Abstract
Acute fibrinous and organizing pneumonia (AFOP) is a rare histologic interstitial pneumonia pattern recently described in the literature with fewer than 120 cases published. AFOP is often difficult to diagnose and may be mistaken for other pulmonary disorders such as interstitial pneumonias or pneumonitides. Patients often present with vague symptoms of cough, dyspnea, hemoptysis, fatigue, and occasionally respiratory failure. Radiological findings show diffuse patchy opacities and ground glass appearance of the lungs. On histologic examination, intra-alveolar fibrin balls are observed. We discuss a case of a man who presented with hemoptysis and dyspnea and whose open lung biopsy revealed AFOP. We will describe the presentation, diagnosis, and post-discharge course, and review the current literature. There are only 4 cases which have reported the patients’ course of disease after 1 year, the longest being 2 years. To our knowledge, this is the only case of AFOP in the literature that describes the course of a patient more than 2 years after the diagnosis of AFOP, and is the most comprehensive review of the current literature.
Keywords: Acute fibrinous and organizing pneumonia, Interstitial lung diseases
1. Introduction
Acute fibrinous and organizing pneumonia (AFOP) is a rare histologic interstitial pneumonia pattern that is histologically characterized by intra-alveolar fibrin “balls” and organizing pneumonia with a patchy distribution. It is associated with viral, bacterial, and fungal infections, connective tissue disorders, autoimmune diseases, certain drugs including amiodarone, everolimus, decitabine, and abacavir, and occupational/environmental exposures such as asbestos, various dusts, exotic animals, aerosol products, and coal; it may also be idiopathic in nature. Patients present with cough, dyspnea, or acute respiratory distress syndrome. Radiological findings demonstrate diffuse patchy opacities. The diagnosis requires a lung biopsy which can be obtained surgically, via bronchoscopy, or under ultrasound- or computed tomographic (CT) scan-guidance. The definitive management of this disease is still unknown; however, there is a role for steroids and other immunosuppressive agents [1].
We present the case of a man diagnosed with AFOP and describe his 30-month post-discharge course, the longest follow-up in the literature to our knowledge. In addition, we will also present the most comprehensive review of the AFOP literature. Patient characteristics, suspected etiology, diagnostic modality, treatments, and outcomes will be reported.
2. Case report
A 60-year-old male smoker was transferred from an outside hospital presenting with progressively worsening dyspnea and blood-tinged sputum over the course of 2 months. He was otherwise healthy and reported a history of working with asbestos and fiberglass from 1975 to 1988, and recent occupational dust exposure. He complained of low grade fevers and sweats and denied weight loss, other significant exposures, recent travel, pets, and sick contacts. At an outside hospital, a chest x-ray showed bilateral diffuse opacities and small bilateral pleural effusions (Fig. 1A). A CT scan of the chest demonstrated diffuse bilateral ground glass densities (Fig. 1B). Bronchoscopy was performed and biopsies were negative for malignancy, granulomas, Pneumocystis jirovecii cysts, and trophozoites. Gram stain and culture, periodic acid–Schiff stain, and acid fast-bacilli culture were negative. Bronchoalveolar lavage (BAL) showed many red blood cells, few white blood cells, and no organisms. The BAL culture grew few Candida albicans. Routine laboratory work up was normal, except for an elevated white blood cell count. Laboratory work for rheumatoid factor, cold agglutinins, antinuclear antibodies, Scl-70 scleroderma antibody, and histone antibody were negative. In addition, HIV-1 and HIV-2, influenza A and B, urine Legionella antigen, and Mycoplasma pneumoniae IgG and IgM were negative. He received a 7-day course of antibiotics (ceftriaxone, azithromycin, and doxycycline) and methylprednisolone 125 mg IV every 6 hours for 6 days. Despite therapy, his symptoms did not improve and he was transferred to our hospital for further work up.
Fig. 1.
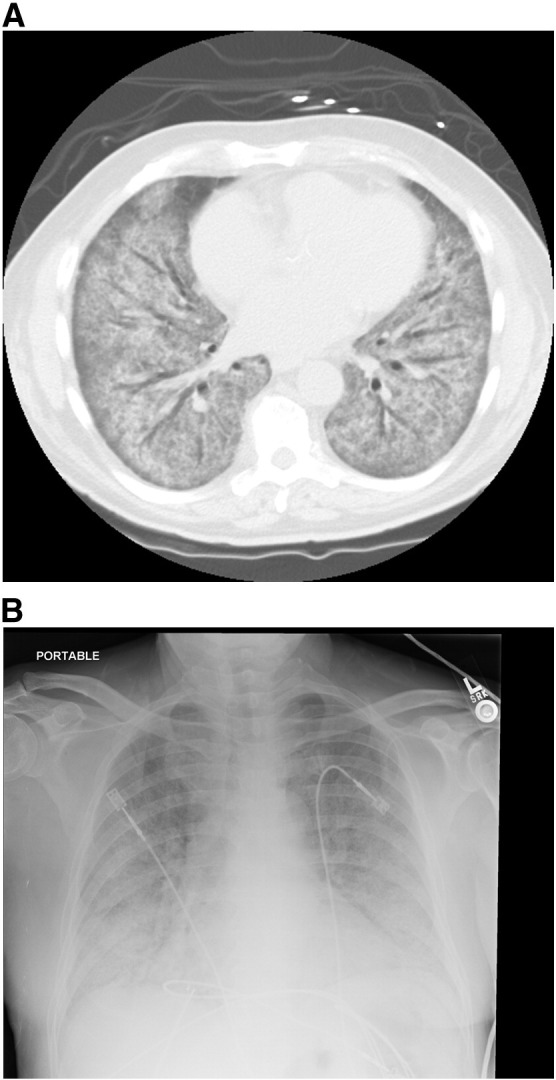
A, Chest x-ray showing bilateral patchy infiltrates.
B, Chest CT scan showing diffuse ground glass opacities.
