Highlights
-
•
Aging can promote changes in the immune system in dogs.
-
•
Nutritional intervention in older dogs aims to increase lifespan.
-
•
The yeast cell wall comprises β-(1,3)-D-glucan, β-(1,6)-D-glucan and mannoproteins.
-
•
Elderly dogs when compared to adult dogs had lower absolute T and B lymphocyte counts.
-
•
Mannoproteins stimulated acquired and innate immune responses in adult and elderly dogs.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; CBC, complete blood count; CD21+, B lymphocyte; CD4+, auxiliary T lymphocyte; CD5+, total T lymphocyte; CD8+, cytotoxic lymphocyte; DCHT, delayed cutaneous hypersensitivity test; AFM, active fraction of mannoproteins; FOSs, fructooligosaccharides; GALT, gut-associated lymphoid tissue; IgA, immunoglobulin A; IL-12, interleukin 12; LPS, bacterial lipopolysaccharide; MOSs, mannanoligosaccharides; NADPH, reduced nicotinamide adenine dinucleotide phosphate; NO, nitrogen monoxide; NOS, nitric oxide synthase; OD, optical density; PMA, phorbol myristate acetate; CO, cells only; Th1, helper T lymphocyte; TNF-α, tumour necrosis factor alpha
Keywords: Ageing, Canine, Immunosenescence, Saccharomyces cerevisiae, Senescence
Abstract
Nutritional intervention in older dogs aims to increase lifespan and improve life quality as well as delay the development of diseases related to ageing. It is believed that active fractions of mannoproteins (AFMs) obtained through extraction and fractionation of yeast cell walls (Saccharomyces cerevisiae) may beneficially modulate the immune system. However, studies that have evaluated this component and the effects of ageing on the immune system of dogs are scarce. This study aimed to evaluate the immunological effects of AFMs in adult and elderly dogs. Three extruded iso-nutrient experimental diets were formulated: without addition of AFM (T0); with AFM at 400 mg/kg (T400); and with AFM at 800 mg/kg (T800). Thirty-six beagle dogs were used, and six experimental treatments, resulting in combinations of age (adult and elderly) and diet (T0, T400, and T800), were evaluated. On days zero, 14, and 28, blood samples were obtained for leucocyte phenotyping and phagocytosis assays. On days zero and 28, a lymphoproliferation test, quantification of reactive oxygen (H2O2) and nitrogen (NO) intermediate production, evaluation of faecal immunoglobulin A (IgA) content, and a delayed cutaneous hypersensitivity test (DCHT) were performed. Statistical analyses were performed with SAS software. Repeated measure variance analyses were performed, and means were compared by the Tukey test. Values of P ≤ 0.05 were considered significant, and values of P ≤ 0.10 were considered tendencies. Dogs fed T400 tended to have higher neutrophilic phagocytic activity than dogs fed T800 (P = 0.073). Regarding reactive oxygen intermediates, bacterial lipopolysaccharide (LPS)-stimulated neutrophils from animals that were fed T400 had a tendency to produce more H2O2 than those from animals fed the control diet (P = 0.093). Elderly dogs, when compared to adult dogs, had lower absolute T and B lymphocyte counts, lower auxiliary T lymphocyte counts, and higher cytotoxic T lymphocyte counts (P < 0.05). A significant effect of diet, age, and time with saline inoculation was noted for the DCHT. There was no effect of diet or age on faecal IgA content in dogs. This study suggests beneficial effects of mannoproteins on the specific and nonspecific immune responses in adult and elderly dogs.
1. Introduction
Dietary interventions that can favour health and mitigate physiological changes of senescence in dogs have become an interesting study topic due to an increase in animal companion lifespan (Swanson et al., 2004; Laflamme and Gunn-Moore, 2014; Larsen and Farcas, 2014; Tian et al., 2017; Li, 2018; Mittendorfer, 2018). During the process of ageing, alterations in the immunological system lead to increased morbidity and mortality because of infections and age-related diseases, a process called immunosenescence. Among these changes are decreased a proliferative response of blood mononuclear cells, reduced peripheral blood lymphocyte numbers, including those of B, T, and CD4 + T helper cells, increased CD8+ cytotoxic T lymphocytes, and a consequent decrease in the CD4+/CD8+ ratio (Franceschi et al., 2000; Day, 2010; Bauer and Fuente, 2016). Although the focus of immunosenescence characterization is acquired immunity, changes in innate immunity components may also contribute to a generalized decline in immune function during senescence (Greeley et al., 2001).
The immune system consists of a set of structures and biological processes formed by a network of specialized cells, tissues, and organs (Tizard, 2013) that are projected to detect and destroy foreign particles and their products (Janeway et al., 1999). This system may be divided into innate and adaptative immunity. Innate immunity acts concurrently with adaptative immunity and is characterized by a rapid response to injury, regardless of previous stimulus (Sigal and Ron, 1994). Adaptative immunity is induced by cellular memory as a result of exposure to microorganisms or antigens and is very effective at preventing infection propagation (Samartin and Chandra, 2001).
Food components and their digestive products are in close contact with the gut-associated lymphoid tissue (GALT), influencing its immune function and systemic immunity (Field et al., 1999; Schley and Field, 2002; Delgado et al., 2010). Among the dietary components with the potential to modulate and stimulate the immune response, the yeast cell wall of Saccharomyces cerevisiae (Swanson et al., 2002; Grieshop, 2002; Han et al., 2017) and its active fractions, such as mannanoligosaccharides (MOSs) and beta-glucans (Brown and Gordon, 2001; Goodridge et al., 2009; Middelbos et al., 2007), can be highlighted. The yeast cell wall comprises β-(1,3)-d-glucan, β-(1,6)-d-glucan, chitin, and mannoproteins (Kollár et al., 1997), all of which have potential action on the immune system. Due to differences in the structures and proportions of functional carbohydrates, it is believed that these fractions have diverse immunoregulatory properties (Che et al., 2012).
Mannoproteins and their carbohydrate component, α-d-mannose, are responsible for the recognition, cell-to-cell interaction, interaction with the environment, and antigen specificity of yeasts (Ruiz-Herrera, 1991). In broilers, the addition of mannoproteins to diets may lead to immune system benefits (Hooge et al., 2013), increasing the expression of genes favourable to cellular and antimicrobial responses in the intestine (Xiao et al., 2011) and the area of goblet cells in the jejunum, which suggests an enhanced innate response due to mucin production (Lea et al., 2013). In weaned pigs experimentally infected with porcine reproductive and respiratory syndrome virus, the supplementation of mannoproteins increased serum concentrations of inflammatory mediators (interleukin-1, interleukin-12, and haptoglobin) that are important in boosting innate and acquired cell-mediated immunity (Che et al., 2012).
Thus, the hypothesis of this study is that dietary supplementation with mannoproteins from the yeast cell wall may alter the immune response of dogs, and based on physiological changes in ageing, the response induced by mannoprotein intake can differ in adults and especially in elderly dogs if the tested ingredient enhances immunological conditions. Thus, mannoproteins might be of interest as senior dog food additives. Therefore, this study aimed to evaluate the effects of two levels of mannoprotein intake on the immunological parameters of adult and elderly dogs.
2. Materials and methods
This study was approved by the Ethics Committee of the College of Agrarian and Veterinary Sciences of Sao Paulo State University (approval number: 019122/12).
2.1. Animals
The study was carried out at the Laboratory of Research in Nutrition and Nutritional Diseases of Dogs and Cats of Sao Paulo State University, Jaboticabal, Brazil. Thirty-six non-neutered beagle dogs, both males (n = 12) and females (n = 24), with a body condition score of 5 (on a scale from 1 to 9; Laflamme, 1997) were used. The treatments resulted from the combination of age (adults and elderly) and diet (T0, T400, and T800), generating six experimental treatments. Six adult dogs (4.0 ± 2.0 years old and 11.79 ± 0.05 kg) and six elderly dogs (11 ± 1.0 years old and 12.10 ± 0.07 kg) were used for each diet. The health status of the dogs was verified at the beginning of the study through a physical exam, complete blood count, and copro-parasitological examinations. Immediately before the experiment, animals were vaccinated with a polyvalent vaccine (Duramune Max - 5CvK/4 L, Fort Dodge Saúde Animal, Campinas, Brazil; a polyvalent vaccine against parvovirus, distemper, adenovirus-2, hepatitis, parainfluenza, coronavirus, and leptospirosis comprising L. canicola, L. icterohaemorrhagiae, L. gripppotyphosa, and L. pomona) and an antirabic vaccine (Canigen R, Virbac Saúde Animal, Sao Paulo, Brazil).
