Abstract
Equine proliferative enteropathy (EPE) is a disease of foals caused by the obligate intracellular organism Lawsonia intracellularis. This organism is unique in that it causes proliferation of infected enterocytes, resulting in thickening of the intestinal epithelium, most often the small intestine. This disease affects mainly weanling foals and causes fever, lethargy, peripheral edema, diarrhea, colic and weight loss. The diagnosis of EPE may be challenging and relies on the presence of hypoproteinemia, thickening of segments of the small intestinal wall observed on abdominal ultrasonography, positive serology and molecular detection of L. intracellularis in feces. The epidemiology and genetic basis for pathogenesis for this disease is beginning to be elucidated. Phenotypic traits, genomic features, and gene expression profiles during L. intracellularis infection in vitro and in vivo are presented. In addition, this article reviews the epidemiology, pathological and clinicopathological findings, diagnosis, and control of EPE.
Keywords: Lawsonia intracellularis, Equine proliferative enteropathy, Epidemiology, Serology, Real-time PCR, Horse
1. Etiology
Lawsonia intracellularis is the etiologic agent of the intestinal disease in horses called equine proliferative enteropathy (EPE). L. intracellularis is an obligate intracellular, motile, curved, gram-negative bacterium that resides freely within the apical cytoplasm of infected intestinal enterocytes (Lawson and Gebhart, 2000). The Genus Lawsonia was placed within the Desulfovibrionaceae Family based on phylogenetic analysis of 16S rDNA sequences and, to date, the Lawsonia Genus contains only one species (McOrist and Gebhart, 2005). Similar to the Desulfovibrionaceae, L.intracellularis is anaerobic but differs from other members of the Family in its inability to reduce sulfate. In vivo, it causes proliferation of the affected enterocytes, resulting in a thickened small and sometimes large intestine. L. intracellularis can only be grown in vitro in cell culture and requires a specific atmosphere and dividing cells for growth (Lawson et al., 1993, Lawson and Gebhart, 2000). The peak of infection occurs five to seven days post inoculation. A single polar flagellum and darting motility are observed with extracellular L. intracellularis organisms in cell culture.
Besides horses, L. intracellularis infects many species of domestic and wild animals, including pigs, hamsters, rabbits, foxes, deer, ferrets, ostriches, and non-human primates. Equine proliferative enteropathy was first reported in horses in 1982 by Duhamel and Wheeldon (1982). Since 1996, several reports of sporadic cases and outbreaks on breeding farms have been described (Williams et al., 1996, Frank et al., 1998, Brees et al., 1999, Lavoie et al., 2000, Schumacher et al., 2000, Bihr, 2003, McClintock and Collins, 2004, Deprez et al., 2005, Dauvillier et al., 2006, Sampieri et al., 2006, Wuersch et al., 2006, Feary et al., 2007, Frazer, 2008, McGurrin et al., 2008, Pusterla et al., 2008, Pusterla et al., 2009a, Guimarães-Ladeira et al., 2009, Merlo et al., 2009, Shimizu et al., 2010, Van Den Wollenberg et al., 2011). In the last few years, reported cases of EPE have been increasing, occurring primarily in post-weaning foals and occasionally in adult horses. It is not known whether the increased numbers of reports are due to increased awareness or to increased incidence of EPE. The disease has almost reached a worldwide occurrence and has been reported in the USA, Canada, Europe, South Africa, Australia, Brazil and Japan.
Molecular investigations of L. intracellularis isolates from proliferative enteropathy lesions of a variety of animal species, including horses and hamsters, showed 98% homology of the 16S-rDNA gene to pig isolates (Cooper and Gebhart, 1998). Moreover, phenotypic characterization of outer membrane proteins and immunoblots of different L. intracellularis isolates using several antibodies and more sensitive molecular characterizations of the L. intracellularis genome demonstrated only minor differences among isolates (Cooper and Gebhart, 1998). None of these differences appear to be antigenically relevant.
The whole genome of the porcine L. intracellularis isolate PHE/MN1-00 (Guedes and Gebhart, 2003b) was sequenced and is publically available (http://www.ncbi.nlm.nih.gov/genome?term=lawsonia). This genome contains a total of 1,719,014 base pairs distributed into one small chromosome and three plasmids. The presence of expression of numerous bacterial genes encoding hypothetical proteins during L. intracellularis infection in vitro suggests that this organism has adopted mechanisms of survival and pathogenesis that are unique among bacterial pathogens (Vannucci et al., 2012a).
2. Epidemiology
L. intracellularis causes PE in a wide range and variety of animal species, highlighting the necessity for universal diagnostic tests to understand the importance of the disease in these various species and to determine the dynamics of potential inter-species transmission. In pig populations, the chronic form of the disease is maintained by subclinically affected pigs or is established environmentally in some sites, allowing transmission of L. intracellularis to serial flows of pigs through the feces (Jordan et al., 2004). Porcine PE has been transmitted to hamsters using mucosal homogenates (McOrist and Lawson, 1987) and cell cultured L. intracellularis (Jasni et al., 1994); however, the lesions tend to be milder than in the originating host. Mice and rats have been shown to be important reservoirs of L. intracellularis on pig farms with prevalences of PCR positive animals varying substantially between farms (4–83%; Friedman et al., 2008, Collins et al., 2011, Backhans et al., 2012). Rodents appear to be suitable reservoir hosts due to their susceptibility to L. intracellularis, their close contact to domestic animals and their high reproductive rate. Though EPE is transmitted by the fecal oral route once introduced into a herd of foals, the source(s) of these infections has not been determined. Exposure to pig feces has been speculated as a potential source of infection for horses since the first reported cases of EPE. However, in most cases of EPE, no history or evidence of direct or indirect exposure to pigs or pig feces has been reported. A recent experimental study has shown host specificity for L. intracellularis isolates cultured from pig or horse intestines (Vannucci et al., 2012b). The study showed that clinical signs, longer periods of shedding and stronger serologic immune responses were observed in animals infected with species-specific isolates. Therefore, host susceptibility is driven by the origin of the isolated L. intracellularis strain. Sampieri et al. (2013a) showed that this host adaptation demonstrated in pigs and horses seems to extend to hamsters and rabbits, respectively, which are used as experimental infection models. Rabbits were more susceptible to equine-derived isolates than to porcine variants and hamsters were more susceptible to porcine-derived isolates than to equine variants. Taken together, this evidence suggests that L. intracellularis variants may have evolved to be adapted to more than one host species. However, the evolutionary ecology responsible for driving this host adaptation remains to be determined.
