Abstract
The objectives were to separate canine seminal plasma proteins (with SDS-PAGE) and to determine the correlation between specific proteins and semen characteristics. Three ejaculates from 20 mixed-breed dogs, of unknown fertility, were collected by digital manipulation. Ejaculate volume and color, sperm motility, sperm vigor, percentage of morphologically normal spermatozoa, and membrane integrity (hypoosmotic swelling test and fluorescent staining) were assessed. For each dog, seminal plasma was pooled from all three ejaculates and proteins were separated with SDS-PAGE, using polyacrylamide concentrations of 13% and 22% in the separation gels. After staining, gel images were digitized to estimate molecular weights (MW) and integrated optical density (IOD) of each lane and of individual bands. Total seminal plasma protein concentration was 2.19 ± 1.56 g/dL (mean ± SD; range 1.12–5.19 g/dL). A total of 37 protein bands were identified (although no dog had all 37 bands). In the 13% gel, molecular weights ranged from 100.6 to 17.1 kDa, with four bands (49.7, 33.2, 26.4, and 19.5 kDa) present in samples from all dogs. In the 22% gel, molecular weights ranged from 15.6 to 3.6 kDa, with nine bands (15.6, 13.5, 12.7, 11.7, 10.5, 8.7, 7.8, 5.6, and 4.9 kDa) present in samples from all dogs. Combined for both gels, the majority of bands (85%) had molecular weights <17 kDa, with B20 (15.6 kDa) in high concentrations in samples from all dogs. There were positive correlations (P ≤ 0.01) between two bands, B4 (67 kDa) and B5 (58.6 kDa), and sperm motility (r = 0.66 and r = 0.46), sperm vigor (r = 0.56 and r = 0.66), percentage of morphologically normal spermatozoa (r = 0.55 and r = 0.59), the hypoosmotic swelling test (r = 0.76 and r = 0.68), and fluorescent staining (r = 0.56 and r = 0.59), respectively. In conclusion, 37 proteins were identified in seminal plasma; two were significantly correlated with semen characteristics.
Keywords: Semen, Seminal plasma protein, Fertility, Dog, Canine
1. Introduction
In recent years, many seminal plasma proteins have been identified and characterized [1], [2], [3]; several have been associated with fertility in various species [1], [4], [5], [6], [7], [8], [9]. Four seminal plasma proteins associated with bull fertility were identified with two-dimensional polyacrylamide gel electrophoresis [5]. Two of these proteins, 26 kDa (pI 6.2) and 55 kDa (pI 4.5), were associated with high-fertility bulls, whereas the other two, 16 kDa (pI 4.1 and 6.7, respectively), were more frequent in low-fertility bulls. Based on the four fertility-associated proteins in seminal plasma, a regression model that provided the best empirical prediction of fertility was developed. Estimated fertility values, based on four proteins, were compared to actual in vivo bull fertility; there was a positive association (r = 0.89), indicating that the predictive model was valid. In subsequent studies, the 55 kDa (pI 4.5) bovine seminal plasma protein was identified as an osteopontin [10] and the 26 kDa (pI 6.2) protein as a lipocalin-type prostaglandin-D synthase [11]. In another study, a seminal plasma ribonuclease (14 kDa) was present in higher concentrations in bulls with poor post-thaw semen quality [12]. In stallions, SP-1 (72 kDa, pI 5.6), a seminal plasma protein was positively correlated with fertility, whereas SP-2 (75 kDa, pI 6.0), SP-3 (18 kDa, pI 4.3), and SP-4 (16 kDa, pI 6.5) were negatively correlated with fertility. Based on Western blot analysis, there was a similarity between SP-1 and the bovine protein (55 kDa, pI 4.5) previously described [8].
Seminal plasma proteins in dogs have not been well characterized. In two studies that used electrophoresis [13], [14], three protein fractions were identified. A study of the protein composition of secretions from the canine prostate [15], six protein bands (86, 73, 28, 25, 19, and 16 kDa, respectively) were identified. Furthermore, the 86 kDa band was also present in castrated dogs. In a preliminary study using polyacrylamide gel (SDS-PAGE) under denaturing conditions, 25 bands with molecular weights ranging from 3.5 to 136 kDa were identified in five dogs of unknown fertility [16].
The objectives of the present study were to separate canine seminal plasma proteins (with SDS-PAGE) and to determine the correlation between individual proteins and semen characteristics.