2.2. Experimental diets
Experimental diets were formulated according to the nutritional recommendations for dog maintenance by the European Pet Food Industry Federation (FEDIAF, 2019). The ingredients and calculated chemical composition of the experimental diets are described in Table 1 . Ingredients were mixed and ground comprising only one production lot. Afterwards, the ingredient lot was divided into three treatments according to the addition of active fractions of mannoproteins (AFMs) obtained through extraction and fractionation of Saccharomyces cerevisiae yeast cell walls (Actigen, Alltech, Lexington, Kentucky, USA; the product is available as basic brewer's yeast composition and yeast cell wall, composed of 140 g/kg of mannanoligosaccharide with a volume density of 700 kg/m3): T0 – control, without addition of AFM; T400 – addition of 400 mg/kg AFM; T800 - addition of 800 mg/kg AFM. After mixing and grinding, the experimental diets were extruded in a single-screw extruder in the Feed Facility of the College of Agrarian and Veterinary Sciences, Sao Paulo State University, Jaboticabal, Sao Paulo, Brazil.
Table 1.
Ingredients and chemical composition of the experimental diets.
| Item | Treatment1 |
||
|---|---|---|---|
| T0 | T400 | T800 | |
| Ingredients, g/kg (as fed) | |||
| Mannoproteins2 | – | 0.4 | 0.8 |
| Maize, grain | 100 | 99.6 | 99.2 |
| Poultry by-product, meal | 360 | 360 | 360 |
| Ground rice | 400 | 400 | 400 |
| Poultry fat | 80.5 | 80.5 | 80.5 |
| Beet pulp | 20 | 20 | 20 |
| Palatant, liquid3 | 25 | 25 | 25 |
| Potassium chloride | 4 | 4 | 4 |
| Refined salt | 4 | 4 | 4 |
| Vitamin-mineral premix4 | 3 | 3 | 3 |
| Choline chloride | 2 | 2 | 2 |
| Mould inhibitor5 | 1 | 1 | 1 |
| Antioxidant6 | 0.5 | 0.5 | 0.5 |
| Chemical composition | |||
| Dry matter, g/kg (as fed) | 936 | 933 | 941 |
| Crude protein, g/kg (DM) | 319 | 307 | 320 |
| Acid-hydrolized fat, g/kg (DM) | 123 | 124 | 127 |
| Crude fibre, g/kg (DM) | 16 | 17 | 17 |
| Ash, g/kg (DM) | 91 | 83 | 88 |
| Calcium, g/kg (DM) | 17 | 16 | 17 |
| Phosphorus, g/kg (DM) | 11 | 10 | 11 |
T0 – control, with no addition of mannoprotein [six adult (three males and three females) and six elderly dogs (three males and three females)]; T400 – addition of 400 mg/kg mannoproteins [six adult (three males and three females) and six elderly dogs (three males and three females)]; T800 - addition of 800 mg/kg mannoproteins [six adult (three males and three females) and six elderly dogs (three males and three females)].
ActigenTM, Alltech, Lexington, KY (CP =280 g/kg; recommended addition: 200–800 g/ton – information provided by the manufacturer).
Liquid palatant enhancer, SPF do Brasil, Descalvado, Brazil.
Provided per kg of diet: Fe, 100 mg as iron sulfate; Cu, 10 mg as copper sulfate; Mg, 10 mg as magnesium oxide; Zn, 150 mg as zinc sulfate; I, 2 mg as potassium iodide; vitamin A (retinol), 18,000 IU; vitamin D (colecalciferol), 1,200 IU; vitamin E (α-tocoferol), 200 IU; thiamine, 6 mg; riboflavin, 10 mg; pantothenic acid, 40 mg; niacin, 60 mg; pyridoxine, 6 mg; folic acid, 0.30 mg; and cobalamin, 0.01 mg.
MoldZap Aquativa, Alltech do Brasil Agroindustrial Ltd., Curitiba, PR, Brazil: ammonium dipropionate, propanediol, propionic acid, acetic acid, lactic acid, ascorbic acid, formic acid, and potassium sorbate.
Banox, Alltech Brasil Agroindustrial Ltd.: BHA, BHT, propyl gallate and calcium carbonate.
Before the initiation of the experiment, dogs received a yeast-free commercial dry dog food (Herói Adulto Carne, Mogiana Alimentos S.A., Campinas, Brazil) for 30 days; the composition according to the manufacturer was maximum moisture: 120 g/kg; minimal crude protein: 200 g/kg; minimal fat: 80 g/kg; and maximum ash: 110 g/kg, with the aim of standardizing the nutritional condition of the animals and eliminating potential interference of previous intake of the tested ingredient.
2.3. Experimental design
The experiment was conducted as a 2 × 3 factorial arrangement of treatment, with two ages (adult and elderly dogs) and three diets (T0, T400, and T800), totalling six experimental treatments. The experimental design was in randomized blocks, with three blocks of twelve dogs each, six adult and six elderly dogs in each block. These animals were first fed the yeast-free commercial diet for 30 days, followed by 28 days of experimental diet feeding. The amount of food offered to the dogs was calculated according to the metabolizable energy concentration of the diets estimated by their chemical composition and the individual energy requirement of each animal (NRC, 2006). The daily amount was divided into two equal meals. Dogs were weighed weekly, and the quantity of food was adjusted to maintain a steady body weight. Water was provided ad libitum.
2.4. Blood collection and testing
On days 1, 14, and 28 after the introduction of the experimental diets, blood samples were collected via jugular puncture and then submitted to immune phenotype characterization and phagocytic activity analysis. Assays examining lymphocyte proliferation and the production of hydrogen peroxide and nitric oxide were carried out on days 1 and 28.
2.4.1. Phagocytic activity
Phagocytic activity was measured on days 1 and 28 using a commercial kit (pHrodo E. coli BioParticles, Molecular Probes Inc., Oregon, USA). The protocol consisted of incubation of 100 μL of a heparinized blood sample with 20 μL of pHrodo E. coli BioParticles reagent provided by the commercial kit (bioparticle:phagocyte ratio of 20:1). For each blood sample, two tubes were prepared with the bioparticles, with one tube placed on ice and the other kept at 37 °C in a water bath for 15 min. Then, the incubated samples were lysed, followed by centrifugation and washing using the proper reagents. Two negative control samples were analysed together on each collection day, both tubes with no bioparticles but one placed on ice and the other kept at 37 °C. Samples were analysed using flow cytometry (FACSCanto®, Becton Dickinson Immunocytometry System, Mountain View, CA, USA), and the results are shown as the percentage of fluorescence signal inside the desired population of phagocytosing neutrophils and monocytes.
2.4.2. Determination of reactive oxygen intermediates (H2O2) and nitrogen monoxide (NO) production by neutrophils and monocytes
Cells were suspended in complete medium, RPMI 1640 plus 40 mg/mL gentamicin and 10 % foetal bovine serum (FBS), and the concentration was adjusted to 3 × 106 neutrophils/mL or 3 × 106 monocytes/mL. Subsequently, the cells were placed in 96-well flat plates (100 μL/well). Mononuclear cells were kept at 37 °C in a humidified 5 % CO2 incubator for 1 h for adherence of monocytes in the well, then the supernatant was carefully discarded with a pipette, and complete medium was added to each well. For monocytes, one plate was incubated for H2O2 production and one for NO production. For neutrophils, the supernatant from the H2O2 production assay was used to conduct the NO analysis.
For H2O2 production, a total of 12 wells received 100 μL of sample each, six of them were kept as non-stimulated cells, and the other six wells were stimulated with E. coli lipopolysaccharide (LPS, 1 μg/well, Sigma Aldrich, St. Louis, USA). The plates were maintained at 37 °C in a humidified 5 % CO2 incubator for 36 h. The H2O2 production was measured according to the method described by Pick and Keisari (1980) and modified by Pick and Mizel (1981). The buffer solution (100 μL/well) consisted of 7.8 mL of distilled water (dH2O), 0.8 mL of solution A (800 mL of dH2O; 80 g of NaCl; 2.0 g of KCl; 2.0 g of KH2PO4; 11.5 g of Na2HPO4), 0.1 mL of solution B (100 mL of dH2O; 1.0 g of CaCl2), 0.1 mL of solution C (100 mL of dH2O; 1.0 g of MgCl2), 0.1 mL of phenol red (100 mL of dH2O; 1.0 g of phenol red), 0.1 mL of peroxidase (10 mg of horseradish peroxidase; 2.0 mL of PBS) and 1 mL of glucose (100 mL of dH2O; 1.0 g of glucose), which was added to each well. Phorbol myristate acetate (PMA; 10 μL/well) was added to half of the non-stimulated wells and to half of the LPS-stimulated wells, after which the wells were kept at 37 °C in a humidified 5 % CO2 incubator for 1 h. Consequently, there were three replications for each cell condition: three wells for non-stimulated cells, three wells for non-stimulated cells + PMA, three wells for LPS-stimulated cells, and three wells for LPS-stimulated cells + PMA. After 1 h of incubation, the reaction was stopped with 10 μL of 1 N NaOH. The absorbance was read in a microplate reader (iMarkMicroplate Absorbance Reader 168–1135, Bio-Rad, Hercules, California, USA) at 630 nm. The results were expressed as nM H2O2/3 × 105 cells. An H2O2 standard curve was built for each plate, with a range of 0.25–16.00 nM H2O2.