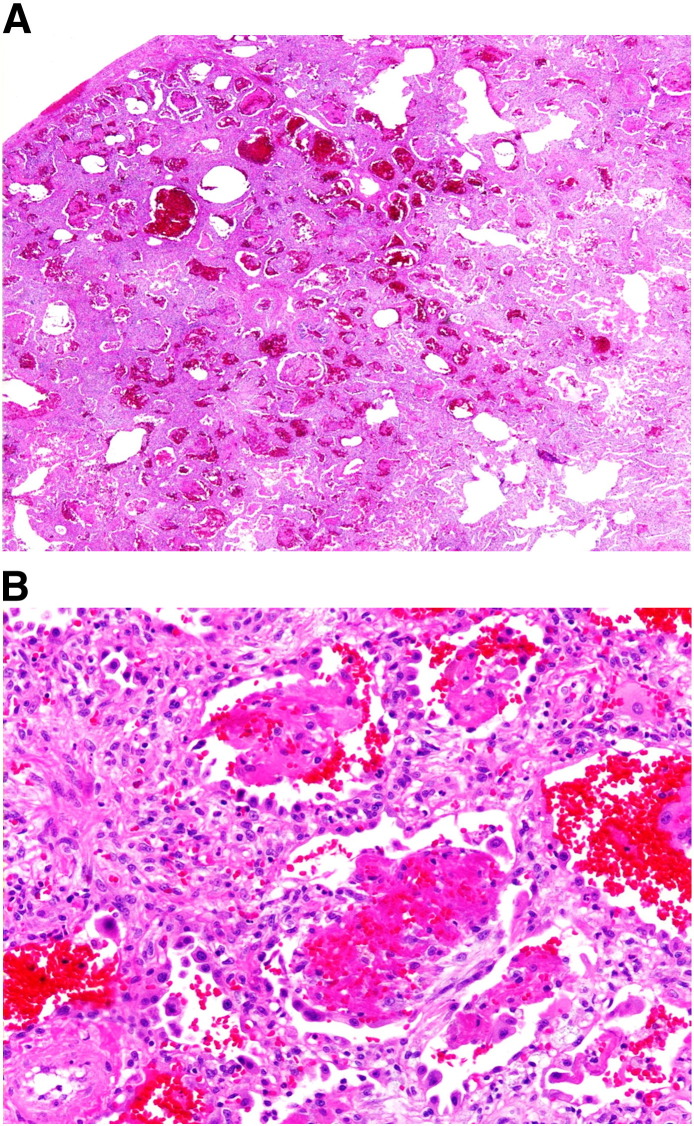
On initial physical examination he was afebrile, tachypneic, and hypoxic, requiring 80% oxygen to achieve a SpO2 of 92%. Lung auscultation revealed diffuse rhonchi and rales. Additional tests performed at our institution included blood and sputum cultures, ANCA, Jo-1 antibody, glomerular basement membrane antibody, cyclic citrullinated peptide IgG and IgA, and creatine phosphokinase, which were negative. On admission to our hospital, methylprednisolone 60 mg IV every 6 hours was given for 2 days, and the dose was later increased to 125 mg IV every 6 hours. Two days later a steroid taper was initiated, decreasing the methylprednisolone dose to 60 mg IV every 6 hours for 5 days, and then prednisone 60 mg PO daily was started. He required oxygen with a high flow nasal cannula. On the fourth day of hospitalization, he underwent an anterior thoracotomy, right wedge lung biopsies, and right chest tube placement. He was brought intubated from the operating room for ventilator weaning in the intensive care unit and he was extubated on postoperative day 1. Specimens of the lung biopsy were sent for pathology, microbiology, and virology. The pathology demonstrated AFOP with a background of chronic interstitial pneumonia (Fig. 2A and B). A trichrome stain revealed fibrosis. Tissue specimens were negative for acid fast-bacilli, mycobacteria, fungi and cytomegalovirus. Gram stain and culture were also negative. However, an anaerobic and aerobic culture grew a coagulase negative Staphylococci. Sulfamethoxazole/trimethoprim was initiated on postoperative day 3. On repeat chest x-ray, the lungs appeared unchanged. The chest tube was eventually removed and he was discharged to pulmonary rehabilitation on prednisone 60 mg PO daily and continuous oxygen via nasal cannula at 3 L/min.
Fig. 2.
A, Histology of AFOP with diffuse and patchy involvement.
B, Histology of AFOP with intraalveolar fibrin balls.
Upon discharge, the patient was followed by the pulmonology team. Eight months after his hospital discharge, he experienced arthralgias and Raynaud’s syndrome, and developed telangiectasias. On further evaluation, blood work was positive for antinuclear antibodies and Sjogren’s syndrome antibody and he was diagnosed with an unspecified connective tissue disease (CTD). He was started on azathioprine, but because of an adverse reaction, his therapy was changed to mycophenolate mofetil 500 mg PO 3 tablets in the morning and 2 tablets at night. Over 2 years, several steroid tapers were attempted unsuccessfully. His symptoms worsened at lower doses. Thirty months after discharge he remains dependent on mycophenolate mofetil, prednisone 10 mg PO daily, and oxygen, requiring 4 L/min via nasal cannula at rest and during sleep, and 8 to 10 L/min via an oxymizer pendant with activity. He is currently on disability. Although his exercise tolerance improved after completing pulmonary rehabilitation, his functional status has not returned to his pre-illness baseline. He has been evaluated and listed for lung transplantation.
3. Discussion
Acute fibrinous and organizing pneumonia (AFOP) is a rare histologic interstitial pneumonia pattern; as of 2015, there have been 111 cases reported in the literature (Table 1 ). Of these cases, 43 were male, 35 were female; gender was not specified in 33 cases. It most commonly occurred in patients in their fifth and sixth decade of life; however, there were 5 reports in children. In most cases, the etiology was unknown. Possible causes of AFOP include connective tissue disease, infections, environmental or occupational exposure, drug reactions, autoimmune disease, after organ transplantation, and cancers (Table 1). In our case, possible etiologies include asbestos and fiberglass exposure, as well as connective tissue disease.
Table 1.
