Abstract
Background
Alzheimer's disease (AD), the most common disease causing dementia, is linked to increased mortality. However, the effect of antipsychotic use on specific causes of mortality has not yet been investigated thoroughly.
Methods
Utilizing the Danish nationwide registers, we defined a cohort of patients diagnosed with AD. Utilizing separate Cox regressions for specific causes of mortality, we investigated the effects of cumulative antipsychotic dosage after diagnosis and current antipsychotic exposure in the time period 2000–2011.
Results
In total, 45,894 patients were followed for 3,803,996 person-years. A total of 6129 cardiovascular related deaths, 2088 cancer related deaths, 1620 infection related deaths, and 28 intentional self-harm related deaths are presented. Current antipsychotic exposure increased mortality rate with HR between 1.92 and 2.31 for cardiovascular, cancer, and infection related death. Cumulative antipsychotic dosages were most commonly associated with increased rates of mortality for cardiovascular and infection as cause of death, whereas the associations were less clear with cancer and intentional self-harm as cause of death.
Conclusions
We showed that cumulative antipsychotic drug dosages increased mortality rates for cardiovascular and infection as cause of death. These findings highlight the need for further investigations of long-term effects of treatment and of possible sub-groups who could benefit from treatment.
Keywords: Dementia, Psychopharmacology, Mortality, Epidemiology
1. Introduction
Alzheimer's disease (AD) has been linked to increased mortality in several studies [1]. Autopsy studies in dementia have shown infection and cardiovascular events as the most common causes of death, with cancer being a less common cause [2], [3]. Some data suggests that cancer is less common in patients diagnosed with or suspected of suffering from AD [4]. Suicide as cause of death in AD is less investigated, as well as passive forms of self-harm, although small studies [5], [6] have shown both to be more frequent in patients with AD.
Current antipsychotic treatment in patients diagnosed with AD increases all-cause mortality, as well as death from specific causes, e.g. cerebrovascular events in which an increased risk has been demonstrated using evidence from randomized controlled trials [7], [8]. Nevertheless, other studies have shown benefits from short-term antipsychotic treatment in reducing Behavioural and Psychological Symptoms in Dementia (BPSD), although studies with long follow-up have been sparse [9]. We have previously shown that lifetime cumulative antipsychotic drug dosages and all-cause mortality are associated [10], suggesting that the negative effects on mortality by antipsychotics could not be outweighed by the effects of antipsychotics on BPSD. So far, no studies have investigated the effects of cumulative antipsychotic treatment in patients diagnosed with AD for specific causes of death. As cardiovascular events and infection are the two most common causes of death in this population, we chose to include these as outcome measures. The choice to include cancer was undertaken, as antipsychotics have been proposed to increase the risk of cancer development, and as cancer is a common cause of death in elderly patients. Lastly, we chose to include intentional self-harm, as this outcome is very rare, and would most likely only be investigated utilizing larger datasets, as ours.
In this study, we examine the effect of cumulative antipsychotic drug dosages, as well as the effect of current antipsychotic exposure, on the rate of different causes of death in patients diagnosed with AD.
2. Methods
2.1. Design
A nationwide, population-based, retrospective cohort study of specific cause mortality (cardiovascular, cancer, infection, and intentional self-harm) in patients diagnosed with dementia in AD.
2.2. Sample
We formed a cohort of newly diagnosed patients with AD, which we defined as either receiving an ICD-10 F00x. (Dementia in Alzheimer's disease) or a G30x. (Alzheimer's disease) diagnosis or picking up a prescription for anti-dementia drugs (ATC N06D), from January 1, 2000 to December 31, 2011 from the total Danish population. Persons previously diagnosed with an ICD-8 dementia diagnosis in the period from January 1, 1980 throughout 1993, or an ICD-10 diagnosis of dementia in the period from January 1, 1994 throughout 1999 were excluded from the analysis, as well as patients picking up a prescription for anti-dementia drugs in the period January 1, 1998 to December 31, 1999.
2.3. Measures
2.3.1. Medication
All medication variables were coded as time-dependent variables, with patients being coded as non-exposed until first prescription.
Antipsychotics were defined as ATC N05A, excluding lithium (ATC N05AN).
The current exposure variable was based on the use of antipsychotics over the previous year and grouped as present or absent, with index defined as a prescription for an antipsychotic drug. The cumulative antipsychotic dosages from dementia diagnosis until end of study for each participant were calculated and divided into 5 groups:
-
•
baseline: no antipsychotic treatment at all;
-
•
more than zero daily defined dosages (DDD) < 90;
-
•
≥ 90 DDDs < 365;
-
•
≥ 365 DDDs < 730;
-
•
≥ 730 DDDs.
Because of the definitions, no patients currently exposed to antipsychotics could be in the baseline cumulative exposure group. The groups were defined arbitrarily, but the definition has been utilized before in a similar study [10].
2.4. Co-variables
2.4.1. Severity of AD
The current Danish registers do not include data on severity of AD, and cognitive function. To compensate for these deficits, we used the following variables as proxy markers of disease severity: number of psychiatric bed days, number of psychiatric outpatient contacts, and accommodation status after diagnosis. All markers were examined after incident dementia diagnosis (as defined by either the ICD-10 diagnosis or the first prescription of anti-dementia drug).
Accommodation status was dichotomized as a time-dependent variable (never previously lived in an institution or lived in an institution at some point in time after the diagnosis).
2.4.2. Psychiatric co-morbid disorder
We defined a psychiatric co-morbidity score ranging from zero to five and determined by the aggregated diagnosis status in the following categories: psychosis, affective disorders, substance misuse, other psychiatric diagnosis, and intentional self-harm. The score was computed for each patient, and could increase over time, if more diagnoses were given. The score was cumulative for all patients since the initiation of the Danish Psychiatric Central Research Registry in 1969. The score has previously been utilized in a similar study [10].
2.4.3. Somatic co-morbid disease
We divided somatic diseases into the following groups: cardiovascular disease, cancer, infection, diabetes, epilepsy, lower respiratory disease, and other somatic diseases. For the somatic co-morbid score, one point was added for a diagnosis in each of the groups unless the diagnosis occurred before the age of 51 years for males and 56 years for females, where two points were added due to an increased risk associated with early onset of disease. The score was computed for each patient, and could increase over time, if more diagnoses were given. The score was cumulative for all patients since the initiation of the Danish Nation Patient Registry in 1977. The score has previously been utilized in a similar study [10].
