1. Introduction
This is the first in a series of two articles dealing with viral infections of importance to elderly populations. Respiratory viral infections have long been recognized as important contributors to death and disability in older adults. The specific reasons why these infectious agents, which usually cause self-limited disease in healthy young adults, are associated with enhanced disease with aging are not completely known. Analysis of this problem is complicated by the difficulties in separating the effects of age itself from those of the chronic diseases that often accompany aging. However, more limited physiologic reserves with aging and potentially blunted immune responses may play a role.
Influenza virus has traditionally received the greatest attention and remains a significant problem in aging populations, while respiratory syncytial virus and parainfluenza viruses have also been implicated as potential causes of serious illness in some elderly individuals. In this brief review, the disease impact of infections with influenza, respiratory syncytial, and parainfluenza viruses will be reviewed, and approaches to control of these agents will be outlined.
2. Influenza
2.1. Disease impact
Influenza is unique among respiratory viruses in that epidemics are regularly associated with excess morbidity and mortality (Glezen, 1982), manifested as excess rates of pneumonia and influenza associated hospitalization during influenza epidemics (Glezen, 1982, Perrotta et al., 1985). For older individuals, the yearly estimate is 4.1–4.4 million excess respiratory illnesses associated with influenza each year (Sullivan et al., 1993). Recent, non-pandemic influenza seasons have been associated, on average, with 5600 excess pneumonia and influenza deaths and 21 300 all-cause excess deaths in the USA (Simonsen et al., 1997). Both influenza A and B can be associated with severe illness (Blaine et al., 1980), although the highest mortality rates are generally seen during years with significant H3 virus activity. Complications and deaths from influenza are of particular concern in those with certain ‘high-risk’ medical conditions, primarily the elderly and adults and children with cardiovascular and pulmonary conditions (Barker and Mullooly, 1980) The presence of such conditions significantly increases the risk of influenza-related hospitalizations above that associated with age alone (Barker and Mullooly, 1982; Table 1 ). Although only about one-fourth of hospitalizations for influenza occur in individuals over the age of 65, three-fourths of influenza-related deaths occur in this age group (Perrotta et al., 1985). It is important to recognize that the majority of elderly high-risk individuals who are hospitalized during influenza epidemics were ambulatory and leading productive lives prior to their acute illness (Barker and Mullooly, 1980).
Table 1.
Effect of age and high-risk conditions on deaths during influenza epidemicsa
| Age group | HR status | No. of P and I deaths | P and I deaths per 100(000±SEM |
|---|---|---|---|
| 15–44 | No HR | 0 | 0 |
| 1 HR condition | 0 | 0 | |
| (2 HR conditions | 0 | 0 | |
| 15–64 | No HR | 1 | 2±2 |
| 1 HR condition | 7 | 10±13 | |
| (2 HR conditions | 4 | 377±195 | |
| (65 | No HR | 1 | 9±9 |
| 1 HR condition | 14 | 217±59 | |
| (2 HR conditions | 11 | 306±257 |
Adapted from Barker and Mullooly (1982).
In addition to causing serious illness and deaths, influenza in the elderly results in significant decreases in functional abilities. In recent studies, 10–12% of individuals over 65 admitted to hospital with acute respiratory disease required a higher level of assistance with daily living then they required prior to illness (Gladman et al., 1991, Falsey et al., 1995a). In one nursing home outbreak, 25% of those surviving acute influenza experienced a significant decline in functional status (Barker et al., 1998).
2.2. Treatment and prevention
2.2.1. Current inactivated influenza vaccine
The most effective measure currently available for the control of influenza is the annual administration of inactivated influenza vaccine. The vaccines are well-tolerated and increases in hemagglutination inhibition (HAI) antibody are seen in most young adult recipients (Cate et al., 1983, LaMontagne et al., 1983, Quinnan et al., 1983). Inactivated influenza vaccine has been shown to be effective in the prevention of influenza A in several controlled studies conducted in young adults, with levels of protection of 70–90% when there is a good antigenic match between vaccine and epidemic viruses (Meiklejohn et al., 1978, Meiklejohn, 1983, Ruben, 1987).
Relatively few prospective trials of protective efficacy have been conducted in elderly populations. In one recent randomized placebo-controlled trial, inactivated vaccine was approximately 58% effective in preventing laboratory-documented influenza in an otherwise healthy elderly population (Fig. 1 ; Govaert et al., 1994). In addition, retrospective case–control studies have documented the effectiveness of inactivated influenza vaccines in the elderly. For example, the study by Barker and Mulooly documented a 72% reduction in hospitalization and 87% reduction in mortality with influenza vaccine in the elderly (Barker and Mullooly, 1980). A recent meta-analysis of cohort observational studies resulted in pooled estimates of efficacy of vaccine of 56% for prevention of influenza-related respiratory illness, 53% for prevention of pneumonia, 50% for prevention of hospitalization, and 68% for prevention of death (Gross et al., 1995). Vaccination was 43–65% effective in preventing pneumonia and influenza-related hospital deaths, and 27–30% effective in preventing deaths from all causes during influenza outbreak periods in non-institutionalized persons 45 and older in Manitoba (Fedson et al., 1993). It has been estimated that among elderly persons living in the community, influenza vaccination is associated with a direct savings of $117 per year per person vaccinated (Nichol et al., 1994).
Fig. 1.
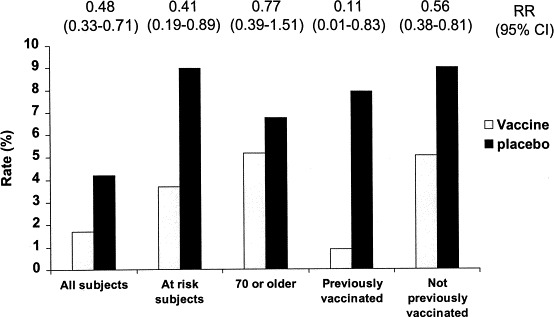
Rates of serologically documented influenza infection in adults over 60 randomized to receive inactivated influenza vaccine or placebo (data from Govaert et al., 1994).
However, the protective efficacy of vaccination appears to be lower in individuals over age 65 than it is in younger adults. The explanation for this decreased efficacy in the elderly is not completely clear, but is at least in part related to decreased antibody responsiveness to influenza vaccine in this age group (Quinnan et al., 1983, Coles et al., 1992, Nicholson et al., 1992, Powers and Belshe, 1993, Remarque et al., 1993, Treanor et al., 1994). For example, the antibody responses to inactivated influenza vaccine in two groups of elderly subjects, individuals living independently at home, and residents of chronic care facilities are compared with the responses of healthy adults in Table 2 (unpublished studies). In addition, the levels of antibody associated needed for protection against influenza illness in the elderly may be higher than the 1:40 titer traditionally associated with protection in young adults (Bennett et al., 1994, Betts et al., 1993).
Table 2.
Immunogenicity of inactivated influenza vaccine
| Group (n) | Mean age (years) | Serum HI antibody responses to the following antigens | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H1N1 responses | H3N2 responses | B responses | ||||||||
| Log 2 HI±SD | % Responding | Log 2 HI±SD | % Responding | Log 2 HI±SD | % Responding | |||||
| Pre | Post | Pre | Post | Pre | Post | |||||
| Healthy adults (52) | 34 | 5.1±2.0 | 6.7±1.7 | 50 | 4.9±1.5 | 6.7±1.7 | 48 | 5.9±1.6 | 7.9±1.8 | 54 |
| Healthy elderly (52) | 75 | 4.2±1.3 | 5.3±1.8 | 25 | 4.0±1.3 | 4.8±1.6 | 25 | 5.9±1.4 | 6.9±1.6 | 23 |
| Nursing home elderly (46) | 85 | 4.5±1.3 | 5.5±1.8 | 33 | 4.2±1.5 | 5.3±2.0 | 32 | 5.3±1.8 | 6.3±2.0 | 26 |
Not all studies have shown diminished responses to influenza vaccination in the elderly, with the affect of the specific antigen occasionally outweighing the effect of age (Glathe et al., 1993). The effect of age may be primarily in effecting the subclass of HA-specific IgG produced (Powers, 1994), consistent with an affect of aging on numbers of naive T cells. In some studies, the presence of chronic disability, rather than age of the subject, appears better able to predict lack of responsiveness (Gross et al., 1989). Malnutrition has been identified as a common problem in the disabled elderly, but does not appear to contribute to the reduced response to vaccination in most people (Pozzetto et al., 1993).
2.2.2. Vaccine development
Efforts to improve the performance of influenza vaccine in the elderly have been focused on three basic areas, improvement in the level and duration of systemic immune responses to vaccine, improvement in mucosal immune responses, and addition of cellular immune responses to the spectrum of potentially protective responses induced by vaccination. However, two features of the current inactivated vaccines are important to keep in mind while considering new approaches to influenza prevention in any population. First, the current vaccine is very well-tolerated, and any significant increase in local or systemic side-effects over that seen with current vaccine would likely be accepted poorly. Second, the current vaccine is relatively inexpensive, and improvements in efficacy might support only a modest increase in cost.
2.2.2.1. High dose vaccine
Current vaccines are administered at a standard dose of 15 μg of HA per antigen. Recently, improved purification techniques have allowed exploration of much higher doses of vaccine. Administration of 405 μg of highly purified monovalent H1 antigen to healthy young adults, or of trivalent vaccine containing 135 μg of each antigen was well-tolerated and produced 2- to 4-fold higher geometric mean serum HAI and neutralizing antibody titers 4 weeks after vaccination, compared with standard doses (Keitel et al., 1994). Improved nasal secretion antibody responses with high dose vaccines were also seen (Keitel et al., 1994).
Studies of high dose vaccines in elderly subjects have generally demonstrated a less robust dose–response curve than seen in young adults (Gross et al., 1988a, Palache et al., 1993). Evaluation of high dose recombinant HA expressed in baculovirus reached similar conclusions. A dose of 135 μg of an H3 HA resulted in significantly increased post-immunization antibody titers in young adults compared with standard inactivated vaccine (Treanor et al., 1996). However, the dose–response curve was flat in elderly recipients, and there was little increase, even at 135 μg, compared with standard vaccine. The enhancement of protective efficacy, if any, resulting from use of very high doses of HA in this population remains to be evaluated.
2.2.2.2. Adjuvants and alternative formulations
The most commonly used adjuvant for vaccines in humans is alum (aluminum hydroxide or aluminum phosphate), but alum does not adsorb the HA of all influenza viruses efficiently, and alum adjuvants are not used for inactivated influenza vaccine. Other adjuvants that have been evaluated with influenza vaccine in animals or phase I studies have consisted of agents which modify the immune response directly, such as the squalene derivative MF59, monophosphoryl lipid A (MPL), the purified soap-bark derivatives Quil A or QS-21, dihydroepiandosterone (DHEA) (Daynes and Araneo, 1994), or cytokines. Another strategy is to physically modify the vaccine to improve antigen presentation, such as formulation in liposomes (el Guink et al., 1989, Kaji et al., 1992), or multimeric complexes such as ISCOMs (immunostimulating complexes) (Sundquist et al., 1988, Lovgren et al., 1990).
Several studies have been performed in elderly humans which have compared responses between adjuvanted and non-adjuvanted formulations of inactivated influenza vaccine. While some studies have shown enhanced serum antibody responses, no single formulation has emerged as clearly superior. Inactivated influenza vaccine formulated with MF59 (FLUAD, Chiron vaccines) has resulted in slightly higher levels of post-immunization antibody in elderly recipients compared with non-adjuvanted vaccine, but at the expense of higher rates of mild local and systemic side-effects (Martin, 1997, Minutello et al., 1999). This vaccine formulation has been licensed in Italy. Dehydroepiandrosterone (DHEA) has been reported to increase post-vaccination antibody titers in elderly subjects, primarily in those without a history of previous vaccination (Ben-Yehuda et al., 1998). Liposomal inactivated vaccine was well-tolerated and induced more frequent CTL responses but did not enhance antibody responses to vaccine in a small study of elderly subjects (Powers, 1997). In contrast, a virosomal preparation incorporating liposomes with phosphatidylcholine and phosphatidylethanolamine did result in enhanced antibody responses in elderly subjects (Gluck et al., 1994), and has been licensed in Switzerland. Conjugation of the hemagluttinin to a protein carrier has been reported to enhance immune responses and protective efficacy (Gravenstein et al., 1994). The use of thymosin-alpha 1 has improved antibody responsiveness in elderly men (Gravenstein et al., 1989), as did the cytokine IL-2 (Provinciali et al., 1994). As additional potential adjuvants are described, it is likely that further improvements will be forthcoming.
2.2.2.3. Live virus vaccines
Studies in a variety of animal models have confirmed the importance of mucosal immunity in protection against influenza (Renegar and Small, 1991). More limited studies in humans have also suggested that the very effective protective immunity that is induced by influenza virus infection is mediated predominantly by the local HA-specific IgA (Clements et al., 1986a, Clements et al., 1986b). Thus, methods to induce mucosal immunity are attractive options for improving influenza vaccine performance.
Live, attenuated influenza virus vaccines, generated by genetic reassortment with cold-adapted influenza A and B viruses (Maassab and DeBorde, 1985) have been extensively evaluated in children, young adults, and elderly subjects (reviewed in Murphy, 1993). Safety has also been demonstrated in high-risk individuals who would not be able to tolerate even minor lower respiratory tract inflammation, such as in individuals with cystic fibrosis or asthma (Miyazaki et al., 1993, Gruber et al., 1994). In adults, such ‘cold-adapted’ vaccines are generally more effective than parenterally administered inactivated influenza vaccine at inducing nasal HA-specific IgA, while inducing levels of serum HAI and HA-specific IgG antibody that are slightly lower than seen with inactivated vaccine. Although not studied extensively, limited data suggests that cold-adapted influenza vaccines may also induce antibody and cytotoxic T cells with more broadened recognition within a subtype than seen after inactivated vaccine (Gorse and Belshe, 1990, Clover et al., 1991).