2. Materials and methods
2.1. Reagents
All reagents were of highest purity-grade available and were purchased from the following companies: GE Healthcare (Uppsala, Sweden), Sigma (St. Louis, MO, USA) or Merck S.A. (São Paulo, SP, Brazil).
2.2. Collection and evaluation of semen
Twenty clinically healthy, adult (age range, 2–6 years), mixed-breed dogs were used. The dogs were chosen on the basis of size (15–20 kg) and good temperament, but their fertility was unknown. All dogs were sero-negative for brucellosis (Canine Brucellosis Antibody Test Kit, D-Tec® CB, Synbiotics Corporation, San Diego, CA, USA) and leptospirosis (Microscopic serum-agglutination, Department of Infectious Diseases, UNESP, Botucatu, SP, Brazil). Starting 3–6 weeks prior to semen collection, the dogs were housed in concrete-floored kennels with access to outside runs and fed commercial dog food. All dogs were vaccinated against rabies, canine distemper, hepatitis, parainfluenza, laryngotracheitis, parvovirus, coronavirus and leptospirosis (L. canicola e L. icterohaemorrhagiae) infection (Duramune DA2PP + CvK/LCI® and Rai-Vac I®, FortDodge, Campinas, SP, Brazil) and treated for external and internal parasites (Ivomec®, Merial, Campinas, SP, Brazil).
The penis was stimulated by digital manipulation in the absence of a teaser bitch. The entire second fraction and a portion of the third fraction were collected with a funnel and attached tube. Three ejaculates were collected from each dog, with a minimum interval of 48 h between successive collections. Semen analysis was done immediately after collection [17]. Any ejaculate that was not opaque white was discarded and another ejaculate subsequently collected as a replacement.
Semen volume was determined with a graduated plastic tube. Sperm motility and vigor were evaluated by placing a drop of semen on a pre-warmed slide (38–40 °C), applying a coverslip, and observing spermatozoa with a phase contrast microscope (Carl Zeiss, Jena, Germany), at 200×. Motility (range 0–100%) was subjectively estimated in increments of 5%. Vigor was scored on a scale of 0–5, where 0 and 5 represent no movement versus rapid, vigorous movement, respectively.
Semen was diluted 1:20 in distilled water and the mixture put in a Neubauer hemocytometer chamber (Bright Line, Labor Optik, Shanghai, China). Spermatozoa were counted under a phase contrast microscope (Carl Zeiss, Jena, Germany) at 200× and the total number of cells per ejaculate was calculated.
For sperm morphology, semen smears were fixed in saline solution warmed to 37 °C for 10 min and stained by the modified KARRAS method [18]. For each ejaculate, 200 spermatozoa were examined under phase contrast microscope (Carl Zeiss), at 1000× (oil immersion), and were classified as normal or as a major or minor defect [19].
Sperm membrane integrity was assessed by the hypoosmotic swelling test and staining with fluorescent probes. The hypoosmotic swelling test was performed by mixing 0.1 mL of semen with 0.9 mL of hypoosmotic solution at 150 mOsmol [20]. An isoosmotic solution at 300 mOsmol was used as control [21]. After incubating for 30 min at 37 °C, a drop of each mixture was placed on a slide and a coverslip applied. For each solution (hypoosmotic and isoosmotic) and sample, 200 spermatozoa were viewed under phase contrast microscope (Carl Zeiss) and the proportion with tail coiling was recorded. The number of spermatozoa with coiled tails in isoosmotic solution was subtracted from the number in hypoosmotic solution.
Sperm membrane integrity was assessed using 6-carboxyfluorescein diacetate and propidium iodide [22], [23]. The semen was diluted (10 μL) with staining medium (40 μL) and incubated in the dark for 10–15 min at 30 °C. One drop was placed on a slide and covered with a covership, and 200 spermatozoa were observed at 400× magnification with epifluorescence (Leica, DMIRB, Aotec Instrumentos Científicos Ltda., São Paulo, SP, Brazil). Fluorescent green cells were considered intact and red or red and green cells were considered damaged. The proportion of intact cells was determined.
2.3. Preparation of seminal plasma samples
Seminal plasma was separated by centrifuging at 800 × g for 10 min (Excelsa Baby II, Fanem, Model 206-R, São Paulo, SP, Brazil) and stored at −20 °C. The samples were thawed, re-centrifuged at 4200 × g for 1 h at 4 °C (MLW, K23, Engelsdorf, Leipzig, Germany) and total protein, in duplicate, was determined by Biuret colorimetric method (Analisa Diagnóstica, Belo Horizonte, MG, Brazil). The seminal plasma was stored again at −20 °C until electrophoresis was performed.