Previous studies have shown that a variety of wild and domestic animals, including dogs, cats, rabbits, opossums, skunks, mice and coyote, can shed L. intracellularis on farms with diagnosed EPE cases (Pusterla et al., 2008, Pusterla et al., 2012a). Further, infection of laboratory rabbits with cell cultured equine L. intracellularis provides an animal model for investigations on the pathogenesis and therapy of EPE. This model simulates natural infection of foals, as typical lesions, immune responses, and fecal shedding patterns were observed in the rabbits (Sampieri et al., 2013b). On a recently identified farm in northern California endemic for EPE, 7.5% of fecal samples and 27% of serum samples from cottontail rabbits tested PCR positive and seropositive for L. intracellularis, respectively (Pusterla et al., 2012a). Of interest was that on this farm, a large population of cottontail rabbits lived in the hay barn and had direct access to the hay fed to the horses. An epidemiological investigation on this farm showed rabbit feces on top of hay bales but also in the feeders of the weanling foals, suggesting that the foals developing EPE were likely exposed to L. intracellularis via the oral ingestion of infected rabbit feces. Similar to rodents, lagomorphs may represent an effective reservoir/amplifier host due to their large population, their close contact to horses and their short reproductive cycle. It still remains to be determined how L. intracellularis became endemic in the rabbit population on this farm.
Feco-oral transmission of L. intracellularis has been documented in naïve foals housed with clinically infected foals experimentally challenged with an equine isolate of L. intracellularis (Pusterla et al., 2010a). A recent study has shown that feces from experimentally infected rabbits with an equine isolate of L. intracellularis served as infectious material to weanling foals (Pusterla et al., 2012b). Although infected rabbits and foals remained asymptomatic, infection was supported by fecal shedding of L. intracellularis and detection of specific antibodies to L. intracellularis. Although the natural infectious dose for foals has not been determined, pigs receiving as low as 105 L. intracellularis have been shown to develop infection (Collins and Love, 2007). Recent work suggested that 1 g of infectious feces would suffice to deliver the above challenge dose (Collins et al., 2011). Amplification of L. intracellularis and environmental contamination leading to exposure rates of up to 100% of resident foals are likely to occur secondary to the shedding of large quantities of L. intracellularis from either clinically or subclinically infected foals.
In piglets, large group size, weaning, transportation, diet change and mixing have been associated with clinical disease (Lawson and Gebhart, 2000). Predisposing factors such as the stress of weaning, overcrowding, decline in L. intracellularis-specific colostral antibodies, endoparasitism and introduction of new animals have been suggested in the development of EPE in foals (Lavoie et al., 2000). In pigs, infection and fecal shedding of L. intracellularis may persist for as long as 12 weeks (Guedes and Gebhart, 2003a). This is in sharp contrast to experimentally infected foals showing onset and duration of fecal shedding ranging from 10 to 14 days and 17 to 27 days, respectively (Pusterla et al., 2010a, Pusterla et al., 2012c). Previous work has shown that L. intracellularis is likely to survive in environmental conditions for 1–2 weeks at 5–15 °C (Collins et al., 2000).
3. Pathogenesis
The pathogenesis of EPE has remained poorly investigated and most of the information available has been extrapolated from experimentally infected hamsters, pigs and rabbits. Comprehensive studies of lesion development and evolution have been conducted in pigs (Guedes and Gebhart, 2003a, Guedes and Gebhart, 2003b, Guedes and Gebhart, 2010) and hamsters (Jacoby, 1978, Jasni et al., 1994). Morphological studies of early lesions in experimentally infected animals indicate that enterocyte hyperplasia is directly preceded by the presence of the intracellular organism (Jacoby, 1978, Guedes and Gebhart, 2003a). In vivo, the onset of hyperplasia associated with proliferative enteropathy follows an increase in numbers of intracellular L. intracellularis in enterocytes. Likewise, resolution of the lesions is closely related to disappearance of the intracellular organisms, indicating a correlation between the two events (Lawson and Gebhart, 2000). Intracellular L.intracellularis are located in the apical membrane of infected enterocytes and are commonly found near the mitochondria, suggesting a symbiotic interaction (Jacoby, 1978). The means by which L. intracellularis produces hyperplasia is unknown. No other cytopathologic effects on infected enterocytes are seen in vivo or in vitro. Inflammation is a factor only in later-stage lesions and is not characteristic of the primary lesion.
Proliferative enteropathy can be reproduced using pure cultures of L. intracellularis at low passages (under 20) in cell culture (McOrist et al., 1993, Guedes and Gebhart, 2003b, Vannucci et al., 2013a). Pigs inoculated with the lower passages (10 and 20 passages) demonstrated typical proliferative lesions with intracellular L. intracellularis. No clinical or pathological affects were seen, however, in the pigs inoculated with the same isolate passed 40 times in cell culture, indicating that the attenuation process occurs between passage 20 and 40 (Vannucci et al., 2013a). These results demonstrate that the virulence properties of L. intracellularis are attenuated between 20 and 40 cell culture passages in vitro. Information on attenuation is useful for design of experimental models and for determining the mechanisms responsible for this loss of virulence. A further study (Vannucci et al., 2013b) compared the whole genome sequences of the same homologous porcine L. intracellularis isolate cultivated for 10 and 60 passages in vitro. An 18 kb prophage-associated genomic island was identified in the passage 10 (pathogenic variant) isolate but was lost in the passage 60 (non-pathogenic variant) isolate. This island comprised 15 genes downstream from a prophage DLP12 integrase gene. Analysis of cell cultured porcine L. intracellularis isolates (12 total) showed that this island was conserved in all porcine isolates cultured for up to 20 passages, but was lost in all isolates cultivated for more than 40 passages. It was present in all (53 total) naturally infected pigs tested. However, no cell cultured equine L. intracellularis isolates harbored this genomic island, regardless of the number of passages in vitro. Nor did fecal samples from naturally infected foals (21 total) or rabbits (4 total) contain this prophage-associated island. We can conclude that the presence of this island is not essential for the virulent phenotype of L. intracellularis, but that this genetic element is porcine isolate-specific and may contribute to the ecological specialization of this organism in pigs (Vannucci et al., 2013b).
