Abstract
The capacity to injure infected cells is a widespread property of viruses. Usually, this cytopathic effect (CPE) is ascribed to viral hijacking of cellular resources to fulfill viral needs. However, evidence is accumulating that CPE is not necessarily directly coupled to viral reproduction but may largely be due to host defensive and viral antidefensive activities. A major part in this virus–cell interaction appears to be played by a putative host-encoded program with multiple competing branches, leading to necrotic, apoptotic, and, possibly, other types of cell suicide. Manifestations of this program are controlled and modulated by host, viral, and environmental factors.
Keywords: virus-induced apoptosis, virus-induced necrosis, picornaviruses
The problem
The ability to injure and often to kill infected cells is a common, although not obligatory, property of viruses. It underlies viral disease-causing capacities. Various pathological cell alterations triggered by eukaryotic viruses are referred to as the cytopathic effect (CPE). Notwithstanding their practical significance, CPE mechanisms are understood rather vaguely. Here, some fundamental aspects of the CPE will be overviewed, based primarily on studies on picornaviruses, a family of RNA-containing animal viruses, including, among others, poliovirus, hepatitis А virus (HAV), rhinoviruses, and foot-and-mouth disease virus.
This analysis leads to a hypothesis according to which viral CPE may largely represent manifestations of a defensive host strategy. Viral antidefensive counter-measures also contribute to, and modulate, development and specific features of the pathological alterations. Changes in cellular metabolism directly associated with the needs of viral reproduction may play a relatively minor part in cellular injuries, at least in some virus–host systems. This hypothesis is also supported by studies with some other viruses (e.g., herpesviruses, see below). If correct and general, it may have important implications for understanding of innate immunity mechanisms as well as pathogenesis and treatment of viral diseases.
Picornavirus reproduction and its outcomes
Some major features of the structure and reproduction of picornaviruses are briefly summarized in Box 1 . In many picornavirus–cell combinations, infection terminates in death of the host cell. Two major types of picornavirus-induced fatal CPE are usually discerned, necrosis, and apoptosis (Box 2 ). It is not obvious, however, to what extent cellular injuries reflect direct needs of viral reproduction, on the one hand, or interaction between host defensive and viral antidefensive activities, on the other. Indeed, certain picornaviruses, for example, HAV, are able to grow without inflicting major damage to the cultured host cells. Moreover, even typically cytocidal viruses, such as poliovirus and some other, are able to establish, in certain cells or under certain conditions, persistent infection not accompanied with overt CPE [1], although it should be noted that viral reproduction in persistently infected cells is usually not as efficient as in productively infected ones.
Box 1. Picornaviruses, their genomes and life cycle.
Picornaviruses possess small (ca. 30 nm in diameter) icosahedral nonenveloped virions typically composed of four capsid proteins (VP1–4) and a ∼7.5–8 kb-long single-stranded RNA molecule of positive polarity (Figure I ) [55].
Figure I.
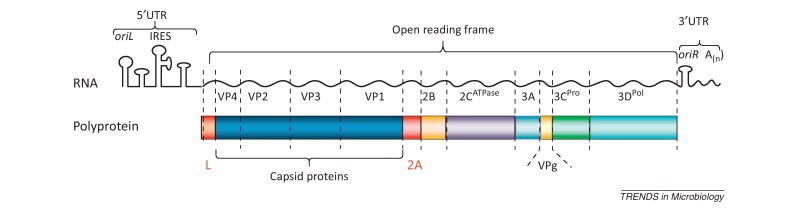
Organization of the picornavirus genome.
The RNA genome usually has a single open reading frame (ORF) as well as 5′- and 3′-terminal untranslated regions (UTRs) harboring replicative (oriL and oriR) and translational (IRES) control elements. After its internalization, the viral RNA is translated in the cytoplasm generating a polyprotein, which is eventually processed by virus-encoded protease(s) into a dozen ‘mature’ proteins, including four capsid proteins (VP1–4), RNA-dependent RNA polymerase 3Dpol, protease 3Cpro (a major player in viral polyprotein processing but it may also cleave some host proteins, including components of the innate immunity system), 2CATPase (believed to function as an RNA helicase but perhaps involved in some other activities too), VPg (a primer for initiation of positive and negative viral RNA strands), and two hydrophobic proteins 2B and 3A that navigate key replicative proteins to their intracellular destinations and help create an optimal environment for viral reproduction. The leader protein (L) is encoded by only certain picornaviruses. L and 2A proteins of picornaviruses may or may not exhibit proteases activities (and in the former case they are referred to as Lpro and 2Apro, respectively). Regardless of this difference, both proteins perform largely antidefensive functions and are called security proteins [2]. RNA of certain picornaviruses contains additional out-of-frame ORFs, giving rise to proteins such as L* and 2B*. After accumulation of a certain level of viral proteins, translation of viral RNA is switched to its exponential replication. The newly synthesized RNA molecules are also translated, and when sufficient amounts of capsid proteins are accumulated, they are assembled with genomic RNA molecules into virions. The viral progeny usually leave the cells owing to their lysis.
