Highlights
-
•
mcr-1-positive ETEC and STEC strains persisted up to four weeks after inoculation.
-
•
O149-F4 ETEC inoculation resulted in hyperthermia and moderate diarrhea.
-
•
Fewer positive samples were obtained from pigs with the F4 resistant genotype.
-
•
An mcr-1 gene transfer to other commensal isolates was observed for O139-F18 STEC.
Keywords: Escherichia coli, Pig, mcr-1, Experimental model, Post-weaning diarrhea
Abstract
Colistin-resistant Escherichia coli are isolated from pigs suffering from post-weaning diarrhea (PWD). This study was designed to develop an experimental model of PWD using mcr-1-carrying shiga toxin-producing E. coli (STEC) or enterotoxigenic E. coli (ETEC), for the future evaluation of control measures. Three groups of eight piglets, kept in high biosecurity units, were orally inoculated with mcr-1-positive STEC or ETEC, and one unchallenged group was used as a control. Clinical signs were recorded. Regularly-collected fecal samples and samples obtained from the digestive tract of animals sacrificed one month after inoculation were cultured in selective media and isolates were characterized. Blood samples were used to genotype the polymorphisms of the pigs’ intestinal receptors for F4 and F18 E. coli adhesins.
Diarrhea was more frequent and more fecal samples contained the inoculated strain in the group inoculated with the O149-F4 ETEC strain than with the O141-F18 or O139-F18 STEC strains. However, fewer positive samples were obtained from the two pigs with the F4 resistant genotype. The three inoculated strains could be re-isolated up to the end of the experiment. Excretion peaked on the first week after inoculation with the O149-F4 ETEC strain, and later for the other two. An mcr-1 gene transfer to other commensal isolates was observed only for O139-F18 STEC, while the loss of mcr-1 from the inoculated strain occurred in all groups. The O149-F4 ETEC challenge may be used to evaluate alternative solutions to combat PWD caused by colistin-resistant E. coli in pigs.
1. Introduction
Post-weaning diarrhea (PWD) and edema disease are common pathological conditions affecting piglets (Rhouma et al., 2017a). Various Escherichia coli strains cause these diseases. Indeed, different serogroups (O138, O139, O141, O149, O157…), adhesive factors (F4 (K88), F8, AIDA, Eae…), and other virulence factors (enterotoxins LT, STa, STb, EAST…) have been reported in these strains (Fairbrother and Gyles, 2012). The control of these infections must be based primarily on optimizing breeding management of the weaning piglets so as to avoid, insofar as possible, stressful conditions as they are separated from the sow, transported, mixed, placed in a new environment and provided with new feed. Prophylactic measures may also include vaccination of the sow (Rhouma et al., 2017a). However, these measures may be ineffective in preventing digestive troubles, and in the past, orally administered antimicrobials were almost systematically used to control colibacillosis. As a result, E. coli strains have developed antimicrobial resistance, particularly to the most frequently administered molecules. Of note, resistance to colistin (CST) had been detected at relatively low levels for years in commensal isolates, but prevalence was sometimes high for pathogenic strains (Kempf et al., 2016), and after a publication on the emerging mcr-1 gene in China (Liu et al., 2016), it soon appeared that this gene was also present in many other countries, particularly in pig E. coli strains (Kempf et al., 2016). As these strains are frequently also resistant to many other antimicrobials (Delannoy et al., 2017), it became necessary to develop and evaluate novel control products or measures. To our knowledge, experimental models of colibacillosis in piglets have been developed with inoculation of CST-susceptible E. coli (Madec et al., 2000; Rhouma et al., 2016, 2017b) but not with CST-resistant strains. However we believe that such models are needed to evaluate the impact or the efficacy of antimicrobials, including colistin, and alternative strategies on infections caused by strains with resistance or reduced susceptibilities to a number of molecules. Furthermore, it has been shown that acquisition and expression of the mcr-1 gene in a E. coli cell may result in gross morphological changes, including cell membrane impairment, and reduced fitness and attenuated virulence in a Galleria mellonella model. Moreover the mcr-1 mediated LPS modification reduces the stimulation of macrophages, and a lower production of IL-6 and TNF was induced with LPS extracted from mcr-1 strains compared to control LPS (Yang et al., 2017). Thus because of these potential differences of susceptibility and virulence of mcr-1 strains, we decided to develop an experimental model of colibacillosis in weaned piglets using multi-drug-resistant E. coli strains isolated from clinical cases and carrying the mcr-1 gene.
2. Material and methods
2.1. Preparation of strains, media and inocula and whole genome sequencing
Three E. coli strains (12–246, 12–269 and 13–220) from our previously characterized collection of mcr-1-positive E. coli isolates from diseased pigs were selected on the basis of their resistances, serogroups and virulence genes as detected by high-throughput real-time PCR microarray technology (Delannoy et al., 2017). The intent was to choose strains representative of CST-resistant isolates obtained from piglets with PWD. All three strains were hemolytic. Strains 12–246, 12–269, and 13–220 belonged to phylogenetic groups (PGG) A, A, and E, and had markers O141-F18, O149-F4, and O139-F18 respectively. All three strains were resistant to colistin, sulfamethoxazole, trimethoprim, tetracycline, and ampicillin. Strains 12–246 and 12–269 were also resistant to gentamicin and chloramphenicol.
A rifampicin-resistant mutant (12–246 M, 12–269 M, and 13–220 M) was prepared from each strain by culturing a large amount of inoculum on rifampicin-supplemented media (250 mg/L). DNA from the three mutants was prepared using QIAmp DNA mini kits (Qiagen, Courtaboeuf, France) according to the manufacturer’s instructions. Whole genome sequencing was performed with the ion proton system (Ion Torrent™, Thermofisher, Villebon sur Yvette, France).
