Graphical abstract
Keywords: Wastewater, Biosolids, Biofuel, Hydrothermal liquefaction
Highlights
-
•
Wastewater treatment plants can become resource recovery centers.
-
•
Metals and nutrients can be recovered from sludges.
-
•
Sludge pathogens are inactivated and organic pollutants are destroyed.
-
•
Thermo-chemical technologies and liquid solvents evaluated for sludge application.
-
•
Hydrothermal liquefaction reduces sludge mass by 50% and produces bio-oil.
Abstract
Limitations on current wastewater treatment plant (WWTP) biological processes and solids disposal options present opportunities to implement novel technologies that convert WWTPs into resource recovery facilities. This review considered replacing or augmenting extensive dewatering, anaerobic digestion, and off-site disposal with new thermo-chemical and liquid extraction processes. These technologies may better recover energy and metals while inactivating pathogens and destroying organic pollutants. Because limited direct comparisons between different sludge types exist in the literature for hydrothermal liquefaction, this study augments the findings with experimental data. These experiments demonstrated 50% reduction in sludge mass, with 30% of liquefaction products converted to bio-oil and most metals sequestered within a small mass of solid bio-char residue. Finally, each technology’s contribution to the three sustainability pillars is investigated. Although limiting hazardous materials reintroduction to the environment may increase economic cost of sludge treatment, it is balanced by cleaner environment and valuable resource benefits for society.
1. Introduction – Sewage sludge issues
Increasingly restrictive regulations for wastewater treatment prior to discharge coupled with the rising costs for sludge disposal pose two interrelated problems: (1) sludges loaded with contaminants may no longer be disposed in traditional ways, and (2) sludge treatment technologies, which have slowly evolved over the past 50 years to adapt to the changing regulations, have increased infrastructure and cost of treatment exponentially. Rather than continuing slow evolution of tweaking of sludge treatment, the authors believe there are existing technologies that provide a new approach to sludge treatment.
The history of sewage sludge treatment and disposal can be viewed as a game of playing catch-up with the regulations. In 1988, Congress passed the Ocean Dumping Ban Act, essentially mandating that all sewage sludge disposal be land-based. The Clean Water Act was amended in 1993 with Code of Federal Regulations Title 40 Part 503 to regulate the use and disposal of treated sewage sludges (U.S. EPA, 1994). Land application of Class B sludges (i.e., treated sludges that still contain pathogens) on agricultural fields was encouraged under the idea that organics in the sludges would promote soil stabilization, enrich soils, and enhance crop growth. However, land application, by which 55% of sludge is disposed, continues to face setbacks ranging from public tolerance for odor to public health and environmental concerns stemming from presence of non-regulated metals and contaminants of emerging concern (CECs). Alternatives to land application include landfill disposal (30% of sludge) and incineration (15% of sludge) (Peccia and Westerhoff, 2015). Incineration poses human toxicity concerns associated with releasing heavy metals and particulates into the air (Hong et al., 2009). Thus, for treated sludges with high metal or CEC content, landfilling is increasingly the only remaining disposal method. These sludges are subject to municipal and hazardous waste landfill regulations set forth by the Resource Conservation and Recovery Act (RCRA). (40 CFR 261, 2011) Landfilling faces several drawbacks – specifically, space is limited due to the growing strain of urbanization, leading to increased cost of hauling to distant locations. Additionally, there is public distaste towards landfill odor, and environmental concerns regarding the release of greenhouse gases and the potential for groundwater contamination from the leachate. (Giusti, 2009).
Wastewater treatment technology has adapted to increasing regulations and concerns regarding the effects of disposal on aquatic life and water reuse applications. Activated sludge technology to reduce biological oxygen demand was first implemented in the mid-20th century. In the 1960s, chemical phosphorous precipitation was added, followed by biological treatment trains with nitrification, denitrification, and enhanced biological phosphorous removal (EBPR). These modifications produced higher quality effluents and increased viability for reuse; however, the biological processes also produce large volumes of low density (98% water) biological solids, chemicals, and inert particles associated with the lipid-rich bio-cellular materials. This ultimately results in a longer solids retention time (SRT) for sludge stabilization during anaerobic digestion, up to 30 days. These very long SRTs result in large reactor volumes with high capital costs and consequently only become economically viable for larger utilities (i.e., WWTPs that serve populations on the order of >100,000) (Peccia and Westerhoff, 2015).
Sewage solids treatments rely on sludge stabilization (e.g., alkaline lime stabilization, anaerobic digestion, aerobic digestion, and composting) to remove pathogens, pollutants, and odor. Anaerobic digestion has been used since the early 1900s and is one of the most popular sludge stabilization technologies. In the absence of oxygen, organic compounds and cells break down to produce biogas (65–70 vol% of methane, CH4, 30–35 vol% CO2). A 56–65.5% reduction in volatile suspended solids (VSS) occurs after a SRT between 15 and 30 days (depending on the operating temperature of the reactor). Aerobic digestion (i.e., in the presence of oxygen) can also stabilize sludges, but it does not allow for energy recovery, and the resulting sludge has poor dewaterability. Stabilized sludges are only 5–10 wt% dry solids and must be mechanically dewatered to 25–35 wt% dry solids using centrifuges and belt presses prior to disposal in order to reduce volume and mass for transportation (Appels et al., 2008, Metcalf and Eddy, 2013).
While biosolids may amend soil and provide plants with beneficial nutrients, they are only applied on <1% of the total agricultural land in the United States (U.S. EPA, 2015). In Germany, only 2.6% of organic fertilizer is composed of sewage sludge (Kruger et al., 2014). Soils are approaching their cumulative heavy metal loading rates (Table 1 ), and the hauling distance required for land application is increasing. The authors argue that land application is merely a preferred sludge disposal alternative when compared to landfill or incineration, and there would be minimal agricultural loss if the total volume of solids produced was reduced and/or a separate, more beneficial use was found for them.
Table 1.
Metal concentrations and loading rates for land-applied and landfilled sewage sludges. Ceiling concentrations are the maximum allowable concentrations of metals in land-applied sludges. Cumulative pollutant loading rates are the maximum allowable concentrations of metals applied over the lifetime of a sludge disposal site. Regulatory levels are maximum concentrations of contaminants in municipal landfills obtained by the Toxicity Characteristic Leaching Procedure.
| Pollutant | Ceiling concentration (mg/kg)a | Cumulative pollutant loading rate (kg/hectare, dry weight)a | Regulatory level (mg/L)b |
|---|---|---|---|
| Arsenic | 75 | 41 | 5 |
| Barium | 100 | ||
| Cadmium | 85 | 39 | 1 |
| Chromium | 3000 | 3000 | 5 |
| Copper | 4300 | 1500 | |
| Lead | 840 | 300 | 5 |
| Mercury | 57 | 17 | 0.2 |
| Molybdenum | 75 | – | |
| Nickel | 420 | 420 | |
| Selenium | 100 | 100 | 1 |
| Silver | 5 | ||
| Zinc | 7500 | 2800 |
It is worthwhile to consider shifting the perspective of WWTPs from being waste treatment and disposal facilities to resource recovery facilities. Human-generated wastes consist of most everything used to nourish health and livelihood – metals, nutrients, organics, and more. Rather than disposing these items and investing time, money, and labor to produce and mine additional resources, society will benefit by seeking more sustainable practices that reuse and recycle these resources. The contents of sludges is a matter of perspective – metals, nutrients, and organics can be viewed as hindering waste disposal or as opportunities for resource recovery, recycling, and sustainability. The goal of this paper is to identify and evaluate alternate sewage sludge treatment trains to enable energy and metal recovery. Thermo-chemical processes and liquid solvents are explored for their potential to convert biomass into reusable forms while simultaneously destroying CECs without releasing harmful pollutants. Specifically, hydrothermal liquefaction (HTL), a thermal process adapted from the algae biofuel industry, is unique in that it can directly convert liquid biomass to energy in the form of bio-oil, thereby avoiding energy and costs associated with sludge dewatering. The technology occupies a minimal land footprint and operates 100 times faster than anaerobic digestion. The dry mass of the HTL product is half of the initial reactants, which will substantially reduce costs for hauling and disposing biosolids, and metals and nutrients are concentrated within this small remaining mass. As such, the remainder of this review identifies techniques adapted from the mining industry to extract these resources (metals and nutrients) for financial gain.
