Highlights
-
•
Bats experience morbidity to many extracellular but few intracellular infections.
-
•
Bats control intracellular pathogens via cellular pathways to apoptosis/autophagy.
-
•
These ROS mitigation pathways promote longevity and tumor avoidance.
-
•
Extracellular pathogen-associated morbidity in bats results from immunopathology.
Keywords: Chiroptera, emerging zoonotic pathogens, immunopathology, immunological tolerance, reactive oxygen species
Abstract
The ongoing West African Ebola epidemic highlights a recurring trend in the zoonotic emergence of virulent pathogens likely to come from bat reservoirs that has caused epidemiologists to ask ‘Are bats special reservoirs for emerging zoonotic pathogens?’ We collate evidence from the past decade to delineate mitochondrial mechanisms of bat physiology that have evolved to mitigate oxidative stress incurred during metabolically costly activities such as flight. We further describe how such mechanisms might have generated pleiotropic effects responsible for tumor mitigation and pathogen control in bat hosts. These synergisms may enable ‘special’ tolerance of intracellular pathogens in bat hosts; paradoxically, this may leave them more susceptible to immunopathological morbidity when attempting to clear extracellular infections such as ‘white-nose syndrome’ (WNS).
An ancient history with a new relevance
The association between bats and human disease has been acknowledged for over a century, since the first identification of rabies Lyssavirus in asymptomatic vampire bats in 1911 [1]. Until recently, rabies dominated the scientific literature on bats and disease; however, following the emergence of horse- and human-infecting Hendra virus from Australian flying foxes in 1994 [2], bats have emerged as potential reservoirs (see Glossary) for a broad variety of zoonotic infections involving particularly virulent – and often fatal – RNA viruses. Although isolation of live virus from bat hosts has proven elusive in certain cases – notably that of Ebola [3] – major evidence supports the role of bats as reservoirs for Hendra and Nipah henipaviruses, Ebola and Marburg filoviruses, and severe acute respiratory syndrome (SARS) and likely Middle East respiratory syndrome (MERS) coronaviruses (CoVs), as well 4, 5. Following the identification of Rhinolophus spp. bats as reservoirs for SARS-CoV [6], Calisher et al. explored links between bats and emerging viruses in a review highlighting various aspects of bat life history and ecology – including ability to fly, dependency on torpor and/or hibernation, long life span, and gregarious social structure – that are likely to influence bats’ roles as viral reservoirs [4]. In an attempt to explain bats’ tolerance of otherwise virulent viruses, these earlier authors emphasized a deep coevolutionary relationship between bat genomes and those of lyssaviruses [7] and henipaviruses [8]. We explore evolutionary mechanisms enabling this immunological tolerance in bats that may be lacking in non-volant mammals, including humans.
Bats as special reservoirs for viral pathogens
Recent studies have confirmed an ancient phylogenetic relationship between bats and a suite of other viral pathogens: in addition to lyssaviruses and henipaviruses, bats are now posited as the most ancestral host taxon for the entire family of paramyxoviruses (of which henipaviruses represent one genus [9]), as well as for CoVs [10], hepadnaviruses related to human hepatitis B virus [11], and hepaciviruses related to hepatitis C virus [12]. Bats also demonstrate deep phylogenetic relationships with influenza A virus [13], filoviruses [14], and simplex viruses [15]. Several authors have compiled informative reviews 16, 17, 18 and meta-analyses 19, 20 from various perspectives, continually asking ‘Are bats special in their reservoir roles for zoonotic pathogens?’ Common threads prevail and, increasingly, the consensus seems to be a complex and qualified ‘yes’.
Although not the most represented mammalian order among zoonotic hosts, bats host more zoonotic viruses per species than do rodents and most of the resulting zoonoses have been high-profile spillover incidents of extreme human pathogenicity [21]. Bats largely host viral pathogens without demonstrating ostensible disease [22], but the pathogenicity is complex and research into impacts of viral infection on bat fitness, particularly with respect to fecundity or longevity, has been critically lacking. Obvious exceptions to viral asymptomaticity in bats include, notably, rabies and Tacaribe virus, a South American Arenavirus that caused widespread bat mortality in the 1950s and in later experimental infections [23]. Adenoviruses have also been linked to bat mortality [24], although such patterns are perhaps unsurprising given the disparity between this gastrointestinal system-infecting DNA virus and other bat-affiliated (mostly RNA) viruses discussed here. One study demonstrated seasonal amplification of RNA viruses, but not DNA viruses, in a monitored insectivorous bat colony in Europe [25], suggesting that different mechanisms of control may be at play for RNA versus DNA viruses in bat hosts. Additionally, a novel filovirus, Lloviu virus, was recently detected in bat tissues following a massive die-off event [26], and although originally cited as the cause of bat mortality Lloviu virus has now been resolved to be a uniquely bat-adapted virus, leading researchers to explore other mechanisms for bat mortality in this incident [27]. In one study examining causes of mortality in 486 deceased bats in Europe, viral infections (lyssaviruses and adenoviruses) were responsible for only five of 144 identified disease-related deaths [28].
Bats as not-so-special reservoirs for non-viral pathogens
Recent work has begun to investigate the role of bats as hosts for non-viral pathogens, with somewhat varied results. Similar to viruses, bats demonstrate coevolutionary associations with intracellular malarial protozoa [29] as well as extracellular trypanosome protozoa including Trypanosoma cruzi, the causative agent in zoonotic Chagas disease [30]. Bats also exhibit coevolutionary specificity with erythrocytic Bartonella spp. bacteria [31]. On a par with viruses, bats appear to host both classes of protozoa and Bartonella spp. without ostensible disease symptoms, yet they exhibit pronounced pathology following infection with certain extracellular pathogens, chiefly Borellia spp. [32] and some enteric bacteria [33]. Bats also experience pathology on infection with the bacterium Pasteurella multocida [34], which can function as both an intra- and extracellular pathogen. Table 1 summarizes bat-hosted pathogens by clade and offers examples to illustrate what is currently known of their affiliated pathogenicity.
Table 1.
Example bat infections and associated immune responses across microbial classes
| Microbial class | Example pathogen | Infection site | Documented pathology in bat host? | Refs |
|---|---|---|---|---|
| Viruses | Henipavirus spp. | Intracellular (blood + tissue) | No | [88] |
| Lyssavirus spp. | Intracellular (central nervous system) | Yes (pathogen induced) | 46, 57 | |
| Bacteria | Bartonella spp. | Intracellular (blood + tissue) | No | [31] |
| Borellia spp. | Extracellular (blood) | Yes | [32] | |
| Protozoa | Plasmodium spp. | Intracellular (blood + tissue) | No | [29] |
| Trypanosoma spp. | Extracellular (blood with intracellular amastigote stage) | No | 30, 42 | |
| Fungi | Histoplasma capsulatum | Intracellular (macrophage) | No (except experimental manipulation) | [40] |
| Pseudogymnoascus destructans | Extracellular (wing surface) | Yes (immunopathology) | [41] | |
| Helminth | Lecithodendrium spp. | Extracellular (intestine) | Minimal | [35] |
In addition to supporting microparasitic viruses, protozoa, and bacteria, bats are hosts for various macroparasitic helminths, chiefly trematodes [35], nematodes (including some filarial species [36]), and cestodes [37]. Bat susceptibility to helminths appears consistent with that of other mammals, which exhibit dose-dependent morbidity rather than mortality. Curiously, some hibernating bats display idiosyncratic patterns of helminth retention that differ from typical patterns of voidance during hibernation in other mammals [38].