We cleaned reads with Trimmomatic (Bolger et al., 2014), then used BWA-MEM (Li, 2013) to align them with the E. coli E455 strain (NCBI Reference Sequence NZ_JEND02000001.1) to obtain an estimated coverage depth of 80. We next performed SPAdes assemblies on cleaned reads (Bankevich et al., 2012) and MIRA assemblies (Chevreux et al., 1999) on related raw reads. Contigs were identified using Megablast on a local nt database (Chen et al., 2015), and those corresponding to “Uncultured soil fungus” were deleted.
Sequences were analyzed using a web tool (https://cge.cbs.dtu.dk/services) to detect antimicrobial resistance genes and verotoxin virulence genes, and to determine the ST of each strain (Thomsen et al., 2016). Other virulence genes, including paa, sfpC, cnf2, AIDA-I, ecf1-1, stcE, and eibG (Tseng et al., 2014), sitA-D, iucA-D, iutA, hlyF, ompT, etsA-C, iss, iroB-E, iroN, cvaA-C, cvi, tsh, and eitA-D (Touzain et al., 2018), terE, ureD, and esc1763 (AE005174.2), hlyA (FM180012.1), orfA and orfB (GU810159), were sought using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
To prepare the inocula, each mutant was cultured in Mueller Hinton (MH) broth containing colistin (2 mg/L) for 18 h at 37 °C, under gentle agitation. Each bacterial pellet obtained after centrifugation (5180 g, 5 min) was re-suspended in peptone buffered solution in order to obtain a titer of 107 colony-forming units (CFU)/mL.
2.2. Experimental design
The experiment was performed in accordance with French animal welfare regulations and the protocol was approved by the ANSES/ENVA/UPEC ethical committee (ComEth authorization 17-059 (APAFiS no. 2017082408183121)). The experiment was conducted in the ANSES Ploufragan animal facilities. Strict biosecurity measures were implemented to avoid contamination of the pigs, including the use of an air filtration system and airlocks for each unit, unit-specific clothes, and compulsory showering after visiting the pigs. The trials were conducted with 29 specific-pathogen-free (SPF) Large White piglets. The SPF piglets are naturally born from hysterectomy-derived sows controlled for presence of classical and african swine fever, Aujesky’s disease, foot-and-mouth disease, H1N1/H3N2 swine influenza, transmissible gastro- enteritis/porcine respiratory coronavirus, porcine parvovirus, reproductive and respiratory syndrome virus, porcine circovirus type 2, Border disease, Mycoplasma hyopneumoniae, Pasteurella multocida, Bordetella bronchiseptica, Actinobacillus pleuropneumoniae, Haemophilus parasuis, Streptococcus suis Type 2, Salmonella, Lawsonia intracellularis, Brachyspira hyodysenteriae, Yersinia, Campylobacter, Listeria monocytogenes, Balantidium coli and Trichomonas. The piglets are left with the sow until weaning and bred in a pathogen-free environment.
The six-week-old piglets were randomized before the experiment. The animals did not receive any antibiotic treatment prior to the trial and were given the same non-supplemented feed. Each room contained two pens (Table 1 ). On Day 0, five piglets in animal room E1 (group 1, five pigs) were orally inoculated with 10 mL of sterile broth. On the same day, eight piglets per room (four piglets per pen) in animal rooms E2, E3, and E4, (respectively named groups G2, G3, and G4, eight pigs each) were orally inoculated with 10 mL of the inocula prepared with strains 12–246 M, 12–269 M, and 13–220 M respectively.
Table 1.
Arrangement of pigs inoculated with the different studied strains.
| Room | Groups- inoculation | Pen D | Pen V |
|---|---|---|---|
| E1 | G1- sterile buffer | 3 control pigs (1 F4-R)a |
2 control pigs (0 F4-R) |
| E2 | G2- E. coli 12-246M | 4 inoculated pigs (0 F4-R) |
4 inoculated pigs (2 F4-R) |
| E3 | G3- E. coli 12-269M | 4 inoculated pigs (1 F4-R) |
4 inoculated pigs (1 F4-R) |
| E4 | G4- E. coli 13-220M | 4 inoculated pigs (0 F4-R) |
4 inoculated pigs (0 F4-R) |
All pigs were susceptible to E. coli F18.
Number of pigs resistant to E. coli F4.
The weight of each animal was recorded once a week. During the week, daily clinical examinations consisted in looking for general clinical signs and taking rectal temperatures. Individual fecal samples were collected from pigs on Days -4, 1, 2, 3, 4, 5, 7, 9, 11, 14, 17, 21, 24, and 28 (groups G2, G3, and G4), and on Days -4, 7, 14, 21 and 28 (group G1). The pigs were sacrificed on Day 35 (group G2), Day 36 (group G3) or Day 37 (group G4). Three pigs from the G1 group were also sacrificed on Day 35, the others being kept as controls for another assay and necropsied on Day 50 following euthanasia. The sacrificed animals were necropsied and samples of the duodenum, jejunum, ileum, cecum, colon, and rectum were collected. The samples collected post-mortem contained both fecal material and scraped mucosa.
2.3. Analysis of fecal and organ samples
For culture analysis, all the samples were diluted 1/10 in peptone buffered solution containing 20% glycerol. The 1/10 diluted aliquots were stored at -70 °C. Before freezing (fecal samples) or after thawing (organ samples), decimal dilutions were prepared. One hundred microliters of decimal dilutions were inoculated using EasySpiral® Dilute (Interscience, Saint-Nom-la-Bretèche, France) onto MacConkey media supplemented with rifampicin (250 mg/L) and incubated overnight at 37 °C. Moreover, 200 μL of the 1/10 thawed dilutions was enriched overnight in 1.8 mL of rifampicin-supplemented LB broth (250 mg/L) before plating on rifampicin-supplemented MacConkey plates. The limit of detection was 100 CFU without enrichment and 50 CFU/g after enrichment. Whenever possible, two colonies (from samples positive only after enrichment) or three colonies (from samples positive without enrichment) per sample were stored. PCR was used to confirm that they belonged to the E. coli species (Bej et al., 1991), to detect the presence of the mcr-1 gene (Liu et al., 2016), and to determine their phylogenetic group (Clermont et al., 2013). For isolates which did not exhibit the expected results (presence of mcr-1 and phylogenetic group of the inoculated strain), ERIC-PCR (Rivera et al., 1995) and PFGE profiles (Ribot et al., 2006) after digestion with XbaI were studied. The susceptibility of some of these isolates was compared to the susceptibility of the inoculated strains by determining the minimum inhibitory concentrations (MIC) using a broth microdilution method on EUVSEC plates (Sensititre, ThermoFisher Scientific, Dardilly, France).
