Abstract
Members of the epidermal growth factor receptor family (ErbB family) possess a wide distribution and diverse functions ranging from cellular growth to migration and apoptosis. Though highly implicated in a variety of cancers, their involvement in infectious disease is less recognised. A growing body of evidence now highlights the importance of the ErbB family in a variety of infections. Their role as growth factor receptors, along with other characteristics, such as surface expression and continuous intracellular trafficking, make this receptor family ideally placed for exploitation by pathogens. Herein, we review our current understanding of the role of the ErbB family in the context of infectious disease, exploring the mechanisms that govern pathogen exploitation of this system.
Keywords: ErbB, EGFR, infection
Trends
A wide and diverse variety of microbes have each evolved distinct mechanisms to exploit ErbB receptors, highlighting this receptor kinase family as a critical factor in initiation and maintenance of pathogen infections.
ErbB family members are utilised by pathogens attempting to gain cellular entry, subvert immune responses, and manipulate the cell cycle of infected host cells. These events support and are necessary for pathogen persistence.
Pathogen-mediated ErbB-exploitation may contribute to cellular transformation and oncogenesis in a variety of cancers.
The use of existing FDA-approved drugs that target ErbB receptors and associated signalling components may offer potential future therapies against infection.
ErbB Receptors, a Gatekeeper of Infectious Disease
The coevolution of host and pathogen has ensured that while the host attempts to maintain immunological homeostasis, invading organisms look to manipulate host biology for their own benefit. A central role in this coevolution is played by host cell receptors which can both recognise microbes and manipulate cellular responses to them. As such, pathogens have developed mechanisms to subvert host cell receptors for their own needs; the ErbB family is one group of host receptors involved in such a relationship.
The ErbB receptor tyrosine kinase family consists of four members, ErbB1 (epidermal growth factor receptor, EGFR), ErbB2, ErbB3, and ErbB4, which are expressed on a plethora of cell types, including epithelial [1], endothelial [2], neuronal and glial [3], bone [4], adipose [5], liver [6], and cardiovascular cells [7]. Following ligand binding and activation, oligomerisation of ErbB family members occurs [8]. ErbB signalling is then induced through phosphorylation of intracellular domains, resulting in the activation of several major intracellular signalling pathways, including mitogen activated protein (MAP) kinase, nuclear factor kappa B (NF-ĸB), phosphoinositide 3 (Pi3) kinase, and janus kinase/signal transducer and activator of transcription (JAK/STAT) pathways. ErbB-associated signalling pathways govern a wide variety of outcomes, including cell survival, proliferation, cell death/apoptosis, angiogenesis, adhesion, differentiation, and migration/invasion.
The majority of studies investigating the role of ErbB receptors in infectious disease focus on pathogens that primarily infect mucosal surfaces. In this context, ErbB expression on epithelial cells, the primary point of pathogen contact, plays a crucial role. As membrane receptors, ErbB functionality and localisation make them well positioned to provide a direct point of contact and entry into host cells. Not only are ErbB family members regularly endocytosed during their normal life cycle, but pathogen–ErbB ligation 9, 10 and ErbB receptor signalling cascades 11, 12, 13, 14 can contribute to cellular entry of a diverse range of microbes. Pathogen-mediated hijacking of ErbB signalling pathways also results in prolonged host cell survival 11, 12, 15, 16, 17 as well as altered immune responses 18, 19, 20, 21, 22, 23, 24, 25, 26, which may, in turn, enhance pathogen persistence. Crucially, however, their role as growth factor receptors may be required by intracellular organisms, dependent on host cell machinery for self-propagation, such as hepatitis C virus (HCV) 15, 27, 28, 29, Epstein–Barr virus (EBV) [30], and human papilloma viruses (HPV) [31]. These pathogens are able to regulate transition through host cell cycle checkpoints, and indeed their association with ErbB receptors is linked to cellular transformation and oncogenesis. A direct correlation, however, between regulation of the cell cycle and oncogenesis, has not been shown.
ErbB receptors clearly exhibit a diverse and important range of functions. This review attempts to detail our current understanding of their role of during infection (summarised in Table 1 ) and contribution towards disease.
Table 1.
ErbB-Dependent Microbes and Their Mechanisms of Receptor Exploitation
| Virusa | Mechanism of ErbB exploitation | Outcomes | Refs |
|---|---|---|---|
| EBV | Induces of EGFR expression | Host cell proliferation | [30] |
| HBV | Induces of surface EGFR expression | Host cell proliferation | 88, 89 |
| HCV | Disrupts EGFR recycling to enhance surface expression through host protein Netrin-1 | Host cell entry | [27] |
| Enhances EGFR and ErbB2 surface expression through NRG-1-dependent mechanism | [29] | ||
| Induces AREG-dependent circumvention of apoptosis; induces proliferation and transformation | Host cell survival and proliferation | [15] | |
| HCMV | Alters EGFR signalling molecules to bypass cell-fate check points and enhance production of cell cycle proteins | Host cell survival | 11, 12 |
| EGFR activation promotes latency by inducing latent, but suppressing lytic, gene expression | Immune evasion | [69] | |
| HPV | Induces EGFR-dependent translocation of AnxA2, results in formation of the HPV-AnxA2-S100A10 binding complex required for viral entry | Host cell entry | [31] |
| HTLV | Induces EGFR activation resulting in cellular transformation in CD4+ T cells | Host cell proliferation | [101] |
| IAV | Induces lipid-raft clustering. Activates EGFR and other RTKs leading to cellular internalisation | Host cell entry | [35] |
| Suppresses IFN-λ and CXCL10 through EGFR activation | Immune modulation | 18, 19 | |
| KSHV | Oncogenic pathogen induces activation of EGFR signalling | Unconfirmed | [100] |
| RV | Suppresses RV-induced IFN-λ and CXCL10 expression via EGFR activation | Immune modulation | 18, 19 |
| RSV | Induces EGFR-dependent macropinocytosis | Host cell entry | 32, 33 |
| Suppresses RSV-induced CXCL10 expression via EGFR activation | Immune modulation | [19] | |
| SARS | Induces EGFR-dependent macropinocytosis | Host cell entry | [34] |
| Bacteria | Mechanism | Outcomes | Refs |
| Brucella abortus | Inhibits MHC-I expression potentially involving EGFR, ErbB2 and/or TACE sheddase | Immune modulation | 22, 67 |
| Campylobacter jejuni | Induces lipid-raft clustering and EGFR activation | Host cell entry | [44] |
| Chlamydia pneumoniae | Bacterial Pmp21 adhesin directly binds EGFR | Host cell entry | [10] |
| Escherichia coli | Disrupts endosome trafficking, resulting in diminished surface EGFR | Unconfirmed | [62] |
| Helicobacter pylori | Induces EGFR-dependent β-catenin nuclear translocation and PI3K/Akt signalling | Host cell survival | [16] |
| Dephosphorylates EGFR to inhibit hBD3 expression and promote infection | Immune modulation | [104] | |
| Klebsiella pneumoniae | Induces EGFR dependent inhibition of NF-kB translocation | Immune modulation | 65, 66 |
| Neisseria gonorrhoeae | Induces redistribution of β-catenin from apical junction to cytoplasm through EGFR activation | Host cell entry | [51] |
| Induces translocation of ErbB2 and ErbB3 to apical surfaces | [61] | ||
| Neisseria meningitidis | Recruits and activates ErbB2 receptors | Host cell entry | [2] |
| Mycobacterium leprae | Directly binds ErbB2 receptors | Host cell entry | [14] |
| Mycobacterium tuberculosis | Prevent proper macrophage function resulting in enhanced infection | Immune modulation | [70] |
| Salmonella Typhimurium | Induces Claudin 2 expression through EGFR and downstream JNK activation | Host cell entry | [50] |
| Shigella flexneri | Induces PI5P production to regulate EGFR trafficking | Host cell survival | [17] |
| Staphylococcus aureus | Cleaves junction proteins occludin and E-cadherin to facilitate transmigration. EGFR activation is required | Host cell entry | [13] |
| Fungi | Mechanism | Outcomes | Refs |
| Candida albicans | EGFR and ErbB2-dependent endocytosis | Host cell entry | 55, 56 |
EBV, Epstein–Barr virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HCMV, human cytomegalovirus; HPV, human papilloma virus; HTLV, human T cell leukaemia virus; IAV, influenza A virus; KSHV, Kaposi’s sarcoma-associated herpesvirus; RV, rhinovirus; RSV, respiratory syncytial virus; SARS, severe acute respiratory syndrome virus.
