Summary
Non-suppurative meningoencephalitis of unknown cause is a frequent finding in dogs and cats. Fifty-three dogs and 33 cats with non-suppurative meningoencephalitis of unknown aetiology were examined immunohistochemically for 18 different infectious agents, including viruses, bacteria and prion proteinSc. In 14 (26%) of the dogs and 13 (39%) of the cats a causative agent was identified in the central nervous system (CNS), two dogs and one cat giving positive results for two infectious agents simultaneously. The study revealed infections with known causative agents (porcine herpes virus 1, feline infectious peritonitis virus, Escherichia coli) and a new disease pattern of parvovirus infection in the CNS of dogs and cats. Infection of the CNS with feline leukaemia virus was found in a cat. Five dogs and four cats gave positive results for West Nile virus (WNV) antigen. In one dog, canine parainfluenza virus antigen was detected in the brain. Four dogs and four cats gave positive results for encephalomyocarditis virus (EMCV). The significance of the detection of WNV and EMCV antigen requires further study. The aetiology remained undetermined in 39 dogs (74%) and 20 cats (61%). Although it is possible that non-infectious causes play a more important role than previously thought, infections with hitherto unrecognized agents cannot be ruled out.
Keywords: bacterial infection, cat, dog, encephalomyocarditis virus, meningoencephalitis, viral infection, West Nile virus
Introduction
Non-suppurative meningoencephalitis in cats and dogs is usually considered to be caused by viral infections (Summers et al., 1995), and the histopathological findings consist mainly of perivascular and parenchymal infiltration with lymphocytes, macrophages and plasma cells, usually associated with meningitis and occasionally with inflammation of the plexus chorioideus and the ependyma (Braund, 1980; Luttgen, 1988). Viruses, due to cell tropism and route of infection, often induce a typical pattern of inflammation in the brain and spinal cord (Braund, 2001).
In newborn animals, infections of the central nervous system (CNS) with bacteria, including various strains of Escherichia coli, are a common sequel to septicaemia, whereas in adult dogs and cats bacteria may follow a neurogenic, otogenic or rhinogenic route to enter the CNS (Meric, 1988; Summers et al., 1995). Whilst most bacterial CNS infections induce a purulent inflammation, some bacteria, particularly Listeria spp., as well as protozoa, fungi and algae, cause a non-suppurative, mainly granulomatous inflammation of the CNS (Rand et al., 1994; Summers et al., 1995; Tipold, 1995; Quesnel et al., 1997). In the brains of beagles with granulomatous leptomeningitis, Maeda et al. (1993) demonstrated E. coli and considered it to be the causative agent. If parasites enter the CNS, they often induce a granulomatous inflammation (Summers et al., 1995). In dogs, the most common parasite to cause infection of the CNS is Toxoplasma gondii, followed by Neospora caninum and, albeit rarely, Cuterebra spp. In cats, Cuterebra spp. are the most common parasites to be found in the CNS, followed by T. gondii in rare cases (Braund, 2001).
There would also seem to be non-infectious causes of non-suppurative meningoencephalitis. Thus, autoimmune and hereditary mechanisms are suspected to be the underlying cause of CNS disorders such as granulomatous meningoencephalitis (GME) in dogs (Harris et al., 1988; Kipar et al., 1998), pug dog encephalitis (Uchida et al., 1999) and non-suppurative meningoencephalitis in greyhounds (Callanan et al., 2002). So far no aetiological agent has been detected for feline “staggering disease” or so-called feline polioencephalomyelitis (Vandevelde and Braund, 1979; Nowotny and Weissenböck, 1995; Braund, 2001), although some suspect staggering disease to be due to Borna disease virus (BDV) infection (Lundgren et al., 1995). Paraneoplastic disorders of the CNS, well known in man, have not been reported in cats and dogs (Braund et al., 1987).
Recent studies on a limited range of infectious agents failed to identify the cause of non-suppurative meningoencephalitis in 47% and 23% of dogs and cats, respectively (Rand et al., 1994; Tipold, 1995; Quesnel et al., 1997; K.Melzer, personal communication). To provide further information, the present study sought to determine the cause of 86 cases of non-suppurative meningoencephalitis in dogs and cats by the immunohistochemical examination of formalin-fixed, paraffin wax-embedded tissue. In addition to examination for known viral and bacterial pathogens and prion proteinSc, West Nile virus (WNV), a recently emerging cause of disease, was included in the present study (Komar, 2000; Lichtensteiger et al., 2003; Austgen et al., 2004), together with encephalomyocarditis virus (EMCV), an agent so far demonstrated only in the CNS of pigs and rodents (Nowotny, 1996; Sue et al., 2003).
Materials and Methods
Animals
Fifty-three canine and 33 feline cases of non-suppurative meningoencephalitis of unknown origin, collected during the period 1998–2003 by the Department of Pathology of the University of Veterinary Medicine Hannover, Germany, were selected. The case selection was made in the light of a preliminary investigation which revealed that 274 of 1209 (23%) dogs and 146 of 741 (20%) cats examined in a 5-year-period (1998–2002) displayed changes in the CNS. Non-suppurative meningoencephalitis accounted for 22% and 49% of the CNS changes in dogs and cats, respectively. The cause of 49 canine and 32 feline cases remained undetermined after examination by routine diagnostic procedures. These 81 animals, together with four dogs and one cat with undetermined non-suppurative meningoencephalitis encountered in 2003, were included in this study.
Due to the retrospective nature of the present study, clinical data were restricted to a short statement from the records of the Department of Pathology in most cases (46 dogs and 30 cats). In seven dogs and three cats, no clinical history was available.
Histology
Archived CNS sections of each case, stained with haematoxylin and eosin (HE), were re-examined for inflammatory changes. From each animal, one section of the cerebral cortex, the hippocampus (missing in three dogs and four cats), the midbrain (missing in five dogs and five cats), the cerebellum (missing in two dogs and two cats), the brain stem (missing in 17 dogs and 13 cats) and the spinal cord (available in only seven dogs and two cats) were examined. In one canine section, a spinal cord ganglion was present. The distribution of the lesions (e.g., polio-, leuco-, and panencephalitis) was categorized according to Summers et al. (1995). The type of inflammation was defined as follows: lymphohistiocytic, if infiltrates consisted mainly of lymphocytes and macrophages; granulomatous, if macrophages predominated; pyogranulomatous, if neutrophils and macrophages were the main cell types; and mixed, if macrophages, lymphocytes, plasma cells and neutrophils appeared in equal quantities.
The degree of histological changes were determined semiquantitatively. Perivascular infiltrates were described as: mild (1–15 perivascular cells per ×100 field, or 1–2 cell layers per vessel, or both); moderate (16–30 perivascular cells per ×100 field or 2–3 cell layers per vessel, or both); and severe (>30 perivascular cells per ×100 field or ⩾3 cell layers per vessel, or both). Meningeal infiltrates were described as mild (one or no more than a few mild infiltrates), moderate (one moderate infiltrate or several mild infiltrates), or severe (several moderate infiltrates or confluent infiltrates).
If suggested by histological results, slides were additionally stained with periodic acid–Schiff (16 dogs, six cats), Ziehl–Neelsen (11 dogs, one cat), Grocott (10 dogs, four cats), luxol-fast blue (nine dogs, one cat), cresyl-echt-violet (two dogs, one cat), alcian-blue (one cat) or Congo-red (one cat), as described by Romeis (1994).
Immunohistochemistry (IHC)
For the detection of 18 infectious agents (Table 1 ), the avidin–biotin-peroxidase complex (ABC) method was performed, as previously described (Hsu et al., 1981; Wünschmann et al., 1997). Due to their known cross-reactivity for the detection of canine and feline herpesvirus, parvovirus and coronavirus antigen, the same antibodies were used for both species. Feline tissue was not examined for canine adenovirus 1 and canine tissue was not examined for feline leukaemia virus (FeLV). IHC for the detection of FeLV antigen was additionally performed on lymphoid tissue; however, such tissue was available from only 20 of the 33 cats. In one dog that showed immunoreactivity for porcine herpesvirus 1, tissue from the gastrointestinal tract was also examined, and in one dog and two cats showing immunoreactivity for EMCV, myocardial tissue was examined.
Table 1.
