Abstract
Severe viral pneumonia is an increasing problem among adults. The incidence and number of viruses known to cause pneumonia and respiratory failure have also expanded in recent years. This article provides an overview of severe respiratory disease caused by coronavirus, respiratory syncytial virus, adenovirus, and hantavirus. These emerging pathogens are easily overlooked and timely diagnosis requires a high index of suspicion and confirmation by molecular testing. Management of individual cases is mainly supportive and requires institution of appropriate infection control measures. Vaccines and effective therapeutics for these potentially devastating respiratory viruses are urgently required.
Keywords: SARS, Coronavirus, RSV, Adenovirus, Hantavirus, Acute respiratory failure, Immunocompromised host
Key points
-
•
Severe acute respiratory syndrome–associated coronavirus and the Middle East respiratory syndrome coronavirus are novel pathogens that can cause severe respiratory infections and acute respiratory distress syndrome, which is associated with high mortality.
-
•
Sustained human-to-human transmission of coronavirus can occur; thus, early case recognition, laboratory diagnosis, isolation, and implementation of appropriate infection control measures in the health care setting are important to prevent disease transmission.
-
•
The diagnosis of respiratory syncytial virus in adults can be challenging and there are no vaccines or antivirals available; these unmet needs should be urgently addressed.
-
•
The diagnosis and surveillance of adenovirus infection has been greatly improved by the development of highly sensitive and quantitative polymerase chain reaction assays of mucosal samples or plasma.
-
•
The diagnosis of hantavirus pulmonary syndrome is primarily based on a history of exposure to potentially infected rodents in endemic areas such as the rural southwestern United States and may be confirmed by polymerase chain reaction or serologic testing.
-
•
Clinical management of hantavirus pulmonary syndrome includes excellent supportive care with particular attention to careful management of fluid status.
Human coronavirus infections
Virology
Most human coronaviruses (eg, hCoV 229E, OC43, NL63) cause mild upper respiratory tract diseases, except occasionally in immunocompromised hosts. However, 2 novel coronaviruses, the severe acute respiratory syndrome–associated coronavirus (SARS-CoV), and a recently identified Middle East respiratory syndrome coronavirus (MERS-CoV) may cause serious viral pneumonitis, leading to hospitalization and death.1, 2 Coronaviruses are large, lipid-enveloped, positive-sense, single-stranded RNA viruses. Viral genome analyses revealed that SARS-CoV and MERS-CoV are Group B and Group C betacoronavirus, respectively, and are closely related to coronaviruses found in bats.1, 2, 3, 4 Intermediate mammalian hosts such as civet cats have been implicated for SARS-CoV before its adaptation for human transmission,1, 3 but no such host has been identified for MERS-CoV. These coronaviruses encode a surface spike glycoprotein (S protein) that attaches the virus to host cells, determining its host range and tropism, and is the target for neutralizing antibodies.1, 3 It has been shown that SARS-CoV uses human angiotensin-converting enzyme 2 (ACE-II) as the primary cellular receptor; the human C-type lectin (DC/L-SIGN) has also been implicated as an alternative receptor.1, 3, 5 MERS-CoV has been shown to bind to dipeptidyl peptidase 4 (DPP4; also called CD26), an interspecies-conserved protein found on the surface of several cell types including the nonciliated cells in human airways,4, 5, 6 and this interaction may explain its broad host range and its ability to cause cross-species zoonotic transmission.4 There is no vaccine available at present for coronaviruses.7
Epidemiology and Disease Transmission
SARS-CoV emerged in Southern China (Guangdong Province) in February 2003; the first victims were those who had direct contact with live animals, either in the wet markets or in restaurants selling these animals as winter food.1, 3 The disease quickly spread to Hong Kong; and within a few weeks, through international air travel, it had reached Vietnam, Singapore, Taiwan, and Canada.1, 3, 8 By July 2003, more than 30 countries were affected, resulting in 8096 confirmed infections and 774 deaths (9.6%).8 Mathematical modeling of the early phase of the outbreak estimated that the basic reproductive number (R0) of SARS-CoV was in the range of 2.2 to 3.7; the primary mode of transmission was via respiratory droplets.9 Two key epidemiologic features of SARS were frequent nosocomial outbreaks and superspreading events, which exacerbated its transmission. Notably, 1706 of 8096 (21%) of SARS victims were health care workers.8 It has been suggested that viral replication was at its peak in SARS patients at the time of hospitalization when symptoms worsen (see later discussion). Transmission was facilitated by close bed proximity and the application of aerosol-generating procedures (eg, intubation, resuscitation) and devices (eg, continuous positive airway pressure and biphasic positive airway pressure [BiPAP] treatments).10 In 1 example, nebulization from a bronchodilator in a SARS patient resulted in a major hospital outbreak involving 138 inpatients, doctors, nurses, allied health workers, and medical students who had worked in the same medical ward.11 Subsequent studies indicated that the attack rates were between 10% and 60% in the hospital settings.10 An example of a community superspreading event that involved more than 300 residents occurred in a private housing estate (Amoy Gardens) in Hong Kong. Drying up of the U-shaped bathroom floor drain and backflow of contaminated sewage (from a SARS patient with diarrhea), coupled with the toilet’s exhaust fan, might have created infectious aerosols that moved upward through the warm air shaft of the building. Computational fluid-dynamics modeling suggested possible dispersion by wind flow, causing long-range transmission to nearby buildings.1 This and other evidence suggested that SARS could be opportunistically airborne.3, 12 Appropriate precautions to prevent airborne transmission should be implemented in the health care setting, particularly when respiratory procedures and devices are used.12 Notably, laboratory-related SARS cases have occurred since the epidemic,13 which highlights the importance of laboratory safety in handling these contagious viral pathogens.
In 2012, MERS-CoV emerged in the Middle East, and by June 2013, a total of 68 people in Saudi Arabia, Qatar, Jordan, the United Arab Emirates, and the United Kingdom were confirmed to have infections caused by the virus.14 Epidemiologic investigations so far have revealed sporadic transmission of the disease. Zoonotic transmission has also been implicated even though most of these cases did not report a history of direct animal contact. It is unknown whether there is a low level of virus circulation in asymptomatic human carriers or if there is an animal reservoir that allows multiple introductions into humans.15 Based on the reports of family and hospital clusters, it is thought that human-to-human transmission is possible.3, 14
Pathogenesis
Humans have no preexisting immunity to these novel coronaviruses. SARS-CoV and MERS-CoV were shown to have the ability to evade innate host defenses (eg, type I interferon responses and related mechanisms), and replicate efficiently in host tissues (SARS-CoV, respiratory and intestinal tract cells; MERS-CoV, respiratory, intestinal, and kidney cells).15, 16, 17 Besides lytic cell damage, uncontrolled replication of SARS-CoV leads to unabated inflammatory cytokine activation (eg, interleukin [IL]-6, IL-8, monocyte chemotactic protein-1, interferon-inducible protein-10, monokine induced by interferon-γ–inducible protein; commonly known as a cytokine storm), which is implicated in the development of progressive pneumonitis, diffuse alveolar damage/acute respiratory distress syndrome (ARDS), and hemophagocytic syndrome.17, 18 Clinically, the respiratory tract viral load peaked around 7 to 11 days and subsequently decreased19; a high viral load and slow viral clearance were associated with progressive disease and fatal outcomes.4 High-level viremia and intestinal tract involvement also predicted adverse outcomes.6, 20 Little is known about the pathogenesis of MERS-CoV. A macaque model has shown active viral replication in lung tissues causing localized to widespread lesions and clinical illness, which starts to decrease after 1 week.21
Clinical Manifestations and Disease Course
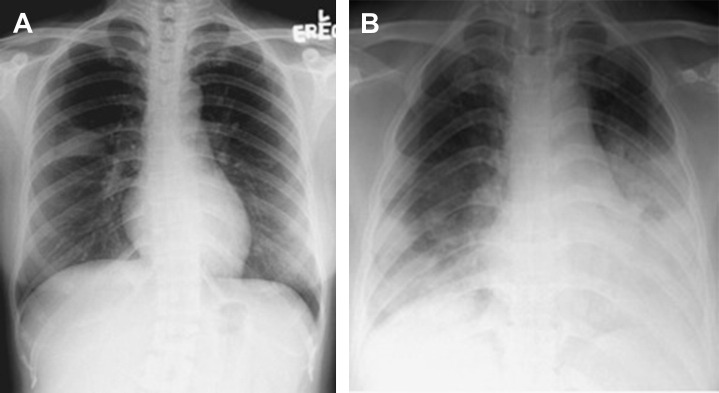
SARS was described as a triphasic illness11, 22 with a typical incubation period of 4 to 6 days (range 2–16 days). In the viremic phase, patients experienced fever, chills, and rigor, which partially subsided in a few days; however, in about 90% of cases, this was followed by a resurgence of fever, cough, and shortness of breath (hyperimmune or pneumonia phase). Chest radiographs first showed patchy consolidation and ground-glass changes that rapidly progressed in the next few days to involve multiple lobes (Fig. 1 ).12 In certain cases, findings on computed tomography scans of the thorax closely resembled bronchiolitis obliterans organizing pneumonia (eg, peripheral air-space consolidation).23 Laboratory features included lymphopenia, thrombocytopenia, increased transaminases, creatinine kinase and lactate dehydrogenase. By the end of the second week (around day 10–14), 15% to 25% of patients deteriorated further and developed refractory respiratory failure and ARDS (pulmonary destruction phase).3, 11, 22 About 20% of patients developed profuse diarrhea containing highly infectious virus particles; nevertheless, renal failure was rare.6 A substantial proportion of intubated and mechanically ventilated patients developed bacterial superinfection (eg, methicillin-resistant Staphylococcus aureus), and some developed complications such as pneumothorax and pneumomediastinum.22, 24 Children typically had a mild disease course that was rarely fatal.1, 3 The overall death rate was 6% to 16%; however, the age-stratified case fatality rate was as follows: less than 25 years, less than 1%; 25 to 44 years, 6%; 45 to 64 years, 15%; and greater than 65 years, greater than 50%.1, 3, 8, 22
Fig. 1.
(A) Subtle right middle zone infiltration in a patient with SARS about 3 days after illness onset; (B) bilateral, peripheral distributed ground-glass opacities about 7 days after illness onset.