2. Sludge composition and recovery potential
2.1. Metals
40 CFR Part 503 established ceiling concentrations for 10 metals in land-applied biosolids (As, Cd, Cu, Cr, Pb, Hg, Mo, Ni, Se, Zn) (U.S. EPA, 1994). However, Cr was removed from the list in 1995 because: (1) Cr appeared primarily in the less toxic, trivalent form rather than the toxic hexavalent form, and (2) field data did not show Cr toxicity to plants at the cumulative loading concentrations (60 Federal Register 206, 1995). RCRA established limits on 8 metals in landfilled solid waste using the Toxicity Characteristic Leaching Potential (As, Ba, Cd, Cr, Pb, Hg, Se, Ag) (Table 1) (40 CFR 261, 2011). There is a wealth of data for concentrations of these metals in sewage sludges in North America, Europe, and Asia (Pathak et al., 2009). Less is known about non-regulated metals such as post-transition and precious metals (Au, Pt, Pd, Te, Bi, Sb, In) used in electronics and platinum group metals (Pt, Pd, Rh) used in chemical, petroleum, and glass industries, jewelry, dentistry, and car catalysts (Chancerel et al., 2009, Saurat and Bringezu, 2009). Recent breakthroughs in nanotechnology have led to metallic nanoparticles incorporated in foods (Ag, TiO2, Si, Pt), textiles (Ag), and medicine (Au, Si) (Chaudhry and Castle, 2011, Gao and Cranston, 2008, Marchesan and Prato, 2013). WWTPs remove these nanoparticles effectively from the liquid effluent and accumulate them within sludges (Westerhoff et al., 2013). Engineered nanomaterials may be detrimental to the environment if disposed on land, therefore identifying methods to limit their entrance into the environment is desirable (Gottschalk et al., 2013).
The widespread use and emerging concern surrounding non-regulated metals led to the EPA surveying 28 metals (19 new metals – Al, Sb, Ba, Be, B, Ca, Cr, Co, Fe, Mg, Mn, P, Ag, Na, Tl, Sn, Ti, V, Y – in addition to 9 regulated by Part 503) in the 2006–2007 Targeted National Sewage Sludge Survey, which was intended to inform exposure and hazard assessments (U.S. EPA, 2009). In a separate survey, sludges across the United States were analyzed for 58 regulated and non-regulated elements and were found to have heavy metals (Al, Ba, Cr, Fe, Mn, Sn, Ti) and precious metals (Ag, Au, Pd, Pt, Rh, Ru) in addition to Part 503 regulated metals. The metals all had enrichment factors above unity, indicating likely anthropogenic sources rather than dust or soil (Westerhoff et al., 2015). Internationally, metal content of sludge ash post-incineration has also been evaluated. In Germany, Si, Ca, Fe, and Al were found in abundance, followed by Zn, Mn, Ba, Cu, Sr, Cr, Pb, and Zr (Kruger et al., 2014). Rare earth element concentrations were calculated for sewage sludge ash in Japan and found to be enriched with Sm, Eu, Tb, Sc, and Gd and slightly enriched by La and Ce (Zhang et al., 2001). Table 2 compares metal concentrations in sewage sludge from the United States, India, and South Africa with sludge ash from Germany and Japan. For nearly all reported metals, incineration increases the metals concentration by up to one order of magnitude. This is likely because incineration reduces the dry mass of solids by more than 65% (Williford et al., 2007).
Table 2.
Concentrations of elements in sewage sludges globally (mg/kg).
| Elements | USA #1a (biosolids) | USA #2b (biosolids) | Germanyc (ash) | Japand (ash) | Indiae(sludge) | South Africaf (sludge) |
|---|---|---|---|---|---|---|
| Li | 23.7 | |||||
| Be | 0.9 | |||||
| Na | 2937 | 952 | 6000 | 17.6 | ||
| Mg | 6041.5 | 4380 | 13,000 | 24.8 | ||
| Al | 18,571 | 11,200 | 48,000 | 117 | 7962 | |
| Si | 121,000 | |||||
| P | 20,966 | 18,750 | 79,000 | 375 | ||
| S | 10,000 | 298 | ||||
| Cl | 95 | |||||
| K | 5104 | 9000 | 16.2 | 911 | ||
| Ca | 32,656 | 27,550 | 105,000 | 100 | 81,166 | |
| Sc | 1.7 | 4.2 | 19.2 | |||
| Ti | 827.5 | 87 | 4000 | 401 | 770 | |
| V | 33.5 | 14 | 54 | 155 | 78 | |
| Cr2 | 88 | 35 | 160 | 226 | 325 | 35.07–134.48 |
| Mn | 9267.5 | 433 | 1307 | 2 | 4035 | |
| Fe | 19,989.5 | 16,300 | 95,000 | 39.8 | 267,975 | |
| Co | 6.6 | 4.6 | 20.7 | 90.6 | ||
| Ni1 | 36 | 24 | 74.8 | 213 | 15 | 18.89–51.43 |
| Cu1 | 440.5 | 468 | 785 | 2838 | 57 | 80.80–626.00 |
| Zn1 | 740 | 803 | 2534 | 3276 | 211 | 303.83–1732 |
| Ga | 14.5 | 11.6 | 179 | |||
| As1,2 | 7.7 | 5.1 | 13.6 | 27 | ||
| Se1,2 | 2 | |||||
| Rb | 12 | 28.9 | ||||
| Sr | 270.5 | 493 | 434 | 86 | ||
| Y | 5.7 | 3.8 | 9.2 | 16.5 | ||
| Zr | 106 | 66.5 | ||||
| Nb | 6.1 | 11 | 11.7 | |||
| Mo1 | 12.5 | 11.2 | 20 | 19.2 | ||
| Ru | 0.2 | |||||
| Pd | 0.3 | 0.109 | ||||
| Ag2 | 35 | 14 | 9.1 | 13.8 | 0.22–21.93 | |
| Cd1,2 | 4.2 | 1.7 | 2.7 | 6.6 | 17.96–171.87 | |
| Sn | 42 | 37 | 76.6 | 552 | ||
| Sb | 3.3 | 1.6 | 12.4 | 54.8 | ||
| Cs | 0.6 | 1.5 | ||||
| Ba2 | 431 | 431 | 1057 | 3295 | 515 | |
| La | 10.8 | 25.5 | 19.3 | |||
| Ce | 18.5 | 42.8 | 35.4 | |||
| Pr | 1.7 | 4.2 | 3.58 | |||
| Nd | 6.8 | 15.6 | 13.7 | |||
| Sm | 1.3 | 2.9 | 10.7 | |||
| Eu | 0.3 | 0.6 | 1.65 | |||
| Gd | 1.4 | 2.8 | 4.06 | |||
| Tb | 0.1 | 0.4 | 0.8 | |||
| Dy | 0.9 | 1.9 | 2.12 | |||
| Ho | 0.2 | 0.4 | 0.43 | |||
| Er | 0.5 | 1 | 1.08 | |||
| Tm | 0.1 | 0.2 | 0.17 | |||
| Yb | 0.5 | 1 | 1.13 | |||
| Lu | 0.1 | 0.2 | 0.19 | |||
| Hf | 0.7 | 3.2 | 3.8 | |||
| Ta | 1.2 | 3.4 | ||||
| W | 1.2 | 41.1 | 11.8 | |||
| Re | 0 | |||||
| Ir | 0 | |||||
| Pt | 0.1 | 0.108 | ||||
| Au | 0.6 | 0.9 | ||||
| Tl | 0.1 | 0.9 | ||||
| Pb1,2 | 71.5 | 49 | 117 | 547 | 171 | 17.96–171.87 |
| Bi | 2.8 | |||||
| Th | 1.5 | 0.1 | 4.9 | 4.8 | ||
| U | 2 | 4.9 | 1.9 |
Median of values reported in Westerhoff et al. (2015).
U.S. EPA Targeted National Sewage Sludge Survey 50th percentile reported in Westerhoff et al. (2015).
Regulated under Title 40 CFR Part 503 for land application.
Regulated under Title 40 CFR Part 261 for toxicity potential for landfill.
Opportunities exist to recover metals from sludges because of the growing public and environmental threats associated with current methods of land disposal, landfill disposal, and incineration of sewage sludges. The metals recovery processes for sludges could be modeled after current recycling programs for glass, paper, and aluminum. In 2013, 1.95 million tons of Al were mined from ore in the United States, and 1.44 million tons were recovered from scrap (Lee Bray, 2014). Using values presented in Table 2, 336,000 tons of Al could be recovered globally from sludge, and 150,000 tons could be recovered within the United States (4.4% of total Al produced). For gold, a high commodity metal, mining from ore produced 2.77 million tons (George, 2014). In comparison, extraction from global sludges can recover 18 tons of Au. The mass is <0.00065% of total Au mined, but it is valued at $20.5/ton of sludge (December 2015 price for pure gold $34,277.66/kg). Resource recovery calculations assume 1.2 billion people living in developed countries generate 30 million tons of sludge/year; the United States alone generates 8 million tons of sludge/year (Kruger et al., 2014, National Research Council, 2002).
