Abstract
Advanced age often is associated with functional and immunologic decline and chronic cardiopulmonary diseases that predispose to pneumonia when viral infection occurs. Influenza virus remains the primary viral pathogen in the elderly, although the impact of the other respiratory viruses remains to be defined. The clinical syndromes associated with respiratory viruses frequently are indistinguishable from one another or bacterial pathogens; often, viral illness in older adults exacerbates underlying conditions, complicating diagnosis. Antiviral therapy is available for influenza A and B; specific viral diagnosis, particularly with the use of rapid antigen detection, may be useful for clinical management. Treatment for other viruses primarily is supportive.
Respiratory viruses are ubiquitous pathogens that cause acute respiratory illnesses in all age groups [1]. Viral pneumonia occurs most frequently at the extremes of age—in young children and elderly adults. When respiratory viruses first are encountered by children who do not have immunity, particularly during infancy when airways are small and immature, pneumonia may result. Although infection in older adults represents reinfection, viruses again become a more frequent cause of pneumonia because of immunosenescence and the presence of chronic medical conditions. Influenza virus is the viral pathogen that is most well recognized in older adults and is the cause of significant morbidity and mortality in this age group [2], [3]. In addition, several common respiratory viruses are implicated in the etiology of pneumonia in older adults ( Table 1). Because of the fastidious nature of several of these viruses and imperfect diagnostic tools, the burden of disease likely is underestimated. Newer investigations using reverse transcription polymerase chain reaction (RT-PCR) and sensitive serologic techniques define the frequency of these agents in adults more accurately. Several other systemic viruses (see Table 1) also can cause pneumonia. Most are uncommon in immunocompetent older adults because of pre-existing immunity. Although viral pneumonia cannot be distinguished reliably on clinical grounds alone, some features can help clinicians judge the likelihood of viral infection and the need for laboratory testing ( Table 2). The focus of this review is on the role of the common respiratory viruses in immunocompetent older adults who have community-acquired pneumonia, with a brief mention of other viruses that cause pneumonia less frequently. Epidemiology, clinical features, methods of diagnosis, and treatments are discussed.
Table 1.
Viral causes of pneumonia
| Respiratory viruses | Other viruses |
|---|---|
| Influenza viruses A and B | Herpes simplex virus type 1 |
| RSV | Cytomegalovirus |
| PIVs 1–4 | Varicella-zoster virus |
| hMPV | Epstein-Barr virus |
| Coronaviruses (CO43, 229, SARS, HKU1, and NL63) | Hantavirus |
| Adenoviruses | |
| Rhinoviruses | |
| Human bocaviruses | |
| Coxsackie viruses |
Table 2.
Common respiratory viruses
| Virus | Season | Clinical clues | Incubation | Treatment |
|---|---|---|---|---|
| Influenza | Winter | Abrupt onset, fever, myalgias | 1–2 days | Oseltamivir, aanamivir |
| RSV | Late fall to late winter | Rhinorrhea, wheezing | 2–8 days | Aerosolized ribavirina |
| hMPV | Late winter | Nonspecific | 5–6 days | None available |
| PIV | Fall to spring | Hoarseness | 2–8 days | None available |
| Coronaviruses | Winter | Nonspecific | 1–3 days | None available |
| Rhinoviruses | All year, fall | Rhinorrhea | 8 hours to 2 days | None available |
Not approved for use in adults.
Epidemiology
The viral contribution to community-acquired pneumonia in adults is difficult to define precisely, because diagnostic tools generally are insensitive. In published series of adults, specifically the elderly, rates of viral pneumonia vary tremendously depending on the type of tests used, populations, and season studied. Viruses are identified in 0.3% to 30% of patients who have community-acquired pneumonia in studies using viral culture and serology for diagnosis ( Table 3) [4], [5]. Recent studies using RT-PCR for diagnosis have improved the ability to detect viruses considerably [6], [7]. In a study of 105 patients who had community-acquired pneumonia, 50% of whom were over age 60, respiratory viruses were detected in 14% of patients using conventional techniques compared with 56% by RT-PCR [7]. In all studies of the etiology of community-acquired pneumonia, regardless of the diagnostic techniques used, influenza A is the viral pathogen identified most commonly, accounting for 4% to 19% of cases [5], [6], [7], [8], [9], [10], [11], [12].
Table 3.
Studies of community-acquired pneumonia, including viral diagnostics
| Year | Location | Methods | Age (years) | % Viral | Most common virus | Second most common virus |
|---|---|---|---|---|---|---|
| 1993–1995 | Spain [9] | Serology | 54 ± 21 | 14 | Influenza | RSV/PIV |
| 1998–1999 | England [12] | Serology | 65 ± 20 | 23 | Influenza | RSV |
| 1998–2000 | Japan [11] | Serology | >65 | 13 | NA | NA |
| 1996–2001 | Spain [8] | Serology | 68 ± 18 | 18 | Influenza | PIV |
| 200–2002 | USA [36] | Serology | 61 | 10 | Influenza | RSV |
| 2000–2002 | Netherlands [7] | Culture, serology, PCR | >60 | 46 | Coronavirus/rhinovirus | Influenza |
| 2002–2004 | Netherlands [6] | Culture, RT-PCR | 64 ± 16 | 29 | Influenza | Coronavirus |
| 2003 | Belgium [5] | Culture, serology | 82 ± 7 | 30 | Influenza | RSV |
| 2003–2004 | Spain [10] | Antigen, culture, RT-PCR | NA | 23 | Influenza | Rhinovirus/adenovirus |
Specific viruses
Influenza virus
Influenza viruses are segmented RNA viruses that are classified as A, B, or C, based on stable internal proteins [13]. Influenza viruses A and B are the most significant human pathogens and are capable of causing serious lower respiratory tract disease, whereas influenza C generally causes mild upper respiratory tract disease. Influenza A viruses are classified further based on the two surface envelope proteins, hemagglutinin (H) and neuraminidase (N). These glycoproteins are the primary targets of neutralizing antibody. At least 16 antigenically distinct hemagglutinin (H1–H16) and nine neuraminidase (N1–N9) proteins are described. H1–H3 viruses currently are the primary pathogens in humans, whereas the others generally are found in other mammals and aquatic birds. As with most RNA viruses, influenza A viruses are prone to a high degree of genomic mutations during replication, which leads to minor changes in the H and N proteins. These changes are referred to as antigenic “drift” and account for seasonal influenza epidemics. Because influenza A has a segmented genome, two influenza viruses can exchange H or N genes, with resultant viruses containing a completely new H or N gene. This phenomenon is referred to as antigenic “shift” and may result in worldwide pandemics. During the past century, three pandemics occurred: 1918 (H1N1), 1957 (H2N2), and 1968 (H3N2). Currently, two type A viruses circulate (H1N1 and H3N2) and influenza B. H3N2 viruses tend to be most severe in the elderly. Partial immunity to H1N1 virus, resulting from exposure in the early part of the twentieth century, is postulated as a mechanism for milder H1N1 disease in this age group.