Preliminary data indicate that the clinical manifestations of MERS-CoV infection closely mimic those of SARS. Patients developed shortness of breath and progressive pneumonia about 1 week after symptom onset; the symptoms were associated with multiple, patchy, consolidation and ground-glass changes on chest imaging, laboratory evidence of lymphopenia, and increased liver enzyme levels. Most strikingly, many patients developed acute renal failure for which there was no clear explanation.2, 5, 14 Most patients with confirmed MERS-CoV infection developed critical illness requiring admission to an intensive care unit (ICU) and, thus far, 38 (55.9%) patients have died despite maximal supportive treatment.14
Diagnosis
Early case recognition of these novel infections requires a high index of suspicion and a combination of detailed clinical and epidemiologic assessments. Based on the experience with SARS, the World Health Organization recommends that laboratory testing for MERS-CoV is indicated if a patient’s pneumonia is otherwise unexplained and there is a history of travel to affected areas.14 Testing should also be considered if there is direct contact with another sick individual, in case clusters of unexplained pneumonia, and if health care workers are involved. Prompt isolation of suspicious cases may greatly assist infection control and prevent hospital outbreaks.
Reverse transcriptase polymerase chain reaction (RT-PCR) is the test of choice for coronavirus infections. For SARS-CoV, a combination of upper respiratory (nasal, pharyngeal, nasopharyngeal), lower respiratory (higher yield because of higher viral levels, eg, sputum, tracheal aspirate, bronchial alveolar lavage), blood, and fecal specimens were required to maximize the chance of detection.1, 3, 20, 25 Plasma RT-PCR may detect viremia as early as 2 to 3 days after symptom onset, and has been shown to have prognostic value.20 A single negative test in an upper respiratory specimen may be insufficient to rule out the diagnosis. Virus culture may provide further confirmation, but it is too slow to assist clinical management and needs to be performed in biosafety level 3 facilities. Serologic diagnosis is largely retrospective but may be useful for epidemiologic surveillance purposes.1, 25 The optimal clinical specimen for MERS-CoV testing is uncertain, but evidence has suggested a better yield with lower respiratory specimens. Given the diversity and periodic emergence of novel coronaviruses that may be extremely challenging to detect, clinicians should consult their local guidelines to determine when to refer cases for coronavirus testing to specialized reference laboratories (eg, pan-coronavirus RT-PCR, specific SARS-CoV and MERS-CoV RT-PCR).3, 14
Treatment
There is no established therapy for coronavirus infection. During the SARS outbreak in 2003, a range of agents was used in patients but their efficacy is questionable. Ribavirin, although shown to be active in vitro, did not seem to provide clinical benefit (>90% of cases progressed despite treatment).3, 7, 11, 22, 26 Lopinavir-ritonavir, a protease inhibitor used to treat human immunodeficiency virus (HIV)/AIDS with evidence of in vitro activity against SARS-CoV, was given to 41 patients in Hong Kong and was associated with viral load reduction and fewer cases of ARDS and fewer deaths; however, the study was uncontrolled.7, 26, 27 Convalescent plasma obtained from recovering individuals that contained neutralizing antibodies had also been used. In 1 study, 19 patients who received such therapy had better survival (100% vs 66.2%) and discharge rates (77.8% vs 23.0%) compared with 21 controls.28 This form of treatment has also been used in fulminant cases of H5N1 and 2009 H1N1 influenza with apparent success.3 Subsequent in vitro and animal (ferrets, hamsters, macaques) studies have shown that monoclonal antibodies targeting the S protein may provide neutralizing activity against SARS-CoV, resulting in viral load reduction and resolution of lung lesions29, 30, 31, 32, 33; however, no clinical data are available. Several in vitro and animal (mice, macaques) studies have consistently shown that type I interferons, if given prophylactically or shortly after exposure, may protect against SARS.34, 35 In humans, it has been shown that interferon-α given within 5 days of illness may result in lower rates of intubation (11.1% vs 23.1%) and death (0.0% vs 7.7%); however, the study was small (9 vs 13 patients) and confounded by corticosteroid use.7 Preliminary data have shown that type I and type III interferons have in vitro activity against MERS-CoV and these observations may deserve further investigation.5, 15, 36 There are no published data on antibody therapies but this approach may also be useful.6 Other potential treatments that have been tested in animal models include short interfering RNA (siRNA),37 proteasome inhibitors,38 prophylactic toll-like receptor agonist administration,39 and immunomodulants.3
Systemic corticosteroid therapy is perhaps the most controversial area in SARS management (Table 1 ). Although favorable clinical and radiological responses have been reported, controlled data were lacking.1, 3, 22, 40 In the only randomized placebo-controlled study performed, early corticosteroid treatment within the first few days was shown to delay viral clearance.41 Metabolic side effects, bacterial and fungal superinfections, avascular osteonecrosis, and even acute psychosis had been reported.22, 24, 26, 42 A systematic review concluded that corticosteroid treatment is not associated with definite benefits and is potentially harmful.26 Similar adverse effects have been reported in severe influenza; notably, an increase in mortality was also found.43 It is unclear whether a lower dose or delayed treatment after viral replication has begun to subside can reduce harm.44 Currently, it is recommended that corticosteroids be limited to SARS cases with refractory septic shock, and given at a low dose (eg, hydrocortisone 50 mg every 8 hours); a similar recommendation has been made for MERS-CoV infections.8, 14
Table 1.
Controversies around the use of corticosteroid therapy in SARS
| Pros | Cons |
|---|---|
| Clinical and radiological responses have been observed Suppression of inflammatory cytokines |
Metabolic complications: hyperglycemia, hypertension, hypokalemia Increased bacterial superinfections Avascular osteonecrosis; but incidence was low (0.6%) if total prednisolone dose was <3 g Reduced viral clearance Others (eg, acute psychosis) |
Respiratory syncytial virus infections in adults
Virology
Respiratory syncytial virus (RSV) is an enveloped, single-stranded RNA paramyxovirus that includes 2 major groups, A and B, each of which consists of 5 to 6 genotypes. The RSV genome encodes 2 nonstructural (NS1 and NS2) and 9 structural proteins, including the F (fusion) and G (attachment) glycoproteins on the viral envelope. Antibodies against the F and G proteins are neutralizing, and have been shown to confer protection against RSV infection in animal models45; these targets are likely important for the development of antiviral agents and vaccines. Immunity after primary infection (which generally occurs by 2 years of age) is partial and short-lived; thus, reinfections can occur throughout life.45, 46 Low serum neutralizing antibody levels in adults predicts infection risk and disease severity.46 Although immunologic mechanisms (eg, cytokine responses) have been implicated in the pathogenesis, emerging evidence suggests that uncontrolled viral replication (indicated by high viral load) drives disease manifestations and leads to severe outcomes.47, 48, 49 Such findings provide an important rationale for the approach to antiviral drug development against RSV.
Epidemiology
RSV is known to be an important cause of lower respiratory tract infection in infants and young children (eg, acute bronchiolitis, wheezy attacks), resulting in hospitalizations and deaths,50 yet its impact in adults has only been appreciated in recent years. It has been estimated that RSV infects 3% to 10% of adults annually; although most infections are mild, increasing evidence suggest that severe lower respiratory tract infections can occur, especially among older adults (eg, >65 years) and those with underlying conditions (eg, chronic lung diseases, chronic cardiovascular diseases).45, 46 RSV may have accounted for 5% to 15% of community-acquired pneumonia, 9% to 10% of hospital admissions for acute cardiorespiratory diseases, and excessive deaths among adults during seasonal peaks.45, 50, 51, 52, 53, 54, 55 Outbreaks among nursing home residents are likely common, but under-recognized.56, 57 The disease burden of RSV has been shown to approach that of seasonal influenza.51 Patients who are profoundly immunosuppressed, such as hematopoietic stem cell transplant (HSCT) recipients, are at particularly high risk for severe RSV infection (2%–17%), which can be rapidly fatal.58
Clinical Manifestations and Outcomes
The clinical manifestations of RSV infection in adults are diverse and mainly determined by host factors such as the underlying chronic medical conditions and degree of immunosuppression. In healthy young adults, RSV may cause self-limiting upper respiratory illnesses; in the profoundly immunosuppressed, progressive lower respiratory tract disease can occur (17%–84%), resulting in fulminant pneumonitis and high mortality (7%–83%).58 Older adults hospitalized for RSV infection may present with fever, cough, sputum production, wheezing, and dyspnea. Although wheezing and dyspnea may be more common with RSV, and the magnitude of fever sometimes lower, such findings could not reliably differentiate it from influenza.51, 59 Radiographically, about 50% to 60% of cases show active pneumonic changes such as consolidations and ground-glass opacities; the remainder may show evidence of congestive heart failure and features of underlying chronic lung conditions.59, 60 Typically, the pneumonic changes are small, patchy, and unilateral and disproportional to the degree of hypoxemia detected in these patients; a diffuse interstitial infiltration pattern is rare.59, 60 Overall, more than 70% of patients hospitalized with RSV develop severe lower respiratory complications, including pneumonia, acute bronchitis, and exacerbations of chronic obstructive pulmonary disease (COPD)/asthma; 10% to 15% have cardiovascular complications such as congestive heart failure or acute coronary syndrome.45, 46, 51, 59, 60 Bacterial superinfection occurred in 12% to 17% of cases,59 10% to 18% of patients required ventilatory support (invasive/noninvasive), and the overall mortality was approximately 8% to 10%.45, 59, 60 Late death might occur as a result of multiple cardiorespiratory complications or exacerbations of underlying medical conditions. Studies have shown that the morbidity and mortality from RSV infection in adults are actually similar to that of seasonal influenza.51, 60
Diagnosis
RSV infection is clinically indistinguishable from other viral respiratory infections and diagnosis requires laboratory testing. Commonly, nasopharyngeal specimens are used; if the patient requires intubation, a lower respiratory tract specimen such as a tracheal aspirate should be obtained. The gold standard for diagnosis is by RT-PCR because of its relative ease and speed; other types of tests such as antigen assays (eg, enzyme immunoassays) and culture have lower sensitivities, which might be related to the lower viral load in adults.45, 61 A negative antigen assay result cannot be used to rule out RSV infection. Serology to detect RSV-specific IgG antibodies may also assist with the diagnosis and, if available, can be used in combination with RT-PCR to maximize the yield.45
Treatment and Prevention
At present, there is no established antiviral therapy or vaccine available for RSV. In immunocompromised adults, ribavirin (aerosolized or systemic administration) and palivizumab (an RSV-specific monoclonal antibody directed against the F glycoprotein) have been used to treat RSV infection with the aim of reducing progression to lower respiratory disease and death58, 62, 63; however, controlled data are lacking. In animal models, palivizumab has been shown to reduce viral titers and replication in pulmonary tissues, and in randomized clinical trials, palivizumab given prophylactically to very young high-risk children was shown to reduce hospitalizations related to RSV infections.45, 58 Currently, it is not known whether these approaches can be applied to older adults. New antiviral agents (eg, fusion protein inhibitors, siRNA) and newer generation antibody therapies are under active research.45, 64, 65
Systemic corticosteroids are commonly used to treat wheezing and exacerbations of COPD/asthma in adults, including those triggered by viral infections. Conversely, randomized controlled trials of corticosteroid therapy in young children with RSV infections have revealed a lack of clinical benefit and inconsistent control of inflammatory cytokine responses.66, 67 A recent study of corticosteroids in adults reported that virus control seemed to be unaffected but humoral immunity against RSV was diminished.68 It is suggested that the decision to treat RSV patients with corticosteroids should be weighed against the potential risks (eg, bacterial superinfections), and be limited to a short course if used.60, 68 Because of the high rates of secondary infections, it is prudent to test and treat bacterial pathogens according to local resistance profiles. In addition to Streptococcus pneumoniae and Hemophilia influenzae, Pseudomonas aeruginosa and other gram-negative bacilli may need to be considered in patients with underlying chronic lung diseases.