Bats have also been long recognized as sources of the globally distributed zoonotic fungus Histoplasma capsulatum, which, as an intracellular parasite of macrophages [39], is asymptomatic in the chiropteran host. By contrast, when experimentally introduced via intraperitoneal inoculation, the pathogen overwhelms the extracellular spaces of bat tissues, causing lesions and severe inflammation [40]. This pronounced immunopathological response to fungal infection is particularly germane to the current widespread infection of North American bats with the extracellular fungus Pseudogymnoascus destructans (the causative agent of WNS). Histological wing lesions characteristic of WNS suggest massive immunopathological inflammation when hibernating bats infected with P. destructans arouse from torpor [41].
Thus, the ubiquitous role of bats as special pathogen reservoirs is called into question when pathogens beyond the ‘virosphere’ are considered; bats exhibit standard-to-extreme pathology following infection with certain bacteria and fungi. In particular, this comparison highlights the unique resilience of bats to infection with intracellular pathogens – a category encompassing all viruses, some protozoa, and some bacteria. By contrast, pathogens that predominantly occupy the extracellular space present considerable challenges for bat immune systems. In the case of trypanosomes, it should be noted that, although largely extracellular, trypanosomes also support an intracellular, amastigote life stage that is subject to the majority of immunological attack and regulation [42]. In the following sections, we explore unique mechanisms linking immune functioning with bat metabolism and longevity to offer a new explanation for how bat physiology and immunology enable a special reservoir role for viral pathogens.
Intra- versus extracellular immunity: where bats succeed and fail
Like other mammals, bat immune systems comprise both innate and adaptive elements (Figure 1 ) [22]. The first line of innate immune defense involves classification of pathogens into broad microbial categories by pattern recognition receptors (PRRs), typically localized in dendritic cells, that enable the host to mount a pathogen-appropriate immune response (Box 1 ) [43]. PRRs are well conserved between bats and other mammalian lineages and the bat immune system capably detects a broad range of viruses, bacteria, and fungi [44]. PRR detection of foreign microbes produces a cascade of cytokine signaling specific to the class of microbe encountered. These cytokines serve as signaling molecules for cell-mediated components of the innate immune system [i.e., recruitment of phagocytic neutrophils and monocytes and cytotoxic natural killer (NK) cells] and drive T cell differentiation in the adaptive immune system. In the humoral immune system, the body's complement cascade constitutes the main innate immune component, while B lymphocytes, their daughter plasma cells, and the antibodies they synthesize represent the adaptive component.
Figure 1.
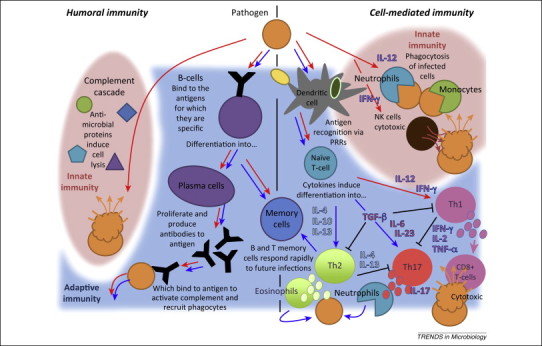
The vertebrate immune system. Pathways to humoral immunity include the innate complement response and the adaptive antibody response, while cell-mediated pathways necessitate action from innate effecter cells [i.e., phagocytes and natural killer (NK) cells] or adaptive T cells. Red arrows indicate possible host immune responses to infection with an intracellular pathogen (i.e., viruses, protozoa, and intracellular bacteria); blue arrows signify immunological pathways to clearance of extracellular pathogens (i.e., extracellular bacteria, fungi, and helminths). It should be noted that extracellular bacteria can also be cleared by the complement cascade.
Box 1. Pathogen recognition, host immune defense, and the mitochondria.
Transmembrane versus cytosolic PRRs
PRRs located within the cellular membrane are capable of recognizing both intra- and extracellular pathogens, while cytosolic PRRs are generally restricted to recognition of intracellular pathogens, chiefly viruses or bacteria [43]. Transmembrane Toll-like receptors (TLRs) have long been acknowledged for their viral recognition role in the mammalian immune system, but the discovery of a new class of cytosolic PRR, the retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), offers an alternative pathway implicated in the immune recognition of RNA viruses [89].
Pathogen recognition and cytokine induction
In humans, pathogen recognition via TLRs induces cytokines such as IL-12, which recruit neutrophils and monocytes of the innate immune system and stimulate the Th1 branch of the cell-mediated components of the adaptive immune system. Pathogen (usually virus) recognition by RLRs also upregulates Th1 pathways of adaptive immunity, but does so through induction of the cytokine IFN-γ, which favors NK cell routes of innate immunity. By contrast, extracellular fungi are detected at dectin-1 PRRs that induce IL-23, the cytokine responsible for upregulation of the Th17 branch of cell-mediated adaptive immunity [43].
A role for the mitochondria
Intriguingly, RLR signaling appears to require the participation of a protein (MAVS) bound in the outer membrane of the cellular mitochondria, thus necessitating a role for mitochondria in viral immunity. Mitochondria have long been recognized for their role in general cell maintenance, damage repair, and apoptosis, a process initiated by PRR recognition of damage-associated molecular patterns (DAMPs), which include mitochondrial ROS. PRRs recognize and respond to pathogen-associated molecular patterns (PAMPs) in a similar way [90]. With the recent discovery of RLRs and their localization to the mitochondria, awareness of the underappreciated role of the mitochondria in immune function is growing.
Until recently, most insights into bat immunity were derived from studies measuring humoral adaptive immune responses, mainly because the tools for antibody detection are available [16]. Bats do mount antibody responses to various infections, including lyssaviruses, filoviruses, and henipaviruses, but these responses are sometimes idiosyncratic [16]. In particular, antibody recruitment has been demonstrated to be delayed post-immune challenge in bat cells compared with other vertebrates [45] and repeated rabies inoculations over a longitudinal time series produce a discontinuous pattern of adaptive immunity in which antibodies in seemingly immune bats sometimes drop below detectable levels altogether or virus and antibody (but not disease) are detected concurrently [46]. Other work demonstrates heightened seroprevalence in female bats during periods of pregnancy and lactation with waning antibodies across the rest of the year, as well as heightened seroprevalence in bats of both sexes under nutritionally stressed conditions [47]. These findings suggest that bats may exhibit increased reliance on adaptive immunity when normal immunological pathways used to control infections are otherwise compromised. As heightened viral shedding and concomitant spillover events have been linked to bat reproduction for both henipaviruses and filoviruses 47, 48, it is possible that humoral adaptive immunity is less effective at controlling viral transmission than bats’ standard mechanism of immune regulation.
