Abstract
Our knowledge of cat family biology was recently expanded to include a genomics perspective with the completion of a draft whole genome sequence of an Abyssinian cat. The utility of the new genome information has been demonstrated by applications ranging from disease gene discovery and comparative genomics to species conservation. Patterns of genomic organization among cats and inbred domestic cat breeds have illuminated our view of domestication, revealing linkage disequilibrium tracks consequent of breed formation, defining chromosome exchanges that punctuated major lineages of mammals and suggesting ancestral continental migration events that led to 37 modern species of Felidae. We review these recent advances here. As the genome resources develop, the cat is poised to make a major contribution to many areas in genetics and biology.
Introduction
Cats and their wild progenitors have existed for a long time – fossil records suggest they first appeared ∼35 million years ago (MYA). Although the great saber-tooth tigers of those times have long been extinct, modern cats are descended from a midsize cat that existed ∼10–11 MYA 1, 2, 3. Humans seem to be fascinated with the feline species – a rich literature and artistic imagery of lions, tigers, cheetahs and leopards testifies to this – and domestic cats rank among the world's most venerated companion animals.
Our affection toward cats, complemented by accounts of cats’ behavior and biology, has resulted in their contribution to a diverse range of disciplines (ranging from disease to conservation studies) that are suitable for genetic and genomic enquiry. For example, cats, like dogs, enjoy extensive veterinary medical surveillance (second only to human medicine) and have ∼250 genetic diseases analogous to human disorders 4, 5, 6, 7, 8. Feline infectious agents offer powerful natural models to human diseases including HIV-AIDS [feline immunodeficiency virus (FIV)], SARS (feline coronavirus-FCoV), avian influenza, neurotropic viruses [canine distemper virus (CDV)] and cancers [feline leukemia virus-feline sarcoma virus (FeLV/FeSV)] 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16. Cats are a domesticated representative of the Felidae family, which includes some of the most successful, but now the most threatened, predator species on earth 1, 2, 17.
With a rapidly growing literature on cat behavior, coat colors, reproductive advances and breed development, the domestic cat is poised to benefit from the recently completed draft whole genome sequence of an Abyssinian cat named Cinnamon 8, 18. Cats present a powerful model in development, physiology and neuroscience (some of which are listed in Table 1 ). Here we review the insights achieved from the cat genome sequence and discuss the wealth of knowledge about genomic organization that is being shown through comparative genomics. Furthermore, we trace the natural history of the wild cats, discuss the origins of cat domestication and review the many aspects of cats that make them a useful animal ‘model’ for human diseases and for comparative genomics.
Table 1.
The cat as a useful model species
| Application | Evidence |
|---|---|
| Medical models of human diseases | There is a rich literature of veterinary disease in cats; >250 hereditary diseases in cats are analogous to human genetic diseases. Eighteen of these have a known gene mutation in cats suitable for pathogenesis and therapeutic study 4, 5, 6, 7, 8, 32. |
| Infectious agents | Cats have well-described models for several deadly human viral diseases, notably HIV-AIDS-feline immunodeficiency virus, which is endemic in 14 free ranging species of Felidae including domestic cats [11]. Cats also harbor feline leukemia virus and feline sarcoma virus, which have laid groundwork for oncogene discoveries and a virulent model for SARS, feline coronavirus, and many others 10, 11, 12, 13, 14, 15, 16. |
| Neuroscience and physiology | Cat are traditional subjects for neurophysiology studies that have led to important insight on ocular and neural physiological processes 43, 45, 97, 98, 99, 100, 101, 102. |
| Behaviors | Cats have many curious behaviors associated with nurturing, defense, allusiveness and tameness that seem to have genetic influences. Social organization in lions and in domestic cats seems to involve co-adaptation of behavior and reproductive strategies 5, 88. |
| Reproduction | The reproductive physiology of cats is relatively advanced; for example, artificial insemination, in vitro fertilization, embryo transfer, chineric embryos and nuclear cloning have been performed in cats. Promising advances have occurred in embryonic stem cell isolation, leading to hopes of gene knockouts and gene replacement in laboratory settings. |
| Domestication | The process of cat domestication represents one of the more fascinating natural experiments. It seems to have happened in a single locale in the Fertile Crescent when humans began agriculture. Cat domestication has led to the growth of 600 million domestic cats worldwide. The adaptive aspects of this process in genetic terms await resolution [42]. |
| Breed development | Approximately 68 certified cat breeds current exist; most are younger than a few hundred years. Each was selected artificially for appearances and behavior, raising the question of what was selected and how well 5, 88. |
| Coat-color variation | The cat breeds are fixed in different combinations for some ∼12 coat color genes that have been described and tracked in pedigrees. Resolution of their genetic bases will lead not only to more precise breed improvement but also to a better understanding of pigmentation, hair development and ocular albinism. We know the basis for ten coat color hair length genes, but others (such as Orange) remain elusive 31, 46, 47, 48, 49, 50, 51, 88. |
| Forensic development | Cats have led the way in establishing the legal precedent for introduction of short tandem repeat–based cat individual identification of hairs and other biospecimens found at crime scenes. Cats hairs are easily picked up, so suspects with cats or dogs can implicate themselves through their pets 91, 103, 104. |
| Felidae evolution, adaption and natural history | The cat family Felidae has shown much in the application of sophisticated tools of molecular evolution to phylogeny reconstruction. Multidisciplinary interpretation of felid phylogeny, geography, paleontology and geology has allowed inference around their historic continental migrations and origins 1, 2, 17. |
| Comparative genomics | The highly conserved synteny of the cat genome map with that of human, dolphin and other mammal species has given us a glimpse of the ancestral genome organization of all mammals. Recent sequence analyses of cat and other mammal genomes are beginning to reveal a view of the pattern of genome organization that has punctuated the mammalian radiations 8, 22, 60. |
| Felidae conservation | Humans’ fascination with the cat species has produced a plethora of ecological and behavioral descriptions of the plight of the many endangered Felidae species. Genetics in conservation became widely accepted with the finding of the cheetahs’ genetic uniformity and progressed to studies on leopards, pumas, tigers, wildcats, lynxes and other free-ranging species [17]. |
Developing and applying a whole genome sequence for cats
The study of feline genetics had its roots in early gene-mapping strategies using linkage and physical mapping approaches 19, 20, 21, 22. Over the past few decades, several important biological and informatics resources required for genetic investigations in cats were developed. The resources are comparable to those developed in other genome projects and include the following: (i) somatic cell and radiation hybrid panels used to build a framework physical map of comparative anchor tagged sequence (CATS) markers for cats 19, 23, 24, 25, 26, 27, 28; (ii) three large cat pedigrees used to build a cat linkage map 29, 30, 31, 32; (iii) several genomic libraries (BAC, PAC, cosmid and fosmid) for gene discovery and genome assembly validation 33, 34, 35 and (iv) finished DNA sequence of three subregions of the cat genome [i.e. FLA, the major histocompatibility complex (3.3 Mbp), mitochondrial DNA and counterpart nuclear mitochondrial DNA (mtDNA; numt) sequence; and 30 Mbp of the ENCODE Project cat genome sequence (http://www.genome.gov/10005107, 33, 34, 35, 36, 37, 38, 39, 40). The application of these important tools led to significant advances in cat genetics including gene discovery and regional gene annotation and to anticipation for a whole genome sequence.
