Abstract
Background
Data on symptoms and radiographic changes in patients with pandemic 2009 influenza A(H1N1) (A[H1N1]) pneumonia during convalescence have not been reported.
Methods
During October 26, 2009, and January 23, 2010, adult patients with pneumonia with laboratory-confirmed or clinically suspected A(H1N1) infections were observed for clinical characteristics, high-resolution chest CT scan, and lung function test changes during acute and 3-month convalescent phases.
Results
Of the 65 case subjects, the median age was 41 (interquartile range [IQR], 28-57) years, 60.0% were men, and 55.4% had at least one underlying medical condition. Sixty-two patients started oseltamivir therapy within a median of 5 (IQR, 4-6) days from the onset of illness, and 31 received IV corticosteroids. ARDS developed in 33 patients, and 24 were treated initially with noninvasive positive pressure ventilation (NPPV). In this group, NPPV was successful in 13 patients (54.2%). Nine patients died at a median of 16 (IQR, 10-24) days after onset of illness. Multivariate Cox regression identified two independent risk factors for death: progressive dyspnea after resolution of fever (relative risk, 5.852; 95% CI, 1.395-24.541; P = .016) and a higher APACHE (Acute Physiology and Chronic Health Evaluation) II score on presentation (relative risk for each point, 1.312; 95% CI, 1.140-1.511; P < .001). At 3-month follow-up of survivors with A(H1N1), ground-glass opacities were still present, although diminished, in 85.7%, and diffusing capacity for carbon monoxide was mildly reduced in 61.5%.
Conclusions
Ground-glass opacities and decreased diffusing capacity were the main abnormalities observed at 3-month follow-up of survivors of A(H1N1).
Abbreviations
- A(H1N1)
pandemic 2009 influenza A(H1N1)
- APACHE
Acute Physiology and Chronic Health Evaluation
- Dlco
diffusing capacity for carbon monoxide
- GGOs
ground-glass opacities
- HRCT
high-resolution CT
- IQR
interquartile range
- LFT
lung function test
- NPPV
noninvasive positive pressure ventilation
- RT-PCR
reverse transcriptase polymerase chain reaction
- SARS
severe acute respiratory syndrome
Outbreaks of novel 2009 influenza A(H1N1) (A[H1N1]) virus infection occurred in April 2009 in the United States and Mexico. The clinical spectrum of this disease has ranged from self-limited illness to respiratory failure and death.1 In our initial report of the A(H1N1) virus infection in China, the majority of patients had mild illness.2, 3 Since the first report of pneumonia caused by the A(H1N1) virus in Mexico,4 severe cases have been documented throughout the world. As of March 7, 2010, ≥ 16,713 laboratory-confirmed cases of death have been reported by the six world regions.5 In mainland China, there were > 127,000 confirmed cases up to February 28, 2010, including 793 deaths.6
Many studies have been published on the clinical manifestations of A(H1N1) pneumonia during the acute phase of illness,7, 8, 9, 10, 11, 12, 13, 14, 15 but no information has been reported on symptoms and radiographic and lung function changes in convalescence. We studied clinical manifestations during the acute phase, antiviral and corticosteroid therapy, noninvasive positive pressure ventilation (NPPV), and the histopathologic changes of a fatal case. Survivors were followed up after discharge for a period of 3 months. We believe our work can help optimize treatment, and also lead to a better understanding of the symptomatic, radiologic, and lung functional changes during the convalescent period.
Materials and Methods
Study Patients and Case Definition
Data were collected retrospectively and prospectively on all patients with confirmed A(H1N1)-related pneumonia treated at Beijing Chao-Yang Hospital between October 26, 2009, and January 23, 2010. The diagnosis of pneumonia was based on respiratory symptoms combined with a new infiltrate on chest radiograph. Real-time reverse transcriptase polymerase chain reaction (RT-PCR) assay was used to confirm the diagnosis of A(H1N1) infection. Patients presenting with pneumonia with high clinical suspicion of A(H1N1) infection but negative RT-PCR test results for A(H1N1) were also included in this study. Children younger than 14 years of age were excluded. Most patients were hospitalized for treatment, whereas those who presented with less serious illness and did not need oxygen supplementation were treated as outpatients under home quarantine.
Treatment decisions for all patients were made by their attending physicians. Hospitalized patients were discharged when their temperatures had returned to normal for at least 3 days, most influenza-like symptoms had disappeared, and they were clinically stable.
Data Collection During Hospitalization and Follow-up
Information recorded included demographic data, underlying medical conditions, symptoms, signs, laboratory and chest radiograph findings before therapy and during follow-up, and the clinical course, treatment, and adverse events during hospital stay. APACHE (Acute Physiology and Chronic Health Evaluation) II scores were determined in all patients to assess the severity of illness. During hospitalization, clinical data were collected retrospectively from medical records.
Patient follow-up and further investigation at follow-up were carried out according to clinical need, so written informed consent was not sought. High-resolution CT (HRCT) scanning was ordered only in those with persisting symptoms, chest signs, or radiologic findings on discharge or on last visit. No contrast was given with CT scan, and the possible radiation harm was also explained to patients. Lung function tests (LFTs) were ordered in those patients still attending at 3 months. The ethics board committee at Beijing Chao-Yang Hospital approved the study design.
Radiologic Evaluation
The severity of lung changes was evaluated initially and on follow-up examinations. Each lung was divided into three zones.16 The number of abnormal zones and the changes in ground-glass opacities (GGOs), consolidation, reticular-nodules, and interlobular septal thickening were evaluated by HRCT scanning. All evaluations were performed by two radiologists who were blinded to the clinical information.