An NO assay was assessed by the colorimetric method of the Griess reaction (Green et al., 1982). The analyses were conducted in triplicate for both non-stimulated and LPS-stimulated cells (1 μg/well), totalling 6 wells per animal sample. One hundred microliters of Griess reagents diluted 1:1 (n-(1-naftil)-etil-enediamin diluted to 0.1 % in dH2O; 1 % sulfonamide diluted in 5 % H2PO4; Sigma Aldrich, St. Louis, MO, USA) were added to the supernatant. The absorbance was read in a microplate reader (iMarkMicroplate Absorbance Reader 168–1135, Bio-Rad, Hercules, CA, USA) at 550 nm. The results were expressed as μM NO/3 × 105 cells. A nitric oxide standard curve was built for each plate, with a range of 0.78–100 μM NO.
2.4.3. Leucocyte separation
After collection of heparinized blood samples via jugular puncture on days 1 and 28, 4.5 mL of Histopaque 1119, 3 mL of Histopaque 1077 (Sigma Aldrich, St. Louis, MO, USA) and 6 mL of blood sample were added to a 15 mL conical centrifuge tube, one at a time. Tubes were centrifuged at 700 x g for 30 min at room temperature. After centrifugation, there were two distinct opaque layers, mononuclear and granulocyte cells, and each layer was collected separately, transferred to a 50 mL conical centrifuge tube and washed at least twice with an isotonic phosphate-buffered saline solution (centrifugation: 360 x g for 10 min at room temperature). Erythrocyte lysis was conducted when necessary using 2 mL of ACK solution (0.15 M ammonium chloride; 10 mM potassium bicarbonate; 0.1 mM EDTA) for a maximum of 2 min. Cells were suspended in complete medium or a phosphate-buffered saline (PBS) solution according to each analysis.
2.4.4. Lymphocyte proliferation assay
Lymphocyte proliferation was measured using the commercial Cell Trace CFSE Cell Proliferation kit (Molecular Probes, Eugene, OR, USA) on samples from days 1 and 28. Cells were suspended in RPMI 1640 medium (Sigma-Aldrich, St. Louis, MO, USA), and the cellular concentration was adjusted to 5 × 106 cells/mL. After running the CFSE protocol according to the instructions in the commercial kit, 100 μL of cells were placed in duplicate in 96-well round-bottom plates, as follows: 100 μL of cells with no CFSE incubation (negative control) plus 100 μL of complete medium (RPMI-1640 medium plus 40 mg/mL gentamicin and 10 % foetal bovine serum (FBS); Life Technologies, Carlsbad, CA, USA); 100 μL of cells with CFSE incubation (positive control) plus 100 μL of complete medium; or 100 μL of cells with CFSE incubation plus 100 μL of Concanavalin A solution (ConA, 100 mg/mL diluted 1:10 in a complete medium). Plates were kept at 37 °C in a humidified 5 % CO2 incubator (Thermo Fisher Scientific Revco, Mod. RCO3000T-9-ABC, Waltham, MA, USA) for 5 d. Later, cells were transferred to flow cytometry tubes in duplicate. Tubes were centrifuged, the supernatant was discarded and 1 mL of 1 % formaldehyde PBS was added to each tube. Samples were read with flow cytometer (FACSCanto®, Becton Dickinson Immunocytometry System, Mountain View, CA, USA), and data were analysed with FlowJo® Software (Tree Star Inc., Ashland, OR, USA).
2.4.5. Immune phenotype characterization of leucocytes
Lymphocyte subpopulations were quantified by flow cytometry on days 1, 14, and 28. The cells evaluated included total T cells (CD5+), helper T cells (CD5+CD4+), cytotoxic T lymphocytes (CD5+CD8+), B cells (CD21+), and monocytes (CD14+), as defined by Cobbold and Metcalfe (1994). To quantify these cells, monoclonal antibodies (AbDSerotec, Raleigh, NC, USA) against surface markers were used. After adding 100 μL of total blood to polystyrene tubes, 2 μL of each antibody was added, homogenized and incubated for 20 min. Then, each tube received 1 mL of 1:10 Lysing Solution® (BD, San Jose, CA, USA), and after homogenization, tubes were incubated for 10 min. Tubes were then centrifuged at 700 x g for 3 min. The supernatant was discarded, and the cells were suspended in 2 mL of PBS. This washing procedure was repeated one more time. After centrifugation, the cells were suspended in 500 μL of PBS plus 1 % formaldehyde. Labelled cells were counted with a FACS Canto (Becton Dickinson Immunocytometry System, San Jose, CA, USA). The proportion of cells obtained by flow cytometry was applied to absolute leucocyte and lymphocyte counts from a CBC to obtain subset absolute values.
2.5. Delayed-type hypersensitivity response
On day 28, animals were injected intradermally in the flank area with 100 μL of the following solutions: saline 0.9 %; 2.0 mg/mL phytohemagglutinin solution (Sigma-Aldrich, St. Louis, MO, USA); or polyvalent vaccine against parvovirus, distemper, adenovirus-2, hepatitis, parainfluenza, coronavirus, and leptospirosis (Duramune® Max - 5CvK/4 L, Fort Dodge Saude Animal, Campinas, Brazil). Skin thickness was measured with a digital micrometer (Mitutoyo, model 700-122, Tokyo, Japan) calibrated to exert an automatic pressure of 2 N at the site of injection. Measurements were performed immediately after injections and after 24, 48, and 72 h. The results were compared to those at the time of injection and expressed as a percentage change in skin thickness. The procedures were adapted from Kim et al. (2000).
2.6. Faecal IgA
On days 1 and 28, fresh faecal samples (immediately after defecation) were collected. Faecal IgA extraction was performed by saline extraction (Peters et al., 2004). Approximately 1 g of faeces was weighed and diluted in 10 mL of extraction buffer composed of 0.01 M PBS (pH 7.4), 0.5 % Tween (Sigma-Aldrich, St. Louis, MO, USA), and 0.05 % sodium azide. After homogenization, faecal suspensions were centrifuged at 1500 × g for 20 min at 5 °C. Then, 1 mL of the supernatant was transferred to a sterile microtube containing 20 μL of a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). To remove residues, samples were centrifuged at 15,000 x g for 15 min at 5 °C, and the supernatants were kept in microtubes at −20 °C until analysis.
Quantification of IgA was performed by an ELISA kit for canine IgA determination (Bethyl Laboratories, Montgomery, TX, USA). Optical density (OD) was read at 450 nm with a microplate reader (MRX TC Plus, Dynex Technology, Chantilly, VA, USA). To calculate the IgA concentration, the OD of the samples was compared to the OD of a standard sample with a known concentration of IgA. The standard canine IgA sample was provided in the kit, and seven dilutions of the standard were made to develop a regression curve between OD and IgA concentration. All samples were tested in duplicate, and measurements were repeated when more than 5 % variation between results was found.
2.7. Statistical analysis
Data were submitted to analysis of variance with means repeated over time. The DCHT was considered at the time in hours after intradermal inoculations. Statistical analyses were performed using the MIXED procedure of the Statistical Analysis System (version 8.2, SAS Institute Inc., Cary, NC, USA) (SAS, 1996), considering the effects of age and diet and their interactions. Prior to statistical analysis, data were screened for normality using the Shapiro-Wilk test, and means were compared by the Tukey test. Results were considered significant at P ≤ 0.05 and trends at P ≤ 0.10.
3. Results
3.1. Phagocytic activity
For the phagocytic activity of leucocyte cells, there was an effect of time (P < 0.001) independent of age and diet, with higher activity of monocytes on day 28 than on days 1 and 14 (Table 2 ). For the percentage of neutrophil phagocytic activity, there was a tendency for diet x age interaction (P = 0.087), with 400 mg AFM/kg tending to have a higher neutrophil phagocytic activity than dogs fed diets with 800 mg AFM/kg in adult dogs.
Table 2.