In 1997, influenza A (H5N1), previously seen only in birds, crossed the species barrier and human infection occurred in Southeast Asia [14]. This highly pathogenic avian influenza has spread in bird populations throughout Asia and into Europe. To date, human infection is rare and transmission has occurred primarily by direct contact with infected birds. Transmission between humans is limited but the possibility of mutation allowing efficient person-to-person spread makes avian influenza (H5N1) the greatest pandemic threat since 1918.
Seasonal influenza virus is a predictable cause of wintertime respiratory disease. Epidemics usually last 6 to 8 weeks and can occur anytime between November and April, with highly variable severity. The virus is transmitted most efficiently by small particle aerosols generated by coughing and sneezing; thus, explosive outbreaks can occur in closed settings, such as nursing homes [15]. Influenza results in approximately 36,000 deaths and more than 200,000 hospital admissions in the United States every year [16]. Age-specific hospitalization rates resulting from influenza form a J-shaped curve in which rates are high in ages below 5, decline in ages 5 to 49, and rise significantly for those age 50 or older [3]. Rates of hospitalization rise significantly with each decade over age 60, rising from 190 per 100,000 for ages 65 to 69 to 1195 per 100,000 for those over age 85. Increases in mortality are more dramatic, rising from 19 to 358 per 100,000 for the previously noted age groups.
The classic description of influenza pneumonia was by Louria and colleagues [17] after the 1957 to 1958 H2N2 pandemic. Lower tract disease was classified into four categories: (1) no radiographic pneumonia; (2) viral infection followed by bacterial pneumonia; (3) rapidly progressive viral pneumonia; and (4) concomitant viral-bacterial pneumonia. Diffuse infiltrates previously were seen in patients who had rheumatic heart disease–associated mitral stenosis ( Fig. 1). Although age is a significant risk factor for the development of lower respiratory tract complications to influenza, pure viral pneumonia is uncommon outside pandemic settings in nonimmunocompromised hosts [18], [19]. Most elderly persons have partial immunity resulting from previous vaccination or natural infections. In a recent study of highly vaccinated elderly in the community who subsequently were documented to have influenza, approximately 5% developed pneumonia [20]. In a survey of 193 patients hospitalized with influenza A, approximately half (101) had radiographic findings consistent with acute disease [21]. Of these, 33% had definitive infiltrates, 45% had atelectasis versus pneumonia, 15% had edema, and 7% had edema versus infiltrates. Most infiltrates were subtle and unilateral and involved the left lower lobe ( Fig. 2). Documented bacterial infection was observed in 8% of cases. Pneumonia and progression to acute respiratory distress syndrome (ARDS) and death are common with influenza A (H5N1). Limited microbiologic data indicate that this syndrome is a primary viral pneumonia. Unlike epidemic influenza, avian influenza to date is most severe in children under age 15.
Fig. 1.
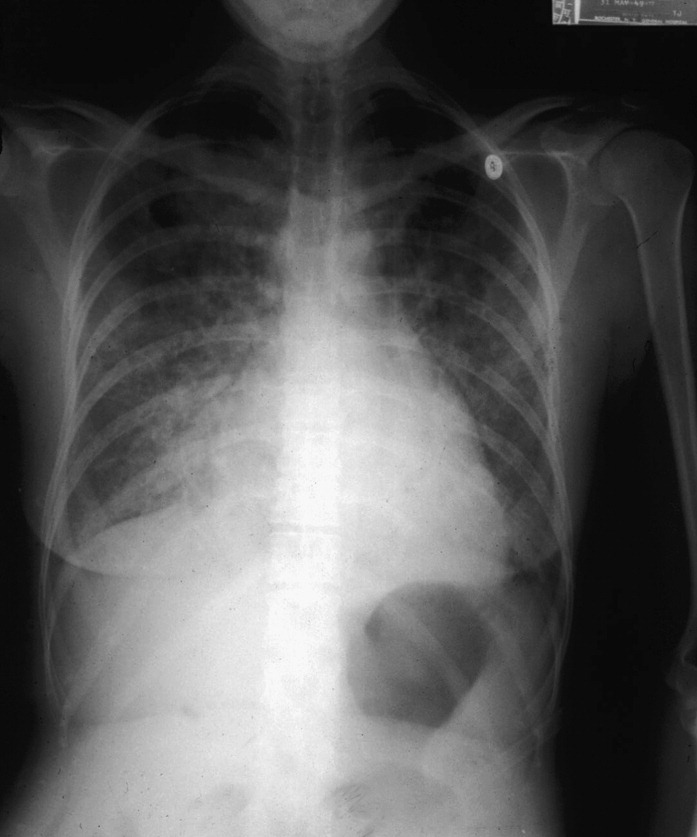
Chest radiograph of a 63-year-old woman who had mitral stenosis admitted to the hospital with severe respiratory distress and culture positive influenza A infection. Diffuse pulmonary infiltrates are illustrated.
Fig. 2.
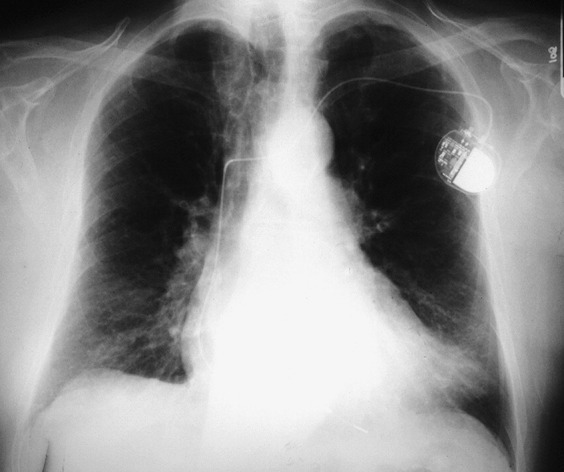
Chest radiograph of a 75-year-old man admitted to the hospital with fever, cough, and dyspnea. Influenza A was diagnosed by RT-PCR and confirmed by serology. A patchy opacity is demonstrated in the left lung base.