Summary of patient characteristics, diagnosis, treatment, and outcome in AFOP from literature
| Publication | Patient characteristics | Proposed cause | Diagnostic method | Treatment | Outcome | Duration of Follow-up | Still on treatment at follow-up? |
|---|---|---|---|---|---|---|---|
| Lopez-Cuenca et al [2] | 27 yo F | Marden-Walker syndrome/sepsis/ARDS | Autopsy | Corticosteroids, antibiotics, mechanical ventilation | Death | 15 days | NA |
| Bhatti et al [3] | 56 yo M | Idiopathic | SB | Corticosteroids, mycophenolate mofetil, mechanical ventilation | Improved | 12 months | Yes |
| Beasley et al [1] | 77 yo M | Haemophilus influenza | SB or autopsy⁎ | Antibiotics | Death | NS | NA |
| 33 yo M | Occupational exposure (construction worker)/Acinetobacter baumanii | SB | Antibiotics | Improved | NS | NS | |
| 55 yo M | Ankylosing spondylitis/occupational exposure (zoologist exposed to exotic animals)/amiodarone | SB | Antibiotics | Improved | NS | NS | |
| 76 yo F | Environmental (excessive hair spray) | SB | Corticosteroids, antibiotics | Improved | NS | NS | |
| 74 yo M | Environmental exposure (coalminer) | SB or autopsy⁎ | Furosemide, dopamine, mechanical ventilation | Death | NS | NA | |
| 78 yo F | Polymyositis | SB or autopsy⁎ | Corticosteroids | Death (unrelated cause) | NS | NA | |
| 58 yo F | Possible fibromyalgia | SB | Antibiotics | Improved | NS | NS | |
| 47 yo M | Idiopathic | SB | Antibiotics | Improved | NS | NS | |
| 39 yo M | Ki-1 lymphoma | SB or autopsy⁎ | Corticosteroids, mechanical ventilation | Death | NS | NA | |
| 59 yo F | Idiopathic | SB or autopsy⁎ | Antibiotics, corticosteroids, mechanical ventilation | Death | NS | NA | |
| 70 yo M | Idiopathic | SB | Antibiotics | Improved | NS | NA | |
| 72 yo F | Idiopathic | SB | Antibiotics, corticosteroids | Improved | NS | NS | |
| 36 yo M | Idiopathic | SB or autopsy⁎ | Mechanical ventilation | Death | NS | NA | |
| 76 yo M | Idiopathic | SB or autopsy⁎ | Antibiotics, corticosteroids | Death | NS | NA | |
| 65 yo F | Idiopathic | SB or autopsy⁎ | Antibiotics | Death | NS | NA | |
| 68 yo F | Idiopathic | SB or autopsy⁎ | NA | Death | NS | NA | |
| 66 yo M | Idiopathic | SB or autopsy⁎ | Antibiotics, corticosteroids, mechanical ventilation | Death | NS | NA | |
| Guimaraes et al [4] | 55yo F | Primary biliary cirrhosis | SB | Corticosteroids | Improved | 14 months | Yes |
| Rapaka et al [5] | 38 yo M | HIV | FTBB | Corticosteroids | Improved | NS | NS |
| Kobayashi et al [6] | 55 yo M | Chronic glomerulonephritis | SB | Corticosteroids | Improved | 3 months | NS |
| Tzouvelekis et al [7] | 65 yo F | Idiopathic | SB | Corticosteroids, antibiotics | Improved | 3 months | Yes |
| Zhang et al [8] | 73 yo M | Idiopathic | Ultrasound-guided percutaneous lung biopsy | Corticosteroids | Improved | 1.5 months | Yes |
| Valim et al [9] | 39 yo F | Systemic sclerosis | SB | Cyclophosph-amide, corticosteroids, mechanical ventilation | Death | 3 days | NA |
| Damas et al [10] | 66 yo M | Idiopathic | SB | Antibiotics, corticosteroids, cyclophosphamide | Improved | NS | NS |
| Yokogawa et al [11] | 52 yo F | Abacavir | SB | Antibiotics, corticosteroids, discontinue Abacavir | Improved | NS | NS |
| Balduin et al [12] | 47 yo M | Collagen vascular disease | SB | Corticosteroids, azathioprine, noninvasive mechanical ventilation | Improved | NS | NS |
| Lee et al [13] | 60 yo M | Hematopoietic stem cell transplantation/Acute myelogenous leukemia | FTBB | Corticosteroids | Death | 61 days | NA |
| Canessa et al [14] | 60 yo F | Whipple’s disease | SB | Antibiotics | Improved | NS | NS |
| Vasu et al [15] | 64 yo M | Decitabine/Acute myelogenous leukemia/myelodysplastic syndrome | SB | Corticosteroids, discontinuation of decitabine | Improved | NS | NS |
| Hariri et al [16] | 47 yo M | Systemic lupus erythematosus | SB | Corticosteroids, cyclophosphamide | Improved | NS | Yes |
| Heo et al [17] | 40 yo M | HIV/Pneumocystisjirovecii | SB | Antibiotics, corticosteroids | Improved | 8 months | No |
| Santos et al [18] | 44 yo M | Idiopathic | SB | Surgical resection | Improved | NS | |
| Otto et al [19] | 66 yo F | Influenza virus/history of double lung transplant | Autopsy | Antiviral, antibiotics, mechanical ventilation, ECMO | Death | 11 months | NA |
| Ribera et al [20] | 69 yo F | Chlamydia pneumoniae | FTBB | Antibiotics, steroids, mechanical ventilation | Death | 20 days | NA |
| Cincotta et al [21] | 38 day old F | ARDS/RSV | SB | Mechanical ventilation | Death | NS | NA |
| 10 month old | ARDS/severe combined immunodeficiency/Pneumocystis carinii pneumonia | SB | Mechanical ventilation | Death | NS | NA | |
| Sverzellati et al [22] | 62 yo F | Pulmonary mycosis fungoides | SB | Corticosteroids | Improved | NS | NS |
| Cho et al [23] | 79 yo M | Idiopathic | SB | Corticosteroids | Improved | NS | NS |
| Sauter et al [24] | 66 yo F | Anti-synthetase syndrome | SB | Azathioprine, mycophenolate, corticosteroids | Improved | 2 years | Yes |
| White et al [25] | 1 patient | Everolimus | FTBB | NS | NS | NS | NS |
| Prahalad et al [26] | 14 yo F | Juvenile dermatomyositis | SB | Antibiotics, corticosteroids, IV immunoglobulin, cyclosporine, cyclophosphamide, oscillating mechanical ventilation | Death | 2 weeks | NA |
| Hwang et al [27] | 6 patients with mean age of 68 yo | Severe acute respiratory syndrome (SARS) | Autopsy | NS | Death | NS | NA |
| Qiu et al [28] | 5 patients, 2 male and 3 female, age 43-61 yo | NS | CT-guided percutaneous lung biopsy | Corticosteroids | Improved | NS | NS |
| Al-Khouzaie et al [29] | 45 yo M | NS | Lung biopsy | Corticosteroids | Improved | NS | NS |
| Labarinas et al [30] | 10 yo M | Severe aplastic anemia/fulminant hepatic failure (suspected to be autoimmune) | Lung biopsy | Antithymocyte globulin, cyclosporine, hematopoietic stem cell transplant | Improved | NS | NS |
| Moreira et al [31] | 44 yo M | NS | SB | Surgical resection | Improved | NS | No |
| Bawa et al [32] | 31 yo F | Idiopathic | Lung biopsy | Corticosteroids, antibiotics | Improved | 9 months | Yes |
| Jarbou et al [33] | 70 yo M | Idiopathic | SB | Corticosteroids | Improved | 6 months | Yes |
| Alici et al [34] | 48 yo F | Grade 2 primary graft dysfunction of lung transplant | FTBB | Corticosteroids | Improved | 1 week | Yes |