In the realm of cell-mediated immunity, bats are known to produce cytokines in response to infection, chiefly high levels of interferon (IFN), a class of signaling molecules that regulate pathways to apoptosis in virus- and tumor-infected cells 49, 50. Type I interferons include IFN-α and -β, which are secreted by various cells to recruit macrophages and NK cells against viral infections or tumors. Many viruses work to antagonize signaling pathways for type I interferons in infected hosts. Antagonism of type I and complementary type III interferons has been demonstrated in both human and bat cell lines in response to Henipavirus infection [51], suggesting that bats might control Henipavirus replication by some non-IFN-mediated mechanism. Bats also possess type II interferon, IFN-γ, which generally functions to coordinate a systemic response involving both innate and adaptive immune pathways against microparasitic infections; IFN-γ was the first-recognized IFN in early viral studies in bat hosts [50]. Although much work remains to be done in this field, several major mammalian cytokines have now been documented in bats, including all major inducers of T helper (Th) cell-mediated adaptive immunity: Th1 [interleukin (IL)-12 and IFN-γ], Th2 (IL-4 and IL-10), and Th17 (IL-6 and IL-23) 23, 52.
Studies of T lymphocyte activity in bats have been limited to date and often rely on crude measures of T cell-associated inflammation that can be easily misinterpreted, as the reagents needed to identify and quantify actual T cell populations in bats do not currently exist [22]. The recent development of bat-specific antibodies to IFN-γ induced in response to viral infection in the Australian flying fox, Pteropus alecto, offers great potential for quantification of bat T lymphocyte activity in the future [53]. Early investigations of cell-mediated immunity in bats report a lack of histological inflammation in individuals infected with H. capsulatum [54], as well as a lack of inflammatory response in most Pteropus giganteus subjected to skin sensitivity tests [45]. Lymphocyte culture reactions conducted on cells of these same bats mixed with stimulator cells also demonstrated a delay in reaction compared with the cells of other vertebrates [45].
In a recent review, O'Shea et al. posit that bats have evolved to favor incomplete viral clearance to evade immunopathologically induced morbidity and mortality [18]. Reported delays in recruitment of B and T lymphocytes in bat immune systems post-infection are consistent with these hypotheses of truncated immune response, or tolerance – an immunological strategy typical of an asymptomatic pathogen reservoir [55]. Bats appear capable of restricting immune responses to avoid immunopathology on infection with certain pathogens with which they share a deep evolutionary history. The recent discovery of endogenous viral elements in animal genomes indicates that bat immune systems might be able to tolerate these pathogens on recognition as elements of ‘self’ [56]. This bat pathogen tolerance is not universal, however, and bats experience severe morbidity and mortality as a result of infection with some viral [23], bacterial 32, 33, and fungal pathogens [41]. In most cases, it is the host immunopathological response to infection, rather than the pathogen itself, that is most responsible for mortality [55]. One major possible exception to this is rabies-related mortality, in which the virus may cause direct pathology in the central nervous system while simultaneously evading immune detection altogether [57]. In the case of WNS, bats experience uncontrolled neutrophilic inflammation, an immunopathological response, on arousal from hibernation [41].
Why, then, have bat immune systems adapted to tolerate certain pathogens while remaining aggressively – and dangerously – vigilant with respect to others? We review evidence that bats have evolved intracellular mitochondrial adaptations to minimize oxidative stress accrued during metabolically costly activities such as flight. As recent research highlights growing acknowledgement of the central role of mitochondria in cellular signaling and immune defense [58], we suggest that bats may control pathogenesis in microbe-invaded cells via processes of autophagy and apoptosis that originally evolved to regulate metabolic stress, thus avoiding the usual immunopathological consequences of heightened immune response. By definition, these control mechanisms will be limited to intracellular pathways, leaving bats vulnerable to suffering the immunopathological consequences of attempts to clear extracellular infections.
‘Flight-as-fever’ hypothesis in bat–virus coevolution
The evolution of flight has been suggested to provide a mechanism for viral control in bats 18, 21, 50. O'Shea et al. posit that higher body temperatures and metabolic rates attained by bats in flight stimulate immune functioning to facilitate viral clearance and that viral adaptation to febrile conditions in the bat host might explain the high pathogenicity of these agents following spillover into other mammalian hosts, which are unable to support a fever response comparable to bat body temperatures in flight [18]. Bats’ minimal thermoregulatory abilities have been acknowledged for some time [59]; bat body temperatures appear to directly approximate ambient air temperature when at rest, meaning that bats in flight regularly maintain temperatures above 40°C, a level typifying high fever in other mammals [59]. Fever is an acknowledged adaptive mechanism through which the body elevates temperatures above those optimal for invading pathogens while also accelerating metabolic and immune processes [60]. Empirical work demonstrates that virus-inoculated bats preferentially maintain viruses in the blood stream rather than in tissue at elevated ambient temperatures; viral clearance then proceeds without demonstrable antibody response, thus suggesting a role for some adaptive, non-humoral form of immune control [50].
Although an attractive theory, O'Shea et al.’s flight-as-fever hypothesis falls short of explaining why bats are particularly well-suited hosts for viral pathogens [18]. If high temperatures and elevated metabolic rates stimulate bat immune functioning for pathogen control, why do bats experience widespread immunopathology when this immune vigilance is directed at some classes of pathogen (i.e., fungi) but not others (i.e., viruses)? If we accept O'Shea et al.’s argument that ancient coevolutionary bat–virus relationships have evolved to favor incomplete viral clearance (i.e., tolerance) in order to mitigate immunopathology, it is possible that North American bats susceptible to WNS may still be in the process of immunological adaptation to P. destructans, the so-called ‘novel pathogen’ hypothesis 18, 61. That said, interspecies sharing of viruses is extensive [20], suggesting that – for viral pathogens at least – generalist host immune responses should predominate over adaptive defenses. In the following section, we examine how metabolic processes adopted for flight might enable modulation of antiviral immune mechanisms more readily than mechanisms directed toward other classes of pathogens.