The typical manifestations of influenza (abrupt fever, cough, myalgias, and headache) may be altered in somewhat older persons [22], [23]. Fever, although still common, may be lower and the presence of underlying cardiac and pulmonary conditions may obscure the diagnosis. In addition, patients who have cognitive impairment may be unable to articulate their symptoms. Among elderly outpatients, cough, fever, and acute onset of symptoms had a positive predictive value for influenza of 30% in contrast to young adults, in whom this triad of symptoms had a value of 78% [23]. The presence of gastrointestinal complaints, fever, and myalgias, with a lack of significant rhinorrhea and wheezing, may help distinguish influenza from respiratory syncytial virus (RSV), another common winter virus [24], [25]. Because of infection control issues and potential therapeutic interventions, clinicians should have a high index of suspicion for influenza during the winter months, particularly when viral activity is high.
The diagnosis of influenza frequently is made on the basis of clinical presentation when viral activity is high in the community. Because of cocirculation of several other respiratory viruses, however, laboratory confirmation generally is recommended. Viral diagnostics include culture, antigen detection, RT-PCR, and serology [26]. Nasal swabs are believed better specimens than throat swabs to detect influenza viruses [13]. Sputum, endotracheal secretions, and bronchoalveolar lavage specimens also can be used to detect virus. Viral culture is the time-honored technique for identifying influenza infection. Influenza virus is hardy and grows well in culture but generally 2 to 3 days is required for cytopathic effect to be evident. Thus, culture is of limited value for making therapeutic or isolation decisions. The use of shell viral culture can shorten time to detection to approximately 24 hours [27]. Rapid antigen testing is available for influenza A and B and offers immediate results. These tests offer a sensitivity of approximately 50% to 60% and sensitivity of 90% or greater in adults [28]. Because false-negative results are common, patients who have pneumonia and negative test in whom a high index of suspicion of influenza based on clinical grounds exists should be considered for empiric treatment. Molecular testing, such as RT-PCR, offers the best combination of sensitivity and specificity for diagnosis of acute influenza but is labor intensive, expensive, and currently not widely available. Most assays use primers complementary to the conserved matrix gene [26]. RT-PCR can be useful in patients who are immunocompromised. Because all adults have antibody, a single serologic test is not useful for the acute diagnosis of influenza. Serologic testing demonstrating a fourfold or greater rise in influenza-specific antibody can be used to make a diagnosis as long as vaccine effect does not complicate interpretation.
Four antivirals—amantadine, rimantadine, zanamivir, and oseltamavir—are approved for the treatment of influenza infection [13]. Amantadine and rimantadine are active against influenza A, whereas zanamivir and oseltamavir are active against influenza A and B. These agents are 70% to 90% effective for prophylaxis and reduce illness severity, duration of symptoms, and viral shedding when given within 48 hours of symptom onset in uncomplicated influenza. Data are limited regarding the effectiveness of antiviral treatment of influenza pneumonia. The duration of patients' symptoms before admission usually presents a dilemma, because the average number of days most are ill is 3 to 4 days, falling outside the recommended 48-hour window of symptom duration for drug treatment [29]. Although no official recommendation can be made because risks and benefits must be weighed in individual clinical situations, many authorities believe treatment of patients who are antigen positive is reasonable because a significant viral load in secretions is needed to generate a positive rapid test. If a decision is made to use antiviral therapy, it is important to consider the type of influenza being treated (A or B), issues of drug resistance, and the possible adverse reactions of specific medications. The Centers for Disease Control and Prevention recently reported that 92% of influenza A (H3N2) and 25% of influenza A (H1N1) were resistant to the adamantanes [13]. Although only a total of 217 isolates was tested, isolates were obtained from 26 states and for this reason, use of this class of drugs is not recommended at the present time. Zanamivir and oseltamivir are effective for influenza A and B and, currently, rates of resistance are low. Each antiviral agent has different side-effect profiles that must be considered in treatment selection ( Table 4). Zanamivir is associated with bronchospasm and decline in forced expiratory volume in 1 second in some patients who have underlying lung disease and is not recommended for patients who have asthma or chronic obstructive pulmonary disease (COPD) [30]. Oseltamivir generally is well tolerated, although approximately 10% of recipients experience associated nausea and vomiting. The precise rate of bacterial complications of nonpandemic influenza is difficult to ascertain. Observational studies report ranges of 8% to 36% [31]. Physicians treating frail, elderly patients who have pneumonia and who have documented influenza face a challenge when deciding if antibiotics are needed. Decisions must be individualized based on duration of symptoms, patient stability, white blood cell count, and results of blood and sputum cultures.
Table 4.
Influenza antivirals
| Drug/use | Route | Treatment dose | Renal impairment (creatine clearance 10–30 mL/min) | Adverse effects |
|---|---|---|---|---|
| Zanamivir | ||||
| Treatment | Inhaled | 10 mg (2 inhalations) twice daily for 5 days | No adjustment | Bronchospasm; inhaler system may be difficult for older adults to use |
| Chemoprophylaxis | Inhaled | 10 mg (2 inhalations) twice daily for 5 days | No adjustment | |
| Oseltamivir | ||||
| Treatment | Oral | 75 mg twice daily for 5 days | 75 mg once daily | Nausea and vomiting |
| Chemoprophylaxis | Oral | 75 mg twice daily for 5 days | 75 mg every other day | |
Adapted from Prevention and control of influenza: recommendations of the advisory committee of immunization practices (ACIP). MMWR Recomm Rep 2006;55:1–48.
Respiratory syncytial virus
RSV is a common wintertime respiratory virus that affects persons of all ages and is the major cause of serious lower respiratory tract infections in young children [32]. For many years after its discovery in 1956, RSV was considered strictly a pediatric pathogen; however, it recently has been recognized increasingly as a serious adult pathogen [20]. Human RSV is an enveloped RNA virus and is a member of the family, Paramyxoviridae, classified within the genus, Pneumovirus [33]. RSV can be classified into two major groups, A and B, based primarily on antigenic differences found in one of the surface glycoproteins but, unlike in influenza, major antigenic shifts do not occur. RSV is a predictable cause of yearly epidemics of winter respiratory illnesses in temperate climates and activity typically begins in late fall and generally lasts 4 to 5 months, ending in late spring. Unlike influenza, which tends to produce a sharp peak in respiratory illness over 6 to 8 weeks, the epidemic curve of RSV generally is broader.