2.4.4. Cardiovascular risk factors
The current Danish registers do not include data on blood pressure or blood test results, such as lipid and glucose levels. Besides, general practitioners examining and treating the majority of patients diagnosed with hypertension, increased lipids, or type II diabetes do not register diagnoses in the Danish registers. To make up for this information gap, we used the following proxy measures of arterial hypertension, increased cholesterol, and diabetes: data on the prescription of antihypertensive drugs (ATC C02), drugs used to lower lipids (ATC C10), and drugs for treatment of diabetes (blood glucose lowering drugs (excl. insulins): ATC A10B, A10XA; insulins: ATC A10A), in addition to the actual diagnoses (see above under somatic co-morbid disease). All medication variables were coded as time-dependent variables, with patients being coded as non-exposed until first prescription.
2.5. Statistical analysis
Cox regressions with event defined as specific cause of mortality were conducted using cumulative antipsychotic dosage and current antipsychotic exposure as explanatory variables, and adjusting for variables defined above. All variables were coded as time-dependent.
In order to investigate the effects of an association between cumulative exposure and current exposure, secondary analyses were conducted for the different specific causes of death listed above, excluding current exposure as an explanatory variable.
Statistical analyses were performed with Stata 13 at the Statistics Denmark server with remote access.
3. Results
3.1. Demography
In total, 45,894 patients (17,082 males and 28,812 females) were followed for 3,803,996 person-years, presenting 27,894 deaths (10,818 males and 17,076 females) in the study population. Patients were mainly treated with second generation antipsychotic drug (12,216 patients, 4850 male, 7366 female), with fewer treated with typical antipsychotic drugs (4760 patients, 1943 male and 2817 female). The most commonly prescribed antipsychotic drugs were risperidone (40% of all treated), quetiapine (36% of all treated), olanzapine (21% of all treated), haloperidole (15% of all treated), zuclopenthixole (6% of all treated), and chlorprothixene (4% of all treated).
3.1.1. Cardiovascular events as cause of death
A total of 6129 cardiovascular related deaths (13.6% of all deaths) (2391 males and 3738 females) were presented in the study population.
In total, 2100 patients (884 males and 1216 females) with cardiovascular related deaths were exposed to antipsychotics at some point. The mean age at death in the exposed group was 84.64 years (SD = 6.63), [males 83.22 years (SD = 6.83); females 85.68 years (SD = 6.29)].
3.2. Antipsychotic medication
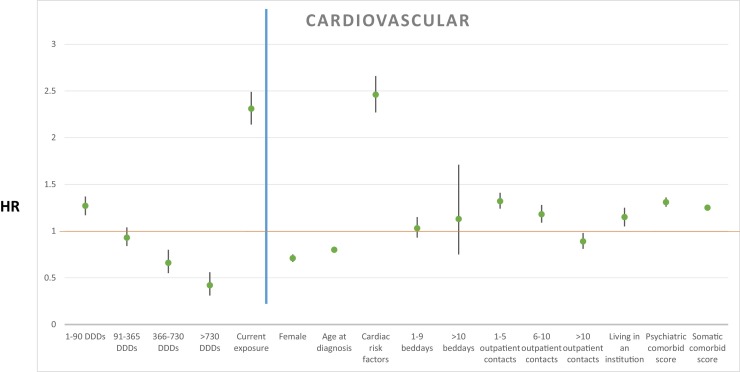
The cumulative dosages of antipsychotic treatment after AD diagnosis were associated with rate of mortality as follows: more than 0 DDDs < 90: HR 1.27, 95% CI (1.17–1.37), P < 0.001; ≥ 90 DDDs < 365: HR 0.93, 95% CI (0.84–1.04), P = 0.203; ≥ 365 DDDs < 730: HR 0.66, 95% CI (0.55–0.80), P < 0.001; ≥ 730 DDDs: HR 0.42, 95% CI (0.31–0.56), P < 0.001. Current antipsychotic treatment exposure increased mortality rate with HR 2.31, 95% CI (2.14–2.49), P < 0.001. Results from the primary analysis are depicted in Fig. 1 . Secondary analyses without current antipsychotic exposure as explanatory variable are shown in Table 1 .
Fig. 1.
Hazard ratio for explanatory variables with cardiovascular event as cause of death shown left to the blue line, and covariates shown right to the blue line. A green point illustrates the specific HR, with 95% confidence intervals illustrated with black lines.
Table 1.
Cox regression analyses for all investigated causes of death, utilizing explanatory variables as in the primary analysis, excluding current antipsychotic exposure.