Comparative transcriptional analyses of homologous pathogenic (low passage) and non-pathogenic (high passage) L. intracellularis isolates in vitro in infected porcine cells was performed using high-throughput sequencing technology. A wider transcriptional landscape was identified in the pathogenic variant and included many plasmid-encoded genes, genes involved in membrane transport, adaptation, and stress responses. In fact, the entire gene repertoire of one plasmid was repressed in the non-pathogenic variant, suggesting this plasmid's role in virulence. Transcription levels of genes commonly expressed in both pathogenic and non-pathogenic variants suggest the involvement of gene silencing mechanisms in attenuation of the virulence properties of the pathogenic variant during multiple passages in cell culture (Vannucci et al., 2012a).
Convalescent pigs have a degree of immunity to reinfection (Collins and Love, 2007). Animals challenged a second time, after cessation of fecal shedding, were evaluated clinically and their feces were tested by PCR to detect shedding. Animals previously infected did not shed detectable numbers of L. intracellularis and had no clinical signs. The cell-mediated immune response appears to be an important feature in protecting animals from reinfection with L. intracellularis (Guedes and Gebhart, 2003a, Guedes and Gebhart, 2010). Descriptive immunocytological studies of intestinal tissue sections of pigs affected by proliferative enteropathy reveal a mild infiltration of cytotoxic T cells, macrophages, and B lymphocytes carrying MHC class II structure at the beginning of the cell-mediated immune response (McOrist et al., 1992).
Immunohistochemical studies of intestinal sections of naturally infected pigs also demonstrated a large accumulation of IgA in the apical cytoplasm of proliferating enterocytes (McOrist et al., 1992). Further, interferon gamma is produced by PBMCs of both pigs and horses following specific stimulation (Guedes and Gebhart, 2003a, Page et al., 2011, Pusterla et al., 2012d), and L.intracellularis-specific IgA is detected in intestinal lavages of challenged pigs (Guedes and Gebhart, 2010). Similarly, interferon-gamma played a role in limiting intracellular infection and increased cellular proliferation in experimentally infected mice (Smith et al., 2000).
4. Clinical and clinicopathological findings
There are characteristic signalment, seasonality, clinical signs and blood work abnormalities associated with EPE. The disease is generally manifested in foals less than one year of age and in North America is often seen between August and January (Frazer, 2008). Although the disease is commonly seen in weanling foals 4–7 months of age, cases of EPE have been seen in young adults (Shimizu et al., 2010). Lethargy, anorexia, fever (>38.5 °C), peripheral edema (ventrum, sheath, throatlatch and distal limbs; Fig. 1 ), weight loss (Fig. 2 ) colic and diarrhea are amongst the most common clinical findings in affected foals. Early clinical signs are generally unspecific and include mild depression, partial anorexia and fever. Although diarrhea is commonly seen in affected foals and can vary from cow pie to watery, some affected foals may have normal fecal character. Foals with EPE may also have concurrent disorders such as respiratory tract infections, gastric ulcerations and intestinal parasitism. One must keep in mind that signs of EPE may resemble those of other gastrointestinal disorders such as parasitism, bacterial infections (Clostridium spp., Salmonella spp., Rhodococcus equi, Neorickettsia risticii), rotavirus, coronavirus, ulcerations, sand accumulation, intestinal obstruction and intoxication with plants, chemicals and pharmacologic agents such as nonsteroidal anti-inflammatory drugs or antimicrobials. Similar to pigs, the disease can be subclinical in foals. Subclinical disease is characterized by a self-limiting and transient decrease of total serum protein concentration coupled with decreased daily weight gain when compared to unaffected foals (Pusterla et al., 2010a, Pusterla et al., 2012a). It will remain to be determined if growth retardation or unthriftiness are associated with subclinical infection.
Fig. 1.
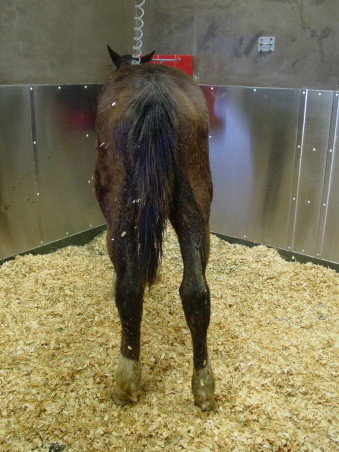
Seven-month-old Quarter Horse colt with proliferative enteropathy displaying distal limb edema and fecal staining of the distal limb due to diarrhea.
Fig. 2.

Severe weight loss in a 5-month-old Quarter Horse colt with proliferative enteropathy.
The most consistent laboratory finding of clinical EPE is hypoproteinemia due to hypoalbuminemia. Total protein is generally less than 5.0 g/dl and albumin is usually less than 2.0 g/dl. In a recent case report (Frazer, 2008), hypoalbuminemia was the only consistent clinicopathologic abnormality of 57 affected foals with albumin concentrations ranging from 0.9 to 3.3 g/dl (normal reference range 2.7–4.2 g/dl). The exact mechanisms by which hypoalbuminemia develops in affected foals has not been investigated. It appears that a combination of decreased feed intake, coupled with malabsorption and protein-losing enteropathy due to the proliferative nature of the disease may represent likely mechanisms by which low albumin occurs (Wong et al., 2009). Affected foals may also demonstrate non-specific blood abnormalities such as anemia or hemoconcentration, leukocytosis or neutropenia, hyperfibrinogenemia, increased activity of muscle enzymes and electrolyte abnormalities (hypocalcemia, hypochloremia and hyponatremia). Urine analysis to rule out protein-losing nephropathy and cytological evaluation of abdominal fluid to rule out protein lost into the peritoneal or pleural cavities are generally unremarkable.