Box 2. Major types of cellular death.
Several types of the death of eukaryotic cells are now recognized [34], among which the most common are apoptosis and necrosis. Apoptosis results from implementation of a cell-encoded program aimed at elimination of cells unwanted for various reasons. Two major mechanisms of apoptosis, extrinsic (initiated by the engagement of ‘death’ receptors on the plasma membrane) and intrinsic (caused by intracellular disturbances), are distinguished. Both mechanisms converge in a cascade of serine proteases (caspases) attacking essential cellular targets. The hallmarks of apoptosis are so-called ‘blebbing’ of the plasma membrane not accompanied with enhanced permeability, strong condensation of chromatin within shrinking nuclei, degradation of chromosomal DNA into oligonucleosome-sized fragments with eventual fragmentation of the cells into apoptotic bodies, which are destined to elimination by phagocytosis. Until relatively recently, necrosis was considered to be passive fatal cellular damage due to exhaustion of cellular resources and destruction of intracellular infrastructure caused by various extrinsic and intrinsic factors. Typical features of necrotic cells include swelling, increased permeability of the plasma membrane with its ultimate rupture, nuclear pyknosis. More recently, the existence of host-encoded necrotic programs is well established, which may also be initiated from the plasma membrane receptors or in response to intracellular disturbances, and may have different underlying mechanisms. Both apoptotic and necrotic cell-encoded pathways may be controlled by the same upstream elements and may compete with each other 34, 37. Pathogen-induced necrotic death is usually accompanied with emitting various proinflammatory signals. In addition to apoptosis and necrosis, at least two other cell death mechanisms were reported to be implemented in viral infection. Cells dying of pyroptosis (a program involving activation of proinflammatory caspase-1) exhibit certain features of both apoptosis and necrosis, for example, permeabilization of the plasma membrane and DNA fragmentation. Autophagy, usually a prosurvival cell reaction, which is characterized by the formation of multiple double-membrane vesicles (autophagosomes) delivering their content to lysosomes for degradation, may be exploited by a virus for its reproduction, but in certain cases may result in death of infected cells.
Uncoupling CPE and viral reproduction in lytic viruses
To evaluate the significance of fighting between the virus and its host in the development of CPE, it is illuminating to look at the outcomes of these fighters’ disarmament. Partial virus disarmament may be achieved by inactivating their security proteins, a set of proteins specifically dedicated to antidefensive functions [2]. In picornaviruses, this set comprises the leader (L) and 2A proteins as well as L* protein of certain cardioviruses. Inactivation of security proteins does not kill viruses but usually decreases their reproductive potential. The latter effect is probably due to decreased viral resistance to the cellular defenses, because viruses with impaired security proteins exhibit milder deficiency in hosts with compromised innate immunity (reviewed in [2]).
Experiments with mutual disarmament of mengovirus (MV), a strain of encephalomyocarditis virus (EMCV, a cardiovirus), and its host HeLa cells were particularly informative. Infection with wild type (wt) virus terminated in necrosis, whereas MV mutants with inactivated L induced apoptosis [3]. However, in L– mutant-infected cells, a chemical inhibitor of apoptosis prevented not only apoptosis but also suppressed manifestations of major signs of necrotic CPE or delayed them until well after the completion of the viral reproduction [4]. The yield and time course of the reproduction of L– mutants were unaffected by this inhibitor. Thus, an adequate level of reproduction of a lytic virus can be maintained without immediate killing or even severely damaging its host cell, indicating that cells have enough resources to fulfill the needs of the virus and retain their own viability. Admittedly, the harvest of the L– mutants was somewhat lower compared with that of its wt counterpart. However, the prolonged survival of L–-infected cells with pharmacologically switched-off apoptosis was not due to this lowered reproduction, because an MV with mutated RNA-dependent RNA polymerase generated the same amount of progeny but induced necrotic CPE with a wt-like time course.
Thus, the major injures of MV-infected HeLa cells come from the fight between host defenses and viral counter-defenses rather than from the bold expropriation of cellular property by the virus.
The uncoupling viral reproduction and cellular pathology is not unique to the MV–HeLa system. Some lag between the CPE appearance and the peak of picornaviral reproduction under certain experimental conditions had been previously noted by several investigators 5, 6, 7 but this observation did not attract much attention.