Lung Function Testing
LFTs, including lung volume, spirometry, and diffusing capacity for carbon monoxide (Dlco) were performed 3 months after the onset of symptoms. All LFTs were performed in accordance with recommended standards. Dlco was measured with a single-breath technique, adjusted for hemoglobin and alveolar volume. LFT measurements were considered abnormal if they were < 80% of the predicted value.
Statistical Analysis
Continuous variables were summarized as means (± SD) or medians (interquartile range [IQR]). Differences between groups were assessed using the χ2 test or Fisher exact test for categorical variables and the Mann-Whitney U test for continuous variables. We used univariate and multivariate Cox regression to identify independent predictors of mortality. All analyses were performed by SPSS software, version 13.0 (SPSS Inc; Chicago, Illinois). A P value ≤ .05 was considered statistically significant.
Results
From October 26, 2009, to January 23, 2010, a total of 2,415 cases of influenza-like illness were reported in our hospital, of which 516 were laboratory-confirmed A(H1N1) cases. During the epidemic, a total of 65 patients were eligible for this study, including 62 patients with laboratory-confirmed A(H1N1) and three patients with high clinical suspicion for A(H1N1) infection. Among the 65 patients, 50 were hospitalized, and 15 were treated as outpatients.
Clinical Characteristics
Median age was 41 years, 60.0% were men, and 55.4% had at least one underlying medical condition (Table 1 ). Dyspnea persisted in 13.8% of patients after resolution of fever. Smokers were more common in the ARDS group (P = .067), and moist rales and wheezing were significantly more frequent in this group. Although leukocyte counts were similar in the two groups, lymphocyte counts were significantly lower and serum potassium levels significantly higher in the ARDS group. Patients with ARDS also required more frequent use of higher doses of oseltamivir, longer duration of oseltamivir treatment, and more frequent use of corticosteroids and vasopressors, and more frequently had positive bacterial and fungal cultures. The most common initial radiologic findings on HRCT scan were bilateral GGOs involving several zones with or without associated multifocal areas of consolidation. Centrilobular nodules were also common, and small pleural effusions were present in 25.7% of patients (Fig 1 ). In patients without ARDS, those who were hospitalized more frequently had diarrhea (P = .003), moist rales (P = .001), a lower serum albumin level (P = .011), and more involved lung zones on chest radiograph (P = .002) than did those who were outpatients.
Table 1.
Characteristics, Symptoms and Signs, Laboratory and Radiographic Findings on Admission, Clinical Course, and Outcome of Patients Who Developed ARDS Compared With Those Who Did Not
| Patients |
||||
|---|---|---|---|---|
| Variable | Total (N = 65)a | Without ARDS (n = 32)a | With ARDS (n = 33)a | P Value |
| Characteristic | ||||
| Male sex | 39 (60.0) | 19 (59.4) | 20 (60.6) | .919 |
| Age, y | 41 (28–57) | 36 (28–53) | 46 (34–60) | .069 |
| Range | 14–75 | 18–67 | 14–75 | … |
| BMI ≥ 30 kg/m2 | 15 (23.1) | 6 (18.8) | 9 (27.3) | .415 |
| Underlying medical condition | 36 (55.4) | 13 (40.6) | 23 (69.7) | .018 |
| Asthma | 3 (4.6) | 2 (6.3) | 1 (3.0) | .613 |
| COPD | 2 (3.1) | 1 (3.1) | 1 (3.0) | 1.000 |
| Chronic bronchitis | 3 (4.6) | 1 (3.1) | 2 (6.1) | 1.000 |
| Bronchiectasis | 2 (3.1) | 1 (3.1) | 1 (3.0) | 1.000 |
| Obstructive sleep apnea syndrome | 4 (6.2) | 1 (3.1) | 3 (9.1) | .613 |
| Hypertension | 17 (26.2) | 5 (15.6) | 12 (36.4) | .090 |
| Coronary heart disease | 3 (4.6) | 1 (3.1) | 2 (6.1) | 1.000 |
| Chronic heart failure | 3 (4.6) | 1 (3.1) | 2 (6.1) | 1.000 |
| Cerebrovascular disease | 2 (3.1) | 0 | 2 (6.1) | .492 |
| Diabetes mellitus | 10 (15.4) | 3 (9.4) | 7 (21.2) | .303 |
| Chronic renal disease | 3 (4.6) | 0 | 3 (9.1) | .238 |
| Cirrhosis | 1 (1.5) | 1 (3.1) | 0 | .492 |
| Mental disorderb | 2 (3.1) | 1 (3.1) | 1 (3.0) | 1.000 |
| Immune suppressionc | 4 (6.2) | 0 | 4 (12.1) | .114 |
| Pregnancy | 2 (3.1) | 0 | 2 (6.1) | .492 |
| Postpartum | 1 (1.5) | 0 | 1 (3.0) | 1.000 |
| Current smoker | 19 (29.2) | 6 (18.8) | 13 (39.4) | .067 |
| Symptoms and signs (on presentation) | ||||
| Feverd | 65 (100) | 32 (100) | 33 (100) | … |