ErbB-Dependent Pathogen Entry and Invasion
Influenza A virus (IAV), respiratory syncytial virus (RSV), and coronaviruses are respiratory pathogens that have been extensively studied in airway epithelial cells with regard to their replication cycle and the host response they elicit. More recently, it has been shown that all three viruses are able to utilise EGFR (ErbB1) for host cell entry. While RSV 32, 33 and coronaviruses [34] induce EGFR-dependent macropinocytosis, a nonselective mechanism of internalising large bodies of extracellular material, IAV induces lipid-raft clustering on surface membranes, resulting in EGFR activation and internalisation of both virus and EGFR [35]. Interestingly, lipid rafts are also important for IAV viral budding and exit from host cells 36, 37, 38, 39. Newly synthesised structural IAV proteins, hemagglutinin (HA), and neuraminidase (NA), can assemble at the cytoplasmic leaflet of lipid rafts 40, 41, providing a platform for nascent viral proteins to cluster at, form progeny virus, and from which to bud [42]. Given that EGFR localises at lipid rafts [43], a potential role for EGFR during viral exit may also exist (see Outstanding Questions). Additionally, the intestinal bacterial pathogen Campylobacter jejuni [44] also requires lipid-raft clustering and activation of raft-associated proteins, integrin-β1, EGFR, and platelet-derived growth factor receptor (PDGFR) for induction of filopodia formation and bacterial invasion.
While such pathogens exploit host internalisation mechanisms, some large microbes appear to have adopted a different mechanism of EGFR-mediated host cell entry involving manipulation of epithelial junction proteins. The integrity of the epithelium is critical for host defence and is maintained by a variety of proteins located at the interface of adjacent epithelial cells, which include tight junctions, adherens, desmosomes, and gap junctions. Such proteins help to maintain intimate contact between neighbouring cells, cellular anchorage, and polarity, as well as the paracellular flux of solutes 45, 46, 47, 48.
With this in mind, it becomes apparent that targeting such proteins may provide a means of breaching this primary defence barrier, and indeed EGFR has been implicated in modulation of such junction proteins. The bacterial species Salmonella enterica serovar Typhimurium is able to activate EGFR and induce expression of Claudin-2, a channel-forming tight-junction protein [49] which results in gut epithelium invasion [50]. Claudin-2 activation during infection is dependent on EGFR phosphorylation, as evidenced by a reduction in bacterial load following siRNA-mediated EGFR downregulation [50]. An alternative mechanism targeting junction proteins for cell entry is utilised by Staphylococcus aureus. Cleavage of occludin and E-cadherin, following EGFR activation, facilitates transmigration of S. aureus through cell–cell junctions, and inhibition of EGFR activity prevents S. aureus migration through an epithelial monolayer [13]. Additionally, Neisseria gonorrhoeae induces an EGFR-dependent mechanism of β-catenin redistribution from apical junctions to the cytoplasm, resulting in weakened apical junctions and gonococcal transmigration across the epithelium [51].
Unsurprisingly, enhanced epithelial cell proliferation and improved barrier integrity can provide protective functions against pathogen invasion 52, 53, 54, and interestingly, both factors can be regulated by ErbB receptors. A protective role of ErbB receptors against infection would be interesting to explore in this context (see Outstanding Questions).
The second ErbB family member, ErbB2, is also targeted by pathogens for cellular entry. Mycobacterium leprae directly binds and activates ErbB2 to induce downstream Erk1/Erk2 signalling and bacterial invasion of Schwann cells. Trastuzumab, a potent ErbB2-targeted monoclonal antibody, abrogated both in vitro and in vivo M. leprae-induced myelin damage, highlighting ErbB2 as a crucial factor for leprosy disease [14]. The bacterium Neisseria meningitidis also induces recruitment and aggregation of ErbB2, but not other ErbB family members, on endothelial cell membranes, directly beneath sites of bacterial colony formation. Subsequent activation of ErbB2 and downstream Src-dependent signalling led to internalisation of N. meningitidis and invasion of target cells [2]. Additionally, the fungus Candida albicans utilises both EGFR and ErbB2 to induce endocytosis and invasion into epithelial cells 55, 56. Reduced severity of oropharangeal candidiasis is observed in mice following treatment with a dual EGFR/ErbB2 kinase inhibitor [55]. Liu et al. also document ErbB2 activation during vulvovaginal candidiasis in humans [57].