2.2. Nutrients
2.2.1. Phosphorous
An estimated 16% of the total mined phosphorous is digested by humans and relayed into the waste stream. Wastewater phosphorous concentration can be between 4 and 16 mg/L (Rittmann et al., 2011). Half of this phosphorous integrates into the cellular biomass of sludges, while the other half discharges into waterways from the WWTPs. Phosphorous concentrations in wastewater effluent disposed to surface waters is limited to less than 2 mg/L across the United States to deter eutrophication. Common treatment processes to achieve these limits are chemical precipitation with iron and biological phosphorous removal. Both approaches accumulate phosphorous in sludges. Phosphorous bound to iron oxides is generally not bio-available to plants and is difficult to recover, but biologically sequestered phosphorous remains bio-available and may be easier to recover upon cellular oxidation. EBPR processes, which stimulate growth of bacteria likely to uptake phosphorous, currently operate at full scale and enhance phosphorous removal compared against conventional activated sludge treatment processes. EBPR increases sludge phosphorous concentrations from 0.02 to 0.06–0.15 mg/mg VSS. This increased sludge phosphorus concentration coupled with the potential for land-applied treated sewage sludges to be subject to erosion and runoff can amplify phosphorus exposure in the environment (Rittmann et al., 2011, Wentzel et al., 2008).
2.2.2. Nitrogen
Nitrogen enters the waste stream through proteins metabolized within the human body. In wastewater, nitrogen can be present as ammonia (40%), organic nitrogen (60%), or nitrate nitrogen (<1%). The influent total nitrogen concentration varies between 20 and 85 mg/L. Because ammonia can be toxic to aquatic life due to its oxygen consumption, nitrogen can be a nutrient to algae and cause eutrophication, and nitrate can cause blue-baby syndrome within infants, wastewater treatment processes must reduce concentration of nitrogenous compounds within the liquid waste effluent. Biological nitrification converts ammonia to nitrate, and the nitrifying bacteria settle through sedimentation, increasing the mass of sludge produced. Ultimately, 2.4–6.7% of the activated sludge dry mass consists of nitrogen (i.e., 24–67 g N/kg dry solids), and the remaining N is denitrified to become N2 gas (Sedlak, 1991, Shammas and Wang, 2007).
2.2.3. Potassium
Potassium is present in sewage sludges between 0.5% and 0.7% K2O/weight of dry solids (Shammas and Wang, 2007). Wastewater effluent leaving a WWTP has potassium concentrations between 10 and 30 mg/L. Potassium applied to soil can increase the reserve of potassium bound to minerals within the soil and can be taken up by plants beneficially. There is, however, a risk of potassium leaching if applied excessively, but as there are no known adverse health or environmental risks, potassium regulatory limits generally do not exist (Arienzo et al., 2009).
2.2.4. Nutrient recovery potential
The 2016 forecast for global fertilizer nutrient demand is 45 million tons of phosphate (as P2O5), 116 million tons of nitrogen (as N), and 33 million tons of potassium (as K2O). With 30 million tons of sludge generated globally annually, complete nutrient recovery and reuse from sludge can amount to 5% of phosphorus demand, 1.7% of nitrogen demand, and 0.64% of potassium demand (FAO, 2012, Shammas and Wang, 2007).
2.3. Energy potential
The rising cost of energy and environmental pollution created by its production confirm the need for green, sustainable energy sources. Energy can be chemically or thermally bound with sludge. Anaerobically digested sewage sludge has a high storage of chemical energy, with carbon content of 67%, higher heating value of 32 MJ/kg (Vardon et al., 2011). Temperature changes during sludge treatment create thermal energy, which can be collected as heat and reused within the treatment system. Anaerobic digestion produces 0.75–1.12 m3 of gas (i.e., CH4 and CO2) per kg VSS destroyed, or 0.03–0.04 m3/person/day. However, this energy produced by anaerobic treatment processes does not balance the total energy used by WWTPs (140–1400 KWh/person/day or 13–130 m3/person/day) (Energy Star, 2015). Moreover, a 10–30 day SRT does not allow anaerobic digestion to be an option at treatment plants with capacity less than 10 million gallons/day (Metcalf and Eddy, 2013).
2.4. Organic pollutants and pathogens
The consolidation and concentration of pharmaceuticals, personal care products, and other CECs within wastewater solids is a significant cause for concern. Antibiotics are particularly scrutinized as they can increase the risk of antibiotic resistance through genetic mutation or gene transfer. Antibiotics and their metabolites enter sewage through feces, urine, or direct medication disposal and will ultimately enter the environment if not removed during sewage treatment. In particular, pharmaceuticals classified as fluoroquinolones, tetracyclines, and sulfonamides can be taken up by flora (McClellan and Halden, 2010). A Swedish study (Lindberg et al., 2005) showed two fluoroquinolones, ciprofloxacin and norfloxacin, were present in all 10 samples collected from five different treatment plants at concentrations between 0.1 and 4.8 mg/kg (dry weight). The adsorption of these antibiotics to the sludge during treatment was 87% (Lindberg et al., 2005). Average concentrations for these fluoroquinolones in sludges in the United States were 6.8 and 0.42 mg/kg for ciprofloxacin and norfloxacin, respectively (Golet et al., 2002, McClellan and Halden, 2010). Other CECs found in sludges to date are brominated flame retardants (BFRs), perfluoroalkyl substances (PFAS), and alkylphenol ethoxylates (APEO). BFRs can persist in soil for at least 3 years, PFAS are resistant to biodegradation, and APEO metabolites have been shown to mimic hormones and can induce endocrine disruption within organisms exposed to the contaminant. These CECs are dangerous when released into the environment as their inability to completely decompose can lead to spreading within soil, air, and water and can bioaccumulate in microbes and animals (Venkatesan and Halden, 2014, Venkatesan and Halden, 2013a, Venkatesan and Halden, 2013b).
A wide variety of pathogens from human waste streams deposit within sewage sludges. Viruses are particularly challenging as their wide genotypic variety creates a plethora of shapes, sizes, infection potential, and fate and transport in the environment and human body. Recent metagenomic data from 12 sewage sludges sampled at 5 WWTPs across the United States identified 43 different forms of human viruses. In particular, the DNA viruses Adenovirus, Herpesvirus, and Papillomavirus were found in more than 90% of the samples, and the RNA viruses Coronavirus, Klassevirus, and Rotavirus were found in more than 80% of samples (Bibby and Peccia, 2013). Bacteria found in class B sludges include fecal coliforms, Escherichia coli, Enterococcus spp., and Clostridium spp. When land-applying these sludges, the resulting aerosols subject humans to airborne exposure of pathogens. The inhalation risks for disaggregated and aggregated norovirus are 10−1 and 10−3 when standing 30 m away from the land application site (Viau et al., 2011).
3. Recovery treatment processes
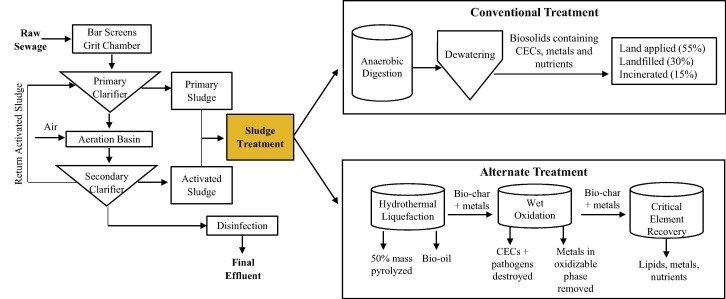
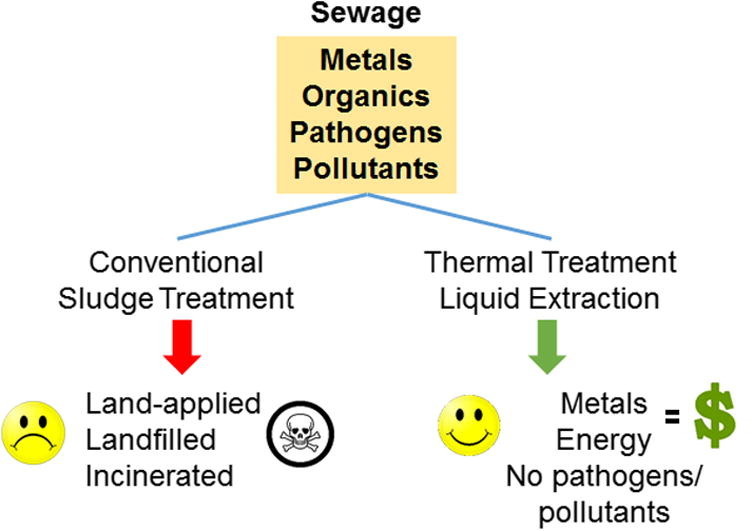
With the evolution of sludge treatment technologies constantly adapting to changing regulations, treatment cost has increased while efficiency has decreased. There is an immediate need for new technologies that will convert wastewater treatment plants to resource recovery centers. Guided by the three pillars of sustainability (economy, environment, and society), resource recovery creates economic opportunities, limits reintroduction of hazardous metals and CECs into the environment when solids are disposed, and potentially reduces public concerns related to odors emanating from sludges or “not in my backyard” apprehension for siting incineration facilities. Instead, metals will be recycled for reuse and economic gain and CECs will be destroyed. Resource recovery technologies can be inspired by and adapted from fields outside of conventional treatment such as the algal biofuel, petroleum, and mining industries. This paper proposes several existing sludge treatment alternatives within the categories of thermal processes and liquid solvent application prior to precipitation or adsorption/extraction of the valuable products. Additionally, results are presented for bench-scale evaluations of HTL, a thermal treatment process. Table 3 summarizes the thermal and liquid solvent processes discussed in this review for sludge treatment and resource recovery. The advantages and disadvantages are outlined in terms of energy use, cost, products, and feasibility for sludge application. Fig. 1 shows a likely alternate treatment train to conventional sludge treatment.