In 2005, the cat was included in a selection of 24 mammals for whole genome sequencing by National Human Genome Research Institute, to facilitate interpretation of the finished human genome sequence. The species were chosen to capture the evolutionary divergence across the 4500 living species of mammals initially with a ‘light’ (i.e. twofold) genome coverage 18, 41. The principal criteria for selecting these species were (i) to discover short conserved sequence regions among mammals that would include gene regulatory motifs; (ii) to provide a platform for reconstruction of ancestral genomes that preceded the divergence nodes of the mammalian radiations and (iii) to stimulate the development and application of new animal models for human medicine and biology. In 2006, the whole genome sequence of a female Abyssinian cat named Cinnamon was determined by Agencourt Bioscience (http://www.agencourt.com); Abyssinian cats are the most inbred, making genome assembly easier. A consortium of scientists assembled, mapped and annotated the cat genome sequence using a comparative approach that involved cross-reference to NCBI-annotated genome assembles of six index mammals (human, chimpanzee, mouse, rat, dog and cow) [8]. For details of the annotation strategy and highlights of genomic features that were discovered, see Box 1 .
Box 1. Annotation of the cat whole genome sequence.
Here we give an overview of the strategy and main conclusions from the cat genome sequence study; full details can be found in Ref. [8]. The ‘light’ (1.9-fold coverage) of Cinnamon was assembled, mapped and annotated using a comparative approach to finished whole genomes sequence/maps of six mammals (human, chimpanzee, mouse, rat, dog and cow). Briefly, 8 027 672 sequence reads (84% plasmid and 16% fosmid) were assembled to 817 956 overlapping contigs and 217 790 scaffolds using the ARACHNE and PHUSION genome assembly algorithms 93, 94, 95. The contigs were aligned to the index mammals’ sequence maps and anchored with 1682 ordered markers from the cat radiation–hybrid map, assuming that the order of reciprocal best match (RBM) sequences between the radiation hybrid (RH) anchor markers for cats was the same as for a their dog and human counterparts. The cat genome sequence is accessible on a web-based genome sequence browser, GARFIELD http://lgd.abcc.ncifcrf.gov 8, 96, which includes the following annotated features:
-
o
817 956 contigs and 217 790 scaffolds across 2.7 Gbp of cat genome sequence
-
o
definition and mapping of >1 million RBM alignments between cat and six index mammals, revealing a set of 133 499 conserved sequence blocks (CSBs) present in all seven genomes. CSBs and RBMs were used to annotate cat genes and homologous synterny blocks (HSBs)
-
o
identification of 20 285 cat genes based on alignment and conserved syntenic orthology with orthologous genes in index mammals
-
o
sequence, iteration and mapping of cat repeat sequence families
-
o
detection and mapping of 201 microRNA loci
-
o
definition of 2814 HSBs across six index mammal genomes
-
o
detection of previously undiscovered nuclear mitochondrial DNA (numt) sequences distributed across cat chromosomes
-
o
description of undiscovered endogenous retroviral sequences and phylogenetic lineages, ten times more abundant than previously known FeLV and RD114 endogenous retroviral sequences
-
o
detection of 327 037 single nucleotide polymorphisms, 34 850 deletion insertion polymorphisms and 200 177 short tandem repeat (or microsatellite) loci
About 327 037 single nucleotide polymorphism (SNP) variants were heterozygous in Cinnamon's genome, and a sampling of 200 SNPs validated ∼85% of them as being variable in additional domestic cats from various breeds. Cinnamon's genomic history was molded by three episodes of historic inbreeding; the original domestication event for cats (∼10 000 years ago), Abyssinian breed formation and disease (rdAc) pedigree establishment 42, 43. Her genome is a patchwork of short regions of SNP homozygosity interspersed with regions of heterozygosity (more than one SNP per 600 bp), perhaps a reflection of historic inbreeding. In total, there are 275 alternating homozygous/heterozygous segments in Cinnamon's genome, with 57% of her genome being largely homozygous. Similar alternating homozygous genome segments occur in dogs, likely for the same reason [44].
Indeed, the potential of cat phenotypic and medical surveillance in the genomic era is beginning to be realized. Currently there are 38 cat genes for which mutational variants responsible for feline metabolic diseases or for morphological phenotypes are described [8]. This includes 18 hereditary diseases, where the mutation in cat occurs in the homologous gene to the analogous human syndrome. The single exception, spinal muscular atrophy (SMA), a leading cause of human infant mortality, involved a large 140-kb deletion of the LIX1 gene in the cat model [45]. The LIX1 gene is located on chromosome A1 within 25 cm (Mbp) of the SMN1 gene, whose human homolog carries causal mutations in 97% of human SMA patients. In addition, the resolution of the molecular genetic bases of ten coat color (or hair length) variants now establishes cats as important species for study of pigmentation, hair development and ocular albinism 8, 21, 31, 46, 47, 48, 49, 50, 51.
Recently, the NHGRI Committee for the Annotation of the Human Genome promoted the cat to an additional sixfold genome coverage, due to be completed in late 2008 [18]. The planned supplement of SNP discovery using ‘light’ resequence coverage of 30 cats from ten cat breeds assures a large store of SNPs and short tandem repeats (STRs) suitable for gene association discovery using SNP genotyping arrays. The decay of linkage disequilibrium (LD) already measured among 24 cat breeds predicts that a SNP genotypic array including ∼45 000 evenly placed SNPs is appropriate for gene association discovery in cats, similar to the approach successfully applied to dogs 8, 44, 52, 53. However, cats have shorter LD stretches than dogs (because they were domesticated more recently than dogs; see below) and require three times more SNPs for gene association studies. We anticipate that these efforts will lead to the discovery of 2 million new SNPs, which will aid the development of an eagerly awaited genotype array chip of 80 000–100 000 SNPs, suitable for genome-wide association studies. The dog genome sequence led to scores of new gene discoveries 44, 52, 53; the availability of these promising cat genome resources portends that cat advances should be close behind.
Comparative genome organization in mammals
Cat genomics has benefited from and also played a key role in the emerging field of comparative genomics. The earliest comparisons of gene order in mammalian chromosomes revealed long stretches of conserved synteny between human chromosomes and homologous chromosome segments in developing gene maps of mouse, cat and cow 9, 19, 54, 55. Conserved syntenic region homologies between mammal species were confirmed by precise G-banding pattern identities across chromosome arms and in some cases over entire chromosomes 20, 56, 57. These large segments of chromosome homology were explicitly defined by florescent in situ hybridization (FISH) or chromosome painting, whereby metaphase chromosomes of various mammals were decorated by flow-sorted and fluorescent-labeled whole chromosome probes 9, 22, 58, 59.
Chromosome painting studies gave us the first look at the details of genome rearrangements that characterized the divergence of mammals. With the caveat that intrachromosomal rearrangements (inversions) were invisible by these methods, a paradigm emerged that chromosomal exchange followed a dichotomous mode in different mammal groups 22, 58. In most lineages, a low or ‘default’ rate was observed where species groups displayed few translocation exchanges, on the order of one new rearrangement every 10–15 MY (exemplified by cat, human, dolphin, pig and other species). These default (slow or conserved) lineages were in sharp contrast to lineages whose chromosomes were reshuffled extensively (examples of reshuffled lineages were gibbons, new world monkeys, murid rodents, canids and ursids) 22, 56, 57, 58. The global reorganization in reshuffled lineages was three to five times more extensive than was seen in the slower ‘default’ lineages for inexplicable reasons.