Another pathogen-mediated mechanism that promotes host cell entry may involve increased numbers of ErbB receptors at the cell surface. ErbB receptors spend the majority of their life span at the surface of cell membranes but continually undergo internalisation as part of normal processing. The fate of internalised receptors has important consequences for the cell. Potential receptor outcomes, following endocytosis, include recycling back to the surface (associated with proliferation), transport to other cellular compartments such as multivesicular bodies, the nucleus, or mitochondria (related to homeostasis), or being targeted for degradation (thought to promote cell death) 58, 59, 60. Pathogens appear to target these trafficking pathways for their own benefit. For example, HCV induces the upregulation of host-derived Netrin-1, which suppresses EGFR recycling, resulting in enhanced levels of surface EGFR, EGFR activation, and subsequent viral endocytosis [27], which occurs via virus-induced EGFR and host-derived CD81 protein binding [28]. Additionally, the ErbB ligand neuregulin-1 has been implicated in enhancing EGFR and ErbB2 surface expression during HCV infection [29]. N. gonorrhoeae-induced upregulation of several ErbB ligands was also found to coincide with translocation of both EGFR and ErbB2 receptors to apical surfaces, following infection. This disruption of normal receptor distribution within polarised epithelial cells led to enhanced bacterial invasion, and upregulation of EGFR-ligand mRNA was also observed [61]. Conversely, however, Escherichia coli-mediated disruption of endosome recycling leads to reduced surface EGFR, transferrin, and β1-integrin expression [62]. Additional examples of EGFR-dependent mechanisms of host cell entry include Chlamydia pneumoniae Pmp21-mediated recruitment of EGFR [10], and EGFR-dependent formation of HPV type 16 (HPV16), Annexin A2 and S100A10 heterotetramers to facilitate HPV16 infection [31]. Interestingly, malignant tissue from patients with HPV-associated cancers often poorly express EGFR 63, 64. Further understanding of the connection between HPV and EGFR is required to fully appreciate this relationship.
ErbB-dependent host-directed internalisation and targeting of barrier integrity, as well as ErbB trafficking, appear to be commonly used mechanisms of cellular entry by a diverse range of pathogens. The development of such distinct pathogen-specific mechanisms to achieve varied methods of internalisation, all of which utilise EGFR, clearly highlights the importance of these receptors as significant points of entry into host cells.
Prolonged Host-Cell Survival through Pathogen-Mediated EGFR Activation
ErbB-mediated host cellular entry is not the only function of this receptor family that is of potential benefit to invading organisms. ErbB receptors are potent mediators of cellular growth and survival, and unsurprisingly they have become a commonly exploited target for this purpose also. The association between human cytomegalovirus (HCMV) and EGFR has been well studied. HCMV employs multiple mechanisms of modulating EGFR signalling for its own propagative benefit, including rapid induction of protein kinase B (PKB, alias Akt) activation, a protein kinase downstream of Pi3K with important roles in cell cycle regulation. Cojohari et al. showed that the use of MK-2206, a highly selective Akt inhibitor, during HCMV infection results in decreased viability of infected monocytes [11]. While uninfected cells utilise the primary isoform of Pi3K (P110δ) for Akt signalling, a switch occurs in HCMV-infected cells to allow use of the less dominant P110β isoform, resulting in prolonged cell survival past the 48 h cell-fate check point. While cell viability was dependent on P110δ activity in uninfected cells, inhibition of this predominant isoform did not cause apoptosis in HCMV-infected cells, which instead succumbed to cell death following P110β inhibition [11]. Furthermore, evidence of EGFR-associated promotion of antiapoptotic proteins, myeloid leukemia sequence (Mcl-1), and heat-shock protein 27 (HSP27) [12], as well as modulation of negative regulators of cell survival, such as phosphatase and tensin homolog (PTEN), and SH2 domain-containing inositol 5-phosphatase 1 (SHIP1) [11] has also been shown. In summary, HCMV appears able to activate EGFR signalling, utilise alternative signalling molecules in this pathway, and manipulate EGFR to selectively prevent cell apoptosis for its own propagative benefit.
Additional mechanisms to modulate the cell cycle via ErbB receptors include HCV-induced cell survival through amphiregulin release (AREG, an EGFR ligand) [15], Helicobacter pylori-induced cell survival and hyperproliferation via EGFR activation [16], and also Shigella flexneri promotion of host cell survival through use of phosphatidylinositol 5-phosphate (PI5P), to impair lysosomal degradation and regulation of EGFR trafficking, as well as downstream signalling [17].
Mechanisms of increasing host infected cell survival appear to involve enhanced ‘growth’ stimulants while suppressing cell death mechanisms. The observation that HCMV, HCV, H. pylori and S. flexneri are all capable of invading and surviving within host cells suggests that their ability to prolong host cell survival is a specific and evolutionary targeted effect, induced for pathogen benefit.
Pathogen-Induced Modulation of Immune Responses via EGFR
The ability of pathogens to modulate host immune responses has been long established. However, the importance of ErbB receptors in this context is less known. A number of pathogens have been found to manipulate EGFR to promote immune evasion. IAV and Rhinovirus (RV)-16 are both capable of inhibiting interferon (IFN)-λ production, a critical antiviral cytokine of the airways, through an EGFR-dependent mechanism that results in enhanced viral titres [18]. Suppression of CXCL10 (IFN-γ-induced protein 10 (IP-10)), a monocyte and T cell chemokine, has also been shown following IAV, RV, or RSV infection, through virus-induced EGFR signalling [19]. Additionally, NF-kB, a primary transcription factor governing immune responses, is targeted during Klebsiella pneumoniae infection of bronchial epithelial cells [65]. Here, the EGFR/Pi3K/Erk signalling pathway is activated to result in diminished nuclear translocation of NF-kB [66], likely resulting in the suppressed activation of NF-kB and inflammatory responses observed during K. pneumoniae infection [65].
EGFR activation may also be involved in airway remodelling, which is a hallmark feature of chronic obstructive pulmonary disease (COPD) and asthma. Airway remodelling refers to the structural modifications of the airway tissue and can involve increased smooth muscle mass, subepithelial fibrosis, and mucous gland hyperplasia which contribute to airflow obstruction and impairment of lung function. In a model of epithelial cell infection, stimulation with synthetic double-stranded RNA [poly(I:C)] led to induction of EGFR- and downstream ERK/p38 MAPK-dependent mucin (MUC)-5AC, transforming growth factor (TGF)-β1, matrix metalloproteinase (MMP)-9, and vascular endothelial growth factor (VEGF) cytokine expression, all of which contribute to airway remodelling [21]. Pseudomonas aeruginosa [25] and poly(I:C)-mediated [26] activation of EGFR also enhanced MUC5AC and subsequent mucus hypersecretion, which contributed to airway mucus obstruction during respiratory disease 25, 26.
Retention of MHC-I molecules at the Golgi, by the bacterium Brucella abortus is another example of immune evasion via ErbB receptors and results in inhibited MHC-I surface expression 22, 67. Inhibition of EGFR, ErbB2, or the ErbB ligand sheddase protein, tumour necrosis factor-α-converting enzyme (TACE/ADAM17), was each able to modestly recover MHC-I surface expression, whilst Erk1/2 inhibition led to significant restoration of MHC-I surface expression [22]. Furthermore, exposure of the monocyte/macrophage cell-line THP-1, to exogenous EGF, TGF-α, or a combination of the two EGFR ligands, was able to mimic B. abortus-induced retention of MHC-I at the Golgi and reduced surface MHC-I expression.