In the hospital setting, RSV may spread via contact (eg, hands or via fomites) and droplets, and appropriate infection control measures such as hand washing and use of surgical masks (for both health care workers and infected patients) should be implemented. RSV particles have been detected in air samples from health care facilities, suggesting airborne transmission.69 Nelson Lee has experienced an RSV outbreak related to the use of BiPAP ventilation (unpublished), and suggests appropriate isolation precautions when aerosol-generating procedures or devices are being used.
Adenovirus infection
Microbiology
Adenoviruses are medium-sized DNA viruses that attach to host cells through the coxsackie B virus-adenovirus receptor.70 The variation in tissue tropism of individual adenovirus species or serotypes may be attributable to differential receptor expression or binding to alternative receptors. Following endocytosis and nuclear localization, early gene expression inhibits various host immune responses and initiates viral replication, whereas late gene expression directs viral assembly and release through host cell lysis. Human adenoviruses have no animal reservoir and do not infect other species; nonhuman adenoviruses that infect other species do not cause zoonotic disease. The traditional classification of human adenoviruses is complex and includes at least 52 serotypes. Adenoviruses have also been divided into 7 species (A–G) based on various biological and biochemical characteristics; each species includes more than 1 serotype and each serotype includes multiple genotypes. Although there is no strict correlation between species or serotype and disease manifestation, it is possible to make certain generalizations; for example, respiratory disease is caused by species B, C, and E, whereas species F causes gastroenteritis. A comprehensive review of serotype associations with disease has recently been published.71
Adenoviruses lack an outer envelope and are relatively resistant to dessication, low pH, and gastric or biliary secretions. The predominant routes of transmission include direct contact with aerosolized droplets in military settings, fecal-oral spread among children or those with prolonged shedding in the stool, and exposure to infected fomites, blood, or tissues. After an incubation period that varies from 2 days to 2 weeks, adenoviruses may be cleared or may cause long-standing asymptomatic infection of lymphoid or other mucosal cells, particularly in children.72, 73 In some cases, adenoviruses may become latent while retaining the potential to undergo endogenous reactivation at a later time point in a highly immune-suppressed host. A combination of innate and adaptive immune responses is required for control or prevention of adenovirus infection in vivo.74 Innate antiviral immunity is mediated by natural killer (NK) cells, neutrophils, and monocytes/macrophages that are capable of directly killing infected cells or secreting inflammatory cytokines and chemokines that recruit additional effector cell types.75 Optimal adaptive immunity includes neutralizing antibody production by antigen-specific B cells as well as generation and expansion of CD4+ and CD8+ T lymphocytes that are capable of eliminating infected host cells. Adenoviruses dedicate a significant portion of their genome to subversion of these host defense mechanisms to ensure infected cell survival and facilitate virus transmission in vivo.76, 77
Epidemiology
In almost all cases, adenoviral infections are mild and self-limiting episodes that occur during childhood and are estimated to cause 5% to 10% of all febrile illnesses in pediatric populations.72, 73, 78, 79 Compared with children, severe adenoviral disease is much less common in adults and makes timely diagnosis in the critically ill patient quite challenging. Possible epidemiologic clues for adenovirus infection include a cluster of febrile pharyngitis, conjunctivitis, or respiratory disease in individuals living in close quarters, including day-care centers, chronic care facilities, or military barracks. Another clue is the simultaneous presence of circulating adenoviral isolates in the community, particularly during the summer or autumn months when influenza is less common. Several reports from military training centers have shown that serotype 3, 4, or 7 may be associated with severe disease as a result of greater virulence or transmissibility. Prompted by frequent outbreaks beginning in the 1950s that affected up to 10% of all military recruits, enteric-coated live oral vaccines against serotypes 4 and 7 were introduced in 1971 and were successful in diminishing acute respiratory disease. Immunization was progressively stopped from 1996 to 1999 and was followed by a resurgence of epidemic disease caused by the vaccine serotypes 3 and 7.80 The global epidemiology of adenovirus infections in healthy populations includes 2 novel Ad7 genome types that have been shown to cause severe or fatal disease during several civilian and military outbreaks in North America and are closely related to endemic strains in China and South America.81 Similarly, a virulent serotype 14 adenovirus strain that had not previously been observed in the United States was recently shown to cause severe and fatal adenovirus disease in children and adult military recruits.82 Adenovirus infection is also an important emerging pathogen among HSCT recipients; the incidence varies from 4.9% to 29% according to the transplant regimen, age of the study population, and diagnostic testing strategy.83, 84 Relatively little is known about the incidence of adenovirus infection among adult solid-organ transplant recipients; however, plasma DNA was detected in 7.2% of cases during a 1-year prospective surveillance study.85 Most transplant recipients were asymptomatic at the time of adenovirus detection and all recovered spontaneously. The incidence of adenovirus in patients with HIV infection, undergoing cancer chemotherapy, or with congenital immunodeficiency has been comprehensively reviewed.86
Severe Clinical Manifestations in the Healthy Host
In the civilian setting, severe adenoviral disease is extremely unusual in healthy adults with intact immunity. For example, only 18 cases of potentially life-threatening adenoviral pneumonia were published between 1974 and 1998,87 and since then 14 additional cases have been reported and analyzed.88 In the more recent series, two-thirds of the patients were male, the mean age was 32.75 years, and the overall mortality rate was 57%. The clinical and radiological features of severe adenoviral disease were generally nonspecific and could mimic bacterial sepsis, although the presence of conjunctivitis or diarrhea served as potential diagnostic clues. A variety of serotypes have been documented to cause sporadic cases of severe civilian89, 90 or fatal military adenoviral pneumonia91, 92 in healthy adults, although the relationship between genotype and virulence has not been comprehensively investigated.89 The largest reported outbreak occurred in a mental health center and was caused by an unusual adenovirus (serotype 35) that had been associated with disease only among immunocompromised patients.93 In that outbreak, affected individuals presented with fever and cough associated with diffuse interstitial or dense unilateral pulmonary infiltrates. No other distinguishing clinical features were evident; however, in several instances, the development of mild leukopenia was observed. Six of 18 infected cases required ICU admission, 5 required intubation, and 4 developed ARDS with septic shock; all patients recovered except for 1 individual with chronic renal insufficiency who required prolonged intubation and died 2 months after admission. Typical pathologic findings in fatal cases of adenoviral pneumonia include severe necrotizing bronchiolitis with fibrinous obstruction, interstitial inflammation, and hyaline membranes lined by characteristic alveolar smudge cells that have a large rounded nucleus containing basophilic inclusions with a surrounding halo.94
Severe Clinical Manifestations in the Immunocompromised Host
Highly immune-suppressed hosts are susceptible to adenovirus infection of various organ systems (brain, heart, lung, liver, intestine, kidney, pancreas, bladder) (Box 1 ) and may go on to exhibit a spectrum of disease severity that is influenced by their age, underlying disease, clinical interventions, and virus serotype.86, 95 Asymptomatic or clinically evident disease occurs mainly via de novo virus acquisition or through reactivation of latent infection from the host; a minority of cases are attributed to donor-derived infection during stem cell transplantation.84 In most cases, it is not possible to determine the exact mechanism of adenovirus infection. Clinical illness associated with coinfection by multiple viral serotypes is also more frequent in the immune-suppressed host compared with other susceptible individuals.96
Box 1. Severe adenovirus disease manifestations.
Interstitial pneumonitis
Meningoencephalitis
Hemorrhagic cystitis
Nephritis
Gastroenteritis
Hepatitis
Myocarditis
Colitis
Adapted from Kojaoghlanian T, Flomenberg P, Horwitz MS. The impact of adenovirus infection on the immunocompromised host. Rev Med Virol 2003;13(3):155–71.