Viral induction and prevention of apoptosis
The ability of picornaviruses to activate cellular apoptotic pathways was first discovered in poliovirus [8] and then described for numerous representatives of this viral family. The virus-triggered apoptosis appears to be an optional innate immunity reaction suppressing reproduction and spread of the pathogen. Ample literature demonstrates that viral infections may change the balance between proapoptotic and antiapoptotic host factors. Alterations in favor of proapoptotic proteins are sufficient to elicit suicidal reaction [9]. Such a switch can be caused by activation of unspecific innate immunity mechanisms through sensors of viral infection as well as by interaction of viral proteins with components of the host apoptotic pathways.
Viruses can induce apoptosis ‘from without’ (through activation of ‘death’ receptors on the plasma membrane) and ‘from within’ (through intervention into intracellular apoptotic machinery). Although several RNA- and DNA-containing viruses trigger apoptosis from without, only a few such examples are reported for picornaviruses 10, 11. Although other examples of the dependence of the apoptosis-triggering capacity of picornaviruses on the properties of their capsid proteins are known [12], it is unclear whether this dependence is linked to the activation of the extrinsic apoptotic pathway or to the viral competence to efficiently infect the cells. In most known cases apoptotic response is caused by replicating picornaviruses, although modulations of the apoptotic program through poliovirus interaction with its receptor was documented [13].
To suppress the defensive apoptotic reaction, viruses have evolved antiapoptotic tools. Indeed, cells infected with poliovirus 8, 14 and coxsackievirus [15] become resistant to nonviral apoptosis inducers. The dominance of antiapoptotic factors can be achieved by either downregulation of cellular proapoptotic activities or upregulation of antiapoptotic ones.
Picornaviruses may not only activate the intrinsic antiapoptotic machinery (thereby maintaining acceptable conditions for growth in the host cell) but also enhance resistance to death receptor-dependent apoptosis. Thus, inhibition of intracytoplasmic protein transport by enteroviruses results in the depletion of receptors for tumor necrotic factor-α (TNF-α), diminishing sensitivity of the infected cell to this inducer of extrinsic apoptosis 15, 16.
Factors contributing to virus-induced necrosis
Another variant of death of virus-infected cells is necrosis. This type of CPE is usually ascribed to the host unspecific exhaustion and damage caused by the lost competition with the virus for resources and infrastructure. Infection with many picornaviruses leads to inhibition of host translation and transcription, increased plasma membrane permeability, ionic disbalance, and damaged intracellular traffic [17]. However, to what extent these alterations are responsible for the (usually rapid) death of the infected cells is questionable.
Important contributions to the inhibition of host translation and transcription are made by picornavirus proteases 2Apro and 3Cpro, which may target appropriate regulatory factors 18, 19. However, even such picornaviruses as cardioviruses, 2A proteins of which are devoid of protease activity may nevertheless suppress host translation [20] and transcription [21] by exploiting capacities of these nonenzymatic proteins to interact with host components 20, 22, 23. Viral proteins may also cause other types of injuries. Thus, infection with both enteroviruses [24] and cardioviruses [25] leads to enhanced permeability of the nuclear envelope but although this effect is due to proteolysis of nucleoporins by the viral 2Apro of enteroviruses 26, 27, 28, cardioviruses elicit phosphorylation of nucleoporins triggered by the L protein 29, 30. Viral hydrophobic nonenzymatic proteins 2B and 3A are important players in the alteration of the cellular membranes [31].
Links between nonenzymatic viral proteins and cellular injuries hint that these injuries may result from modifications of certain cellular pathways.
Necrotic CPE: a host-encoded program modulated by viruses?
We have recently proposed that not only apoptosis but also virus-triggered necrosis may represent manifestations of host-encoded death programs, bona fide members of the innate immunity system [4]. A strong argument for this hypothesis is provided by a striking multifunctionality of the small (67 amino acids) nonenzymatic cardiovirus L protein. If apoptosis of infected HeLa cells was pharmacologically suppressed, diverse signs of necrotic CPE such as permeabilization of the plasma membrane, rearrangements of microtubule and microfilament networks, changes in the cellular and nuclear shapes, condensation of chromatin, and loss of the general metabolic activity all depend on L functionality [4]. This protein also impairs cellular interferon system [32], formation of stress granules [33], and, as mentioned above, nucleocytoplasmic traffic [25]. In addition, it exhibits antiapoptotic activity [3]. Admittedly, one cannot rigorously exclude the possibility that L directly affects such a multitude of targets by itself but it is much more likely that it modulates the activity of a single (or few) cellular control element(s). We hypothesize that this putative element is a part of the innate immunity system involved in deciding the fate of the virus-infected cell.