| Feng et al [35] | 64 yo M | Mycobacterium tuberculosis | Percutaneous needle lung biopsy | Corticosteroids, anti-tuberculosis antibiotics | Improved | 9 months | NS |
| 84 yo M | Lung adenocarcinoma | Percutaneous needle lung biopsy, surgical lung biopsy | Corticosteroids, antibiotics, surgical resection | Death due to brain metastasis | 10 months | NA | |
| Garcia et al [36] | 46 yo M | Idiopathic | SB | Corticosteroids | Improved | NS | NS |
| Hara et al [37] | 70 yo M | Idiopathic | FTBB | Corticosteroids | Resolved | 3 months | NS |
| 55 yo M | Idiopathic | SB | Corticosteroids | Resolved | 3 months | NS | |
| Kassir et al [38] | 53 yo F | Mycoplasma pneumoniae | Peripheral lung biopsy | Corticosteroids | Improved | 2 weeks | Yes |
| Lococo etal [39] | 65 yo F | Idiopathic | SB | Corticosteroids | Resolved | 6 weeks | NS |
| Piciucchi et al [40] | 79 yo M | Amiodarone | FTBB | Corticosteroids | Resolved | 3 months | NS |
| Mittal et al [41] | 14 yo F | Idiopathic | CT-guided percutaneous transthoracic lung biopsy | Corticosteroids | Resolved | 1 month | No |
| Renaud-Picard et al [42] | 22 yo M | Cystic fibrosis; lung transplant | FTBB | Corticosteroids, antibiotics, re-transplantation of lungs | Improved | 2 years | No |
| Akhtar et al [43] | 68 yo F | Idiopathic | CT-guided biopsy | Corticosteroids | Improved | 2 months | Yes |
| Feinstein et al [44] | 10 patients, 4 M, 6 F, average age 59.6 yo | NS | SB | Corticosteroids | 6 patients improved; Death in 4 patients(unrelated to AFOP) | NS | NS |
| Rajan et al [45] | 42 yo M | Acute myelogenous leukemia/Aspergillosis | SB | Antifungal | Resolved | 5 months | No |
| Paraskeva et al [46] | 22 patients | Lung transplant | FTBB | Antibiotics, antifungals, corticosteroids | Death | Median time to death 94 days after diagnosis | NA |
| Bierach et al [47] | 4 patients | Lung transplant | NS | Corticosteroids | NS | NS | NS |
| Oskuei et al [48] | 71 yo M | Decitabine/myelodysplastic syndrome | SB | Corticosteroids, antibiotics, discontinuation of decitabine | Improved | NS | NS |
| Hankollari et al [49] | 36 yo M | Bleomycin | FTBB | Corticosteroids | Improved | NS | NS |
| Rafii et al [50] | 55 yo F | Idiopathic | SB | Corticosteroids | Improved | NS | NS |
yo, years old; F, female; M, male; SB, surgical biopsy; FTBB, fiberoptic transbronchial biopsy; NS, not specified; NA, not applicable; ARDS, adult respiratory distress syndrome; RSV, respiratory syncytial virus; HIV, human immunodeficiency virus; ECMO, extracorporeal membrane oxygenation.
Beasley et al reported that 15 patients had open lung biopsies to confirm the diagnosis, and 2 patients had the diagnosis made on autopsy. It was not specified which of the patients had the diagnosis made on autopsy.
Patients present with progressive dyspnea, cough, hemoptysis, fever, fatigue, or acute respiratory distress syndrome. Patients can present with an acute, rapidly progressing form associated with poor outcomes, or more commonly, a subacute form, with a gradual onset of symptoms and a more favorable prognosis [1], [2], [3]. Poor outcomes are associated with patients requiring mechanical ventilation [1]. Of the 51 deaths reported in the literature, 6 were unrelated to AFOP [1], [2], [9], [13], [19], [20], [21], [26], [27], [35], [44], [46]. Of those that died, 12 required mechanical ventilation; in 37 cases, the use of mechanical ventilation was not specified. In the cases summarized in Table 1, death resulted in 8 patients with viral infections (SARS, influenza virus, RSV), 2 patients with bacterial infections, 1 patient with environmental exposure, 1 patient with cancer, 1 patient following stem cell transplant, 1 patient with a combined picture of sepsis, ARDS, and developmental disorder, 22 patients after double-lung transplant and chronic allograft dysfunction, 2 patients with autoimmune diseases, and 7 patients in whom AFOP was idiopathic [1], [2], [9], [13], [19], [20], [21], [26], [27], [46]. The time to death was not reported in a majority of the cases; death occurred in less than 1 month in 4 patients, and less than 1 year in 24 patients [2], [9], [13], [19], [20], [26], [46]. Our patient had a subacute onset of the illness, with symptoms developing over 2 months. He required mechanical ventilation for less than 24 hours postoperatively. His symptoms improved with immunosuppressants, but he remains dependent on steroids, oxygen, and immunosuppressive medication for more than 2 years after diagnosis.
The diagnosis of AFOP is challenging. The radiological findings for AFOP are not diagnostic, and may vary. The most common radiological findings are diffuse, patchy opacities with both peripheral and bilateral distribution; it may be limited to the lung bases [1], [2], [3]. CT scan images may show the lesion as a solitary nodule with air bronchograms with progression to diffuse lung opacities [6]. In addition, the images may appear similar to other lung diseases such as interstitial pneumonia, pulmonary edema, and infectious pneumonia [9]. Our patient’s chest x-ray showed bilateral pleural effusions and bilateral diffuse opacities. His chest CT scan showed diffuse bilateral ground glass densities; these findings were nonspecific and suggestive of a broad differential diagnoses. The diagnostic work up of AFOP may include BAL, but a lung biopsy provides the definitive diagnosis [1], [2]. Of the cases reviewed, 24 patients underwent BAL before pathological diagnosis. In all cases, the BAL was inconclusive [3], [4], [6], [7], [10], [11], [13], [15], [16], [18], [19], [20], [26], [33], [34], [36], [37], [38], [40], [42], [48], [49], [50]. In 8 patients, 7 of whom underwent transbronchial biopsy [3], [4], [6], [9], [33], [41], [47] and 1 who had a transthoracic biopsy [31], the results were inconclusive and required further diagnostic work up. The diagnosis of AFOP was made by surgical lung biopsy in 52 patients, transbronchial lung biopsy in 9 patients, percutaneous needle biopsy in 2 patients, image-guided (CT- or ultrasound-guided) percutaneous lung biopsy in 8 patients, peripheral lung biopsy in 1 patient, unspecified lung biopsy techniques in 3 patients, and autopsy in 10 patients [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50]. Our patient’s BAL and bronchoscopic biopsy were inconclusive; the diagnosis was made with surgical lung biopsy. To make a definitive diagnosis of AFOP, histopathologic evaluation is required [1].