Phagocytic activity of neutrophils and monocytes in total blood of adult and elderly dogs before and after consumption of experimental diets on days 1, 14, and 28.
| Item | Treatment1 |
Mean | SEM2 | P-value |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adult |
Elderly |
||||||||||||||
| T0 | T400 | T800 | T0 | T400 | T800 | Diet | Age | Time | Diet x Age | Time x Diet | Time x Age | Time x Age x Diet | |||
| Neutrophils, % | |||||||||||||||
| Day 1 | 56.68 | 74.00 | 61.3 | 68.83 | 64.00 | 68.53 | 66.06 | 1.742 | 0.073 | 0.833 | 0.560 | 0.087 | 0.691 | 0.260 | 0.834 |
| Day 14 | 68.15 | 79.72 | 55.93 | 61.48 | 60.27 | 60.05 | 64.27 | ||||||||
| Day 28 | 72.85 | 72.55 | 58.58 | 78.93 | 70.60 | 68.88 | 70.40 | ||||||||
| Monocytes, % | |||||||||||||||
| Day 1 | 67.75 | 71.93 | 65.03 | 66.33 | 61.73 | 67.60 | 66.73b | 1.291 | 0.359 | 0.841 | <0.001 | 0.602 | 0.269 | 0.669 | 0.946 |
| Day 14 | 66.20 | 69.43 | 60.92 | 67.22 | 69.35 | 63.98 | 66.18b | ||||||||
| Day 28 | 78.73 | 68.00 | 72.80 | 81.00 | 69.70 | 73.60 | 73.97a | ||||||||
1T0 – control, with no addition of mannoprotein [six adult (three males and three females) and six elderly dogs (three males and three females)]; T400 – addition of 400 mg/kg mannoproteins [six adult (three males and three females) and six elderly dogs (three males and three females)]; T800 - addition of 800 mg/kg mannoproteins [six adult (three males and three females) and six elderly dogs (three males and three females)].
2Standard error of the mean.
A, B - The averages followed by different letters in the columns differ from each other, as determined by Student’s t-test (P ≤ 0.05).
3.2. Reactive oxygen intermediates (H2O2) and nitrogen monoxide (NO) production
The results for H2O2 and NO production are described in Table 3 . Regardless of age and diet, a time effect was noted for H2O2 production by neutrophils, which was increased on day 28 compared to day 1 (P < 0.05). NO production in LPS stimulated neutrophils higher on day 1 than day 28 and a tendency for non-treated cells. When neutrophils were stimulated with LPS, dogs fed the T400 diet tended to produce more H2O2 than dogs consuming the control diet (P = 0.093). There were no effects regarding the production of H2O2 or NO by monocytes.
Table 3.
Production of reactive oxygen (H2O2) and nitrogen monoxide (NO) intermediates by neutrophils and monocytes of adult and elderly dogs before and after consumption of experimental diets on days 1, 14, and 28.
| Item | Treatment1 |
Mean | SEM2 | P-value |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adult |
Elderly |
||||||||||||||
| T0 | T400 | T800 | T0 | T400 | T800 | Diet | Age | Time | Diet x Age | Time x Diet | Time x Age | Time x Age x Diet | |||
| Production of H2O2 by neutrophils, nanomoles of H2O2 per 3 × 105 cells | |||||||||||||||
| OC3 | |||||||||||||||
| Day 1 | 2.01 | 2.08 | 2.85 | 1.73 | 2.39 | 2.31 | 2.23b | 0.293 | 0.919 | 0.231 | 0.028 | 0.710 | 0.756 | 0.143 | 0.387 |
| Day 28 | 2.58 | 3.52 | 2.29 | 5.12 | 3.23 | 4.86 | 3.60ª | ||||||||
| LPS4 | |||||||||||||||
| Day 1 | 2.17 | 3.28 | 2.47 | 2.35 | 3.48 | 2.73 | 2.75b | 0.243 | 0.093 | 0.443 | 0.040 | 0.455 | 0.941 | 0.286 | 0.436 |
| Day 28 | 3.34 | 5.67 | 3.47 | 2.89 | 3.32 | 3.96 | 3.78a | ||||||||
| PMA5 | |||||||||||||||
| Day 1 | 3.02 | 2.15 | 2.63 | 2.47 | 4.22 | 2.60 | 2.85b | 0.300 | 0.423 | 0.319 | <0.001 | 0.067 | 0.090 | 0.904 | 0.959 |
| Day 28 | 5.43 | 3.04 | 5.99 | 5.19 | 4.56 | 5.35 | 4.93a | ||||||||
| LPS4 + PMA5 | |||||||||||||||
| Day 1 | 4.10 | 3.11 | 3.86 | 2.88 | 4.67 | 4.06 | 3.78b | 0.271 | 0.474 | 0.671 | <0.001 | 0.348 | 0.337 | 0.355 | 0.187 |
| Day 28 | 5.50 | 4.83 | 6.00 | 5.39 | 4.37 | 5.15 | 5.21a | ||||||||
| Production of H2O2 by monocytes, nanomoles of H2O2 per 3 × 105 cells | |||||||||||||||
| OC3 | |||||||||||||||
| Day 1 | 4.41 | 2.97 | 3.56 | 4.01 | 5.22 | 5.50 | 4.28 | 0.391 | 0.905 | 0.599 | 0.965 | 0.496 | 0.744 | 0.411 | 0.185 |
| Day 28 | 3.56 | 6.35 | 2.93 | 4.50 | 3.15 | 4.47 | 4.16 | ||||||||
| LPS4 | |||||||||||||||
| Day 1 | 4.65 | 4.24 | 3.25 | 4.8 | 3.27 | 3.15 | 3.89 | 0.352 | 0.810 | 0.792 | 0.793 | 0.709 | 0.653 | 0.911 | 0.910 |
| Day 28 | 4.00 | 4.87 | 3.63 | 3.83 | 3.60 | 4.70 | 4.10 | ||||||||
| PMA5 | |||||||||||||||
| Day 1 | 9.20 | 8.77 | 9.94 | 9.13 | 11.62 | 6.27 | 9.16 | 0.674 | 0.408 | 0.247 | 0.415 | 0.246 | 0.868 | 0.384 | 0.611 |
| Day 28 | 11.22 | 11.00 | 10.76 | 9.69 | 9.55 | 7.61 | 9.97 | ||||||||
| LPS4 + PMA5 | |||||||||||||||
| Day 1 | 8.19 | 7.26 | 8.05 | 5.68 | 7.65 | 5.75 | 7.10 | 0.562 | 0.478 | 0.299 | 0.222 | 0.735 | 0.720 | 0.655 | 0.797 |
| Day 28 | 8.55 | 9.59 | 7.7 | 8.52 | 9.22 | 6.32 | 8.32 | ||||||||
| Production of NO by neutrophils, nanomoles of NO per 3 × 105 cells | |||||||||||||||
| OC3 | |||||||||||||||
| Day 1 | 6.48 | 6.57 | 3.66 | 5.26 | 5.33 | 6.25 | 5.59 | 0.361 | 0.777 | 0.573 | 0.083 | 0.448 | 0.656 | 0.573 | 0.671 |
| Day 28 | 4.17 | 3.35 | 3.51 | 5.27 | 3.64 | 4.99 | 4.16 | ||||||||
| LPS4 | |||||||||||||||
| Day 1 | 5.74 | 4.63 | 6.22 | 4.52 | 4.80 | 6.11 | 5.34a | 0.414 | 0.829 | 0.935 | 0.031 | 0.886 | 0.784 | 0.743 | 0.802 |
| Day 28 | 3.11 | 3.71 | 2.54 | 3.52 | 2.76 | 3.79 | 3.24b | ||||||||
| Production of NO by monocytes, nanomoles of NO per 3 × 105 cells | |||||||||||||||
| OC3 | |||||||||||||||
| Day 1 | 8.28 | 4.07 | 11.62 | 17.30 | 9.26 | 4.80 | 9.22 | 1.301 | 0.627 | 0.774 | 0.822 | 0.848 | 0.606 | 0.694 | 0.297 |
| Day 28 | 12.56 | 11.83 | 5.65 | 8.54 | 8.98 | 7.71 | 9.21 | ||||||||
| LPS4 | |||||||||||||||
| Day 1 | 7.60 | 3.26 | 6.66 | 13.25 | 7.55 | 7.25 | 7.60 | 0.632 | 0.158 | 0.257 | 0.084 | 0.767 | 0.386 | 0.156 | 0.671 |
| Day 28 | 5.96 | 3.95 | 6.34 | 4.88 | 4.02 | 6.08 | 5.21 | ||||||||
A, B - The averages followed by different letters in the columns differ from each other, as determined by Student’s t-test (P ≤ 0.05).
T0 – control, with no addition of mannoprotein [six adult (three males and three females) and six elderly dogs (three males and three females)]; T400 – addition of 400 mg/kg mannoproteins [six adult (three males and three females) and six elderly dogs (three males and three females)]; T800 - addition of 800 mg/kg mannoproteins [six adult (three males and three females) and six elderly dogs (three males and three females)].