Antibodies used for the immunohistochemical detection of various infectious agents
| Infectious agent | Antibody | Specificity of antibody |
|---|---|---|
| Rabies virus | Polyclonal goat-anti-rabies virus (Sifin, Berlin, Germany) | Not stated |
| Porcine herpesvirus 1 (PHV 1) | Polyclonal mouse-anti-PHV-1 (Dr Eskens, Veterinär-Untersuchungsamt Mittelhessen, Germany); ESKENS et al., 1991 | Not stated |
| Canine distemper virus (CDV) | 1. Monoclonal mouse-anti-10H3 (Prof. Haas, Department of Virology, University of Veterinary Medicine, Hannover, Germany); 2. Polyclonal rabbit-anti-CDV (Dr Örvell, Huddinge Hospital, Huddinge, Sweden); BAUMGÄRTNER et al., 1989b | 1. Nucleoprotein |
| 2. Nucleoprotein | ||
| Parvovirus (canine and feline) | Mouse-anti-CPV1-2A1 (Custom Monoclonals International, Sacramento, USA); KIPAR et al., 2000 | Unknown |
| Feline infectious peritonitis (FIP) virus and canine coronavirus | Polyclonal cat-anti-FIP-type 1,2, FITC-ligated (Biologo, Kronshagen, Germany) | Not stated |
| Feline leukaemia virus (FeLV)⁎ | Monoclonal mouse-anti-FeLV (Custom Monoclonals International, Sacramento, USA); ELDER et al., 1987 | Glycoprotein 70 |
| Herpesvirus (canine and feline; CHV, FHV) | Monoclonal mouse-anti-4A1 (Prof. Haas, Department of Virology, University of Veterinary Medicine, Hannover, Germany); XUAN et al., 1992; LEBICH et al., 1994 | Glycoprotein complex 143/108 (FHV) and |
| Glycoprotein complex 145/112 (CHV) | ||
| Canine adenovirus 1 (CAV 1)⁎ | Monoclonal mouse-anti-CAV-1 (Chemicon, Hamburg, Germany) | The whole CAV-1 tribe “Mirandola” |
| Tick-borne encephalitis (TBE) virus | Polyclonal rabbit-anti-TBE (Prof. Holzmann, Department of Virology, University of Vienna, Austria); WEISSENBÖCK et al., 1998 | Not stated |
| Borna disease virus (BDV) | 1. Monoclonal anti-p38 (Bo18) | 1. Nucleoprotein |
| 2. Monospecific, polyclonal anti-p24 (Prof. Richt, National Animal Disease Centre, Ames, Iowa, USA); HERDEN et al., 1999 | 2. Phosphoprotein | |
| West Nile virus (WNV) | 1. Monoclonal anti-MEP-E | 1. Major envelope protein E |
| 2. Monoclonal anti-NSP-1 (both Chemicon, Hamburg, Germany); HALL, 2000 | 2. Non-structural protein 1 | |
| Canine parainfluenza virus (CPIV) | Monoclonal mouse-anti-SV5-NP-C (Dr Randall, Department of Biochemistry and Microbiology, University of St Andrews, UK); DURCHFELD et al., 1991 | Nucleoprotein C |
| Encephalomyocarditis virus (EMCV) | Monoclonal mouse-anti-EMCV (mab 4F3, 3E5, 4E4; Prof. Vlemmas, Institute of Veterinary Pathology, Aristotle University Thessaloniki, Greece); VLEMMAS et al., 1998 | Capsid protein |
| Prion proteinSc | Monoclonal mouse-anti-prion-proteinSc (mab L42) (Prof. Groschup, Bundesforschungsanstalt für Viruskrankheiten der Tiere, Insel Riems, Germany); HARDT et al., 2000 | Epitope on first α–helix of bovine prion proteinSc |
| Listeria monocytogenes | Polyclonal rabbit-anti-Listeria monocytogenes-1, 4 (Voigt Global Distribution, Kansas City, USA) | Whole organism |
| Chlamydia spp. | Monoclonal mouse-anti-Chlamydia-species (Chlamydia-Antigen-Vet-IFT, Medac, Wedel, Germany); BAURIEDEL, 1999; | Surface protein 1 |
| Mycoplasma spp. | Monoclonal anti-Mycoplasma-species (Capricorn, Portland, ME, USA) | Whole organism of Mycoplasma pneumoniae |
| Escherichia coli (E. coli) | Polyclonal rabbit-anti-E. coli (Dako, Hamburg, Germany); KELLY et al., 1987 | Majority of the proteins in E. coli-lysates |
CAV 1 antibody was used only in dogs, FeLV antibody was used only in cats. FITC: fluorescein-isothiocyanate.
Briefly, specimens were dewaxed in graded alcohols and endogenous peroxidase was quenched with H2O2 0.5% in ethanol. The pre-treatments applied to sections (Table 2 ) depended on the primary antibody and were performed as previously described (see below). The various pre-treatments were as follows: Pronase E (Merck, Darmstadt, Germany; Bahn, 1988); trypsin (Fluka, Neu Ulm, Germany; Nietfeld et al., 1989); Target Unmasking Fluid (Kreatech Diagnostics, Amsterdam, Netherlands; Kovacevic et al., 1997); pepsin (Dako, Hamburg, Germany; Bahn, 1988), proteinase K and formic acid (Merck, Darmstadt, Germany; Hardt et al., 2000); and microwave pre-treatment (Kahveci et al., 2003). Goat serum, diluted 1 in 5 in phosphate-buffered saline (PBS), served as a blocking serum. Primary antibodies were, unless stated otherwise, diluted in PBS containing bovine serum albumin (BSA) 1%, and incubated overnight (12–16 h) at 4 °C (Table 2). Biotinylated goat-anti-mouse (gam-b), goat-anti-rabbit (gar-b) and goat-anti-fluorescein-isothiocyanate (ga-FITC-b; directed against the FITC-ligated anti-feline infectious peritonitis [FIP] virus antibody) antibodies served as secondary antibodies (Vector Laboratories, Burlingame, CA, USA) and were all diluted 1 in 200 in PBS. Finally, sections were incubated with ABC (Vector) for 30 min at room temperature. Between the incubation steps, sections were thoroughly rinsed with PBS. Positive antigen–antibody reactions were “visualized” by incubation with 3,3′-diaminobenzidine-tetrahydrochloride (DAB) with H2O2 0.5% for 10 min followed by mild counter-staining with haematoxylin. Sections were mounted with Roti-Histokit (Carl Roth KG, Karlsruhe, Germany) under coverslips. In control sections the primary and secondary antibodies were replaced by PBS, control serum or isotype-specific antibodies directed against irrelevant antigens.
Table 2.
Immunohistochemical methods
| Primary antibody⁎ | Dilution† (1 in PBS) | Pretreatment | Secondary antibody |
|---|---|---|---|
| Anti-rabies virus | 3000 | Trypsin | gar-b |
| Anti-PHV-1 | 10 000 | None | gar-b |
| Anti-CDV (mouse-anti-10H3) | 100 | Trypsin | gam-b |
| Anti-CDV (rabbit) | 2000 (PBS contained 20% NSS but no BSA) | None | gar-b (PBS contained 20% NSS) |
| Anti-parvovirus | 500 | None | gam-b |
| Anti-FIP-FITC | 20 | TUF | ga-FITC-b |
| Anti-FeLV | 200 | TUF | gam-b |
| Anti-FHV and -CHV | 200 | None | gam-b |
| Anti-CAV-1 | 1600 | None | gam-b |
| Anti-TBE | 3000 | Pronase E | gar-b |
| Anti-BDV(anti-p38[Bo18]) | 500 | Pronase E (only in cats) | gam-b |
| Anti-BDV (anti-p24) | 8000 (PBS contained 20% NSS but no BSA), adsorbed to rat brain powder | None | gar-b |
| Anti-WNV (anti-NSP-1) | 400 | pronase E | gam-b |
| Anti-WNV (anti-MEP-E) | 300 | pronase E | gam-b |
| Anti-CPIV | 50 000 | None | gam-b |
| Anti-EMCV(4F3) | 40 | Microwave | gam-b |
| Anti-prion proteinSc | 400 (PBS contained 10% goat serum but no BSA); no further blocking with goat serum necessary | Formic acid, proteinase K, microwave | gam-b (PBS contained 10% goat serum) |
| Anti-Listeria monocytogenes | 2000 | Pepsin | gar-b |
| Anti-Chlamydia sp. | 10 | None | ga-FITC-b |
| Anti-Mycoplasma sp. | 500 (PBS contained 30% NSS but no BSA) | None | gam-b |
| Anti-E. coli | 1600 | None | gar-b |
NSS, normal swine serum; TUF, target unmasking fluid; gam-b, biotinylated goat-anti-mouse antibody; gar-b, biotinylated goat-anti-rabbit antibody; ga-FITC-b, biotinylated goat-anti-FITC antibody (see Materials and Methods).
Against agents listed in Table 1.
In phosphate-buffered saline (PBS) containing bovine serum albumin (BSA) 1%.
Results
Animals
Toy breeds constituted 27% (14) of the affected dogs (Yorkshire terrier [3], West Highland white terrier [4], Jack Russell terrier [2], chihuahua [1], shih tzu [1], miniature pinscher [1], Maltese dog [1], miniature poodle [1]). Animals aged less than 1 year accounted for 38% of affected dogs, 37% were 1–5 years old, and 21% were aged>5 years. In 4% of dogs the age was unknown. The domestic shorthair breed accounted for 52% of affected cats. In 11 cats the breed was unknown. Cats less than 1 year of age accounted for 27%, 26% were aged 1–5 years, and 26% were>5 years old. In 21%, the age was unknown. The sexes were represented equally in both species.
Clinical Signs
Neurological signs, including ataxia, seizures and central blindness, were reported in 35 dogs (68%). Nine dogs (17%) showed other symptoms such as vomiting and diarrhoea but lacked neurological signs. One dog died spontaneously and one under anaesthesia during routine surgery, no clinical signs having been reported before death. In seven dogs no clinical data were available.
Neurological signs were reported in 17 cats (52%). Thirteen (39%) showed gastrointestinal or respiratory signs but not neurological signs. In three cats, one of which died spontaneously, no clinical data were available.
Histopathology
Lymphohistiocytic, perivascular infiltrates were found in 40 dogs (75%) and 23 cats (70%). Predominantly granulomatous infiltrates were found in 10 dogs and two cats. Mixed inflammatory infiltrates appeared more often in cats (six animals) than in dogs (two animals). Two cats and one dog exhibited pyogranulomatous inflammation. In 39 dogs (74%), encephalitis was the most prominent finding. In 13 dogs (25%), infiltrates were restricted to the meninx and a ganglioneuritis without encephalitis or meningitis was found in one animal. In 26 cats (79%), encephalitis was observed. In six cats (18%), infiltrates were restricted to the meninx and a chorioiditis without encephalitis was found in one animal.