In addition, fecal samples collected on Days 2, 9 and 17 were inoculated, after thawing, onto the Chromagar Orientation™agar medium (i2a, Montpellier, France) supplemented with colistin (8 mg/L) and vancomycin (8 mg/L) (CAO-CV) as previously described (Mourand et al., 2018) to detect colistin-resistant rifampicin-susceptible E. coli resulting from transfer of the mcr-1 gene to commensal E. coli. When E. coli colonies were observed, two colonies per sample were stored and further analyzed. Their identity and the presence of the mcr-1 gene were checked by PCR, and their resistance to rifampicin was tested by culture on rifampicin-supplemented MH media (250 mg/L). Moreover, 200 μL of the 1/10 dilutions was enriched overnight in 1.8 mL of colistin-supplemented LB broth (2 mg/L) before plating onto rifampicin-supplemented MacConkey plates (limit of detection: 50 CFU/g). The colonies were identified and analyzed for the presence of the mcr-1 gene as previously described.
For molecular analysis, thawed fecal and organ samples were tested with a slightly modified version of the protocol described by Dona et al. (Dona et al., 2017). Briefly, 200 μL of the 1/10 diluted thawed fecal suspension was used to inoculate 1.8 mL of Luria Bertani (LB) broth supplemented with rifampicin (250 mg/L). After overnight culture at 37 °C under agitation, the broths were centrifuged at 5180 g for ten minutes and a cellular lysate was prepared from the pellet. E. coli DNA and the mcr-1 gene were then detected by PCR in the lysate. PCR was performed on lysates obtained from enrichment in the colistin-supplemented broths (2 mg/L), like for samples collected on Days 2, 9, and 17.
2.4. Study of resistance polymorphism to adhesion factors in pigs
Blood was obtained from each pig just before euthanasia. These samples were used to genotype the pigs for the different polymorphisms linked to the intestinal receptors for F4 and F18 E. coli adhesins.
The porcine mucin 4 (muc4) gene has been mapped on the q1 region of the pig chromosome 13, significantly associated with in vitro E. coli F4ac adhesion to enterocytes, and MUC13 is the most likely responsible gene governing susceptibility towards enterotoxigenic E. coli (ETEC) F4ac diarrhea (Ren et al., 2012). The polymorphism of muc4 was analyzed based on the test described by Rasschaert et al. (Rasschaert et al., 2007) to determine the F4ac/ab receptor status. Briefly, a fragment of the mucin 4 gene was amplified using Hemo Klen Taq polymerase (NEB, Evry, France) and digested with XbaI. The 367 bp amplified muc4 PCR fragment of the resistant allele is indigestible, unlike the susceptible allele. MUC13 was genotyped by PCR to detect the presence of amplicons of 151 bp (MUC13 A allele) and 83 pb (MUC13B allele) as described by Ren et al (Ren et al., 2012). Susceptible animals usually carry at least one MUC13B allele.
The E. coli F18 receptor locus is closely linked to the fucosyltransferase gene, and we used the test developed by Meijerink et al. (Meijerink et al., 1997) in which amplification followed by restriction with CfoI enables discrimination between the different alleles, based on the number of amplified fragments: two fragments for homozygous A/A, three for homozygous G/G and four for heterozygous. The resistance is associated with allele A (Meijerink et al., 1997).
2.5. Statistical analysis
The Kruskall-Wallis non-parametric test was used to compare weight gain. The distributions of pigs or isolates were analyzed using the Chi2-test or the Fisher exact test.
2.6. Accession numbers
This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession QZVZ00000000, QZWA00000000 and QZVY00000000 (respectively 12-246M, 12-269M and 13-220M).
3. Results
3.1. Strain sequences
Sequencing enabled us to detect the resistance genes present in the isolates. Thus, beside the previously described mcr-1 gene and the mutations in genes possibly involved in resistance to polymyxins (Delannoy et al., 2017), the strains harbored the genes coding for their other observed resistances: sulfonamides (sul1, sul2 or sul3), trimethoprim (dfrA1), tetracycline (tet(A)), chloramphenicol (catA1, cmlA1), ampicillin (bla TEM1a or bla TEM1b) or aminoglycosides (aac(3)-IIa, aac(3)-IVa, aadA1, aadA3, aadA24, aph(3′)-Ia, aph(3′)-1c, aph(4)-Ia, strA or strB).
The virulence genes are listed in Table 2 . The genes previously detected by microarray (Delannoy et al., 2017) were confirmed to be present. The two F18 stx2-positive strains belonged to ST 1 and 10, whereas the O149 F4 strain containing genes coding for the LT, STa and STb toxins belonged to ST-100.
Table 2.