Standard error of the mean.
OC – Only cells.
LPS – Lipopolysaccharide.
PMA – Phorbol myristate acetate.
3.3. Lymphocyte proliferation
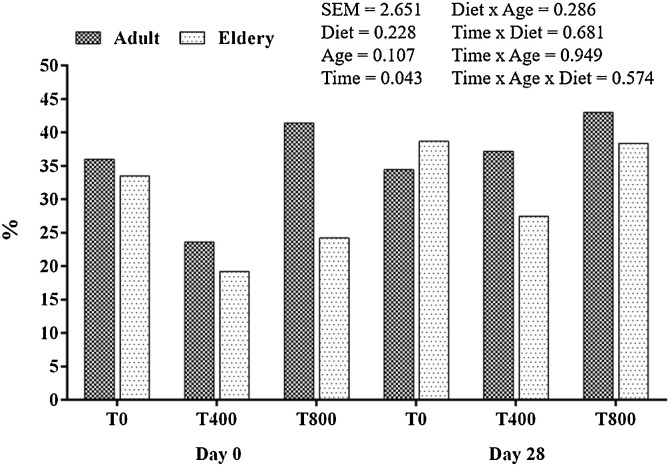
An effect of time on the mean proliferation of lymphocytes, expressed as percentage and regardless of age and diet, was observed, with an increase on day 28 compared to that on day 1 (Fig. 1 ).
Fig. 1.
Lymphocyte proliferation in adult and elderly dogs before and after consumption of experimental diets.
3.4. Leucocyte phenotyping
For absolute counts of CD5+ cells and CD21+ cells and for the CD4+/CD8+ ratio, an age effect was observed, for which adult dogs presented higher values than elderly dogs (Table 4 ).
Table 4.
Leucocyte subpopulations of adult and elderly dogs before and after consumption of experimental diets on days 1, 14, and 28.
| Item | Treatment1 |
Mean | SEM2 | P-value |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adult |
Elderly |
||||||||||||||
| T0 | T400 | T800 | T0 | T400 | T800 | Diet | Age | Time | Diet x Age | Time x Diet | Time x Age | Time x Age x Diet | |||
| CD5+, x 103/μL | |||||||||||||||
| Day 1 | 1.32 | 1.49 | 1.24 | 1.27 | 1.32 | 1.29 | 1.32 | 0.053 | 0.103 | 0.013 | 0.463 | 0.127 | 0.524 | 0.295 | 0.158 |
| Day 14 | 1.24 | 1.32 | 1.31 | 0.87 | 1.26 | 1.21 | 1.20 | ||||||||
| Day 28 | 1.15 | 1.99 | 1.49 | 1.27 | 1.17 | 1.28 | 1.39 | ||||||||
| CD4+, x 103/μL | |||||||||||||||
| Day 1 | 0.67 | 0.67 | 0.54 | 0.49 | 0.49 | 0.60 | 0.58a | 0.022 | 0.774 | 0.345 | <0.001 | 0.608 | 0.370 | 0.395 | 0.164 |
| Day 14 | 0.39 | 0.43 | 0.41 | 0.23 | 0.37 | 0.41 | 0.37b | ||||||||
| Day 28 | 0.44 | 0.81 | 0.60 | 0.47 | 0.42 | 0.46 | 0.53b | ||||||||
| CD8+, x 103/μL | |||||||||||||||
| Day 1 | 0.25 | 0.28 | 0.25 | 0.32 | 0.31 | 0.27 | 0.28a | 0.012 | 0.218 | 0.779 | <0.001 | 0.472 | 0.773 | 0.399 | 0.865 |
| Day 14 | 0.08 | 0.14 | 0.11 | 0.09 | 0.16 | 0.10 | 0.11b | ||||||||
| Day 28 | 0.14 | 0.21 | 0.18 | 0.16 | 0.17 | 0.16 | 0.17b | ||||||||
| CD21+, x 103/μL | |||||||||||||||
| Day 1 | 0.33 | 0.31 | 0.24 | 0.21 | 0.22 | 0.18 | 0.25a | 0.022 | 0.690 | 0.013 | 0.002 | 0.602 | 0.892 | 0.805 | 0.619 |
| Day 14 | 0.05 | 0.07 | 0.10 | 0.06 | 0.04 | 0.04 | 0.06b | ||||||||
| Day 28 | 0.13 | 0.22 | 0.19 | 0.16 | 0.09 | 0.11 | 0.15b | ||||||||
| CD14+, x 103/μL | |||||||||||||||
| Day 1 | 0.46 | 0.42 | 0.52 | 0.42 | 0.44 | 0.44 | 0.45a | 0.021 | 0.73 | 0.490 | <0.001 | 0.988 | 0.650 | 0.845 | 0.186 |
| Day 14 | 0.31 | 0.23 | 0.30 | 0.23 | 0.18 | 0.24 | 0.25b | ||||||||
| Day 28 | 0.37 | 0.38 | 0.35 | 0.33 | 0.24 | 0.35 | 0.34b | ||||||||
| CD4+/CD8+ | |||||||||||||||
| Day 1 | 2.74 | 2.59 | 2.58 | 1.98 | 2.12 | 2.33 | 2.39a | 0.154 | 0.416 | 0.002 | <0.001 | 0.284 | 0.761 | 0.629 | 0.191 |
| Day 14 | 5.48 | 3.11 | 4.15 | 2.95 | 2.83 | 4.02 | 3.76b | ||||||||
| Day 28 | 3.68 | 4.10 | 3.80 | 3.59 | 2.88 | 3.09 | 3.52b | ||||||||
1T0 – control, with no addition of mannoprotein [six adult (three males and three females) and six elderly dogs (three males and three females)]; T400 – addition of 400 mg/kg mannoproteins [six adult (three males and three females) and six elderly dogs (three males and three females)]; T800 - addition of 800 mg/kg mannoproteins [six adult (three males and three females) and six elderly dogs (three males and three females)].
2Standard error of the mean.
A, B - The averages followed by different letters in the columns differ from each other, as determined by Student’s t-test (P ≤ 0.05).
An effect of time, regardless of diet and age, was observed in absolute counts of CD4+, CD8+, CD14+, and CD21+ cells and in the CD4+/CD8+ ratio (P < 0.005). CD21+ and CD8+ cell counts were reduced on day 14 compared to those on day 1 and remained stable on day 28. The CD14+ cell count was reduced on day 14, and despite being increased on day 28, it was still below initial levels. The CD4+ cell count was reduced on day 14, with an increase on day 28, while it was not different from that on day 1. The CD4+/CD8+ ratio increased on day 14 and remained stable on day 28.
3.5. Delayed cutaneous hypersensitivity test (DCHT)
Diet, age, and time had significant effects on the DCHT upon saline inoculation (Table 5 ). For other inoculations, a higher response to phytohemagglutinin in elderly dogs was observed. Diet and age effects were observed for the polyvalent vaccine (P = 0.002), and time and age interactions were observed for intradermal inoculation of phytohemagglutinin (P = 0.002).
Table 5.
Delayed cutaneous hypersensitivity response of adult and elderly dogs after consumption of experimental diets for 28 days.
| Item | Treatment1 |
Mean | SEM2 | P-value |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adult |
Elderly |
||||||||||||||
| T0 | T400 | T800 | T0 | T400 | T800 | Diet | Age | Time | Diet x Age | Time x Diet | Time x Age | Time x Age x Diet | |||
| Saline solution, mm | |||||||||||||||
| 0 Hours | 3.43 | 3.39 | 3.6 | 3.3 | 3.07 | 3.13 | 3.32ª | 0.055 | <0.001 | <0.001 | <0.001 | 0.503 | 0.506 | 0.691 | 0.282 |
| 24 Hours | 2.80 | 2.58 | 2.84 | 2.49 | 2.46 | 2.61 | 2.63b | ||||||||
| 48 Hours | 2.70 | 2.54 | 2.73 | 2.40 | 2.40 | 2.55 | 2.55b | ||||||||
| 72 Hours | 2.78 | 2.54 | 2.89 | 2.56 | 2.39 | 2.53 | 2.62b | ||||||||
| Phytohemagglutinin solution, mm | |||||||||||||||
| 0 Hours | 3.39 | 3.2 | 3.52 | 3.04 | 2.87 | 3.05 | 3.18 | 0.052 | 0.004 | 0.148 | <0.001 | 0.908 | 0.471 | 0.002 | 0.759 |
| 24 Hours | 3.46 | 3.37 | 3.77 | 3.47 | 3.46 | 3.78 | 3.55 | ||||||||
| 48 Hours | 3.19 | 3.06 | 3.4 | 3.27 | 2.97 | 3.65 | 3.26 | ||||||||
| 72 Hours | 3.07 | 3.01 | 3.27 | 2.96 | 2.73 | 2.94 | 3.00 | ||||||||
| Vaccine, mm | |||||||||||||||
| 0 Hours | 3.77 | 3.62 | 3.96 | 3.57 | 3.25 | 3.19 | 3.56c | 0.061 | 0.696 | <0.001 | <0.001 | 0.002 | 0.639 | 0.075 | 0.633 |
| 24 Hours | 4.32 | 4.25 | 4.50 | 4.12 | 4.28 | 4.12 | 4.27ª | ||||||||
| 48 Hours | 4.35 | 4.28 | 4.43 | 4.00 | 4.05 | 3.97 | 4.18b | ||||||||
| 72 Hours | 4.23 | 4.19 | 4.40 | 3.80 | 4.03 | 3.63 | 4.05b | ||||||||
1T0 – control, with no addition of mannoprotein [six adult (three males and three females) and six elderly dogs (three males and three females)]; T400 – addition of 400 mg/kg mannoproteins [six adult (three males and three females) and six elderly dogs (three males and three females)]; T800 - addition of 800 mg/kg mannoproteins [six adult (three males and three females) and six elderly dogs (three males and three females)].