The existence of cell-encoded necrotic programs, called necroptosis or regulated necrosis, which can be triggered by engagement of ‘death’ receptors or by intrinsic stimuli such as DNA damage, was recently well established [34]. Competitive apoptotic and necrotic pathways controlled by a shared upstream element are exemplified by the Ripoptosome, a complex involving protein kinases RIP1 and RIP3 35, 36, 37. However, HeLa cells appear to be deficient in RIP3 [38] and therefore any RIP3-dependent mechanisms are unlikely directly involved in the implementation of CPE in picornavirus-infected HeLa cells. Rather some unknown, RIP3-independent necrotic pathway should operate in this system.
The proposed hypothesis on the existence of host-encoded virus-triggered necrotic pathway(s) by no means negates major effects of viral functions on this pathway. A great variability of morphological and biochemical manifestations of necrotic CPE caused by different picornaviruses unambiguously indicates viral contributions to these manifestations. The capability of viral proteins, both enzymatic (e.g., proteases) and nonenzymatic, to target cellular functions contributes to this virus specificity.
Competition between death programs of virus-infected cells
Different cellular antiviral defensive death programs compete with each other. For example, at early stages of poliovirus infection, HeLa cells appear to become committed to apoptosis but later on the necrotic program prevails [39] (Figure 1 ). Molecular mechanisms underlying this competition are not well elucidated.
Figure 1.
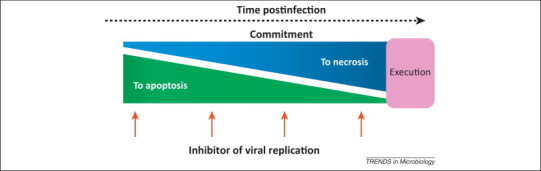
Switch of the commitment to different death programs during poliovirus reproduction in HeLa cells. Early in infection, the cells are committed to apoptosis, whereas in the course of the infectious cycle their commitment changes to necrosis. This switch can be revealed by the addition of inhibitors of viral replication, for example, millimolar concentrations of guanidine hydrochloride, at different times. Addition of the inhibitor at an early step of infection eventually results in the development of apoptosis in the majority of infected cells but inhibition of viral replication at later steps resulted in the prevailing of the necrotic program. Based on data in [39].
In general terms, relationships between apoptotic and necrotic programs may be depicted as follows (Figure 2 a). The incoming virus may activate both apoptotic and necrotic branches of the defensive suicidal program. Activation of these branches may be started from the same or different cellular sensors. It should be admitted that there is no evidence so far that the necrotic CPE starts from some dedicated sensors rather than being initiated by impairment of cellular metabolism. However, nonviral necroptosis is known to be activated from the same sensors as apoptosis 34, 35, 36, 37. Returning to the viral situation, crosstalk between the branches may suppress implementation of one of them by the other. Additional positive or negative stimuli may be sent by newly synthesized viral proteins. And the dominance of one of the branches will depend on the balance of proapoptotic and antiapoptotic factors.
Figure 2.
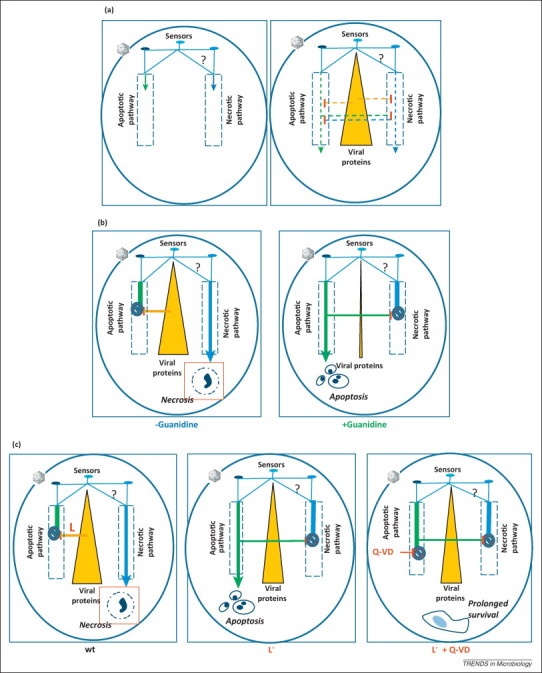
Effects of viral infection on cellular suicidal programs. (a) A general model. Both apoptotic and necrotic branches of the defensive death program may be activated by the incoming virus from the same or different sensors; the sensors leading to the necrotic pathway are yet to be identified (left panel). Crosstalk between these branches may suppress implementation of one of them by the other; additional positive or negative stimuli may be sent by newly synthesized viral proteins (right panel). The dominance of one of the branches will depend on the balance of proapoptotic and antiapoptotic factors. (b) A model for HeLa cells infected with poliovirus. In productively infected cells, implementation of the apoptotic program is suppressed by viral proteins (e.g., 2A), channeling the cells to necrosis (left panel). In the presence of a viral replication inhibitor (e.g., guanidine hydrochloride), the supply of viral antiapoptotic proteins is insufficient to suppress the apoptotic pathway, implementation of which results in the competitive inhibition of the necrotic branch by an unknown mechanism (right panel). (c) A model for HeLa cells infected with mengovirus (MV). In cells infected with wild type (wt) virus, implementation of the apoptotic program is suppressed by the viral leader (L) protein, channeling the cells to necrosis (left panel). In the absence of L upon infection with L– mutants, implementation of the apoptotic program results in the competitive inhibition of necrotic branch by an unknown mechanism (central panel). In cells infected with L– mutants in the presence of a caspase inhibitor (Q-VD), implementation of the apoptotic program is interrupted after a step at which the apoptotic pathway competitively suppresses the necrotic one; as a result, manifestations of major cellular injuries (apoptotic and necrotic) are suppressed or delayed without detrimental effects on viral reproduction (right panel).