Samples were diluted in ultra-purified water at a concentration of 2 μg/μL (for 22% gel) and 5 μg/μL (for 13% gel) of total protein mixed with sample buffer (TRIS-HCl 60 mM, pH 6.8, 50% glycerol, 2% SDS, 23 mM 2-mercapetoethanol, and 0.1% bromophenol blue) at 1:4 buffer/sample dilution, homogenized and boiled (∼100 °C) for 7 min.
2.4. SDS polyacrylamide denaturing gel electrophoresis (SDS-PAGE)
One-dimensional PAGE of seminal plasma was performed under denaturing conditions by methods previously described [16], [24], [25]. Electrophoresis was performed in a vertical system (Mini VE, GE Healthcare, Uppsala, Sweden), using two polyacrylamide concentrations, 13% and 22%. A 5% stacking gel was used in all runs.
The total loaded quantity of protein in each well was 80 μg in 20 μL for the 13% gel and 16 μg in 10 μL for the 22% gel. Each gel was also loaded with a standard protein of high (Recombinant Protein Molecular Weight Markers, 10–250 kDa, GE Healthcare, Uppsala, Sweden) and low (Rainbow Colored Protein Molecular Weight Markers, 2.5–45 kDa, GE Healthcare, Uppsala, Sweden) molecular weight respectively for separation gels at 13% and 22%. The electrophoresis system was connected to a power supply (EPS 300 Power Supply, GE Healthcare, Uppsala, Sweden) with constant current of 25 mA and maximum voltages of 184 V for 3 h (13% gel) or 250 V for 5 h (22% gel).
2.5. Gel staining
Gels were stained1 in 0.1% Coomassie Brilliant Blue R-250 dye, 45% methanol, and 10% acetic acid in ultra-purified water, warmed (∼50 °C), for 1 h, shaking slowly (Rotary Shaker, Biomixer, model MOS-1, São Paulo, SP, Brazil). Gels were destained twice for 15 min in 10% methanol and 10% acetic acid in ultra-purified water, warmed (∼50 °C), shaking slowly (Rotary Shaker, Biomixer, model MOS-1, São Paulo, SP, Brazil). Another destaining was performed in distilled water for 20 min in a microwave oven (CCE, Manaus, AM, Brazil) at 70 W, and for 10 min at 100 W. Gels were stored in plastic containers in distilled water with 5% glycerol at 4 °C (1–7 days).
2.6. Image acquisition and analysis
Gel images were digitized (VDS, GE Healthcare, Uppsala, Sweden). For each band of each gel, an image analyzer (Image Master™ 1D, GE Healthcare) was used to determine molecular weight (MW) and integrated optical density (IOD). The image analyzer corrected the densitometry of each band, discounting the optical density of background. After analysis, the gels were dried between impermeable cellophane papers with gelatin (200 mg/mL) on a glass plate.
2.7. Statistical analysis
For each dog, the mean of all three collections was used. The Pearson's correlation (r) between semen characteristics and IOD of each protein band, as well as total seminal plasma protein concentrations were determined (Excel, Version 6.0; Microsoft Corporation, Redmond, WA, USA). Correlations with P < 0.05 were considered significant.
3. Results
Semen characteristics are shown in Table 1 . Total seminal plasma protein concentration was 2.19 ± 1.56 g/dL (mean ± SD; range 1.12–5.19).
Table 1.