HSCT
The development of extremely sensitive molecular techniques such as quantitative real-time PCR and systematic screening protocols have led to greater recognition of adenovirus infection in HSCT recipients. The infection rates reported in the literature vary widely and are likely due to different diagnostic approaches, host factors, and conditioning regimens; thus, standardization of detection methods is required to definitively quantify the incidence of disease in this patient population.97 In general, pediatric stem cell transplant recipients are much more likely to develop adenovirus infection (20%–26%) compared with adults (9%) because they have limited natural exposure before transplant and lack species cross-reactive T cells.98, 99 Most adenoviral infections are detected during the first 100 days after transplantation although the median time to detection is much shorter in children (<30 days) compared with adults (>90 days).97 Several risk factors for adenoviral infection (Box 2 ) and disease (Box 3 ) after transplant that reflect a state of profound host immune deficiency have been identified. Adenoviral infection may cause subclinical viremia, single organ system dysfunction (interstitial pneumonitis, cholangiohepatitis, hemorrhagic cystitis, or colitis, and so forth) or may disseminate to involve 2 or more organ systems and cause a significantly higher mortality (8%–26%).98 Detection of adenovirus in the blood may precede symptomatic disease by 2 to 3 weeks and provides an opportunity for preemptive intervention95, 98; however, identification of patients who might benefit most from therapy remains challenging. For example, a retrospective study of 26 pediatric HSCT recipients showed that 7 of the 11 patients diagnosed with adenovirus infection cleared the virus without antiviral therapy.100 Coinfection or sequential infection with multiple serotypes has been demonstrated in pediatric HSCT recipients and these individuals may have prolonged viral excretion.
Box 2. Risk factors for adenovirus infection in hematopoietic stem cell transplantation.
Younger age
Mismatched or unrelated grafts
Total body irradiation
Presence of adenovirus antibody in the donor
Use of antithymocyte globulin or anti-CD52 (alemtuzumab)
T-cell depleted or stem cell (CD34+) selected graft
Box 3. Risk factors for development of adenovirus disease in HSCTx.
High dose immunosuppression
Severe or prolonged lymphopenia
Moderate to severe GVHD
Detection of adenovirus in peripheral blood
Rising viral load in peripheral blood
Rising viral load in stool
Solid-Organ Transplantation
Adenovirus infection typically occurs within the first 3 months of solid-organ transplantation and has a predilection to involve the allograft.101 Clinical manifestations of liver infection include jaundice and hepatitis, lung infection presents as obliterative bronchiolitis or respiratory failure, cardiac infection leads to coronary vasculopathy, renal infection presents mainly with hemorrhagic cystitis that may be complicated by pneumonia, and intestinal infection presents with diarrhea. In all these situations, severe adenovirus infection may lead to premature graft loss or death. Risk factors for adenoviral infection include younger age (particularly children <5 years old), intestinal transplantation, augmented immune suppression including antilymphocyte antibodies, and transplantation of a seropositive organ into a seronegative recipient.102 In addition to exogenous acquisition or possible transmission via the allograft,103 organ transplant recipients may reactivate endogenous adenoviral infection after receiving immune suppression. In an older series, the incidence in adult liver transplant patients was 5.8% and most patients had symptomatic disease of varying severity.104 More recent studies using PCR detection have also shown that asymptomatic infection in the blood of adult solid-organ transplant recipients is common (6.5%–22.5%); however, most cases have not been associated with progressive disease or acute graft rejection and for this reason routine screening is not recommended.85, 101, 102 Molecular detection of adenovirus in blood or affected tissue samples is indicated for adult solid-organ transplant recipients with compatible disease manifestations. Conversely, in pediatric solid-organ recipients, serial monitoring of viral load in the blood may precede the development of symptomatic disease and serve as a useful guide for the initiation of antiviral therapy.105 Currently there are no specific measures for prevention of adenovirus infection in the transplant recipient.
Congenital Immune Deficiency
Patients with congenital immune deficiency are prone to infection with a wide array of microbes (bacterial, fungal, viral, and so forth) including adenovirus.86 Because of the exceedingly rare nature of these inherited disorders, the true incidence of adenoviral disease is not known. Adenovirus infection is most commonly described in severe congenital immune deficiency (SCID), a condition in which both cellular and humoral immunity is defective. Adenoviral disease in SCID may involve 1 or more organ systems and be rapidly fatal; in addition, there is a higher incidence of unusual serotypes in these patients compared with immunocompetent children.106
AIDS
Before the development of highly active antiretroviral therapy (HAART) for patients infected with HIV, adenovirus infection was frequently detected and was associated with several other opportunistic pathogens. Despite this potential confounding effect of multiple coinfections, various case reports have described severe pneumonia, hepatitis, meningoencephalitis, nephritis, gastrointestinal, and potentially fatal disseminated disease caused by adenovirus.107, 108 A comprehensive review estimated that 12% of patients with clinical AIDS develop active adenovirus infection. In these studies, the mean age of patients was 31 years and within 2 months of first detection of adenovirus, the associated mortality was 45%. The most remarkable aspects of adenovirus infection in AIDS were the diversity of serotypes and frequency of antigenically intermediate isolates that are presumed to arise through spontaneous mutation within a strain or recombination between coinfecting serotypes during long-term infection. To clarify the epidemiology of adenovirus infection in patients infected with HIV, a prospective longitudinal study was subsequently performed and determined that the 1-year actuarial incidence of adenovirus infection was 17% with a CD4 count greater than 200 mm3 and 38% with a CD4 count less than 200 mm3. Prolonged and generally asymptomatic shedding from the gastrointestinal tract, and to a lesser degree the urinary tract, was documented in most patients. These data suggest that the mortality rate of adenovirus infection in AIDS may be lower than previously stated; however, differences in patient populations and adenovirus detection methods preclude a definitive conclusion of the true attributable mortality. Nonetheless, in the absence of another plausible explanation, adenovirus infection should be considered as a potential causative agent in the critically ill patient infected with HIV who has severe pneumonia, encephalitis, or hepatitis and a high retroviral load and/or a CD4 cell count less than 200 mm3.
Diagnosis
In addition to exclusion of bacterial and atypical organisms, the main differential diagnosis of severe viral pneumonia in the immunocompetent host includes influenza, parainfluenza, and RSV, followed by several less common agents such as measles, varicella, rhinovirus, and others.109, 110 In the immunocompromised host, reactivation of members of the herpesvirus family (cytomegalovirus [CMV], Epstein-Barr virus [EBV], and human herpesvirus 6 [HHV-6]) as well as adenovirus should also be considered in the differential diagnosis of life-threatening pneumonia, meningoencephalitis, or cases of disseminated organ dysfunction. In such cases, adequate sample procurement from all potentially infected sites is required for further analysis. For severe respiratory failure, a nasopharyngeal aspirate, endotracheal secretions, or bronchoalveolar lavage are all suitable and relatively easy to obtain for viral and other microbiological studies. Comprehensive analysis of lung tissue is also extremely valuable if a transbronchial or surgical biopsy has been performed. When disseminated disease is suspected, a blood sample should also be obtained for detection of circulating adenovirus. Until recently, the gold standard for diagnosis of adenoviral disease from clinical samples was identification of a characteristic cytopathic effect using tissue culture–based methods; however, this approach is slow, costly, technically challenging, and may lack sensitivity. Direct antigen detection in respiratory specimens by immunofluorescence or related techniques is a rapid but less sensitive alternative to viral isolation.111, 112 Serology for determination of antibody responses and viral serotyping or genotyping methods are impractical for use in clinical decision making and are mainly used for epidemiologic investigations. All these techniques have now been supplanted by molecular detection methods that can be applied to blood, stool, sputum, or biopsy specimens.113 Qualitative PCR and quantitative real-time PCR are rapid and highly sensitive techniques; however, both are critically dependent on the design of primers and probes that are able to amplify and detect the genomes of all clinically relevant adenoviruses.114, 115 Amplification of 1 or more highly conserved adenovirus hexon gene sequence or the use of multiple primer/probe combinations in a single assay have been successfully used by several investigators to identify all disease-associated serotypes. Medical centers may choose to establish their own local PCR protocols for adenovirus detection or refer their samples to commercial laboratories or other service providers. Regardless of where adenovirus PCR testing is done, it is essential to know the sensitivity and specificity of the testing protocol for each type of clinical sample that is analyzed.
In addition to confirming a diagnosis of severe clinical disease, many bone marrow transplant centers now use weekly quantitative PCR to monitor the DNA load of adenovirus (as well as CMV, EBV, and HHV-6) at mucosal sites or in the blood before and after stem cell transplantation.98 Positive viral identification at a mucosal site in the absence of clinical or laboratory abnormality is consistent with infection but does not necessarily signify disease. On the other hand, adenovirus detection from the same site on multiple occasions or from multiple sites is predictive of disease risk and death.116 It has been suggested that detection of adenovirus in the blood of stem cell transplant patients should be defined as probable disseminated disease and may signify a window of opportunity for early intervention.117 One such approach using serial analysis of viral load for therapeutic decision making in pediatric stem cell transplant patients was recently published; however, it is not known whether this strategy is applicable to adults.98, 118 A decreasing viral copy number may be used as a surrogate for a response to therapy; conversely, a titer greater than 106/mL is associated with a greater likelihood of death.116 The usefulness of early intervention after stem cell transplantation is supported by another series in which delayed initiation of cidofovir seemed to be associated with a poor outcome.119
Management
The management options for severe adenoviral disease are limited because there are no formally approved antiviral agents with proven efficacy and no prospective randomized trials of antiadenoviral therapy. Several studies have correlated clinical recovery with the return of T cells; therefore, a reduction of immune suppression is generally recommended as soon as a diagnosis of adenovirus infection has been established.120 A variety of different antiviral agents including ribavirin, ganciclovir, and cidofovir have been used to treat adenovirus infection; however, none of these compounds have been shown to be particularly efficacious for established disease.102 Cidofovir is an acyclic analogue of deoxycytidine monophosphate, which has in vitro activity against several DNA viruses and is approved for treatment of CMV retinitis. Because of its poor bioavailability, cidofovir must be given intravenously and is converted by host enzymes to an active diphosphate form with a prolonged intracellular half-life. Cidofovir diphosphate exhibits higher binding affinity for viral DNA polymerase compared with the host cell enzyme and inhibits viral replication by competing with host nucleotides for incorporation to viral DNA.121, 122 The most common dosing regimens are 5 mg/kg intravenously weekly for 2 weeks then once every other week until an appropriate clinical response is observed. Cidofovir is filtered and actively secreted by the proximal tubule of the kidney with 90% of the drug excreted unchanged in the urine. Dose-dependent nephrotoxicity is the major adverse effect of cidofovir; it presents as proteinuria, azotemia, glycosuria, metabolic acidosis, and, uncommonly, Fanconi syndrome. Saline prehydration and administration of probenecid are recommended to mitigate cidofovir toxicity; a dose adjustment or discontinuation of cidofovir is recommended in the case of an increasing creatinine level or proteinuria. Lipid analogues such as hexadecyloxypropyl-cidofovir exhibit much better cellular penetration and greater in vitro activity against adenovirus and have shown promising results in an immune-suppressed animal model of disseminated disease.123 Ribavirin, a guanosine analogue used to treat severe RSV pneumonia, has not shown consistent clinical benefits or efficacy for a variety of severe adenovirus infections and is not recommended.124 Similarly, ganciclovir shows in vitro activity against some adenovirus isolates and was reported to reduce the incidence of infection in stem cell transplantation125; however, there is insufficient clinical experience to support its usefulness.