Interestingly, hepatitis B virus (HBV) can promote immune evasion by inducing tolerance. Surface EGFR is upregulated on HBV-infected intrahepatic regulatory T cells (Treg), which enhances their immunosuppressive capacity [68]. HBV-infected mice exhibited increased numbers of Treg cells that expressed immunosuppressive cytokines such as interleukin (IL)-10 and TGF-β, in addition to an enhanced ability to prevent CD8+ T cell proliferation, a requirement for HBV clearance [68]. HCMV-induced latency is another interesting tactic providing immune evasion and is also governed by EGFR activity. During infection, the ability to induce latency-associated UL138, while suppressing IE1/IE2 lytic genes, was shown to be EGFR dependent, by the use of EGFR-specific inhibitors [69]. Additionally, Mycobacterium tuberculosis can utilise EGFR-induced p38/MAPK signalling pathways within macrophages to prevent proper antimicrobial macrophage responses, resulting in enhanced murine infection [70]. However, in contrast to these pathogen-driven functions of EGFR that contribute to infection, some EGFR-induced host protective functions have also been observed. One study highlights the requirement of EGFR in macrophage activation and function against H. pylori, where EGFR-deficient macrophages exhibited impaired T helper (Th)1 and Th17 adaptive responses, resulting in suppressed H. pylori-induced chronic inflammation and disease progression in mice [71]. Additionally, EGFR-dependent S. aureus-induced IL-1α and IL-1β [23], as well as E. coli- [24] and virus-induced 72, 73, 74 IL- 8, may be examples of host-beneficial EGFR-dependent responses induced by pathogens.
Together, these studies highlight a complexity of EGFR functionality, which has the ability to both contribute to and protect against infections. Mechanisms for immune modulation are highly diverse and pathogen-specific, perhaps emphasising the strength of our intricate immune system and the specialisms of each microbial species. The observation that pathogens are able to augment cellular immune responses through EGFR signalling again highlights the importance of this class of receptors during infection.
ErbB-Associated Cancers
ErbB receptor activity is highly associated with oncogenic transformation. Several cancers, including head and neck squamous cell carcinoma (HNSCC), lung, and colon cancers, are known to result from aberrant ErbB expression or signalling. Aberrations leading to overexpressed or constitutively active ErbB receptors 75, 76, 77, overproduced ErbB receptor ligands 15, 78, 79, 80, 81, 82, 83, and sheddases that cleave ErbB precursor ligands into their mature forms 84, 85, 86, 87, have all been shown to cause cellular transformation. Thus, it is perhaps unsurprising that several cancer-inducing pathogens have been found to promote ErbB activation, with evidence of cellular transformation being directly caused by pathogen-induced ErbB signalling. In fact, the ErbB signalling pathway has been implicated in a number of major oncogenic virus infections.
For example, HBV has been shown to induce upregulated expression of EGFR gene and protein, both of which are strongly associated with hepatocellular transformation 88, 89. This virus is also thought to enhance ErbB2 mRNA stability through the HBV-encoded X-protein (HBx), which is itself strongly associated with hepatocellular carcinoma (HCC), resulting in enhanced HCC cell migration [90]. Likewise, HCV infection results in enhanced surface EGFR expression 29, 61, EGFR activation, and consequent viral entry [28], whilst patients exhibiting certain EGFR polymorphisms spontaneously clear HCV infection [91]. HCV-induced AREG overexpression in human hepatoma cells contributes to prolonged infected-cell survival, cirrhosis, and HCC progression [15]. Furthermore, EGFR, which can also be cleaved from apoptotic cells to shut down signalling [92], is found in significantly higher concentrations in HCC patient plasma, in the presence of HCV and HBV infection [93]. Whether this reflects host attempts to curtail aberrant cell proliferation, or is merely a by-product of virus-induced apoptosis, remains unclear. So consistent is the increase in soluble EGFR during virus-induced HCC that it may serve as a marker for such disease [93].
HPV is another virus which has been associated with both EGFR and oncogenesis. Interestingly, in this case, EGFR expression is commonly low 63, 64 and has been an area of detailed investigation. One explanation for the existence of ‘HPV-positive EGFR-negative’ malignancies could lie in the functional redundancy between ErbB receptors as well as an ability of family members to regulate expression of each other. Indeed, several studies have highlighted an overexpression of ErbB2 and ErbB3 receptors, or even ErbB3 dependency [94], in HPV-positive cancers 95, 96, 97, whilst Stindt et al. showed that HCV induced downregulation of ErbB3 and enhanced EGFR and ErbB2 expression on hepatocytes [29]. These results were reproducible following suppression of ErbB3 with siRNAs and suggests that ErbB3 can function to impact on the expression of EGFR and ErbB2 [29]. However, the presence of ‘HPV-positive EGFR-positive’ malignancies has also been observed 98, 99. Together, these examples highlight an association between HPV-induced cancer and ErbB receptors, though the full extent of receptor contribution to such disease remains incompletely understood.
Other oncogenic viruses found to have an association with EGFR include Epstein–Barr virus (EBV), whose virus-encoded LMP1 protein induces EGFR expression [30], Kaposi’s sarcoma-associated herpesvirus (KSHV), which promotes EGFR activation [100], and human T cell leukaemia virus (HTLV), shown to directly induce EGFR-dependent cellular transformation [101].
Additionally, bacteria-induced EGFR-dependent oncogenesis has been documented. The association between H. pylori and gastric cancer is highly acknowledged, with studies suggesting that this pathogen remains the strongest known risk factor for stomach tumorigenesis. Early clearance of infection significantly lowers the risk of malignancies [102], which supports its role as a carcinogen, and crucially, H. pylori infections are strongly linked to the dysregulation of EGFR ligands and/or transactivation of EGFR (reviewed in [103]). The ability of H. pylori to dephosphorylate EGFR is particularly interesting. Pathogen-induced inactivation of EGFR involves activation of host SHP2 tyrosine phosphatase, to result in suppression of the antimicrobial peptide, human beta-defensin 3 (hBD3) expression, and sustained bacterial infection [104].
It is curious to note that the ErbB-dependent oncogenic pathogens mentioned herein are capable of replicating intracellularly. Coupled with their ability to induce hyperproliferation, it would be interesting to investigate the role of host cell turnover in ErbB-dependent pathogen survival. Several viruses (HBV, HCV, EBV, and HPV among others) are known to require host machinery for protein synthesis and subsequent viral replication; these host translational apparatus can be found in greater abundance at specific points during the host cell cycle [105]. As such, subversion of the cell cycle is a common theme where both cycle arrest and induction may occur [106]; however, the effect of either on associated disease remains unclear. Indeed, not all viruses possessing the ability to regulate the cell cycle, such as IAV and SARS viruses, are particularly associated with oncogenesis. Further investigation into the precise requirements of oncogenic pathogens, leading to host cell survival and replication, and the role of ErbB receptors in this context, may provide valuable insights to developing more effective cancer therapies.