5. Diagnosis
A presumptive diagnosis of EPE is generally made based on age of the affected animal, clinical signs, hypoproteinemia/hypoalbuminemia, presence of thickened small intestinal loops on ultrasonographic evaluation and ruling out other causes of enteropathy and protein losses. Abdominal ultrasonography, although not very sensitive, may show segments of thickened small intestine (Fig. 3 ) and excessive abdominal fluid. In these cases, abdominocentesis will yield a non-inflammatory transudate. An ante-mortem diagnosis is generally confirmed via PCR detection of L. intracellularis in feces or rectal swab and/or serology (Pusterla et al., 2009a).
Fig. 3.
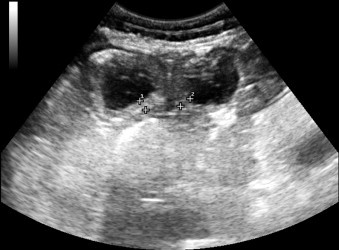
Ultrasound image showing thickened section of small intestinal wall in a 7-month-old Thoroughbred colt with proliferative enteropathy. The wall thickness measured between 5.2 and 5.8 mm (normal wall thickness ≤3 mm).
It is essential to combine both molecular and serologic diagnostic testing, since these assays have high analytical specificity but variable sensitivity depending on the situation. Negative PCR results can be expected if the fecal samples are collected from foals with prior antimicrobial treatment or during advanced disease stage, when L. intracellularis organisms are no longer expected in the feces. Negative serological results can be expected in the early stage of the disease, when humoral immune responses are not yet strong enough to be detectable by serology. Further, differences in sensitivity amongst different PCR and serological assays can lead to divergent results. Amongst PCR assays, the use of real-time PCR methods has been shown to yield the best sensitivity and to reduce the likelihood of cross- or carry-over contamination (i.e. false positive results) (Nathues et al., 2009, Pusterla et al., 2010b, Richter et al., 2010). Several serological assays, including indirect fluorescent antibody test (IFAT), enzyme-linked immunosorbent assay (ELISA) and immunoperoxidase monolayer assay (IPMA), have all been validated and established for pigs (Guedes et al., 2002a, Guedes et al., 2002b, Boesen et al., 2005, Wattanaphansak et al., 2008). A preliminary comparative study using equine serum samples has shown that various serology assays are accurate; however the IPMA is the most specific when determining the presence of anti-L. intracellularis antibodies in adult horses with EPE (Gebhart et al., 2012).
6. Pathologic findings
Lesions are most commonly seen in the ileum, near the ileal–cecal junction, and appear as a thickening of the mucosa. Gross lesions are not evident in all cases of EPE and may often be overlooked. Intestines show an irregular, patchy subserosal edema. The ileal mucosa is thickened with deep folds and chronically affected animals may have patches of pseudomembrane covering the mucosa (Fig. 4 ). Hypertrophy and thickening of the muscularis mucosa may occur in chronically affected or recovering animals (Fig. 4). Histologically, adenomatous proliferation occurs among the epithelial cells in the crypts of the small intestine, in association with the presence of curved, intracellular bacteria in the apical cytoplasm of these enterocytes (Duhamel and Wheeldon, 1982, Williams et al., 1996, Lavoie et al., 2000). Severe PE is diagnosed by the demonstration of hyperplasia of the crypt glands with an increased number of mitotic figures and marked reduction or absence of goblet cells in routine hematoxylin and eosin preparations; however, for visualization of the bacteria in the cytoplasm of enterocytes, special stains are necessary. The histological lesions of PE are unique and inflammation is not normally a hallmark of disease. Warthin–Starry silver stain allows the detection of the bacteria in histologic sections (Fig. 5 ), improving the diagnostic sensitivity, but the technique has limitations when applied to autolyzed and necrotic samples (Lawson and Gebhart, 2000). Immunohistochemistry procedures, using postmortem tissue or biopsy material with an antibody specific for L. intracellularis, are considered the gold standard for histological confirmation of EPE (Fig. 6 ).
Fig. 4.
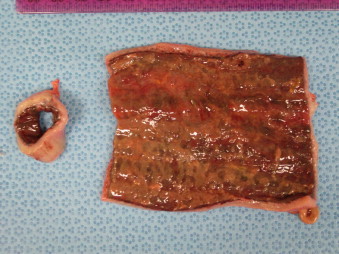
Gross lesions of equine proliferative enteropathy of a 9-month-old Quarter Horse colt with proliferative enteropathy showing diffusely thickened intestinal wall and patches of pseudomembrane covering the mucosa.
Fig. 5.
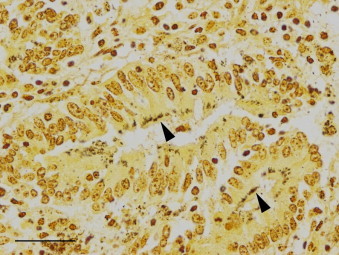
Ileal mucosa from a 9-month-old Quarter Horse colt with proliferative enteropathy. Aggregates of bacteria (arrowheads) are present in the cytoplasm of epithelial cells of hyperplastic crypts (Warthin–Starry silver stain; bar = 30 μm).
Fig. 6.
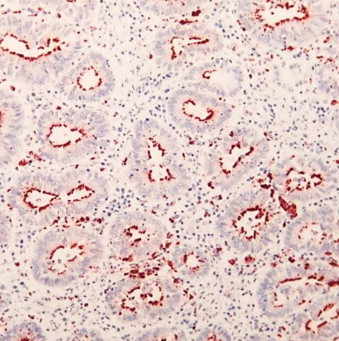
Immunohistochemical stained section of small intestine from a 6-month-old foal with proliferative enteropathy. L. intracellularis specific antibody stains the bacteria lining the apical cytoplasm of the affected crypts (red areas).