Various strategies for immune reconstitution of the host are currently being investigated but are not yet widely available. For example, donor lymphocyte infusion has been associated with temporary clearance of adenovirus; however, this approach is currently limited by the risk of exacerbation of graft-versus-host disease (GVHD). Another approach that has been effective in preventing and treating diseases related to EBV and CMV reactivations in HSCT recipients is reconstitution of the host with in vitro expanded virus-specific cytotoxic T lymphocytes (CTLs).126 Widespread application of this strategy for definitive cure of adenovirus infection is limited by incomplete knowledge of antigen-specific T-cell immunity as well as the practical, technical, regulatory, and financial barriers to generation of sufficient subgroup cross-reactive, adenovirus-specific, cytotoxic T lymphocytes ex vivo.90, 97 Novel CD4 and CD8 T-cell epitopes of the hexon protein have been recently identified and may lead to improved adoptive immunotherapy strategies in the future. Intravenous immunoglobulin has been used to treat severe adenovirus infection; however, its role is currently undefined.102
Hantavirus pulmonary syndrome
Introduction
The genus Hantavirus includes a diverse group of spherical enveloped viruses that all have a trisegmented negative-sense single-strand RNA genome.127 Individual viral strains are maintained through prolonged asymptomatic infection of specific rodent hosts and the distribution of human disease depends on local ecological circumstances. Outside North America, a variety of hantaviruses such as the Hantaan virus are known to cause a serious and fatal acute disease termed hemorrhagic fever with renal syndrome. In North America, the related Sin Nombre virus (SNV) is the most common cause of hantavirus cardiopulmonary syndrome, an acute condition characterized by severe respiratory failure, shock, and mild renal dysfunction. In South America, the Andes virus causes a similar condition and is the only hantavirus to be associated with person to person transmission.128, 129, 130 Zoonotic transmission of SNV and related strains occurs by domestic or occupational exposure to aerosols of infected rodent excreta and is more likely when rodent populations are abundant and enter buildings in search of food or cover. The first outbreak of North American hantavirus pulmonary syndrome was described in the Four Corners region of the United States in 1993 with a subsequent outbreak in 1998; both episodes were attributed to higher rodent densities and greater human exposure as a result of increased precipitation.131, 132
Clinical and Pathologic Features
The clinical manifestations of hantavirus infection follow a long incubation period (7–25 days) and are characterized by a prodrome of fever, chills, myalgia, and gastrointestinal symptoms.131, 133 A mild cough and dyspnea develop after several days and are associated with mild pulmonary disease in one-third of cases.134 On the other hand, most patients rapidly develop severe respiratory distress and profound oxygen desaturation with progressive radiographic evidence of noncardiogenic pulmonary edema.135 The physical findings at the time of presentation are usually nonspecific and include fever, tachypnea, tachycardia, inspiratory crackles, and mild hypotension. Laboratory studies reveal hemoconcentration, mild thrombocytopenia, left shift with circulating myeloblasts and circulating immunoblasts, and mildly abnormal liver function tests. Dizziness, nausea or vomiting, and absence of cough at presentation as well as thrombocytopenia, an increased hematocrit, and a decreased serum bicarbonate level help distinguish hantavirus pulmonary syndrome (HPS) from other common causes of acute respiratory distress such as pneumococcal pneumonia and influenza.136 In severe cases, hemodynamic analysis is consistent with a shock state that is attributable to a combination of fluid redistribution and myocardial depression.137, 138 The outcome of HPS is unpredictable and varies widely; in some series, all patients with a mild clinical course survived whereas the mortality of those with fulminant disease was as high as 46%.139, 140
In 40 of 44 fatal cases of HPS, histopathologic analysis showed interstitial pneumonitis with a variable mononuclear cell infiltrate, edema, and focal hyaline membranes; the remainder had diffuse alveolar damage with variable degrees of severe air-space disorganization.141 Immunohistochemistry and electron microscopy demonstrated the widespread presence of hantavirus antigens and viral inclusions in pulmonary microvascular endothelial cells as well as dendritic cells, macrophages, and lymphocytes. In severe clinical disease, an immunopathologic response has been implicated in the pathogenesis of the capillary leak syndrome that results in severe pulmonary edema.
Diagnosis
HPS is an extremely rare disease in North America; less than 500 cases have been reported in the United States. Historical information such as recent travel to rural areas with endemic hantavirus infection and/or exposure to potentially infected rodents that may have entered human dwellings is crucial to its initial consideration. Patients presenting with severe hantavirus infection invariably have a detectable humoral immune response and positive serologic testing for IgM or IgG is diagnostic of disease in low-prevalence geographic areas.142 Viremia occurs during the first 10 days of illness and may be detected by RT-PCR of blood143; however, isolation of hantavirus requires appropriate containment facilities and is not practical for diagnostic purposes.
Treatment and Prevention
The mainstay of clinical management of HPS is excellent supportive care with particular attention to careful management of fluid status to minimize alveolar edema while maintaining overall organ perfusion.144 The use of rescue therapies such as steroids and extracorporeal life support are of potential benefit127, 145, 146; however, administration of ribavirin was not shown to be efficacious against SNV despite its in vitro activity against hantavirus.147 Prevention of human hantavirus infection includes environmental and ecological measures to control or reduce exposure to rodent reservoirs.148
Acknowledgments
The authors thank Adam Flaczyk for assistance with article preparation and Dr Cedric P. Yansouni for critical review of the article content.
Footnotes
Contributors: N. Lee drafted the sections on coronavirus and RSV; S.T. Qureshi drafted the sections on adenovirus and hantavirus, edited, and assembled the final content of the article.
Potential Conflicts of Interest: The authors declare that they do not have any conflicts of interest.
S.T. Qureshi holds a Tier II Canada Research Chair in Respiratory Infection and receives research funding from the Canadian Institutes of Health Research.
References
- 1.Peiris J.S., Yuen K.Y., Osterhaus A.D. The severe acute respiratory syndrome. N Engl J Med. 2003;349(25):2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 2.Kojaoghlanian T., Flomenberg P., Horwitz M.S. The impact of adenovirus infection on the immunocompromised host. Rev Med Virol. 2003;13(3):155–171. doi: 10.1002/rmv.386. [DOI] [PubMed] [Google Scholar]
- 3.Chan P.K., Tang J.W., Hui D.S. SARS: clinical presentation, transmission, pathogenesis and treatment options. Clin Sci (Lond) 2006;110(2):193–204. doi: 10.1042/CS20050188. [DOI] [PubMed] [Google Scholar]
- 4.Raj V.S., Mou H., Smits S.L. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen J.M., Cooper N., Chakrabarti S. EBV-related disease following haematopoietic stem cell transplantation with reduced intensity conditioning. Leuk Lymphoma. 2007;48(2):256–269. doi: 10.1080/10428190601059837. [DOI] [PubMed] [Google Scholar]
- 6.Gierer S., Bertram S., Kaup F. The spike protein of the emerging betacoronavirus EMC uses a novel coronavirus receptor for entry, can be activated by TMPRSS2, and is targeted by neutralizing antibodies. J Virol. 2013;87(10):5502–5511. doi: 10.1128/JVI.00128-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groneberg D.A., Poutanen S.M., Low D.E. Treatment and vaccines for severe acute respiratory syndrome. Lancet Infect Dis. 2005;5(3):147–155. doi: 10.1016/S1473-3099(05)01307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO). Severe acute respiratory syndrome (SARS). Available at: http://www.who.int/csr/sars/en/. Accessed May 15, 2013.
- 9.Zaki A.M., van Boheemen S., Bestebroer T.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 10.Yu I.T., Xie Z.H., Tsoi K.K. Why did outbreaks of severe acute respiratory syndrome occur in some hospital wards but not in others? Clin Infect Dis. 2007;44(8):1017–1025. doi: 10.1086/512819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee N., Hui D., Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 12.Hui D.S., Chow B.K., Chu L.C. Exhaled air and aerosolized droplet dispersion during application of a jet nebulizer. Chest. 2009;135(3):648–654. doi: 10.1378/chest.08-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim P.L., Kurup A., Gopalakrishna G. Laboratory-acquired severe acute respiratory syndrome. N Engl J Med. 2004;350(17):1740–1745. doi: 10.1056/NEJMoa032565. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO). Coronavirus infections. Available at: http://www.who.int/csr/disease/coronavirus_infections/en/. Accessed June 15, 2013.