Concluding Remarks and Future Perspectives
There exists a growing body of evidence to support the role of the ErbB receptor family as a critical regulator of pathogen invasion and propagation within the human host. Numerous studies have highlighted pathogen-selective exploitation of ErbB receptors as a mechanism that contributes to host cell entry, cell survival, augmentation of host immunity, and cancer (Figure 1 , Key Figure). Their cellular localisation, regulated mode of internalised recycling, and functional capacity make the ErbB family an excellent target for pathogens that require access into host cells in order to self-replicate. Several ErbB-dependent pathogens show the capacity to regulate the cell cycle to access host machinery; however, the direct effects of this on prolonging host cell survival or enhancing proliferation remain undefined. Malignancies do not occur as a result of all infections and suggests that there are likely other factors involved in driving cellular transformation during infection.
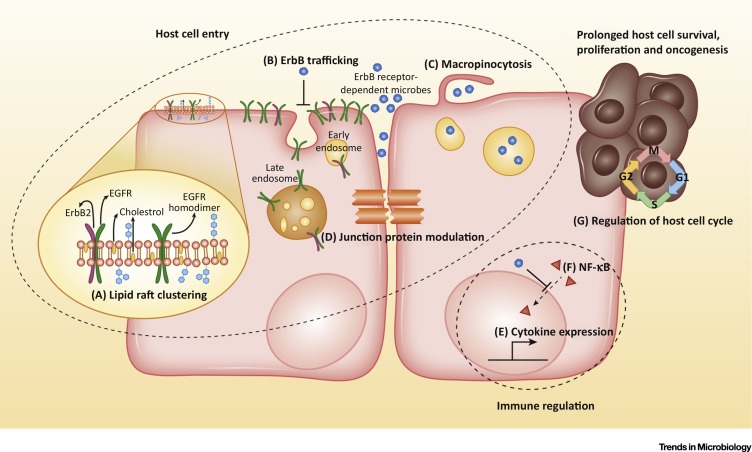
Figure 1.
Key Figure: Primary Mechanisms and Targets Modulated or Induced by Microbes, Involving ErbB Receptors
Pathogen-induced mechanisms of host cell entry can involve (A) ErbB-mediated lipid raft clustering, (B) inhibition of ErbB endocytosis resulting in elevated surface receptor expression, (C) ErbB-mediated macropinocytosis, and (D) modulation of junction proteins. Pathogens can also manipulate factors that govern immune responses through ErbB receptor exploitation. Mechanisms here include (E) altering cytokine expression in infected cells, and (F) inhibition of NF-ĸB nuclear-translocation. Additionally, some pathogens that are able to regulate the cell cycle also appear to be able to induce host cell survival, proliferation or oncogenesis (G).
On a grander scale, the role of host ErbB receptors during infection is an overlooked aspect of disease which warrants deeper consideration. To date, research in this context has focused predominantly on viral pathogens, with much less detail known regarding bacterial and fungal infections. Investigations are also skewed toward the more prominent EGFR, and interest in this host receptor may be warranted as it is the only ErbB member to undergo ligand-induced internalisation, greatly enhancing its appeal for pathogen exploitation. However, given the functional redundancy of ErbB receptors, and their potential to regulate expression of each other [29], it would be remiss not to fully investigate the role of all family members. With drug resistance increasing in a plethora of pathogens, there is an immediate and critical need for the development of innovative therapies that target novel factors. As such, ErbB receptors and associated signalling pathways may possess potential value as novel therapeutic targets during pathogenic infection. This notion is supported by a recent study which validates the efficacy of the specific EGFR inhibitor Erlotinib, in conjunction with a broad-spectrum tyrosine kinase inhibitor, Sunitinib, against a range of evolutionarily distinct viral pathogens [107]. Both drugs have FDA approval as immunomodulatory cancer therapies and would also be exciting prospects as novel antimicrobial drugs. However, toxicity concerns that include adverse skin conditions, diarrhoea, and heart failure, may warrant limitation of their use to treatment of more serious infections.
The future of both research and therapies surrounding ErbB receptors is continually expanding, and the next decade should reveal some intriguing and critical information regarding the interaction between microbes and these receptors.
Outstanding Questions.
The EGFR has been associated with lipid-raft clustering and viral entry mechanisms, but lipid-raft aggregation is also involved in viral egress. Does EGFR, therefore, play a role during budding of nascent viral progeny?
Both enhanced cell turnover and barrier integrity have been shown to be protective against pathogen invasion, factors that ErbB receptors are directly capable of inducing. Is there potential to enhance this protective role of ErbB receptors as a preventative measure against infection?
How efficacious would current ErbB-targeted therapies be against pathogen infection in humans? Toxicity and resistance against such drugs has been observed in cancer patients. Are the mechanisms behind such events relevant in the setting of pathogenic infection?
Is ErbB receptor function capable of directly driving pathogen-induced cellular transformation? If so, at which stage(s) of cancer does this contribute? During initial oncogenesis or maintenance and progression of cancer?
References
- 1.Tao R.-H., Maruyama I.N. All EGF(ErbB) receptors have preformed homo- and heterodimeric structures in living cells. J. Cell Sci. 2008;121:3207–3217. doi: 10.1242/jcs.033399. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann I. Activation of ErbB2 receptor tyrosine kinase supports invasion of endothelial cells by Neisseria meningitidis. J. Cell Biol. 2001;155:133–144. doi: 10.1083/jcb.200106148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torp S.H. Epidermal growth factor receptor expression in human gliomas. Cancer Immunol. Immunother. 1991;33:61–64. doi: 10.1007/BF01742530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oda Y. Expression of growth factors and their receptors in human osteosarcomas. Immunohistochemical detection of epidermal growth factor, platelet-derived growth factor and their receptors: its correlation with proliferating activities and p53 expression. Gen. Diagn. Pathol. 1995;141:97–103. [PubMed] [Google Scholar]
- 5.Donnenberg A.D. The cell-surface proteome of cultured adipose stromal cells. Cytometry. 2015;87:665–674. doi: 10.1002/cyto.a.22682. [DOI] [PubMed] [Google Scholar]
- 6.Scheving L.A. Loss of hepatocyte ERBB3 but not EGFR impairs hepatocarcinogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;309:G942–G954. doi: 10.1152/ajpgi.00089.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brea M.S. Epidermal growth factor receptor silencing blunts the slow force response to myocardial stretch. J. Am. Heart Assoc. 2016;5:e004017. doi: 10.1161/JAHA.116.004017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Needham S.R. EGFR oligomerization organizes kinase-active dimers into competent signalling platforms. Nat. Commun. 2016;7:13307. doi: 10.1038/ncomms13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature. 2003;424:456–461. doi: 10.1038/nature01818. [DOI] [PubMed] [Google Scholar]