Estimates using national health care databases and viral surveillance data indicate that approximately 10,000 deaths in persons over age 65 in the United States each year are attributable to RSV. Several epidemiologic studies and mathematic models indicate that RSV is second to influenza as a cause of serious viral respiratory disease in adults [16], [34]. Several studies have examined the frequency of RSV as a cause of community-acquired pneumonia and estimates vary widely (0–14%) depending on the diagnostic tools used and season of study [35]. In a large study of approximately 1200 adults admitted to the hospital with a diagnosis of pneumonia, investigators found that RSV was identified in 4.4% of cases and was the pathogen identified third most commonly after Streptococcus pneumoniae and influenza [36]. A recent study of community-acquired pneumonia from Spain using a combination of diagnostic techniques found RSV to be the cause of 5 of 198 (3%) of adult patients who had pneumonia [10]. In composite, using data from the past 30 years, RSV accounts for 2% to 5% of pneumonia throughout the year and 5% to 15% during the winter months.
Although the clinical manifestations are difficult to distinguish from influenza, there are a few helpful clinical clues that suggest RSV infection. In a recent study of 118 RSV- and 133 influenza A–infected hospitalized patients, several signs and symptoms were found significantly different. RSV-infected subjects were more likely to have nasal congestion, wheezing, and a productive cough compared with influenza-infected patients and less likely to have high-grade fever [37]. In addition, the duration of symptoms before admission was 1.3 days longer for patients who had RSV compared with those who had influenza. Thus, in elderly patients who present to the hospital with pneumonia and have a low-grade fever and wheezing, particularly if preceded by a “cold,” the diagnosis of RSV infection should be entertained.
The radiographic findings associated with RSV vary from patchy subsegmental alveolar densities to lobar consolidation [36]. In a recent review of chest radiographs from 118 elderly adults hospitalized with RSV in whom known concomitant bacterial infections were excluded, opacities consistent with pneumonia were described in 20% of chest radiographs and an additional 13% had infiltrates believed to be atelectasis or pneumonia. Opacities generally were basilar, unilateral, and relatively subtle ( Fig. 3). Few patients had diffuse interstitial infiltrates considered typical for viral pneumonia. Bacterial pathogens are demonstrated in up to 30% of adult RSV cases, although the adequacy of specimens commonly is not addressed [35]. In Walsh and colleagues' [37] recent study of 132 hospital patients who had RSV, 15% had a potential pathogen identified in an adequate sputum sample and 3% had positive blood cultures. Unlike those who had only virus identified, 5 of 14 of these individuals had large pulmonary infiltrates with consolidation.
Fig. 3.
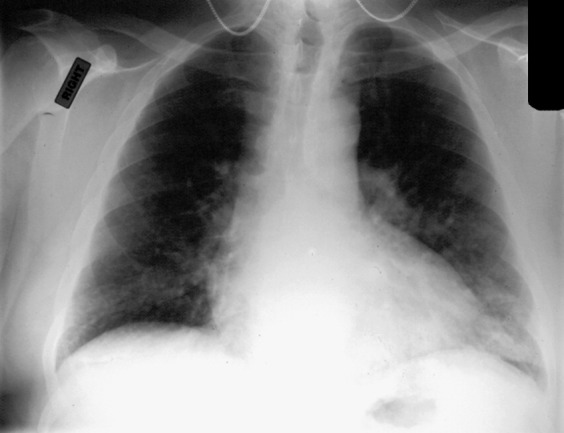
Chest radiograph of a 68-year-old man who had a history of emphysema admitted to the hospital with a history of recent “cold,” low-grade fever, wheezing, and dyspnea. RSV infection was documented by viral culture and serology. Patchy airspace disease is seen in the lung bases bilaterally.
Because RSV does not produce a distinctive clinical syndrome in adults, laboratory testing is required for specific viral diagnosis. The first diagnostic hurdle for RSV is consideration of the diagnosis. Because RSV is known best as a pediatric pathogen, it is not considered frequently in adults. In addition, the labile nature of the virus and low titers of virus in nasal secretions in the elderly make diagnosis of acute RSV infection problematic. Viral culture, rapid antigen tests, and RT-PCR can be used for diagnosis of acute infection and serology for retrospective diagnosis. A variety of specimens can be tested by the means described previously, including nasopharyngeal swabs, nasal washes, sputum, or bronchiolar lavages. Under ideal circumstances, viral culture is only 20% to 50% sensitive compared with serology using enzyme immunoassay (EIA) [35]. Unlike for influenza, commercial rapid antigen tests for RSV have poor sensitivity in adults. In a study of 60 older persons who had RSV documented by serology or RT-PCR, rapid testing by indirect fluorescence assay was positive in only 23% and by EIA in only 10% [38]. RT-PCR is used more successfully to detect RSV in adult populations [39]. In a study of more than 1000 adult nasal samples, RT-PCR was 73% sensitive and 99% specific compared with viral culture, which was 39% sensitive and 100% specific [40]. Although RT-PCR is sensitive and specific, the test currently is limited by expense, labor intensity, and limited commercial availability. The NucliSens EasyQ RSV A+B assay is a commercial real-time nucleic acid sequence amplification system that uses molecular beacons [41]. This assay is simpler to perform than traditional RT-PCR and may represent a method that can be used by clinical laboratories for adult diagnosis. Recent data suggest that sputum may be a better sample than nasal specimens if RT-PCR is used for diagnosis [42]. Infection also can be demonstrated retrospectively by a fourfold or greater rise in RSV-specific IgG, either by complement fixation or EIA. Because RSV in adults always represents reinfection, a single elevated titer is not useful for acute diagnosis. RSV-specific IgM has been detected in 11% to 81% of older subjects who have acute RSV, but its clinical usefulness has yet to be defined [43].
The treatment of RSV pneumonia in elderly adults largely is supportive with antipyretics, intravenous fluids, and oxygen as needed. It may be reasonable to administer corticosteroids and bronchodilators to patients who are wheezing acutely, although no formal controlled trials to use such agents for treatment of RSV-related wheezing have been performed. Antiviral therapy with aerosolized ribavirin and RSV-specific immunoglobulin are approved for high-risk infants, but only anecdotal data are available in adults [32]. Although the general use of ribavirin cannot be recommended, its use may be considered in selected cases, such as for patients who are immunocompromised. Anecdotal experience suggests that high-dose, short-duration therapy (60 mg/mL for 2 hours given by mask 3 times a day) in nonintubated adults is tolerated better than tent treatment [44].