- 15.Kindler E., Jonsdottir H.R., Muth D. Efficient replication of the novel human betacoronavirus EMC on primary human epithelium highlights its zoonotic potential. MBio. 2013;4(1):e00611–e00612. doi: 10.1128/mBio.00611-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J., Subbarao K. The immunobiology of SARS*. Annu Rev Immunol. 2007;25:443–472. doi: 10.1146/annurev.immunol.25.022106.141706. [DOI] [PubMed] [Google Scholar]
- 17.Frieman M., Heise M., Baric R. SARS coronavirus and innate immunity. Virus Res. 2008;133(1):101–112. doi: 10.1016/j.virusres.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong C.K., Lam C.W., Wu A.K. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136(1):95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zielecki F., Weber M., Eickmann M. Human cell tropism and innate immune system interactions of human respiratory coronavirus EMC compared to those of severe acute respiratory syndrome coronavirus. J Virol. 2013;87(9):5300–5304. doi: 10.1128/JVI.03496-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng E.K., Hui D.S., Chan K.C. Quantitative analysis and prognostic implication of SARS coronavirus RNA in the plasma and serum of patients with severe acute respiratory syndrome. Clin Chem. 2003;49(12):1976–1980. doi: 10.1373/clinchem.2003.024125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munster V.J., de Wit E., Feldmann H. Pneumonia from human coronavirus in a macaque model. N Engl J Med. 2013;368(16):1560–1562. doi: 10.1056/NEJMc1215691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sung J.J., Wu A., Joynt G.M. Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax. 2004;59(5):414–420. doi: 10.1136/thx.2003.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong K.T., Antonio G.E., Hui D.S. Thin-section CT of severe acute respiratory syndrome: evaluation of 73 patients exposed to or with the disease. Radiology. 2003;228(2):395–400. doi: 10.1148/radiol.2283030541. [DOI] [PubMed] [Google Scholar]
- 24.Yap F.H., Gomersall C.D., Fung K.S. Increase in methicillin-resistant Staphylococcus aureus acquisition rate and change in pathogen pattern associated with an outbreak of severe acute respiratory syndrome. Clin Infect Dis. 2004;39(4):511–516. doi: 10.1086/422641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan P.K., To W.K., Ng K.C. Laboratory diagnosis of SARS. Emerg Infect Dis. 2004;10(5):825–831. doi: 10.3201/eid1005.030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu C.M., Cheng V.C., Hung I.F. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soo Y.O., Cheng Y., Wong R. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10(7):676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts A., Thomas W.D., Guarner J. Therapy with a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody reduces disease severity and viral burden in golden Syrian hamsters. J Infect Dis. 2006;193(5):685–692. doi: 10.1086/500143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyoshi-Akiyama T., Ishida I., Fukushi M. Fully human monoclonal antibody directed to proteolytic cleavage site in severe acute respiratory syndrome (SARS) coronavirus S protein neutralizes the virus in a rhesus macaque SARS model. J Infect Dis. 2011;203(11):1574–1581. doi: 10.1093/infdis/jir084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ter Meulen J., Bakker A.B., van den Brink E.N. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet. 2004;363(9427):2139–2141. doi: 10.1016/S0140-6736(04)16506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sui J., Li W., Murakami A. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc Natl Acad Sci U S A. 2004;101(8):2536–2541. doi: 10.1073/pnas.0307140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu Z., Chakraborti S., He Y. Potent cross-reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies. Proc Natl Acad Sci U S A. 2007;104(29):12123–12128. doi: 10.1073/pnas.0701000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cinatl J., Morgenstern B., Bauer G. Treatment of SARS with human interferons. Lancet. 2003;362(9380):293–294. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haagmans B.L., Kuiken T., Martina B.E. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med. 2004;10(3):290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan R.W., Chan M.C., Agnihothram S. Tropism of and innate immune responses to the novel human betacoronavirus lineage C virus in human ex vivo respiratory organ cultures. J Virol. 2013;87(12):6604–6614. doi: 10.1128/JVI.00009-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li B.J., Tang Q., Cheng D. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nat Med. 2005;11(9):944–951. doi: 10.1038/nm1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma X.Z., Bartczak A., Zhang J. Proteasome inhibition in vivo promotes survival in a lethal murine model of severe acute respiratory syndrome. J Virol. 2010;84(23):12419–12428. doi: 10.1128/JVI.01219-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao J., Wohlford-Lenane C., Zhao J. Intranasal treatment with poly(I*C) protects aged mice from lethal respiratory virus infections. J Virol. 2012;86(21):11416–11424. doi: 10.1128/JVI.01410-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ho J.C., Ooi G.C., Mok T.Y. High-dose pulse versus nonpulse corticosteroid regimens in severe acute respiratory syndrome. Am J Respir Crit Care Med. 2003;168(12):1449–1456. doi: 10.1164/rccm.200306-766OC. [DOI] [PubMed] [Google Scholar]
- 41.Lee N., Allen Chan K.C., Hui D.S. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffith J.F., Antonio G.E., Kumta S.M. Osteonecrosis of hip and knee in patients with severe acute respiratory syndrome treated with steroids. Radiology. 2005;235(1):168–175. doi: 10.1148/radiol.2351040100. [DOI] [PubMed] [Google Scholar]
- 43.Lee N., Hui D.S. Dexamethasone in community-acquired pneumonia. Lancet. 2011;378(9795):979–980. doi: 10.1016/S0140-6736(11)61440-2. [author reply: 981] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen R.C., Tang X.P., Tan S.Y. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129(6):1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walsh E.E. Respiratory syncytial virus infection in adults. Semin Respir Crit Care Med. 2011;32(4):423–432. doi: 10.1055/s-0031-1283282. [DOI] [PubMed] [Google Scholar]
- 46.Walsh E.E., Peterson D.R., Falsey A.R. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis. 2004;189(2):233–238. doi: 10.1086/380907. [DOI] [PubMed] [Google Scholar]
- 47.Duncan C.B., Walsh E.E., Peterson D.R. Risk factors for respiratory failure associated with respiratory syncytial virus infection in adults. J Infect Dis. 2009;200(8):1242–1246. doi: 10.1086/605948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walsh E.E., Peterson D.R., Kalkanoglu A.E. Viral shedding and immune responses to respiratory syncytial virus infection in older adults. J Infect Dis. 2013;207(9):1424–1432. doi: 10.1093/infdis/jit038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeVincenzo J.P., Wilkinson T., Vaishnaw A. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med. 2010;182(10):1305–1314. doi: 10.1164/rccm.201002-0221OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nair H., Nokes D.J., Gessner B.D. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Falsey A.R., Hennessey P.A., Formica M.A. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 52.Widmer K., Zhu Y., Williams J.V. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis. 2012;206(1):56–62. doi: 10.1093/infdis/jis309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jansen A.G., Sanders E.A., Hoes A.W. Influenza- and respiratory syncytial virus-associated mortality and hospitalisations. Eur Respir J. 2007;30(6):1158–1166. doi: 10.1183/09031936.00034407. [DOI] [PubMed] [Google Scholar]
- 54.van Asten L., van den Wijngaard C., van Pelt W. Mortality attributable to 9 common infections: significant effect of influenza A, respiratory syncytial virus, influenza B, norovirus, and parainfluenza in elderly persons. J Infect Dis. 2012;206(5):628–639. doi: 10.1093/infdis/jis415. [DOI] [PubMed] [Google Scholar]
- 55.Choi S.H., Hong S.B., Ko G.B. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am J Respir Crit Care Med. 2012;186(4):325–332. doi: 10.1164/rccm.201112-2240OC. [DOI] [PubMed] [Google Scholar]
- 56.Caram L.B., Chen J., Taggart E.W. Respiratory syncytial virus outbreak in a long-term care facility detected using reverse transcriptase polymerase chain reaction: an argument for real-time detection methods. J Am Geriatr Soc. 2009;57(3):482–485. doi: 10.1111/j.1532-5415.2008.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ellis S.E., Coffey C.S., Mitchel E.F., Jr. Influenza- and respiratory syncytial virus-associated morbidity and mortality in the nursing home population. J Am Geriatr Soc. 2003;51(6):761–767. doi: 10.1046/j.1365-2389.2003.51254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shah J.N., Chemaly R.F. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood. 2011;117(10):2755–2763. doi: 10.1182/blood-2010-08-263400. [DOI] [PubMed] [Google Scholar]
- 59.Walsh E.E., Peterson D.R., Falsey A.R. Is clinical recognition of respiratory syncytial virus infection in hospitalized elderly and high-risk adults possible? J Infect Dis. 2007;195(7):1046–1051. doi: 10.1086/511986. [DOI] [PubMed] [Google Scholar]
- 60.Lee N., Lui G.C., Wong K.T. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis. 2013 doi: 10.1093/cid/cit471. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 61.de-Paris F., Beck C., Machado A.B. Optimization of one-step duplex real-time RT-PCR for detection of influenza and respiratory syncytial virus in nasopharyngeal aspirates. J Virol Methods. 2012;186(1–2):189–192. doi: 10.1016/j.jviromet.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 62.Park S.Y., Baek S., Lee S.O. Efficacy of oral ribavirin in hematologic disease patients with paramyxovirus infection: analytic strategy using propensity scores. Antimicrob Agents Chemother. 2013;57(2):983–989. doi: 10.1128/AAC.01961-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hynicka L.M., Ensor C.R. Prophylaxis and treatment of respiratory syncytial virus in adult immunocompromised patients. Ann Pharmacother. 2012;46(4):558–566. doi: 10.1345/aph.1Q553. [DOI] [PubMed] [Google Scholar]