Table 3.
Comparison summary of advantages, disadvantages, and costs of various thermal and liquid solvent processes.
| References | |||
|---|---|---|---|
| Conventional sludge stabilization process | |||
| Anaerobic digestion of RAS | Description |
|
Appels et al., 2008, Metcalf and Eddy, 2013 |
| Advantages |
|
||
| Disadvantages |
|
||
| Cost |
|
||
| Thermal processes to lyse cells and release metals | |||
| Hydrothermal liquefaction of RAS/ADS | Description |
|
Jones et al., 2014, Pham et al., 2013, Vardon et al., 2011, Zhang et al., 2010 |
| Advantages |
|
||
| Disadvantages |
|
||
| Cost |
|
||
| Pyrolysis of RAS/ADS | Description |
|
Bridle and Pritchard, 2004, Kim and Parker, 2008 |
| Advantages |
|
||
| Disadvantages |
|
||
| Cost |
|
||
| Combustion/incineration of RAS/ADS | Description |
|
Zhang et al. (2010) |
| Advantages |
|
||
| Disadvantages |
|
||
| Cost |
|
||
| Gasification of RAS/ADS | Description |
|
Worley and Yale, 2012, Zhang et al., 2010 |
| Advantages |
|
||
| Disadvantages |
|
||
| Cost |
|
||
| Oxidation of RAS/ADS or HTL product | Description |
|
Debellefontaine and Foussard, 2000, Hii et al., 2014 |
| Advantages |
|
||
| Disadvantages |
|
||
| Cost |
|
||
| Liquid extraction of critical elements | |||
| Conventional lipid extraction from RAS | Description |
|
Boocock et al., 1992, Dufreche et al., 2007 |
| Advantages |
|
||
| Disadvantages |
|
||
| Cost |
|
||
| Supercritical carbon dioxide extraction for lipids and/or metals | Description |
|
Dufreche et al., 2007, Wang and Wai, 2005 |
| Advantages |
|
||
| Disadvantages |
|
||
| Cost |
|
||
| Acids for metals extraction | Description |
|
Darnall et al., 1986, Yuan et al., 2011 |
| Advantages |
|
||
| Disadvantages |
|
||
| Cost |
|
||
| Thiourea for metal extraction | Description |
|
Marsden and House, 2006, Zuo and Muhammed, 1990 |
| Advantages |
|
||
| Disadvantages |
|
||
| Cost |
|
||
| Thiosulfate for metal extraction | Description |
|
Marsden and House (2006) |
| Advantages |
|
||
| Disadvantages |
|
||
| Cost |
|
||
Fig. 1.
Alternate treatment schematic outlined within this review compared to conventional treatment.
3.1. Thermal processes to lyse cells and release metals
Thermo-chemical processes such as liquefaction, pyrolysis, combustion, and gasification transform biomass organic and inorganic compounds into energy. These processes are more time efficient and have higher conversion efficiencies than biological processes such as anaerobic digestion (Zhang et al., 2010). Thermal processes are evaluated and distinguished by their varying reaction conditions, energy input, use of dry or wet biomass, and value of product.
3.1.1. Hydrothermal liquefaction
HTL is an emerging technology used to extract biofuel from algae. While several thermal technologies (e.g., pyrolysis, combustion, gasification) require dry biomass to maximize energy recovery potential, HTL operates with 5–30% solids. The avoided dewatering and drying costs significantly reduce energy use relative to pyrolysis and other conventional high temperature processes. HTL is all-encompassing, simultaneously achieving multiple sludge stabilization goals by increasing dewaterability, decreasing mass of the reaction product, and removing harmful pathogens and pollutants. Therefore, it is evaluated in depth for its potential to follow and even replace anaerobic digestion.
In HTL, liquid biomass reacts at a high temperature (250–350 °C) and pressure (10–15 MPa), causing cells to lyse and proteins, lipids, and carbohydrates to break down into reactive molecules in the solvent (e.g., water, acetone, ethanol) and to repolymerize into oily compounds (Zhang et al., 2010). The four products of HTL are bio-crude oil, a solid residue termed bio-char, an aqueous component containing water soluble compounds, and CO2 gas. The biochemical composition of the biomass influences bio-oil yield and follows the trend lipids > proteins > carbohydrates (Biller and Ross, 2011). HTL has been used to deactivate antibiotic resistant genes and remove bioactive compounds such as estrone, florfenicol, and ceftiofur in Spirulina algae and swine manure (Pham et al., 2013).
HTL can be adapted for sewage sludges, which is a wet biomass medium similar to algae. Sludge that has been anaerobically digested and dewatered has 20–30% solids and is thus suitable for direct liquefaction. Return activated sludge (∼2% solids) should be dried to achieve at least 5–30% solids prior to HTL. There are few reports of sewage sludge liquefaction to produce oil, and most have used a catalyst (NaOH) and/or liquefaction solvent (acetone, ethanol) to propel the reaction (Leng et al., 2014, Yuan et al., 2011). Vardon et al. showed bio-oil yield of 9.4% from anaerobic sludge without a catalyst and using water as the solvent, while Leng et al. showed 45% bio-oil yield with acetone and 40% with ethanol from dewatered sewage sludge (Leng et al., 2014, Vardon et al., 2011).
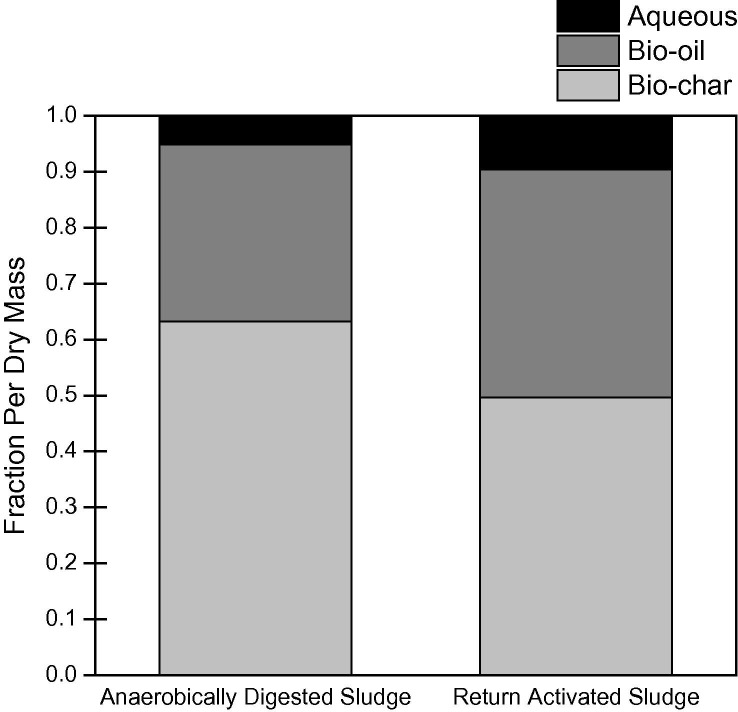
Herein, the authors present data from their lab for application of HTL without a catalyst or solvent to both anaerobically digested sludge (ADS) and return activated sludge (RAS) at 20% solids. The liquefaction reaction was run for 30 min at 300 °C and 10 MPa (see SI for details). HTL reduced the solids mass by 47% and 55% for ADS and RAS, respectively. In the remaining liquefaction product, 64% of ADS and 50% of RAS dry mass was in the bio-char, 32% and 41% in bio-oil, and the remainder in the water soluble aqueous byproduct (Fig. 2 ). HTL efficiency can be compared to anaerobic digestion by calculating the loading rate. With a conservative estimate of 1 gram of solid reacting in a 300 mL vessel for 30 min, the equivalent loading rate is 160 kg/m3/day, or 100 times more efficient than the anaerobic digestion solids loading rate of 1.6–4.8 kg VSS/m3/day (Metcalf and Eddy, 2013). This disparity and the relative similarity in HTL results between ADS and RAS shows HTL is viable to follow secondary treatment directly, thereby removing anaerobic digestion.
Fig. 2.
Phase composition of 20 g sewage sludge (20% solids) after hydrothermal liquefaction (300 °C, 10 MPa, 30 min). The mass reduced by pyrolyzation was 47% and 55% for anaerobically digested sludge (ADS) and return activated sludge, respectively. Data for ADS based on the average of 4 replicates with 13% error.
