ito S., Isemura M., Sanada K. Cystatin S: a cysteine proteinase inhibitor of human saliva. J. Biochem. 1984;96:1311–1314. doi: 10.1093/oxfordjournals.jbchem.a134952. [DOI] [PubMed] [Google Scholar]
- Isemura S., Saitoh E., Sanada K. Characterization of a new cysteine proteinase inhibitor of human saliva, cystatin SN, which is immunologically related to cystatin S. FEBS Lett. 1986;198:145–149. doi: 10.1016/0014-5793(86)81201-7. [DOI] [PubMed] [Google Scholar]
- Isemura S., Saitoh E., Sanada K. Characterization of a new cysteine proteinase inhibitor (cystatin SA) structurally closely related to cystatin S, from human whole saliva. J. Biochem. 1987;102:693–704. doi: 10.1093/oxfordjournals.jbchem.a122107. [DOI] [PubMed] [Google Scholar]
- Isemura S., Saitoh E., Sanada K., Minakata K. Identification of full-sized forms of salivary (S-type) cystatins (cystatin SN, cystatin SA, cystain S, and two phosphorylated forms of cystain S) in human whole saliva and determination of phosphorylation site. J. Biochem. 1991;110:648–654. doi: 10.1093/oxfordjournals.jbchem.a123634. [DOI] [PubMed] [Google Scholar]
- Kato T., Takazoe I., Okuda K. Structual analysis of lipopolysaccharides from Eikenella corrodens by use of murine monoclonal antibodies. Infect. Immun. 1989;57:656–659. doi: 10.1128/iai.57.2.656-659.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Imatani T., Miura T., Minaguchi K., Saitoh E., Okuda K. Cytokine inducing activity of family 2 cystatins. Biol. Chem. 2000;381:1143–1147. doi: 10.1515/BC.2000.141. [DOI] [PubMed] [Google Scholar]
- Kato T., Imatani T., Minaguchi K., Saitoh E., Okuda K. Salivary cystatins induce interleukin-6 expression via cell surface molecules in human gingival fibroblasts. Mol. Immunol. 2002;39:423–430. doi: 10.1016/s0161-5890(02)00144-x. [DOI] [PubMed] [Google Scholar]
- Kato T., Ito T., Imatani T., Minaguchi K., Saitoh E., Okuda K. Cystatin SA, a cysteine proteinase inhibitor, induces gamma interferon expression in CD4 positive T cells. Biol. Chem. 2004;385:419–422. doi: 10.1515/BC.2004.047. [DOI] [PubMed] [Google Scholar]
- Ni J., Abrahamson M., Zhang M., Fernadez M.A., Grubb A., Su J., Yu G.L., Li Y., Parmelee D., Xing L., Coleman T.A., Gentz S., Thotakura R., Nguyen N., Hesselberg M., Gentz R. Cystatin E is a novel human cysteine protease inhibitor with structural resemblance to family 2 cystatins. J. Biol. Chem. 1997;272:10,853–10,858. doi: 10.1074/jbc.272.16.10853. [DOI] [PubMed] [Google Scholar]
- Ni J., Fernandez M.A., Danielsson L., Chillakuru R.A., Zhang J., Grubb A., Su J., Gentz R., Abrahamson M. Cystatin F is a glycosylated human low molecular weight cysteine protease inhibitor. J. Biol. Chem. 1998;273:24,797–24,804. doi: 10.1074/jbc.273.38.24797. [DOI] [PubMed] [Google Scholar]
- Saitoh E., Kim H.-S., Smithies O., Maeda N. Human cysteine proteinase inhibitors: nucleotide sequence analysis of three members of the cystatin gene family. Gene. 1987;61:329–338. doi: 10.1016/0378-1119(87)90196-x. [DOI] [PubMed] [Google Scholar]
- Saitoh E., Isemura S. Molecular biology of human salivary cysteine proteinase inhibitors. Crit. Rev. Oral Biol. Med. 1993;4:487–493. doi: 10.1177/10454411930040033301. [DOI] [PubMed] [Google Scholar]
- Saitoh E., Isemura S. Production of human salivary type cysteine proteinase inhibitors (cystatins) by an Escherichia coli system and partial characterization of recombinant cystatin S and its mutant (117arginine → tryptophan) J. Biochem. 1994;116:399–405. doi: 10.1093/oxfordjournals.jbchem.a124538. [DOI] [PubMed] [Google Scholar]
- Saitoh E., Minaguchi K., Ishibashi O. Production and characterization of two variants of human cystatin SA by two alleles at the CST2 locus of the type 2 cystatin gene family. Arch. Biochem. Biophys. 1998;352:199–206. doi: 10.1006/abbi.1997.0609. [DOI] [PubMed] [Google Scholar]
- Shintani M., Minaguchi K., Isemura S., Saitoh E., Sanada K., Semba T. Genetic polymorphisms of the CST2 locus coding for cystatin SA. Hum. Genet. 1994;94:45–49. doi: 10.1007/BF02272840. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou G., Anisowicz A., Sager R. Identification, cloning, and characterization of cystatin M, a novel cysteine proteinase inhibitor, down regulated in breast cancer. J. Biol. Chem. 1997;272:903–910. doi: 10.1074/jbc.272.2.903. [DOI] [PubMed] [Google Scholar]
- Stenman G., Astrom A.K., Roijer E., Sotiropoulou G., Zhang M., Sager R. Assignment of a novel cysteine protease inhibitor (CST6) to 11q13 by fluorescence in situ hybridization. Cytogenet. Cell Genet. 1997;76:45–46. doi: 10.1159/000134512. [DOI] [PubMed] [Google Scholar]
- Turk B., Turk D., Salvesen G.S. Regulating cysteine protease activity: essential role of protease inhibitors as guardians and regulators. Curr. Pharm. Des. 2002;8:1623–1637. doi: 10.2174/1381612023394124. [DOI] [PubMed] [Google Scholar]