Mean (of three ejaculates) semen characteristics in adult dogs
| Dog | Volume (mL) | Motility (%) | Vigor (0–5) | Sperm concentration (×106) | Sperm morphology |
Hypoosmotic swelling test (%) | Fluorescence (% intact) | ||
|---|---|---|---|---|---|---|---|---|---|
| Normal (%) | Major defect (%) | Minor defect (%) | |||||||
| 1 | 5.6 | 93.3 | 4.7 | 292.3 | 82.8 | 4.9 | 12.4 | 75.7 | 96.3 |
| 2 | 3.5 | 70.0 | 4.0 | 345.7 | 82.3 | 3.8 | 13.8 | 68.3 | 87.7 |
| 3 | 5.3 | 0.0 | 0.0 | 36.7 | 6.0 | 83.3 | 8.8 | 0.0 | 0.0 |
| 4 | 3.5 | 88.3 | 3.7 | 314.9 | 89.0 | 7.3 | 3.7 | 84.3 | 78.3 |
| 5 | 2.2 | 83.3 | 3.7 | 480.3 | 87.3 | 7.5 | 5.2 | 83.0 | 83.0 |
| 6 | 1.7 | 60.0 | 2.7 | 231.1 | 72.7 | 21.2 | 6.2 | 76.7 | 83.3 |
| 7 | 2.0 | 73.3 | 3.3 | 20.2 | 76.0 | 13.8 | 10.2 | 72.3 | 73.3 |
| 8 | 4.5 | 86.7 | 4.3 | 251.3 | 80.8 | 12.2 | 7.0 | 89.7 | 82.0 |
| 9 | 2.5 | 88.3 | 3.7 | 276.3 | 42.5 | 53.3 | 5.2 | 86.3 | 89.7 |
| 10 | 2.7 | 90.0 | 3.3 | 129.5 | 85.7 | 11.0 | 3.3 | 90.7 | 94.0 |
| 11 | 2.8 | 91.7 | 4.0 | 134.2 | 83.5 | 11.7 | 4.8 | 90.0 | 92.0 |
| 12 | 3.5 | 16.7 | 2.3 | 14.2 | 29.2 | 30.2 | 7.3 | 31.7 | 15.3 |
| 13 | 2.8 | 91.7 | 4.3 | 271.3 | 89.8 | 6.0 | 4.2 | 89.0 | 90.0 |
| 14 | 2.8 | 91.7 | 4.0 | 273.4 | 81.3 | 12.7 | 6.0 | 87.0 | 74.3 |
| 15 | 3.5 | 91.7 | 4.0 | 343.7 | 83.5 | 12.8 | 3.7 | 85.3 | 72.0 |
| 16 | 2.5 | 93.3 | 4.3 | 267.0 | 88.2 | 8.7 | 3.2 | 92.3 | 86.7 |
| 17 | 5.0 | 88.3 | 4.0 | 177.0 | 87.3 | 8.7 | 4.0 | 90.0 | 75.0 |
| 18 | 1.5 | 83.3 | 3.7 | 980.6 | 90.5 | 6.5 | 3.0 | 84.7 | 78.0 |
| 19 | 3.2 | 88.3 | 4.7 | 520.6 | 91.0 | 5.7 | 3.3 | 90.7 | 91.7 |
| 20 | 3.2 | 85.0 | 4.0 | 499.2 | 88.2 | 7.7 | 4.2 | 88.0 | 86.3 |
Gel images are shown in Fig. 1, Fig. 2 and the graphic profile of IOD mean bands in seminal plasma of all 20 dogs is shown in Fig. 3 . A total of 37 protein bands were identified, 19 and 18 in the 13% and 22% gels, respectively. However, no individual dog had all 37 bands. In the 13% gel, molecular weights ranged from 100.6 to 17.1 kDa, with four bands (49.7, 33.2, 26.4, and 19.5 kDa) present in all dogs. In the 22% gel, molecular weights ranged from 15.6 to 3.6 kDa, with nine bands (15.6, 13.5, 12.7, 11.7, 10.5, 8.7, 7.8, 5.6, and 4.9 kDa) present in all dogs. Considering both gel concentrations, the majority of bands (85%) had molecular weights below 17 kDa, with the 15.6 kDa (B20) in high concentrations in all dogs.
Fig. 1.
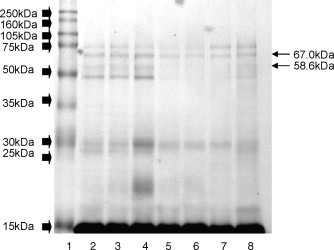
Polyacrylamide electrophoresis gel (SDS-PAGE) at 13% concentration in a seperation gel, in a discontinuous alkaline system of canine seminal plasma proteins. Lane 1, MW marker (full molecular weight range, 10–250 kDa); wide arrows indicate bands formed by MW marker. Lanes 2–8 are canine seminal plasma; fine arrows indicate protein bands B4 (67 kDa) and B5 (58.6 kDa).
Fig. 2.