Six dogs showed, in addition to the inflammatory changes in the CNS, mild-to-moderate vacuolation of the white matter of the cerebrum (three animals) or cerebellum (one animal) or both (two animals). Luxol-fast-blue staining revealed demyelination in two of these dogs. Only one of these six dogs showed an inflammatory infiltrate within the areas of vacuolation.
Two cats showed in addition to the inflammatory changes in the CNS, mild vacuolation of the white matter of the cerebellum (one animal; severe) or both cerebrum and cerebellum (one animal; mild). Luxol-fast-blue staining did not reveal demyelination.
Of the nine dogs without neurological signs, one showed mild, focal meningitis and two showed mild, multifocal meningitis. Moderate inflammation, consisting of multifocal or focal infiltrations in various brain regions (midbrain, cerebellum, meninges, choroid plexus) was observed in six dogs. Inflammatory changes were lymphohistiocytic (six cases), mixed (two cases) or granulomatous (one case).
The 13 cats without neurological signs showed mild, multifocal inflammation of the meninx (two cases), meninx and cerebellum (two), meninx and cerebrum (one), meninx, cerebrum and brainstem (one), meninx and brainstem (one) or choroid plexus (one). Also seen were mild-to-moderate inflammation of the brainstem (one) and of the meninx and cerebral cortex in addition (one). One cat showed moderate, focal meningitis, one a moderate multifocal meningoencephalitis and one a severe, generalized choroiditis. The type of inflammation was lymphohistiocytic in six cases, mixed in three, granulomatous in two, and pyogranulomatous in two.
The gastrointestinal tissue examined in one dog, and the myocardial tissue examined in one dog and two cats, showed no significant histological changes.
Aetiological Diagnosis
In 14 dogs (26%) and 13 cats (39%), viral or bacterial antigens were detected by IHC (Table 3 ), two dogs and one cat giving positive results for two infectious agents simultaneously. Control sections containing the appropriate antigens gave positive signals, in contrast to negative controls. Special histochemical stains invariably gave negative results.
Table 3.
Antigens of various infectious agents detected in the CNS of dogs and cats with non-suppurative meningoencephalitis
|
Number of positive cases in |
||
|---|---|---|
| Infectious agent | dogs | cats |
| Porcine herpesvirus 1 | 1 | 0 |
| Parvovirus | 5 | 1 |
| Feline infectious peritonitis virus | 0 | 3 |
| Feline leukaemia virus | nd | 1 |
| West Nile virus (WNV) | 5 | 4 |
| WNV and canine parainfluenza virus | 1 | 0 |
| WNV and encephalomyocarditis virus (EMCV) | 1 | 1 |
| EMCV | 4 | 4 |
| E. coli | 0 | 1 |
nd, Not done.
In 12 of the 14 positive dogs and nine of the 13 positive cats, reactions were found only in CNS regions infiltrated with inflammatory cells. The remaining two dogs and four cats showed immunoreactivity only in CNS regions without inflammatory infiltration.
Of the 14 positive dogs, 12 (94%), had shown neurological signs, almost always said to be severe. It was not possible, however, to relate the histological or IHC results to the clinical signs. One dog positive for EMCV antigen did not show neurological signs, and for one dog positive for WNV antigen no clinical data were available.
Of the 13 positive cats, eight had shown neurological signs (severe in three cases). In the remaining five cats, no statement regarding the severity of signs was recorded. Four cats with a positive immunoreaction showed no neurological signs. For one positive cat, no clinical data were available.
Clinical and Light Microscopical Findings in Aetiologically Diagnosed Cases
In a 2-year-old male Canadian shepherd dog with diarrhoea and progressive convulsions, porcine herpesvirus-1 (PHV-1) antigen was demonstrated in neuronal perikarya of the gyrus parahippocampalis. No PHV-1 antigen was detected in the hippocampus, cerebellum, brain stem, spinal cord, remaining part of the cerebrum, or the gastrointestinal tract. Histologically, there was mild, lymphohistiocytic polioencephalitis in the gyrus parahippocampalis and occasional neuronal necrosis in the CA1 and CA2 regions of the hippocampus.
Parvovirus antigen was demonstrated in the CNS of five Greek hunting dog puppies from two litters originating from the same breeder, and in a 2-week-old cat. Clinically, the dogs had shown generalized tremor and jumping movements in the hind limbs, and the neurological signs in the cat were characterized by severe ataxia, opisthotonus and convulsions. Neither in the dogs nor in the cat were cerebellar hypoplasia or necrotic Purkinje cells observed. Histopathologically, all affected dogs showed mild to moderate lymphohistiocytic meningitis or leucoencephalitis (or both) as well as mild to moderate, and in one case severe, vacuolation in the white matter of cerebrum and cerebellum. Parvovirus antigen was demonstrated in periventricular cells resembling spongioblasts, in macrophages, microglia and astrocytes, and in cells of the outer granular layer of the cerebellum (Fig. 1 ). The histopathological changes in the cat consisted of mild, lymphohistiocytic inflammation of the meninges and cerebellum, without vacuolation. Parvovirus antigen was detected in the cytoplasm and nucleus of numerous small and large neurons, mainly in the granular layer and outer granular layer of the cerebellum, in the grey matter of the spinal cord, and multifocally in most other brain regions (Fig. 2 ). In addition, parvovirus antigen was observed in macrophages, microglia, astrocytes and ependymal cells.
Fig. 1.
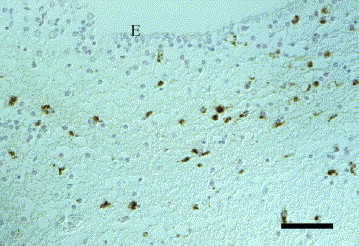
Dog brain, cerebrum. Positive immunoreaction for parvovirus antigen in nucleus and cytoplasm of periventricular cells resembling spongioblasts. E, ependyma. IHC. Bar, 50 μm.
Fig. 2.
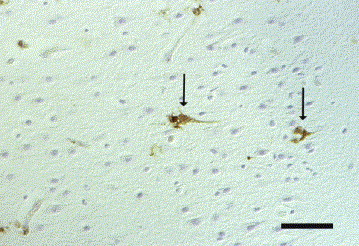
Cat brain, cerebrum. Positive immunoreaction for parvovirus antigen in nucleus and cytoplasm of neurons (arrows). IHC. Bar, 50 μm.
FIP virus antigen was detected in three cats aged 6, 18 and 36 months. Two affected cats had shown increasing apathy and recurrent fever attacks (one animal) or diarrhoea and dyspnoea (one). No clinical data were available for the third cat. Viral antigen was demonstrated in the cytoplasm of macrophages within the inflamed brain regions in all three cases. Histologically, lesions consisted of pyogranulomatous and mixed infiltrates in the plexus chorioideus and the meninges. The third cat showed moderate lymphohistiocytic leucoencephalitis in the caudal regions of the cerebrum.
Weak immunolabelling for feline leukaemia virus antigen was seen in microglia and astrocytes in the hypothalamus of a 9.5-year-old European shorthair cat with progressive apathy and increased salivation (Fig. 3 ). Histopathological changes consisted of mild multifocal lymphohistiocytic meningitis and severe satellitosis in the cerebral cortex. No lymphoid tissues or other organs were available for further investigations.
Fig. 3.
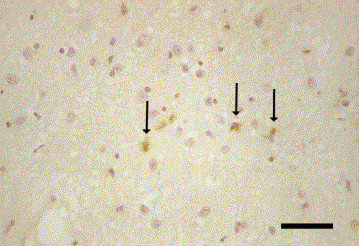
Cat brain, hypothalamus. Positive immunoreaction for feline leukaemia virus antigen in the cytoplasm of microglia and astrocytes (arrows). IHC. Bar, 50 μm.
Five dogs (8.9%) and four cats (11.8%) showed immunoreactivity for West Nile virus (WNV) antigen. In one dog, canine parainfluenza virus (CPIV) antigen was also detected in the same brain region (see also below). One of these dogs, and one cat also gave positive reactions with EMCV-specific antibodies (see also below). In four of the five dogs, severe neurological signs, including ataxia, convulsions and central blindness, had been recorded. No clinical data were available for the fifth dog. All four cats had shown neurological signs, reported in one case to consist of severe circling, hypermetria and blindness. In four of the five dogs and two of the four cats a moderate to severe meningoencephalitis affecting the grey and white matter was observed; this varied from granulomatous, pyogranulomatous, lymphohistiocytic to fibrinopurulent, occasionally associated with neuronal necrosis or malacia. In the remaining dog, inflammation was restricted to the grey matter of the cerebrum and cerebellum, being associated with severe malacia in the hippocampus. One cat showed mild focal, lymphocytic polioencephalitis and moderate focal, lymphohistiocytic meningitis, as well as severe vacuolation of the cerebral white matter and severe generalized neuronal necrosis. In another cat, severe, focal, fibrinopurulent meningitis and severe, acute neuronal necrosis in the hippocampus were observed.