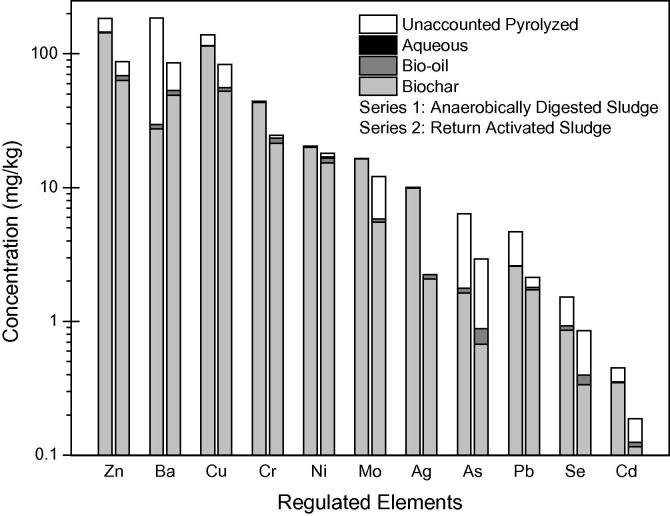
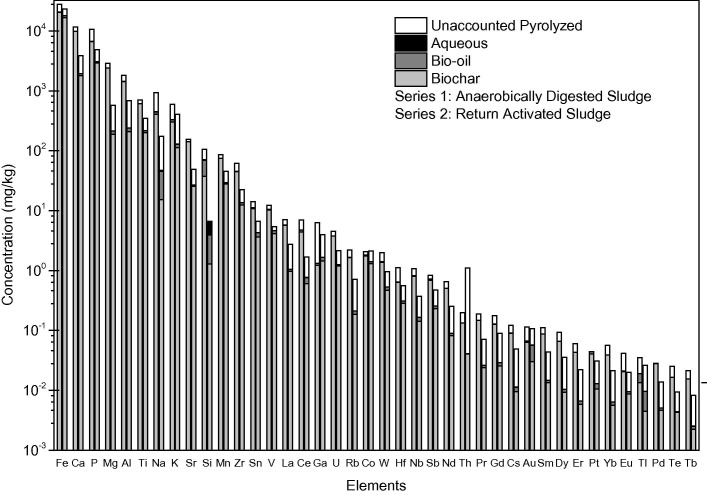
To study the effect of bacteria and organics transformation on metals migration within the liquefaction product, each sludge phase was microwave digested with 16 M nitric acid, 12 M hydrochloric acid, and 29 M hydrofluoric acid and analyzed for 58 elements using inductively coupled plasma mass spectrometry (ICP-MS) (see SI for details). Approximately 60–80% of each element concentrated within the bio-char fraction, the bio-oil fraction contained less than 7% of each metal, and the remaining percentage was unaccounted for within the pyrolyzed mass. Elsewhere, similar results were seen for Cr, Ni, Cu, Zn, Cd, and Pb undergoing liquefaction with acetone or ethanol (Leng et al., 2015, Leng et al., 2014). Fig. 3 shows concentrations of RCRA and 40 CFR Part 503 regulated metals from this work. Fig. 4 shows concentrations of all other metals present at concentrations higher than two times the detection limit of the ICP-MS. The authors hypothesize that significant volatilization is seen for arsenic due to biological formation of methylated arsenic species from organoarsenicals (arsenic ions reacted with carbon). The boiling points of these species vary between 128 and 215 °C, indicating that volatilization is possible at 300 °C (Smithsonian Institution, 1873). In separate work, phosphorous phase partitioning in algae was similar to this study’s sludge experimental data; 80% of P in algae went to the aqueous phase after HTL at 250 °C. Additionally, 75 ± 9% of nitrogen distributed to the aqueous phase in the algae study (Valdez et al., 2012).
Fig. 3.
Concentration of elements in each phase of sewage sludge after hydrothermal liquefaction of 20 g sludges (20% solids). Elements are regulated by 40 CFR Part 503 and 40 CFR Part 261 (Resource Conservation and Recovery Act). Standard deviation for experimental replicates <20% of mean.
Fig. 4.
Concentration of selected elements in each phase of sewage sludge after hydrothermal liquefaction of 20 g sludges (20% solids). Standard deviation for experimental replicates < 20%.
Extracting individual metals from the bio-char may depend on the forms they are present in after stabilization and/or thermal processing. Several studies have utilized the European Commission’s Community Bureau of Reference (BCR) extraction procedure to understand the chemical forms of metals in a complex matrix. Specifically, Yuan et al. determined that after liquefaction with acetone as the solvent and no catalyst, 35% of Cr, 50% of Zn and Ni, 60% of Cd, and 70% of Cu are in the “oxidizable phase” (i.e., bound to organic matter) while the remaining percentage is in the “residual phase” (i.e., not acid soluble, reducible, or oxidizable) (Yuan et al., 2011).
While only a small metals concentration enters the oil phase, it could cause concern with regards to oil purity and toxicity. However, when Leng et al. extracted metals from sewage sludges with a modified BCR method (0.1 M acetic acid, 0.1 M hydroxylammonium chloride, 30% hydrogen peroxide, and 1 M ammonium acetate) prior to liquefaction, bio-oil yield decreased by 20% and the oil’s heating value also decreased (Leng et al., 2015). Nevertheless, oil demetallization technologies can be adapted from the petroleum industry that will conserve oil volume and its properties (Ali and Abbas, 2006).
Overall, HTL is 100× more efficient than anaerobic digestion with regards to loading rate, reduces biomass volume by half, produces energy in the form of bio-oil, and concentrates metals into bio-char fraction. To recover the metals for beneficial use, the metal-laden bio-char fraction must undergo further processing.
3.1.2. Incineration/combustion
Several European and Asian countries incinerate sludges to reduce their final volume by up to 90%. Heating dewatered or dry sludge to higher than 760 °C destroys organic pollutants and pathogens and converts the sludge to carbon dioxide, water, and ash. The potential release of toxic exhaust gases to the environment can be a significant problem; however, installing gas scrubbers at incineration plants has helped reduce emission of these byproducts. In the process of burning, metals concentrate and stabilize within the sludge ash, or slag. Depending on the temperature and reaction time, metal fixation to sludge ash can vary between 50% and 97% (Chen and Yan, 2012).
Incineration is also used to recover energy from waste. Approximately 18% of the total heat input is recovered for sludge at 20% solids. This energy can be recycled into the system and used to dry sludge prior to incineration or used to produce electricity (Williford et al., 2007). Co-incineration of biomass with coal or solid waste is a cost-effective technique to produce energy from multiple sources, but the process dilutes the density and value of metals in sludges that could potentially be extracted (Zhang et al., 2010).
3.1.3. Chemical oxidation
Wet oxidation, also termed wet air oxidation and wet chemical oxidation (WCO), is a thermal process similar to incineration that oxidizes organics and inorganics remaining in anaerobically digested sludge using air or oxygen (T > 150 °C, P > 1 MPa). Oxidation creates hydroxyl and peroxide radicals that transform complex organics to low molecular weight carbon compounds and simultaneously destroy CECs and pathogens. WCO converts 99% of biomass to CO2, H2O, and non-hazardous byproducts, without producing hazardous byproducts such as nitrous oxides, sulfur oxides, dioxins, furans, and ash. Chemical oxygen demand is reduced by 15%. An additional advantage over incineration is its ability to operate using aqueous phase biomass, making it an ideal application for sewage sludge (Fytili and Zabaniotou, 2008, Hii et al., 2014). Oxidation can potentially leach metals from sludge and the HTL bio-char product. In the mining industry, refractory ores with a high carbon content require a pre-oxidation step to assist with releasing tightly bound metals (Marsden and House, 2006). Given that sludge also has a high organic content and that 35–70% of metals are present in the oxidizable phase of the bio-char, oxidation can be used to liberate metal ions and colloids that may be firmly bound to the solids (Fig. 1).
There is limited research testing the effect of WCO on resource recovery from sewage sludge. In a German study, oxidation was applied with nanofiltration to separate phosphorous and form phosphoric acid, resulting in 54% phosphorous recovery. Moreover, the volume of suspended solids was reduced by 75%. However, the process does not function if iron is being used at the plant for phosphorous removal (Blöcher et al., 2012).
3.2. Liquid extraction of critical elements
3.2.1. Conventional lipid extraction
High lipid content sludge can be subject to alternate energy extraction technologies in which extracted lipids are converted to biodiesel by the transesterification of fatty acid methyl ethers. Several classical solvent techniques have been proven for lipid extraction. The Folch method and Bligh and Dyer method both involve a mixture of chloroform and methanol as solvents (Bligh and Dyer, 1959, Folch et al., 1953). The Soxhlet extraction technique, which can use chloroform, methanol, or toluene, has been successfully demonstrated on sewage sludges by extracting 12 wt% lipids (Boocock et al., 1992). These polar solvents target the polar heads of the phospholipid membranes in sludge microorganisms (Dufreche et al., 2007). However, polyunsaturated fatty acids in sludges can undergo thermo-degradation under these extraction conditions, and these chemical solvents are highly toxic to human health and the environment. Therefore, green alternatives for lipid extraction must be identified and evaluated.