More specifically, a model compatible with the observations on poliovirus-infected HeLa cells 39, 40 is presented in Figure 2b. Upon productive infection, both branches are activated but the apoptotic one is suppressed by accumulating viral antiapoptotic protein(s), (e.g., 2A 41, 42). As a result, infected cells die with signs of necrosis. If, however, viral reproduction is inhibited at early steps of infection (e.g., by guanidine hydrochloride), insufficient amounts of viral antiapoptotic protein(s) permit implementation of the apoptotic pathway, one of the consequences of which is competitive suppression of the necrotic branch. Addition of the inhibitor late in infection, when adequate levels of the viral antiapoptotic protein(s) have already been accumulated, results in necrotic CPE.
Suppression of both apoptosis and necrosis in the MV-infected HeLa cells by an antiapoptotic drug [4] suggests the model shown in Figure 2c. Upon wt MV infection, the two branches of the death program are activated but the apoptotic subroutine is suppressed by newly synthesized L protein, thereby channeling the cells to necrosis. Infection with L-deficient mutants permits full implementation of the apoptotic pathway coincidently with competitive inhibition of the necrotic branch. However, if the apoptotic pathway is pharmacologically interrupted after the necrosis-inhibiting step, manifestations of both apoptosis and necrosis are suppressed or delayed.
In line with this reasoning, components of the apoptotic system can suppress manifestations of certain forms of necrosis in uninfected cells and vice versa [37]. Thus, caspase-8 can target CYLD (the cylindromatosis tumor suppressor protein) 43, 44, a deubiquinating enzyme participant of necroptosis, and infection of HeLa cells with L– MV results in some activation of this caspase [4]. However, suppression of necrosis in our system could hardly be due to this activation because it was observed in the presence of a pan-caspase inhibitor.
The generality of this understanding of CPE
The above arguments for the fight between cellular defenses and viral antidefenses as the major cause of CPE and for necrotic CPE as being largely controlled by a host-encoded program came from studies on picornaviruses, relatively small RNA viruses. However, recent evidence demonstrates that large DNA viruses (e.g., herpesviruses) also provide strong support for this concept: they may activate innate immunity reactions leading to either apoptotic or necrotic cell suicide and partial mutual virus–cell disarmament may alleviate pathological symptoms caused by these viruses [44].
The latter observation is of special importance because it demonstrates that the relevant regularities deduced from virus-infected cultured cells may hold true at the level of organism as well. Nevertheless, it should be kept in mind that in vitro experiments, although indispensable for uncovering all the diversity of cell-encoded mechanisms, do not necessarily mirror the variety of in vivo situations, which should be pinpointed in special studies.
Unstable balance between the death programs
The models presented in Figure 2b and c are based on still poorly understood mechanisms of picornaviral CPE. Nor do they take into account dependence of the cellular response on experimental conditions. For example, fetal bovine serum abrogates apoptosis in HeLa cells infected with poliovirus in the presence of guanidine hydrochloride [45], suggesting that variability of host responses to viral infection may partly be due to differences in experimental settings.
The response to a given picornavirus is also host cell-specific. Theiler's murine encephalomyelitis virus, a cardiovirus, elicits necrosis in permissive cells but triggers apoptosis in restrictive ones [46]. Although productive poliovirus infection of HeLa leads, as already said, to necrosis [8], infection of partially permissive promonocytic cells brings about apoptosis [47]. The difference in response to a virus is not necessarily related to the level of host permissiveness: poliovirus [48] and EMCV [49] may elicit apoptosis also in permissive cells. Another type of host-dependence is demonstrated in human rhabdomyosarcoma (RD) cells: inhibition of poliovirus reproduction in these permissive cells does not result in the necrosis-to-apoptosis switch as it does in HeLa cells [50]. Furthermore, the response to picornavirus of a given population is very rarely, if ever, homogeneous. Usually, it is possible only to identify the predominant reaction, and both apoptotic and necrotic cells are present in noticeable proportions in some cases 50, 51.