The major histopathologic features of AFOP are intra-alveolar fibrin “balls”, organizing pneumonia, and a patchy distribution within the lung parenchyma. It involves up to 90% of the alveolar spaces within a tissue sample. Minor features that may be associated with AFOP include inflammatory changes of the alveolar walls surrounding the areas of fibrin, myxoid connective tissue in the alveolar septum, type 2 pneumocyte hyperplasia, and minimal changes in the lung tissue areas without fibrin. It does not demonstrate hyaline membranes and lacks eosinophils, which distinguishes it from diffuse alveolar damage and eosinophilic pneumonia, respectively. Although fibrin deposition has been associated with diffuse alveolar damage, it is not the prominent feature and does not display the intra-alveolar fibrin ball pattern. In addition, diffuse alveolar damage is associated with diffuse changes, while AFOP presents in a patchy distribution. AFOP does not consist of granulomatous inflammation, bronchopneumonia, or abscess formation [1].
An ideal treatment of AFOP has not been reported; it has been treated with antibiotics, corticosteroids, and immunosuppressants (mycophenolate mofetil, azathioprine, and cyclophosphamide) with varying responses [1], [3], [10], [16], [26]. Corticosteroids are the most common and successful treatment modality described in the literature [43]. Akhtar et al [43] recommended methylprednisolone 60 mg every 6 to 8 hours for the first 5 days, followed by a gradual taper and a maintenance dose of 40 mg daily for 3 months. Other therapeutic regimens described included methylprednisolone 0.5 to 1 mg/kg per day IV followed by prednisone 0.5 to 1 mg/kg per day which is gradually tapered [3], [4], [6], [43]. The exact duration of therapy is unknown, and is based on the patient’s clinical course and underlying etiology. Some patients return to baseline within months of treatment [17], [41], [45]. Other patients experienced worsening of symptoms during steroid tapers or noncompliance with treatment; improvement was noted when higher doses were resumed [7], [32], [43]. Treatment may be as long as 2 years [1], [3], [4], [10], [24]. From our literature review, the follow-up course of only 19 patients was reported and ranged from 2 weeks to 2 years [3], [4], [6], [7], [8], [17], [24], [32], [33], [34], [35], [37], [39], [40], [41], [43], [45], [47]. Only 4 reports gave information on the patients’ condition after 1 year [3], [4], [24], [42]. Ten patients were still receiving treatment at the time of follow-up, 3 patients completed treatment and were asymptomatic, and in 6 patients it was not specified whether or not they were still receiving therapy at the time of follow-up. Fifteen patients received only corticosteroids. One patient required corticosteroids and anti-tuberculosis antibiotics [35], and 1 patient improved after 5 months with only oral antifungal therapy [46]. Two patient required immunosuppressive agents such as cyclophosphamide, azathioprine, and mycophenolate mofetil in addition to corticosteroids [3], [24]. Of the 4 cases that reported on the patient’s condition over 2 years, 3 patients remained on therapy at 1 year, 14 months, and 2 years of follow-up [3], [4], [24]; treatment was not specified at 2-year follow-up in 1 patient [42]. None of the cases in the literature specified whether the patients were oxygen-dependent on discharge or at follow-up. Our patient was discharged on a steroid taper; however, he experienced relapses with decreased doses, requiring resumption of higher doses. After his diagnosis of a connective tissue disease, mycophenolate mofetil was added to his therapeutic regimen. At 30 month follow-up, he continues to require immunosuppressant therapy, remains oxygen-dependent, and has been listed for lung transplantation.
Patients diagnosed with autoimmune or collagen vascular diseases may benefit from immunosuppressant therapy in addition to steroids. Cyclophosphamide and steroids were used to treat AFOP in patients with systemic sclerosis and systemic lupus erythematosus [9], [16]. In addition to steroids, azathioprine was used to treat a patient with collagen vascular disease and AFOP [12]. Sauter et al used azathioprine, mycophenolate mofetil, and steroids in the treatment for a patient with AFOP and anti-synthetase syndrome [24]. Prahalad et al described a case of AFOP diagnosed in the setting of juvenile dermatomyositis in which the patient continued to decline despite therapy with azithromycin and increased doses of methylprednisolone. She received intravenous immunoglobulin (2 g/kg), cyclosporine 5 mg/kg IV, and cyclophosphamide 500 mg/kg IV. She eventually required mechanical and oscillating mechanical ventilation but died after a massive pneumothorax [26]. Mycophenolate mofetil and cyclophosphamide have also been reported in idiopathic cases of AFOP, resulting in improved outcomes [3], [10]. Mycophenolate mofetil has been used to treat scleroderma-related interstitial lung disease and cyclophosphamide-refractory lupus nephritis. It is safe and effective in maintaining lung function in interstitial lung diseases associated with CTD [3]. It may be a useful adjunct to steroids in patients with associated autoimmune diseases and CTD.
Other therapeutic interventions include drug discontinuation, surgical resection, and organ transplantation. In cases caused by a medication (amiodarone, decitabine, abacavir, and bleomycin), treatment with corticosteroids and discontinuation of the drug resulted in improvement of symptoms [11], [15], [40], [48], [49]. There were 2 reports of surgical lung resection in the management of AFOP, without medical management, with subsequent improvement of symptoms [18], [31]. Surgical resection may be an additional treatment option in the management of AFOP if the disease is relatively localized.
AFOP has been described following organ transplantation; however, organ transplantation may be indicated to treat AFOP refractory to medical treatment. AFOP developed in 1 patient after an allogenic hematopoietic stem cell transplantation for the treatment of AML and in 23 patients after double-lung transplantation. All of the patients failed treatment with antibiotics, steroids, and immunosuppressants and expired [13], [19], [46]. Four patients developed AFOP after lung transplantation and were treated with immunosuppressive agents and corticosteroids but the long-term outcome was not specified [47]. Alici et al reported a patient with AFOP associated with grade 2 primary graft dysfunction of a lung transplant whose condition improved after corticosteroid treatment; however, the follow-up duration was only 1 week [34]. Labarinas et al described a case of AFOP in the setting of aplastic anemia and fulminant liver failure, suspected to be autoimmune in nature. There was no response to treatment with antithymocyte globulin or cyclosporine. Disease resolution occurred after hematopoietic stem cell transplant [30]. Renaud-Picard et al described a patient with cystic fibrosis who received a lung transplant. After 42 months, he experienced respiratory failure unresponsive to antibiotics and steroids. He underwent a bilateral lung retransplantation, and surgical pathology of the explanted lungs revealed AFOP. At 2-year follow-up, the patient was reportedly doing well [42]. At 30 months of follow-up, our patient remains dependent on immunosuppressive agents, and was listed for lung transplantation after he experienced clinical worsening and increased oxygen requirements.