| Body temperature, °C | 39.5 (39.1–39.7) | 39.5 (39.2–39.7) | 39.5 (38.9–39.8) | .664 |
| Cough and sputum production | 56 (86.2) | 27 (84.4) | 29 (87.9) | .733 |
| Blood in sputum | 30 (46.2) | 12 (37.5) | 18 (54.5) | .168 |
| Dyspnea | 57 (87.7) | 24 (75.0) | 33 (100) | .002 |
| Progressive dyspnea after resolution of fever | 9 (13.8) | 1 (3.1) | 8 (24.2) | .027 |
| Sore throat or rhinorrhea | 36 (55.4) | 19 (59.4) | 17 (51.5) | .524 |
| Myalgia | 37 (56.9) | 22 (68.8) | 15 (45.5) | .058 |
| Fatigue | 60 (92.3) | 30 (93.8) | 30 (90.9) | 1.000 |
| Diarrhea | 18 (27.7) | 8 (25.0) | 10 (30.3) | .633 |
| Moist rales | 52 (80.0) | 21 (65.6) | 31 (93.9) | .005 |
| Wheezing | 19 (29.2) | 4 (12.5) | 15 (45.5) | .006 |
| Laboratory findings (on presentation) | ||||
| Leukocyte count, mm3 | 5,000 (3,400–7,600) | 5,700 (3,500–8,600) | 5,000 (3,300–7,200) | .189 |
| < 4000/mm3 | 21 (32.3) | 10 (31.3) | 11 (33.3) | .857 |
| Lymphocyte count, mm3 | 750 (500–1,150) | 920 (700–1,300) | 560 (360–840) | < .001 |
| < 1000/mm3 | 47 (72.3) | 18 (56.3) | 29 (87.9) | .006 |
| Pao2/Fio2e | 295 (242–374) | 375 (321–412) | 244 (211–290) | < .001 |
| Serum albumin,f g/L | 31.9 (27.0–35.2) | 35.2 (31.5–38.2) | 29.7 (24.0–33.0) | < .001 |
| Creatine kinase,f U/L | 180 (74–544) | 155 (75–644) | 221 (73–520) | .857 |
| Lactate dehydrogenase,f U/L | 345 (272–501) | 290 (201–411) | 404 (314–548) | .004 |
| Alanine aminotransferase,f U/L | 30 (22–44) | 30 (27–53) | 29 (20–42) | .164 |
| Aspartate aminotransferase,f U/L | 55 (34–99) | 45 (27–86) | 57 (40–106) | .245 |
| Potassium,f mmol/L | 3.8 (3.5–4.0) | 3.5 (3.2–4.0) | 3.8 (3.6–4.2) | .033 |
| < 3.5 mmol/L | 14 (21.5) | 10 (31.3) | 4 (12.1) | .028 |
| Sodium,f mmol/L | 133.5 (130.9–136.3) | 133.6 (131.5–135.6) | 133.4 (129.1–136.5) | .572 |
| Procalcitonin,f ng/mL | 0.29 (0.05–0.99) | 0.09 (0.05–0.73) | 0.46 (0.15–1.01) | .170 |
| APACHE II score | 8 (4–11) | 4 (2–6) | 11 (9–13) | < .001 |
| Initial radiographic findings | ||||
| Chest radiographg | ||||
| No. of involved zones ≥ 3 | 38/57 (66.7) | 13/29 (44.8) | 25/28 (89.3) | .001 |
| Bilateral infiltrate | 41/57 (71.9) | 14/29 (48.3) | 27/28 (96.4) | < .001 |
| High-resolution chest CT scanh | ||||
| No. involved zones | 6 (4–6) | 4 (4–5) | 6 (5–6) | < .001 |
| Ground-glass opacities | 31/35 (88.6) | 11/14 (78.6) | 20/21 (95.2) | .279 |
| Consolidation | 27/35 (77.1) | 8/14 (57.1) | 19/21 (90.5) | .039 |
| Centrilobular nodules | 15/35 (42.9) | 5/14 (35.7) | 10/21 (47.6) | .728 |
| Pleural effusion | 9/35 (25.7) | 3/14 (21.4) | 6/21 (28.6) | .712 |
| Clinical course | ||||
| Days from onset of symptoms to ED | 5 (4–6) | 5 (4–6) | 5 (4–7) | .383 |
| Duration of fever, d | 6.0 (4.0–7.0) | 6.0 (4.5–6.8) | 6.0 (4.0–7.3) | .665 |
| Antiviral therapy (oseltamivir) | 62 (95.4) | 29 (90.6) | 33 (100) | .114 |
| 150 mg bid po | 23 (37.1) | 6 (20.7) | 17 (51.5) | .012 |
| Duration of antiviral, d | 5 (5–7) | 5 (5–5) | 6 (5–8) | < .001 |
| Duration of antiviral > 5 d | 21 (33.9) | 0 | 21 (63.6) | < .001 |
| Interval from onset to antiviral ≤ 48 h | 6 (9.7) | 4 (13.8) | 2 (6.1) | .405 |
| Use of antibiotics | 49 (75.4) | 20 (62.5) | 29 (87.9) | .023 |
| Use of corticosteroids | 31 (47.7) | 5 (15.6) | 26 (78.8) | < .001 |
| Mechanical ventilation | 25 (38.5) | 1 (3.1) | 24 (72.7) | < .001 |
| Invasive | 10 (15.4) | 0 | 10 (30.3) | .001 |
| Noninvasive | 15 (23.1) | 1 (3.1) | 14 (42.4) | < .001 |
| Extracorporeal membrane oxygenation | 2 (3.1) | 0 | 2 (6.1) | .492 |
| Renal replacement therapy | 3 (4.6) | 0 | 3 (9.1) | .238 |
| Acute liver function failure | 1 (1.5) | 0 | 1 (3.0) | 1.000 |
| Hypotension needed vasopressor | 7 (10.8) | 0 | 7 (21.2) | .011 |
| Positive culture on presentation or during hospitalization | 13 (20.0) | 1 (3.1) | 12 (36.4) | .001 |
| Bacterial | 7 (10.8) | 1 (3.1) | 6 (18.2) | .105 |
| Fungal | 2 (3.1) | 0 | 2 (6.1) | .492 |
| Bacterial and fungal | 4 (6.2) | 0 | 4 (12.1) | .114 |
| Length of stay in hospital for survivors (n = 41), d | 7.0 (5.3–11.0) | 5.0 (4.0–6.5) | 11.0 (7.0–13.0) | < .001 |
| Death | 9 (13.8) | 0 | 9 (27.3) | .002 |
| Days from onset of symptoms to death | 16 (10–24) | … | 16 (10–24) | … |
| Days from admission to death | 9 (4–16) | … | 9 (4–16) | … |
Data are presented as No. (%) or median (interquartile range). APACHE = Acute Physiology and Chronic Health Evaluation.