7. Therapy
Treatment of EPE in horses involves the use of antimicrobials such as macrolides, alone or in combination with rifampin, chloramphenicol, oxytetracycline, doxycycline or minocycline administered for 2–3 weeks. The choice of antimicrobial in the treatment of EPE should take into account the risk of inducing disturbance of the gastrointestinal flora and renal toxicity. This is especially a concern when treating older foals with severe hypoalbuminemia. In addition, supportive care such as intravenous fluids, plasma transfusion, parenteral nutrients and anti-ulcer drugs are commonly used to treat affected foals. Concurrent medical conditions should also be addressed. It is important to treat affected animals early, before lesions become advanced and result in marked weight loss and critically low serum protein values. Rapid clinical improvement following treatment is to be expected; however, it may take weeks for the hypoproteinemia to resolve (Pusterla and Gebhart, 2009). Spontaneous recovery of clinically infected foals has not been documented, and up to 93% treated foals usually survive the disease (Frazer, 2008). However, in more recent years there have been reports of more severe cases presented to veterinary hospitals with a poorer outcome (Page et al., 2012). These cases do not demonstrate the classic, chronic clinical signs of EPE, but occur acutely with severe secondary complications, often leading to death. Long-term sequelae have not been reported; however, clinically affected and successfully treated foals sell for an average of 68% of the average price of unaffected foals by the same stallion (Frazer, 2008). However, their monetary earnings from racing were not significantly different from other horses (Frazer, 2011).
8. Monitoring and prevention
Early recognition of clinical cases and separating them from the rest of the susceptible foals until full recovery or cessation of fecal shedding appears to be a logical biosecurity measure to prevent spread and environmental contamination. The monitoring of a herd with endemic EPE status includes the regular physical evaluation of resident foals and the monthly or bimonthly assessment of total protein concentration and monthly serological status. Monitoring for exposure to L. intracellularis and hypoproteinemia/hypoalbuminemia should begin at least 4 weeks prior to the historical first detection of clinical cases.
The lack of epidemiological data regarding potential natural reservoir hosts as well as the lack of information pertaining to the biology of L. intracellularis precludes the institution of any management changes on endemic farms. Further, maintaining good pest control and preventing non-equine domestic and wild animals to gain access to feed and feeding areas may potentially minimize the risk of disease spread.
Prevention strategies have been best described in pigs using in-feed antimicrobials and a commercially available L. intracellularis vaccine (Guedes and Gebhart, 2003a, Kroll et al., 2004, McOrist and Smits, 2007). Recent work has shown that detectable humoral and cellular responses can be measured in foals administered an avirulent live L. intracellularis vaccine (Pusterla et al., 2009b, Pusterla et al., 2012d). The L. intracellularis vaccine has been shown to be safe and the administration well tolerated by the foals. A field efficacy trial performed on EPE endemic farms in central Kentucky in 2008 showed that vaccinated foals maintained higher daily weight gains and higher total protein concentrations when compared to a non-vaccinated, naturally seroconverted group (Nogradi et al., 2012). Under experimental conditions, weanling foals vaccinated intra-rectally with an avirulent live vaccine against L. intracellularis were protected against clinical and subclinical EPE following challenge exposure with a virulent L. intracellularis isolate of equine origin (Pusterla et al., 2012c). This was determined by lack of clinical disease, absence of hypoproteinemia and ultrasonographic abnormalities compatible with EPE, and a significant reduction in L. intracellularis fecal shedding in vaccinated foals compared to non-vaccinated foals. Further, average daily weight gains from the vaccinated foals over the entire study period were similar to the control foals and significantly higher when compared to the non-vaccinated foals, highlighting the benefit of the vaccine in the prevention of subclinical disease. The extra-label use of the L. intracellularis vaccine should be considered on naïve and endemic farms in an attempt to reduce or prevent EPE. Timing of vaccine administration should again be synchronized with historical disease occurrence. Further, routine monitoring for clinical signs and hypoproteinemia/hypoalbuminemia is still recommended even when vaccine prophylaxis is used.
References
- Backhans A., Jacobson M., Hansson I., Lebbad M., Lambertz S.T., Gammelgård E., Saager M., Akande O., Fellström C. Occurrence of pathogens in wild rodents caught on Swedish pig and chicken farms. Epidemiol. Infect. 2012 doi: 10.1017/S0950268812002609. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihr T.P. Protein-losing enteropathy caused by Lawsonia intracellularis in a weanling foal. Can. Vet. J. 2003;44:65–66. [PMC free article] [PubMed] [Google Scholar]
- Boesen H.T., Jensen T.K., Møller K., Nielsen L.H., Jungersen G. Evaluation of a novel enzyme-linked immunosorbent assay for serological diagnosis of porcine proliferative enteropathy. Vet. Microbiol. 2005;109:105–112. doi: 10.1016/j.vetmic.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Brees D.J., Sondhoff A.H., Kluge J.P., Andreasen C.B., Brown C.M. Lawsonia intracellularis-like organism infection in a miniature foal. J. Am. Vet. Med. Assoc. 1999;215:511–515. [PubMed] [Google Scholar]