WNV immunolabelling was seen in the cytoplasm of various types of neurons, macrophages, astrocytes and microglia (Fig. 4 ). Strong immunolabelling of neutrophils was observed in two cats with pyogranulomatous inflammation (Fig. 5 ). These immunohistochemical reactions were in general more intense with the anti-NSP-1 WNV antibody than with the anti-MEP-E WNV antibody. In some cases, immunoreactivity was observed with only one of these two WNV monoclonal antibodies. One dog showed immunoreactivity in pyramidal cells of the CA1 and CA2 sector of the hippocampus, and another in the frontal cortex, respectively. A positive reaction in numerous macrophages and microglial cells in the midbrain, pons and frontal cerebrum was detected only with the anti-NSP-1 antibody. One dog showed WNV immunolabelling in numerous macrophages and microglial cells, and pyramidal cells in the cerebrum, hippocampus and midbrain, but only with the anti-NSP-1 antibody. Another dog, in contrast, showed immunoreactivity in a few neurons and macrophages in an inflamed part of the midbrain, but only with the anti-MEP-E antibody. One cat showed immunolabelling with both WNV antibodies in numerous neutrophils and occasional macrophages in the plexus chorioideus and laterally to the third ventricle. In this cat, small neurons, macrophages and microglial cells in the same brain region reacted only with the anti-NSP-1 antibody. In another cat, astrocytes and perivascular macrophages in the lobus parietalis reacted with both antibodies, whereas the anti-NSP-1 antibody additionally labelled pyramidal cells in the same brain region. In one cat, astrocytes and oligodendrocytes of the medulla oblongata displayed more abundant positive signals with the anti-NSP-1 antibody than with anti-MEP-E. Additionally, in this animal, the anti-NSP-1 antibody labelled large neurons in the medulla oblongata which failed to react with the anti-MEP-E antibody.
Fig. 4.
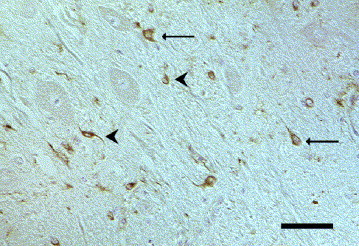
Dog brain, nucleus caudatus. Positive immunoreaction for West Nile virus antigen (anti-NSP-1) in the cytoplasm of small neurons (arrows) and various cells (arrowheads), probably microglia/macrophages and astrocytes. IHC. Bar, 50 μm.
Fig. 5.
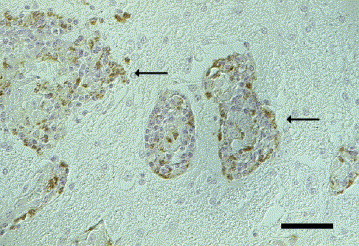
Cat brain, cerebrum. Positive immunoreaction for West Nile virus antigen (anti-NSP-1) in periventricular macrophages and neutrophil granulocytes (arrows). IHC. Bar, 50 μm.
Canine parainfluenza virus (CPIV) antigen was detected in the brain of a 9-year-old male collie that was also positive for West Nile virus antigen (see also WNV, above). This dog had exhibited changed behaviour and was generally weak. Histopathologically, severe periventricular granulomatous meningoencephalitis was found. Viral antigen was demonstrated in the cytoplasm of occasional macrophages in the hypothalamus (Fig. 6 ).
Fig. 6.
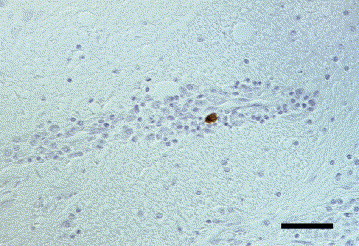
Dog brain, cerebrum. Severe, periventricular, granulomatous encephalitis. Positive immunoreaction for canine parainfluenza virus antigen in the cytoplasm of a macrophage. IHC. Bar, 50 μm.
IHC revealed encephalomyocarditis virus (EMCV) antigen in the brains of four dogs (7.1%) and four cats (11.8%). In one dog and one cat, WNV-antigen was detected in addition (see also WNV, above). The dogs were aged 0.3–8 years and the cats 0.2–10 years. Two domestic shorthairs, one Persian and one cat of unknown breed as well as a Landseer, a West Highland white terrier, a Bordeaux Dogge and an Entlebucher Sennenhund were affected. A granulomatous immune response was found in three of the four dogs. The remaining dog displayed a lymphohistiocytic chorioiditis and meningitis. Infiltration with macrophages, neutrophils, lymphocytes and plasma cells or pyogranulomatous inflammation was present in two of the four cats, and lymphohistiocytic inflammation in the remaining two cats. In three of these four dogs, severe neurological signs had been recorded; the fourth dog had shown dyspnoea and anaemia but no neurological signs. In three of the cats, neurological signs were recorded without reference to their severity. The fourth cat showed respiratory signs only. The eight animals showed different patterns of immunoreactivity. DAB precipitates were observed in the cytoplasm of perivascular and parenchymal macrophages, in the perikarya and processes of various neurons distant from or within the inflammatory lesions (Fig. 7 ), and in periventricular and submeningeal astrocytic foot processes (Fig. 8 ). In one dog and two cats, vessel-associated cells, probably pericytes or endothelial cells, showed immunolabelling (Fig. 9 ). Seven of the eight animals showed immunolabelling with the EMCV-specific monoclonal antibody (mab) 4F3; mab 3E5 gave results in selected cases only. One cat which was negative with the 4F3 antibody gave EMCV-specific reactions with mab 3E5. In one dog the results with mab 4F3 were confirmed with the mab 3E5. The mab 4E4, which was applied only in one cat, confirmed the reactions observed with the 4F3 antibody in numerous cells of the medulla oblongata and in perivascular macrophages and occasional neurons of the mediocaudal thalamus. The histologically intact myocardial tissue available from one dog and two cats was not immunolabelled with the mab 4F3.
Fig. 7.
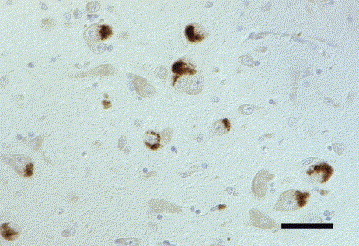
Dog brain, hypothalamus. Positive immunoreaction for encephalomyocarditis virus antigen (mab 4F3) in perikarya of large neurons. IHC. Bar, 50 μm.
Fig. 8.
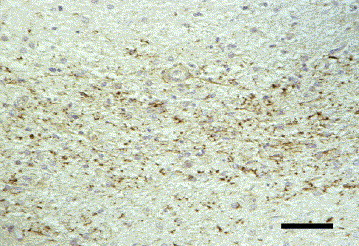
Dog brain, cerebrum. Positive immunoreaction for encephalomyocarditis virus antigen (mab 4F3) in periventricular astrocytes. IHC. Bar, 50 μm.
Fig. 9.
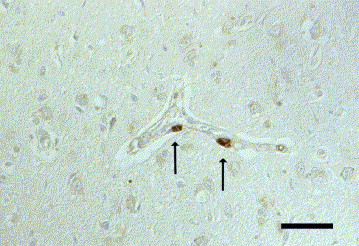
Cat brain, cerebrum. Positive immunoreaction for encephalomyocarditis virus antigen (mab 4F3) in vessel-associated cells (arrows). IHC. Bar, 50 μm.
E. coli antigen was demonstrated in the brain of a 5-month old male cat. Histopathologically, mild to moderate, multifocal, perivascular infiltration with lymphocytes, plasma cells, macrophages and neutrophilic granulocytes was observed in the cerebellum and meninges. Moderate numbers of bacterial emboli in various vessels were visible in HE-stained sections. E. coli antigen was demonstrated immunohistochemically in the cytoplasm of numerous perivascular, meningeal and periventricular macrophages, and in intravascular bacteria.
Cases remaining unclear included 39 (74%) dogs and 20 (61%) cats, no infectious agent being identified as the underlying cause of the observed non-suppurative meningoencephalitis.
Discussion
In the present study, CNS tissue of 53 dogs and 33 cats with non-suppurative meningoencephalitis of unknown cause was investigated for the presence of 18 different infectious agents. Former studies either investigated at most six agents or were based on cases selected only on the presence of neurological signs (Tipold, 1995; Quesnel et al., 1997; Bradshaw et al., 2004; K. Melzer, personal communication).
As in other studies (Sorjonen, 1992; Tipold, 1995), no specific canine breed predisposition was observed. Cases were seen more often in toy breeds than in other breeds, but this may merely have reflected the normal distribution of dog breeds in Germany. So far, no breed predisposition for non-suppurative meningoencephalitis has been described in respect of the cat (Sorjonen, 1992). In the present study, domestic shorthair cats accounted for more than half of the feline cases, probably due to the normal feline breed distribution in Germany.
The CNS of the majority of dogs (40/53) and cats (23/33) examined showed lymphohistiocytic perivascular infiltrates. This is generally suggestive of a viral aetiology (Summers et al., 1995) but has also been reported following hypoxic or toxic damage, which also sometimes produces demyelinating lesions (Braund, 1980; Lee et al., 2002).
