Keywords: Zoonoses, One health, Spillover, Trypanosoma spp., Trichinella, Echinococcus spp., Giardia, Toxoplasma
Highlights
-
•
Wildlife is not always the original source of human infections.
-
•
We redress the balance in a one-health philosophy using parasite zoonoses.
-
•
Human activity is central to zoonotic transmission.
-
•
Spillover of ‘human’ parasites to naïve species of wildlife is an emerging threat.
-
•
Increased surveillance of wildlife populations is essential.
Abstract
This review examines parasite zoonoses and wildlife in the context of the One Health triad that encompasses humans, domestic animals, wildlife and the changing ecosystems in which they live. Human (anthropogenic) activities influence the flow of all parasite infections within the One Health triad and the nature and impact of resulting spillover events are examined. Examples of spillover from wildlife to humans and/or domestic animals, and vice versa, are discussed, as well as emerging issues, particularly the need for parasite surveillance of wildlife populations. Emphasis is given to Trypanosoma cruzi and related species in Australian wildlife, Trichinella, Echinococcus, Giardia, Baylisascaris, Toxoplasma and Leishmania.
1. Introduction
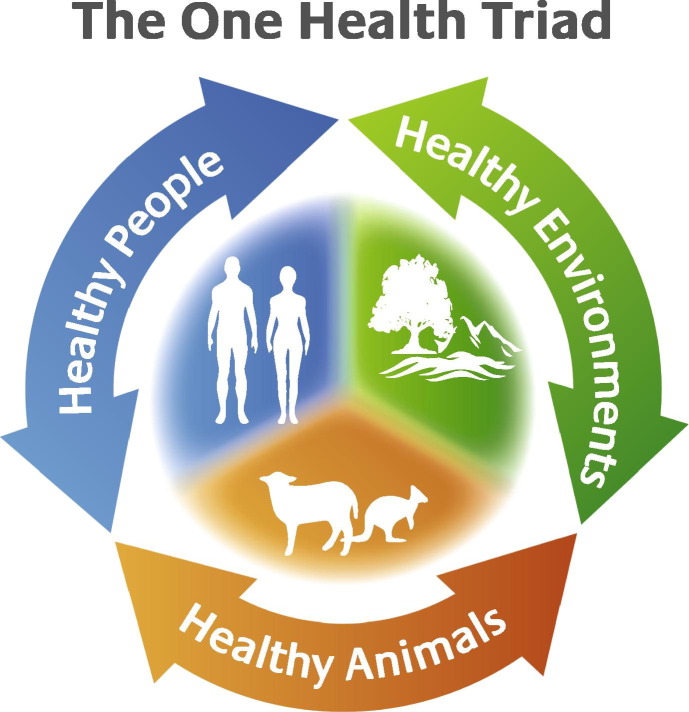
Nearly 30 years ago, Calvin Schwabe referred to the dynamic state of flux and new discovery that has always characterised studies on zoonoses (Schwabe, 1984). This statement still holds true today, although the emphasis has been on the emergence of infectious diseases from wildlife that threaten human health and the role of wildlife as originators of the infectious agents (Polley, 2005, Jones et al., 2008, Rhyan and Spraker, 2010, Plowright et al., 2011, Wood et al., 2012, Kooriyama et al., 2013). As such, models fail to include spillover from humans to wildlife (e.g., Lloyd-Smith et al., 2009). This is understandable but at the same time unfortunate as it tends to cloud the broader issues which, in theory, make up the so-called ‘One Health’ triad (Fig. 1 ). Diseases of human and domestic animal origin do infect wildlife (Thompson et al., 2009, Thompson et al., 2010a, Salyer et al., 2012, Kooriyama et al., 2013). However, in practice the balance is often skewed towards demonstrating the ‘source’ of ‘new’ human diseases rather than determining ‘why’ in terms of One Health. As such, we need to better understand the factors that enable zoonotic transmission to humans from wildlife, and which may lead to outbreaks of disease. Is it purely a question of spillover from wildlife to humans, or are wildlife reservoirs resulting from spillover from a non-wildlife source? The impact of spillover of ‘human’ parasites to naïve species of wildlife is another emerging threat that is not well understood yet such spillovers are likely to increase in the future, establishing novel spill-back reservoirs of potential public health and economic significance, as well as threatening wildlife (Thompson et al., 2009, Thompson et al., 2010a).
Fig. 1.
The ‘One Health’ triad, encompassing the collaborative goals of providing optimal health for people, animals (domestic and wild) and the environment by considering interactions between all three systems.
The situation is compounded by how little we know of what infectious agents occur naturally in wildlife and which of these could have the potential to establish infection in domestic hosts. There is a pronounced lack of knowledge about pathogen diversity and susceptibility in wildlife (MacPhee and Greenwood, 2013). Without improved and ongoing surveillance of wildlife hosts (Polley, 2005), not only will we always be behind in terms of predicting the possibility of reservoirs being established and/or outbreaks occurring, but also at a disadvantage in preventing declines of native fauna resulting from infectious disease and understanding the circumstances promoting an infection to a disease state. Much of the information on wildlife parasites has been obtained opportunistically, often at the level of individual animals. Furthermore, all extinction and many population decline studies are retrospective and this lack of knowledge affects our understanding of parasites that effect wildlife (MacPhee and Greenwood, 2013, Wayne et al., 2013a, Wayne et al., 2013b).
Much of the commentary in this area has focussed on viral diseases such as HIV, Severe Acute Respiratory Syndrome (SARS), Hendra, Nipah and bird flu (H5N1) (Jones et al., 2008, Quammen, 2012). Generalisations made in the context of these emerging infectious diseases (EIDs) are often not applicable to the broad range of other infectious zoonotic agents, both prokaryotes and eukaryotes. In this respect, a consideration of parasite zoonoses caused by protozoa, helminths and arthropods is timely considering the diversity of the life cycle patterns and modes of transmission. In addition, considerations related to EIDs have often been reactionary (understandable due to the serious nature and rapid spread of viral diseases) with little consideration given to the ecology of the causative factors, such as the impact of biodiversity loss, human encroachment, the role of invasive species, and of course the conservation issues.
Furthermore, although some zoonotic infections cause frank disease in wildlife hosts, many infections of humans and domestic animals exist silently in wildlife species as infections which are not apparent (Schwabe, 1969). This usually reflects a long evolutionary history of adaptation and the acquisition of a balanced host parasite relationship. However, zoonotic infections are not always ‘silent’ in wildlife hosts and it is important to understand how some infections become diseases.
When such parasite zoonoses infect humans it is usually as a consequence of human influence or activity (anthropogenic). This may be passive as a result of poverty and other socioeconomic factors that enhance the risk of spillover, for example poor housing and Chagas disease; human encroachment on wildlife habitats and Baylisascaris; or climate change and waterborne diseases such as giardiasis. In contrast, there are numerous human activities, such as hunting, modifying wildlife populations by vaccination or translocation, tourism etc. (Table 1 ) that may increase the risk of zoonotic transmission.
Table 1.
Human activities that may influence the risk of zoonotic transmission involving wildlife.
| Human activity impact | Impact | Parasite examples |
|---|---|---|
| Poor housing | Poor hygiene; encourage vectors/IHs | Trypansoma cruzi |
| Socioeconomic factors | Poor hygiene; lack of education/awareness | Many |
| Lack of surveillance | Lack of control/awareness | Trichinella; Echinococcus |
| Climate change | Distribution of vectors/IHs | Leishmania; Trypanosoma; Echinococcus multilocularis; Screworm/myiasis e.g., Cochliomyia hominivorax, Dermatobia hominis, Chrysomya bezziana, Cordylobia anthrophoga, Callitroga spp. etc. |
| Hunting (recreational/subsistence) | Exposure/ingestion of parasites by humans or domestic hosts | Trichinella; Toxoplasma; Echinococcus; Sarcocystis |
| Vaccination-wildlife host control | Changes to host distribution | Potentially many |
| Vaccination-disease control | Rabies control and increase in fox numbers | E. multilocularis |
| Therapeutic interventions in wildlife hosts | Altered host–parasite relationship/polyparasitism | Various but e.g., Baylisascaris; E. multilocularis |
| Migration of humans | Exposure/introduction to novel parasites | T. cruzi; Leishmania spp; Plasmodium knowlesi |
| Tourism | Exposure to wildlife parasites | Trichinella; Sarcocystis |
| Pet travel | Exposure to exotic parasites | Leishmania; Echinococcus |
| Lack of control of domestic hosts (diet/roaming) | Exposure of wildlife to domestic parasites | Echinococcus; Giardia; Toxoplasma; Sarcoptes |
| Farming | Keeping wildlife/pasture management | Echinococcus canadensis/E. multilocularis |
| Landscape changes | Deforestation/expansion of vectors/IHs | T. cruzi; E. multilocularis; P. knowlesi; Giardia |
| Translocation of wildlife hosts | Introduction/altered distribution of zoonotic agents | E. multilocularis; Baylisascaris |
| Wildlife introductions | Introduction/altered distribution of zoonotic agents | Echinococcus spp; Leishmania; Trypanosoma |
| Culture/traditions | Dietary factors | Trichinella; Toxoplasma |
| Importation of food | Introduction of parasites to non-endemic regions | Trichinella |
| Livestock trade | Exposure to exotic parasites | Screworm/myiasis e.g., C. hominivorax, D. hominis, C. bezziana, C. anthrophoga, Callitroga spp. etc. |
| Environmental contamination | Aquatic, marine, terrestrial | Giardia; Toxoplasma |
| Feeding | Urban wildlife | Toxoplasma |
In this review, I want to embrace the One Health philosophy and examine the circumstances promoting spillover of parasite zoonoses from wildlife to humans and domestic animals, as well as from domestic foci of transmission to wildlife, as a result of human activity (Fig. 2 , Table 1). Fig. 2 illustrates the possible flow of parasite transmission between humans, domestic animals and wildlife in different host ecosystems. In urban areas, wildlife may be synanthropes (ecologically associated with domestic environments) or adapters (able to adapt to urban conditions but can utilise natural resources) (Blair, 1996, Shochat et al., 2006).
Fig. 2.
Illustration of the possible flow of parasite transmission between humans, domestic animals and wildlife in different host ecosystems.