Unless otherwise indicated.
One patient had schizophrenia, and the other had alcohol withdrawal syndrome.
Two patients were taking oral corticosteroids equal to prednisolone 15 mg per day for > 2 mo; one patient was receiving immunosuppressant after kidney transplant; one patient had aplastic anemia.
The highest temperature before presentation.
Total (N = 59); without ARDS group (n = 26), with ARDS group (n = 33).
Total (N = 58); without ARDS group (n = 25); with ARDS group (n = 33).
Chest radiograph was performed within 1 wk after onset of symptoms. Total (N = 57); without ARDS group (n = 29); with ARDS group (n = 28).
High-resolution chest CT scanning was performed at a median 6 (interquartile range, 4–9) days after onset of symptoms. Total (N = 35); without ARDS group (n = 14); with ARDS group (n = 21).
Figure 1.
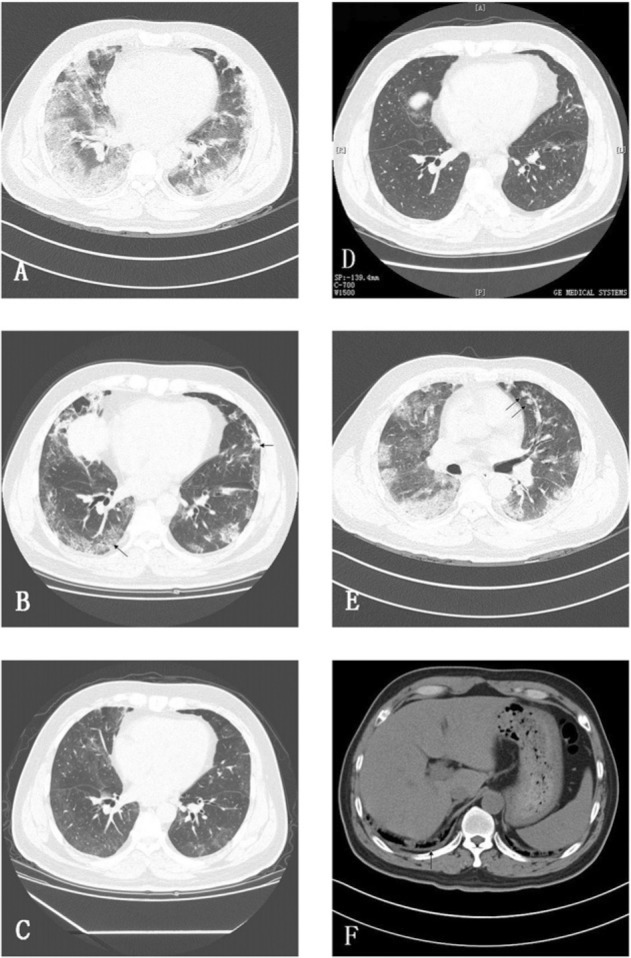
Radiologic findings during follow-up of a 49-year-old male discharged patient with 2009 influenza A(H1N1) (A[H1N1]) (patient No. 5 in Table 4), who was treated successfully with oseltamivir and noninvasive positive pressure ventilation. A, Initial high-resolution CT (HRCT) scan obtained 12 days after onset of illness shows bilateral extensive ground-glass opacities (GGOs) and multifocal consolidation that had a predominant subpleural distribution. B, HRCT scan obtained 29 days after onset of illness shows GGOs, interlobular septal thickening, and reticular nodules pattern (arrows). C, On day 54, only GGOs are seen. D, At a 3-month visit, GGOs are still present but are much improved. E and F, The same scan as A shows centrilobular nodules in the left upper lobe (arrows in E) and a very small amount of right pleural effusion (arrow in F).
Medication Treatment
Sixty-two of 65 patients started oseltamivir therapy within a median of 5 (IQR, 4-6) days from the onset of illness. Dosages and duration of antiviral therapy are listed in Table 1. Thirty-one patients received IV corticosteroids for a median duration of 3 (IQR, 3-6) days, with a dose of methylprednisolone, 1-3 mg/kg/d.
Adverse effects involving hallucinations and disorientation occurred in three male hospitalized patients 24 to 36 h after beginning corticosteroids or oseltamivir. Two of the three patients received both drugs, and the other one received only oseltamivir. Symptoms disappeared 1 to 2 days after stopping corticosteroids and oseltamivir or lowering the dose of oseltamivir.
Ventilation Support
Among 33 patients with ARDS, 24 required ventilation support, all of whom were initially treated with NPPV. In this group, NPPV succeeded in 13 (54.2%) (duration 5.1 ± 2.8 days) and 10 (41.7%) failed and were intubated at a median of 16 (IQR, 10-84) h after admission; the last one refused intubation and died. Among the 10 patients who were intubated, eight died. Patients who failed NPPV treatment had higher APACHE II scores on presentation (median 13 [IQR, 11-14]) compared with those who succeeded (median 10 [IQR, 9-11]; P = .020). Barotrauma occurred in two patients, one during extracorporeal membrane oxygenation therapy.
Coinfections
Sputum or transtracheal aspirate specimens obtained for bacterial culture were positive in 13 patients (Table 1), including Acinetobacter baumannii, four; Klebsiella pneumoniae, four; Pseudomonas aeruginosa, two; Enterobacter aerogenes, one; Escherichia coli, one; Staphylococcus aureus, one; and Aspergillus spp, six. Only one patient had a positive sputum culture within the first 48 h of hospitalization (Klebsiella pneumoniae). All other positive bacterial or fungal cultures were obtained ≥ 48 h after hospitalization.