2Standard error of the mean.
A, B - The averages followed by different letters in the columns differ from each other, as determined by Student’s t-test (P ≤ 0.05).
3.6. Evaluation of faecal IgA concentration
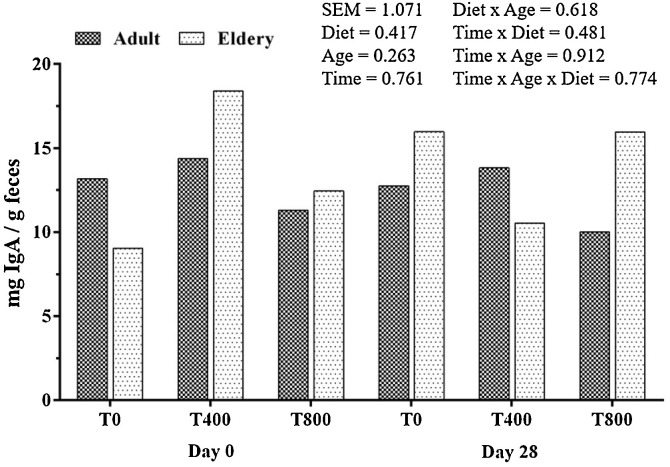
There was no effect of age, diet or time on faecal IgA concentrations in dogs (Fig. 2 ). The mean values were 12.94 ± 2.42, 14.10 ± 2.43, and 10.72 ± 1.88 mg/g of faeces (dry matter basis) for adult dogs and 12.50 ± 2.98, 14.47 ± 3.39 and 14.36 ± 2.0 mg/g faeces for elderly dogs fed the T0, T400, and T800 diets, respectively.
Fig. 2.
Faecal IgA concentration in adult and elderly dogs before and after consumption of experimental diets.
4. Discussion
The animals had good diet acceptance and, regardless of age and specific diet, the dogs maintained their weight throughout the study, with individual adjustments of food intake according to metabolizable energy content of experimental diets and energy requirements of each animal (NRC, 2006).
The use of AFM itself is safe for dogs since there were no alterations in the quality of faeces or the clinical profiles of the animals, and the haematological and biochemistry parameters were within reference ranges. Recent studies have demonstrated that a dog’s immune system is affected by age (Strasser et al., 2000; Greeley et al., 2001; Heaton et al., 2002; Day, 2010; Gomes et al., 2011; Fujiwara et al., 2012; Bauer and Fuente, 2016), and according to Alexander et al. (2018), the immune response declines with ageing, a phenomenon called immunosenescence.
The results of the monocyte phagocytic activity tests do not seem relevant since there was no effect of age or diet. Dogs fed the diet with 0.04 % AFM had a tendency towards higher neutrophil phagocytic activity than dogs fed the diet with 0.08 % AFM. However, there was no difference in this parameter when the control and T400 diets were compared. Therefore, further studies on phagocytic activities and AFM are recommended.
Regarding the production of reactive intermediates of oxygen, it was observed that LPS-stimulated neutrophils of animals that consumed the T400 diet tended to produce more H2O2 than those of dogs fed the control diet. AFM may favour neutrophil activity, which is important to innate immunity. Other authors also observed AFM effects on the innate immune system of dogs and other species (Abbas et al., 2011; Che et al., 2012; Lea et al., 2013).
Neutrophils and macrophages of elderly humans have impaired capacity to produce oxidative bursts and nitrogenated oxidants. As a result, these cells have a lower capacity than those of a younger individual to destroy ingested bacteria (Plackett et al., 2004). Such alterations were not verified in the present study, as an age effect was not detected in these cells. Only a lower segmented neutrophil count in elderly dogs was observed, but the functional activity of these phagocytic cells was not impaired. However, LPS-stimulated neutrophils of animals fed the diet with 0.04 % AFM had a tendency for increased H2O2 production, indicating potential beneficial effects of addition of mannoproteins in the diet of elderly dogs.
Development of the acquired immune response depends on rapid expansion of lymphocyte numbers. In the present study, the lymphoproliferation assay presented important variation in results among animals, and only a time effect was observed (P < 0.05), which makes discussing these results difficult because they were independent of diet, age, or interactions.
Regarding leucocyte subpopulations, compared to adult dogs, elderly dogs presented reduced absolute counts of CD5+ (total T lymphocytes), CD21+ (B lymphocytes), CD4+ (auxiliary) cells, elevation in CD8+ (cytotoxic) cell counts, and reduction in the CD4:CD8 ratio; these results were similar to those obtained by Day (2010). A decrease in CD5+ cells may be attributed to a loss of CD4+ cells, although CD21+ and CD8+ cells also decreased but in a slower rhythm than CD4+ cells (Blount et al., 2005). Fujiwara et al. (2012) evaluated 44 beagles ranging from 2.5–10 years and observed decreased absolute counts of CD4+ and CD8+ cells with ageing. Similar results were described by Greeley et al. (2001), who observed a decrease in the absolute numbers of CD5+, CD4+, and CD8+ cells and the percentage of CD21+ cells with ageing; Heaton et al. (2002) observed a decrease in CD4+ cells and the CD4+/CD8+ ratio with ageing; Faldyna et al. (2001), who evaluated dogs of different breeds, observed both increases and decreases in CD8+ lymphocytes; and Gomes et al. (2011) observed a decrease in CD5+, CD8+, and CD21+ cells and maintenance of CD4+ cells in older dogs.
The delayed cutaneous hypersensitivity test (DCHT) is an intradermal test that evaluates the reaction ability of the cell-mediated immune response (Kim et al., 2000). It is known that mannoproteins induce hypersensitivity reactions and the production of cytokines (Chaka et al., 1997; Pietrella et al., 2001). After 28 d, regardless of age, a significant effect of time was observed when inoculations of phytohemagglutinin and vaccines were performed. Saline intradermal inoculation was an efficient negative control since it promoted cutaneous swelling on site soon after inoculation (0 h), had a rapid absorption, and then returned to basal levels.
As in the study by Kim et al. (2000), dogs previously immunized with the same vaccine were used to ensure previous sensitization. However, those authors used dogs at 1 yr of age, and therefore, the maximum immune response due to immunization stimulation may have differed from that of the present study.
When an antigen is inoculated, it is phagocytosed by Langerhans cells, which migrate to the regional lymph node and present the antigen to memory T cells. The T cells then respond with the production of helper T lymphocytes (Th1) that recognize the antigen in the skin and accumulate around it, and then, an inflammatory response develops (Tizard, 2009). It is known that elderly dogs present less naïve T cells and more memory T cells than young dogs (Fujiwara et al., 2012; Tizard, 2009). However, Fujiwara et al. (2012) did not find differences between memory T cell levels when comparing dogs of 2, 6, and 10 years of age.
Regardless of age, the diet with 0.08 % AFM was efficient in promoting increases in skin thickness (mm) of animals, suggesting increased sensitization of nonspecific immune responses caused by the T800 diet. According to Tizard (2009), phytohemagglutinin is a T cell-stimulating lectin that causes local tissue reactions with many aspects of delayed hypersensitivity responses. However, its response is not specific, and its interpretation may be difficult.
Regarding the mucosal immunity evaluation, no difference was observed between adult and elderly dogs in faecal immunoglobulin A (IgA), similar to results found by Zaine et al. (2011). However, those authors observed that young Beagles presented lower concentrations of faecal IgA than adult and elderly dogs. Regarding diet, it was observed that despite variation in faecal IgA concentrations throughout time course, the treatments did not influence this parameter, and this result may be related to individual variation.