- 64.Bonavia A., Franti M., Pusateri Keaney E. Identification of broad-spectrum antiviral compounds and assessment of the druggability of their target for efficacy against respiratory syncytial virus (RSV) Proc Natl Acad Sci U S A. 2011;108(17):6739–6744. doi: 10.1073/pnas.1017142108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DeVincenzo J., Lambkin-Williams R., Wilkinson T. A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc Natl Acad Sci U S A. 2010;107(19):8800–8805. doi: 10.1073/pnas.0912186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Woensel J.B., Vyas H., Group S.T. Dexamethasone in children mechanically ventilated for lower respiratory tract infection caused by respiratory syncytial virus: a randomized controlled trial. Crit Care Med. 2011;39(7):1779–1783. doi: 10.1097/CCM.0b013e318218a030. [DOI] [PubMed] [Google Scholar]
- 67.Fernandes R.M., Bialy L.M., Vandermeer B. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2010;(10) doi: 10.1002/14651858.CD004878.pub3. CD004878. [DOI] [PubMed] [Google Scholar]
- 68.Lee F.E., Walsh E.E., Falsey A.R. The effect of steroid use in hospitalized adults with respiratory syncytial virus-related illness. Chest. 2011;140(5):1155–1161. doi: 10.1378/chest.11-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lindsley W.G., Blachere F.M., Davis K.A. Distribution of airborne influenza virus and respiratory syncytial virus in an urgent care medical clinic. Clin Infect Dis. 2010;50(5):693–698. doi: 10.1086/650457. [DOI] [PubMed] [Google Scholar]
- 70.Roelvink P.W., Lizonova A., Lee J.G. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol. 1998;72(10):7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lynch J.P., 3rd, Fishbein M., Echavarria M. Adenovirus. Semin Respir Crit Care Med. 2011;32(4):494–511. doi: 10.1055/s-0031-1283287. [DOI] [PubMed] [Google Scholar]
- 72.Fox J.P., Brandt C.D., Wassermann F.E. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. VI. Observations of adenovirus infections: virus excretion patterns, antibody response, efficiency of surveillance, patterns of infections, and relation to illness. Am J Epidemiol. 1969;89(1):25–50. doi: 10.1093/oxfordjournals.aje.a120913. [DOI] [PubMed] [Google Scholar]
- 73.Fox J.P., Hall C.E., Cooney M.K. The Seattle virus watch. VII. Observations of adenovirus infections. Am J Epidemiol. 1977;105(4):362–386. doi: 10.1093/oxfordjournals.aje.a112394. [DOI] [PubMed] [Google Scholar]
- 74.Sumida S.M., Truitt D.M., Kishko M.G. Neutralizing antibodies and CD8+ T lymphocytes both contribute to immunity to adenovirus serotype 5 vaccine vectors. J Virol. 2004;78(6):2666–2673. doi: 10.1128/JVI.78.6.2666-2673.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ginsberg H.S., Moldawer L.L., Sehgal P.B. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci U S A. 1991;88(5):1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mahr J.A., Gooding L.R. Immune evasion by adenoviruses. Immunol Rev. 1999;168:121–130. doi: 10.1111/j.1600-065x.1999.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 77.Burgert H.G., Ruzsics Z., Obermeier S. Subversion of host defense mechanisms by adenoviruses. Curr Top Microbiol Immunol. 2002;269:273–318. doi: 10.1007/978-3-642-59421-2_16. [DOI] [PubMed] [Google Scholar]
- 78.Cooper R.J., Hallett R., Tullo A.B. The epidemiology of adenovirus infections in Greater Manchester, UK 1982-96. Epidemiol Infect. 2000;125(2):333–345. doi: 10.1017/s0950268899004550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brandt C.D., Kim H.W., Vargosko A.J. Infections in 18,000 infants and children in a controlled study of respiratory tract disease. I. Adenovirus pathogenicity in relation to serologic type and illness syndrome. Am J Epidemiol. 1969;90(6):484–500. doi: 10.1093/oxfordjournals.aje.a121094. [DOI] [PubMed] [Google Scholar]
- 80.Ryan M.A., Gray G.C., Smith B. Large epidemic of respiratory illness due to adenovirus types 7 and 3 in healthy young adults. Clin Infect Dis. 2002;34(5):577–582. doi: 10.1086/338471. [DOI] [PubMed] [Google Scholar]
- 81.Erdman D.D., Xu W., Gerber S.I. Molecular epidemiology of adenovirus type 7 in the United States, 1966-2000. Emerg Infect Dis. 2002;8(3):269–277. doi: 10.3201/eid0803.010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Binn L.N., Sanchez J.L., Gaydos J.C. Emergence of adenovirus type 14 in US military recruits–a new challenge. J Infect Dis. 2007;196(10):1436–1437. doi: 10.1086/522969. [DOI] [PubMed] [Google Scholar]
- 83.Leen A.M., Rooney C.M. Adenovirus as an emerging pathogen in immunocompromised patients. Br J Haematol. 2005;128(2):135–144. doi: 10.1111/j.1365-2141.2004.05218.x. [DOI] [PubMed] [Google Scholar]
- 84.Howard D.S., Phillips I.G., Reece D.E. Adenovirus infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 1999;29(6):1494–1501. doi: 10.1086/313514. [DOI] [PubMed] [Google Scholar]
- 85.Humar A., Kumar D., Mazzulli T. A surveillance study of adenovirus infection in adult solid organ transplant recipients. Am J Transplant. 2005;5(10):2555–2559. doi: 10.1111/j.1600-6143.2005.01033.x. [DOI] [PubMed] [Google Scholar]
- 86.Hierholzer J.C. Adenoviruses in the immunocompromised host. Clin Microbiol Rev. 1992;5(3):262–274. doi: 10.1128/cmr.5.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Komshian S.V., Chandrasekar P.H., Levine D.P. Adenovirus pneumonia in healthy adults. Heart Lung. 1987;16(2):146–150. [PubMed] [Google Scholar]
- 88.Hakim F.A., Tleyjeh I.M. Severe adenovirus pneumonia in immunocompetent adults: a case report and review of the literature. Eur J Clin Microbiol Infect Dis. 2008;27(2):153–158. doi: 10.1007/s10096-007-0416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barker J.H., Luby J.P., Sean Dalley A. Fatal type 3 adenoviral pneumonia in immunocompetent adult identical twins. Clin Infect Dis. 2003;37(10):e142–e146. doi: 10.1086/379127. [DOI] [PubMed] [Google Scholar]
- 90.Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 6-1979. N Engl J Med. 1979;300(6):301–308. doi: 10.1056/NEJM197902083000609. [DOI] [PubMed] [Google Scholar]
- 91.Levin S., Dietrich J., Guillory J. Fatal nonbacterial pneumonia associated with Adenovirus type 4. Occurrence in an adult. JAMA. 1967;201(12):975–977. [PubMed] [Google Scholar]
- 92.Dudding B.A., Wagner S.C., Zeller J.A. Fatal pneumonia associated with adenovirus type 7 in three military trainees. N Engl J Med. 1972;286(24):1289–1292. doi: 10.1056/NEJM197206152862403. [DOI] [PubMed] [Google Scholar]
- 93.Klinger J.R., Sanchez M.P., Curtin L.A. Multiple cases of life-threatening adenovirus pneumonia in a mental health care center. Am J Respir Crit Care Med. 1998;157(2):645–649. doi: 10.1164/ajrccm.157.2.9608057. [DOI] [PubMed] [Google Scholar]
- 94.Landry M.L., Greenwold J., Vikram H.R. Herpes simplex type-2 meningitis: presentation and lack of standardized therapy. Am J Med. 2009;122(7):688–691. doi: 10.1016/j.amjmed.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 95.Echavarria M. Adenoviruses in immunocompromised hosts. Clin Microbiol Rev. 2008;21(4):704–715. doi: 10.1128/CMR.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gray G.C., McCarthy T., Lebeck M.G. Genotype prevalence and risk factors for severe clinical adenovirus infection, United States 2004-2006. Clin Infect Dis. 2007;45(9):1120–1131. doi: 10.1086/522188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leen A.M., Bollard C.M., Myers G.D. Adenoviral infections in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12(3):243–251. doi: 10.1016/j.bbmt.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 98.Lindemans C.A., Leen A.M., Boelens J.J. How I treat adenovirus in hematopoietic stem cell transplant recipients. Blood. 2010;116(25):5476–5485. doi: 10.1182/blood-2010-04-259291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Walls T., Shankar A.G., Shingadia D. Adenovirus: an increasingly important pathogen in paediatric bone marrow transplant patients. Lancet Infect Dis. 2003;3(2):79–86. doi: 10.1016/s1473-3099(03)00515-2. [DOI] [PubMed] [Google Scholar]
- 100.Walls T., Hawrami K., Ushiro-Lumb I. Adenovirus infection after pediatric bone marrow transplantation: is treatment always necessary? Clin Infect Dis. 2005;40(9):1244–1249. doi: 10.1086/429235. [DOI] [PubMed] [Google Scholar]
- 101.Ison M.G. Adenovirus infections in transplant recipients. Clin Infect Dis. 2006;43(3):331–339. doi: 10.1086/505498. [DOI] [PubMed] [Google Scholar]
- 102.Ison M.G., Green M. Adenovirus in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S161–S165. doi: 10.1111/j.1600-6143.2009.02907.x. [DOI] [PubMed] [Google Scholar]
- 103.Koneru B., Atchison R., Jaffe R. Serological studies of adenoviral hepatitis following pediatric liver transplantation. Transplant Proc. 1990;22(4):1547–1548. [PMC free article] [PubMed] [Google Scholar]
- 104.McGrath D., Falagas M.E., Freeman R. Adenovirus infection in adult orthotopic liver transplant recipients: incidence and clinical significance. J Infect Dis. 1998;177(2):459–462. doi: 10.1086/517375. [DOI] [PubMed] [Google Scholar]
- 105.Seidemann K., Heim A., Pfister E.D. Monitoring of adenovirus infection in pediatric transplant recipients by quantitative PCR: report of six cases and review of the literature. Am J Transplant. 2004;4(12):2102–2108. doi: 10.1111/j.1600-6143.2004.00631.x. [DOI] [PubMed] [Google Scholar]
- 106.South M.A., Dolen J., Beach D.K. Fatal adenovirus hepatic necrosis in severe combined immune deficiency. Pediatr Infect Dis. 1982;1(6):416–419. doi: 10.1097/00006454-198211000-00012. [DOI] [PubMed] [Google Scholar]