Although case reports are considered to be at the bottom of the hierarchy of what is regarded as reliable evidence for basing clinical decisions, there is important knowledge that can be obtained from them [51]. The value of publishing case series and reviews is to educate providers on conditions which are rare, but may be more prevalent than expected. It informs clinicians of the diagnostic tools necessary to make the diagnosis, and provides them with treatment options that cannot be tested by randomized controlled trials because of the small number of subjects who have the rare disease [52]. It provides information on the clinical course of the disease and what therapeutic interventions have failed and succeeded. The goal of this case report and review is to provide clinicians with the presentation, diagnosis, and management of AFOP, a condition which may be more prevalent than we suspect. We presented the clinical presentation, radiological findings, diagnostic approach, associated conditions and how they affect management, and reported treatment options which have failed and succeeded. The literature does not clearly define the duration of the disease course or treatment, especially when it is associated with autoimmune, collagen vascular, or connective tissue disease. In addition, we describe that the disease course may be lengthy and may not resolve with medical management alone, and surgical intervention may be necessary. Through our literature review, we discovered localized disease may be curative by surgical resection, and lung transplantation may be considered in disease refractory to medical treatment with good long-term outcome.
4. Conclusion
The treatment and long-term outcome of acute fibrinous and organizing pneumonia are not well defined. In the case presented, the patient was admitted with progressively worsening dyspnea and cough over 2 months and a ground glass appearance of lungs on CT scan. The diagnosis of AFOP was made by open lung biopsy and histology. His condition was complicated by a diagnosis of CTD. Thirty months after diagnosis he remains dependent on steroids, immunosuppressive agents, oxygen, and has been listed for lung transplantation. Our extensive literature review describes the associated medications and conditions with the disease, diagnostic modalities, various treatment strategies, and outcomes of AFOP. Definitive management of this illness is not yet clearly defined. Treatment includes steroids, immunosuppressive agents, and potentially surgical lung resection and organ transplantation. There are few studies which report the long-term outcomes of these patients. To date, we present the longest follow-up of a patient diagnosed with AFOP. It is important to raise awareness of this disease, so symptoms and radiological findings may be identified and lead to appropriate diagnostic tests and management. Further reports of therapeutic management and long-term outcomes are required.
Acknowledgment
We greatly appreciate the assistance of Ms Christine Ford, Dr Kristine Cornejo, MD, and Dr Thomas Stockl, MD, from the Department of Pathology for providing us with histologic images and interpretation. We also thank Dr Peggy Wu, MD, and Dr Raymond Pertusi, MD, from the Department of Rheumatology for their role in the patient’s care. We wish to thank Dr Mark Dershwitz, MD, from the Department of Anesthesiology for his help in preparing this manuscript.
References
- 1.Beasley M.B., Franks T.J., Galvin J.R., Gochuico B., Travis W.D. Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med. 2002;126(9):1064–1070. doi: 10.5858/2002-126-1064-AFAOP. [PubMed PMID:12204055] [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Cuenca S., Morales-Garcia S., Martin-Hita A., Frutos-Vivar F., Fernandez-Segoviano P., Esteban A. Severe acute respiratory failure secondary to acute fibrinous and organizing pneumonia requiring mechanical ventilation: a case report and literature review. Respir Care. 2012;57(8):1337–1341. doi: 10.4187/respcare.01452. [PubMed PMID:22348347] [DOI] [PubMed] [Google Scholar]
- 3.Bhatti S., Hakeem A., Torrealba J., McMahon J.P., Meyer K.C. Severe acute fibrinous and organizing pneumonia (AFOP) causing ventilatory failure: successful treatment with mycophenolate mofetil and corticosteroids. Respir Med. 2009;103(11):1764–1767. doi: 10.1016/j.rmed.2009.07.009. [PubMed PMID:19666216] [DOI] [PubMed] [Google Scholar]
- 4.Guimaraes C., Sanches I., Ferreira C. Acute fibrinous and organising pneumonia. BMJ Case Rep. 2012;2012:1–4. doi: 10.1136/bcr.01.2011.3689. [PubMed PMID: 22605688. Pubmed Central PMCID: 3316785] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rapaka V., Hussain M.A., Niazi M., Diaz-Fuentes G. Severe acute fibrinous and organizing pneumonia causing acute respiratory distress syndrome and shock. J Bronchol Interv Pulmonol. 2011;18(3):269–273. doi: 10.1097/LBR.0b013e318222a4f2. [PubMed PMID: 23208573] [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi H., Sugimoto C., Kanoh S., Motoyoshi K., Aida S. Acute fibrinous and organizing pneumonia: initial presentation as a solitary nodule. J Thorac Imaging. 2005;20(4):291–293. doi: 10.1097/01.rti.0000168600.78213.85. [PubMed PMID:16282908] [DOI] [PubMed] [Google Scholar]
- 7.Tzouvelekis A., Koutsopoulos A., Oikonomou A., Froudarakis M., Zarogoulidis P., Steiropoulos P. Acute fibrinous and organising pneumonia: a case report and review of the literature. J Med Case Rep. 2009;3:74–79. doi: 10.1186/1752-1947-3-74. [PubMed PMID: 19946550. Pubmed Central PMCID: 2783073] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J., Fang Q.H., Feng R.E., Ma Y.M., Cao Y., Wang R.G. Acute fibrinous and organizing pneumonia: a case report and review of the literature. Zhonghua Jie He He Hu Xi Za Zhi. 2010;33(12):892–895. [PubMed PMID: 21211407] [PubMed] [Google Scholar]