Constant IgA production, which is secreted in large amounts on the intestinal mucosal surface, occurs because of continuous stimulation by the intestinal microbiota (Gomes et al., 2011). Beneficial bacteria indirectly affect the immune system by producing substances with immunostimulatory properties that interact with the immune system in many ways, including cytokine production, mononuclear cell proliferation, macrophagic phagocytosis, and induction of immunoglobulin synthesis (Macfarlane and Cummings, 1999). Therefore, the influence of diet may be better understood by evaluating the microbiota present in the intestinal lumen after addition of mannoproteins. Although current research has not evaluated this parameter, a study performed by Swanson et al. (2002) demonstrated that supplementation with 0.3 % MOS positively influenced microbial populations, with a tendency for increasing the abundance of Lactobacillus spp., along with a tendency to increase serum IgA and ileal IgA levels when associated with intake of fructooligosacharides (FOSs). Middelbos et al. (2007) reported increased ileal IgA with the addition of 0.25 % yeast cell wall. By studying the oral intake of 225 mg of beta-1,3/1,6-glucans in the form of tablets, Stuyven et al. (2010) found a significant reduction in salivary and lacrimal IgA and suggested that this decrease was due to production of Th1 cells induced by the cytokines IL-12 and TNF-α secreted after interaction of beta-glucan with the dectin-1 receptor.
The present study demonstrates that mannoproteins may have effects on innate and acquired immune responses. Both complement each other to guarantee defence against infectious agents and foreign substances, and both take part in an integrated immune system, acting in cooperation for defence (Abbas et al., 2011). Characteristic mechanisms by which active mannoprotein fractions act on immunity, however, are not known. Immune modulation as a result of prebiotics intake appears to act on GALT, secondary lymphoid tissues, and peripheral cells, according to Schley and Field (2002), possibly because of direct contact with lactic acid bacteria, products of the cell wall or cytoplasmic components of intestinal cells, by production of short-chain fatty acids derived from fermentation, or through mucin production changes. Mansour et al. (2002) established that AFMs act by binding to mannose receptors in macrophages, which serve as a link between the innate and acquired immune responses, and the MOS fraction acts to activate macrophages by occupying mannose receptor sites of glycoproteins on their cell surface. Once these sites are occupied, a chain reaction begins, resulting in the activation of macrophages and cytokine secretion.
All of the observed effects are relevant; however, there are few studies with mannoproteins and the effect of ageing on the immune system of dogs. Therefore, additional studies are necessary to better understand the mechanism of action of mannoproteins and how they can modulate the immune system in both adult and elderly dogs.
5. Conclusion
In the conditions of the present study, active fractions of mannoproteins derived from yeast cell wall stimulated innate and acquired immunity of adult and elderly dogs, with beneficial immunomodulating effects. Active fractions of mannoproteins had effects on some parameters of the specific and nonspecific immune responses mediated by cells, such as a increased percentage of phagocytic activity by neutrophils, increased production of H2O2, and enhanced cutaneous responses in elderly dogs to vaccine and phytohemagglutinin stimuli. Elderly dogs are affected by the immunosenescence process, as evidenced by a decrease in lymphocytic counts and the proliferation ability of these cells. Therefore, the immune responses of dogs may benefit from the addition of active fractions of mannoproteins in the diet.
CRediT authorship contribution statement
F.S.A. Kroll: Validation, Investigation, Formal analysis. T.C. Putarov: Formal analysis, Resources. L. Zaine: Formal analysis, Resources. K.S. Venturini: Formal analysis, Resources. C.G. Aoki: Formal analysis, Resources. J.P.F. Santos: Formal analysis, Resources. V. Pedrinelli: Formal analysis, Writing - review & editing. T.H.A. Vendramini: Formal analysis, Investigation, Writing - original draft. M.A. Brunetto: Conceptualization, Methodology, Visualization, Supervision. A.C. Carciofi: Conceptualization, Methodology, Supervision.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest to the current manuscript
Acknowledgements
The authors are grateful to FAPESP (process 2011/17036-2) for a studentship awarded to the first author and to Alltech for financial support.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.anifeedsci.2020.114392.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Abbas A.K., Lichtman A.H., Pillai S. 7th. Elsevier Health Sciences.; Philadelphia: 2011. Cellular and Molecular Immunology. [Google Scholar]
- Alexander J.E., Colyer A., Haydock R.M., Hayek M.G., Park J. Understanding how dogs age: longitudinal analysis of markers of inflammation, immune function, and oxidative stress. J. Gerontol. A Biol. Sci. Med. Sci. 2018;73:720–728. doi: 10.1093/gerona/glx182. [DOI] [PubMed] [Google Scholar]
- Bauer M.E., Fuente M. The role of oxidative and inflammatory stress and persistent viral infections in immunosenescence. Mech. Ageing Dev. 2016;158:27–37. doi: 10.1016/j.mad.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Blount D.G., Pritchard D.I., Heaton P.R. Age-related alterations to immune parameters in Labrador retriever dogs. Vet. Immunol. Immunopathol. 2005;108:309–407. doi: 10.1016/j.vetimm.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Brown G.D., Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- Chaka W., Verheul A.F., Vaishnav V.V., Cherniak R., Scharringa J., Verhoef J., Snippe H., Hoepelman A.I. Induction of TNF-α in human peripheral blood mononuclear cells by the mannoprotein of Cryptococcus neoformans involves human mannose binding protein. J. Immunol. 1997;159:2979–2985. [PubMed] [Google Scholar]
- Che T.M., Song M., Liu Y., Johnson R.W., Kelley K.W., van-Alstine W.G., Dawson K.A., Pettigrew J.E. Mannan oligosaccharide increases serum concentrations of antibodies and inflammatory mediators in weanling pigs experimentally infected with porcine reproductive and respiratory syndrome virus. J. Anim. Sci. 2012;90:2784–2793. doi: 10.2527/jas.2011-4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold S.P., Metcalfe S. Monoclonal antibodies that define canine homologues of human CD antigens: summary of the First International Canine Leukocyte Antigen Workshop (CLAW) Tissue Antigens. 1994;43:137–154. doi: 10.1111/j.1399-0039.1994.tb02315.x. [DOI] [PubMed] [Google Scholar]
- Day M.J. Ageing, immunosenescence and inflammageing in the dog and cat. J. Comp. Pathol. 2010;142:60–69. doi: 10.1016/j.jcpa.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Delgado G.T.C., Tamashiro W., Pastore G.M. Immunomodulatory effects of fructans. Food Res. Int. 2010;43:1231–1236. [Google Scholar]
- Faldyna M., Leva L., Knotigova P., Toman M. Lymphocyte subsets in peripheral blood of dogs – a flow cytometric study. Vet. Immunol. Immunopathol. 2001;82:23–37. doi: 10.1016/s0165-2427(01)00337-3. [DOI] [PubMed] [Google Scholar]
- FEDIAF . European Pet Food Industry Federation; Brussels, Belgium: 2019. Nutritional Guidelines for Complete and Complementary Pet Food for Cats and Dogs. [Google Scholar]
- Field C.J., McBurney M.I., Massimino S., Hayek M.G., Sunvold G.D. The fermentable fiber content of the diet alters the function and composition of canine gut associated lymphoid tissue. Vet. Immunol. Immunopathol. 1999;72:325–341. doi: 10.1016/s0165-2427(99)00148-8. [DOI] [PubMed] [Google Scholar]
- Franceschi C., Bonafè M., Valensin S., Olivieri F., De Luca M., Ottaviani E., De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Fujiwara M., Yonezawa T., Arai T., Yamamoto I., Ohtsuka H. Alterations with age in peripheral blood lymphocytes subpopulations and cytokine synthesis in beagles. Vet. Med. Res. Rep. 2012;3:79–84. doi: 10.2147/VMRR.S32590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes M.O.S., Beraldo M.C., Putarov T.C., Brunetto M.A., Zaine L., Gloria M.B.A., Carciofi A.C. Old beagle dogs have lower fecal concentrations of some fermentation products and lower peripheral lymphocyte counts than young adult beagles. Br. J. Nutr. 2011;106:187–190. doi: 10.1017/S0007114511002960. [DOI] [PubMed] [Google Scholar]