- Collins A., Love R.J., Pozo J., Smith S.H., McOrist S. Studies on the ex vivo survival of Lawsonia intracellularis. J. Swine Health Prod. 2000;8:211–215. [Google Scholar]
- Collins A.M., Love R.J. Re-challenge of pigs following recovery from proliferative enteropathy. Vet. Microbiol. 2007;120:381–386. doi: 10.1016/j.vetmic.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Collins A.M., Fell S., Pearson H., Toribio J.A. Colonisation and shedding of Lawsonia intracellularis in experimentally inoculated rodents and in wild rodents on pig farms. Vet. Microbiol. 2011;150:384–388. doi: 10.1016/j.vetmic.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Cooper D.M., Gebhart C.J. Comparative aspects of proliferative enteritis. J. Am. Vet. Med. Assoc. 1998;212:1446–1451. [PubMed] [Google Scholar]
- Dauvillier J., Picandet V., Harel J., Gottschalk M., Desrosiers R., Jean D., Lavoie J.P. Diagnostic and epidemiological features of Lawsonia intracellularis enteropathy in 2 foals. Can. Vet. J. 2006;47:689–691. [PMC free article] [PubMed] [Google Scholar]
- Deprez P., Chiers K., Gebhart C.J., Ducatelle R., Lefère L., Vanschandevijl K., van Loon G. Lawsonia intracellularis infection in a 12-month-old colt in Belgium. Vet. Rec. 2005;157:774–776. doi: 10.1136/vr.157.24.774. [DOI] [PubMed] [Google Scholar]
- Duhamel G.E., Wheeldon E.B. Intestinal adenomatosis in a foal. Vet. Pathol. 1982;19:447–449. doi: 10.1177/030098588201900410. [DOI] [PubMed] [Google Scholar]
- Feary D.J., Gebhart C.J., Pusterla N. Lawsonia intracellularis proliferative enteropathy in a foal. Schweiz. Arch. Tierheilkd. 2007;149:129–133. doi: 10.1024/0036-7281.149.3.129. [DOI] [PubMed] [Google Scholar]
- Frank N., Fishman C.E., Gebhart C.J. Lawsonia intracellularis proliferative enteropathy in a weanling foal. Equine Vet. J. 1998;30:549–552. doi: 10.1111/j.2042-3306.1998.tb04533.x. [DOI] [PubMed] [Google Scholar]
- Frazer M.L. Lawsonia intracellularis infection in horses: 2005-2007. J. Vet. Intern. Med. 2008;22:1243–1248. doi: 10.1111/j.1939-1676.2008.0160.x. [DOI] [PubMed] [Google Scholar]
- Frazer M. Sale price and race earnings in horses previously diagnosed by Lawsonia intracellularis. J. Vet. Intern. Med. 2011;25:677–678. [Google Scholar]
- Friedman M., Bednár V., Klimes J., Smola J., Mrlík V., Literák I. Lawsonia intracellularis in rodents from pig farms with the occurrence of porcine proliferative enteropathy. Lett. Appl. Microbiol. 2008;47:117–121. doi: 10.1111/j.1472-765X.2008.02394.x. [DOI] [PubMed] [Google Scholar]
- Gebhart C.J., Page A.E., Kelley M., Chander Y., Mapes S., White A., Horohov D.W., Pusterla N. Comparative study of serology assays for equine proliferative enteropathy. Proc. Am. Assoc. Equine Pract. 2012;58:503. [Google Scholar]
- Guedes R.M., Gebhart C.J., Winkelman N.L., Mackie-Nuss R.A., Marsteller T.A., Deen J. Comparison of different methods for diagnosis of porcine proliferative enteropathy. Can. J. Vet. Res. 2002;66:99–107. [PMC free article] [PubMed] [Google Scholar]
- Guedes R.M., Gebhart C.J., Winkelman N.L., Mackie-Nuss R.A. A comparative study of an indirect fluorescent antibody test and an immunoperoxidase monolayer assay for the diagnosis of porcine proliferative enteropathy. J. Vet. Diagn. Invest. 2002;14:420–423. doi: 10.1177/104063870201400512. [DOI] [PubMed] [Google Scholar]
- Guedes R.M., Gebhart C.J. Onset and duration of fecal shedding, cell-mediated and humoral immune responses in pigs after challenge with a pathogenic isolate or attenuated vaccine strain of Lawsonia intracellularis. Vet. Microbiol. 2003;91:35–145. doi: 10.1016/s0378-1135(02)00301-2. [DOI] [PubMed] [Google Scholar]
- Guedes R.M., Gebhart C.J. Comparison of intestinal mucosa homogenate and pure culture of the homologous Lawsonia intracellularis isolate in reproducing proliferative enteropathy in swine. Vet. Microbiol. 2003;93:159–166. doi: 10.1016/s0378-1135(03)00013-0. [DOI] [PubMed] [Google Scholar]
- Guedes R.M., Gebhart C.J. Evidence of cell-mediated immune response and specific local mucosal immunoglobulin (Ig) A production against Lawsonia intracellularis in experimentally infected swine. Can. J. Vet. Res. 2010;74:97–101. [PMC free article] [PubMed] [Google Scholar]
- Guimarães-Ladeira C.V., Palhares M.S., Oliveira J.S., Ramirez M.A., Guedes R.M. Faecal shedding and serological cross-sectional study of Lawsonia intracellularis in horses in the state of Minas Gerais, Brazil. Equine Vet. J. 2009;41:593–596. doi: 10.2746/042516409x407639. [DOI] [PubMed] [Google Scholar]
- Jacoby R.O. Transmissible ileal hyperplasia of hamsters. Am. J. Pathol. 1978;91:433–444. [PMC free article] [PubMed] [Google Scholar]
- Jasni S., McOrist S., Lawson G.H. Reproduction of proliferative enteritis in hamsters with a pure culture of porcine ileal symbiont intracellularis. Vet. Microbiol. 1994;41:1–9. doi: 10.1016/0378-1135(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Jordan D.M., Knittel J.P., Schwartz K.J., Roof M.B., Hoffman L.J. A Lawsonia intracellularis transmission study using a pure culture inoculated seeder-pig sentinel model. Vet. Microbiol. 2004;104:83–90. doi: 10.1016/j.vetmic.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Kroll J.J., Roof M.B., McOrist S. Evaluation of protective immunity in pigs following oral administration of an avirulent live vaccine of Lawsonia intracellularis. Am. J. Vet. Res. 2004;65:559–565. doi: 10.2460/ajvr.2004.65.559. [DOI] [PubMed] [Google Scholar]