Assembled whole genome sequences, including the cat genome, have allowed the detection of intrachromosomal inversions, because ∼100 000 conserved sequence block (CSB) markers of homology between species (see Box 1) enabled an extremely high-density genome resolution of conserved syntenic segments 8, 60, 61. Using sophisticated breakpoint parsimony algorithms [genome rearrangements in man and mouse (GRIMM) synteny and multiple genome rearrangement algorithm (MGR)] 62, 63, 64 that resolved the breakpoint coordinates joining 500 homologues synteny blocks [HSBs; previously called smallest combined evolutionary unit segments (SCEUS); Figure 1 ], the paradigm of dichotomous rates of genome exchange was unseated 8, 26. The wrinkle came from the occurrence of numerous intrachromosomal rearrangements but at different tempos in the slow default translocation lineages versus the reshuffled lineages (Figure 1). A preliminary analysis of six species genome sequences showed that lineages with few translocation exchanges display considerably more intrachromosomal inversions, whereas species with reshuffled and increased translocations showed fewer inversions (Table 2 ). The total number of translocations plus inversions is similar in all lineages, because in the slow ‘default’ lineages (human and cat), inversions are increased, whereas in the shuffled translocation-rich lineages (murids and dogs), inversions are infrequent. It almost seems that interchromosomal and intrachromosomal exchanges are compensating in different lineages.
Figure 1.
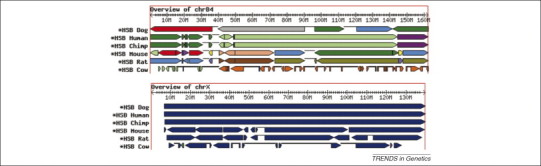
Homologous synteny blocks (HSBs) relative to cat chromosomes B4 and X defined by conserved sequence blocks across six index mammalian species’ genome sequences 8, 26, 60. HSBs reflect the chromosome segments that are retained across divergent mammals, allowing one to reconstruct the chromosome exchanges and breakpoint that punctuate genome organization throughout the mammalian radiation (see main text and Table 2 for more details).
Table 2.
Counting ancestral translocations and inversions (breakpoints) that discriminate the genome of cat from index mammals
| Counts | Number of chromosome breakpoint events in comparing cat to |
||||
|---|---|---|---|---|---|
| Human | Chimpanzee | Mouse | Rat | Dog | |
| Breakpoints (BP) | 135 | 136 | 256 | 258 | 100 |
| Translocations | 29 | 29 | 110 | 106 | 58 |
| Inversions | 106 | 107 | 146 | 152 | 42 |
| Translocation:inversion | 0.27 | 0.27 | 0.75 | 0.70 | 1.38 |
| BP/MY | 0.71 | 0.74 | 1.36 | 1.4 | 0.89 |
aThe number of interchromosomal exchanges (translocations) between the genome of cat versus primates is two to five times lower than that of cat versus ‘shuffled’ taxa mouse, rat and dog, whereas intrachromosomal exchanges (inversions) are fourfold higher in cat versus primates than translocations. This difference is apparent in low translocation:inversion ratios in cat-primate versus cat-rodent or cat-dog, and results in a balanced overall rate (BP/MY) among all comparisons (see Refs. 8, 26, 60).
Additional fascinating observations have emerged from comparing available mammal species genomes [60]. First, the overall rates of chromosome exchanges within mammalian orders have increased (more than tenfold) since the Cretaceous–Tertiary boundary (∼65 MYA), coincident with the advancement and diversification of most mammalian orders at the time. Second, ∼20% of chromosome breakpoint regions defined by comparative alignments were not only reused more than once during mammalian evolution, but the reused sites are clustered nonrandomly around centromeres. Third, chromosomal breakpoints defined between mammals show a marked enrichment in gene density compared with the genome-wide average; they seem to cluster in gene-rich areas. Fourth, primate-defined breakpoints (translocation and inversion) occur in regions of human segmental duplications, suggesting that such local aneuploidy plays a part in chromosomal exchange. Finally, evolutionary breakpoints in mammal lineages seem to coincide with breakpoints that occur in human cancers, suggestive of a common underlying mechanism.
These conclusions emerged from comparisons of species that have a whole genome sequence and a dense radiation hybrid (RH) map. The inferences must be affirmed by future comparisons, particularly with the additional 24 species selected for whole genome sequence [18]. The puzzle is not completely solved, but the prospect of richer whole genome sequence data across mammals in the future holds high anticipation for both resolving the effective chromosomal dynamics and also for inferring the genome organization of the ancestral bears, felids, at other mammal groups in high definition.
Genomic natural history of the world's cats
Nestled in the genome of every individual are cryptic footprints of historic episodes encountered by their ancestral progenitors. The emerging field of genomic archeology (or genomic prospecting) aims to mine the ancient DNA sequence to enable better interpretation of the divergence, migration and demographic perturbations that occurred in the silence of prehistory 65, 66, 67, 68. The cat family Felidae – an assemblage of 37 successful predatory carnivore species on five continents (Figure 2 ) – provides a cogent example of how genomic data can complement geographic, paleontological and geological aspects to characterize a species group's natural history 1, 2 (Figure 3 ).
Figure 2.
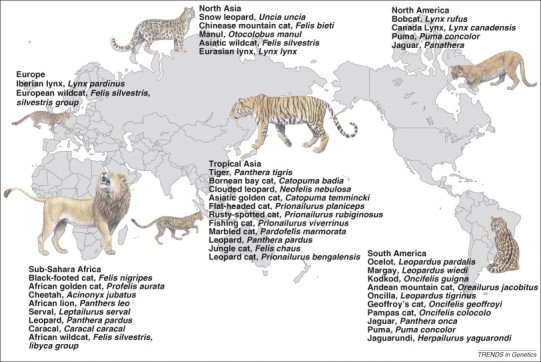
Geographic distribution and Latin names for the 37 living species of the Felidae family. All these species except domestic cats are considered threatened or endangered by the conservation organization of the world.
Figure 3.
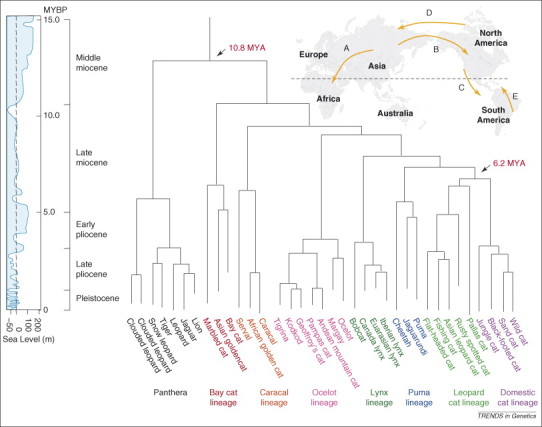
Molecular phylogeny of Felidae 1, 2. The branching hierarchy of the cat family as discerned from an analysis of 30 genes from members of each species. Modern cat species assort into eight lineages, each representing a close relative relationship among species within each group. The time scale is imputed from several dates of fossils that define certain portions of the tree (arrows). The geological periods during the 11 million year interval of cat evolution are presented beside the time scale. The global sea levels relative to current sea level are shown in meters. Arrows A–E depict five imputed ancestral migration routes discussed in text that we infer were traversed by ancestors of modern cat species.