Postmortem Findings
An autopsy was performed on a 44-year-old previously healthy man who was admitted 7 days after onset of symptoms and died of severe ARDS on day 18 of hospitalization (Fig 2 ). Gross examination of lung tissue revealed prominent congestion and consolidation, with increased weight (left, 860 g; right, 1,178 g). An abscess was seen in the right lower lobe that contained a large number of Aspergillus spp hyphae on microscopic examination. Microscopically, the lungs showed diffuse alveolar damage with hyaline membrane formation, intraalveolar edema and/or fibrin, necrotizing bronchiolitis, hemorrhage, secondary infection, focal alveolar necrosis, multifocal proliferation of pneumocytes, and fibrosis of the interstitium. Bacterial culture of lung tissue was positive for E coli, K pneumoniae, and Aspergillus spp. Lung tissue was also positive for A(H1N1) virus by real-time RT-PCR. No significant lesions were seen in other organs.
Figure 2.
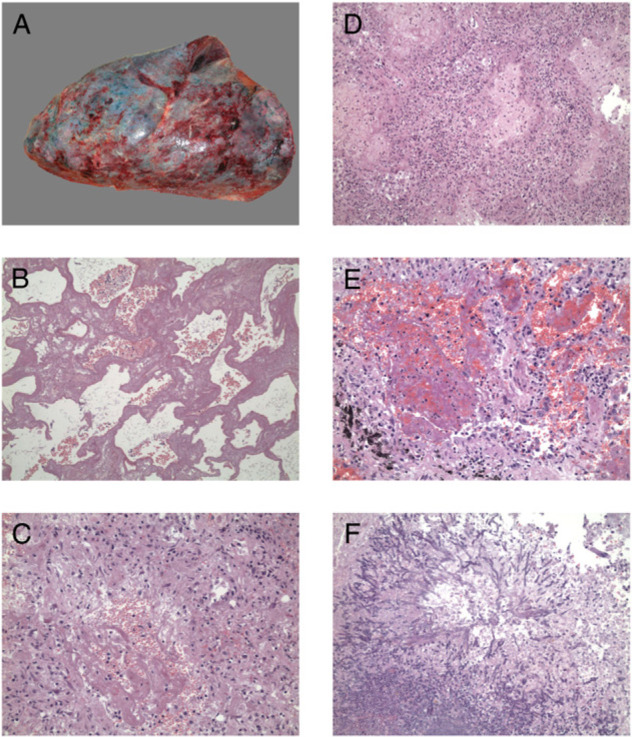
Histopathologic changes of lung tissue sample of one fatal case. A, Gross findings of right lung. B-E, Microscopic findings of the specimen (hematoxylin and eosin, original magnification × 100 [B, D] or original magnification × 400 [C, E]) show diffuse alveolar damage, formation of hyaline membrane, thickening of alveolar septa, and inflammatory cell infiltration with fibrinous exudates. F, The right lower lobe abscess shows Aspergillus spp hyphae microscopically (hematoxylin and eosin, original magnification × 100).
Outcome and Predictors of Mortality
Among the 65 patients, nine died, of whom eight had hemorrhagic respiratory secretions. One 19-year-old man died of severe hemoptysis within 24 h of admission. The death rate among patients with ARDS was 27.3% (9/33). The main cause of death was refractory hypoxemia. Two factors were found to be independently associated with death: progressive dyspnea after resolution of fever (relative risk, 5.852; 95% CI, 1.395-24.541; P = .016) and a higher APACHE II score on presentation (relative risk for each point, 1.312; 95% CI, 1.140-1.511; P < .001) (Table 2 ).
Table 2.
Analysis of Predictors for Fatal A(H1N1) Viral Pneumonia by Univariate and Multivariate Cox Regression
| Univariate Analysis |
Multivariate Analysis |
|||||
|---|---|---|---|---|---|---|
| Variable | Patients Who Survived (n = 56) | Patients Who Died (n = 9) | Relative Risk (95% CI) | P Value | Relative Risk (95% CI) | P Value |
| Hemoptysis, No. (%) | 22 (39.3) | 8 (88.9) | 10.288 (1.286–82.309) | .028 | … | .217 |
| Progressive dyspnea after resolution of fever, No. (%) | 5 (8.9) | 4 (44.4) | 5.173 (1.386–19.304) | .014 | 5.852 (1.395–24.541) | .016 |
| Lymphocyte count, mm3 | 800 (605–1,300) | 330 (300–640) | 0.028 (0.002–0.328) | .004 | … | .182 |
| Pao2/Fio2a | 302 (246–382) | 215 (164–277) | 0.990 (0.982–0.997) | .006 | … | .860 |
| Serum albumin,b g/L | 32.3 (29.9–35.6) | 25.7 (20.8–27.1) | 0.850 (0.774–0.933) | .001 | … | .447 |
| Lactate dehydrogenase,b U/L | 325 (246–474) | 546 (454–637) | 1.005 (1.002–1.008) | .001 | … | .528 |
| Imaging finding involved all six zones, No. (%) | 16 (28.6) | 7 (77.8) | 7.548 (1.566–36.388) | .012 | … | .389 |
| APACHE II score on presentation | 7 (3–10) | 13 (11–14) | 1.276 (1.130–1.441) | < .001 | 1.312 (1.140–1.511) | < .001 |
| Days from onset of symptoms to ED | 5 (4–6) | 6 (5–8) | 1.319 (1.103–1.578) | .002 | … | .455 |
Data are presented as median (interquartile range) unless otherwise indicated. A(H1N1) = 2009 influenza A(H1N1). See Table 1 for expansion of the other abbreviation.
Total (N = 59); survived group (n = 50); died group (n = 9).
Total (N = 58); survived group (n = 49); died group (n = 9).