Field trial evaluations have demonstrated the protective efficacy of ca reassortant influenza vaccines in children and adults. A recently completed field trial in 1600 children ages 18–71 months demonstrated a greater than 90% efficacy of trivalent cold-adapted influenza vaccine in prevention of culture positive influenza A (H3N2) and influenza B (Belshe et al., 1997). In adults, a randomized, double-blind trial conducted in 5210 individuals over 5 years showed that mono- or bivalent cold-adapted vaccines was approximately equal to inactivated vaccine in prevention of laboratory confirmed influenza illness (Edwards et al., 1994). The trivalent formulation of cold-adapted vaccine (FluMist) was of approximately equal effectiveness to inactivated vaccine in prevention of infection-associated illness due to all three components in experimentally infected adults (Treanor et al., 1999). In a recent study, FluMist was 23% effective in preventing febrile upper respiratory tract illnesses during the influenza season in a population of working adults (Nichol et al., 1999). Live, attenuated, cold-adapted influenza vaccines thus represent a promising approach to influenza vaccination.
Studies of cold-adapted vaccines in elderly and high-risk subjects have shown these vaccines to be well-tolerated in these populations also (Gorse et al., 1988, Gorse et al., 1991, Powers et al., 1989, Atmar et al., 1990, Gorse and Belshe, 1990, Powers et al., 1991, Powers et al., 1992). No changes in oxygen saturation or pulmonary mechanics have been seen when cold-adapted vaccines have been given to elderly subjects or to those with chronic lung diseases (Gorse et al., 1988, Gorse et al., 1991, Atmar et al., 1990). A minority of vaccine recipients shed detectable virus in nasal secretions, generally at low titer and for a short duration.
Serum antibody responses to cold-adapted vaccines in elderly subjects have been less than those seen in younger subjects (Gorse et al., 1988, Gorse et al., 1991, Powers et al., 1989, Powers et al., 1991, Powers et al., 1992). Systemic antibody responses to cold-adapted influenza A virus vaccines as measured by hemagglutinin-specific IgG enzyme immunoassays of various types have been observed in from 0 to 48% of subjects, significantly less than the rate of systemic antibody responses to inactivated vaccines in these studies. In addition, geometric mean serum antibody titers are higher following inactivated influenza vaccines than after cold-adapted vaccine. Local HA-specific IgA responses have been seen in up to 60% of elderly recipients of cold-adapted vaccines, while slightly lower rates of local antibody response have been seen following inactivated vaccine. Mean post-vaccination nasal antibody levels have often been difficult to interpret because of a high degree of variability in the level of pre-immunization antibody and the inherent difficulty in making these types of measurements, but appear to be somewhat higher in those receiving cold-recombinant vaccines. Relatively less work has been performed to evaluate cold-adapted influenza B virus vaccines in the elderly. However, one study in ambulatory elderly adults demonstrated that cold-adapted influenza B virus vaccines to be were well-tolerated, but less immunogenic than inactivated vaccine (Treanor et al., 1994). Nasal wash antibody responses were not seen very frequently with any of the vaccines given in that study.
The reasons for the apparently decreased infectivity of cold-adapted influenza vaccine viruses in the elderly may be related to the extensive previous exposure of older adults to influenza virus infections. However, this immunologic experience is not simply reflected by serum HAI titers, because the infectivity of cold-recombinant vaccines in older adults is low even with low pre-vaccination serum HAI antibody titers (Powers et al., 1992). In that study, only 36% of elderly adults with pre-vaccination serum HI titers of ≤1:8 responded to vaccination with the CR A/Kawasaki/86 (H1N1) virus, compared with 100% of young adults (Powers et al., 1992).
The relative lack of serum HAI antibody responses to vaccination with cold-adapted influenza vaccines in older adults has suggested that such vaccine may not be optimal when used alone in this population. However, live vaccination does appear to potentially offer a benefit in induction of more frequent local antibody responses than seen with inactivated vaccine. This has led to the hypothesis that the use of both vaccine might have a synergistic effect, with inactivated vaccines providing systemic immunity and the cold-adapted vaccine augmenting the local immune response. Preliminary evaluation of the immunogenicity of parenteral inactivated vaccine given with intranasal cold-adapted vaccine has suggested that combined live and inactivated vaccines resulted in modest, but consistent, increases in the frequency of immune responses compared with either inactivated vaccine or cold-adapted vaccine alone (Powers et al., 1989, Powers et al., 1991, Gorse et al., 1996, Treanor and Betts, 1998).
These promising results were the basis of a small field trial to evaluate the combination of intranasal cold-adapted vaccine and intramuscular inactivated influenza vaccine in the prevention of influenza A in residents of nursing homes (Treanor et al., 1992). All participants received trivalent inactivated influenza vaccine parenterally and were randomly assigned to receive either live attenuated influenza A (H3N2) vaccine or placebo intranasally. Influenza A activity was relatively quiescent during this study, and attack rates of influenza in the participating nursing homes were generally low. However, over the 3-year period of the study, participants who received intranasal vaccine and who were subsequently exposed to influenza A had significantly lower rates of laboratory-documented influenza A and outbreak associated (i.e. temporally related to institutional influenza outbreaks) respiratory illness (Fig. 2 ; Treanor et al., 1992). A similar study comparing intramuscular trivalent inactivated vaccine combined with intranasal cold-adapted influenza B/Yamagata/88 to inactivated vaccine alone also showed reductions in laboratory-documented influenza B with combined vaccination, but the rates of infection were too low to allow conclusions to be drawn about the efficacy of this approach for influenza B (Treanor and Betts, 1998).
Fig. 2.
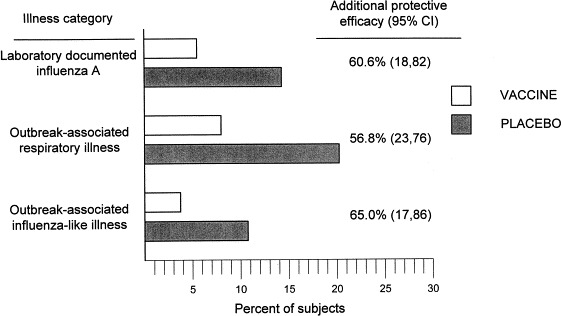
Rates of laboratory-documented influenza illness, outbreak-associated respiratory illness, and outbreak-associated influenza-like illness in nursing home residents randomized to receive parenteral inactivated influenza vaccine with intranasal cold-adapted influenza vaccine or intranasal placebo (from Treanor et al., 1992).
In summary, currently available data suggests that cold-adapted influenza A vaccines are safe in elderly and chronically ill individuals. Both local and systemic antibody responses are clearly less than those seen in young adults, even in elderly individuals with relatively low pre-vaccination serum HAI titers. In addition, systemic antibody responses to cold-adapted vaccines are generally less frequent and of lower magnitude than seen with inactivated vaccine, while local antibody responses are only marginally greater. However, combined intranasal cold-adapted and intramuscular inactivated vaccine provided significantly greater protection in this population than inactivated vaccine alone, despite the very modest apparent increase in immunogenicity. These data are promising, and should be confirmed in a larger population during a period of more significant influenza activity.
2.2.3. Antiviral agents
2.2.3.1. M2 channel inhibitors
The antiviral drugs amantadine and rimantadine are both licensed in the USA and in some other countries for the treatment and prevention of influenza A in adults. Both drugs act by inhibiting the action of the viral M2 protein, an ion channel that plays an important role in viral uncoating. Because only influenza A viruses contain an M2 protein that interacts with these drugs, amantadine and rimantadine are only active against influenza A viruses.
Studies to demonstrate the efficacy of M2 channel inhibitors in acute influenza A have largely been carried out in younger adult populations and in children. These studies have demonstrated that therapy within 48 h of onset of symptoms is associated with reductions in symptom scores, in more rapid resolution of fever, and return to normal activities, and in some studies, reductions in viral shedding (Van Voris et al., 1981, Younkin et al., 1983, Hayden and Monto, 1986). Efficacy has been demonstrated in infections due to H1N1, H2N2, and H3N2 influenza A viruses. Neither drug has been subjected to extensive efficacy evaluation in high-risk subjects. One placebo-controlled blinded study carried out in nursing home patients showed more rapid reduction in fever and in symptoms in rimantadine recipients. Furthermore, physicians who were caring for these patients, but who were blinded to the study drug status, prescribed significantly fewer antipyretics, antitussives, and antibiotics, and obtained fewer chest X-rays for the rimantadine recipients (Betts et al., 1987). The effectiveness of early therapy of high-risk individuals with amantadine or rimantadine in reducing the frequency of subsequent complications is unknown.
M2 channel inhibitors are also effective in the prevention of influenza. In large-scale studies of seasonal prophylaxis in otherwise healthy adults, the drugs have had efficacy rates of 70–90% against laboratory-documented influenza A (Dolin et al., 1982, Douglas, 1990). Amantadine and rimantadine have similar levels of efficacy in this setting, but rimantadine is associated with significantly fewer side-effects (Dolin et al., 1982). Efficacy has also been demonstrated in post-exposure prophylaxis in the family setting (Galbraith et al., 1969). These findings suggest that prophylaxis of contacts in other closed settings, such as nursing homes, would also effectively terminate influenza outbreaks. Evidence of the effectiveness of outbreak-initiated prophylaxis in nursing homes is largely observational, but does suggest that early initiation can reduce the rate of influenza in institutions (Atkinson et al., 1986, Arden et al., 1988). Use of such prophylaxis for 14 days is as effective as are longer courses of administration (Drinka et al., 1998). It is currently recommended that when institutional outbreaks of influenza occur, chemoprophylaxis should be administered to all residents, and continued for approximately 2 weeks, or for 1 week after termination of the outbreak (CDC, 1998).
The development of antiviral resistance in treated individuals, with subsequent transmission to contacts, can significantly limit the effectiveness of post-exposure prophylaxis in either the family setting (Hayden et al., 1989) or in institutions (Mast et al., 1991, Degelau et al., 1992). For this reason it is recommended that treated and prophylaxed individuals should be isolated from each other if possible (CDC, 1998). However, resistant influenza A virus has been identified in this setting even before the onset of amantadine or rimantadine administration (Houck et al., 1995).
Both amantadine and rimantadine should be administered at a maximum dose of 100 mg per day in individuals over the age of 65. The daily dose of amantadine may need to be further reduced in individuals with impaired renal function. At 100 mg per day, the incidence of CNS side-effects in the elderly is substantially lower with rimantadine than amantadine (Keyser et al., 1998). However, side-effects of rimantadine in the elderly occur at a higher rate than in young adults (Tominack and Hayden, 1987). Administration of amantadine to individuals with motor seizures has been reported to increase the frequency of seizures (Atkinson et al., 1986). This may also occur with rimantadine, but appears to be less frequent (Soo, 1989). In one study, long-term prophylactic administration of rimantadine in a nursing home population was well-tolerated and had an estimated efficacy of 58% in prevention of flu in a nursing home (Monto et al., 1995).
2.2.3.2. Neuraminidase inhibitors
The determination of the crystal structure of the neuraminidase complexed with sialic acid (Varghese et al., 1983) has allowed the development of a series of analogs with neuraminidase-inhibiting activity (von Itzstein et al., 1993). The first of these compounds to be evaluated in clinical studies, 4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid, or zanamivir (Relenza) is a highly potent and selective inhibitor of the neuraminidase of influenza A and B viruses. Zanamivir has significant antiviral activity against a wide variety of laboratory and clinical influenza A and B isolates in cell culture, and in the mouse and ferret models (von Itzstein et al., 1993, Woods et al., 1993). The drug is not orally bioavailable and is administered topically. Intranasal zanamivir was effective in the therapy of influenza in experimentally infected adult volunteers in whom both early treatment (beginning 26–32 h after challenge) and late treatment (beginning 50 h after challenge) were effective in reducing virus shedding compared with placebo, and early treatment also reduced symptom scores and reduced the frequency of middle ear abnormalities (Hayden et al., 1996, Walker et al., 1997). Subsequently, inhaled zanamivir was shown to be effective in the early treatment of naturally acquired acute influenza A and B in man, resulting in an approximately 24-h decrease in the duration of symptoms when administered within the first 48 h of illness (Hayden et al., 1997). Zanamivir also reduced the frequency of complications in a small number of high-risk subjects (MIST, 1998). This drug thus represents the first antiviral with clear-cut clinical efficacy against both influenza A and B in man. Zanamivir was recently licensed for treatment of acute, uncomplicated influenza in adults. An oral antineuraminidase inhibitor, oseltamivir (Tamiflu), is in development, and has also demonstrated therapeutic efficacy in experimentally infected adults (Hayden et al., 1999a).
Relatively less information is available regarding the use of zanamivir for prophylaxis. In one study, once daily zanamivir inhalation was associated with a 67% reduction in influenza illness in healthy adults during the epidemic season (Monto et al., 1999) Use in the elderly has not been evaluated extensively. In one small study in a nursing home which experienced sequential outbreaks of influenza A and B, the use of outbreak-initiated prophylaxis with either rimantadine or zanamivir was compared during the influenza A outbreak, and of zanamivir or no treatment during the influenza B outbreak. One case of laboratory confirmed influenza A influenza occurred in study subjects, in an individual receiving rimantadine, and one case of influenza B, in an individual receiving no treatment (Schilling et al., 1998)
3. Respiratory syncytial virus (RSV)
3.1. Disease impact
Respiratory syncytial virus (RSV) is one of the most important causes of lower respiratory tract infection in infants and young children, causing an estimated 90 000 hospitalizations and 4500 deaths annually in the USA (Glezen et al., 1971, Mufson et al., 1973, Parrott et al., 1973) Immunity to RSV is incomplete and re-infection occurs throughout life, although re-infection has always been considered a mild illness (Hall et al., 1976, Hall et al., 1978) Traditionally, severe RSV infection has been considered a problem of childhood. However, RSV has recently been identified as a common cause of serious disease in a number of adult populations, including immunocompromised persons, frail elderly, and those with underlying cardiopulmonary disease (Englund et al., 1988, Englund et al., 1991, Martin et al., 1988, Hertz et al., 1989, Falsey et al., 1992a, Falsey et al., 1995a, Harrington et al., 1992, Whimbey et al., 1995) Despite recent advances in the understanding of RSV infections in infants, three important areas regarding RSV infection in the adult remain relatively unexplored. These include (1) detailed knowledge about the epidemiology and clinical impact of RSV in various adult populations; (2) an understanding of the mechanisms of disease pathogenesis and (3) development of the correlates of immunity to RSV in the adult.