Linking metabolism, longevity, and immunity
Going fast in the slow lane
Bats are among the longest-lived mammalian species, with lifespans 3.5 times those of non-flying eutherian mammals of comparable body size [62]. Traditional ‘rate-of-living’ theories suggest an inverse correlation between metabolic activity and longevity, and because large-bodied animals often have lower basal metabolic rates this is argued to correspond to shorter lifespans for smaller animals [63]. Typically, metabolically costly activities such as flight yield byproducts in the form of reactive oxygen species (ROS), which damage cell structure and mitochondrial DNA. Basal metabolic rates are not well characterized in bats, but bats are known to experience high variation in metabolic rate, being capable of achieving rates up to 34 times their resting levels when in flight compared with an eightfold increase in metabolic rate in exercising rodents [64]. Despite this, some bats species live for over 40 years in the wild [62] and average lifespans in bats significantly exceed those of comparably sized non-volant mammals [65].
Many bats from temperate zones lower metabolic rates while undergoing hibernation through the winter and several studies demonstrate a link between extended longevity, slower reproductive rate, and proclivity for hibernation [62]. Nonetheless, even non-hibernating bats have extended lifespans for their body size, although recent work suggests that, among tropical and subtropical bats, reduction of metabolic activity during episodes of periodic diurnal torpor might be more common than has been previously acknowledged [66]. Luis et al. demonstrate diminished viral hosting among torpor- or hibernation-dependent bats, a pattern seemingly at odds with theories of extended longevity from torpor [20]. The authors suggest this viral depletion to result from lowered contact rates, while O'Shea et al. argue that diminished immunological vigilance during torpor could explain the pattern 18, 20. As most bats have not been thoroughly studied with respect to daily torpor energetics, more research in this arena is needed.
One possible mechanism of torpor-linked pathogen control in bats may be related to high levels of interscapular brown adipose tissue (BAT), one of two types of mammalian lipid tissue, found in both hibernating and non-hibernating bats [67]. In humans, BAT plays a role in thermogenesis in neonates, and in both hibernating rodents and bats it is thought to aid in torpor arousal, as the mitochondrion-rich tissue (called ‘brown fat’ because of the color induced from heavy mitochondrial loading) can exceed the temperature of the surrounding tissue by as much as 3°C during the late stages of hibernation arousal [67]. As brown fat loading in bats is known to vary seasonally and reach a minimum during periods of lactation [68], this reduction in mitochondrial load could help explain bats’ increased reliance on adaptive immune defenses during the reproductive period. Early studies investigating impacts of hibernation on pathogenesis of rabies in bat hosts have demonstrated the presence of rabies virus in chiropteran BAT when absent in other tissues, thus leading scientists to posit a role for long-term viral storage in chiropteran brown fat [69].
Antioxidants and bat metabolism
On average, bats expend double the amount of metabolic energy in a lifetime compared with non-flying eutherian mammals [70], yet they appear to accrue remarkably little oxidative stress as a result [71]. One study demonstrated that mitochondria in the little brown bat (Myotis lucifugus) produced significantly less free radical oxygen (H2O2) per unit oxygen consumed during metabolism than shrews and mice [72]. Measures of the important mammalian antioxidant superoxide dismutase (SOD) in the same analysis did not vary among species, causing the author to suggest that bats might simply generate fewer ROS in more-efficient mitochondria than comparable non-volant mammals. Alternatively, a different endogenous antioxidant enzyme may be responsible for immediate scavenging of free radicals at the mitochondrial production site. Some role for antioxidant defense in bat mitigation of oxidative stress is indicated by demonstrations of higher antioxidant levels in torpid versus active bats [73]; most antioxidant-mediated ROS scavenging occurs during periods of high metabolic activity, allowing antioxidant levels to recover during torpor.
The human antioxidant literature supports the idea of multiple roles for cellular ROS, some negative and some positive [74]. Mitigation of mitochondrial ROS at the site of production is important in both extending longevity and avoiding tumorigenesis, which results from oxidative damage to mitochondrial DNA (mtDNA) (Box 2 ) [75]. Somewhat paradoxically, mitochondrial ROS also upregulate cellular pathways to autophagy and apoptosis, helping the cell repair this damage or self-destruct when necessary [76]. Enzymatic antioxidants are thought to act most directly on ROS within the mitochondria, while dietary antioxidants play a significant role against exogenous forms of ROS or metabolic ROS escaped from mitochondria [74]. Bats may be able to recruit enzymatic antioxidants to scavenge ROS at the site of mitochondrial production more efficiently than comparably sized non-volant mammals, thus avoiding oxidative damage to mitochondrial DNA and its negative impacts on longevity and tumorigenesis.
Box 2. Mitochondria, oxidative stress, and aging.
The mitochondrial theory of aging
Numerous pathogenic mutations have been identified in the mitochondrial genome as causative agents in neurodegenerative and aging-related human diseases [91] and the accumulation of mutations in the mitochondrial genome of cells has been unequivocally linked to accelerated aging in mouse models via pathways of mitochondrial apoptosis [92]. One popular theory suggests that the accumulation of cellular damage from ROS produced as a byproduct of metabolism are responsible for most mtDNA damage, largely due to the physical proximity of the site of most respiration-induced ROS production (i.e., the mitochondrial electron transport chain) to the relatively unprotected mitochondrial genome [93]. However, despite decades of research, the causal role of ROS in mtDNA damage and its consequences for aging remain to be fully elucidated 76, 94.
Replicative senescence and telomere shortening
An alternative aging theory postulates the concept of replicative senescence, which proposes a finite lifespan of 50 ± 10 mitotic divisions (the so-called ‘Hayflick limit’) for somatic cells, negating the theory of ROS-accrued progressive cellular damage [95]. Shortening of telomeres, the end caps of nuclear chromosomal DNA, indeed correlates with aging in mammalian fibroblasts and was originally posited to be a sort of replicometer, or counting device, of mitotic division [96]. More recent work indicates that the rate of telomere degradation is not constant, being hastened by oxidative stress [97] and halted or reversed via production of the enzyme telomerase [98], which functions as a prerequisite for cellular immortalization in cancer [99].
Mitochondrial–nuclear crosstalk
Ongoing research highlights the role of ROS-mediated mitochondrial–nuclear crosstalk in the processes of cell maintenance, aging, and apoptosis. Mitochondrial signaling, probably via changes in intracellular calcium concentration, is posited to activate certain nuclear genes involved in the induction of replicative senescence [94]. ROS also appear to function as signaling molecules, upregulating pathways of autophagy and mitophagy at low levels to remove damaged mitochondria from the cell and inducing cellular apoptosis at high levels (Figure I ) [76]. Autophagy is believed to play an important role in cell tumor regulation via mitigation of genome damage to limit tumor necrosis and inflammation; however, defective autophagy is known to enable tumor survival in starved cells by downregulating apoptosis [100].
Figure I.