Follow-up in Survivors
Of the 56 survivors, 39 had one or more follow-up visits. Among 14 who completed the 3-month visits, symptoms reported at the last visit included exertional dyspnea (four), hair loss (two), and cough (one). The duration of symptoms was as follows: sputum 19.6 ± 6.6 days, bloody sputum 11.0 ± 4.1 days, fatigue 16.0 ± 7.7 days. A 31-year-old female patient who was previously healthy still had a low platelet count of 34,000 per mm3 at 75 days after the onset of illness.
Changes in lung abnormalities from initial to follow-up HRCT scan examinations are shown in Table 3 . Among the 14 patients who completed their 3-month visit, 12 still showed lesser degrees of GGOs (Fig 1). In those who had ARDS (n = 9), “involved zones” were significantly (P = .002) more frequent than in those without ARDS (n = 5).
Table 3.
Analysis of HRCT Scanning During Follow-up
| Follow-up Number | Days From Onset of Illness, mean ± SD | Involved Zones, mean ± SD | GGOs | Consolidation | Interlobular Septal Thickening | Reticular Nodules Pattern |
|---|---|---|---|---|---|---|
| Initial (n = 20) | 6.6 ± 2.8 | 5.1 ± 1.2 | 20 (100) | 16 (80.0) | 0 | 9 (45.0) |
| Visit 1 (n = 12) | 24.4 ± 3.8 | 5.3 ± 1.4 | 12 (100) | 4 (33.3) | 9 (75.0) | 6 (50.0) |
| Visit 2 (n = 9) | 49.8 ± 6.3 | 4.6 ± 1.7 | 9 (100) | 1 (11.1) | 5 (55.6) | 3 (33.3) |
| Visit 3 (n = 14) | 93.9 ± 14.0 | 3.4 ± 2.2 | 12 (85.7) | 0 | 3 (21.4) | 2 (14.3) |
Data are presented as No. (%) of patients, unless otherwise indicated. Of the 20 patients who had ≥ 1 follow-up visits, four made all three visits, one made visits one and two, four made visits one and three, and two made visits two and three. The other 9 patients made only one visit (visit one for three of them, visit two for two of them, and visit three for four of them). GGOs = ground-glass opacities; HRCT = high-resolution CT.
LFTs were performed at visit 3 for 13 patients (Table 4 ). All 13 had been hospitalized, and there was no statistical difference in clinical and laboratory characteristics between these patients and those in whom LFTs were not obtained. Impairment of Dlco was the most common (8/13 [61.5%]) abnormality detected.
Table 4.
LFT Results and HRCT Scanning Patterns for 13 Discharged Patients With A(H1N1) Pneumonia, Performed at Visit Three (3 Mo After Onset of Illness)
| Patient No. | Sex/Age | Underlying Condition | ARDS/NPPV | FEV1, % | FEV1/FVC, % | TLC, % | Dlco, % | Dlco/VA, % | HRCT Scan Findings the Same Day LFTs Were Performed (Involved Zones) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M/55 | Chronic bronchitis, diabetes mellitus, hypertension, smoke (35 pack-y) | Yes/No | 78.1 | 76.55 | 97.6 | 66.5 | 98.0 | GGOs + interlobular septal thickening (6) |
| 2 | M/18 | Asthma, smoke (5 pack-y) | No/No | 45.6 | 56.04 | 93.6 | 81.1 | 129.8 | Normal |
| 3 | M/34 | Smoke (2.5 pack-y) | Yes/No | 109 | 95.68 | 97.9 | 91.8 | 104.5 | Normal |
| 4 | M/49 | Smoke (20 pack-y) | Yes/Yes | 69.3 | 76.88 | 68.8 | 64.3 | 90.8 | GGOs (4) |
| 5 | M/41 | Diabetes mellitus, hypertension, OSAS | Yes/Yes | 92.4 | 92.85 | 87.6 | 71.3 | 116.6 | GGOs + interlobular septal thickening (6) |
| 6 | M/57 | Emphysema, smoke (40 pack-y) | Yes/No | 62.3 | 56.87 | 96.7 | 41.1 | 57.3 | GGOs + nodules (2) |
| 7 | M/35 | None | No/No | 94.7 | 84.06 | 91.7 | 99.6 | 120.8 | GGOs (1) |
| 8a | F/56 | None | No/No | 62.1 | 87.92 | 51.6 | 57.5 | 103.5 | GGOs (4) |
| 9 | M/28 | None | No/No | 94.4 | 82.41 | 97.9 | 115.0 | 127.2 | GGOs (1) |
| 10 | M/58 | Rheumatoid arthritis, smoke (20 pack-y) | Yes/Yes | 102.1 | 81.55 | 102.4 | 58.6 | 75.6 | GGOs + interlobular septalthickening (6) |
| 11 | F/62 | Emphysema, asthma, hypertension | Yes/No | 44.1 | 41.55 | 145.2 | 11.3 | 18.6 | GGOs (4) |
| 12 | M/42 | Kidney transplant, hypertension, smoke (5 pack-y) | Yes/Yes | 89.1 | 86.06 | 81.8 | 67.9 | 96.7 | GGOs (2) |
| 13 | M/25 | Smoke (1.5 pack-y) | Yes/Yes | 83.7 | 85.44 | 80.2 | 80.5 | 107.8 | GGOs (4) |
Dlco = diffusing capacity for carbon monoxide; Dlco/VA = Dlco adjusted for alveolar volume; F = female; LFT = lung function test; M = male; NPPV = noninvasive positive pressure ventilation; OSAS = obstructive sleep apnea syndrome; TLC = total lung capacity. See Table 2, Table 3 for expansion of other abbreviations.
HRCT scan of this 56-y-old woman also presented diffuse pleural thickening and calcification, which indicated the pleural disease she had in the past, but she could not remember the details.