3.2.2. Super critical carbon dioxide for lipids and/or metal extraction
Super critical carbon dioxide (scCO2) extraction has successfully extracted lipids from algae and sludges. Carbon dioxide at temperature and pressure above supercritical values (T > 31.1 °C, P > 7.38 MPa) exhibits increased transport properties and ability to extract thermolabile compounds without degradation (Martínez and Carolina De Aguiar, 2014). Super critical carbon dioxide extraction alone can yield 3.55 wt% oil from sewage sludge, and adding a polar co-solvent such as methanol can increase lipid yield to 13.56 wt%. However, although increasing polar solvent volume results in higher oil yield by weight, the transesterifiable material volume may decrease (Dufreche et al., 2007).
In electronic waste application, scCO2 dissolves precious metals (Au, Cu, Pd) when oxidized by HNO3, followed by chelation with hexafluoroacetylacetone, to form CO2 soluble metal β-diketonate complexes. These complexes are then reduced to their elemental state for pure element recovery, either by using supercritical fluid immersion deposition to form a thin film of metal on a silicon surface, adding a reducing agent (e.g., H2, NaBH3CN), or using a trap solution at ambient conditions (Wang and Wai, 2005). While these techniques were developed for a different industry and waste, there is potential to adapt them for sewage sludges and other organic compounds.
3.2.3. Acids and green solvents for metal extraction
At neutral pH, metal ions are likely to be strongly bound to cell surfaces. Reducing the pH can desorb metals from cells and will facilitate metal recovery. Strong and weak acids (H2SO4, HNO3, HCl) used in heap leaching processes can be applied to sludges and chars for complete recovery of metals, much like in acid digestion. Oxidizing agents such as nitric acid and hydrogen peroxide decompose complex organic material into carbon dioxide. In fact, the BCR sequential extraction procedure, which was used by Yuan et al. to identify speciation of metals after HTL, uses nitric acid, perchloric acid, and hydrogen peroxide in its final step to extract metals still bound to organics after the initial three extractions (acetic acid, hydroxylammonium chloride at pH 2, and hydrogen peroxide followed by ammonium acetate at pH 2) (Yuan et al., 2011). However, this process is likely to completely remove all organic content from char and produce acid waste that is difficult to dispose. Additionally, some metal ions, such as Au3+ and Ag+ do not detach from cells in this acidic environment, and a ligand is required to chelate the metals (Darnall et al., 1986).
Au and Ag are soft acids and have a tendency to be selective towards sulfur and nitrogen functional groups. While cyanide (CN-) has been used universally to extract these precious metals from ore, it has toxic effects, and using it to treat sludges would not bode well for environmental and public health and safety. Alternate solvents that have potential for success but have not yet been tested on sewage sludges are thiourea and thiosulfate. Thiourea (CH4N2S) is a viable alternative ligand as it is nontoxic, has the ability to dissolve a wide array of metals, and behaves as a plant fertilizer in the environment. The ligand has both S and N atoms and can attract other metals that are soft acids such as Pt, Pd, and Cd. Thiosulfate (S2O3 2−), too, is a fertilizer and green alternative to cyanide, but has specificity towards Au, Ag, Zn, and Cu (Marsden and House, 2006, Zuo and Muhammed, 1990).
3.3. Critical element recovery from liquid concentrate streams
Metals recovery from sewage sludges has not yet been explored within the literature beyond the acid digestions and extractions described in Section 3.2.3. Acids are prone to generate hazardous waste that will increase, rather than decrease, waste treatment and disposal issues. In addition, application of acids will decrease the purity or “reusability” of the sludges or chars once the metals have been extracted. Instead, the following processes that have been successfully applied to other mediums such as ore and electronic waste are suggested for adaptation to sequester, concentrate, and recover elements from sewage sludge or any of its processed forms (e.g., post-liquefaction, post-oxidation).
Metals can be recovered from liquid chelating agents and solvents using ion-exchange resins, activated carbon, or precipitation. For example, Au in a thiosulfate solution can load onto a commercial, basic ion-exchange resin at pH 11. However, other metals in the solution such as Cu may compete for attachment (Zhang and Dreisinger, 2004). Using this technology will add an additional step of separating various metals once they have adsorbed to the resin. Alternatively, a selective ion-exchange resin can be developed for individual element recovery.
Extraction of metals can also occur through precipitation. Two different metal ions in solution can be precipitated separately into nanoparticles through liquid–liquid extraction and separate from each other and the leaching solution. Subsequently, a reducing agent is applied to stabilize the particle (Park and Fray, 2009). These nanomaterials can be directly recycled into the nano-manufacturing industries.
While metals recovery is still under exploration, phosphorous recovery is being implemented at WWTPs. Phosphorous can be recovered as struvite (MgNH4PO4·6H2O) once its concentration reaches 100–200 mg/L in the presence of ammonium and magnesium ions. In the case of complete ammonium removal, potassium struvite could also form (KMgPO4·6H2O). While struvite is actually a scalant that builds in pipes and near the anaerobic digester, it contains valuable nutrients that can be recycled as fertilizers. Struvite recovery efficiency from the waste stream is greater than 60%, and the process adaptation and implementation for commercial use has increased since the first full-scale tests in 2000 (Rittmann et al., 2011).
4. Sustainability assessment
Because explicit cost comparisons are difficult to assess in the early stages of process development, processes are discussed herein using the triple bottom line framework of sustainable development (i.e., environment, economy, and society). New resource recovery centers can improve environmental quality by eliminating hauling of biosolids for land application, which will decrease carbon dioxide emissions and ensure hazardous materials are not reintroduced to the environment. Westerhoff et al. determined that for a community of 1 million people, metals in biosolids are valued up to $13 million per year. Extracting the 13 most valuable elements (Ag, Cu, Au, P, Fe, Pd, Mn, Zn, Ir, Al, Cd, Ti, Ga, and Cr) from sewage sludges could amount to a relative potential economic value of $280/ton of sludge produced. Nitrogen in sludges amounts to $24/ton of sludge, while phosphorous is valued at $7/ton of sludge (Peccia and Westerhoff, 2015, Westerhoff et al., 2015). These extracted elements would return directly into society for reuse, ensuring minimization of waste and maximization of resources. Society pays to keep the environment clean by funding municipal solid waste programs, including recycling and reuse of glass, aluminum, and plastic. For example, a 2016 notice of beverage container recycling processing fees in California shows that recycling bimetal cost of $677.40/ton is an order of magnitude greater than the scrap value of $53.37/ton. Glass had no monetary recycling value – it cost $99.97/ton to process, and in turn the scrap value paid to recyclers amounted to negative $1.10 (Smithline, 2015).
The current wastewater treatment cost (primary and secondary treatment, anaerobic digestion, and dewatering) is approximately $300/ton. This number can skyrocket to $800/ton when the cost of hauling processed biosolids for land or landfill disposal and the energy cost of incineration, sludge treatment, and handling are considered (Peccia and Westerhoff, 2015). Novel thermal processes and liquid solvent technologies described herein can eliminate the need for anaerobic digestion and significantly reduce capital costs for land. In terms of sludge loading, HTL is 100 times faster than anaerobic digestion (see Section 3.1.1), thereby decreasing SRT for sludge stabilization, energy production, and resource concentration. Recovery will also contribute to decreased cost. These benefits outweigh initial capital costs for installation of new HTL and oil processing technologies (∼$450 million for 440,000 tons/yr reactor) and further operation and maintenance (O&M) costs (∼$60/ton, estimate adapted from algae biofuel analysis) (Jones et al., 2014). Ultimately, the goal of a new system is to be closer to net-neutral or even become net-positive in terms of energy use and costs endured in comparison to conventional systems.
5. Conclusions
Recent data shows metals (non-regulated transition, post-transition, and precious), nutrients, pathogens, and organic pollutants in sewage sludges. Alternative sludge treatments such as thermal processes and solvent application can be used to recover energy, metals, and nutrients. HTL showed 50% mass reduction, with 30–40% of the liquefaction product converting to oil, and metals sequestering within the bio-char residue. Transitioning to element recovery using the technologies outlined may evolve due to local sites’ specific situations (e.g., phasing out land application). After pilot-scale technology demonstration, implementation is possible at large facilities where space limitations and public input reduces suitability for incineration.
Acknowledgments
This work is supported by the Arizona State University Fulton Schools of Engineering Deans Fellowship, Central Arizona Phoenix Long-Term Ecological Research (BCS-1026865), U.S. Environmental Protection Agency through the STAR program (RD83558001), and the National Science Foundation through the Nano-Enabled Water Treatment Technologies Nanosystems Engineering Research Center (EEC-1449500). We thank Drs. Kiril Hristovski and Pierre Herckes for their insight in data interpretation and Stan Klonowski for his assistance with hydrothermal liquefaction setup.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.biortech.2016.03.075.