Characteristics of the studied strains.
| Strains | PGG | O and F antigens and ST | Resistances | Resistance determinants | Virulence or marker genes |
|---|---|---|---|---|---|
| 12-246M | A | O141 F18 ST-10 |
CST SMX TMP TET CHL AMP GEN |
mcr-1 (deletion in pmrB gene)a sul1, sul2 dfrA1 tetA catA1 blaTEM-1a aph3’1c, aac3IIa, aadA1, strA, strB |
fedA, fedF, gad, hlyA, iha, iss, orfA, orfB, paa, stx2, terE, ureD |
| 12-269M | A | O149 F4 ST-100 |
CST SMX TMP TET CHL AMP GEN |
mcr-1, (mutations in PhoQ and PhoP)a sul1, sul2, sul3 dfrA1 tetA cat, cmlA1 blaTEM-1a aac(3)-IIa, aac(3)-IVa, aadA1, aadA3, aph(3')-Ia, aph(4)-Ia, strA, strB |
astA, capU, esc1763 (317/408 nt), gad, hlyA, iha, K88ab, ltcA, sta1, stb, terE, ureD |
| 13-220M | E | O139 F18 ST-1 |
CST SMX TMP TET AMP |
mcr-1, (mutations in PmrB and PhoQ)a sul1, sul2, sul3 dfrA1 tetA blaTEM-1b aadA1, aadA24, strA, strB |
AIDA (94.5% identity), eilA, esc1763 (317/408 nt), fedA, fedF, gad, hlyA, lpfA, orfA, orfB, stx2, terE |
PGG: phylogenetic group (Clermont et al., 2013); CST: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline; CHL: chloramphenicol; AMP: ampicillin; GEN: gentamicin; AA: amino acid.
as determined previously, impact on colistin resistance unknown (Delannoy et al., 2017).
3.2. Inoculum titers
The inoculum titers were 1.06 × 107 CFU/mL, 7.68 × 106 CFU/mL, and 9.28 × 106 CFU/mL for 12–246 M, 12–269 M and 13–220 M respectively.
3.3. Analysis of pig genotypes
Only 27 pigs could be tested as no blood was obtained from the two control pigs sacrificed on Day 50. As expected, muc4 and muc13 gene results were in agreement, and only five animals were found to be of the genotype resistant to E. coli F4ac/ab: one in group G1, two in G2, and two in G3 (Table 1). For the F18 receptor, ten pigs were heterozygous AG and the other 17 were homozygous GG. Thus all pigs were susceptible to E. coli F18.
3.4. Clinical signs, growth and lesions
All pigs of group G1 had normal rectal temperature during the assay. Moderate pyrexia (40.1 °C–41.0 °C) was observed during the three days after inoculation in 4, 3 and 1 pigs in groups G2 to G4 respectively.
No clinical signs were observed in group G1. In groups G2 to G4, moist, cow-dung appearance feces were observed in all pigs during the third week after inoculation. Moreover, in group G3, two pigs had diarrhea during the four days following inoculation.
The mean weight gains of groups G1 to G4 from Day 0 to Day 4 were respectively 2.9 ± 0.3, 3.0 ± 0.4, 2.1 ± 0.9, and 2.0 ± 0.6 kg (P = 0.005). The Day 0-Day 4 weight gain of group G4, but not those of groups G2 and G3, was significantly different from the weight gain of the control group (P = 0.01). The weight gains from Day 0 to Day 32 of groups G1 to G4 were respectively 29.5 ± 3.1, 26.6 ± 3.4, 25.1 ± 3.6, and 27.2 ± 3.5 kg and did not differ significantly (P > 0.05).
No lesions could be observed post-mortem.
3.5. Analysis of fecal samples
3.5.1. Cultures on rifampicin-supplemented media
The results are presented in Table 3, Table 4 . All cultures from non-inoculated pigs or from samples collected before inoculation were negative on rifampicin-supplemented media. Some of these samples yielded E. coli colonies after enrichment, but none of the isolates tested had the mcr-1 gene. For cultures on rifampicin-supplemented media, with or without enrichment, and after mcr-1-PCR control of isolates, all inoculated pigs were observed to be positive at least once. In groups G2, G3, and G4, out of 12 sampling days after inoculation, the pigs were positive on 1 to 7 (mean 4.1), 3 to 11 (mean 6.9) and 1 to 8 (mean 4.5) different days respectively. The total positive samples were 33 out of 102, 55 out of 103, and 40 out of 104 tested samples in groups G2 to G4 respectively, the proportion in G3 being significantly higher than the other two groups (Chi-2 test, P < 0.05). In all groups, at least one pig was positive on the last sampling day, four weeks after inoculation. The maximum mean titers were observed on Day 11 (1.84) in group G2, on Days 4 and 5 (5.75 on each day) in group G3, and on Day 17 (1.73) for group G4.
Table 3.
Culture on rifampicin media (with or without enrichment in rifampicin-supplemented broth).
| Day (D) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group (strain) | D1 | D2 | D3 | D4 | D5 | D7 | D9 | D11 | D14 | D17 | D21 | D24 | D28 | Total | mcr+ isolates |
| G2 (12-246 M) | 3/8a | 3/8 | 1/6 | 0/8 | 2/8 | 3/8 | 7/8 | 7/8 | 3/8 | 1/8 | 1/8 | 1/8 | 1/8 | 33/102a | 52/53c |
| 0.96b | 0.62 | 0.48 | 0.00 | 0.65 | 1.07 | 1.42 | 1.84 | 1.09 | 0.36 | 0.21 | 0.21 | 0.25 | |||
| G3 (12-269 M) | 4/8 | 6/8 | 6/7 | 7/8 | 8/8 | 8/8 | 2/8 | 1/8 | 3/8 | 2/8 | 2/8 | 3/8 | 3/8 | 55/103 | 98/107 |
| 1.55 | 4.89 | 4.41 | 5.75 | 5.75 | 4.16 | 0.84 | 0.37 | 0.89 | 0.46 | 0.54 | 0.61 | 0.97 | |||
| G4 (13-220 M) | 1/8 | 1/8 | 1/8 | 0/8 | 4/8 | 3/8 | 4/8 | 3/8 | 3/8 | 5/8 | 5/8 | 6/8 | 4/8 | 40/104 | 68/69 |
| 0.37 | 0.21 | 0.25 | 0.00 | 1.28 | 0.90 | 0.50 | 0.88 | 0.85 | 1.73 | 1.06 | 1.71 | 1.02 | |||
aNumber of samples containing rifampicin-resistant mcr-1-positive E. coli/number of tested pigs; bMean titer obtained on rifampicin- supplemented MacConkey media; cNumber of mcr-1-positive E. coli/number of tested E. coli (obtained without enrichment).