| Cardiovascular | ||||
|---|---|---|---|---|
| HR | CI (95%) | P | ||
| Antipsychotic medication | ||||
| Cumulative exposure | ||||
| 0 DDDs | Reference | |||
| 0 > DDDs ≥ 90 | 1.95 | 1.83 | 2.09 | < 0.001 |
| 90 > DDDs ≥ 365 | 1.68 | 1.53 | 1.84 | < 0.001 |
| 365 > DDDs ≥ 730 | 1.24 | 1.04 | 1.48 | < 0.05 |
| 730 > DDDs | 0.89 | 0.67 | 1.18 | 0.429 |
| Explanatory variables | ||||
| Demographic factors | ||||
| Female | 0.69 | 0.65 | 0.73 | < 0.001 |
| Age at diagnosis | 0.80 | 0.79 | 0.81 | < 0.001 |
| Other medication | ||||
| Cardiac risk factors | 2.47 | 2.29 | 2.68 | < 0.001 |
| Proxy markers of severity | ||||
| Psychiatric bed days = 0 | Reference | |||
| Psychiatric bed days 1–9 | 1.01 | 0.91 | 1.13 | 0.836 |
| Psychiatric bed days ≥ 10 | 1.06 | 0.70 | 1.60 | 0.783 |
| Psychiatric outpatient contacts = 0 | Reference | |||
| Psychiatric outpatient contacts: 1–5 | 1.35 | 1.27 | 1.43 | < 0.001 |
| Psychiatric outpatient contacts: 6–10 | 1.20 | 1.10 | 1.30 | < 0.001 |
| Psychiatric outpatient contacts > 10 | 0.88 | 0.80 | 0.97 | < 0.01 |
| Living in an institution | 1.14 | 1.04 | 1.24 | < 0.01 |
| Co-morbidity | ||||
| Psychiatric co-morbid score | 1.32 | 1.27 | 1.37 | < 0.001 |
| Somatic co-morbid score | 1.26 | 1.23 | 1.29 | < 0.001 |
| Cancer | ||||
|---|---|---|---|---|
| HR | CI (95%) | P | ||
| Antipsychotic medication | ||||
| Cumulative exposure | ||||
| 0 DDDs | Reference | |||
| 0 > DDDs ≥ 90 | 1.85 | 1.65 | 2.08 | < 0.001 |
| 90 > DDDs ≥ 365 | 1.19 | 1.00 | 1.43 | 0.057 |
| 365 > DDDs ≥ 730 | 0.97 | 0.69 | 1.37 | 0.878 |
| 730 > DDDs | 0.46 | 0.25 | 0.85 | 0.013 |
| Explanatory variables | ||||
| Demographic factors | ||||
| Female | 0.69 | 0.63 | 0.76 | < 0.001 |
| Age at diagnosis | 0.79 | 0.78 | 0.81 | < 0.001 |
| Other medication | ||||
| Cardiac risk factors | 0.53 | 0.48 | 0.59 | < 0.001 |
| Proxy markers of severity | ||||
| Psychiatric bed days = 0 | Reference | |||
| Psychiatric bed days 1–9 | 0.92 | 0.75 | 1.12 | 0.401 |
| Psychiatric bed days ≥ 10 | 0.68 | 0.28 | 1.64 | 0.393 |
| Psychiatric outpatient contacts = 0 | Reference | |||
| Psychiatric outpatient contacts: 1–5 | 1.42 | 1.28 | 1.58 | < 0.001 |
| Psychiatric outpatient contacts: 6–10 | 1.21 | 1.06 | 1.39 | < 0.01 |
| Psychiatric outpatient contacts > 10 | 0.63 | 0.52 | 0.76 | < 0.001 |
| Living in an institution | 0.94 | 0.79 | 1.11 | 0.454 |
| Co-morbidity | ||||
| Psychiatric co-morbid score | 1.34 | 1.26 | 1.42 | < 0.001 |
| Somatic co-morbid score | 1.84 | 1.79 | 1.90 | < 0.001 |
| Infection | ||||
|---|---|---|---|---|
| HR | CI (95%) | P | ||
| Antipsychotic medication | ||||
| Cumulative exposure | ||||
| 0 DDDs | Reference | |||
| 0 > DDDs ≥ 90 | 2.37 | 2.09 | 2.68 | < 0.001 |
| 90 > DDDs ≥ 365 | 2.05 | 1.73 | 2.44 | < 0.001 |
| 365 > DDDs ≥ 730 | 1.77 | 1.31 | 2.38 | < 0.001 |
| 730 > DDDs | 1.02 | 0.62 | 1.67 | 0.946 |
| Explanatory variables | ||||
| Demographic factors | ||||
| Female | 0.54 | 0.49 | 0.60 | < 0.001 |
| Age at diagnosis | 0.78 | 0.77 | 0.80 | < 0.001 |
| Other medication | ||||
| Cardiac risk factors | 0.80 | 0.70 | 0.91 | 0.001 |
| Proxy markers of severity | ||||
| Psychiatric bed days = 0 | Reference | |||
| Psychiatric bed days 1–9 | 1.12 | 0.93 | 1.36 | 0.233 |
| Psychiatric bed days ≥ 10 | 1.38 | 0.74 | 2.59 | 0.314 |
| Psychiatric outpatient contacts = 0 | Reference | |||
| Psychiatric outpatient contacts: 1–5 | 1.17 | 1.03 | 1.32 | 0.013 |
| Psychiatric outpatient contacts: 6–10 | 1.07 | 0.92 | 1.25 | 0.382 |
| Psychiatric outpatient contacts > 10 | 0.72 | 0.60 | 0.86 | < 0.001 |
| Living in an institution | 1.17 | 1.00 | 1.38 | 0.053 |
| Co-morbidity | ||||
| Psychiatric co-morbid score | 1.32 | 1.23 | 1.42 | < 0.001 |
| Somatic co-morbid score | 1.54 | 1.48 | 1.60 | < 0.001 |
| Intentional self-harm | ||||
|---|---|---|---|---|
| HR | CI (95%) | P | ||
| Antipsychotic medication | ||||
| Cumulative exposure | ||||
| 0 DDDs | Reference | |||
| 0 > DDDs ≥ 90 | 0.76 | 0.24 | 2.41 | 0.640 |
| 90 > DDDs ≥ 365 | 0.24 | 0.03 | 2.02 | 0.189 |
| 365 > DDDs ≥ 730 | 0.00 | 0.00 | 1.000 | |
| 730 > DDDs | 0.87 | 0.09 | 8.57 | 0.902 |
| Explanatory variables | ||||
| Demographic factors | ||||
| Female | 0.49 | 0.23 | 1.04 | 0.063 |
| Age at diagnosis | 0.82 | 0.74 | 0.91 | < 0.001 |
| Other medication | ||||
| Cardiac risk factors | 1.92 | 0.71 | 5.18 | 0.199 |
| Proxy markers of severity | ||||
| Psychiatric bed days = 0 | Reference | |||
| Psychiatric bed days 1–9 | 2.70 | 0.79 | 9.23 | 0.113 |
| Psychiatric bed days ≥ 10 | 10.48 | 1.12 | 98.42 | < 0.05 |
| Psychiatric outpatient contacts = 0 | Reference | |||
| Psychiatric outpatient contacts: 1–5 | 1.51 | 0.58 | 3.90 | 0.400 |
| Psychiatric outpatient contacts: 6–10 | 1.70 | 0.54 | 5.35 | 0.368 |
| Psychiatric outpatient contacts > 10 | 0.53 | 0.10 | 2.96 | 0.471 |
| Living in an institution | 1.09 | 0.25 | 4.82 | 0.909 |
| Co-morbidity | ||||
| Psychiatric co-morbid score | 2.94 | 2.02 | 4.27 | < 0.001 |
| Somatic co-morbid score | 0.91 | 0.66 | 1.25 | 0.552 |
HR: hazard ratio; CI: confidence interval; DDD: daily defined dosage.