- Lavoie J.P., Drolet R., Parsons D., Leguillette R., Sauvageau R., Shapiro J., Houle L., Hallé G., Gebhart C.J. Equine proliferative enteropathy: a cause of weight loss, colic, diarrhoea and hypoproteinemia in foals on three breeding farms in Canada. Equine Vet. J. 2000;32:418–425. doi: 10.2746/042516400777591110. [DOI] [PubMed] [Google Scholar]
- Lawson G.H., McOrist S., Jasni S., Mackie R.A. Intracellular bacteria of porcine proliferative enteropathy: cultivation and maintenance in vitro. J. Clin. Microbiol. 1993;31:1136–1142. doi: 10.1128/jcm.31.5.1136-1142.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson G.H., Gebhart C.J. Proliferative Enteropathy. J. Comp. Pathol. 2000;122:77–100. doi: 10.1053/jcpa.1999.0347. [DOI] [PubMed] [Google Scholar]
- McClintock S.A., Collins A.M. Lawsonia intracellularis proliferative enteropathy in a weanling foal in Australia. Aust. Vet. J. 2004;82:750–752. doi: 10.1111/j.1751-0813.2004.tb13236.x. [DOI] [PubMed] [Google Scholar]
- McGurrin M.K., Vengust M., Arroyo L.G., Baird J.D. An outbreak of Lawsonia intracellularis infection in a standardbred herd in Ontario. Can. Vet. J. 2008;48:927–930. [PMC free article] [PubMed] [Google Scholar]
- McOrist S., Lawson G.H. Possible relationship of proliferative enteritis in pigs and hamsters. Vet. Microbiol. 1987;15:293–302. doi: 10.1016/0378-1135(87)90017-4. [DOI] [PubMed] [Google Scholar]
- McOrist S., MacIntyre N., Stokes C.R., Lawson G.H. Immunocytological responses in porcine proliferative enteropathies. Infect. Immun. 1992;60:4184–4191. doi: 10.1128/iai.60.10.4184-4191.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McOrist S., Jasni S., Mackie R.A., MacIntyre N., Neef N., Lawson G.H. Reproduction of porcine proliferative enteropathy with pure cultures of ileal symbiont intracellularis. Infect. Immun. 1993;61:4286–4292. doi: 10.1128/iai.61.10.4286-4292.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McOrist S., Gebhart C.J. Genus Lawsonia. In: Garrity G.E., editor. Bergey's Manual of Systematic Bacteriology, 2nd ed. Williams and Wilkins; Baltimore, MD: 2005. [Google Scholar]
- McOrist S., Smits R.J. Field evaluation of an oral attenuated Lawsonia intracellularis vaccine for porcine proliferative enteropathy (ileitis) Vet. Rec. 2007;161:26–28. doi: 10.1136/vr.161.1.26. [DOI] [PubMed] [Google Scholar]
- Merlo J.L., Sheats M.K., Elce Y., Hunter S., Breuhaus B.A. Outbreak of Lawsonia intracellularis on a standardbred farm in North Carolina. Equine Vet. Educ. 2009;21:179–182. [Google Scholar]
- Nathues H., Holthaus K., Beilage E. Quantification of Lawsonia intracellularis in porcine faeces by real-time PCR. J. Appl. Microbiol. 2009;107:2009–2016. doi: 10.1111/j.1365-2672.2009.04389.x. [DOI] [PubMed] [Google Scholar]
- Nogradi N., Slovis N.M., Gebhart C.J., Wolfsdorf K.E., McCracken J.L., Scoggin C.F., Kass P.H., Mapes S.M., Toth B., Lundquist M.L., Pusterla N. Evaluation of the field efficacy of an avirulent live Lawsonia intracellularis vaccine in foals. Vet. J. 2012;192:511–513. doi: 10.1016/j.tvjl.2011.05.026. [DOI] [PubMed] [Google Scholar]
- Page A.E., Loynachan A.T., Bryant U., Stills H.F., Adams A.A., Gebhart C.J., Pusterla N., Horohov D.W. Characterization of the interferon gamma response to Lawsonia intracellularis using an equine proliferative enteropathy challenge (EPE) model. Vet. Immunol. Immunopathol. 2011;143:55–65. doi: 10.1016/j.vetimm.2011.06.023. [DOI] [PubMed] [Google Scholar]
- Page A.E., Fallon L.H., Bryant U.K., Horohov D.W., Luna T.W., Marsh P.S., Slovis N.M., Sprayberry K.A., Loynachan A.T. Acute deterioration and death with necrotizing enteritis associated with Lawsonia intracellularis in 4 weanling horses. J. Vet. Int. Med. 2012;26:1476–1480. doi: 10.1111/j.1939-1676.2012.01002.x. [DOI] [PubMed] [Google Scholar]
- Pusterla N., Mapes S., Rejmanek D., Gebhart C. Detection of Lawsonia intracellularis by real-time PCR in the feces of free-living animals from equine farms with documented occurrence of equine proliferative enteropathy. J. Wildl. Dis. 2008;44:992–998. doi: 10.7589/0090-3558-44.4.992. [DOI] [PubMed] [Google Scholar]
- Pusterla N., Gebhart C. Equine proliferative enteropathy caused by Lawsonia intracellularis. Eq. Vet. Edu. 2009;21:415–419. doi: 10.2746/095777309X453119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusterla N., Jackson R., Wilson R., Collier J., Mapes S., Gebhart C. Temporal detection of Lawsonia intracellularis using serology and real-time PCR in Thoroughbred horses residing on a farm endemic for equine proliferative enteropathy. Vet. Microbiol. 2009;136:173–176. doi: 10.1016/j.vetmic.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Pusterla N., Hilton H., Wattanaphansak S., Collier J., Mapes S.M., Stenbom R.M., Gebhart C. Evaluation of the humoral immune response and fecal shedding in weanling foals after oral and intra-rectal administration of an avirulent live vaccine of Lawsonia intracellularis. Vet. J. 2009;182:458–462. doi: 10.1016/j.tvjl.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Pusterla N., Wattanaphansak S., Mapes S., Collier J., Hill J., DiFrancesco M., Gebhart C. Oral infection of weanling foals with an equine isolate of Lawsonia intracellularis, agent of equine proliferative enteropathy. J. Vet. Intern. Med. 2010;24:622–627. doi: 10.1111/j.1939-1676.2010.0482.x. [DOI] [PubMed] [Google Scholar]
- Pusterla N., Mapes S., Johnson C., Slovis N., Page A., Gebhart C. Comparison of feces versus rectal swabs for the molecular detection of Lawsonia intracellularis in foals with equine proliferative enteropathy. J. Vet. Diagn. Invest. 2010;22:741–744. doi: 10.1177/104063871002200513. [DOI] [PubMed] [Google Scholar]