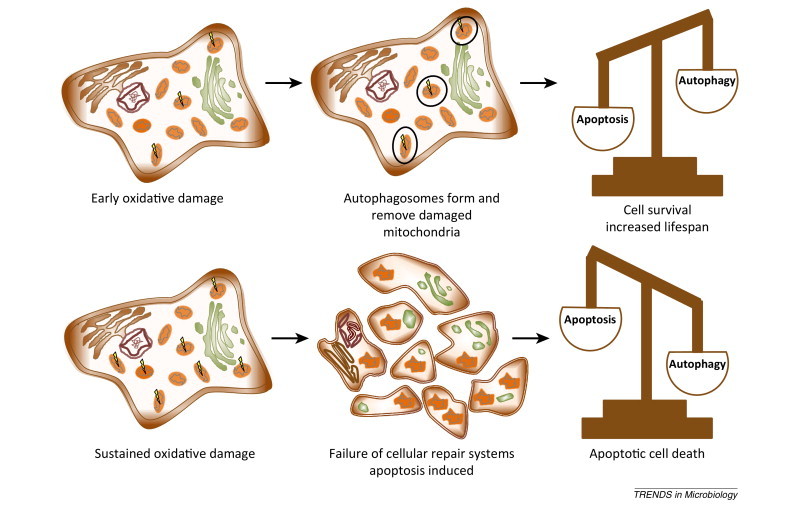
Reactive oxygen species (ROS) levels can control cell fate. When low levels of ROS are produced, autophagic and mitophagic processes remove damaged mitochondria and allow cell survival (top). As ROS levels increase and cell repair systems fail, apoptotic cell death occurs (bottom). Adapted, with permission, from [76].
In conjunction with retarded aging, it is relevant to note anecdotal observations suggesting lower rates of tumorigenesis in bats versus other mammals [17]. This pattern is supported by similar trends of longevity linked with tumor avoidance in other taxa: both echidnas and naked mole rats are surprisingly long lived for their body size and experience markedly low rates of cancer 77, 78, 79, 80. By contrast, marsupials in the Didelphidae and Dasyuridae families exhibit patterns of diminished longevity with body size and high susceptibility to tumors 81, 82, 83, a pattern today most obvious in the case of Tasmanian devil facial tumor disease [84]. Future studies investigating host immune responses to intra- versus extracellular pathogens across these diverse taxa will be valuable in testing our theories.
It's all in the mitochondrial genes
Exciting new research describes rapid evolution of mtDNA damage and repair pathways in bat genomes concordant with the timing of metabolically costly flight emergence. Other work has highlighted the underappreciated role of the mitochondria in signaling pathways of the immune system (Box 1) [85], again linking bats’ flying abilities with pathogen control. As noted above, free radical oxidation upregulates cellular damage and repair pathways (via autophagy) and induces apoptosis in cells damaged beyond repair. Intracellular microparasitic pathogens are known to cause oxidative damage both directly and via the induction of cytokines that recruit monocytes, neutrophils, cytotoxic T cells, and NK cells [which generate nitric oxide (NO), a common form of ROS] at the site of infection [86]. Thus, if bat immune systems interpret intracellular pathogen infection in a manner similar to oxidative damage incurred via metabolic processes or tumorigenesis, bats’ abilities to regulate these infections – via both autophagy and apoptosis – are unsurprising. The developing recognition of the mitochondria as a recruitment platform in antiviral signaling further supports this connection [58].
Finally, it is worth noting that analysis of the genomes of P. alecto and Myotis davidii indicates a striking absence of the entire locus (and associated genes) of the PYHIN gene family, a critical actor in innate immune responses in all other eutherian mammals [87]. Also absent or severely reduced are the typical receptor cell complexes for NK cells [87]. As NK cells are heavily involved in viral innate immunity and tumor mediation, it seems plausible that these bat genome inconsistencies are further linked to mechanisms enabling viral control; however, further study across a wider diversity of genomes will be necessary to assess the extent to which these patterns are universal.
Concluding remarks
The field of bat-borne zoonosis remains very much in its infancy and opportunities for future research abound. It seems safe to conclude that bats are special in their capacity to act as reservoir hosts for intracellular pathogens and this review represents a first attempt to identify some of the immunological mechanisms that underlie this ability. Bats are distinguished from other mammals by their ability to fly and processes critically involved in the evolution of this trait have yielded inadvertent consequences on their longevity and immune functioning. In particular, because flight requires bats to regularly endure wide variations in both temperature and metabolic rate, bats have developed altered mitochondrial genomes and corresponding pathways enabling the mediation of mitochondrial oxidative stress that simultaneously support long life and tumor and intracellular pathogen mediation. By contrast, bats remain vulnerable to immunopathological responses to extracellular infections and, in the case of WNS, are experiencing widespread mortality as a result. Future in vitro work characterizing and quantifying the mechanisms (i.e., PRRs, cytokines, and T lymphocytes) by which bats recognize and mount immune responses to diverse microbial classes will be influential in testing hypotheses presented here (Box 3 ). In addition, in vivo studies investigating the role of bat life history – particularly with respect to periods of hibernation and torpor – in immune functioning are likely to prove enlightening to researchers hoping to understand when and why bats operate as special pathogen reservoirs.
Box 3. Outstanding questions.
-
•
Which PRRs identify the different classes of microbes in bat immune systems?
-
•
What is the nature of cell-mediated immunity in bats? Can we characterize and quantify induction of specific cytokines and differentiation of Th cells in response to challenge with different classes of pathogen?
-
•
How do such profiles in bat immunity vary across periods of hibernation and torpor?
-
•
Can we characterize and quantify these immune parameters throughout the pathogenesis of WNS? How might they vary among species and geographical locale? What immunocompromising factors (i.e., roost temperature, duration of hibernation, and coinfection with other pathogens) might differ between exposed European and North American bats? Speculatively, can we demonstrate a role for Th17-associated antifungal immunopathology in the histological symptoms of WNS?
-
•
Do non-chiropteran taxa with similar life-history strategies to bats (i.e., naked mole rats and echidnas) display comparable tolerance for intracellular pathogens?
Acknowledgments
The authors thank A.L. Graham, B.T. Grenfell, and C.J.E. Metcalf for stimulating conversations and helpful comments throughout the development of the manuscript. They thank T. O'Shea and two anonymous reviewers for useful critiques of the final product. This work was partly supported by a Princeton University graduate fellowship and a National Science Foundation Graduate Research Fellowship to C.E.B.
Glossary
- Adaptive immune system
one of the two main immunity strategies of vertebrates. Relies on acquired immunological memory (circulating antibodies and T and B memory cells) left over from previous encounters with a specific pathogen to promote B cell proliferation and appropriate T cell differentiation to clear a new infection. Comprises both humoral and cell-mediated components.
- Antibodies
also known as immunoglobulins, these Y-shaped glycoproteins are produced by plasma cells (the daughter cells of B cells) in the humoral immune system in response to an encounter with a specific pathogen. Antibodies are found both free floating and attached to B cells and are retained in the humor after a pathogen is cleared in case of future infections.