3.2.1. Covariate variables
Female gender (HR 0.71, 95% CI [0.67–0.75], P < 0.001), as well as older age at dementia diagnosis (HR 0.80, 95% CI [0.79–0.80], P < 0.001) decreased rate of mortality.
3.2.2. Other medication
Prescription of antihypertensive drugs, lipid-lowering drugs, and oral antidiabetics or insulin increased the rate of mortality: HR 2.46, 95% CI [2.27–2.66], P < 0.001.
3.2.3. Severity of AD
Mortality increased with increasing severity of AD as measured by some of the proxy markers. The number of bed days hospitalized was not associated with mortality rate, with one to nine bed days resulting in a HR of 1.03 95% CI [0.93–1.15], P < 0.551, and more than nine bed days resulting in a HR of 1.13, 95% CI [0.75–1.71], P = 0.554, as compared to never having been admitted. The rate of mortality increased with lower number of outpatient contacts as compared to no outpatient contacts: one to five outpatient contacts resulted in a HR of 1.32, 95% CI (1.24–1.41), P < 0.001; six to ten outpatient contacts: HR 1.18, 95% CI (1.09–1.341), P < 0.001; and more than ten outpatient contacts: HR 0.89, 95% CI (0.81–0.98), P < 0.05. Living in an institution after being diagnosed with dementia increased the mortality rate (HR 1.15, 95% CI [1.05–1.25], P < 0.005).
3.2.4. Co-morbid psychiatric and somatic disorders
The presence of previous or concurrent psychiatric disorders increased the rate of mortality: HR 1.31, 95% CI (1.26–1.36), P < 0.001, as well as the presence of diagnosed somatic diseases: HR 1.25, 95% CI (1.22–1.28), P < 0.001.
3.3. Cancer as cause of death
A total of 2088 cancer related deaths (4.5% of all deaths) (923 males and 1165 females) were presented in the study population.
In total, 626 (291 males and 335 females) patients with cancer related deaths had been exposed to antipsychotic medication. The mean age at death in the exposed group was 82.00 years (SD = 6.87), [males 81.51 years (SD = 6.43); females 82.43 years (SD = 7.21)].
3.3.1. Antipsychotic medication
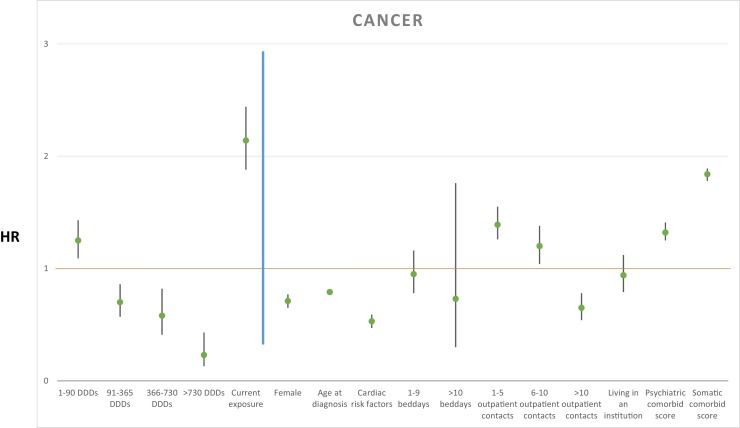
The cumulative dosages of antipsychotic treatment after AD diagnosis were associated with rate of mortality as follows: more than 0 DDDs < 90: HR 1.25, 95% CI (1.09–1.43), P < 0.005; ≥ 90 DDDs < 365: HR 0.70, 95% CI (0.57–0.86), P < 0.005; ≥ 365 DDDs < 730: HR 0.58, 95% CI (0.41–0.82), P < 0.005; ≥ 730 DDDs: HR 0.23, 95% CI(0.13–0.43), P < 0.001. Current antipsychotic treatment exposure increased mortality rate with HR 1.90, 95% CI (2.14–2.44), P < 0.001. Results from the primary analysis are depicted in Fig. 2 . Secondary analyses without current antipsychotic exposure as explanatory variable are shown in Table 1.
Fig. 2.
Hazard ratio for explanatory variables with cancer as cause of death shown left to the blue line, and covariates shown right to the blue line. A green point illustrates the specific HR, with 95% confidence intervals illustrated with black lines.
3.4. Covariate variables
Female gender (HR 0.71, 95% CI [0.65–0.77], P < 0.001), as well as older age at dementia diagnosis (HR 0.79, 95% CI [0.78–0.80], P < 0.001) decreased rate of mortality.
3.5. Other medication
Prescription of antihypertensive drugs, lipid-lowering drugs, and oral antidiabetics or insulin decreased the rate of mortality: HR 0.53, 95% CI (0.47–0.59), P < 0.001.
3.6. Severity of AD
Mortality from cancer was associated to some proxy measures of AD severity. The number of bed days hospitalized did not increase the mortality rate, with one to nine bed days resulting in a HR of 0.95, 95% CI (0.78–1.16), P = 0.610, and more than nine bed days resulting in a HR of 0.73, 95% CI (0.30–1.76), P = 0.483, as compared to never having been admitted. The rate of mortality increased with any number of outpatient contacts as compared to no outpatient contacts, with one to five outpatient contacts resulting in a HR of 1.39, 95% CI [1.26–1.55], P < 0.001; six to ten outpatient contacts: HR 1.20, 95% CI (1.04–1.38), P < 0.05; more than ten outpatient contacts: HR 0.65, 95% CI (0.54–0.78), P < 0.001. Living in an institution was not associated to rate of mortality (HR 0.94, 95% CI [0.79–1.12], P = 0.496).
3.7. Co-morbid psychiatric and somatic disorders
The presence of previous or concurrent psychiatric disorders increased the rate of mortality: HR 1.32, 95% CI (1.25–1.41), P < 0.001, as well as the presence of diagnosed somatic diseases: HR 1.84, 95% CI (1.78–1.89), P < 0.001.
3.7.1. Infection as cause of death
A total of 1620 infection related deaths (3.5% of all deaths) (743 males and 877 females) were presented in the study population.