Discussion
Our series of 65 cases of A(H1N1) identified two independent risk factors associated with fatal pneumonia: progressive dyspnea after resolution of fever and a higher APACHE II score on presentation. Three months later, GGOs of less severity were still present on chest radiographs in 85.7% of patients (12/14). LFTs revealed decreased Dlco (< 80% predicted) in eight (61.5%) of the 13 patients tested.
The clinical characteristics of A(H1N1) pneumonia we described during the acute phase were similar to those reported by others.8, 9, 10, 11, 12, 13, 14, 15 In this report, most patients complained of dyspnea, which usually occurred within 1 week after illness onset. Dyspnea continued to progress after resolution of fever in 13.8% of the patients, a finding that has not been reported by others.
In this report, the success rate for NPPV was 54.2%, which is much higher than that reported by others (14.5%-27.3%).7, 12, 13, 14 Although the death rate (8/10 [80%]) in patients who received invasive ventilation in our study was higher than that reported in another study,7 among patients with ARDS, the death rate was 27.3%, similar to other reports.7, 8, 11, 12, 13, 14, 15 Moreover, although NPPV was used widely in the ward specifically set aside for patients infected with A(H1N1), none of the 28 doctors and nurses who were in direct contact with these patients developed respiratory symptoms or influenza-like illnesses. Therefore, we believe that with proper infection-control procedures, NPPV can be used successfully and safely for treating patients with A(H1N1) pneumonia complicated by ARDS.
It has been reported that 29% to 55% of autopsied patients with A(H1N1) had evidence of bacterial coinfection.17, 18 Streptococcus pneumoniae, Streptococcus pyogenes, and S aureus were the most predominant pathogens. However, in our study, community-acquired bacterial infection (defined as sputum collected within 48 h of hospitalization) was detected in only one of 50 patients (K pneumoniae). The low yield of gram-positive bacteria before or within 48 h of hospitalization may be due to the widespread use of prophylactic antibiotic therapy. In contrast, nosocomial infection was common in the patients (12/50 [24.0%]), and gram-negative bacilli were the predominant causative pathogens. Aspergillus spp was also seen. Progressive A(H1N1) infection, intubation, a prolonged hospital stay, IV antibiotic use, and use of oral or IV corticosteroids may be risk factors for nosocomial infection caused by gram-negative bacilli and Aspergillus spp.
We showed that symptoms and laboratory abnormalities in survivors of A(H1N1) virus infection returned to normal within 1 month of the onset of illness. Nonetheless, GGOs were still found at 3 months, although no fibrotic changes were seen. In survivors of A(H5N1) virus infection, persistent radiologic abnormalities including GGOs, often with a reticular pattern, have been seen as long as 1 year after illness onset.19 In survivors of severe acute respiratory syndrome (SARS) followed for 1 year, marked improvements in pulmonary fibrosis have been seen, but some patients still had residual changes.20 Because this kind of fibrosis was reversible, it has been suggested that these findings were partially caused by postinflammatory atelectasis rather than by genuine fibrosis alone.21 The resolution of lung abnormalities in patients with A(H1N1) viral pneumonia seemed better than that seen in patients with SARS and influenza A(H5N1) infection.
Impairment of Dlco was the most common (8/13 [61.5%]) abnormality in lung function testing 3 months after the onset of illness, followed by restrictive defects (2/13 [15.4%]). The Dlco findings were similar to the findings of one study of patients with SARS at 3-month follow-up visits.22 The impairment of Dlco in survivors of SARS persisted for 1 year in 23.7% of patients reported by other investigators.23 Although the number of cases with LFTs in our series is limited (only 13 cases, of whom eight had a reduction of Dlco), it seemed that patients who had bilateral GGOs on HRCT scan were more likely to have an impaired Dlco. During the convalescent period of ARDS, GGOs may consist of intralobular fibrosis that is below the limits of resolution of HRCT scanning.24 A longer follow-up study is needed to determine whether lung function abnormalities in patients infected with A(H1N1) are irreversible and radiologic changes persist over time.
To our knowledge, this is the first report of symptoms and radiographic changes in patients with A(H1N1) pneumonia during the convalescent period. There were several limitations. First, it is a single-center study with a limited number of patients. Second, monthly follow-up visits were offered to all patients when they were discharged but some of the patients felt that was inconvenient and did not come back. As a result, 39 out of 56 survivors had one or more follow-up visits. Third, most patients had underlying medical conditions that could have contributed to the lung function abnormalities.
Conclusions
In conclusion, we found that progressive dyspnea after resolution of fever and a higher APACHE II score on presentation were independent risk factors associated with death in patients with A(H1N1) viral pneumonia. At the 3-month follow-up visit of survivors of A(H1N1) pneumonia, some degree of GGOs persisted in most patients and decreased Dlco was common.
Acknowledgments
Author contributions: Dr Bai: contributed to data collection and analysis and the first draft of the manuscript.
Dr Gu: contributed to data collection and analysis.
Dr Cao: contributed to data collection and analysis, the first draft of the manuscript, and revision of the manuscript.
Dr Zhai: contributed to radiologic evaluation and review and revision of the manuscript.
Dr M. Lu: contributed to postmortem examination and review and revision of the manuscript.
Dr Y. Lu: contributed to lung function testing and review and revision of the manuscript.
Dr Liang: contributed to statistical analysis and review and revision of the manuscript.
Dr Zhang: contributed to radiologic evaluation and review and revision of the manuscript.
Dr Gao: contributed to postmortem examination and review and revision of the manuscript.
Dr Huang: contributed to lung function testing and review and revision of the manuscript.
Dr Liu: contributed to influenza virus testing and review and revision of the manuscript.
Dr Song: contributed to data collection and review and revision of the manuscript.
Dr Wu: contributed to data collection and review and revision of the manuscript.