2. Spillover from wildlife to humans and/or domestic animals
The majority of parasite zoonoses for which wildlife are the main reservoir, and probably represent the hosts in which the parasite relationship evolved, are characterised by having little clinical impact on their wildlife hosts in terms of overt disease. However, there are a few zoonotic parasites such as Echinococcus with indirect life cycles that require the ingestion of larval stages in their intermediate hosts. This may enhance the chance of predation by the carnivore definitive host through a predilection for lung involvement by the larval cystic stage in the intermediate host, and thus a reduction in exercise tolerance (Durie and Riek, 1952; Barnes et al., 2007).
Such zoonoses are primarily perpetuated in natural life cycle patterns with spillover to humans resulting from human activity (Table 1).
2.1. Trypanosoma cruzi
Chagas disease is one of the most important of the neglected diseases, largely confined to poorer areas of many countries of South America where it results in a broad spectrum of disease states, both acute and chronic, exerting extreme morbidity in approximately 10 million people (Coura and Vinas, 2010, Hotez et al., 2012). The disease is difficult to treat and the few drugs that are available are often toxic (Keenan et al., 2013). The causative agent is T. cruzi, a vector-borne stercorarian trypanosome that exhibits pronounced genetic diversity reflected in differences in host specificity of both the vector and a large range of wildlife hosts (Zingales et al., 2012). In terms of zoonotic transmission, T. cruzi has established several cycles of transmission involving numerous sylvatic, peridomestic and domestic triatomid insect vectors. It is thought that humans would have first been exposed to T. cruzi when they migrated to the New World approximately 30,000 years ago and first came into contact with the arthropod vectors of the parasite (Hamilton et al., 2012).
Thus the principal determinant for zoonotic transmission is proximity to infected vectors. This is usually associated with socioeconomic factors, changes in land use and poor living conditions that provide suitable resting sites for vectors, plus close proximity to wildlife habitats which provide the reservoirs of T. cruzi infection (Acha and Szyfres, 2003, Karesh et al., 2012). Improved housing would thus do much to lower the incidence of Chagas disease in endemic regions by reducing exposure to the vectors. A recent study in Columbia has also found evidence that domestic dogs are susceptible to infection with genotypes of T. cruzi normally associated with sylvatic transmission (Ramirez et al., 2013). These authors therefore consider that dogs provide a link between domestic and wildlife cycles of transmission.
The emerging issue with Chagas disease is its globalisation (Hotez et al., 2012) as a result of migration of millions of Chagas sufferers to non-endemic regions of the world (Coura and Vinas, 2010). This is a particular problem in parts of the USA where triatomid vectors occur and local transmission is possible with wild rodents acting as reservoirs, often of multiple strains (Charles et al., 2013). The major problem in non-endemic regions is a lack of awareness among the medical profession of a chronic disease, usually with a late onset of symptoms that are often vague and non-specific (WHO, 2011, and see Section 4.1.1). It is therefore important for authorities in non-endemic regions to recognise the importance of raising awareness among the medical profession and evaluating the potential for local transmission, whether this is through vectors or directly in blood transfusions (WHO, 2011).
2.2. Echinococcus multilocularis
The cestode E. multilocularis is principally maintained in wild animal cycles involving foxes as definitive hosts and microtine rodents as intermediate hosts. Spillover to humans results in severe and frequently incurable disease (Hegglin and Deplazes, 2013) as a consequence of the metastasising nature of infection with the larval parasite (Thompson, 1995). In the past, zoonotic transmission was associated with occupational activities such as fox trapping (Schantz et al., 1995) but over the last two decades it has emerged as a major urban zoonosis as a consequence of various human activities (Table 1) and exacerbated by the fox, to a lesser extent the coyote and the arvicolid rodent intermediate hosts as very successful urban adaptors (Deplazes et al., 2004, Romig et al., 2006, Catalano et al., 2012). Another urban adaptor, the raccoon dog (Nyctereutes procyonoides), which is invading western Europe from the east, is an excellent definitive host for E. multilocularis, comparable to the fox (Kapel et al., 2006, Thompson et al., 2006a), but its future role in transmission is uncertain given differences in the ecology of foxes and raccoon dogs (Bružinskaitė-Schmidhalter et al., 2012).
The urbanisation of the life cycle of E. multilocularis is now a major public health issue in Europe and Japan, and an emerging issue in Canada. Anthropogenic landscape changes such as deforestation and overgrazing have resulted in an expansion of known intermediate hosts in Europe, North America and Tibet (Schantz et al., 1995, Deplazes et al., 2004, Romig et al., 2006, Wang et al., 2007, Davidson et al., 2012) and new intermediate hosts, such as the southern red-backed vole (Myodes gapperi), have been identified in urban areas in Canada (Liccioli et al., 2012). Since the 1980s the autochthonous presence of E. multilocularis has been reported in 17 European countries that were previously considered non-endemic (Davidson et al., 2012). In other areas of the world such as the Circumpolar North/Arctic, the public health significance of the parasite is an emerging issue in villages and communities since once infection spills over from infected wildlife, free roaming domestic dogs with access to stable and abundant populations of intermediate hosts are an effective definitive host and the main source of human infection (Davidson et al., 2012, Jenkins et al., 2013).
Translocations of wildlife have also contributed to the expansion of the range of E. multilocularis, including the accidental introduction of microtine rodents to Svalbard in the Norwegian Arctic, deliberate translocation of foxes within the southeastern USA for recreational hunting purposes, and the movement of foxes for rodent control from endemic Kuriles to Rebun Island off the northwestern coast of Hokkaido, Japan (Davidson et al., 1992; Schantz et al., 1995, Romig et al., 2006, Davidson et al., 2012). Echinococcus multilocularis is not endemic in the UK and thus the recent report of infection in a captive European beaver, 4 years after being imported from Germany (Barlow et al., 2011), raises concerns should such imported animals escape or be released.
2.3. Echinococcus canadensis
Echinococcus canadensis presents a similar picture to that of E. multilocularis, although zoonotic transmission is much more restricted. This species of Echinococcus is also maintained in a wild animal cycle involving wolves and large cervids (moose and caribou) that harbour the non-invasive cystic larval stage, usually in their lungs. This cycle occurs in northern Canada, Alaska and parts of Scandinavia. Domestic or free-roaming dogs are important “bridging hosts” between wildlife and people (Rausch, 2003). Spillover from sylvatic foci most commonly occurs as a result of subsistence hunting within indigenous communities where dogs have access to offal and carcasses (Jenkins et al., 2013). In this respect, indigenous patients are over-represented as being infected. A domestic cycle of transmission involving domestic dogs and farmed elk was also recently identified in western Canada (Thompson et al., 2006b).
Unlike Echinococcus granulosus, E. canadensis appears to be less virulent in humans, often giving rise to asymptomatic cases which may remain undiagnosed. However, there are two genotypes of E. canadensis, G8 and G10, and there is some evidence that G8 may be more virulent than G10 (McManus et al., 2002, Thompson et al., 2006b). This needs to be further examined as there is growing awareness of the occurrence of E. canadensis in dogs in communities in northern Canada (Himsworth et al., 2010).
2.4. Trichinella
In terms of zoonotic considerations and the public health impact of Trichinella, much of the emphasis has been on the control of Trichinella spiralis in domestic environments, principally involving pigs and synanthropic hosts such as the rat. However, it is now realised that the maintenance of Trichinella spp. in a variety of wildlife transmission cycles and the impact of humans on these cycles is central to the zoonotic transmission of the parasite (Pozio et al., 2009).
Globally, most Trichinella infections occur in wildlife (Pozio, 2013). Infections in wildlife have been documented in 66 (33%) countries worldwide compared with 43 (21.9%) countries for domestic animals (Pozio, 2007). Thus the spillover from wildlife to domestic foci represents a constant challenge for control in both endemic regions and from a biosecurity viewpoint for those countries which are considered to be Trichinella-free.
The situation is exacerbated by the limitations of current detection procedures used for meat inspection. This is an emerging, politically driven issue as market forces become more competitive and demanding with respect to demonstrating pigs are Trichinella-free. For example, in the UK there is a perceived need for data gathering and assessment of Trichinella in UK wildlife even though it has rarely been detected in the past (Zimmer et al., 2009). However, although there is increasing awareness that wildlife, particularly rats, can serve as reservoirs of infection for pig producers, particularly in free-ranging situations, surveillance capability is limited by the lack of diagnostic sensitivity of current meat inspection procedures (Newell et al., 2010, van der Giessen et al., 2013). Pig farms need to show negligible risk of their animals becoming infected from wildlife. Similarly in Australia, the main concern regarding Trichinella is the ability of wildlife to become reservoirs and thus synanthropes and adaptors to be sources of infection for domestic animals and humans (AWHN, 2011) yet few surveys have been undertaken. Wildlife surveys are considered essential to truly determine the presence or absence of Trichinella but surveys in high-risk regions of incursion are largely lacking on the mainland of Australia (Cuttell et al., 2012). However, a recent research survey led to the first report of Trichinella papuae in a wild pig from an Australian island in the Torres Strait (Cuttell et al., 2012), a parasite not only infective to other mammals, including humans, but also to crocodiles (Pozio et al., 2009).
In areas such as the Arctic, tradition and hunter-based life styles enhance the potential for zoonotic infections in people, a situation exacerbated by a paucity of meat inspection services (Davidson et al., 2011, Jenkins et al., 2013). In other areas of the world, human infections from hunted wildlife are on the rise. These are not associated with subsistence but usually recreational hunting and often related to tourism activities (Houze et al., 2009). The latter is a particular cause for concern when the meat from hunted animals is taken to other countries with returning tourists, leading to local outbreaks (Ancelle et al., 2005).
3. Spillover from humans and/or domestic animals to wildlife
3.1. Giardia
Giardia serves as the archetypal example of a zoonotic parasite that is principally found in wildlife as a result of human activities. The role of wildlife such as beavers as amplifiers of Giardia of human origin following contamination of freshwater is well documented (reviewed in Thompson, 2004). Giardia has also been reported in numerous other species of wildlife, both terrestrial and aquatic, and in the majority of cases, zoonotic species have been recovered rather than a species of Giardia that is host-specific for a wildlife host (Ash et al., 2010, Johnston et al., 2010, Thompson et al., 2010a, Thompson et al., 2010b). Very few species of Giardia have been described in terrestrial wildlife. In contrast, zoonotic species have not been identified in birds which do harbour their own species of Giardia (Thompson and Monis, 2004, Thompson and Monis, 2012).