Biological relevance of CPE
Destruction of the infected cell may be useful for the virus, in particular, to help externalization of the progeny. However, the virus should take care not to harm its host prematurely and to ‘consider’ another potentially rewarding option, to keep the infected cell alive, and exploit this ‘platform’ for long-term reproduction.
The innate immune system should also select between two possible choices: to keep the infected cell alive, even if this permits efficient viral reproduction, or to push cell sacrifice to limit viral growth and spread. Furthermore, different modes of sacrifice are available: to die peacefully, occluding as many virus particles as possible (apoptosis) or to permit the viral progeny to go out together with various cell components, emitting loud alarm signals (necrosis).
Each of these options is biologically relevant. The choice depends on a multitude of factors, such as the genetic background of the interacting partners, the multiplicity of infection, physiological state of the host, and environmental conditions.
The evolutionary pressure, however, appears to force the fighting parties (viruses and hosts) to negotiate for a peaceful coexistence. ‘Old’ viruses are likely to exhibit low or even no pathogenicity. The so-called ‘newly emerging’ viruses usually are very harmful. Remarkably, predecessors of such dangerous human pathogens as the human immunodeficiency virus (HIV) or severe acute respiratory syndrome (SARS) virus are relatively harmless in their earlier hosts, non-human primates and bats, respectively 52, 53. The severe human diseases inflicted by these viruses do not result from the acquisition by them of new pathogenic factors. Rather they appear to be due to miscalculated innate immunity mechanisms. A classical example of the subsequent trend of ‘newly emerged’ viruses toward peaceful coexistence with their hosts is the relationships between the fibroma/myxoma virus introduced to Australia and local rabbits [54].
Concluding remarks
Growing evidence from studies on both small RNA viruses and large DNA viruses strongly suggests that various types of CPE may represent manifestations of the virus-modulated host-encoded innate immunity program(s). In other words, virus-induced injuries, both necrotic and apoptotic, may largely reflect fighting between cellular defenses and viral counter-defenses, the efficiency of viral reproduction being not necessarily directly coupled to the level of cellular injury.
Further work is required for the validation of this viewpoint, assessment of its generality, elucidation of the underlying mechanisms, and utilization of its consequences for the treatment of viral diseases (Box 3 ).
Box 3. Outstanding questions.
-
•
How general is the ability of viruses to activate host-encoded necrotic (and other nonapoptotic) death pathway(s)?
-
•
What are upstream (induction) and downstream (execution) mechanism(s) of virus-triggered necrotic cell death?
-
•
What are mechanisms underlying crosstalk between different virus-induced cell suicide pathways?
-
•
What and how are cell death mechanisms operating in virus-infected organisms and what is their biological relevance?
-
•
How can intervention with host cell suicidal reactions be exploited for amelioration of virus-induced pathology?
Acknowledgment
Current work of our group is supported by a grant from the Russian Foundation for Basic Research. I apologize for not citing many relevant papers owing to journal limits.