Among elderly adults, RSV is a significant cause of excess morbidity and mortality. RSV infections have been documented in attendees of senior daycare centers, the community-dwelling and institutionalized elderly (Falsey et al., 1992a, Falsey et al., 1995a, Falsey et al., 1995b). RSV outbreaks are well-documented in nursing homes, with attack rates ranging from 2 to 89% (Mathur et al., 1980, Sorvillo et al., 1984, Agius et al., 1990, Falsey et al., 1990). However, it should also be noted that RSV is consistently identified each winter as a cause of sporadic respiratory illness in nursing homes indicating that RSV infections are not limited to nosocomial outbreaks (Nicholson et al., 1990, Osterweil et al., 1990, Falsey et al., 1992a). Even during documented influenza virus epidemics, RSV infections can be identified in this population (Falsey et al., 1990). In one surveillance study in a 550-bed nursing home in Rochester, New York, RSV caused 27% of 149 acute respiratory tract infections during a single winter season (Falsey et al., 1992a). In this study, rhinovirus (9%) and parainfluenza viruses (6%) were the next most commonly identified agents. Of the 40 RSV infected patients, four (10%) developed pneumonia and two (5%) died.
The severity of RSV infection in institutionalized persons can be quite variable. Serious complications are well-recognized with rates of pneumonia ranging from 5 to 67%, and mortality from 0 to 53% (Mathur et al., 1980, Sorvillo et al., 1984, Agius et al., 1990, Falsey et al., 1990, Falsey et al., 1992a). Symptoms are often indistinguishable from those of influenza virus infection, although, the presence of nasal congestion and wheezing are more characteristic of RSV (Mathur et al., 1980).
In addition to the institutionalized elderly, RSV has also been recovered from attendees at adult day care centers (Falsey et al., 1995b). During a 15-month period encompassing two winter seasons in Rochester, NY, RSV was identified in 16 of the 165 participants, slightly less than the 22 influenza A and B virus infections which occurred during the same period. Although there were no deaths directly attributable to RSV, one infected patient required hospitalization for pneumonia. There is little information available on the incidence of RSV infection in the community dwelling elderly, although significant illness clearly occurs each winter. Three cases of culture positive RSV lower respiratory tract infection were identified in one analysis of community acquired pneumonia (Zaroukian et al., 1988), and 57 cases were reported among elderly patients (average age 75) in a retrospective 10-year review of hospitalization for pneumonia in Sweden, representing 2.3% of all pneumonia cases (Vikerfors et al., 1987, Vikerfors et al., 1988). In Rochester, NY, 10% of 1600 admissions of persons 65 years of age or older for acute cardiopulmonary or flu-like illness over a 3-year period were caused by RSV, and 13% were due to influenza virus (Falsey et al., 1995a). Underlying COPD or congestive heart failure was present in 43 and 63% of the RSV infected patients, respectively. Mortality among RSV infected patients was 10%, comparable with the 6% mortality in influenza virus infected patients, and 14% were discharged to a higher level of care. Among 1195 hospitalized adults in Ohio, RSV was the third most common identifiable cause (4.4%), behind Streptococcus pneumoniae (6.2%) and influenza virus (5.4%) (Dowell et al., 1996). Of the 57 RSV cases, 55% were over 65 years of age. Slightly more than half had underlying COPD, coronary artery disease or congestive heart failure.
In addition to clinical reports describing acute lower respiratory tract infections, there is growing evidence to implicate RSV as an important contributor to excess morbidity within the geriatric population during the winter, as has been shown for influenza virus. Although the impact of RSV in the elderly is less readily apparent than in the infant, evidence continues to accumulate that among community-dwelling older persons, RSV may be as serious as non-pandemic influenza (Nicholson et al., 1975, Fleming et al., 1993). In the United Kingdom during 1991–1994, excess mortality in older persons was temporally associated with peak RSV activity in children (Fleming et al., 1993). In a detailed statistical analysis of data spanning 1975–1990, also from the UK, RSV had a greater impact than influenza virus on excess morbidity and mortality in persons over age 65 (Nicholson et al., 1975).
3.2. Treatment and prevention
There is little known about immunity and disease pathogenesis in the elderly or high-risk adult. It is not known if the increase in severe RSV infections in this age group is a result of underlying frailty of the host, global immunosenescence, or an age related decline in one or another of RSV-specific immune functions. Very little is known about the role of antibody to RSV in the elderly, with the exception that all adults have detectable serum antibody (Falsey et al., 1992). Elderly adults are capable of mounting a brisk serum IgA and IgG response to RSV infection (Angius et al., 1990). This is important, because relative lack of serum neutralizing antibody was recently found to be a risk factor for RSV disease in a study of frail elderly adults (Falsey et al., 1998). Baseline blood samples were obtained from subjects attending a senior daycare center and volunteers were followed over the next 26 months and evaluated for any acute respiratory tract illness. Samples from 22 subjects who developed symptomatic RSV infection were compared with 22 control subjects who did not become infected with RSV. The mean serum IgG titer to RSV fusion protein (F) was significantly lower in the RSV-infected group compared with the controls as were the neutralizing titers to group A RSV (Fig. 3 ). These data suggest that older adults with low levels of serum neutralizing antibody may be at greater risk of developing symptomatic RSV and supports the view that boosting titers by vaccination may be beneficial.
Fig. 3.
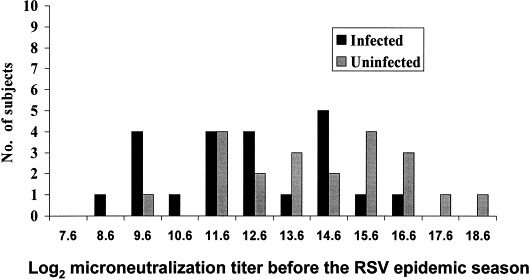
Pre-illness levels of serum neutralizing antibody to group A RSV in elderly individuals enrolled in adult day care who were or were not subsequently infected with RSV during RSV outbreaks (from Falsey et al., 1995b).
Currently, several RSV vaccine candidates are in human trials, with the potential for development of numerous additional vaccine candidates. One approach of potential benefit for the elderly is the use of purified RSV F protein (PFP-2, Wyeth–Lederle vaccines). Results in previously infected children who were vaccinated with PFP-2 demonstrated stimulation of neutralizing antibody to both group A and B strains, although large-scale efficacy studies have not been performed (Belshe et al., 1993). PFP-2 was also safe and reasonably immunogenic in both healthy and frail elderly subjects. Twenty-nine of 33 (87%) healthy elderly recipients of PFP-2 vaccine had a 4-fold or greater rise in serum IgG to the F protein at 8 weeks post-vaccination and twenty of 33 (61%) had a 4-fold or greater rise in serum neutralizing titer to group A or group B RSV. In that study, response to vaccination was inversely correlated with pre-immunization serum neutralizing titers. Subjects were followed throughout the ensuing winter, and 3 RSV infections were identified in the placebo group and none in the vaccine group (Falsey and Walsh, 1996). In a second study, PFP-2 was evaluated in 37 institutionalized persons over age 65 (Falsey and Walsh, 1997). Vaccination was well-tolerated and 36 of 37 subjects completed the study. Nineteen of 36 (53%) of vaccinees had a 4-fold or greater rise in IgG to F protein at 4 weeks and 17 (47%) had a significant rise in neutralizing titers to either group A or B virus. The response rate to PFP-2 again correlated with pre-immunization neutralization titers, although PFP-2 appeared to be slightly less immunogenic in the institutionalized elderly than in the healthy elderly
Recent animal studies suggest that adjuvants such as QS-21 and MPL (monophosphoryl lipid A) can alter the primary immune response to FG towards a Th1 pattern rather than the Th2 pattern seen when alum is used as the adjuvant (Neuzil et al., 1997). This vaccine could potentially induce greater neutralizing antibody titers than an F protein vaccine due to the induction of antibody to the G protein.
Incorporation of specific RSV genes, most notably F, into virus vectors, such as vaccinia virus and adenovirus, have been successfully completed and could be used as a vaccine. Although promising in rodents, these vaccines did not reach clinical trials in infants because of lack of immunogenicity and efficacy in subhuman primates (Wertz et al., 1987, Hsu et al., 1992, Hsu et al., 1994). However, it is possible that in already primed elderly subjects, these vectored vaccines may be immunogenic.
Live attenuated vaccines represent an attractive approach towards the development of an RSV vaccine because they should restimulate local nasal immunity and a balanced cellular immune response. Initial results with temperature-sensitive (ts) or cold-adapted (ca) mutants were disappointing in infants, as vaccines were either poorly immunogenic, excessively virulent, or genetically unstable (Kim et al., 1973, Wright et al., 1982). Currently, several new live virus vaccines are being evaluated in infants. Genetically stable, attenuated viruses have been produced from previous cold passaged and temperature-sensitive (cpts) mutants by additional chemical mutagenesis and the precise genetic alterations defined by sequence analysis of viral RNA (Crowe et al., 1994, Firestone et al., 1996). The cpts-248/404 virus is one of several candidate temperature-sensitive vaccines and has a shut-off temperature of 38°C (Firestone et al., 1996). Studies in rodents and chimpanzees show the virus to be phenotypically stable, and despite restricted replication are immunogenic and protective against wild-type challenge (Crowe et al., 1995, Crowe et al., 1996). Ts-1C is another live virus vaccine candidate produced by chemical mutagenesis with 5-fluorouracil (McKay et al., 1988, Pringle et al., 1993). Virus replication is shut off at 37°C and, in studies of adult volunteers, the vaccine was found to be stable, attenuated, and modestly immunogenic. The recent development of full length infectious cDNA clones of RSV, which produce live virus upon transfection of cells in tissue culture, offers the potential of a limitless supply of specifically altered live vaccine products (Collins et al., 1995).
4. Parainfluenza virus
4.1. Disease impact
Parainfluenza viruses are common causes of respiratory tract illness in infants and young children. Although each of the serotypes has distinctive epidemiology and clinical characteristics, in individual cases it is generally not possible to distinguish between the viruses. Although the epidemiology of PIV infection is relatively constant on a national level, there is substantial regional variation in the distribution of serogroups each year (Chanock and McIntosh, 1990). Each of the viruses can cause a wide spectrum of disease from mild upper respiratory symptoms to life threatening pneumonia. PIV1 tends to occur in the fall every other year and is associated with half of all croup cases, but also causes bronchiolitis and pneumonia. PIV2, like PIV1 may occur every other year, although also is seen annually, and is associated with croup. Infection with PIV1 and PIV2 can occur at any age, although severe disease is uncommon under age 1 year. Most children demonstrate serologic evidence of infection with PIV1 and PIV2 by the age of 5 years. In contrast, PIV3 tends to infect infants less than 6 months of age and half of all infants are infected in the first year of life, with most of the rest infected by age 2 years. PIV3 is not specifically associated with croup, and is second to RSV as a cause of bronchiolitis and pneumonia in young infants. The virus is endemic and infection occurs throughout the year including winter, spring and summer months.
Although generally considered a disease of childhood, the parainfluenza viruses, like RSV, have recently been recognized as causes of serious respiratory illness in certain adult populations such as the elderly and the severely immunocompromised. Parainfluenza infection in an adult always represents re-infection, and generally symptoms are limited to the upper respiratory tract, or produce mild lower tract symptoms (Cooney et al., 1975). Within families, up to 25% of young adult parents develop evidence of infection when parainfluenza virus is introduced into the household (Cooney et al., 1975). PIV infections have been documented in community dwelling elderly with pneumonia or signs of lower respiratory tract infection (Mufson et al., 1967, Fransen et al., 1969, Cooney et al., 1975, Stanek and Heinz, 1988, Greenberg, 1991, Monto and Sullivan, 1993). However, the incidence and clinical significance of PIV in these groups appears to be variable. In one report of community acquired PIV lower respiratory tract infection, PIV3 was isolated from two of 361 patients and documented seroconversion to PIV in 23 of 346 subjects (6.6%) (Mufson et al., 1967). In persons between 60–70, 70–80, and >80 years of age, seroconversion to PIV was associated with 11, 14 and 21% of the lower respiratory tract illnesses, respectively, in contrast to rates of 3, 4 and 6% in controls without respiratory symptoms. In another serologic study of respiratory illness from Sweden, 69 cases of PIV infection were detected out of 598 patients with pneumonia (Fransen et al., 1969). Of the 189 patients greater than 60 years of age, 20 (11%) had serologic evidence of PIV infection. More recently, PIV infection was identified in 2.1% of acute respiratory illness in persons over age 40 years during 11 years of community surveillance in Tecumseh, MI (Monto and Sullivan, 1993). For comparison, influenza A and B viruses were identified as the cause of illness in 10.3%. However, not all investigators have described parainfluenza virus as a cause of lower respiratory tract illness in adults (Cooney et al., 1975, MacFarlane et al., 1993, Marrie et al., 1996).
Outbreaks of parainfluenza types 1 and 3 have also been reported from long-term care facilities Anon, 1983. Illnesses were characterized by fever, sore throat, rhinorrhea, and cough. Rates of pneumonia were high (17–29%) with several deaths. Attack rates of 22 and 28% for residents and employees, respectively, were noted in an Alabama nursing home outbreak. In prospective studies in nursing home populations, parainfluenza viruses have been responsible for 5–6% of respiratory illnesses (Arroyo et al., 1988, Gross et al., 1988b, Falsey et al., 1992a, Falsey et al., 1995b).