Granulomatous CNS infiltrates, observed in 10 dogs and two cats, are often considered to be associated with fungal, parasitic or bacterial infections. Fungal infections occur mainly in warmer climates (Gerds-Grogan and Dayrell-Hart, 1997) and, like parasitic infections, were not detected in the present study. In the 10 dogs referred to above, granulomatous meningoencephalitis (GME) was suggested by the histopathological findings and distribution of lesions. In six of these cases, toy breeds were affected (two West Highland white terriers, two chihuahuas, a shih tzu and a rehpinscher). Interestingly, three of the affected dogs belonged to large breeds (Landseer, Bordeaux Dogge and collie), in which GME is only rarely reported (Braund, 1985). GME has been thought to be associated with viral infections including canine distemper, but no viral agent has been demonstrated in the CNS so far (Thomas and Eger, 1989; Kipar et al., 1998). E. coli antigen, as found in the brains of beagles with granulomatous leptomeningitis (Maeda et al., 1993) or in association with human cases of granulomatous encephalitis (Rickert et al., 2000), was not observed in the present cases with suspected GME. The cat with abundant E. coli antigen in the brain in the present study showed perivascular infiltrations, indicating septicaemia.
Pyogranulomatous and mixed inflammatory infiltration in the CNS occurred less often in dogs than in cats. In cats, this type of change is reminiscent of FIP (Rand et al., 1994). In the present study, FIP virus antigen was also detected in a cat showing lymphohistiocytic leucoencephalitis, a rare consequence of FIP (Summers et al., 1995; Braund, 2001). In dogs, pyogranulomatous or mixed inflammatory changes in the CNS are occasionally described, but no infectious agents have so far been detected (Meric, 1988).
It cannot be ruled out that aetiologically undetermined feline cases were due to feline immunodeficiency virus (FIV), which leads to lymphocytic encephalitis in 30% of naturally infected cats (Gunn-Moore et al., 1996). Only five of the 33 cats had been examined serologically for FIV, a positive result having being obtained in one animal.
In a Canadian shepherd dog, porcine herpesvirus 1 (PHV 1) antigen was detected in neurons of the gyrus parahippocampalis. Due to a retrograde neural route of infection, PHV 1 antigen usually occurs in the brain stem or spinal cord ganglions (Quiroga et al., 1998; Honavar and Meldrum, 2002). The dog in this study showed diarrhoea for 3 weeks, reminiscent of the rare alimentary form of Aujeszky disease (Pensaert and Maes, 1987); however, no PHV 1 antigen was detected in the gastrointestinal tract. Only later did the animal develop neurological signs, and an unusual portal of entry of the virus to the brain remains a possibility.
Neither the five dogs nor the cat with parvovirus infection of the brain showed typical CNS manifestations, such as cerebellar hypoplasia or death of Purkinje cells (Sharp et al., 1999), or encephalomalacia (Johnson and Castro, 1984; Agungpriyono et al., 1999). The findings indicated, however, a potential association between parvovirus and spongiform lesions in the CNS.
In chronic feline leukaemia virus (FeLV) infection, lymphocytic infiltration of peripheral nerves of the spinal cord has been reported occasionally (Pedersen, 1987). In the brain, however, FeLV infection has been associated only with degenerative CNS disease (Carmichael et al., 2002). In one cat, FeLV infection of microglia and astrocytes was detected. Histologically, this animal showed mild, multifocal, lymphohistiocytic meningitis. Since FeLV regularly persists for years in lymphocytes and macrophages (Rohn and Overbaugh, 1999), a persistent infection of glial cells seemed possible but unfortunately no lymphoid tissue, including bone marrow (in which FeLV-antigen can typically be demonstrated), was available.
Surprisingly, five (9.4%) dogs and four (12%) cats showed immunolabelling for WNV. Histopathologically, these animals showed mild to moderate meningoencephalitis in the grey and white matter, mostly with granulomatous or pyogranulomatous inflammation. In contrast, previous reports of WNV infection in birds and in mammals indicated that the grey matter was mainly affected and that the histopathology was characterized by lymphoplasmahistiocytic inflammation with small numbers of neutrophils (Steele et al., 2000; Cantile et al., 2001; Buchweitz et al., 2003; Kelly et al., 2003; Lichtensteiger et al., 2003). Polyclonal WNV-specific antibodies used for immunolabelling often cross-react with other flaviviruses (Steele et al., 2000; Kelly et al., 2003; Chvala et al., 2004). The monoclonal antibodies specific for the major envelope protein E (anti-MEP) and non-structural protein 1 (anti-NSP-1) used in this study (Table 1, Table 2) cross-react with Kunjin virus, also a member of the Flaviviridae (Scherret et al., 2001). Presumably, the anti-NSP-1 antibody is more specific than the anti-MEP antibody (Hall, 2000). This might explain its stronger immunoreactivity in the present study and might support the identification of a WNV infection. The affected animals did not react positively for tick-borne encephalitis (TBE) virus antigen with a polyclonal specific antibody that may cross-react with other flaviviruses (H. Weissenböck, personal communication). However, it is still possible that the positive WNV reaction was due to infection with other cross-reactive agents or represented molecular mimicry of host-derived antigens (Oldstone, 1998). The latter possibility receives some support from studies indicating that dogs and cats can be infected with WNV but usually develop only a low viraemia, without clinical signs or histopathological changes in the brain (Blackburn et al., 1989; Lichtensteiger et al., 2003; Austgen et al., 2004). Furthermore, no evidence of WNV infections in domestic animals has so far been detected in Germany. Therefore, the significance of the present findings requires further studies.
Natural infections of dogs with canine parainfluenza virus (CPIV) are typically associated with hydrocephalus and necrotizing periventricular encephalitis, but neither viral antigen nor RNA has been detected so far in spontaneous cases (Baumgärtner et al., 1982b; Vieler et al., 1994; Cantile et al., 1997). After experimental infection with CPIV, viral antigen was detected in ependymal cells and neurons and in macrophages, in ferrets and dogs respectively (Baumgärtner et al., 1989a, Baumgärtner et al., 1982a, Baumgärtner et al., 1982b; W. Baumgärtner, unpublished). CPIV infection of brain macrophages was also observed in the present study in a collie with granulomatous encephalitis characteristic of GME. Interestingly, this dog also showed immunolabelling of WNV antigen. It remains unclear, therefore, whether the detection of CPIV antigen represented a true infection, or whether immunoreactivity was due to a cross-reaction of the antibody with degraded cellular antigens, or to molecular mimicry (Oldstone, 1998).
A further surprising finding was the detection of EMCV antigen in the CNS of four dogs and four cats. EMCV-specific antibodies have been detected in primates, mice, rats, horses and elephants in various parts of the world, the rat being the natural host (Nowotny et al., 1993; Nowotny, 1996; Psalla et al., 2006). In Austria, a low seroprevalence has been reported in cats (Nowotny, 1996), but not dogs. In Germany, EMCV-associated non-suppurative meningoencephalitis is reported occasionally in man, but the virus has been isolated from the CNS in only a few cases (Nowotny et al., 1993; LaRue et al., 2003). The histopathological changes reported here varied (neuronal necrosis, mild lymphohistiocytic meningitis, severe generalized granulomatous or pyogranulomatous meningoencephalitis). In pigs with natural EMCV infections, lymphohistiocytic infiltrates in the CNS have been described (Gainer et al., 1968). Experimentally infected mice and pigs also develop lymphohistiocytic, perivascular infiltrates in the CNS, poliomeningoencephalomyelitis being the predominant feature in mice (Topham et al., 1991; LaRue et al., 2003). In the present study, EMCV immunolabelling was observed in perikarya and processes of neurons in some animals, indicating neurotropism similar to that observed in experimental EMCV infection. The EMCV-positive cells, associated with cerebral vessels, may have been endothelial cells or pericytes. Neither cell type has been reported to be susceptible to EMCV, but endothelial and mesenchymal cells in organs other than the CNS are regularly infected with EMCV, as shown by Papaioannou et al. (2003) in pigs. The detection of EMCV antigen in macrophages in the CNS of dogs and cats may support their role in viral replication and distribution, as proposed by Papaioannou et al. (2003). In natural EMCV infection of pigs, interstitial myocarditis and myocardial degeneration represent the main features (Papaioannou et al., 2003). Neither change was observed in any of the present cases, but myocardial tissue was available from only one dog and two cats.
Despite the broad spectrum of antibodies used, the aetiology of the CNS lesions could be determined in only 26% and 39% of the cases in dogs and cats, respectively. Due to the retrospective character of the study, a standardized clinical and neurological investigation and serum and CSF laboratory procedures were not possible. In the remaining cases, infection cannot be ruled out. Other agents that might have caused the observed histopathological changes in dogs include Ehrlichia spp. (Glaus and Jaggy, 1992; Maretzki et al., 1994), Leptospira spp. (Rentko et al., 1992) and Borrelia spp. (Mandel et al., 1993; Chang et al., 2001), and in cats Bartonella henselae (Breitschwerdt et al., 1999) cannot be excluded. However, the high percentage of cases in which no infectious agent was identified suggests that non-infectious causes are more common than anticipated. Various factors, ranging from autoimmune processes to intoxication, have already been suggested as causes of non-suppurative changes in the CNS (Kipar et al., 1998; Uchida et al., 1999, Lee et al., 2002).