References
- 1.Colbère-Garapin F., Lipton H.L. Persistent infection. In: Ehrenfeld E., editor. The Picornaviruses. ASM Press; 2010. pp. 321–335. [Google Scholar]
- 2.Agol V.I., Gmyl A.P. Viral security proteins: counteracting host defences. Nat. Rev. Microbiol. 2010;8:867–878. doi: 10.1038/nrmicro2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romanova L.I. Antiapoptotic activity of the cardiovirus leader protein, a viral “security” protein. J. Virol. 2009;83:7273–7284. doi: 10.1128/JVI.00467-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mikitas O.V. Suppression of injuries caused by a lytic RNA virus (mengovirus) and their uncoupling from viral reproduction by mutual cell/virus disarmament. J. Virol. 2012;86:5574–5583. doi: 10.1128/JVI.07214-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gosselin A.S. Poliovirus-induced apoptosis is reduced in cells expressing a mutant CD155 selected during persistent poliovirus infection in neuroblastoma cells. J. Virol. 2003;77:790–798. doi: 10.1128/JVI.77.1.790-798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deszcz L. Apoptotic events induced by human rhinovirus infection. J. Gen. Virol. 2005;86:1379–1389. doi: 10.1099/vir.0.80754-0. [DOI] [PubMed] [Google Scholar]
- 7.Wahid R. Dendritic cells and macrophages are productively infected by poliovirus. J. Virol. 2005;79:401–409. doi: 10.1128/JVI.79.1.401-409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tolskaya E.A. Apoptosis-inducing and apoptosis-preventing functions of poliovirus. J. Virol. 1995;69:1181–1189. doi: 10.1128/jvi.69.2.1181-1189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willis S.N. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 10.Peng J.M. VP1 of foot-and-mouth disease virus induces apoptosis via the Akt signaling pathway. J. Biol. Chem. 2004;279:52168–52174. doi: 10.1074/jbc.M403686200. [DOI] [PubMed] [Google Scholar]
- 11.Jin H. Induction of immature dendritic cell apoptosis by foot and mouth disease virus is an integrin receptor mediated event before viral infection. J. Cell. Biochem. 2007;102:980–991. doi: 10.1002/jcb.21332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gullberg M. A single coxsackievirus B2 capsid residue controls cytolysis and apoptosis in rhabdomyosarcoma cells. J. Virol. 2010;84:5868–5879. doi: 10.1128/JVI.02383-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blondel B. Apoptotic signaling cascades operating in poliovirus-infected cells. Front. Biosci. 2009;14:2181–2192. doi: 10.2741/3370. [DOI] [PubMed] [Google Scholar]
- 14.Koyama A.H. Suppression of apoptotic and necrotic cell death by poliovirus. J. Gen. Virol. 2001;82:2965–2972. doi: 10.1099/0022-1317-82-12-2965. [DOI] [PubMed] [Google Scholar]
- 15.Salako M.A. Coxsackievirus protein 2BC blocks host cell apoptosis by inhibiting caspase-3. J. Biol. Chem. 2006;281:16296–16304. doi: 10.1074/jbc.M510662200. [DOI] [PubMed] [Google Scholar]
- 16.Neznanov N. Poliovirus protein 3A inhibits tumor necrosis factor (TNF)-induced apoptosis by eliminating the TNF receptor from the cell surface. J. Virol. 2001;75:10409–10420. doi: 10.1128/JVI.75.21.10409-10420.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dougherty J.D. Interference with cellular gene expression. In: Ehrenfeld E., editor. The Picornaviruses. ASM Press; 2010. pp. 165–180. [Google Scholar]
- 18.Kuechler E. Picornavirus protease-mediated shutoff of host translation: direct cleavage of a cellular initiation factor. In: Semler B.L., Wimmer E., editors. Molecular Biology of Picornaviruses. ASM Press; 2002. pp. 301–311. [Google Scholar]
- 19.Dasgupta A. Effects of picornavirus proteinases on host cell transcription. In: Semler B.L., Wimmer E., editors. Molecular Biology of Picornaviruses. ASM Press; 2002. pp. 321–335. [Google Scholar]
- 20.Svitkin Y.V. Rapamycin and wortmannin enhance replication of a defective encephalomyocarditis virus. J. Virol. 1998;72:5811–5819. doi: 10.1128/jvi.72.7.5811-5819.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krupina K.A. Variability in inhibition of host RNA synthesis by entero- and cardioviruses. J. Gen. Virol. 2010;91:1239–1244. doi: 10.1099/vir.0.017723-0. [DOI] [PubMed] [Google Scholar]
- 22.Aminev A.G. Encephalomyocarditis virus (EMCV) proteins 2A and 3BCD localize to nuclei and inhibit cellular mRNA transcription but not rRNA transcription. Virus Res. 2003;95:59–73. doi: 10.1016/s0168-1702(03)00163-1. [DOI] [PubMed] [Google Scholar]
- 23.Groppo R. Mutational analysis of the EMCV 2A protein identifies a nuclear localization signal and an eIF4E binding site. Virology. 2011;410:257–267. doi: 10.1016/j.virol.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belov G.A. Early alteration of nucleo-cytoplasmic traffic induced by some RNA viruses. Virology. 2000;275:244–248. doi: 10.1006/viro.2000.0427. [DOI] [PubMed] [Google Scholar]
- 25.Lidsky P.V. Nucleo-cytoplasmic traffic disorder induced by cardioviruses. J. Virol. 2006;80:2705–2717. doi: 10.1128/JVI.80.6.2705-2717.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belov G.A. Bidirectional increase in permeability of nuclear envelope upon poliovirus infection and accompanying alterations of nuclear pores. J. Virol. 2004;78:10166–10177. doi: 10.1128/JVI.78.18.10166-10177.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castelló A. RNA nuclear export is blocked by poliovirus 2A protease and is concomitant with nucleoporin cleavage. J. Cell Sci. 2009;122:3799–3809. doi: 10.1242/jcs.055988. [DOI] [PubMed] [Google Scholar]