- 9.Valim V., Rocha R.H., Couto R.B., Paixao T.S., Serrano E.V. Acute fibrinous and organizing pneumonia and undifferentiated connective tissue disease: a case report. Case Rep Rheumatol. 2012;2012:1–6. doi: 10.1155/2012/549298. [549298], [PubMed PMID: 22957292. Pubmed Central PMCID: 3420729] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damas C., Morais A., Moura C.S., Marques A. Acute fibrinous and organizing pneumonia. Rev Port Pneumol. 2006;12(5):615–620. [PubMed PMID:17117329] [PubMed] [Google Scholar]
- 11.Yokogawa N., Alcid D.V. Acute fibrinous and organizing pneumonia as a rare presentation of abacavir hypersensitivity reaction. AIDS. 2007;21(15):2116–2117. doi: 10.1097/QAD.0b013e3282f08c5a. [PubMed PMID: 17885309] [DOI] [PubMed] [Google Scholar]
- 12.Balduin R., Giacometti C., Saccarola L., Marulli G., Rea F., Bartoli M. Acute fibrinous and organizing pneumonia in a patient with collagen vascular disease "stigma". Sarcoidosis Vasc Diffuse Lung Dis. 2007;24(1):78–80. [PubMed PMID:18069424] [PubMed] [Google Scholar]
- 13.Lee S.M., Park J.J., Sung S.H., Kim Y., Lee K.E., Mun Y.C. Acute fibrinous and organizing pneumonia following hematopoietic stem cell transplantation. Korean J Intern Med. 2009;24(2):156–159. doi: 10.3904/kjim.2009.24.2.156. [PubMed PMID: 19543497. Pubmed Central PMCID: 2698626] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canessa P.A., Prattico L., Sivori M., Magistrelli P., Fedeli F., Cavazza A. Acute fibrinous and organising pneumonia in Whipple's disease. Monaldi Arch Chest Dis. 2008;69(4):186–188. doi: 10.4081/monaldi.2008.382. [PubMed PMID:19350842] [DOI] [PubMed] [Google Scholar]
- 15.Vasu T.S., Cavallazzi R., Hirani A., Marik P.E. A 64-year-old male with fever and persistent lung infiltrate. Respir Care. 2009;54(9):1263–1265. [PubMed PMID:19712502] [PubMed] [Google Scholar]
- 16.Hariri L.P., Unizony S., Stone J., Mino-Kenudson M., Sharma A., Matsubara O. Acute fibrinous and organizing pneumonia in systemic lupus erythematosus: a case report and review of the literature. Pathol Int. 2010;60(11):755–759. doi: 10.1111/j.1440-1827.2010.02586.x. [PubMed PMID:20946526] [DOI] [PubMed] [Google Scholar]
- 17.Heo J.Y., Song J.Y., Noh J.Y., Yong H.S., Cheong H.J., Kim W.J. Acute fibrinous and organizing pneumonia in a patient with HIV infection and Pneumocystis jiroveci pneumonia. Respirology. 2010;15(8):1259–1261. doi: 10.1111/j.1440-1843.2010.01845.x. [PubMed PMID:20920123] [DOI] [PubMed] [Google Scholar]
- 18.Santos C., Fradinho F., Catarino A. Acute fibrinous and organizing pneumonia. Rev Port Pneumol. 2010;16(4):607–616. doi: 10.1016/S0873-2159(15)30055-6. [PubMed PMID: 20700558. Pneumonia aguda fibrinosa e organizante] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otto C., Huzly D., Kemna L., Huttel A., Benk C., Rieg S. Acute fibrinous and organizing pneumonia associated with influenza A/H1N1 pneumonia after lung transplantation. BMC Pulm Med. 2013;13:30–34. doi: 10.1186/1471-2466-13-30. [PubMed PMID: 23683442. Pubmed Central PMCID: 3662564] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribera A., Llatjos R., Casanova A., Santin M. Chlamydia pneumoniae infection associated to acute fibrinous and organizing pneumonia. Enferm Infecc Microbiol Clin. 2011;29(8):632–634. doi: 10.1016/j.eimc.2011.01.018. [PubMed PMID:21775026] [DOI] [PubMed] [Google Scholar]
- 21.Cincotta D.R., Sebire N.J., Lim E., Peters M.J. Fatal acute fibrinous and organizing pneumonia in an infant: The histopathologic variability of acute respiratory distress syndrome. Pediatr Crit Care Med. 2007;8(4):378–382. doi: 10.1097/01.PCC.0000269375.10806.60. [PubMed PMID:17545929] [DOI] [PubMed] [Google Scholar]
- 22.Sverzellati N., Poletti V., Chilosi M., Casoni G., Hansell D., Zompatori A.M. The crazy-paving pattern in granulomatous mycosis fungoides: high-resolution computed tomography-pathological correlation. J Comput Assist Tomogr. 2006;30(5):843–845. doi: 10.1097/01.rct.0000214269.72180.64. [PubMed PMID:16954940] [DOI] [PubMed] [Google Scholar]
- 23.Cho J.Y., Lee H.K., Lee S.S., Lee K.K., Lee Y.M., Lee H.P. A case of acute fibrinous and organizing pneumonia. Tuberc Respir Dis. 2006;61:479–483. [Google Scholar]
- 24.Sauter J.L., Butnor K.J. Expanding the spectrum of pulmonary histopathological manifestations of anti-synthetase syndrome: anti-EJ-associated acute fibrinous and organizing pneumonia. Histopathology. 2014;65(4):581–582. doi: 10.1111/his.12420. [PubMed PMID:24660769] [DOI] [PubMed] [Google Scholar]
- 25.White D.A., Camus P., Endo M., Escudier B., Calvo E., Akaza H. Noninfectious pneumonitis after everolimus therapy for advanced renal cell carcinoma. Am J Respir Crit Care Med. 2010;182(3):396–403. doi: 10.1164/rccm.200911-1720OC. [PubMed PMID:20194812] [DOI] [PubMed] [Google Scholar]
- 26.Prahalad S., Bohnsack J.F., Maloney C.G., Leslie K.O. Fatal acute fibrinous and organizing pneumonia in a child with juvenile dermatomyositis. J Pediatr. 2005;146(2):289–292. doi: 10.1016/j.jpeds.2004.09.023. [PubMed PMID:15689928] [DOI] [PubMed] [Google Scholar]
- 27.Hwang D.M., Chamberlain D.W., Poutanen S.M., Low D.E., Asa S.L., Butany J. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod Pathol. 2005;18(1):1–10. doi: 10.1038/modpathol.3800247. [PubMed PMID:15272286] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu Y.Y., Miao L.Y., Cai H.R., Xiao Y.L., Ye Q., Meng F.Q. The clinicopathological features of acute fibrinous and organizing pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2013;36(6):425–430. [PubMed PMID: 24103205] [PubMed] [Google Scholar]