The immunohistochemical reactions for WNV, CPIV and EMCV, which were unexpected, were possibly due to molecular mimicry, a phenomenon reported for various paramyxoviruses and for Japanese encephalitis virus (JPEV; Shesberadaran and Norrby, 1984), which belongs to the Flaviviridae. In most of the cases showing these unexpected reactions the histopathological changes were severe and often resembled GME. Autoimmune mechanisms have already been discussed as a possible cause of canine GME and pug dog encephalitis (Kipar et al., 1998; Uchida et al., 1999). Other factors that may have played a role in the findings described include the following: epitope spreading (Bluestone and Miller, 1998; Vanderlugt et al., 1998); hereditary factors, as already suspected in pug dog encephalitis and non-suppurative meningoencephalitis in greyhounds (Hinrichs et al., 1996; Uchida et al., 1999; Callanan et al., 2002); intoxication (Lee et al., 2002); increased permeability of the blood-brain barrier in systemic illness, due to prostaglandins and interleukin (IL)-1, IL-6 or tumour necrosis factor (TNF)-α, leading to infiltration of the CNS by inflammatory cells (Reiss et al., 2000). In addition, it should be mentioned that, unlike paraneoplastic encephalitis, which has been described in man but not in dogs or cats (Scaravilli et al., 1999), paraneoplastic polyneuropathy has been described in dogs (Braund, 1990; Wagner, 2002). As tumours in the CNS or other organs were present in only one of the cases examined, paraneoplastic encephalitis seemed unlikely to have played any role in the findings.
In conclusion, in a high proportion of dogs and cats with non-suppurative meningoencephalitis, immunohistochemical examination with antibodies against 18 different infectious agents failed to reveal a cause. The significance of positive immunoreactions obtained with WNV and EMCV antibodies requires further investigation. In addition, primary or virus-triggered secondary immune-mediated mechanisms should be considered in many inflammatory CNS disorders of dogs and cats.
Acknowledgments
For providng antibodies, the authors thank: Prof. Haas, Institute of Virology, University of Veterinary Medicine Hannover, Germany, Prof. Richt, National Animal Disease Center, Ames, Iowa, USA; Dr Eskens, Veterinär-Untersuchungsamt Mittelhessen; Prof. Holzmann, Institute of Virology, University of Vienna; Prof. Groschup, Bundesforschungsanstalt für Viruskrankheiten der Tiere, Insel Riems; Dr Randall, Department of Biochemistry and Microbiology, University of St Andrews, UK; and Dr Örvell, Huddinge Hospital, Huddinge, Sweden. Thanks are also due to Mrs Petra Grünig and Mrs Danuta Waschke for excellent technical support.
References
- Agungpriyono D.R., Uchida K., Tabaru H., Yamaguchi R., Tateyama S. Subacute massive necrotizing myocarditis by canine parvovirus type 2 infection with diffuse leukoencephalomalacia in a puppy. Veterinary Pathology. 1999;36:77–80. doi: 10.1354/vp.36-1-77. [DOI] [PubMed] [Google Scholar]
- Austgen L.E., Bowen R.A., Bunning M.L., Davis B.S., Mitchell C.J., Chang G. Experimental infection of dogs and cats with West Nile virus. Emerging Infectious Diseases. 2004;10:82–86. doi: 10.3201/eid1001.020616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn H. The proteolytic pretreatment of formalin-fixed tissue in immunohistochemical diagnosis. Acta Histochemica, Supplements. 1988;35:151–158. [PubMed] [Google Scholar]
- Baumgärtner W., Krakowka S., Gorham J.R. Canine parainfluenza virus-induced encephalitis in ferrets. Journal of Comparative Pathology. 1989;100:67–76. doi: 10.1016/0021-9975(89)90091-1. [DOI] [PubMed] [Google Scholar]
- Baumgärtner W., Krakowka S., Koestner A., Evermann J. Acute encephalitis and hydrocephalus in dogs caused by canine parainfluenza virus. Veterinary Pathology. 1982;19:79–92. doi: 10.1177/030098588201900111. [DOI] [PubMed] [Google Scholar]
- Baumgärtner W., Krakowka S., Koestner A., Evermann J. Ultrastructural evaluation of acute encephalitis and hydrocephalus in dogs caused by canine parainfluenza virus. Veterinary Pathology. 1982;19:305–314. doi: 10.1177/030098588201900308. [DOI] [PubMed] [Google Scholar]
- Baumgärtner W., Örvell C., Reinacher M. Naturally occurring canine distemper virus encephalitis: distribution and expression of viral polypeptides in nervous tissues. Acta Neuropathologica. 1989;78:504–512. doi: 10.1007/BF00687712. [DOI] [PubMed] [Google Scholar]
- Bauriedel G.L. Chlamydia pneumoniae in koronarem Plaquegewebe: Vermehrter Nachweis bei akutem Koronarsyndrom. (In German.) Deutsche Medizinische Wochenschrift, 1999;124:375–380. doi: 10.1055/s-2007-1024309. [DOI] [PubMed] [Google Scholar]
- Blackburn N.K., Reyers F., Berry W.L., Shepherd A.J. Susceptibility of dogs to West Nile virus: a survey and pathogenicity trial. Journal of Comparative Pathology. 1989;100:59–66. doi: 10.1016/0021-9975(89)90090-x. [DOI] [PubMed] [Google Scholar]
- Bluestone J.A., Miller S.D. The functional significance of epitope spreading and its regulation by co-stimulatory molecules. Immunological Reviews. 1998;164:63–72. doi: 10.1111/j.1600-065x.1998.tb01208.x. [DOI] [PubMed] [Google Scholar]
- Bradshaw J.M., Pearson G.R., Gruffydd-Jones T.J. A retrospective study of 238 cases of neurological disorders of the cat. Journal of Comparative Pathology. 2004;131:112–120. doi: 10.1016/j.jcpa.2004.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braund K.G. Encephalitis and meningitis. Veterinary Clinics of North America: Small Animal Practice. 1980;10:31–56. doi: 10.1016/s0195-5616(80)50002-1. [DOI] [PubMed] [Google Scholar]
- Braund K.G. Granulomatous meningoencephalomyelitis. Journal of the American Veterinary Medical Association. 1985;186:138–141. [PubMed] [Google Scholar]
- Braund K.G. Remote effects of cancer on the nervous system. Seminars in Veterinary Medicine and Surgery (Small Animals) 1990;5:262–270. [PubMed] [Google Scholar]
- Braund, K.G. (2001). Inflammatory diseases of the central nervous system. In: Clinical Neurology in Small Animals—Localization, Diagnosis and Treatment, K.G. Braund, Ed., International Veterinary Information Service, Ithaca.
- Braund K.G., McGuire J.A., Amling K.A., Henderson R.A. Peripheral neuropathy associated with malignant neoplasms in dogs. Veterinary Pathology. 1987;24:16–21. doi: 10.1177/030098588702400104. [DOI] [PubMed] [Google Scholar]
- Breitschwerdt E.B., Atkins C.E., Brown T.T., Kordick D.L., Bucheler J. Fading kitten syndrome and neonatal isoerythrolysis. Veterinary Clinics of North America: Small Animal Practice. 1999;29:853–870. [PubMed] [Google Scholar]
- Buchweitz S., Kleiboecker S., Marioni K., Ramos-Vara J., Rottinghaus A., Schwabenton B., Johnson G. Serological, reverse transcriptase-polymerase chain reaction, and immunohistochemical detection of West Nile virus in a clinically affected dog. Journal of Veterinary Diagnostic Investigation. 2003;15:324–329. doi: 10.1177/104063870301500404. [DOI] [PubMed] [Google Scholar]
- Callanan J.J., Mooney C.T., Mulkahy G., Fatzer R., Vandevelde M., Ehrensperger F., McElroy M., Toolan D., Raleigh P. A novel non-suppurative meningoencephalitis in young greyhounds in Ireland. Veterinary Pathology. 2002;39:56–65. doi: 10.1354/vp.39-1-56. [DOI] [PubMed] [Google Scholar]
- Cantile C., Arispici M., Modenato M., Fatzer R. Hydrocephalus with periventricular encephalitis in the dog. Journal of Veterinary Medicine A. 1997;44:595–601. doi: 10.1111/j.1439-0442.1997.tb01145.x. [DOI] [PubMed] [Google Scholar]
- Cantile C., Del Piero F., Di Guardo G., Arispici M. Pathologic and immunhistochemical findings in naturally occurring West Nile infection in horses. Veterinary Pathology. 2001;38:414–421. doi: 10.1354/vp.38-4-414. [DOI] [PubMed] [Google Scholar]
- Carmichael K.P., Bienzle D., McDonnell J.J. Feline leukaemia virus-associated myelopathy in cat. Veterinary Pathology. 2002;39:536–545. doi: 10.1354/vp.39-5-536. [DOI] [PubMed] [Google Scholar]
- Chang Y.F., Novosel V., Chang C., Summers B.A., Ma D., Chiang Y., Acree W.M., Chu H., Shin S., Lein D.H. Experimental induction of chronic borreliosis in adult dogs exposed to Borrelia burgdorferi-infected ticks and treated with dexamethasone. American Journal of Veterinary Research. 2001;62:1104–1112. doi: 10.2460/ajvr.2001.62.1104. [DOI] [PubMed] [Google Scholar]
- Chvala S., Kolodziejek J., Nowotny N., Weissenböck H. Pathology and viral distribution in fatal Usutu virus infections in birds from the 2001 and 2002 outbreaks in Austria. Journal of Comparative Pathology. 2004;131:176–185. doi: 10.1016/j.jcpa.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Durchfeld B., Baumgärtner W., Krakowka S. Intranasal infection of ferrets (Mustela putorius furo) with canine parainfluenza virus. Zentralblatt für Veterinärmedizin B. 1991;38:505–512. doi: 10.1111/j.1439-0450.1991.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Elder J., McGee J.S., Munson M., Houghten R.A., Kloetzer W., Bittle J.L., Grant C.K. Localization of neutralizing regions of the envelope gene of feline leukaemia virus by using anti-synthetic peptide antibodies. Journal of Virology. 1987;61:8–15. doi: 10.1128/jvi.61.1.8-15.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskens U., Hamann H.P., Kilz P., Zschöck M. Zur Diagnostik der Aujeszkyschen Krankheit—eine vergleichende Untersuchung immunhistologischer und virologisch-kultureller Nachweismethoden. (In German.) Tierärztliche Umschau, 1991;46:502–513. [Google Scholar]
- Gainer J.H., Sandefur J.R., Bigler W.J. High mortalitiy in a Florida swine herd infected with the encephalomyocarditis virus. An accompanying epizootiological survey. Cornell Veterinarian. 1968;58:31–47. [PubMed] [Google Scholar]
- Gerds-Grogan S., Dayrell-Hart B. Feline cryptococcosis: a retrospective evaluation. Journal of the American Animal Hospital Association. 1997;33:118–122. doi: 10.5326/15473317-33-2-118. [DOI] [PubMed] [Google Scholar]
- Glaus T., Jaggy A. Ehrlichiose beim Hund: Literaturübersicht und Fallbeschreibung (In German.) Schweizer Archiv für Tierheilkunde. 1992;134:319–323. [PubMed] [Google Scholar]
- Gunn-Moore D.A., Pearson G.R., Harbour D.A., Whiting C.V. Encephalitis associated with giant cells in a cat with naturally occurring feline immunodeficiency virus infection demonstrated by in situ -hybridization. Veterinary Pathology. 1996;36:699–703. doi: 10.1177/030098589603300610. [DOI] [PubMed] [Google Scholar]
- Hall R.A. The emergence of West Nile virus: the Australian connection. Viral Immunology. 2000;13:447–461. doi: 10.1089/vim.2000.13.447. [DOI] [PubMed] [Google Scholar]
- Hardt M., Baron T., Groschup M.H. A comparative study of immunohistochemical methods for detecting abnormal prion protein with monoclonal and polyclonal antibodies. Journal of Comparative Pathology. 2000;122:43–53. doi: 10.1053/jcpa.1999.0343. [DOI] [PubMed] [Google Scholar]
- Harris C.W., Didier P.J., Parker A.J. Simultaneous central nervous system reticulosis in two related Afghan hounds. Compendium on Continuing Education for the Practicing Veterinarian. 1988;10:308–310. [Google Scholar]
- Herden, C., Herzog, S., Wehner, T., Richt, J.A. and Frese, K. (1999). Comparison of different methods of diagnosing Borna disease in horses post mortem. In: Equine Infectious Diseases, U. Wernery, J. Wade, J. A. Mumford and O.R. Kaaden, Eds, R&W Publications, Newmarket, pp. 286–290.