The sources of zoonotic Giardia infection in terrestrial wildlife is considered to be, as with beavers, the result of environmental contamination with Giardia from humans or domestic animals, principally cattle. This appears to be the case even in presumed pristine habitats such as the Canadian Arctic where muskoxen (Ovibos moschatus) have been found to be infected with two species of zoonotic Giardia, Giardia duodenalis and Giardia enterica (Kutz et al., 2008). Human infection is endemic in some communities in the area (Kutz et al., 2009; Hotez, 2010). It is thought that people introduced the Giardia and contaminated the ecosystem shared with muskoxen and as a result Giardia is now maintained in a sylvatic cycle in muskoxen and possibly cervids (Kutz et al., 2009, Jenkins et al., 2013). Thus a wildlife reservoir for human infection may have been established. The impact of infection on muskoxen, presumably a naïve host, and the consequences of coinfection with other parasites is not understood but should, as with other parasite systems (Johnson and Hoverman, 2012), form the basis for future studies.
Wolves have been found to be commonly infected with zoonotic species of Giardia in remote coastal British Columbia and domestic dogs have been suggested as a possible source of infection through environmental contamination (Bryan et al., 2011, Bryan et al., 2012). It is also possible wolves may have contracted infection by eating beavers infected with Giardia.
Giardia has been reported in several species of non-human primates in Africa and is considered to be a cause of morbidity (Teichroeb et al., 2009, Johnston et al., 2010). Where humans and their domestic animals living in close proximity to the infected primates have been examined, Giardia infection has been found. In these cases, molecular epidemiological investigations have demonstrated that humans and/or their livestock are the likely source of infection in the primates (Teichroeb et al., 2009, Johnston et al., 2010). In one study, polyparasitism in colobus monkeys with Giardia and Isospora belli resulting from anthropozoonotic transmission was reported (Teichroeb et al., 2009).
Giardia duodenalis has been reported in marine mammals on a number of occasions with the most likely source of contamination being sewage effluent and surface run-off (Appelbee et al., 2005, Appelbee et al., 2010, Robertson, 2007). Bivalve molluscs are excellent sentinels of environmental contamination with parasitic protozoa including Giardia and are a likely source of infection in some seal species (Appelbee et al., 2005).
In Australia, zoonotic Giardia spp. of human or domestic animal origin appear to be the most common source of infection in native wildlife (Thompson et al., 2010b). Only one host-adapted species of Giardia has been identified in Australian native animals, in bandicoots (Isoodon obesulus) (Adams et al., 2004). There is evidence that urban expansion and the re-colonisation of wildlife habitats in urban areas by species such as bandicoots and brush-tailed possums (Trichosurus vulpecula), will expose naïve wildlife to infection with zoonotic protozoa such as Giardia and Toxoplasma with unknown consequences for their health (Thompson et al., 2009).
3.2. Echinococcus spp.
Echinococcus granulosus represents the ‘European form’ of this species as defined by Rausch, and is considered to be distinct from the proposed ancestral ‘Northern form’, E. canadensis (Rausch, 1986). Throughout most of its geographic range, E. granulosus is perpetuated by domestic animals with the incidental infection of humans being infrequent. It exists almost invariably under conditions modified by humans (Rausch, 1986).
Echinococcus felidis is confined to Africa and may represent an indigenous form perpetuated in wildlife cycles involving the lion as the principal definitive host and the warthog as an intermediate host, although the role of other intermediate hosts is as yet unknown (Schantz et al., 1995, Huttner and Romig, 2009, Huttner et al., 2009). With the introduction and domestication of livestock in Africa, E. granulosus has become widespread in domestic livestock including sheep, cattle and goats, with Echinococcus intermedius also occurring in camels (Huttner et al., 2009, Addy et al., 2012). Over 18 species of wild herbivores from different parts of southern, central and eastern Africa have been found to be infected with hydatid cysts (Macpherson, 1983). These include the most common prey species of the lion (reviewed in Schantz et al., 1995) but without molecular characterisation of isolates from intermediate hosts it is not possible to determine the extent of transmission cycles of E. felidis in African wildlife. The existence of an independent cycle in wild mammals in Africa has been proposed (Macpherson et al., 1983, Rausch, 1986). This is supported on ecological grounds and if the lion is the principal definitive host, E. felidis is the species most likely to be involved since apart from hyaenas there is no evidence canids are susceptible to this species of Echinococcus. Molecular epidemiological studies are required to determine the species of Echinococcus in wild ungulates in Africa (Huttner et al., 2009). If it is E. granulosus then perhaps it has displaced the genetically closely related (Saarma et al., 2009) E. felidis over time, with E. felidis now confined to a lion warthog cycle.
In Australia, the situation is more clear-cut. There is no evidence of an indigenous species of Echinococcus or that E. granulosus was present in Australia before the arrival of European settlers, with whom E. granulosus was introduced in sheep (Thompson et al., 2010a). Indigenous macropodid marsupials are highly susceptible to infection with the cystic stage of E. granulosus and are commonly infected throughout the mainland in a wild animal cycle with the dingo as the definitive host. Cystic infection may seriously impair respiratory function and thus enhance predation of infected marsupials, particularly small species of wallabies (Barnes et al., 2007). This is thus a serious conservation issue. In addition, this artificial, man-made cycle, makes it very difficult to control or eradicate infection in sheep on the mainland of Australia due to infected dingoes contaminating sheep pasture (Jenkins et al., 2005).
Echinococcus multilocularis occurs in two distinct and geographically separated populations in central and North America, and in the northern Arctic tundra (Schantz et al., 1995, Jenkins et al., 2013). It is therefore of some concern that an apparently non-indigenous form of E. multilocularis has recently been identified in central British Colombia, Canada (Jenkins et al., 2012). This has been regarded as a non-endemic region for E. multilocularis and it is not known what impact the introduction of a novel form of the parasite could have on native wildlife. The parasite was identified in a domestic dog and characterised as the so-called ‘European strain’ of E. multilocularis (Jenkins et al., 2012). Further research is required to definitively determine the origin of this isolate of E. multilocularis, prospects for establishment, if not already present, and likely impact on wildlife. In Europe, inter-country pet travel presents an emerging risk for the introduction of zoonotic parasites such as E. multilocularis (Davidson and Robertson, 2012).
3.3. Baylisascaris
Baylisascaris procyonis is a well recognised zoonotic ascarid for which raccoons (Procyon lotor) are the principal definitive host (Kazacos, 2001). The eggs are highly resistant and can remain viable in the environment for years. The parasite has a very low specificity in its paratenic hosts with humans one of many hosts in which larvae can migrate, often leading to serious disease. The parasite seems to thrive better in terms of prevalence in association with human-dominated landscapes which put paratenic hosts under pressure and where domestic dogs can become infected and extend the range of environmental contamination (Page, 2013).
The synanthropic behaviour of raccoons has exacerbated the public health significance of B. procyonis in the USA (Page et al., 2009; Kellner et al., 2012). However, the impact of the parasite on conservation is an emerging issue that should not be overshadowed by the public health issue. Human factors leading to habitat loss and fragmentation, as well as translocation of raccoons, are the main drivers (Page, 2013). The low host specificity for paratenic hosts and the pathogenic potential of the migratory larval parasite is considered to be a potential contributor to the extinction of vulnerable species of wildlife (Page, 2013). As such, B. procyonis is considered to have contributed to local declines and extinctions of the Allegheny woodrat (Neotoma magister) (LoGiudice, 2006). Endangered Allegheny woodrats became infected with Baylisascaris when translocated into contaminated environments (LoGiudice, 2003). A recent intervention using anthelmintic baits was shown to facilitate woodrat recovery (Smyser et al., 2013). Other wildlife species are also thought to be at risk and not just in the USA, but also in Europe and Japan where raccoons have been introduced (Bauer, 2013, Page, 2013).
3.4. Toxoplasma
As with Trichinella, research over the last decade has demonstrated that Toxoplasma is far more prevalent in wildlife than previously considered, and affects a huge diversity of species, both terrestrial and marine mammals and birds, in all environments studied including the Arctic (Wendte et al., 2011, Pan et al., 2012, Jenkins et al., 2013). In addition, as more isolates from wildlife are characterised genetically using multiple loci, what is also apparent is the great genetic diversity of Toxoplasma in wildlife and the occurrence of novel genotypes which differ from the ‘standard’ domestic strains (Ajzenberg et al., 2004, Wendte et al., 2011, Pan et al., 2012). These observations raise many questions about the ecology of Toxoplasma infections in wildlife ecosystems and how it differs from that in domestic ecosystems from which we have obtained most of the information on which we have based our understanding of the population dynamics and transmission.
Firstly, little is known about the virulence of strains of Toxoplasma that are maintained in wildlife populations. Available evidence would suggest that they are largely avirulent in natural ecosystems (Thompson et al., 2010a, Pan et al., 2012). Toxoplasma is prevalent in wildlife ecosystems and exhibits a high level of genetic variation. Large-scale die offs have not been reported and most cases of clinical disease in wildlife are in captive animals (Thompson et al., 2010a). This may be as a result of stress and/or exposure of immunologically naïve animals to ‘domestic’ strains of Toxoplasma to which they have not previously been exposed. Interestingly, it has been proposed that biodiversity loss in wildlife populations may promote disease occurrence and heightened virulence of infectious agents that are characterised by having high genetic diversity (Johnson and Hoverman, 2012; Bolzoni et al., 2013; Johnson et al., 2013). This may well apply to Toxoplasma in wildlife ecosystems. Novel wildlife strains isolated from Australian native wildlife have proved to be avirulent in experimentally infected mice (Parameswaran et al., 2010). However, the novel Type X strain caused severe disease in stranded sea otters in California (Miller et al., 2008). Wild felid hosts in the same area were demonstrated to be infected with the same strain of Toxoplasma and it has been proposed that run-off resulted in coastal contamination with the exposure of filter feeding shellfish from which the sea otters became infected (Miller et al., 2008). However, it is not known whether this strain is normally perpetuated in wildlife cycles and/or whether the sea otters were immunologically compromised due to environmental stressors that have yet to be identified.
The maintenance of Toxoplasma in wildlife ecosystems is considered to result from environmental contamination with oocysts from wild or domestic felid hosts. For example, a recent study of Toxoplasma in moose and reindeer in Sweden found a higher seroprevalence in adult animals than in yearlings, indicating that most moose and reindeer did not become infected in utero but by environmental exposure to oocysts originating from domestic cats and/or lynx, as the animals browse (Malmsten et al., 2011). However, in other environments it is difficult to understand how environmental contamination can be the sole source of infection in wildlife ecosystems. This is certainly the case in arid areas where felid hosts are not common, for example in parts of Australia where high levels of infection occur in native marsupials (Pan et al., 2012). Under such circumstances, the maintenance of infection by vertical transmission cannot be dismissed in the face of mounting epidemiological evidence from both wild and domestic ecosystems (Hide et al., 2009, Parameswaran et al., 2009).