Among these, 647 patients (325 males and 322 females) of this population had been exposed to antipsychotic medication. The mean age at death in the exposed group was 83.79 years (SD = 6.87), [males 82.94 years (SD = 6.86); females 84.65 years (SD = 6.78)].
3.8. Antipsychotic medication
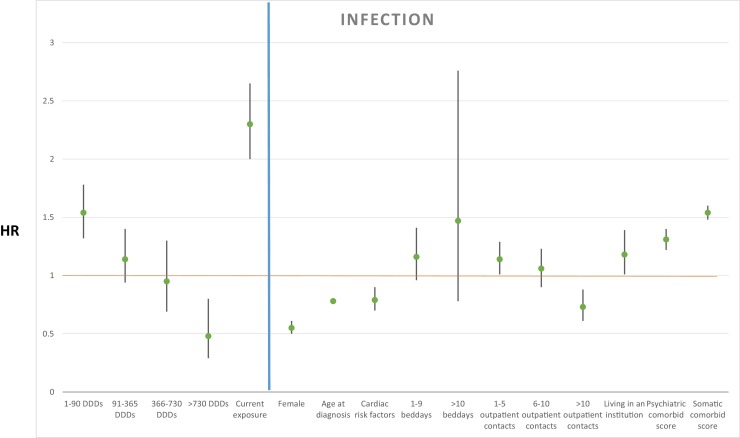
Regarding death from infection, we found that cumulative dosages of antipsychotic treatment after diagnosis of AD compared to no exposure were associated to the rate of mortality as follows: more than 0 DDDs < 90: HR 1.54, 95% CI (1.32–1.78), P < 0.001; ≥ 90 DDDs < 365: HR 1.14, 95% CI (0.94–1.40), P = 0.191; ≥ 365 DDDs < 730: HR 0.95, 95% CI (0.69–1.30), P = 0.754; ≥ 730 DDDs: HR 0.48, 95% CI (0.29–0.80), P < 0.01. Current antipsychotic treatment exposure increased mortality rate with HR 2.30, 95% CI (2.00–2.65), P < 0.001. Results from the primary analysis are depicted in Fig. 3 . A secondary analysis without current antipsychotic exposure as explanatory variable is shown in Table 1.
Fig. 3.
Hazard ratio for explanatory variables with infection as cause of death shown left to the blue line, and covariates shown right to the blue line. A green point illustrates the specific HR, with 95% confidence intervals illustrated with black lines.
3.9. Covariate variables
Female gender (HR 0.55, 95% CI [0.50–0.61], P < 0.001), as well as older age at dementia diagnosis (HR 0.78, 95% CI [0.77–0.79], P < 0.001) decreased rate of mortality.
3.10. Other medication
Prescription of antihypertensive drugs, lipid-lowering drugs, and oral antidiabetics or insulin decreased the rate of mortality: HR 0.79, 95% CI(0.70–0.90), P < 0.001.
3.11. Severity of AD
Mortality was associated with increasing severity of AD as measured by some of the proxy markers. The number of bed days hospitalized did not increase the mortality rate significantly, with one to nine bed days resulting in a HR of 1.16, 95% CI (0.96–1.41), P = 0.134, as well as more than nine bed days resulting in a HR of 1.47, 95% CI (0.78–2.76), P = 0.230, as compared to never having been admitted. The rate of mortality increased with low number of outpatient contacts as compared to no outpatient contacts, with one to five outpatient contacts resulting in a HR of 1.14, 95% CI (1.01–1.29), P < 0.05; six to ten outpatient contacts: HR 1.06, 95% CI (0.90–1.23), P = 0.493; more than ten outpatient contacts: HR 0.73, 95% CI (0.61–0.88), P < 0.005. Living in an institution increased the mortality rate (HR 1.18, 95% CI [1.01–1.39], P < 0.05).
3.12. Co-morbid psychiatric and somatic disorders
The presence of previous or concurrent psychiatric disorders increased the rate of mortality: HR 1.31, 95% CI (1.22–1.40), P < 0.001, as well as the presence of diagnosed somatic diseases: HR 1.54, 95% CI (1.48–1.60), P < 0.001.
3.12.1. Intentional self-harm as cause of death
Twenty-eight intentional self-harm related deaths (0.06% of all deaths) were presented in the study population, and six patients with intentional self-harm related deaths were exposed to antipsychotic medication. The mean age at death in the exposed group was 71.64 years (SD = 11.28). Following the rules of Statistics Denmark, we do not report gender distribution due to the very low numbers encountered.
3.13. Antipsychotic medication
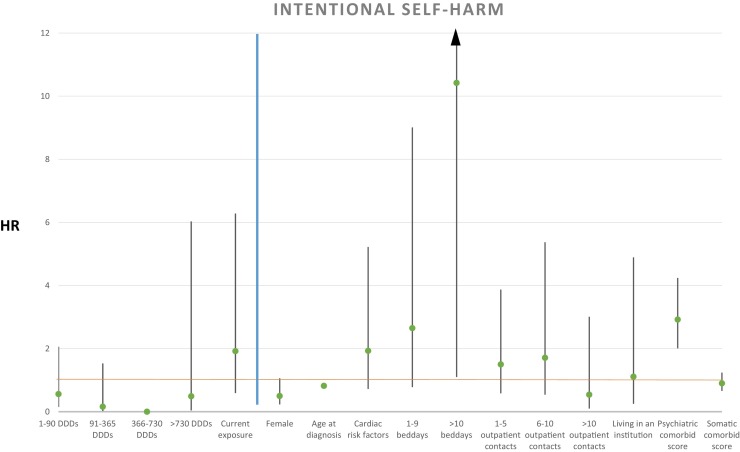
Regarding death from intentional self-harm, we did not find that cumulative dosages of antipsychotic treatment after AD diagnosis compared to no exposure were associated to the rate of mortality: more than 0 DDDs < 90: HR 0.56, 95% CI (0.23–2.06), P = 0.383; ≥ 90 DDDs < 365: HR 0.16, 95% CI (0.02–1.53), P = 0.111; ≥ 730 DDDs: HR 0.49, 95% CI (0.04–6.03), P = 0.576. Current antipsychotic treatment exposure did not increase the mortality rate significantly (HR 1.92, 95% CI [0.59–6.28], P = 0.279) as compared to no exposure. Results from the primary analysis are depicted in Fig. 4 . The secondary analysis without current antipsychotic exposure as explanatory variable is shown in Table 1.