- Hinrichs U., Tobias R., Baumgärtner W. Ein Fall von nekrotisierender Meningoencephalitis beim Mops (pug dog encephalitis—PDE) (In German.) Tierärztliche Praxis, 1996;24:489–492. [PubMed] [Google Scholar]
- Honavar, M. and Meldrum, B. S. (2002). Epilepsy. In: Greenfield's Neuropathology, D. I. Graham and P. L. Lantos, Eds, Arnold, London, pp. 899–931.
- Hsu S.M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex, ABC, in immunoperoxidase techniques. Journal of Histochemistry and Cytochemistry. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Johnson B.J., Castro A.E. Isolation of canine parvovirus from a dog brain with severe necrotizing vasculitis and encephalomalacia. Journal of the American Veterinary Medical Association. 1984;184:1398–1399. [PubMed] [Google Scholar]
- Kahveci Z., Minbay F.Z., Noyan S.U., Cavusoglu I. A comparison of microwave heating and proteolytic pretreatment antigen retrieval techniques in formalin fixed, paraffin embedded tissues. Biotechnic and Histochemistry. 2003;78:119–128. doi: 10.1080/10520290310001593775. [DOI] [PubMed] [Google Scholar]
- Kelly J.K., Pai C.H., Jadusingh I.H., Macinnis M.L., Shaffer E.A., Hershfield N.B. The histopathology of rectosigmoid biopsies from adults with bloody diarrhea due to verotoxin-producing E. coli. American Journal of Clinical Pathology. 1987;88:78–82. doi: 10.1093/ajcp/88.1.78. [DOI] [PubMed] [Google Scholar]
- Kelly T., Prayson R.A., Ruiz A.I., Isada C.M., Gordon S.M. The neuropathology of West Nile virus meningoencephalitis. American Journal of Clinical Pathology. 2003;119:749–754. doi: 10.1309/PU4R-76JJ-MG1F-81RP. [DOI] [PubMed] [Google Scholar]
- Kipar A., Baumgärtner W., Vogl C., Gaedke K., Wellmann M. Immunohistochemical characterization of inflammatory cells in brains of dogs with granulomatous meningoencephalomyelitis. Veterinary Pathology. 1998;35:43–52. doi: 10.1177/030098589803500104. [DOI] [PubMed] [Google Scholar]
- Kipar A., Kremendahl J., Grant C.K., von Bothmer I., Reinacher M. Expression of viral proteins in feline leukaemia virus-associated enteritis. Veterinary Pathology. 2000;37:129–136. doi: 10.1354/vp.37-2-129. [DOI] [PubMed] [Google Scholar]
- Komar N. West Nile viral encephalitis. Revue Scientifique et Technique (International Office of Epizootics) 2000;19:166–176. doi: 10.20506/rst.19.1.1201. [DOI] [PubMed] [Google Scholar]
- Kovacevic S., Kipar A., Kremendahl J., Teebken-Schuler D., Grant C.K. Immunohistochemical diagnosis of feline leukaemia virus infection in formalin-fixed tissue. European Journal of Pathology. 1997;3:13–19. [Google Scholar]
- LaRue R., Myers S., Brewer L., Shaw D.P., Brown C., Seal B.S., Njenga M.K. A wild-type porcine encephalomyocarditis virus containing a short poly C tract is pathogenic to mice, pigs and cynomolgus macaques. Journal of Virology. 2003;177:9136–9146. doi: 10.1128/JVI.77.17.9136-9146.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebich M., Harder T.C., Frey H.R., Visser I.K., Osterhaus A.D., Liess B. Comparative immunological characterization of type-specific and conserved B-cell epitopes of pinniped, felid and canid herpesviruses. Archives of Virology. 1994;136:335–347. doi: 10.1007/BF01321062. [DOI] [PubMed] [Google Scholar]
- Lee R.Z., Hardiman O., O`Connell P.G. Ibuprofen-induced aseptic meningoencephalitis. Rheumatology. 2002;41:353–355. doi: 10.1093/rheumatology/41.3.353. [DOI] [PubMed] [Google Scholar]
- Lichtensteiger C.A., Heinz-Taheny K., Osborne T.S., Novak R.J., Lewis B.A., Firth M. West Nile encephalitis and myocarditis in wolf and dog. Emerging Infectious Diseases. 2003;9:1303–1306. doi: 10.3201/eid0910.020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren A.L., Zimmermann W., Bode L., Czech G., Gosztonyi G., Lindberg R., Ludwig H. Staggering disease in cats: isolation and characterizaton of the feline borna disease virus. Journal of General Virology. 1995;76:2215–2222. doi: 10.1099/0022-1317-76-9-2215. [DOI] [PubMed] [Google Scholar]
- Luttgen P.J. Inflammatory disease of the central nervous system. Veterinary Clinics of North America: Small Animal Practice. 1988;18:623–640. doi: 10.1016/S0195-5616(88)50059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H., Ozaki K., Horikiri K., Narama I. Granulomatous leptomeningitis in beagle dogs. Veterinary Pathology. 1993;30:566–573. doi: 10.1177/030098589303000611. [DOI] [PubMed] [Google Scholar]
- Mandel N.S., Schneider E.M., Bosler E.M. Intrathecal production of Borrelia burgdorferi-specific antibodies in a dog with central nervous system lyme disease. Compendium on Continuing Education for the Practicing Veterinarian. 1993;15:581–586. [Google Scholar]
- Maretzki C.H., Fisher D.J., Green C.E. Granulocytic ehrlichiosis and meningitis in a dog. Journal of the American Veterinary Medical Association. 1994;205:1554–1556. [PubMed] [Google Scholar]
- Meric S.M. Canine meningitis—a changing emphasis. Journal of Veterinary Internal Medicine. 1988;2:26–35. doi: 10.1111/j.1939-1676.1988.tb01974.x. [DOI] [PubMed] [Google Scholar]
- Nietfeld J.C., Rakich P.M., Rtyler D.E., Bauer R.W. Rabies-like inclusions in dogs. Journal of Veterinary Diagnostic Investigation. 1989;1:333–338. doi: 10.1177/104063878900100410. [DOI] [PubMed] [Google Scholar]
- Nowotny N. Serological studies of domestic cats for potential human pathogenic virus infections from wild rodents. (In German.) Zentralblatt für Hygiene und Umweltmedizin. 1996;196:452–461. [PubMed] [Google Scholar]
- Nowotny N., Holzmann A., Loupal G., Schilcher F. Encephalomyocarditis-Virusinfektion beim Schwein. (In German.) Wiener Tierärztliche Monatsschrift. 1993;80:287–288. [Google Scholar]
- Nowotny N., Weissenböck H. Description of feline meningoencephalomyelitis (“staggering disease”) and studies of its aetiology. Journal of Clinical Microbiology. 1995;33:1668–1669. doi: 10.1128/jcm.33.6.1668-1669.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M.B.A. Molecular mimicry and immune-mediated diseases. FASEB Journal. 1998;12:1255–1265. doi: 10.1096/fasebj.12.13.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou N., Billinis C., Psychas V., Papadopoulos O.U., Vlemmas I. Pathogenesis of encephalomyocarditis virus (EMCV) infection in piglets during the viraemia phase: a histopathological, immunohistochemical and virological study. Journal of Comparative Pathology. 2003;129:161–168. doi: 10.1016/s0021-9975(03)00026-4. [DOI] [PubMed] [Google Scholar]
- Pedersen, N.C. (1987). Feline leukaemia virus. In: Virus Infections of Carnivores, M. J. Appel, Ed., Elsevier, Amsterdam, pp. 299–320.