- 29.Al-Khouzaie T.H., Dawamneh M.F., Hazmi A.M. Acute fibrinous and organizing pneumonia. Ann Saudi Med. 2013;33(3):301–303. doi: 10.5144/0256-4947.2013.301. [PubMed PMID:23793436] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labarinas S., Gumy-Pause F., Rougemont A.L., Baerlocher G., Leibundgut E.O., Porret N. Is acute fibrinous and organizing pneumonia the expression of immune dysregulation? J Pediatr Hematol Oncol. 2013;35(2):139–143. doi: 10.1097/MPH.0b013e31827e5782. [PubMed PMID:23337545] [DOI] [PubMed] [Google Scholar]
- 31.Moreira C.S., Fradinho F.C., Catarino A.C., Acute Fibrinous and Organizing Pneumonia . American Thoracic Society International Conference Abstracts: American Thoracic Society. 2010. C43 Case reports in interstitial lung disease and more II; p. A4515-A. [Google Scholar]
- 32.Bawa A.S., Delaney M.D., Potter B.M. Acute fibrinous and organizing pneumonia: A case report of newly identified entity with follow-up data. Chest. 2004;126(4_MeetingAbstracts):992S–993S. [Google Scholar]
- 33.Jarbou M., Yusof M., Coberly E., Johnson J., Tabba M.K. Acute fibrinous and organizing pneumonia. Chest. 2006;130(4_MeetingAbstracts):300S-c-1S. [Google Scholar]
- 34.Alici I.O., Yekeler E., Yazicioglu A., Turan S., Tezer-Tekce Y., Demirag F. A case of acute fibrinous and organizing pneumonia during early postoperative period after lung transplantation. Transplant Proc. 2015;47(3):836–840. doi: 10.1016/j.transproceed.2015.02.002. [PubMed PMID: 25891742] [DOI] [PubMed] [Google Scholar]
- 35.Feng A.N., Cai H.R., Zhou Q., Zhang Y.F., Meng F.Q. Diagnostic problems related to acute fibrinous and organizing pneumonia: misdiagnosis in 2 cases of lung consolidation and occupying lesions. Int J Clin Exp Pathol. 2014;7(7):4493–4497. [PubMed PMID: 25120840. Pubmed Central PMCID: 4129075] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia B.A., Goede T., Mohammed T.L. Acute fibrinous organizing pneumonia: a case report and literature review. Curr Probl Diagn Radiol. 2015;44(5):469–471. doi: 10.1067/j.cpradiol.2015.02.006. [PubMed PMID: 25817128] [DOI] [PubMed] [Google Scholar]
- 37.Hara Y., Shinkai M., Kanoh S., Kawana A., Rubin B.K., Matsubara O. Clinico-pathological analysis referring hemeoxygenase-1 in acute fibrinous and organizing pneumonia patients. Respir Med Case Rep. 2015;14:53–56. doi: 10.1016/j.rmcr.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kassir M.M., Tran T.C., Bagdasarian N. Acute fibrinous and organizing pneumonia after mycoplasma pneumoniae infection. Infect Dis Clin Pract. 2014;22(4):e63–e65. [PubMed PMID: 00019048-201407000-00029] [Google Scholar]
- 39.Lococo F., Rossi G., Filice A., Prandini N., Rapicetta C., Paci M. A woman with progressive dyspnea and multiple lung consolidative lesions. Am J Respir Crit Care Med. 2014;190(6):e22–e23. doi: 10.1164/rccm.201310-1759IM. [PubMed PMID:25221891] [DOI] [PubMed] [Google Scholar]
- 40.Piciucchi S., Dubini A., Tomassetti S., Casoni G., Ravaglia C., Poletti V. A case of amiodarone-induced acute fibrinous and organizing pneumonia mimicking mesothelioma. Am J Respir Crit Care Med. 2015;191(1):104–106. doi: 10.1164/rccm.201405-0844IM. [PubMed PMID:25551348] [DOI] [PubMed] [Google Scholar]
- 41.Mittal V., Kulshrestha R., Arya A., Bajaj P. Acute fibrinous and organising pneumonia presenting as complete lung consolidation. Singap Med J. 2011;52(5):e88–e90. [PubMed PMID:21633758] [PubMed] [Google Scholar]
- 42.Renaud-Picard B., Degot T., Biondini D., Weingertner N., Reeb J., Chenard M.P. Successful lung retransplantation in a patient with acute fibrinous and organizing pneumonia: a case report. Transplant Proc. 2015;47(1):182–185. doi: 10.1016/j.transproceed.2014.08.039. [PubMed PMID:25600847] [DOI] [PubMed] [Google Scholar]
- 43.Aktar A., UI Abideen Z. Acute fibrinous and organizing pneumonia masquerading as a lower respiratory tract infection: a case report and review of the literature. BMC Res Notes. 2015;8:38–43. doi: 10.1186/s13104-015-0984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feinstein M.B., DeSouza S.A., Moreira A.L., Stover D.E., Heelan R.T., Iyriboz T.A. A comparison of the pathological, clinical and radiographical, features of cryptogenic organising pneumonia, acute fibrinous and organising pneumonia and granulomatous organising pneumonia. J Clin Pathol. 2015;68(6):441–447. doi: 10.1136/jclinpath-2014-202626. [PubMed PMID: 25742910] [DOI] [PubMed] [Google Scholar]
- 45.Rajan S., Schaefer O.P., Smyrnios N.A., Fraire A.E. Acute fibrinous and organizing pneumonia in association with invasive pulmonary aspergillosis and underlying acute myeloid leukemia. Chest. 2008;134(4_MeetingAbstracts):c65002-c. [Google Scholar]
- 46.Paraskeva M., McLean C., Ellis S., Bailey M., Williams T., Levvey B. Acute fibrinoid organizing pneumonia after lung transplantation. Am J Respir Crit Care Med. 2013;187(12):1360–1368. doi: 10.1164/rccm.201210-1831OC. [PubMed PMID:23614642] [DOI] [PubMed] [Google Scholar]
- 47.Bierach J.B., Meyer K.C., Kanne J., Torrealba J. Acute fibrinous and organizing pneumonia following lung transplantation: a report of four cases. Chest. 2010;138(4_MeetingAbstracts):548A. [Google Scholar]
- 48.Oskuei A., Alvarez M. Acute fibrinous and organizing pneumonia: a severe side effect of decitabine. Chest. 2014;146(4_MeetingAbstracts):306A. [Google Scholar]
- 49.Hankollari E., Sallem S., Jones J., Torrealba J., Girod C., Scaglioni P. Acute fibrinous and organizing pneumonia: a rare histopathologic variant of bleomycin-induced lung injury. Chest. 2013;144(4_MeetingAbstracts):456A. [Google Scholar]
- 50.Rafii R., Murin S., Morrissey B.M. A case report of idiopathic acute fibrinous pneumonia and a review of the literature. Chest. 2010;138(4_MeetingAbstracts):47A. [Google Scholar]
- 51.Martyn C. Case reports, case series, and systematic reviews. Q J Med. 2002;95:197–198. doi: 10.1093/qjmed/95.4.197. [DOI] [PubMed] [Google Scholar]
- 52.Hoffman J.R. Rethinking case reports. West J Med. 1999;170(5):253–254. [PMC free article] [PubMed] [Google Scholar]