Parainfluenza viruses
Parainfluenza viruses (PIVs) are single-stranded RNA viruses belonging to the paramyxovirus family. Four distinct serotypes are recognized, termed 1, 2, 3, and 4A and 4B [45]. These viruses cause croup, bronchitis, and pneumonia in young children. PIV-3 is endemic year round, whereas PIV-1 and PIV-2 tend to peak during the fall months. PIV-3 is associated most often with pneumonia in children [1]. Infection recurs throughout adulthood, accounting for 1% to 15% of acute respiratory illnesses with occasional reports of pneumonia in young adults. The burden of disease resulting from PIV in the elderly is not well studied, but pneumonia is reported [46]. Investigators in Sweden found that 11% of elderly persons who had community-acquired pneumonia had serologic evidence of recent PIV-1 or PIV-3 [47]. Prospective studies in nursing homes have documented PIV infection in 4% to 14% of respiratory illnesses and fatal cases of bronchopneumonia are described [48]. PIV also is implicated as a precursor to an outbreak of invasive pneumococcal disease in a long-term care facility [49]. Clinical syndromes, characterized by fever, rhinorrhea, hoarseness, cough, and sore throat, are not distinctive. Viral culture, RT-PCR, and serologic tests can be used to diagnose PIV infection. At the present time there are no commercially available rapid antigen tests. Ribavirin has activity in vitro against PIV but is not approved for treatment of PIV pneumonia [45].
Human metapneumovirus
Human metapneumovirus (hMPV) was identified by Dutch researchers, in 2001, in young children who had bronchiolitis [50]. hMPV also is an RNA virus in the paramyxovirus family. The virus is shown to have worldwide distribution; by age 5, all children are infected. In temperate climates, the virus circulates predominantly in the winter months overlapping with activity of influenza virus and RSV. Several studies suggest there may be significant year-to-year variation in incidence rates [51]. Infection with hMPV in young children causes a syndrome similar to RSV, with bronchiolitis and pneumonia the most common manifestations. Asymptomatic infection, colds, asthma exacerbations, and flu-like illnesses are documented in older children and healthy young adults [52]. Pneumonia and exacerbation of COPD are documented in elderly adults; however, comprehensive studies have not yet been published [53]. In a 2-year study of elderly and high-risk adults, hMPV infection was identified in 4.1%, using RT-PCR and serology for diagnosis [51]. Twenty-five percent of patients hospitalized with hMPV had infiltrates on chest radiographs. Illness may be more severe in frail elderly, as evidenced by a Canadian study in which pneumonia was documented in 40% of hMPV-infected nursing home residents [54]. The clinical characteristics of hMPV pneumonia in older adults do not appear distinctive from the other wintertime respiratory viruses, although rates of fever seem higher than with RSV and are similar to influenza. Diagnosis of hMPV outside research settings is difficult. The virus has special growth requirements and cytopathic effect can take up to 3 weeks to be detectable [50]. RT-PCR and serology are used successfully in research settings and transplant units, but these assays are not widely available commercially. Similar to PIV, ribavirin has activity in vitro but is not approved for treatment of hMPV infection.
Coronaviruses
Coronaviruses are RNA viruses, discovered in 1965, and are the second most frequent cause of the common cold after rhinoviruses [55]. Similar to hMPV, studies of human illness are limited by the inability to grow the virus under routine conditions. Two groups of coronaviruses are identified and four strains identified as causes of acute respiratory illnesses, ranging from colds to pneumonia, and include group 1 (229E and NL63) and group 2 (OC43 and HKU1) [56]. A novel coronavirus, SARS-CoV, which represents an early split from group 2, was identified in 2002 as the cause of the severe acute respiratory syndrome (SARS) epidemic, which originated in China and spread quickly to distant locations around the world [57].
OC43 and 229E occur most often in late winter and early spring demonstrating 2- to 3-year periodicity. Infection in healthy adults is characterized by low-grade fever, malaise, and nasal symptoms. Pneumonia is described in young children, patients who are immunocompromised, and the elderly [58], [59]. Using RT-PCR, coronaviruses were identified in 17% of community elderly who had acute respiratory illnesses in a recent Dutch study and 50% had lower respiratory tract symptoms [59]. Coronavirus lower respiratory tract disease is described in frail elderly attending senior daycare, where 66% of infected persons complained of a productive cough and 34% were short of breath [60]. In a study from China, investigators examined the frequency and clinical features of the newly described coronavirus HKU1 [61]. Of 418 patients admitted to the hospital with community-acquired pneumonia, 2.4% had evidence of coronavirus HKU1 by RT-PCR. All cases occurred between January and May. Of the 10 patients identified, eight were over age 65. Preceding upper respiratory tract symptoms were noted in only two patients. Clinically, illnesses were not distinguishable from other causes of community-acquired pneumonia. Two patients who had multiple chronic medical problems died. Another new strain of coronavirus discovered recently, HCOV-NL63, is found to circulate in summer and autumn and is associated with upper respiratory infection, bronchiolitis, and asthma exacerbations in children [62]. Little data on H COV-NL63 in the elderly exist. Nineteen of 525 respiratory specimens collected from patients who had winter respiratory illnesses in Canada were positive by RT-PCR for this pathogen [63]. Nine of 19 (47%) were elderly persons. Symptoms primarily were fever, sore throat, and cough. Most coronavirus studies to date do not describe radiographic findings; thus, the frequency of coronavirus pneumonia remains unknown.
Although SARS-CoV currently is quiescent, the clinical syndrome is worth describing, because older age is a significant risk factor for death [57]. Infected persons present initially with fever, myalgias, cough, and chills. Unlike other respiratory viruses, rhinorrhea and sore throat are uncommon symptoms. Approximately two thirds of patients develop persistent fever, tachypnea, hypoxia, and diarrhea. Serial chest radiographs reveal progressive multifocal airspace disease. Age and coexisting medical conditions are independent risk factors for risk for death, and in patients older than 65, the mortality rate exceeds 50%. Although therapies, such as ribavirin, corticosteroids, and intravenous gamma globulin, are used for treatment of SARS, there are no randomized placebo controlled trials by which to assess benefit.
Rhinoviruses
Rhinoviruses, the most frequent cause of the common cold, circulate throughout the year but have peaks in the fall and spring [64]. Infections are common at all ages, including the elderly, and account for approximately 25% to 50% of respiratory illnesses in community-dwelling elderly [65]. Outbreaks also are documented in long-term care facilities and senior daycare centers [60], [66]. Prominent nasal congestion, cough, and constitutional symptoms characterize illnesses. The role of rhinoviruses in pneumonia remains somewhat controversial. Replication of rhinoviruses is restricted at core body temperature and for this reason rhinoviruses once were dismissed as a cause of pneumonia. Recently, rhinoviruses have been recovered from lower airways after experimental challenge and cases of pneumonia described in very young children and patients who are severely immunocompromised [67]. The role of rhinoviruses as a cause of community-acquired pneumonia in older adults remains to be determined.