Fig. 4.
Hazard ratio for explanatory variables with intentional self-harm as cause of death shown left to the blue line, and covariates shown right to the blue line. A green point illustrates the specific HR, with 95% confidence intervals illustrated with black lines.
3.14. Other medication
Prescription of antihypertensive drugs, lipid-lowering drugs, and oral antidiabetics or insulin was not associated to rate of mortality: HR 1.93, 95% CI (0.72–5.22), P = 0.193.
3.15. Severity of AD
One to nine bed days resulted in a HR of 2.65, 95% CI (0.78–9.01) P = 0.120, and more than nine bed days resulted in a HR of 10.42, 95% CI (1.10–98.35), P < 0.05, as compared to not being admitted. The rate of mortality was not increased with any number of outpatient contacts as compared to no outpatient contacts, with one to five outpatient contacts resulting in a HR of 1.50, 95% CI (0.58–3.87), P = 0.405; six to ten outpatient contacts: HR 1.71, 95% CI (0.54–5.37), P = 0.484; more than ten outpatient contacts: HR 0.54, 95% CI (0.10–3.01), P = 0.484. Likewise, living in an institution was not associated with a higher mortality rate (HR 1.11, 95% CI [0.25–4.89], P = 0.893).
3.16. Co-morbid psychiatric and somatic disorders
The presence of previous or concurrent psychiatric disorders increased the rate of mortality: HR 2.92, 95% CI [2.01–4.24], P < 0.001, while the presence of diagnosed somatic diseases did not: HR 0.90, 95% CI (0.66–1.24), P = 0.528.
4. Discussion
The current study allowed us to investigate the effects of antipsychotic exposure in a nationwide population, including all newly diagnosed with AD where clinicians were able to treat the individual patient as they judged to be optimal. In this population, we confirmed the results from previous randomized controlled trials, cohort studies, and meta-analyses that have shown an increased risk of death associated with current exposure to antipsychotic treatment [7], [11], [12], [13]. The finding is consistent and significant for all investigated causes of death – except intentional self-harm –, which could suggest that current antipsychotic exposure affects mortality similarly on all investigated causes of death, or could also suggest that current antipsychotic exposure is a marker of severity of illness (Table 2 ).
Table 2.
Definition of specific causes of death.
| ICD-10 diagnostic groups |
|---|
| Cardiovascular disease |
| (I05-I09) Chronic rheumatic heart diseases |
| (I10-I15) Hypertensive diseases |
| (I20-I25) Ischaemic heart diseases |
| (I26-I28) Pulmonary heart disease and diseases of pulmonary circulation |
| (I30-I52) Other forms of heart disease |
| (I60-I69) Cerebrovascular diseases |
| (I70-I79) Diseases of arteries, arterioles and capillaries |
| (I80-I89) Diseases of veins, lymphatic vessels and lymph nodes, not elsewhere classified |
| (I95-I99) Other and unspecified disorders of the circulatory system |
| Cancer |
| (C00-C14) Malignant neoplasms of lip, oral cavity and pharynx |
| (C15-C26) Malignant neoplasms of digestive organs |
| (C30-C39) Malignant neoplasm of respiratory and intrathoracic organs |
| (C40-C41) Malignant neoplasm of bone and articular cartilage |
| (C43-C44) Melanoma and other malignant neoplasms of skin |
| (C45-C49) Malignant neoplasms of mesothelial and soft tissue |
| (C50-C50) Malignant neoplasm of breast |
| (C51-C58) Malignant neoplasms of female genital organs |
| (C60-C63) Malignant neoplasms of male genital organs |
| (C64-C68) Malignant neoplasm of urinary tract |
| (C69-C72) Malignant neoplasms of eye, brain and other parts of central nervous (C73-C75) Malignant neoplasms of thyroid and other endocrine glands |
| (C76-C80) Malignant neoplasms of ill-defined, secondary and unspecified sites |
| (C81-C96) Malignant neoplasm of lymphoid, haematopoietic and related tissue |
| (C97-C97) Malignant neoplasms of independent (primary) multiple sites |
| (D37-D48) Neoplasms of uncertain or unknown behaviour |
| Infections |
| (A00-A09) Intestinal infectious diseases |
| (A15-A19) Tuberculosis |
| (A20-A28) Certain zoonotic bacterial diseases |
| (A30-A49) Other bacterial diseases |
| (A50-A64) Infections with a predominantly sexual mode of transmission |
| (A65-A69) Other spirochaetal diseases |
| (A70-A74) Other diseases caused by chlamydiae |
| (A75-A79) Rickettsioses |
| (A80-A89) Viral diseases of the central nervous system |
| (A90-A99) Arthropod-borne viral fevers and viral haemorrhagic fevers |
| (B00-B09) Viral infections characterised by skin and mucous membrane lesions |
| (B15-B19) Viral hepatitis |
| (B20-B24) Human immunodeficiency virus [HIV] disease |
| (B35-B49) Mycoses |
| (B50-B64) Protozoal diseases |
| (B65-B83) Helminthiases |
| (B85-B89) Pediculosis, acariasis and other infestations |
| (B90-B94) Sequelae of infectious and parasitic diseases |
| (B95-B97) Bacterial, viral and other infectious agents |
| (B99-B99) Other infectious diseases |
| G00 Bacterial meningitis, not elsewhere classified |
| G01 Meningitis in bacterial diseases classified elsewhere |
| G02 Meningitis in other infectious and parasitic diseases classified elsewhere |
| G03 Meningitis due to other and unspecified causes |
| G04 Encephalitis, myelitis and encephalomyelitis |
| G05 Encephalitis, myelitis and encephalomyelitis in diseases classified elsewhere |
| G06 Intracranial and intraspinal abscess and granuloma |
| G07 Intracranial and intraspinal abscess and granuloma in diseases classified elsewhere |
| (J00-J06) Acute respiratory infections |
| (J10-J18) Influenza and pneumonia |
| (J20-J22) Other acute lower respiratory infections |
| (M00-M03) Infectious arthropathies |
| U04 Severe acute respiratory syndrome [SARS] |
| Intentional self-harm |
| (X60-X84) Intentional self-harm |
| (Y10-Y34) Event of undetermined intent |