- Pusterla N., Mapes S., Gebhart C. Further investigation of exposure to Lawsonia intracellularis in wild and feral animals captured on horse properties with equine proliferative enteropathy. Vet. J. 2012;194:253–255. doi: 10.1016/j.tvjl.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Pusterla N., Sanchez-Migallon, Guzman D., Vannucci F.A., Mapes S., White A., DiFrancesco M., Gebhart C. Transmission of Lawsonia intracellularis to weanling foals using feces from experimentally infected rabbits. Vet. J. 2012;195:241–243. doi: 10.1016/j.tvjl.2012.05.028. [DOI] [PubMed] [Google Scholar]
- Pusterla N., Vannucci F.A., Mapes M.S., Nogradi N., Collier J.R., Hill J.A., DiFrancesco M., White A.M., Akana N.K., Simonek G., Gebhart C.J. Efficacy of an avirulent live vaccine against Lawsonia intracellularis in the prevention of proliferative enteropathy in experimentally infected weanling foals. Am. J. Vet. Res. 2012;73:741–746. doi: 10.2460/ajvr.73.5.741. [DOI] [PubMed] [Google Scholar]
- Pusterla N., Mapes S., Gebhart C. Lawsonia intracellularis-specific interferon γ gene expression by peripheral blood mononuclear cells in vaccinated and naturally infected foals. Vet. J. 2012;192:249–251. doi: 10.1016/j.tvjl.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Richter B., Ladinig A., Nedorost N., Weissenböck H. A TaqMan quantitative polymerase chain reaction assay for the detection of Lawsonia intracellularis in fecal and tissue samples from pigs. J. Vet. Diagn. Invest. 2010;22:70–73. doi: 10.1177/104063871002200112. [DOI] [PubMed] [Google Scholar]
- Sampieri F., Hinchcliff K.W., Toribio R.E. Tetracycline therapy of Lawsonia intracellularis enteropathy in foals. Equine Vet. J. 2006;38:89–92. doi: 10.2746/042516406775374270. [DOI] [PubMed] [Google Scholar]
- Sampieri F., Vannucci F.A., Allen A.L., Pusterla N., Antonopoulos A.J., Ball K.R., Thompson J., Dowling P.M., Hamilton D.L., Gebhart C.J. Species-specificity of equine and porcine L. intracellularis isolates in laboratory animals. Can. J. Vet. Res. 2013 in press. [PMC free article] [PubMed] [Google Scholar]
- Sampieri F., Allen A.L., Pusterla N., Vannucci F.A., Anatonopoulos A.J., Hamilton D.L., Gebhart C.J. The rabbit as an infection model for equine proliferative enteropathy. Can. J. Vet. Res. 2013 in press. [PMC free article] [PubMed] [Google Scholar]
- Schumacher J., Schumacher J., Rolsma M., Brock K.V., Gebhart C.J. Surgical and medical treatment of an Arabian filly with proliferative enteropathy caused by Lawsonia intracellularis. J. Vet. Intern. Med. 2000;14:630–632. [PubMed] [Google Scholar]
- Shimizu C., Shibahara T., Takai S., Kasuya K., Chikuba T., Murakoshi N., Kobayashi H., Kubo M. Lawsonia intracellularis and virulent Rhodococcus equi infection in a thoroughbred colt. J. Comp. Pathol. 2010;143:303–308. doi: 10.1016/j.jcpa.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Smith D.G., Mitchell S.C., Nash T., Rhind S. Gamma interferon influences intestinal epithelial hyperplasia caused by Lawsonia intracellularis infection in mice. Infect. Immun. 2000;68:6737–6743. doi: 10.1128/iai.68.12.6737-6743.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Wollenberg L., Butler C.M., Houwers D.J., de Grootv M.W., Panhuijzen H., van Maanen C., van Oldruitenborgh-Oosterbaan M.M. Lawsonia intracellularis-associated proliferative enteritis in weanling foals in the Netherlands. Tijdschr. Diergeneeskd. 2011;136:565–570. [PubMed] [Google Scholar]
- Vannucci F.A., Foster D.N., Gebhart C.J. Comparative transcriptional analysis of homologous pathogenic and non-pathogenic Lawsonia intracellularis isolates in infected porcine cells. PLoS One. 2012;7:e46708. doi: 10.1371/journal.pone.0046708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci F.A., Pusterla N., Mapes S.M., Gebhart C. Evidence of host adaptation in Lawsonia intracellularis infections. Vet. Res. 2012;43:53–65. doi: 10.1186/1297-9716-43-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci F.A., Beckler D., Pusterla N., Mapes S.M., Gebhart C.J. Attenuation of virulence of Lawsonia intracellularis after in vitro passages and its effects on the experimental reproduction of porcine proliferative enteropathy. Vet. Microbiol. 2013;162:265–269. doi: 10.1016/j.vetmic.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Vannucci F.A., Kelley M.R., Gebhart C.J. Comparative genome sequencing identifies a prophage-associated genomic island linked to host adaptation of Lawsonia intracellularis infections. Vet. Res. 2013 doi: 10.1186/1297-9716-44-49. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattanaphansak S., Asawakarn T., Gebhart C.J., Deen J. Development and validation of an enzyme-linked immunosorbent assay for the diagnosis of porcine proliferative enteropathy. J. Vet. Diagn. Invest. 2008;20:170–177. doi: 10.1177/104063870802000205. [DOI] [PubMed] [Google Scholar]
- Williams N.M., Harrison L.R., Gebhart C.J. Proliferative enteropathy in a foal caused by Lawsonia intracellularis-like bacterium. J. Vet. Diagn. Invest. 1996;8:254–256. doi: 10.1177/104063879600800220. [DOI] [PubMed] [Google Scholar]
- Wong D.M., Alcott C.J., Sponseller B.A., Young J.L., Sponseller B.T. Impaired intestinal absorption of glucose in 4 foals with Lawsonia intracellularis infection. J. Vet. Intern. Med. 2009;23:940–944. doi: 10.1111/j.1939-1676.2009.0334.x. [DOI] [PubMed] [Google Scholar]
- Wuersch K., Huessy D., Koch C., Oevermann A. Lawsonia intracellularis proliferative enteropathy in a filly. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2006;53:17–21. doi: 10.1111/j.1439-0442.2006.00776.x. [DOI] [PubMed] [Google Scholar]