- 10.Mölleken K. The Chlamydia pneumoniae invasin protein Pmp21 recruits the EGF receptor for host cell entry. PLoS Pathog. 2013;9:e1003325. doi: 10.1371/journal.ppat.1003325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cojohari O. Human cytomegalovirus induces an atypical activation of Akt to stimulate the survival of short-lived monocytes. J. Virol. 2016;90:6443–6452. doi: 10.1128/JVI.00214-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peppenelli M.A. Human cytomegalovirus stimulates the synthesis of select Akt-dependent antiapoptotic proteins during viral entry to promote survival of infected monocytes. J. Virol. 2016;90:3138–3147. doi: 10.1128/JVI.02879-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soong G. Staphylococcus aureus protein A mediates invasion across airway epithelial cells through activation of RhoA GTPase signaling and proteolytic activity. J. Biol. Chem. 2011;286:35891–35898. doi: 10.1074/jbc.M111.295386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tapinos N. ErbB2 receptor tyrosine kinase signaling mediates early demyelination induced by leprosy bacilli. Nat. Med. 2006;12:961–966. doi: 10.1038/nm1433. [DOI] [PubMed] [Google Scholar]
- 15.Pei R. Hepatitis C virus infection induces the expression of amphiregulin, a factor related to the activation of cellular survival pathways and required for efficient viral assembly. J. Gen. Virol. 2011;92:2237–2248. doi: 10.1099/vir.0.032581-0. [DOI] [PubMed] [Google Scholar]
- 16.Himaya S.W.A. EGFR tyrosine kinase inhibitory peptide attenuates Helicobacter pylori-mediated hyper-proliferation in AGS enteric epithelial cells. Toxicol. Appl. Pharmacol. 2013;269:205–214. doi: 10.1016/j.taap.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Ramel D. Shigella flexneri infection generates the lipid PI5P to alter endocytosis and prevent termination of EGFR signaling. Sci. Signal. 2011;4:ra61. doi: 10.1126/scisignal.2001619. [DOI] [PubMed] [Google Scholar]
- 18.Ueki I.F. Respiratory virus-induced EGFR activation suppresses IRF1-dependent interferon λ and antiviral defense in airway epithelium. J. Exp. Med. 2013;210:1929–1936. doi: 10.1084/jem.20121401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalinowski A. EGFR activation suppresses respiratory virus-induced IRF1-dependent CXCL10 production. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014;307:L186–L196. doi: 10.1152/ajplung.00368.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koff J.L. Multiple TLRs activate EGFR via a signaling cascade to produce innate immune responses in airway epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;294:L1068–L1075. doi: 10.1152/ajplung.00025.2008. [DOI] [PubMed] [Google Scholar]
- 21.Jiang J. Regulation of viral infection-induced airway remodeling cytokine production by the TLR3-EGFR signaling pathway in human bronchial epithelial cells. COPD. 2016;13:750–755. doi: 10.3109/15412555.2016.1168391. [DOI] [PubMed] [Google Scholar]
- 22.Velásquez L.N. Inhibition of MHC-I by Brucella abortus is an early event during infection and involves EGFR pathway. Immunol. Cell Biol. 2016 doi: 10.1038/icb.2016.111. Published online November 29, 2016. [DOI] [PubMed] [Google Scholar]
- 23.Simanski M. The Inflammasome and the epidermal growth factor receptor (EGFR) are involved in the Staphylococcus aureus-mediated induction of IL-1alpha and IL-1beta in human keratinocytes. PLoS One. 2016;11:e0147118. doi: 10.1371/journal.pone.0147118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser-Pitt D.J. Phosphorylation of the epidermal growth factor receptor (EGFR) is essential for interleukin-8 release from intestinal epithelial cells in response to challenge with Escherichia coli O157:H7 flagellin. Microbiology. 2011;157:2339–2347. doi: 10.1099/mic.0.047670-0. [DOI] [PubMed] [Google Scholar]
- 25.Yu H. Flagellin/TLR5 responses induce mucus hypersecretion by activating EGFR via an epithelial cell signaling cascades. Exp. Cell Res. 2012;318:723–731. doi: 10.1016/j.yexcr.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Shishikura Y. Extracellular ATP is involved in dsRNA-induced MUC5AC production via P2Y2R in human airway epithelium. Respir. Res. 2016;17:121. doi: 10.1186/s12931-016-0438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plissonnier M.-L. Epidermal growth factor receptor-dependent mutual amplification between Netrin-1 and the hepatitis C virus. PLoS Biol. 2016;14:e1002421. doi: 10.1371/journal.pbio.1002421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diao J. Hepatitis C virus induces epidermal growth factor receptor activation via CD81 binding for viral internalization and entry. J. Virol. 2012;86:10935–10949. doi: 10.1128/JVI.00750-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stindt S. Hepatitis C virus activates a neuregulin-driven circuit to modify surface expression of growth factor receptors of the ErbB family. PLoS One. 2016;11:e0148711. doi: 10.1371/journal.pone.0148711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller W.E. The Epstein–Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor. J. Virol. 1995;69:4390–4398. doi: 10.1128/jvi.69.7.4390-4398.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dziduszko A., Ozbun M.A. Annexin A2 and S100A10 regulate human papillomavirus type 16 entry and intracellular trafficking in human keratinocytes. J. Virol. 2013;87:7502–7515. doi: 10.1128/JVI.00519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Currier M.G. EGFR interacts with the fusion protein of respiratory syncytial virus strain 2-20 and mediates infection and mucin expression. PLoS Pathog. 2016;12:e1005622. doi: 10.1371/journal.ppat.1005622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krzyzaniak M.A. Host cell entry of respiratory syncytial virus involves macropinocytosis followed by proteolytic activation of the F protein. PLoS Pathog. 2013;9:e1003309. doi: 10.1371/journal.ppat.1003309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freeman M.C. Coronaviruses induce entry-independent, continuous macropinocytosis. mBio. 2014;5:01340–1414. doi: 10.1128/mBio.01340-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eierhoff T. The epidermal growth factor receptor (EGFR) promotes uptake of Influenza A viruses (IAV) into host cells. PLoS Pathog. 2010;6:e1001099. doi: 10.1371/journal.ppat.1001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mariani C. Role of Gag and lipids during HIV-1 assembly in CD4(+) T cells and macrophages. Front. Microbiol. 2014;5:312. doi: 10.3389/fmicb.2014.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Licata J.M. Overlapping motifs (PTAP and PPEY) within the Ebola virus VP40 protein function independently as late budding domains: involvement of host proteins TSG101 and VPS-4. J. Virol. 2003;77:1812–1819. doi: 10.1128/JVI.77.3.1812-1819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marty A. Association of matrix protein of respiratory syncytial virus with the host cell membrane of infected cells. Arch. Virol. 2004;149:199–210. doi: 10.1007/s00705-003-0183-9. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen D.H., Hildreth J.E. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 2000;74:3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohkura T. Influenza A virus hemagglutinin and neuraminidase mutually accelerate their apical targeting through clustering of lipid rafts. J. Virol. 2014;88:10039–10055. doi: 10.1128/JVI.00586-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeda M. Influenza virus hemagglutinin concentrates in lipid raft microdomains for efficient viral fusion. Proc. Natl. Acad. Sci. U. S. A. 2003;100:14610–14617. doi: 10.1073/pnas.2235620100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeda M. Lipid raft and influenza virus – viral glycoproteins on a raft. Uirusu. 2004;54:9–15. doi: 10.2222/jsv.54.9. [DOI] [PubMed] [Google Scholar]