In the analyses of cumulative antipsychotic dosages after AD diagnosis, we initially included current exposure as an explanatory variable, showing that the lowest cumulative antipsychotic dosage increased rate of mortality, whereas the higher dosages decreased rate of mortality for most causes of death. No patients currently exposed would be in the baseline cumulative dosage group with zero DDDs, causing current exposure and cumulative drug dosage groups to be associated. When conducting the analyses without current exposure as an explanatory variable, we showed higher cumulative drug dosages being associated with an increased mortality rate for infection and cardiovascular related deaths. We believe the divergent findings are a result of a collider bias, which could have led to false conclusions, had the secondary analyses without current exposure as explanatory variable not been conducted. The findings of increased mortality rate associated with cumulative dosages of antipsychotic drugs for cardiovascular and infection related deaths are in line with results from other studies [2], [3]. In our study, cancer related deaths are not associated with higher cumulative dosages of antipsychotic drug treatment. This finding is reassuring, although previous studies have proposed antipsychotics as carcinogenic [14], [15]. As opposed to the other disorders investigated, our study population is selected given that they are generally older and have not yet died from a cancer related illness. Studies have suggested cancer to be less common in patients diagnosed with AD [4], perhaps resulting in the lack of association between cumulative antipsychotic dosages and mortality related to cancer. Secondly, the possible effects of antipsychotics on the risk of developing cancer would most likely not be dramatic, and time from exposure to a possible cancer related death could be long, resulting in some patients dying from other causes. This would be opposed to the effects of antipsychotic drugs on cardiovascular related deaths, where time from exposure to effects is shorter, as shown in studies on patients with dementia, as well as other psychiatric disorders [8], [16]. The specific background for the increased occurrence of cardiovascular events are unknown, but antipsychotic drugs are known to increase the risk of hypotension, increase blood lipids, as well as increase the risk of lowered insulin sensitivity or even diabetes, all being associated with an increase in cardiovascular events longer term [17], [18].
When reviewing both statistical models (with and without current exposure), we see a trend towards an inverse relationship between antipsychotic cumulative dosage and mortality. This finding is likely caused by a survival bias, in which survivors of treatment are being exposed for a longer period, and thereby are able to receive higher dosages of medication. An alternative interpretation could be that clinicians are able to determine which patients benefit from antipsychotic treatment and continue treatment in patients where the risk-benefit ratio is positive. Several short-term randomized controlled trials have been conducted on the effects of antipsychotics on aggression and psychosis in patients diagnosed with Alzheimer's disease, predominantly showing decreased levels of symptoms after treatment [19]. An explanation for our results could be that in patients with severe BPSD, where antipsychotic drugs are efficacious, the increased mortality rate of current antipsychotic exposure could be partly balanced by a lowered mortality rate caused by the lowered level of BPSD. No previous studies have investigated the effects of cumulative dosages of antipsychotic drug on specific cause mortality in AD, but findings of decreased mortality appear in studies of schizophrenia and bipolar affective disorders where the effects of cumulative drug dosages have been investigated [20], [21].
We show that psychiatric co-morbidities increase the rate of mortality in all investigated causes of death, including intentional self-harm, when controlling for remaining variables. The finding is similar to smaller studies showing an increased mortality associated with some psychiatric co-morbidities, on suicide, as we do, but also on all-cause mortality [22], [23]. In a parallel manner, we show that a known somatic disease increases the rate of mortality in all investigated causes of death, except for intentional self-harm, to some degree supporting the face-validity of our statistical model.
Lastly, we show that older age at diagnosis as well as female gender decreases rate of mortality in the investigated causes of death. These findings are most likely caused by age at diagnosis being a prognostic factor for severity of dementia [24], whereas the latter finding could be explained by females generally living longer, as compared to males, in the Danish population [25].
The most important limitation of the study is the lack of randomization, which increases the risk of systematic biases between the exposed and non-exposed groups. Although we added explanatory variables to minimize the bias from psychiatric and somatic co-morbidities, as well as severity of the disorder, there are unmeasured differences between the exposed and unexposed groups that may have affected the results.
Despite these limitations, the main strengths of the study are the long follow-up time and the inclusion of all eligible patients in a nationwide sample where the current data are systematically gathered and patients are not lost to follow-up.
In conclusion, we found that lifetime cumulative antipsychotic drug dosages as compared to no exposure are associated with increased mortality rates in cardiovascular and infection related deaths, especially for lower cumulative dosages. We also showed an inverse dose to mortality relationship, with the highest investigated cumulative drug dosage not being associated with or decreasing rate of mortality. These findings confirm the established concern about the use of antipsychotic drugs in patients diagnosed with AD, but also highlight the need for further investigations of long-term effects of treatment, and possible sub-groups who could benefit from treatment.
Funding
The study has been funded by the affiliated research institutions, as well as a grant from the Psychiatric Research Fund of North Jutland. Funding sources did not have a role in study design, analyses of data, presentation of data, or discussion of results. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Disclosure of interest
RE Nielsen has received research grants from H. Lundbeck for clinical trials, received speaking fees from Bristol-Myers Squibb, Astra Zeneca, Janssen & Cilag, Lundbeck, Servier, Otsuka Pharmaceuticals, and Eli Lilly and has acted as advisor to Astra Zeneca, Eli Lilly, Lundbeck, Otsuka Pharmaceuticals, Takeda, and Medivir. K Andersen has received a research grant from the Lundbeck Foundation and a travel grant from H. Lundbeck. The other authors declare that they have no competing interest.
Acknowledgements
None.