The first step in reconstructing cat origins was to establish a robust molecular phylogeny, which informed the hierarchical relationship and divergence nodes that define the Felidae radiation. This was accomplished by selecting 35 cat genes (a total of 22 789 bp chosen from autosomal, mitochondrial, X- and Y-linked loci) that were sequenced in all cat species [1]. Available tree-building algorithms converged on a phylogenetic tree on which the nodes could be dated with a dozen well-calibrated fossils. The earliest predecessor of living cats, Pseudaelurus, lived in Eurasia during the Miocene and spawned the Asian ancestor of modern cats ∼11 MYA. Molecular phylogeny defined eight principal Felidae lineages (Figure 3), descended from major Miocene divergence events that form the axiom for Felidae genus recognition [69]. Armed with robust and high resolution molecular phylogeny, the fossil-calibrated dates for the branches, the current and historic locations of cat species and a record of global sea level changes and continental movements, we postulated a plausible sequence of 11 historic felid migrations to explain available data 1, 2. This scenario describes our current understanding of how cats came to be (Figure 3).
The first split from the Pseudaelurus occurred ∼10.8 MYA in Asia, producing the five great roaring cats of the genus Panthera (lion, tiger, leopard, jaguar and snow leopard) and the earliest offshoot (the two clouded leopard species). Interestingly, a novel species of clouded leopards, Neofelis diardi, the first new Felidae species described in a century, was defined recently on the basis of genomic analysis 70, 71. A second major separation, also in Asia, led to the Bornean bay cat lineage, a group that evolved, speciated and currently resides in Southeast Asia. The next divergence and first intercontinental migration founded the caracal lineage, three African cat species whose progenitors crossed into Africa 6–10 MYA (migration route A; Figure 3). During the Miocene, sea levels were ∼60 m below current levels such that Africa and the Arabian Peninsula were connected by land bridges across the Red Sea, facilitating the first felid migration to Africa. During the same period, cats dispersed across Asia and traversed the Bering Straits to Alaska (migration route B; Figure 3). The earliest progenitors of three subsequent cat lineages, (the ocelot, lynx and puma) were found in North America. Thus, cats were present in Asia, Europe, Africa and North America. Subsequently the sea levels rose, separating the continents; the changing habitats of the now ‘isloated’ cats contributed to the evolution of new cat species we are familiar with today.
A dynamic evolutionary process in North America produced the lynx and puma lineages 8.0 and 7.2 million years ago, respectively, to generate pumas, jaguarundi, American cheetahs, three lynx species and bobcats, species whose fossil remains in American deposits affirm their Western Hemisphere origins. Eurasian lynx species’ progenitors and American cheetahs would later migrate back to Asia during the Pliocene (3–4 MYA) when the sea levels dropped once more (migration route D; Figure 3).
Toward the end of the Pliocene (2–3 MYA), the oceans receded once again, and the North and South American continents were connected by the Isthmus of Panama. North American cat species were able to migrate south via the Isthmus of Panama (migration route C; Figure 3), where they encountered a continent with no placental carnivores (i.e. no bears, dogs, cats, skunks) 72, 73. South America had been isolated from northern land masses for tens of millions of years (since Australia, Africa and South American parted from the southern supercontinent Gondwanaland > 100 MYA). Several marsupial species, including a few successful carnivorous varieties, had evolved in South America. These carnivorous marsupials were no match for the newly arrived cats 72, 73. Outcompeted in their habitat and ecological niches, nearly all marsupials were quickly replaced by migrant carnivores such as the cats from the ocelot lineage, a divergent group of seven feline species that survive in South America. Contemporaneous with the cats arrival in South America, North American dogs took the empty southern continent as a terrific opportunity to exploit. New South American Canidae (dog family) species rapidly evolved and, similar to the Felidae, they would display a wide breadth of morphological and physiological adaptations 44, 74.
More recently, cheetahs and pumas became extinct in North America, after the last ice age ∼12 000 years ago. A major Pleistocene extinction of mammals eliminated 40 species of mammals from North America, including 75% of the large vertebrates living there 75, 76. Mammoths, mastodons, dire wolves, massive short-faced bears, giant ground sloth, American lions, saber-tooth cats, puma and cheetahs abruptly disappeared from North America. Much earlier, ancestral populations of cheetahs living in Asia would migrate to Africa where they survive today. Pumas avoided complete annihilation as some had moved to South America and returned to North America many generations later (migration route E; Figure 3) [77]. The other large mammal species would never return. The series of migration events needs to be confirmed by more extensive paleontological and geographical precision in the future; if it is, it would provide one of the most detailed accounts of natural history dispersal for any mammalian species group.
Origins of cat domestication
The next act in the cat's journey, from the wild to human settlements, occurred ∼10 000 years ago in Southwest Asia. At that time, a handful of diminutive cat species intermingled within dense forests of the Mediterranean basin: jungle cat, desert cat and a ubiquitous wildcat species, Felis silvestris, which had three recognized wildcat subspecies – F. silvestris silvestris (European wildcat), F.s. ornata.(Middle Eastern wildcat) and F.s. lybica (Asian and Near Eastern wildcat). From one of these progenitors, perhaps the most successful experiment in cat natural history began – that of cat domestication.
A recent phylogeographic study of wildcats and domestic cats (N = 979) from three continents (Figure 4 ) used 36 STR loci and 2604 bp of mitochondrial DNA to resolve subspecies structure of F. silvestris and their relationship to domestic cats [42]. Although it was well known that modern wildcat populations often include ongoing admixture of domestic cats and wildcats 42, 78, 79, 80, 81, subspecies-specific nuclear and mtDNA lineages in each population were clearly resolved. For example, in sub-Saharan Africa, two versions of mtDNA genotypes were apparent (domestic and African wildcat; beige and blue pie charts, respectively, in Figure 4). The derivative molecular phylogeny for five geographically distinct subspecies (F.s. silvestris, F. cafra, F.s. ornata, F.s.lybica and F.s. bieti) were monophyletic and well resolved using both mtDNA sequence (Figure 5a) and composite STR genotypes [42]. The analysis also showed that a large assemblage of domestic cats from across the globe (including 112 fancy breed cats) had genotypes that were indistinguishable from the clades defined by the Near East subspecies F.s. lybica. Furthermore, a STRUCTURE-based population genetic analysis identified a discrete population of wildcats from the Near East (Israel, United Arab Emirates and Saudi Arabia; Figure 5b) that probably reflects the ancestral founder population for the world's domestic cats.
Figure 4.