Parainfluenza virus has also been implicated as a cause of exacerbation of chronic obstructive pulmonary disease (COPD) (Carilli et al., 1964, Buscho et al., 1978, Hudgel et al., 1979, Smith et al., 1985). In composite, PIV is generally second or third behind influenza virus and respiratory syncytial virus as a cause of acute deterioration of lung function in this group (Fagan and Chastre, 1996).
4.2. Treatment and prevention
The earliest attempt at immunization for PIV used a formalin inactivated trivalent whole virus vaccine (Fulginiti et al., 1967, Weibel et al., 1967, Fulginiti et al., 1969). Although immunogenic, it was not efficacious in infants and was thus abandoned. However, in contrast to the findings with inactivated RSV vaccine discussed above, there was no evidence of enhanced disease in infants who received the PIV vaccine. Purified HN and F or virus vectors expressing HN or F have provided materials for either parenteral or topical immunization, and have been tested in animal models of parainfluenza virus infection (Blaine et al., 1980, Ray et al., 1985, Spriggs et al., 1987, Ray et al., 1988, Sullivan et al., 1993). Two live attenuated parainfluenza type 3 vaccines have also been developed for use in infants and children (Karron et al., 1995a, Karron et al., 1995b). One is a bovine PIV which has significant antigenic relatedness to human PIV3, and which is attenuated for primates based on its long evolution in a heterologous bovine host. The second is a cold-passaged (cp), cold-adapted (ca), temperature-sensitive (ts) mutant of the JS strain of human PIV3. In seronegative children this vaccine is well-tolerated and highly immunogenic (81% seroconversion) at the highest dose given (105 TCID50). However, in seropositive children who are perhaps more predictive of the performance of such a vaccine in adults, only 4 of 34 (11%) developed 4-fold rises in hemagglutination titers, and only 22% developed nasal IgA responses.
5. Rhinovirus and coronavirus infections
5.1. Disease impact
The common cold is one of the most common disease experiences in all age groups. Although there is no single clinical entity defined as a ‘cold’, generally colds are considered to be predominantly respiratory illnesses including symptoms of rhinitis with variable degrees of pharyngitis (Gwaltney et al., 1967, Rao et al., 1995). Patients often report chills, but true fever is unusual. Cough and hoarseness are occasionally also part of the illness, but lower respiratory tract signs and symptoms are not characteristics of colds. Recognized complications of colds in adults include secondary bacterial infections of the paranasal sinuses and middle ear, and exacerbations of asthma, chronic bronchitis, and emphysema.
Although there are many viral etiologies of the common cold, two viruses that are often associated with this syndrome, rhinoviruses and coronaviruses, are emerging as potentially important pathogens in the frail elderly. Rhinoviruses infections are extremely common, and in the USA have been observed at rates of 0.74–0.77 infections per person per year in adults (Gwaltney et al., 1966, Hamre and Procknow, 1966). In the USA, RV infections are most prevalent in the early fall and late spring. The major reservoir for RV is school children, who transmit RV infection among their peers in the classroom (Beem et al., 1969) and introduce it into their homes, infecting other family members (Hendley et al., 1969).
In addition to causing cold symptoms, rhinoviruses have been implicated in the development of acute sinusitis and in the precipitation of exacerbations of chronic bronchitis and asthma. The virus has been recovered from aspirates obtained by direct puncture of the maxillary sinuses of patients with acute sinusitis (Pitkaranta et al., 1997), and mucosal thickening and/or sinus exudates have been observed in as many as 77% of subjects with acute colds (Turner et al., 1992, Gwaltney et al., 1994). Clinically manifest acute sinusitis is seen in a small (0.5–5%) proportion of individuals with naturally occurring colds (Wald et al., 1991). As many as 40% of exacerbations of chronic bronchitis have been associated with RV infection (Eadie et al., 1966, Stenhouse, 1967, McNamara et al., 1969, Lambert et al., 1972). However, the effect of RV infection on pulmonary function in patients with chronic obstructive pulmonary disease is less marked than that of influenza infection (Smith et al., 1985). It is not known whether rhinovirus invades the bronchial tree directly, but several studies indicate that direct invasion may occur (Halperin et al., 1983, Gern et al., 1999). Rhinovirus is also among the respiratory viruses shown to precipitate attacks of asthma, predominantly in children (Minor et al., 1974, Minor et al., 1976, Horn et al., 1979 but also in adults Teichtahl et al., 1997). Experimental RV infection of varying severity led to the development of wheezing and increased bronchial reactivity, as demonstrated by histamine provocation, in 20% of adults with mild to moderate asthma (Halperin et al., 1985).
Coronaviruses are moderate-sized (100–150 nm), round viruses containing positive-sense single-stranded RNA. Distinctive club-shaped projections are present on the virus surface, giving the virus particle the appearance of having a crown or corona, from which it derives its name. Two antigenic types of human coronavirus have been recognized: the 229E-related strains that have been isolated in cell cultures such as human embryonic lung cell fibroblasts, and the OC43-related strains that require organ culture. The coronavirus group also appears to be an important cause of common colds, although the overall contribution to annual respiratory disease morbidity is uncertain. In longitudinal studies in adults, coronaviruses have accounted for 4–15% of acute respiratory disease annually and up to 35% during peak periods (Hamre and Beem, 1972, Hendley et al., 1972). Annual illness rates in children have been measured at 8%, with peak rates of up to 20% (Kaye et al., 1971, Kaye and Dowdle, 1975). The reported frequency of infection in adults for 229E and OC43 viruses has ranged from 15 to 25 per 100 persons per year, with up to 80% of infections occurring in persons with prior antibody to the infecting virus (Cavallaro and Monto, 1970, Hamre and Beem, 1972, Hendley et al., 1972, Monto and Lim, 1974). Most large peaks of coronavirus activity have occurred in winter and early spring, but infections have been detected throughout the year Macnaughton, 1982, Monto, 1997).
Recent data have implicated these viruses as common causes of moderately debilitating acute respiratory disease in older adults. Prospective studies have identified rhinoviruses as associated with 7% of acute respiratory illnesses in elderly adults attending supervised day care (Falsey et al., 1997) and as many as one-fourth of episodes occurring among ambulatory elderly adults (Nicholson et al., 1997). In the same studies, coronaviruses were identified as responsible for 8 and 10% of episodes, respectively. Rhinovirus outbreaks have also been reported in long-term care settings (Nicholson et al., 1990, Falsey et al., 1992b, Wald et al., 1995).
A striking feature of these illnesses has been significant lower respiratory tract involvement in many cases. Signs and symptoms such as cough, sputum production, dyspnea, and wheezing have been reported in from 40 to 90% of individuals, and are significantly more common in the elderly than in younger health care workers (Table 3 ) (Falsey et al., 1997). Clinical illness caused by coronavirus and rhinovirus in ambulatory elderly adults was similar to that associated with influenza (Nicholson et al., 1997), and was associated with significant mobidity. Restriction of activity was seen in almost one-fifth of those with rhinovirus illnesses, and 43% of infections resulted in medical consultation. The presence of chronic medical conditions and smoking may be risk factors for the development of lower respiratory tract symptoms from rhinovirus infection (Nicholson et al., 1996). Of note, however, rhinovirus and coronavirus infections have not yet been established convincingly as causes of increased levels of hospital admission or enhanced mortality among elderly populations.
Table 3.
Clinical features of rhinovirus and coronavirus infectoins among staff compared with older participants
| Symptom | [2]No. with indicated symptom within the following group (%) | P value comparing rates in elderly and younger individuals | |
|---|---|---|---|
| Elderly participants in senior daycare (n=66, mean age: 78.5 years) | Staff members at the daycare center (n=44, mean age: 35 years) | ||
| Nasal congestion | 56 (85) | 42 (95) | NS |
| Sore throat | 23 (35) | 23 (52) | NS |
| Hoarseness | 30 (45) | 21 (48) | NS |
| Cough | 61 (92) | 28 (64) | <.001 |
| Sputum | 38 (58) | 10 (23) | <.001 |
| Dyspnea | 24 (36) | 11 (25) | NS |
| Constitutional | 58 (94 | 39 (61) | <.001 |
| Fever | 15 (23) | 10 (23) | NS |
5.2. Treatment and prevention
The only effective therapy for RV colds currently available is symptomatic treatment of individual complaints. However, because of their high incidence, development of effective antiviral therapy for rhinovirus infections has been an object of considerable interest for many years. A detailed review of all of the efforts over many years to generate such a treatment is beyond the scope of this review, but many such studies have utilized experimental infection of human adult volunteers for this purpose. Benefit has been seen in this model with early treatment with a combination of interferon α2 and ipratroprium bromide given nasally and naproxen given orally (Gwaltney, 1992), and capsid binding agents such as pirodavir (Hayden et al., 1992) and pleconaril. Because 90% of rhinovirus serotypes use the same cellular receptor, receptor blockade with soluble ICAM-1 has also been evaluated with minor benefit (Turner et al., 1999). Pleconaril has been reported to reduce the duration of symptoms by 21% in naturally occuring colds (Hayden et al., 1999). Although serotype-specific immunity does develop after infection (Fleet et al., 1965), prospects for a rhinovirus vaccine are poor because of the multiplicity of rhinovirus serotypes.
Antiviral agents with clinical usefulness for coronavirus infection are currently unavailable, so that treatment of coronavirus colds also depends upon the use of symptomatic therapies. Intranasal interferon is protective against experimental coronavirus colds (Higgins et al., 1983), and intranasal nedocromil sodium was also effective in reducing nasal mucus weights in this model (Barrow et al., 1990). Little attention has been given to the development of coronavirus vaccines. In volunteer experiments, infection with a 229E-like coronavirus induced effective short-term immunity appeared to rechallenge with the homotypic virus, but challenge with other strains of the 229E serotype resulted in infection and illness (Reed, 1984).
6. Summary
Viral respiratory infections represent a significant challenge for those interested in improving the health of the elderly. Influenza continues to result in a large burden of excess morbidity and mortality. Two effective measures, inactivated influenza vaccine, and the antiviral drugs rimantadine and amantadine, are currently available for control of this disease. Inactivated vaccine should be given yearly to all of those over the age of 65, as well as younger individuals with high-risk medical conditions and individuals delivering care to such persons. Live, intranasally administered attenuated influenza vaccines are also in development, and may be useful in combination with inactivated vaccine in the elderly. The antiviral drugs amantadine and rimantadine are effective in the treatment and prevention of influenza A, although rimantadine is associated with fewer side-effects. Recently, the inhaled neuraminidase inhibitor zanamivir, which is active against both influenza A and B viruses, was licensed for use in uncomplicated influenza. The role of this drug in treatment and prevention of influenza in the elderly remains to be determined. Additional neuraminidase inhibitors are also being developed. In addition, to influenza, respiratory infections with respiratory syncytial virus, parainfluenza virus, rhinovirus, and coronavirus have been identified as potential problems in the elderly. With increasing attention, it is probable that the impact of these infections in this age group will be more extensively documented. Understanding of the immunology and pathogenesis of these infections in elderly adults is in its infancy, and considerable additional work will need to be performed towards development of effective control measures.
References
- Agius G, Dindinaud G, Bigger R.J, et al. An epidemic of respiratory syncytial virus in the elderly: Clinical and serological findings. J. Med. Virol. 1990;30:117–127. doi: 10.1002/jmv.1890300208. [DOI] [PubMed] [Google Scholar]
- Angius G, Dindinaud G, Bigger R.J, et al. An epidemic of respiratory syncytial virus in the elderly: Clinical and serological findings. J. Med. Virol. 1990;30:117–127. doi: 10.1002/jmv.1890300208. [DOI] [PubMed] [Google Scholar]
- Anon Parainfluenza infections in the elderly 1976–1982. Can. Med. Assoc. J. 1983;287(6405):1619. doi: 10.1136/bmj.287.6405.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden N.H, Patriarca P.A, Fasano M.B, et al. The roles of vaccination and amantadine prophylaxis in controlling an outbreak of influenza A (H3N2) in a nursing home. Arch. Intern. Med. 1988;148:865–868. [PubMed] [Google Scholar]
- Arroyo J.C, Jordan A.W, Milligan L. Upper respiratory tract infection and serum antibody responses in nursing home patients. Infect. Cont. 1988;16:152–158. doi: 10.1016/0196-6553(88)90026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson W.L, Arden N.H, Patriarca P.A, Leslie N, Lui K.-J, Gohd R. Amantadine prophylaxis during an institutional outbreak of type A (H1N1) influenza. Arch. Intern. Med. 1986;146:1751–1756. [PubMed] [Google Scholar]
- Atmar R.L, Bloom K, Keitel W, Couch R.B, Greenberg S.B. Effect of live attenuated, cold recombinant (CR) influenza virus vaccines on pulmonary function in heathy and asthmatic adults. Vaccine. 1990;8:217–224. doi: 10.1016/0264-410x(90)90049-r. [DOI] [PubMed] [Google Scholar]
- Barker W.H, Mullooly J.P. Impact of epidemic type A influenza in a defined adult population. Am. J. Epidemiol. 1980;112(6):798–813. doi: 10.1093/oxfordjournals.aje.a113052. [DOI] [PubMed] [Google Scholar]
- Barker W.H, Mullooly J.P. Pneumonia and influenza deaths during epidemics: implications for prevention. J. Am. Med. Assoc. 1982;142:85–89. [PubMed] [Google Scholar]
- Barker W.H, Borisute H, Cox C. A study of the impact of influenza on the functional status of frail older people. Arch. Intern. Med. 1998;158:645–650. doi: 10.1001/archinte.158.6.645. [DOI] [PubMed] [Google Scholar]
- Barrow G.I, Higgins P.G, Al-Nakib W, Smith A.P, Wenham R.B.M, Tyrrell D.A.J. The effect of intranasal nedocromil sodium on viral upper respiratory tract infections in human volunteers. Clin. Exp. Allergy. 1990;20:45–51. doi: 10.1111/j.1365-2222.1990.tb02774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beem M.O. Acute respiratory illness in nursery school children: A longitudinal study of the occurrence of illness and respiratory viruses. Am. J. Epidemiol. 1969;90:30–44. doi: 10.1093/oxfordjournals.aje.a121047. [DOI] [PubMed] [Google Scholar]
- Belshe R.B, Anderson E.L, Walsh E.E. Immunogenicity of purified F glycoprotein of respiratory syncytial virus: clinical and immune responses to subsequent natural infection in children. J. Infect. Dis. 1993;168(4):1024–1029. doi: 10.1093/infdis/168.4.1024. [DOI] [PubMed] [Google Scholar]
- Belshe, R., Iacuzio, D., Mendelman, P., Wolff, M., 1997. Efficacy of a trivalent live attenuated intranasal influenza vaccine in children. Infectious Diseases Society of America 35th Annual Meeting, San Francisco, CA.