As with Giardia, urban expansion and the re-colonisation of wildlife habitats in urban areas by wildlife species will expose them to Toxoplasma (Lehrer et al., 2010), some of which are likely to be novel strains not circulating in wildlife habitats. Sources of infection include environmental contamination by domestic cats but also food, particularly meat scraps left in gardens to encourage wildlife. In Perth, cases of neurological disease in bandicoots taken to wildlife carers have been shown to be due to Toxoplasma infection, most likely as a result of eating household scraps (personal observation).
4. Emerging issues
4.1. Surveillance
We know very little about the normal parasite fauna of wildlife populations (Mathews, 2009, Thompson et al., 2010a). Much of what we do know is fragmented and often obtained opportunistically or as part of targeted investigations. It is becoming increasingly important to understand the impact and transmission of parasites in wildlife populations. In addition to zoonotic potential, parasites can have a negative impact on the regulation of wildlife populations (Watson, 2013) and have been identified as causative agents in the population declines of a growing number of wildlife species (Abbott, 2006, Wyatt et al., 2008, Robinson et al., 2010, Cameron et al., 2011, Ewen et al., 2012, MacPhee and Greenwood, 2013, Wayne et al., 2013a, Wayne et al., 2013b). We need to better understand how stress, as a result of captivity, translocation or environmental changes, may affect the normal host–parasite relationship in terms of disease as well as susceptibility to novel pathogens (Harrington et al., 2013). Surveillance of native fauna in natural ecosystems should thus be a priority in order to document parasite diversity in terms of the host parasite relationship and their potential importance in the aetiology of disease in their wildlife hosts (Smith et al., 2009a, Smith et al., 2009b, Thompson et al., 2010a). Such studies will also provide information on the zoonotic potential of wildlife parasites as well as the likely susceptibility of wildlife to exotic pathogens.
Such surveillance can be more readily achieved with the advent of molecular tools from which information on parasite identity can be obtained using non-invasive sampling of live animals (Lymbery and Thompson, 2012). Such tools also enable greater discrimination at the species and intraspecific/genotypic level. For example, the zoonotic potential of the primate malaria species Plasmodium knowlesi was suspected as early as 1932 (Schwabe, 1984, Conlan et al., 2011) but human cases were greatly underestimated until the advent of appropriate molecular tools (Singh et al., 2004, Conlan et al., 2011). In addition, the value of undertaking detailed necropsies of wildlife, not necessarily motivated by a specific investigation of cause of death but for disease surveillance, can yield valuable information on the occurrence of parasite zoonoses. For example, detailed histopathology of a variety of wildlife species recently identified the emergence of Parastrongylus (=Angiostrongylus)cantonensis in mammals and birds in the Sydney area of Australia (Ma et al., 2013).
4.1.1. Trypanosomes in Australian wildlife
Recent research on trypanosomes in Australian wildlife has not only demonstrated the need for parasite surveillance but also the value of applying molecular tools. The discovery of a novel, indigenous species of Leishmania in macropodid marsupials, followed by the identification of a new, non-sand fly vector, the day feeding midge (Forcipomyia (Lasiohelea) sp.) (Rose et al., 2004, Dougall et al., 2009, Dougall et al., 2011), raises critical issues with respect to the zoonotic transmission of Leishmania spp. in Australia. Australia has always been considered to be non-endemic for Leishmania and the parasite a minimal biosecurity risk in terms of establishment. The regular introduction of zoonotic pathogenic species of Leishmania such as Leishmania infantum in tourists, army personnel and migrants, as well as domestic dogs (Thompson et al., 2010a, Anon, 2011), raises the possibility of transmission to wild or domestic hosts via midges. More importantly, the fact that macropodids are susceptible to infection with their own species of Leishmania means they could be susceptible to infection with exotic, introduced species which could cause disease and/or establish reservoirs of zoonotic infection.
As with Leishmania, Australia has never been considered an endemic country for Chagas disease caused by T. cruzi. However, the question of local vectorial transmission has been raised by the World Health Organization (WHO, 2011) due to the increasing numbers of people with chronic Chagas disease migrating to non-endemic countries, including Australia (Coura and Vinas, 2010). Little research has been undertaken in Australia on the host range of triatomes nor whether any bite and feed on the blood of mammals, although Triatoma leopoldi is thought to occur in northern Australia and is a vector of T. cruzi in South America (Monteith, 1974, Thompson et al., 2010a). However, recent research on trypanosomes in Australian native mammals has demonstrated that they are frequently infected with a diversity of species and genotypes of Trypanosoma, some of which are genetically very closely related to T. cruzi (Smith et al., 2008, Averis et al., 2009, Botero et al., 2013, Thompson et al., 2013). Importantly, a detailed investigation of the host–parasite relationship in one species of Australian marsupial, the brush-tailed bettong (Bettongia penicillata), which is presently undergoing a rapid population decline (Wayne et al., 2013a), has demonstrated that at least one genotype of Trypanosoma copemani has an intracellular amastigote stage and that infections are associated with tissue changes in smooth and cardiac muscle, very similar to those characteristic of Chagas disease in humans (Botero et al., 2013). As with Leishmania, these findings raise the possibility of Australian native fauna acting as reservoirs for T. cruzi introduced in Chagas patients from South America, and which can be transmitted in the same vectors of wildife trypanosomes such as T. copemani.
4.2. ‘New’ parasite zoonoses
It is to be expected that new parasite zoonoses will be identified as a result of human activity (Schwabe, 1984). For example, ongoing reports of zoonotic Sarcocystis infection in humans visiting remote locations of Malaysia are probably of wildlife origin (Esposito et al., 2012, Freedman and Caumes, 2012, von Sonnenburg et al., 2012, Tappe et al., 2013) and may represent a newly identified species of Sarcocystis. Humans can become intermediate hosts for at least some of the 130 known species of Sarcocystis (von Sonnenburg et al., 2012), so it is not surprising that zoonotic infections with new species will occur as humans venture into more remote locations.
In Australia, so little is known about the parasites of native fauna that a multitude of new species await discovery, particularly with the ease with which molecular characterisation of tissues, blood and faeces can now be undertaken. It is unlikely that many of these will be of zoonotic significance since they evolved and adapted to their wildlife hosts long before humans arrived in Australia. However, this may not always be the case, especially if host susceptibility is compromised as with two recent reports of Babesia microti in human patients in Australia (Fuller et al., 2012, Senanayake et al., 2012). The first was believed to be locally acquired, whereas the history of the latter case suggested it was imported. These are the first reports of B. microti in humans in Australia and may not necessarily be imported since too few studies have been undertaken of Australian native wildlife to rule out the occurrence of B. microti, especially considering the genetic diversity of the B. microti sp. complex. For example, the recent description of a new species of Babesia in kangaroos (Dawood et al., 2013) may only be the ‘tip of the iceberg’ in describing new species as more molecular-based surveillance of wildlife parasites is undertaken.
The nematode, Haycocknema perplexum, remains a curiosity in terms of its zoonotic potential. It has been identified in five human patients in Australia since 1998 (Spratt, 2005, Basuroy et al., 2008). Clinical features common to infection are limb wasting and weakness associated with myositis, dysphagia and persistent eosinophilia, suggesting a long period of subclinical infection (Basuroy et al., 2008). The source of infection is not known but related species do occur in native wildlife. Thus the possibility of a wildlife reservoir and zoonotic transmission cannot yet be excluded.
4.3. Climate change
Climate change is well recognised as having a major influence on the transmission and distribution of many parasites, including parasite zoonoses, and particularly on their vectors and intermediate hosts (Polley, 2005, Harrus and Baneth, 2005, Polley and Thompson, 2009, Mills et al., 2010). This will be no more greatly felt than in the Arctic where we are currently only beginning to understand the complex ecology of parasite systems, especially zoonoses (Jenkins et al., 2013).
Food and water safety are likely to become more important as climate changes exacerbate the potential for transmission of waterborne parasites (Davidson et al., 2011). As such, waterborne infections with Giardia in wildlife will be affected by climate change (Jenkins et al., 2013). Trichinella, Toxoplasma and Echinococcus are all considered to be affected in terms of range expansion, host switching, disease emergence or reduction (Davidson et al., 2011). For example, it is proposed that the transmission of Toxoplasma from lynx may increase, and that the distribution of rodent intermediate hosts of E. multilocularis will expand (Davidson et al., 2012, Jenkins et al., 2013). As the climate becomes warmer the habitat range of both lynx and their prey are predicted to move northward and environmental conditions will be more conducive to oocyst survival (Jenkins et al., 2013).
5. Conclusions
I hope this review has helped to focus attention on the need to consider the One Health triad holistically, within which wildlife can be victims of human activities resulting in exposure to novel infections or conditions that may affect their response to infections they already harbour. This requires increased surveillance of wildlife populations and an ecological approach to studying host, wild and domestic, and environmental ecosystems. It is not just a matter of cataloguing which parasites infect wildlife. Acquired data must be interpreted in a true One Health philosophy in which parasite biodiversity in wildlife hosts is considered in terms of its impact on non-wildlife hosts and vice versa, but also the potential impact of parasite biodiversity perturbations, or loss, on wildlife health.
For example, with E. multilocularis, it is proposed that effective control will only be achieved by a concerted effort with sustained, long-term cestocidal baiting programmes of foxes in highly endemic areas (Hegglin and Deplazes, 2013). The cestocide used is praziquantel, which will have no effect on the nematode or protozoal intestinal fauna of foxes. Such control programmes will therefore alter the parasite community of a wild host. Is this something that should be considered without knowing the effects of treatment on other fox parasites? A recent study of the effects of anthelmintic treatment in wild mice (Peromyscus maniculatus) resulted in an unexpected increase of non-targeted parasites, coccidia and cestodes (Pedersen and Antonovics, 2013). Although further studies are required to determine the long-term consequences of this alteration to the parasite community, if any, in wild mice, coccidia have been associated with decreased mass and lower over-wintering survival in P. maniculatus (Vandegrift et al., 2008, Pedersen and Antonovics, 2013). Thus foxes receiving cestocidal treatment with praziquantel may experience alterations to their intestinal parasite fauna with unknown consequences. It is unlikely to be of public health significance but could adversely impact the health of foxes if the removal of cestodes causes an increase in non-targeted parasites, nematodes and protozoa. This could, in turn, alter the ecological balance between foxes, rodent intermediate hosts and raccoon dogs with the latter emerging in prominence as a definitive host for E. multilocularis. Unlikely, I concede, but this serves to demonstrate the ecological considerations that should form part of our approach to parasite zoonoses and wildlife.