Herpes simplex virus
Herpes simplex virus (HSV) rarely causes lower respiratory tract disease in adults despite the fact the mucocutaneous reactivation of HSV during periods of stress is common [68]. Patients at risk for HSV pneumonia include those who have immunosuppression, severe burns, AIDS, or trauma. Age per se is not a specific risk factor, but older adults may reactivate HSV because of any of these factors. Because the isolation of HSV is possible as a result of asymptomatic reactivation, interpretation of cultures may be difficult. For example, HSV was isolated in 74% and 65% of upper and lower respiratory tract samples in one series of patients who had ARDS; however, pathogenicity was unclear [69].
Herpetic pneumonia can present either as localized or a disseminated pneumonia. Focal pneumonia is attributed to direct spread or a virus from the upper tract with tracheitis or esophagitis to the lower tract from aspiration of infected secretion [70]. The disseminated form results from viremia and occurs in patients who are severely immunocompromised. Fever is common as are cough and dyspnea. Chest pain and hemoptysis also may occur and mucocutaneous lesions are present in all patients. Because oral lesions in the majority of patients are not associated with lung involvement and contamination of bronchoalveolar lavage, specimens from upper airway secretions are a problem, definitive diagnosis requires the finding of histologic or cytologic examination of lower tract specimen showing necrotizing or hemorrhagic pneumonia with viral inclusion bodies [70]. Infection is localized mainly to the trachea and large bronchi, and a thick inflammatory membrane and mucosal ulcerations may be seen. Endobronchial biopsy is preferred for diagnosis compared with open lung biopsy. Acyclovir is active against HSV and has decreased mortality in disseminated HSV and pneumonia in children; however, in adults who have ARDS, the benefit is less clear [69]. No controlled trials of acyclovir for pneumonia have been done, and risks and benefits must be weighed in individual cases.
Other viruses
Several other viruses are implicated as causes of pneumonia in children or young adults; however, data in the elderly are lacking. Adenoviruses, in particular types 4 and 7, are linked to large outbreaks of respiratory disease and severe pneumonia in young adults living in congregate settings [71]. In the author's experience, adenovirus is not a frequent pathogen in elderly adults. Human bocavirus is a newly discovered parvovirus [72]. The virus has been identified by RT-PCR in respiratory samples from children who have lower respiratory tract illnesses; however, no data are available in adults. Several systemic viruses, such as varicella zoster and measles, not uncommonly cause pneumonia when acquired in adulthood, yet are uncommon in the elderly because of pre-existing immunity. Hantaviruses primarily are pathogens of rodents but may be transmitted to humans by contact with rodent excrement or bites [73]. Cases are reported in the southwestern United States. Human infection results in a febrile prodrome followed by pulmonary edema and shock. Progression may be rapid, and laboratory findings include elevated hematocrit and white blood cell count with thrombocytopenia. Treatment is supportive. Although primary Epstein-Barr virus is uncommon in the elderly, when it occurs, clinical syndromes can be atypical and pneumonia is described [74].
Summary
Aging can be associated with functional and immunologic decline and chronic cardiopulmonary diseases that predispose to pneumonia when viral infection occurs. Influenza virus remains the primary viral pathogen in the elderly, although the true impact of the other respiratory viruses remains to be defined as more sensitive diagnostic tools are developed. Unfortunately, the clinical syndromes associated with respiratory viruses frequently are indistinguishable from one another and bacterial pathogens. Because healthy elderly persons may visit exotic locations, which place them at risk for emerging pathogens, a travel history is important in the workup of pneumonia of older adults. Antiviral therapy is available for influenza A and B; thus, specific viral diagnosis may be useful for clinical management. Rapid antigen tests, although not as sensitive as viral culture or new molecular techniques, are widely available and offer quick turnaround times. RSV, PIV, hMPV, and coronaviruses in composite contribute to a substantial proportion of the community-acquired pneumonia cases in the elderly but at the present time treatment primarily is supportive.
References
- 1.Treanor J.J. Viral infections of the respiratory tract: prevention and treatment. Int J Antimicrob Agents. 1994;4:1–22. doi: 10.1016/0924-8579(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 2.Barker W.H., Mullooly J.P. Impact of epidemic a influenza in a defined adult population. Am J Epidemiol. 1980;112:798–811. doi: 10.1093/oxfordjournals.aje.a113052. [DOI] [PubMed] [Google Scholar]
- 3.Thompson W.W., Shay D.K., Weintraub E. Influenza-associated hospitalizations in the united states. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 4.Fang G., Fine M., Orloff J. New and emerging etiologies for community-acquired pneumonia with implications for therapy. A prospective multicenter study of 359 cases. Medicine. 1990;69:307–315. doi: 10.1097/00005792-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Flamaing J., Engelmann I., Joosten E. Viral lower respiratory tract infection in the elderly: a prospective in-hospital study. Eur J Clin Microbiol Infect Dis. 2003;22:720–725. doi: 10.1007/s10096-003-1042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oosterheert J.J., van Loon A.M., Schuurman R. Impact of rapid detection of viral and atypical bacterial pathogens by real-time polymerase chain reaction for patients with lower respiratory tract infection. Clin Infect Dis. 2005;41:1438–1444. doi: 10.1086/497134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Templeton K.E., Scheltinga S.A., van den Eeden W.C. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005;41:345–351. doi: 10.1086/431588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Roux A., Marcos M.A., Garcia E. Viral community-acquired pneumonia in nonimmunocompromised adults. Chest. 2004;125:1343–1351. doi: 10.1378/chest.125.4.1343. [DOI] [PubMed] [Google Scholar]
- 9.Almirall J., Bolibar I., Vidal J. Epidemiology of community-acquired pneumonia in adults: a population-based study. Eur Respir J. 2000;15:757–763. doi: 10.1034/j.1399-3003.2000.15d21.x. [DOI] [PubMed] [Google Scholar]
- 10.Angeles Marcos M., Camps M., Pumarola T. The role of viruses in the aetiology of community-acquired pneumonia in adults. Antivir Ther. 2006;11:351–359. [PubMed] [Google Scholar]
- 11.Kobashi Y., Okimoto N., Matsushima T. Clinical analysis of community-acquired pneumonia in the elderly. Intern Med. 2001;40:703–707. doi: 10.2169/internalmedicine.40.703. [DOI] [PubMed] [Google Scholar]
- 12.Lim W.S., Macfarlane J.T., Boswell T.C. Study of community acquired pneumonia aetiology (SCAPA) in adults admitted to hospital: implications for management guidelines. Thorax. 2001;56:296–301. doi: 10.1136/thorax.56.4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prevention and control of influenza: recommendations of the advisory committee of immunization practices (ACIP) MMWR Recomm Rep. 2006;55:1–48. [PubMed] [Google Scholar]
- 14.World health organization Current concepts: avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 15.Gravenstein S., Miller B.A., Drinka P. Prevention and control of influenza a outbreaks in long term care facilities. Infect Control Hosp Epidemiol. 1992;13:49–54. doi: 10.1086/646422. [DOI] [PubMed] [Google Scholar]
- 16.Thompson W.W., Shay D.K., Weintraub E. Mortality associated with influenza and respiratory syncytial virus in the united states. J Am Med Assoc. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 17.Louria D.E., Blumenfeld H.L., Ellis J.T. Studies on influenza in the pandemic of 1957–1958. II. Pulmonary complications of influenza. J Clin Invest. 1959;38:213–265. doi: 10.1172/JCI103791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fry J. Influenza, 1959: the story of an epidemic. Br Med J. 1959;2:135–138. doi: 10.1136/bmj.2.5144.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foy H.H., Cooney M.K., Allan I. Rates of pneumonia during influenza epidemics in seattle 1964–1975. JAMA. 1979;241:253–258. [PubMed] [Google Scholar]
- 20.Falsey A.R., Hennessey P.A., Formica M.A. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 21.Murata Y, Walsh EE, Falsey AR. Pulmonary complication of influenza a infections in the inter-pandemic era. J Infect Dis, in press.