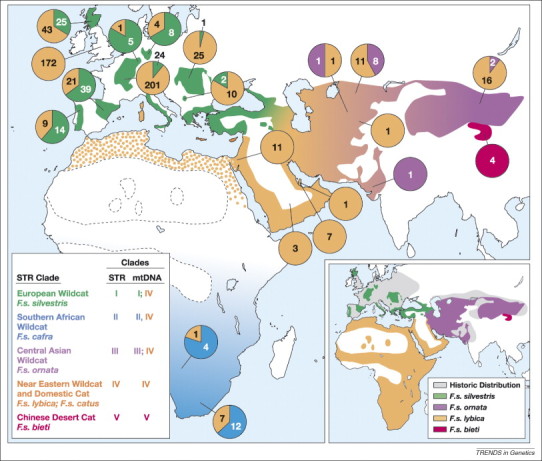
The current range of Felis silvestris and areas of sample collection are shown [42]. The colored regions reflect the location of capture of individuals with different short tandem repeat and mitochondrial DNA (mtDNA) clade genotypes (defined in the bottom left). mtDNA haplotype frequencies are indicated in pie charts specifying the number of specimens carrying each mtDNA haplotype clade. Domestic cats, F. s. catus, are distributed world wide and overwhelmingly carry Clade IV mtDNA haplotypes (beige). The inset on the right shows the current and historic range of F. silvestris subspecies on the basis of traditional morphology-based taxonomy. The Chinese desert cat is considered a wildcat subspecies, F. silvestris bieti as supported by data presented in Ref. [42].
Figure 5.
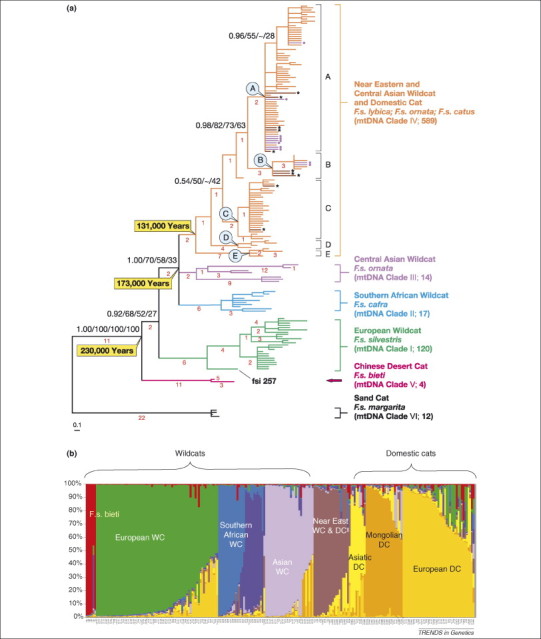
Resolving the origins of cat domestication. (a) Phylogenetic tree of mitochondrial DNA sequence (minimum evolution/neighbor joining phylogram of 2604 bp of the genes NADH5 and NADH6) of 176 haplotypes discerned from 742 cats sampled across the range of the domestic cat, European, Asian and African wildcat, Chinese desert cat and sand cat. Trees created from Bayesian, maximum likelihood (ML) and maximum parsimony (MP) methods result in identical topologies for clade groupings. Confidence/bootstrap values [Bayes/MP/ML/minimum evolution (ME)] are based on 1000 iterations and are adjacent to nodes. The number of single nucleotide differences is indicated in red below the corresponding branch. Clade designations and number of individuals is indicated in parentheses after the corresponding common name and taxonomic trinomial. Beige Clade IV bearing mtDNA haplotypes are found among domestic cats, in wild potentially admixed populations in Europe, Asia, or Africa (see Figure 4) and in Near Eastern wildcats (see main text for further details). Nodes A–E are mtDNA lineages occurring in modern domestic cats that they retain from their wildcat forbearers, F.s. lybica; see text. (b) STRUCTURE-based populations resolved 851 cats into several wildcat groups, three domestic cat groups and one group (brown) that included both domestic cats and Near East wildcats 42, 92. y-axis represent Q-value, the percent representation of resolved populations (colors) within each individual (listed on x-axis) 42, 92.
The oldest known archaeological deposits that show co-occurrence of cat and human remains date to 9500 years ago in Cyprus [82], some 5000–6000 years before the ancient Egyptian civilization existed, which had been thought to be the site of cat domestication 83, 84, 85, 86. Alternatively, the combined genomic archaeological data seem to point to a domestication event in the Near East around the same time as the first agricultural village settlements in the Fertile Crescent (∼10 000 years ago).Perhaps the wild cats made themselves useful to early farmers by dispatching rodents from the early grain stores.
Evidence suggests that cats were probably domesticated on multiple occasions in separate locations, because at least five much older (>100 000 years old) mtDNA lineages were found in extant domestic cats (Clades A–E in domestic cat cluster in Figure 5a). From the Near East origins of domestication, subsequent gradual movements of cats with their human companions would spread domestic cats across the globe. The 600 million domestic cats alive today comprise the only Felidae species (Felis catus) that is not considered to be threatened or endangered by the world's conservation bodies [87].
By the time of the industrial revolution (late 18th–early 19th century), pet cat owners were selectively mating their pet tabbies to produce fancy breeds. The American Cat Fanciers Association (http://www.acfacats.com) and the International Cat Association (http://www.tica.org) currently recognize 68 official cat breeds, from Maine Coon, Siamese, Persian to Korat; all of their roots can be traced to the origins of human and feline civilization in the Fertile Crescent.
Modern cat breeds derive from the earliest fancy breeds (Siamese, Persian, Korat, Egyptian Mau, Manx, Turkish Angora and others) established around the 17th century to the most recent (American Curl, Selkirk Rex, Singapora) established during the late 20th century [88]. A comprehensive SNP and STR survey of 38 cat breeds (∼27 individuals per breed, 1040 cats in total) revealed only modest phylogenetic and population genetic partitions caused by the recent timing of breed initiation and (unlike dog breeds) the allowable intercrossing between certain breeds in recent generations 89, 90. Nonetheless, there is a recognizable and diagnostic population structure that permitted confirmation of 96% breed assignment on the basis of a forensic panel of ten highly informative, tetra-nucleotide STR loci [91]. In Figure 6 , we present a phylogenetic topology of 34 cat breeds based on composite STR genotype, population genetic–based STRUCTURE algorithm output of the same individuals. The results provide a genomic view of the slight but useful genetic differentiation among modern breeds of domestic cats 89, 90.
Figure 6.
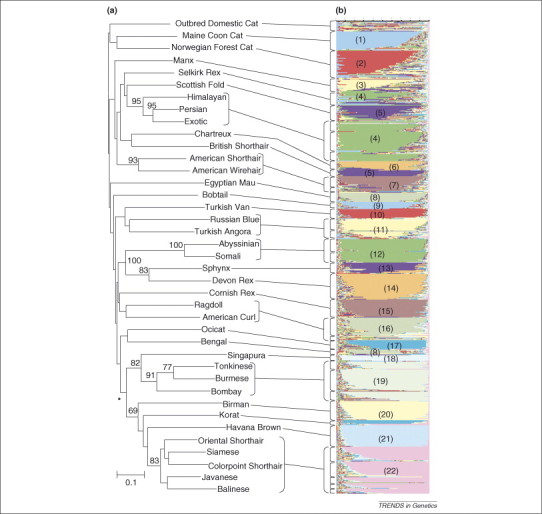
Phylogenetic neighbor-joining tree (a) of individuals from 38 cat breeds based on distance matrices generated from proportion of shared alleles algorithm (Dps) from composite genotypes [83]. Bootstrap support for branches that are supported in >60% of 100 replicates are indicated. The asterisk indicates a group of breeds that was derived completely or in part from Southeast Asian ancestors. The histogram (b), generated from STRUCTURE analysis of 1040 cats, shows the proportion of each individual's genome that originated from 22 populations 83, 92. The numbers in colored blocks refer to 22 distinct cluster groups that were resolved. Some populations are composed of multiple breeds [83].