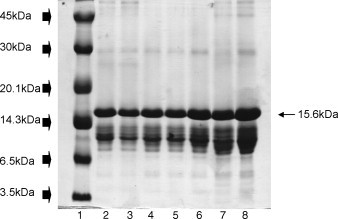
Polyacrylamide electrophoresis gel (SDS-PAGE) at 22% concentration in a seperation gel, in a discontinuous alkaline system of canine seminal plasma proteins. Lane 1, MW marker (low molecular weight range, 2.5–45 kDa); wide arrows indicate bands formed by MW marker. Lanes 2–8 are canine seminal plasma; fine arrow indicates protein band B20 (15.6 kDa).
Fig. 3.
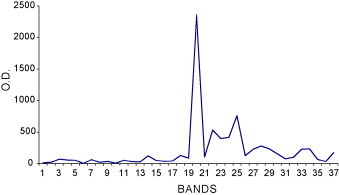
Optical density (OD) profile of seminal plasma proteins (mean of 20 adult dogs). Each peak represents the quantity of protein in each band.
There was no significant correlation between total protein concentration and semen characteristics. There was a positive correlation between the IOD of two bands (B4, 67 kDa and B5, 58.6 kDa) of seminal plasma and semen characteristics (Table 2 ).
Table 2.
Correlation coefficients (r) and probability (P) between seminal protein bands B4 (67.0 kDa) and B5 (58.6 kDa) and semen characteristics in adult dogs
| Semen characteristic | B4 (67.0 kDa) |
B5 (58.6 kDa) |
||
|---|---|---|---|---|
| Correlation (r) | P | Correlation (r) | P | |
| Sperm motility | 0.66 | 0.0016 | 0.46 | 0.0011 |
| Sperm vigor | 0.56 | 0.011 | 0.66 | 0.0015 |
| Morphologically normal | 0.55 | 0.012 | 0.59 | 0.0065 |
| Hypoosmotic test | 0.76 | 0.00009 | 0.68 | 0.0011 |
| Fluorescence | 0.56 | 0.009 | 0.59 | 0.0063 |
4. Discussion
There was nearly a fourfold variation among individual dogs in seminal plasma protein concentrations, consistent with a previous study [26]. Furthermore, the mean concentrations were similar to those previously reported [13], [14], [26], [27], [28]. The high molecular weight bands were a small fraction of total protein, whereas those with a low molecular weight were at much higher concentrations. Therefore, although we were using gradient gels, it was not possible to separate low and high molecular weight proteins in the same gel, necessitating the use of two separate gels.
The first study to characterize canine seminal plasma proteins used electrophoresis; three protein fractions were identified [13]. These fractions arranged themselves from the starting line solely in the direction of the negative pole, in the area of gamma-globulins. Dogs with reduced fertility also had the three fractions; the migration path and protein concentration were identical to those found in the seminal plasma of fertile dogs. However, ejaculates from old dogs with azoospermia did not separate, but remained as a single fraction at a variable distance from the starting line, becoming diffuse towards the cathode. Bruschi et al. [14] also reported three fractions of proteins separated from canine seminal plasma, but did not determine associations between these proteins and fertility or seminal analysis. In the present study, using SDS-PAGE, we identified 37 bands. These findings extend our previous report, in which 25 bands (molecular weights ranged from 3.5 to 136 kDa) were identified in five dogs of unknown fertility [16].
Band B20 (15.6 kDa) had the highest concentration of all 37 bands identified. Perhaps this band is an arginine esterase subunit. High concentrations (∼10 ng/mL) of arginine esterase are present in canine prostatic secretions [29]; it accounted for >90% of protein secreted by the canine prostate and ∼30% of canine seminal plasma proteins [30]. The molecular weight of this protein was estimated at 29.5 kDa by Sephadex G-100 gel filtration and at 25 kDa by SDS-PAGE [31]. Under denaturing conditions it formed two sub-units, H and L, with molecular weights of 15 and 12–14 kDa, respectively [32].
Canine arginine esterase is considered a specific immunological marker to assess the normality of the prostate gland [33] and is very similar to human Prostatic Specific Antigen (PSA), an important marker of prostrate cancer [34]. Furthermore, canine arginine esterase has considerable homology to a family of heparin-binding proteins from equine seminal plasma (SSPs-7) [35]. We have investigated the affinity by heparin of canine seminal plasma proteins; Band B20 (15.6 kDa) was a heparin-binding protein [36]. The current data provided additional evidence that band B20 is a canine arginine esterase subunit; however, that requires confirmation with other methods.