As Schwabe predicted in 1984, there are many situations yet to discover about how humans’ changing activities may influence the transmission of zoonoses (Schwabe, 1984). How such activities will influence wildlife ecosystems as well as public health remain to be discovered.
Acknowledgements
The work reported here on parasites of wildlife in Western Australia was supported by the Australian Research Council and the Western Australian Department of Environment and Conservation. I am very grateful to Mark Preston and Adam White (Murdoch University, Australia) for producing the graphics.
References
- Abbott I. Mammalian faunal collapse in Western Australia, 1875–1925: the hypothesised role of epizootic disease and a conceptual model of its origin, introduction, transmission and spread. Aust. Zool. 2006;33:530–561. [Google Scholar]
- Acha P.N., Szyfres B. vol. III. Pan American Health Organization; Washington: 2003. (Zoonoses and Communicable Diseases Common to Man and Animals: Parasitoses). [Google Scholar]
- Adams P.J., Monis P.T., Elliot A.D., Thompson R.C.A. Cyst morphology and sequence analysis of the small subunit rDNA and ef1a identifies a novel Giardia genotype in a quenda (Isoodon obesulus) from Western Australia. Infect. Genet. Evol. 2004;4:365–370. doi: 10.1016/j.meegid.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Addy F., Alakonya A., Wamae N., Magambo J., Mbae C., Mulinge E., Zeyhle E., Wassermann M., Kern P., Romig T. Prevalence and diversity of cystic echinococcosis in livestock in Masailand, Kenya. Parasitol. Res. 2012;111:2289–2294. doi: 10.1007/s00436-012-3082-8. [DOI] [PubMed] [Google Scholar]
- Ajzenberg D., Banuls A.L., Su C., Dumetre A., Demar M., Carme B., Darde M.L. Genetic diversity, clonality and sexuality in Toxoplasma gondii. Int. J. Parasitol. 2004;34:1185–1196. doi: 10.1016/j.ijpara.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Ancelle, T., De Bruyn, A., Poisson, D.M., Dupouy-Camet, J. 2005. Outbreak of trichinellosis due to consumption of bear meat from Canada, France, September 2005. Euro Surveill. 10, (10):E051013.3. [DOI] [PubMed]
- Anon Exotic leishmaniasis detected. NSW Anim. Health Surveill. 2011;1:1. [Google Scholar]
- Appelbee A.J., Thompson R.C., Olson M.E. Giardia and Cryptosporidium in mammalian wildlife – current status and future needs. Trends Parasitol. 2005;21:370–376. doi: 10.1016/j.pt.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbee A.J., Thompson R.C.A., Measures L.M., Olson M.E. Giardia and Cryptopsoridium in harp and hooded seals from the Gulf of St. Lawrence, Canada. Vet. Parasitol. 2010;173:19–23. doi: 10.1016/j.vetpar.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Ash A., Lymbery A.J., Lemon J., Vitali S., Thompson R.C.A. Molecular epidemiology of Giardia duodenalis in an endangered carnivore – the African painted dog. Vet. Parasitol. 2010;174:206–212. doi: 10.1016/j.vetpar.2010.08.034. [DOI] [PubMed] [Google Scholar]
- Averis S., Thompson R.C.A., Lymbery A.J., Wayne A.F., Morris K.D., Smith A. The diversity, distribution and host–parasite associations of trypanosomes in Western Australian wildlife. Parasitology. 2009;136:1269–1279. doi: 10.1017/S0031182009990801. [DOI] [PubMed] [Google Scholar]
- AWHN 2011. Exotic – Trichinellosis. Australian Wildlife Health Network, Fact Sheet 20 Jul, 1-5. http://www.wildlifehealth.org.au/Portals/0/Documents/FactSheets/EXOTIC%20-%20Trichinellosis%2020%20Jul%202011%20(1.0).pdf.
- Barlow A.M., Gottstein B., Mueller N. Echinococcus multilocularis in an imported captive beaver (Castor fiber) in Great Britain. Vet. Rec. 2011;169:339. doi: 10.1136/vr.d4673. [DOI] [PubMed] [Google Scholar]
- Barnes T.S., Morton J.M., Coleman G.T. Clustering of hydatid infection in macropodids. Int. J. Parasitol. 2007;37:943–952. doi: 10.1016/j.ijpara.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Basuroy R., Pennisi R., Robertson T., Norton R., Stokes J., Reimers J., Archer J. Parasitic myositis in tropical Australia. Med. J. Aust. 2008;188:254–256. doi: 10.5694/j.1326-5377.2008.tb01601.x. [DOI] [PubMed] [Google Scholar]
- Bauer C. Baylisascariosis – infections of animals and humans with ‘unusual’ roundworms. Vet. Parasitol. 2013;193:404–412. doi: 10.1016/j.vetpar.2012.12.036. [DOI] [PubMed] [Google Scholar]
- Blair R.B. Land use and avian species diversity along an urban gradient. Ecol. Appl. 1996;6:506–519. [Google Scholar]
- Bolzoni L., De Leo G.A. Unexpected consequences of culling on the eradication of wildlife diseases: the role of virulence evolution. Am. Nat. 2013;181:301–313. doi: 10.1086/669154. [DOI] [PubMed] [Google Scholar]
- Botero A., Thompson C.K., Peacock C.S., Clode P.L., Nicholls P.K., Wayne A.F., Lymbery A.J., Thompson R.C.A. Trypanosomes genetic diversity, polyparasitism and the population decline of the critically endangered Australian marsupial, the brush tailed bettong or woylie (Bettongia penicillata) Int. J. Parasitol. Parasites Wildlife. 2013;2:77–89. doi: 10.1016/j.ijppaw.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bružinskaitė-Schmidhalter R., Šarkūnas M., Malakauskas A., Mathis A., Torgerson P.R., Deplazes P. Helminths of red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) in Lithuania. Parasitology. 2012;139:120–127. doi: 10.1017/S0031182011001715. [DOI] [PubMed] [Google Scholar]
- Bryan H.M., Darimont C.T., Paquet P.C., Ellis J.A., Goji N., Gouix M., Smits J.E. Exposure to infectious agents in dogs in remote coastal British Columbia: possible sentinels of diseases in wildlife and humans. Can. J. Vet. Res. 2011;75:11–17. [PMC free article] [PubMed] [Google Scholar]
- Bryan H.M., Darimont C.T., Hill J.E., Paquet P.C., Thompson R.C.A., Wagner B., Smits J.E. Seasonal and biogeographical patterns of gastrointestinal parasites in large carnivores: wolves in a coastal archipelago. Parasitology. 2012;139:781–790. doi: 10.1017/S0031182011002319. [DOI] [PubMed] [Google Scholar]
- Cameron S.A., Lozier J.D., Strange J.P., Koch J.B., Cordes N., Solter L.F., Griswold T.L. Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. USA. 2011;108:662–667. doi: 10.1073/pnas.1014743108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano S., Lejeune M., Liccioli S., Verocai G.G., Gesy K.M., Jenkins E.J., Kutz S.J., Fuentealba C., Duignan P.J., Massolo A. Echinococcus multilocularis in Urban Coyotes, Alberta, Canada. Emerg. Infect. Dis. 2012;18:1625–1628. doi: 10.3201/eid.1810.120119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles R.A., Kjos S., Ellis A.E., Barnes J.C., Yabsley M.J. Southern plains woodrats (Neotoma micropus) from Southern Texas are important reservoirs of two genotypes of Trypanosoma cruzi and host of a putative novel Trypanosoma species. Vector Borne Zoon. Dis. 2013;13:22–30. doi: 10.1089/vbz.2011.0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan J.V., Sripa B., Attwood S., Newton P.N. A review of parasitic zoonoses in a changing Southeast Asia. Vet. Parasitol. 2011;182:22–40. doi: 10.1016/j.vetpar.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Coura J.R., Vinas P.A. Chagas disease: a new worldwide challenge. Nature. 2010;465:S6–S7. doi: 10.1038/nature09221. [DOI] [PubMed] [Google Scholar]
- Cuttell L., Cookson B., Jackson L.A., Gray C., Traub R.J. First report of a Trichinella papuae infection in a wild pig (Sus scrofa) from an Australian island in the Torres Strait region. Vet. Parasitol. 2012;185:343–345. doi: 10.1016/j.vetpar.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Davidson R.K., Robertson L.J. European pet travel: misleading information from veterinarians and government agencies. Zoonoses Public Health. 2012;59:575–583. doi: 10.1111/j.1863-2378.2012.01499.x. [DOI] [PubMed] [Google Scholar]
- Davidson W.R., Appel M.J., Doster G.L., Baker O.E., Brown J.F. Diseases and parasites of red foxes, gray foxes, and coyotes from commercial sources selling to fox-chasing enclosures. J. Wildl. Dis. 1992;28:581–589. doi: 10.7589/0090-3558-28.4.581. [DOI] [PubMed] [Google Scholar]