Concluding remarks and future perspectives
Humans’ fascination with cats seems to have spread to the science and genetic community. We have attempted to highlight here some of the genetic advances that provide new opportunities for better understanding cat biology and the evolutionary processes that created this exquisitely successful group of predators. The tools have applications in every aspect of cat biology, and it is exhilarating for us to witness the invigoration of science potential for the species.
Whether pursuing genetic diseases, behavior or species conservation, the cat has provided powerful examples and lessons of evolutionary, developmental and adaptive processes. We anticipate a bright future for the cat's entry among the company of major animal models of genetic biology.
Acknowledgements
Work in our laboratory is funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
References
- 1.O’Brien S.J., Johnson W.E. Big cat genomics. Annu. Rev. Genomics Hum. Genet. 2005;6:407–429. doi: 10.1146/annurev.genom.6.080604.162151. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien S.J., Johnson W.E. The evolution of cats. Genomic paw prints in the DNA of the world's wild cats have clarified the cat family tree and uncovered several remarkable migrations in their past. Sci. Am. 2007;297:68–75. [PubMed] [Google Scholar]
- 3.Savage D.E., Russell D.E. Addison-Wesley; 1993. Mammalian Paleofaunas of the World. [Google Scholar]
- 4.Lenffer J. OMIA (Online Mendelian Inheritance in Animals): an enhanced platform and integration into the Entrez search interface at NCBI. Nucleic Acids Res. 2006;34:D599–D601. doi: 10.1093/nar/gkj152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffin B., Baker H.J. Academic Press; 2000. Domestic Cats as Laboratory Animals. [Google Scholar]
- 6.O’Brien S.J. The feline genome project. Annu. Rev. Genet. 2002;36:657–686. doi: 10.1146/annurev.genet.36.060602.145553. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien S.J. Cats. Curr. Biol. 2004;14:R988–R989. doi: 10.1016/j.cub.2004.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pontius J.U. Initial sequence and comparative analysis of the cat genome. Genome Res. 2007;17:1675–1689. doi: 10.1101/gr.6380007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien S.J. Comparative genomics: lessons from cats. Trends Genet. 1997;13:393–399. doi: 10.1016/s0168-9525(97)01297-3. [DOI] [PubMed] [Google Scholar]
- 10.Willett B.J. FIV infection of the domestic cat: an animal model for AIDS. Immunol. Today. 1997;18:182–189. doi: 10.1016/s0167-5699(97)84665-8. [DOI] [PubMed] [Google Scholar]
- 11.Troyer J.L. Seroprevalence and genomic divergence of circulating strains of feline immunodeficiency virus among Felidae and Hyaenidae species. J. Virol. 2005;79:8282–8294. doi: 10.1128/JVI.79.13.8282-8294.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown M.A. Genetic characterization of feline leukemia virus from Florida panther. Emerg. Infect. Dis. 2008;14:252–259. doi: 10.3201/eid1402.070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearks-Wilkerson A.J. Coronavirus outbreak in cheetahs: lessons for SARS. Curr. Biol. 2004;14:R227–R228. doi: 10.1016/j.cub.2004.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy W.D. Elsevier; 1980. Feline Leukemia Virus. [Google Scholar]
- 15.Roelke-Parker M.E. A canine distemper virus epidemic in Serengeti lions (Panthera leo) Nature. 1996;379:441–445. doi: 10.1038/379441a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuiken T. Avian H5N1 influenza in cats. Science. 2004;306:241. doi: 10.1126/science.1102287. [DOI] [PubMed] [Google Scholar]
- 17.Sunquist M., Sunquist F. University of Chicago Press; 2002. Wild Cats of the World. [Google Scholar]
- 18.Green P. 2x genomes–does depth matter? Genome Res. 2007;17:1547–1549. doi: 10.1101/gr.7050807. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien S.J., Nash W.G. Genetic mapping in mammals: chromosome map of domestic cat. Science. 1982;216:257–265. doi: 10.1126/science.7063884. [DOI] [PubMed] [Google Scholar]
- 20.Nash W.G., O’Brien S.J. Conserved regions of homologous G-banded chromosomes between orders in mammalian evolution: carnivores and primates. Proc. Natl. Acad. Sci. U. S. A. 1982;79:6631–6635. doi: 10.1073/pnas.79.21.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien S.J. Chromosomal mapping of beta-globin and albino loci in the domestic cat. A conserved mammalian chromosome group. J. Hered. 1986;77:374–378. doi: 10.1093/oxfordjournals.jhered.a110264. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien, S.J. et al. (1999) The promise of comparative genomics in mammals. Science 286, 458-462, 479–481 [DOI] [PubMed]
- 23.O’Brien S.J. Comparative gene mapping in the domestic cat (Felis catus) J. Hered. 1997;88:408–414. doi: 10.1093/oxfordjournals.jhered.a023127. [DOI] [PubMed] [Google Scholar]
- 24.Murphy W.J. Development of a feline whole genome radiation hybrid panel and comparative mapping of human chromosome 12 and 22 loci. Genomics. 1999;57:1–8. doi: 10.1006/geno.1998.5695. [DOI] [PubMed] [Google Scholar]
- 25.Menotti-Raymond M. Radiation hybrid mapping of 304 novel microsatellites in the domestic cat genome. Cytogenet. Genome Res. 2003;102:272–276. doi: 10.1159/000075762. [DOI] [PubMed] [Google Scholar]
- 26.Murphy W.J. A 1.5-Mb-resolution radiation hybrid map of the cat genome and comparative analysis with the canine and human genomes. Genomics. 2007;89:189–196. doi: 10.1016/j.ygeno.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyons L.A. Development of comparative anchor tagged sequences (CATS) for canine genome mapping. J. Hered. 1999;90:15–26. doi: 10.1093/jhered/90.1.15. [DOI] [PubMed] [Google Scholar]
- 28.Murphy W.J., O’Brien S.J. Designing and optimizing comparative anchor primers for comparative gene mapping and phylogenetic inference. Nat. Protoc. 2007;2:3022–3030. doi: 10.1038/nprot.2007.429. [DOI] [PubMed] [Google Scholar]
- 29.Menotti-Raymond M. A genetic linkage map of microsatellites in the domestic cat (Felis catus) Genomics. 1999;57:9–23. doi: 10.1006/geno.1999.5743. [DOI] [PubMed] [Google Scholar]
- 30.Menotti-Raymond M. Second-generation integrated genetic linkage/radiation hybrid maps of the domestic cat (Felis catus) J. Hered. 2003;94:95–106. doi: 10.1093/jhered/esg008. [DOI] [PubMed] [Google Scholar]
- 31.Eizirik E. Molecular genetics and evolution of melanism in the cat family. Curr. Biol. 2003;13:448–453. doi: 10.1016/s0960-9822(03)00128-3. [DOI] [PubMed] [Google Scholar]