- Pensaert, M. and Maes, L. (1987). Pseudorabies virus (Aujeszky′s disease). In: Virus Infections of Carnivores, M. J. Appel, Ed., Elsevier, Amsterdam, pp. 17–26.
- Psalla D., Psychas V., Spyrou V., Billinis C., Papaioannou N., Vlemmas I. Pathogenesis of experimental encephalomyocarditis: a histopathological, immunohistochemical and virological study in rats. Journal of Comparative Pathology. 2006;134:30–39. doi: 10.1016/j.jcpa.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Quesnel A.D., Parent J.M., McDonnel W., Percyand D., Lumsden J.H. Diagnostic evaluation of cats with seizure disorders: 30 cases. 1991–1993. Journal of the American Veterinary Medical Association. 1997;210:65–77. [PubMed] [Google Scholar]
- Quiroga M.I., Nieto J.M., Sur J., Osorio F. Diagnosis of Aujeszky′s disease virus infections in dogs by use of immunohistochemistry and in-situ-hybridization. Zentralblatt für Veterinärmedizin A. 1998;45:75–81. doi: 10.1111/j.1439-0442.1998.tb00803.x. [DOI] [PubMed] [Google Scholar]
- Rand J.S., Parent J., Percyand D., Jacobs R. Clinical, cerebrospinal fluid and histological data from twenty-seven cats with primary inflammatory disease of the central nervous system. Canadian Veterinary Journal. 1994;35:103–109. [PMC free article] [PubMed] [Google Scholar]
- Reiss, C.S., Chesler, D.A., Hodges, J., Ireland, D.D.C. and Chen, N. (2000). Innate immune responses in viral encephalitis. In: Protective and Pathological Immune Response, B. Dietzschold and J.A. Richt, Eds, Springer, Berlin, pp. 64–94.
- Rentko V.T., Clark N., Ross L.A., Schelling S.H. Canine leptospirosis: a retrospective study of 17 cases. Journal of Veterinary Internal Medicine. 1992;6:235–244. doi: 10.1111/j.1939-1676.1992.tb00345.x. [DOI] [PubMed] [Google Scholar]
- Rickert C.H., August C., Brandt M., Wagner V., Paulus W. Cerebral malakoplakia associated with E. coli infection. Acta Neuropathologica. 2000;99:595–598. doi: 10.1007/s004010051167. [DOI] [PubMed] [Google Scholar]
- Rohn, J.L. and Overbaugh, J. (1999). Pathogenic feline retroviruses: feline leukaemia virus and feline immunodeficiency virus. In: Persistent Viral Infections, S. Y. Chen and R. Ahmed, Eds, John Wiley and Sons, New York, pp. 379–408.
- Romeis, B. (1994). Mikroskopische Technik, 17th Edit., Urban and Schwarzenberg, Munich. (In German.)
- Scaravilli F., An S.F., Groves M., Thom M. The neuropathology of paraneoplastic syndromes. Brain Pathology. 1999;9:251–260. doi: 10.1111/j.1750-3639.1999.tb00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherret J.H., Poidinger M., Mackenzie J.S., Broom A.K., Deubel V., Lipkin W.I., Briese T., Gould E.A., Hall R.A. The relationships between West Nile and Kunjin Viruses. Emerging Infectious Diseases. 2001;7:697–705. doi: 10.3201/eid0704.010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp N.J.H., Davis B.J., Guy J.S., Cullen J.M., Steingold F.S., Kornegay J.N. Hydranencephaly and cerebellar hypoplasia in two kittens attributed to intrauterine parvovirus infection. Journal of Comparative Pathology. 1999;121:39–53. doi: 10.1053/jcpa.1998.0298. [DOI] [PubMed] [Google Scholar]
- Shesberadaran H., Norrby E. Three monoclonal antibodies against measles virus F protein cross-react with cellular stress proteins. Journal of Virology. 1984;52:995–999. doi: 10.1128/jvi.52.3.995-999.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorjonen D.J. Myelitis and meningitis. Veterinary Clinics of North America: Small Animal Practice. 1992;22:951–964. doi: 10.1016/S0195-5616(92)50086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele K.E., Linn M.J., Schopp R.J., Komar N., Geisbert T.W., Manduca R.M., Calle P.P., Raphael B.L., Clippinger T.L., Larsen T., Smith J., Lanciotti R.S., Panella N.A., McNamara T.S. Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City, New York. Veterinary Pathology. 2000;37:208–224. doi: 10.1354/vp.37-3-208. [DOI] [PubMed] [Google Scholar]
- Sue W., Ikegami H., Nakayama Y., Suzuki K., Katayama K., Nakayama D.K. Susceptibility of primary cell culture neurones from rats of different ages to encephalomyocarditis, EMC, virus infection. Experimental Molecular Pathology. 2003;75:160–164. doi: 10.1016/s0014-4800(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Summers, B.A., Cummings, J.F. and A. De Lahunta (Eds) (1995). Veterinary Neuropathology, Mosby, St Louis.
- Thomas J.B., Eger C. Granulomatous meningoencephalomyelitis in 21 dogs. Journal of Small Animal Practice. 1989;30:287–293. [Google Scholar]
- Tipold A. Diagnosis of inflammatory and infectious diseases of the central nervous system in dogs: a retrospective study. Journal of Veterinary Internal Medicine. 1995;9:304–314. doi: 10.1111/j.1939-1676.1995.tb01089.x. [DOI] [PubMed] [Google Scholar]
- Topham D.J., Adesina A., Shenoy M., Craighead J.E., Sriam S. Indirect role of T cells in development of polioencephalitis and encephalomyelitis induced by encephalomyocarditis virus. Journal of Virology. 1991;65:3238–3245. doi: 10.1128/jvi.65.6.3238-3245.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K., Hasegawa T., Ikeda M., Yamaguchi R., Tateyama S. Detection of an autoantibody from pug dogs with necrotizing encephalitis (pug dog encephalitis) Veterinary Pathology. 1999;36:301–307. doi: 10.1354/vp.36-4-301. [DOI] [PubMed] [Google Scholar]
- Vanderlugt C.L., Begolka W.S., Neville K.L., Katz-Levy Y., Howard L.M., Eagar T.N., Bluestone J.A., Miller S.D. The functional significance of epitope spreading and its regulation by co-stimulatory molecules. Immunological Reviews. 1998;164:63–72. doi: 10.1111/j.1600-065x.1998.tb01208.x. [DOI] [PubMed] [Google Scholar]
- Vandevelde M., Braund K.G. Polioencephalomyelitis in cats. Veterinary Pathology. 1979;16:420–427. doi: 10.1177/030098587901600404. [DOI] [PubMed] [Google Scholar]
- Vieler E., Herbst W., Baumgärtner W., Breuker S. Isolation of parainfluenzavirus type 2 from the prostatic fluid of a dog. Veterinary Record. 1994;135:384–385. doi: 10.1136/vr.135.16.384. [DOI] [PubMed] [Google Scholar]
- Vlemmas J., Papaioannou N., Psychas V., Billinis C., Paschaleri-Papadopoulou E., Brocchi E., Cara E., Papadopoulos O. Immunohistochemical detection of encephalomyocarditis virus (EMCV) both in formalin and ethanol-fixed, paraffin-embedded tissues of pigs. European Journal of Pathology. 1998;4:23–27. [Google Scholar]
- Wagner, H. (2002). Untersuchungen zur paraneoplastischen Polyneuropathie des Hundes. (In German.) Doctoral Thesis. University of Veterinary Medicine Hannover, Germany.
- Weissenböck H., Suchy A., Holzmann A. Tick-borne encephalitis in dogs: neuropathological findings and distribution of antigen. Acta Neuropathologica. 1998;95:361–366. doi: 10.1007/s004010050811. [DOI] [PubMed] [Google Scholar]
- Wünschmann A., Alldinger S., Kremmer E., Baumgärtner W. Identification of CD4+ and CD8+ T cell subsets and B cells in the brain of dogs with spontaneous acute, subacute, and chronic-demyelinating distemper encephalitis. Veterinary Immunology and Immunopathology. 1997;67:101–116. doi: 10.1016/s0165-2427(98)00216-5. [DOI] [PubMed] [Google Scholar]
- Xuan X., Horimoto T., Limcumpao J.A., Tohya Y., Takahashi E., Mikami T. Glycoprotein specific immune response in canine herpesvirus infection. Archives of Virology. 1992;122:359–365. doi: 10.1007/BF01317197. [DOI] [PubMed] [Google Scholar]