- Bennett N.M, Lewis B, Doniger A.S, et al. A coordinated, community wide program in Monroe County, New York, to increase influenza immunization rates in the elderly. Arch. Intern. Med. 1994;154(15):1741–1745. [PubMed] [Google Scholar]
- Ben-Yehuda A, Danenberg H.D, Zakay-Rones Z, Gross D.J, Friedman G. The influence of sequential annual vaccination and of DHEA administration on the efficacy of the immune response to influenza vaccine in the elderly. Mech. Ageing Dev. 1998;102(2–3):299–306. doi: 10.1016/s0047-6374(98)00017-7. [DOI] [PubMed] [Google Scholar]
- Betts R.F, Treanor J, Braman P, Bentley D, Dolin R. Antiviral agents to prevent or treat influenza in the elderly. J. Respir. Dis. 1987;8:S56–S59. [Google Scholar]
- Betts, R.F., O’Brien. D., Menegus, M., et al., 1993. A comparison of the protective benefit of influenza (FLU) vaccine in reducing hospitalization of patients infected with FLU A or FLU B. Clin. Infect. Dis. 17(3), 573 (A257).
- Blaine W.B, Luby J.P, Martin S.M. Severe illness with influenza B. Am. J. Med. 1980;68:181–189. doi: 10.1016/0002-9343(80)90352-6. [DOI] [PubMed] [Google Scholar]
- Buscho R.O, Saxtan D, Shultz P.S, Finch E, Mufson M.A. Infections with viruses and Mycoplasma pneumoniae during exacerbations of chronic bronchitis. J. Infect. Dis. 1978;137:377–383. doi: 10.1093/infdis/137.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carilli A.D, Gohd R.S, Grodon W. A virologic study of chronic bronchitis. N. Engl. J. Med. 1964;270:123. doi: 10.1056/NEJM196401162700303. [DOI] [PubMed] [Google Scholar]
- Cate T.R, Couch R.B, Parker D, Baxter B. Reactogenicity, immunogenicity, and antibody persistence in adults given inactivated influenza virus vaccines — 1978. Rev. Infect. Dis. 1983;5(4):737–747. doi: 10.1093/clinids/5.4.737. [DOI] [PubMed] [Google Scholar]
- Cavallaro J.J, Monto A.S. Community-wide outbreak of infection with a 229E-like coronavirus in Tecumseh, Michigan. J. Infect. Dis. 1970;122:272–279. doi: 10.1093/infdis/122.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 1998. Prevention and control of influenza: recommendations of the advisory committee on immunization practices (ACIP). MMWR 47 (RR-6), 1–26. [PubMed]
- Chanock R.M, McIntosh D. In: Fields B.N, Knipe D.M, editors. Vol. 1. Raven Press; New York: 1990. Parainfluenza viruses; pp. 963–988. (Virology). [Google Scholar]
- Clements M.L, Betts R.F, Tierney E.L, Murphy B.R. Resistance of adults to challenge with influenza A wild-type virus after receiving live or inactivated virus vaccine. J. Clin. Microbiol. 1986;23:73–76. doi: 10.1128/jcm.23.1.73-76.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements M.L, Betts R.F, Tierney E.L, Murphy B.R. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J. Clin. Microbiol. 1986;24(1):157–160. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clover R.D, Crawford S, Glezen W.P, Taber L.H, Matson C.C, Couch R.B. Comparison of heterotypic protection against influenza A/Taiwan/86 (H1N1) by attenuated and inaactivated vaccines to A/Chile/83-like viruses. J. Infect. Dis. 1991;163:300–304. doi: 10.1093/infdis/163.2.300. [DOI] [PubMed] [Google Scholar]
- Coles F.B, Balzano G.J, Morse D.L. An outbreak of influenza A(H3N2) in a well immunized nursing home population. J. Am. Geriatr. Soc. 1992;40(6):589–592. doi: 10.1111/j.1532-5415.1992.tb02108.x. [DOI] [PubMed] [Google Scholar]
- Collins P.L, Hill M.G, Camargo E, Grosfeld H, Chanock R.M, Murphy B.R. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc. Natl. Acad. Sci. USA. 1995;92(25):11563–11567. doi: 10.1073/pnas.92.25.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney M.K, Fox J.P, Hall C.E. The Seattle virus watch. VI. Observations of infections with and illness due to parainfluenza, mumps, and respiratory syncytial viruses and Mycoplasma pneumoniae. Am. J. Epidemiol. 1975;101:532–551. doi: 10.1093/oxfordjournals.aje.a112125. [DOI] [PubMed] [Google Scholar]
- Crowe J.E, Jr., Bui P.T, London W.T, et al. Satisfactorily attenuated and protective mutants derived from a partially attenuated cold-passaged respiratory syncytial virus mutant by introduction of additional attenuating mutations during chemical mutagenesis. Vaccine. 1994;12(8):691–699. doi: 10.1016/0264-410x(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Crowe J.E, Jr., Bui P.T, Siber G.R, Elkins W.R, Chanock R.M, Murphy B.R. Cold-passaged, temperature-sensitive mutants of human respiratory syncytial virus (RSV) are highly attenuated, immunogenic, and protective in seronegative chimpanzees, even when RSV antibodies are infused shortly before immunization. Vaccine. 1995;13(9):847–855. doi: 10.1016/0264-410x(94)00074-w. [DOI] [PubMed] [Google Scholar]
- Crowe J.E, Jr., Bui P.T, Firestone C.Y, et al. Live subgroup B respiratory synyctial virus vaccines that are attenuated, genetically stable, and immunogenic in rodents and non-human primates. J. Infect. Dis. 1996;173:829–839. doi: 10.1093/infdis/173.4.829. [DOI] [PubMed] [Google Scholar]
- Daynes R.A, Araneo B.A. The development of effective vaccine adjuvants employing natural regulators of T-cell lymphokine production in vivo. Ann. N.Y. Acad. Sci. 1994;730:144–161. doi: 10.1111/j.1749-6632.1994.tb44246.x. [DOI] [PubMed] [Google Scholar]
- Degelau J, Somani S.K, Cooper S.L, Guay D.R.P, Crossley K.B. Amantadine-resistant influenza A in a nursing facility. Arch. Intern. Med. 1992;152:390–392. [PubMed] [Google Scholar]
- Dolin R, Reichman R.C, Madore H.P, Maynard R, Linton P.N, Webber-Jones J. A controlled trial of amantadine and rimantadine in the prophylaxis of influenza A in humans. N. Engl. J. Med. 1982;307:580–584. doi: 10.1056/NEJM198209023071002. [DOI] [PubMed] [Google Scholar]
- Douglas R.G., Jr. Prophylaxis and treatment of influenza. N. Engl. J. Med. 1990;322(7):443–450. doi: 10.1056/NEJM199002153220706. [DOI] [PubMed] [Google Scholar]
- Dowell S.F, Anderson L.J, Gary H.E, Jr., et al. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J. Infect. Dis. 1996;174(3):456–562. doi: 10.1093/infdis/174.3.456. [DOI] [PubMed] [Google Scholar]
- Drinka P.J, Gravenstein S, Schilling M, Krause P, Miller B.A, Shult P. Duration of antiviral prophylaxis during nursing home outbreaks of influenza A: a comparison of two protocols. Arch. Intern. Med. 1998;158:2155–2159. doi: 10.1001/archinte.158.19.2155. [DOI] [PubMed] [Google Scholar]
- Eadie M.B, Stott E.J, Grist N.R. Virological studies in chronic bronchitis. Br. Med. J. 1966;2:671–673. doi: 10.1136/bmj.2.5515.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K.M, Dupont W.D, Westrich M.K, Plummer W.D.J, Palmer P.S, Wright P.F. A randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J. Infect. Dis. 1994;169(1):68–76. doi: 10.1093/infdis/169.1.68. [DOI] [PubMed] [Google Scholar]
- el Guink N, Kris R.M, Goodman-Snitkoff G, Small P.A.J, Mannino R.J. Intranasal immunization with proteoliposomes protects against influenza. Vaccine. 1989;7(2):147–151. doi: 10.1016/0264-410x(89)90055-8. [DOI] [PubMed] [Google Scholar]
- Englund J.A, Sullivan C.J, Jordan M.C. Respiratory syncytial virus infection in immunocompromised adults. Ann. Intern. Med. 1988;109:203–208. doi: 10.7326/0003-4819-109-3-203. [DOI] [PubMed] [Google Scholar]
- Englund J.A, Anderson L.J, Rhame F.S. Nosocomial transmission of respiratory syncytial virus in immunocompromised adults. J. Clin. Microbiol. 1991;29(1):115–119. doi: 10.1128/jcm.29.1.115-119.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan J.-Y, Chastre J. Severe exacerbations of COPD patients: the role of pulmonary infections. Sem. Respir. Infect. 1996;11:109–118. [PubMed] [Google Scholar]
- Falsey A.R, Walsh E.E. Safety and immunogenicity of a respiratory syncytial virus subunit vaccine (PFP-2) in ambulatory adults over age 60. Vaccine. 1996;14(13):1214–1218. doi: 10.1016/s0264-410x(96)00030-8. [DOI] [PubMed] [Google Scholar]
- Falsey A.R, Walsh E.E. Safety and immunogenicity of a respiratory syncytial virus subunit vaccine (PFP-2) in the institutionalized elderly. Vaccine. 1997;15(10):1130–1132. doi: 10.1016/s0264-410x(97)00002-9. [DOI] [PubMed] [Google Scholar]
- Falsey A.R, Walsh E.E, Betts R.F. Serologic evidence of respiratory syncytial virus infection in nursing home patients. J. Infect. Dis. 1990;162:568–569. doi: 10.1093/infdis/162.2.568. [DOI] [PubMed] [Google Scholar]
- Falsey A.R, Walsh E.E. Humoral immunity to respiratory syncytial virus infection in the elderly. J. Med. Virol. 1992;36:39–43. doi: 10.1002/jmv.1890360108. [DOI] [PubMed] [Google Scholar]
- Falsey A.R, Treanor J.J, Betts R.F, Walsh E.E. Viral respiratory infections in the institutionalized elderly: clinical and epidemiologic findings. J. Am. Geriatr. Soc. 1992;40:115–119. doi: 10.1111/j.1532-5415.1992.tb01929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey A.R, Treanor J.J, Betts R.F, Walsh E.E. Viral respiratory infections in the institutionalized elderly: clinical and epidemiologic findings. J. Am. Geriatr. Soc. 1992;40(2):115–119. doi: 10.1111/j.1532-5415.1992.tb01929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey A.R, Cunningham C.K, Barker W.H, et al. Respiratory syncytial virus and influenza A virus infections in the hospitalized elderly. J. Infect. Dis. 1995;172:389–394. doi: 10.1093/infdis/172.2.389. [DOI] [PubMed] [Google Scholar]
- Falsey A.R, McCann R.M, Hall W.J, et al. Acute respiratory tract infection in daycare centers for older persons. J. Am. Geriatr. Soc. 1995;43:30–36. doi: 10.1111/j.1532-5415.1995.tb06238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey A.R, McCann R.M, Hall W.J, et al. The ‘common cold’ in frail older persons: impact of rhinovirus and coronavirus in a senior daycare center. J. Am. Geriatr. Soc. 1997;45(6):706–711. doi: 10.1111/j.1532-5415.1997.tb01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey A.R, Walsh E.E. Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. J. Infect. Dis. 1998;177(2):463–466. doi: 10.1086/517376. [DOI] [PubMed] [Google Scholar]
- Fedson D.S, Wajda A, Nicol J.P, Hammond G.W, Kalser D.L, Roos L.L. Clinical effectivenss of influenza vaccination in Manitoba. J. Am. Med. Assoc. 1993;270(16):1956–1961. [PubMed] [Google Scholar]
- Firestone C.Y, Whitehead S.S, Collins P.L, Murphy B.R, Crowe J.E., Jr. Nucleotide sequence analysis of the respiratory syncytial virus subgroup A cold-passaged (cp) temperature-sensitive (ts) cpts-248/404 live attenuated virus vaccine candidate. Virology. 1996;225(2):419–422. doi: 10.1006/viro.1996.0618. [DOI] [PubMed] [Google Scholar]
- Fleet W.J, Couch R.B, Cate T.R, Knight V. Homologous and hetrologous resistance to rhinovirus common cold. Am. J. Epidemiol. 1965;82:185–196. doi: 10.1093/oxfordjournals.aje.a120543. [DOI] [PubMed] [Google Scholar]
- Fleming D.M, Cross K.W. Respiratory syncytial virus or influenza? Lancet. 1993;342:1507–1510. doi: 10.1016/s0140-6736(05)80082-0. [DOI] [PubMed] [Google Scholar]
- Fransen H, Heigl Z, Wolontis S, Forsgren M, Svedmyr A. Infections with viruses in patients hospitalized with acute respiratory illness, Stockholm, 1963–1967. Scand. J. Infect. Dis. 1969;1:127–136. doi: 10.3109/inf.1969.1.issue-2.09. [DOI] [PubMed] [Google Scholar]
- Fulginiti V.A, Eller J.J, Joyner J.W, Askin P. Parainfluenza virus immunization. Am. J. Dis. Child. 1967;114:26–28. doi: 10.1001/archpedi.1967.02090220032005. [DOI] [PubMed] [Google Scholar]
- Fulginiti V.A, Eller J.J, Sieber O.F, Joyner J.W, Minamitani M, Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines: an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respriratory syncytial virus vaccine. Am. J. Epidemiol. 1969;89:435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- Galbraith A.W, Oxford J.S, Schild G.C. Protective effect of 1-adamantanamine hydrochloride on influenza A2 in the family environment. Lancet. 1969;2:1026–1028. doi: 10.1016/s0140-6736(69)90639-4. [DOI] [PubMed] [Google Scholar]
- Gern J.E, Busse W.W. Association of rhinovirus infections with asthma. Clin. Microbiol. Rev. 1999;12(1):9–18. doi: 10.1128/cmr.12.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladman J.R.F, Barer D, Venkatesan P, et al. The outcome of pneumonia in the elderly: a hospital survey. Clin. Rehab. 1991;5:201–205. [Google Scholar]
- Glathe H, Bigl S, Grosche A. Comparison of humoral immune responses to trivalent influenza split vaccine in young, middle-aged and elderly people. Vaccine. 1993;11(7):702–705. doi: 10.1016/0264-410x(93)90252-s. [DOI] [PubMed] [Google Scholar]