- 22.Cate T.R. Clinical manifestations and consequences of influenza. Am J Med. 1987;82:15–19. doi: 10.1016/0002-9343(87)90555-9. [DOI] [PubMed] [Google Scholar]
- 23.Govaert T.M.E., Dinant G.J., Aretz K. The predictive value of influenza symptomatology in elderly peole. Fam Pract. 1998;15:16–22. doi: 10.1093/fampra/15.1.16. [DOI] [PubMed] [Google Scholar]
- 24.Mathur U., Bentley D.W., Hall C.B. Concurrent respiratory syncytial virus and influenza a infections in the institutionalized elderly and chronically ill. Ann Intern Med. 1980;93:49–52. doi: 10.7326/0003-4819-93-1-49. [DOI] [PubMed] [Google Scholar]
- 25.Wald T.G., Miller B.A., Shult P. Can respiratory syncytial virus and influenza a be distinguished clinically in institutionalized older persons? J Am Geriatr Soc. 1995;43:170–174. doi: 10.1111/j.1532-5415.1995.tb06384.x. [DOI] [PubMed] [Google Scholar]
- 26.Petric M., Comanor L., Petti C.A. Role of the laboratory in diagnosis of influenza during seasonal epidemics and potential pandemics. J Infect Dis. 2006;194(Suppl 2):S98–S110. doi: 10.1086/507554. [DOI] [PubMed] [Google Scholar]
- 27.Schirm J., Luijt D.S., Pastoor G.W. Rapid detection of respiratory viruses using mixtures of monoclonal antibodies on shell viral cultures. J Med Virol. 1992;38:147–151. doi: 10.1002/jmv.1890380214. [DOI] [PubMed] [Google Scholar]
- 28.Storch G.A. Rapid tests for influenza. Curr Opin Pediatr. 2003;15:77–84. doi: 10.1097/00008480-200302000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Walsh E.E., Cox C., Falsey A.R. Clinical features of influenza a virus infection in elderly hospitalized persons. J Am Geriatr Soc. 2002;50:1498–1503. doi: 10.1046/j.1532-5415.2002.50404.x. [DOI] [PubMed] [Google Scholar]
- 30.Williamson J.C., Pegram P.S. Respiratory distress associated with zanamivir. N Engl J Med. 2000;342:661–662. doi: 10.1056/NEJM200003023420914. [DOI] [PubMed] [Google Scholar]
- 31.Falsey AR, Murata Y, Walsh EE. Impact of rapid diagnosis on management of adults hospitalized with influenza. Arch Intern Med 2007;167:354–60. [DOI] [PubMed]
- 32.Hall C.B. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;334:1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 33.Collins P. The molecular biology of human respiratory syncytial viruses (RSV) of the genus pneumovirus. In: Kingsbury D.W., editor. The paramyxoviruses. Plenum Publishing; New York: 1991. pp. 103–162. [Google Scholar]
- 34.Nicholson K.G. Impact of influenza and respiratory syncytial virus on mortality in England and Wales from january 1975 to december 1990. Epidemiol Infect. 1996;116:51–63. doi: 10.1017/s0950268800058957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falsey A.R., Walsh E.E. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000;13:371–384. doi: 10.1128/cmr.13.3.371-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dowell S.F., Anderson L.J., Gary H.E.J. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J Infect Dis. 1996;174:456–462. doi: 10.1093/infdis/174.3.456. [DOI] [PubMed] [Google Scholar]
- 37.EE Walsh, DR Peterson, AR Falsey. Clinical recognition of respiratory syncytial virus infection in hospitalized elderly and high-risk adults possible? J Infect Dis 2007;195:1046–51. [DOI] [PubMed]
- 38.Casiano-Colon A.E., Hulbert B.B., Mayer T.K. Lack of sensitivity of rapid antigen tests for the diagnosis of respiratory syncytial infection in adults. J Clin Virol. 2003;28:169–174. doi: 10.1016/s1386-6532(03)00002-7. [DOI] [PubMed] [Google Scholar]
- 39.Zambon M.C., Stockton J.D., Clewley J.P. Contribution of influenza and respiratory syncytial virus to community cases of influenza-like illness: an observational study. Lancet. 2001;358:1410–1416. doi: 10.1016/s0140-6736(01)06528-x. [DOI] [PubMed] [Google Scholar]
- 40.Falsey A.R., Formica M.A., Walsh E.E. Diagnosis of respiratory syncytial virus infection: comparison of reverse transcription-PCR to viral culture and serology in adults with respiratory illness. J Clin Microbiol. 2002;40:817–820. doi: 10.1128/JCM.40.3.817-820.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore C., Valappil M., Corden S. Enhanced clinical utility of the NucliSens EasyQ RSV A+B assay for tapid detection of respiratory syncytial virus in clinical samples. Eur J Clin Microbiol Infect Dis. 2006;25:167–174. doi: 10.1007/S10096-006-0112-4. [DOI] [PubMed] [Google Scholar]
- 42.Falsey A.R., Formica M.A., Hennessey P.A. Detection of respiratory syncytial virus in adults with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;173:639–643. doi: 10.1164/rccm.200510-1681OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vikerfors T., Grandien M., Johansson M. Detection of an immunoglobulin M response in the elderly for early diagnosis of respiratory syncytial virus infection. J Clin Microbiol. 1988;26:808–811. doi: 10.1128/jcm.26.5.808-811.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Englund J.A., Piedra P., Jefferson L.S. High-dose, short duration ribavirin aerosol therapy in children with suspected respiratory syncytial virus infection. J Pediatr. 1990;117:313–320. doi: 10.1016/s0022-3476(05)80554-2. [DOI] [PubMed] [Google Scholar]
- 45.Henrickson K.J. Parainfluenza viruses. Clin Micro Rev. 2003;16:242–264. doi: 10.1128/CMR.16.2.242-264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marx A., Gary H.E., Martston B.J. Parainfluenza virus infection among adults hospitalized for lower respiratory tract infection. Clin Infect Dis. 1999;29:134–140. doi: 10.1086/520142. [DOI] [PubMed] [Google Scholar]
- 47.Fransen H., Heigl Z., Wolontis S. Infections with viruses in patients hospitalized with acute respiratory illness, stockholm 1963–1967. Scand J Infect Dis. 1969;1:127–136. doi: 10.3109/inf.1969.1.issue-2.09. [DOI] [PubMed] [Google Scholar]