- 32.Menotti-Raymond M., O’Brien S.J. The domestic cat, Felis catus, as a model of hereditary and infectious disease. In: Conn P.M., editor. Source Book for Models for Biomedical Research. Humana Press; 2008. pp. 221–232. [Google Scholar]
- 33.Beck T.W. The feline major histocompatibility complex is rearranged by an inversion with a breakpoint in the distal class I region. Immunogenetics. 2005;56:702–709. doi: 10.1007/s00251-004-0742-6. [DOI] [PubMed] [Google Scholar]
- 34.Beck T.W. Comparative feline genomics: a BAC/PAC contig map of the major histocompatibility complex class II region. Genomics. 2001;71:282–295. doi: 10.1006/geno.2000.6416. [DOI] [PubMed] [Google Scholar]
- 35.Yuhki N. Comparative genome organization of human, murine, and feline MHC class II region. Genome Res. 2003;13:1169–1179. doi: 10.1101/gr.976103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez J.V. Complete nucleotide sequences of the domestic cat (Felis catus) mitochondrial genome and a transposed mtDNA tandem repeat (Numt) in the nuclear genome. Genomics. 1996;33:229–246. doi: 10.1006/geno.1996.0188. [DOI] [PubMed] [Google Scholar]
- 37.Lopez J.V. Numt, a recent transfer and tandem amplification of mitochondrial DNA to the nuclear genome of the domestic cat. J. Mol. Evol. 1994;39:174–190. doi: 10.1007/BF00163806. [DOI] [PubMed] [Google Scholar]
- 38.Kim J.H. Evolutionary analysis of a large mtDNA translocation (numt) into the nuclear genome of the Panthera genus species. Gene. 2006;366:292–302. doi: 10.1016/j.gene.2005.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ENCODE Project Consortium (2004)The ENCODE (encyclopedia of DNA elements) project. Science 306, 636–640 [DOI] [PubMed]
- 40.Guigó R. EGASP: the human ENCODE genome annotation assessment project. Genome Biol. 2006;7:1–31. doi: 10.1186/gb-2006-7-s1-s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Brien S.J. On choosing mammalian genomes for sequencing. Science. 2001;292:2264–2266. doi: 10.1126/science.1059393. [DOI] [PubMed] [Google Scholar]
- 42.Driscoll C.A. The Near Eastern origin of cat domestication. Science. 2007;317:519–523. doi: 10.1126/science.1139518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menotti-Raymond M. Mutation in CEP290 discovered for cat model of human retinal degeneration. J. Hered. 2007;98:211–220. doi: 10.1093/jhered/esm019. [DOI] [PubMed] [Google Scholar]
- 44.Lindblad-Toh K. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–819. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- 45.Fyfe J.C. An approximately 140-kb deletion associated with feline spinal muscular atrophy implies an essential LIX1 function for motor neuron survival. Genome Res. 2006;16:1084–1090. doi: 10.1101/gr.5268806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imes D.L. Albinism in the domestic cat (Felis catus) is associated with a tyrosinase (TYR) mutation. Anim. Genet. 2006;37:175–178. doi: 10.1111/j.1365-2052.2005.01409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt-Kuntzel A. Tyrosinase and tyrosinase related protein 1 alleles specify domestic cat coat color phenotypes of the albino and brown loci. J. Hered. 2005;96:289–301. doi: 10.1093/jhered/esi066. [DOI] [PubMed] [Google Scholar]
- 48.Giebel L.B. A tyrosinase gene missense mutation in temperature-sensitive type I oculocutaneous albinism. A human homologue to the Siamese cat and the Himalayan mouse. J. Clin. Invest. 1991;87:1119–1122. doi: 10.1172/JCI115075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyons L.A. Tyrosinase mutations associated with Siamese and Burmese patterns in the domestic cat (Felis catus) Anim. Genet. 2005;36:119–126. doi: 10.1111/j.1365-2052.2005.01253.x. [DOI] [PubMed] [Google Scholar]
- 50.Ishida Y. A homozygous single-base deletion in MLPH causes the dilute coat color phenotype in the domestic cat. Genomics. 2006;88:698–705. doi: 10.1016/j.ygeno.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Kehler J.S. Four independent mutations in the feline fibroblast growth factor 5 gene determine the long-haired phenotype in domestic cats. J. Hered. 2007;98:555–566. doi: 10.1093/jhered/esm072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karlsson E.K. Efficient mapping of mendelian traits in dogs through genome-wide association. Nat. Genet. 2007;39:1321–1328. doi: 10.1038/ng.2007.10. [DOI] [PubMed] [Google Scholar]
- 53.Ostrander E.A. Cold Spring Harbor Press; 2006. The Dog and Its Genome. [Google Scholar]
- 54.Lalley P.A. Conservation of autosomal gene synteny groups in mouse and man. Nature. 1978;274:160–163. doi: 10.1038/274160a0. [DOI] [PubMed] [Google Scholar]
- 55.Womack J.E., Moll Y.D. Gene map of the cow: conservation of linkage with mouse and man. J. Hered. 1986;77:2–7. doi: 10.1093/oxfordjournals.jhered.a110160. [DOI] [PubMed] [Google Scholar]
- 56.Nash W.G., O’Brien S.J. A comparative chromosome banding analysis of the Ursidae and their relationship to other carnivores. Cytogenet. Cell Genet. 1987;45:206–212. doi: 10.1159/000132455. [DOI] [PubMed] [Google Scholar]
- 57.Nash W.G. The pattern of phylogenomic evolution of the Canidae. Cytogenet. Cell Genet. 2001;95:210–224. doi: 10.1159/000059348. [DOI] [PubMed] [Google Scholar]
- 58.Graves J.A.M., Vanderberg J.L. Comparative gene mapping: introduction. ILAR J. 1998;39:47–260. [Google Scholar]
- 59.O’Brien S.J., Stanyon R. Phylogenomics. Ancestral primate viewed. Nature. 1999;402:365–366. doi: 10.1038/46450. [DOI] [PubMed] [Google Scholar]
- 60.Murphy W.J. Dynamics of mammalian chromosome evolution inferred from multispecies comparative maps. Science. 2005;309:613–617. doi: 10.1126/science.1111387. [DOI] [PubMed] [Google Scholar]
- 61.Murphy W.J. Mammalian phylogenomics comes of age. Trends Genet. 2004;20:631–639. doi: 10.1016/j.tig.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 62.Bourque G., Pevzner P.A. Genome-scale evolution: reconstructing gene orders in the ancestral species. Genome Res. 2002;12:26–36. [PMC free article] [PubMed] [Google Scholar]
- 63.Tesler G. GRIMM: genome rearrangements web server. Bioinformatics. 2002;18:492–493. doi: 10.1093/bioinformatics/18.3.492. [DOI] [PubMed] [Google Scholar]
- 64.Tesler G. Efficient algorithms for multichromosomal genome rearrangements. J. Comput. Syst. Sci. 2002;65:587–609. [Google Scholar]