- Glezen W.P, Loda F.A, Clyde W.A, Jr., et al. Epidemiologic patterns of acute lower respiratory disease of children in a pediatric group practice. J. Pediatr. 1971;78(3):397–406. doi: 10.1016/s0022-3476(71)80218-4. [DOI] [PubMed] [Google Scholar]
- Glezen W.P. Serious morbidity and mortality associated with influenza epidemics. Epidemiol. Rev. 1982;4:24–44. doi: 10.1093/oxfordjournals.epirev.a036250. [DOI] [PubMed] [Google Scholar]
- Gluck R, Mischler R, Finkel B, Que J.U, Scarpa B, Cryz S.J., Jr. Immunogenicity of new virosome influenza vaccine in elderly people. Lancet. 1994;344(8916):160–163. doi: 10.1016/s0140-6736(94)92758-8. [DOI] [PubMed] [Google Scholar]
- Gorse G.J, Belshe R.B. Enhancement of anti-influenza A virus cytotoxicity following influenza A vaccination in older, chronically ill adults. J. Clin. Microbiol. 1990;28:2539–2550. doi: 10.1128/jcm.28.11.2539-2550.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorse G.J, Belshe R.B, Munn N.J. Local and systemic antibody responses in high-risk adults given live attenuated and inactivated influenza A virus vaccines. J. Clin. Microbiol. 1988;26:911–918. doi: 10.1128/jcm.26.5.911-918.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorse G.J, Belshe R.B, Munn N.J. Superiority of live attenuated compared with inactivated influenza A virus vaccines in older, chronically ill adults. Chest. 1991;100:977–984. doi: 10.1378/chest.100.4.977. [DOI] [PubMed] [Google Scholar]
- Gorse G.J, Otto E.E, powers D.C, Chambers G.W, Eickhoff C.S, Newman F.K. Induction of mucosal antibodies by live attenuated and inactivated influenza vaccines in the chronically ill elderly. J. Infect. Dis. 1996;173:285–290. doi: 10.1093/infdis/173.2.285. [DOI] [PubMed] [Google Scholar]
- Govaert T.M, Thijs C.T, Masurel N, Sprenger M.J, Dinant G.J, Knottnerus J.A. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. J. Am. Med. Assoc. 1994;270(16):1956–1961. [PubMed] [Google Scholar]
- Gravenstein S, Miller B.A, et al. Augmentation of influenza antibody response in elderly men by thymosin alpha one: a double-blind placebo-controlled clinical study. J. Am. Geriatr. Soc. 1989;37:1–8. doi: 10.1111/j.1532-5415.1989.tb01561.x. [DOI] [PubMed] [Google Scholar]
- Gravenstein S, Drinka P, Duthie E.H, et al. Efficacy of an influenza hemagglutinin-diphtheria toxoid conjugate vaccine in elderly nursing home subjects during an influenza outbreak. J. Am. Geriatr. Soc. 1994;42(3):245–251. doi: 10.1111/j.1532-5415.1994.tb01746.x. [DOI] [PubMed] [Google Scholar]
- Greenberg S.B. Viral pneumonia. Infect. Dis. Clin. North Am. 1991;5(3):603–621. [PubMed] [Google Scholar]
- Gross P.A, Quinnan G.V, Weksler M.E, Gaerlan P.F, Cenning C.R. Immunization of elderly people with high doses of influenza vaccine. J. Am. Geriatr. Soc. 1988;36:209–212. doi: 10.1111/j.1532-5415.1988.tb01802.x. [DOI] [PubMed] [Google Scholar]
- Gross P.A, Rodstein M, LaMontagne J.R, et al. Epidemiology of acute respiratory illness during an influenza outbreak in a nursing home: a prospective study. Arch. Intern. Med. 1988;148:559–561. [PubMed] [Google Scholar]
- Gross P.A, Quinnan G.V, Weksler M.E, Setia U, Douglas R.G., Jr. Relation of chronic disease and immune response to influenza vaccine in the elderly. Vaccine. 1989;7:303. doi: 10.1016/0264-410x(89)90190-4. [DOI] [PubMed] [Google Scholar]
- Gross P.A, Hermogenes A.W, Sacks H.S, Lau J, Levandowski R.A. The efficacy of influenza vaccine in elderly persons: a meta-analysis and review of the literature. Ann. Intern. Med. 1995;123:518–527. doi: 10.7326/0003-4819-123-7-199510010-00008. [DOI] [PubMed] [Google Scholar]
- Gruber W.C, Campbell P.W, Thompson J.M, Reed G.W, Roberts B, Wright P.F. Comparison of live attenuated and inactivated influenza vaccines in cystic fibrosis patients and their families: results of a 3-year study. J. Infect. Dis. 1994;169:241–247. doi: 10.1093/infdis/169.2.241. [DOI] [PubMed] [Google Scholar]
- Gwaltney J.M., Jr. Combined antiviral and antimediator treatment of rhinovirus colds. J. Infect. Dis. 1992;166(4):776–782. doi: 10.1093/infdis/166.4.776. [DOI] [PubMed] [Google Scholar]
- Gwaltney J.M, Jr., Hendley J.O, Simon G, Jordan W.S., Jr. Rhinovirus infections in an industrial population. I. the occurrence of illness. N. Engl. J. Med. 1966;275:1261–1268. doi: 10.1056/NEJM196612082752301. [DOI] [PubMed] [Google Scholar]
- Gwaltney J.M, Jr., Hendley J.O, Simon G, Jordan W.S., Jr. Rhinovirus infections in an industrialized population II characteristics of illness and antibody response. J. Am. Med. Assoc. 1967;202(6):158–164. [PubMed] [Google Scholar]
- Gwaltney J.M, Jr., Phillips C.D, Miller R.D, Riker D.K. Computed tomographic study of the common cold. N. Engl. J. Med. 1994;330(1):25–30. doi: 10.1056/NEJM199401063300105. [DOI] [PubMed] [Google Scholar]
- Hall C.G, Geiman J.M, Biggar R, Kotok D, Hogan P, Douglas R.G., Jr. Respiratory syncytial virus infections within families. N. Engl. J. Med. 1976;294:414–419. doi: 10.1056/NEJM197602192940803. [DOI] [PubMed] [Google Scholar]
- Hall W.J, Hall C.B, Speers D.M. Respiratory syncytial virus infection in adults. Ann. Intern. Med. 1978;88:203–205. doi: 10.7326/0003-4819-88-2-203. [DOI] [PubMed] [Google Scholar]
- Halperin S.A, Eggleston P.A, Hendley J.O, Suratt P.M, Groschel D.H.M, Gwaltney J.M., Jr. Pathogenesis of lower respiratory tract symptoms in experimental rhinovirus infection. Am. Rev. Respir. Dis. 1983;128:806–810. doi: 10.1164/arrd.1983.128.5.806. [DOI] [PubMed] [Google Scholar]
- Halperin S.A, Eggleston P.A, Beasley P, et al. Exacerbations of asthma in adults during experimental rhinovirus infection. Am. Rev. Respir. Dis. 1985;132:976–980. doi: 10.1164/arrd.1985.132.5.976. [DOI] [PubMed] [Google Scholar]
- Hamre D, Beem M. Virologic studies of acute respiratory disease in young adults. V. Coronavirus 229E infections during six years of surveillance. Am. J. Epidemiol. 1972;96:94–106. doi: 10.1093/oxfordjournals.aje.a121445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamre D, Procknow J.J. A new virus isolated from the human respiratory tract. Proc. Soc. Exp. Biol. Med. 1966;121:190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- Harrington R.D, Hooton R.D, Hackman R.C, et al. An outbreak of respiratory syncytial virus in a bone marrow transplant center. J. Infect. Dis. 1992;165:987–993. doi: 10.1093/infdis/165.6.987. [DOI] [PubMed] [Google Scholar]
- Hayden F.G, Monto A.S. Oral rimantadine hydrochloride therapy of influenza A virus H3N2 subtype infection in adults. Antimicrob. Agents Chemother. 1986;29(2):339–341. doi: 10.1128/aac.29.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F.G, Belshe R.B, Clover R.D, Hay A.J, Oakes M.G, Soo W. Emergence and apparent transmission of rimantadine-resistant influenza A virus in families. N. Engl. J. Med. 1989;321:1696–1702. doi: 10.1056/NEJM198912213212502. [DOI] [PubMed] [Google Scholar]
- Hayden F.G, Andries K, Jannssen P.A.J. Safety and efficacy of intranasal pirodavir (R77975) in experimental rhinovirus infection. Antimicrob. Agents Chemother. 1992;36(4):727–732. doi: 10.1128/aac.36.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F.G, Treanor J.J, Betts R.F, Lobo M, Esinhart J.D, Hussey E.K. Safety and efficacy of the neuraminidase inhibitor GG167 in experimental human influenza. J. Am. Med. Assoc. 1996;275:295–299. [PubMed] [Google Scholar]
- Hayden F.G, Osterhaus A.D.M.E, Treanor J.J, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza virus infections. N. Engl. J. Med. 1997;337(13):874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- Hayden, F.G., Treanor, J.J., Lobo, M., et al., 1999. Randomized, double-blind studies of the oral neuramindase inhibitor GS4104 (Ro64-0796) in experimental human influenza. J. Am. Med. Assoc. (in press).
- Hayden, F.G., Hassman, H.A., Coats, T., Menendez, R., Bock, T., 1999. Pleconaril treatment shortens the duration of piconavirus respiratory illness in adults. Abstract LB-3. In: Thirty-ninth Interscience Conference of Antimicrobial Agents and Chemotherapy, San Francisco, CA, September, 1999.
- Hendley J.O, Gwaltney J.M, Jordan W.S., Jr. Rhinovirus infections in an industrial population. IV. infections within families of employeees during two fall peaks of respiratory illness. Am. J. Epidemiol. 1969;89(2):184–196. doi: 10.1093/oxfordjournals.aje.a120928. [DOI] [PubMed] [Google Scholar]
- Hendley J.O, Fishburne H.B, Gwaltney J.M., Jr. Coronavirus infections in working adults. Eight year study with 229E and OC43. Am. Rev. Respir. Dis. 1972;105:805–811. doi: 10.1164/arrd.1972.105.5.805. [DOI] [PubMed] [Google Scholar]
- Hertz M.I, Englund J.A, Snover D, Bitterman P.B, McGlave P.B. Respiratory syncytial virus-induced acute lung injury in adult patients with bone marrow transplants: a clinical approach and review of the literature. Medicine. 1989;68(5):269–281. doi: 10.1097/00005792-198909000-00002. [DOI] [PubMed] [Google Scholar]
- Higgins P.G, Phillpotts R.J, Scott G.M, Wallace J, Bernhardt L.L, Tyrrell D.A.J. Intranasal interferon as protection against experimental respiratory coronavirus infection in volunteers. Antimicrob. Agents Chemother. 1983;24:713–715. doi: 10.1128/aac.24.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn M.E.C, Reed S.E, Taylor P. Role of viruses and bacteria in acute wheezy bronchitis in childhood. Arch. Dis. Child. 1979;54:587–592. doi: 10.1136/adc.54.8.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck P, Hemphill M, SlCroix S, Hirsh D, Cox N. Amantadine-resistant influenza A in nursing homes: identification of a resistant virus prior to drug use. Arch. Intern. Med. 1995;155:533–537. [PubMed] [Google Scholar]
- Hsu K.-H, Lubeck M.D, Davis A.R, et al. Immunogenicity of recombinant adenovirus-respiratory syncytial virus vaccines with adenovirus types 4, 5, and 7 vectors in dogs and a chimpanzee. J. Infect. Dis. 1992;199:769–775. doi: 10.1093/infdis/166.4.769. [DOI] [PubMed] [Google Scholar]
- Hsu K.H, Lubeck M.D, Bhat B.M, et al. Efficacy of adenovirus-vectored respiratory syncytial virus vaccines in a new ferret model. Vaccine. 1994;12(7):607–612. doi: 10.1016/0264-410x(94)90264-x. [DOI] [PubMed] [Google Scholar]
- Hudgel D.W, Lanston L, Selner J.C, McIntosh K. Viral and bacterial infections in adults with chronic asthma. Am. Rev. Respir. Dis. 1979;120:393–397. doi: 10.1164/arrd.1979.120.2.393. [DOI] [PubMed] [Google Scholar]
- Kaji M, Kaji Y, Kaji M, et al. Phase I clinical tests of influenza MDP-virosome vaccine (KD-5382) Vaccine. 1992;10(10):637–663. doi: 10.1016/0264-410x(92)90086-y. [DOI] [PubMed] [Google Scholar]
- Karron R.A, Wright P.F, Hall S.L, et al. A live attenuated bovine parainfluenza virus type 3 vaccine is safe, infectious, immunogenic, and phenotypically stable in infants and children. J. Infect. Dis. 1995;171:1107–1114. doi: 10.1093/infdis/171.5.1107. [DOI] [PubMed] [Google Scholar]
- Karron R.A, Wright P.F, Newman F.K, et al. A live human parainfluenza virus type 3 virus vaccine is attenuated and immunogenic in healthy infants and children. J. Infect. Dis. 1995;172:1445–1450. doi: 10.1093/infdis/172.6.1445. [DOI] [PubMed] [Google Scholar]
- Kaye H.S, Dowdle W.R. Seroepidemiologic survey of coronavirus (strain 229E) infections in a population of children. Am. J. Epidemiol. 1975;101:238–244. doi: 10.1093/oxfordjournals.aje.a112091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye H.S, Marsh H.B, Dowdle W.R. Seroepidemiologic survey of coronavirus (strain OC43) related infections in a children's population. Am. J. Epidemiol. 1971;94:43–49. doi: 10.1093/oxfordjournals.aje.a121293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitel W.A, Couch R.B, Cate T.R, et al. High doses of purified influenza A virus hemagglutinin significantly augment serum and nasal secretion antibody responses in healthy young adults. J. Clin. Microbiol. 1994;32(10):2468–2473. doi: 10.1128/jcm.32.10.2468-2473.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser, L.A., Karl, M., Nafziger, A.N., Bertino, J.S., Jr., 1998. Comparison of adverse events (AEs) of amantadine (AM) and rimantadine (RM) used as sequential prophylaxis of influenza A (FLU) in elderly nursing home residents. 38th ICAAC, San Francisco, CA: American Society of Microbiology.