- Davidson R., Simard M., Kutz S.J., Kapel C.M.O., Hamnes I.S., Robertson L.J. Arctic parasitology: why should we care? Trends Parasitol. 2011;27:238–244. doi: 10.1016/j.pt.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Davidson R.K., Romig T., Jenkins E., Tryland M., Robertson L.J. The impact of globalisation on the distribution of Echinococcus multilocularis. Trends Parasitol. 2012;28:239–247. doi: 10.1016/j.pt.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Dawood K.E., Morgan J.A.T., Busfield F., Srivastava M., Fletcher T.I., Sambono J., Jackson L.A., Venus B., Philbey A.W., Lew-Tabor A.E. Observation of a novel Babesia spp. in Eastern Grey Kangaroos (Macropus giganteus) in Australia. Int. J. Parasitol. Parasites Wildlife. 2013;2:54–61. doi: 10.1016/j.ijppaw.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplazes P., Gloor S., Hegglin D., Romig T. Wilderness in the city – the urbanization of Echinococcus multilocularis. Trends Parasitol. 2004;20:77–84. doi: 10.1016/j.pt.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Dougall A., Shilton C., Low Choy J., Alexander B., Walton S. New reports of Australian cutaneous leishmaniasis in northern Australian macropods. Epidemiol. Infect. 2009;137:1516–1520. doi: 10.1017/S0950268809002313. [DOI] [PubMed] [Google Scholar]
- Dougall A., Alexander B., Holt D.C., Harris T., Sultan A.H., Bates P.A., Rose K., Walton S. Evidence incriminating midges (Diptera: Ceratopogonidae) as potential vectors of Leishmania in Australia. Int. J. Parasitol. 2011;41:571–579. doi: 10.1016/j.ijpara.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Durie P.H., Riek R.F. The role of the dingo and wallaby in the infestation of cattle with hydatids (Echinococcus granulosus (Batsch, 1786) Rudolphi, 1805) in Queensland. Aust. Vet. J. 1952;28:249–254. [Google Scholar]
- Esposito D.H., Freedman D.O., Neumayr A., Parola P. Ongoing outbreak of an acute muscular Sarcocystis-like illness among travellers returning from Tioman Island, Malaysia, 2011–2012. Euro Surveill. 2012;17(45) doi:pii: 20310. [PMC free article] [PubMed] [Google Scholar]
- Ewen J.G., Acevedo-Whitehouse K., Alley M.R., Carraro C., Sainsbury A.W., Swinnerton K., Woodroffe R. Empirical consideration of parasites and health in reintroduction. In: Ewen J.G., Armstrong D.P., Parker K.A., Seddon P.J., editors. Reintroduction Biology. Wiley-Blackwell; Hoboken, NJ, USA: 2012. pp. 320–335. [Google Scholar]
- Freedman D.O., Caumes E. Sarcocystosis, human – Malaysia (Tioman Island) new outbreak confirmed. GeoSentinel. 2012;19(16):12. [Google Scholar]
- Fuller A., Manitta J., Marks R., Tencic S., Gordon C.L. First reported case of imported human Babesia microti infection in Australia. Pathology. 2012;44:580–582. doi: 10.1097/PAT.0b013e328358349a. [DOI] [PubMed] [Google Scholar]
- Hamilton P.B., Teixeira M.M.G., Stevens J.R. The evolution of Trypanosoma cruzi: the ‘bat seeding’ hypothesis. Trends Parasitol. 2012;28(4):136–141. doi: 10.1016/j.pt.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Harrington L.A., Moehrenschlager A., Gelling M., Atkinson R.P.D., Hughes J., Macdonald D.W. Conflicting and complementary ethics of animal welfare considerations in reintroductions. Conserv. Biol. 2013;27:486–500. doi: 10.1111/cobi.12021. [DOI] [PubMed] [Google Scholar]
- Harrus S., Baneth G. Drivers for the emergence and re-emergence of vector-borne protozoal diseases. Int. J. Parasitol. 2005;35:1309–1318. doi: 10.1016/j.ijpara.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Hegglin D., Deplazes P. Control of Echinococcus multilocularis: strategies, feasibility and cost benefit analyses. Int. J. Parasitol. 2013;43:327–337. doi: 10.1016/j.ijpara.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Hide G., Morley E.K., Hughes J.M., Gerwash O., Elmahaishi M.S., Elmahaishi K.H., Thomasson D., Wright E.A., Williams R.H., Murphy R.G., Smith J.E. Evidence for high levels of vertical transmission in Toxoplasma gondii. Parasitology. 2009;136:1877–1885. doi: 10.1017/S0031182009990941. [DOI] [PubMed] [Google Scholar]
- Himsworth C.G., Jenkins E., Hill J., Nsungu M., Ndao M., Thompson R.C.A., Ash A., McConnel A., Leighton F.A., Skinner S. The emergence of sylvaticEchinococcus granulosus as a parasitic zoonosis of public health concern in an indigenous Canadian community. Am. J. Trop. Med. Hyg. 2010;82:643–645. doi: 10.4269/ajtmh.2010.09-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez, P.J., 2010. Neglected infections of poverty among the Indigenous peoples of the Arctic. PLoS Negl. Trop. Dis. 4 (1), 1–6, (e606). [DOI] [PMC free article] [PubMed]
- Hotez P.J., Dumonteil E., Woc-Colburn L., Serpa J.A., Bezek S., Edwards M.S., Hallmark C.J., Musselwhite L.W., Flink B.J., Bottazzi M.E. Chagas disease: “the new HIV/AIDS of the Americas”. PLoS Negl. Trop. Dis. 2012;6:e1498. doi: 10.1371/journal.pntd.0001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houze S., Ancelle T., Matra R., Boceno C., Carlier Y., Gajadhar A.A., Dupouy-Camet J. Trichinellosis acquired in Nunavut, Canada in September 2009: meat from grizzly bear suspected. Euro Surveill. 2009;14(44) doi:pii=19383. [PubMed] [Google Scholar]
- Huttner M., Romig T. Echinococcus species in African wildlife. Parasitology. 2009;136:1089–1095. doi: 10.1017/S0031182009990461. [DOI] [PubMed] [Google Scholar]
- Huttner M., Siefert L., Mackenstedt U., Romig T. A survey of Echinococcus species in wild carnivores and livestock in East African. Int. J. Parasitol. 2009;39:1269–1276. doi: 10.1016/j.ijpara.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Jenkins D.J., Romig T., Thompson R.C.A. Emergence/re-emergence of Echinococcus spp. – a global update. Int. J. Parasitol. 2005;35:1205–1219. doi: 10.1016/j.ijpara.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Jenkins E.J., Peregrine A.S., Hill J.E., Somers C.M., Gesy K.M., Barnes B., Gottstein B., Polley L. Detection of a European strain of Echinococcus multilocularis in North America. Emerg. Infect. Dis. 2012;18:1010–1012. doi: 10.3201/eid1806.111420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins E.J., Castrodale L.J., de Rosemond S.J.C., Dixon B.R., Elmore S.A., Gesy K.M., Hoberg E.P., Polley L., Schurer J.M., Simard M., Thompson R.C.A. Tradition and transition: parasitic zoonoses of people and animals in Alaska, northern Canada, and Greenland. Adv. Parasitol. 2013;82:33–204. doi: 10.1016/B978-0-12-407706-5.00002-2. [DOI] [PubMed] [Google Scholar]
- Johnson P.T.J., Hoverman J.T. Parasite diversity and coinfection determine pathogen infection success and host fitness. PNAS. 2012;109:9006–9011. doi: 10.1073/pnas.1201790109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P.T.J., Preston D.L., Hoverman J.T., Richgels K.L.D. Biodiversity decreases disease through predictable changes in host community competence. Nature. 2013;494:230–234. doi: 10.1038/nature11883. [DOI] [PubMed] [Google Scholar]
- Johnston A.R., Gillespie T.R., Rwego I.B., McLachlan T.L., Kent A.D., Goldberg T.L. Molecular epidemiology of cross-species Giardia duodenalis transmission in Western Uganda. PLoS. Negl. Trop. Dis. 2010;11:4. doi: 10.1371/journal.pntd.0000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapel C.M., Torgerson P.R., Thompson R.C.A., Deplazes P. Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. Int. J. Parasitol. 2006;36:79–86. doi: 10.1016/j.ijpara.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Karesh W.B., Dobson A., Lloyd-Smith J.O., Lubroth J., Dixon M.A., Bennett M., Aldrich S., Harrington T., Formenty P., Loh E.H., Machalaba C.C., Thomas M.J., Heymann D.L. Ecology of zoonoses: natural and unnatural histories. Lancet. 2012;380:1936–1945. doi: 10.1016/S0140-6736(12)61678-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazacos, K.R., 2001. Baylisascaris procyonis and related species. In: Samuel, W.M., Pybus, M.J., Kocan, K.K. (Eds.), Parasitic Diseases of Wild Mammals. Iowa State University Press, Ames, pp. 301–341 (559 pp.).