- 43.Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–672. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- 44.Krause-Gruszczynska M. The signaling pathway of Campylobacter jejuni-induced Cdc42 activation: role of fibronectin, integrin beta1, tyrosine kinases and guanine exchange factor Vav2. Cell Commun. Signal. 2011;9:32. doi: 10.1186/1478-811X-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guichard A. RAB11-mediated trafficking in host–pathogen interactions. Nat. Rev. Microbiol. 2014;12:624–634. doi: 10.1038/nrmicro3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammad H., Lambrecht B.N. Barrier epithelial cells and the control of Type 2 immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Lu R.-Y. The role of epithelial tight junctions involved in pathogen infections. Mol. Biol. Rep. 2014;41:6591–6610. doi: 10.1007/s11033-014-3543-5. [DOI] [PubMed] [Google Scholar]
- 48.Zihni C. Signalling at tight junctions during epithelial differentiation and microbial pathogenesis. J. Cell Sci. 2014;127:3401–3413. doi: 10.1242/jcs.145029. [DOI] [PubMed] [Google Scholar]
- 49.Rosenthal R., Milatz S., Krug S.M., Oelrich B., Schulzke J.D., Amasheh S., Günzel D., Fromm M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J. Cell Sci. 2010;123:1913–1921. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y. Salmonella infection upregulates the leaky protein claudin-2 in intestinal epithelial cells. PLoS One. 2013;8:e58606. doi: 10.1371/journal.pone.0058606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edwards V.L. Neisseria gonorrhoeae breaches the apical junction of polarized epithelial cells for transmigration by activating EGFR. Cell. Microbiol. 2013;15:1042–1057. doi: 10.1111/cmi.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu G.-Q. AIM2 contributes to the maintenance of intestinal integrity via Akt and protects against Salmonella mucosal infection. Mucosal Immunol. 2016;9:1330–1339. doi: 10.1038/mi.2015.142. [DOI] [PubMed] [Google Scholar]
- 53.Sim S., Hibberd M.L. Caenorhabditis elegans susceptibility to gut Enterococcus faecalis infection is associated with fat metabolism and epithelial junction integrity. BMC Microbiol. 2016;16:6. doi: 10.1186/s12866-016-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burgener A. HIV and mucosal barrier interactions: consequences for transmission and pathogenesis. Curr. Opin. Immunol. 2015;36:22–30. doi: 10.1016/j.coi.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Solis N.V. The aryl hydrocarbon receptor governs epithelial cell invasion during oropharyngeal candidiasis. mBio. 2017;8 doi: 10.1128/mBio.00025-17. e00025-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu W. EGFR and HER2 receptor kinase signaling mediate epithelial cell invasion by Candida albicans during oropharyngeal infection. Proc. Natl. Acad. Sci. U. S. A. 2012;109:14194–14199. doi: 10.1073/pnas.1117676109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y. New signaling pathways govern the host response to C. albicans infection in various niches. Genome Res. 2015;25:679–689. doi: 10.1101/gr.187427.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tomas A. EGF receptor trafficking: consequences for signaling and cancer. Trends Cell. Biol. 2014;24:26–34. doi: 10.1016/j.tcb.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiley H.S. Trafficking of the ErbB receptors and its influence on signaling. Exp. Cell Res. 2003;284:78–88. doi: 10.1016/s0014-4827(03)00002-8. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y.-N. Nuclear functions and subcellular trafficking mechanisms of the epidermal growth factor receptor family. Cell Biosci. 2012;2:13. doi: 10.1186/2045-3701-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swanson K.V. Neisseria gonorrhoeae-induced transactivation of EGFR enhances gonococcal invasion. Cell. Microbiol. 2011;13:1078–1090. doi: 10.1111/j.1462-5822.2011.01603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clements A. Enterohaemorrhagic Escherichia coli inhibits recycling endosome function and trafficking of surface receptors. Cell. Microbiol. 2014;16:1693–1705. doi: 10.1111/cmi.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Almadori G. Human papillomavirus infection and epidermal growth factor receptor expression in primary laryngeal squamous cell carcinoma. Clin. Cancer Res. 2001;7:3988–3993. [PubMed] [Google Scholar]
- 64.Yu J.J. HPV infection and EGFR activation/alteration in HIV-infected East African patients with conjunctival carcinoma. PLoS One. 2010;5:e10477. doi: 10.1371/journal.pone.0010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Regueiro V. Klebsiella pneumoniae subverts the activation of inflammatory responses in a NOD1-dependent manner. Cell. Microbiol. 2011;13:135–153. doi: 10.1111/j.1462-5822.2010.01526.x. [DOI] [PubMed] [Google Scholar]
- 66.Frank C.G. Klebsiella pneumoniae targets an EGF receptor-dependent pathway to subvert inflammation. Cell. Microbiol. 2013;15:1212–1233. doi: 10.1111/cmi.12110. [DOI] [PubMed] [Google Scholar]
- 67.Barrionuevo P. Brucella abortus induces intracellular retention of MHC-I molecules in human macrophages down-modulating cytotoxic CD8+ T cell responses. Cell. Microbiol. 2013;15:487–502. doi: 10.1111/cmi.12058. [DOI] [PubMed] [Google Scholar]
- 68.Dai K. Amphiregulin promotes the immunosuppressive activity of intrahepatic CD4+ regulatory T cells to impair CD8+ T cell immunity against hepatitis B virus infection. Immunology. 2014;144:506–517. doi: 10.1111/imm.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim J.H. Human cytomegalovirus requires epidermal growth factor receptor signaling to enter and initiate the early steps in the establishment of latency in CD34(+) human progenitor cells. J. Virol. 2017;91 doi: 10.1128/JVI.01206-16. e01206-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stanley S.A. Identification of host-targeted small molecules that restrict intracellular Mycobacterium tuberculosis growth. PLoS Pathog. 2014;10:e1003946. doi: 10.1371/journal.ppat.1003946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hardbower D.M. EGFR regulates macrophage activation and function in bacterial infection. J. Clin. Invest. 2016;126:3296–3312. doi: 10.1172/JCI83585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ito Y. Influenza induces IL-8 and GM-CSF secretion by human alveolar epithelial cells through HGF/c-Met and TGF-α/EGFR signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015;308:L1178–L1188. doi: 10.1152/ajplung.00290.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Monick M.M. Activation of the epidermal growth factor receptor by respiratory syncytial virus results in increased inflammation and delayed apoptosis. J. Biol. Chem. 2005;280:2147–2158. doi: 10.1074/jbc.M408745200. [DOI] [PubMed] [Google Scholar]
- 74.Liu K. Epidermal growth factor receptor signaling to Erk1/2 and STATs control the intensity of the epithelial inflammatory responses to rhinovirus infection. J. Biol. Chem. 2008;283:9977–9985. doi: 10.1074/jbc.M710257200. [DOI] [PubMed] [Google Scholar]