- Kim H.W, Arrobio J.O, Brandt C.D, et al. Safety and antigenicity of temperature-sensitive (TS) mutant respiratory syncytial virus (RSV) in infants and children. Pediatrics. 1973;52:56–63. [PubMed] [Google Scholar]
- Lambert H.P, Stern H. Infective factors in exacerbations of bronchitis and asthma. Br. Med. J. 1972;3:323–327. doi: 10.1136/bmj.3.5822.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMontagne J.R, Noble G.R, Quinnan G.V, et al. Summary of clinical trials of inactivated influenza vaccine — 1978. Rev. Infect. Dis. 1983;5(4):723–736. doi: 10.1093/clinids/5.4.723. [DOI] [PubMed] [Google Scholar]
- Lovgren K, Kaberg H, Morein B. An experimental influenza subunit vaccine (ISCOM): induction of protective immunity to challenge infection in mice after intranasal or subcutaneous administration. Clin. Exp. Immunol. 1990;82(3):435–439. doi: 10.1111/j.1365-2249.1990.tb05467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maassab H.F, DeBorde D.C. Development and characterization of cold-adapted viruses for use as live virus vaccines. Vaccine. 1985;3:335–369. doi: 10.1016/0264-410x(85)90124-0. [DOI] [PubMed] [Google Scholar]
- MacFarlane J.T, Colville A, Guion A, MacFarlane R.M, Rose D.H. Prospective study of aetiology and outcome of adults lower respiratory tract infections in the community. Lancet. 1993;34:511–514. doi: 10.1016/0140-6736(93)90275-l. [DOI] [PubMed] [Google Scholar]
- Macnaughton M.R. Occurrence and frequency of coronavirus infections in humans as determined by enzyme linked immunosorbent assay. Infect. Immunol. 1982;38:419–423. doi: 10.1128/iai.38.2.419-423.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie T.J, Peeling R.W, Fine M.J, singer D.E, Coley C.M, Kappor W.N. Ambulatory patients with community-acquired pneumonia: the frequency of atypical agents and clinical course. Am. J. Med. 1996;101:508–515. doi: 10.1016/s0002-9343(96)00255-0. [DOI] [PubMed] [Google Scholar]
- Martin M.A, Block M.J, Peallen M.A, Wenzel R.P. Respiratory syncytial virus infections in adult bone marrow transplant recipients. Lancet. 1988;i:1341–1343. doi: 10.1016/s0140-6736(88)92208-8. [DOI] [PubMed] [Google Scholar]
- Martin J.T. Development of an adjuvant to enhance the immune response to influenza vaccine in the elderly. Biologicals. 1997;25(2):209–213. doi: 10.1006/biol.1997.0086. [DOI] [PubMed] [Google Scholar]
- Mast E.E, Harman M.W, Gravenstein S, et al. Emergence and possible transmission of amantadine-resistant viruses during nursing home outbreaks of influenza A (H3N2) Am. J. Epidemiol. 1991;134(9):988–997. doi: 10.1093/oxfordjournals.aje.a116184. [DOI] [PubMed] [Google Scholar]
- Mathur U, Bentley D.W, Hall C.B. Concurrent respiratory syncytial virus and influenza A infections in the institutionalized elderly and chronically ill. Ann. Intern. Med. 1980;93:49–52. doi: 10.7326/0003-4819-93-1-49. [DOI] [PubMed] [Google Scholar]
- McKay E, Higgins P, Tyrrell D, Pringle C. Immunogenicity and pathogenicity of temperature-sensitive modified respiratory syncytial virus in adult volunteers. J. Med. Virol. 1988;25:411. doi: 10.1002/jmv.1890250405. [DOI] [PubMed] [Google Scholar]
- McNamara M.J, Phillips I.A, Williams O.B. Viral and Mycoplasma pneumoniae infections in exacerbations of chronic lung disease. Am. Rev. Respir. Dis. 1969;100:19–24. doi: 10.1164/arrd.1969.100.1.19. [DOI] [PubMed] [Google Scholar]
- Meiklejohn G, Eickhoff T.C, Graves P.I.J. Antigenic drift and efficacy of influenza virus vaccines, 1976–1977. J. Infect. Dis. 1978;138(5):618–624. doi: 10.1093/infdis/138.5.618. [DOI] [PubMed] [Google Scholar]
- Meiklejohn G. Viral respiratory disease at Lowry Air Force Base in Denver, 1952–1982. J. Infect. Dis. 1983;148(5):775–783. doi: 10.1093/infdis/148.5.775. [DOI] [PubMed] [Google Scholar]
- Minor T.E, Dick E.C, DeMeo A.N, Ouellette J.J, Cohen M, Reed C.E. Viruses as precipitants of asthmatic attacks in children. J. Am. Med. Assoc. 1974;21:292–298. [PubMed] [Google Scholar]
- Minor T.E, Dick E.C, Baker J.W, Ouellette J.J, Cohen M, Reed C.E. Rhinovirus and influenza type A infections as precipitants of asthma. Am. Rev. Respir. Dis. 1976;113:149–153. doi: 10.1164/arrd.1976.113.2.149. [DOI] [PubMed] [Google Scholar]
- Minutello M, Senatore F, Cecchinelli G, et al. Safety and immunogenicity of an inactivated subunit influenza virus vaccine combined with MF59 adjuvant emulsion in elderly subjects, immunized for three consecutive influenza seasons. Vaccine. 1999;17(2):99–104. doi: 10.1016/s0264-410x(98)00185-6. [DOI] [PubMed] [Google Scholar]
- Miyazaki C, Nakayama M, Tanaka Y, et al. Immunization of institutionalized asthmatic children and patients with psychomotor retardation using live attenuated cold-adapted reassortment influenza A H1N1, H3N2, and B vaccines. Vaccine. 1993;11(8):853–858. doi: 10.1016/0264-410x(93)90361-z. [DOI] [PubMed] [Google Scholar]
- Monto A.S, Lim S.K. The Tecumseh study of respiratory illness. VI. Frequency of and relationship between outbreaks of coronavirus infection. J. Infect. Dis. 1974;129:271–276. doi: 10.1093/infdis/129.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto A.S, Sullivan K.M. Acute respiratory illness in the community: frequency of illness and the agents involved. Epidemiol. Infect. 1993;110:145–160. doi: 10.1017/s0950268800050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto A.S, Ohmit S.E, Hornbuckle K, Pearce C.L. Safety and efficacy of long-term use of rimantadine for prophylaxis of type A influenza in nursing homes. Antimicrob. Agents Chemother. 1995;39:2224–2228. doi: 10.1128/aac.39.10.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto A.S, Robinson D.P, Herlocher M.L, Hinson J.M, Jr., Elliot M.J, Crisp A. Zanamivir in the prevention of influenza among healthy adults: a randomized controlled trial. J. Am. Med. Assoc. 1999;282(1):31–35. doi: 10.1001/jama.282.1.31. [DOI] [PubMed] [Google Scholar]
- Monto A.S. In: Viral Infections of Humans. Evans A.S, editor. Plenum Publishing; New York: 1997. Coronaviruses; pp. 211–227. [Google Scholar]
- Mufson M.A, Chang V, Gill V, et al. The role of viruses, Mycoplasma and bacteria in acute pneumonia in civilian adults. Am. J. Epidemiol. 1967;86:526–587. doi: 10.1093/oxfordjournals.aje.a120763. [DOI] [PubMed] [Google Scholar]
- Mufson M.A, Mocega H.E, Krause H.E. Acquisition of parainfluenza 3 virus infection by hospitalized children. I. Frequencies, rates, and temporal data. J. Infect. Dis. 1973;128:141–147. doi: 10.1093/infdis/128.2.141. [DOI] [PubMed] [Google Scholar]
- Murphy B.R. Use of live, attenuated cold-adapted influenza A reassortant virus vaccines in infants, children, young adults, and elderly adults. Infect. Dis. Clin. Pract. 1993;2:176–181. [Google Scholar]
- Neuzil K.M, Johnson J.E, Tang Y.W, et al. Adjuvants influence the quantitative and qualitative immune response in BALB/c mice immunized with respiratory syncytial virus FG subunit vaccine. Vaccine. 1997;15(5):525–532. doi: 10.1016/s0264-410x(97)00218-1. [DOI] [PubMed] [Google Scholar]
- Nichol K.L, Margolis K.L, Wuorenma J, Von Sternberg T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N. Engl. J. Med. 1994;331(12):778–784. doi: 10.1056/NEJM199409223311206. [DOI] [PubMed] [Google Scholar]
- Nichol K.L, Mendelman P.M, Mallon K.P, et al. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. J. Am. Med. Assoc. 1999;282(2):137–144. doi: 10.1001/jama.282.2.137. [DOI] [PubMed] [Google Scholar]
- Nicholson K.G. Impact of influenza and respiratory syncytial virus on mortality in England and Wales from Jaunary to December 1990. Epidemiol. Infect. 1975;116:51–63. doi: 10.1017/s0950268800058957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson K.G, Baker D.J, Farquhar A, Hurd D, Kent J, Smith S.H. Acute upper respiratory tract viral illness and influenza immunization in homes for the elderly. Epidemiol. Infect. 1990;105(3):609–618. doi: 10.1017/s0950268800048251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson K.G, Baker D.J, Chakraverty P, et al. Immunogenicity of inactivated influenza vaccine in residential homes for elderly people. Age Ageing. 1992;21:182–188. doi: 10.1093/ageing/21.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson K.G, Kent J, Hammersley V, Cancio E. Risk factors for lower respiratory complications of rhinovirus infections in elderly people living in the community: prospective cohort study. Br. Med. J. 1996;313(7065):1119–1123. doi: 10.1136/bmj.313.7065.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson K.G, Kent J, Hammersley V, Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. Br. Med. J. 1997;315(7115):1060–1064. doi: 10.1136/bmj.315.7115.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterweil D, Norman D. An outbreak of an influenza-like illness in a nursing home. J. Am. Geriatr. Soc. 1990;38:659–662. doi: 10.1111/j.1532-5415.1990.tb01425.x. [DOI] [PubMed] [Google Scholar]
- Palache A.M, Beyer W.E.P, Sprenger M.J.W, et al. Antibody response after influenza immunization with various vaccine doses: a double-blind, placebo-controlled, multi-centre, dose–respones study in elderly nursing home residents and young volunteers. Vaccine. 1993;11(1):3–9. doi: 10.1016/0264-410x(93)90333-s. [DOI] [PubMed] [Google Scholar]
- Parrott R.H, Kim H.W, Arrobio J.O. Epidemiology of respiratory syncytial virus infection in Washington, DC. Am. J. Epidemiol. 1973;98:289–300. doi: 10.1093/oxfordjournals.aje.a121558. [DOI] [PubMed] [Google Scholar]
- Perrotta D.M, Decker M, Glezen W.P. Acute respiratory disease hospitalizations as a measure of impact of epidemic influenza. Am. J. Epidemiol. 1985;122(3):468–476. doi: 10.1093/oxfordjournals.aje.a114128. [DOI] [PubMed] [Google Scholar]
- Pitkaranta A, Arruda E, Malmberg H, Hayden F.G. Detection of rhinovirus in sinus brushings of patients with acute community-acquired sinusitis by reverse transcription-PCR. J. Clin. Microbiol. 1997;35(7):1791–1793. doi: 10.1128/jcm.35.7.1791-1793.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers D.C, Belshe R.B. Effect of age on cytotoxic T lymphocyte memory as well as serum and local antibody responses elicited by inactivated influenza vaccine. J. Infect. Dis. 1993;197:584–592. doi: 10.1093/infdis/167.3.584. [DOI] [PubMed] [Google Scholar]
- Powers D.C, Sears S.D, Murphy B.R, Thuman B, Clements M.L. Systemic and local antibody responses in elderly subjects given live or inactivated influenza A virus vaccines. J. Clin. Microbiol. 1989;27:2666–2671. doi: 10.1128/jcm.27.12.2666-2671.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers D.C, Fries L.F, Murphy B.R, Thumar J.B, Clements M.L. In elderly persons live attenuated influenza A virus vaccines do not offer an advantage over inactivated virus vaccine in inducing serum or secretory antibodies or local immunologic memory. J. Clin. Microbiol. 1991;29:498–505. doi: 10.1128/jcm.29.3.498-505.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers D.C, Murphy B.R, Fries L, Adler W.H, Clements M.L. Reduced infectivity of cold-adapted influenza A H1N1 viruses in the elderly: correlation with serum and local antibodies. J. Am. Geriatr. Soc. 1992;40:163–167. doi: 10.1111/j.1532-5415.1992.tb01938.x. [DOI] [PubMed] [Google Scholar]
- Powers D.C. Effect of age on serum immunoglobulin G subclass antibody responses to inactivated influenza virus vaccine. J. Med. Virol. 1994;43(1):57–61. doi: 10.1002/jmv.1890430111. [DOI] [PubMed] [Google Scholar]