- Keenan M., Alexander P.W., Diao H., Best W.M., Khong A., Kerfoot M., Thompson R.C.A., White K.L., Shackleford D.M., Ryan E., Gregg A.D., Charman S.A., von Geldern T.W., Scandale I., Chatelain E. Design, structure–activity relationship and in vivo efficacy of piperazine analogues of fenarimol as inhibitors of Trypanosoma cruzi. Bioorg. Med. Chem. 2013;21:1756–1763. doi: 10.1016/j.bmc.2013.01.050. [DOI] [PubMed] [Google Scholar]
- Kellner K.F., Page L.K., Downey M., McCord S.E. Effects of urbanization on prevalence of Baylisascaris procyonis in intermediate host populations. J. Wildl. Dis. 2012;48:1083–1087. doi: 10.7589/2011-09-267. [DOI] [PubMed] [Google Scholar]
- Kooriyama T., Okamoto M., Yoshida T., Nishida T., Tsubota T., Saito A., Tomonaga M., Matsuzawa T., Akari H., Nishimura H., Miyabe-Nishiwaki T. Epidemiological study of zoonoses derived from humans in captive chimpanzees. Primates. 2013;54:89–98. doi: 10.1007/s10329-012-0320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutz S.J., Thompson R.A., Polley L., Kandola K., Nagy J., Wielinga C.M., Elkin B.T. Giardia assemblage A: human genotype in muskoxen in the Canadian Arctic. Parasit. Vectors. 2008;1:32. doi: 10.1186/1756-3305-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutz S.J., Thompson R.C.A., Polley L. Wildlife with Giardia: villain or victim and vector? In: Ortega-Pierres G., Caccio S., Fayer R., Mank T.G., Smith H.V., Thompson R.C.A., editors. Giardia and Cryptosporidium: From Molecules to Disease. CABi; Wallingford, UK: 2009. pp. 94–106. [Google Scholar]
- Lehrer E.W., Fredebaugh S.L., Schooley R.L., Mateus-Pinilla N.E. Prevalence of antibodies to Toxoplasma gondii in woodchucks across an urban–rural gradient. J. Wildl. Dis. 2010;46:977–980. doi: 10.7589/0090-3558-46.3.977. [DOI] [PubMed] [Google Scholar]
- Liccioli S., Duignan P.J., Lejeune M., Deunk J., Majid S., Massolo A. A new intermediate host for Echinococcus multilocularis: the Southern Red-backed Vole (Myodes gapperi) in urban landscape in Calgary, Canada. Parasitol. Int. 2012;62:355–357. doi: 10.1016/j.parint.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Lloyd-Smith J.O., George D., Pepin K.M., Pitzer V.E., Pulliam J.R.C., Dobson A.P., Hudson P.J., Grenfell B.T. Epidemic dynamics at the human–animal interface. Science. 2009;326:1362–1367. doi: 10.1126/science.1177345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoGiudice K. Trophically transmitted parasites and the conservation of small populations: raccoon roundworm and the imperiled Allegheny Woodrat. Conserv. Biol. 2003;17:258–266. [Google Scholar]
- LoGiudice K. Toward a synthetic view of extinction: a history lesson from a 647 North American rodent. BioScience. 2006;56:687–693. [Google Scholar]
- Lymbery A.J., Thompson R.C.A. The molecular epidemiology of parasite infections: tools and applications. Mol. Biochem. Parasitol. 2012;181:102–116. doi: 10.1016/j.molbiopara.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Ma G., Dennis M., Rose K., Spratt D., Spielman D. Tawny frogmouths and brushtail possums as sentinels for Angiostrongylus cantonensis, the rat lungworm. Vet. Parasitol. 2013;192:158–165. doi: 10.1016/j.vetpar.2012.11.009. [DOI] [PubMed] [Google Scholar]
- MacPhee R.D.E., Greenwood A.D. Infectious disease, endangerment, and extinction. Int. J. Evol. Biol. 2013;2013:571939. doi: 10.1155/2013/571939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson C.N.L. An active intermediate host role for man in the life cycle of Echinococcus granulosus in Turkana, Kenya. Am. J. Trop. Med. Hyg. 1983;32:397–404. doi: 10.4269/ajtmh.1983.32.397. [DOI] [PubMed] [Google Scholar]
- Macpherson C.N.L., Karstad L., Stevenson P., Arundel J.H. Hydatid disease in the Turkana district of Kenya. III. The significance of wild animals in the transmission of Echinococcus granulosus, with particular reference to Turkana and Masailand in Kenya. Ann. Trop. Med. Parasitol. 1983;77:61–73. [PubMed] [Google Scholar]
- Malmsten J., Jakubek E.-B., Bjorkman C. Prevalence of antibodies against Toxoplasma gondii and Neospora caninum in moose (Alces alces) and roe deer (Capreolus capreolus) in Sweden. Vet. Parasitol. 2011;177:275–280. doi: 10.1016/j.vetpar.2010.11.051. [DOI] [PubMed] [Google Scholar]
- Mathews F. Zoonoses in wildlife: integrating ecology into management. Adv. Parasitol. 2009;68:185–208. doi: 10.1016/S0065-308X(08)00608-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus D.P., Zhang L., Castrodale L.J., Le T.H., Pearson M., Blair D. Short report: molecular genetic characterization of an unusually severe case of hydatid disease in Alaska caused by the cervid strain of Echinococcus granulosus. Am. J. Trop. Med. Hyg. 2002;67:296–298. doi: 10.4269/ajtmh.2002.67.296. [DOI] [PubMed] [Google Scholar]
- Miller M.A., Miller W.A., Conrad P.A., James E.R., Melli A.C., Leutenegger C.M., Dabritz H.A., Packham A.E., Paradies D., Harris M., Ames J., Jessup D.A., Worcester K., Grigg M.E. Type X Toxoplasma gondii in a wild mussel and terrestrial carnivore from coastal California: new linkages between terrestrial mammals, runoff and toxoplasmosis of sea otters. Int. J. Parasitol. 2008;38:1319–1328. doi: 10.1016/j.ijpara.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Mills J.N., Gage K.L., Khan A.S. Potential influence of climate change on vector-borne and zoonotic diseases: a review and proposed research plan. Environ. Health Perspect. 2010;118:1507–1514. doi: 10.1289/ehp.0901389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteith G.B. Confirmation of the presence of Triatominae (Hemiptera: Reduviidae) in Australia, with notes on Indo-Pacific species. J. Aust. Entomol. Soc. 1974;13:89–94. [Google Scholar]
- Newell D.G., Koopmans M., Verhoef L., Duizer E., Aidara-Kane A., Sprong H., Opsteegh M., Langelaar M., Threfall J., Scheutz F., van der Giessen J., Kruse H. Food-borne diseases – the challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 2010;139:S3–15. doi: 10.1016/j.ijfoodmicro.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page K. Parasites and the conservation of small populations: the case of Baylisascaris procyonis. Int. J. Parasitol. Parasites Wildlife. 2013;2:203–210. doi: 10.1016/j.ijppaw.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page L.K., Anchor C., Luy E., Kron S., Larson G., Madsen L., Kellner K., Smyser T.J. Backyard raccoon latrines and risk for Baylisascaris procyonis transmission to humans. Emerg. Infect. Diseas. 2009;15:1530–1531. doi: 10.3201/eid1509.090128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S., Thompson R.C.A., Grigg M.E., Sundar N., Smith A., Lymbery A.J. Western Australian marsupials are multiply infected with genetically diverse strains of Toxoplasma gondii. PLoS One. 2012;7:e45147. doi: 10.1371/journal.pone.0045147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran N., O’Handley R.M., Grigg M.E., Wayne A., Thompson R.C.A. Vertical transmission of Toxoplasma gondii in Australian marsupials. Parasitology. 2009;136:939–944. doi: 10.1017/S0031182009006453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran N., Thompson R.C.A., Sundar N., Pan S., Johnson M., Smith N.C., Grigg M.E. Non-archetypal type-II and atypical strains of Toxoplasma gondii infecting marsupials of Australia. Int. J. Parasitol. 2010;40:635–640. doi: 10.1016/j.ijpara.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen A.B., Antonovics J. Anthelmintic treatment alters the parasite community in a wild mouse host. Biol. Lett. 2013;9:20130205. doi: 10.1098/rsbl.2013.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright R.K., Foley P., Field H.E., Dobson A.P., Foley J.E., Eby P., Daszak P. Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.) Proc. R. Soc. B. 2011;278:3703–3712. doi: 10.1098/rspb.2011.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley L. Navigating parasite webs and parasite flow: emerging and re-emerging parasitic zoonoses of wildlife origin. Int. J. Parasitol. 2005;35:1279–1294. doi: 10.1016/j.ijpara.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Polley L., Thompson R.C.A. Parasite zoonoses and climate change: molecular tools for tracking shifting boundaries. Trends Parasitol. 2009;25:285–291. doi: 10.1016/j.pt.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Pozio E. World distribution of Trichinella spp. infections in animals and humans. Vet. Parasitol. 2007;149:3–21. doi: 10.1016/j.vetpar.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Pozio E. The opportunistic nature of Trichinella – Exploitation of new geographies and habitats. Vet. Parasitol. 2013;194:128–132. doi: 10.1016/j.vetpar.2013.01.037. [DOI] [PubMed] [Google Scholar]
- Pozio E., Hoberg E., La Rosa G., Zarlenga D.S. Molecular taxonomy, phylogeny and biogeography of nematodes to the Trichinella genus. Infect. Genet. Evol. 2009;9:606–616. doi: 10.1016/j.meegid.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Quammen D. The Bodley Head; London: 2012. Spillover. Animal Infections and the Next Human Pandemic. [Google Scholar]
- Ramirez, J.D., Turriago, B., Taipa-Calle, G., Guhl, F., 2013. Understanding the role of dogs (Canis lupus familiaris) in the transmission dynamics of Trypanosoma cruzi genotypes in Colombia. Vet. Parasitol. (in press). [DOI] [PubMed]
- Rausch R.L. Life-cycle patterns and geographic distribution of Echinococcus species. In: Thompson R.C.A., editor. The Biology ofEchinococcus and Hydatid Disease. Allen and Unwin; London, UK: 1986. pp. 45–80. [Google Scholar]
- Rausch R.L. Cystic echinococcosis in the Arctic and sub-Arctic. Parasitology. 2003;127:S73–S85. doi: 10.1017/s0031182003003664. [DOI] [PubMed] [Google Scholar]
- Rhyan J.C., Spraker T.R. Emergence of disease from wildlife reservoirs. Vet. Pathol. 2010;47:34–39. doi: 10.1177/0300985809354466. [DOI] [PubMed] [Google Scholar]
- Robertson L.J. The potential for marine bivalve shellfish to act as transmission vehicles for outbreaks of protozoan infections in humans: a review. Int. J. Food Microbiol. 2007;120:201–216. doi: 10.1016/j.ijfoodmicro.2007.07.058. [DOI] [PubMed] [Google Scholar]
- Robinson R.A., Lawson B., Toms M.P., Peck K.M., Kirkwood J.K., Chantrey J., Clatworthy I.R., Evans A.D., Hughes L.A., Hutchinson O.C., John S.K., Pennycott T.W., Perkins M.W., Rowley P.S., Simpson V.R., Tyler K.M., Cunningham A.A. Emerging infectious disease leads to rapid population declines of common British birds. PLoS One. 2010;5:e12215. doi: 10.1371/journal.pone.0012215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romig T., Thoma D., Weible A.-K. Echinococcus multilocularis – a zoonosis of anthropogenic environments? J. Helminthol. 2006;80:207–212. doi: 10.1079/joh2006347. [DOI] [PubMed] [Google Scholar]