There was no significant correlation between total protein concentration and semen characteristics, in contrast with previous reports in other species [37], [38], [39], [40]. It is noteworthy that this is the first study in dogs to correlate protein band densitometry to semen characteristics. Sperm motility, as well as other semen characteristics, were correlated to the IOD of two bands (B4 and B5, 67 and 58.6 kDa). Similarly, in stallions, there was a linear correlation between motility of both fresh and frozen-thawed spermatozoa and the relative concentration of two protein bands (19.6 and 15.3 kDa) [41]. Furthermore, Killian et al. [5] reported that differences in nonreturn rates of reproductively normal bulls were correlated with the amounts of four proteins in seminal plasma. Moreover, in previous studies we showed the ∼58.6 kDa band to be a heparin-binding protein [36]. Thus, since motility and other seminal characteristics are correlated to in vitro and in vivo fertility in dogs [20], [42], the proteins identified in the present study may be valuable markers for fertility.
Identification of seminal plasma proteins has considerable merit; they can potentially be used to predict fertility, and perhaps to either increase fertility or as a contraceptive [5]. Studies in dogs are important, both as a model for assisted reproductive technologies in endangered species as well as for advances in medical research (since dogs are an important model for the study of human prostate function).
The fertility of the dogs used in the present study was unknown. Furthermore, although we detected significant correlations between seminal plasma proteins and semen characteristics, it is noteworthy that correlation does not imply cause. Therefore, these findings should be interpreted with caution, pending further studies that directly relate seminal plasma proteins and fertility. In addition, more sophisticated studies that allow higher-resolution separation of seminal plasma proteins and more detailed characterization of those proteins, as well as investigation of their physiological role, will further advance knowledge in this area.
Acknowledgement
We acknowledge financial support from FAPESP (98/16388-6).
Footnotes
Dr. Paulo Roberto Rodrigues Ramos, Department of Physical and Biophysical, IB, UNESP, Botucatu, SP, Brazil—Personal Communication, 2000.
References
- 1.Ayyagari R.R., Fazleabas A.T., Dawood M.Y. Seminal plasma proteins of fertile and infertile men analyzed by two-dimensional electrophoresis. Am J Obstet Gynecol. 1987;157:1528–1533. doi: 10.1016/s0002-9378(87)80257-0. [DOI] [PubMed] [Google Scholar]
- 2.Frazer G.S., Bucci D.M. Characterization of the major polypeptides of equine seminal plasma by two-dimensional polyacrilamide gel electrophoresis. Theriogenology. 1996;46:1389–1402. [Google Scholar]
- 3.Moreau R., Manjunath P. Characterization of lipid efflux particles generated by seminal phospholipid-binding proteins. Biochem Biophys Acta. 1999;1438:175–184. doi: 10.1016/s1388-1981(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 4.Manjunath P., Marcel Y.L., Uma J., Seidah N.G., Chretien M., Chapdelaine A. Apolipoprotein A-1 binds to a family of bovine seminal plasma proteins. J Biol Chem. 1989;264:16853–16857. [PubMed] [Google Scholar]
- 5.Killian G.J., Chapman D.A., Rogowski L.A. Fertility-associated proteins in Holstein bull seminal plasma. Biol Reprod. 1993;49:1202–1207. doi: 10.1095/biolreprod49.6.1202. [DOI] [PubMed] [Google Scholar]
- 6.Manjunath P., Chandonnet L., Baillargeon L., Roberts K.D. The calmodulin-binding proteins of bovine semen. J Reprod Fertil. 1993;97:75–81. doi: 10.1530/jrf.0.0970075. [DOI] [PubMed] [Google Scholar]