- Powers D.C. Summary of a clinical trial with liposome-adjuvanted influenza A virus vaccine in elderly adults. Mech. Aging Dev. 1997;93(1–3):179–188. doi: 10.1016/s0047-6374(96)01809-x. [DOI] [PubMed] [Google Scholar]
- Pozzetto B, Odelin M.F, Bienvenu J, Defayolle M, Aymard M. Is there a relationship between malnutrition, inflammation, and post-vaccinal antibody response to influenza viruses in the elderly? J. Med. Virol. 1993;41(1):39–43. doi: 10.1002/jmv.1890410109. [DOI] [PubMed] [Google Scholar]
- Pringle C.R, Filipiuk A.H, Robinson B.S, Watt P.J, Higgins P, Tyrrell D.A.J. Immunogenicity and pathogenicity of a triple temperature-sensitive modified respiratory syncytial virus in adult volunteers. Vaccine. 1993;11(4):473–478. doi: 10.1016/0264-410x(93)90290-e. [DOI] [PubMed] [Google Scholar]
- Provinciali M, Di Stefano G, Colombo M, et al. Adjuvant effect of low-dose interleukin-2 on antibody response to influenza virus vaccination in healthy elderly subjects. Mech. Ageing Dev. 1994;77(2):75–82. doi: 10.1016/0047-6374(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Quinnan G.V, Schooley R, Dolin R, Ennis F.A, Gross P, Gwaltney J.M., Jr. Serologic respones and systemic reactions in adults after vaccination with monovalent A/USSR/77 and trivalent A/USSR/77, A/Texas/77, B/Hong Kong/72 influenza vaccines. Rev. Infect. Dis. 1983;5(4):748–757. doi: 10.1093/clinids/5.4.748. [DOI] [PubMed] [Google Scholar]
- MIST, 1998. Randomised trial of efficacy and safety of inhaled zanamivir in treatment of influenza A and B virus infections. Lancet 352, 1877–1881. [PubMed]
- Rao S.S, Hendley J.O, Hayden F.G, Gwaltney J.M. Symptom expression in natural and experimental rhinovirus colds. Am. J. Rhinol. 1995;9:49–52. [Google Scholar]
- Ray R, Brown V.E, Compans R.W. Glycoproteins of human parainfluenza virus type 3: characterization and evaluation as a subunit vaccine. J. Infect. Dis. 1985;152(6):1219–1229. doi: 10.1093/infdis/152.6.1219. [DOI] [PubMed] [Google Scholar]
- Ray R, Glaze B.J, Compans R.W. The role of individual glycoproteins of human parainfluenza virus type 3 in the induction of a protective immune response. J. Virol. 1988;62:783–787. doi: 10.1128/jvi.62.3.783-787.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S.E. The behavior of recent isolates of human respiratory coronavirus in vitro in volunteers: Evidence of heterogeneity among 229E-related strains. J. Med. Virol. 1984;13:179–192. doi: 10.1002/jmv.1890130208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remarque E.J, van Beek W.C, Ligthart G.J, et al. Improvement of the immunoglobulin subclass response to influenza vaccine in elderly nursing-home residents by the use of high dose vaccines. Vaccine. 1993;11(6):649–654. doi: 10.1016/0264-410x(93)90311-k. [DOI] [PubMed] [Google Scholar]
- Renegar K.B, Small P.A.J. Passive transfer of local immunity to influenza virus by IgA antibody. J. Immunol. 1991;146(6):1972–1978. [PubMed] [Google Scholar]
- Ruben F.L. Prevention and control of influenza: role of vaccine. Am. J. Med. 1987;82(6A):31–33. doi: 10.1016/0002-9343(87)90558-4. [DOI] [PubMed] [Google Scholar]
- Schilling M, Povinelli L, Krause P, et al. Efficacy of zanamivir for chemoprophylaxis of nursing home influenza outbreaks. Vaccine. 1998;16(18):1771–1774. doi: 10.1016/s0264-410x(98)00141-8. [DOI] [PubMed] [Google Scholar]
- Simonsen L, Clarke M.J, Williamson D.W, Stroup D.F, Arden N.H, Schonberger L.B. The impact of influenza epidemics on mortality: introducing a severity index. Am. J. Pub. Health. 1997;87:1944–1950. doi: 10.2105/ajph.87.12.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.B, Kanner R.E, Golden C.A, Klauber M.R, Renzetti A.D.J. Effect of viral infections on pulmonary function in patients with chronic obstructive pulmonary diseases. J. Infect. Dis. 1985;141:271–280. doi: 10.1093/infdis/141.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo W. Adverse effects of rimantadine: summary from clinical trials. J. Respir. Dis. 1989;10:S26–S31. [Google Scholar]
- Sorvillo F.J, Huie S.F, Strassburg M.A, Butsumyo A, Shandera W.X, Fannin S.L. An outbreak of respiratory syncytial virsu pneumonia in a nursing home for the elderly. J. Infect. 1984;9:252–256. doi: 10.1016/s0163-4453(84)90530-9. [DOI] [PubMed] [Google Scholar]
- Spriggs M.K, Murphy B.R, Prince G.A, Olmsted R.A, Collins P.L. Expression of the F and HN glycoproteins of human parainfluenza virus type 3 by recombinant vaccinia virus: contributions of the individual proteins to host immunity. J. Virol. 1987;61:3416–3423. doi: 10.1128/jvi.61.11.3416-3423.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek J, Heinz F. On the epidemiology and etiology of pneumonia in adults. J. Hyg. Epidemiol. Microbiol. Immunol. 1988;32:31–38. [PubMed] [Google Scholar]
- Stenhouse A.C. Rhinovirus infection in acute exacerbation of chronic bronchitis: a controlled prospective study. Br. Med. J. 1967;3:461. doi: 10.1136/bmj.3.5563.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K.M, Monto A.S, Longini I.M. Estimates of the US health impact of influenza. Am. J. Pub. Health. 1993;83(12):1712–1716. doi: 10.2105/ajph.83.12.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundquist B, Lovgren K, Hoglund S, Morein B. Influenza virus ISCOMs: biochemical characterization. Vaccine. 1988;6:44–48. doi: 10.1016/0264-410x(88)90013-8. [DOI] [PubMed] [Google Scholar]
- Teichtahl H, Buckmaster N, Pertnikovs E. The incidence of respiratory tract infection in adults requiring hospitalization for asthma. Chest. 1997;112(3):591–596. doi: 10.1378/chest.112.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominack R.L, Hayden F.G. Rimantadine hydrochloride and amantadine hydrochloride use in influenza A virus infections. Infect. Dis. Clin. North Am. 1987;1(2):459–478. [PubMed] [Google Scholar]
- Treanor J.J, Betts R.F. Evaluation of live, cold-adapted influenza A and B virus vaccines in elderly and high-risk subjects. Vaccine. 1998;16:1756–1760. doi: 10.1016/s0264-410x(98)00136-4. [DOI] [PubMed] [Google Scholar]
- Treanor J.J, Mattison H.R, Dumyati G, et al. Protective efficacy of combined live intranasal and inactivated influenza A virus vaccines in the elderly. Ann. Intern. Med. 1992;117(8):625–633. doi: 10.7326/0003-4819-117-8-625. [DOI] [PubMed] [Google Scholar]
- Treanor J, Dumyati G, O’Brien D, et al. Evaluation of cold-adapted, reassortant influenza B virus vaccines in elderly and chronically ill adults. J. Infect. Dis. 1994;169(2):402–407. doi: 10.1093/infdis/169.2.402. [DOI] [PubMed] [Google Scholar]
- Treanor J.J, Betts R.F, Smith G.E, et al. Evaluation of a recombinant hemagglutinin expressed in insect cells as an influenza vaccine in young and elderly adults. J. Infect. Dis. 1996;173(6):1467–1470. doi: 10.1093/infdis/173.6.1467. [DOI] [PubMed] [Google Scholar]
- Treanor, J.J., Betts, R.F., Kotloff, K., et al., 1999. Efficacy of trivalent, live, cold-adapted influenza vaccine (CAIV-T) in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine (in press). [DOI] [PubMed]
- Turner B.W, Cail W.S, Hendley J.O, et al. Physiologic abnormalities in the paranasal sinuses during experimental rhinovirus colds. J. Allergy Clin. Immunol. 1992;90(3 pt. 2):474–478. doi: 10.1016/0091-6749(92)90172-x. [DOI] [PubMed] [Google Scholar]
- Turner R.B, Wecker M.T, Pohl G, et al. Efficacy of tremacamra, a soluble intercellular adhesion molecule 1, for experimental rhinovirus infection. J. Am. Med. Assoc. 1999;281(19):1797–1804. doi: 10.1001/jama.281.19.1797. [DOI] [PubMed] [Google Scholar]
- Van Voris L.P, Betts R.F, Hayden F.G, Christmas W.A, Douglas R.G., Jr. Successful treatment of naturally occurring influenza A/USSR/77 H1N1. J. Am. Med. Assoc. 1981;245(11):1128–1131. doi: 10.1001/jama.245.11.1128. [DOI] [PubMed] [Google Scholar]
- Varghese J.N, Laver W.G, Colman P.M. Structure of the influenza glycoprotein antigen neuraminidase at 2.9A resolution. Nature. 1983;303:35–40. doi: 10.1038/303035a0. [DOI] [PubMed] [Google Scholar]
- Vikerfors T, Grandien M, Olan P. Respiratory syncytial virus infections in adults. Am. Rev. Respir. Dis. 1987;136:561–564. doi: 10.1164/ajrccm/136.3.561. [DOI] [PubMed] [Google Scholar]
- Vikerfors T, Grandien M, Johansson M, Pettersson C.-A. Detection of an immunoglobulin M response in the elderly for early diagnosis of respiratory syncytial virus infection. J. Clin. Microbiol. 1988;26:808–811. doi: 10.1128/jcm.26.5.808-811.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Itzstein M, Wu W.-Y, Kok G.B, et al. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363:418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- Wald E.R, Guerra N, Byers C. Upper respiratory tract infections in young children: duration of and frequency of complications. Pediatrics. 1991;87:129–133. [PubMed] [Google Scholar]
- Wald T.G, Shult P, Krause P, Miller B.A, Drinka P, Gravenstein S. A rhinovirus outbreak among residents of a long-term care facility. Ann. Intern. Med. 1995;123(8):588–593. doi: 10.7326/0003-4819-123-8-199510150-00004. [DOI] [PubMed] [Google Scholar]
- Walker J.B, Hussey E.K, Treanor J.J, Montalvo A, Jr., Hayden F.G. Effects of the neuraminidase inhibitor Zanamivir on otologic manifestations of experimental human influenza. J. Infect. Dis. 1997;176(6):1417–1422. doi: 10.1086/514136. [DOI] [PubMed] [Google Scholar]
- Weibel, R.E., Stokes, J., Mascoli, C.C., et al., 1967. Respiratory virus vaccines: VII. Field evaluation of respriatory syncytial, parainfluenza 1, 2, 3, and Mycoplasma pneumoniae vaccines, 1965 to 1966. Am. Rev. Respir. Dis. 96, 724–739. [DOI] [PubMed]
- Wertz G.W, Stott E.J, Young K.K, Anderson K, Ball L.A. Expression of the fusion protein of human respiratory sysncytial virus from recombinant vaccinia virus vectors and protection of vaccinated mice. J. Virol. 1987;61(2):293–301. doi: 10.1128/jvi.61.2.293-301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whimbey E, Champlin R.E, Englund J.A, et al. Combination therapy with aerosolized ribavirin and intravenous immunoglobulin for respiratory syncytial virus disease in adult bone marrow transplant recipients. Bone Marrow Transplant. 1995;16:393–399. [PubMed] [Google Scholar]
- Woods J.M, Bethell R.C, Coates J.A.V, et al. 4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid is a highly effective inhibitor both of the sialidase (neuraminidase) and of growth of a wide range of influenza A and B viruses in vitro. Antimicrob. Agents Chemother. 1993;37(7):1473–1479. doi: 10.1128/aac.37.7.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P.F, Belshe R.B, Kim H.W, Van Voris L.P, Chanock R.M. Administration of a highly attenuated, live respiratory syncytial virus vaccine to adults and children. Infect. Immun. 1982;37:397–400. doi: 10.1128/iai.37.1.397-400.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younkin S.W, Betts R.F, Roth F.K, Douglas R.G., Jr. Reduction in fever and symptoms in young adults with influenza A/Brazil/78 H1N1 infection after treatment with aspirin or amantadine. Antimicrob. Agents Chemother. 1983;23(4):577–582. doi: 10.1128/aac.23.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaroukian M.H, Kashyap G.H, Wentworth B. Case report: respiratory syncytial virus infection — a cause of respiratory distress syndrome and pneumonia in adults. Am. J. Med. Sci. 1988;295:218–222. doi: 10.1097/00000441-198803000-00011. [DOI] [PubMed] [Google Scholar]