All pigs were negative before inoculation and all pigs from the G1 non-inoculated group were negative on all sampling days.
Table 4.
Isolation from individual pigs.
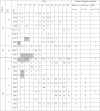 |
A: sample containing rifampicin-resistant, mcr-1-positive E. coli of the expected phylogenetic group, obtained directly.
B: sample containing rifampicin-resistant, mcr-1-positive E. coli of the expected phylogenetic group, obtained after enrichment.
C: sample containing rifampicin-resistant, mcr-1-positive E. coli of an unexpected phylogenetic group and PFGE profile, obtained directly.
D: sample containing rifampicin-resistant, mcr-1-positive E. coli of an unexpected phylogenetic group and PFGE profile, obtained after enrichment (possibly transconjugant).
E: sample containing rifampicin-resistant, mcr-1-negative E. coli of the expected phylogenetic group and PFGE profile, obtained directly (possibly inoculated strain having lost the mcr-1 gene).
F: sample containing rifampicin-resistant, mcr-1-positive E. coli detected on CAO-CV medium.
NS: no sample.
Grey cells: clinical signs (diarrhea or temperature >40.0 °C).
*: pig resistant to E. coli F4.
3.5.2. Analysis of isolates obtained from fecal samples directly or after enrichment on rifampicin-supplemented media
Most isolates obtained directly on rifampicin-supplemented media (218/229, 95%) or after enrichment in rifampicin-supplemented media (70/83, 84%) harbored the mcr-1 gene and showed the expected characteristics of the inoculated strains. Considering only isolates obtained without enrichment, for group G2, 52 out of 53 isolates shared the characteristics of inoculated strain 12–246 M, and one isolate obtained on Day 14, without enrichment, belonged to PGG A, had the ERIC and PFGE profiles and the antimicrobial susceptibility of 12–246 M, including resistance to colistin, although it was negative for the mcr-1 gene.
In group G3, 98 out of 107 isolates obtained without enrichment shared the characteristics of inoculated strain 12–269 M, but nine isolates lacked the mcr-1 gene. Six were fully analyzed and were found to belong to PGG A, and have the ERIC and PFGE profiles of the 12–269 M isolate. They were resistant to tetracycline and gentamicin but, contrary to 12–269 M, susceptible to sulfamethoxazole, trimethoprim, colistin and ampicillin. The mcr-1-negative isolates had been obtained on Day 3, 4, 5, 9 and 28 from three pigs bred in the two pens of the animal room.
In group G4, 62 out of 69 isolates obtained without enrichment had the expected characteristics (presence of the mcr-1 gene and phylogenetic group E). Six had the mcr-1 gene but belonged to PGG A, and their ERIC and PFGE profiles were identical to each other but different to that of 13–220 M. They were obtained on Day 2 and Day 5 from three pigs placed in the two pens of the animal room. One isolate obtained on Day 24 was mcr-1-negative. Its PFGE profile was identical to the inoculated strain and it shared the susceptibility of 13–220 M except for colistin resistance.
3.5.3. Culture on CAO-CV media
The results obtained for samples collected on Days 2, 9 and 17 and inoculated on CAO-CV agar plates showed that respectively 15, 13 and 19 samples out of 24 contained E. coli. However, after analysis of the isolates, only 16 out of 80 contained the mcr-1 gene and all 16 were rifampicin-resistant. Thus, for group G2, only two pigs were positive on Day 9; for G3, five pigs were positive on Day 2, and another one on Day 9; and for G4, three pigs were positive on Day 17. Positive pigs were detected in the two pens for groups G3 and G4 (Table 4). There was no difference between the proportion of positive samples in the inoculated groups (Fisher's Exact Test, P > 0.05).
3.5.4. mcr-1 PCR on enrichment broths in rifampicin-supplemented LB
All the samples collected before inoculation were negative. For group G2, nine out of 102 samples were positive; they were obtained on Days 1, 7, 11, and 24 from seven different pigs. For group G3, 14 samples out of 103 obtained on Days 4, 5, 7, 21, 24, and 28 were positive; seven pigs were positive. For the E4 group, 12 out of 104 samples were positive; they were obtained on Days 5, 11, 21, 24, and 28 from six different pigs. There was no difference between the proportion of positive samples in the inoculated groups (Chi 2 test, P > 0.05).
3.5.5. mcr-1 PCR on enrichment broths in colistin-supplemented LB
Only samples collected on Days 2, 9, and 17 were analyzed. All the results were negative except three fecal samples collected on Day 17 from two pigs in group G3 and one in G4. For the same Days 2, 9 and 17, culture on rifampicin media detected 31 positive samples out of 72 samples from inoculated groups and was thus significantly more sensitive (P < 0.001).
3.6. Analysis of organs
The results obtained on rifampicin-supplemented MacConkey plates after enrichment in rifampicin-supplemented broth, are given in Table 5 .
Table 5.
Number of samples positive for rifampicin-resistant mcr-1-positive E. coli and number of mcr-1-positive lysates.
| Group (strain) | Jejunum | Ileum | Colon | Cecum | Rectum | Total (organs) | Total (pigs) | |
|---|---|---|---|---|---|---|---|---|
| G2 (12-246 M) | Culture PCR |
0/8a 1/8 |
5/8 5/8 |
3/8 1/8 |
4/8 4/8 |
1/8 1/8 |
13/48 12/48 |
6/8 6/8 |
| G3 (12-269 M) | Culture PCR |
0/8 0/8 |
1/8 1/8 |
1/8 0/8 |
0/8 0/8 |
1/8 1/8 |
3/48 2/48 |
3/8 2/8 |
| G4 (13-220 M) | Culture PCR |
0/8 0/8 |
4/8 7/8 |
5/8 4/8 |
4/8 4/8 |
4/8 0/8 |
17/48 15/48 |
6/8 7/8 |
All samples from non-inoculated pigs were negative.