- Apoptosis
a process of programmed cell death in which the mitochondria of stressed or damaged cells initiate a signaling cascade that induces the damaged cell to burst. Resulting apoptotic bodies are then cleared by phagocytes with no harm to the host. NO and other forms of mitochondrial ROS are heavily implicated in the initiation of pathways to apoptosis.
- Autophagy
a catabolic process by which lysosomes recycle internal cellular components that are unnecessary or damaged. When cell damage exceeds the ameliorating capabilities of autophagy, pathways to apoptosis are induced.
- Cell-mediated immunity
the components of the immune response performed by immune cells rather than antibodies. In the innate immune system, this refers primarily to phagocytes such as neutrophils and monocytes and to cytotoxic NK cells. In the adaptive immune system, this involves cytotoxic T lymphocytes (also known as CD8+ T cells), which are specific to a remembered pathogen.
- Cytokines
a class of small proteins involved in cellular signaling. Cytokines are particularly important in the immune system and signal for the proliferation or recruitment of various immune cells to an infection site. They are produced by a broad range of immune cells that they signal, including macrophages, B lymphocytes, and T lymphocytes.
- Humoral immunity
the aspects of immunity mediated by macromolecules rather than cells, found in extracellular fluid. In the innate immune system, this refers to the body's complement system of antimicrobial peptides; in the adaptive immune system, it primarily describes the functions of secreted antibodies.
- Immunological tolerance
the failure of the immune system to mount an immune response against a recognized antigen. There is both ‘self’ tolerance, in which the immune system fails to attack its own proteins, and induced tolerance resulting from previous exposure to an exogenous antigen. Hosts and pathogens with deep coevolutionary relationships may share elements of their genomes, sometimes allowing the host to tolerate the pathogen as self.
- Immunopathology
the detrimental effects that the host immune system inflicts on the host itself as a result of overzealous attempts to clear infection with a given pathogen. Immunopathology typically involves extensive inflammation as immune cells are over-recruited to an infection site.
- Innate immune system
the second of the two main immunity strategies of vertebrates. The innate immune system is the body's first line of nonspecific defense against pathogen attack and comprises both humoral components (the complement cascade) and cell-mediated components (recruitment of nonspecific phagocytes and NK cells to infection sites).
- Lymphocyte
refers to any of three types of white blood cell localized in the lymphatic fluid of the vertebrate immune system: (i) NK cells of the innate immune system; and (ii) T cells and (iii) B cells of the adaptive immune system. They are distinct from other white blood cells (chiefly macrophages) that are localized in the blood.
- Mitophagy
the process by which autophagic processes are targeted to mitochondria.
- Nitric oxide synthase (NOS)
an enzyme that catalyzes the production of NO from the amino acid L-arginine. NO is an important cellular signaling molecule as well as a form of endogenous ROS. NOS is produced by host immune cells recruited during induction of the cytokine IFN-γ.
- Reactive oxygen species (ROS)
chemically reactive molecules containing oxygen that are important in intracellular signaling yet also cause damage to cell structures and both mitochondrial and nuclear DNA. Endogenous forms of ROS are produced by the mitochondria as a byproduct of metabolism and exogenous forms accrue in the cell from contact with pollutants or radiation.
- Reservoir host
a species that serves primarily as a maintenance host for a pathogen. The species typically experiences minimal-to-no morbidity or mortality as a result of infection.
References
- 1.Carini A. Sur une grande épizootie de rage. Ann. Inst. Pasteur (Paris) 1911;25:843–846. (in French) [Google Scholar]
- 2.Field H.E. A fatal case of Hendra virus infection in a horse in north Queensland: clinical and epidemiological features. Aust. Vet. J. 2000;78:279–280. doi: 10.1111/j.1751-0813.2000.tb11758.x. [DOI] [PubMed] [Google Scholar]
- 3.Leroy E.M. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 4.Calisher C.H. Bats: important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006;19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Memish Z.A. Coronavirus in bats, Saudi Arabia. Emerg. Infect. Dis. 2013;19:1819–1823. doi: 10.3201/eid1911.131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 7.Badrane H., Tordo N. Host switching in Lyssavirus history from the Chiroptera to the Carnivora orders. J. Virol. 2001;75:8096–8104. doi: 10.1128/JVI.75.17.8096-8104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gould A.R. Comparison of the deduced matrix and fusion protein sequences of equine morbillivirus with cognate genes of the Paramyxoviridae. Virus Res. 1996;43:17–31. doi: 10.1016/0168-1702(96)01308-1. [DOI] [PubMed] [Google Scholar]
- 9.Drexler J.F. Bats host major mammalian paramyxoviruses. Nat. Commun. 2012;3:796. doi: 10.1038/ncomms1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui J. Evolutionary relationships between bat coronaviruses and their hosts. Emerg. Infect. Dis. 2007;13:1526–1532. doi: 10.3201/eid1310.070448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drexler J.F. Bats carry pathogenic hepadnaviruses antigenically related to hepatitis B virus and capable of infecting human hepatocytes. Proc. Natl. Acad. Sci. U.S.A. 2013;110:16151–16156. doi: 10.1073/pnas.1308049110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quan P. Bats are a major natural reservoir for hepaciviruses and pegiviruses. Proc. Natl. Acad. Sci. U.S.A. 2012;110:8194–8199. doi: 10.1073/pnas.1303037110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tong S. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. U.S.A. 2012;109:4269–4274. doi: 10.1073/pnas.1116200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor D.J. Filoviruses are ancient and integrated into mammalian genomes. BMC Evol. Biol. 2010;10:193. doi: 10.1186/1471-2148-10-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki M. Isolation and characterization of a novel alphaherpesvirus in fruit bats. J. Virol. 2014;88:9819–9829. doi: 10.1128/JVI.01277-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayman D.T.S. Ecology of zoonotic infectious diseases in bats: current knowledge and future directions. Zoonoses Public Health. 2013;60:2–21. doi: 10.1111/zph.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L-F. Mass extinctions, biodiversity and mitochondrial function: are bats “special” as reservoirs for emerging viruses? Curr. Opin. Virol. 2011;1:649–657. doi: 10.1016/j.coviro.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Shea T.J. Bat flight and zoonotic viruses. Emerg. Infect. Dis. 2014;20:741–745. doi: 10.3201/eid2005.130539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turmelle A.S., Olival K.J. Correlates of viral richness in bats (order Chiroptera) Ecohealth. 2009;6:522–539. doi: 10.1007/s10393-009-0263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luis A.D. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc. R. Soc. B. 2013;280 doi: 10.1098/rspb.2012.2753. 20122753, http://dx.doi.org/10.1098/rspb.2012.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobson A.P. What links bats to emerging infectious diseases? Science. 2005;310:628–629. doi: 10.1126/science.1120872. [DOI] [PubMed] [Google Scholar]