- 28.Park N. Specific cleavage of the nuclear pore complex protein Nup62 by a viral protease. J. Biol. Chem. 2010;285:28796–28805. doi: 10.1074/jbc.M110.143404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bardina M.V. Mengovirus-induced rearrangement of the nuclear pore complex: hijacking cellular phosphorylation machinery. J. Virol. 2009;83:3150–3161. doi: 10.1128/JVI.01456-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porter F.W., Palmenberg A.C. Leader-induced phosphorylation of nucleoporins correlates with nuclear trafficking inhibition by cardioviruses. J. Virol. 2009;83:1941–1951. doi: 10.1128/JVI.01752-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Kuppeveld F. Remodeling cellular membranes. In: Ehrenfeld E., editor. The Picornaviruses. ASM Press; 2010. pp. 181–193. [Google Scholar]
- 32.Hato S.V. The mengovirus leader protein blocks interferon-α/β gene transcription and inhibits activation of interferon regulatory factor 3. Cell. Microbiol. 2007;9:2921–2930. doi: 10.1111/j.1462-5822.2007.01006.x. [DOI] [PubMed] [Google Scholar]
- 33.Borghese F., Michiels T. The leader protein of cardioviruses inhibits stress granule assembly. J. Virol. 2011;85:9614–9622. doi: 10.1128/JVI.00480-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galluzzi L. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tenev T. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol. Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Feoktistova M. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han J. Programmed necrosis: backup to and competitor with apoptosis in the immune system. Nat. Immunol. 2011;12:1143–1149. doi: 10.1038/ni.2159. [DOI] [PubMed] [Google Scholar]
- 38.He S. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Agol V.I. Competing death programs in poliovirus-infected cells: commitment switch in the mid of infectious cycle. J. Virol. 2000;74:5534–5541. doi: 10.1128/jvi.74.12.5534-5541.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belov G.A. The major apoptotic pathway activated and suppressed by poliovirus. J. Virol. 2003;77:45–56. doi: 10.1128/JVI.77.1.45-56.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burgon T.B. Bypass suppression of small-plaque phenotypes by a mutation in poliovirus 2A that enhances apoptosis. J. Virol. 2009;83:10129–10139. doi: 10.1128/JVI.00642-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Igarashi H. 2A protease is not a prerequisite for poliovirus replication. J. Virol. 2010;84:5947–5957. doi: 10.1128/JVI.02575-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Donnell M.A. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat. Cell Biol. 2011;13:1437–1442. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mocarski E.S. Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways. Nat. Rev. Immunol. 2012;12:79–88. doi: 10.1038/nri3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tolskaya E.A. A final сheckpoint in the drug-promoted and poliovirus-promoted apoptosis is under post-translational control by growth factors. J. Cell. Biochem. 1996;63:422–431. doi: 10.1002/(SICI)1097-4644(19961215)63:4%3C422::AID-JCB4%3E3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 46.Jelachich M.L., Lipton H.L. Theiler's murine encephalomyelitis virus kills restrictive but not permissive cells by apoptosis. J. Virol. 1996;70:6856–6861. doi: 10.1128/jvi.70.10.6856-6861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopez-Guerrero J.A. Poliovirus induces apoptosis in the human U937 promonocytic cell line. Virology. 2000;272:250–256. doi: 10.1006/viro.2000.0405. [DOI] [PubMed] [Google Scholar]
- 48.Ammendolia M.G. Poliovirus infection induces apoptosis in CaCo-2 cells. J. Med. Virol. 1999;59:122–129. [PubMed] [Google Scholar]
- 49.Schwarz E.M. NF-κB-mediated inhibition of apoptosis is required for encephalomyocarditis virus virulence: a mechanism of resistance in p50 knockout mice. J. Virol. 1998;72:5654–5660. doi: 10.1128/jvi.72.7.5654-5660.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romanova L.I. Variability in apoptotic response to poliovirus infection. Virology. 2005;331:292–306. doi: 10.1016/j.virol.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 51.Arslan S.Y. The anti-apoptotic Mcl-1 protein controls the type of cell death in Theiler's virus-infected BHK-21 cells. J. Virol. 2012;86:1922–1929. doi: 10.1128/JVI.06516-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hemelaar J. The origin and diversity of the HIV-1 pandemic. Trends Mol. Med. 2012;18:182–192. doi: 10.1016/j.molmed.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Li W. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 54.Kerr P.J. Myxomatosis in Australia and Europe: a model for emerging infectious diseases. Antiviral Res. 2012;93:387–415. doi: 10.1016/j.antiviral.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 55.Palmenberg A. Genome organization and encoded proteins. In: Ehrenfeld E., editor. The Picornaviruses. ASM Press; 2010. pp. 3–17. [Google Scholar]