References
- 1.Brodaty H., Seeher K., Gibson L. Dementia time to death: a systematic literature review on survival time and years of life lost in people with dementia. Int Psychogeriatr. 2012;24(7):1034–1045. doi: 10.1017/S1041610211002924. [DOI] [PubMed] [Google Scholar]
- 2.Magaki S., Yong W.H., Khanlou N., Tung S., Vinters H.V. Comorbidity in dementia: update of an ongoing autopsy study. J Am Geriatr Soc. 2014;62(9):1722–1728. doi: 10.1111/jgs.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunnstrom H.R., Englund E.M. Cause of death in patients with dementia disorders. Eur J Neurol. 2009;16(4):488–492. doi: 10.1111/j.1468-1331.2008.02503.x. [DOI] [PubMed] [Google Scholar]
- 4.Romero J.P., Benito-Leon J., Louis E.D., Bermejo-Pareja F. Alzheimer's disease is associated with decreased risk of cancer-specific mortality: a prospective study (NEDICES) J Alzheimers Dis. 2014;40(2):465–473. doi: 10.3233/JAD-132048. [DOI] [PubMed] [Google Scholar]
- 5.Erlangsen A., Zarit S.H., Conwell Y. Hospital-diagnosed dementia and suicide: a longitudinal study using prospective, nationwide register data. Am J Geriatr Psychiatry. 2008;16(3):220–228. doi: 10.1097/JGP.0b013e3181602a12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Draper B., Brodaty H., Low L.F., Richards V. Prediction of mortality in nursing home residents: impact of passive self-harm behaviors. Int Psychogeriatr. 2003;15(2):187–196. doi: 10.1017/s1041610203008871. [DOI] [PubMed] [Google Scholar]
- 7.Schneider L.S., Dagerman K.S., Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 8.Corbett A., Burns A., Ballard C. Don’t use antipsychotics routinely to treat agitation and aggression in people with dementia. BMJ. 2014;349:g6420. doi: 10.1136/bmj.g6420. [DOI] [PubMed] [Google Scholar]
- 9.Ma H., Huang Y., Cong Z., Wang Y., Jiang W., Gao S. The efficacy and safety of atypical antipsychotics for the treatment of dementia: a meta-analysis of randomized placebo-controlled trials. J Alzheimers Dis. 2014;42(3):915–937. doi: 10.3233/JAD-140579. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen R.E., Lolk A., Valentin J.B., Andersen K. Cumulative dosages of antipsychotic drugs are associated with increased mortality rate in patients with alzheimer's dementia. Acta Psychiatr Scand. 2016;134(4):314–320. doi: 10.1111/acps.12614. http://dx.doi.org/10.1111/acps.12614 [Epub 2016 Jun 30] [DOI] [PubMed] [Google Scholar]
- 11.Maust D.T., Kim H.M., Seyfried L.S., Chiang C., Kavanagh J., Schneider L.S. Antipsychotics, other psychotropics and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72(5):438–445. doi: 10.1001/jamapsychiatry.2014.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeste D.V., Blazer D., Casey D., Meeks T., Salzman C., Schneider L. ACNP white paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957–970. doi: 10.1038/sj.npp.1301492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballard C., Hanney M.L., Theodoulou M., Douglas S., McShane R., Kossakowski K. The dementia antipsychotic withdrawal trial (DART-AD): long-term follow-up of a randomised placebo-controlled trial. Lancet Neurol. 2009;8(2):151–157. doi: 10.1016/S1474-4422(08)70295-3. [DOI] [PubMed] [Google Scholar]
- 14.Wang P.S., Walker A.M., Tsuang M.T., Orav E.J., Glynn R.J., Levin R. Dopamine antagonists and the development of breast cancer. Arch Gen Psychiatry. 2002;12(59):1147–1154. doi: 10.1001/archpsyc.59.12.1147. [0003-990; 12] [DOI] [PubMed] [Google Scholar]
- 15.Fond G., Macgregor A., Attal J., Larue A., Brittner M., Ducasse D. Antipsychotic drugs: pro-cancer or anti-cancer? A systematic review. Med Hypotheses. 2012;79(1):38–42. doi: 10.1016/j.mehy.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Emul M., Kalelioglu T. Etiology of cardiovascular disease in patients with schizophrenia: current perspectives. Neuropsychiatr Dis Treat. 2015;11:2493–2503. doi: 10.2147/NDT.S50006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stahl S.M., Mignon L., Meyer J.M. Which comes first: atypical antipsychotic treatment or cardiometabolic risk? Acta Psychiatr Scand. 2009 03;119(3):171–179. doi: 10.1111/j.1600-0447.2008.01334.x. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen R.E., Nielsen J. Antipsychotic drug treatment for patients with schizophrenia. Clin Med Therap. 2009;1:1053–1068. [Google Scholar]
- 19.Ballard C., Waite J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer's disease. Cochrane Database Syst Rev. 2006;1(1):CD003476. doi: 10.1002/14651858.CD003476.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiihonen J., Lonnqvist J., Wahlbeck K., Klaukka T., Niskanen L., Tanskanen A. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study) Lancet. 2016:620–627. doi: 10.1016/S0140-6736(09)60742-X. [08/22;374(1474–547); 9690] [DOI] [PubMed] [Google Scholar]
- 21.Kessing L.V., Gerds T.A., Feldt-Rasmussen B., Andersen P.K., Licht R.W. Use of lithium and anticonvulsants and the rate of chronic kidney disease: a nationwide population-based study. JAMA Psychiatry. 2015;4:1–10. doi: 10.1001/jamapsychiatry.2015.1834. [DOI] [PubMed] [Google Scholar]
- 22.Vilalta-Franch J., Lopez-Pousa S., Calvo-Perxas L., Garre-Olmo J. Psychosis of alzheimer disease: prevalence, incidence, persistence, risk factors, and mortality. Am J Geriatr Psychiatry. 2013;21(11):1135–1143. doi: 10.1016/j.jagp.2013.01.051. [DOI] [PubMed] [Google Scholar]
- 23.Garcez M.L., Falchetti A.C., Mina F., Budni J. Alzheimer s disease associated with psychiatric comorbidities. An Acad Bras Cienc. 2015;87(Suppl. 2):1461–1473. doi: 10.1590/0001-3765201520140716. [DOI] [PubMed] [Google Scholar]
- 24.Larson E.B., Shadlen M.F., Wang L., McCormick W.C., Bowen J.D., Teri L. Survival after initial diagnosis of alzheimer disease. Ann Intern Med. 2004;140(7):501–509. doi: 10.7326/0003-4819-140-7-200404060-00008. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen R.E., Uggerby A.S., Jensen S.O., McGrath J.J. Increasing mortality gap for patients diagnosed with schizophrenia over the last three decades: a Danish nationwide study from 1980 to 2010. Schizophr Res. 2013;146(1–3):22–27. doi: 10.1016/j.schres.2013.02.025. [DOI] [PubMed] [Google Scholar]