- 22.Baker M.L. Antiviral immune responses of bats: a review. Zoonoses Public Health. 2013;60:104–116. doi: 10.1111/j.1863-2378.2012.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cogswell-Hawkinson A. Tacaribe virus causes fatal infection of an ostensible reservoir host, the Jamaican fruit bat. J. Virol. 2012;86:5791–5799. doi: 10.1128/JVI.00201-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonntag M. New adenovirus in bats, Germany. Emerg. Infect. Dis. 2009;15:2052–2055. doi: 10.3201/eid1512.090646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drexler J.F. Amplification of emerging viruses in a bat colony. Emerg. Infect. Dis. 2011;17:449–456. doi: 10.3201/eid1703.100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maruyama J. Characterization of the envelope glycoprotein of a novel filovirus, Lloviu virus. J. Virol. 2014;88:99–109. doi: 10.1128/JVI.02265-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olival K., Hayman D. Filoviruses in bats: current knowledge and future directions. Viruses. 2014;6:1759–1788. doi: 10.3390/v6041759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mühldorfer K. Diseases and causes of death in European bats: dynamics in disease susceptibility and infection rates. PLoS ONE. 2011;6:e29773. doi: 10.1371/journal.pone.0029773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaer J. High diversity of West African bat malaria parasites and a tight link with rodent Plasmodium taxa. Proc. Natl. Acad. Sci. U.S.A. 2013;110:17415–17419. doi: 10.1073/pnas.1311016110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamilton P.B. The evolution of Trypanosoma cruzi: the “bat seeding” hypothesis. Trends Parasitol. 2012;28:136–141. doi: 10.1016/j.pt.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Kosoy M. Bartonella spp. in bats, Kenya. Emerg. Infect. Dis. 2010;16:1875–1881. doi: 10.3201/eid1612.100601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans N.J. Fatal borreliosis in bat caused by relapsing fever spirochete, United Kingdom. Emerg. Infect. Dis. 2009;15:1330–1331. doi: 10.3201/eid1508.090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mühldorfer K. Bats and bacterial pathogens: a review. Zoonoses Public Health. 2013;60:93–103. doi: 10.1111/j.1863-2378.2012.01536.x. [DOI] [PubMed] [Google Scholar]
- 34.Blehert D.S. Acute pasteurellosis in wild big brown bats (Eptesicus fuscus) J. Wildl. Dis. 2014;50:136–139. doi: 10.7589/2012-02-063. [DOI] [PubMed] [Google Scholar]
- 35.Lord J.S. Gastrointestinal helminths of pipistrelle bats (Pipistrellus pipistrellus/Pipistrellus pygmaeus) (Chiroptera: Vespertilionidae) of England. Parasitology. 2012;139:366–374. doi: 10.1017/S0031182011002046. [DOI] [PubMed] [Google Scholar]
- 36.Lichtenfels J.R. Filaroid nematodes in olfactory mucosa, olfactory bulb, and brain ventricular system of bats. Trans. Am. Microsc. Soc. 1981;100:216–219. [Google Scholar]
- 37.Ubelaker J.E. Some observations on ecto- and endoparasites of Chiroptera. In: Slaughter B.H., Walton D.W., editors. About Bats. Southern Methodist University Press; 1970. pp. 247–261. [Google Scholar]
- 38.Coggins J.R. Seasonal changes and overwintering of parasites in the bat, Myotis lucifugus (Le Conte) in a Wisoconsin hibernaculum. Am. Midl. Nat. 1982;107:305–315. [Google Scholar]
- 39.Sebghati T.S. Intracellular parasitism by Histoplasma capsulatum: fungal virulence and calcium dependence. Science. 2000;290:1368–1372. doi: 10.1126/science.290.5495.1368. [DOI] [PubMed] [Google Scholar]
- 40.Greer D.L., McMurray A.N. Pathogenesis of experimental histoplasmosis in the bat, Artibeus lituratus. Am. J. Trop. Med. Hyg. 1981;30:653–659. doi: 10.4269/ajtmh.1981.30.653. [DOI] [PubMed] [Google Scholar]
- 41.Meteyer C.U. Pathology in euthermic bats with white nose syndrome suggests a natural manifestation of immune reconstitution inflammatory syndrome. Virulence. 2012;3:1–6. doi: 10.4161/viru.22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cabral H.R.A. The tumoricidal effect of Trypanosoma cruzi: its intracellular cycle and the immune response of the host. Med. Hypotheses. 2000;54:1–6. doi: 10.1054/mehy.1998.0808. [DOI] [PubMed] [Google Scholar]
- 43.Palm N.W., Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol. Rev. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 44.Cowled C. Molecular characterisation of Toll-like receptors in the black flying fox Pteropus alecto. Dev. Comp. Immunol. 2011;35:7–18. doi: 10.1016/j.dci.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chakraborty A.K., Chakravarty A.K. Dichotomy of lymphocyte population and cell-mediated immune responses in a fruit bat. J. Ind. Inst. Sci. 1983;64:157–168. [Google Scholar]
- 46.Turmelle A.S. Host immunity to repeated rabies virus infection in big brown bats. J. Gen. Virol. 2010;91:2360–2366. doi: 10.1099/vir.0.020073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plowright R.K. Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus) Proc. R. Soc. B. 2008;275:861–869. doi: 10.1098/rspb.2007.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amman B.R. Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection. PLoS Pathog. 2012;8:e1002877. doi: 10.1371/journal.ppat.1002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chawla-Sarkar M. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8:237–249. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- 50.Sulkin S.E., Allen R. S. Karger; 1974. Virus Infections in Bats. [PubMed] [Google Scholar]
- 51.Virtue E.R. Interferon production and signaling pathways are antagonized during Henipavirus infection of fruit bat cell lines. PLoS ONE. 2011;6:e22488. doi: 10.1371/journal.pone.0022488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iha K. Molecular cloning and expression analysis of bat Toll-like receptors 3, 7 and 9. J. Vet. Med. Sci. 2010;72:217–220. doi: 10.1292/jvms.09-0050. [DOI] [PubMed] [Google Scholar]
- 53.Janardhana V. Cloning, expression and antiviral activity of IFNγ from the Australian fruit bat, Pteropus alecto. Dev. Comp. Immunol. 2012;36:610–618. doi: 10.1016/j.dci.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hasenclever H. The use of cultural and histologic methods for the detection of Histoplasma capsulatum in bats: absence of a cellular response. Am. J. Epidemiol. 1969;90:77–83. doi: 10.1093/oxfordjournals.aje.a121052. [DOI] [PubMed] [Google Scholar]
- 55.Graham A.L. Evolutionary causes and consequences of immunopathology. Annu. Rev. Ecol. Evol. Syst. 2005;36:373–397. [Google Scholar]