Number of positive samples/number of tested samples.
All the samples collected from the non-inoculated pigs and all the duodenum samples were negative. The proportion of positive samples in group G3 (3/48) was significantly lower than the proportion in G2 (13/48) and G4 (17/48) (Fisher exact test, P < 0.01). Besides the isolates with the characteristics of the inoculated strains, a few rifampicin-resistant mcr-1-negative isolates belonging to the phylogenetic group of the respective inoculated strains were obtained in the jejunum or cecum from three different pigs in group G2; in the ileum or cecum from three different pigs in group G3; and in the ileum, colon, rectum or cecum from three different pigs in group G4.
4. Discussion – conclusion
The three strains proposed as an experimental model are relevant because they have been reported in literature and seem to be frequently involved in pig diarrhea. Indeed sequencing confirmed that they were Shiga toxin-producing (STEC) (12–246 M and 13–220 M) or ETEC (12–269 M). O141:F18 12–246 M is of type ST-10, which has already been isolated from many different sources, such as humans, animals (including swine in Italy and China), food (including pork in the USA) and the environment (water in France) (http://enterobase.warwick.ac.uk/species/ecoli/search_strains). An ST-10 mcr-1 and bla NDM-5 E. coli of human origin was also characterized by Zhang et al. (Zhang et al., 2017) and several mcr-1 ST-10 E. coli of healthy swine origin have also been reported in China (Wang et al., 2016). The O149:F4 12–269 M E. coli belongs to ST-100, like several pig or bovine pathogenic isolates of serogroups O149:H10:F4ac reported in different European, American or Asian countries, or an O149 poultry APEC isolated in Germany. The O139:F18 13–220 M strain belongs to ST-1, like O139:F18 isolates obtained from pig diarrhea in Hungary in 1988 (http://enterobase.warwick.ac.uk/species/ecoli/search_strains ?query=st_search). An analysis of the different contigs carrying mcr-1 did not reveal other resistance or virulence genes or notifiable genes.
Symptoms, including hyperthermia and diarrhea, were more severe in group G3. This group also had the highest excretion titers in the first week, and more fecal samples were found positive. However, at necropsy—more than one month after inoculation—the O149:F4+ inoculated strain was less frequently isolated than the other two inoculated strains, both of type F18+ fimbriae. This may be related to the fact that susceptibility to F18+ or F4+ E. coli may vary with the pig’s age. Verdonck et al. (Verdonck et al., 2002) previously observed a similar delay, with F4+ ETEC peak excretion 2 days post-infection (PI), but no excretion 7 days PI, in contrast to the peak excretion of F18+ VTEC infection observed between 3 and 5 days PI, and excretion persisting up to 9 days PI. Interestingly, in group G3, the only two pigs found to be genetically resistant to E. coli F4 had no diarrhea and gave only a total of eight positive fecal samples out of 26, whereas 47 out of 77 samples were positive for the six pigs which were genetically susceptible to E. coli F4 (P = 0.007); their mean log10 titer was 0.6 compared to a mean of 3.0 (P = 0.0004) for the other pigs in group G3. Although all the pigs were genetically susceptible to E. coli F18, the symptoms remained relatively moderate in groups G2 and G4. The experimental model could be improved by using higher inocula doses, intra-gastric inoculation, withdrawing feed before inoculation, cold stress, pretreatment with antibiotics, co-inoculation of viruses, or inoculating piglets when younger (Madec et al., 2000), or using pigs known to be genetically susceptible to the inoculated strains. It is acknowledged that post-weaning digestive disorders are generally encountered in inadequate breeding conditions, contrary to the high-standard environment offered to our weaning piglets.
Using rifampicin-supplemented media, we could evaluate the recovery of the inoculated strain, and search for the possible loss of the mcr-1 gene from the inoculated strains. The use of colistin-supplemented media was used to try to detect the transfer of the mcr-1 gene from the inoculated strains to commensal ones. Detection of the mcr-1 gene in colistin-enrichment broths was also performed but the sensitivity of this method was poor as previously observed in experiments with pigs inoculated with a commensal mcr-1-positive E. coli strain (Mourand et al., 2018), and needs further improvements. The three inoculated strains could be recovered from fecal and post-mortem samples up to four and five weeks respectively after inoculation. For group G4 only, a few isolates carrying the mcr-1 gene were also obtained, but their other characteristics (phylogenetic group, PFGE or ERIC-PCR profiles, and antimicrobial susceptibility) were different from those of the inoculated strain. As no mcr-1-containing E. coli was present in the non-inoculated animals, the uncharacteristic isolates are very probably commensal isolates having acquired the mcr-1 gene or plasmid from E. coli 13–220 M. Other analyses are necessary to determine the precise mechanisms of this transfer, which can occur through either conjugation or transposon mobilization. Interestingly, in vitro, the mcr-1 gene was shown to be on a conjugative plasmid in E. coli 13–220, but not in E. coli 12–246 or E. coli 12–269. The transconjugant obtained in vitro from E. coli 13–220 acquired resistance to colistin, tetracycline, sulfamethoxazole and trimethoprim. Different studies have shown that the in vitro acquisition of an mcr-1-containing plasmid does not lead to a considerable fitness cost in E. coli as measured by growth kinetics, cytotoxicity, and virulence in a Galleria mellonella model (Tietgen et al., 2018; Zhang et al., 2017), but a high level of mcr-1 expression compromises growth rate, fitness, and the membrane’s structural integrity while increasing cellular death (Yang et al., 2017). In our experiment, most isolates from samples collected from the E. coli 13–220 M-inoculated pigs shared the characteristics of this strain, suggesting that the commensal strains which had acquired the mcr-1 gene were perhaps not as competitive as E. coli 13–220 M.