Appendix A. Supplementary data
References
- 40 CFR 261 . 2011. Identification and Listing of Hazardous Waste. [Google Scholar]
- 60 FR 54764 Standards for the Use or Disposal of Sewage Sludge. Fed. Regist. 1995;60(206):54771–54792. [Google Scholar]
- Ali M.F., Abbas S. A review of methods for the demetallization of residual fuel oils. Fuel Process. Technol. 2006;87:573–584. doi: 10.1016/j.fuproc.2006.03.001. [DOI] [Google Scholar]
- Appels L., Baeyens J., Degrève J., Dewil R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008;34:755–781. doi: 10.1016/j.pecs.2008.06.002. [DOI] [Google Scholar]
- Arienzo M., Christen E.W., Quayle W., Kumar A. A review of the fate of potassium in the soil–plant system after land application of wastewaters. J. Hazard. Mater. 2009;164:415–422. doi: 10.1016/j.jhazmat.2008.08.095. [DOI] [PubMed] [Google Scholar]
- Bibby K., Peccia J. Identification of viral pathogen diversity in sewage sludge by metagenome analysis. Environ. Sci. Technol. 2013;47:1945–1951. doi: 10.1021/es305181x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller P., Ross A.B. Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour. Technol. 2011;102:215–225. doi: 10.1016/j.biortech.2010.06.028. [DOI] [PubMed] [Google Scholar]
- Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Physiol. Pharmacol. 1959;37:911–917. doi: 10.2307/2738252. [DOI] [PubMed] [Google Scholar]
- Blöcher C., Niewersch C., Melin T. Phosphorus recovery from sewage sludge with a hybrid process of low pressure wet oxidation and nanofiltration. Water Res. 2012;46:2009–2019. doi: 10.1016/j.watres.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Boocock D.G.B., Konar S.K., Leung A., Ly L.D. Fuels and chemicals from sewage sludge: 1. The solvent extraction and composition of a lipid from a raw sewage sludge. Fuel. 1992;71:1283–1289. doi: 10.1016/0016-2361(92)90055-S. [DOI] [Google Scholar]
- Bridle T.R., Pritchard D. Energy and nutrient recovery from sewage sludge via pyrolysis. Water Sci. Technol. 2004;50:169–175. [PubMed] [Google Scholar]
- Chancerel P., Meskers C.E.M., Hageluken C., Rotter V.S. Assessment of precious metal flows during preprocessing of waste electrical and electronic equipment. J. Ind. Ecol. 2009;13:791–810. doi: 10.1111/j.1530-9290.2009.00171.x. [DOI] [Google Scholar]
- Chaudhry Q., Castle L. Food applications of nanotechnologies: an overview of opportunities and challenges for developing countries. Trends Food Sci. Technol. 2011;22:595–603. doi: 10.1016/j.tifs.2011.01.001. [DOI] [Google Scholar]
- Chen T., Yan B. Fixation and partitioning of heavy metals in slag after incineration of sewage sludge. Waste Manage. 2012;32:957–964. doi: 10.1016/j.wasman.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Darnall D.W., Greene B., Henzl M.T., Hosea J.M., McPherson R.A., Sneddon J., Alexander M.D. Selective recovery of gold and other metal ions from an algal biomass. Environ. Sci. Technol. 1986;20:206–208. doi: 10.1021/es00144a018. [DOI] [PubMed] [Google Scholar]
- Debellefontaine H., Foussard J.N. Wet air oxidation for the treatment of industrial wastes. Chemical aspects, reactor design and industrial applications in Europe. Waste Manage. 2000;20:15–25. doi: 10.1016/S0956-053X(99)00306-2. [DOI] [Google Scholar]
- Dufreche S., Hernandez R., French T., Sparks D., Zappi M., Alley E. Extraction of lipids from municipal wastewater plant microorganisms for production of biodiesel. J. Am. Oil Chem. Soc. 2007;84:181–187. doi: 10.1007/s11746-006-1022-4. [DOI] [Google Scholar]
- Energy Star Energy use in wastewater treatment plants [WWW Document] Energy Star Portf. Manag. DataTrends. 2015 URL https://www.energystar.gov/sites/default/files/tools/DataTrends_Wastewater_20150129.pdf (accessed 12.31.15) [Google Scholar]
- FAO . Food Agric. Organ; United Nations: 2012. Current World Fertilizer Trends and Outlook to 2016. [Google Scholar]
- Folch J., Lees M., Stanley G. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1953;226:497–509. [PubMed] [Google Scholar]
- Fytili D., Zabaniotou A. Utilization of sewage sludge in EU application of old and new methods—A review. Renew. Sustain. Energy Rev. 2008;12:116–140. doi: 10.1016/j.rser.2006.05.014. [DOI] [Google Scholar]
- Gao Y., Cranston R. Recent advances in antimicrobial treatments of textiles. Text. Res. J. 2008;78:60–72. doi: 10.1177/0040517507082332. [DOI] [Google Scholar]
- George M.W. Gold. US Geol. Surv. Miner. Yearb. 2014:66–67. doi: 10.1126/science.1247727. [DOI] [Google Scholar]
- Giusti L. A review of waste management practices and their impact on human health. Waste Manage. 2009;29:2227–2239. doi: 10.1016/j.wasman.2009.03.028. [DOI] [PubMed] [Google Scholar]
- Golet E.M., Strehler A., Alder A.C., Giger W. Determination of fluoroquinolone antibacterial agents in sewage sludge and sludge-treated soil using accelerated solvent extraction followed by solid-phase extraction. Anal. Chem. 2002;74:5455–5462. doi: 10.1021/ac025762m. [DOI] [PubMed] [Google Scholar]
- Gottschalk F., Sun T., Nowack B. Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies. Environ. Pollut. 2013;181:287–300. doi: 10.1016/j.envpol.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Hii K., Baroutian S., Parthasarathy R., Gapes D.J., Eshtiaghi N. A review of wet air oxidation and thermal hydrolysis technologies in sludge treatment. Bioresour. Technol. 2014;155:289–299. doi: 10.1016/j.biortech.2013.12.066. [DOI] [PubMed] [Google Scholar]
- Hong J., Hong J., Otaki M., Jolliet O. Environmental and economic life cycle assessment for sewage sludge treatment processes in Japan. Waste Manage. 2009;29:696–703. doi: 10.1016/j.wasman.2008.03.026. [DOI] [PubMed] [Google Scholar]
- Jones S.B., Zhu Y., Anderson D.B., Hallen R.T., Elliott D.C., Schmidt A.J., Albrecht K.O., Hart T.R., Butcher M.G., Drennan C., Snowden-Swan L.J., Davis R., Kinchin C. 2014. Process design and economics for the conversion of algal biomass to hydrocarbons: whole algae hydrothermal liquefaction and upgrading. PNNL-23227. [Google Scholar]
- Kim Y., Parker W. A technical and economic evaluation of the pyrolysis of sewage sludge for the production of bio-oil. Bioresour. Technol. 2008;99:1409–1416. doi: 10.1016/j.biortech.2007.01.056. [DOI] [PubMed] [Google Scholar]
- Kruger O., Grabner A., Adam C. Complete survey of german sewage sludge ash. Environ. Sci. Technol. 2014;48:11811–11818. doi: 10.1021/es502766x. [DOI] [PubMed] [Google Scholar]
- Lee Bray E. Aluminum. US Geol. Surv. Miner. Yearb. 2014;1:16–17. [Google Scholar]
- Leng L., Yuan X., Huang H., Jiang H., Chen X., Zeng G. The migration and transformation behavior of heavy metals during the liquefaction process of sewage sludge. Bioresour. Technol. 2014;167:144–150. doi: 10.1016/j.biortech.2014.05.119. [DOI] [PubMed] [Google Scholar]
- Leng L., Yuan X., Shao J., Huang H., Wang H., Li H., Chen X., Zeng G. Study on demetalization of sewage sludge by sequential extraction before liquefaction for the production of cleaner bio-oil and bio-char. Bioresour. Technol. 2015;200:320–327. doi: 10.1016/j.biortech.2015.10.040. [DOI] [PubMed] [Google Scholar]
- Lindberg R.H., Wennberg P., Johansson M.I., Tysklind M., Andersson B.A.V. Screening of human antibiotic substances and determination of weekly mass flows in five sewage treatment plants in Sweden. Environ. Sci. Technol. 2005;39:3421–3429. doi: 10.1021/es048143z. [DOI] [PubMed] [Google Scholar]
- Marchesan S., Prato M. Nanomaterials for (Nano)medicine. ACS Med. Chem. Lett. 2013;4:147–149. doi: 10.1021/ml3003742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden J., House I. second ed. 2006. The Chemistry of Gold Extraction. [Google Scholar]
- Martínez J., Carolina De Aguiar A. Extraction of triacylglycerols and fatty acids using supercritical fluids – Review. Curr. Anal. Chem. 2014;10:67–77. doi: 1875-6727/14 $58.00+.00. [Google Scholar]