- 7.Manjunath P., Chandonnet L., Leblond E., Desnoyers L. Major proteins of bovine seminal vesicles bind to spermatozoa. Biol Reprod. 1993;49:27–37. doi: 10.1095/biolreprod50.1.27. [DOI] [PubMed] [Google Scholar]
- 8.Brandon C.I., Heusner G.L., Caudle A.B., Fayrer-Hosken R.A. Two-dimensional polyacrylamide gel electrophoresis of equine seminal plasma proteins and their correlation with fertility. Theriogenology. 1999;52:863–873. doi: 10.1016/S0093-691X(99)00178-8. [DOI] [PubMed] [Google Scholar]
- 9.Kraus M., Tichá M., Jonáková V. Heparin-binding proteins of human seminal plasma homologous with boar spermadhesins. J Reprod Immunol. 2001;51:131–144. doi: 10.1016/s0165-0378(01)00072-9. [DOI] [PubMed] [Google Scholar]
- 10.Cancel A.M., Chapman D.A., Killian G.J. Osteopontin is the 55-kilodalton fertility-associated protein in Holstein bull seminal plasma. Biol Reprod. 1997;57:1293–1301. doi: 10.1095/biolreprod57.6.1293. [DOI] [PubMed] [Google Scholar]
- 11.Gerena M.R.L., Irikuda D., Urade Y., Eguchi N., Chapman D.A., Killian G.J. Identification of a fertility associated protein in bull seminal plasma as lipocalin-type prostaglandin D synthetase. Biol Reprod. 1998;58:826–833. doi: 10.1095/biolreprod58.3.826. [DOI] [PubMed] [Google Scholar]
- 12.Roncoletta M., Morani E.S.C., FranceschinI P.H. 14 kDa seminal plasma protein identification and its relation with bull semen freezability. Theriogenology. 2002;57:479. [Google Scholar]
- 13.Dubiel A. Electrophoretic studies of dog's semen plasma in both fertile and sterile dogs. Polskie Archiwum Weterynaryjne. 1974;17:699–706. [abstract] [PubMed] [Google Scholar]
- 14.Bruschi J.H., Mendes M.C., Viana E.S., Abreu J.J., Megale F. Teores de ácido cítrico, frutose, proteína total e seu fracionamento eletroforético no sêmen do cão pastor alemão normal. Arquivos da Escola de Veterinária da UFMG. 1979;31:13–17. [Google Scholar]
- 15.Stubbs A.J., Resnick M.I. Protein electrophoresis patterns of canine prostatic fluid—effect of hormonal manipulation. Invest Urol. 1978;16:175–178. [PubMed] [Google Scholar]
- 16.de Souza F.F., Lopes M.D. Massa molar das proteínas do plasma seminal canino: dados preliminares. Revista Brasileira de Reprodução Animal. 2002;26:75–77. [Google Scholar]
- 17.Seager S.W.J., Platz C.C. Artificial insemination and frozen semen in the dog. Vet Clin North Am. 1977;7:757–764. doi: 10.1016/s0091-0279(77)50088-3. [DOI] [PubMed] [Google Scholar]
- 18.Papa F.O., Alvarenga M.A., Bicudo S.D., Lopes M.D., Ramires P.R.N. Coloração espermática, segundo KARRAS modificada pelo emprego do Barbatimão (Stryphnodendrum barbatiman). Proceedings of the V Congresso Brasileiro de Biologia Celular, III Congresso Ibero-Americano de Biologia Celular; Rio de Janeiro, Brazil; 1986. [Google Scholar]
- 19.Blom E. The ultrastructure of some characteristic sperm defects and a proposal for a new classification of the bull spermiogram. Proceedings of the VII Symposio Internationale de Zootechnia; Milão, Itália; 1972. pp. 125–139. [Google Scholar]
- 20.Jeyendran R.S., van der Ven H.H., Perez-Pelaez M. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil. 1984;47:219–228. doi: 10.1530/jrf.0.0700219. [DOI] [PubMed] [Google Scholar]
- 21.Inamassu A., Uechi E., Lopes M.D. Viabilização do teste hiposmótico em cães e sua relação com outras variáveis espermáticas. Revista Brasileira de Reprodução Animal. 1999;23:302–304. [Google Scholar]
- 22.Harrison R.A.P., Vickers S.E. Use of fluorescent probes to assess membrane integrity in mammalian spermatozoa. J Reprod Fert. 1990;88:343–352. doi: 10.1530/jrf.0.0880343. [DOI] [PubMed] [Google Scholar]
- 23.Cunha I.C.N., Lopes M.D., Zuccari C.E.S.N. Padronização da técnica fluorescente para a avaliação da integridade de membranas espermáticas na espécie canina. Proceedings of the XV Congresso Panamericano de Ciências Veterinárias; Campo Grande, Mato Grosso, Brazil; 1996. p. 411. [Google Scholar]