In the three inoculated groups, few of the isolates obtained were resistant to rifampicin, shared the phylogenetic group and PFGE profile of the inoculated strain but lacked the mcr-1 gene. Wu et al. (2018) studied the stability of various mcr-1-harboring plasmids and showed that the IncHI2, IncI2, IncX4 and IncY plasmids were maintained after 14 days of passage in a colistin-free medium, but the IncFII plasmid was lost from the E. coli DH5alpha transformant after day 5. The in vivo loss of the mcr-1 gene has already been detected for other strains (Mourand et al., 2018). Interestingly, the loss of the mcr-1 gene was not accompanied by a loss of resistance to colistin for 12–246 M, suggesting that the previously described deletion in the PmrB protein, or other unnoticed mutations, probably played a role in colistin resistance. For E. coli 12–269 M, the isolates lacking mcr-1 were no longer resistant to colistin, trimethoprim, sulfamethoxazole, and ampicillin, suggesting that these resistance genes may be present on the same genetic element, but further analysis is needed to confirm this hypothesis, as the genes were present on contigs different from the mcr-1 contig. The loss of the colistin resistance consecutive to the absence of the mcr-1 gene also suggests that the previously detected PhoQ and PhoP mutations (Delannoy et al., 2017) have probably no impact on colistin resistance). Finally in group G4, the isolate lacking the mcr-1 gene had only lost colistin resistance.
Globally, comparison of the clinical signs and culture results obtained in the inoculated and control groups, shows that the O149-F4 ETEC challenge, resulting in more clinical signs and excretion, particularly in pigs with a F4 susceptible genotype, may be used to evaluate alternative solutions to combat PWD caused by colistin-susceptible or colistin-resistant E. coli in pigs.
Conflict of interest statement
The authors indicate that there are no conflicts of interest.
Funding
This work was supportedby grant ANR-15-CE21-0015-02 from the French National Research Agency, COMPARE, in the framework of the European Union’s Horizon 2020 program under grant agreement No. 643476, ANSES (the French Agency for Food, Environmental and Occupational Health & Safety), and the Côtes d’Armor General Council.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgments
The authors would like to sincerely thank Yann Bailly (ANSES Ploufragan) for technical assistance. The authors thank Labocea 22 for providing the three E. coli strains through RESAPATH network.
References
- Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S., Lesin V.M., Nikolenko S.I., Pham S., Prjibelski A.D., Pyshkin A.V., Sirotkin A.V., Vyahhi N., Tesler G., Alekseyev M.A., Pevzner P.A. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bej A.K., DiCesare J.L., Haff L., Atlas R.M. Detection of Escherichia coli and Shigella spp. in water by using the polymerase chain reaction and gene probes for uid. Appl. Environ. Microbiol. 1991;57:1013–1017. doi: 10.1128/aem.57.4.1013-1017.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Ye W., Zhang Y., Xu Y. High speed BLASTN: an accelerated MegaBLAST search tool. Nucleic Acids Res. 2015;43:7762–7768. doi: 10.1093/nar/gkv784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevreux B., Wetter T., Suhai S. Proceedings of the German Conference on Bioinformatics (GCB) 99. Presented at the German Conference on Bioinformatics (GCB) 1999. Genome sequence assembly using trace signals and additional sequence information; pp. 45–56. [Google Scholar]
- Clermont O., Christenson J.K., Denamur E., Gordon D.M. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Reports. 2013;5:58–65. doi: 10.1111/1758-2229.12019. [DOI] [PubMed] [Google Scholar]
- Delannoy S., Le Devendec L., Jouy E., Fach P., Drider D., Kempf I. Characterization of colistin-resistant Escherichia coli isolated from diseased pigs in France. Front. Microbiol. 2017;8:2278. doi: 10.3389/fmicb.2017.02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dona V., Bernasconi O.J., Kasraian S., Tinguely R., Endimiani A. A SYBR(R) Green-based real-time PCR method for improved detection of mcr-1-mediated colistin resistance in human stool samples. J. Glob. Antimicrob. Resist. 2017;9:57–60. doi: 10.1016/j.jgar.2017.01.007. [DOI] [PubMed] [Google Scholar]
- Fairbrother J.M., Gyles C.L. Colibacillosis. In: Zimmerman J.J., Karriker L.A., Ramirez A., Schwartz K.J., Stevenson G.W., editors. Diseases of Swine. 10th edit. Wiley-Blackwell; 2012. [Google Scholar]
- Kempf I., Jouy E., Chauvin C. Colistin use and colistin resistance in bacteria from animals. Int. J. Antimicrob. Agents. 2016;48:598–606. doi: 10.1016/j.ijantimicag.2016.09.016. [DOI] [PubMed] [Google Scholar]
- Li H. 2013. Aligning Sequence Reads, Clone Sequences and Assembly Contigs with BWA-MEM ».https://arxiv.org/abs/1303.3997 arXiv preprint arXiv:1303.3997. [Google Scholar]
- Liu Y.Y., Wang Y., Walsh T.R., Yi L.X., Zhang R., Spencer J., Doi Y., Tian G., Dong B., Huang X., Yu L.F., Gu D., Ren H., Chen X., Lv L., He D., Zhou H., Liang Z., Liu J.H., Shen J. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- Madec F., Bridoux N., Bounaix S., Cariolet R., Duval-Iflah Y., Hampson D.J., Jestin A. Experimental models of porcine post-weaning colibacillosis and their relationship to post-weaning diarrhoea and digestive disorders as encountered in the field. Vet. Microbiol. 2000;72:295–310. doi: 10.1016/s0378-1135(99)00202-3. [DOI] [PubMed] [Google Scholar]