- McClellan K., Halden R.U. Pharmaceuticals and personal care products in archived U.S. biosolids from the 2001 EPA national sewage sludge survey. Water Res. 2010;44:658–668. doi: 10.1016/j.watres.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf, Eddy . fifth ed. McGraw-Hill Education; 2013. Wastewater Engineering: Treatment and Resource Recovery. [Google Scholar]
- National Research Council . National Academy Press; 2002. Biosolids Applied to Land: Advancing Standards and Practices. [Google Scholar]
- Park Y.J., Fray D.J. Separation of zinc and nickel ions in a strong acid through liquid–liquid extraction. J. Hazard. Mater. 2009;163:259–265. doi: 10.1016/j.jhazmat.2008.06.085. [DOI] [PubMed] [Google Scholar]
- Pathak A., Dastidar M.G., Sreekrishnan T.R. Bioleaching of heavy metals from sewage sludge: a review. J. Environ. Manage. 2009;90:2343–2353. doi: 10.1016/j.jenvman.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Peccia J., Westerhoff P. We should expect more out of our sewage sludge. Environ. Sci. Technol. 2015;49:8271–8276. doi: 10.1021/acs.est.5b01931. [DOI] [PubMed] [Google Scholar]
- Pham M., Schideman L., Sharma B.K., Zhang Y., Chen W.-T. Effects of hydrothermal liquefaction on the fate of bioactive contaminants in manure and algal feedstocks. Bioresour. Technol. 2013;149:126–135. doi: 10.1016/j.biortech.2013.08.131. [DOI] [PubMed] [Google Scholar]
- Ramteke S., Patel K.S., Nayak Y., Jaiswal N.K. Contamination of heavy metals and nutrients in sediment, sludge and sewage of India. Int. J. Geosci. 2015;6:1179–1192. [Google Scholar]
- Rittmann B.E., Mayer B., Westerhoff P., Edwards M. Capturing the lost phosphorus. Chemosphere. 2011;84:846–853. doi: 10.1016/j.chemosphere.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Saurat M., Bringezu S. Platinum group metal flows of Europe, Part II. J. Ind. Ecol. 2009;13:406–421. doi: 10.1111/j.1530-9290.2008.00106.x. [DOI] [Google Scholar]
- Sedlak R. second ed. CRC Press; 1991. Phosphorous and Nitrogen Removal from Municipal Wastewater: Principles and Practice. [Google Scholar]
- Shammas N.K., Wang L.K. Characteristics and quantity of biosolids. In: Wang L.K., Shammas N.K., Hung Y.-T., editors. Biosolids Treatment Processes. The Humana Press Inc; 2007. [Google Scholar]
- Shamuyarira K.K., Gumbo J.R. Assessment of heavy metals in municipal sewage sludge: a case study of Limpopo province, South Africa. Int. J. Environ. Res. Public Health. 2014;11:2569–2579. doi: 10.3390/ijerph110302569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithline S. CalRecycle; 2015. 2016 Processing Fees: Beverage Container Recycling. [Google Scholar]
- Smithsonian Institution . 1873. Specific Gravities; Boiling and Melting Points; and Chemical Formula Part 1. [Google Scholar]
- U.S. EPA . 1994. Land Application of Sewage Sludge: A Guide for Land Appliers on the Requirements of the Federal Standard for the Use or Disposal of Sewage Sludge, 40 CFR Part 503 Washington, D.C. doi: EPA-831-B-93-002b. [Google Scholar]
- U.S. EPA Targeted National Sewage Sludge Survey Sampling and Analysis Technical Report. 2009 doi: EPA-822-R-08-016. [Google Scholar]
- U.S. EPA . 2015. Sewage Sludge (Biosolids) Frequently Asked Questions [WWW Document] URL http://www.epa.gov/biosolids/frequently-asked-questions-about-biosolids (accessed 12.28.15) [Google Scholar]
- Valdez P.J., Nelson M.C., Wang H.Y., Lin X.N., Savage P.E. Hydrothermal liquefaction of Nannochloropsis sp.: systematic study of process variables and analysis of the product fractions. Biomass Bioenergy. 2012;46:317–331. doi: 10.1016/j.biombioe.2012.08.009. [DOI] [Google Scholar]
- Vardon D.R., Sharma B.K., Scott J., Yu G., Wang Z., Schideman L., Zhang Y., Strathmann T.J. Chemical properties of biocrude oil from the hydrothermal liquefaction of Spirulina algae, swine manure, and digested anaerobic sludge. Bioresour. Technol. 2011;102:8295–8303. doi: 10.1016/j.biortech.2011.06.041. [DOI] [PubMed] [Google Scholar]
- Venkatesan A.K., Halden R.U. National inventory of perfluoroalkyl substances in archived U.S. biosolids from the 2001 EPA national sewage sludge survey. J. Hazard. Mater. 2013;252–253:413–418. doi: 10.1016/j.jhazmat.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan A.K., Halden R.U. National inventory of alkylphenol ethoxylate compounds in U.S. sewage sludges and chemical fate in outdoor soil mesocosms. Environ. Pollut. 2013;174:189–193. doi: 10.1016/j.envpol.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan A.K., Halden R.U. Brominated flame retardants in U.S. biosolids from the EPA national sewage sludge survey and chemical persistence in outdoor soil mesocosms. Water Res. 2014;55:133–142. doi: 10.1016/j.watres.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau E., Bibby K., Paez-Rubio T., Peccia J. Toward a consensus view on the infectious risks associated with land application of sewage sludge. Environ. Sci. Technol. 2011;45:5459–5469. doi: 10.1021/es200566f. [DOI] [PubMed] [Google Scholar]
- Wang J.S., Wai C.M. Dissolution of precious metals in supercritical carbon dioxide. Ind. Eng. Chem. Res. 2005;44:922–926. doi: 10.1021/ie040198m. [DOI] [Google Scholar]
- Wentzel M.C., Comeau Y., Ekama G., van Loosdrecht M.C., Brdjanovic D. Biological Wastewater Treatment: Principles, Modelling and Design. 2008. Enhanced biological phosphorous removal. [Google Scholar]
- Westerhoff P.K., Kiser A., Hristovski K. Nanomaterial removal and transformation during biological wastewater treatment. Environ. Eng. Sci. 2013;30:109–117. doi: 10.1089/ees.2012.0340. [DOI] [Google Scholar]
- Westerhoff P., Lee S., Yang Y., Gordon G.W., Hristovski K., Halden R.U., Herckes P. Characterization, recovery opportunities, and valuation of metals in municipal sludges from U.S. wastewater treatment plants nationwide. Environ. Sci. Technol. 2015;150127115347007 doi: 10.1021/es505329q. [DOI] [PubMed] [Google Scholar]
- Williford C., Chen W.-Y., Wang L.K., Shammas N.K. High temperature thermal processes. In: Shammas N.K., Hung Y.-T., editors; Wang L.K., editor. The Humana Press Inc.; 2007. pp. 613–643. (Biosolids Treatment Processes). [Google Scholar]
- Worley M., editor. 2012. (Biomass Gasification Technology Assessment Consolidated Report). [Google Scholar]
- Yuan X., Huang H., Zeng G., Li H., Wang J., Zhou C., Zhu H., Pei X., Liu Z., Liu Z. Total concentrations and chemical speciation of heavy metals in liquefaction residues of sewage sludge. Bioresour. Technol. 2011;102:4104–4110. doi: 10.1016/j.biortech.2010.12.055. [DOI] [PubMed] [Google Scholar]
- Zhang H., Dreisinger D.B. The recovery of gold from ammoniacal thiosulfate solutions containing copper using ion exchange resin columns. Hydrometallurgy. 2004;72:225–234. doi: 10.1016/S0304-386X(03)00183-X. [DOI] [Google Scholar]
- Zhang F.-S., Yamasaki S., Kimura K. Rare earth element content in various waste ashes and the potential risk to Japanese soils. Environ. Int. 2001;27:393–398. doi: 10.1016/S0160-4120(01)00097-6. [DOI] [PubMed] [Google Scholar]
- Zhang F.-S., Yamasaki S., Kimura K. Waste ashes for use in agricultural production: II. Contents of minor and trace metals. Sci. Total Environ. 2002;286:111–118. doi: 10.1016/S0048-9697(01)00968-8. [DOI] [PubMed] [Google Scholar]
- Zhang F.-S., Yamasaki S., Nanzyo M. Waste ashes for use in agricultural production: I. Liming effect, contents of plant nutrients and chemical characteristics of some metals. Sci. Total Environ. 2002;284:215–225. doi: 10.1016/S0048-9697(01)00887-7. [DOI] [PubMed] [Google Scholar]
- Zhang L., Xu C. (Charles), Champagne P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manage. 2010;51:969–982. doi: 10.1016/j.enconman.2009.11.038. [DOI] [Google Scholar]
- Zuo G., Muhammed M. Extraction of gold and silver by thiourea-based reagents. Sep. Sci. Technol. 1990;25:1785–1802. doi: 10.1080/01496399008050424. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.