- 65.O’Brien S.J. Genomic prospecting. Nat. Med. 1995;1:742–744. doi: 10.1038/nm0895-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dominguez I. Plant genome archaeology: evidence for conserved ancestral chromosome segments in dicotyledonous plant species. Plant Biotechnol. J. 2003;1:91–99. doi: 10.1046/j.1467-7652.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 67.Cherry M. Genetics to unlock secrets of our African past. Nature. 2003;422:460. doi: 10.1038/422460a. [DOI] [PubMed] [Google Scholar]
- 68.Hoft D.F., Belshe R.B. The genetic archaeology of influenza. N. Engl. J. Med. 2004;351:2550–2551. doi: 10.1056/NEJMcibr043708. [DOI] [PubMed] [Google Scholar]
- 69.Eizirik, E., et al. Molecular systematics and revised classification of the family Felidae (Mammalia, Carnivora). J Mammal. (in press)
- 70.Buckley-Beason V.A. Molecular evidence for species-level distinctions in clouded leopards. Curr. Biol. 2006;16:2371–2376. doi: 10.1016/j.cub.2006.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilting A. Clouded leopard phylogeny revisited: support for species recognition and population division between Borneo and Sumatra. Front. Zool. 2007;4:15. doi: 10.1186/1742-9994-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Novacek M.J. A biogeographic event: the great American biotic interchange. Science. 1986;231:1021–1022. doi: 10.1126/science.231.4741.1021. [DOI] [PubMed] [Google Scholar]
- 73.Marshall L.G. Mammalian evolution and the great American interchange. Science. 1982;215:1351–1357. doi: 10.1126/science.215.4538.1351. [DOI] [PubMed] [Google Scholar]
- 74.Macdonald D.W., Silero-Zubiri C. Oxford University Press; 2004. The Biology and Conservation of Wild Canids. [Google Scholar]
- 75.Barnosky A.D. Assessing the causes of late Pleistocene extinctions on the continents. Science. 2004;306:70–75. doi: 10.1126/science.1101476. [DOI] [PubMed] [Google Scholar]
- 76.Hofreiter M. Pleistocene extinctions: haunting the survivors. Curr. Biol. 2007;17:R609–R611. doi: 10.1016/j.cub.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 77.Culver M. Genomic ancestry of the American puma (Puma concolor) J. Hered. 2000;91:186–197. doi: 10.1093/jhered/91.3.186. [DOI] [PubMed] [Google Scholar]
- 78.Beaumont M. Genetic diversity and introgression in the Scottish wildcat. Mol. Ecol. 2001;10:319–336. doi: 10.1046/j.1365-294x.2001.01196.x. [DOI] [PubMed] [Google Scholar]
- 79.Randi E. Genetic identification of wild and domestic cats (Felis silvestris) and their hybrids using Bayesian clustering methods. Mol. Biol. Evol. 2001;18:1679–1693. doi: 10.1093/oxfordjournals.molbev.a003956. [DOI] [PubMed] [Google Scholar]
- 80.Lecis R. Bayesian analyses of admixture in wild and domestic cats (Felis silvestris) using linked microsatellite loci. Mol. Ecol. 2006;15:119–131. doi: 10.1111/j.1365-294X.2005.02812.x. [DOI] [PubMed] [Google Scholar]
- 81.Macdonald D.W. Oxford University Press; 2004. The Scottish Wildcat: Analyses for Conservation and an Action Plan. [Google Scholar]
- 82.Vigne J.D. Early taming of the cat in Cyprus. Science. 2004;304:259. doi: 10.1126/science.1095335. [DOI] [PubMed] [Google Scholar]
- 83.Serpell J.A. Cambridge University Press; 2000. The Domestic Cat: the Biology of its Behaviour. [Google Scholar]
- 84.Clutton-Brock J. Cambridge University Press; 1999. A Natural History of Domesticated Mammals. [Google Scholar]
- 85.Clutton Brock J. Harvard University Press; 1993. Cats, Ancient and Modern. [Google Scholar]
- 86.Zeuner F.E. Harper & Row; 1963. A History of Domesticated Animals. [Google Scholar]
- 87.Nowell K., Jackson P. International Union for Conservation of Nature and Natural Resources; 1996. Status Survey and Conservation Action Plan, Wild Cats. [Google Scholar]
- 88.Vella C., Robinson R. Oxford University Press; 1999. Robinson's Genetics for Cat Breeders and Veterinarians. [Google Scholar]
- 89.Menotti-Raymond M. Patterns of molecular genetic variation among cat breeds. Genomics. 2008;91:1–11. doi: 10.1016/j.ygeno.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 90.Lipinski M.J. The ascent of cat breeds: genetic evaluations of breeds and worldwide random-bred populations. Genomics. 2008;91:12–21. doi: 10.1016/j.ygeno.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Menotti-Raymond M.A. An STR forensic typing system for genetic individualization of domestic cat (Felis catus) samples. J. Forensic Sci. 2005;50:1061–1070. [PubMed] [Google Scholar]
- 92.Pritchard J.K. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mullikin J.C., Ning Z. The phusion assembler. Genome Res. 2003;13:81–90. doi: 10.1101/gr.731003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Batzoglou S. ARACHNE: a whole-genome shotgun assembler. Genome Res. 2002;12:177–189. doi: 10.1101/gr.208902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jaffe D.B. Whole-genome sequence assembly for Mamm. Genomes: Arachne 2. Genome Res. 2003;13:91–96. doi: 10.1101/gr.828403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pontius J.U., O’Brien S.J. Genome Annotation Resource Fields–GARFIELD: a genome browser for Felis catus. J. Hered. 2007;98:386–389. doi: 10.1093/jhered/esm055. [DOI] [PubMed] [Google Scholar]
- 97.Payne B.R., Peters A. Academic Press; 2002. The Cat Primary Visual Cortex. [Google Scholar]
- 98.Kind P.C. Correlated binocular activity guides recovery from monocular deprivation. Nature. 2002;416:430–433. doi: 10.1038/416430a. [DOI] [PubMed] [Google Scholar]
- 99.Anderson J.S. The contribution of noise to contrast invariance of orientation tuning in cat visual cortex. Science. 2000;290:1968–1972. doi: 10.1126/science.290.5498.1968. [DOI] [PubMed] [Google Scholar]
- 100.Henson M.S., O’Brien T.D. Feline models of type 2 diabetes mellitus. ILAR J. 2006;47:234–242. doi: 10.1093/ilar.47.3.234. [DOI] [PubMed] [Google Scholar]
- 101.Rah H. Early-onset, autosomal recessive, progressive retinal atrophy in Persian cats. Invest. Ophthalmol. Vis. Sci. 2005;46:1742–1747. doi: 10.1167/iovs.04-1019. [DOI] [PubMed] [Google Scholar]
- 102.Ryugo D.K. Restoration of auditory nerve synapses in cats by cochlear implants. Science. 2005;310:1490–1492. doi: 10.1126/science.1119419. [DOI] [PubMed] [Google Scholar]
- 103.Menotti-Raymond M. Pet cat hair implicates murder suspect. Nature. 1997;386:774. doi: 10.1038/386774a0. [DOI] [PubMed] [Google Scholar]
- 104.Menotti-Raymond M. Genetic individualization of domestic cats using feline STR loci for forensic applications. J. Forensic Sci. 1997;42:1039–1051. [PubMed] [Google Scholar]