- Goodridge H.S., Wolf A.J., Underhill D.M. Beta-glucan recognition by the innate immune system. Immunol. Rev. 2009;230:38–50. doi: 10.1111/j.1600-065X.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeley E.H., Ballam J.M., Harrison J.M., Kealy R.D., Lawler D.F., Segre M. The influence of age and gender on the immune system: a longitudinal study in Labrador retriever dogs. Vet. Immunol. Immunopathol. 2001;82:57–71. doi: 10.1016/s0165-2427(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Grieshop C.M. the interaction of nutrition and the immune system: the role of fatty acids, antioxidants and carbohydrates. Alltech´s Annual Symposium. Proceedings from the Alltech 18th Annual Symposium. Lexington; Nottingham University Press; 2002. pp. 481–487. [Google Scholar]
- Han F., Fan H., Yao M., Yang S., Han J. Oral administration of yeast β-glucan ameliorates inflammation and intestinal barrier in dextran sodium sulfate-induced acute colitis. J. Funct. Foods. 2017;35:115–126. [Google Scholar]
- Heaton P.R., Blount D.G., Devlin P., Koelsch S., Mann S.J., Smith B.H.E., Stevenson J., Harper J.E. Assessing age-related changes in peripheral blood leukocyte phenotypes in labrador retriever dogs using flow cytometry. J. Nutr. 2002;132:1655–1657. doi: 10.1093/jn/132.6.1655S. [DOI] [PubMed] [Google Scholar]
- Hooge D.M., Kiers A., Connolly A. Meta-analysis summary of broiler chicken trials with dietary actigenTM (2009-2012) Int. J. Poult. Sci. 2013;12:01–08. [Google Scholar]
- Janeway C.A., Jr, Travers P., Walport M., Capra J.D. Elsevier Science Ltd./Garland Publishing; New York: 1999. Immunobiology: The Immune System in Health and Disease. [Google Scholar]
- Kim H.W., Chew B.P., Wong T.S., Park J.S., Weng B.B.C., Byrne K.M., Hayek M.G., Reinhart G.A. Dietary lutein stimulates immune response in dogs. Vet. Immunol. Immunopathol. 2000;74:315–327. doi: 10.1016/s0165-2427(00)00180-x. [DOI] [PubMed] [Google Scholar]
- Kollár R., Reinhold B.B., Petrakova E., Yeh H.J., Ashwell G., Drgonova J., Kapteyn J.C., Klis F.M., Cabib E. Architecture of the yeast cell wall: β(1-6)-glucan interconnects mannoprotein, β(1-3)-glucan, and chitin. J. Biol. Chem. 1997;272(17762-):17775. doi: 10.1074/jbc.272.28.17762. [DOI] [PubMed] [Google Scholar]
- Laflamme D.P. Development and validation of body condition score system for dogs. Canine Pract. 1997;22:10–15. [Google Scholar]
- Laflamme D.P., Gunn-Moore D. Nutrition of aging cats. Vet. Clin. Small Anim. 2014;44:761–774. doi: 10.1016/j.cvsm.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Larsen J.A., Farcas A. Nutrition of aging dogs. Vet. Clin. Small Anim. 2014;44:741–759. doi: 10.1016/j.cvsm.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Lea H., Spring P., Taylor-Pickard J., Burton E. A natural carbohydrate fraction Actigen™ from Saccharomyces cerevisiae cell wall: Effects on goblet cells, gut morphology and performance of broiler chickens. J. Appli. Anim. Nutr. 2013;1:1–7. [Google Scholar]
- Li J. Companion Animal Nutrition Summit, Gerontology An Inside Out Perspective, Charleston, SC, Clearwater Charleston. 2018. Searching for nutrition targets: multi-omics study in early-stage myxomatous mitral valve disease in dogs; pp. 25–30. [Google Scholar]
- Macfarlane G.T., Cummings J.H. Probiotics and prebiotics: can regulating the activities of intestinal bacteria benefit health? Br. Med. J. 1999;18:999–1003. doi: 10.1136/bmj.318.7189.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour M.K., Schlesinger L.S., Levitz S.M. Optimal T cell responses to Cryptococcus neorformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. J. Immunol. 2002;168:2872–2879. doi: 10.4049/jimmunol.168.6.2872. [DOI] [PubMed] [Google Scholar]
- Middelbos I.S., Godoy M.R., Fastinger N.D., Fahey G.C., Jr A dose-response evaluation of spray-dried yeast cell wall supplementation of diets fed to adult dogs: effects on nutrient digestibility, immune indices, and fecal microbial populations. J. Anim. Sci. 2007;85:3022–3032. doi: 10.2527/jas.2007-0079. [DOI] [PubMed] [Google Scholar]
- Mittendorfer B. Companion Animal Nutrition Summit, Gerontology An Inside Out Perspective, 2018, Charleston, SC, Clearwater Charleston. 2018. The role of n-3 PUFA on muscle mass and function in aging humans; pp. 37–42. [Google Scholar]
- NRC . National Academic Press.; Washinton, D.C: 2006. Nutrient Requirements of Dogs and Cats. [Google Scholar]
- Peters I.R., Calvert E.L., Hall E.J., Day M.J. Measurement of immunoglobulin concentrations in the feces of healthy dogs. Clin. Diagn. Lab. Immunol. 2004;11:841–848. doi: 10.1128/CDLI.11.5.841-848.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick E., Keisari Y.A. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Methods. 1980;38:161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J. Immunol. Methods. 1981;46:211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Pietrella D., Cherniak R., Strappini C., Perito S., Mosci P., Bistoni F., Vecchiarelli A. Role of mannoprotein in induction and regulation of immunity to Crytococcus neoformans. Infect. Immun. 2001;69:2808–2814. doi: 10.1128/IAI.69.5.2808-2814.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plackett T.P., Boehmer E.D., Faunce D.E., Kovacs E.J. Aging and innate immune cells. J. Leukoc. Biol. 2004;76:291–299. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- Ruiz-Herrera J. CRC Press; Boca Raton: 1991. Fungal Cell Wall: Structure, Synthesis, and Assembly. [Google Scholar]
- Samartin S., Chandra R.K. Obesity, overnutrition and the immune system. Nutr. Res. 2001;21:243–262. [Google Scholar]
- SAS . SAS Institute; Cary: 1996. Stastistical Analysis System: Users Guide. [Google Scholar]
- Schley P.D., Field C.J. The immune-enhancing effects of dietary fibers and prebiotics. Br. J. Nutr. 2002;87:221–230. doi: 10.1079/BJNBJN/2002541. [DOI] [PubMed] [Google Scholar]
- Sigal L.H., Ron Y. McGraw-Hill; New York: 1994. Immunology and Inflammation. Basic Mechanisms and Clinical Consequences. [Google Scholar]
- Strasser A., Teltscher A., May B., Sanders C., Niedermuller H. Age-associated changes in the immune system of German Shepherd dogs. J. Vet. Med. 2000;47:181–192. doi: 10.1046/j.1439-0442.2000.00278.x. [DOI] [PubMed] [Google Scholar]
- Stuyven E., Verdonck F., Van Hoek I., Daminet S., Duchateau L., Remon J.P., Goddeeris B.M., Cox E. Oral administration of beta-1,3/1,6-glucan to dogs temporally changes total and antigen 1 specific IgA and IgM. Clin. Vaccine Immunol. 2010;17:281–285. doi: 10.1128/CVI.00344-09. https://dx.doi.org/10.1128%2FCVI.00344-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson K.S., Grieshop C.M., Flickinger E.A., Merchen N.R., Fahey G.C., Jr Effects of supplemental fructooligosaccharides and mannanoligosaccharides on colonic microbial populations, immune function and fecal odor components in the canine. J. Nutr. 2002;132:1717–1719. doi: 10.1093/jn/132.6.1717S. [DOI] [PubMed] [Google Scholar]
- Swanson K.S., Kuzmuk K.N., Schook L.B., Fahey G.C., Jr Diet affects nutrient digestibility, hematology, and serum chemistry of senior and weanling dogs. J. Anim. Sci. 2004;82:1713–1724. doi: 10.2527/2004.8261713x. [DOI] [PubMed] [Google Scholar]
- Tian X., Seluanov A., Gorbunova V. Molecular mechanisms determining lifespan in short and long lived species. Trends Endocrinol. Metab. 2017;28:722–734. doi: 10.1016/j.tem.2017.07.004. https://dx.doi.org/10.1016%2Fj.tem.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizard I.R. 9th ed. Elsevier; St Louis, MO: 2013. Veterinary Immunology: An Introduction. [Google Scholar]
- Tizard I.R. 8th ed. Elsevier; Rio de Janeiro, R.J: 2009. Imunologia Veterinária. [Google Scholar]
- Xiao W.D., Chen W., Sun L.H., Wang W.S., Zhou S.W., Yang H. The protective effect of enteric glial cells on intestinal epithelial barrier function is enhanced by inhibiting inducible nitric oxide synthase activity under lipopolysaccharide stimulation. Mol. Cell. Neurosci. 2011;46:527–534. doi: 10.1016/j.mcn.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Zaine L., Ferreira C., Gomes M.O., Monti M., Tortola L., Vasconcellos R.S., Carciofi A.C. Faecal IgA concentration is influenced by age in dogs. Br. J. Nutr. 2011;106:183–186. doi: 10.1017/S0007114511000559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.