- 107.Khoo S.H., Bailey A.S., de Jong J.C. Adenovirus infections in human immunodeficiency virus-positive patients: clinical features and molecular epidemiology. J Infect Dis. 1995;172(3):629–637. doi: 10.1093/infdis/172.3.629. [DOI] [PubMed] [Google Scholar]
- 108.Schnurr D., Bollen A., Crawford-Miksza L. Adenovirus mixture isolated from the brain of an AIDS patient with encephalitis. J Med Virol. 1995;47(2):168–171. doi: 10.1002/jmv.1890470210. [DOI] [PubMed] [Google Scholar]
- 109.Vigil K.J., Adachi J.A., Chemaly R.F. Viral pneumonias in immunocompromised adult hosts. J Intensive Care Med. 2010;25(6):307–326. doi: 10.1177/0885066610377969. [DOI] [PubMed] [Google Scholar]
- 110.Chemaly R.F., Ghosh S., Bodey G.P. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine. 2006;85(5):278–287. doi: 10.1097/01.md.0000232560.22098.4e. [DOI] [PubMed] [Google Scholar]
- 111.August M.J., Warford A.L. Evaluation of a commercial monoclonal antibody for detection of adenovirus antigen. J Clin Microbiol. 1987;25(11):2233–2235. doi: 10.1128/jcm.25.11.2233-2235.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shetty A.K., Treynor E., Hill D.W. Comparison of conventional viral cultures with direct fluorescent antibody stains for diagnosis of community-acquired respiratory virus infections in hospitalized children. Pediatr Infect Dis J. 2003;22(9):789–794. doi: 10.1097/01.inf.0000083823.43526.97. [DOI] [PubMed] [Google Scholar]
- 113.Fox J.D. Nucleic acid amplification tests for detection of respiratory viruses. J Clin Virol. 2007;40(Suppl 1):S15–S23. doi: 10.1016/S1386-6532(07)70005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Heim A., Ebnet C., Harste G. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J Med Virol. 2003;70(2):228–239. doi: 10.1002/jmv.10382. [DOI] [PubMed] [Google Scholar]
- 115.Lion T., Baumgartinger R., Watzinger F. Molecular monitoring of adenovirus in peripheral blood after allogeneic bone marrow transplantation permits early diagnosis of disseminated disease. Blood. 2003;102(3):1114–1120. doi: 10.1182/blood-2002-07-2152. [DOI] [PubMed] [Google Scholar]
- 116.Claas E.C., Schilham M.W., de Brouwer C.S. Internally controlled real-time PCR monitoring of adenovirus DNA load in serum or plasma of transplant recipients. J Clin Microbiol. 2005;43(4):1738–1744. doi: 10.1128/JCM.43.4.1738-1744.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Suparno C., Milligan D.W., Moss P.A. Adenovirus infections in stem cell transplant recipients: recent developments in understanding of pathogenesis, diagnosis and management. Leuk Lymphoma. 2004;45(5):873–885. doi: 10.1080/10428190310001628176. [DOI] [PubMed] [Google Scholar]
- 118.Leruez-Ville M., Minard V., Lacaille F. Real-time blood plasma polymerase chain reaction for management of disseminated adenovirus infection. Clin Infect Dis. 2004;38(1):45–52. doi: 10.1086/380450. [DOI] [PubMed] [Google Scholar]
- 119.Neofytos D., Ojha A., Mookerjee B. Treatment of adenovirus disease in stem cell transplant recipients with cidofovir. Biol Blood Marrow Transplant. 2007;13(1):74–81. doi: 10.1016/j.bbmt.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 120.Chakrabarti S., Mautner V., Osman H. Adenovirus infections following allogeneic stem cell transplantation: incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood. 2002;100(5):1619–1627. doi: 10.1182/blood-2002-02-0377. [DOI] [PubMed] [Google Scholar]
- 121.Lenaerts L., De Clercq E., Naesens L. Clinical features and treatment of adenovirus infections. Rev Med Virol. 2008;18(6):357–374. doi: 10.1002/rmv.589. [DOI] [PubMed] [Google Scholar]
- 122.Lenaerts L., Naesens L. Antiviral therapy for adenovirus infections. Antiviral Res. 2006;71(2–3):172–180. doi: 10.1016/j.antiviral.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 123.Toth K., Spencer J.F., Dhar D. Hexadecyloxypropyl-cidofovir, CMX001, prevents adenovirus-induced mortality in a permissive, immunosuppressed animal model. Proc Natl Acad Sci U S A. 2008;105(20):7293–7297. doi: 10.1073/pnas.0800200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gavin P.J., Katz B.Z. Intravenous ribavirin treatment for severe adenovirus disease in immunocompromised children. Pediatrics. 2002;110(1 Pt 1):e9. doi: 10.1542/peds.110.1.e9. [DOI] [PubMed] [Google Scholar]
- 125.Bruno B., Gooley T., Hackman R.C. Adenovirus infection in hematopoietic stem cell transplantation: effect of ganciclovir and impact on survival. Biol Blood Marrow Transplant. 2003;9(5):341–352. doi: 10.1016/s1083-8791(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 126.Leen A.M., Christin A., Khalil M. Identification of hexon-specific CD4 and CD8 T-cell epitopes for vaccine and immunotherapy. J Virol. 2008;82(1):546–554. doi: 10.1128/JVI.01689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Peters C.J., Khan A.S. Hantavirus pulmonary syndrome: the new American hemorrhagic fever. Clin Infect Dis. 2002;34(9):1224–1231. doi: 10.1086/339864. [DOI] [PubMed] [Google Scholar]
- 128.Ferres M., Vial P., Marco C. Prospective evaluation of household contacts of persons with hantavirus cardiopulmonary syndrome in Chile. J Infect Dis. 2007;195(11):1563–1571. doi: 10.1086/516786. [DOI] [PubMed] [Google Scholar]
- 129.Wells R.M., Sosa Estani S., Yadon Z.E. An unusual hantavirus outbreak in southern Argentina: person-to-person transmission? Hantavirus Pulmonary Syndrome Study Group for Patagonia. Emerg Infect Dis. 1997;3(2):171–174. doi: 10.3201/eid0302.970210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wells R.M., Young J., Williams R.J. Hantavirus transmission in the United States. Emerg Infect Dis. 1997;3(3):361–365. doi: 10.3201/eid0303.970314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Khan A.S., Khabbaz R.F., Armstrong L.R. Hantavirus pulmonary syndrome: the first 100 US cases. J Infect Dis. 1996;173(6):1297–1303. doi: 10.1093/infdis/173.6.1297. [DOI] [PubMed] [Google Scholar]
- 132.Hjelle B., Glass G.E. Outbreak of hantavirus infection in the Four Corners region of the United States in the wake of the 1997-1998 El Nino-southern oscillation. J Infect Dis. 2000;181(5):1569–1573. doi: 10.1086/315467. [DOI] [PubMed] [Google Scholar]
- 133.Young J.C., Hansen G.R., Graves T.K. The incubation period of hantavirus pulmonary syndrome. Am J Trop Med Hyg. 2000;62(6):714–717. doi: 10.4269/ajtmh.2000.62.714. [DOI] [PubMed] [Google Scholar]
- 134.Zavasky D.M., Hjelle B., Peterson M.C. Acute infection with Sin Nombre hantavirus without pulmonary edema. Clin Infect Dis. 1999;29(3):664–666. doi: 10.1086/598649. [DOI] [PubMed] [Google Scholar]
- 135.Ketai L.H., Williamson M.R., Telepak R.J. Hantavirus pulmonary syndrome: radiographic findings in 16 patients. Radiology. 1994;191(3):665–668. doi: 10.1148/radiology.191.3.8184043. [DOI] [PubMed] [Google Scholar]
- 136.Moolenaar R.L., Dalton C., Lipman H.B. Clinical features that differentiate hantavirus pulmonary syndrome from three other acute respiratory illnesses. Clin Infect Dis. 1995;21(3):643–649. doi: 10.1093/clinids/21.3.643. [DOI] [PubMed] [Google Scholar]
- 137.Hallin G.W., Simpson S.Q., Crowell R.E. Cardiopulmonary manifestations of hantavirus pulmonary syndrome. Crit Care Med. 1996;24(2):252–258. doi: 10.1097/00003246-199602000-00012. [DOI] [PubMed] [Google Scholar]
- 138.Saggioro F.P., Rossi M.A., Duarte M.I. Hantavirus infection induces a typical myocarditis that may be responsible for myocardial depression and shock in hantavirus pulmonary syndrome. J Infect Dis. 2007;195(10):1541–1549. doi: 10.1086/513874. [DOI] [PubMed] [Google Scholar]
- 139.Verity R., Prasad E., Grimsrud K. Hantavirus pulmonary syndrome in northern Alberta, Canada: clinical and laboratory findings for 19 cases. Clin Infect Dis. 2000;31(4):942–946. doi: 10.1086/318137. [DOI] [PubMed] [Google Scholar]
- 140.Boroja M., Barrie J.R., Raymond G.S. Radiographic findings in 20 patients with Hantavirus pulmonary syndrome correlated with clinical outcome. AJR Am J Roentgenol. 2002;178(1):159–163. doi: 10.2214/ajr.178.1.1780159. [DOI] [PubMed] [Google Scholar]
- 141.Nolte K.B., Feddersen R.M., Foucar K. Hantavirus pulmonary syndrome in the United States: a pathological description of a disease caused by a new agent. Hum Pathol. 1995;26(1):110–120. doi: 10.1016/0046-8177(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 142.Bharadwaj M., Nofchissey R., Goade D. Humoral immune responses in the hantavirus cardiopulmonary syndrome. J Infect Dis. 2000;182(1):43–48. doi: 10.1086/315657. [DOI] [PubMed] [Google Scholar]
- 143.Terajima M., Hendershot J.D., 3rd, Kariwa H. High levels of viremia in patients with the Hantavirus pulmonary syndrome. J Infect Dis. 1999;180(6):2030–2034. doi: 10.1086/315153. [DOI] [PubMed] [Google Scholar]
- 144.Levy H., Simpson S.Q. Hantavirus pulmonary syndrome. Am J Respir Crit Care Med. 1994;149(6):1710–1713. doi: 10.1164/ajrccm.149.6.8004332. [DOI] [PubMed] [Google Scholar]
- 145.Mertz G.J., Hjelle B., Crowley M. Diagnosis and treatment of new world hantavirus infections. Curr Opin Infect Dis. 2006;19(5):437–442. doi: 10.1097/01.qco.0000244048.38758.1f. [DOI] [PubMed] [Google Scholar]
- 146.Crowley M.R., Katz R.W., Kessler R. Successful treatment of adults with severe Hantavirus pulmonary syndrome with extracorporeal membrane oxygenation. Crit Care Med. 1998;26(2):409–414. doi: 10.1097/00003246-199802000-00047. [DOI] [PubMed] [Google Scholar]
- 147.Mertz G.J., Miedzinski L., Goade D. Placebo-controlled, double-blind trial of intravenous ribavirin for the treatment of hantavirus cardiopulmonary syndrome in North America. Clin Infect Dis. 2004;39(9):1307–1313. doi: 10.1086/425007. [DOI] [PubMed] [Google Scholar]
- 148.Mills J.N., Corneli A., Young J.C. Hantavirus pulmonary syndrome–United States: updated recommendations for risk reduction. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002;51(RR-9):1–12. [PubMed] [Google Scholar]
- 149.Leen A.M., Heslop H.E. Cytotoxic T lymphocytes as immune-therapy in haematological practice. Br J Haematol. 2008;143(2):169–179. doi: 10.1111/j.1365-2141.2008.07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]