- 24.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Bollag D.M., Rozycki M.D., Edelstein S.J. Wiley-Liss; New York: 1996. Protein methods. p. 107–54. [Google Scholar]
- 26.Wales R.G., White I.G. Some observations on the chemistry of dog semen. J Reprod Fertil. 1965;9:69–77. doi: 10.1530/jrf.0.0090069. [DOI] [PubMed] [Google Scholar]
- 27.Bartlett D.J. Biochemical characteristics of dog semen. Nature. 1958;182:1605–1606. doi: 10.1038/1821605a0. [DOI] [PubMed] [Google Scholar]
- 28.Bartlett D.J. Studies on dog semen. II. Biochemical characteristics. J Reprod Fertil. 1962;3:190–205. doi: 10.1530/jrf.0.0030190. [DOI] [PubMed] [Google Scholar]
- 29.Frenette G., Dubé J.Y., Marcotte J.R. Arginine esterase from isolated dog prostate secretory granules is fully active enzymatically. Can J Physiol Pharm. 1985;63:1603–1607. doi: 10.1139/y85-264. [DOI] [PubMed] [Google Scholar]
- 30.Dubé J.Y. Prostatic kallikreins: biochemistry and physiology. Comp Biochem Physiol. 1994;107:13–20. [Google Scholar]
- 31.Chapdelaine P., Dube J.Y., Frenette G., Tremblay R.R. Identification of arginine esterase as the major androgen-dependent protein secreted by dog prostate and preliminary molecular characterization in seminal plasma. J Androl. 1984;5:206–210. doi: 10.1002/j.1939-4640.1984.tb02395.x. [DOI] [PubMed] [Google Scholar]
- 32.Isaacs W.B., Shaper J.H. Immunological localization and quantification of the androgen-dependent secretory protease of the canine prostate. Endocrinology. 1985;117:1512–1520. doi: 10.1210/endo-117-4-1512. [DOI] [PubMed] [Google Scholar]
- 33.McEntee M., Isaacs W., Smith C. Adenocarcinoma of the canine prostate: immunohistochemical examination for secretory antigens. Prostate. 1987;11:163–170. doi: 10.1002/pros.2990110207. [DOI] [PubMed] [Google Scholar]
- 34.Dubé J.Y., Lazure C., Tremblay R.R. Dog prostate arginine esterase is related to human prostate specific antigen. Clin Invest Med. 1986;9:51–54. [PubMed] [Google Scholar]
- 35.Calvete J., Sanz L., Reinert M., Dostalova Z., Topfer-Petersen E. Heparin-binding proteins on bull, boar, stallion, and human spermatozoa. Mem Mus Nat Hist Nat. 1995;166:515–524. [abstract] [Google Scholar]
- 36.Souza F.F., Martins M.I.M., Fernandes C.E.S., Ribolla P.E.M., Lopes M.D. Heparin-binding proteins of canine seminal plasma. Theriogenology. 2006;66:1606–1609. doi: 10.1016/j.theriogenology.2006.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore H.D., Hibbitt K.G. The binding of labelled basic proteins by boar spermatozoa. J Reprod Fertil. 1976;46:71–76. doi: 10.1530/jrf.0.0460071. [DOI] [PubMed] [Google Scholar]
- 38.Dott H.M., Harrison R.A.P., Foster G.C.A. The maintenance of motility and the surface properties of epididymal spermatozoa from bull, rabbit and ram in homologous seminal and epididymal plasma. J Reprod Fertil. 1979;55:113–124. doi: 10.1530/jrf.0.0550113. [DOI] [PubMed] [Google Scholar]
- 39.Baas J.W., Molan P.C., Shannon P. Factors in seminal plasma of bulls that affect the viability and motility of spermatozoa. J Reprod Fertil. 1983;68:275–280. doi: 10.1530/jrf.0.0680275. [DOI] [PubMed] [Google Scholar]
- 40.de Souza F.F., Rodrigues A.L.R., Tutida L., Bicudo S.D. Proceedings of the II Congreso Latinoamericano de Especialistas em Pequeños Ruminantes y Camélidos Sudamericanos y el XI Congreso Nacional de Ovinocultura Mérida. 2001. Efeito de dez colheitas sucessivas de sêmen sobre a concentração de proteínas totais do plasma seminal de carneiros. [Google Scholar]
- 41.Amann R.P., Cristanelli M.J., Squires E.L. Proteins in stallion seminal plasma. Equine reproduction IV. J Reprod Fertil Suppl. 1987;35:113–120. [PubMed] [Google Scholar]
- 42.Revell S.G., Mrode R.A. An osmotic resistance test for bovine semen. Anim Reprod Sci. 1994;36:200–203. [Google Scholar]