- 56.Katzourakis A., Gifford R.J. Endogenous viral elements in animal genomes. PLoS Genet. 2010;6:e1001191. doi: 10.1371/journal.pgen.1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Z.W. Attenuated rabies virus activates, while pathogenic rabies virus evades the host innate immune responses in the central nervous system. J. Virol. 2005;79:12554–12565. doi: 10.1128/JVI.79.19.12554-12565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arnoult D. The role of mitochondria in cellular defense against microbial infection. Semin. Immunol. 2009;21:223–232. doi: 10.1016/j.smim.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 59.Hock R.J. The metabolic rates and body temperatures of bats. Biol. Bull. 1951;101:289–299. [Google Scholar]
- 60.Kluger M.J. Role of fever in disease. Ann. N. Y. Acad. Sci. 1998;856:224–233. doi: 10.1111/j.1749-6632.1998.tb08329.x. [DOI] [PubMed] [Google Scholar]
- 61.Warnecke L. Inoculation of bats with European Geomyces destructans supports the novel pathogen hypothesis for the origin of white-nose syndrome. Proc. Natl. Acad. Sci. U.S.A. 2012;109:6999–7003. doi: 10.1073/pnas.1200374109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilkinson G.S., South J.M. Life history, ecology and longevity in bats. Aging Cell. 2002;1:124–131. doi: 10.1046/j.1474-9728.2002.00020.x. [DOI] [PubMed] [Google Scholar]
- 63.Pearl R. University of London Press; 1928. The Rate of Living. [Google Scholar]
- 64.Thomas B.Y.S.P., Suthers R.A. The physiology and energetics of bat flight. J. Exp. Biol. 1972;57:317–335. [Google Scholar]
- 65.O'Shea T.J. Adult survival and population growth rate in Colorado big brown bats (Eptesicus fuscus) J. Mammal. 2011;92:433–443. [Google Scholar]
- 66.Geiser F., Stawski C. Hibernation and torpor in tropical and subtropical bats in relation to energetics, extinctions, and the evolution of endothermy. Integr. Comp. Biol. 2011;51:337–348. doi: 10.1093/icb/icr042. [DOI] [PubMed] [Google Scholar]
- 67.Smalley R.L., Dryer R.L. Brown fat: thermogenic effect during arousal from hibernation in the bat. Science. 1963;140:1333–1334. doi: 10.1126/science.140.3573.1333. [DOI] [PubMed] [Google Scholar]
- 68.Kurta A., Baker R.H. Eptesicus fuscus. Mamm. Spec. 1990;356:1–10. [Google Scholar]
- 69.Sulkin S.E. Studies on the pathogenesis of rabies in insectivorous bats: role of brown adipose tissue. J. Exp. Med. 1959;110:369–388. doi: 10.1084/jem.110.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Austad S.N., Fischer K.E. Mammalian aging, metabolism, and ecology: evidence from the bats and marsupials. J. Gerontol. 1991;46:B47–B53. doi: 10.1093/geronj/46.2.b47. [DOI] [PubMed] [Google Scholar]
- 71.Brunet-Rossinni A.K., Austad S.N. Ageing studies on bats: a review. Biogerontology. 2004;5:211–222. doi: 10.1023/B:BGEN.0000038022.65024.d8. [DOI] [PubMed] [Google Scholar]
- 72.Brunet-Rossinni A.K. Reduced free-radical production and extreme longevity in the little brown bat (Myotis lucifugus) versus two non-flying mammals. Mech. Ageing Dev. 2004;125:11–20. doi: 10.1016/j.mad.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 73.Filho D.W. Antioxidant defenses, longevity and ecophysiology of South American bats. Comp. Biochem. Physiol. 2007;146:214–220. doi: 10.1016/j.cbpc.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 74.Chandel N.S. The promise and perils of antioxidants for cancer patients. N. Engl. J. Med. 2014;371:177–178. doi: 10.1056/NEJMcibr1405701. [DOI] [PubMed] [Google Scholar]
- 75.Koopal S. Viral oncogene-induced DNA damage response is activated in Kaposi sarcoma tumorigenesis. PLoS Pathog. 2007;3:1348–1360. doi: 10.1371/journal.ppat.0030140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marchi S. Mitochondria–ROS crosstalk in the control of cell death and aging. J. Signal. Transduct. 2012;2012:329635. doi: 10.1155/2012/329635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gentz E.J. Splenic lymphoma in a short-beaked echidna (Tachyglossus aculeatus) Aust. Vet. J. 2009;87:273–274. doi: 10.1111/j.1751-0813.2009.00445.x. [DOI] [PubMed] [Google Scholar]
- 78.Perez V.I. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc. Natl. Acad. Sci. U.S.A. 2009;106:3059–3064. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J. Comp. Physiol. B. 2008;178:439–445. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- 80.Seluanov A. Distinct tumor suppressor mechanisms evolve in rodent species that differ in size and lifespan. Aging Cell. 2008;7:813–823. doi: 10.1111/j.1474-9726.2008.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Canfield P.J. Spontaneous proliferations in Australian marsupials: a survey and review. J. Comp. Pathol. 1990;103:147–158. doi: 10.1016/s0021-9975(08)80171-5. [DOI] [PubMed] [Google Scholar]
- 82.Kusewitt D.F. Ultraviolet radiation-induced skin tumors in a South American opossum (Monodelphis domestica) Vet. Pathol. 1991;28:55–65. doi: 10.1177/030098589102800108. [DOI] [PubMed] [Google Scholar]
- 83.Fisher D.O. The ecological basis of life history variation in marsupials. Ecology. 2001;82:3531–3540. [Google Scholar]
- 84.McCallum H., Jones M. To lose both would look like carelessness: Tasmanian devil facial tumour disease. PLoS Biol. 2006;4:e342. doi: 10.1371/journal.pbio.0040342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krysko D.V. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32:157–164. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 86.Bogdan C. Nitric oxide and the immune response. Nat. Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 87.Zhang G. Comparative analysis of bat genomes provides insight into the evolution of flight and immunity. Science. 2013;339:456–460. doi: 10.1126/science.1230835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Halpin K. Pteropid bats are confirmed as the reservoir hosts of henipaviruses: a comprehensive experimental study of virus transmission. Am. J. Trop. Med. Hyg. 2011;85:946–951. doi: 10.4269/ajtmh.2011.10-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koyama S. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J. Immunol. 2007;179:4711–4720. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- 90.West A.P. Mitochondria in innate immune responses. Nat. Rev. Immunol. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wallace D.C. Mitochondrial DNA mutations in disease and aging. Environ. Mol. Mutagen. 2010;51:440–450. doi: 10.1002/em.20586. [DOI] [PubMed] [Google Scholar]
- 92.Kujoth G.C. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 93.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 94.Passos J.F., von Zglinicki T. Mitochondria, telomeres and cell senescence. Exp. Gerontol. 2005;40:466–472. doi: 10.1016/j.exger.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 95.Hayflick L., Moorhead P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 96.Harley C.B. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 97.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 98.Bodnar A.G. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 99.Kiyono T. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:6–10. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- 100.Mathew R. Role of autophagy in cancer. Nature. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]