Dr Yin: contributed to data collection and review and revision of the manuscript.
Dr Wang: contributed to data analysis and careful revision of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: All work was performed at the Beijing Chao-Yang Hospital, Capital Medical University and the School of Basic Medical Sciences, Peking University, Bejing, China. We thank Ran Li, BS; Chen Ma, BS; Cui-Lian Li, MS; Fang Li, BS; Bin-Bin Li, BS; Shan-Shan Wang, BS; and Chun-Lei Wang, BS, for their help with the collection of specimens and clinical data. We thank Jun Zhang, BS; Bao-Mei Wu, BS; and Xiao-Hong Chang, BS, for their help with lung function testing.
Footnotes
Drs Bai, Gu, and Cao contributed equally to this article.
Funding/Support: This study was supported by grants from Beijing Science and Technology [Grant Z08050700020801] and the Beijing Nova Programme [Grant 2007A037], and the Major State Basic Research Development Program [Grant 2009CB522107].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.World Health Organization (WHO) Clinical management of human infection with pandemic (H1N1 2009: revised guidance. http://www.who.int/csr/resources/publications/swineflu/clinical_management_h1n1.pdf Accessed November 2009.
- 2.Bin C, Xingwang L, Yuelong S, National Influenza A Pandemic (H1N1) 2009 Clinical Investigation Group Clinical and epidemiologic characteristics of 3 early cases of influenza A pandemic (H1N1) 2009 virus infection, People's Republic of China, 2009. Emerg Infect Dis. 2009;15(9):1418–1422. doi: 10.3201/eid1509.090794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao B, Li XW, Mao Y, National Influenza A Pandemic (H1N1) 2009 Clinical Investigation Group of China Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med. 2009;361(26):2507–2517. doi: 10.1056/NEJMoa0906612. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, INER Working Group on Influenza Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361(7):680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) Pandemic (H1N1 2009. World Health Organization Web site. Update 91. http://www.who. int/csr/don/2010_03_12/en/index.html Accessed March 12, 2010.
- 6.Ministry of Health of the People's Republic of China Pandemic H1N1 in China. Ministry of Health Web site. http://www.moh.gov.cn/publicfiles/business/htmlfiles/mohwsyjbgs/s7863/201003/46150.htm Updated February 2010. Accessed March 2, 2010.
- 7.Rello J, Rodríguez A, Ibañez P, H1N1 SEMICYUC Working Group Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit Care. 2009;13(5):R148. doi: 10.1186/cc8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain S, Kamimoto L, Bramley AM, 2009 Pandemic Influenza A (H1N1) Virus Hospitalizations Investigation Team Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361(20):1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 9.Denholm JT, Gordon CL, Johnson PD. Hospitalised adult patients with pandemic (H1N1) 2009 influenza in Melbourne, Australia. Med J Aust. 2010;192(2):84–86. doi: 10.5694/j.1326-5377.2010.tb03424.x. [DOI] [PubMed] [Google Scholar]
- 10.Louie JK, Acosta M, Winter K, California Pandemic (H1N1) Working Group Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302(17):1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 11.Chien YS, Su CP, Tsai HT. Predictors and outcomes of respiratory failure among hospitalized pneumonia patients with 2009 H1N1 influenza in Taiwan. J Infect. 2010;60(2):168–174. doi: 10.1016/j.jinf.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Kumar A, Zarychanski R, Pinto R, Canadian Critical Care Trials Group H1N1 Collaborative Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302(17):1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 13.Domínguez-Cherit G, Lapinsky SE, Macias AE. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302(17):1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 14.Miller RR, 3rd, Markewitz BA, Rolfs RT. Clinical findings and demographic factors associated with ICU admission in Utah due to novel 2009 influenza A(H1N1) infection. Chest. 2010;137(4):752–758. doi: 10.1378/chest.09-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webb SA, Pettilä V, Seppelt I, ANZIC Influenza Investigators Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361(20):1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 16.Ooi GC, Khong PL, Müller NL. Severe acute respiratory syndrome: temporal lung changes at thin-section CT in 30 patients. Radiology. 2004;230(3):836–844. doi: 10.1148/radiol.2303030853. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1)-United States, May-August 2009. MMWR Morb Mortal Wkly Rep. 2009;58(38):1071–1074. [PubMed] [Google Scholar]
- 18.Gill JR, Sheng ZM, Ely SF. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med. 2010;134(2):235–243. doi: 10.5858/134.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu PX, Wang YX, Zhou BP. Radiological features of lung changes caused by avian influenza subtype A H5N1 virus: report of two severe adult cases with regular follow-up. Chin Med J (Engl) 2010;123(1):100–104. [PubMed] [Google Scholar]
- 20.Xie L, Liu Y, Fan B. Dynamic changes of serum SARS-coronavirus IgG, pulmonary function and radiography in patients recovering from SARS after hospital discharge. Respir Res. 2005;6:5. doi: 10.1186/1465-9921-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong KT, Antonio GE, Hui DS. Severe acute respiratory syndrome: thin-section computed tomography features, temporal changes, and clinicoradiologic correlation during the convalescent period. J Comput Assist Tomogr. 2004;28(6):790–795. doi: 10.1097/00004728-200411000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Ong KC, Ng AW, Lee LS. Pulmonary function and exercise capacity in survivors of severe acute respiratory syndrome. Eur Respir J. 2004;24(3):436–442. doi: 10.1183/09031936.04.00007104. [DOI] [PubMed] [Google Scholar]
- 23.Hui DS, Wong KT, Ko FW. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128(4):2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desai SR. Acute respiratory distress syndrome: imaging of the injured lung. Clin Radiol. 2002;57(1):8–17. doi: 10.1053/crad.2001.0889. [DOI] [PubMed] [Google Scholar]