- 75.Shigematsu H. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J. Natl. Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 76.Lynch T.J. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 77.Okabe T. Differential constitutive activation of the epidermal growth factor receptor in non-small cell lung cancer cells bearing EGFR gene mutation and amplification. Cancer Res. 2007;67:2046–2053. doi: 10.1158/0008-5472.CAN-06-3339. [DOI] [PubMed] [Google Scholar]
- 78.Kuramochi H. Amphiregulin and epiregulin mRNA expression in primary colorectal cancer and corresponding liver metastases. BMC Cancer. 2012;12:88. doi: 10.1186/1471-2407-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Neufert C. Tumor fibroblast–derived epiregulin promotes growth of colitis-associated neoplasms through ERK. J. Clin. Invest. 2013;123:1428–1443. doi: 10.1172/JCI63748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khambata-Ford S. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J. Clin. Oncol. 2007;25:3230–3237. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 81.Harada K. Transforming growth factor-alpha and epidermal growth factor receptor in chronic liver disease and hepatocellular carcinoma. Liver. 1999;19:318–325. doi: 10.1111/j.1478-3231.1999.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 82.Qiao Q. Over expression of transforming growth factor-alpha and epidermal growth factor receptor in human hepatic cirrhosis tissues. Hepatogastroenterology. 2016;55:169–172. [PubMed] [Google Scholar]
- 83.Inui Y. Expression of heparin-binding epidermal growth factor in human hepatocellular carcinoma. Gastroenterology. 1994;107:1799–1804. doi: 10.1016/0016-5085(94)90823-0. [DOI] [PubMed] [Google Scholar]
- 84.Nakagawa M. Up-regulated expression of ADAM17 in gastrointestinal stromal tumors: coexpression with EGFR and EGFR ligands. Cancer Sci. 2009;100:654–662. doi: 10.1111/j.1349-7006.2009.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mullooly M. ADAM10: a new player in breast cancer progression? Br. J. Cancer. 2015;113:945–951. doi: 10.1038/bjc.2015.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sung S.-Y. Oxidative stress induces ADAM9 protein expression in human prostate cancer cells. Cancer Res. 2006;66:9519–9526. doi: 10.1158/0008-5472.CAN-05-4375. [DOI] [PubMed] [Google Scholar]
- 87.Ohtsuka T. ADAM28 is overexpressed in human non-small cell lung carcinomas and correlates with cell proliferation and lymph node metastasis. Int. J. Cancer. 2006;118:263–273. doi: 10.1002/ijc.21324. [DOI] [PubMed] [Google Scholar]
- 88.Menzo S. Trans-activation of epidermal growth factor receptor gene by the hepatitis B virus X-gene product. Virology. 1993;196:878–882. doi: 10.1006/viro.1993.1550. [DOI] [PubMed] [Google Scholar]
- 89.Miyaki M. Malignant transformation and EGFR activation of immortalized mouse liver epithelial cells caused by HBV enhancer-X from a human hepatocellular carcinoma. Int. J. Cancer. 2000;85:518–522. doi: 10.1002/(sici)1097-0215(20000215)85:4<518::aid-ijc12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 90.Hung C.-M. Hepatitis B virus X upregulates HuR protein level to stabilize HER2 expression in hepatocellular carcinoma cells. BioMed Res. Int. 2014;2014:1–9. doi: 10.1155/2014/827415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carapito R. Polymorphisms in EGFR and IL28B are associated with spontaneous clearance in an HCV-infected iranian population. Genes Immun. 2015;16:514–518. doi: 10.1038/gene.2015.38. [DOI] [PubMed] [Google Scholar]
- 92.He Y.-Y. Cleavage of epidermal growth factor receptor by caspase during apoptosis is independent of its internalization. Oncogene. 2006;25:1521–1531. doi: 10.1038/sj.onc.1209184. [DOI] [PubMed] [Google Scholar]
- 93.Divella R. Circulating transforming growth factor-beta and epidermal growth factor receptor as related to virus infection in liver carcinogenesis. Anticancer Res. 2012;32:141–145. [PubMed] [Google Scholar]
- 94.Brand T.M. Human papillomavirus regulates HER3 expression in head and neck cancer: implications for targeted HER3 therapy in HPV(+) patients. Clin. Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-2203. Published online December 21, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 95.Narisawa-Saito M. HPV16 E6-mediated stabilization of ErbB2 in neoplastic transformation of human cervical keratinocytes. Oncogene. 2007;26:2988–2996. doi: 10.1038/sj.onc.1210118. [DOI] [PubMed] [Google Scholar]
- 96.Pollock N.I. Increased expression of HER2, HER3, and HER2:HER3 heterodimers in HPV-positive HNSCC using a novel proximity-based assay: implications for targeted therapies. Clin. Cancer Res. 2015;21:4597–4606. doi: 10.1158/1078-0432.CCR-14-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stankiewicz E. Alternative HER/PTEN/Akt pathway activation in HPV positive and negative penile carcinomas. PLoS One. 2011;6:e17517. doi: 10.1371/journal.pone.0017517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reimers N. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int. J. Cancer. 2007;120:1731–1738. doi: 10.1002/ijc.22355. [DOI] [PubMed] [Google Scholar]
- 99.Kong C.S. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int. J. Radiat. Oncol. 2009;74:553–561. doi: 10.1016/j.ijrobp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheng F. Screening of the human kinome identifies MSK1/2-CREB1 as an essential pathway mediating Kaposi’s sarcoma-associated herpesvirus lytic replication during primary infection. J. Virol. 2015;89:9262–9280. doi: 10.1128/JVI.01098-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen L. Foxp3-dependent transformation of human primary CD4+ T lymphocytes by the retroviral protein tax. Biochem. Biophys. Res. Commun. 2015;466:523–529. doi: 10.1016/j.bbrc.2015.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wong B.C.-Y. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China. JAMA. 2004;291:187. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 103.Polk D.B., Peek R.M. Helicobacter pylori: gastric cancer and beyond. Nat. Rev. Cancer. 2010;10:403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bauer B. The Helicobacter pylori virulence effector CagA abrogates human β-defensin 3 expression via inactivation of EGFR signaling. Cell Host Microbe. 2012;11:576–586. doi: 10.1016/j.chom.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 105.Walsh D. Tinkering with translation: protein synthesis in virus-infected cells. Cold Spring Harb. Perspect. Biol. 2013;5:a012351. doi: 10.1101/cshperspect.a012351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bagga S., Bouchard M.J. Cell cycle regulation during viral infection. Methods Mol. Biol. 2014;1170:165–227. doi: 10.1007/978-1-4939-0888-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bekerman E. Anticancer kinase inhibitors impair intracellular viral trafficking and exert broad-spectrum antiviral effects. J. Clin. Invest. 2017;127:1338–1352. doi: 10.1172/JCI89857. [DOI] [PMC free article] [PubMed] [Google Scholar]