- Meijerink E., Fries R., Vogeli P., Masabanda J., Wigger G., Stricker C., Neuenschwander S., Bertschinger H.U., Stranzinger G. Two alpha(1,2) fucosyltransferase genes on porcine chromosome 6q11 are closely linked to the blood group inhibitor (S) and Escherichia coli F18 receptor (ECF18R) loci. Mamm. Genome. 1997;8:736–741. doi: 10.1007/s003359900556. [DOI] [PubMed] [Google Scholar]
- Mourand G., Jouy E., Chauvin C., Le Devendec L., Paboeuf F., Kempf I. Dissemination of the mcr-1 colistin resistance gene among pigs: an experimental study. Vet. Microbiol. 2018;221:122–128. doi: 10.1016/j.vetmic.2018.06.006. [DOI] [PubMed] [Google Scholar]
- Rasschaert K., Verdonck F., Goddeeris B.M., Duchateau L., Cox E. Screening of pigs resistant to F4 enterotoxigenic Escherichia coli (ETEC) infection. Vet. Microbiol. 2007;123:249–253. doi: 10.1016/j.vetmic.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Ren J., Yan X., Ai H., Zhang Z., Huang X., Ouyang J., Yang M., Yang H., Han P., Zeng W., Chen Y., Guo Y., Xiao S., Ding N., Huang L. Susceptibility towards enterotoxigenic Escherichia coli F4ac diarrhea is governed by the MUC13 gene in pigs. PLoS One. 2012;7:e44573. doi: 10.1371/journal.pone.0044573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhouma M., Beaudry F., Thériault W., Bergeron N., Beauchamp G., Laurent-Lewandowski S., Fairbrother J.M., Letellier A. In vivo therapeutic efficacy and pharmacokinetics of colistin sulfate in an experimental model of Enterotoxigenic Escherichia coli infection in weaned pigs. Vet. Res. 2016;47:58. doi: 10.1186/s13567-016-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhouma M., Fairbrother J.M., Beaudry F., Letellier A. Post weaning diarrhea in pigs: risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017;59:31. doi: 10.1186/s13028-017-0299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhouma M., Fairbrother J.M., Thériault W., Beaudry F., Bergeron N., Laurent-Lewandowski S., Letellier A. The fecal presence of enterotoxin and F4 genes as an indicator of efficacy of treatment with colistin sulfate in pigs. BMC Microbiol. 2017;17:6. doi: 10.1186/s12866-016-0915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot E.M., Fair M.A., Gautom R., Cameron D.N., Hunter S.B., Swaminathan B., Barrett T.J. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne pathogens and disease. 2006;3:59–67. doi: 10.1089/fpd.2006.3.59. [DOI] [PubMed] [Google Scholar]
- Rivera I.G., Chowdhury M.A.R., Huq A., Jacobs D., Martins M.T., Colwell R.R. Enterobacterial repetitive intergenic consensus sequences and the PCR to generate fingerprints of genomic DNAs from vibrio cholerae O1, O139, and non- O1 strains. Appl. Environ. Microbiol. 1995;61:2898–2904. doi: 10.1128/aem.61.8.2898-2904.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M.C., Ahrenfeldt J., Cisneros J.L., Jurtz V., Larsen M.V., Hasman H., Aarestrup F.M., Lund O. A bacterial analysis platform: an integrated system for analysing bacterial whole genome sequencing data for clinical diagnostics and surveillance. PLoS One. 2016;11:e0157718. doi: 10.1371/journal.pone.0157718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietgen M., Semmler T., Riedel-Christ S., Kempf V.A.J., Molinaro A., Ewers C., Göttig S. Impact of the colistin resistance gene mcr-1 on bacterial fitness. Internat. J. Antimic. Agents. 2018;51:554–561. doi: 10.1016/j.ijantimicag.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Touzain F., Le Devendec L., de Boisseson C., Baron S., Jouy E., Perrin-Guyomard A., Blanchard Y., Kempf I. Characterization of plasmids harboring blaCTX-M and blaCMY genes in E. coli from French broilers. PLoS One. 2018;13:e0188768. doi: 10.1371/journal.pone.0188768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng M., Fratamico P.M., Bagi L., Delannoy S., Fach P., Manning S.D., Funk J.A. Diverse virulence gene content of Shiga toxin-producing Escherichia coli from finishing swine. Appl. Environ. Microbiol. 2014;80:6395–6402. doi: 10.1128/AEM.01761-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdonck F., Cox E., van Gog K., Van der Stede Y., Duchateau L., Deprez P., Goddeeris B.M. Different kinetic of antibody responses following infection of newly weaned pigs with an F4 enterotoxigenic Escherichia coli strain or an F18 verotoxigenic Escherichia coli strain. Vaccine. 2002;20:2995–3004. doi: 10.1016/s0264-410x(02)00220-7. [DOI] [PubMed] [Google Scholar]
- Wang Q., Li Z., Lin J., Wang X., Deng X., Feng Y. Complex dissemination of the diversified mcr-1-harbouring plasmids in Escherichia coli of different sequence types. Oncotarget. 2016;7:82112–82122. doi: 10.18632/oncotarget.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Li M., Spiller O.B., Andrey D.O., Hinchliffe P., Li H., MacLean C., Niumsup P., Powell L., Pritchard M., Papkou A., Shen Y., Portal E., Sands K., Spencer J., Tansawai U., Thomas D., Wang S., Wang Y., Shen J., Walsh T. Balancing mcr-1 expression and bacterial survival is a delicate equilibrium between essential cellular defence mechanisms. Nat. Commun. 2017;8:2054. doi: 10.1038/s41467-017-02149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liao K., Gao H., Wang Q., Wang X., Li H., Wang R., Wang H. Decreased fitness and virulence in ST10 Escherichia coli harboring blaNDM-5 and mcr-1 against a ST4981 strain with blaNDM-5. Front. Cell. Infect. Microbiol. 2017;7:242. doi: 10.3389/fcimb.2017.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]