- Rose K., Curtis J., Baldwin T., Mathis A., Kumar B., Sakthianandeswaren A., Spurck T., Low Choy J., Handman E. Cutaneous leishmaniasis in red kangaroos: isolation and characterisation of the causative organisms. Int. J. Parasitol. 2004;34:655–664. doi: 10.1016/j.ijpara.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Saarma U., Jogisalu I., Moks E., Varcasia A., Lavikainen A., Oksanen A., Simsek S., Andresiuk V., Denegri G., Gonzalez L.M., Ferrer E., Garate T., Rinaldi L., Maravilla P. A novel phylogeny for the genus Echinococcus, based on nuclear data, challenges relationships based on mitochondrial evidence. Parasitology. 2009;136:317–328. doi: 10.1017/S0031182008005453. [DOI] [PubMed] [Google Scholar]
- Salyer S., Gillespie T.R., Rwego I.B., Chapman C.A., Goldberg T.L. Epidemiology and molecular relationships of Cryptosporidium spp. in people, primates, and livestock from Western Uganda. PLoS Negl. Trop. Dis. 2012;6(4):e1597. doi: 10.1371/journal.pntd.0001597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz P.M., Chai J., Craig P.S., Eckert J., Jenkins D.J., Macpherson C.N.L., Thakur A. Epidemiology and control of hydatid disease. In: Thompson R.C.A., Lymbery A.J., editors. Echinococcus and Hydatid Disease. CAB International; Wallingford, Oxon, UK: 1995. pp. 233–331. [Google Scholar]
- Schwabe C.W. second ed. Williams and Wilkins; Baltimore: 1969. Veterinary Medicine and Human Health. [Google Scholar]
- Schwabe C.W. third ed. Williams and Wilkins; Baltimore: 1984. Veterinary Medicine and Human Health. [Google Scholar]
- Senanayake S.N., Paparini A., Latimer M., Andiolo K., Dasilva A.J., Wilson H., Xayavong M.V., Collignon P.J., Jeans P., Irwin P. First report of human babesiosis in Australia. Med. J. Aust. 2012;196:350–352. doi: 10.5694/mja11.11378. [DOI] [PubMed] [Google Scholar]
- Shochat E., Warren P.S., Faeth S.H., McIntryre N.E., Hope D. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 2006;21:186–191. doi: 10.1016/j.tree.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Singh B., Sung L.K., Matusop A., Radhakrishnan A., Shamsul S.S.G., Cox-Singh J., Thomas A., Conway D.J. Lancet. 2004;363:1017–1024. doi: 10.1016/S0140-6736(04)15836-4. [DOI] [PubMed] [Google Scholar]
- Smith A., Clark P., Averis S., Lymbery A.J., Wayne A.F., Morris K.D., Thompson R.C.A. Trypanosomes in a declining species of threatened Australian marsupial, the brush-tailed bettong Bettongia penicillata (Marsupialia: Potoroidae) Parasitology. 2008;135:1329–1335. doi: 10.1017/S0031182008004824. [DOI] [PubMed] [Google Scholar]
- Smith K.F., Behrens M.D., Sax D.F. Local scale effects of disease on biodiversity. Ecosyst. Health. 2009;6:287–295. doi: 10.1007/s10393-009-0254-9. [DOI] [PubMed] [Google Scholar]
- Smith K.F., Acevedo-Whitehouse K., Pedersen A.B. The role of infectious diseases in biological conservation. Animal Conserv. 2009;12:1–12. [Google Scholar]
- Smyser T.J., Johnson S.A., Page L.K., Hudson C.M., Rhodes O.E. Use of experimental translocations of Allegheny woodrat to decipher causal agents of decline. Cons. Biol. 2013;27:752–762. doi: 10.1111/cobi.12064. [DOI] [PubMed] [Google Scholar]
- von Sonnenburg F., Freedman D.O., Esposito D.H., Lankau E.W. Notes from the field: acute muscular sarcocystosis among returning travellers – Tioman Island, Malaysia, 2011. Morb. Mort. Wkly. Rep. 2012;61:37–38. [PubMed] [Google Scholar]
- Spratt D.M. Australian ecosystems, capricious food chains and parasitic consequences for people. Int. J. Parasitol. 2005;35:717–724. doi: 10.1016/j.ijpara.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Tappe D., Ernestus K., Rauthe S., Schoen C., Frosch M., Muller A., Stich A. Initial patient cluster and first positive biopsy finding in an ourbreak of acute muscular Sarcosystis-like infection in travellers returning from Tioman Island, Peninsular Malaysia, in 2011. J. Clin. Microbiol. 2013;51(2):725–726. doi: 10.1128/JCM.03063-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichroeb J.A., Kutz S.J., Parkar U., Thompson R.C.A., Sicotte P. Ecology of the gastrointestinal parasites of Colobus vellerosus at Boabeng-Fiema, Ghana: possible anthropozoonotic transmission. Am. J. Phys. Anthropol. 2009;140:498–507. doi: 10.1002/ajpa.21098. [DOI] [PubMed] [Google Scholar]
- Thompson R.C.A. Biology and systematics of Echinococcus. In: Thompson R.C.A., Lymbery A.J., editors. Echinococcus and Hydatid Disease. CAB International; Wallingford, Oxon, UK: 1995. pp. 1–50. [Google Scholar]
- Thompson R.C.A. The zoonotic significance and molecular epidemiology of Giardia and giardiasis. Vet. Parasitol. 2004;126:15–35. doi: 10.1016/j.vetpar.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Thompson R.C.A., Monis P.T. Variation in Giardia: implications for taxonomy and epidemiology. Adv. Parasitol. 2004;58:69–137. doi: 10.1016/S0065-308X(04)58002-8. [DOI] [PubMed] [Google Scholar]
- Thompson R.C.A., Monis P. Giardia – from genome to proteome. Adv. Parasitol. 2012;78:57–95. doi: 10.1016/B978-0-12-394303-3.00003-7. [DOI] [PubMed] [Google Scholar]
- Thompson R.C.A., Kapel C.M., Hobbs R.P., Deplazes P. Comparative development of Echinococcus multilocularis in its definitive hosts. Parasitology. 2006;19:1–8. doi: 10.1017/S0031182005009625. [DOI] [PubMed] [Google Scholar]
- Thompson R.C.A., Boxell A.C., Ralston B.J., Constantine C.C., Hobbs R.P., Shury T., Olson M.E. Molecular and morphological characterization of Echinococcus in cervids from North America. Parasitology. 2006;132:439–447. doi: 10.1017/S0031182005009170. [DOI] [PubMed] [Google Scholar]
- Thompson R.C.A., Kutz S.J., Smith A. Parasite zoonoses and wildlife: Emerging issues. Int. J. Env. Res. Publ. Hlth. 2009;6:678–693. doi: 10.3390/ijerph6020678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.C.A., Lymbery A.J., Smith A. Parasites, emerging disease and wildlife conservation. Int. J. Parasitol. 2010;40:1163–1170. doi: 10.1016/j.ijpara.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Thompson R.C.A., Smith A., Lymbery A.J., Averis S., Morris K.D., Wayne A.F. Giardia in Western Australian wildlife. Vet. Parasitol. 2010;170:207–211. doi: 10.1016/j.vetpar.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Thompson C.K., Botero A., Wayne A.F., Godfrey S.S., Lymbery A.J., Thompson R.C.A. Morphological polymorphism of Trypanosoma copemani and description of the genetically diverse T. vegrandis sp. nov. from the critically endangered Australian potoroid, the brush-tailed bettong (Bettongia penicillata (Gray, 1837)) Parasit. Vectors. 2013;6:121. doi: 10.1186/1756-3305-6-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Giessen J., Franssen F., Fonville M., Kortbeek T., Beckers P., Tolsma P., Stenvers O., Teunis P., Takumi K. How safe is the meat inspection based on artificial digestion of pooled samples for Trichinella in pork? A scenario from wildlife to a human patient in a non-endemic region of Europe. Vet. Parasitol. 2013;194:110–112. doi: 10.1016/j.vetpar.2013.01.032. [DOI] [PubMed] [Google Scholar]
- Vandegrift K.J., Raffel T.R., Hudson P.J. Parasites prevent summer breeding in white-footed mice, Peromyscus leucopus. Ecology. 2008;89:2251–2258. doi: 10.1890/07-1935.1. [DOI] [PubMed] [Google Scholar]
- Wang Q., Xiao Y.F., Vuitton D.A., Schantz P.M., Raoul F., Budke C., Campos-Ponce M., Craig P.S., Giraudoux P. Impact of overgrazing on the transmission of Echinococcus multilocularis in Tibetan pastoral communities of Sichuan Province, China. Chin. Med. J. 2007;120:237–242. [PubMed] [Google Scholar]
- Watson M.J. The costs of parasites-What drives population-level effects? Meta-analysis meets life-history. Int. J. Parasitol. Parasites Wildlife. 2013;2:190–196. doi: 10.1016/j.ijppaw.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne, A.F., Maxwell, M., Ward, C.G., Vellios, C.V., Wilson, I., Wayne, J.C., Matthew, W., 2013a. Sudden, rapid and catastrophic decline of an abundant marsupial, Bettongia penicillata: characterizing the decline and identifying the potential causes. Oryx (in press).
- Wayne, A.F., Maxwell, M., Ward, C.G., Vellios, C.V., Ward, B., Liddelow, G.L., Wilson, I., Wayne, J.C., Williams, M.R., 2013b. The importance of getting the numbers right: quantifying the rapid and substantial decline of an abundant marsupial, Bettongia penicillata. Wild. Res. (in press).
- Wendte J.M., Gibson A.K., Grigg M.E. Population genetics of Toxoplasma gondii: new perspectives from parasite genotypes in wildlife. Vet. Parasitol. 2011;182:96–111. doi: 10.1016/j.vetpar.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2011. Informal Consultation on Chagas Disease in the Western Pacific. Nagasaki, Japan, 29–30 June 2011. World Health Organization, Regional Office for the Western Pacific, Manila, Philippines.
- Wood J.L.N., Leach M., Waldman L., MacGregor H., Fooks A.R., Jones K.E., Restif O., Dechmann D., Hayman D.T.S., Baker K.S., Peel A.J., Kamins A.O., Fahr J., Ntiamoa-Baidu Y., Suu-Ire R., Breiman R.F., Epstein J.H., Field H.E., Cunningham A.A. A framework for the study of zoonotic disease emergence and its drivers: spillover of bat pathogens as a case study. Philos. Trans. R. Soc. Lond B. 2012;367:2881–2892. doi: 10.1098/rstb.2012.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt K.B., Campos P.F., Gilbert M.T.P., Kolokotronis S.-O., Hynes W.H., DeSalle R., Daszak P., MacPhee R.D.E., Greenwood A.D. Historical mammal extinction on Christmas Island (Indian Ocean) correlates with introduced infectious disease. PLoS One. 2008;3:1–9. doi: 10.1371/journal.pone.0003602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer I.A., Fee S.A., Spratt-Davison S., Hunter S.J., Boughtflower V.D., Morgan C.P., Hunt K.R., Smith G.C., Abernethy D., Howell M., Taylor M.A. Report of Trichinella spiralis in a red fox (Vulpes vulpes) in Northern Ireland. Vet. Parasitol. 2009;159:300–303. doi: 10.1016/j.vetpar.2008.10.066. [DOI] [PubMed] [Google Scholar]
- Zingales B., Miles M.A., Campbell D.A., Tibayrenc M., Macedo A.M., Teixeira M.M.G., Schijman A.G., Llewellyn M.S., Lages-Silva E., Machado C.R., Andrade S.G., Sturm N.R. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect. Genet. Evol. 2012;12:240–253. doi: 10.1016/j.meegid.2011.12.009. [DOI] [PubMed] [Google Scholar]