- 48.Public Health Laboratory Service Communicable Disease Surveillance Centre Parainfluenza infections in the elderly 1976–82. Br Med J. 1983;287:1619. doi: 10.1136/bmj.287.6405.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiore A.E., Iverson C., Messmer T. Outbreak of pneumonia in a long-term care facility: antecedent human parainfluenza virus 1 infection may predispose to bacterial pneumonia. J Am Geriatr Soc. 1998;46:1112–1117. doi: 10.1111/j.1532-5415.1998.tb06649.x. [DOI] [PubMed] [Google Scholar]
- 50.van den Hoogen B.G., DeJong J.C., Groen J. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Falsey A.R., Erdman D., Anderson L.J. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–790. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 52.Hamelin M.E., Abed Y., Boivin G. Human metapneumovirus: a new player among respiratory viruses. Clin Infect Dis. 2004;38:983–990. doi: 10.1086/382536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamelin M.E., Cote S., Laforge J. Human metapneumovirus infection in adults with community-acquired pneumonia and exacerbation of chronic obstructive pulmonary disease. Clin Infect Dis. 2005;41:498–502. doi: 10.1086/431981. [DOI] [PubMed] [Google Scholar]
- 54.Boivin G., Abed Y., Pelletier G. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis. 2002;186:1330–1334. doi: 10.1086/344319. [DOI] [PubMed] [Google Scholar]
- 55.Larson H.E., Reed S.E., Tyrrell D.A.J. Isolation of rhinoviruses and coronaviruses from 38 colds in adults. J Med Virol. 1980;5:221–229. doi: 10.1002/jmv.1890050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lau S.K., Woo P.C., Yip C.C. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol. 2006;44:2063–2071. doi: 10.1128/JCM.02614-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peiris J.S.M., Yuen K.Y., Osterhaus A.D. The severe acute respiratory syndrome. N Engl J Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 58.El-Sahly H.M., Atmar R.L., Glezen W.P. Spectrum of clinical illness in hospitalized patients with “common cold” virus infections. Clin Infect Dis. 2000;31:96–100. doi: 10.1086/313937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Graat J.M., Schouten E.G., Heijnen M.A. A prospective, community-based study on virologic assessment among elderly people with and without symptoms of acute respiratory infection. J Clin Epidemiol. 2003;56:1218–1223. doi: 10.1016/S0895-4356(03)00171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Falsey A.R., McCann R.M., Hall W.J. The “common cold” in frail older persons: impact of rhinovirus and coronavirus in a senior daycare center. J Am Geriatr Soc. 1997;45:706–711. doi: 10.1111/j.1532-5415.1997.tb01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woo P.C., Lau S.K., Tsoi H.W. Clinical and molecular epidemiological features of coronavirus HKU1-associated community-acquired pneumonia. J Infect Dis. 2005;192:1898–1907. doi: 10.1086/497151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiu S.S., Chan K.H., Chu K.W. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis. 2005;40:1721–1729. doi: 10.1086/430301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bastien N., Anderson K., Hart L. Human coronavirus NL63 infection in canada. J Infect Dis. 2005;191:503–506. doi: 10.1086/426869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gwaltney J.M., Hendley J.O., Simon G. Rhinovirus infections in an industrial population–I. The occurrence of illness. N Engl J Med. 1966;275:1261–1268. doi: 10.1056/NEJM196612082752301. [DOI] [PubMed] [Google Scholar]
- 65.Nicholson K.G., Kent J., Hammersley V. Acute viral infections of upper respiratory tract in elderly people living in the community; comparative, prospective, population based study of disease burden. Br Med J. 1997;315:1060–1064. doi: 10.1136/bmj.315.7115.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wald T.G., Shult P., Krause P. A rhinovirus outbreak among residents of a long-term care facility. Ann Intern Med. 1995;123:588–593. doi: 10.7326/0003-4819-123-8-199510150-00004. [DOI] [PubMed] [Google Scholar]
- 67.Papadopoulos N.G., Bates P.J., Bardin P.G. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181:1875–1884. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 68.Cherr G.S., Meredith J.W., Chang M. Herpes simplex virus pneumonia in trauma patients. J Trauma. 2000;49:547–549. doi: 10.1097/00005373-200009000-00024. [DOI] [PubMed] [Google Scholar]
- 69.Tuxen D.V., Wilson J.W., Cade J.F. Prevention of lower respiratory herpes simplex virus infection with acyclovir in patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1987;136:402–405. doi: 10.1164/ajrccm/136.2.402. [DOI] [PubMed] [Google Scholar]
- 70.Ruben F.L., Nguyen M. Viral pneumonitis. Clin Chest Med. 1991;12:223–235. [PubMed] [Google Scholar]
- 71.Dudding B.A., Wagner S.C., Zeller J.A. Fatal pneumonia associated with adenovirus type 7 in three military trainees. N Engl J Med. 1972;286:1289–1292. doi: 10.1056/NEJM197206152862403. [DOI] [PubMed] [Google Scholar]
- 72.Manning A., Russell V., Eastick K. Epidemiological profile and clinical associations of human bocavirus and other human parvoviruses. J Infect Dis. 2006;194:1283–1290. doi: 10.1086/508219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miedzinski L. Community-acquired pneumonia: new facets of an old disease—hantavirus pulmonary syndrome. Respir Care Clin N Am. 2005;11:45–58. doi: 10.1016/j.rcc.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 74.Schmader K., van der Horst, Charles M. Epstein-Barr virus and the elderly host. Rev Infect Dis. 1989;11:64–73. doi: 10.1093/clinids/11.1.64. [DOI] [PubMed] [Google Scholar]


